Abstract
The Epstein-Barr virus (EBV) can establish at least four different forms of latent infection. Previously, we have shown that the level of methylation of the EBV genome varies, depending on the form of latency. The methylation status of CpGs was analyzed by the bisulfite genomic sequencing technique in four different cell types representing different forms of latency. The dyad symmetry element of the origin of replication (oriP) region and the latent membrane protein 1 (LMP-1) regulatory sequence (LRS) were studied. The dyad symmetry element has four binding sites for EBNA-1. In a cell with type I latency, a region upstream of the dyad symmetry element was highly methylated, whereas the dyad symmetry element was unmethylated in the EBNA-1-binding region. The LRS was extensively methylated in the LMP-1-negative cell line Rael, in contrast to a LMP-1-expressing nasopharyngeal carcinoma tumor (NPC C15), which was almost completely unmethylated. The methylation pattern of LRS in type I and type III Burkitt lymphoma cells of similar parental origins confirmed that demethylation of some regions takes place upon phenotypic drift.
Methylcytosine (5-meC) may be considered a fifth base, but with a different flexibility in its utility than the other four bases (20). It offers unique possibilities of regulation of a genome at an epigenetic level, in particular by modifying the interactions of DNA with proteins. It may also act as a temporary mutation. In the study of cancer progression, much concern has been focused on the theory that methylation may act similarly to truncation or deletion mutations in silencing gene expression (4). Methylation seems to be involved in the control of viral genomes, e.g., latent Epstein-Barr virus (EBV) infection (21, 24, 25).
EBV is carried by 95% of human adults in a latent form (23). EBV is associated with several human malignancies. In healthy carriers and in tumor cells, at least four different latency forms have been detected. In latency I, only the EBV nuclear antigen 1 (EBNA-1) is expressed; in latency II, EBNA-1 and latent membrane proteins 1 and 2 (LMP-1 and LMP-2, respectively) are expressed; and in latency III, EBNA-1 to EBNA-6 are expressed together with LMP-1, LMP-2A, and LMP-2B (23). Recently, a fourth form was described in which only EBNA-1 together with LMP-2A is expressed (10), with or without EBER-I (36). LMP-2A expression without EBNA-1 has also been described (26, 27). Burkitt lymphoma (BL) tumors in vivo resemble the latency I program. When these tumor cells are explanted in vitro, they tend to drift to a more lymphoblastoid phenotype expressing all of the EBNAs and LMPs (18). In nasopharyngeal carcinoma (NPC) biopsies, EBNA-1 is always expressed and in 65% of the tumors LMP-1 is also expressed (13). EBNA-1 is essential for maintenance of the viral episomes and for virus DNA replication in latency (39). EBNA-1 binds in multiple copies to two regions within the origin of replication (oriP): the family of repeats (FR) and the dyad symmetry (DS) region (2, 28, 38). The DS region is the site of initiation of replication of the episome (18).
The LMP-1 protein is transcribed in a leftward direction from the EDL1 promoter that is controlled by the LMP regulatory sequence (LRS). The promoter is located in the BamHI Nhet fragment (3, 15). Previously, we have shown that the EBV genome is almost unmethylated in lymphoblastoid cell lines. Conversely, the virus genome in BL and NPC cells is highly methylated except for three regions: oriP, LRS, and the Qp promoter (1, 21, 24, 25). It is well known that specific methylation patterns are established in different cell types in vertebrates during development and that methylation of CpGs is involved in promoter control (9, 12). In the case of EBV, it has been shown that the Cp promoter is silent when a particular CpG site is methylated (29).
We have shown that a 4.5-kb region centered on oriP is unmethylated in all EBV-derived cell lines, including those derived from EBV-carrying tumors, by using methylation-sensitive enzymes (14). However, by this method it was possible to analyze only about 10% of all CpG sites. We have now applied a genomic sequencing technique that allows determination of the methylation status of all of the cytosines in either DNA strand. We have mapped in detail the locations of methylated CpGs in and around the EBNA-1-binding sites in the DS element of the oriP. As expected, the methylation pattern was much more varied than that detected by the restriction enzyme-based method. In one cell line, Rael, in which EBV genomes are highly methylated, CpGs in the EBNA-1-binding sites and two additional CpGs were unmethylated while surrounding CpGs were partially methylated. The methylation status of LRS correlates strongly with LMP-1 expression, as judged by restriction enzyme analysis (21). This pattern was confirmed by analyzing the LMP-1-negative cell line Rael and the LMP-1-positive NPC tumor C15. In the cell line Mutu, with two in vitro phenotypes corresponding to type I and type III latencies, the overall methylation pattern also correlated with phenotype, although this was less pronounced.
MATERIALS AND METHODS
Cell lines.
