Abstract
The replication sites of the recently discovered hepatitis G virus (HGV) remain unknown. Using highly strand-specific Tth-based reverse transcriptase PCR, we searched for the presence of viral RNA negative strand in multiple autopsy tissues from four patients with AIDS and in peripheral blood mononuclear cells from six other human immunodeficiency virus-positive patients. Negative-strand HGV RNA was detected in three of four bone marrow samples, in two of two spleen samples, and in one of four liver tissue samples. However, the specific cellular site of replication within the positive tissues was not determined. This study does not support HGV as a primary hepatotropic virus.
Recently, two independent groups of investigators described two isolates of the same novel flavivirus and named the virus hepatitis G virus (HGV) and hepatitis GB virus C (10, 13). Since the nomenclature of this new agent has not yet been decided, for the purpose of this article it will be referred to as HGV. HGV RNA sequences have been detected by reverse transcriptase (RT) PCR in 1 to 2% of volunteer blood donors and at significantly higher rates in persons with repeated parenteral exposure such as intravenous drug addicts (10, 14) or patients receiving multiple transfusions (4, 10, 16). Furthermore, HGV infection was found to be common in subjects with various forms of chronic liver disease, being particularly prevalent in subjects with chronic hepatitis C (2, 3, 10, 15). However, the association between hepatitis and HGV infection is unclear since the vast majority of infected individuals do not show liver injury unless simultaneously infected with another hepatotropic virus (1, 11, 16). This raises the possibility that HGV is not a strictly hepatotropic virus but rather one which causes hepatitis only occasionally.
Although studies on the clinical effects of HGV infection are abundant, studies addressing the issue of viral replication sites are missing. In a previous article (7), we reported on the lack of evidence for HGV replication in the liver in a group of HGV-HCV-coinfected patients with cirrhosis, which implies that the liver is not the primary replication site for this virus. However, no other cell compartments have been studied so far.
The major obstacle to a study of HGV replication sites is the lack of availability of multiple tissue samples from infected individuals since such samples can be obtained only during autopsy. We reasoned that such an investigation could be conducted on postmortem tissues from intravenous drug addicts who died from AIDS, since HGV infection in this group is common and viral titers are expected to be elevated, facilitating positive identification of replication sites.
HGV genome organization was found to be similar to that of hepatitis C virus (HCV), with a single open reading frame and 5′ and 3′ untranslated regions (10, 13). In addition, analysis of the predicted amino acid sequences indicated the presence of structural and nonstructural proteins as well as a number of putative proteolytic cleavage sites in a relative position found in HCV (9). Taking into account these similarities, it can be assumed that HGV replicates through negative-strand RNA, the presence of which could be regarded as direct evidence of viral replication.
However, standard RT-PCR is not strand specific due to false priming of the incorrect strand or self-priming related to RNA secondary structures (5). An efficient way of avoiding these mispriming events is by conducting cDNA synthesis at high temperature with the thermostable enzyme Tth (5, 6, 8). In the current study, we employed this technique in the search for negative-strand HGV RNA in peripheral blood mononuclear cells (PBMCs) and multiple organs from HGV-positive patients with AIDS. The sensitivity and strand specificity of our assay were determined on synthetic RNA templates.
MATERIALS AND METHODS
Biological samples.
PBMCs were collected from six human immunodeficiency virus type 1 (HIV-1)-positive drug addicts whose sera were found to be HGV RNA positive. All were HCV positive and hepatitis B surface antigen negative; none had received any antiviral therapy prior to the study. PBMCs were isolated by Ficoll-Hypaque (Pharmacia, Uppsala, Sweden) density gradient centrifugation, washed three times with phosphate-buffered saline (pH 7.4), and stored frozen at −80°C until use. RNA was extracted from 5 × 106 to 1 × 107 cells or 100 μl of serum by means of a modified guanidinium thiocyanate-phenol-chloroform technique using commercially available kits (Ultraspec 2 and Ultraspec 3; Biotecx Laboratories, Houston, Tex.) and finally dissolved in 30 μl of water. Ten microliters of this RNA solution was reverse transcribed as further described; in the case of serum, the amount of extracted RNA loaded into the reaction mixture corresponded to 20 μl.
