Abstract
The recent introduction of the innovative therapy, onasemnogene abeparvovec (Zolgensma®), has revolutionized the spinal muscular atrophy (SMA) therapeutic landscape. Although Zolgensma® therapy has proven to lead to functional improvements in SMA children, some gaps in its safety profile still need to be investigated. To better characterize the Zolgensma® safety profile, we conducted a retrospective observational study, analyzing all the Individual Case Safety Reports (ICSRs) referred to it and collected in the European pharmacovigilance database between 1 January 2019 and 22 September 2023. We found 661 ICSRs related to Zolgensma®, with a growing trend in the annual reporting. The majority of the reports were sent by healthcare professionals and referred to infant females. In more than 90% of the cases, Zolgensma® was the only reported suspected drug. Out of a total of 2744 reported ADRs, increased hepatic enzymes, pyrexia, vomiting, and thrombocytopenia were the most commonly reported adverse reactions. Of these adverse reactions (ADRs), 56.9% were serious, causing or prolonging the patient’s hospitalization. A total of 39 ICSRs related to cases with a fatal outcome. Alterations in the heart rhythm, acute hepatic failure, and hepatic cytolysis emerged among the cardiac and hepatic disorders, respectively.
Keywords: gene therapy, orphan drug, safety data, pharmacovigilance, spinal muscular atrophy
1. Introduction
Spinal muscular atrophy (SMA) is a leading genetic cause of infant mortality [1]. It is a rare neuromuscular disease, with an estimated incidence of approximately 1 in 10,000 live births and a prevalence of 1–2 in every 100,000 people [2]. Muscle weakness and atrophy, particularly affecting the lower limbs and respiratory muscles, are the typical SMA symptoms. In the most serious forms, this evolves into reduced or absent abilities to move, swallow, and breathe [3]. The progressive degeneration of the α-motor neurons in the spinal cord and defects in neuromuscular junction development are characteristic of SMA and are due to the reduced expression or deficiency of the survival motor neuron (SMN) protein [4]. This ubiquitous protein is essential for motor system and motoneuron functionality [1]. SMN is coded by two genes mapped to chromosome 5q13: the telomeric SMN1 and the centromeric SMN2 [4]. The genetic cause of SMA has been identified as deletions or mutations in the SMN1 gene, the only gene able to code for a fully functional SMN protein. The SMN2 gene mainly produces an SMN protein with diminished functions, known as SMNΔ7. This truncated isoform, representing 85–90% of SMN2 products, is highly unstable and more susceptible to degradation by the ubiquitin–proteasome pathway. Only 10–15% of SMN2-coded product is a functional protein. The age of symptom onset and the severity of clinical course in SMA are inversely correlated with the SMN2 copy number [5]. Based on these three correlated variables, SMA is categorized into five types, from the prenatal-onset and fatal one (type 0) to adult-onset and less invaliding form (type IV). However, there are degrees of severity even within an individual type, and as many as 25% of patients elude precise classification [6]. The infantile form (type I SMA) is the most frequent, accounting for approximately half of patients. Children with type I SMA show the following symptoms in the first 6 months of life: hypotonia, delayed motor milestones, feeding difficulties, and never sitting independently [7]. Moreover, infants with type I SMA usually develop respiratory failure before 2 years of life, requiring permanent ventilation and nutritional support [6]. Regarding the therapeutic possibilities, until 2016, the therapy only supported vital functions. The identification of the molecular mechanisms underlying SMA’s onset and progression allow us to identify some SMN-dependent or -independent therapeutic strategies. To date three SMA treatments are available, all aimed at increasing SMN protein levels in different ways [8]. In particular, both the antisense oligonucleotide nusinersen (Spinraza®) and risdiplam (Evrysdi®) work by changing SMN2 splicing, while onasemnogene abeparvovec (Zolgensma®) works by replacing the SMN1 gene [9].
Zolgensma® was the first SMA gene therapy approved by the Food and Drug Administration (FDA) and the European Medicine Agency (EMA)—in 2017 and 2020, respectively [9]. The recent introduction of this innovative therapy revolutionized the SMA therapeutic landscape [10]. To date, Zolgensma® is indicated for the treatment of patients with 5q SMA with a bi-allelic mutation in the SMN1 gene and a clinical diagnosis of SMA type 1, or patients with 5q SMA with a bi-allelic mutation in the SMN1 gene and up to three copies of the SMN2 gene [9]. The used AAV9 vector delivers a functional copy of the SMN1 gene to the motoneuron nucleus, as a primary source of the functional SMN. In addition to the innovative mechanism of action, the great innovation of Zolgensma® is the method of administration, consisting of a single intravenous infusion over 60 min.
This one-shot and lifetime gene replacement therapy directly works on the monogenic cause of SMA [11]. This feature represents a huge step forward compared with the pharmacological alternatives nusinersen, which requires intrathecal administration, and risdiplam, which needs daily oral administration. Even if Zolgensma® has been proven to lead to functional improvements in treated SMA children, reducing pulmonary and nutritional support requirements as well as hospitalization rate and improving motor function [12], some gaps in its safety profile still need to be investigated [9,10]. There are still some unanswered questions that need to be addressed: the long-term benefits remain to be determined and the pre-approval safety data need to be confirmed/refused in a real-world setting. Moreover, its cardiac toxicity, as well as the dorsal root ganglion toxicity and the tumorigenicity due to chromosomal integration are important potential risks that require investigation [13].
In this context, analysis of post-marketing data is particularly important as a source of new information that has not yet emerged, especially in specific populations [14]. In light of this, the aim of this study was a post-marketing evaluation of the safety profile of Zolgensma® through the analysis of data retrieved from the European pharmacovigilance database Eudravigilance (EV).