Rael is a BL cell line with a stable type I phenotype, expressing only EBNA-1 (22). Mutu BL I clone 148 is a BL-derived cell line with a type I phenotype. To exclude the possibility that the cells used in this study had drifted to a type III phenotype, the cells were analyzed by immunofluorescence and their type I phenotype was confirmed. Mutu BL III clone 99 was obtained by in vitro culturing, and the cells have a type III phenotype (18). NPC C15 is a nude mouse-passaged, EBV DNA-positive African NPC tumor expressing EBNA-1, LMP-1, LMP-2A, and LMP-2B (7, 8, 10). The levels of EBNA and LMP expression in the cell lines and tumors used in this study are summarized in Table 1.
TABLE 1.
EBV protein expression patterns in cell lines and tumors used for this study
| Cell line or tumor | Expression with:
|
Type of latency | |||
|---|---|---|---|---|---|
| EBNA-1 | EBNA-2 to EBNA-6 | LMP-1 | LMP-2A and LMP-2B | ||
| Rael | + | − | − | − | I |
| NPC C15 | + | − | + | + | II |
| Mutu I | + | − | − | − | I |
| Mutu III | + | + | + | + | III |
Preparation of DNA and treatment with sodium bisulfite.
High-molecular-weight DNA was prepared by standard methods (30). The bisulfite conversion reaction was adopted according to the method described by Clark et al. (11). DNA was digested with the restriction enzyme BamHI and then precipitated with EtOH. DNA was denatured in 0.3 M NaOH for 15 min at 37°C. Denatured DNA was incubated in a solution containing 3.1 M sodium bisulfite (pH 5.0) and 0.5 mM hydroquinone. The mixture was then overlaid with mineral oil and incubated for 20 h at 55°C. The bisulfite was removed, and the DNA was purified with a desalting column (Wizard DNA Clean-Up system; Promega, Madison, Wis.) according to the manufacturer’s instructions. The DNA was desulfonated by alkali treatment by adding 3 M NaOH to a final concentration of 0.3 M, and the mixture was incubated for 15 min at 37°C. The DNA was precipitated in 3 M ammonium acetate and ethanol and was amplified by PCR (see below).
PCR amplification. (i) The oriP region.
The following primers were used for amplification within the oriP region: 5′-ACCTCACATACACCTTACTG-3′ (EBV-6), 5′-CTGACTGTAGTTGACATCCT-3′ (EBV-7), 5′-GAGTATTTTATATATATTTTA-3′ (EBV-8), and 5′-AAATTCTCTAACTATAATTAA-3′ (EBV-9), corresponding to coordinates 8789 to 8808, 9399 to 9418, 8785 to 8805, and 9405 to 9425 in the B95-8 genome, respectively. The bisulfite-treated genomic DNA was amplified with the strand (sense)-specific primer pair EBV-8 and EBV-9. DNA primers EBV-6 and EBV-7 were used to amplify the untreated genomic DNA. Amplifications were performed in a 50-μl reaction mixture containing 5 μl of untreated or, alternatively, bisulfite-treated DNA, 300 μM deoxynucleoside triphosphates (dNTPs), 20 pmol of primers, 1.5 mM MgCl2, 50 mM KCl, 10 mM Tris-HCl (pH 8.3), 0.001% gelatin, and 1 U of Ampli Taq (Perkin-Elmer Cetus) in a Perkin-Elmer PCR machine. Twenty-five cycles of amplification were performed under the following conditions: denaturation (95°C for 3 min in the first cycle; 94°C for 1 min in cycles 2 to 25), annealing (53°C for EBV-6 and EBV-7; 43°C for EBV-8 and EBV-9), and extension (72°C for 1 min; at the end, 72°C for 7 min). Five microliters from the first round of PCR was amplified a second time with the same primers and under the same conditions.
(ii) LMP regulatory region.
The following primers were used for amplification of the LRS region: 5′-ATTCCAGAGAGCGATGAGCAG-3′ (LRS-1), 5′-AGCCCACACCCTTTTCGCCT-3′ (LRS-2), 5′-TTGAAGATAAAGATGATTAAAATT-3′ (LRS-3), and 5′-ACCTCATTCTAAAATTCCCAT-3′ (LRS-4), corresponding to coordinates 169100 to 169120, 170030 to 170049, 169257 to 169280, and 170000 to 170020 in the B95-8 genome, respectively. For untreated genomic DNA primers, LRS-1 and LRS-2 were used in both rounds of PCR. The bisulfite-treated genomic DNA was first amplified with the primer pair LRS-1 and LRS-2 and was then reamplified with a strand (sense)-specific primer pair (LRS-3 and LRS-4). Amplifications were performed in a 50-μl reaction mixture containing 5 μl of untreated or, alternatively, bisulfite-treated DNA, 0.3 mM dNTPs, 20 pmol of primers, 1.5 mM MgCl2, 10× reaction buffer supplied by the manufacturer, and 1 U of Red Hot DNA polymerase (Advanced Biotechnologies, Ltd., Learthead Surrey, United Kingdom) in a Perkin-Elmer PCR machine. Thirty-one cycles of amplification were performed under the following conditions: denaturation (95°C for 5 min in the first cycle; 95°C for 30 s in cycles 2 to 31), annealing (40°C for 1 min), and extension (72°C for 2 min; at the end, 72°C for 7 min). Two to five microliters from the first round of PCR was amplified a second time.
