Abstract
Mouse mammary tumor virus (MMTV) infects a number of different cell types, including mammary gland and lymphoid cells, in vivo. To identify the cellular receptor for this virus, a mouse cDNA expression library was transfected into Cos-7 monkey kidney cells, and those transfected cells able to bind virus were selected by using antibody against the virus’s cell surface envelope protein, gp52. One clone isolated from a library prepared from newborn thymus RNA, called MTVR, was able to confer virus binding to both monkey and human cells; this binding was blocked by anti-MTVR antibody. Moreover, transfection of MTVR into CV1 cells rendered them susceptible to infection by a murine leukemia virus-based retrovirus vector pseudotyped with the MMTV envelope protein. An epitope-tagged MTVR cofractionated with cellular membranes. Coimmunoprecipitation of the MMTV envelope protein and a MTVR-rabbit Fc fusion protein showed that these two proteins bound to each other. The MTVR sequence clone is unique, shows no homology to known membrane proteins, and is transcribed in many tissues.
Mouse mammary tumor virus (MMTV) is a causative agent of mammary carcinomas in vivo and is acquired as an exogenous virus when newborns suckle on the milk of viremic mothers (14). Like other retroviruses, MMTV encodes an envelope protein, consisting of two chains generated by processing a precursor polyprotein, a cell surface (SU) domain of 52 kDa and a transmembrane domain of 36 kDa (22). It is the SU protein that binds the cellular receptor for the virus, since anti-SU antibody blocks MMTV infection of cultured cells (10).
Although the ultimate target for MMTV is the mammary gland, cells of the immune system play a role in milk-borne virus infection (2, 7, 9; for a review, see reference 16). MMTV encodes a superantigen protein in its long terminal repeat that is presented by the major histocompatibility complex class II proteins and interacts with the Vβ portion of the T-cell receptor (reviewed in reference 16). During the course of milk-borne MMTV transmission, the virus is first acquired by B cells in the Peyer’s patches (2, 9). These B cells act as antigen-presenting cells and present the superantigen to T cells. Subsequent to the activation of the B and T cells, both types become MMTV infected and are capable of shedding virions, at least in vitro (5). Whether both B and T cells transmit virus to the mammary gland has not yet been resolved, since adoptive transfer studies of the different lymphocyte subsets from infected mice into nude mice indicated that only T cells transmitted virus (20), whereas similar studies with immunocompetent mice showed that transfer of either B or T cells resulted in transmission of the virus to both cell types of an uninfected host (21).
In spite of our knowledge of the cell types involved in transmission of MMTV from milk to the mammary gland, the molecular steps involved in this process have not yet been elucidated. For example, it is not known how the virus gets into the cells of the lymphoid system or how it is transferred to mammary gland cells. One critical component of this process is the cellular receptor, the molecule(s) present on the cell surface that binds to the viral envelope protein. Previously, it has been shown that the MMTV receptor maps to chromosome 16 in the mouse (10). It was also reported that MMTV virions could bind to cells from many different tissues, but that mammary gland and spleen were able to bind higher amounts than salivary gland, ovary, adrenal gland, and liver (3). If this binding activity represents virus interaction with the actual MMTV receptor, mammary gland and lymphoid cells might be the most efficiently infected because they have the highest receptor levels.
To identify the cellular receptor for MMTV, we used virus binding to cells transfected with a mouse cDNA expression library to enrich for clones that coded for this receptor. Using this method, we isolated the gene for a novel membrane-associated protein that confers both MMTV binding and infectability. This gene, which is also found in humans and other mammals, not only is likely to be important for MMTV infection of mice but also must play a role in normal cell function.
MATERIALS AND METHODS
Receptor cloning.
A cDNA library was prepared from RNA isolated from the thymi of Swiss Webster mice in the pcDNA1 vector (Stratagene, Inc., La Jolla, Calif.), containing the cytomegalovirus (CMV) promoter and simian virus 40 origin of replication, using the Superscript plasmid system (Gibco/BRL, Bethesda, Md.). A total of 2 × 106 independent clones were transfected into Cos-7 cells by spheroplast fusion (1). After transfection, the cells were incubated first with MMTV(C3H) particles (0.5 μg/ml) at 37°C for 1 h and then washed and incubated with monospecific goat anti-SU polyclonal antiserum (8) for 1 h on ice. The transfected cells which bound virus were isolated by panning on dishes coated with rabbit anti-goat polyclonal antiserum as described previously (1). DNA was isolated by Hirt fractionation from the transfected cells, amplified in bacteria, and retransformed into Cos-7 cells by spheroplast fusion. After six rounds of selection, individual clones were isolated. All of the clones were individually transiently transfected into Cos-7 or stably transfected into CV1 cells along with a hygromycin resistance gene. The MTVR clone was also stably transfected into the human mammary carcinoma line T47D with a plasmid bearing the neomycin resistance gene and selected in G418.