Tissue samples were collected from four HIV-1-infected drug addicts who died from AIDS-related complications between March and May 1997. All four patients were anti-HCV positive and hepatitis B surface antigen negative; their CD4 cell count was below 200 cells/mm3. Tissue samples were obtained from each patient during routine autopsy conducted within 48 h of death and stored at −80°C until analysis.
Samples of the following tissues were collected postmortem from each patient: liver, bone marrow, spleen, mediastinal lymph node, pancreas, thyroid, adrenal gland, kidney, lung, skeletal muscle, skin, and spinal cord. RNA was extracted after tissue homogenization as described above. For each tissue, two different amounts of extracted RNA (6 and 1 μg, as determined by spectrophotometry) were initially used for RT-PCR. If the sample was positive in the amount of 1 μg of RNA, the RNA template was serially 10-fold diluted for the purpose of titer determination.
Synthetic HGV RNA.
To generate synthetic positive- and negative-strand HGV RNA, PCR product encompassing the 5′ untranslated region of the virus was cloned into a plasmid vector and subsequently transcribed with T7 polymerase as described elsewhere (7).
Strand-specific RT-PCR with Tth.
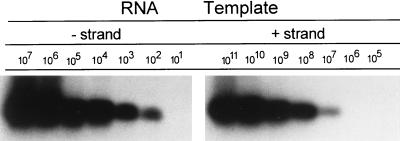
The Tth-based RT-PCR detection of the negative-strand HGV RNA was described in detail previously (7); the final product was analyzed by agarose gel electrophoresis and Southern hybridization with a 32P-labeled internal oligoprobe. As described in our previous report (7) and as illustrated in Fig. 1, this assay was capable of detecting approximately 100 genomic equivalent molecules (genomic eq) of the correct strand while unspecifically detecting ≥107 genomic eq of the incorrect strand. When 1 or 6 μg of total cellular RNA extracted from normal human liver tissue was added, the sensitivity of the reactions was lowered by no more than 1 log, while the specificity of the assay was not affected.
FIG. 1.
Sensitivity and specificity of RT-PCR using the Tth assay. Synthetic positive and negative strands were generated by in vitro runoff transcription with T7 RNA polymerase from a vector (pGEM-3Z) containing the 5′ untranslated sequence of HCV and serially diluted in water. The number of target template copies was calculated from optical density readings. A positive-sense primer was present during cDNA synthesis, after which the enzyme was inactivated by chelation with Mn2+ and then negative-sense primer was added. Samples were amplified as described in the text. Twenty microliters (20%) of the reaction mixture was fractionated on agarose, transferred to a nylon membrane by Southern blotting, and subsequently hybridized to a 32P-labeled probe. When 1 or 6 μg of total cellular RNA extracted from normal human liver tissue was added, the sensitivity of the reactions was lowered by no more than 1 log, while the specificity of the assay was not affected.
RT-PCR with MMLV RT.
Moloney murine leukemia virus (MMLV) RT-based detection of HGV RNA has been described in detail elsewhere (7). This assay was found to be very sensitive but totally unspecific. As reported previously (7), the assay was capable of detecting 10 genomic eq of the correct template but at the same time also unspecifically detected ≥101 to 103 genomic eq of the positive strand.
The detection of HCV sequences with MMLV RT and Tth-based RT-PCR was described previously (8). The sensitivity and specificity of HCV RNA detection were similar to those for the detection of HGV RNA (8).
To increase the specificity and sensitivity of our assays, wax beads (Ampliwax; Perkin-Elmer) were employed for “hot start” of all PCRs after the RT step. All RT-PCR runs included positive controls consisting of end-point dilutions of respective RNA strands, and negative controls included normal liver tissue and normal sera.
