Abstract
Dry rot of potato tubers is a harmful disease caused by species of the Fusarium genus. Studies on the composition and features of Fusarium spp. that cause the disease in Russia are limited. Thirty-one Fusarium strains belonging to the F. sambucinum species complex (FSAMSC) and F. solani species complex (FSSC) were accurately identified using multilocus phylogenetic analysis of the tef and rpb2 loci, and their physiological characteristics were studied in detail. As a result, 21 strains of F. sambucinum s. str. and 1 strain of F. venenatum within the FSAMSC were identified. Among the analyzed strains within the FSSC, one strain of F. mori, four strains of F. noneumartii, and two strains of both F. stercicola and F. vanettenii species were identified. This is the first record of F. mori on potato as a novel host plant, and the first detection of F. noneumartii and F. stercicola species in Russia. The clear optimal temperature for the growth of the strains belonging to FSAMSC was noted to be 25 °C, with a growth rate of 11.6–15.0 mm/day, whereas, for the strains belonging to FSSC, the optimal temperature range was between 25 and 30 °C, with a growth rate of 5.5–14.1 mm/day. The distinctive ability of F. sambucinum strains to grow at 5 °C has been demonstrated. All analyzed Fusarium strains were pathogenic to potato cv. Gala and caused extensive damage of the tuber tissue at an incubation temperature of 23 °C for one month. Among the fungi belonging to the FSAMSC, the F. sambucinum strains were more aggressive and caused 23.9 ± 2.2 mm of necrosis in the tubers on average compared to the F. venenatum strain—17.7 ± 1.2 mm. Among the fungi belonging to the FSSC, the F. noneumartii strains were the most aggressive and caused 32.2 ± 0.8 mm of necrosis on average. The aggressiveness of the F. mori, F. stercicola, and especially the F. vanettenii strains was significantly lower: the average sizes of damage were 17.5 ± 0.5 mm, 17.2 ± 0.2 mm, and 12.5 ± 1.7 mm, respectively. At an incubation temperature of 5 °C, only the F. sambucinum strains caused tuber necroses in the range of 6.7 ± 0.5–15.9 ± 0.8 mm.
Keywords: Fusarium sambucinum, F. venenatum, F. noneumartii, F. mori, F. stercicola, F. vanettenii, phylogeny, growth rate, Solanum tuberosum, aggressiveness
1. Introduction
A serious problem in potato growing is crop losses due to fungal diseases. Dry rot of potato caused by Fusarium fungi is widespread and reduces the food and seed quality of tubers [1,2,3]. Fusarium fungi infect tubers in the field and during storage [4]. The vegetative propagation method of potato contributes to the spread of pathogens residing on tubers. Fusarium dry rot on tubers appear as brown spots, sometimes with a dark border, and the internal tissues of the tuber under the influence of fungi often leads to the formation of concentric rings and wrinkling of the periderm [5,6].
According to published information, about 11–13 different species of the Fusarium genus are associated with potato dry rot, but the species composition of the fungi depends on environmental conditions [3,7,8]. The F. sambucinum species belonging to the F. sambucinum species complex (FSAMSC) is often identified in the complex of pathogens of tubers: it dominates among other Fusarium fungi in Europe [2,9,10], Asia [1,11,12,13], Africa [3], and North America [14,15,16]. Other predominant species in the mycobiota of potato tubers are members of the F. solani species complex (FSSC), F. oxysporum species complex, and F. tricinctum species complex, as well as fungi from the F. redolens and F. equiseti-incarnatum species complexes, which were sporadically isolated [2,10,11,12,16,17,18,19].
The aggressiveness of different Fusarium spp. to potato plants varies significantly [12,19,20]. The most common pathogenicity assay is the tuber plugging method, which is widely applicable because it allows to obtain unambiguous information on the aggressiveness of strains and reveals the susceptibility of potato cultivars to fungal infection [1,2,3,12,17,21,22,23,24].
New knowledge about Fusarium species’ diversity and the effect of environmental conditions on the physiological characters of these fungi is essential to recognize prevention opportunities and develop methods of disease control.
The aim of this study was the molecular identification of Fusarium strains isolated from potato tubers with dry rot symptoms and to characterize their physiological characters.
2. Materials and Methods
2.1. Fusarium Strains
In 2021–2022, tubers with symptoms of dry rot were selected from 46 batches of seed potatoes collected in the Northwest, Central European, South European, Volga, and West Siberian regions of Russia. The isolation of fungi from plant tissues was carried out on a nutrient medium using the conventional mycological method. The tissues were placed on potato–sucrose agar (PSA) containing a mixture of 1 mL/L penicillin–streptomycin solution (HyClone™, GE Healthcare Life Sciences, Wien, Austria) and 0.4 μL/L Triton X-100 (Panreac, Barcelona, Spain). Following incubation at 25 °C, the cultures resembling Fusarium fungi were transferred to fresh PSA and synthetic nutrient agar (SNA).
The single-spore Fusarium strains (MFG) were deposited in the fungal culture collection of the Laboratory of Mycology and Phytopathology at the All-Russian Institute of Plant Protection (St. Petersburg, Russia). The 31 isolates of Fusarium spp. that phenotypically belong to the FSAMSC (22 strains) and the FSSC (9 strains) were selected for a detailed investigation (Table 1). In our study, we also included 12 strains from the collection that were isolated from potato tubers in 2020.
Table 1.
Fusarium spp. included in the study.
| Species | Species Complex |
Strain ID | Origin | Year | GenBank Accessions | |
|---|---|---|---|---|---|---|
| tef | rpb2 | |||||
| F. sambucinum | FSAMSC * | MFG 60833 | Russia: Vologda region | 2020 | OR020701 | OR727754 |
| F. sambucinum | FSAMSC | MFG 60834 | Russia: Vologda region | 2020 | OR020702 | OR727755 |
| F. sambucinum | FSAMSC | MFG 70102 | Russia: Novgorod region | 2020 | OR020704 | OR727756 |
| F. sambucinum | FSAMSC | MFG 70133 | Russia: Yaroslavl region | 2020 | OR020710 | OR727758 |
| F. sambucinum | FSAMSC | MFG 70134 | Russia: Yaroslavl region | 2020 | OR020711 | OR727759 |
| F. sambucinum | FSAMSC | MFG 70135 | Russia: Stavropol region | 2020 | OR020712 | OR727760 |
| F. sambucinum | FSAMSC | MFG 70149 | Russia: Samara region | 2020 | OR020717 | OR727761 |
| F. sambucinum | FSAMSC | MFG 70160 | Russia: Moscow region | 2021 | OR020724 | OR727762 |
| F. sambucinum | FSAMSC | MFG 70162 | Russia: Chuvashia | 2021 | OR020725 | OR727763 |
| F. sambucinum | FSAMSC | MFG 70166 | Russia: Chuvashia | 2021 | OR020728 | OR727764 |
| F. sambucinum | FSAMSC | MFG 70175 | Russia: Kaluga region | 2021 | OR020730 | OR727753 |
| F. sambucinum | FSAMSC | MFG 70201 | Russia: Vologda region | 2021 | OR020734 | OR727765 |
| F. sambucinum | FSAMSC | MFG 70202 | Russia: Udmurtia | 2021 | OR020735 | OR727766 |
| F. sambucinum | FSAMSC | MFG 70208 | Russia: Ryazan region | 2022 | OR020736 | OR727767 |
| F. sambucinum | FSAMSC | MFG 70210 | Russia: Ryazan region | 2022 | OR020737 | OR727768 |
| F. sambucinum | FSAMSC | MFG 80005 | Russia: Novgorod region | 2020 | OR020738 | OR727769 |
| F. sambucinum | FSAMSC | MFG 80204 | Russia: Moscow region | 2021 | OR020739 | OR727770 |
| F. sambucinum | FSAMSC | MFG 80337 | Russia: Tula region | 2022 | OR020741 | OR727771 |
| F. sambucinum | FSAMSC | MFG 80361 | Russia: Omsk region | 2022 | OR020742 | OR727772 |
| F. sambucinum | FSAMSC | MFG 80362 | Russia: Omsk region | 2022 | OR020743 | OR727773 |
| F. sambucinum | FSAMSC | MFG 80365 | Russia: Omsk region | 2022 | OR020744 | OR727774 |
| F. venenatum | FSAMSC | MFG 70118 | Russia: Leningrad region | 2020 | OR020706 | OR727757 |
| F. mori | FSSC ** | MFG 70147 | Russia: Samara region | 2020 | OR020716 | OR727777 |
| F. noneumartii | FSSC | MFG 70154 | Russia: Bashkiria | 2021 | OR020719 | OR727778 |
| F. noneumartii | FSSC | MFG 70155 | Russia: Bashkiria | 2021 | OR020720 | OR727779 |
| F. noneumartii | FSSC | MFG 70176 | Russia: Moscow region | 2021 | OR020731 | OR727781 |
| F. noneumartii | FSSC | MFG 70177 | Russia: Moscow region | 2021 | OR020732 | OR727782 |
| F. stercicola | FSSC | MFG 70108 | Russia: Pskov region | 2020 | OR020705 | OR727775 |
| F. stercicola | FSSC | MFG 70141 | Russia: Stavropol region | 2020 | OR020715 | OR727776 |
| F. vanettenii | FSSC | MFG 70164 | Russia: Chuvashia | 2021 | OR020726 | OR727780 |
| F. vanettenii | FSSC | MFG 80216 | Russia: Pskov region | 2021 | OR020740 | OR727783 |
* FSAMSC—Fusarium sambucinum species complex; ** FSSC—Fusarium solani species complex.