Virus binding assay.
The different cell lines (Cos-7, CV1, T47D, BT474, MCF-7, transiently transfected Cos-7, and stably transfected CV1 and T47D) were briefly incubated in [3H]thymidine (Fig. 1A) or [35S]methionine (Fig. 1B and C) and removed from the plates with EDTA (1 mM). The labeled cells were incubated with or without MMTV(C3H) followed by goat anti-SU antibody and panned on plates coated with rabbit anti-goat antiserum as described above. After extensive washing, the cells remaining on the plate were removed with detergent and the number of counts per minute bound was determined.
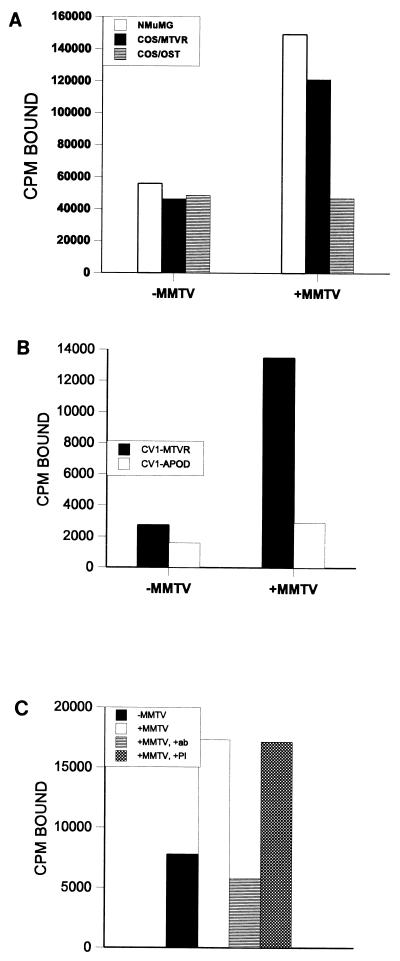
FIG. 1.
Expression cloning of the MMTV receptor. (A) Virus binding to NMuMG, Cos-7, and 44T(MTVR)- and osteopontin-transiently transfected Cos-7 cells. (B) Virus binding to NMuMG and MTVR- and ApoD-stably transfected CV1 cells. (C) Anti-44T antibody blocking of binding to NMuMG cells. A 1:600 dilution of immune (+ab) or preimmune (+PI) serum was used.
Production of the His-tagged bacterial protein and antiserum production.
To make anti-MTVR antisera, the cDNA was cloned into the pET vector (Novagen, Inc., Madison, Wis.) and transfected into Escherichia coli, and bacterially produced His-tagged fusion protein was purified on nickel-agarose columns as specified by the manufacturer (Qiagen, Inc.). The purified protein was injected into rabbits by Cocalico, Inc. (Reamstown, Pa.), and the bleeds were tested for the ability to recognize the bacterially produced protein by Western blotting. For the blocking experiments, the ammonium sulfate-precipitated immunoglobulin G (IgG) fraction was used at the indicated concentrations.
Pseudotyped MLV virion production.
The pseudotyped murine leukemia viruses (MLVs) were created by transient transfection of 293T cells with plasmids pHIT111 (containing the MLV packaging sequence and the lacZ gene), pHIT60 (containing the MLV gag-pol genes under the control of the CMV promoter), and pENV [containing the MMTV(C3H) envelope gene under the control of the CMV promoter] (4) and used for infection of CV1-MTVR cells as described by Soneoka et al. (19). Virions lacking envelope protein were produced by 293T cells cotransfected with pHIT111 and pHIT60 alone and used as controls for infection.
Coimmunoprecipitation of Env and MTVR/Ig.
The MTVR coding region was amplified by PCR and ligated in frame with the rabbit IgG heavy-chain Fc coding region (25; a gift from John Young) in the pcDNA1 expression vector. This plasmid was transfected into 293T cells either alone or with the pEnv plasmid described above. The cells were lysed in radioimmunoprecipitation assay buffer without sodium dodecyl sulfate or deoxycholate and immunoprecipitated with either goat anti-MMTV antiserum (Quality Biotech., Inc., Camden, N.J.), anti-MTVR antiserum, or protein A-Sepharose (Gibco/BRL). After blotting onto nitrocellulose membranes, the Env protein was detected with goat anti-MMTV followed by horseradish peroxidase-conjugated mouse anti-goat antiserum and enhanced chemiluminescence reagents as recommended by the manufacturer (Amersham, Inc.).
Cell fractionation.
A T7 tag from the pET28 vector (Novagen) was ligated in frame to the N terminus of the MTVR cDNA, and the construct was subcloned into pcDNA1. The plasmid was transiently transfected into 293T cells, and the cells were fractionated by previously described methods (15). Equal amounts of protein (50 μg) from each of the different fractions were trichloroacetic acid precipitated, subjected to electrophoresis sodium dodecyl sulfate–12% polyacrylamide gels, and analyzed by Western blotting.
Immunoprecipitations, immunohistochemistry, and Western blots.
The anti-His-tagged-MTVR polyclonal antiserum was used to immunoprecipitate [35S]methionine-labeled protein synthesized by using an in vitro transcription-translation kit (Gibco/BRL). Western blot analysis was performed with a monoclonal antibody against the T7 epitope (Novagen) on the tagged MTVR protein. For immunohistochemistry, HeLa cells were transiently transfected with the T7-tagged MTVR, fixed with 4% paraformaldehyde, and stained with the anti-T7 monoclonal antibody or a monoclonal antibody against the transferrin receptor (a kind gift from Morrie Birnbaum).
RESULTS
Cloning of the MMTV receptor.
The first step in viral entry is binding of the virus to a cell surface protein. To clone the MMTV receptor, we used a modification of the technique developed by Aruffo and Seed (1, 18) to look for a cloned protein that would confer virus binding to cells. A cDNA expression library prepared from newborn mouse thymus was transfected into Cos-7 cells, which cannot be infected with MMTV (not shown). The transfected cells were first incubated with purified MMTV and then panned with an anti-MMTV polyclonal antibody. Plasmid DNA was isolated from the Cos-7 cells that bound to the plates, amplified, and retransfected into Cos-7 cells. After six rounds of transfection and panning, plasmid DNAs from the bound Cos-7 cells were individually introduced into Cos-7 or CV1 (monkey kidney) cells by transient or stable transfection, respectively. Approximately 10 different clones were tested in this manner.
The cells transfected with the individual plasmids were tested for the ability to bind to MMTV virions. As can be seen in Fig. 1A, when the NMuMG murine mammary gland cell line, which can be infected by MMTV, was subjected to this analysis, there were approximately three times more cells bound in the presence of MMTV. In contrast, with Cos-7 cells, there was no binding of MMTV, since equal numbers of cells bound to the antibody coated plates in the presence or absence of virus. When the cloned cDNAs were subjected to this analysis, only 1 (44T) of the 10 clones isolated from the thymus cDNA library was able to confer binding of virus to transiently transfected Cos-7 cells (Fig. 1A) or to stably transfected CV1 cells (Fig. 1B). In both cases, the ratio of bound to unbound cells in the presence and absence of virus was similar to what was seen with the NMuMG cells. No other clones identified in the library conferred binding of MMTV to either Cos-7 cells, as shown for osteopontin, or CV1 cells, as shown for apolipoprotein D (ApoD). These data showed that 44T conferred virus binding to cells that could not be infected with virus; hereafter it is called MTVR.
MTVR confers MMTV infectability.
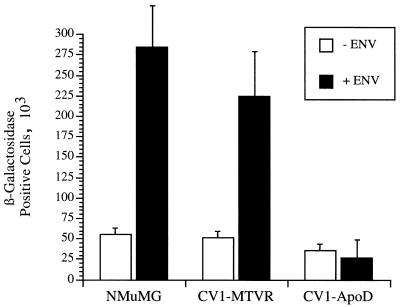
To determine if expression of MTVR also made the CV1 cells permissive for virus infection, we created a pseudotyped MLV-based retroviral vector with the MMTV envelope proteins. The MTVR-transfected CV1 cells were infected with these pseudotyped viruses. Both of the mouse mammary gland cell lines, NMuMG and MTVR-transfected CV1, but not the ApoD-transfected cells were infected with the pseudotyped viruses (Fig. 2). Thus, MTVR is a bona fide MMTV receptor.
FIG. 2.
Infection of MTVR-transfected CV1 cells by MLV pseudotyped with MMTV envelope proteins. The numbers of β-galactosidase-positive colonies from three independent transfections were averaged; shown are the average numbers with standard deviations.
MTVR is a novel protein.
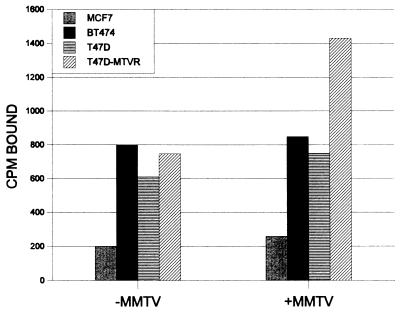
The complete nucleotide and predicted amino acid sequences of the cloned MTVR cDNA are shown in Fig. 3A. The clone is 1,141 nucleotides in length, excluding the poly(A) tail. By low-stringency Southern blot analysis, homologous sequences were seen in all mammalian species examined but not in Drosophila or birds (not shown). When either the amino acid or nucleic acid sequence of MTVR was compared to entries in GenBank, no homology to any known gene was found, using either the Blast or the FastA algorithm. However, partial clones of MTVR were recently cloned from both mouse and human fetal liver EST (expressed sequence tag) libraries. The region of the human gene cloned (approximately 733 bp) showed 83.5% homology at the nucleotide level (not shown) and 86% homology in the predicted protein sequence (Fig. 3B). However, previous studies, including our own, have shown, that MMTV does not infect human cells (11, 16a, 24). For example, as seen in Fig. 4, no human mammary carcinoma cultured cells (T47D, MCF-7, and BT474) bound MMTV although they all produced an MTVR-hybridizing transcript (not shown). However, when the T47D cell line was transfected with the mouse MTVR clone, virus binding was detected. These results imply that the differences in the amino acid sequence of the human MTVR alter its ability to interact with the MMTV envelope protein.
FIG. 3.
Sequence of MTVR. (A) Nucleotide and predicted amino acid sequences of MTVR cDNA. The overlined amino acids indicate the hydrophobic domain. Underlined G’s represent putative myristoylation sites; the italicized N represents a potential N-glycosylation site. The sites were determined by using the PROSITE program. The overlapping putative polyadenylation signals are double underlined. (B) Homology between the mouse (M) and human (H) MTVR coding regions. The human sequence was derived from sequences under accession no. T95780 and H68224 in the EST database.
FIG. 4.
MMTV binding to human mammary carcinoma cell lines. MCF-7, BT474, T47D, and T47D/MTVR cells were panned with anti-MMTV antibody as described in Materials and Methods.
A Kyte-Doolittle hydrophobicity plot of the predicted MTVR protein indicates that there is one hydrophobic domain (amino acids [aa] 10 to 28) (12). There are one putative N-linked glycosylation site (aa 32), three O-linked glycosylation sites (aa 7, 129, and 147), and six putative myristoylation sites (aa 11, 12, 14, 26, 29, 70, and 100).
We screened an independent cDNA library with MTVR cDNA probe and isolated a clone that had an additional 15 nucleotides at the 5′ end; moreover, using rapid amplification of cDNA ends to amplify the 5′ end, we showed that the bona fide mRNA contained these additional nucleotides (not shown). The cDNA contains a single contiguous open reading frame that codes for a protein of 18,704 Da. The protein is shown initiating at the first in-frame methionine in Fig. 3A; this AUG conforms to the Kozak consensus sequence rules, and in vitro transcription-translation studies indicate that the protein is approximately this size (see below). However, the open reading frame continues upstream of this first methionine. Because there are two transcripts that hybridize to the MTVR cDNA probe in some tissues (see below), it is possible that there is more than one form of the protein.
MTVR is found on the cell surface.
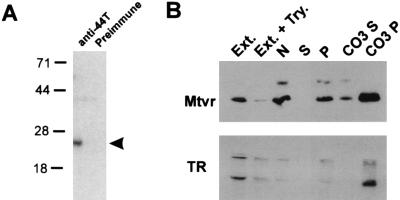
A histidine-tagged MTVR bacterial fusion protein was constructed, and a polyclonal rabbit antiserum against this protein was made. In vitro transcription-translation of MTVR indicates that it encodes an approximately 19-kDa protein that is immunoprecipitated by the anti-MTVR polyclonal antiserum (Fig. 5A). This antiserum blocked binding of the virus to NMuMG cells, while preimmune serum from the same rabbit did not (Fig. 1C). However, we have been unable to detect endogenous protein by Western blot or immunoprecipitation analyses, indicating that the expression levels are low.
FIG. 5.
(A) Immunoprecipitation of in vitro-transcribed, translated MTVR. Sizes are indicated in kilodaltons. (B) Cell fractionation studies. Lanes: Ext., total extract; Ext. + Try., extract from trypsin-treated cells; N, nuclear pellet; S, S100 supernatant; P, S100 pellet; CO3 S, supernatant from carbonate-extracted S100 pellet; CO3 P, pellet from carbonate-extracted S100 pellet.
To prove that the MTVR was found on the cell surface, as is predicted for a viral receptor, we epitope tagged the cDNA at the N terminus with a peptide from the T7 gene. This construct was put into a mammalian expression vector (pcDNA1) and transiently transfected into 293T cells. Western blot analysis using a monoclonal antibody against the T7 epitope (Fig. 5B, lane 1) or the anti-His-tagged MTVR (not shown) detected this protein. Cell fractionation studies of 293T cells transiently transfected with the MTVR, including carbonate extraction of the S100 pellet, showed that the tagged cDNA copurified with a known transmembrane protein, the transferrin receptor in the membrane fraction (Fig. 5B). Trypsin treatment of the transfected cells greatly diminished the amount of MTVR and the transferrin receptor (Fig. 5B) but not the simian virus 40 large T protein made in these cells (not shown).
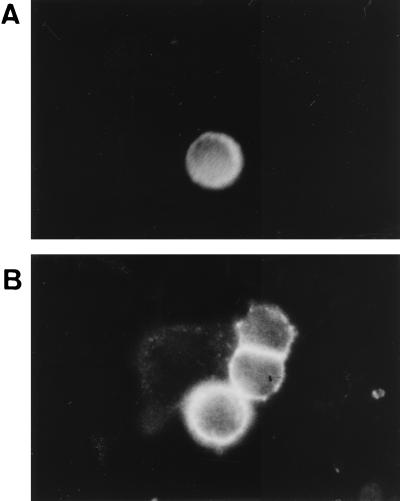
We also performed immunohistochemistry to show that the MTVR is a cell surface protein. HeLa cells were transiently transfected with the T7-tagged MTVR construct and fixed but not permeabilized. The cells were stained with an anti-T7 monoclonal antibody (Fig. 6A) or an anti-transferrin receptor antibody (Fig. 6B). Whereas all cells showed cell surface staining for the transferrin receptor, only those cells that were transfected stained with the anti-T7 antibody. Taken together, these results indicate that the N terminus of the MTVR is located on the cell exterior.
FIG. 6.
Immunohistochemistry of MTVR-transfected cells. HeLa cells were transiently transfected with the T7-tagged MTVR construct, fixed with 4% paraformaldehyde, and stained with anti-T7 antibody (A) or anti-transferrin receptor antibody (B).
The MTVR clone binds the MMTV envelope proteins.
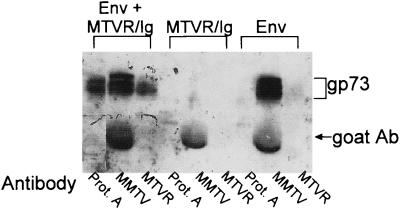
To determine whether the MTVR clone bound to the MMTV envelope proteins, we constructed an immunoadhesin in which the entire MTVR was inserted upstream of the rabbit IgG Fc coding region in a mammalian expression vector (MTVR/Ig). This construct was transiently transfected into 293T cells either alone or with a plasmid containing the coding region for the MMTV gp73 polyprotein precursor to the gp52 and gp36 proteins. Crude cell extracts were prepared and precipitated either with anti-MTVR antiserum, anti-MMTV antiserum, or protein A-Sepharose alone. In all three cases, the envelope precursor protein gp73 was detected (Fig. 7). In contrast, neither anti-MTVR antiserum nor protein A-agarose was able to precipitate the Env protein in the absence of MTVR protein. The gp52 protein is difficult to detect in these immunoprecipitations because the MTVR immunoadhesin and goat anti-MMTV antibodies migrate at the same apparent molecular weight. The MTVR/Ig protein also bound the Env proteins when the extracts from cells separately transfected with these plasmids were mixed together (not shown). Thus, the MTVR protein binds to MMTV envelope proteins.
FIG. 7.
Coimmunoprecipitation of the MMTV envelope protein and MTVR/Ig. 293T cells were transfected either separately or together with plasmids containing the MMTV envelope coding region (Env) and the MTVR/Ig construct. Lysates prepared from these cells were immunoprecipitated either with goat anti-MMTV antiserum followed by rabbit anti-goat immunoglobulin (MMTV) or rabbit anti-MTVR antisera (MTVR) or with protein A-agarose alone (Prot. A). Western blot analysis was carried out with goat anti-MMTV antiserum followed by horseradish peroxidase-linked mouse anti-goat antibodies and enhanced chemiluminescence detection. The gp73 protein is the envelope polyprotein precursor. Because the gp52 SU protein is about 10-fold less abundant in the cells and migrates at approximately the same apparent molecular weight as the goat anti-MMTV antibody (goat Ab) and MTVR/Ig proteins, it is obscured on these gels.
MTVR is ubiquitously transcribed.
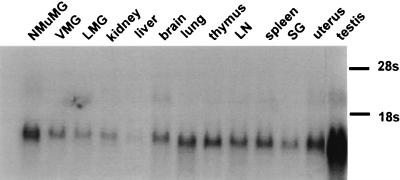
MMTV infects only a limited number of cell types in vivo and in vitro (16). To determine in which tissues the receptor was transcribed, we hybridized the MTVR cDNA clone to Northern blots containing RNA from different tissues (Fig. 8). The receptor mRNA was widely expressed, and two transcripts were detected in some tissues (e.g., brain and testis lanes in Fig. 8).
FIG. 8.
Northern blot analysis of MTVR RNA from different tissues. Twenty-microgram aliquots of total RNA from the tissues and cell lines were analyzed. Abbreviations: VMG, virgin mammary gland; LMG, lactating mammary gland; LN, lymph node; SG, salivary gland.
MTVR maps to chromosome 19.
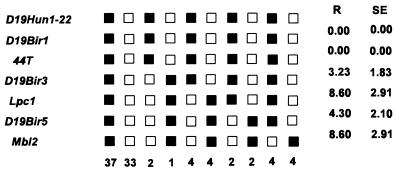
To determine the chromosomal location of the MTVR, we used DNAs from a panel of 93 animals ([C57BL6/Ei × SPRET/Ei] × SPRET/Ei backcross panel) from the Jackson Laboratory to map the MTVR chromosomal location (17). This analysis mapped MTVR (Mtvr2) to the proximal end of chromosome 19 (Fig. 9).
FIG. 9.
Haplotype figure for the MTVR-mapping data from the proximal part of chromosome 19 from the Jackson Laboratory BSS backcross panel. Loci are listed in the left column in order with the centromere at the top. Black boxes represent the C57BL/6J allele, and white boxes represent the SPRET/Ei allele. The number below each column of boxes indicates the number of N2 animals with that haplotype. R is recombination frequency in each interval, and SE is the standard error for that R.
DISCUSSION
The method used to clone the MMTV receptor, MTVR, relied on both virus binding to cells and the recognition of virus by antibodies. MMTV virion stocks have many noninfectious particles, and there is no assay for detecting MMTV infection of single tissue culture cells. Moreover, it is difficult to obtain high-titer recombinant MMTVs. These technical difficulties have previously prevented the identification of the receptor for this virus. These problems were circumvented here, since high infectious virus titers were not required to assay for binding. This technique should be applicable to other viruses for which similar low titers and lack of biological assays have prevented identification of the receptor.
RNA tumor viruses exhibit a high degree of host range and cell type specificity. This specificity is partially determined by the presence of the appropriate cell surface receptors on target cells. MMTV is different from most other murine retroviruses in that it infects only a limited range of cell types. However, we showed that the MTVR is ubiquitously expressed, at least at the RNA level. The lack of infectivity could be due to variable levels of receptor protein in different cell types. It is also possible that there are post-receptor binding steps that are blocked in some cell types. Alternatively, MMTV may utilize a coreceptor for membrane fusion that is not ubiquitously present.
Another block to tissue-specific infection by MMTV is at the transcriptional level. It has been shown that MMTV expresses only in mammary gland epithelial cells, lymphoid cells, and a few other epithelial cell types (for a review, see reference 16). Thus, even if MMTV infects and integrates into the chromosomes of cell types besides the mammary gland, expression of the virus may not occur and infection of the tissue would be limited. Whether MTVR is the only receptor for the virus on all permissive cell types (i.e., lymphoid and mammary gland cells) will be addressed in future experiments, using antibody blocking and targeted mutagenesis.
The MMTV receptor was previously mapped to mouse chromosome 16, using vesicular stomatitis virus pseudotypes containing envelope glycoproteins to infect mouse-hamster somatic cell hybrids (10). In this study, we found that the MTVR maps to the proximal end of chromosome 19. Either there is more than one receptor for MMTV or the original mapping of the receptor to chromosome 16 was incorrect; these studies used only one marker per chromosome, and there may have been recombination events in the somatic cell hybrids. In support of this, the presence of chromosome 19 in the mouse-hamster somatic cell hybrids showed the second-highest correlation with infectability in the MMTV/vesicular stomatitis virus pseudotypes, using a marker that was located on the distal end of this chromosome.
Several retroviral receptors have been previously identified. At least three of these receptors, the human immunodeficiency virus receptor CD4 and the avian sarcoma virus subgroup A and subgroup B receptors, have single membrane-spanning domains, whereas a number of other retroviral receptors have multiple membrane-spanning domains (reviewed in reference 23). The MTVR identified here is unique among retroviral receptors in that it is relatively small and it has no strong homology to any known transmembrane protein. Thus, MTVR-SU interactions may be different from those that occur between other retroviral Env proteins and their receptors. For example, unlike the case for other retroviruses, expression of endogenous MMTVs does not appear prevent superinfection by exogenous MMTVs through a receptor interference mechanism. GR mice have a functional endogenous MMTV, Mtv-2, that is expressed in mammary gland, yet mammary tumors that acquire multiple new virus integrants derived from Mtv-2 arise in this mouse strain (13). In addition, most MMTV-induced mammary tumors have multiple copies of newly integrated proviruses, supporting a lack of receptor interference for this virus. We have also shown that cultured mammary cells transfected with a molecular clone of MMTV, HYB PRO, can be infected with MLV pseudotypes containing the same HYB PRO Env on the virion (6). How the MMTV envelope protein binds to this receptor is therefore likely to provide new information about virus-cell interactions.
Retroviruses take advantage of host-encoded cell surface proteins that play important roles in cellular metabolism, and MTVR is likely to also play such a role. The identification of the receptor for this virus should allow us to determine not only the cellular function of this novel protein but also the mechanism(s) by which MMTV binds and enters cells, to determine how the cell type restriction occurs, and to understand how this virus utilizes cells of both the immune system and mammary gland in its infection pathway.
ACKNOWLEDGMENTS
The first three authors contributed equally to the work, which was begun at the University of Illinois School of Medicine, Chicago.
We thank Hans Stauss for initially suggesting this approach for cloning the receptor, Paul Gadeu for help with construction of the pENV construct, Wei Zhu for help with the MTVR/Ig construct, Marta de Olano Vela and Valsamma Abraham for technical assistance, Paul Bates and Lijin Rong for the MLV packaging plasmids and for advice on creating the pseudotyped virions, and Lucy Rowe for help with the chromosome mapping analysis.
This work was supported by grants from the National Cancer Institute.
REFERENCES
- 1.Aruffo A, Seed B. Molecular cloning of a CD28 cDNA by a high-efficiency COS cell expression system. Proc Natl Acad Sci USA. 1987;84:8573–8577. doi: 10.1073/pnas.84.23.8573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beutner U, Draus E, Kitamura D, Rajewsky K, Huber B T. B cells are essential for murine mammary tumor virus transmission, but not for presentation of endogenous superantigens. J Exp Med. 1994;179:1457–1466. doi: 10.1084/jem.179.5.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bolander F F, Blackstone M E. Tissue distribution of the cellular binding protein for the mouse mammary tumor virus. J Endocrinol. 1991;7:169–174. doi: 10.1677/jme.0.0070169. [DOI] [PubMed] [Google Scholar]
- 4.Dzuris, J. Unpublished data.
- 5.Dzuris J, Golovkina T, Ross S R. Both T and B cells shed infectious mouse mammary tumor virus. J Virol. 1997;71:6044–6048. doi: 10.1128/jvi.71.8.6044-6048.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dzuris, J. L., W. Zhu, T. V. Golovkina, and S. R. Ross. Unpublished data.
- 7.Golovkina T V, Chervonsky A, Dudley J P, Ross S R. Transgenic mouse mammary tumor virus superantigen expression prevents viral infection. Cell. 1992;69:637–645. doi: 10.1016/0092-8674(92)90227-4. [DOI] [PubMed] [Google Scholar]
- 8.Golovkina T V, Chervonsky A, Prescott J A, Janeway C A, Ross S R. The mouse mammary tumor virus envelope gene product is required for superantigen presentation to T cells. J Exp Med. 1994;179:439–446. doi: 10.1084/jem.179.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Held W, Waanders G, Shakhov A N, Scarpellino L, Acha-Orbea H, Robson-MacDonald H. Superantigen-induced immune stimulation amplifies mouse mammary tumor virus infection and allows virus transmission. Cell. 1993;74:529–540. doi: 10.1016/0092-8674(93)80054-i. [DOI] [PubMed] [Google Scholar]
- 10.Hilkens J, van der Zeust B, Buijs F, Kroezen V, Bluemink N, Hilgers J. Identification of a cellular receptor for mouse mammary tumor virus and mapping of its gene to chromosome 16. J Virol. 1983;45:140–147. doi: 10.1128/jvi.45.1.140-147.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Howard D K, Schlom J. Isolation of a series of novel variants of murine mammary tumor viruses with broadened host range. Int J Cancer. 1980;25:647–654. doi: 10.1002/ijc.2910250515. [DOI] [PubMed] [Google Scholar]
- 12.Kyte J, Doolittle R F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982;157:105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- 13.Michalides R, van Deemter L, Nusse R, van Nie R. Identification of the Mtv-2 gene responsible for early appearance of mammary tumors in the GR mouse by nucleic acid hybridization. Proc Natl Acad Sci USA. 1978;75:2368–2372. doi: 10.1073/pnas.75.5.2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nandi S, McGrath C M. Mammary neoplasia in mice. Adv Cancer Res. 1973;17:353–414. [Google Scholar]
- 15.Platt E J, Firestone G L. Expression of mouse mammary tumor virus glycoprotein trucations defines roles for the transmembrane domain and ectodomain hydrophobic region in constitutive exocytic trafficking and proteolytic processing. J Biol Chem. 1991;266:19384–19395. [PubMed] [Google Scholar]
- 16.Ross S R. MMTV and the immune system. Adv Pharmacol. 1996;37:21–46. doi: 10.1016/s1054-3589(08)60068-x. [DOI] [PubMed] [Google Scholar]
- 16a.Ross, S. R., et al. Unpublished data.
- 17.Rowe L B, Nadeau J H, Turner R, Frankel W N, Letts V A, Eppig J T, Ko M S H, Thurston S J, Birkenmeier E H. Maps from two interspecific backcross DNA panels available as a community genetic mapping resource. Mamm Genet. 1994;5:253–274. doi: 10.1007/BF00389540. [DOI] [PubMed] [Google Scholar]
- 18.Seed B, Aruffo A. Molecular cloning of the CD2 antigen, the T cell erythrocyte receptor by rapid immunoselection procedure. Proc Natl Acad Sci USA. 1987;84:3365–3369. doi: 10.1073/pnas.84.10.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soneoka Y, Cannon P M, Ramsdale E E, Griffiths J C, Romano G, Kingsman S M, Kingsman A J. A transient three-plasmid expression system for the production of high titer retroviral vectors. Nucleic Acids Res. 1995;23:628–633. doi: 10.1093/nar/23.4.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsubura A, Inaba M, Imai S, Murakami A, Oyaizu N, Yasumizu R, Ohnishi Y, Tanaka H, Morii S, Ikehara S. Intervention of T-cells in transportation of mouse mammary tumor virus (milk factor) to mammary gland cells in vivo. Cancer Res. 1988;48:6555–6559. [PubMed] [Google Scholar]
- 21.Waanders G A, Shakhov A N, Held W, Karapetian P, Acha-Orbea H, MacDonald H R. Peripheral T cell activation and deletion induced by transfer of lymphocyte subsets expressing endogenous or exogenous mouse mammary tumor virus. J Exp Med. 1993;177:1359–1366. doi: 10.1084/jem.177.5.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weiss R, Teich N, Varmus H, Coffin J. RNA tumor viruses. In: Weiss R, Teich N, Coffin J M, editors. Molecular biology of tumor viruses. Cold Spring Harbor, N.Y: CSH Press; 1984. pp. 590–591. [Google Scholar]
- 23.Weiss R A, Tailor C S. Retrovirus receptors. Cell. 1995;82:531–533. doi: 10.1016/0092-8674(95)90024-1. [DOI] [PubMed] [Google Scholar]
- 24.Zavada J, Dickson C, Weiss R. Pseudotypes of vesicular stomatitis virus with envelope antigens provided by murine mammary tumor virus. Virology. 1977;82:221–331. doi: 10.1016/0042-6822(77)90045-9. [DOI] [PubMed] [Google Scholar]
- 25.Zingler K, Young J A T. Residue Trp-48 of Tva is critical for viral entry but not for high-affinity binding to the SU glycoprotein of subgroup A avian leukosis and sarcoma viruses. J Virol. 1996;70:7510–7516. doi: 10.1128/jvi.70.11.7510-7516.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]