Abstract
Wounds, which are becoming more common as a result of traumas, surgery, burns, and chronic illnesses like diabetes, remain a critical medical problem. Infectious bacteria impact the healing process, particularly if its biofilm (biological films) leads to a prolonged effect. Nanomaterials have emerged as promising candidates in the field of wound healing due to their unique properties and versatile applications. New insights into the interactions between nanomaterials and wound microenvironments have shed light on the mechanisms underlying their therapeutic effects. However, a significantly minimal amount of research has been carried out to see if these nanomaterials significantly promote the wound healing process. In this review, we provided an outline of the various types of nanomaterials that have been studied for healing wounds and infection prevention. Overall, the utilization of nanomaterials in wound healing holds great promise and continues to evolve, providing new opportunities for the development of effective and efficient wound care therapies.
Keywords: nanomaterials, wound healing, wound management, nanoparticles, antimicrobial
1. Introduction
The skin is the body’s primary organ, having a multitude of activities that tend to range from the immunologic and sensory to insulating and regulating moisture [1]. Because of the documented frequency of accidents, surgeries, burns, and chronic illnesses like diabetes, the prevalence of various injuries remains a critical problem [2]. According to the World Health Organization’s (WHO) most recent data, burn wounds alone cause more than 300,000 fatalities per year. After skin injury, the skin’s immune and defensive processes are compromised; as a result, germs can readily infect the wound site, resulting in chronic infection as well as septic shock unless the invaders spread throughout the body [3]. Furthermore, pathogenic microbes can induce healing delays by forming a biofilm, which can lead to treatment problems owing to resistance to antibiotics and life-threatening consequences [4]. Simultaneously, due to limited antibiotic treatment choices, the emergence of multidrug-resistant (MDR) pathogens has posed a serious therapeutic issue [5]. For a wound, the longer the duration that it takes to heal, the greater the chance of a substantial adverse occurrence, such as amputation, organ loss, or death [6].
Antimicrobial-resistance (AMR) and multidrug-resistance (MDR) illnesses will cause more deaths than all forms of cancer combined, as according to the Centers for Disease Control and Prevention (CDCP). AMR is predicted to cause more than 10 million deaths per year by 2050 [7,8]. Traditional antibacterial medications are being used to treat wound infections, which pose a number of clinical problems. As a result, it is vital to create innovative dressing materials that do not rely on current antibiotics [8].
Over the last two decades, the use of nanomaterials (NMs), specifically novel formulations such as nanoparticles formulated for wound healing processes and infection management, has expanded dramatically [9]. The scaffolding of nanoparticles for wound healing has recently gained a lot of attention as a targeted approach to advanced dressings [10]. Antimicrobial NMs currently come in a range of forms, including polymers such as lipids in liposomes, cellulose, and mesoporous silica, which serve as carriers for antimicrobial and wound healing agents (Figure 1) [11]. Metal/metalloid-based nanoparticles (MNPs) are another type of antibacterial NM that has been shown to help with wound healing in various studies [12]. Their great antibacterial efficacy could indeed, or might not, be intimately correlated to wound healing. Although the concept of nano-antibiotics (nanobots) has been synthesized for at least a decade, and there are several recent evaluations of nanomaterial utilization as antimicrobials or antiseptics available, there has been less focus on whether they truly enhance wound healing results [13]. Although there are several evaluations on the antibacterial effectiveness of different nanomaterials, only a few articles on nanomaterials specifically utilized to enhance wound healing have been published [14].
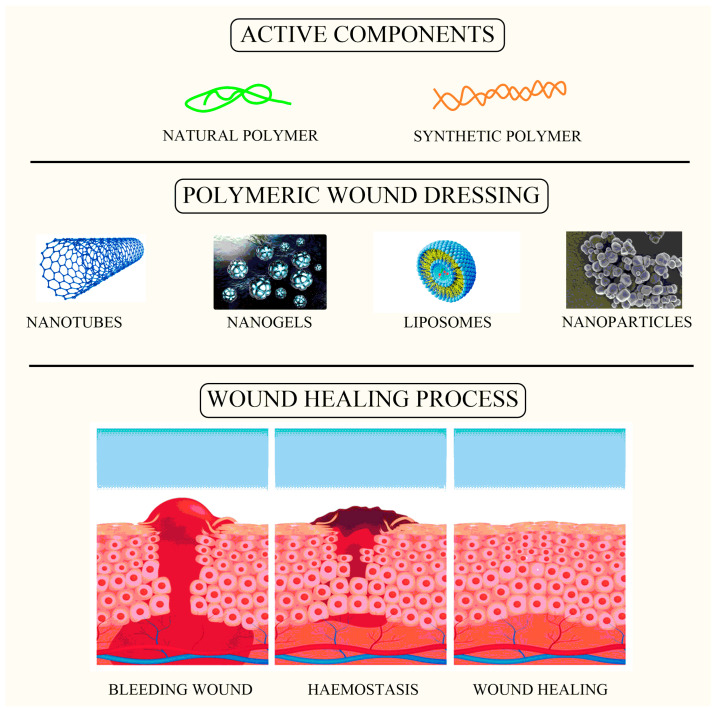
Figure 1.
Image showing active components loaded within wound dressing as a carrier for the wound healing process.
2. Wound Processes
To illustrate how nanomaterials could impact healing in context, here is a brief overview of wound subtypes and the healing process [15]. Disruption to the viability of underlying biologic and organic tissues, including skin and mucous membranes, is characterized as a wound. Burns, incisions, lacerations, contusions, abrasions, and combinations of these forms of trauma are all conceivable, and the maintenance of wound dressings for each type is vital to prevent infection and additional damage [16]. Wound categorization is carried out using a variety of approaches depending on cleanliness, wound state, and healing period [17]. Based on wound cleanliness and infection level, the CDC divides wounds into four categories. (1) Clean wounds: closed and free of infection and inflammation. (2) Cleanly contaminated or uncommonly contaminated; there is no remarkable contamination. (3) Infected: fresh, open, and also without exudate inflammation. (4) Highly infected wounds: the most frequent microorganisms are found in these wounds, which often originate from bad care of traumatic wounds [18,19,20].
Wounds are further divided into two groups: those that are infected and those that are not.
3. Wound Healing Process
Healing is multifaceted, and it can go forward as well as backward depending on a variety of factors, including the patient’s external and internal surroundings/conditions [23]. Wound healing is split into four stages that overlap [24].
3.1. Hemostasis
This step occurs quickly, in order to create a blood clot and block blood flow [25].
3.2. Inflammation
Inflammation is managed during this phase, and organisms and cell debris are evacuated from the affected area. When activated by thrombin, platelets release a slew of growth factors including signals for storing white blood cells, nutrients, and more growth factors that further promote rapid wound and skin healing as well as strengthen immunity against infection [26].
3.3. Proliferation
Beginning with proangiogenic chemicals generated by inflammatory blood platelets, this phase repairs the wound. After that, angiogenesis takes place, fibroblasts multiply, and elastin is formed. By holding the wound edge, fibroblasts develop into myofibroblasts, forcing the wound region to constrict [27].
3.4. Maturation
With the aid of collagen fibers, the wound becomes completely closed. The debris and damaged cells that were used to heal the wound are cleared away by a process called apoptosis and thus collagen cross-linking strengthens the skin of the wounded region [28].
Hemostasis, inflammation, proliferation, and remodeling are the four phases of the complex wound healing process [29,30]. The therapeutic impact, dose, depth, and various approaches are the key factors for rational. Scientists have been investigating technologies that can both maintain a wet environment and kill bacteria [31]. There are several different engineered wound healing routes using a wide range of natural and synthetic polymeric materials and their amalgamation [32].
Here too, natural polymers with important biological roles have found their way into a wide variety of wound healing approaches [33]. For its ability to maintain a moist, protective environment for the tissues to heal in, collagen has been heralded as a superior, mechanically strong scaffold [34]. Fibrin is another protein that helps stop bleeding by entrapping platelets during the formation of the primary clot [35]. The mechanical strength of silk fibroin also makes it efficient. Keratin is another nanomaterial that sees extensive use in the wound healing process [36]. There is tremendous potential in the fact that nanomaterials can be used to facilitate regenerative self-healing mechanisms. However, due to the diverse character of wounds, it is necessary to learn more about the fundamental processes and cellular cascades in order to tailor those nanomaterials to specific wound healing needs [37].
