STRUCTURED ABSTRACT
Background:
Whether COVID-19 vaccination and the associated immune response increases susceptibility to immune related adverse events (IRAEs) among patients treated with immune checkpoint inhibition (ICI) remains unknown. Short-term follow-up can assess the safety of concurrent administration of the vaccine and ICI treatment.
Methods:
Electronic health record analysis of a cohort of 408 patients with cancer on ICI therapy who received COVID-19 vaccination from January to March, 27, 2021. Patients were followed from 90 days from day of first dose in this single-institution tertiary care center. We evaluated incidence of IRAE as well as frequency of each event type and grade among patients who experienced an IRAE. We also evaluated the incidence of IRAE in patients who began a new immunotherapy agent following vaccination.
Results:
Among 408 patients with cancer on ICI therapy (Median age 71, 217 (53%) male), administration of a COVID-19 mRNA vaccine within 90 days of ICI treatment was not associated with an increased incidence of IRAEs, including among those with previous IRAEs from ICI. No excess risk of COVID-19 diagnosis was seen in this subset of patients on ICI therapy, and no breakthrough COVID-19 cases were seen following full COVID-19 vaccination.
Conclusions:
These findings should reassure providers that COVID-19 vaccination during ICI therapy is safe and efficacious.
Keywords: COVID-19, immunotherapy, mRNA vaccine
INTRODUCTION
Recently introduced COVID-19 vaccines are a critical advance against the COVID-19 pandemic 1, 2. The post-vaccine reduction in COVID-19-related hospitalization heralds the immense promise of these tools to reduce the impact of the COVID-19 pandemic among those at most significant risk, including cancer patients3.
Active cancer treatment predisposes to severe COVID-19 disease, and subdued vaccine immune responses are expected with certain cancer treatments. Among cancer therapies, immune checkpoint inhibitors (ICI) have a negligible effect on vaccine-elicited immune responses. For example, influenza vaccine studies demonstrate comparable immunogenicity in ICI-treated patients and healthy individuals and the overall safety of immunization4. Still, with higher rates of immediate post-vaccine adverse reactions with mRNA vaccines5, concerns have emerged about the risk for immune-related adverse events (IRAEs) following COVID-19 immunization. In addition, patients on active cancer treatment were excluded from key COVID-19 vaccine trials1, 2. Therefore, establishing the safety and efficacy of mRNA vaccines in patients on ICI therapy by extended monitoring for post-vaccine IRAE is critical.
This study characterizes IRAE and COVID-19 incidence in patients who received an mRNA vaccine within 90 days of ICI treatment.
METHODS
Memorial Sloan Kettering Cancer Center (MSKCC) is a 574-bed tertiary care cancer center in New York City. The study institution began vaccinating patients with the two SARS CoV-2 mRNA vaccines on January 16, 2021 following a priority scheme developed by New York State. Electronic pharmacy records were used to identify all patients vaccinated with a first dose between January 16 and March 27, 2021 who received FDA-approved ipilimumab, pembrolizumab, or nivolumab within 90 days before or after first vaccination dose. Clinicopathologic parameters related to any grade new onset IRAE occurring after first vaccine dose and ICI initiation, as defined by the Common Terminology Criteria for Adverse Events, version 4.0, were collected along with data on vaccine manufacturer, age at vaccination, sex, tumor type, dates and type(s) of ICI administration, and death during the follow-up period
We determined the incidence of IRAE in patients who received immunotherapy within 90 days before or after the first dose of an mRNA SARS-CoV-2 vaccine, following patients for 90 days from day of vaccination. For patients who experienced an IRAE during the follow-up period, we summarized the frequency of each event type and grade. In bivariate analyses (chi-square test, Fisher’s exact test, Wilcoxon rank sum test) we compared patients who did versus did not experience an IRAE during the follow-up period with respect to age, sex, tumor type, previous IRAE, previous SARS-CoV-2 infection, vaccine type, immunotherapy type, proximity of immunotherapy administration to vaccine, initiation of new immunotherapy following vaccination, death during follow-up, and total days of follow-up observed. We also calculated the incidence rate of IRAE in patients who began a new immunotherapy agent following vaccination.
The MSKCC institutional review board granted a HIPAA waiver of authorization to conduct this study.
Results
Four hundred and eight patients received a first dose of SARS-CoV-2 mRNA vaccine within 90 days before or after treatment with an ICI agent. Patients ranged in age from 55 to 93 years (median=71) and the cohort was 53% male (n=217). Patients were in treatment for thoracic (n=122, 30%), genitourinary (n=84, 21%), upper gastrointestinal (n=50, 12%), melanoma (n=47, 12%), gynecologic (n=41, 10%), sarcoma (n=20, 5%), head and neck (n=19, 5%), lower gastrointestinal (n=13, 3%), glioblastoma (n=5, 1%), lymphoma (n=4, 1%), and breast (n=3, 1%) cancers. Within the 90 day windows before and after first vaccine dose, patients were treated with pembrolizumab only (n=264, 65%), nivolumab only (n=99, 24%), ipilimumab and nivolumab (n=41, 10%), ipilimumab (n=3, 0.7%), or all three agents (n=1, 0.3%). The majority of patients (95%, n=389) received the Pfizer-BioNTech vaccine.
Twenty-seven patients (7%) experienced a new IRAE during the follow-up period. Among patients with a history of IRAE prior to vaccination (n=54), 3 (6%) experienced an IRAE during the post-vaccination follow-up period. The majority of new IRAE were mild (n=21 Grade 1, 78%; Table 1). All four patients who experienced a severe (Grade 3) IRAE had gastrointestinal events (colitis or diarrhea). Table 2 compares characteristics of patients who did versus did not experience an IRAE. There were no significant differences in baseline characteristics or treatment between these groups, except that patients beginning a new immunotherapy agent during the follow-up period were more likely to experience an IRAE. Of 28 patients who received the first vaccine dose and ICI therapy on the same day, none developed an IRAE during the follow-up period. Among patients who began a new immunotherapy agent following vaccination (n=52), 17% developed an IRAE (n=9; incidence rate=2.7 per 1,000 patient days observed). The median follow-up after initiation of the new immunotherapy agent in these patients was 78.5 days (range: 33, 88). Figure 1 presents the swimmer’s plot describing the treatment history and trajectory of patients who experienced an IRAE during the follow-up period.
Table 1.
Type and grade of immune-related adverse events experienced by patients who received SARS-CoV-2 mRNA vaccine and immune checkpoint inhibitor therapy within 90 days
| Grade 1 | Grade 2 | Grade 3 | |
|---|---|---|---|
|
| |||
| Arthralgias | 3 | - | - |
| Arthritis plus rash | 1 | 1 | - |
| Colitis | - | - | 1 |
| Dermatitis | 10 | - | - |
| Diarrhea | 2 | - | 3 |
| Pneumonitis | 2 | 1 | - |
| Thyroiditis | 1 | - | - |
| Transaminitis | 2 | - | - |
|
| |||
| Total | 21 | 2 | 4 |
Table 2.
Characteristics of patients who did and did not experience immune-related adverse events following receipt of SARS-CoV-2 mRNA vaccine and immune checkpoint inhibitor therapy within 90 days
| Patients who experienced IRAE | Patients who did not experience IRAE | p-value | |
|---|---|---|---|
| N | 27 | 381 | |
| Male sex | 12 (44) | 205 (54) | 0.35 |
| Age (median, interquartile range) | 75 (66, 78) | 71 (66, 78) | 0.76 |
| Tumor type | * | ||
| Breast | 2 (7) | 1 (1) | |
| Gastrointestinal, lower | 0 | 13 (3) | |
| Gastrointestinal, upper | 4 (15) | 46 (12) | |
| Genitourinary | 6 (22) | 78 (20) | |
| Glioblastoma | 0 | 5 (1) | |
| Gynecologic | 4 (15) | 37 (10) | |
| Head and neck | 2 (7) | 17 (4) | |
| Lymphoma | 1 (4) | 3 (1) | |
| Melanoma | 3 (11) | 44 (12) | |
| Sarcoma | 1 (4) | 19 (5) | |
| Thoracic | 4 (15) | 118 (31) | |
| Previous infection with SARS CoV-2 | 0 | 11 (3) | 0.99 |
| Previous immune-related adverse event | 3 (11) | 51 (13) | 0.99 |
| Received Pfizer-BioNTech vaccine | 27 (100) | 362 (89) | 0.24 |
| Received nivolumab | 11 (41) | 130 (34) | 0.48 |
| Received pembrolizumab | 16 (59) | 249 (65) | 0.52 |
| Received ipilimumab | 5 (19) | 40 (10) | 0.20 |
| Died during follow-up | 0 | 18 (5) | 0.62 |
| Receipt of vaccine first dose and immunotherapy within same week | 8 (30) | 189 (50) | 0.04 |
| Receipt of vaccine second dose and immunotherapy within same week (date imputed) | 1 (4) | 22 (6) | 0.99 |
| Started new immunotherapy agent during follow-up period | 9 (33%) | 43 (11%) | <0.001 |
| Days observed (median, interquartile range) | 90 (71, 90) | 90 (90, 90) | 0.01 |
Data are no. (%) unless otherwise indicated.
P-value not reported due to cell sizes <5.
Figure 1.
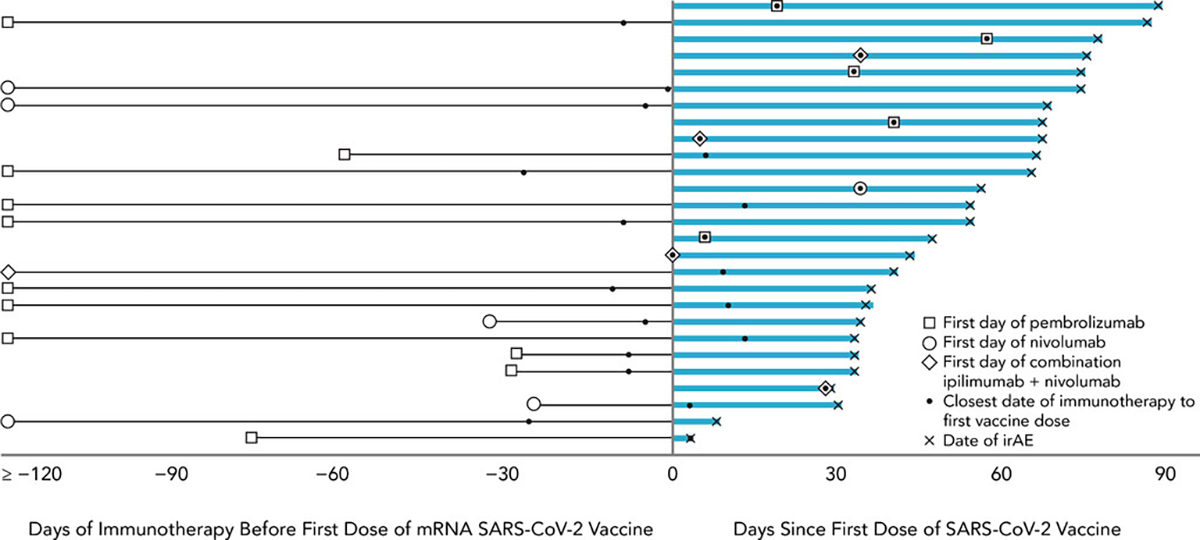
Swimmer’s plot depicting days from first dose of mRNA SARS-CoV-2 vaccine to immune-related adverse events (IRAE) among patients who received an immune checkpoint inhibitor within 90 days before or after vaccination and experienced an IRAE following receipt of immunotherapy and vaccine (N=27).
Two patients in our cohort developed COVID-19 infection following vaccination (through August, 31, 2021)
Discussion
The overall incidence of IRAE was 7 % in our cohort of 408 ICI-treated patients who received the COVID-19 vaccine. In 52 patients who began a first ICI therapy after vaccination, the incidence of IRAE (17%) is similar to published reports6, 7. For example, a recent study of pembrolizumab as adjuvant therapy in 509 patients with resected stage III melanoma found an IRAE incidence of 19% within 3 months of treatment initiation6. The vast majority of the IRAE were mild. No differences were observed in the risk of IRAE in those with and without preexisting immune toxicities. Furthermore, there were 11 individuals with prior COVID-19 infection in our cohort, and severe immediate reactogenicity from the vaccine or IRAE was not observed in any of them.
ICI is a standard treatment option for many cancers. In contrast to conventional chemotherapy, vaccine responses are not significantly impaired in those treated with ICI. Studies with influenza vaccine report a high serological response rate among ICI recipients without an exaggerated risk of IRAE4, 7. Similarly, emerging studies in mRNA vaccinated cancer patients show high antibody levels among ICI treated individuals that are comparable to healthy controls and superior to humoral responses in chemotherapy recipients8, 9.
Despite proven efficacy in larger populations, there are substantial lingering concerns on the long-term safety of vaccines with ICI. Early experience from Israel in patients on ICI treatment found that 19% were vaccine reluctant due to toxicity concerns. This report examined the short-term safety of ICI with the vaccine in 134 patients and over a brief median follow-up period of 19 days after vaccination5. They did not observe higher immediate side-effects, risk of new IRAE, or exacerbation of preexisting IRAE. Similarly, in a study of 81 ICI-treated patients where vaccine and immunotherapy were administered within 30 days10, no patients experienced new adverse events. Our findings add a sizable number of newly treated patients and a longer follow-up period to capture IRAE. We note that any conclusions about comparative incidence must be interpreted with caution given biases implicit within cross trial comparisons. An important limitation of our study is the absence of a temporally aligned comparison group of patients at our center on ICI therapy who did not receive the Covid-19 vaccine. We further note that the sample size of our study is not appropriately powered to capture the risk of post-vaccine rare events such as Guillain-Barré syndrome and others.
In summary, our data do not show a higher risk of immune toxicity among ICI-treated patients who received the COVID-19 vaccine, including those newly started on therapy. The findings should encourage new and third dose vaccine uptake among ICI-treated cancer patients without interruption of cancer therapy.
REFERENCES
- 1.Baden LR, El Sahly HM, Essink B, et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. Feb 4 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Polack FP, Thomas SJ, Kitchin N, et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. Dec 31 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tenforde MW, Olson SM, Self WH, et al. Effectiveness of Pfizer-BioNTech and Moderna Vaccines Against COVID-19 Among Hospitalized Adults Aged >/=65 Years - United States, January-March 2021. MMWR Morb Mortal Wkly Rep. May 7 2021;70(18):674–679. doi: 10.15585/mmwr.mm7018e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keam B, Kang CK, Jun KI, et al. Immunogenicity of Influenza Vaccination in Patients with Cancer Receiving Immune Checkpoint Inhibitors. Clin Infect Dis. Jul 11 2020;71(2):422–425. doi: 10.1093/cid/ciz1092 [DOI] [PubMed] [Google Scholar]
- 5.Waissengrin B, Agbarya A, Safadi E, Padova H, Wolf I. Short-term safety of the BNT162b2 mRNA COVID-19 vaccine in patients with cancer treated with immune checkpoint inhibitors. Lancet Oncol. May 2021;22(5):581–583. doi: 10.1016/S1470-2045(21)00155-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eggermont AMM, Kicinski M, Blank CU, et al. Association Between Immune-Related Adverse Events and Recurrence-Free Survival Among Patients With Stage III Melanoma Randomized to Receive Pembrolizumab or Placebo: A Secondary Analysis of a Randomized Clinical Trial. JAMA Oncol. Apr 1 2020;6(4):519–527. doi: 10.1001/jamaoncol.2019.5570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chong CR, Park VJ, Cohen B, Postow MA, Wolchok JD, Kamboj M. Safety of Inactivated Influenza Vaccine in Cancer Patients Receiving Immune Checkpoint Inhibitors. Clin Infect Dis. Jan 2 2020;70(2):193–199. doi: 10.1093/cid/ciz202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Massarweh A, Eliakim-Raz N, Stemmer A, et al. Evaluation of Seropositivity Following BNT162b2 Messenger RNA Vaccination for SARS-CoV-2 in Patients Undergoing Treatment for Cancer. JAMA Oncol. Aug 1 2021;7(8):1133–1140. doi: 10.1001/jamaoncol.2021.2155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yazaki S, Yoshida T, Kojima Y, et al. Difference in SARS-CoV-2 Antibody Status Between Patients With Cancer and Health Care Workers During the COVID-19 Pandemic in Japan. JAMA Oncol. Aug 1 2021;7(8):1141–1148. doi: 10.1001/jamaoncol.2021.2159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen YW, Tucker MD, Beckermann KE, Iams WT, Rini BI, Johnson DB. COVID-19 mRNA vaccines and immune-related adverse events in cancer patients treated with immune checkpoint inhibitors. Eur J Cancer. Jul 28 2021;doi: 10.1016/j.ejca.2021.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]