Cloning and sequencing.
Amplified DNA was ligated into a SmaI-linearized pUC18 vector (Pharmacia) and transformed into competent Escherichia coli (JM 109; SDS Promega). Plasmid DNA was recovered from individual clones by PCR with primers (RIT 28 and RIT 29; Pharmacia) within the M13 region of the vector. The individual clones were sequenced by using the AutoReadTm sequencing kit (Pharmacia) or, alternatively, a thermo Sequenase fluorescence-labelled primer cycle sequencing kit (Amersham), and the samples were analyzed on an ALF sequencer (Pharmacia). Alternatively, an ABI DNA sequencing kit (Perkin-Elmer) was used, and the samples were analyzed on a 373 A automated DNA sequencer (Applied Biosystems).
RESULTS
Methylation status of the DS region in a type I BL cell line.
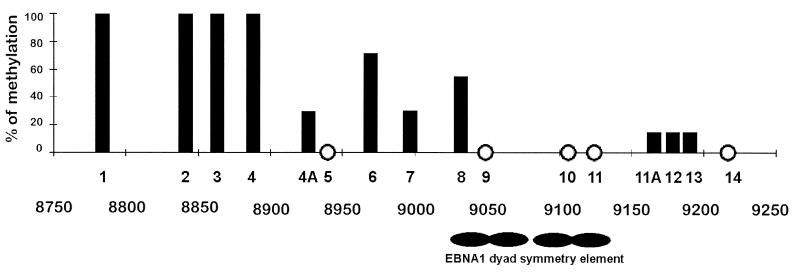
A total of 16 CpG sites in Rael were analyzed for methylation in the oriP region. Untreated DNA from the Rael cell line was also sequenced. The positions of the CpG sites were identical when the B95-8 genome was compared to the Rael genome, with two exceptions. The Rael genome had two additional sites, one at coordinate 8926 (site 4A) and one at coordinate 9166 (site 11A; Fig. 1). Of the eight sites upstream of the EBNA-1 binding, sites 1 to 4 were completely methylated, whereas site 4A and site 7 were methylated only in two of seven clones. Five of seven clones were methylated at site 6, while site 5 was unmethylated. We found that three (sites 9, 10, and 11) of four CpGs present in the region where EBNA-1 binds were unmethylated in all of the clones studied, in contrast to site 8, in which four of seven clones were methylated (Fig. 1). The four CpG sites downstream of the EBNA-1-binding region were found to be almost unmethylated; one clone of seven was methylated at three of these sites.
FIG. 1.
The percentage of CpG methylation in the DS region and surrounding regions in the cell line Rael. A schematic drawing of the oriP region is shown. The positions of the following four EBNA-1-binding sites in the DS region are indicated: site 1, coordinates 9104 to 9129 in the B95-8 genome; site 2, coordinates 9083 to 9108; site 3, coordinates 9049 to 9074; and site 4, coordinates 9029 to 9055. CpG sites 4A and site 11A are not present in the B95-8 genome. The y axis shows the percentage of methylation in all clones analyzed at this CpG site. The x axis shows the individual CpG sites analyzed. Three to seven clones at each site were analyzed. Open circles, unmethylated sites.
Methylation status of the LRS region in LMP-1-positive and LMP-1-negative cells.
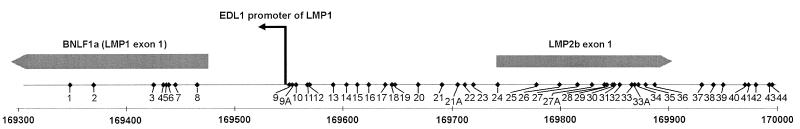
We analyzed three different cell lines and one NPC tumor for the presence of CpG methylation in a part of the LMP-1 exon and the LRS region. The positions and numbers of CpG sites in the four isolates varied compared to those of the prototype B95-8 (Fig. 2). The sequence from the Rael genome differed from that for B95-8 at eight positions: sites 1, 8, 24, and 38 were not present, while four other sites were present instead, i.e., sites 9A, 21A, 27A, and 33A (for details, see Fig. 2 and 3). When the sequence obtained from NPC C15 was compared with that for B95-8, they were identical. The sequences from Mutu I and Mutu III were identical with that for B95-8, except for one additional CpG site (site 27A; Fig. 2 and 3).