2.2. Genomic DNA Isolation, Sequencing, and Phylogenetic Analysis
The Fusarium strains were cultured on PSA for 7 days. Genomic DNA was isolated from the fungal mycelium (10–50 mg per strain) using the Genomic DNA Purification Kit (Thermo Fisher Scientific, Vilnius, Lithuania). Amplification of part of the translation elongation factor 1-α gene (tef) and RNA polymerase second-largest subunit gene (rpb2) was performed as described previously [25,26,27]. Amplicons were sequenced on the ABI Prism 3500 sequencer (Applied Biosystems, Hitachi, Japan) using the BigDye Terminator 3.1 cycle sequencing kit (Applied Biosystems, Foster City, CA, USA).
Consensus sequences of each strain were obtained and manually edited using the Vector NTI Advance 10 program (Thermo Fisher Scientific, Carlsbad, CA, USA). The Basic Local Alignment Search Tool (BLAST) was used to search for similar sequences in the NCBI GenBank database. Sequences mainly representing cultures from the Agricultural Research Service (NRRL, Peoria, IL, USA), the CBS-KNAW culture collection (CBS, Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands) and other collections were incorporated into the phylogenetic analysis (Supplementary Materials).
Sequence alignment was performed using MEGA X 10.1 [28]. The FSAMSC and FSSC data sets were analyzed separately. Determination of the best-fitting substitution model and the maximum likelihood (ML) analysis was conducted using IQ-TREE 2 v.2.1.3 program [29]. The TIM2e + I + G4 and TIM2e + R4 models were chosen for the multilocus analysis of the FSAMSC and FSSC data sets, respectively. To infer the phylogenetic relationships among the taxa, maximum parsimony (MP) analysis was conducted using MEGA X 10.1 [28]. Nodal support was assessed by bootstrap analysis based on 1000 replicates. To further infer the phylogenetic relationships among the taxa, Bayesian analysis was conducted with MrBayes 3.2.1 on the Armadillo 1.1 platform [30] using 2,000,000 generations of Markov chain Monte Carlo (MCMC), and the trees were sampled every 1000th generation. The sequence data obtained in this study were deposited in GenBank (Table 1).
2.3. Determination of Growth
Twenty of the FSAMSC strains and nine of the FSSC strains were previously grown on PSA for 7 days in the dark at 25 °C. The plates, 85 mm in diameter, with pure PSA were inoculated, and 5 mm disks were taken with a sterile cork-borer from the margin of actively growing fungal culture. The disks were placed, mycelium down, on the surface of the medium in the center of the dish. Each isolate was cultured on PSA at 5, 10, 15, 20, 25, 30, and 35 °C in the dark for 5 days in the Innova 44R thermostat (Eppendorf, Hamburg, Germany).
The size of each colony was measured in two perpendicular directions, and the average diameter of the colony minus the diameter of the inoculation disk was calculated. The growth rate of the strain was calculated as the ratio of the diameter of the fungal colony to the number days of cultivation (mm/day).
Microscopic examination and photography were carried out for colonies grown on SNA with an Olympus BX53 and an Olympus SZX16 microscope (Olympus, Tokyo, Japan).
2.4. Pathogenicity Test
The pathogenicity of all strains was assessed by inoculation of the potato tubers under storage conditions at temperatures of 5 °C and 23 °C.
For the experiments, tubers of potato cv. Gala, widely cultivated in Russia, were chosen. Tubers without visible damage and weighing ~40 g were selected, surface-sterilized with 5% sodium hypochlorite for 5–10 min, washed with water, and then air-dried.
Then, the tubers were wounded by a cork borer with a diameter of 5 mm to a depth of 20 mm. The agar disks (4 mm in diameter) were cut out from the fungal cultures grown on PSA for 7 days and placed in the hole, which was subsequently sealed with the excised plug of tuber tissue. At least five tubers were inoculated with one fungal strain, then they were placed in plastic containers (8.5 cm × 19 cm × 31 cm), capped loosely, and incubated at temperatures of 5 °C or 23 °C for 4 weeks. In the control, a disk of pure PSA was used.
After two weeks of incubation, the grown sprouts were removed from the tubers. After 4 weeks, each tuber was cut across the hole, and the width and depth of rotted tissue were measured (mm), calculating the average value. The size of the damage caused by the fungi was assessed for each variant, excluding the average size of the inoculation hole in the control.
2.5. Statistical Analysis
The data were analyzed using Microsoft Office Excel 2010 (Microsoft, Redmond, WA, USA) and Statistica 12.0 (StatSoft, Tulsa, OK, USA). The significance of differences among the mean values of groups was estimated by Tukey’s test (95% confidence level).
3. Results
3.1. Molecular Phylogeny
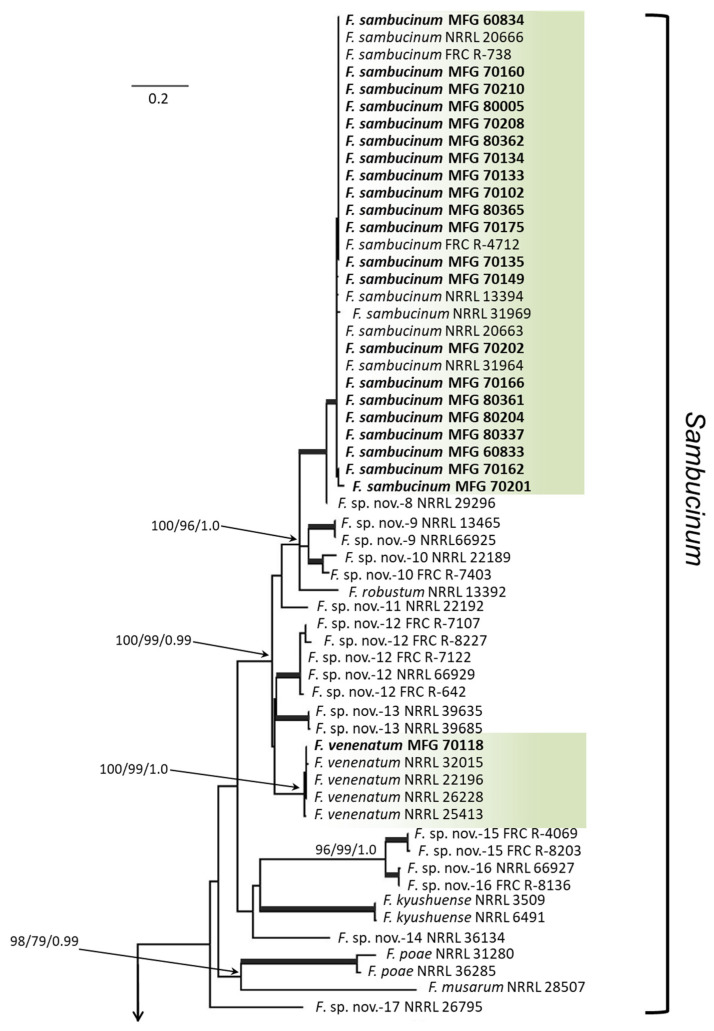
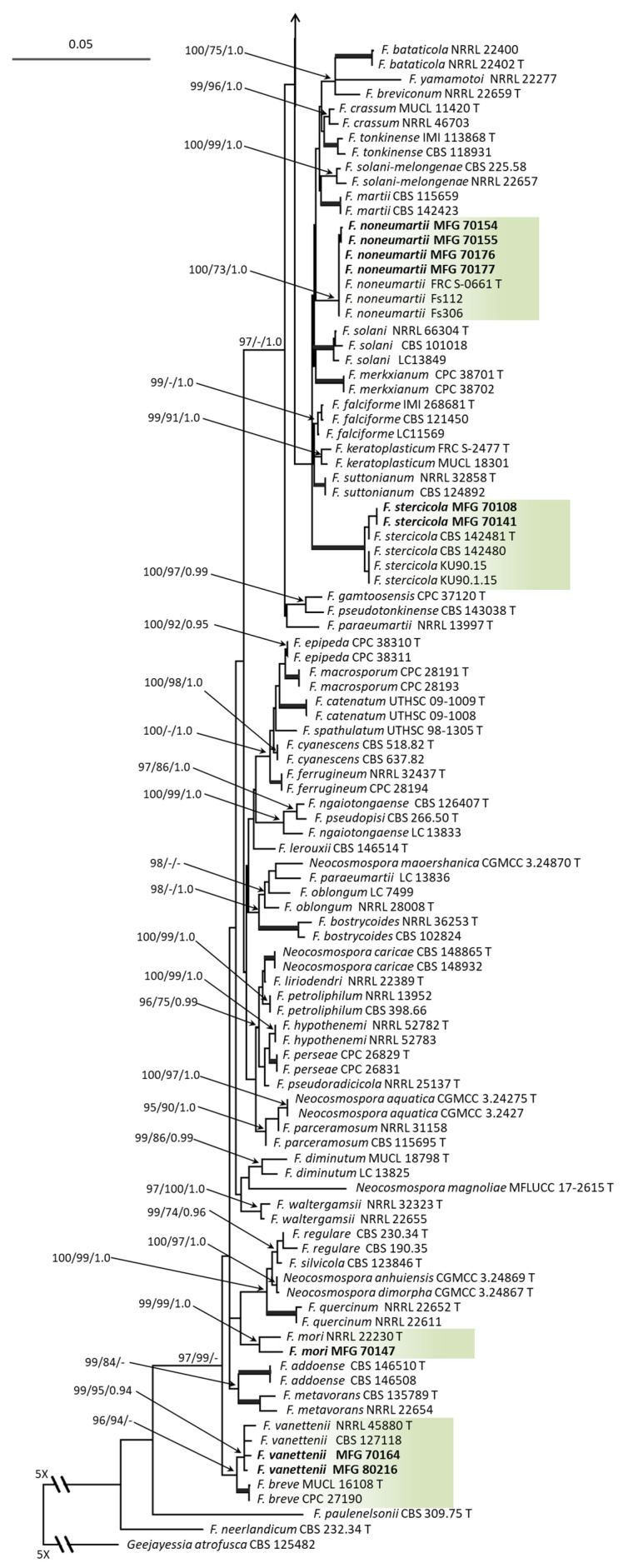
Multilocus analysis of the tef and rpb2 sequences was used to infer the genetic relationships among Fusarium strains. The FSAMSC data set included combined sequences of the 22 analyzed strains as well as 89 reference sequences of Fusarium spp. strains and consisted of a total of 1587 characters (666 bp from tef, and 921 bp from rpb2), among which 963 characters were conserved and 589 were variable (37.1%); 526 characters were parsimony informative (33.1%). Fusarium nelsonii strain NRRL 13338 was used as an outgroup (Figure 1). The FSSC data set included combined sequences of 9 analyzed strains and 166 reference sequences of Fusarium spp. strains and consisted of a total of 1485 characters (663 bp from tef, and 822 bp from rpb2), among which 838 characters were conserved and 645 were variable (43.4%); 491 characters were parsimony informative (33.1%). Geejayessia atrofusca CBS 125482 was used as an outgroup (Figure 2).