2. Results
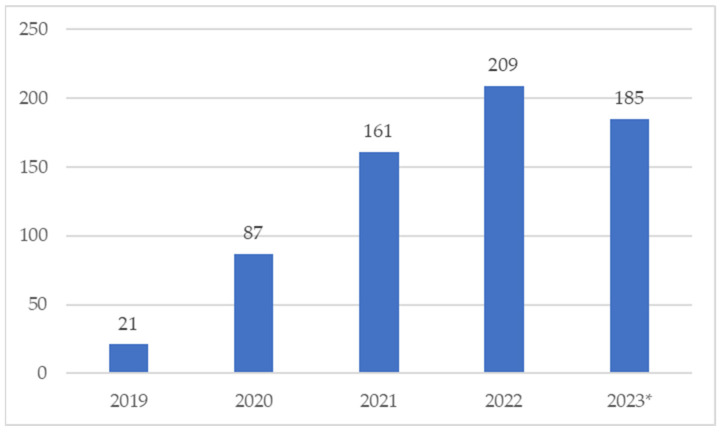
During our study period, 661 ICSRs related to the use of onasemnogene abeparvovec were collected into the EV, describing 2744 suspected adverse reactions (ADRs). Starting from 2019, a constant increase in ICSR reporting was observed, with a peak of 209 ICSRs sent in 2022, as shown in Figure 1.
Figure 1.
Distribution of Individual Case Safety Reports (ICSRs) reporting onasemnogene abeparvovec (Zolgensma®) as a suspected drug by year (2019–2023). * Up to 22 September 2023.
The demographic characteristics of the patients are described in Table 1. Considering the distribution of ICSRs for age groups, a higher proportion (n = 396; 59.9%) emerged for the infants group (2 months–2 years), followed by children (3–11 years), which comprised 76 ICSRs (11.5%). Neonates were represented in 9.2% of total ICSRs (n = 61). Among all cases, 287 ICSRs (43.4%) were related to female patients, while males accounted for 249 ICSRs (37.7%). In 125 ICSRs the patient sex was not specified. Healthcare professionals were the most represented reporter type, as they submitted the majority of onasemnogene-related ICSRs (88.5%) collected in the EV. The majority of the ICSRs reported only onasemnogene as a suspected drug (92.1%), with no concomitant drugs reported in 61.6% of cases. Prednisolone and nusinersen were the other suspected drugs more represented, described in 26 (35.1%) and 23 (31.1%) ICSRs, respectively. Risdiplam was indicated as suspected drug in only one case. All the other suspected drugs and their therapeutic indication are reported in Supplementary Table S1.
Table 1.
Demographic characteristics of Individual Case Safety Reports (ICSRs) having onasemnogene abeparvovec (Zolgensma®) as the suspected drug sent through the EV database from January 2019 to September 2023.
| Overall (n = 661) | |
|---|---|
| Age Group | |
| 0–1 month (neonates) | 61 (9.2%) |
| 2 months–2 years (infants) | 396 (59.9%) |
| 3–11 years (children) | 76 (11.5%) |
| 12–17 years (adolescents) | 1 (0.2%) |
| 18–64 years (adults) | 1 (0.2%) |
| Not specified | 126 (19.1%) |
| Patient Sex | |
| Female | 287 (43.4%) |
| Male | 249 (37.7%) |
| Not specified | 125 (18.9%) |
| Reporter Type | |
| Healthcare professional | 585 (88.5%) |
| Non-healthcare professional | 76 (11.5%) |
| Country | |
| European Economic Area | 319 (48.3%) |
| Non-European Economic Area | 342 (51.7%) |
| Concomitant Drugs per ICSR | |
| 0 | 407 (61.6%) |
| 1 | 143 (21.6%) |
| 2 | 49 (7.4%) |
| 3 | 33 (5.0%) |
| 4 | 12 (1.8%) |
| ≥5 | 17 (2.6%) |
| Suspected Drugs per ICSR | |
| 1 | 609 (92.1%) |
| 2 | 42 (6.4%) |
| 3 | 8 (1.2%) |
| 4 | 1 (0.2%) |
| ≥5 | 1 (0.2%) |
Regarding the seriousness distribution of ADRs, 43% were not serious (n = 1185), and more than 50% of reported adverse events were classified as serious. In particular, serious ADRs were mainly categorized as other medically important conditions (n = 721; 26.3%). Moreover, serious adverse events caused/prolonged the patient’s hospitalization or were life-threatening in 21% (n = 588) and 4.3% (n = 118) of the cases, respectively. Although in 54.7% of the cases the ADR outcome was unknown (n = 1502), the most reported outcomes were favorable, resulting as recovered/resolved (n = 676; 24.6%) or recovering/resolving (n = 238; 8.7%). On the other hand, 6.9% of ADRs did not resolve (n = 188) and a fatal outcome occurred for 130 out of all reported ADRs (4.7%) (Table 2). The 130 ADRs with the fatal outcome were described in a total of 39 ICSRs, mainly related to patients aged 2 months–2 years (n = 33). Only one fatal case was related to a patient aged 0–1 month and no fatal cases were related to the children’s group (3–11 years). Three ICSRs with fatal outcomes included nusinersen as another suspected drug. We have reported all adverse events with fatal outcomes in Supplementary Table S2. Fatal outcomes were mainly due to cardiac arrest (n = 8; 6.2%), respiratory arrest or respiratory failure (both n = 5; 3.8%), or acute hepatic failure (n = 4; 3.1%).
Table 2.
Characteristics of the suspected adverse drug reactions (ADRs) related to onasemnogene abeparvovec (Zolgensma®) sent to and collected in the European pharmacovigilance EudraVigilance database from 2019 to 22 September 2023.
| Overall ADRs (n = 2744) | |
|---|---|
| ADR Seriousness Criteria | |
| Caused/prolonged hospitalization | 588 (21.4%) |
| Disabling | 2 (0.1%) |
| Life-threatening | 118 (4.3%) |
| Not serious | 1185 (43.1%) |
| Other medically important condition | 721 (26.3%) |
| Results in death | 130 (4.7%) |
| ADR Outcome | |
| Fatal | 130 (4.7%) |
| Not recovered/not resolved | 188 (6.9%) |
| Recovered/resolved | 676 (24.6%) |
| Recovered/resolved with sequelae | 10 (0.4%) |
| Recovering/resolving | 238 (8.7%) |
| Unknown | 1502 (54.7%) |
In Table 3 we describe the ADRs most frequently reported, primarily resulting in pyrexia (n = 173; 6.3%), vomiting (141; 5.10%), increased aspartate and alanine aminotransferase (n = 129; 4.70% and n = 120; 4.40%), and thrombocytopenia (n = 118; 4.30%). In Table 3 we also compare the single reported ADRs with the total number of ICSRs. However, by adding those ADRs which were indicative of the same clinical adverse event but reported differently by the reporters with different terms, we found that 505 ADRs indicated an increase in hepatic enzyme, 195 ADRs indicated an increase in body temperature, and a decrease in count platelet was described 167 times, accounting in 76.40%, 29.50%, and 25.26% of the ICSRs, respectively.
Table 3.
The top twenty adverse events reported as suspected adverse drug reactions (ADRs) related to onasemnogene abeparvovec (Zolgensma®) sent to and collected in the European pharmacovigilance EudraVigilance database from 2019 to 22 September 2023.
| Reported Adverse Events | Overall ADRs (n = 2744) |
Overall ICSRs (n = 661) |
|
|---|---|---|---|
| n | % per Total ADRs | % per Total ICSRs | |
| Pyrexia | 173 | 6.30% | 26.17% |
| Vomiting | 141 | 5.10% | 21.33% |
| Aspartate aminotransferase increased | 129 | 4.70% | 19.52% |
| Alanine aminotransferase increased | 120 | 4.40% | 18.15% |
| Thrombocytopenia | 118 | 4.30% | 17.85% |
| Transaminases increased | 91 | 3.30% | 13.77% |
| Hepatic enzyme increased | 77 | 2.80% | 11.65% |
| Decreased appetite | 50 | 1.80% | 7.56% |
| Platelet count decreased | 49 | 1.80% | 7.41% |
| Troponin I increased | 40 | 1.50% | 6.05% |
| Pneumonia | 36 | 1.30% | 5.45% |
| Liver function test increased | 35 | 1.30% | 5.30% |
| Hypertransaminasaemia | 30 | 1.10% | 4.54% |
| Asthenia | 29 | 1.10% | 4.39% |
| Dyspnoea | 25 | 0.90% | 3.78% |
| Gamma-glutamyltransferase increased | 23 | 0.80% | 3.48% |
| Blood lactate dehydrogenase increased | 22 | 0.80% | 3.33% |
| Body temperature increased | 22 | 0.80% | 3.33% |
| Nausea | 21 | 0.80% | 3.18% |
| Apathy | 19 | 0.70% | 2.87% |
| Thrombotic microangiopathy | 19 | 0.70% | 2.87% |
In Figure 2, the reported adverse events are categorized in System Organ Classes (SOCs). Of these SOCs, “Investigations” (35.8%), “General disorders and administration site condition” (11.9%), “Gastrointestinal disorders” (9.3%), “Blood and lymphatic system disorders” (7.5%), and “Respiratory, thoracic and mediastinal disorders” (6.5%) were more frequently reported.
Figure 2.
Distribution of reported adverse Zolgensma®-related reactions categorized as System Organ Classes (SOCs).
Among the events belonging to the investigation SOC (Table 4), the most common ones indicated qualitative results of the conducted hepatic, hematopoietic, and cardiac clinical laboratory tests. In particular, increased hepatic values, like aspartate and alanine aminotransferase (n = 129 and n = 120) and gamma-glutamyl transferase (n = 23), as well as blood bilirubin (n = 11) or generic liver function tests increased (n = 35), accounting for more than 40% of the investigations. The decrease in platelet count (n = 49) and the increase in monocyte count (n = 13) emerged as hemopoietic disturbances. Other alterations, such as increased values of blood lactate dehydrogenase (n = 22) and troponin I (n = 40) and T (n = 15), were signs of tissue damage. Finally, alterations in the heart rhythm, acute hepatic failure, and hepatic cytolysis emerged among the cardiac and hepatic disorders, respectively, as described in Table 5 and Table 6. Regarding the neoplasms (benign, malignant, or unspecified (including cysts and polyps)) SOC, only one ICSR reported an adverse event belonging to this category. This was an astrocytoma malignant occurrence in a male patient in the 2 months–2 years group, which caused or prolonged his hospitalization, but for which the outcome was not available.
Table 4.
Adverse events reported in Zolgesma®-related ICSRs collected in the EudraVigilance spontaneous reporting system from 2019 to 22 September 2023, belonging to the Investigations MedDRA System Organ Class (frequency > 1.00%).
| Adverse Events Belonging to the Investigation MedDRA SOC (n = 982) | n | % |
|---|---|---|
| Aspartate aminotransferase increased | 129 | 13.10% |
| Alanine aminotransferase increased | 120 | 12.20% |
| Transaminases increased | 91 | 9.30% |
| Hepatic enzyme increased | 77 | 7.80% |
| Platelet count decreased | 49 | 5.00% |
| Troponin I increased | 40 | 4.10% |
| Liver function test increased | 35 | 3.60% |
| Gamma-glutamyltransferase increased | 23 | 2.30% |
| Blood lactate dehydrogenase increased | 22 | 2.20% |
| Body temperature increased | 22 | 2.20% |
| Troponin increased | 18 | 1.80% |
| Oxygen saturation decreased | 15 | 1.50% |
| Troponin T increased | 15 | 1.50% |
| C-reactive protein increased | 14 | 1.40% |
| Heart rate increased | 13 | 1.30% |
| Monocyte count increased | 13 | 1.30% |
| Blood bilirubin increased | 11 | 1.10% |
Table 5.
Adverse events reported in Zolgensma®-related ICSRs collected in the EudraVigilance spontaneous reporting system from 2019 to 22 September 2023, belonging to the Cardiac disorders MedDRA System Organ Class.
| Adverse Events Belonging to the Cardiac Disorders MedDRA SOC (n = 59) |
n | % |
|---|---|---|
| Tachycardia | 14 | 23.70% |
| Bradycardia | 11 | 18.60% |
| Cardiac arrest | 9 | 15.30% |
| Cardio-respiratory arrest | 4 | 6.80% |
| Tachyarrhythmia | 3 | 5.10% |
| Arrhythmia | 2 | 3.40% |
| Cardiac failure | 2 | 3.40% |
| Pericardial effusion | 2 | 3.40% |
| Pericarditis | 2 | 3.40% |
| Bradyarrhythmia | 1 | 1.70% |
| Cardiac disorder | 1 | 1.70% |
| Cardiomegaly | 1 | 1.70% |
| Myocardial hypoxia | 1 | 1.70% |
| Myocardial injury | 1 | 1.70% |
| Pulseless electrical activity | 1 | 1.70% |
| Sinus tachycardia | 1 | 1.70% |
| Toxic cardiomyopathy | 1 | 1.70% |
| Ventricular extrasystoles | 1 | 1.70% |
| Ventricular hypertrophy | 1 | 1.70% |
Table 6.
Adverse events reported in Zolgensma®-related ICSRs collected in the EudraVigilance spontaneous reporting system from 2019 to 22 September 2023, belonging to the Hepatobiliary disorders MedDRA System Organ Class (SOC).
| Adverse Events Belonging to the Hepatobiliary Disorders MedDRA SOC | n | % |
|---|---|---|
| Hypertransaminasaemia | 30 | 25.90% |
| Liver disorder | 10 | 8.60% |
| Acute hepatic faliure | 9 | 7.80% |
| Hepatic cytolysis | 9 | 7.80% |
| Abnormal hepatic function | 8 | 6.90% |
| Hepatitis | 8 | 6.90% |
| Hepatotoxicity | 6 | 5.20% |
| Drug-induced liver injury | 5 | 4.30% |
| Jaundice | 4 | 3.40% |
| Cholestasis | 3 | 2.60% |
| Hepatic failure | 3 | 2.60% |
| Hepatomegaly | 3 | 2.60% |
| Liver injury | 3 | 2.60% |
| Gallbladder enlargement | 2 | 1.70% |
| Hepatic fibrosis | 2 | 1.70% |
| Hyperbilirubinaemia | 2 | 1.70% |
| Ocular icterus | 2 | 1.70% |
| Autoimmune hepatitis | 1 | 0.90% |
| Cholangitis | 1 | 0.90% |
| Hepatic steatosis | 1 | 0.90% |
| Hepatosplenomegaly | 1 | 0.90% |
| Ischaemic hepatitis | 1 | 0.90% |
| Liver tenderness | 1 | 0.90% |
| Subacute hepatic failure | 1 | 0.90% |
3. Discussion
Our study aimed to describe the Zolgensma® safety profile emerging from a real-world context by analyzing data collected in the European pharmacovigilance database. Our choice was based on the consideration that, even if pre-marketing clinical trials allow the acquisition of the majority of important drug-safety data, only use in a real-world context can better define the safety aspects, allowing for the identification of rarer ADRs [15,16,17,18]. For these reasons, continuous post-marketing monitoring of drug safety is essential. Long-term safety evaluation is always considered necessary, especially for drugs that have been authorized with conditional and/or accelerated approvals, as often happens for innovative drugs [19,20,21,22]. This necessity is amplified for some particular drug classes, like the orphan ones used for rare diseases [23]. Zolgensma® meets all these mentioned characteristics, as an innovative drug, supported through EMA’s PRIority MEdicines (PRIME) scheme [24], which received a conditional marketing authorization for the rare disease, SMA [24]. The efficacy and safety data obtained in post-marketing can be particularly important for possible confirmation or re-evaluation of a drug’s safety and efficacy profiles and innovativeness, as well as its price [25,26]. Also, this aspect is particularly relevant for Zolgensma®, considering its high cost (approximately $2 million per course of treatment) [27]. However, some pharmacoeconomic studies in the literature support the cost-effectiveness of this gene therapy compared with the other therapeutic possibilities, when used in pre-symptomatic patients [28,29,30,31,32].
From our analysis, 661 safety reports related to Zolgensma® and collected in the European pharmacovigilance database emerged. Excluding the partial data referred to 2023, the annual reporting trend was growing up to 2022. Our reporting trend differed compared with the trend that emerged from a recent study conducted on the US pharmacovigilance database [33]. Following the surge in the Zolgensma®-related ADR reporting in 2020 compared with 2019, the authors found a decreasing trend in the number of reports collected until 2022 in the US database, the Food and Drug Administration Adverse Event Reporting System (FAERS) [33]. Regarding the sex distribution, although some evidence suggests that males may be more vulnerable to SMA than females [34,35], our analysis revealed a greater reporting of ADRs in female patients. This result, as well as the major distribution in terms of the reporter type in favor of HPs, is in line with the analysis conducted by Zhuang et al. on the US database [33]. As described in other studies, HPs represent the major reporting source, even if, to date, citizens can also send safety reports to regulatory authorities [36,37]. The distribution of reports by age group of patients is also comparable, although not completely coincident. Our analysis revealed that the majority of reports referred to patients aged between 2 months and 2 years, while the analysis of FAERS data revealed that the majority of adverse events reported for onasemnogene abeparvovec referred to patients less than 1 year of age. However, this slight discrepancy between the two results could be traced back to the different methods of data extraction and categorization related to the age of the patients. The data extraction method used for the present pharmacovigilance study did not allow us to trace the precise age of the patient, but only the age group to which it referred.
As regards the types of adverse events, our results were in line with the recently published Zhuang et al. study [33]. Both pharmacovigilance studies highlighted a higher reporting of pyrexia, vomiting, increased aspartate aminotransferase, increased alanine aminotransferase, thrombocytopenia, increased transaminases, and increased liver enzymes, as ADRs related to the SMA gene therapy. Hepatotoxicity, transient thrombocytopenia, and thrombotic microangiopathy have been identified as possible Zolgensma® toxicities [13]. These safety aspects emerged among the top twenty ADRs reported in our dataset. In addition to possible liver damage, signs of cardiac damage also emerged among the most frequently adverse events reported, such as the increase in troponin I. The heart and the liver had also been found to be the main target organs of Zolgensma® toxicity in pre-clinical studies [38]. According to the main safety results derived from non-clinical studies, inflammation, edema, fibrosis, and characteristics of widespread myocardial degeneration/regeneration in the ventricles emerged at the cardiac level in mice. These findings were present at all studied doses and were dose-related in terms of seriousness [38].