4. Available Therapeutic Approaches in Wound Healing
Biological-based techniques such as immune-based antimicrobial compounds (for example, polypeptides including defensins), therapeutic microorganisms (for example, bacterial phages and probiotics), stem cell treatments, and skin tissue engineering are presently available therapeutic possibilities [38,39]. Reactive species and nitric oxide generators, different topical antiseptics including antibiotics, negative pressure, and ultrasonic treatment, along with manual wound debridement, various surgical techniques, and skin replacements are among the classic and non-traditional non-biological approaches [40]. Owing to variations in the molecular and cellular pathways between wound types, there is not yet a universally effective treatment for wounds [41]. The evaluation and management procedures in the field of wound healing research have been greatly enriched by recent breakthroughs and technological advancements [42]. Changes in the way wounds are treated, such as the introduction of moist dressings, stimuli-responsive dressings, as well as growth factor-based therapy, skin alternative treatments (tissue-engineered and bio-engineered), gene treatment, nanotherapeutics, as well as stem cell therapy, resulted in a new era of progress [43,44,45,46]. Emerging technologies, such as three-dimensional bioprinting (3D bioprinting), novel platelet-rich plasma therapy, including extracellular matrix (ECM)-based techniques, have also paved the way for tailored wound care [47]. However, there is still cause for concern regarding the evaluation and management of chronic wounds. As a result, we need cutting-edge approaches to wound care that take into account efficacy, benefit-to-risk, and cost-effectiveness [48].
5. Applications of Different Nanomaterials in Wound Healing
Nanoparticles, with their high surface area-to-volume ratio, are well-suited for use in this type of study. Wound dressings could benefit from the incorporation of metal nanoparticles like silver, gold, and zinc due to their exceptional features, such as wound healing stimulation and antibacterial properties [49]. Rapid wound closure with little scarring has been reported after treatment with silver nanoparticles, which have been shown to influence the release of anti-inflammatory cytokines [50]. They can also promote keratinocyte proliferation, which is essential for epidermal re-epithelization. This last influence appears to be dose-dependent as well. Gold nanoparticles (AuNPs) can boost keratinocyte development and differentiation at low concentrations while also promoting healing and preventing microbial colonization [51]. When incorporated into hydrogel-based wound dressings, zinc oxide nanoparticles (ZnONPs) indicate an efficacious antibacterial agent by triggering bacterial cell membrane perforations, and by increasing the overall contact time, they promote keratinocyte migration and improve re-epithelialization [52]. In addition, the antibacterial and re-epithelialization characteristics of biopolymeric nanoparticles as treatments for wounds or as delivery vectors are impressive [53]. In particular, biopolymer’s high water absorptivity makes it ideal for setting up a humid wound environment and controlling exudation [54]. Hyaluronic acid, by virtue of its hygroscopicity, controls cell adhesion as well as attachment in the course of wound healing. Endothelial cell proliferation, motility, and angiogenesis were all reported to be boosted by hyaluronan oligosaccharides due to elevated levels of vascular endothelial growth factor [55,56].
Infection prevention, skin regeneration, and therapy for wounds are all intricate parts of the healing process. Both topical and systemic treatments are used to aid with wound recovery [57]. However, local therapy may be preferable since it reduces the risk of complications, boosts efficiency, and helps combat antibiotic resistance [58]. Studies have shown that many different pharmacological NPs have remarkable effectiveness in promoting skin regeneration, preventing infections, and inhibiting the growth of multidrug-resistant bacteria [59]. Significant improvements in wound care may result from the synthesis, manufacture, and therapeutic relevance of these NM compounds [60].
By increasing the degree of antibiotic concentration, local antimicrobial therapy helps eliminate wound infections [61]. To reduce the risk of antibiotic-related side effects and resistance, antibiotics should be limited to site applications rather than systemic and long-term therapy [62]. Injuries to the skin’s protective barrier increases the risk that drugs applied topically, including NPs, will be absorbed systemically [63]. Therefore, studies of these NPs as antimicrobials and carriers are essential in order to discover more about their kinetics as well as dynamics, cytotoxicity (eukaryotes), and likely systematic adverse reactions [64]. Furthermore, when employing topical treatments, it is important to consider the effect that cleanup techniques have on the NPs’ half-life within the wound site [65]. Below, we present the many nanomaterials that can be used for wound care. While we do provide a sample of case studies demonstrating the applicability of each type of nanomaterial, we do not publish an exhaustive catalogue of all studies involving any given nanomaterial.
6. Carbon-Based Nanomaterial
Deoxyribonucleic Acid (DNA) is the primary vital component of all known forms of life on Earth. It could be made available in a number of different formats. Carbon’s peculiar electron configuration (1s2, 2s2, 2p2) allows for the possibility of both crystalline and amorphous forms of the element. Carbon is a highly versatile element since it may exist in a wide variety of allotropes and crystal structures. The type of carbon allotrope depends on whether the carbon is hybridized (sp3, sp2, or sp1) or bound to other atoms. Carbon-based nanoparticles (CBNs) have garnered interest from several disciplines due to their distinctive physicochemical properties and structural properties [66]. Nanomaterials made of carbon are becoming increasingly popular due to their novel size and promising features, which include mechanical, chemical, thermal, electrical, as well as optical properties. Nanotubes (carbon-based), notably their nanocomposites, have been shown to have broad antibacterial activity [67,68]. Burn wound infections are triggered by bacteria, fungus, and viruses. Knowing the specific mechanisms that govern CNMs’ antibacterial activity is crucial for ensuring their safe and effective inclusion into biomaterials. Carbon-based nanomaterials offer attractive physical and chemical characteristics that could have uses in biomedicine [69,70]. In addition to improved optical activity and a substantial multifunctional surface area, these materials have showed increased drug-loading capacity, greater biocompatibility, and lower immunogenicity. Research into enriched carbon-based nanomaterials has yielded significant benefits for the development of biologically compatible scaffolds and nanomedicines [71,72].
6.1. Carbon Nanotubes
The most common form of carbon nanotubes (CNTs) is a tube or cylinder. Size, shape, thickness, and chirality are all possible distinctions between them. They displayed numerous desirable characteristics because of their unusual blend of pliancy, toughness, and rigidity. Single-walled, double-walled, multi-walled, and functionalized CNTs exist because of their varied structural configurations. CNTs can be synthesized using a variety of techniques, including chemical vapor deposition, laser ablation, arc discharge, or, for high-pressure regions, carbon monoxide disproportionate. As Sumiolijima in 1991 mentioned, CNTs constitute an allotropic type of carbon with similar qualities to graphene [73]. CNTs have a cylinder shape and are made up of rolled graphene sheets that are sp2-hybridized. CNTs are often manufactured via arc discharge, laser ablation, or chemical vapor deposition (CVD) [74]. While the carbon source was heated at 3000–4000 °C in the arc discharge as well as during the laser ablation procedures to create cylindrical CNTs, the carbon source was pyrolyzed at 600–1100 °C in the CVD method. The synthetic route taken has a significant impact on the resulting CNTs’ physicochemical qualities [75]. Because of their unique structure and fabrication method, CNTs have exceptional thermal characteristics. Depending on whether they are single-walled or multi-walled, the conductivity of CNTs can range from 6000 to 0.1 W/mK [76,77]. Collective atomic vibration, encompassing phonon and electron transport, is responsible for thermal conductivity [78,79]. The conductivity of CNTs is also dependent on their length [80]. Therefore, in order to achieve a targeted thermal conductivity, it is necessary to optimize synthetic parameters [81]. Based on the Tersoff–Brenner potential, which is analogous to a hypothetical isolated graphene monolayer [82], Berber et al. used molecular dynamics simulation to calculate the thermal conductivity (k = 6600 W/mK) of CNTs. As a counterexample, Osman et al. investigated how CNTs’ physical characteristics affected their heat conductivity. They looked at the fact that SWCNTs’ thermal conductivity varies with temperature. Armchair (10,10) arranged SWCNTs like monolayered graphene and showed a drop in thermal conductivity over 400 K. Maximum conductivity is found in CNTs of equal diameter but differing chirality at 300 K [83,84], with the armchair CNTs having a relatively sharper peak than the zigzag CNTs. A composite film made of bacterial cellulose and multi-walled carbon nanotubes (MWCNTs) was created by Khalid and her colleagues. The antibacterial properties of the finished films were tested. All of the bacteria strains put to the test were successfully inhibited by the composite film. Furthermore, macroscopic inspection of the wound showed that the BC-MWCNT group healed their diabetic wounds more quickly than the negative control group (77% vs. 99% recovery in 21 days). Histological tests corroborated the findings, showing that the BC-MWCNT-treated group had full re-epithelization of the epidermis as well as normal granulation tissue. Studies at the molecular level suggested that the faster healing time in the BC-MWCNT group was likely due to the lower expression of pro-inflammatory cytokines IL-1 and TNF- and the increased expression of VEGF compared to the control [85]. To regulate the gradual release of isoniazid, Chen and his colleagues developed a nanoparticle formulation composed of chitosan and carbon nanotubes. The dimension of chitosan/carbon nanotube nanoparticles has been found to be between 150 and 250 nm using transmission electron microscopy and nanoparticle tracking as well as analysis. The release of isoniazid was greatly extended by chitosan/carbon nanotube nanoparticles, by 48 h. In vitro studies demonstrated that isoniazid cytotoxicity and inflammation could be mitigated by chitosan/carbon nanotube nanoparticles without destroying the drug’s biological function. The wound area was reduced by 94.6% in the isoniazid group and by 89.8% in the isoniazid/carbon nanotubes group. Immunohistochemistry analysis revealed a marked reduction in CD3+ and CD4+ T cell count in the isoniazid/chitosan/carbon nanotubes group [86]. Kittana et al. [87] have synthesized complexes of chitosan with both single- and multi-walled carbon nanotubes (C-SWCNTs and C-MWNTs, respectively). After assessing the produced compounds using TEM, it was found that a structure composed of carbon nanotubes had successfully formed, interlaced within the chitosan polymer. Fibroblast viability and the ability to structure and bind the extracellular matrix were confirmed in tests of synthetic connective tissue. Both forms of complexes were found to enhance re-epithelialization of healed wounds in vivo, but they also resulted in a higher rate of fibrosis in some wounds. These complexes, including the chitosan–multi-wall carbon nanotube composite in particular, were found to increase collage deposition in vitro, correlating with in vivo results that indicated a concentration-dependent increase in fibrosis extent. As a result of these findings, inflammatory symptoms in the bed of the wound increased.
6.2. Single-Walled Nanotube
SWCNTs, or single-walled carbon nanotubes, are essentially a long, folded sheet of graphene. Graphene sheet patterns are determined by the diameter as well as the C-C orientation [88]. The length-to-diameter ratio in this case is close to 1000-to-1. They have been built with sp2 hybridization and have one-dimensional hollow as well as cylindrical shapes. These CNTs can grow to lengths of thousands of times their original diameter [89]. They have numerous uses in nanotechnology. To obtain SWCNTs of a high quality and purity, regulated catalytic conditions are needed. SWCNTs have found widespread application as scaffolding for cellular culture growth and as diagnostic devices in the biomedical sector. Due to their strong interaction, SWCNTs have a low solubility and are challenging to distribute in the aqueous phase. Solubilizing and dispersing SWCNTs in water through chemical modification has been proposed to boost its biological and pharmacological interest [90,91].
6.3. Double-Walled Nanotube
DWCNTs, or double-walled carbon nanotubes, are originated by joining together two single-walled CNTs. About 2–4 nm is the outer tube diameter, and 1–3 nm is that of the inner tube. DWCNTs possess the same small diameters, mechanical properties, and electrical properties as SWCNTs. Double-walled carbon nanotubes (DWCNTs) have a graphene sheet folded on top of itself twice, creating a double-layer structure. It is a newly discovered type of CBN [92]. DWCNTs excel in these areas compared to SWCNT parameters such as mechanical strength, thermal stability, as well as chemical resistance. The optical and electrical properties of this group are also rather intriguing. As an electrode material, anti-salmonella was injected into DWCNT bundles. DWCNTs, as opposed to the SiO2 surface, are preferable for the growth of neuron cells in tissue engineering. After neuron growth on DWCNTs, cell differentiation was enhanced [93].
6.4. Multi-Walled Nanotube
The diameter of a multi-wall carbon nanotube (MWCNT) ranges from 2 to 50 nm; the diameter of the outermost layer can be as high as 100 nm, while the diameter of the innermost layer is less than 1 nm. The amount of rolled-up graphene sheets in MWCNTs increases the number of structural flaws that benefit surface functionalization. As a result of their exceptional chemical, thermal, mechanical, electrical, and changeable structural properties and functions, MWCNTs are being evaluated as possible materials for biomedical applications. The Russian-doll and parchment model [94] works well to describe the structure of MWCNTs. When contrasted with DWCNTs and SWCNTs, they are found to have a better signal-to-noise ratio. Sensitivity, however, is conditional on the nature of the matrix (biological). The scaffolds for pancreatic cancer cells have been developed using MWCNTs. The proliferation and growth of fibers/sheets based on validated materials show greater potential in killing cancer cells [95].
7. Nanostructured Carriers
Nanomaterials may be made in many different sizes and structures and are generally spherical and have a diameter of 10–100 nm [96]. A wide range of organic compounds and metals can be used to synthesize nanomaterials. Most NPs are made up of two parts: the core compounds or atoms and a surface “cap” or shell that affects the NPs’ stability and also their function [97,98]. The efficacy of NPs is influenced by the interaction of an infectious organism with damaged cells in the wounded region, which is influenced by different biomaterial assets including their nature, dosage form, particle size, as well as the intensity of infectious injury [99]. NPs act as drug carriers that mainly operate as a vehicle to transport medicine to the targeted site more effectively in comparison to the standard topical medicament [100]. Organic (such as surfactant/lipid), carbohydrate-based (e.g., cellulose), or inorganic carrier NPs may be used (e.g., silicate or metal-based). The antimicrobial/drug may be encapsulated in the carrier NP, as in liposome-based nanomaterials, or the antimicrobials may be part of the shell, making the NP the carrier [101]. Surface nanoengineering, which is associated with core–shell nanomaterials, aims to minimize biofilm development in wounds and infections on implantable devices that are medicated [102]. The goal of surface nanoengineering is to develop a coating material that either passively inhibits bacterial growth or actively inhibits bacterial development by permanently attaching antimicrobials to the polymer (non-release-based) or releasing them (either with or without bacterial stimulation) [103,104,105,106].
7.1. Liposomal Delivery
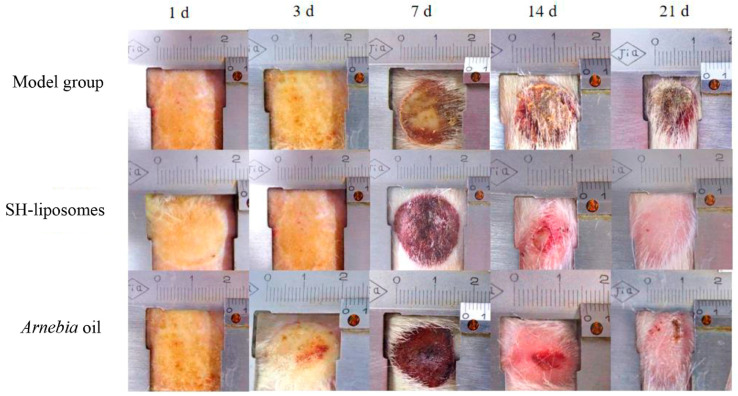
Liposomal drug delivery helps in the facile transfer of hydrophilic compounds including small molecules as well as non-Lipinski-based macromolecules in varied applications, especially wound healing [107,108,109]. The phospholipid building components are responsible for the amphipathic character of liposomes (LP). The amphiphilic lipid spontaneously forms its bilayer with an aqua base when dispersed in aqueous solutions. The ultimate size of the liposome is determined by the lipid type and extrusion across membranes of varying pore sizes [110,111]. These LPs can also be derivatized with biopolymers to improve their stability and targeting abilities [112]. Towards this direction, Eid et al. in 2022 [113] explored the viable usage of citicoline-tailored chitosan-engulfed liposomes for efficacious topical wound healing, especially in the case of streptozocin-induced diabetic animal models. The study acquired optimized formulations with the average particle size at 211 nm, EE as 50%, and surface area as +32 mV approximately. These liposomes resulted in sustained-release behavior and decreased inflammatory process, thus initiating a VEGF immune reaction, fibroblast development, as well as enhanced epithelialization. Recently, Daodu and coworkers [114] developed curcumin-loaded liposomal delivery inside lysine and collagen-based hydrogel for the improvement of surgical wounds by thin-film hydration process. These infused dosage forms were of 5–10 microns in particle size, EE was 99.9% approximately, and they exhibited 79% of increased wound contraction after the third day of operation. Hemmingsen et al. in 2022 [115] designed another chitosan-based chlorhexidine delivery system for antimicrobial action. The inflammatory pathways in treated rodent macrophages showed a 60% reduction in comparison to the non-treated animals. The novel formulation has indicated an excellent antimicrobial action against Staphylococcus aureus. Shu and coworkers in 2022 [116] fabricated a herbal antimicrobial wound healing preparation bearing shikonin-based LP using a film development process against methicillin-resistant S. aureus (MRSA). The LPs were stable, exhibited an EE of 62%, were negatively charged, and had a sustained-release effect. The preparations damaged the bacterial cell membrane, alleviated the inflammatory pathway, and showed better repair activity (Figure 2).
Figure 2.
Out of the model group, Shikonin (SH)-based liposomal group, and Arnebia oil® group, applied topically twice daily for 21 days, SH-liposomes observed a better wound healing effect on the 21st day compared to the others (reprinted from [116], copyright (2024), with permission from Elsevier).
Another group investigated chitosan-coated liposomal compositions for delivering substance P (SP), an 11-amino-acid neuropeptide that aids in mucosal wound healing. Liposomes were produced using lecithin and cholesterol, then loaded with SP and resuspended in water before being coated with chitosan. The diameter of the uncoated liposomes (UN-LP) was 151 ± 27 nm, whereas the diameter of the coated liposomes (CH-LP) was 243–248 nm. The SP-infused chitosan-functionalized liposomes were tested in vitro against HaCaT keratinocyte cells to see if they could repair wounds. When cell migration was monitored, it was shown that SP incorporated in chitosan-coated liposomes led in wound repairs and closure was of 85.512%, which was much greater than untreated cells [117]. Notably, the plant origin extract curcumin has revealed wound healing capabilities. Deformable liposomes (DL) in chitosan hydrogel were used for its therapeutic action. Both DLs and chitosan hydrogel assured the drug’s long-term release and penetration. A longer retention duration at the targeted skin location, better localized wound treatment, and increased medication effectiveness were its positives [118].
7.2. Nanohydrogels
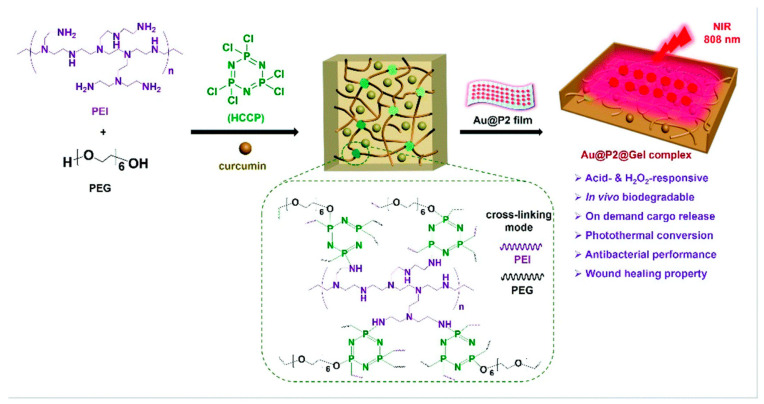
Interestingly, hydrogels have proved to have an advanced efficacy with the incorporation of nanomaterials and have made wound dressings more pragmatic based on research studies. Merging nanotechnology with hydrogels has shown improvements in the adhesive properties of these gels and the potency of drug delivery through covalent coupling and noncovalent interactions. Henceforth, nanohydrogels can be employed directly on the wounded site and can cover the damaged area to enhance wound healing as well as the growth of hair follicles and capillaries [119]. Many research studies have observed that different functionalities bearing hydrogels have enhanced pharmacological healing activity in comparison to the standard formulations. The modern advantages of hydrogel-based dressings are their antibacterial, surface adhesion, hemostasis, anti-inflammatory, antioxidant, target delivery, self-healing, stimuli-responsive, and wound diagnostic features [120]. Bilayer hydrogels have shown tremendous applicability, especially in wound healing action [121,122]. In fact, a few compounds, such as hyaluronic acid, have demonstrated excellent flexibility in the pharmaceutical industry owing to their gel-based nature [123]. Furthermore, Sun et al. in 2022 [124] discovered that a novel oxidized konjac glucomannan (OKGM), γ-poly(glutamic acid), could be modified with dopamine and L-cysteine (γ-PGA-DA-Cys) and ε-polylysine (ε-PL) to produce an OKGM/γ-PGA-DA-Cys/ε-PL (OKPP) bioadhesive hydrogel. The formulation was active against P. aeruginosa and S. aureus, showing better antibacterial as well as antioxidant activity. The animal model observed efficacious wound repair properties and decreased inflammatory parameters, leading to a potential wound dressing for burn infections. To our knowledge, Shang et al. in 2022 [125] demonstrated a versatile nanohydrogel-based wound dressing that observed biodegradability and multiple biological effects including antimicrobial, antioxidant, and healing action against MRSA. This construct was an acid-cleavable antibacterial formulation bearing a hydrogen peroxide (H2O2)-responsive polymer/gold hybrid film with a photothermal conversion ability based on polyethylenimine (PEI), polyethylene glycol (PEG), hexachlorocyclictriphosphonitrile (HCCP), as well as gold nanoparticles. These gels showed notable surface adhesion and an easy-to-remove feature, which may lead to patient compliance (Figure 3).
Figure 3.
Schematic representation of hydrogen peroxide (H2O2)-responsive nanohydrogel with a photothermal conversion ability based on polyethylenimine (PEI), polyethylene glycol (PEG), hexachlorocyclic triphosphonitrile (HCCP), as well as gold nanoparticles. Reproduced from [125] with permission from the Royal Society of Chemistry.
7.3. Metals and Metal Nanoparticles as Antimicrobials
Bacterial infectious illnesses are a major public health concern because they indicate a global pandemic. Around the world, resistant diseases kill at least 700,000 people per year. Since antibiotics are so powerful against these illnesses, they have become the standard for both prevention and treatment [126]. However, numerous studies have found that antibiotics currently in widespread use are the leading sources of life-threatening multidrug-resistant bacteria. Between 2017 and 2019, the FDA authorized eight novel antibiotics developed to combat WHO priority infections. Many of these medicines are variants of traditional antibiotic classes already in use. Nanotechnology, defined as nanoscale materials (1–100 nm), is a promising field in modern biomedical applications. These materials can improve physicochemical and biological qualities because of their unique properties [127,128,129].
Metal-based nanoparticles (MBNPs) are being examined extensively for a variety of biological applications, and they provide hope in the fight against antibiotic resistance. Additionally, they target several biomolecules, which further hinders the evolution of resistant strains of bacteria, and their mechanisms of action are completely distinct from those outlined for conventional antibiotics [130]. Nanomaterials have been shown to be hazardous to multiple bacterial strains in in vitro tests, raising hopes that they may one day be used in medicine delivery as well as tissue engineering [131].
Antibacterial wound dressings, including a variety of intermetallic NPs and polymeric biomaterials, have been reported in a number of investigations. In this direction, researchers have developed antibacterial cellulose-PMMA fibers with a pressurized gyration process for epidermal wound dressing applications. Dressings with the highest NP concentrations (1 wt.%) for both blends were tested by in vitro co-culturing S. aureus and keratinocytes to determine the cellular response and the concomitant bactericidal effectiveness. Compared to samples containing UHNP-1 intermetallic NPs, samples containing AVNP-2 intermetallic NPs demonstrated superior antibacterial activity and keratinocyte cell survival. The fibers showed great potential as a wound treatment for the epidermis. The aforementioned research suggests that wound dressings composed of intermetallic NPs–polymeric biomaterial systems can efficiently kill many bacterial strains [132,133,134].
Antibacterial wound dressings based on intermetallic NPs–antibiotic/polymeric biomaterial complexes are currently poorly understood. Using intermetallic NPs to decrease antibiotic dosage and polymeric biomaterials to increase their sustained bactericidal activity would be an exciting field of study [135,136]. One of the most prevalent ways in which MBNPs inhibit the growth of bacteria is by inducing oxidative stress through the release of reactive oxygen species (ROS). Overproduction of reactive oxygen species (ROS) causes cell death in bacteria by damaging their membranes, DNA, ribosomes, and proteins, and by inhibiting their electron transport chain, enzymes, and DNA transcription and translation [137,138]. It has been established that most MBNPs can enter cells and trigger the production of reactive oxygen species (ROS), while ROS production outside of cells has also been recorded. The microbial membrane experienced elevated oxidative stress because free electrons from Ag NPs that were embedded in Ti reacted directly with O2 in the liquid culture medium. The levels of reactive oxygen species (ROS) in bacteria can be increased by NPs, but bacteria have a defense mechanism termed hormesis that can respond to ROS [139,140].
Hormesis is a short- and long-term defense mechanism against reactive oxygen species (ROS), with the former being activated by a sudden spike in ROS concentration and leading to the development of ROS scavenger enzymes, and the latter being increased at the transcriptional level. So, hormesis should be taken into account while designing MBNPs–antibiotic/polymeric biomaterial systems. In addition, MBNPs can bind to the cell membrane and cause damage, which can lead to the release of intracellular material or the clogging of transport channels. Furthermore, MBNPs can dissociate into ions in solution that can cross the cell membrane. The production of proteins and DNA can be hampered by ions because of their ability to bind to functional groups. Many different modes of action have been proposed, and it is believed that several of these processes can operate in combination [141,142,143,144].
7.4. Silver Nanoparticles
Healing a wound requires a series of coordinated events, including clotting, inflammation, tissue deposition, fibroplasia, differentiation of the extracellular matrix, cellular contraction, tissue remodeling, and, finally, epithelialization. In order to combat bacterial growth in long-standing wounds and burns, doctors frequently turn to silver compounds like silver nitrate as well as silver sulfadiazine [145,146]. The faster healing of wounds caused by diabetes is achieved with the use of AgNPs, which promote the differentiation of fibroblasts into myofibroblasts. By promoting keratinocyte proliferation and migration, AgNPs hasten the healing process. It is possible that AgNPs will interact with sulfate-containing proteins in bacteria membrane cells, leading to an attack on the respiratory chain and, ultimately, apoptosis. Neutrophil apoptosis is triggered upon contact with the wounded area because mitochondrial function is reduced, which in turn decreases cytokine production [147,148]. This results in a less inflammatory response, which in turn promotes quicker recovery. However, AgNPs are able to cause DNA damage, suppress cell proliferation, and stifle cellular ATP generation because of their small size, which allows them to easily infiltrate biofilms and cell membranes. The presence of silver nanoparticles in a wound alters the local concentration of messenger RNA. Collagen deposition, which is associated with the migration of macrophages and fibroblasts, increases as the concentration of AgNPs in the dressings rises. Decreased silver ion toxicity and increased local antibacterial activity can be achieved through the use of sustained-release mechanisms [149,150,151,152].
Using a box behnken design, Salem et al. 2022 [153] developed and optimized RVS-loaded nanocubics encapsulated with AgNPs to test their efficacy in wound treatment. Various pharmacological studies were conducted on rats to determine the efficacy of wound healing when the optimal formulation topped with AgNPs was added to a gel foundation. The combination of RVS-loaded nanocubic vesicles as well as AgNPs-loaded hydrogel has shown promise as a platform for improving wound healing and tissue restoration. Wound dressings with silver nanoparticles and antibiotics (ciprofloxacin) were developed by Massey et al. in 2022 [154]. Cross-linking the hemicellulose from Lallemantiaroyleana seeds with chitosan/chitin and glutaraldehyde improved the hemicellulose’s absorptive capabilities. It was determined that the dressings were permeable and that the silver nanoparticles as well as the drug particles were dispersed evenly throughout the polymeric matrix. Because of its high swelling index, porosity, and spongy texture, it has been found useful in the creation of wound dressings with enhanced characteristics.
7.5. Zinc Oxide Nanoparticles
A number of beneficial effects of zinc oxide nanoparticles (ZnO NPs) have been reported. Cell migration, re-epithelialization, as well as angiogenesis are all boosted by ZnO NPs. These characteristics are crucial for the recovery process after an injury. The extraordinary antibacterial capabilities of ZnO-NPs have contributed to their development as potent agents against MDR. ZnO-NPs also exhibit outstanding photo-oxidation and photocatalytic features [155,156]. It is widely believed that ZnO-NPs’ antibacterial efficiency can be attributed to oxidative stress, DNA damage, and photocatalytic activity, even though the mechanism of ZnO-NPs’ antimicrobial action is not fully established. Oxygen annealing of ZnO enhances the amount of oxygen atoms on its surface, which increases oxygen atom adsorption as well as leads to the formation of more ROS, which boosts the efficiency of oxidation and, by extension, facilitates the material’s antibacterial property, as reported by Sirelkhatim et al. It has been discovered that ZnO-NPs directly interact there with the cell membrane of bacteria, disrupting its integrity, making them a potent bactericidal agent versus both Gram-positive and Gram-negative bacteria [157].
7.6. Titanium Oxide Nanoparticles
TiO2 has antibacterial characteristics, making it a promising biomaterial for use in wound treatment. Because of its high biocompatibility, TiO2 is used in a wide variety of medicinal fields [158]. Additionally, there is probably a low danger of bacterial resistance acquisition with the clinical usage of TiO2 in dressings for wounds. Soft silicone and alginate were used as hydrophobic and hydrophilic dressing materials, respectively, and TiO2 was deposited on them, employing the atomic layer deposition technique. Flexible dressings were maintained without sacrificing durability thanks to a nanoscale TiO2 covering [159].
In 2022, Aleem et al. [160] cross-linked chitosan and cellulose with sulfur-doped titanium oxide nanoparticles using triethyl orthoformate. In comparison to the other tested materials, membranes loaded with titanium oxide nanoparticles (CLHTS-5 wt%) showed the highest level of angiogenesis. Furthermore, it was concluded that a decrease in lactate dehydrogenase enzyme release was observed for up to 72 h after the wound developed, highlighting the promising ability of these nanoparticles in applications related to wound healing. Ismail et al. 2021 [161] developed a biofilm wound dressing using an evaporative casting method that included titanium dioxide nanoparticles integrated in gellan gum (GG + TiO2-NPs). Within 14 days, the GG + TiO2-NPs biofilm-treated wound was completely healed, and there were no harmful effects on the mouse fibroblast cells. Titanium nanoparticles (TiNPs@Ziziphora) were developed by Mahdavi et al. (2019) [162], utilizing an aqueous extract of Ziziphoraclinopodioides Lam leaves and TiO2 nanoparticles at Ziziphuschandleri. The antibacterial and antifungal activities of synthesized nanoparticles were shown to be superior to those of all currently available conventional antibiotics. When compared to the control groups, those given the TiNPs@Ziziphora ointment saw significant improvements in wound closure, hydroxyl proline, hexosamine, hexuronic acid, and fibrocytes/fibroblast rate, and a decrease in wound area, total cells, neutrophils, and lymphocytes (all p ≤ 0.01).
7.7. Gold Nanoparticles
Due to their high absorption of near-infrared (NIR) light, facile manufacturing, and chemical stability, gold nanoparticles (AuNPs) have found applications in tissue restoration, wound healing, and intelligent drug delivery. To aid in the in vivo transcutaneous co-delivery of other medications, gold nanoparticles traversed the subcutaneous layer of skin and disseminated across the epidermis [163]. When applied to open wounds in Wistar Albino rats, AUNPs boosted collagen as well as granulation tissue deposition, accelerated re-epithelialization, and hastened wound healing. Adenosine triphosphate (ATP) is the main molecule for storing and transmitting energy in cells, and gold nanoparticles stopped the activity of P. aeruginosa as well as S. aureus by inhibiting their energy metabolism and the activity of the enzyme that produces ATP, adenosine triphosphate synthase [164]. The biocompatibility, biodegradability, and free radical scavenging activity of AuNPs are enhanced by bridging between collagen, gelatin, and chitosan. When compared to chitosan alone, the chitosan–AuNPs composite enhanced hemostasis and epithelial tissue development in injured rats. Cryopreserved human fibroblasts, which were treated with AuNPs, were applied topically to burn sites, where they expedited healing by decreasing inflammation and increasing collagen deposition [165,166].
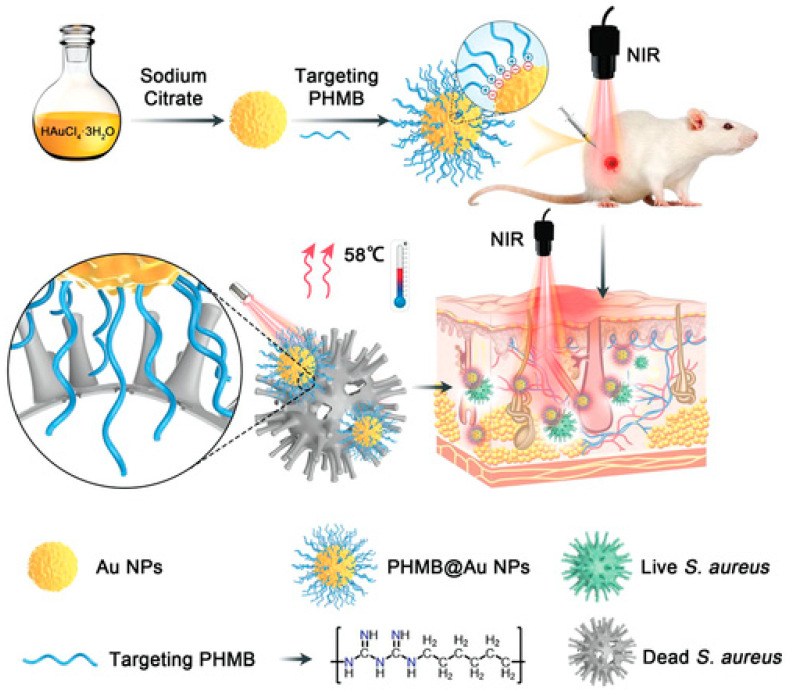
Polyhexamethylene biguanide–gold nanoparticle (PHMB@Au NPs) hybrids were developed as an antibacterial agent by He et al. in 2022 [167]. This platform is a promising antibacterial agent in wound healing since its synergistic action greatly improves the photothermal bactericidal activity against Staphylococcus aureus when exposed to near-infrared irradiation (Figure 4).
Figure 4.
Diagram depicting the production of Au NPs and PHMB@Au NPs, as well as an analysis of the mechanism of their bactericidal impact in vivo and their ability to expedite the healing of infected wounds [167]. (https://doi.org/10.1002%2Fadvs.202105223 is licensed under https://creativecommons.org/licenses/by/4.0./, accessed on 15 November 2023).
Hydrogels based on gold nanoparticles (Au NPs) were synthesized by Batool et al. [168] from Cydonia oblonga seed powder, which has been shown to have improved antibacterial and wound healing properties. In vivo treatment of wounds with hydrogel-based Au NPs indicated that Au NPs in murine models demonstrated a 99% closure of the wound within 5 days, and the hydrogel-based Au NPs suppressed microbial development in zones with a diameter of 12 mm.
7.8. Nanofibers
In addition to the utilization of NP, nanofibers are promising for wound healing due to their unique advantages. Their high surface area facilitates enhanced cell attachment, proliferation, and migration, fostering expedited wound closure and tissue regeneration. Additionally, their high porosity ensures breathability, prevents fluid accumulation, and inhibits bacterial growth, fostering a moist wound healing environment crucial for optimal recovery. Nanofibers, crafted from biocompatible materials like collagen and chitosan, mitigate the risk of allergic reactions, ensuring safe application on the skin. Moreover, their ability to carry drugs and bioactive molecules enables targeted delivery, enhancing treatment efficacy [169,170].
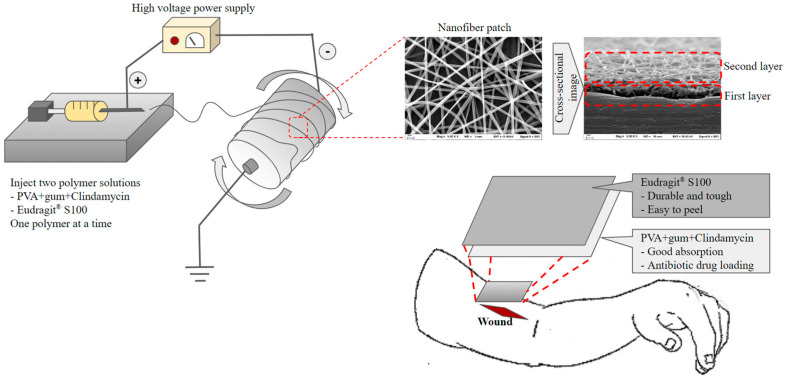
Kampanart and colleagues [106] have successfully engineered a bilayer nanofiber wound dressing patch using the electrospinning technique (Figure 5). The initial layer included modified tamarind seed gum encapsulating PVA and clindamycin HCl, whereas the subsequent layer consisted of Eudragit® S100. The results obtained from the scanning electron microscopy (SEM) analysis revealed the presence of nanofibers, exhibiting a wide range of diameters, spanning from 153 to 1830 nm. These variations in diameter were observed to be impacted by sprayed solution formulation and equipment setting. The patch was effectively created by utilizing optimum electrospinning conditions. The drug analysis results have proven the presence of an amorphous state. The biological experiments conducted demonstrated effectiveness against Staphylococcus aureus. The utilization of Eudragit® S100 and modified tamarind seed gum exhibits promising prospects in augmenting the strength of nanofiber patches.
Figure 5.
Preparation and application of the bilayer wound healing patch nanofiber fabricated by electrospinning.
8. Metal Nanoparticles with Biomaterials in Wound Healing
Biomaterials made from metals have been shown to play an active role in wound healing and infection prevention. Research into metal-based biomaterials is an exciting new frontier since they show promise in a wide range of applications beyond simple wound hemostasis, including management of inflammation, encouragement of angiogenesis and re-epithelialization, antibacterial activity, and even multistage combination therapy. Accordingly, we categorize metal-based biomaterials into 0D, 1D, 2D, and 3D categories and review the most recent findings about their use in wound repair based on these dimensions [171].
8.1. Zero-Dimensional Metal-Based Biomaterial Platforms in Wound Repair
In the field of biomaterials based on metals, OD materials are those whose dimensions vary only on the nanoscale scale. The biomedical profession makes considerable use of common OD metal-based biomaterials, including angstrom-scale materials as well as metal quantum dots, because of their high biological or photocatalytic activity. OD metal-based biomaterials have potent antibacterial effects during wound repair because of their elevated electron mobility and unique specific surface area. Compared to conventional antibacterial drugs, the therapeutic effects of OD metal-based biomaterials are significantly higher [172]. By penetrating the bacterial membrane and making close contact with the cell, materials with ultrafine structures can reduce bioburden and accomplish their goal of lysing bacteria. Nanosilver has gained a lot of attention as a potentially effective broad-spectrum antibacterial material for treating wounds. To this end, Chen et al. [173] employed a safe as well as rapid physical method to synthesis angstrom-scale Ag nanoparticles (AgPs) and then utilized them with carbomer gel (L-AgPs-gel) to assess their broad-spectrum antibacterial impact on wounds. AgPs cause bacteria to produce more reactive oxygen species (ROS) and hence perish. The infection and inflammation induced by bacterial colonization in the wound are greatly reduced because the L-AgP gel has a higher suppressive inhibitory impact on the biofilm development of numerous pathogens than the readily accessible AgNP gel.
8.2. One-Dimensional Metal-Based Biomaterial Platforms in Wound Repair
The term “1D biomaterials” is employed to describe substances that only have a single dimension of extension, with the other two dimensions being on the nanoscale scale. In contrast to OD biomaterials, its strong specific surface area, exceptional photothermal action, catalytic properties, stability, and biological activity render it a suitable material for directing electron transport. Wound repair is a major use case for 1D biomaterials like metal nanorods and nanowires. One-dimensional (1D) biomaterials based on metals are a novel material for facilitating wound repair and angiogenesis [174]. The human body’s tissues rely on angiogenesis to bring in oxygen and nutrients. Biomaterials based on lanthanide metals are increasingly used in the medical industry because of their superior biological and fluorescent properties and because their proangiogenic qualities have been demonstrated experimentally [175,176]. A blend of polycaprolactone (PCL) and TiO2 nanorods (TNRs), electrospun nanocomposite mesh was developed by Augustine et al. [177] for use as a wound dressing. The composite material net mimics the ECM and promotes cell adhesion and proliferation, creating an optimal setting for wound healing. TNRs in the PCL grid have been shown to enhance cell migration, proliferation, and angiogenesis in wound healing in both in vivo and in vitro evaluation.
8.3. Two-Dimensional Metal-Based Biomaterial Platforms in Wound Repair
High-efficiency biocatalytic activity is provided by two-dimensional metal-based biomaterials, which are characterized by their two-dimensionality (the other dimension having been on the nanometer scale) and their ability to allow molecules of a substrate to interact onto active sites on the outermost layer with a minimal energy barrier [178]. Recent years have seen an uptick in studies examining their potential in the fields of tissue engineering and wound healing. The exceptional mechanical properties of 2D metal-based biomaterials such as nanocoatings/films, 2D transition metal dihalides (2D TMDs), and 2D metal–organic frameworks (2D MOFs) have made them desirable for use in wound healing. When it comes to treating wounds, 2D metal-based biomaterials have shown to be highly successful as novel antibacterial agents, penetrating biofilms and preventing infection [179,180].
8.4. Three-Dimensional Metal-Based Biomaterial Platforms in Wound Repair
Three-dimensional biomaterials are composites made up of one or more low-dimensional biomaterial’s basic structural elements [181]. Metal-based biomaterials in the third dimension (compared to other dimensions) typically combine metal-based compounds with non-metallic materials [182]. Because of their complexity, they are more versatile and biocompatible. The regulatory effect of metal elements on wound healing has been mentioned previously, and in recent years, 3D metal-based composite biomaterials such as bioactive glass (BG), mesoporous nanoparticles (MNs), and metal–organic frameworks (MOFs) have been demonstrated to play essential functions in wound management by metal ions and to exhibit better biocompatibility [183,184]. Hu et al. [185] developed copper-doped borate BG/poly (lactic-co-glycolic acid) dressings infused with vitamin E (VE) to stimulate blood vessel formation and skin cell renewal. Enhanced wound angiogenesis, collagen remodeling, and re-epithelialization were observed in studies using the dressing, suggesting its potential utility in wound repair. Feng et al. [186] designed a multilayer composite dressing loaded with BG to promote angiogenesis, with a Janus membrane, which comprises a microporous array for biologically active ions active pumping, an absorbent with effective wound exudate absorption capacity, and a bioactive layer containing BG to stimulate tissue repair and new blood vessel formation.
9. Metal Nanoparticle Potential Cytotoxicity
Unlike common metals (such as Li and Na), metal-based NPs display toxicities and biochemical activity that are distinct from those of these elements. Because metal-based NPs are about the same size as protein molecules, they can easily cross cell membranes and accumulate in subcellular organelle structures, where they can trigger a cascade of harmful effects including neurotoxicity, immunotoxicity, and genotoxicity [187]. Numerous studies have demonstrated that many factors, including surface area, particle size, crystal conformity, exposure manner, and chemical components, contribute to the cytotoxicity generated by metal-based NPs. Extensive and well-designed experiments are thus necessary to provide a convincing explanation for the cytotoxic properties of metal-based NPs [188]. Furthermore, the consequences of each parameter must be independently validated when working with metal-based NPs. In order to assess the cytotoxicity of metal-based NPs in a thorough and accurate manner, it is necessary to build a structure–activity correlation model linking the attributes of metal-based NPs and compounds in the body. In order to evaluate the connections between various physical and chemical factors and cytotoxicity, for instance, high-throughput screening technology has been developed. Nanoparticle (NP) and nanobiological system (NBS) interactions can be understood and predicted with the help of structure-based computational molecular modeling [189].
9.1. Neurotoxic Effects
Metal-based NPs cause neurotoxicity, which manifests primarily in changes to neurobehavior, histopathology, and neurotransmitters. Changes in neurobehavioral functioning are frequently utilized as a proxy for neurotoxicity caused by metal-based NPs. For example, Ag NPs impaired the social behavior, memory retention, and motor coordination of mice administered with Ag NPs [190]. Cognitive dysfunction and pathological abnormalities in the hippocampus (a brain area directly connected with learning and memory) were demonstrated by Tian et al. In this instance, the cognitive deficits seen in elderly mice were more severe than those seen in adult animals. Despite their widespread application as MRI contrast agents and theragnostic drug transporters, SPIONs may also cause neurotoxicity in the dorsal striatum, including the hippocampus. Metal-based NPs can cause neurotoxicity in children as well as adults. Neurotoxicity has been reported to be a concern for infants and adolescents exposed to metal-based NPs. Children born to moms who have been exposed to NPs made from metals also run the risk of experiencing neurotoxic consequences [191].
9.2. Immunotoxicity
The immune system is highly attuned to the presence of foreign chemicals and capable of rapidly mounting a defense against them. Immunity encompasses a wide variety of interactions between innate and adaptive responses, which vary in speed and specificity. Nanoparticles made of metals are more probable to enter organisms as well as interact with one another than other materials. Metal-based NPs, once inside an organism, would have an effect and be removed by the immune system in a way that is determined by their physical and chemical properties [192,193].
9.3. Genotoxicity
Substances with deleterious impacts on gene material (DNA and RNA) and on cell integrity are deemed to be genotoxic. Cancer research and the risk assessment of possible carcinogens rely heavily on the study of genotoxicity. It is common to confuse mutagenicity and genotoxicity. Even though genotoxic compounds can cause mutations, not all mutagens do so. This is because not all mutagens cause permanent changes in the genetic material. While a mutagen might temporarily damage DNA or RNA, these damages can sometimes be repaired by cellular mechanisms without leaving a lasting alteration. In contrast, genotoxic substances may also induce other forms of harm that do not necessarily directly alter the sequence of nucleotides, such as chromosomal breaks, rearrangements, or epigenetic modifications. These effects, even if not directly mutational, can still disrupt normal cellular processes and potentially lead to cancerous development. Genotoxicity is caused by metal-based NPs because of their nanoscale size, which allows them to penetrate the nucleus and interact with the arrangement and functioning of genomic DNA. Unfortunately, the precise cause of this genotoxic impact is still unknown. The direct effects of NPs on genomic materials, including ROS production and DNA/chromosome damage, are currently the focus of much study [194,195]. A study discovered that the presence of gold nanoparticles measuring 10 nm altered these markers significantly in rat organs, indicating that they interfere with the antioxidant defense mechanism. The observed rise in free radical concentrations and decline in antioxidant activity may be ascribed to the nanoparticles’ generation of reactive oxygen species (ROS), which may result in detrimental consequences [196]. However, gold nanoparticles with a size of 20 nm, at a concentration of 2.5 mg/mL for 21 days, showed potential therapeutic benefits without toxicity [197].
10. Future Challenges
Nanomaterials have emerged as promising candidates for wound healing applications due to their unique properties and potential therapeutic benefits, especially biopolymer nanoformulation. These nanoformulations, composed of biocompatible and biodegradable polymers, offer controlled drug delivery, enhanced wound closure, and reduced scarring. However, as we look ahead to the future, several challenges need to be addressed to maximize the efficacy and clinical translation of these biopolymeric nanoformulations. One of the primary challenges is the optimization of drug release kinetics. Biopolymeric nanoformulations can encapsulate a wide range of therapeutic agents, including growth factors, antimicrobials, and anti-inflammatory drugs. However, achieving the desired release profile of these drugs is critical for effective wound healing. Future research should focus on developing innovative strategies to control the release kinetics, such as stimuli-responsive systems that can respond to the wound microenvironment or external triggers. Additionally, understanding the pharmacokinetics and pharmacodynamics of these nanoformulations in vivo is essential for determining the optimal dosage and frequency of administration. Another significant challenge is improving the stability and shelf-life of biopolymeric nanoformulations. Many biopolymers are prone to degradation and can undergo changes in their physicochemical properties over time, leading to a loss of drug encapsulation efficiency and compromised therapeutic efficacy. Future efforts should concentrate on developing robust formulation techniques and storage conditions that can ensure the long-term stability of these nanoformulations without compromising their structural integrity or drug release properties. This includes exploring novel cross-linking methods, lyophilization techniques, and protective coatings to enhance stability and extend shelf-life. Furthermore, the scalability and manufacturing of biopolymeric nanoformulations remain a challenge. The transition from laboratory-scale synthesis to large-scale production is often hindered by the complexity and variability of the manufacturing processes. Future research should focus on developing scalable and reproducible manufacturing techniques that can ensure the consistent production of high-quality nanoformulations. This may involve the optimization of formulation parameters, the development of continuous manufacturing processes, and the integration of process analytical technologies for real-time monitoring and control. In addition to technical challenges, regulatory and safety considerations are crucial for the clinical translation of biopolymeric nanoformulations. The regulatory landscape for nanomedicine is rapidly evolving, and it is essential to address concerns related to biocompatibility, toxicity, immunogenicity, and long-term safety. Rigorous preclinical and clinical studies are necessary to evaluate the biocompatibility and safety profiles of these nanoformulations. Furthermore, standardization of characterization techniques and guidelines for evaluating the quality, safety, and efficacy of biopolymeric nanoformulations are needed to facilitate regulatory approval and clinical translation. Lastly, the cost-effectiveness of biopolymeric nanoformulations needs to be addressed. While the potential therapeutic benefits of these nanoformulations are significant, their high production costs can limit their widespread adoption and accessibility. Future research should focus on developing cost-effective manufacturing processes, optimizing material selection, and exploring alternative strategies, such as 3D printing, to reduce production costs and enhance affordability.
11. Conclusions
The use of nanoparticles in the treatment of wounds has been on the rise in recent years. The most recent advances in nanomaterials that aid wound healing as well as the underlying mechanisms have been covered here. The majority of the published works focus on improving the hemostasis, infection, and immunoregulation, including proliferation; however, there is a knowledge gap about the correct processes and post-wound adjustments. Due to their unique combination of physicochemical and biological features, NPs have shown promise as a means of sustained therapeutic drug delivery and release in wound dressings. Wound healing NPs can also absorb light and convert it to heat or reactive oxygen species, killing any bacteria that may be present in the wound. To treat microbial infections, NPs may be combined with other endogenous triggers including pH level, temperature, enzymes, and toxins released by the bacteria to create an effective wound dressing. Although there has been significant progress in the use of NP-based wound dressings for bacterial detection and treatment, many obstacles, including reliability, stability, toxicity, and histocompatibility, remain in the manner of NPs rendering the transition from the laboratory to clinical. Furthermore, animal trials are typically used to investigate the behavior of NP-based wound dressings in vivo. Since there are significant differences between human and animal models, it is critical to locate a different approach for preclinical investigations. More and more people are interested in and making progress towards developing intelligent wound dressings that can identify and cure bacterial infections without detaching the protective covering from the wound. In this way, bacterial infections can be immediately monitored and treated using the multimodal approach, which provides synergistic and successful therapy. Therefore, NP-based wound healing offers promise for the future diagnosis and treatment of wound infections.
Acknowledgments
The authors would like to express gratitude to Rajnish Sharma, Vice-Chancellor of Chitkara University in Himachal Pradesh, India; Nitin Verma, Principal of the Chitkara School of Pharmacy at Chitkara University in Himachal Pradesh, India; and Ashok Chitkara, Chancellor of Chitkara University in Punjab, India. Divya Dheerwould like to express her deepest gratitude and appreciation for the Teachers Associateship for Research Excellence (TARE) award (Grant No. TAR/2022/000526) for supporting and recognizing the significance of her research work. The TARE award has provided invaluable encouragement, enabling her to conduct extensive research, analyze data, and synthesize valuable insights. Their support has greatly contributed to the advancement of knowledge in her field of study and has paved the way for future scientific endeavors.
Author Contributions
Conceptualization, V.P. and A.S.; writing—original draft preparation, T.S., V.P., D.D. and D.N.V.; writing—review and editing, K.H. and A.S.; supervision, T.S. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Zarei M., Lee G., Lee S.G., Cho K. Advances in Biodegradable Electronic Skin: Material Progress and Recent Applications in Sensing, Robotics, and Human–Machine Interfaces. Adv. Mater. 2023;35:e2203193. doi: 10.1002/adma.202203193. [DOI] [PubMed] [Google Scholar]
- 2.Sharma A., Chopra H., Singh I., Emran T.B. Physically and Chemically Crosslinked Hydrogels for Wound Healing Applications. Int. J. Surg. 2022;106:106915. doi: 10.1016/j.ijsu.2022.106915. [DOI] [PubMed] [Google Scholar]
- 3.Vinaik R., Barayan D., Jeschke M.G. Essential Burn Care for Non-Burn Specialists. Springer; Cham, Switzerland: 2023. Pathophysiology and Hypermetabolic Response to Burn. [DOI] [Google Scholar]
- 4.Nosrati H., Heydari M., Tootiaei Z., Ganjbar S., Khodaei M. Delivery of Antibacterial Agents for Wound Healing Applications Using Polysaccharide-Based Scaffolds. J. Drug Deliv. Sci. Technol. 2023;84:104516. doi: 10.1016/j.jddst.2023.104516. [DOI] [Google Scholar]
- 5.Hetta H.F., Ramadan Y.N., Al-Harbi A.I., Ahmed E.A., Battah B., Abd Ellah N.H., Zanetti S., Donadu M.G. Nanotechnology as a Promising Approach to Combat Multidrug Resistant Bacteria: A Comprehensive Review and Future Perspectives. Biomedicines. 2023;11:413. doi: 10.3390/biomedicines11020413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wenthen R., Landers Z.A. Traumatic Injury and Traumatic Brain Injury. In: Hemphill M., Nathanson A., editors. The Practice of Clinical Social Work in Healthcare. Springer International Publishing; Cham, Switzerland: 2023. pp. 215–239. [DOI] [Google Scholar]
- 7.Jan B., Jan R., Afzal S., Ayoub M., Masoodi M.H. Treatment Strategies to Combat Multidrug Resistance (MDR) in Bacteria. In: Wani M.Y., Ahmad A., editors. Non-Traditional Approaches to Combat Antimicrobial Drug Resistance. Springer Nature; Singapore: 2023. pp. 79–100. [DOI] [Google Scholar]
- 8.Chopra H., Kumar S., Singh I. Strategies and Therapies for Wound Healing: A Review. Curr. Drug Targets. 2021;23:87–98. doi: 10.2174/18735592MTE1cMzQ0y. [DOI] [PubMed] [Google Scholar]
- 9.Sadeghi A., Ebrahimi M., Kharazmi M.S., Jafari S.M. Role of Nanomaterials in Improving the Functionality of Probiotics; Integration of Nanotechnology onto Micro-Structured Platforms. Food Biosci. 2023;53:102843. doi: 10.1016/j.fbio.2023.102843. [DOI] [Google Scholar]
- 10.Chhabra J., Chopra H., Pahwa R., Raina N., Wadhwa K., Saini S., Negi P., Gupta M., Singh I., Dureja H., et al. Potential of Nanoemulsions for Accelerated Wound Healing: Innovative Strategies. Int. J. Surg. 2023;109:2365–2377. doi: 10.1097/JS9.0000000000000460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharma R., Borah S.J., Bhawna N., Kumar S., Gupta A., Kumari V., Kumar R., Dubey K.K., Kumar V. Emerging Trends in Nano-Based Antidiabetic Therapeutics: A Path to Effective Diabetes Management. Mater. Adv. 2023;4:3091–3113. doi: 10.1039/D3MA00159H. [DOI] [Google Scholar]
- 12.Roy M., Roy A., Rustagi S., Pandey N. An Overview of Nanomaterial Applications in Pharmacology. BioMed Res. Int. 2023;2023:4838043. doi: 10.1155/2023/4838043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhattacharjee R., Negi A., Bhattacharya B., Dey T., Mitra P., Preetam S., Kumar L., Kar S., Das S.S., Iqbal D., et al. Nanotheranostics to Target Antibiotic-Resistant Bacteria: Strategies and Applications. OpenNano. 2023;11:100138. doi: 10.1016/j.onano.2023.100138. [DOI] [Google Scholar]
- 14.Abbasi R., Shineh G., Mobaraki M., Doughty S., Tayebi L. Structural Parameters of Nanoparticles Affecting Their Toxicity for Biomedical Applications: A Review. J. Nanoparticle Res. 2023;25:43. doi: 10.1007/s11051-023-05690-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang G., Yang F., Zhou W., Xiao N., Luo M., Tang Z. The Initiation of Oxidative Stress and Therapeutic Strategies in Wound Healing. Biomed. Pharmacother. 2023;157:114004. doi: 10.1016/j.biopha.2022.114004. [DOI] [PubMed] [Google Scholar]
- 16.Sung S., Steele L.A., