Abstract
A series of silica-supported catalysts containing molybdenum, tungsten, and vanadium oxides as the active phase was investigated in the process of oxidative desulfurization with sodium hypochlorite. It was shown for the first time that catalysts containing vanadium oxide as the active phase are more stable under oxidation conditions with sodium hypochlorite and retain their effectiveness at increased dosages of the oxidant and at high initial sulfur contents. The catalysts were characterized in detail by a complex of methods: Fourier transform infrared, X-ray spectral fluorescence, transmission electron microscopy, scanning electron microscopy, and low-temperature nitrogen adsorption/desorption. Key factors affecting the oxidation of dibenzothiophene (DBT) were investigated: oxidant and catalyst amount, oxidation time, initial sulfur content, and acetonitrile amount. Under optimized conditions, the DBT conversion rate was 100% in 5 min at room temperature (25 °C), NaClO/S molar ratio 6:1, catalyst amount 2 wt %. In the real sample of the straight-run diesel fraction, the sulfur content was reduced from 10,100 to 3030 ppm. The V(10%)/SiO2 catalyst retains its activity for 5 oxidation–regeneration cycles.
1. Introduction
Modern motor fuels are the subject of strict requirements that are regulated by environmental standards. One of the key parameters is the total sulfur content in motor fuels because sulfur dioxide generated during the combustion of sulfur-containing compounds has a significant negative impact on the environment: toxic effects on humans and living organisms, the cause of acid rain, the greenhouse effect, etc. Therefore, in recent decades, there is a tendency to tighten the requirements for the sulfur content in motor fuels up to ultralow values. Thus, the level of sulfur content in diesel fuel to less than 15 ppm has been introduced since 2006 in the United States, less than 10 ppm since 2005 in Europe, and less than 10 ppm since 2008 in Beijing and Shanghai in China.1,2 Moreover, given the tendency to deplete the reserves of light low-sulfur oils and involve heavy high-sulfur hydrocarbon raw materials in processing (high-viscosity oil, bituminous oil, shale oil, etc.), the task of desulfurization of petroleum distillates is of particular relevance.
Reductive desulfurization (hydrotreating) is highly effective for removing thiols, sulfides, and disulfides, and less effective for dibenzothiophene3 derivatives. Harsh operating conditions, such as high temperatures, high pressure, and high hydrogen consumption, are necessary for this process to achieve ultradeep desulfurization of motor fuel components. High capital and operating costs are unavoidable. Therefore, it is necessary to develop alternative processes for ultradeep desulfurization. One of the methods is bio-desulfurization-bacteria are mixed with oil (liquid phase) at ambient temperature and pressure to selectively remove organosulfide compounds from oil fractions, but microorganisms are very sensitive to the pH of the medium and it should be noted that this method is significantly longer in comparison with other methods-about several days.4 Oxidative demercaptanization is used on an industrial scale in practice, but this method is effective only for removing hydrogen sulfide and low-molecular-weight mercaptans.5 For extractive desulfurization, such solvents as acetonitrile, dimethylformamide,6 and dimethyl sulfoxide7 are used. This method is easy to implement due to the mild process conditions, but in most cases, extractants have low selectivity, which leads to losses of hydrocarbon components. Adsorptive desulfurization is also carried out at atmospheric pressure and moderate temperature using various adsorbents: zeolites,8 silica gel,9 aluminum oxide,10 activated carbon,11 etc. Despite the high degree of sulfur removal, several disadvantages limit its practical application: low sorption capacity and short operating time, labor-intensive regeneration process.
In this regard, the method of oxidative desulfurization is of great interest and, in comparison with the applied technology of desulfurization–hydrotreating, has a number of advantages. For example, inert substituted dibenzothiophenes are easily oxidized at low temperatures and pressures, so expensive hydrogen is not required, and therefore the capital cost of an oxidative desulfurization unit is significantly lower than for a deep hydrotreating unit.12 Another important advantage is the minimal waste formation since the oxidation products–sulfones–can be isolated and used.13 Since the use of expensive hydrogen is eliminated, oxidation processes are more suitable for small and medium-sized refineries. In this connection, not only regenerated catalysts but also regenerated oxidizing agents have recently attracted increasing attention. It should be noted, that for the process of oxidative desulfurization, various oxidizing agents can be used such as oxygen,14 ozone,15 hydrogen peroxide,16 sodium hypochlorite, etc. As an oxidant, sodium hypochlorite is readily available and has many applications, including the processes of alcohols acidification,17 nitrogen-and sulfur-containing compounds oxidation,18,19 epoxidation,20 oxidation of wood,21 starch,22 desulfurization, and denitrogenation of fuel oils.23 It is known that sodium hypochlorite can be regenerated in situ by electrolysis, which opens up wide opportunities for its use in oxidation processes.24 However, the use of pure sodium hypochlorite can lead to the occurrence of side processes of oxidation of hydrocarbon components, as well as the formation of organochlorine compounds.25 Therefore, the development of catalysts that allow the oxidation process to be carried out under the mildest possible conditions in order to minimize side processes plays a key role in using sodium hypochlorite as an oxidant. In the literature, there are known data on the use of sodium hypochlorite as an oxidant for sulfur-containing compound oxidation with catalysts containing nickel, molybdenum, manganese, and cobalt oxides on an aluminum oxide support. Under the selected optimal conditions, the dibenzothiophene oxidation reaction proceeds completely in 5 min at room temperature (25 °C).26 However, it should be noted that the sodium hypochlorite solution has an alkaline pH, which can lead to the destruction of the aluminum oxide-based catalyst. In other example dibenzothiophene was oxidized by sodium hypochlorite in 30 min in the presence of ionic liquid [C16MIM] [PMoO] (C16MIM—1-hexadecyl-3-methylimidazolium).27 However, the use of expensive ionic liquids and a time-consuming regeneration procedure limits the possibilities for practical application of the results obtained.
In this paper, systematic studies of the oxidation of the main classes of sulfur-containing compounds by sodium hypochlorite in the presence of heterogeneous catalysts, which are molybdenum, tungsten, and vanadium oxides deposited on silica gel, were carried out for the first time. The use of these oxides, which are prone to the formation of active peroxocomplexes, allows the oxidation reaction to proceed at room temperature (25 °C). The main process parameters affecting the conversion of sulfur-containing substrates are studied in detail. The results obtained in this work can be used in the development of environmentally friendly processes for cleaning motor fuels where the only consumable component is electricity, which is necessary for oxidant regeneration.
2. Experimental Methods
2.1. Materials and Reagents
The following reagents were used for the synthesis of catalysts: silica gel fraction, 2–0.5 mm.
The following reagents were used for the synthesis and modification of silica gel-based catalysts: ammonium orthovanadate (NH4VO3, “PrimeChemicalsGroup”, 99%), ammonium paratungstate [(NH4)10(H2W12O42)·4H2O, “PrimeChemicalsGroup”, 99%], and ammonium heptamolybdate [(NH4)6Mo7O24, “PrimeChemicalsGroup”, 99%].
The following reagents were used for the oxidation reactions: dibenzothiophene (DBT, Sigma-Aldrich, 98%), benzothiophene (BT, Sigma-Aldrich, 98%), 4-methyldibenzothiophene (MeDBT, Sigma-Aldrich, 96%), 4,6-dimethyldibenzothiophene (Me2DBT, Sigma-Aldrich, 95%), dodecane (Sigma-Aldrich), acetonitrile (MeCN, “PrimeChemicalsGroup”, 99%), sodium hypochlorite (NaClO, “PrimeChemicalsGroup”, 15% aqueous solution).
To carry out the oxidative desulfurization reaction, a straight-run diesel fraction with an initial sulfur content of 10,100 ppm and methanol (CH3OH, “PrimeChemicalsGroup”, 99%) were used.