Figure 1.
Maximum likelihood (ML) phylogenetic tree based on DNA sequence data from tef and rpb2 loci of Fusarium spp. from Fusarium sambucinum species complex. ML and maximum parsimony (MP) bootstrap support values > 70%, followed by Bayesian posterior probability (BP) scores > 0.95 are shown at the nodes. Thickened lines indicate ML/MP of 100 and a BP of 1.0. The studied strains are in bold. The tree was rooted on sequences of F. nelsonii strain NRRL 13338.
Figure 2.
Maximum likelihood (ML) phylogenetic tree based on DNA sequence data from tef and rpb2 loci of Fusarium spp. from Fusarium solani species complex. ML and maximum parsimony (MP) bootstrap support values > 70%, followed by Bayesian posterior probability (BP) scores > 0.95 are shown at the nodes. Thickened lines indicate ML/MP of 100 and BP of 1.0. The studied strains are in bold. The tree was rooted on sequences of Geejayessia atrofusca strain CBS 125482.
All analyzed strains from the FSAMSC were distributed into the Sambucinum clade (Figure 1). Moreover, 21 strains clustered together with the F. sambucinum reference strains with high bootstrap support (ML/MP/BP 100/100/1.0). One strain, MFG 70118, formed a clade with the F. venenatum reference strains (ML/MP/BP 100/99/1.0).
On the FSSC phylogenetic tree (Figure 2), the four analyzed strains formed a clade with the F. noneumartii reference strains (ML/MP/BP 100/73/1.0); two strains, MFG 70108 and MFG 70141, clustered together with the F. stercicola reference strains (ML/MP/BP 100/100/1.0); two strains, MFG 70164 and MFG 80216, formed a clade with the F. vanettenii reference strains (ML/MP/BP 99/95/0.95); another strain, MFG 70147, formed a clade with the F. mori type strain (ML/MP/BP 99/99/1.0).
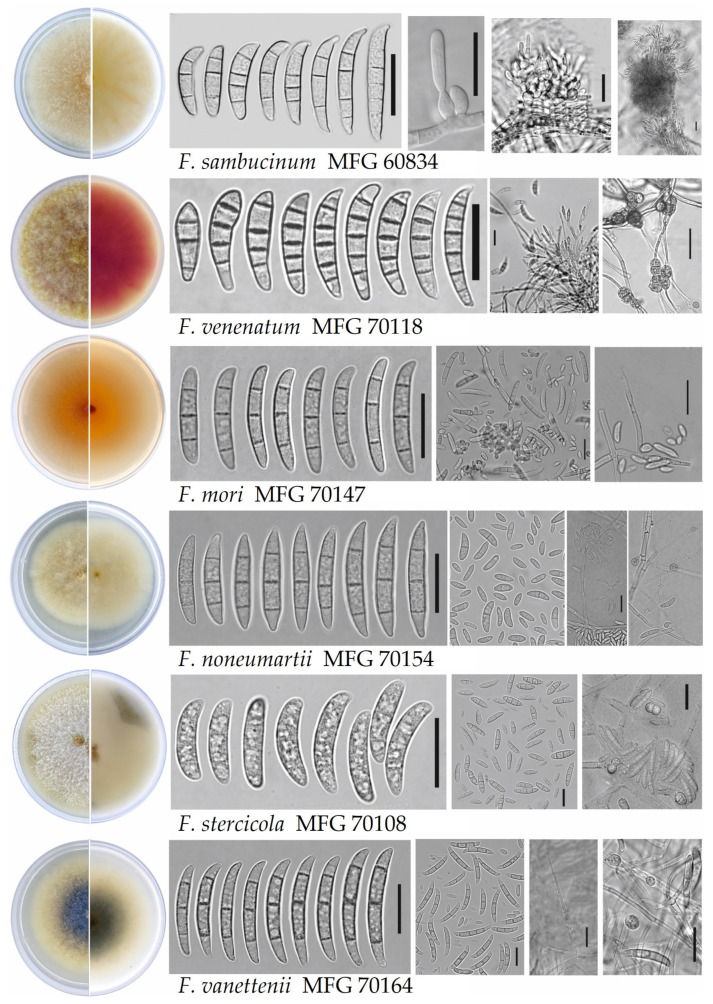
The appearance of colonies and some micromorphological features of the different Fusarium spp. identified in our study are shown in Figure 3. They corresponded to the ranges of morphology characteristics, which are specific to these species [31].
Figure 3.
Colony morphology on potato sucrose agar and the micromorphological characteristic on synthetic nutrient agar of analyzed Fusarium fungi isolated from potato tubers (25 °C in the darkness). Scale bars = 20 μm.
3.2. Effect of Temperature on Fungal Growth
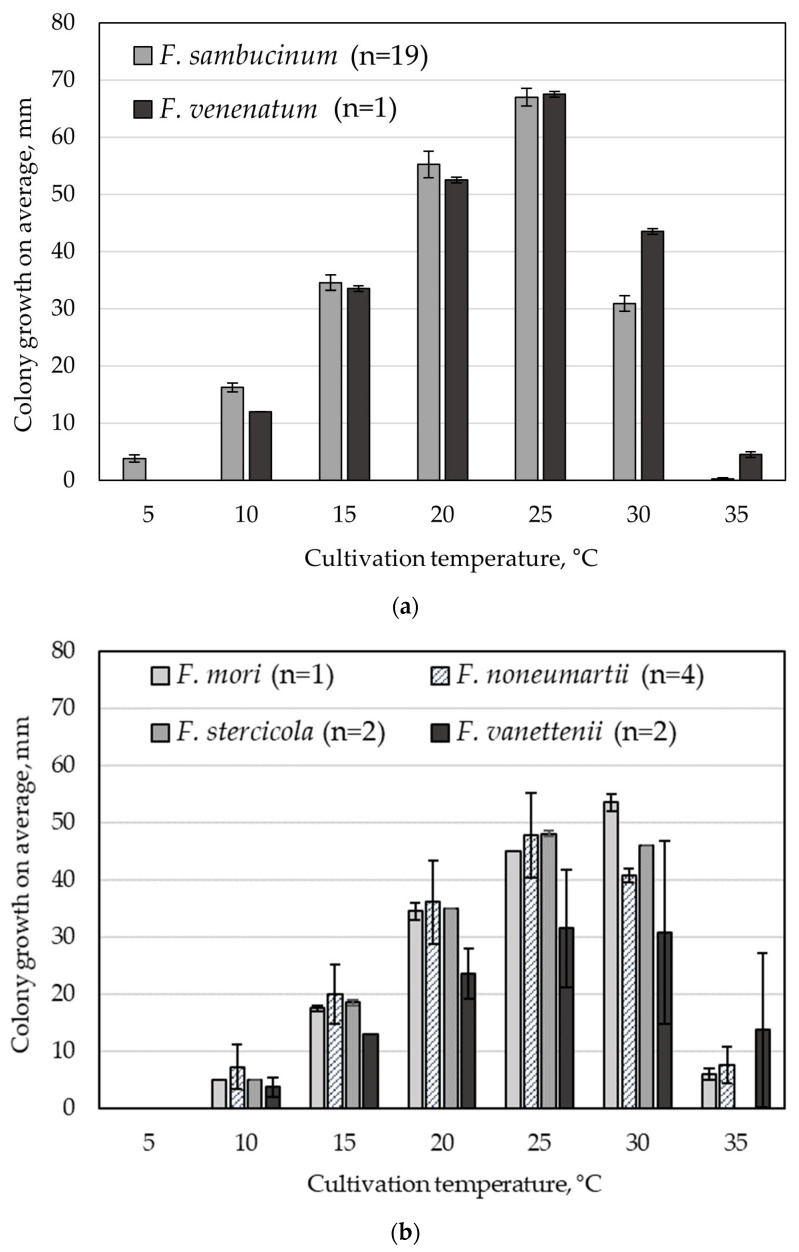
At a temperature of 5 °C, only 84% of F. sambucinum strains were able to grow on PSA (Figure 4a), and their growth rate averaged 0.9 ± 0.1 mm/day. A single F. venenatum strain, like the nine strains of four different species from the FSSC (Figure 4b), did not grow at such low temperature.
