Abstract
Background:
Concern has been expressed by some that sodium reduction could lead to increased prevalence of hyponatremia and hyperkalemia for specific population subgroups. Current concentrations of serum sodium and potassium in the US population can help address this concern.
Methods:
We used data from the National Health and Nutrition Examination Survey 2009–2016 to examine mean and selected percentiles of serum sodium and potassium by sex and age group among 25 520 US participants aged 12 years or older. Logistic regression models with predicted residuals were used to examine the age-adjusted prevalence of low serum sodium and high serum potassium among adults aged 20 or older by selected sociodemographic characteristics and by health conditions or medication use.
Results:
The distributions of serum sodium and potassium concentrations were within normal reference intervals overall and across Dietary Reference Intake life-stage groups, with a few exceptions. Overall, 2% of US adults had low serum sodium (<135 mmol/L) and 0.6% had high serum potassium (>5 mmol/L). Prevalence of low serum sodium and high serum potassium was higher among adults aged 71 or older (4.7 and 2.0%, respectively) and among adults with chronic kidney disease (3.4 and 1.9%), diabetes (5.0 and 1.1%), or using certain medications (which varied by condition), adjusted for age; whereas, prevalence was <1% among adults without these conditions or medications.
Conclusions:
Most of the US population has normal serum sodium and potassium concentrations; these data describe population subgroups at higher risk of low serum sodium and high serum potassium and can inform clinical care.
INTRODUCTION
Sodium and potassium are essential electrolytes for human physiological function. However, evidence shows that dietary sodium intake is too high in the US, and reductions would lower the risk of hypertension and cardiovascular diseases such as heart disease and stroke, leading causes of death (1, 2). Because most of US adults’ dietary sodium intake comes from sodium added to commercially processed food, (3) public health agencies recommend gradual, widespread sodium reduction in the US food supply. Sodium reduction strategies include product reformulation such as increased use of salt substitutes/enhancers, particularly potassium chloride. Large, rapid reductions in dietary sodium intake, to levels below sodium recommendations (i.e., <1500 mg) for the US population, (1) can result in statistically significant declines in mean serum sodium levels (4–7). As a result, some researchers have concerns (8, 9) that population sodium reduction could result in hyponatremia and hyperkalemia, electrolyte disorders that can be fatal (10, 11). Current data on the distributions of serum sodium and potassium in the general US population are lacking (12–14) and could provide baseline data to monitor concerns and inform sodium reduction strategies and clinical practice. Changes to serum levels do not occur with a more moderate and gradual reduction in dietary sodium intake or increase in dietary potassium intake from foods because compensatory responses in the kidneys ensure serum osmolality is maintained (15, 16). For this reason, serum levels are not used as indicators of sodium deficiency and potassium excess (1). Impaired serum sodium and potassium could result, however, from a number of clinical conditions (e.g., chronic kidney disease) or medications (e.g., antihypertensive medications) (17–20). Clinical management of hyponatremia and hyperkalemia requires diagnosis and treatment of the underlying cause(s) (17, 18).
Updated estimates of the prevalence of low levels of serum sodium and high levels of potassium, especially among subgroups at risk for hyponatremia and hyperkalemia, can help clinicians put individual patient levels in the context of the US population. Thus, our primary objective was to describe current mean and selected percentiles of serum sodium and potassium concentrations in the US population. Our secondary objectives were to describe among US adults (a) the mean and selected percentiles of serum sodium and potassium by demographic characteristics, chronic health conditions, and medication use and (b) the prevalence of low serum sodium and high serum potassium in the overall population and by these subgroups.
MATERIALS AND METHODS
Survey Design and Study Participants
We used data from the National Health and Nutrition Examination Survey (NHANES), a complex, multistage survey of the noninstitutionalized US civilian population. NHANES protocols have been approved by the National Center for Health Statistics Ethics Review Board, and participants have provided written informed consent (parental consent was obtained for participants <18 years). Data from NHANES is publicly released in 2-year cycles, and survey methods and procedures have been described fully elsewhere (21). For this analysis, we used data from participants aged 12 years or older who participated in NHANES cycles 2009–2010 through 2015–2016. Participants missing serum sodium/potassium measurements or females whose pregnancy status was uncertain at the exam or who responded “I don’t know” or “refused” on lactating status were excluded from all analyses, leaving 25 520 participants for our main analyses.
Serum Sodium and Potassium Concentrations
Serum samples for sodium and potassium measurement were obtained from participants aged 12 years or older in the Mobile Examination Center as part of the standard biochemistry profile. Detailed collection and processing methods can be found in the NHANES Laboratory procedure manuals (22). Specimens were then processed, stored, and shipped to the Collaborative Laboratory Services for analysis. The DxC800 system was used to measure serum sodium and potassium through indirect (or diluted) ion-selective electrode (ISE) methodology.
The analytical range for reportable samples was 100.0–200.0 mmol/L for sodium and 1.0–15.0 mmol/L for potassium (23, 24). The normal reference interval for serum sodium is 135–145 mmol/L (130–140 mmol/L for pregnant women) and for serum potassium is 3.5–5.0 mmol/L (12). Low serum sodium was defined as <135 mmol/L for adults and <130 mmol/L for pregnant women (plasma concentrations are lower during a normal pregnancy), and high serum potassium was defined as >5 mmol/L for adults (12). Because clinical symptoms usually occur with more severe hyponatremia and hyperkalemia, we analyzed prevalence at a range of concentrations of serum sodium (moderate and severe hyponatremia defined as serum sodium ≤130 and ≤125 mmol/L, respectively) and potassium (mild, moderate, and severe hyperkalemia defined as serum potassium ≥5.5, ≥6.0, and ≥6.5 mmol/L, respectively) in supplemental analyses (25, 26).
Covariates
Potential covariates examined included age, sex, race and Hispanic origin, smoking status, and body mass index. These variables may be associated with differences in serum sodium and potassium concentrations. Impaired sodium and/or potassium may arise from a number of medical conditions or prescription drugs (12, 17–20). Using NHANES questionnaire and laboratory data available for adults aged 20 or older, we identified participants with the following chronic conditions: heart failure, coronary heart disease (CHD), stroke, cardiovascular disease (CVD), chronic obstructive pulmonary disease (COPD), cancer, liver disease, hypertension, diabetes, thyroid dysfunction, hyperlipidemia, and chronic kidney disease (CKD). Information on prescription drug use during to the month before the survey was obtained from all participants. Reported medications were matched to drugs in the Lexicon Plus database (Cerner Multum, Inc.), a comprehensive database of all prescription and some nonprescription drug products available in the United States (27). The Multum Lexicon uses a 3-level nested category system that assigns a therapeutic classification to each drug and each ingredient of the drug [e.g., losartan has 2 classification levels: cardiovascular agents (level 1) and angiotensin-II inhibitors (level 2)]. We identified adults aged 20 or older who reported taking one or more medications in one of the therapeutic drug classes examined. Detailed definitions used to identify people with a chronic condition(s) and/or using medication(s) examined can be found in the online Data Supplement in Supplemental Table 1. We also evaluated the prevalence of low serum sodium and high serum potassium among participants without any chronic conditions and who were not taking any of the prescription medications in supplemental analyses.