As regards a possible hepatotoxicity risk, our results confirmed the possible hepatic damage evidenced by increased hepatic enzymes or tests (ALT and AST). This safety aspect, already found in pre-clinical studies [38], emerged from the first patients enrolled in pre-marketing clinical trials [39]. In post-mortem tissue samples from two patients treated with onasemnogene abeparvovec, the highest concentrations of vector DNA were found in the liver [39]. Safety signals have also emerged in the post-marketing context about the liver and heart organs [38]. Similarly, our analysis revealed reports of possible liver damage (hypertransaminasemia, liver disorder, acute liver failure, and liver cytolysis) and cardiac rhythm alterations. The increase in hepatic enzymes can be caused by the immune response induced the gene therapy [40]. Both hepatobiliary and hematologic abnormalities can be considered the immune response to the viral capsid. Moreover, hepatoxicity risk is complicated by the major predisposition of patients with SMA to acute liver injury [39]. Abnormal fatty acid metabolism, as the reported cause of liver failure, was reported in children with SMA [40]. Their increased risk of dyslipidemia and fatty liver could predispose them to hepatotoxicity [41]. Zolgensma®-related hepatoxicity can generally be mitigated with prophylactic prednisolone. This latter emerged among the most frequently reported other concomitant drug in our study. These hepatic and heart rhythm disorders also emerged from the analysis of the US FAERS pharmacovigilance database. Furthermore, it is important to note that, in both studies, a risk of damage to the heart was highlighted, evidenced by an increase in troponin blood concentrations. According to Zhuang et al., such increased levels of troponin I and T have been identified as idiosyncratic adverse reactions to onasemnogene abeparvovec [33]. However, the possible causal relationship and the biological plausibility between onasemnogene abeparvovec treatment and cardiotoxicity is still unclear. An increased risk of cardiac events may be related to SMA itself. Out of all the suspected ADRs reported, some may be symptoms of progression of SMA, as a disease with a very broad and subjective spectrum of symptoms, which also change based on the type of SMA. Heart and liver function should be monitored before, during, and after use of the drug to prevent more serious manifestations. In addition to hepatic and cardiac adverse events, serious respiratory adverse events, such as bronchiolitis, pneumonia, respiratory distress, and respiratory syncytial virus bronchiolitis, were also observed in the STR1VE trial. However, they were considered unrelated to onasemnogene abeparvovec [41]. Pneumonia and dyspnea emerged among the top twenty ADRs in our analysis. Regarding the possible tumorgenicity due to chromosomal integration as a potential Zolgensma® adverse event, only one case describing a tumoral adverse event emerged in our analysis. This potential risk certainly requires more long-term monitoring and careful analysis with more appropriate methodologies. The same applies to the hypothetical risk of dorsal root ganglion toxicity, which is more difficult to identify through pharmacovigilance database analysis. However, none of the retrieved ICSRs involved either areflexia or hyporeflexia, which have emerged in other studies as sensory abnormalities suggestive of gangliopathy [42].
Our study showed some strengths as well as some limitations. The use of a low-cost, broad database, coming from the real world setting, overcame several limitations of data collection during the clinical and pre-approval trials, and helped in characterizing drug safety profiles. However, this study also had several limitations, particularly the underreporting phenomenon, which characterizes all the spontaneous reporting systems [43,44,45,46]. This phenomenon can have repercussions for public health, interfering with the ADRs’ incidence quantification and risk estimates, as well as delaying the identification of safety signals. Only 6–10% of all adverse events are reported to regulatory authorities [43,47]. We cannot exclude the fact that some safety cases may not have been reported to the drug authorities and thus not collected in the EV. In addition to the underreporting, possible missing information can also be a difficulty in analysis based on spontaneous reporting systems. The quality of information reported may be incomplete and lack useful clinical information. This can delay a complete evaluation of confounding factors such as clinical history and concomitant comorbidities and medications. From our data source, we also could not retrieve information on the exact dates of administration and event onset—useful for a long-term safety evaluation. Moreover, our data source did not report the exact number of patients exposed to the treatment as real users of Zolgensma®; this would be necessary for reporting-rate evaluation. Considering these limitations, our study only aimed to analyze ICSRs related to Zolgensma® and to show its adverse event characteristics; it refrained from asserting any direct causal association between the SMA gene therapy and the adverse events reported as suspected ADRs. Considering the peculiarities of the rare disease, SMA, further investigations with appropriate methodologies are needed. However, analysis of the post-marketing database often represents the first step or the primum movens in identifying new safety aspects needing deeper examination.
4. Materials and Methods
On 22 September 2023, we retrieved data on Individual Case Safety Reports (ICSRs) reporting Zolgensma® as a suspected drug from the EV, the EMA pharmacovigilance database (www.adrreports.eu, accessed on 22 September 2023). This is a publicly available database, that contains the spontaneous safety reports submitted to the EMA [15]. Analysis of pharmacovigilance databases allows for early detection of safety signals and quantification of the association between drugs and reported adverse events (AEs) [16,17]. A wide variety of sources can send safety reports, both healthcare professionals (HPs) (e.g., physicians, pharmacists, and nurses) and non-healthcare professionals (patients, citizens, lawyers, and consumers) [18,19,20,21,22,23]. ICSRs reporting onasemnogene as a suspected drug were searched and a list of cases was exported. Our study period was 1 January 2019–22 September 2023. We checked for duplicates highlighted by the same ICSR code. We conducted an observational retrospective study, verifying the reporting annual trend, the distribution of the reports by sex and age group, the source country, and the reporter type, as well as the number of concomitant or suspected drugs. We analyzed the downloaded data. In the EV, the age groups are categorized as neonates, including preterm and term newborns (0–1 month), infants (2 months–2 years), and children (3–11 years). The reporter types are classified as healthcare professional and non-healthcare professional. The source country is not specified, but it is distinguished as belonging to the European Economic Area or the Non-European Economic Area. The suspected adverse drug reactions (ADRs) were analyzed in terms of seriousness, outcome, and type of event. According to current pharmacovigilance regulations, an ADR is categorized as serious if it induces death, hospitalization or prolongation of hospitalization, severe or permanent disability, threat to life, congenital abnormalities/birth defects, or is considered clinically relevant.
In the EV, the reported ADRs are coded according to the Medical Dictionary for Regulatory Activities (MedDRA). We analyzed ADRs based on Preferred Terms (PTs) and corresponding System Organ Class (SOC). We focused on the most representative SOC, and the events belonging to Hepatobiliary disorders and Cardiac disorders SOCs. Since each ICSR can contain more than one ADR, the total number of ADRs can be higher than the overall ICSR number. Moreover, cases with fatal outcomes were investigated as a sub-analysis, describing their distribution in terms of sex and age group, as well as the type of fatal event. All data manipulation and statistical analysis were performed using R Statistical Software (version 4.0.3).