2.2. Synthesis of Catalysts
Modification of silica gel with a molybdenum compound was carried out by the method of impregnation by moisture capacity. To do this, the calculated amount is (NH4)6Mo7O24 [0.0934 (1%), 0.4973 (5%), 1.0824 (10%)] it was dissolved in 30 mL of distilled water. 5 g of silica gel was added to the solution, the flask was placed on a rotary evaporator and kept first for 2 h at a temperature of 40 °C, then at a temperature of 60 °C until the water was completely evaporated. The resulting silica gel with deposited sodium heptamolybdate was dried in the following mode; 80 °C—4 h, 90 °C—4 h, 100 °C—4 h, 110 °C—4 h. To form molybdenum oxide on the surface of the support, the resulting substance was calcined after drying at a temperature of 550 °C for 5 h. The obtained MoO3/SiO2 catalyst is designated as Mo/SiO2.
The synthesis of tungsten and vanadium-containing catalysts (W/SiO2 and V/SiO2) was carried out by a similar procedure, using paratungstate (NH4)10(H2W12O42)·4H2O, and ammonium orthovanadate NH4VO3 as the corresponding metal source. To determine the optimal active phase content, 9 catalysts were synthesized with a metal content of 1, 5, and 10 wt %.
2.3. Materials Characterization Methods
The elemental composition was studied by X-ray spectral fluorescence analysis (XRF) using a Thermo ARL Perform’x Sequential XRF X-ray wave spectrometer with an X-ray tube with a power of 2500 V. Powdered samples were pressed into tablets on a boric acid substrate and covered with a dacron film, which was pressed with a ring-shaped rim to the cuvette.
The characteristics of the porous structure of the samples were determined using a Gemini VII 2390 (V1.02 t) Micromeritics analyzer according to the standard procedure. Before analysis, the samples were evacuated at 350 °C for 12 h to a pressure of 3 × 10–3 atm. The nitrogen adsorption–desorption isotherm was removed at a temperature of 77 K. The characteristics of the porous structure were calculated using standard software. The specific surface area was calculated using the BET model at a relative partial pressure of P/P0 = 0.2. The total pore volume was calculated using the BJH (Barrett–Joyner–Halenda) model at a relative partial pressure of P/P0 = 0.95.
Fourier transform infrared (FTIR) spectroscopy was performed on a Nicolet IR2000 (Thermo Scientific) instrument using the method of multiple disturbed total internal reflection using the Multireflection HATR prefix containing a ZnSe 45° crystal for various wavelength ranges with a resolution of 4 nm, in the range of 500–4500 cm–1.
Transmission electron microscopy (TEM) investigations were performed using a JEOL JEM-2100 UHR (Japan) microscope operated at a 200 kV acceleration voltage. The microphotographs were acquired in bright-field mode with using an Olympus Quemesa 11 megapixel CCD camera.
Determination of the total sulfur content in hydrocarbon fractions was carried out using a sulfur analyzer in petroleum products ASE-2. The principle of operation of the device is based on the X-ray fluorescence energy-dispersive spectrometry method, which is an arbitration method for determining the mass fraction of sulfur in diesel fuel and an acceptable method for determining the mass fraction of sulfur in unleaded gasoline. The device allows you to determine the sulfur content in the range from 7 to 50,000 ppm.
2.4. Oxidation of Model Fuel
Model mixtures of dibenzothiophene (DBT) in dodecane with sulfur contents of 100, 500, 1000, 2000, 4000, and 8000 ppm were used for the oxidation reactions. To do this, the calculated amount of DBT was added to the measured volume of dodecane and mixed until it was completely dissolved. Model mixtures with BT, 4-MeDBT, and 4,6-Me2DBT with a sulfur content of 500 ppm were prepared in the same way. For the oxidation reaction, 5 mL of the model mixture was used, and calculated amounts of sodium hypochlorite solution, catalyst, and acetonitrile were added. The reaction was carried out under constant stirring at a speed of 1200 rpm. Chromatograph sampling was performed at the 10th, 20th, and 60th minutes.
2.5. Oxidation of Real Fuel
Oxidative desulfurization of the diesel fraction was carried out according to the following procedure. Calculated amounts of sodium hypochlorite solution, catalyst, and acetonitrile were added to 10 mL of diesel fuel sample. The resulting mixture was stirred at 1200 rpm at room temperature (25 °C) for 30 min. After the oxidation reaction, a layer of organic phase (diesel fuel) was taken from the reaction mixture, which was then mixed in a 1:1 ratio (by volume) with methyl alcohol and centrifuged to separate the diesel fuel and methanol phase. A sample of diesel fuel was taken, and the sulfur content was measured on an ASE-2 device.
3. Results and Discussion
3.1. Characterization of Supports and Catalysts
Quantitative determination of metals in the catalyst was performed by X-ray diffraction analysis using a Thermo ARL Perform’x Sequential XRF X-ray fluorescence wave spectrometer. X-ray fluorescence (XRF) is an analytical method that utilizes X-rays to determine the elemental composition of materials. The analysis determines the types of elements in the sample based on the characteristic wavelengths of the X-rays emitted by the atoms.28 The data of the elementary analysis are listed in Table 1. The obtained values of the mass content of metal correspond well with the calculated values, which indicates the successful application of the required amount of the active phase. It should be noted that after 5 cycles of oxidation regeneration, the molybdenum content decreases sharply, while the vanadium content remains close to the initial value. These results indicate greater stability of the vanadium-containing catalyst to the sodium hypochlorite solution.
Table 1. Elemental Analysis of the Catalysts.
| name of catalysts | mass fraction of metal (calculation), % | mass fraction of metal (analysis), % | ||
|---|---|---|---|---|
| Mo | W | V | ||
| Me(1%)/SiO2 | 1.0 | 0.95 | 0.92 | 0.97 |
| Me(5%)/SiO2 | 5.0 | 4.93 | 4.92 | 4.97 |
| Me(10%)/SiO2 | 10.0 | 9.87 | 9.95 | 10.02 |
| Me(10%)/SiO2 | 10.0 (regenerated 5 cycles) | 3.71 | 9.62 |
The textural characteristics of materials were studied using the method of low-temperature nitrogen adsorption/desorption (Figure 1). Low temperature nitrogen adsorption–desorption was used to calculate the surface area and determine the pore size and pore volume.29
Figure 1.
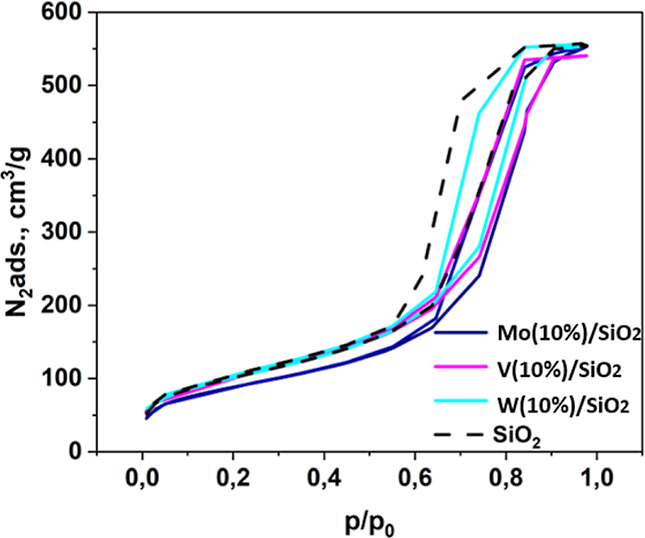
N2 adsorption of the synthesized materials.
The isotherms have a hysteresis loop in the relative pressure range of 0.6–1, which indicates the presence of meso-and macropores in the structure of the catalysts. Table 2 shows the surface characteristics of the catalysts. It should be noted that according to the data, after modification of the support, there is no sharp decrease in the surface area; that is, all catalysts retain the developed surface of silica gel.