Statistical Analyses
Statistical analyses were conducted using SAS callable SUDAAN, version 11 to account for the complex survey design. Eight-year sample weights, constructed from 2-year weights for participants examined in the Mobile Examination Center for NHANES 2009–2016 were used to account for unequal sampling probabilities, nonresponse, non-coverage, and sample design. The DESCRIPT procedure was used to produce mean, percentiles, and standard error estimates of serum sodium and potassium among participants aged 12 years or older and by sex and age group (28) and among participants aged 20 years or older by sociodemographic or health characteristics. Because of small sample sizes, analyses examining serum sodium and potassium among pregnant or lactating women were considered exploratory.
The CROSSTAB procedure was used to estimate the proportion of people aged 20 or older with low serum sodium or high serum potassium, overall and by subgroup. Since serum sodium and potassium concentrations increased with age in initial analyses, and medication use and medical conditions are more prevalent in older age groups, we adjusted for age to determine if there was a difference in the prevalence of low serum sodium and high serum potassium among people with and without chronic conditions or using prescription medications. Logistic regression models with predicted marginals were used to examine these differences adjusted for age (29). We also calculated unadjusted prevalence estimates of low serum sodium and high serum potassium among these subgroups. We defined unstable estimates as a relative standard error >30% but ≤40% and suppressed estimates as a relative standard error >40% (30). Reported P values are based on the Rao–Scott modified chi-square test. All tests were two-sided, and a P < 0.05 was considered significant.
RESULTS
The mean [95% confidence interval (CI)] serum sodium concentration for nonpregnant and nonlactating adults aged 19 or older (n = 21 092) was 139.2 (139.0, 139.4) mmol/L and for the small number of pregnant women (n = 235) was 137.1 (136.7, 137.5) mmol/L (Supplemental Table 1). The estimated means and percentiles of serum sodium concentration were within normal reference intervals overall and across subgroups examined, with a few exceptions (Fig. 1). First percentile estimates and 95% CIs of serum sodium concentrations for US males aged 31 or older (n = 8288) (133.1 mmol/L, 95% CI: 132.5, 133.7) and females aged 14 or older (n = 12 029) (134.1 mmol/L, 95% CI: 133.3, 134.9) were below the normal interval. Fifth percentile estimates of serum sodium for females 71 or older (n = 1562) (133.9 mmol/L, 95% CI: 133.3, 134.5) and males 51 or older (n = 4856) (134.5 mmol/L, 95% CI: 134.1, 134.9) also were below the normal interval. In exploratory analyses of pregnant NHANES participants (n = 235), 97.5th percentile estimates of serum sodium were above the normal interval (140.8 mmol/L, 95% CI: 140.0, 141.6).
Fig. 1.
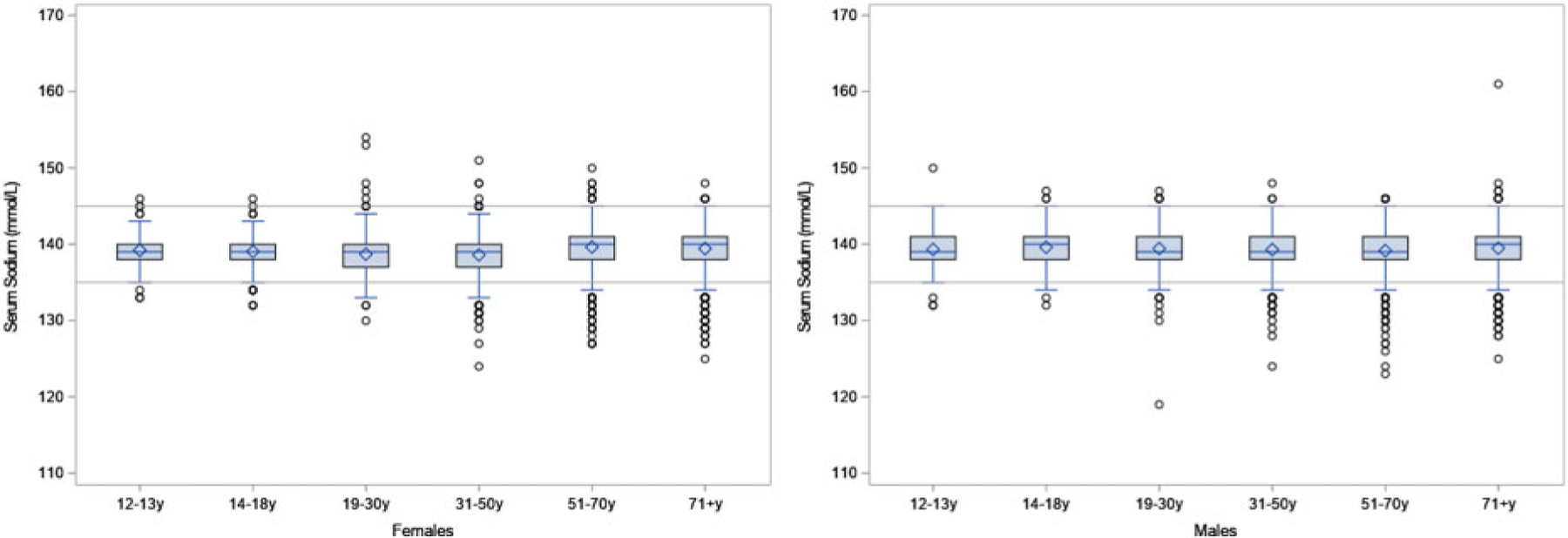
Boxplot of serum sodium (mmol/L) by sex and age group.
The mean (95% CI) serum potassium concentration for nonpregnant and nonlactating adults aged 19 or older (n = 21 092) was 3.98 (3.96, 4.00) mmol/L and for pregnant women (n = 235) was 3.76 (3.72, 3.80) mmol/L (Supplemental Table 2). The means and percentiles of serum potassium concentration were within normal reference intervals overall and across subgroups examined, with a few exceptions (Fig. 2). First percentile estimates of serum potassium for US males aged 19 or older (n = 21 092) (3.29 mmol/L, 95% CI: 3.21, 3.37) and females aged 14 or older (n = 12 029) (3.39 mmol/L, 95% CI: 3.31, 3.47) were below normal. Fifth percentile estimates of serum potassium for males aged 19–30 (n = 2189) (3.50 mmol/L, 95% CI: 3.50, 3.50) and females aged 31 or older (n = 8581) (3.40 mmol/L, 95% CI: 3.38, 3.42) were below normal. Tenth percentile estimates of serum potassium for pregnant women (n = 235) in NHANES were below normal. Ninety-ninth percentile estimates of serum potassium for males aged 71 or older (n = 1509) were above normal (5.35 mmol/L, 95% CI: 5.13, 5.57).
Fig. 2.