5. Conclusions
Our study aimed to better define the safety profile of drugs, by using the spontaneous reports of safety data in routine clinical practice. The results of our study confirmed hepatoxicity as the principal issue emerging from Zolgensma® use in clinical practice. Considering that SMA children are predisposed to liver dysfunction, hepatic monitoring is necessary during the treatment. In the same way, cardiac monitoring is also important, considering the possible cardiotoxicity induced by Zolgensma®, even if this latter requires more investigations to better define it and its plausible correlation to the treatment. From our analysis, alterations in the heart rhythm, acute hepatic failure, and hepatic cytolysis emerged among the cardiac and hepatic disorders, respectively.
Approval of Zolgensma® for the treatment of SMA has shown evident benefits. Nevertheless, some safety concerns remain unresolved, especially long-term ones. These latter include the potential dorsal root ganglion (DRG) toxicity and late-onset motor dysfunction that emerged as long-term AAV9-mediated SMN overexpression in mouse models [48]. In this context, systematic data collection and long-term follow-up could better define Zolgensma’s long-term safety profile. This is particularly important for the hypothetical tumorgenicity due to chromosomal interaction, which has been identified as an important potential risk. In conclusion, further studies and continuous monitoring are therefore necessary for SMA gene therapy, as an important and expensive therapeutic strategy for a rare disease. The systematic and spontaneous collection of post-marketing data is even more fundamental in the context of such rare diseases and orphan and innovative drugs such as Zolgensma®.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ph17030394/s1, Table S1: Other suspected drugs reported in the Individual Case Safety Reports related to Zolgensma® (onasemnogene abeparvovec) collected in the EudraVigilance database from 2019 to 22 September 2023 and their distribution by therapeutic indications. Table S2: MedDRA Preferred Terms with fatal outcome reported in the Individual Case Safety Reports related to Zolgensma® (onasemnogene abeparvovec) collected in the EudraVigilance database from 2019 to 22 September 2023 and their distribution by patient sex and age groups.
Author Contributions
R.R., conceptualization, methodology, data curation, and writing—original draft; N.B., methodology, data curation, and writing—original draft; M.M.N., data curation; G.d.M., methodology, and data curation; F.F., methodology, data curation, and writing—original draft; M.R.C., investigation, writing—review and editing, and supervision; F.R., conceptualization, validation, and project administration; A.C., validation, supervision, and funding acquisition. Final approval of manuscript: all authors. Accountable for all aspects of the work: all authors. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
EV data are publicly available at https://www.adrreports.eu/, accessed on 22 September 2023.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Chaytow H., Huang Y.T., Gillingwater T.H., Faller K.M.E. The Role of Survival Motor Neuron Protein (SMN) in Protein Homeostasis. Cell. Mol. Life Sci. 2018;75:3877–3894. doi: 10.1007/s00018-018-2849-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Verhaart I.E.C., Robertson A., Wilson I.J., Aartsma-Rus A., Cameron S., Jones C.C., Cook S.F., Lochmüller H. Prevalence, Incidence and Carrier Frequency of 5q-Linked Spinal Muscular Atrophy-a Literature Review. Orphanet J. Rare Dis. 2017;12:124. doi: 10.1186/s13023-017-0671-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ross L.F., Kwon J.M. Spinal Muscular Atrophy: Past, Present, and Future. Neoreviews. 2019;20:e437–e451. doi: 10.1542/neo.20-8-e437. [DOI] [PubMed] [Google Scholar]
- 4.Oliveira D., Sarkar P.S., Zeng C.-W., Tedesco B., Dimitriadi M., Rashid S. Autophagy in Spinal Muscular Atrophy: From Pathogenic Mechanisms to Therapeutic Approaches. Front. Cell. Neurosci. 2024;17:1307636. doi: 10.3389/fncel.2023.1307636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Younger D.S., Mendell J.R. Childhood Spinal Muscular Atrophy. Handb. Clin. Neurol. 2023;196:43–58. doi: 10.1016/B978-0-323-98817-9.00030-2. [DOI] [PubMed] [Google Scholar]
- 6.Kolb S.J., Kissel J.T. Spinal Muscular Atrophy. Neurol. Clin. 2015;33:831–846. doi: 10.1016/j.ncl.2015.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arnold E.S., Fischbeck K.H. Spinal Muscular Atrophy. Handb. Clin. Neurol. 2018;148:591–601. doi: 10.1016/B978-0-444-64076-5.00038-7. [DOI] [PubMed] [Google Scholar]
- 8.Lefebvre S., Sarret C. Pathogenesis and Therapeutic Targets in Spinal Muscular Atrophy (SMA) Arch. Pédiatrie. 2020;27:7S3–7S8. doi: 10.1016/S0929-693X(20)30269-4. [DOI] [PubMed] [Google Scholar]
- 9.Crisafulli S., Boccanegra B., Vitturi G., Trifirò G., De Luca A. Pharmacological Therapies of Spinal Muscular Atrophy: A Narrative Review of Preclinical, Clinical–Experimental, and Real-World Evidence. Brain Sci. 2023;13:1446. doi: 10.3390/brainsci13101446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hagenacker T., Schara-Schmidt U. Gene Replacement Therapy in Spinal Muscular Atrophy: Filling the Data Gaps. Lancet Reg. Health—Eur. 2024;37:100822. doi: 10.1016/j.lanepe.2023.100822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mendell J.R., Al-Zaidy S., Shell R., Arnold W.D., Rodino-Klapac L.R., Prior T.W., Lowes L., Alfano L., Berry K., Church K., et al. Single-Dose Gene-Replacement Therapy for Spinal Muscular Atrophy. N. Engl. J. Med. 2017;377:1713–1722. doi: 10.1056/NEJMoa1706198. [DOI] [PubMed] [Google Scholar]
- 12.Al-Zaidy S., Pickard A.S., Kotha K., Alfano L.N., Lowes L., Paul G., Church K., Lehman K., Sproule D.M., Dabbous O., et al. Health Outcomes in Spinal Muscular Atrophy Type 1 Following AVXS-101 Gene Replacement Therapy. Pediatr. Pulmonol. 2019;54:179–185. doi: 10.1002/ppul.24203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.European Medicines Agency (EMA) Summary of the Risk Management for Zolgensma® (onasemnogene abeparvovec). Last updated:18/10/2023. [(accessed on 3 March 2024)]; Available online: https://www.ema.europa.eu/en/documents/rmp-summary/zolgensma-epar-risk-management-plan-summary_en.pdf.
- 14.Zinzi A., Gaio M., Liguori V., Ruggiero R., Tesorone M., Rossi F., Rafaniello C., Capuano A. Safety Monitoring of MRNA COVID-19 Vaccines in Children Aged 5 to 11 Years by Using EudraVigilance Pharmacovigilance Database: The CoVaxChild Study. Vaccines. 2023;11:401. doi: 10.3390/vaccines11020401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ruggiero R., Balzano N., Di Napoli R., Fraenza F., Pentella C., Riccardi C., Donniacuo M., Tesorone M., Danesi R., Del Re M., et al. Do Peripheral Neuropathies Differ among Immune Checkpoint Inhibitors? Reports from the European Post-Marketing Surveillance Database in the Past 10 Years. Front. Immunol. 2023;14:1134436. doi: 10.3389/fimmu.2023.1134436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rogers J.R., Sarpatwari A., Desai R.J., Bohn J.M., Khan N.F., Kesselheim A.S., Fischer M.A., Gagne J.J., Connolly J.G. Effect of Lawyer-Submitted Reports on Signals of Disproportional Reporting in the Food and Drug Administration’s Adverse Event Reporting System. Drug Saf. 2019;42:85–93. doi: 10.1007/s40264-018-0703-x. [DOI] [PubMed] [Google Scholar]
- 17.Toki T., Ono S. Spontaneous Reporting on Adverse Events by Consumers in the United States: An Analysis of the Food and Drug Administration Adverse Event Reporting System Database. Drugs—Real World Outcomes. 2018;5:117–128. doi: 10.1007/s40801-018-0134-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sienkiewicz K., Burzyńska M., Rydlewska-Liszkowska I., Sienkiewicz J., Gaszyńska E. The Importance of Direct Patient Reporting of Adverse Drug Reactions in the Safety Monitoring Process. Int. J. Environ. Res. Public Health. 2022;19:413. doi: 10.3390/ijerph19010413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herdeiro M.T., Figueiras A., Polónia J., Gestal-Otero J.J. Physicians’ Attitudes and Adverse Drug Reaction Reporting: A Case-Control Study in Portugal. Drug Saf. 2005;28:825–833. doi: 10.2165/00002018-200528090-00007. [DOI] [PubMed] [Google Scholar]
- 20.Hoffman K.B., Demakas A.R., Dimbil M., Tatonetti N.P., Erdman C.B. Stimulated Reporting: The Impact of US Food and Drug Administration-Issued Alerts on the Adverse Event Reporting System (FAERS) Drug Saf. 2014;37:971–980. doi: 10.1007/s40264-014-0225-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Castel E.S., Ginsburg L.R., Zaheer S., Tamim H. Understanding Nurses’ and Physicians’ Fear of Repercussions for Reporting Errors: Clinician Characteristics, Organization Demographics, or Leadership Factors? BMC Health Serv. Res. 2015;15:326. doi: 10.1186/s12913-015-0987-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bihan K., Lebrun-Vignes B., Funck-Brentano C., Salem J.E. Uses of Pharmacovigilance Databases: An Overview. Therapie. 2020;75:591–598. doi: 10.1016/j.therap.2020.02.022. [DOI] [PubMed] [Google Scholar]
- 23.Lucas S., Ailani J., Smith T.R., Abdrabboh A., Xue F., Navetta M.S. Pharmacovigilance: Reporting Requirements throughout a Product’s Lifecycle. Ther. Adv. Drug Saf. 2022;13:20420986221125006. doi: 10.1177/20420986221125006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.European Medicine Agency . New Gene Therapy to Treat Spinal Muscolar Atrophy. Volume 31 European Medicine Agency; Amsterdam, The Netherlands: 2020. [Google Scholar]
- 25.Martini N., Trifirò G., Capuano A., Corrao G., Corrao G., Racagni G., Pani L. Expert Opinion on Real World Evidence RWE in Drug Development and Usage. Pharmadvances. 2020;2:41–50. doi: 10.36118/pharmadvances.02.2020.01. [DOI] [Google Scholar]
- 26.Pani L., Cicchetti A., De Luca A., Mennini F.S., Mini E., Nocentini G., Racagni G., Jommi C. Pricing for Multi Indication Medicines: A Discussion with Italian Experts. Pharmadvances. 2022;4:163–170. doi: 10.36118/pharmadvances.2022.28. [DOI] [Google Scholar]
- 27.Nuijten M. Pricing Zolgensma—The World’s Most Expensive Drug. J. Mark. Access Health Policy. 2022;10:2022353. doi: 10.1080/20016689.2021.2022353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dean R., Jensen I., Cyr P., Miller B., Maru B., Sproule D.M., Feltner D.E., Wiesner T., Malone D.C., Bischof M., et al. An Updated Cost-Utility Model for Onasemnogene Abeparvovec (Zolgensma®) in Spinal Muscular Atrophy Type 1 Patients and Comparison with Evaluation by the Institute for Clinical and Effectiveness Review (ICER) J. Mark. Access Heal. Policy. 2021;9:1889841. doi: 10.1080/20016689.2021.1889841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mitsue Ivama-Brummell A., Wagner A.K., Lúcia V., Pepe E., Naci H. Ultraexpensive Gene Therapies, Industry Interests and the Right to Health: The Case of Onasemnogene Abeparvovec in Brazil Commentary. BMJ Glob. Health. 2022;7:8637. doi: 10.1136/bmjgh-2022-008637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dias Fernandes B., D’Athayde Rodrigues F., Cardoso Cirilo H.N., Borges S.S., Krug B.C., Fernandes Probst L., Zimmermann I. Economic Evaluation Cost-Effectiveness of Onasemnogene Abeparvovec Compared with Nusinersen and Risdiplam in Patients with Spinal Muscular Atrophy Type 1 in Brazil. Value Health Reg. Issues. 2024;40:108–117. doi: 10.1016/j.vhri.2023.11.004. [DOI] [PubMed] [Google Scholar]
- 31.Dangouloff T., Botty C., Beaudart C., Servais L., Hiligsmann M. Systematic Literature Review of the Economic Burden of Spinal Muscular Atrophy and Economic Evaluations of Treatments. Orphanet J. Rare Dis. 2021;16:47. doi: 10.1186/s13023-021-01695-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Malone D.C., Dean R., Arjunji R., Jensen I., Cyr P., Miller B., Maru B., Sproule D.M., Feltner D.E., Dabbous O. Cost-Effectiveness Analysis of Using Onasemnogene Abeparvocec (AVXS-101) in Spinal Muscular Atrophy Type 1 Patients. J. Mark. Access Health Policy. 2019;7:1601484. doi: 10.1080/20016689.2019.1601484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhuang W., Lu M., Wu Y., Chen Z., Wang M., Wang X., Guan S., Lin W. Safety Concerns with Nusinersen, Risdiplam, and Onasemnogene Abeparvovec in Spinal Muscular Atrophy: A Real-World Pharmacovigilance Study. Clin. Drug Investig. 2023;43:949–962. doi: 10.1007/s40261-023-01320-4. [DOI] [PubMed] [Google Scholar]
- 34.Sun J., Harrington M.A., Porter B. Sex Difference in Spinal Muscular Atrophy Patients—Are Males More Vulnerable? J. Neuromuscul. Dis. 2023;10:847. doi: 10.3233/JND-230011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bianco A., Antonacci Y., Liguori M. Sex and Gender Differences in Neurodegenerative Diseases: Challenges for Therapeutic Opportunities. Int. J. Mol. Sci. 2023;24:6354. doi: 10.3390/ijms24076354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rossi C., Ruggiero R., Sportiello L., Pentella C., Gaio M., Pinto A., Rafaniello C. Did the COVID-19 Pandemic Affect Contrast Media-Induced Adverse Drug Reaction’s Reporting? A Pharmacovigilance Study in Southern Italy. J. Clin. Med. 2022;11:5104. doi: 10.3390/jcm11175104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scavone C., Carnovale C., Ruggiero R., Radice S., Scatigna M., Racagni G., Mugelli A., Rossi F., Clementi E., Capuano A. On the Policy of the Italian Government in the Discovery, Development, and Access to Medicines. Clin. Ther. 2018;40:1931–1940. doi: 10.1016/j.clinthera.2018.09.015. [DOI] [PubMed] [Google Scholar]
- 38.Day J.W., Mendell J.R., Mercuri E., Finkel R.S., Strauss K.A., Kleyn A., Tauscher-Wisniewski S., Tukov F.F., Reyna S.P., Chand D.H. Clinical Trial and Postmarketing Safety of Onasemnogene Abeparvovec Therapy. Drug Saf. 2021;44:1109. doi: 10.1007/s40264-021-01107-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blair H.A. Onasemnogene Abeparvovec: A Review in Spinal Muscular Atrophy. CNS Drugs. 2022;36:995–1005. doi: 10.1007/s40263-022-00941-1. [DOI] [PubMed] [Google Scholar]
- 40.Chand D., Mohr F., Mcmillan H., Tukov F.F., Montgomery K., Kleyn A., Sun R., Tauscher-Wisniewski S., Kaufmann P., Kullak-Ublick G. Hepatotoxicity Following Administration of Onasemnogene Abeparvovec (AVXS-101) for the Treatment of Spinal Muscular Atrophy. J. Hepatol. 2020;74:560–566. doi: 10.1016/j.jhep.2020.11.001. [DOI] [PubMed] [Google Scholar]
- 41.Day J.W., Finkel R.S., Chiriboga C.A., Connolly A.M., Crawford T.O., Darras B.T., Iannaccone S.T., Kuntz N.L., Peña L.D.M., Shieh P.B., et al. Onasemnogene Abeparvovec Gene Therapy for Symptomatic Infantile-Onset Spinal Muscular Atrophy in Patients with Two Copies of SMN2 (STR1VE): An Open-Label, Single-Arm, Multicentre, Phase 3 Trial. Artic. Lancet Neurol. 2021;20:284–293. doi: 10.1016/S1474-4422(21)00001-6. [DOI] [PubMed] [Google Scholar]
- 42.Strauss K.A., Farrar M.A., Muntoni F., Saito K., Mendell J.R., Servais L., Mcmillan H.J., Finkel R.S., Swoboda K.J., Kwon J.M., et al. Onasemnogene Abeparvovec for Presymptomatic Infants with Two Copies of SMN2 at Risk for Spinal Muscular Atrophy Type 1: The Phase III SPR1NT Trial. Nat. Med. 2022;28:1381–1389. doi: 10.1038/s41591-022-01866-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.García-Abeijon P., Costa C., Taracido M., Herdeiro M.T., Torre C., Figueiras A. Factors Associated with Underreporting of Adverse Drug Reactions by Health Care Professionals: A Systematic Review Update. Drug Saf. 2023;46:625–636. doi: 10.1007/s40264-023-01302-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Biagi C., Montanaro N., Buccellato E., Roberto G., Vaccheri A., Motola D. Underreporting in Pharmacovigilance: An Intervention for Italian GPs (Emilia-Romagna Region) Eur. J. Clin. Pharmacol. 2013;69:237–244. doi: 10.1007/s00228-012-1321-7. [DOI] [PubMed] [Google Scholar]
- 45.Pellegrino P., Carnovale C., Cattaneo D., Perrone V., Antoniazzi S., Pozzi M., Napoleone E., Filograna M.R., Clementi E., Radice S. Pharmacovigilance Knowledge in Family Paediatricians. A Survey Study in Italy. Health Policy. 2013;113:216–220. doi: 10.1016/j.healthpol.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 46.Kumar A., Shariff M., Bhat V., DeSimone C., Deshmukh A. Atrial Fibrillation after Vaccination for COVID-19: Analysis of the Vaccine Adverse Event Reporting System. J. Interv. Card. Electrophysiol. 2022;65:1–2. doi: 10.1007/s10840-022-01263-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lopez-Gonzalez E., Herdeiro M.T., Figueiras A. Determinants of Under-Reporting of Adverse Drug Reactions A Systematic Review. Drug Saf. 2009;32:19–31. doi: 10.2165/00002018-200932010-00002. [DOI] [PubMed] [Google Scholar]
- 48.Van Alstyne M., Tattoli I., Delestrée N., Recinos Y., Workman E., Shihabuddin L.S., Zhang C., Mentis G.Z., Pellizzoni L. Gain of Toxic Function by Long-Term AAV9-Mediated SMN Overexpression in the Sensory-Motor Circuit. Nat. Neurosci. 2021;24:930. doi: 10.1038/s41593-021-00827-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
EV data are publicly available at https://www.adrreports.eu/, accessed on 22 September 2023.