Table 2. Textural Characteristics of the Synthesized Catalysts.
| catalyst | BET surface area (m2/g) | pore volume (cm3/g) | pore size (Å) |
|---|---|---|---|
| support (SiO2) | 385 | 1.12 | 122 |
| Mo(1%)/SiO2 | 365 | 0.89 | 118 |
| V(1%)/SiO2 | 364 | 0.99 | 120 |
| W(1%)/SiO2 | 365 | 0.96 | 110 |
| Mo(5%)/SiO2 | 353 | 0.87 | 102 |
| V(5%)/SiO2 | 351 | 0.87 | 116 |
| W(5%)/SiO2 | 357 | 0.88 | 99 |
| Mo(10%)/SiO2 | 340 | 0.85 | 92 |
| V(10%)/SiO2 | 316 | 0.86 | 107 |
| W(10%)/SiO2 | 316 | 0.84 | 94 |
Silica gel and catalysts were characterized by FTIR spectroscopy. FTIR spectroscopy allows you to obtain information about the presence of certain bonds in the composition of the catalyst.30 In the literature data, there is information that the signals of transition metal oxides (Mo, W, and V) and the support are in the same regions and overlap each other. When comparing the spectra, it is noticeable that −OH groups do not appear in the catalysts (974 cm–) due to the overlap of transition metal oxide signals, which confirms their adsorption on the support (Figure 2). In the range 900–980 cm–1, a signal corresponding to the Mo–O–Mo bond valence vibrations (925 cm–1) can be detected in a molybdenum-modified catalyst, which confirms the presence of molybdenum oxide in the catalyst Mo(10%)/SiO2. The symmetric and antisymmetric stretching of the Mo–O–Mo bond is superimposed on the strain and valence signals of the Si–O–Si bond, which occur at wave numbers 796 and 1060 cm–1, respectively. Thus, it can be concluded that the process of deposition of oxides did not lead to the destruction SiO231 structure. The same results were obtained for catalysts containing tungsten and vanadium. Due to the weak interaction, there is only a slight shift compared to the support.32
Figure 2.
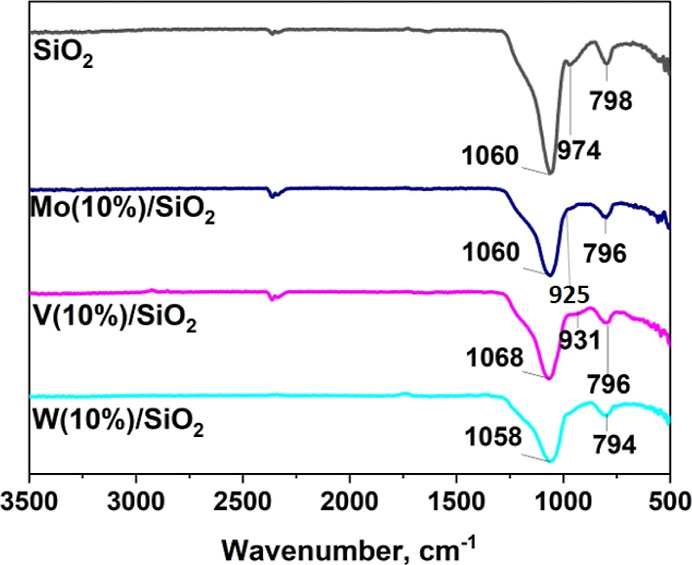
FTIR spectra of the support (silica gel) and synthesized catalysts.
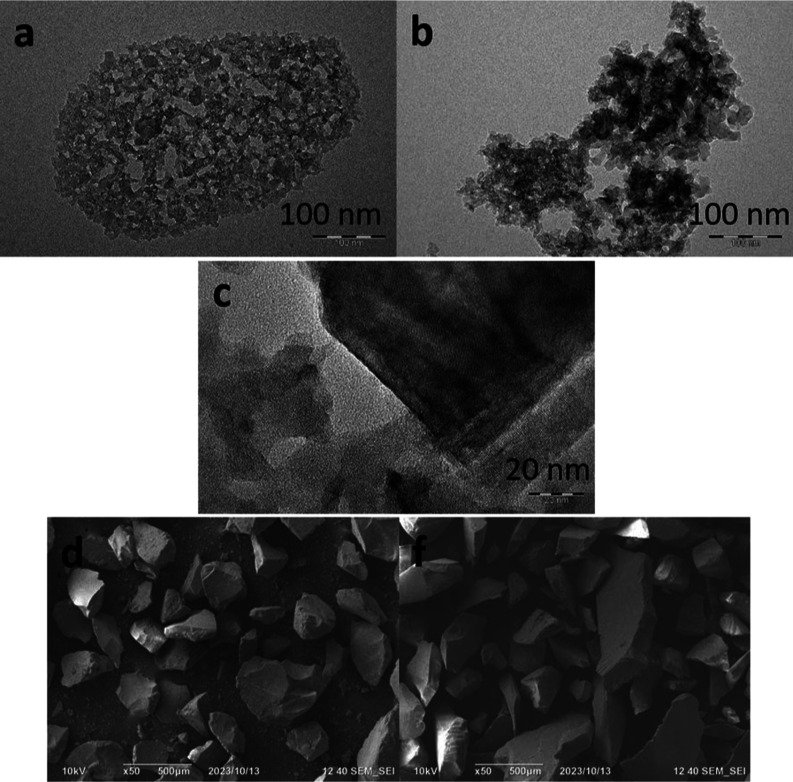
Analysis of the catalyst and support by SEM and TEM was carried out to compare the morphologies of the original silica gel and the resulting catalyst. SEM and TEM provide important information about the surface morphology, shape, and distribution of materials.33 TEM and SEM images are shown in Figure 3. As can be seen, the support has a porous structure, while the modification preserves the structure of the support, and darkening appears evenly distributed throughout the volume, which indicates the successful deposition of the active phase.
Figure 3.
TEM and SEM images of the support (a,d) and catalyst V(10%)/SiO2 (b,c,f).
3.2. Oxidation of Model Sulfides
The activity of the catalysts was compared during the oxidation of a model mixture of dibenzothiophene in dodecane (Figure 4). Acetonitrile was added to the reaction mixture in order to prevent the catalyst particles from sticking together in excess aqueous phase. This approach was used in previous34,35 studies and showed its effectiveness.
Figure 4.
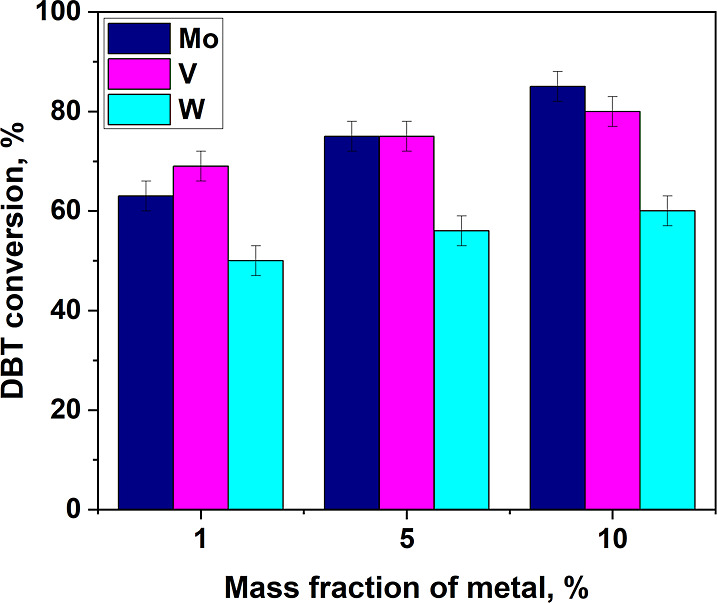
Dependence of DBT conversion on the amount of metal deposited on the support. Oxidation conditions: ω(cat.) = 2%, molar ratio 4:1, 1 mL of CH3CN, 25 °C, 10 min.