All titers were determined by analyzing 10-fold serial dilutions of the RNA template since at this dilution the results were reliably reproducible from run to run. The titers were calculated by assuming that the end-point dilution contains 10 genomic eq when tested by the MMLV RT-based assay and 102 genomic eq when tested with the Tth-based assay.
To prevent contamination, pre-PCR and post-PCR steps were carried out in separate rooms. To detect carryover contamination, negative controls were included in all reaction series: one negative sample was processed for every three to four tested specimens, and nontarget controls were included in each run. Under these conditions, none of the negative samples or controls was positive.
Since we were concerned about the integrity of the RNA in the samples, which were collected up to 48 h after the patient’s death, all liver samples were tested for the presence of β2-microglobulin RNA by RT-PCR using sense primer 5′TTAGCTGTGCTCGCGCTACTCTCTC3′ and antisense primer 5′GTCGGATTGATGAAACCCAGACACA3′. A product of the expected size, 144 bp, was amplified from as little as 0.1 ng of total liver RNA from each of the four patients.
RESULTS AND DISCUSSION
HGV RNA in PBMCs.
All six patients from whom PBMCs were collected were positive for the presence of positive-strand HGV RNA in serum and in PBMCs at titers ranging from 5 × 103 to 5 × 107 genomic eq/ml and 101 to 103 genomic eq/2 × 106 to 3 × 106 cells, respectively (Table 1). However, all serum and PBMC samples were negative for the presence of the negative-strand HGV RNA when tested with the Tth-based assay in two independent experiments.
TABLE 1.
Detection of positive and negative strands of HGV RNA in serum samples and PBMCs in six HIV-infected patients
| Patient | CD4 cells/mm3 | HGV RNA titera in:
|
|||
|---|---|---|---|---|---|
| Serum (genomic eq/ml)
|
PBMCs (genomic eq/2 × 106 to 3 × 106 cells)
|
||||
| Positive strand | Negative strand | Positive strand | Negative strand | ||
| 1 | 132 | 5 × 107 | Negb | 103 | Neg |
| 2 | 270 | 5 × 105 | Neg | 102 | Neg |
| 3 | 198 | 5 × 104 | Neg | 102 | Neg |
| 4 | 309 | 5 × 104 | Neg | 102 | Neg |
| 5 | 545 | 5 × 103 | Neg | 101 | Neg |
| 6 | 33 | 5 × 103 | Neg | 101 | Neg |
The presence and titers of the positive strand were determined by the MMLV RT-based assay, while the presence of the negative strand was determined by the Tth-based assay.
Neg, negative.
HGV RNA in various organs.
Almost all analyzed tissues, with the exception of a few spinal cord and muscle tissue samples, were positive for the presence of HGV RNA when tested with the MMLV RT-based assay, although the actual titer varied from tissue to tissue, the highest being in the bone marrow (Table 2).
TABLE 2.
Detection of positive (+) and negative (−) strands of HGV RNA in serum samples and various tissues from four patients with AIDS
| Patient | HGV RNA titera in:
|
|||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serum (genomic eq/ml)
|
Tissue (genomic eq of RNA/μg)
|
|||||||||||||||||||||||||
| Liver
|
Spleen
|
Bone marrow
|
Lymph node
|
Pancreas
|
Thyroid
|
Adrenal gland
|
Kidney
|
Lung
|
Muscle
|
Skin
|
Spinal cord
|
|||||||||||||||
| + | − | + | − | + | − | + | − | + | − | + | − | + | − | + | − | + | − | + | − | + | − | + | − | + | − | |
| 1 | 5 × 104 | N | P | N | ND | ND | 103 | 102 | 103 | N | 102 | N | 101 | N | 101 | N | 101 | N | 102 | N | 101 | N | ND | ND | 101 | N |
| 2 | 5 × 104 | N | 102 | N | 103 | 102 | 105 | 103 | 101 | N | 101 | N | P | N | P | N | 101 | N | 101 | N | N | N | 101 | N | N | N |
| 3 | 5 × 105 | N | 103 | 102 | 103 | 102 | 103 | P | 101 | N | P | N | P | N | 101 | N | 101 | N | 101 | N | N | N | 101 | N | N | N |
| 4 | 5 × 104 | N | 102 | N | ND | ND | 102 | N | 103 | N | 102 | N | 101 | N | 102 | N | P | N | 102 | N | 101 | N | ND | ND | N | N |
The presence and titers of the positive strand were determined by the MMLV RT-based assay, while the presence and titers of the negative strand were determined by the Tth-based assay. N, negative; P, positive in 6 μg of total RNA; ND, not done.