Figure 4.
Effect of temperature on the growth of the strains identified within the Fusarium sambucinum species complex (a) and Fusarium solani species complex (b) (potato–sucrose agar, 5 days, in the darkness). The bars indicate the average values, and the intervals are the confidence interval at a significance level of p ˂ 0.05.
In the temperature range of 10–25 °C, the strains of both species from the FSAMSC grew more actively compared to the strains from the FSSC. On the contrary, at 30–35 °C, the diameters of the colonies of the FSSC strains, on average, were significantly larger than those of the colonies of the FSAMSC strains.
The clear optimal temperature for the growth of the FSAMSC strains was noted at 25 °C, when the diameter of the fungal colonies varied in the range of 60.0–79.5 mm. On average, it was 1.2–4.2 times larger than the average size of the colonies determined at a temperature of 10–30 °C. Increasing the temperature to 30 °C led to the slowed down growth of all FSAMSC strains, and at 35 °C, it stopped, with the exception of two strains—F. sambucinum MFG 70135 and F. venenatum MFG 70118, which continued to grow at rates of 0.8 and 0.9 mm/day, respectively (Table 2).
Table 2.
Growth rates of strains within the Fusarium sambucinum species complex and Fusarium solani species complex (mm/day) at different temperatures (PSA, in the darkness).
| SC * |
Fusarium spp. (No. of Strains) |
Temperature, °C | ||||||
|---|---|---|---|---|---|---|---|---|
| 5 | 10 | 15 | 20 | 25 | 30 | 35 | ||
| FSAMSC | F. sambucinum | 0.8 ± 0.1 ** | 3.2 ± 0.2 | 6.9 ± 0.3 | 11.0 ± 0.5 | 13.4 ± 0.3 | 6.2 ± 0.3 | 0 |
| (n = 19) | (0–1.8) | (2.0–4.4) | (5.1–8.8) | (8.0–13.8) | (11.6–15.0) | (3.6–8.3) | (0–0.8) | |
| F. venenatum | 0 | 2.4 | 6.7 ± 0.1 | 10.5 ± 0.1 | 13.5 ± 0.1 | 8.7 ± 0.1 | 0.9 ± 0.1 | |
| (n = 1) | (2.4; 2.4) | (6.6; 6.8) | (10.4; 10.6) | (13.4; 13.6) | (8.6; 8.8) | (0.8; 1.0) | ||
| FSSC | F. noneumartii | 0 | 1.5 ± 0.8 | 4.0 ± 1.0 | 7.2 ± 1.5 | 9.6 ± 1.5 | 8.2 ± 0.2 | 1.5 ± 0.6 |
| (n = 4) | (0.6–3.8) | (3.0–7.2) | (5.5–11.7) | (8.0–14.1) | (7.5–8.6) | (0–3.2) | ||
| F. stercicola | 0 | 1.0 | 3.7 ± 0.1 | 7.0 | 9.6 ± 0.1 | 9.2 | 0 | |
| (n = 2) | (1.0; 1.0) | (3.6; 3.8) | (7.0; 7.0) | (9.5; 9.7) | (9.2; 9.2) | |||
| F. vanettenii | 0 | 0.8 ± 0.3 | 2.6 | 4.7 ± 0.9 | 6.3 ± 2.1 | 6.2 ± 3.2 | 2.8 ± 2.7 | |
| (n = 2) | (0.4; 1.1) | (2.6; 2.6) | (3.8; 5.6) | (4.2; 8.4) | (2.9; 9.4) | (0; 5.5) | ||
| F. mori | 0 | 1.0 | 3.5 ± 0.1 | 6.9 ± 0.3 | 9.0 | 10.7 ± 0.3 | 1.2 ± 0.2 | |
| (n = 1) | (1.0; 1.0) | (3.4; 3.6) | (6.6; 7.2) | (9.0; 9.0) | (10.4; 11.0) | (1.0; 1.4) | ||
* SC—species complex: FSAMSC—Fusarium sambucinum species complex; FSSC—Fusarium solani species complex. ** The average value with a confidence interval at a significance level of p ˂ 0.05; the range of values is indicated in parentheses.
The optimal growth temperature of the FSSC strains was in the range of 25–30 °C, without significant differences. However, the F. vanettenii strains were the slowest-growing compared to the strains of three other species. At the temperature of 35 °C, a significant decrease in the growth rate of all analyzed FSSC strains was detected, up to its complete cessation in 44% of the strains. Both strains of F. stecicola included in this study, as well as F. noneumartii strain MFG 70176 and F. vanettenii strain MFG 80216, were not able grow at the highest temperature in this experiment. The F. vanettenii strain MFG 70164 turned out to be relatively tolerant to 35 °C, because its growth rate at that temperature was 5.5 mm/day, which is 1.7–4.2 times higher than the rate of the other growing strains.
3.3. Pathogenicity of the Strains
The control tubers remained asymptomatic, and the size of mechanical damage at 23 °C was, on average, 13.5 ± 0.1 mm, and at 5 °C—14.1 ± 0.2 mm.
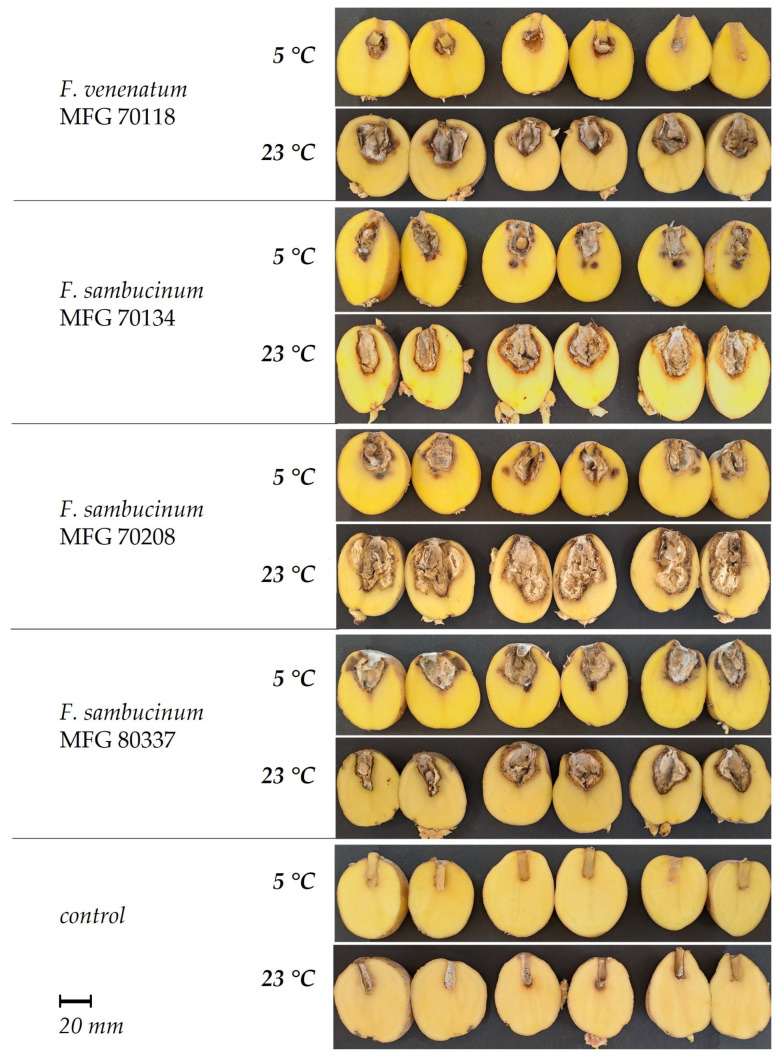
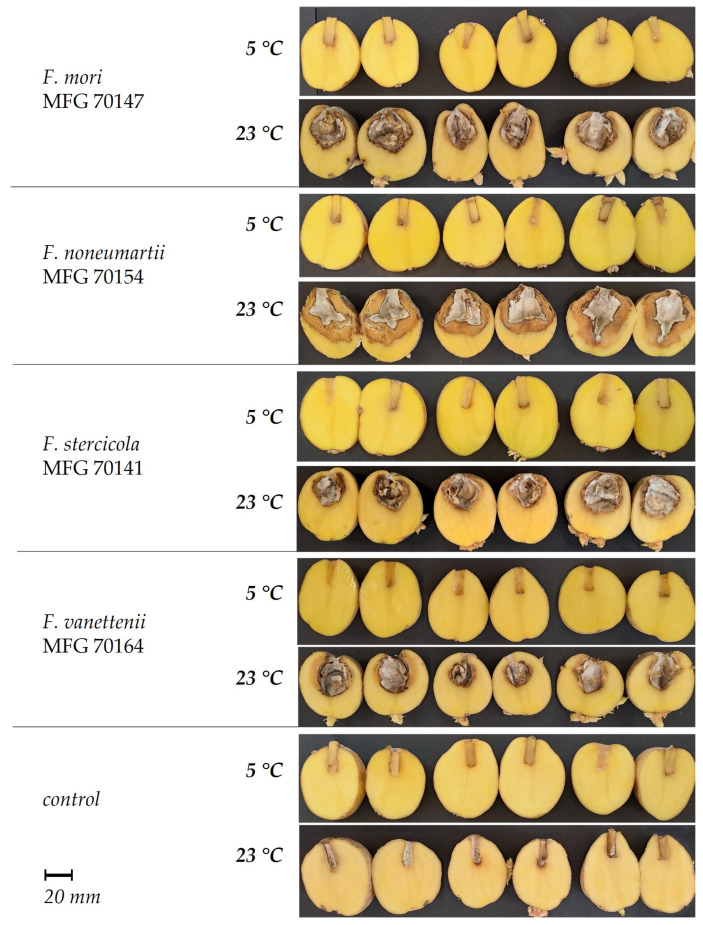
Upon visual inspection of the tubers inoculated with the FSAMSC, dark depressions of varying sizes were noted around the inoculation hole. Aerial mycelium formulated on the surface of the tuber, and as the affected tissue dried, the surface of the tuber wrinkled in concentric rings. The tuber tissue dried out, and depending on the aggressiveness of the strain, internal cavities of different sizes were formed, separated by a light to dark brown border from the apparently healthy tissue (Figure 5). The surface of the resulting cavity was lined with mycelium and sporulation of the fungus; the color of the aerial mycelium varied from white to salmon-gray. Some FSSC strains, in addition to a distinct lesion cavity, caused extensive maceration of the surrounding tuber tissue, reaching the periderm (Figure 6).