In general, an increase in the metal content in the catalyst composition leads to an increase in the substrate conversion. The best results were achieved in the presence of molybdenum- and vanadium-containing catalysts with a metal content of 10%.
It should be noted that the results of comparing different metals differ from the data obtained by the authors earlier in the oxidation of dibenzothiophene with hydrogen peroxide, where tungsten-containing catalysts performed better than their analogues containing vanadium.11 This fact is probably related to the peculiarities of the type of oxidant–nethane hypochlorite, which has an alkaline environment that promotes the leaching of the active phase. This assumption will be further studied in more detail.
The effect of the oxidation time on DBT conversion was studied in the presence of Mo(10%)/SiO2, V(10%)/SiO2, and W(10%)/SiO2 catalysts (Figure 5). According to the experimental data, oxidation occurs almost completely in 10 min, which indicates a high rate of oxidation. Further, the conversion curve reaches a plateau, which may be due to a decrease in the concentration of sodium hypochlorite. In the case of the W(10%)/SiO2 catalyst, the time-dependent DBT conversion curve reaches a plateau already at the substrate conversion rate of 60%, which is difficult to explain by a drop in the oxidant concentration. In this case, the contribution of the process of leaching the active sites of the catalyst under the action of an alkaline solution of sodium hypochlorite is apparently noticeable, which leads to a drop in the concentration of active sites and slows the oxidation process. During idle oxidation with sodium hypochlorite without a catalyst, a smooth increase in the conversion of dibenzothiophene is observed, which indicates a high activity of sodium hypochlorite as an oxidizer during desulfurization compared, for example, with hydrogen peroxide, which without a catalyst, even when heated, practically does not show activity.36 However, the use of sodium hypochlorite without a catalyst is impractical due to a significant increase in the reaction time. Two catalysts were selected for further experiments due to their better activity: Mo(10%)/SiO2 and V(10%)/SiO2.
Figure 5.
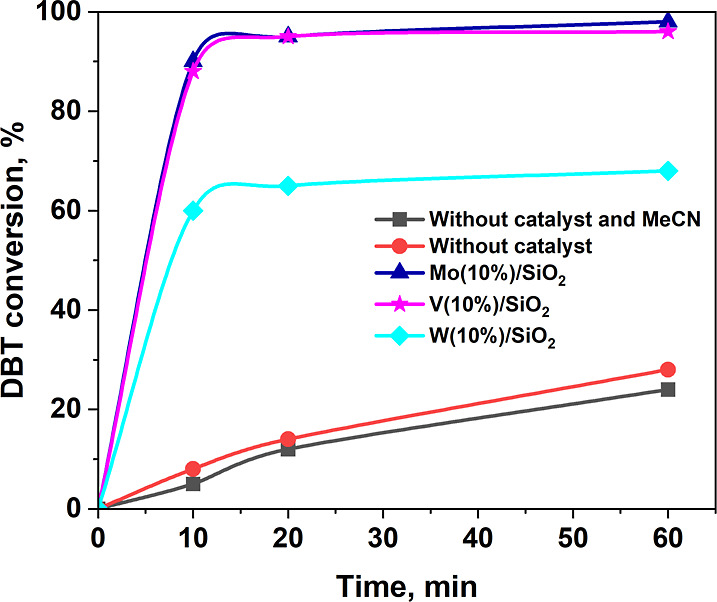
Time dependence of the DBT conversion. Oxidation conditions: ω(cat.) = 2%, O/S molar ratio 4:1, 1 mL of CH3CN, 25 °C.
The study of the effect of the amount of acetonitrile on the DBT conversion showed that the values of DBT conversion pass through an extremum when the volume of acetonitrile is equal to 0.5 mL (Figure 6). At lower amounts of acetonitrile, the catalyst particles stick together in excess of the aqueous phase, which leads to a decrease in the contact area between the model mixture and the catalyst. With large amounts of acetonitrile, the oxidizer is diluted, which leads to a decrease in the oxidation rate. Therefore, 0.5 mL of acetonitrile volume was used for further experiments.
Figure 6.
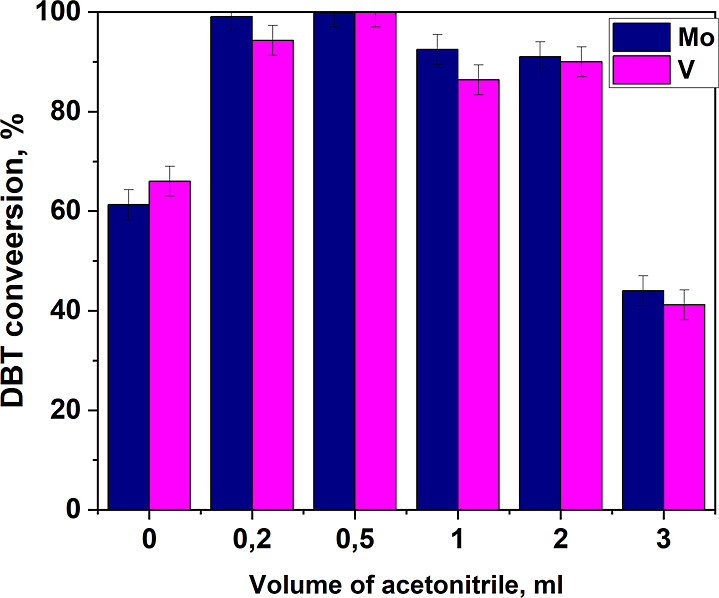
Dependence of DBT conversion on the volume of acetonitrile. Oxidation conditions: ω(cat. 10%) = 2%, O/S molar ratio, 25 °C, 10 min.
The study of the dependence of the DBT conversion on the mass of the catalyst revealed an optimal value of 2 wt % (Figure 7). Conducting reactions with a mass fraction of the catalyst greater than 2 wt % does not reduce the conversion rate. This, in turn, shows that sodium hypochlorite practically does not decompose at increased dosages of the catalyst and indicates the stability of the oxidative catalytic system. When using a smaller amount of catalyst, the contact area between the phases decreases, which leads to a decrease in the oxidation rate and a decrease in DBT conversion.
Figure 7.
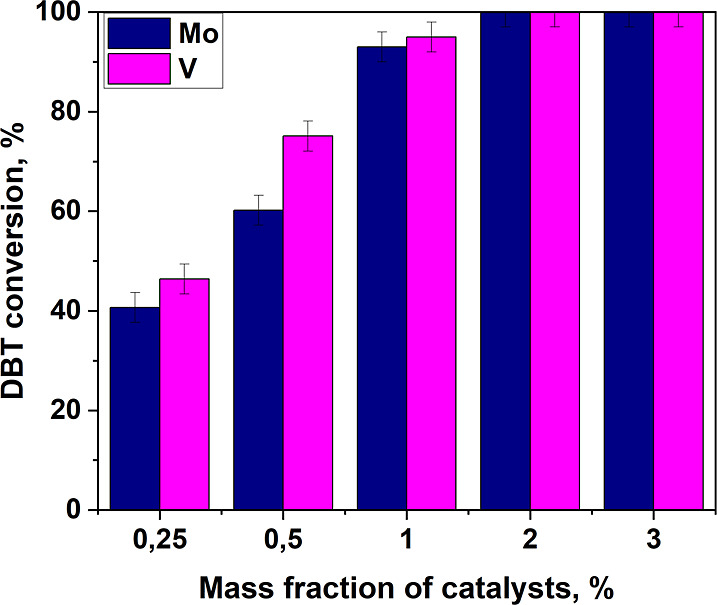
Dependence of DBT conversion on the amount of catalyst. Oxidation conditions: O/S molar ratio 4:1, 0.5 mL CH3CN, 25 °C, 10 min.