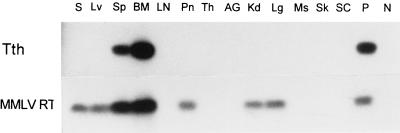
By using the Tth-based strand-specific assay, the presence of negative-strand HGV RNA was documented in some bone marrow, spleen, and liver tissue samples at titers which were 1 to 2 logs lower than the titers of the positive-strand HGV RNA (Table 2). In patient 1, negative-strand HGV RNA was found in bone marrow only; in patient 2, it was found in bone marrow and spleen; in patient 3, it was present in bone marrow, spleen, and liver; while in the remaining patient, all studied samples were persistently negative for negative-strand HGV RNA (Table 2; Fig. 2). These results were confirmed in two independent experiments using two separate extraction procedures.
FIG. 2.
Detection of negative-strand HCV RNA in various tissue samples from patient 2 by the Tth-based strand-specific RT-PCR assay and by the non-strand-specific MMLV RT-based assay. The amount of RNA loaded into each reaction mixture was 1 μg; in the case of serum (S), it corresponded to 20 μl. The examined tissues included liver (Lv), spleen (Sp), bone marrow (BM), lymph node (LN), pancreas (Pn), thyroid (Th), adrenal gland (AG), kidney (Kd), lung (Lg), muscle (Ms), Skin (Sk), and spinal cord (SC). Positive or sensitivity controls (lane P) consisted of end-point dilutions of the correct synthetic strand (10 genomic eq for MMLV RT assay and 100 genomic eq for the Tth assay), and negative controls (lane N) consisted of RNA extracted from livers from uninfected subjects. While the majority of samples were positive by the MMLV RT-based RT-PCR, only bone marrow and spleen samples were positive by the strand-specific Tth-based assay.
In contrast, positive-strand HCV RNA was detected in liver tissue from all four patients at titers ranging from 103 to 107 genomic eq of RNA/μg, and negative-strand HCV RNA strand, as determined by Tth assay, was present in all liver samples at titers that were 1 to 2 logs lower than those of the positive strand. Titers of positive-strand HCV RNA in serum samples ranged from 5 × 103 to 5 × 105 genomic eq/ml (Table 3).
TABLE 3.
Detection of positive and negative strands of HCV RNA in serum and liver tissue samples from four patients with AIDS
| Patient | HCV RNA titera in:
|
|||
|---|---|---|---|---|
| Serum (genomic eq/ml)
|
Liver tissue (genomic eq of RNA/μg)
|
|||
| Postive strand | Negative strand | Positive strand | Negative strand | |
| 1 | 5 × 105 | Negb | 107 | 106 |
| 2 | 5 × 103 | Neg | 105 | 104 |
| 3 | 5 × 104 | Neg | 104 | 102 |
| 4 | 5 × 103 | Neg | 103 | 102 |
The presence and titers of the positive strand were determined by the MMLV RT-based assay, while the presence and titers of the negative strand were determined by the Tth-based assay.
Neg, negative.
The present study is the first to positively identify HGV replication sites in humans. By examining autopsy material from AIDS patients, we found the presence of viral negative-strand RNA, a putative viral replicative form, in bone marrow, spleen, and liver tissue. Moreover, negative-strand HGV RNA titers were 1 to 2 logs lower than titers of the positive strand, which is the same proportion as that found for another flavivirus, HCV, at its respective replication site. However, negative-strand HGV RNA detection was not consistent from patient to patient—in one case, no negative-strand RNA was found in any of the organs tested. Since the tissue samples were collected at the time of autopsy, some RNA might have been degraded and low-level replication could have been missed. Nevertheless, detection of high titers of positive- and negative-strand HCV RNA in all four liver samples suggests the presence of relatively well preserved template.