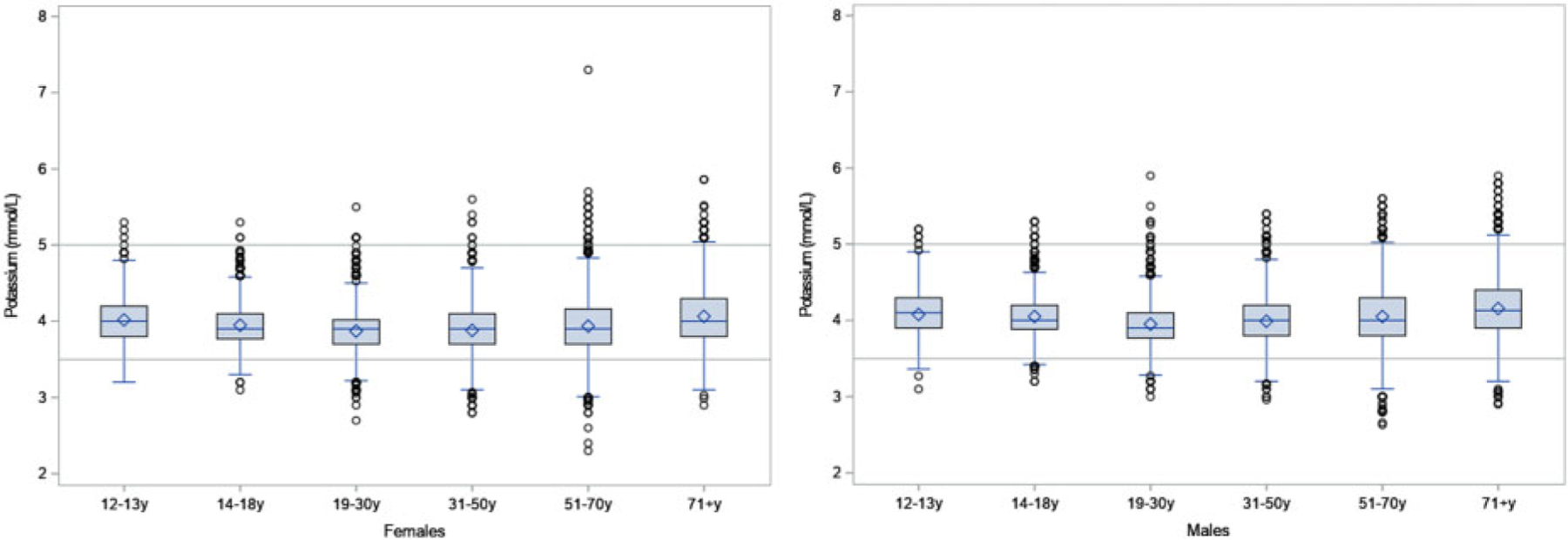
Boxplot of serum potassium (mmol/L) by sex and age group.
Overall, the unadjusted prevalence of low serum sodium was 2.3% among US adults aged 20 or older (n = 20 924) (Table 1) and was significantly higher among older age groups (P < 0.001) and among non-Hispanic whites (n = 8600) compared with non-Hispanic black (n = 4285) and Hispanic participants (n = 5375) (P = 0.04). Among adults with a health condition, the age-adjusted prevalence of low serum sodium was highest among those with diabetes (n = 3438, 5.0%), liver disease (n = 813, 4.3%), COPD (n = 1340, 3.9%), CKD (n = 3693, 3.4%), and hypertension (n = 10029, 3.2%) (Table 2). Adults with these conditions had significantly higher age-adjusted prevalence of low serum sodium compared to adults who did not report having the condition (P < 0.003). Adults taking one or more of the medications in the medication classes examined had a significantly higher age-adjusted prevalence of low serum sodium compared with adults not taking a medication, with the following exceptions: angiotensin-II receptor blockers, loop diuretics, proton pump inhibitors, and nonsteroidal antiinflammatory drugs. By types of medication, the age-adjusted prevalence of low serum sodium was highest among adults taking phenothiazine antipsychotics (n = 363, 6.9%), followed by tricyclic antidepressants (n = 224, 5.9%), potassium-sparing diuretics (n = 390, 5.2%, statistically unreliable), and thiazide diuretics (n = 2126, 4.2%). The age-adjusted prevalence of low serum sodium was also significantly higher among adults taking angiotensin-converting enzyme (ACE) inhibitors (n = 2918), selective serotonin receptor inhibitors (SSRIs) (n = 1420), beta blockers (n = 2583), combination antihypertensive medications (n = 1338), and calcium channel blockers (n = 1935) compared adults who did not report taking these medications. These results were similar to those seen in unadjusted analyses (Supplemental Table 4).
Table 1.
Mean (SE) and selected percentiles of serum sodium concentration (mmol/L) and prevalence of low serum sodium by demographic and health-related characteristics among adults aged 20 years or older, NHANES, 2009–2016 (N = 20 924).
| Demographic and health-related characteristics | Serum sodium mmol/L |
P valuea | |||||
|---|---|---|---|---|---|---|---|
| N | Mean | Percentiles |
Prevalence of low serum sodium (<135 mmol/L) | ||||
| 25th | 50th | 75th | |||||
| Overall | 20 924 | 139.2 (0.1) | 137.3 (0.1) | 138.7 (0.1) | 140.1 (0.1) | 2.3 (0.2) | <0.001 |
| Age group | <0.001 | ||||||
| 20–30 y | 3901 | 139.1 (0.1) | 137.3 (0.1) | 138.6 (0.1) | 139.9 (0.1) | 0.9 (0.3)* | |
| 31–50 y | 7198 | 138.9 (0.1) | 137.2 (0.1) | 138.5 (0.1) | 139.7 (0.1) | 2.0 (0.3) | |
| 51–70 y | 6754 | 139.4 (0.1) | 137.5 (0.1) | 139.1 (0.1) | 140.4 (0.1) | 2.6 (0.3) | |
| 71+ y | 3071 | 139.5 (0.1) | 137.5 (0.1) | 139.3 (0.1) | 140.7 (0.1) | 4.7 (0.4) | |
| Sex | 0.23 | ||||||
| Male | 10 199 | 139.3 (0.1) | 137.5 (0.1) | 138.9 (0.1) | 140.2 (0.1) | 2.2 (0.2) | |
| Female | 10 725 | 139.0 (0.1) | 137.2 (0.1) | 138.6 (0.1) | 140.0 (0.1) | 2.4 (0.2) | |
| Race and Hispanic originb | 0.04 | ||||||
| NH, White | 8600 | 139.2 (0.1) | 137.4 (0.1) | 138.8 (0.1) | 140.1 (0.1) | 2.4 (0.2) | |
| NH, Black | 4285 | 139.2 (0.1) | 137.4 (0.1) | 138.8 (0.1) | 140.2 (0.1) | 1.9 (0.3) | |
| Hispanic | 5375 | 139.0 (0.1) | 137.3 (0.1) | 138.6 (0.1) | 139.8 (0.1) | 2.0 (0.2) | |
| NH, Asian | 1874 | 139.0 (0.1) | 137.2 (0.1) | 138.5 (0.1) | 139.9 (0.1) | 2.2 (0.5) | |
| BMI | 0.06 | ||||||
| <25 kg/m2 | 6051 | 139.2 (0.1) | 137.4 (0.1) | 138.8 (0.1) | 140.1 (0.1) | 2.0 (0.3) | |
| 25–29.9 kg/m2 | 6794 | 139.2 (0.1) | 137.4 (0.1) | 138.8 (0.1) | 140.2 (0.1) | 2.1 (0.3) | |
| ≥30 kg/m2 | 7849 | 139.0 (0.1) | 137.2 (0.1) | 138.6 (0.1) | 140.0 (0.1) | 2.5 (0.3) | |
| Smoking status | 0.22 | ||||||
| Yes | 4223 | 139.0 (0.1) | 137.2 (0.1) | 138.6 (0.1) | 139.9 (0.1) | 3.0 (0.5) | |
| No | 4948 | 139.2 (0.1) | 137.3 (0.1) | 138.8 (0.1) | 140.2 (0.1) | 2.3 (0.2) | |
Abbreviations. NH, non-Hispanic; SE, standard error.
The reported P value is from the Rao–Scott chi-squared test. P < 0.05 is considered significant.
Data on NH, Asian is from NHANES 2011–2016 and was not evaluated in 2009–2010 and data on NH, other is not presented (n = 2664). The P value assesses the difference in the prevalence of persons with low serum sodium among NH, White, NH, Black, and Hispanics.
