Abstract
Persistent infection with hepatitis C virus (HCV) is associated with the development of liver cirrhosis and hepatocellular carcinoma. To examine the oncogenic potential of the HCV core gene product, primary rat embryo fibroblasts (REFs) were transfected with the core gene in the presence or absence of the H-ras oncogene. In contrast to a previous report (R. B. Ray, L. M. Lagging, K. Meyer, and R. Ray, J. Virol. 70:4438–4443, 1996), HCV core proteins from two different genotypes (type 1a and type 1b) were not found to transform REFs to tumorigenic phenotype in cooperation with the H-ras oncogene, although the core protein was successfully expressed 20 days after transfection. In addition, REFs transfected with E1A- but not core-expressing plasmid showed the phenotype of immortalized cells when selected with G418. The biological activity was confirmed by observing the transcription activation from two viral promoters, Rous sarcoma virus long terminal repeat and simian virus 40 promoter, which are known to be activated by the core protein from HCV-1 isolate. In contrast to the result with primary cells, the Rat-1 cell line, stably expressing HCV core protein, exhibited focus formation, anchorage-independent growth, and tumor formation in nude mice. HCV core protein was able to induce the transformation of Rat-1 cells with various efficiencies depending on the expression level of the core protein. These results indicate that HCV core protein has an oncogenic potential to transform the Rat-1 cell line but is not sufficient to either immortalize primary REFs by itself or transform primary cells in conjunction with the H-ras oncogene.
Hepatitis C virus (HCV) is a major etiologic agent of non-A, non-B hepatitis and is known to be associated with a high frequency of chronic infection (1, 2). Although the action mechanism of transformation is still unknown, persistent infection with HCV is highly correlated with the development of liver cirrhosis and hepatocellular carcinoma (HCC) (7, 11). The viral genome and replication have been detected in liver cells with pathologic changes (14).
Earlier studies suggested that the HCV core gene product could regulate the growth of hepatocytes by affecting the transcription of cellular proto-oncogenes and tumor suppressor genes (23, 24). Also, it has been reported that the core protein of the HCV-1 strain in cooperation with H-ras can transform primary rat embryo fibroblasts (REFs) to the tumorigenic phenotype (25). In contrast, other groups have reported that transgenic mice expressing the full-length core protein show no histologic or biochemical evidence of liver disease or HCC (9, 20), suggesting that the HCV core protein may be not cytopathic for the hepatocytes in vivo.
Nucleotide sequences of the HCV core gene are well conserved among all identified isolates (5). The core protein is produced as a polyprotein and matured by a host signal peptidase. The HCV core gene of several different isolates was shown to be expressed as both a 21-kDa core protein (191 amino acids) and a 19-kDa core protein (173 amino acids) (8, 15–17, 28). A third HCV core protein, approximately 16 kDa in size, was also reported to be produced in the HCV-1 isolate (17). Noticeably, several studies have produced controversial results in that HCV core proteins have been described as either cytoplasmic or nuclear, depending on the size of the core protein and the genotype of the virus (4, 15, 17, 19, 22, 28, 31). The various subcellular localizations of HCV core proteins could imply that different core proteins have distinct biological properties. Therefore, it remains to be determined whether the core proteins of other isolates can have oncogenic potential to transform primary cells in cooperation with the H-ras oncogene.
In this study, HCV core proteins from two different genotypes (HCV-K for type 1b and HCV-RH for type 1a) were tested for the ability to transform primary REFs in cooperation with H-ras. No significant cooperative effect for the transformation of REFs was observed upon cotransfection of the HCV core gene and H-ras DNA. In addition, they did not show any phenotypic change, such as immortalization, when the core-expressing cells were selected by G418. In contrast, Rat-1 cells transfected with the HCV core gene exhibited transformed phenotypes and the frequency of transformation was directly proportional to the expression level of the core protein. These results suggest that the HCV core protein appears to have oncogenic potential to transform the Rat-1 cell line but is not sufficient for either immortalizing by itself after G418 treatment or transforming primary cells in cooperation with H-ras oncogene.
MATERIALS AND METHODS
Cells and plasmids.
Primary REFs were isolated from 13.5-day-old embryos of F344/DuCrj rats (Charles River Japan, Inc.) by the standard method (21) and were grown in Dulbecco’s modified Eagle’s medium (Gibco BRL) supplemented with 10% fetal calf serum (Biological Industries). Rat-1 cells, an immortalized REF cell line, were maintained in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal calf serum. Plasmid pCI-neo-core K was constructed by inserting the PCR-amplified core region of the HCV-K isolate (genotype 1b [6]) into the mammalian expression vector pCI-neo (Promega) bearing the strong human cytomegalovirus immediate-early promoter. A termination codon was introduced at the end of the core protein-coding sequence. Plasmid pCI-neo-ST contains the entire structural gene of HCV-K, core-E1-E2, in the pCI-neo backbone. pCMV-RC containing the entire core gene sequence of the HCV RH isolate (genotype 1a) is described elsewhere (16). Plasmid pEJ6.6 containing activated H-rasVal-12 was obtained from American Type Culture Collection and used in the cotransfection with HCV core gene plasmids. pRSV-luc and pGL2 (Promega) contain the luciferase gene under the control of the Rous sarcoma virus long terminal repeat (RSV LTR) and the simian virus 40 (SV40) early promoter, respectively.
Focus formation assays.
Secondary cultures of REFs (3 × 105 cells per 60-mm-diameter dish) were transfected with the HCV core gene plasmid with or without the oncogene plasmid by the calcium phosphate coprecipitation method as described previously (18). Briefly, 2 μg of each plasmid was transfected with carrier DNA, and the cells were treated with 10% dimethyl sulfoxide at 18 h posttransfection. The cells were washed three times with phosphate-buffered saline and fed with fresh medium. After 24 h, the transfected cultures were split in a ratio of 1:6. When the cells reached confluence, the serum concentration was lowered from 10 to 5%. The cultures were refed every 3 to 4 days, and focus formation was assessed 2 to 5 weeks later. The adenovirus E1A and H-ras genes were used as a positive control. For drug selection, the transfected cells were treated with medium containing 500 μg of G418 sulfate (Gibco BRL) per ml. When drug-resistant colonies appeared at 10 to 14 days posttransfection, colonies were transferred into six-well plates (first passage). Proliferative potential was tested by further passage onto 100-mm-diameter dishes (second passage) and continuous cultivation, i.e., splitting cells at a ratio of 1:10 up to the fourth passage. For the focus formation assay of the Rat-1 cell line, cells were transfected with 20 μg of pCI-neo or pCI-neo-core K and individual G418-resistant clones were selected. The relative level of the expressed core protein in each selected clone was examined by immunoblotting. The individual clones expressing the core protein at higher or lower levels, designated pCI-neo-core K (H) or pCI-neo-core K(L), respectively, were mixed with Rat-1 control cells at a ratio of 1:10 and incubated for 6 days.
Luciferase assay.
A total of 4 × 105 REFs were transfected with 2 μg of HCV core expression plasmids and 1 μg of pRSV-luc or pGL2 reporter plasmid by the calcium phosphate coprecipitation method. After 48 h, luciferase assays were performed as recommended by the manufacturer (Promega). The difference in transfection efficiency was normalized by cotransfecting a reporter plasmid, pNEB-SEAP, expressing human secreted alkaline phosphatase. Relative light units were measured in a luminometer (Lumat LB 9501; Berthold). The means of three independent experiments were calculated.
Western blotting.
The cells were resuspended in sodium dodecyl sulfate-polyacrylamide gel electrophoresis loading buffer. The proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (12% polyacrylamide) and electrotransferred onto a Hybond-C membrane (Amersham). HCV core proteins were detected with HCV-positive human serum and horseradish peroxidase-conjugated anti-human immunoglobulin G as the primary and secondary antibodies, respectively.
Growth in soft agar.
Soft agar assays were performed as described previously (12). Soft agar dishes were prepared with an underlayer of 0.75% agarose (Sigma) in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal calf serum. Core-expressing Rat-1 clones were plated in the same medium containing 0.25% agarose. The dishes were examined microscopically for colony formation after incubation for 14 days. The data was expressed as the percentage of colonies containing more than 200 cells 2 weeks after plating.
Tumorigenicity of transfected Rat-1 cells.
The cells were trypsinized, washed with phosphate-buffered saline, and inoculated (5 × 105 cells per injection) subcutaneously into 4-week-old female athymic nude mice. The animals were examined for tumor formation over a period of 4 weeks.
RESULTS
Oncogenic potential of the HCV core protein in REFs.
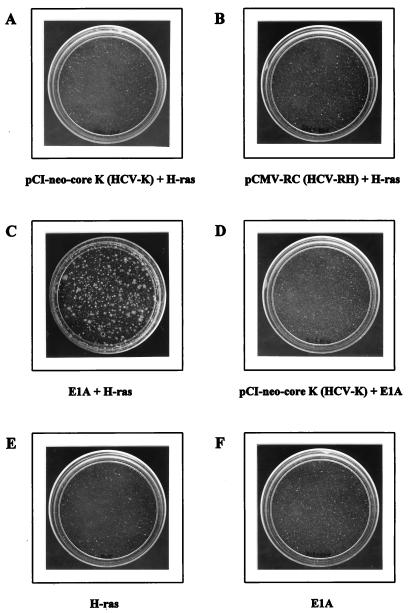
HCV infection was shown to be strongly linked to the development of HCC in epidemiological studies (7, 11). To test the transforming activity of the HCV core protein, we examined its ability to transform primary REFs in conjunction with H-ras oncogene. In contrast to the previous report (25), the transformed focus was not detected up to 30 days after cotransfection with pCI-neo-core K (HCV-K core gene; genotype 1b) and the H-ras gene (Fig. 1A). In addition, cotransfection of pCI-neo-core K and E1A could not generate any transformed foci, indicating that the HCV core cannot substitute for the function of the H-ras oncogene (Fig. 1D). Similar observations were reproducibly made when seven different batches of REF cultures were tested. As a positive control, adenovirus E1A cotransfected with H-ras readily gave more than 200 foci per 3 × 105 REFs 14 days after transfection (Fig. 1C). Although the core protein is highly conserved among all identified HCV isolates (5, 6), the difference in our results and those in the previous report (25) might be due to the use of different genotypes of HCV to obtain the core protein used in the transformation assay. Since the previous study (25) had used the core gene from HCV-1 (genotype 1a), we also tested a core from the same genotype, such as the HCV-RH isolate, for its ability to transform REFs in cooperation with the H-ras oncogene. Four amino acids were found to be different between the HCV-1 and the HCV-RH core protein sequences (16). As shown in Fig. 1B, we did not detect any transforming activity, suggesting that the failure of the core-mediated transformation in the presence of H-ras is not due to different genotypes of the HCV core gene, genotype 1a and 1b. As a negative control, transfection with either adenovirus E1A construct or H-ras plasmid, by itself, did not show any focus formation (Fig. 1E and F).
FIG. 1.
The lack of transforming activity by HCV core and H-ras. E1A-plus-H-ras transfectants were photographed 14 days after transfection. The other plates were examined 30 days after transfection.
It was previously reported that H-ras transfectants are unable to generate actively tumorigenic colonies but grow strongly and manifest a transformed morphology when untransfected adjacent cells are removed by cytocidal drug selection such as G418 treatment (13). To investigate whether the core protein alone can affect cell growth, REFs transfected with the HCV core gene (pCI-neo-core K or pCMV-RC) were selected by G418 treatment. Several G418-resistant colonies were picked and subcultured. Upon further passaging, they went into a crisis, during which most of the cells ceased to proliferate (Table 1). In contrast, six G418-resistant REF colonies cotransfected with E1A and pCI-neo appeared to show continuous growth up to the fourth passage. As a positive control, colonies from H-ras-transfected REFs were shown to continuously grow up to four passages (Table 1). These results demonstrate that HCV core proteins from two different genotypes do not appear to be equivalent to E1A, which is sufficient for inducing the immortalization of primary cells under G418 selection.
TABLE 1.
Comparison of the ability of the HCV core gene, adenovirus E1A, and H-ras oncogenes to induce in vitro immortalizationa
| Plasmid DNA transfected | No. of clones surviving 4th passage/no. tested |
|---|---|
| pCI-neo | 0/6 |
| pCI-neo-core K (HCV-K) | 0/12 |
| pCMV-RC (HCV-RH) | 0/12 |
| pCI-neo + adenovirus E1A | 6/6 |
| pCI-neo + H-ras | 6/6 |
Secondary cultures of REFs were cotransfected with each plasmid DNA. G418 selection was applied 48 h after transfection. When drug-resistant colonies had developed, single colonies were isolated and transferred into six-well dishes (first passage). The proliferative potential was then tested by further passage onto 100-mm dishes (second passage) and subsequent passages (third and fourth passages), splitting cells at a ratio of 1:10. Clones which survived the fourth passage were scored as surviving.
Expression and biological activity of HCV core protein in REFs.
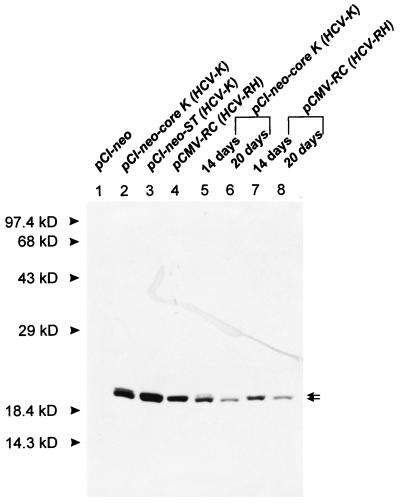
To investigate whether the HCV core is successfully expressed in transfected REF cells, immunoblotting analyses were performed at various time intervals. As shown in Fig. 2, two forms of HCV-K core protein, of 19 and 21 kDa, were specifically detected even 20 days after transfection. A similar experiment with pCI-neo-transfected REFs as a negative control did not show any corresponding band (Fig. 2, lane 1). The expression pattern of the core from HCV-1 was previously reported to generate both the 19- and 21-kDa proteins and a 16-kD protein, depending on the downstream E1 sequence (16, 17). Both pCI-neo-core K and pCI-neo-ST, containing the core, E1, and E2 genes of the HCV-K isolate (6), were shown to produce the 19- and 21-kDa polypeptides as the major and minor products, respectively (lanes 2 and 3). These results indicate that the expression pattern of the core gene from HCV-K is not affected by the envelope sequence downstream of the core gene, which is the same observation as that obtained with HCV-BK isolates (15). Expression of the HCV-RH core gene resulted in the synthesis of a 21-kDa protein, which is consistent with the previous report (lane 4) (16).
FIG. 2.
Immunoblot analysis of the HCV core protein from transfected REFs. The REF transfectants were harvested 2 (lanes 1 to 4), 14 (lanes 5 and 7), or 20 (lanes 6 and 8) days after transfection, and cell lysates were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (12% polyacrylamide). The core protein was detected by immunoblotting with anti-HCV human immunoglobulin and horseradish peroxidase-conjugated anti-human immunoglobulin G. Two forms of the HCV core protein (19 and 21 kDa) are indicated by arrows. The positions of molecular mass standards are indicated.
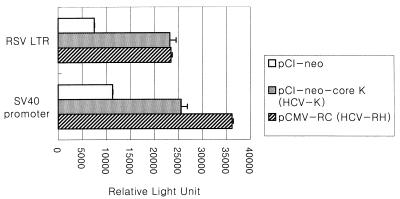
The HCV core protein from the HCV-1 isolate was shown to activate the human c-myc promoter, RSV LTR, and the SV40 early promoter by four- to sixfold (23). To test whether HCV core proteins in our study are able to transcriptionally activate RSV LTR and the SV40 early promoter, the core expression plasmid was cotransfected with either pRSV-luc or pGL2 as a reporter plasmid into REFs and the relative luciferase activities were measured. As shown in Fig. 3, the promoter activities of RSV LTR and the SV40 early promoter were increased by two- to fourfold, respectively. This result indicates that the HCV core protein expressed in REFs retains its biological activity in terms of transcriptional activation.
FIG. 3.
Biological activity of HCV core protein expressed in REFs. Each HCV core plasmid (2 μg) was cotransfected with a reporter plasmid (1 μg) into secondary cultures of REFs. The resulting luciferase activity, normalized to secreted alkaline phosphatase activity, is presented as relative light units.
Tumorigenic conversion of Rat-1 cell lines by the HCV core protein.
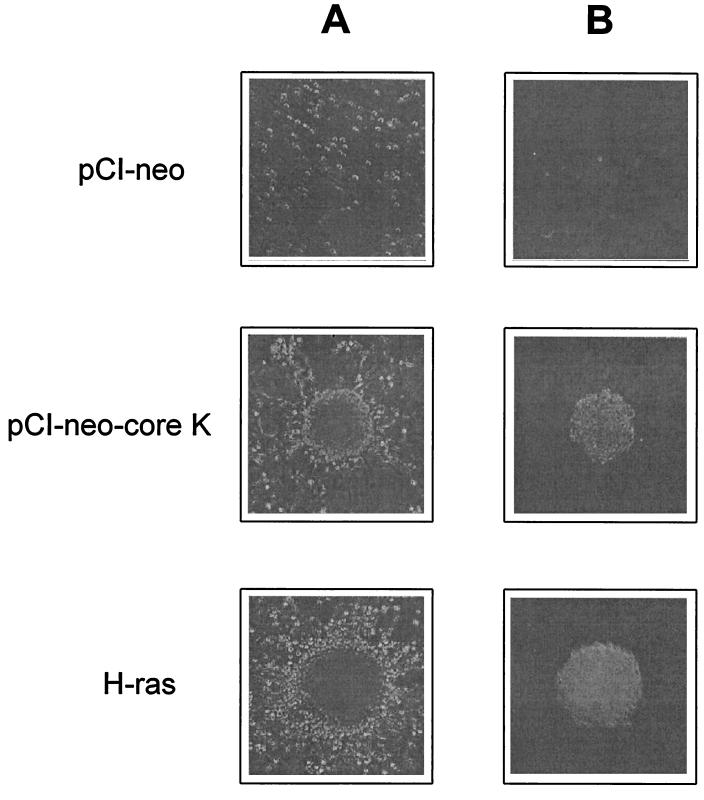
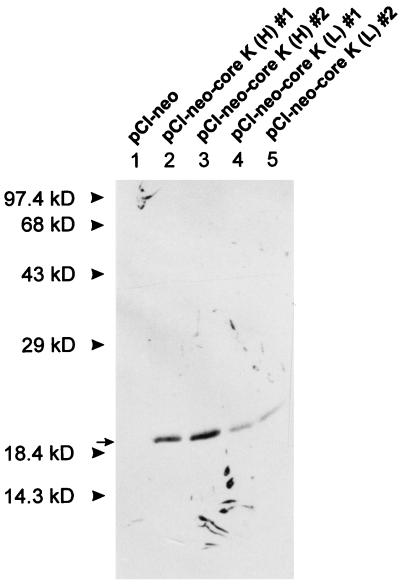
The transforming potential of the HCV core gene was further investigated by using the Rat-1 cell line, since the established cell line may harbor unidentified mutations which facilitate its transforming ability. Rat-1 cells, the established REFs, were transfected with either pCI-neo control plasmid or pCI-neo-core K plasmid and the resulting colonies were selected by G418 treatment. The drug-resistant colonies were isolated and tested for their ability to generate transformed foci. As shown in Fig. 4A and Table 2, Rat-1 cells were readily transformed by the HCV-K core protein and exhibited focus formation. When Rat-1 cell lines transfected with pCI-neo-core K were inoculated into soft agar to assess anchorage-independent growth, they formed large colonies with high frequency (Fig. 4B; Table 2). As a negative control, Rat-1 cells transfected with pCI-neo plasmid could not induce any foci in the monolayer cultures and produced only small colonies (fewer than 50 cells) at a very low frequency in the soft agar assay. The expression of HCV core in the selected Rat-1 clone was identified by immunoblotting experiments (Fig. 5). In contrast to the core expression in primary REFs, the single 19-kDa species was detected in transfected Rat-1 clones, implying that different transforming potentials may be due to the distinct expression patterns in the different cell types. Interestingly, the frequency of transformation was proportional to the expression level of HCV-K core protein (Table 2). To confirm the tumorigenic potential of HCV core gene transfectants, nude mice were inoculated subcutaneously with either pCI-neo- or pCI-neo-core K-transfected cells. At 4 weeks after injection, tumors developed in all mice inoculated with pCI-neo-core K transfectants (Table 2) but none of the mice inoculated with pCI-neo transfectants developed a detectable tumor. These results indicate that the HCV core protein of HCV-K isolate can induce tumorigenic transformation of established Rat-1 fibroblasts.
FIG. 4.
(A) Focus morphology of pCI-neo- or pCI-neo-core K-transfected Rat-1 clones. (B) Colony formation in a soft agar assay by pCI-neo- or pCI-neo-core K-transfected Rat-1 clones. H-ras-transformed clones are also shown as a positive control. Magnification, ×100.
TABLE 2.
Tumorigenicity of Rat-1 cells transfected with the HCV-K core gene
| Clone | No. of foci formeda | Colony formation in soft agar assay (%)b | Size of tumors on nude mice (mm) |
|---|---|---|---|
| pCI-neo | 0 | 0.05 ± 0.03 | NDc |
| pCI-neo-core K(H)d | 124 ± 6 | 13.19 ± 0.57 | 6.5 ± 0.5 |
| pCI-neo-core K(L)d | 3 ± 1 | 3.20 ± 0.08 | 4.5 ± 0.5 |
| H-ras | 231 ± 7 | 22.96 ± 0.78 | NTe |
A total of 5 × 104 cells of each clone were mixed with Rat-1 control cells at a ratio of 1:10 and incubated for 6 days.
The data is expressed as the percentage of colonies containing more than 200 cells 2 weeks after plating. Colony numbers are means of three independent experiments.
ND, not detected.
pCI-neo-core K(H) clones express the HCV-K core protein at higher level than pCI-neo-core K(L) clones. The data is represented as the mean of the results for three independent clones.
NT, not tested.
FIG. 5.
Immunoblot analysis of the HCV core protein from G418-selected Rat-1 clones. The experiment was performed as described in the legend to Fig. 2. pCI-neo-core K (H) (expressing the HCV-K core protein at a high level) clones express the HCV-K core protein at higher level than do pCI-neo-core K (L) clones (expressing the HCV-K core protein at a low level).
DISCUSSION
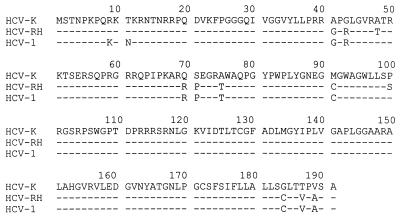
We have demonstrated that the HCV core gene encodes a weakly oncogenic protein, just oncogenic enough to transform the established Rat-1 cell line with regard to focus formation, anchorage independence, and tumor formation in nude mice. However, the core proteins used in this study are not sufficient to transform primary REFs in cooperation with H-ras, which is inconsistent with the previous report (25). A possible explanation for the discrepancies may be inferred from the following observations. First, the difference in the tumorigenic potential of HCV core protein may result from different core gene products. It was reported that both the processing and the ratio of alternative forms of HCV core protein are different for different isolates (15–17), although the amino acid sequences are highly conserved (Fig. 6). In our study, the core gene of genotype 1b (HCV-K isolate) resulted in distinct core protein products: Rat-1 cells produced 19-kDa species, while primary REFs generated both 19- and 21-kDa species with a ratio of approximately 10:1 in the presence and absence of the downstream E1 sequence. In contrast, it was shown that the core of the HCV-1 isolate produced both the 21- and 19-kDa products and a 16-kDa product, depending on the downstream E1 sequence (17). The 21- and 19-kDa proteins appear to be associated with the membrane of the endoplasmic reticulum, and the 16-kDa protein showed predominant nuclear localization (17). The relationship between proteolytic processing and subcellular localization of the core protein remains unclear. Liu et al. have suggested that the interaction between the 19- and 21-kDa proteins in the cytoplasm may prevent nuclear translocation of the 19-kDa protein, resulting in the cytoplasmic localization of both forms (15). However, it is likely that a small portion of the 19-kDa protein is accumulated in the nucleus because the core protein of HCV-K and HCV-RH in our study could activate the transcription of other promoters (Fig. 3). Although the biological significance of the nuclear translocation of the HCV core protein is uncertain, previous studies have proposed that the core protein in the nucleus may have a regulatory function in host gene expression (10, 23). On the basis of these observations, it is likely that the ratio of two or three alternative forms (21, 19, and/or 16 kDa) and their subcellular localization will be important for the transforming potential of HCV core protein. Ray et al. have shown a predominantly nuclear localization of the HCV-1 core protein in the transformed REFs (25), which may result in higher transformation activity, strong enough to induce the transformation of primary cells in cooperation with H-ras. However, it is unknown whether the HCV core protein is accumulated in the nucleus during natural viral infection and whether it affects the transcription of cellular genes. Second, it is possible that some REFs used in the previous study (25) were capable of undergoing secondary events after transfection under certain culture conditions, such as chromosomal translocation or deletions, which predispose transfected cells to exhibit a transformed phenotype. Alternatively, overexpression of the HCV-1 core protein in vitro may generate an artificial condition in which some REFs were transformed to the tumorigenic phenotype. However, it is known that the HCV core is expressed at a low level during natural infection, just enough to be detected by immunohistochemistry (34).
FIG. 6.
Comparison of the core protein-coding sequences of the HCV-K, HCV-RH, and HCV-1 isolates. The amino acid sequence of HCV-K is shown at the top. Identical sequences are represented by dashes.
The fact that the Rat-1 cell line was readily transformed by the HCV core protein indicates that the core gene of HCV-K and HCV-RH isolates may code for an oncogenic protein. However, the oncogenic activity of the core protein in our study was not strong enough for the protein to readily transform primary REFs in cooperation with H-ras, compared with other immortalizing oncogenes such as E1A, c-myc, and human papillomavirus 16 E6, or E7 (3, 12, 13, 26, 30, 33). It is known that the established cell line may retain the altered cellular genes as well as the activated oncogene(s) generated during the establishment and passages of the cell line, which may facilitate its transformation by a weak oncogene. Since the development of HCC is a long-term process, taking more than 20 years (7), it is reasonable to speculate that the HCV core protein is just one of multiple factors required for carcinogenesis and/or has a weakly oncogenic activity that is sufficient to stimulate only part of a complex multistep pathway.
The continuous regeneration of hepatocytes as a result of chronic hepatitis may increase the incidence of genetic alterations. According to a previous report (29), the rate of nucleotide substitutions in the HCV core gene was significantly greater for isolates from HCC patients than for those from individuals with chronic hepatitis. Also, it was proposed that chronic inflammation and cirrhosis, accompanied by regenerative processes, may function as a tumor promoter which is providing a pathway from chronic HCV infection to HCC and that p53 tumor suppressor gene mutations in HCV-associated HCC may induce tumor progression (32). In addition, other HCV gene products may have oncogenic potential. The observation that the HCV nonstructural protein NS3 was able to transform NIH 3T3 cells suggests the involvement of protease activity in cellular transformation (27). Further studies are necessary to elucidate the oncogenic potential of the HCV core gene in cooperation with other HCV genes.
ACKNOWLEDGMENTS
Jun Chang and Se-Hwan Yang contributed equally to this work.
This research was supported by grants N96318-1 and 1NI9732001 from Ministry of Science and Technology and by grant 1NN9715701 from National Institute of Health, Ministry of Health and Welfare, Korea.
REFERENCES
- 1.Aach R D, Stevens C E, Hollinger F B, Mosley J W, Peterson D A, Taylor P E, Johnson R G, Barbosa L H, Nemo G J. Hepatitis C virus infection in post-transfusion hepatitis. An analysis with first- and second-generation assays. N Engl J Med. 1991;325:1325–1329. doi: 10.1056/NEJM199111073251901. [DOI] [PubMed] [Google Scholar]
- 2.Alter H J, Purcell R, Shih J, Melpolder J, Choo Q L, Kuo G. Detection of antibody to hepatitis C virus in prospectively followed transfusion recipients with acute and chronic non-A, non-B hepatitis. N Engl J Med. 1989;321:1494–1500. doi: 10.1056/NEJM198911303212202. [DOI] [PubMed] [Google Scholar]
- 3.Band V, Zajchowski D, Kulesa V, Sager R. Human papilloma virus DNAs immortalize normal human mammary epithelial cells and reduce their growth factor requirements. Proc Natl Acad Sci USA. 1990;87:463–467. doi: 10.1073/pnas.87.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barba G, Harper F, Harada T, Kohara M, Goulinet S, Matsuura Y, Eder G, Schaff Z, Chapman M J, Miyamura T, Brechot C. Hepatitis C virus core protein shows a cytoplasmic localization and associates to cellular lipid storage droplets. Proc Natl Acad Sci USA. 1997;94:1200–1205. doi: 10.1073/pnas.94.4.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bukh J, Purcell R H, Miller R H. Sequence analysis of the core gene of 14 hepatitis C virus genotypes. Proc Natl Acad Sci USA. 1994;91:8239–8243. doi: 10.1073/pnas.91.17.8239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cho Y-G, Yoon J-W, Jang K-L, Kim C-M, Sung Y-C. Full genome cloning and nucleotide sequence analysis of hepatitis C virus from sera of chronic hepatitis patients in Korea. Mol Cells. 1993;3:195–202. [Google Scholar]
- 7.Di Bisceglie A M, Simpson L H, Lotze M T, Hoofnagle J H. Development of hepatocellular carcinoma among patients with chronic liver disease due to hepatitis C viral infection. J Clin Gastroenterol. 1994;19:222–226. doi: 10.1097/00004836-199410000-00011. [DOI] [PubMed] [Google Scholar]
- 8.Hüssy P, Langen H, Mous J, Jacobsen H. Hepatitis C virus core protein: carboxy-terminal boundaries of two processed species suggest cleavage by a signal peptide peptidase. Virology. 1996;224:93–104. doi: 10.1006/viro.1996.0510. [DOI] [PubMed] [Google Scholar]
- 9.Kawamura T, Furusaka A, Koziel M J, Chung R T, Wang T C, Schmidt E V, Liang T J. Transgenic expression of hepatitis C virus structural proteins in the mouse. Hepatology. 1997;25:1014–1021. doi: 10.1002/hep.510250437. [DOI] [PubMed] [Google Scholar]
- 10.Kim D W, Suzuki R, Harada T, Saito I, Miyamura T. Trans-suppression of gene expression by hepatitis C viral core protein. Jpn J Med Sci Biol. 1994;47:211–220. doi: 10.7883/yoken1952.47.211. [DOI] [PubMed] [Google Scholar]
- 11.Kiyosawa K, Sodeyama T, Tanaka E, Gibo Y, Yoshizawa K, Nakano Y, Furuta S, Akahane Y, Nishioka K, Purcell R H, Alter H J. Interrelationship of blood transfusion, non-A, non-B hepatitis and hepatocellular carcinoma: analysis by detection of antibody to hepatitis C virus. Hepatology. 1990;12:671–675. doi: 10.1002/hep.1840120409. [DOI] [PubMed] [Google Scholar]
- 12.Land H, Parada L F, Weinberg R A. Tumorigenic conversion of primary embryo fibroblasts requires at least two cooperating oncogenes. Nature (London) 1983;304:596–602. doi: 10.1038/304596a0. [DOI] [PubMed] [Google Scholar]
- 13.Land H, Chen A C, Morgenstern J P, Parada L F, Weinberg R A. Behavior of myc and ras oncogenes in transformation of rat embryo fibroblasts. Mol Cell Biol. 1986;6:1917–1925. doi: 10.1128/mcb.6.6.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lanford R E, Chavez D, Chisari F V, Sureau C. Lack of detection of negative-strand hepatitis C virus RNA in peripheral blood mononuclear cells and other extrahepatic tissues by the highly strand-specific rTth reverse transcriptase PCR. J Virol. 1995;69:8079–8083. doi: 10.1128/jvi.69.12.8079-8083.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Q, Tackney C, Bhat R A, Prince A M, Zhang P J. Regulated processing of hepatitis C virus core protein is linked to subcellular localization. J Virol. 1997;71:657–662. doi: 10.1128/jvi.71.1.657-662.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lo S Y, Selby M, Tong M, Ou J-H. Comparative studies of the core gene products of two different hepatitis C virus isolates: two alternative forms determined by a single amino acid substitution. Virology. 1994;199:124–131. doi: 10.1006/viro.1994.1104. [DOI] [PubMed] [Google Scholar]
- 17.Lo, S. Y., F. Masiarz, S. B. Hwang, M. M. Lai, and J. H. Ou. Differential subcellular localization of hepatitis C virus core gene products. Virology 213:455–461. [DOI] [PubMed]
- 18.Lowy D R, Rands E, Scolnick E M. Helper-independent transformation by unintegrated Harvey sarcoma virus DNA. J Virol. 1978;26:291–298. doi: 10.1128/jvi.26.2.291-298.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsuura Y, Harada T, Makimura M, Sato M, Aizaki H, Suzuki T, Miyamura T. Characterization of HCV structural proteins expressed in various animal cells. Intervirology. 1994;37:114–118. doi: 10.1159/000150365. [DOI] [PubMed] [Google Scholar]
- 20.Pasquinelli C, Shoenberger J M, Chung J, Chang K M, Guidotti L G, Selby M, Berger K, Lesniewski R, Houghton M, Chisari F V. Hepatitis C virus core and E2 protein expression in transgenic mice. Hepatology. 1997;25:719–727. doi: 10.1002/hep.510250338. [DOI] [PubMed] [Google Scholar]
- 21.Pollack R, Risser R, Conlon S, Rifkin D. Plasminogen activator production accompanies loss of anchorage regulation in transformation of primary rat embryo cells by simian virus 40. Proc Natl Acad Sci USA. 1974;71:4792–4796. doi: 10.1073/pnas.71.12.4792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ravaggi A, Natoli G, Primi D, Albertini A, Levrero M, Cariani E. Intracellular localization of full-length and truncated hepatitis C virus core protein expressed in mammalian cells. J Hepatol. 1994;20:833–836. doi: 10.1016/s0168-8278(05)80157-6. [DOI] [PubMed] [Google Scholar]
- 23.Ray R B, Lagging L M, Meyer K, Steele R, Ray R. Transcriptional regulation of cellular and viral promoters by the hepatitis C virus core protein. Virus Res. 1995;37:209–220. doi: 10.1016/0168-1702(95)00034-n. [DOI] [PubMed] [Google Scholar]
- 24.Ray R B, Steele R, Meyer K, Ray R. Transcriptional repression of p53 promoter by hepatitis C virus core protein. J Biol Chem. 1997;272:10983–10986. doi: 10.1074/jbc.272.17.10983. [DOI] [PubMed] [Google Scholar]
- 25.Ray R B, Lagging L M, Meyer K, Ray R. Hepatitis C virus core protein cooperates with ras and transforms primary rat embryo fibroblasts to tumorigenic phenotype. J Virol. 1996;70:4438–4443. doi: 10.1128/jvi.70.7.4438-4443.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ruley H E. Adenovirus early region 1A enables viral and cellular transforming genes to transform primary cells in culture. Nature (London) 1983;304:602–606. doi: 10.1038/304602a0. [DOI] [PubMed] [Google Scholar]
- 27.Sakamuro D, Furukawa T, Takegami T J. Hepatitis C virus nonstructural protein NS3 transforms NIH 3T3 cells. J Virol. 1995;69:3893–3896. doi: 10.1128/jvi.69.6.3893-3896.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Santolini E, Migliaccio G, La Monica N. Biosynthesis and biochemical properties of the hepatitis C virus core protein. J Virol. 1994;68:3631–3641. doi: 10.1128/jvi.68.6.3631-3641.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shimizu I, Yao D F, Horie C, Yasuda M, Shiba M, Horie T, Nishikado T, Meng X Y, Ito S J. Mutations in a hydrophilic part of the core gene of hepatitis C virus in patients with hepatocellular carcinoma in China. Gastroenterology. 1997;32:47–55. doi: 10.1007/BF01213296. [DOI] [PubMed] [Google Scholar]
- 30.Storey A, Banks L. Human papillomavirus type 16 E6 gene cooperates with EJ-ras to immortalize primary mouse cells. Oncogene. 1993;8:919–924. [PubMed] [Google Scholar]
- 31.Suzuki R, Matsuura Y, Suzuki T, Ando A, Chiba J, Harada S, Saito I, Miyamura T. Nuclear localization of the truncated hepatitis C virus core protein with its hydrophobic C terminus deleted. J Gen Virol. 1995;76:53–61. doi: 10.1099/0022-1317-76-1-53. [DOI] [PubMed] [Google Scholar]
- 32.Tabor E. Hepatocarcinogenesis: hepatitis viruses and altered tumor suppressor gene function. Princess Takamatsu Symp. 1995;25:151–161. [PubMed] [Google Scholar]
- 33.Watanabe S, Kanda T, Yoshiike K. Human papillomavirus type 16 transformation of primary human embryonic fibroblasts requires expression of open reading frames E6 and E7. J Virol. 1989;63:965–969. doi: 10.1128/jvi.63.2.965-969.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yap S H, Willems M, Van den Oord J, Habets W, Middeldorp J M, Hellings J A, Nevens F, Moshage H, Desmet V, Fevery J. Detection of hepatitis C virus antigen by immunohistochemical staining: a histological marker of hepatitis C virus infection. J Hepatol. 1994;20:275–281. doi: 10.1016/s0168-8278(05)80069-8. [DOI] [PubMed] [Google Scholar]