Negative-strand HGV RNA was detected in only one of four studied liver samples, although the overall HGV titers in liver tissue were significantly higher than those of previously studied HIV-negative patients (7). In striking contrast, significant titers of negative-strand HCV RNA were detected in liver tissue from all four patients. These findings support our previous conclusion that HGV is not a strictly hepatotropic virus and that even in the presence of severe HIV-related immunosuppression, its replication in the liver is low or absent.
However, we cannot exclude the possibility of a very low level of HGV replication in the liver in the remaining three subjects. In cells supporting HCV replication, negative RNA strands are generally detected at a level that is 1 to 2 logs lower than the levels of positive strands (6, 7). Since the same seems likely to be true for HGV, it would mean that replication is below the sensitivity level of our Tth-based strand-specific assay. Obviously, the same applies to other tissues and PBMCs, where low-level replication or replication confined to a small subset of cells would remain undetected.
It is currently unclear which particular cells are infected at the identified replication sites. Replication in bone marrow precursor cells could manifest itself clinically, but so far no hematological disturbances have been associated with HGV infection. Alternate candidates for supporting bone marrow viral replication are stromal endothelial cells, fibroblasts, and macrophages. Interestingly, macrophages are richly represented at the sites where HGV replication was detected and are known to be permissive for a wide range of viruses, including some other flaviviruses (12). Alternatively, HGV could infect various cells at different locations.
In summary, by using a strand-specific Tth-based assay on a variety of autopsy samples from AIDS patients, we identified the presence of negative-strand HGV RNA in bone marrow, spleen, and liver tissue. However, the cell lineage supporting viral replication at these sites remains to be determined.
REFERENCES
- 1.Alter H J. The cloning and clinical implications of HGV and HGBV-C. N Engl J Med. 1996;334:1536–1537. doi: 10.1056/NEJM199606063342310. [DOI] [PubMed] [Google Scholar]
- 2.Berenguer M, Terrault N A, Piatak M, Yun A, Kim J P, Lau J Y N, Lake J R, Roberts J R, Ascher N, Ferrell L, Wright T. Hepatitis G virus infection in patients with hepatitis C virus infection undergoing liver transplantation. Gastroenterology. 1996;111:1569–1575. doi: 10.1016/s0016-5085(96)70019-7. [DOI] [PubMed] [Google Scholar]
- 3.Fiordalisi G, Zanella I, Mantero G, Bettinardi A, Stellini R, Paraninfo G, Cadeo G, Primi D. High prevalence of GB virus C infection in a group of Italian patients with hepatitis of unknown etiology. J Infect Dis. 1996;174:181–183. doi: 10.1093/infdis/174.1.181. [DOI] [PubMed] [Google Scholar]
- 4.Jarvis L M, Davidson F, Hanley J P, Yap P L, Ludlam C A, Simmonds P. Infection with hepatitis G virus among recipients of plasma products. Lancet. 1996;348:1352–1355. doi: 10.1016/s0140-6736(96)04041-x. [DOI] [PubMed] [Google Scholar]
- 5.Lanford R E, Sureau C, Jacob J R, White R, Fuerst T R. Demonstration of in vitro infection of chimpanzee hepatocytes with hepatitis C virus using strand-specific RT/PCR. Virology. 1994;202:606–614. doi: 10.1006/viro.1994.1381. [DOI] [PubMed] [Google Scholar]