FIG. 2.
Schematic drawing of the LRS of the EBV genome, with the coordinates referring to the B95-8 map analyzed by the bisulfite genomic sequencing method. Forty-eight individual CpG sites are indicated. CpG sites 9A, 21A, 27A, and 33A are not present in the B95-8 genome. The LMP-1 promoter and the LMP-1 and LMP-2B exons are indicated. The coordinates in the B95-8 genome are given.
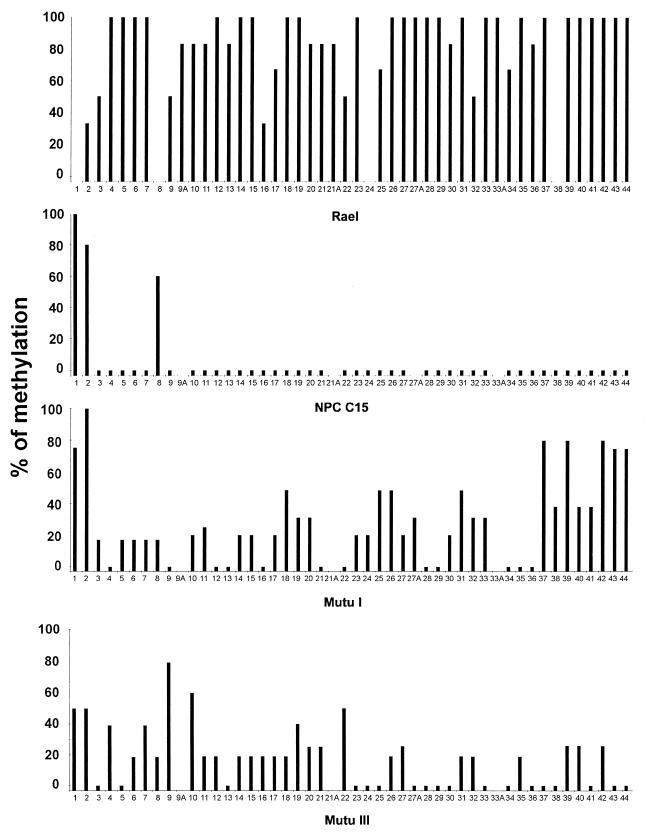
FIG. 3.
A summary of the bisulfite genomic sequencing of the LRS region. The y axis shows the percentage of methylation in all clones analyzed at this CpG site. The x axis shows the individual CpG sites in the LMP-1 promoter region and the 5′ region of the exon analyzed. Each bar represents one CpG site. A small bar below the zero level indicates that the CpG site is present and unmethylated. A CpG site with no bar on top shows that the site is not present in the isolate. A, additional CpG sites (for details, see the legend to Fig. 2). Three to six individual clones for each CpG site in bisulfite-treated Rael DNA were analyzed. Five NPC C15 clones were analyzed at each individual CpG site. Two to six clones from the Mutu I and III cell lines were analyzed.
The LMP-1-negative cell line Rael contains 44 CpG sites, like B95-8, in the region analyzed, and all of the sites showed some degree of methylation. Twenty-six of these sites were completely methylated in all clones tested.
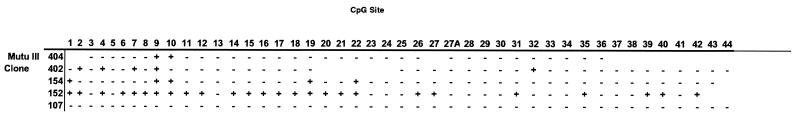
A total of five clones were sequenced from the LMP-1-positive NPC C15 tumor, and 44 CpG sites were analyzed for methylation. Of the eight sites localized in the LMP-1 exon, sites 3 to 7 were unmethylated. Site 1 was methylated in all clones. Sites 2 and 8 showed methylation in four of five and in three of five clones, respectively. Sites 9 to 44, all of which are in the LRS region, showed a complete lack of methylation. A total of 45 CpG sites were included in the sequence from the LMP-1-negative cell line Mutu I; 12 of these sites were completely unmethylated, while the remaining sites showed a variable degree of methylation (Fig. 3). The LMP-1-positive cell line Mutu III showed a mixed methylation pattern (Fig. 3). Eighteen sites were completely unmethylated, while the percentage of methylation varied at the other sites by between 20 and 80% (Fig. 3 and 4).
FIG. 4.
CpG methylation of the LMP-1 regulatory region in five individual DNA clones from Mutu III. +, the CpG site was methylated; −, the CpG site was unmethylated.
DISCUSSION
In latent EBV infections, there are examples of expression-related methylation of promoters. We have also described hypomethylation in another type of regulatory region, the origin of DNA replication (14).