Figure 5.
Pathogenicity of the strains within Fusarium sambucinum species complex in tubers of potato cv. Gala at two incubation temperatures (4 weeks).
Figure 6.
Pathogenicity of the strains within Fusarium solani species complex in tubers of potato cv. Gala at two incubation temperatures (4 weeks).
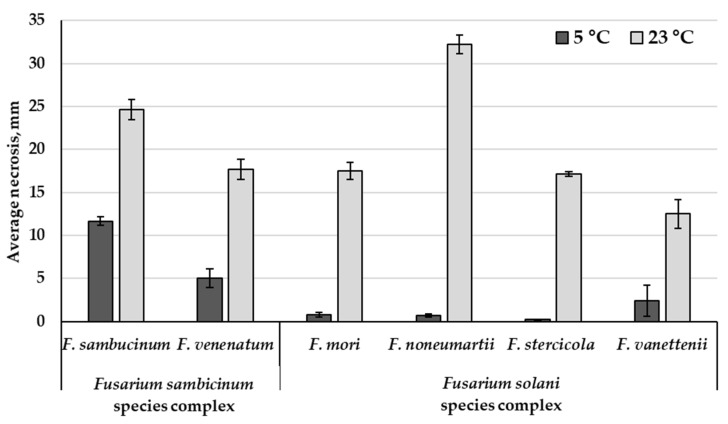
At an incubation temperature of 23 °C, extensive damage to the tuber tissue was observed. The size of tissue necroses caused by the FSAMSC and FSSC strains varied in the ranges of 12.9 ± 1.3–32.5 ± 4.1 mm and 12.5 ± 1.7–33.9 ± 1.0 mm, respectively. The aggressiveness of the strains belonging to two species from the FSAMSC was similar: the F. sambucinum strains caused 23.9 ± 2.2 mm of necrosis on average, and F. venenatum strain MFG 70118—17.7 ± 1.2 mm (Figure 7).
Figure 7.
Pathogenicity of Fusarium fungi in tubers at two incubation temperatures (potato cv. Gala, 4 weeks, in the darkness). The bars indicate the average values, and the intervals are the confidence interval at a significance level of p ˂ 0.05.
Among the FSSC strains, the largest tissue necrosis was noted as a result of inoculation of tubers with F. noneumartii strains—32.2 ± 0.8 mm on average, compared to the significantly less aggressive strains of F. mori, F. stercicola, and F. vanettenii: the average sizes of the damage caused by these fungi were 17.5 ± 0.5 mm, 17.2 ± 0.2 mm, and 12.5 ± 1.7 mm, respectively.
At an incubation temperature of 5 °C, the sizes of tuber necroses inoculated with F. sambucinum strains varied in the range of 6.7 ± 0.5–15.9 ± 0.8 mm, which is, on average, 2.1 times less than that at 23 °C. Only three strains of this species of different geographic origins demonstrated similar aggressiveness at the contrasting temperatures. At 5 °C, the F. sambucinum strains turned out to be significantly more aggressive compared to the FSSC strains, which caused necroses that did not exceed 0.3 ± 0.1–3.0 ± 0.7 mm.
4. Discussion
To date, information on the species diversity of the pathogens causing Fusarium dry rot of potato tubers in Russia remains scarce. Previously, analysis of the species composition of Fusarium fungi isolated from potato tubers with dry rot symptoms revealed four species: F. sambucinum, F. solani, F. sporotrichioides, and F. oxysporum, and one more strain could not be identified [32]. Subsequently, the analysis of potato samples collected from storage facilities using the sequencing of tef revealed at least 15 different Fusarium species, most of which belonged to the Fusarium oxysporum species complex [33]. According to our research conducted in 2021–2022, the most common pathogens causing the dry rot of potato tubers in Russia were the species within the FSAMSC [34].
In this study, 31 Fusarium strains isolated from potato tubers with symptoms of dry rot, which belong to the FSAMSC and FSSC according to preliminary morphological analysis, were randomly chosen from the fungal culture collection. Multilocus analysis of the combined tef and rpb2 sequences was used to infer the taxonomic status of these strains. The topologies of the constructed trees for the FSAMSC and FSSC by different methods are concordant with the phylogenetic relationships among Fusarium species reconstructed previously [35,36,37,38,39,40,41,42]. The phylogenetic analysis allowed for accurately identifying the Fusarium species: among the analyzed FSAMSC strains, most of them belong to F. sambucinum and one to F. venenatum, and among the FSSC strains, four species (F. mori, F. noneumartii, F. stercicola, and F. vanettenii) were detected.
Fusarium sambucinum was one of the first described species in the Fusarium genus. Later, the significant intraspecific diversity among F. sambucinum strains was revealed, and three morphologically similar species—F. sambucinum s. str., F. venenatum, and F. torulosum—were described [31,43,44]. Currently, the Sambucinum clade within the FSAMSC includes 6 described species and at least 10 phylogenetic lineages [40].
Among the analyzed fungi within the FSAMSC, 21 strains of F. sambucinum s. str. and one strain of F. venenatum were identified. The last species was previously isolated from potato tubers in Algeria [3] and Poland [2] and was also found in the soil and mycobiota of various cereals [40]. In Russia, this is the first finding of F. venenatum in the mycobiota of potato; it was previously identified only in oat and wheat grain [45].
Fusarium solani was first isolated from potato with dry rot in 1842 in Germany and described as the pathogen causing this disease. Currently, there are more than 70 recognized phylogenetic species of the FSSC, while some of them do not yet have formal descriptions or Latin binomial names [46,47,48,49]. The Fusarium solani species complex is the most controversial group of fungi, both in terms of its intraspecific diversity and its degree of relatedness to the genus Fusarium s. str. [39,47,50,51]. These fungi are cosmopolitan and not confined to one species or family of host plants [38,52].
In this study, among the strains distinguished within the FSSC, one strain of F. mori, four strains of F. noneumartii, and two strains of both F. stercicola and F. vanettenii species were identified. This is the first record of F. mori on potato as a novel host plant and the first finding of this species in Russia. In previous studies, F. mori was isolated from mulberry in Japan [52] and China [53] and was also found in the mycobiota of tomato stems in Japan [54]. Fusarium noneumartii and F. stercicola were also identified in Russia for the first time. Previously, F. noneumartii was isolated from potatoes in Israel and from tomatoes in the USA [52], as well as from the roots of orange trees in South Africa [55]. F. stercicola has been isolated from plant debris in Switzerland [56], from soil in Europe [38,57] and in China [41], and also has been found on Solanum tuberosum and other host plants in New Zealand [58] and in nematodes [59]. F. vanettenii was previously isolated from potato tubers in Russia [33,60], as well as from tomato [61], legumes [42], and soil [38]. The existing information indicates a potentially wide distribution of F. mori, F. noneumartii, F. stercicola, and F. vanettenii and other FSSC species in the mycobiota of potato and widely cultivated crops.
Temperature significantly affects the adaptability of fungi in nature. Our results demonstrate that a temperature of 5 °C was favorable for the growth of only the F. sambucinum strains, whereas all the strains of the other analyzed five species were not able grow at this low temperature. These results are in agreement with previously obtained data in Tunisia showing the ability of F. sambucinum to grow at temperatures below 5 °C, contrary to F. solani [62]. This tolerance to extremely low temperatures can give F. sambucinum an advantage over other representatives of tuber mycobiota under storage conditions, where the temperature does not exceed 4–10 °C.
In our study, 25 °C was the optimal temperature for growth of the F. sambucinum and F. venenatum strains, and 25–30 °C for the growth of FSSC strains. Thus, this confirms the previous data showing that at temperatures below 25 °C, F. sambucinum grows faster than F. solani, but high temperatures (30–35 °C) are more favorable for the growth of the latter [63]. Increasing the temperature to 35 °C led to a stop in the growth of 95% of the F. sambucinum strains; only one strain, MFG 70135, from Stavropol Krai, which is the most southern region in this study, was able to grow at a rate of 0.8 mm/day. The cultivation of the FSSC strains at 35 °C can be used as an additional marker for the separation of F. stercicola from phylogenetically related species of the FSSC; both strains of this species completely stopped their growth at this high temperature. Inconsistency of the F. vanettenii strains in response to 35 °C was established; one strain, MFG 70164, turned out to be relatively tolerant, but the other strain, MFG 80216, did not grow.