Table 2.
Mean (SE) and selected percentiles of serum sodium concentration (mmol/L) and age-adjusted prevalence of low serum sodium by chronic health conditions and selected medications among adults aged 20 years or older, NHANES, 2009–2016 (N = 20 924).
| Conditions and health-related medication use | Serum sodium mmol/L |
P valuea | |||||
|---|---|---|---|---|---|---|---|
| N | Mean | Percentiles |
Age-adjusted prevalence of low serum sodium (<135 mmol/L) | ||||
| 25th | 50th | 75th | |||||
| Health conditions (yes) | |||||||
| Hypertension | 10 029 | 139.1 (0.1) | 137.2 (0.1) | 138.8 (0.1) | 140.2 (0.1) | 3.2 (0.3) | <0.001 |
| Chronic kidney disease | 3693 | 139.1 (0.1) | 137.2 (0.1) | 138.8 (0.1) | 140.4 (0.1) | 3.4 (0.5) | <0.001 |
| Diabetes mellitus | 3438 | 138.6 (0.1) | 136.6 (0.2) | 138.4 (0.2) | 139.8 (0.2) | 5.0 (0.7) | <0.001 |
| Cardiovascular disease | 2199 | 139.3 (0.1) | 137.2 (0.1) | 138.9 (0.1) | 140.6 (0.1) | 2.7 (0.4) | 0.19 |
| Thyroid problem | 2122 | 139.3 (0.1) | 137.3 (0.1) | 138.9 (0.1) | 140.4 (0.1) | 2.5 (0.4) | 0.46 |
| Cancer | 1927 | 139.4 (0.1) | 137.5 (0.2) | 139.1 (0.1) | 140.5 (0.1) | 2.1 (0.3) | 0.61 |
| Coronary heart disease | 1467 | 139.3 (0.1) | 137.2 (0.2) | 138.9 (0.1) | 140.6 (0.1) | 2.8 (0.4) | 0.18 |
| Chronic obstructive pulmonary disease | 1340 | 139.0 (0.1) | 137.1 (0.2) | 138.8 (0.1) | 140.3 (0.1) | 3.9 (0.6) | <0.001 |
| Liver Disease | 813 | 138.7 (0.2) | 136.6 (0.2) | 138.4 (0.2) | 139.8 (0.2) | 4.3 (0.9) | 0.003 |
| Stroke | 759 | 139.2 (0.1) | 137.2 (0.2) | 138.8 (0.1) | 140.5 (0.2) | 2.1 (0.5) | 0.66 |
| Hyperlipidemia | 740 | 139.4 (0.1) | 137.5 (0.2) | 139.1 (0.2) | 140.5 (0.1) | 2.8 (0.9)* | 0.49 |
| Heart failure | 671 | 139.1 (0.2) | 137.1 (0.2) | 138.7 (0.2) | 140.4 (0.3) | 2.5 (0.5) | 0.67 |
| Medication use | |||||||
| Anti-HTN medication(s)b | 6760 | 139.1 (0.1) | 137.1 (0.1) | 138.8 (0.1) | 140.3 (0.1) | 3.7 (0.5) | <0.001 |
| ACE inhibitors | 2918 | 138.9 (0.1) | 136.7 (0.1) | 138.6 (0.1) | 140.1 (0.1) | 3.9 (0.6) | <0.001 |
| Beta-blockersc | 2583 | 139.1 (0.1) | 137.1 (0.1) | 138.9 (0.1) | 140.5 (0.1) | 3.3 (0.6) | 0.01 |
| Thiazide diuretics | 2126 | 138.7 (0.1) | 136.7 (0.1) | 138.5 (0.1) | 139.9 (0.1) | 4.2 (0.6) | <0.001 |
| Calcium channel blockers | 1935 | 139.3 (0.1) | 137.3 (0.1) | 139.0 (0.1) | 140.5 (0.1) | 3.0 (0.5) | 0.04 |
| ARBs | 1561 | 139.2 (0.1) | 137.3 (0.1) | 138.9 (0.1) | 140.4 (0.1) | 2.4 (0.5) | 0.79 |
| Combinationd | 1338 | 138.8 (0.1) | 136.7 (0.1) | 138.5 (0.1) | 140.0 (0.1) | 3.7 (0.7) | 0.01 |
| Loop diuretics | 692 | 139.4 (0.1) | 137.3 (0.1) | 138.9 (0.2) | 140.6 (0.2) | 1.9 (0.5) | 0.57 |
| Potassium-sparing diuretics | 390 | 138.4 (0.2) | 136.6 (0.2) | 138.1 (0.2) | 139.7 (0.3) | 5.2 (1.8)* | 0.01 |
| Antidepressantse | 2229 | 139.0 (0.1) | 137.1 (0.1) | 138.6 (0.1) | 140.0 (0.1) | 3.5 (0.5) | 0.001 |
| SSRI | 1420 | 139.0 (0.1) | 137.1 (0.1) | 138.7 (0.1) | 140.0 (0.1) | 3.8 (0.7) | 0.005 |
| Tricyclic | 224 | 138.6 (0.3) | 136.4 (0.4) | 138.4 (0.4) | 140.0 (0.2) | 5.9 (1.6) | 0.001 |
| Proton pump inhibitors | 1955 | 139.2 (0.1) | 137.3 (0.1) | 138.9 (0.1) | 140.2 (0.1) | 2.7 (0.4) | 0.18 |
| NSAIDs | 1114 | 139.1 (0.1) | 137.2 (0.2) | 138.7 (0.1) | 140.1 (0.2) | 2.5 (0.5) | 0.57 |
| Phenothiazine antipsychotics | 363 | 138.8 (0.2) | 137.2 (0.2) | 138.7 (0.2) | 140.0 (0.2) | 6.9 (1.9) | <0.001 |
Abbreviations. ACE, angiotensin-converting enzyme; ARB, angiotensin-II receptor blockers; HTN, hypertension; NSAID, nonsteroidal antiinflammatory drugs; SE, standard error; SSRI, selective serotonin receptor inhibitors.
30<RSE ≤ 40; RSE > 40 suppressed according to NCHS standards.
The reported P value is from the Rao–Scott chi-squared test. P < 0.05 is considered significant.
Adults who reported taking at least one blood pressure medication in one of the following drug/ingredient therapeutic categories: antihypertensive emergency agents, diuretics, vasodilators, antiadrenergic agents (peripherally or centrally acting), beta blockers, calcium channel blockers, ACE inhibitors, aldosterone receptor antagonists, renin inhibitors, or a combination blood pressure drugs.
All beta-adrenergic agents reported were beta blockers.
A combination antihypertensive medication is a multiple ingredient drug that contains more than one therapeutic class drug with different modes of action (e.g., ACE inhibitors with thiazides or ACE inhibitors with calcium channel blocking agents).
Adults who reported taking at least one antidepressant in one of the following therapeutic drug-class categories: miscellaneous, SSRI, tricyclic, monoamine oxidase inhibitors (MOI), phenylpiperazine, tetracyclic, and selective serotonin-norepinephrine reuptake inhibitor antidepressants.