In conjunction with earlier observations on the importance of methylation of the EBV genome in different forms of latent infection, we have studied methylation of two regulatory regions in DNA from different types of EBV-infected cells. The analysis of the LRS region extended over 600 bp, including the LMP-1 promoter, the first LMP-2B exon 1, and the first exon of LMP-1. In the LMP-1-negative cell line Rael and the LMP-1-positive NPC C15 tumor, we confirmed our earlier observation of a negative correlation between methylation and expression. The analysis could be extended from four CpG sites with the earlier enzyme method to 44 CpG sites. A total of 35 of the 44 CpG sites in Rael showed ≥80% methylation. No CpG sites in Rael were completely unmethylated in the clones analyzed. In the NPC-derived C15 cells, the pattern was reversed, with 41 of 44 CpG sites being unmethylated. Interestingly, the three sites which were methylated (≥60%) in C15 cells were among those least methylated in Rael cells (30 to 50%), suggesting some regulatory role for these sites. This is puzzling, since the sites are located within the first exon of LMP-1 more than 100 bp downstream of the EDL1 promoter and outside the upstream regulatory region of the promoter. Methylation of exons is not known to affect transcriptional or replicative functions (5), in contrast to the modulation of promoter activity. Two sites (sites 20 and 21) are located in a region of LRS which is regulated by EBNA-2 in B cells (bp −136 to −176 relative to the promoter) (32). This region contains several transcription factor binding sites: a POU-binding site (distinct from oct-1 or oct-2) and a Pu.1-binding site. This region is modified by methylation in Rael, which may contribute to the LMP-1 downregulation in conjunction with the lack of EBNA-2 in this cell line. However, LRS must be regulated differently in epithelial cells compared to B cells, in that EBNA-2 is not expressed and the accessible pools of transcription factors differ. Further upstream of the promoter (bp −201 to −260), another regulatory region which contains an RBP-Jk-binding site is located. It includes two CpGs (sites 23 and 24), which are located in protein-DNA interacting domains (33). Site 23 is completely methylated in Rael cells, while site 24 is not present. Both sites are unmethylated in C15 cells.
The LRS region in the Mutu I and III cell lines, which have opposite LMP-1 expression patterns, was also analyzed. The methylation pattern was less distinct when Mutu I and III were compared. At this high level of resolution in the analysis, it is unavoidable that both cell lines to some extent are mixtures of type I and III latencies, i.e., they are contaminated by a few cells with the other phenotype. Mutu I cells are constantly drifting to the group-type III phenotype (18). To illustrate this problem, we show the sequence data obtained from four individual DNA clones derived from Mutu III. Only one of these shows a high level of methylation and may represent a genome from a type I cell among the type III cells. Although the overall patterns of methylation in Mutu I and III are similar, the demethylation is extensive in Mutu III all the way from the POU-binding region and upstream to CpG site 44. This is far upstream in the LRS and beyond the LMP-2B exon, close to the terminal repeats. Altogether, another 12 sites were unmethylated in Mutu III versus Mutu I, while 4 sites were more methylated. If the single highly methylated cloned sequence in Mutu III is omitted, the level of methylation is very low and would be restricted to 8 sites, of which half are outside the LRS. Methylation of site 9 in Mutu III looks specific in that it is localized precisely at the EDLI promoter in LMP-1-expressing cells. In the oriP region, two specific EBNA-1-binding regions with different functions have been identified, i.e., the FR and the DS regions, which are separated by approximately 960 bp (19, 28). It has been shown that the DS region is involved both in replication and in control of the downstream Cp and Wp promoters (17, 35). It contains two opposed pairs of EBNA-1-binding sites that can loop and interact with the FR through EBNA-1 protein-protein interaction (16, 34). Little is known about the binding of proteins other than EBNA-1 to this region. We have shown that while EBNA-1 is bound, it interacts with oct-1 and oct-2 and other unidentified proteins by electrophoretic mobility shift assays (1a). In the type I latent Rael cell line, which has, on average, a high level of methylation, we found a low level of methylation in and around the DS region. In 2 of 12 sites (sites 4A to 14), the methylation level was greater than 40%. However, the three CpGs within the EBNA-1 core binding region were not methylated. One of these sites (site 10) was previously shown to be completely unmethylated by MspI/HpaII restriction enzyme analysis (14). In this region of oriP, only 1 of 16 CpG sites could be analyzed by the previous method. The region closer to the FR was 100% methylated in four of four sites. The previously described 4.5-kb unmethylated island covering the oriP (14) thus seems to be a mixture of unmethylated and methylated regions when analyzed in detail. Since multiple copies of the EBV genome are present within a single cell, methylation may vary between copies in one cell.
By this sequencing method, it is necessary to sequence several clones to establish the level of partial methylation in some sites. Partial methylation must reflect that the degree of methylation in such CpG sites varies between the EBV genome copies and may reflect that the genomes are differentially active within the cell. Each copy may serve two functions in a latently infected cell, i.e., as template for replication and for transcription. As each episome undergoes one round of replication during S phase, they must all be able to use the oriP for DNA replication to maintain the copy number. The variation in methylation levels in some sites may reflect different activities in the role of the DS region and the surrounding region as a promoter regulatory region. However, in Rael cells it has been shown that the Cp promoter adjacent to oriP is silent in favor of EBNA-1 expression from the Qp promoter (31).
Toth et al. (37) have shown in experimental systems with the adenovirus major late promoter that DNA methylation may spread horizontally over the genome from CpG sites which are initially methylated. The varying degrees of methylation may reflect an ongoing process of this kind. Methylation patterns are usually conserved in the cells by maintenance methylases that act following the synthesis of the new DNA strand (6). It is most likely that this mechanism also operates on the EBV episomes, since they replicate during S phase by the cellular replication machinery. EBV episomes with methylation in the oriP region will give rise to episomal copies with the same methylation patterns. If the efficiency of replication varies with the degree of methylation, DNA episome imbalances may occur over time in the cell. This may affect the fate of the cell unless the copy number is corrected by some mechanism that allows several copies to be made during S phase from a single master episome. A trivial explanation of sites with partial methylation, i.e., variation between clones, could be that the chemical method to convert the DNA from the cells by bisulfite varies in efficiency in different DNA regions. However, this can be controlled by the conversion of Cs in nonmethylatable sites, and it was shown to be more than 98% in this study.
It is an open question as to whether the unmethylated sites can be caused by an enzyme-specific mechanism or whether their methylation is blocked by constant protection from de novo methylation by binding of proteins, e.g., EBNA-1. This issue can be addressed only by functional studies. The low level of methylation in sites 11A to 14 and site 5 (Fig. 1) may reflect blocking by DNA-binding proteins other than EBNA-1. These sites may be helpful in the identification of cofactor proteins that bind together with EBNA-1 to this control region.
Latent EBV infection with its natural methylation spectrum lends itself to studies of the impact of methylation on viral gene expression as well as to studies of more general aspects of the role of methylation. The role of methylation in functional studies of oriP and LRS will be easier to address now as detailed information on methylation patterns becomes available.
ACKNOWLEDGMENTS
This study was funded by the Swedish Cancer Society, the Medical Research Council, the Swedish Children Cancer Foundation, and the Cornell Foundation.
We are grateful to Tamarra Cadd for correcting the English.
REFERENCES
- 1.Altiok E, Minarovits J, Hu L F, Contreras-Brodin B, Klein G, Ernberg I. Host-cell-phenotype-dependent control of the BCR2/BWR1 promoter complex regulates the expression of Epstein-Barr virus nuclear antigens 2-6. Proc Natl Acad Sci USA. 1992;89:905–909. doi: 10.1073/pnas.89.3.905. . (Erratum, 89:6225.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1a.Altiok, E., Y. Öztürk, E. Bermek, and I. Ernberg. Octamer transcription factors and Epstein-Barr virus nuclear antigen (EBNA)-1 form complex with OriP tandom repeats. Submitted for publication.
- 2.Ambinder R F, Shah W A, Rawlins D R, Hayward G S, Hayward S D. Definition of the sequence requirements for binding of the EBNA-1 protein to its palindromic target sites in Epstein-Barr virus DNA. J Virol. 1990;64:2369–2379. doi: 10.1128/jvi.64.5.2369-2379.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baer R, Bankier A T, Biggin M D, Deininger P L, Farrell P J, Gibson T J, Hatfull G, Hudson G S, Satchwell S C, Seguin C, Tuffnell P S, Baer B G. DNA sequence and expression of the B95-8 Epstein-Barr virus genome. Nature. 1984;310:207–211. doi: 10.1038/310207a0. [DOI] [PubMed] [Google Scholar]