All analyzed strains caused significant damages of the tubers at 23 °C, but the largest tissue necroses were induced by the F. noneumartii and F. sambucinum strains. The F. venenatum, F. mori, F. stercicola, and especially the F. vanetenii strains can be characterized as less aggressive. Previously, the higher aggressiveness of the F. sambucinum strains to potato tubers, compared to the other fungi, including members of the FSSC, has been repeatedly noted [1,2,3,12,19,23,62,63].
The characterization of the aggressiveness of the FSSC isolates (F. solani f. sp. eumartii, F. coeruleum, and F. eumartii) to potato revealed their differences: some isolates caused sunken dry lesions and vascular discoloration on tubers, while others did not cause lesions on any of the inoculated tubers and showed minimal growth on plant tissue [64]. In that study, F. sambucinum isolate caused sunken lesions on the tubers. Our observations showed, under inoculation with FSSC fungi, softening and moistening of the internal tissue of tubers sometimes occurred, which is not very similar to the classic symptom of Fusarium dry rot and should be taken into account when visually diagnosing potato diseases.
The aggressiveness of the strains belonging to four species of the FSSC varied significantly at 23 °C. Previously, the significant differences in the aggressiveness among F. solani s. lat. strains to potato tubers (20 °C, 3 weeks) was revealed [21,24]. According to [65], the pathogenicity of F. solani strains varies from highly aggressive to completely non-pathogenic. However, the lack of molecular identification of the strains in those studies does not allow us to conclude on the interspecific differences in F. solani s. lat. However, in our study, the significantly higher aggressiveness of the F. noneumartii strains compared to the F. mori, F. stercicola, and F. vanetenii strains was revealed.
At 5 °C, the F. sambucinum strains turned out to be significantly more aggressive compared to the other analyzed fungi, which is consistent with earlier results [20,62]. Thus, the F. sambucinum strains turned out to be consistently aggressive and capable of causing dry rot in tubers at a wide range of temperatures close to growing and storage conditions. The inability of the FSSC strains to grow at 5 °C indicates the lack of infection development in the inoculated tubers. This highlights the key effect of environmental conditions on the physiological characters of pathogens and their interaction with host plants.
The pathogenicity of fungi is a complex process that depends on the expression of many genes and plant resistance mechanisms [5], e.g., the cutinase activity in the F. solani strains correlated with their pathogenicity to potato tubers [66]. Fusarium species associated with potato dry rot are known to produce different metabolites, some of which are phytotoxins [67,68]. Previously, in the extract obtained from potato tubers infected with the F. sambucinum strain, 18 compounds were found, including 5 mycotoxins/phytotoxins (aurofusarin, beauvericin, sambutoxin, emodin, and fusaric acid) and 2 specific fungal compounds (bikaverin and deoxygerfelin), whereas after F. sambucinum cultivation on malt extract agar, only 8 compounds, including 2 mycotoxins (beauvericin and diacetoxyscirpenol (DAS)), were detected [69]. The accumulation of DAS in the tubers inoculated with F. sambucinum was analyzed under different storage conditions, and an increase temperature from 10 °C to 20 °C over a period of 30 and 50 days led to an increase in the DAS amounts found in the tubers [70]. Enniatins are mycotoxins produced by different Fusarium fungi, including those that are associated with potato diseases, causing necrosis of the potato tissue in vitro, but these mycotoxins are not essential for the successful infection of tuber tissue by fungal strains [5]. Loss of the ability to produce enniatins in the strain was correlated with it causing less tuber necrosis. Conversely, the ESYN1 gene overexpression led to an increase in enniatin production, as well as more tuber necrosis [71].
Thus, with a large collection of Fusarium strains isolated from potato tubers obtained from different regions, it would be useful to establish the proportions of fungal species in the distant regions with different environmental conditions. In the future, it would also be interesting to reveal the specialization of the identified Fusarium species not only to different potato varieties but also to other crops, as well as to determine the profiles of metabolites and their role in the interaction with the host plant.
5. Conclusions
Fusarium strains isolated from potato tubers with dry rot symptoms collected from different regions of Russia were accurately identified using multilocus phylogenetic analysis of the tef and rpb2 loci. The Fusarium sambucinum and F. venenatum species were identified among the FSAMSC strains, and the F. mori, F. noneumartii, F. stercicola, and F. vanettenii species were present among the FSSC strains. This is the first finding of F. mori on potato as a novel host plant, and the first detection of the F. noneumartii and F. stercicola species in Russia. The clear optimal temperature for the growth of the FSAMSC strains was determined at 25 °C, whereas for the FSSC strains, the optimum was in the range of 25–30 °C. The ability of the F. sambucinum strains to grow at 5 °C distinguished them from the other analyzed fungi. All analyzed Fusarium strains were pathogenic to potato cv. Gala and caused extensive lesions in the tuber tissue at an incubation temperature of 23 °C. At an incubation temperature of 5 °C, only the F. sambucinum strains caused necroses, whereas the other Fusarium strains were not able to infect the tuber tissue. Low-temperature storage can slow down the process of infection by Fusarium species, but violation of storage conditions can lead to significant potato losses. Fusarium species producing a wide spectrum of metabolites makes it is necessary to pay special attention to the contamination of potato seed tubers with these fungi, since, even if storage conditions are met, the presence of infection poses a serious threat to future yield.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microorganisms12030598/s1, Table S1: Fusarium strains whose sequences were used in the phylogenetic study.
Author Contributions
Conceptualization, O.G., A.O. and T.G.; methodology, O.G., A.O. and T.G.; validation, O.G., A.O. and I.T.; formal analysis, O.G. and A.O.; investigation, O.G., A.O., I.T. and T.G.; data curation, O.G. and A.O.; writing—original draft preparation, O.G. and A.O.; writing—review and editing, O.G., A.O. and T.G.; visualization, O.G. and A.O.; supervision, O.G.; project administration, T.G. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
The data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research was funded by the Russian Science Foundation, grant no. 23-26-00105.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Du M., Ren X., Sun Q., Wang Y., Zhang R. Characterization of Fusarium spp. causing potato dry rot in China and susceptibility evaluation of Chinese potato germplasm to the pathogen. Potato Res. 2012;55:175–184. doi: 10.1007/s11540-012-9217-6. [DOI] [Google Scholar]
- 2.Stefańczyk E., Sobkowiak S., Brylińska M., Śliwka J. Diversity of Fusarium spp. associated with dry rot of potato tubers in Poland. Eur. J. Plant Pathol. 2016;145:871–884. doi: 10.1007/s10658-016-0875-0. [DOI] [Google Scholar]
- 3.Azil N., Stefańczyk E., Sobkowiak S., Chihat S., Boureghda H., Śliwka J. Identification and pathogenicity of Fusarium spp. associated with tuber dry rot and wilt of potato in Algeria. Eur. J. Plant Pathol. 2021;159:495–509. doi: 10.1007/s10658-020-02177-5. [DOI] [Google Scholar]
- 4.Wharton P., Hammerschmidt R., Kirk W. Fusarium Dry Rot. Michigan State University; East Lansing, MI, USA: 2007. [(accessed on 22 January 2024)]. Available online: https://archive.lib.msu.edu/DMC/extension_publications/e2992/e2992.pdf. [Google Scholar]
- 5.Herrmann M., Zocher R., Haese A. Enniatin production by Fusarium strains and its effect on potato tuber tissue. Appl. Environ. Microbiol. 1996;62:393–398. doi: 10.1128/aem.62.2.393-398.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Secor G.A., Salas B. Fusarium dry rot and Fusarium wilt. In: Stevenson W.R., Loria R., Franc G., Weingartner D.P., editors. Compendium of Potato Diseases. 2nd ed. The American Phytopathological Society; St. Paul, MN, USA: 2001. pp. 23–25. [Google Scholar]
- 7.Cullen D.W., Toth I.K., Pitkin Y., Boonham N., Walsh K., Barker I., Lees A.K. Use of quantitative molecular diagnostic assays to investigate Fusarium dry rot in potato stocks and soil. Phytopathol. 2005;95:1462–1471. doi: 10.1094/PHYTO-95-1462. [DOI] [PubMed] [Google Scholar]
- 8.Tiwari R.K., Kumar R., Sharma S., Sagar V., Aggarwal R., Naga K.C., Lal M.K., Chourasia K.N., Kumar D., Kumar M. Potato dry rot disease: Current status, pathogenomics and management. 3 Biotech. 2020;10:503. doi: 10.1007/s13205-020-02496-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choiseul J., Allen L., Carnegie S.F. Fungi causing dry tuber rots of seed potatoes in storage in Scotland. Potato Res. 2007;49:241–253. doi: 10.1007/s11540-007-9020-y. [DOI] [Google Scholar]
- 10.Heltoft P., Brurberg M.B., Skogen M., Le V.H., Razzaghian J., Hermansen A. Fusarium spp. causing dry rot on potatoes in Norway and development of a real-time PCR method for detection of Fusarium coeruleum. Potato Res. 2016;59:67–80. doi: 10.1007/s11540-015-9313-5. [DOI] [Google Scholar]
- 11.Esfahani M.N. Susceptibility assessment of potato cultivars to Fusarium dry rot species. Potato Res. 2005;48:215–226. doi: 10.1007/BF02742378. [DOI] [Google Scholar]
- 12.Yikilmazsoy G., Tosun N. Characterization of Fusarium sambucinum isolates associated with potato dry rot and evaluation of cultivar susceptibility and fungicides. Turk. J. Agric. For. 2021;45:10. doi: 10.3906/tar-2006-100. [DOI] [Google Scholar]
- 13.Erper I., Alkan M., Zholdoshbekova S., Turkkan M., Yildirim E., Özer G. First report of dry rot of potato caused by Fusarium sambucinum in Kyrgyzstan. J. Plant Dis. Prot. 2022;129:189–191. doi: 10.1007/s41348-021-00505-3. [DOI] [Google Scholar]
- 14.Hanson L.E., Schwager S.J., Loria R. Thiabendazole resistance in 22 Fusarium species associated with dry rot of potato. Phytopathology. 1996;86:378–384. doi: 10.1094/Phyto-86-378. [DOI] [Google Scholar]
- 15.Estrada Jr R., Gudmestad N.C., Rivera V.V., Secor G.A. Fusarium graminearum as a dry rot pathogen of potato in the USA: Prevalence, comparison of host isolate aggressiveness and factors affecting aetiology. Plant Pathol. 2010;59:1114–1120. doi: 10.1111/j.1365-3059.2010.02343.x. [DOI] [Google Scholar]
- 16.Christian C.L. Ph.D. Thesis. College of Graduate Studies University of Idaho; Moscow, ID, USA: 2023. Characterization of Fusarium Dry Rot Pathogens of Potato and Fusarium Dry Rot Disease Management in the Pacific Northwest of the United States. A Dissertation Presented in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy with a Major in Plant Science in the College of Graduate Studies University of Idaho. [Google Scholar]
- 17.Peters J.C., Lees A.K., Cullen D.W., Sullivan L., Stroud G.P., Cunnington A.C. Characterization of Fusarium spp. responsible for causing dry rot of potato in Great Britain. Plant Pathol. 2008;57:262–271. doi: 10.1111/j.1365-3059.2007.01777.x. [DOI] [Google Scholar]
- 18.Daami-Remadi M. Potato Fusarium dry rot in Tunisia: Current status and future prospects. Pest. Technol. 2012;6:15–22. [Google Scholar]
- 19.Gashgari R.M., Gherbawy Y.A. Pathogenicity of some Fusarium species associated with superficial blemishes of potato tubers. Pol. J. Microbiol. 2013;62:59–66. doi: 10.33073/pjm-2013-007. [DOI] [PubMed] [Google Scholar]
- 20.Gachango E., Hanson L.E., Rojas A., Hao J.J., Kirk W.W. Fusarium spp. causing dry rot of seed potato tubers in Michigan and their sensitivity to fungicides. Plant Dis. 2012;96:1767–1774. doi: 10.1094/PDIS-11-11-0932-RE. [DOI] [PubMed] [Google Scholar]
- 21.Chehri K., Ghasempour H.R., Karimi N. Molecular phylogenetic and pathogenetic characterization of Fusarium solani species complex (FSSC), the cause of dry rot on potato in Iran. Microb. Pathog. 2014;67–68:14–19. doi: 10.1016/j.micpath.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 22.Baturo-Ciesniewska A., Lenc L., Grabowski A., Lukanowski A. Characteristics of Polish isolates of Fusarium sambucinum: Molecular identification, pathogenicity, diversity and reaction to control agents. Am. J. Potato Res. 2015;92:49–61. doi: 10.1007/s12230-014-9410-z. [DOI] [Google Scholar]
- 23.Aydin M.H., İnal B. Comparative susceptibility of some commercial potato cultivars to Fusarium sambucinum and F. solani isolates causing tuber dry rot. Appl. Ecol. Environ. Res. 2018;16:4879–4892. doi: 10.15666/aeer/1604_48794892. [DOI] [Google Scholar]
- 24.Gherbawy Y.A., Hussein M.A., Hassany N.A., Shebany Y.M., Hassan S., El-Dawy E.G.A.E. Phylogeny and pathogenicity of Fusarium solani species complex (FSSC) associated with potato tubers. J. Basic Microbiol. 2021;61:1133–1144. doi: 10.1002/jobm.202100393. [DOI] [PubMed] [Google Scholar]
- 25.O’Donnell K., Cigelnik E., Caspe H.H. Molecular phylogenetic, morphological, and mycotoxin data support reidentification of the Quorn mycoprotein fungus as Fusarium venenatum. Fungal Genet. Biol. 1998;23:57–67. doi: 10.1006/fgbi.1997.1018. [DOI] [PubMed] [Google Scholar]
- 26.Liu Y.J., Whelen S., Hall B.D. Phylogenetic relationships among ascomycetes: Evidence from an RNA polymerase II subunit. Mol. Biol. Evol. 1999;16:1799–1808. doi: 10.1093/oxfordjournals.molbev.a026092. [DOI] [PubMed] [Google Scholar]
- 27.Jewell L.E., Hsiang T. Multigene differences between Microdochium nivale and Microdochium majus. Botany. 2013;91:99–106. doi: 10.1139/cjb-2012-0178. [DOI] [Google Scholar]
- 28.Kumar S., Stecher G., Li M., Knyaz C., Tamura K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018;35:1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Minh B.Q., Schmidt H.A., Chernomor O., Schrempf D., Woodhams M.D., von Haeseler A., Lanfear R. IQ-TREE 2: New models and efficient methods for phylogenetic inference in the genomic era. Mol. Biol. Evol. 2020;37:1530–1534. doi: 10.1093/molbev/msaa015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lord E., Leclercq M., Boc A., Diallo A.B., Makarenkov V. Armadillo 1.1: An original workflow platform for designing and conducting phylogenetic analysis and simulations. PLoS ONE. 2012;7:e29903. doi: 10.1371/journal.pone.0029903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nirenberg H.I. Morphological differentiation of Fusarium sambucinum Fuckel sensu stricto, F. torulosum (Berk. & Curt.) Nirenberg comb. nov. and F. venenatum Nirenberg sp. nov. Mycopathology. 1995;129:131–141. doi: 10.1007/BF01103337. [DOI] [PubMed] [Google Scholar]
- 32.Khadieva G.F., Lutfullin M.T., Akosakh Y.A., Malova A.V., Mochalova N.K., Vologin S.G., Staszevski Z., Mardanova A.M. Analysis of micromycetes of the genus Fusarium isolated from infected potato tubers grown in the Republic of Tatarstan. Dostizheniya Nauk. I Teh. APK. 2018;32:34–39. doi: 10.24411/0235-2451-2018-10307. (In Russian) [DOI] [Google Scholar]
- 33.Belosokhov A.F., Yarmeeva M.M., Dolgov A.M., Mislavsky S.M., Albantov G.P., Kurchaev M.L., Kokaeva L.Y., Chudinova E.M., Elansky S.N. Fungi of the genus Fusarium on potato tubers. Sovrem. Mikol. V Ross. 2022;9:250–252. (In Russian) [Google Scholar]
- 34.Gagkaeva T., Orina A., Trubin I., Gavrilova O., Khiutti A. Fusarium sambucinum: Causing dry tuber rot of potatoes. Plant Prot. News. 2023;106:137–145. doi: 10.31993/2308-6459-2023-106-3-16041. (In Russian) [DOI] [Google Scholar]
- 35.Lombard L., Sandoval-Denis M., Lamprecht S.C., Crous P.W. Epitypification of Fusarium oxysporum—Clearing the taxonomic chaos. Persoonia. 2019;43:1–47. doi: 10.3767/persoonia.2019.43.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang M.M., Chen Q., Diao Y.Z., Duan W.J., Cai L. Fusarium incarnatum-equiseti complex from China. Persoonia. 2019;43:70–89. doi: 10.3767/persoonia.2019.43.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xia J.W., Sandoval-Denis M., Crous P.W., Zhang X.G., Lombard L. Numbers to names—Reappraisal of the Fusarium incarnatum-equiseti species complex. Persoonia. 2019;43:186–221. doi: 10.3767/persoonia.2019.43.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Crous P.W., Lombard L., Sandoval-Denis M., Seifert K.A., Schroers H.-J., Chaverri P., Gené J., Guarro J., Hirooka Y., Bensch K., et al. Fusarium: More than a node or a foot-shaped basal cell. Stud. Mycol. 2021;98:100116. doi: 10.1016/j.simyco.2021.100116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Geiser D.M., Al-Hatmi A.M.S., Aoki T., Arie T., Balmas V., Barnes I., Bergstrom G.C., Bhattacharyya M.K., Blomquist C.L., Bowden R.L., et al. Phylogenomic analysis of a 55.1-kb 19-gene dataset resolves a monophyletic Fusarium that includes the Fusarium solani Species Complex. Phytopathol. 2021;111:1064–1079. doi: 10.1094/PHYTO-08-20-0330-LE. [DOI] [PubMed] [Google Scholar]