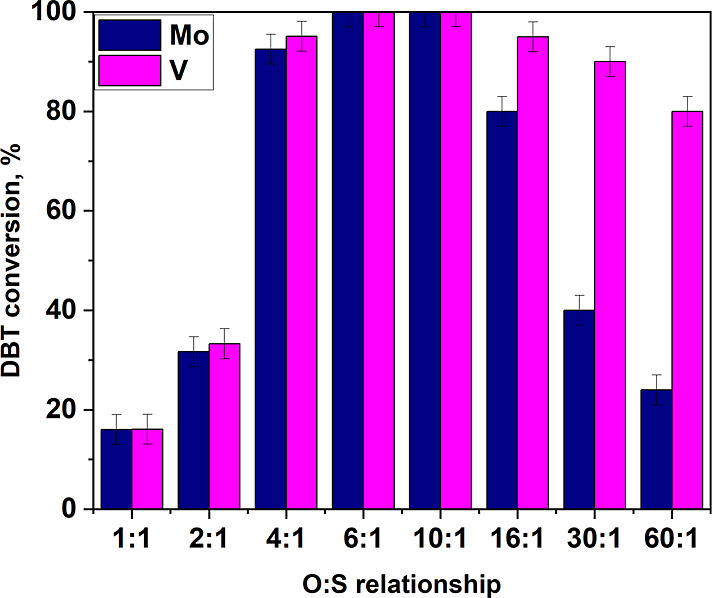
The effect of the oxidant: sulfur ratio on the conversion of dibenzothiophene was also studied (Figure 8). Thus, at a stoichiometric dosage of 2:1, the reaction proceeds relatively slowly in the presence of both catalysts, and the DBT conversion barely exceeds 30% for both catalysts. This may be due to the fact that the reaction is carried out in acetonitrile and, at low dosages, dilution of hypochlorite negatively affects the speed of the process. In general, the dependence of the DBT conversion on the amount of oxidant is also extreme, with a maximum in the range of molar ratios of sodium hypochlorite: sulfur 6–10:1. It should be noted that with a 6-fold molar excess of sodium hypochlorite, 100% DBT conversion can be achieved within 5 min of oxidation. Interesting results were obtained with a sharp increase in the amount of oxidant in the presence of a vanadium-containing catalyst, the DBT conversion decreases by 20%, while in the presence of Mo(10%)/SiO2, the conversion decreases by a factor of 5. This fact may be related to the leaching of transition metal oxides with an excess of hypochlorite since an aqueous solution of hypochlorite has an alkaline medium (pH = 11–12) and the following reactions can occur
| 1 |
| 2 |
| 3 |
Figure 8.
Dependence of DBT conversion depends on the amount of oxidant. Oxidation conditions: ω(cat. 10%) = 2%, 0.5 mL of CH3CN, 25 °C, 5 min.
At the same time, a sharp decrease in the DBT conversion in the presence of Mo(10%)/SiO2 indicates that eq 2 proceeds much more intensively than eq 3. New data obtained for the first time show that catalysts based on vanadium oxide are more preferable than molybdenum compounds in the process of oxidative desulfurization with sodium hypochlorite due to their greater stability in the process of oxidative desulfurization.
The sulfur content in oil fractions can vary widely and exceed 1%. Therefore, a series of experiments was conducted on desulfurization of model mixtures with high sulfur content.
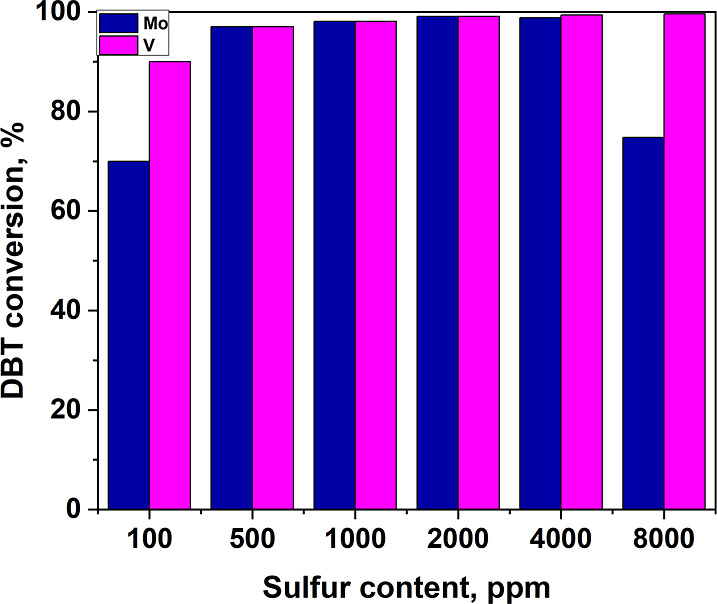
As a result, both catalysts showed high efficiency in a wide range of initial sulfur contents (Figure 9). The low conversion rate at a sulfur content of 100 ppm seems to be related to a slowdown in the oxidation rate due to dilution of the substrate and oxidant (in experiments, the amount of catalyst did not change and the actual amount of oxidant added was tied to the initial sulfur content). The results on the oxidation of model mixtures with an increased initial sulfur content correlate well with previous data on varying the amount of the oxidizer. The higher the initial sulfur content, the more oxidizing agent is actually added–the more the conversion decreases in the case of a molybdenum-containing catalyst. That is, when desulfurization of high-sulfur raw materials is oxidized with sodium hypochlorite, it is preferable to use vanadium-containing catalysts.
Figure 9.
Dependence of DBT conversion on the sulfur content of the model mixture. Oxidation conditions: ω(cat.) = 2%, molar ratio 4:1, 0.5 mL of CH3CN, 25 °C, 10 min.
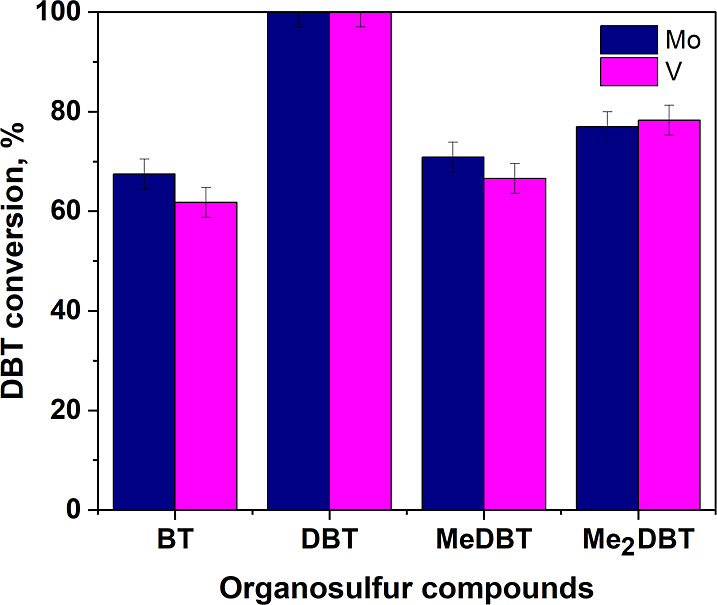
Real hydrocarbon fractions contain various types of sulfur-containing compounds, which may be more difficult to enter into oxidation reactions for several reasons: steric hindrances for alkyl-substituted substrates (methyl-DBT, dimethyl-DBT) or lower electron density (benzothiophene).33 Thus, the ability to oxidize methyldibenzothiophene, dimethyldibenzothiophene, and benzothiophene under other equal conditions was investigated (Figure 10). Benzothiophene showed the lowest conversion (67 and 62% on catalysts Mo(10%)/SiO2 and V(10%)/SiO2, respectively). At the same time, the presence of methyl substituents also leads to a decrease in the conversion. The results obtained correlate well with the literature data.1
Figure 10.
Oxidation of various organosulfur compounds. Oxidation conditions: ω(cat.) = 2%, O/S molar ratio 4:1, 0.5 mL CH3CN, 25 °C, 10 min.
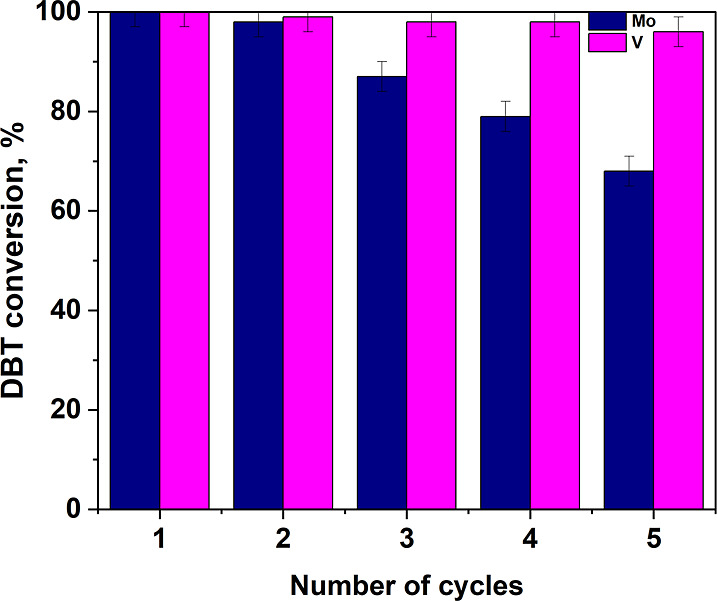
When reused after the reaction, the catalyst was washed with acetone to remove the adsorbed sulfones and then dried at 110 °C. The catalyst obtained after drying was collected and used in the next oxidation of a new portion of model fuel (Figure 11).
Figure 11.
Dependence of the DBT conversion on the number of oxidation cycles. Oxidation conditions: ω(cat.) = 2%, O/S molar ratio 4:1, 0.5 mL CH3CN, 25 °C, 10 min.
According to the results obtained, the Mo(10%)/SiO2 catalyst can withstand 4 cycles without significantly reduce in DBT conversion, while V(10%)/SiO2—can withstand 5 cycles. The decrease in DBT conversion can be attributed to the leaching of metal by sodium hypochlorite.
3.3. Oxidation of Diesel Fraction
In the presence of Mo(10%)/SiO2 and V(10%)/SiO2 catalysts, a series of experiments were performed on the oxidative desulfurization of a real straight-run diesel fraction with an initial sulfur content of 10,100 ppm (Figure 12). As a result of sequential oxidation–extraction, the sulfur content was reduced from 10,100 to 3030 ppm in the presence of V(10%)/SiO2, with the degree of desulfurization being 70% and from 10,100 to 5760 ppm in the presence of Mo(10%)/SiO2, the degree of desulfurization was 43%. The results obtained on real raw materials correspond well with experiments on a model mixture.
Figure 12.
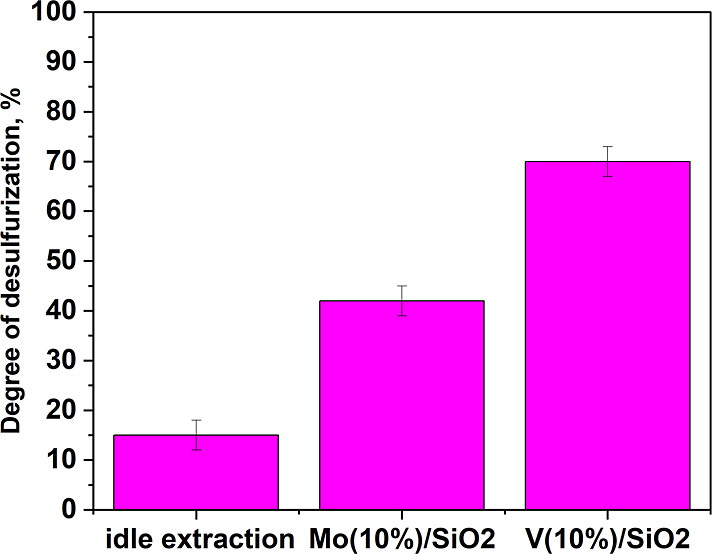
Oxidative desulfurization of the straight-run diesel fraction. Oxidation conditions: ω(cat. 10%) = 2%, molar ratio 4:1, 1 mL of CH3CN, 25 °C, 30 min, methanol extraction.
The high content of sulfur and, as a consequence, the high dosage of the oxidant lead to intensive leaching of the active phase in the case of Mo(10%)/SiO2, which explains the low degree of oxidation. The degree of desulfurization in the presence of V(10%)/SiO2 is more than 30% higher than in the presence of molybdenum-containing analogue, which confirms the conclusion that the use of vanadium-containing catalysts in desulfurization with sodium hypochlorite is preferable. It should be noted that the relatively lower degree of desulfurization compared to the model mixtures is explained by several factors: first, diesel fuel contains compounds that are oxidized under more severe conditions, for example, benzothiophene, alkyl-substituted derivatives of benzo- and dibenzothiophene, and second, a large volume of sodium hypochlorite was used for the oxidation of diesel fuel, which means that this can lead to leaching of the catalyst, and third, extraction may not take place completely, as a result of which some sulfones may remain in the diesel fuel phase.
Idle extraction with methanol without preoxidation reduced the sulfur content from 10,100 to 8680 ppm (14% desulfurization rate). Such a low degree of sulfur recovery from diesel fuel is explained by the low polarity of organosulfur compounds in diesel fuel; therefore, most of them remain in the nonpolar phase of diesel fuel.
Table 3 shows known catalytic systems that allow the oxidation of organosulfur compounds with sodium hypochlorite at room temperature (25 °C). In the works,18,25,36,37 expensive ionic liquids were used as a catalyst, which makes it difficult to further practical application of these catalysts. Good results were obtained in work,24 but aluminum oxides are unstable in a highly alkaline environment of sodium hypochlorite. This work shows for the first time that when purifying high-sulfur raw materials, molybdenum-containing catalysts are washed out when sodium hypochlorite is used as an oxidizing agent. Moreover, catalysts containing vanadium oxide exhibit greater stability in the presence of sodium hypochlorite. This opens up prospects for the use of vanadium-containing catalytic systems for the oxidative desulfurization of high-sulfur raw materials, using sodium hypochlorite as an oxidizing agent. Under the selected optimal conditions, it is possible to achieve 100% conversion of dibenzothiophene for a model mixture with an initial sulfur content of 8000 ppm.
Table 3. Comparison of Catalytic Systems for Oxidative Desulfurization at Room Temperature (25 °C) with Sodium Hypochlorite.
| catalyst | substrate | initial sulfur content, ppm | O/S | time, min | conversion, % | ref |
|---|---|---|---|---|---|---|
| Mn–Co–Mo/Al2O3 and IL([Bmim]Cl/ZnCl2) | DBT and pyridine | 800 | 4:1 | 15 | 99 | (18) |
| MnO2/UiO-66 composite | DBT and pyridine | 2000 | 4:1 | 5 | 100 | (19) |
| IL ([EimC4SO3H]NTf2) | DBT | 1600 | 5:1 | 180 | 99 | (38) |
| IL ([C16MIM][PMoO]) | DBT | 500 | 5:1 | 30 | 100 | (27) |
| Mn(Co–Mo/Al2O3 and Ni–Mo/Al2O3) | DBT | 2000 | 6:1 | 5 | 100 | (26) |
| V(10%)/SiO2 | DBT | 500 | 6:1 | 5 | 100 | current work |
| V(10%)/SiO2 | DBT | 8000 | 4:1 | 10 | 100 | current work |
4. Conclusions
Catalysts based on silica with different contents of molybdenum, tungsten, and vanadium were synthesized by the impregnation method. It was shown that the synthesized catalysts allow dibenzothiophene to be oxidized with sodium hypochlorite already at room temperature (25 °C) and almost completely proceed in 5 min, which indicates a high oxidation rate. It was shown that tungsten-containing catalysts are less active than analogues containing vanadium and molybdenum. The study of the effect of the acetonitrile amount on the DBT conversion showed that the DBT conversion values pass through an extremum when the volume of acetonitrile is equal to 0.5 mL. Reactions with a mass content of the catalyst greater than 2 wt % do not lead to a decrease in conversion, which shows that these catalytic systems do not lead to sodium hypochlorite decomposition. The dependence of the DBT conversion on the amount of oxidant has an extremum, with a maximum in the range of molar ratios of sodium hypochlorite: sulfur 6–10:1. It should be noted that with a 6-fold molar excess of sodium hypochlorite, 100% DBT conversion can be achieved within 5 min of oxidation. For the first time it was shown that with a sharp increase in the amount of oxidant in the presence of a vanadium-containing catalyst, the DBT conversion decreases by 20%, while in the presence of Mo(10%)/SiO2, the conversion decreases by a factor of 5, so vanadium-containing catalyst is much more resistant to leaching. The vanadium-containing catalyst, in comparison with the molybdenum-containing one, remains active even at high sulfur contents. The activity of catalysts on various types of sulfur-containing compounds was studied, and benzothiophene had the lowest conversion. In addition, the vanadium-containing catalysts remained active without significantly reducing the DBT conversion to 5 cycles. For a real straight-run diesel fraction with an initial sulfur content of 10,100 ppm, the conversion reaches 70% for the V(10%)/SiO2 catalyst and 43% for Mo(10%)/SiO2 at room temperature (25 °C). Thus, optimal conditions for ultrafast oxidation of dibenzothiophene were revealed: 2 wt % of catalyst, 0.5 mL of acetonitrile, and 6:1 oxidant to sulfur molar ratio, under which 100% conversion was achieved in 5 min. The results obtained can be used to develop catalysts for the oxidative desulfurization process that are stable to hypochlorite, which will make a significant contribution to the development of the method of oxidative desulfurization using hypochlorite as an oxidant. The obtained data can also be used in the development of stable catalysts for other oxidative processes, where sodium hypochlorite is used as a regenerable oxidant.
Acknowledgments
The study was financially supported by the Russian Science Foundation project no. 22-79-00063, https://rscf.ru/en/project/22-79-00063/.
Glossary
Abbreviations
- FT-IR
Fourier-transform infrared spectroscopy
- SEM
scanning electron microscopy
- TEM
transmission electron microscopy
- XRF
X-ray fluorescence
- BET
Brunauer–Emmett–Teller
- BJH
Barrett–Joyner–Halenda
- DBT
dibenzothiophene
- BT
benzothiophene
- 4-MeDBT
4-methyldibenzothiophene
- 4,6-Me2DBT
4,6-dimethyldibenzothiophene
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
References
- Song C.; Ma X. Ultra-deep desulfurization of liquid hydrocarbon fuels: Chemistry and Process. Int. J. Green Energy 2004, 1, 167–191. 10.1081/GE-120038751. [DOI] [Google Scholar]
- Song C. An overview of new approaches to deep desulfurization for ultra-clean gasoline, diesel fuel and jet fuel. Catal. Today 2003, 86, 211–263. 10.1016/S0920-5861(03)00412-7. [DOI] [Google Scholar]
- Röthlisberger A.; Prins R. Intermediates in the hydrodesulfurization of 4,6-dimethyl-dibenzothiophene over Pd/γ-Al2O3. J. Catal. 2005, 235, 229–240. 10.1016/j.jcat.2005.08.007. [DOI] [Google Scholar]
- Stylianou M.; Vyrides I.; Agapiou A. Oil biodesulfurization: A review of applied analytical techniques. J. Chromatogr. B 2021, 1171, 122602. 10.1016/j.jchromb.2021.122602. [DOI] [PubMed] [Google Scholar]
- Rezvani M. A.; Shokri Aghbolagh Z.; Hosseini Monfared H.; Khandan S. Mono Mn(II)-substituted phosphotungstate@modified graphene oxide as a high-performance nanocatalyst for oxidative demercaptanization of gasoline. J. Ind. Eng. Chem. 2017, 52, 42–50. 10.1016/j.jiec.2017.03.021. [DOI] [Google Scholar]
- Baaqel H.; Hallett J. P.; Guillén-Gosálbez G.; Chachuat B.; Chachuat B. Sustainability assessment of alternative synthesis routes to aprotic ionic liquids: The case of 1-Butyl-3-Methylimidazolium Tetrafluoroborate for fuel desulfurization. ACS Sustainable Chem. Eng. 2022, 10, 323–331. 10.1021/acssuschemeng.1c06188. [DOI] [Google Scholar]
- Singh S.; Srivastava V. C.; Gautam S. Oxidative-extractive desulfurization of liquid fuel by dimethyl sulfoxide and ZnCl2 based ionic liquid. Int. J. Chem. React. Eng. 2016, 14, 539–545. 10.1515/ijcre-2015-0026. [DOI] [Google Scholar]
- Dehghan R.; Anbia M. Zeolites for adsorptive desulfurization from fuels: A review. Fuel Process. Technol. 2017, 167, 99–116. 10.1016/j.fuproc.2017.06.015. [DOI] [Google Scholar]
- Dong L.; Miao G.; Ren X.; Liao N.; Anjum A. W.; Li Z.; Xiao J. Desulfurization kinetics and regeneration of silica gel-supported TiO2 extrudates for reactive adsorptive desulfurization of real diesel. Ind. Eng. Chem. Res. 2020, 59, 10130–10141. 10.1021/acs.iecr.0c00942. [DOI] [Google Scholar]
- Srivastav A.; Srivastava V. C. Adsorptive desulfurization by activated alumina. J. Hazard. Mater. 2009, 170, 1133–1140. 10.1016/j.jhazmat.2009.05.088. [DOI] [PubMed] [Google Scholar]
- Yaseen M.; Ullah S.; Ahmad W.; Subhan S.; Subhan F. Fabrication of Zn and Mn loaded activated carbon derived from corn cobs for the adsorptive desulfurization of model and real fuel oils. Fuel 2021, 284, 119102. 10.1016/j.fuel.2020.119102. [DOI] [Google Scholar]
- Polikarpova P.; Akopyan A.; Shigapova A.; Glotov A.; Anisimov A.; Karakhanov E. Oxidative desulfurization of fuels using heterogeneous catalysts based on MCM-41. Energy Fuels 2018, 32, 10898–10903. 10.1021/acs.energyfuels.8b02583. [DOI] [Google Scholar]
- Alba A. R.; Companyó X.; Rios R. Sulfones: new reagents in organocatalysis. Chem. Soc. Rev. 2010, 39, 2018–2033. 10.1039/B911852G. [DOI] [PubMed] [Google Scholar]