- 6.Lanford R E, Chavez D, Chisari F V, Sureau C. Lack of detection of negative-strand hepatitis C virus RNA in peripheral blood mononuclear cells and other extrahepatic tissues by the highly strand-specific rTth reverse transcriptase PCR. J Virol. 1995;69:8079–8083. doi: 10.1128/jvi.69.12.8079-8083.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laskus T, Radkowski M, Wang L-F, Vargas H, Rakela J. Lack of evidence for hepatitis G virus replication in the livers of patients coinfected with hepatitis C and G viruses. J Virol. 1997;71:7804–7806. doi: 10.1128/jvi.71.10.7804-7806.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laskus T, Radkowski M, Wang L-F, Cianciara J, Vargas H, Rakela J. Hepatitis C virus negative strand is not detected in peripheral blood mononuclear cells and viral sequences are identical to those in serum: a case against extrahepatic replications. J Gen Virol. 1997;78:2747–2750. doi: 10.1099/0022-1317-78-11-2747. [DOI] [PubMed] [Google Scholar]
- 9.Leary T P, Muerhoff A S, Simons J N, Pilot-Matias T J, Erker J C, Chalmers M L, Schlauder G G, Dawson G J, Desai S M, Mushahwar I K. Sequence and genomic organization of GBV-C: a novel member of the Flaviviridae associated with human non-A-E hepatitis. J Med Virol. 1996;48:60–67. doi: 10.1002/(SICI)1096-9071(199601)48:1<60::AID-JMV10>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 10.Linnen J, Wages J, Zhank-Keck Z-Y, Fry K E, Krawczynski K Z, Alter H, Koonin E, Gallaher M, Alter M, Hadziyannis S, Karayiannis P, Fung K, Nakatsuji Y, Shih J W K, Young L, Piatak M, Hoover C, Fernandez J, Chen S, Zou J-C, Morris T, Hyams K C, Ismay S, Lifson J D, Hess G, Foung S K H, Thomas H, Bradley D, Margolis H, Kim J P. Molecular cloning and disease association of hepatitis G virus: a transfusion-transmissible agent. Science. 1996;271:505–508. doi: 10.1126/science.271.5248.505. [DOI] [PubMed] [Google Scholar]
- 11.Masuko K, Mitsui T, Iwano K, Yamazaki C, Okuda K, Meguro T, Murayama N, Inoue T, Tsuda K, Okamoto H, Miyakawa Y, Mayumi M. Infection with hepatitis GB virus C in patients on maintenance hemodialysis. N Engl J Med. 1996;334:1465–1490. doi: 10.1056/NEJM199606063342301. [DOI] [PubMed] [Google Scholar]
- 12.Mogensen S C. Role of macrophages in natural resistance to virus infections. Microbiol Rev. 1979;43:1–26. doi: 10.1128/mr.43.1.1-26.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simons J N, Leary T P, Dawson G J, Pilot-Matias T J, Muerhoff A S, Schlauder G G, Desai S M, Mushahwar I K. Isolation of novel virus-like sequences associated with human hepatitis. Nat Med. 1995;1:564–569. doi: 10.1038/nm0695-564. [DOI] [PubMed] [Google Scholar]
- 14.Stark K, Bienzle U, Hess G, Engel A M, Hagenschied B, Schluter V. Detection of hepatitis G virus genome among injecting drug users, homosexual and bisexual men, and blood donors. J Infect Dis. 1996;174:1320–1323. doi: 10.1093/infdis/174.6.1320. [DOI] [PubMed] [Google Scholar]
- 15.Tanaka E, Alter H J, Nakatsuji Y, Shih W-K, Kim J P, Matsumoto A, Kobayashi M, Kiyosawa K. Effect of hepatitis G virus infection on chronic hepatitis C. Ann Intern Med. 1996;125:772–773. doi: 10.7326/0003-4819-125-9-199611010-00007. [DOI] [PubMed] [Google Scholar]
- 16.Wang J T, Tsai F C, Lee C-Z, Chen P-J, Sheu J C, Wang T-H, Chen D-S. A prospective study of transfusion associated GB virus C infection: similar frequency but different clinical presentation compared with hepatitis C virus. Blood. 1996;88:1881–1886. [PubMed] [Google Scholar]