- 40.Laraba I., McCormick S.P., Vaughan M.M., Geiser D.M., O’Donnell K. Phylogenetic diversity, trichothecene potential, and pathogenicity within Fusarium sambucinum species complex. PLoS ONE. 2021;16:e0245037. doi: 10.1371/journal.pone.0245037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang M.M., Crous P.W., Sandoval-Denis M., Han S.L., Liu F., Liang J.M., Duan W.J., Cai L. Fusarium and allied genera from China: Species diversity and distribution. Persoonia. 2022;48:1–53. doi: 10.3767/persoonia.2022.48.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Akhmetova G.K., Knapp D.G., Özer G., O’Donnell K., Laraba I., Kiyas A., Zabolotskich V., Kovács G.M., Molnár O. Multilocus molecular phylogenetic-led discovery and formal recognition of four novel root-colonizing Fusarium species from northern Kazakhstan and the phylogenetically divergent Fusarium steppicola lineage. Mycologia. 2023;115:16–31. doi: 10.1080/00275514.2022.2119761. [DOI] [PubMed] [Google Scholar]
- 43.Altomare C., Logrieco A., Bottalico A., Mulé G., Moretti A., Evidente A. Production of type A trichothecenes and enniatin B by Fusarium sambucinum Fuckel sensu lato. Mycopathology. 1995;129:177–181. doi: 10.1007/BF01103344. [DOI] [PubMed] [Google Scholar]
- 44.Desjardins A.E. Gibberella from A (venaceae) to Z (eae) Annu. Rev. Phytopathol. 2003;41:177–198. doi: 10.1146/annurev.phyto.41.011703.115501. [DOI] [PubMed] [Google Scholar]
- 45.Stakheev A.A., Samokhvalova L.V., Mikityuk O.D., Zavriev S.K. Phylogenetic analysis and molecular typing of trichothecene-producing Fusarium fungi from Russian collections. Acta Nat. 2018;10:79–92. doi: 10.32607/20758251-2018-10-2-79-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.O’Donnell K., Humber R.A., Geiser D.M., Kang S., Park B., Robert V.A.R.G., Crous P.W., Johnston P.R., Aoki T., Rooney A.P., et al. Phylogenetic diversity of insecticolous fusaria inferred from multilocus DNA sequence data and their molecular identification via FUSARIUM-ID and Fusarium MLST. Mycologia. 2012;104:427–445. doi: 10.3852/11-179. [DOI] [PubMed] [Google Scholar]
- 47.O’Donnell K., Al-Hatmi A.M.S., Aoki T., Brankovics B., Cano-Lira J.F., Coleman J.J., de Hoog G.S., Di Pietro A., Frandsen R.J.N., Geiser D.M., et al. No to Neocosmospora: Phylogenomic and practical reasons for continued inclusion of the Fusarium solani species complex in the genus Fusarium. mSphere. 2020;5:e00810-20. doi: 10.1128/mSphere.00810-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sandoval-Denis M., Guarnaccia V., Polizzi G., Crous P.W. Symptomatic Citrus trees reveal a new pathogenic lineage in Fusarium and two new Neocosmospora species. Persoonia. 2018;40:1–25. doi: 10.3767/persoonia.2018.40.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sandoval-Denis M., Crous P.W. Removing chaos from confusion: Assigning names to common human and animal pathogens in Neocosmospora. Persoonia. 2018;41:109–129. doi: 10.3767/persoonia.2018.41.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gräfenhan T., Schroers H.J., Nirenberg H.I., Seifert K.A. An overview of the taxonomy, phylogeny, and typification of nectriaceous fungi in Cosmospora, Acremonium, Fusarium, Stilbella, and Volutella. Stud. Mycol. 2011;68:79–113. doi: 10.3114/sim.2011.68.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schroers H.J., Samuels G.J., Zhang N., Short D.P., Juba J., Geiser D.M. Epitypification of Fusisporium (Fusarium) solani and its assignment to a common phylogenetic species in the Fusarium solani species complex. Mycologia. 2016;108:806–819. doi: 10.3852/15-255. [DOI] [PubMed] [Google Scholar]
- 52.Sandoval-Denis M., Lombard L., Crous P.W. Back to the roots: A reappraisal of Neocosmospora. Persoonia. 2019;43:90–185. doi: 10.3767/persoonia.2019.43.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhu Z., Dong Z., Mo R., Liu X., Zuo Y., Hu X., Zhang C., Yu C. First report of Neocosmospora mori causing root rot and stem blight of mulberry in Nanzhang, Hubei, China. Plant Dis. 2023;108:206. doi: 10.1094/PDIS-04-23-0661-PDN. [DOI] [Google Scholar]
- 54.Imazaki I., Kadota I. Molecular phylogeny and diversity of Fusarium endophytes isolated from tomato stems. FEMS Microbiol. Ecol. 2015;91:fiv098. doi: 10.1093/femsec/fiv098. [DOI] [PubMed] [Google Scholar]
- 55.Guarnaccia V., Van Niekerk J., Crous P., Sandoval-Denis M. Neocosmospora spp. associated with dry root rot of Citrus in South Africa. Phytopathol. Mediter. 2021;60:79–100. doi: 10.36253/phyto-12183. [DOI] [Google Scholar]
- 56.Šišić A., Al-Hatmi A.M.S., Baćanović-Šišić J., Ahmed S.A., Dennenmoser D., de Hoog G.S., Finckh M.R. Two new species of the Fusarium solani species complex isolated from compost and hibiscus (Hibiscus sp.) Antonie Van. Leeuwenhoek. 2018;111:1785–1805. doi: 10.1007/s10482-018-1068-y. [DOI] [PubMed] [Google Scholar]
- 57.Crous P.W., Hernández-Restrepo M., van Iperen A.L., Starink-Willemse M., Sandoval-Denis M., Groenewald J.Z. Citizen science project reveals novel fusarioid fungi (Nectriaceae, Sordariomycetes) from urban soils. Fungal Syst. Evol. 2021;8:101–127. doi: 10.3114/fuse.2021.08.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Biota of New Zealand Names and Classification of Bacteria, Fungi, Land Invertebrates and Plants. [(accessed on 19 January 2024)]. Available online: https://biotanz.landcareresearch.co.nz/scientific-names/3bbf4ec3-6c71-40bf-88bf-3873f2a5357f.
- 59.Zhang H., Yang Z., Jiang Z., Zhang X., Nizamani M.M., Wu Y., Wei S., Wang Y., Xie X. Diversity of fungi isolated from potato nematode cysts in Guizhou province, China. J. Fungi. 2023;9:247. doi: 10.3390/jof9020247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Prudnikova S.V., Churakov A.A., Ovsyankina S.V., Khizhnyak S.V. Biotechnology of New Materials–Environment–Quality of Life, Proceedings of the IV International Scientific Conference, Krasnoyarsk, Russia, 10–13 October 2021. Siberian Federal University; Krasnoyarsk, Russia: 2021. Isolation and identification of autochthonous pathogens of potato diseases common in the regions of Siberia; pp. 174–177. (In Russian) [Google Scholar]
- 61.Debbarma R., Kamil D., Maya Bashyal B., Choudhary S.P., Thokla P. First report of root rot disease on Solanum lycopersicum L. caused by Fusarium vanettenii in India. J. Phytopathol. 2021;169:752–756. doi: 10.1111/jph.13047. [DOI] [Google Scholar]
- 62.Daami-Remadi M., Jabnoun-Khiareddine H., Ayed F., El Mahjoub M. Effect of temperature on aggressivity of Tunisian Fusarium species causing potato (Solanum tuberosum L.) tuber dry rot. J. Agron. 2006;5:350–355. doi: 10.3923/ja.2006.350.355. [DOI] [Google Scholar]
- 63.Lenc L. Pathogenicity and potential capacity for producing mycotoxins by Fusarium sambucinum and Fusarium solani isolates derived from potato tubers. Plant Breed. Seed Sci. 2011;64:23–34. doi: 10.2478/v10129-011-0025-y. [DOI] [Google Scholar]
- 64.Romberg M.K., Davis R.M. Host range and phylogeny of Fusarium solani f. sp. eumartii from potato and tomato in California. Plant Dis. 2007;91:585–592. doi: 10.1094/PDIS-91-5-0585. [DOI] [PubMed] [Google Scholar]
- 65.El-Hassan K.I., El-Saman M.G., Mosa A.A., Mostafa M.H. Variation among Fusarium spp. the causal of potato tuber dry rot in their pathogenicity and mycotoxins production. Egypt. J. Phytopathol. 2007;35:53–68. [Google Scholar]
- 66.Morid B., Zare R., Rezaee S., Zamani-Zadeh H., Hajmansour S. The relationship between cutinases and the pathogenicity/virulence of Fusarium solani in potato tubers. Phytopathol. Mediter. 2009;48:403–410. [Google Scholar]
- 67.Xue H., Liu Q., Yang Z. Pathogenicity, mycotoxin production, and control of potato dry rot caused by Fusarium spp.: A review. J. Fungi. 2023;9:843. doi: 10.3390/jof9080843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bojanowski A., Avis T.J., Pelletier S., Tweddell R.J. Management of potato dry rot. Postharvest Biol. Technol. 2013;84:99–109. doi: 10.1016/j.postharvbio.2013.04.008. [DOI] [Google Scholar]
- 69.Steglińska A., Sulyok M., Janas R., Grzesik M., Liszkowska W., Kręgiel D., Gutarowska B. Metabolite formation by fungal pathogens of potatoes (Solanum tuberosum L.) in the presence of bioprotective agents. Int. J. Environ. Res. Public Health. 2023;20:5221. doi: 10.3390/ijerph20065221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ellner F.M. Mycotoxins in potato tubers infected by Fusarium sambucinum. Mycotox. Res. 2002;18:57–61. doi: 10.1007/BF02946697. [DOI] [PubMed] [Google Scholar]
- 71.Eranthodi A., Schneiderman D., Harris L.J., Witte T.E., Sproule A., Hermans A., Overy D.P., Chatterton S., Liu J., Li T., et al. Enniatin production influences Fusarium avenaceum virulence on potato tubers, but not on durum wheat or peas. Pathogens. 2020;9:75. doi: 10.3390/pathogens9020075. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data are contained within the article.