Overall, the unadjusted prevalence of high serum potassium among US adult aged 20 or older was 0.62% (n = 200 924) (Table 3). The prevalence of high serum potassium was significantly higher among older age groups (n = 3071) P < 0.001) and among non-Hispanic whites (n = 8600) compared to non-Hispanic Black (n = 4285) and Hispanic participants (n = 5375) (P = 0.04). Among adults with a health condition, the age-adjusted prevalence of high serum potassium was highest among those with CKD (n = 3693, 1.86%), stroke (n = 759, 1.54%), diabetes (n = 3438, 1.10%), and heart failure (n = 671, 1.04%) (Table 4). Adults with CKD, diabetes, CVD, and stroke had significantly higher age-adjusted prevalence of high serum potassium compared to adults who did not report having one of these conditions (P < 0.004). The age-adjusted prevalence of high serum potassium was highest among adults taking loop diuretics (n = 692, 1.31%), followed by beta blockers (n = 2583, 1.11%) and ACE inhibitors (n = 2918, 1.00%), and was significantly higher than that of adults who did not report taking at least one medication in these classes of medications. These results were similar to those seen in unadjusted analyses (Supplemental Table 4).
Table 3.
Mean (SE) and selected percentiles of serum potassium concentration (mmol/L) and the prevalence of high serum potassium by demographic and health-related characteristics among adults aged 20 years or older, NHANES, 2009–2016 (N = 20 924).
| Demographic and health-related characteristics | Serum potassium mmol/L |
P valuea | |||||
|---|---|---|---|---|---|---|---|
| N | Mean | Percentiles |
Prevalence of high serum potassium (>5.0 mmol/L) | ||||
| 25th | 50th | 75th | |||||
| Overall | 20 924 | 3.98 (0.01) | 3.79 (0.01) | 3.99 (0.01) | 4.19 (0.00) | 0.62 (0.09) | <0.001 |
| Age group | <0.001 | ||||||
| 20–30 y | 3901 | 3.92 (0.01) | 3.70 (0.00) | 3.90 (0.00) | 4.09 (0.00) | 0.40 (0.13)* | |
| 31–50 y | 7198 | 3.94 (0.01) | 3.74 (0.02) | 3.90 (0.02) | 4.10 (0.01) | 0.23 (0.07)* | |
| 51–70 y | 6754 | 4.01 (0.01) | 3.79 (0.00) | 4.00 (0.00) | 4.20 (0.01) | 0.70 (0.17) | |
| 71+ y | 3071 | 4.10 (0.01) | 3.80 (0.02) | 4.09 (0.01) | 4.30 (0.03) | 2.02 (0.32) | |
| Sex | 0.21 | ||||||
| Male | 10 199 | 4.03 (0.01) | 3.80 (0.00) | 4.00 (0.00) | 4.20 (0.00) | 0.68 (0.12) | |
| Female | 10 725 | 3.93 (0.01) | 3.70 (0.00) | 3.90 (0.00) | 4.10 (0.00) | 0.56 (0.09) | |
| Race and Hispanic originb | 0.04 | ||||||
| NH, White | 8600 | 4.00 (0.01) | 3.79 (0.00) | 3.99 (0.00) | 4.20 (0.00) | 0.71 (0.12) | |
| NH, Black | 4285 | 3.93 (0.01) | 3.70 (0.00) | 3.90 (0.00) | 4.10 (0.02) | 0.51 (0.11) | |
| Hispanic | 5375 | 3.93 (0.01) | 3.70 (0.00) | 3.90 (0.00) | 4.10 (0.00) | 0.46 (0.12) | |
| NH, Asian | 1874 | 3.89 (0.01) | 3.69 (0.00) | 3.89 (0.01) | 4.09 (0.01) | 0.19 (0.09) | |
| BMI | 0.44 | ||||||
| <25 kg/m2 | 6051 | 3.95 (0.01) | 3.70 (0.00) | 3.90 (0.01) | 4.11 (0.02) | 0.73 (0.14) | |
| 25–29.9 kg/m2 | 6794 | 3.98 (0.01) | 3.79 (0.02) | 3.99 (0.01) | 4.19 (0.01) | 0.52 (0.13) | |
| ≥30 kg/m2 | 7849 | 4.00 (0.01) | 3.79 (0.00) | 3.99 (0.00) | 4.19 (0.00) | 0.56 (0.13) | |
| Smoking status | 0.22 | ||||||
| Yes | 4223 | 3.99 (0.01) | 3.79 (0.01) | 3.99 (0.01) | 4.19 (0.00) | 0.50 (0.12) | |
| No | 4948 | 4.02 (0.01) | 3.80 (0.00) | 4.00 (0.00) | 4.20 (0.01) | 0.72 (0.15) | |
Abbreviations. NH, non-Hispanic; SE, standard error. *30<RSE ≤ 40; RSE > 40 suppressed according to NCHS recommendations.
The reported P value is from the Rao–Scott chi-squared test. P < 0.05 is considered significant.
Data on NH, Asian is from NHANES 2011–2016 and was not evaluated in 2009–2010 and data on NH, other is not presented (N = 2664). The P value assesses the difference in the prevalence of persons with low serum sodium among NH, White, NH, Black, and Hispanics.
Table 4.
Mean (SE) and selected percentiles of serum potassium concentration (mmol/L) and the age-adjusted prevalence of high serum potassium by chronic health conditions and selected medications among adults aged 20 years or older, NHANES, 2009–2016 (N = 20 954).
| Conditions and health-related medication use | Serum potassium mmol/L |
P valuea | |||||
|---|---|---|---|---|---|---|---|
| N | Mean | Percentiles |
Age-adjusted prevalence of high serum potassium (>5.0 mmol/L) | ||||
| 25th | 50th | 75th | |||||
| Health conditions (yes) | |||||||
| Hypertension | 10 029 | 3.99 (0.01) | 3.78 (0.02) | 3.99 (0.01) | 4.20 (0.00) | 0.68 (0.11) | 0.12 |
| Chronic kidney disease | 3693 | 4.08 (0.01) | 3.80 (0.00) | 4.00 (0.03) | 4.30 (0.00) | 1.86 (0.30) | <0.001 |
| Diabetes mellitus | 3438 | 4.08 (0.01) | 3.80 (0.03) | 4.09 (0.01) | 4.29 (0.00) | 1.10 (0.19) | 0.001 |
| Cardiovascular disease | 2199 | 4.09 (0.01) | 3.80 (0.02) | 4.09 (0.02) | 4.30 (0.00) | 1.04 (0.22) | 0.004 |
| Thyroid | 2122 | 3.98 (0.01) | 3.75 (0.02) | 3.99 (0.02) | 4.19 (0.00) | 0.48 (0.10) | 0.55 |
| Cancer | 1927 | 4.04 (0.01) | 3.79 (0.00) | 4.00 (0.01) | 4.25 (0.03) | 0.56 (0.11) | 0.60 |
| Coronary heart disease | 1467 | 4.09 (0.01) | 3.87 (0.02) | 4.09 (0.01) | 4.30 (0.00) | 0.72 (0.21) | 0.54 |
| Chronic obstructive pulmonary disease | 1340 | 4.01 (0.02) | 3.79 (0.01) | 3.99 (0.01) | 4.20 (0.03) | 0.45 (0.14)* | 0.33 |
| Stroke | 759 | 4.06 (0.02) | 3.78 (0.02) | 4.00 (0.03) | 4.29 (0.04) | 1.54 (0.43) | 0.001 |
| Heart failure | 671 | 4.12 (0.02) | 3.82 (0.03) | 4.10 (0.03) | 4.33 (0.04) | 1.04 (0.33) | 0.08 |
| Medication use | |||||||
| Anti-HTN medication(s)b | 6760 | 4.01 (0.01) | 3.78 (0.02) | 4.00 (0.00) | 4.24 (0.02) | 0.80 (0.13) | 0.052 |
| ACE inhibitors | 2918 | 4.06 (0.01) | 3.80 (0.00) | 4.04 (0.02) | 4.29 (0.00) | 1.00 (0.18) | 0.004 |
| Beta-blockersc | 2583 | 4.08 (0.01) | 3.80 (0.01) | 4.09 (0.01) | 4.30 (0.00) | 1.12 (0.22) | 0.001 |
| Thiazide diuretics | 2126 | 3.84 (0.01) | 3.59 (0.01) | 3.80 (0.02) | 4.02 (0.03) | 0.63 (0.17) | 0.95 |
| Calcium channel blockers | 1935 | 3.99 (0.01) | 3.70 (0.03) | 3.99 (0.02) | 4.20 (0.03) | 0.98 (0.26) | 0.052 |
| ARBs | 1561 | 3.99 (0.02) | 3.70 (0.03) | 3.99 (0.02) | 4.20 (0.02) | 0.58 (0.18)* | 0.81 |
| Combinationd | 1338 | 3.90 (0.02) | 3.64 (0.03) | 3.89 (0.02) | 4.10 (0.03) | 0.64 (0.21)* | 0.92 |
| Loop diuretics | 692 | 4.09 (0.02) | 3.78 (0.02) | 4.07 (0.03) | 4.37 (0.03) | 1.31 (0.42) | 0.02 |
| Antidepressantse | 2229 | 3.99 (0.01) | 3.79 (0.01) | 3.99 (0.01) | 4.19 (0.00) | 0.53 (0.16)* | 0.58 |
| SSRI | 1420 | 4.00 (0.01) | 3.79 (0.00) | 3.99 (0.00) | 4.20 (0.00) | 0.68 (0.23)* | 0.75 |
| Proton pump inhibitors | 1959 | 4.02 (0.01) | 3.79 (0.01) | 4.00 (0.00) | 4.23 (0.02) | 0.69 (0.21)* | 0.12 |
Abbreviations. ACE, angiotensin-converting enzyme; ARB, angiotensin-II receptor blockers; HTN, hypertension; SE, standard error; SSRI, selective serotonin reuptake inhibitor.