- Eseva E. A.; Akopyan A. V.; Anisimov A. V.; Maksimov A. L. Oxidative desulfurization of hydrocarbon feedstock using oxygen as oxidizing agent: A Review. Pet. Chem. 2020, 60, 979–990. 10.1134/S0965544120090091. [DOI] [Google Scholar]
- Akopyan A. V.; Grigoriev D. A.; Polikarpova P. L.; Eseva E. A.; Litvinova V. V.; Anisimov A. V. Ozone-assisted oxidative desulfurization of light oil fractions. Pet. Chem. 2017, 57, 904–907. 10.1134/S0965544117100024. [DOI] [Google Scholar]
- Fraile M.; Gil C.; Mayoral J. A.; Muel B.; Roldán L.; Vispe E.; Calderón S.; Puente F. Heterogeneous titanium catalysts for oxidation of dibenzothiophene in hydrocarbon solutions with hydrogen peroxide: On the road to oxidative desulfurization. Bull. Chem. React. Eng. Catal. 2016, 180, 680–686. 10.1016/j.apcatb.2015.07.018. [DOI] [Google Scholar]
- Kirihara M.; Suzuki K.; Nakakura K.; Saito K.; Nakamura R.; Tujimoto K.; Sakamoto Y.; Kikkawa Y.; Shimazu H.; Kimura Y. Oxidation of fluoroalkyl alcohols using sodium hypochlorite pentahydrate. J. Fluorine Chem. 2021, 243, 109719. 10.1016/j.jfluchem.2020.109719. [DOI] [Google Scholar]
- Subhan S.; Muhammad Y.; Sahibzada M.; Subhan F.; Tong Z. Studies on the selection of a catalyst–oxidant system for the energy-efficient desulfurization and denitrogenation of fuel oil at mild operating conditions. Energy Fuels 2019, 33, 8423–8439. 10.1021/acs.energyfuels.9b01950. [DOI] [Google Scholar]
- Subhan S.; Yaseen M.; Ahmad B.; Tong Z.; Subhan F.; Ahmad W.; Sahibzada M. Fabrication of MnO2 NPs incorporated UiO-66 for the green and efficient oxidative desulfurization and denitrogenation of fuel oils. J. Environ. Chem. Eng. 2021, 9, 105179. 10.1016/j.jece.2021.105179. [DOI] [Google Scholar]
- Teixeira J.; Silva A. R.; Branco L. C.; Afonso C. A. M.; Freire C. Asymmetric alkene epoxidation by Mn(III)salen catalyst in ionic liquids. Inorg. Chim. Acta 2010, 363, 3321–3329. 10.1016/j.ica.2010.06.018. [DOI] [Google Scholar]
- Okita Y.; Saito T.; Isogai A. TEMPO-mediated oxidation of softwood thermomechanical Pulp. Holzforschung 2009, 63, 529–535. 10.1515/HF.2009.096. [DOI] [Google Scholar]
- Vanier N. L.; El Halal S. L. M.; Dias A. R. G.; da Rosa Zavareze E. Molecular structure, functionality and applications of oxidized starches: A review. Food Chem. 2017, 221, 1546–1559. 10.1016/j.foodchem.2016.10.138. [DOI] [PubMed] [Google Scholar]
- Yaseen M.; Subhan S.; Subhan F.; Ur Rahman A.; Naeem A.; Ahmad Z.; Tong Z. Hausmannite Mn3O4 functionalized graphene Oxide-NaClO system for oxidative desulfurization and denitrogenation of fuel oils. Fuel 2022, 321, 124017. 10.1016/j.fuel.2022.124017. [DOI] [Google Scholar]
- Zhao R.; Tang Y.; Wei S.; Xu X.; Shi X.; Zhang G. Electrosynthesis of sodium hypochlorite in room temperature ionic liquids and in situ electrochemical epoxidation of olefins. Mech. Catal. 2012, 106, 37–47. 10.1007/s11144-011-0403-3. [DOI] [Google Scholar]
- Emmanuel E.; Keck G.; Blanchard J. M.; Vermande P.; Perrodin Y. Toxicological effects of disinfections using sodium hypochlorite on aquatic organisms and its contribution to AOX formation in hospital wastewater. Environ. Int. 2004, 30, 891–900. 10.1016/j.envint.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Subhan S.; Ur Rahman A.; Yaseen M.; Ur Rashid H.; Ishaq M.; Sahibzada M.; Tong Z. Ultra-fast and highly efficient catalytic oxidative desulfurization of dibenzothiophene at ambient temperature over low Mn loaded Co-Mo/Al2O3 and Ni-Mo/Al2O3 catalysts using NaClO as oxidant. Fuel 2019, 237, 793–805. 10.1016/j.fuel.2018.10.067. [DOI] [Google Scholar]
- Li A.; Song H.; Meng H.; Lu Y.; Li C. Ultrafast desulfurization of diesel oil with ionic liquid based PMoO catalysts and recyclable NaClO oxidant. Chem. Eng. J. 2020, 380, 122453. 10.1016/j.cej.2019.122453. [DOI] [Google Scholar]
- Li H.; Wu Y.; Xu Z.; Wang Y. In situ anchoring Cu nanoclusters on Cu-MOF: A new strategy for a combination of catalysis and fluorescence toward the detection of H2O2 and 2, 4-DNP. Chem. Eng. J. 2024, 479, 147508. 10.1016/j.cej.2023.147508. [DOI] [Google Scholar]
- Zheng Y.; Liu Y.; Guo X.; Chen Z.; Zhang W.; Wang Y.; Tang X.; Zhang Y.; Zhao Y. Sulfur-doped g-C3N4/rGO porous nanosheets for highly efficient photocatalytic degradation of refractory contaminants. J. Mater. Sci. Technol. 2020, 41, 117–126. 10.1016/j.jmst.2019.09.018. [DOI] [Google Scholar]
- Rozali N. L.; Azizan K. A.; Singh R.; Syed Jaafar S. N.; Othman A.; Weckwerth W.; Ramli U. S. Fourier transform infrared (FTIR) spectroscopy approach combined with discriminant analysis and prediction model for crude palm oil authentication of different geographical and temporal origins. Food Control 2023, 146, 109509. 10.1016/j.foodcont.2022.109509. [DOI] [Google Scholar]
- Alisha G. D.; Trisunaryanti W.; Syoufian A.; Larasati S. Synthesis of high stability Mo/SiO2 catalyst utilizing Parangtritis beach sand for hydrocracking waste palm oil into biofuel. Biomass Convers. Biorefin. 2023, 13, 11041–11055. 10.1007/s13399-021-02064-x. [DOI] [Google Scholar]
- Murgia V.; Torres E. M. F.; Gottifredi J. C.; Sham E. L. Sol–gel synthesis of V2O5–SiO2 catalyst in the oxidative dehydrogenation of n-butane. Appl. Catal., A 2006, 312, 134–143. 10.1016/j.apcata.2006.06.042. [DOI] [Google Scholar]
- Kong L.; Liu Y.; Dong L.; Zhang L.; Qiao L.; Wang W.; You H. Enhanced red luminescence in CaAl 12 O 19: Mn 4+ via doping Ga 3+ for plant growth lighting. Dalton Trans. 2020, 49, 1947–1954. 10.1039/C9DT04086B. [DOI] [PubMed] [Google Scholar]
- Haw K. G.; Bakar W. A.; Ali R.; Chong J.; Kadir A. Catalytic oxidative desulfurization of diesel utilizing hydrogen peroxide and functionalized-activated carbon in a biphasic diesel–acetonitrile system. Fuel Process. Technol. 2010, 91, 1105–1112. 10.1016/j.fuproc.2010.03.021. [DOI] [Google Scholar]
- Tian Y.; Yao Y.; Zhi Y.; Yan L.; Lu S. Combined extraction–oxidation system for oxidative desulfurization (ODS) of a model fuel. Energy Fuels 2015, 29, 618–625. 10.1021/ef502396b. [DOI] [Google Scholar]
- Akopyan A. V.; Shlenova A. O.; Cherednichenko K. A.; Polikarpova P. D. Immobilized multifunctional ionic liquids for highly efficient oxidation of sulfur-containing compounds in model fuels. Energy Fuels 2021, 35, 6755–6764. 10.1021/acs.energyfuels.1c00172. [DOI] [Google Scholar]
- Houda S.; Lancelot C.; Blanchard P.; Poinel L.; Lamonier C. Oxidative desulfurization of heavy oils with high sulfur content: A review. Catalysts 2018, 8, 344–369. 10.3390/catal8090344. [DOI] [Google Scholar]
- Yansheng C.; Changping L.; Qingzhu J.; Qingshan L.; Peifang Y.; Xiumei L.; Welz-Biermann U. Desulfurization by oxidation combined with extraction using acidic room temperature ionic liquids. Green Chem. 2011, 13, 1224. 10.1039/C0GC00745E. [DOI] [Google Scholar]