- 4.Bayliss, S. B., J. G. Herman, J. R. Graff, P. M. Vertino, and J.-P. Issa. Alterations in DNA methylation—a fundamental aspect of neoplasia. Adv. Cancer Res., in press. [PubMed]
- 5.Bird A P. CpG-rich islands and the function of DNA methylation. Nature. 1986;321:209–213. doi: 10.1038/321209a0. . (Review.) [DOI] [PubMed] [Google Scholar]
- 6.Bolden A H, Nalin C M, Ward C A, Poonian M S, Weissbach A. Primary DNA sequence determines sites of maintenance and de novo methylation by mammalian DNA methyltransferases. Mol Cell Biol. 1986;6:1135–1140. doi: 10.1128/mcb.6.4.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brooks L, Yao Q Y, Rickinson A B, Young L S. Epstein-Barr virus latent gene transcription in nasopharyngeal carcinoma cells: coexpression of EBNA1, LMP1, and LMP2 transcripts. J Virol. 1992;66:2689–2697. doi: 10.1128/jvi.66.5.2689-2697.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Busson P, Ganem G, Flores P, Mugneret F, Clausse B, Caillou B, Braham K, Wakasugi H, Lipinski M, Tursz T. Establishment and characterization of three transplantable EBV-containing nasopharyngeal carcinomas. Int J Cancer. 1988;42:599–606. doi: 10.1002/ijc.2910420422. [DOI] [PubMed] [Google Scholar]
- 9.Cedar H. DNA methylation and gene activity. Cell. 1988;53:3–4. doi: 10.1016/0092-8674(88)90479-5. . (Review.) [DOI] [PubMed] [Google Scholar]
- 10.Chen F, Hu L F, Ernberg I, Klein G, Winberg G. Coupled transcription of Epstein-Barr virus latent membrane protein (LMP)-1 and LMP-2B genes in nasopharyngeal carcinomas. J Gen Virol. 1995;76:131–138. doi: 10.1099/0022-1317-76-1-131. [DOI] [PubMed] [Google Scholar]
- 11.Clark S J, Harrison J, Paul C L, Frommer M. High sensitivity mapping of methylated cytosines. Nucleic Acids Res. 1994;22:2990–2997. doi: 10.1093/nar/22.15.2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doerfler W. DNA methylation and gene activity. Annu Rev Biochem. 1983;52:93–124. doi: 10.1146/annurev.bi.52.070183.000521. . (Review.) [DOI] [PubMed] [Google Scholar]
- 13.Fåhraeus R, Jansson A, Sjoblom A, Nilsson T, Klein G, Rymo L. Cell phenotype-dependent control of Epstein-Barr virus latent membrane protein 1 gene regulatory sequences. Virology. 1993;195:71–80. doi: 10.1006/viro.1993.1347. [DOI] [PubMed] [Google Scholar]
- 14.Falk K, Ernberg I. An origin of DNA replication (oriP) in highly methylated episomal Epstein-Barr virus DNA localizes to a 4.5-kb unmethylated region. Virology. 1993;195:608–615. doi: 10.1006/viro.1993.1412. [DOI] [PubMed] [Google Scholar]
- 15.Fennewald S, van Santen V, Kieff E. Nucleotide sequence of an mRNA transcribed in latent growth-transforming virus infection indicates that it may encode a membrane protein. J Virol. 1984;51:411–419. doi: 10.1128/jvi.51.2.411-419.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frappier L, O’Donnell M. Epstein-Barr nuclear antigen 1 mediates a DNA loop within the latent replication origin of Epstein-Barr virus. Proc Natl Acad Sci USA. 1991;88:10875–10879. doi: 10.1073/pnas.88.23.10875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gahn T A, Schildkraut C L. The Epstein-Barr virus origin of plasmid replication, oriP, contains both the initiation and termination sites of DNA replication. Cell. 1989;58:527–535. doi: 10.1016/0092-8674(89)90433-9. [DOI] [PubMed] [Google Scholar]
- 18.Gregory C D, Rowe M, Rickinson A B. Different Epstein-Barr virus-B cell interactions in phenotypically distinct clones of a Burkitt’s lymphoma cell line. J Gen Virol. 1990;71:1481–1495. doi: 10.1099/0022-1317-71-7-1481. [DOI] [PubMed] [Google Scholar]
- 19.Harrison S, Fisenne K, Hearing J. Sequence requirements of the Epstein-Barr virus latent origin of DNA replication. J Virol. 1994;68:1913–1925. doi: 10.1128/jvi.68.3.1913-1925.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hotchkiss R D. The quantitative separation of purines, pyrimidines, and nucleosides by paper chromatography. J Biol Chem. 1948;168:315–332. [PubMed] [Google Scholar]
- 21.Hu L F, Minarovits J, Cao S L, Contreras-Salazar B, Rymo L, Falk K, Klein G, Ernberg I. Variable expression of latent membrane protein in nasopharyngeal carcinoma can be related to methylation status of the Epstein-Barr virus BNLF-1 5′-flanking region. J Virol. 1991;65:1558–1567. doi: 10.1128/jvi.65.3.1558-1567.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klein G, Dombos L, Gothoskar B. Sensitivity of Epstein-Barr virus (EBV) producer and non-producer human lymphoblastoid cell lines to superinfection with EB-virus. Int J Cancer. 1972;10:44–57. doi: 10.1002/ijc.2910100108. [DOI] [PubMed] [Google Scholar]
- 23.Masucci M G, Ernberg I. Epstein-Barr virus: adaptation to a life within the immune system. Trends Microbiol. 1994;2:125–130. doi: 10.1016/0966-842x(94)90599-1. [DOI] [PubMed] [Google Scholar]
- 24.Minarovits J, Minarovits-Kormuta S, Ehlin-Henriksson B, Falk K, Klein G, Ernberg I. Host cell phenotype-dependent methylation patterns of Epstein-Barr virus DNA. J Gen Virol. 1991;72:1591–1599. doi: 10.1099/0022-1317-72-7-1591. [DOI] [PubMed] [Google Scholar]
- 25.Minarovits J, Hu L-F, Marcsek Z, Minarovits-Kormuta S, Klein G, Ernberg I. RNA polymerase III-transcribed EBER1 and 2 transcription units are expressed and hypomethylated in the major Epstein-Barr virus carrying cell types. J Gen Virol. 1992;73:1687–1692. doi: 10.1099/0022-1317-73-7-1687. [DOI] [PubMed] [Google Scholar]
- 26.Miyashita E M, Yang B, Babcock G J, Thorley-Lawson D A. Identification of the site of Epstein-Barr virus persistence in vivo as a resting B cell. J Virol. 1997;71:4882–4891. doi: 10.1128/jvi.71.7.4882-4891.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qu L, Rowe D T. Epstein-Barr virus latent gene expression in uncultured peripheral blood lymphocytes. J Virol. 1992;66:3715–3724. doi: 10.1128/jvi.66.6.3715-3724.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rawlins D R, Milman G, Hayward S D, Hayward G S. Sequence-specific DNA binding of the Epstein-Barr virus nuclear antigen (EBNA-1) to clustered sites in the plasmid maintenance region. Cell. 1985;42:859–868. doi: 10.1016/0092-8674(85)90282-x. [DOI] [PubMed] [Google Scholar]
- 29.Robertson K D, Hayward S D, Ling P D, Samid D, Ambinder R F. Transcriptional activation of the Epstein-Barr virus latency C promoter after 5-azacytidine treatment: evidence that demethylation at a single CpG site is crucial. Mol Cell Biol. 1995;15:6150–6159. doi: 10.1128/mcb.15.11.6150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sambrook J, Fritsch E, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 31.Schaefer B C, Strominger J L, Speck S H. Redefining the Epstein-Barr virus-encoded nuclear antigen EBNA-1 gene promoter and transcription initiation site in group I Burkitt lymphoma cell lines. Proc Natl Acad Sci USA. 1995;92:10565–10569. doi: 10.1073/pnas.92.23.10565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sjoblom A, Jansson A, Yang W, Lain S, Nilsson T, Rymo L. PU box-binding transcription factors and a POU domain protein cooperate in the Epstein-Barr virus (EBV) nuclear antigen 2-induced transactivation of the EBV latent membrane protein 1 promoter. J Gen Virol. 1995;76:2679–2692. doi: 10.1099/0022-1317-76-11-2679. [DOI] [PubMed] [Google Scholar]
- 33.Sjoblom A, Nerstedt A, Jansson A, Rymo L. Domains of the Epstein-Barr virus nuclear antigen 2 (EBNA2) involved in the transactivation of the latent membrane protein 1 and the EBNA Cp promoters. J Gen Virol. 1995;76:2669–2678. doi: 10.1099/0022-1317-76-11-2669. [DOI] [PubMed] [Google Scholar]
- 34.Su W, Middleton T, Sugden B, Echols H. DNA looping between the origin of replication of Epstein-Barr virus and its enhancer site: stabilization of an origin complex with Epstein-Barr nuclear antigen 1. Proc Natl Acad Sci USA. 1991;88:10870–10874. doi: 10.1073/pnas.88.23.10870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sugden B, Warren N. A promoter of Epstein-Barr virus that can function during latent infection can be transactivated by EBNA-1, a viral protein required for viral DNA replication during latent infection. J Virol. 1989;63:2644–2649. doi: 10.1128/jvi.63.6.2644-2649.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tierney R J, Steven N, Young L S, Rickinson A B. Epstein-Barr virus latency in blood mononuclear cells: analysis of viral gene transcription during primary infection and in the carrier state. J Virol. 1994;68:7374–7385. doi: 10.1128/jvi.68.11.7374-7385.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Toth M, Lichtenberg U, Doerfler W. Genomic sequencing reveals a 5-methylcytosine-free domain in active promoters and the spreading of preimposed methylation patterns. Proc Natl Acad Sci USA. 1989;86:3728–3732. doi: 10.1073/pnas.86.10.3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Woodcock D M, Crowther P J, Jefferson S, Diver W P. Methylation at dinucleotides other than CpG: implications for human maintenance methylation. Gene. 1988;74:151–152. doi: 10.1016/0378-1119(88)90273-9. . (Review.) [DOI] [PubMed] [Google Scholar]
- 39.Yates J L, Warren N, Sugden B. Stable replication of plasmids derived from Epstein-Barr virus in various mammalian cells. Nature. 1985;313:812–815. doi: 10.1038/313812a0. [DOI] [PubMed] [Google Scholar]