30<RSE ≤ 40; RSE > 40 suppressed according to NCHS recommendations.
The reported P value is from the Rao–Scott chi-squared test. P < 0.05 is considered significant.
Adults who reported taking at least one blood pressure medication in one of the following drug/ingredient therapeutic categories: thiazide diuretics, loop diuretics, potassium-sparing diuretics, beta blockers, calcium channel blockers, ARBs, ACE inhibitors, aldosterone receptor antagonists, renin inhibitors, or a combination blood pressure drug.
All beta-adrenergic agents reported were beta blockers.
A combination antihypertensive medication is a multiple ingredient drug that contains more than one therapeutic class drug with different modes of action (e.g., ACE inhibitors with thiazides or ACE inhibitors with calcium channel blocking agents).
Adults who reported taking at least one antidepressant in one of the following therapeutic drug-class categories: miscellaneous, SSRI, tricyclic, monoamine oxidase inhibitor (MOI), phenylpiperazine, tetracyclic, and selective serotonin-norepinephrine reuptake inhibitor antidepressants.
Overall, the unadjusted prevalence of moderate hyponatremia (serum sodium ≤130 mmol/L) among US adults aged 20 or older (n = 20 924) was 0.28% (Supplemental Table 5). Adults with COPD (1.02%, statistically unreliable) or hypertension (0.38%) or who reported taking phenothiazine antipsychotics (2.23%, statistically unreliable), tricyclic antidepressants (1.16%), or thiazide diuretics (0.58%) had significantly higher age-adjusted prevalence of moderate hyponatremia compared to adults who did not report having these conditions or taking these medications (Supplemental Table 5). Overall, the unadjusted prevalence of mild hyperkalemia (≥5.5 mmol/L) among US adults aged 20 or older was 0.11% (Supplemental Table 5). Age-adjusted prevalence of mild hyperkalemia was significantly higher for adults with some conditions or taking some medications (hyperlipidemia, CKD, hypertension, tricyclic antidepressants, ACE inhibitors) compared to adults who did not report having these conditions or taking these medications; however, these results were statistically unreliable (Supplemental Table 5). Data on the prevalence of severe hyponatremia and moderate/severe hyperkalemia could not be presented because of statistical unreliability. Among the 6294 participants aged 20 years or older without chronic conditions or who were not taking any prescription medications, the unadjusted prevalence of serum sodium <135 mmol/L was 0.67% and serum potassium >5.0 mmol/L was 0.28% (Supplemental Table 6).
DISCUSSION
During 2009–2016, serum sodium and potassium concentrations were within normal reference intervals for most of the US population aged 12 years or older. Our results suggest that the prevalence of low serum sodium was <3% among adults aged 20 or older and <7% among adults with chronic conditions or using certain medications. The prevalence of high serum potassium was <1 and <2% among these groups, respectively. Prevalence estimates of low serum sodium and high serum potassium were <1% for healthy people (i.e., those without chronic conditions or who were not taking prescription medications) and prevalence of moderate hyponatremia or mild hyperkalemia were also <1% overall. Further, prevalence of low serum sodium and high serum potassium was higher among older age groups, among people with diabetes and CKD independent of age, and among people using certain medication classes, suggesting that these groups may be at higher risk for impaired serum sodium and/or potassium and may need individualized recommendations in the clinical setting.
Consistent with data from NHANES 1988–1994, mean and selected percentiles of serum sodium and potassium in the current analysis were within the normal interval (12). US males aged 71 or older had the highest mean and median serum potassium concentration of either sex and all age groups (4.16 vs. 4.26 mmol/L in 1988–1994). However, mean and percentile estimates of serum sodium and potassium concentrations in the present analysis were slightly lower than serum concentrations reported using data from NHANES 1988–1994 (~1–2 and ~0.1 mmol/L, respectively) (12). Lower concentrations of serum sodium and potassium could potentially be due to an instrument change from the Hitachi 737 used in NHANES III to the Beckman DxC800 used in NHANES 2009–2016. Although both instruments use ISE methodology, the College of American Pathologists Chemistry/Therapeutic Drug Monitoring Survey C-C from 2018 indicated that between platforms, serum potassium and sodium may have differences of ~0.2 and 5 mmol/L, respectively (31) (personal communication, Christine Pfeiffer, January 2019, CAP Chemistry PT Survey). Another possible explanation could be that the distributions of serum sodium and potassium have shifted over time among some subgroups. For example, among males aged 71 or older, serum sodium in the 10th percentile was 3.8 mmol/L less, the median was 2.3 mmol/L less, and the 90th percentile was 1.3 mmol/L less than concentrations reported in 1988–1994, whereas serum potassium in the 10th percentile was 0.22 mmol/L less, the median was 0.08 mmol/L less, and the 90th percentile was 0.02 mmol/L less than concentrations reported in 1988–1994 (12). Although the reason behind these shifts is not known, it may be related to changes in population characteristics (i.e., increases in chronic diseases such as CKD and diabetes or increases in use of prescription medications such as antihypertensive or antidepressant agents) (31–33).
Overall, ~2% of adults aged 20 or older had a low serum sodium, which is consistent with the prevalence reported in 1999–2004 among US adults aged 18 or older (1.72%), despite differences in definitions of low serum sodium and age of the population (13). In general, our results are consistent with this previous study in that adults who were older; were non-Hispanic white; had hypertension, diabetes, or COPD; or were taking thiazide diuretics, SSRIs, or antipsychotics had higher prevalence of low serum sodium (13). However, contrary to the previous study, we found that people with CKD or liver disease, or taking ACE inhibitors or tricyclic antidepressants also had a higher prevalence of low serum sodium, whereas people who had a stroke or CHD, or taking angiotensin-II receptor blockers (ARBs) did not. Several clinical conditions that predispose people to hyponatremia were observed in our study, including CKD, diabetes, COPD, and liver disease (12, 18, 26, 34, 35). Similarly, people taking antidepressants (e.g., tricyclic, SSRIs), antipsychotics, thiazide diuretics, and ACE inhibitors have been found to be at higher risk for hyponatremia (12, 19, 36, 37).
In our study, the prevalence of high serum potassium among adults aged 20 or older was lower than the prevalence reported among adult patients with health insurance using data obtained from the Marketscan database of patients with a serum potassium measurement between 2010 and 2014 (0.68% vs. 1.57% mmol/L) (14). Similar to the few studies that have evaluated prevalence of hyperkalemia in the US population and by sociodemographic and health characteristics, high serum potassium was higher for adults who were older and those with CKD, heart failure, or diabetes (14, 38). Further, consistent with a study evaluating the risk of hyperkalemia among certain medication classes, we found that people taking ACE inhibitors and beta blockers had a higher prevalence of high serum potassium than those not taking these medications (12, 20). Contrary to previous evidence, in our study people taking loop diuretics also had a higher prevalence of high serum potassium. While loop diuretics increase potassium excretion, people taking loop diuretics could have been at increased risk of hyperkalemia because they could have one or more comorbid conditions (e.g., heart failure, CKD, diabetes) and/or be using one or more medications (e.g., spironolactone, ACE inhibitor) associated with high serum potassium (39, 40).
Our study has some limitations. People who participated in NHANES may systematically differ from those who did not participate, although data were weighted to account for nonresponse. We examined low serum sodium or high serum potassium in our study because assessments of serum osmolality and volume status are needed to classify and differentially diagnose hyponatremia (18, 36), and repeated measurements on separate occasions are required to clinically diagnose hyperkalemia (25). This could result in misclassification of some participants in these analyses, potentially resulting in a higher proportion of participants with low serum sodium and high serum potassium concentrations based on a single measure. Further, we did not correct estimates of low serum sodium for potential cases of pseudo-hyponatremia or hyperglycemia as we could not determine the appropriate dilution factor to use to correct for elevated protein or lipid concentrations in using indirect ISE method (13). We could also not estimate instances of pseudohyperkalemia that could occur due to thrombocytosis, refrigeration during centrifugation of the blood sample, or due to temperature variations such as seasonality or geographic location (41). Some chronic conditions and medications were based on self-reported data and could be subject to response bias. We could not investigate several known causes of hyponatremia or hyperkalemia because appropriate measurements were not available in NHANES (e.g., syndrome of inappropriate antidiuretic hormone secretion, pituitary disorders, Addison disease, beer potomania syndrome, hypoaldosteronism) (18, 25, 36). We did not evaluate the association of single drugs with low serum or high serum potassium, nor did we evaluate the association of drug interactions (e.g., ACE inhibitors, ARBs, and/or renin inhibitors) or drug-disease interactions (e.g., CKD + potassium-sparing diuretics) (38) with high serum potassium. Last, to comply with National Center for Health Statistics reporting standards, (30) we suppressed the prevalence of low serum sodium among people who reported taking certain prescriptions because of small sample size and unreliable estimates.
CONCLUSION
To our knowledge, this is the first study to update distributions of serum sodium and potassium reported in the 2004 Dietary Reference Intake (12) and to examine prevalence of low serum sodium and high serum potassium among a representative US population (13, 14). While most participants had normal serum sodium and potassium concentrations, a small proportion of people may be at higher risk for low serum sodium and high serum potassium: those with conditions and/or taking medications that alter the homeostatic process, such as CKD, diabetes, heart failure, ACE inhibitors, beta blockers, diuretics, antidepressants, or antipsychotics. This study provides baseline data for monitoring potential adverse changes in prevalence of hyponatremia and hyperkalemia in the US population. As recommended by the National Academies of Science and Medicine, future research should focus on evaluating the effects of sodium reduction and potassium supplementation (at levels based on population sodium recommendations) on balance (i.e., intakes and losses), serum concentrations of sodium and potassium, and adverse outcomes (i.e., CVD, hypertension) in subpopulations prone to disorders of serum sodium and potassium (1).
Supplementary Material
IMPACT STATEMENT.
Hyponatremia and hyperkalemia (i.e., altered serum sodium and potassium concentrations) are electrolyte disorders that can be fatal. There is concern from some researchers that strategies to reduce population dietary sodium intake may result in hyponatremia and hyperkalemia. Additionally, imbalances in serum sodium and potassium could result from a number of clinical conditions or medications and some population subgroups may be more at risk for these disorders. Current data on the distributions of serum sodium and potassium in the general US population are lacking and could provide baseline data to monitor concerns, inform sodium reduction strategies, and clinical practice.
Nonstandard Abbreviations:
- NHANES
National Health and Nutrition Examination Survey
- US
United States
- CHD
coronary heart disease
- CVD
cardiovascular disease
- COPD
chronic obstructive pulmonary disease
- CKD
chronic kidney disease
- CI
confidence intervals
- ACE inhibitors
angiotensin-converting enzyme inhibitors
- ARB
angiotensin-II receptor blockers
- SSRI
selective serotonin receptor inhibitors
Footnotes
Publisher's Disclaimer: Disclaimer: The findings and conclusions presented in this article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC).
Authors’ Disclosures or Potential Conflicts of Interest: No authors declared any potential conflicts of interest.
Role of Sponsor: No sponsor was declared.
SUPPLEMENTAL MATERIAL
Supplemental material is available at The Journal of Applied Laboratory Medicine online.
REFERENCES
- 1.National Academies of Science, Engineering, and Medicine. Dietary reference intakes for sodium and potassium. Washington (DC): The National Academies Press; 2019. [PubMed] [Google Scholar]
- 2.Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, et al. Heart disease and stroke statistics—2018 update: a report from the American Heart Association. Circulation 2018;137:e67–492. [DOI] [PubMed] [Google Scholar]
- 3.Harnack LJ, Cogswell ME, Shikany JM, Gardner CD, Gillespie C, Loria CM, et al. Sources of sodium in US adults from 3 geographic regions. Circulation 2017;135: 1775–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.He FJ, Markandu ND, Sagnella GA, de Wardener HE, MacGregor GA. Plasma sodium ignored and underestimated. Hypertension 2005;45:98–102. [DOI] [PubMed] [Google Scholar]
- 5.Kawano Y, Yoshida K, Kawamura M, Yoshimi H, Ashida T, Abe H, et al. Sodium and noradrenaline in cerebrospinal fluid and blood in salt-sensitive and non-salt-sensitive essential hypertension. Clin Exp Pharmacol Physiol 1992; 19:235–41. [DOI] [PubMed] [Google Scholar]
- 6.Suckling RJ, He FJ, Markandu ND, MacGregor GA. Dietary salt influences postprandial plasma sodium concentration and systolic blood pressure. Kidney Int 2012;81:407–11. [DOI] [PubMed] [Google Scholar]
- 7.Kirkendell WM, Connor WE, Abboud F, Rastogi SP, Anderson TA, Fry M. The effect of dietary sodium chloride on blood pressure, body fluids, electrolytes, renal function, and serum lipids of normotensive man. J Lab Clin Med 1979;87:418–34. [PubMed] [Google Scholar]
- 8.Kovesdy CP, Appel LJ, Grams ME, Gutekunst L, McCullough PA, Palmer BF, et al. Potassium homeostasis in health and disease: a scientific workshop cosponsored by the National Kidney Foundation and the American Society of Hypertension. Am J Kidney Dis 2017;70:844–58. [DOI] [PubMed] [Google Scholar]
- 9.DiNicolantonio JJ, Niazi AK, Sadaf R, O’ Keefe JH, Lucan SC, Lavie CJ. Dietary sodium restriction: take it with a grain of salt. Am J Med 2013;126:951–5. [DOI] [PubMed] [Google Scholar]
- 10.Henry DA. In the clinic: hyponatremia. Ann Intern Med 2015;163:ITC1–19. [DOI] [PubMed] [Google Scholar]
- 11.Gumz ML, Rabinowitz L, Wingo CS. Disorders of fluids and electrolytes. N Engl J Med 2015;373:60–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Institute of Medicine. Panel on dietary intakes for electrolytes and water. Dietary reference intakes for water, potassium, sodium, chloride, and sulfate. Washington (DC): The National Academies Press; 2004. [Google Scholar]
- 13.Mohan S, Gu S, Parikh A, Radhakrishnan J. Prevalence of hyponatremia and association with mortality: results from NHANES. Am J Med 2013;126:1127–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fang J, Madhavan S, Cohen H, Alderman MH. Serum potassium and cardiovascular mortality. J Gen Intern Med 2000;15:885–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Institute of Medicine. Strategies to reduce sodium intake in the United States. Washington (DC): The National Academies of Press; 2010. [Google Scholar]
- 16.He FJ, Li J, MacGregor GA. Effect of a longer term modest salt reduction on blood pressure: Cochrane systematic review and meta-analysis of randomized trials. BMJ 2013; 346:f1325. [DOI] [PubMed] [Google Scholar]
- 17.Viera AJ, Wouk N Potassium disorders: hypokalemia and hyperkalemia. Am Fam Physician 2015;92:487–95. [PubMed] [Google Scholar]
- 18.Braun MM, Barstow CH, Pyzocha NJ. Diagnosis and management of sodium disorders: hyponatremia and hypernatremia. Am Fam Physician 2015;91:299–307. [PubMed] [Google Scholar]
- 19.Liamis G, Milionis H, Elisaf M. A review of drug-induced hyponatremia. Am J Kidney Dis 2008;52:144–53. [DOI] [PubMed] [Google Scholar]
- 20.Ben Salem C, Badreddine A, Fathallah N, Slim R, Hmouda H. Drug-induced hyperkalemia. Drug Saf 2014;37: 677–92. [DOI] [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey: analytic guidelines, 2011–2014 and 2015–2016. https://wwwn.cdc.gov/nchs/data/nhanes/2011-2012/analyticguidelines/analytic_guidelines_11_16.pdf (Accessed September 2019).
- 22.Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey (NHANES): MEC laboratory procedures manual. 2013. https://wwwn.cdc.gov/nchs/data/nhanes/2013-2014/manuals/2013_MEC_Laboratory_Procedures_Manual.pdf (Accessed February 2020).
- 23.Collaborative Laboratory Services, L.L.C. Laboratory procedure manual: sodium in refrigerated serum. NHANES; 2013–2014. https://wwwn.cdc.gov/nchs/data/nhanes/2013-2014/labmethods/BIOPRO_H_MET_SODIUM.pdf (Accessed September 2019). [Google Scholar]
- 24.Collaborative Laboratory Services, L.L.C. Laboratory procedure manual: potassium in refrigerated serum. NHANES; 2013–2014. https://wwwn.cdc.gov/nchs/data/nhanes/2013-2014/labmethods/BIOPRO_H_MET_POTASSIUM.pdf (Accessed September 2019). [Google Scholar]
- 25.DynaMed. Ipswich (MA): EBSCO Information Services. 1995–. Record No. T115641, Hyperkalemia—Approach to the Patient. https://www.dynamed.com/topics/dmp~AN~T115641 (Accessed September 2019).
- 26.DynaMed. Ipswich (MA): EBSCO Information Services. 1995–. Record No. T113706, Hyponatremia—Approach to the Patient. https://www.dynamed.com/topics/dmp~AN~T113706 (Accessed September 2019).
- 27.Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey: 1988–2016 data documentation, codebook and frequencies. Prescription medications—drug information (RXQ_DRUG). https://wwwn.cdc.gov/Nchs/Nhanes/1999-2000/RXQ_DRUG.htm (Accessed September 2019).
- 28.Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. Dietary reference intakes for calcium, phosphorus, magnesium, vitamin D, and fluoride. Washington (DC). National Academies Press; 1997. [PubMed] [Google Scholar]
- 29.Smith KW, Sasaki MS. Decreasing multicollinearity: a method for models with multiplicative function. Sociol Methods Res 1979;8:35–56. [Google Scholar]
- 30.Parker JD, Talih M, Malec DJ. National Center for Health Statistics data presentation standards for proportions. Vital Health Stat 2017;2:1–22. [PubMed] [Google Scholar]
- 31.Murphy D, McCulloch CE, Lin F, Banerjee T, Bragg-Gresham JL, Eberhardt MS, et al. Trends in prevalence of chronic kidney disease in the United States. Ann Intern Med 2016;165:473–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kantor ED, Rehm CD, Haas JS, Chan AT, Giovannucci EL. Trends in prescription drug use among adults in the United States from 1999–2012. JAMA 2015;314:1818–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Centers for Disease Control and Prevention. Long term trends in diabetes. Division of Diabetes Translation. 2017. https://www.cdc.gov/diabetes/statistics/slides/long_term_trends.pdf (Accessed February 2020).
- 34.Bernardi M, Ricci CS, Santi L. Hyponatremia in patients with cirrhosis of the liver. J Clin Med 2014;4:85–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liamis G, Liberopolous E, Barkas F, Elisaf M. Diabetes mellitus and electrolyte disorders. World J Clin Cases 2014;2:488–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Verbalis JG, Goldsmith SR, Greenberg A, Korzelius C, Schrier RQ, Sterns RH, Thompson CJ. Diagnosis, evaluation, and treatment of hyponatremia: expert panel recommendations. Am J Med 2013;126:S1–42. [DOI] [PubMed] [Google Scholar]
- 37.Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, et al. ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Hypertension 2018;138: e426–83. [DOI] [PubMed] [Google Scholar]
- 38.Betts KA, Woolley JM, Mu F, McDonald E, Tang W, Wu EQ. The prevalence of hyperkalemia in the United States. Curr Med Res Opin 2018;34:971–8. [DOI] [PubMed] [Google Scholar]
- 39.DynaMed. Ipswich (MA): EBSCO Information Services. 1995–. Record No. T921915, Overview of Diuretics. https://www.dynamed.com/topics/dmp~AN~T921915 (Accessed September 2019).
- 40.Aldahl M, Caroline Jensen A-S, Davidsen L, Alida Eriksen M, Hansen SM, Nielsen BJ, et al. Associations of serum potassium levels with mortality in chronic heart failure patients. Eur Heart J 2017;38:2890–6. [DOI] [PubMed] [Google Scholar]
- 41.Sinclair D, Briston P, Young R, Pepin N. Seasonal pseudohyperkalemia. J Clin Pathol 2003;56:385–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


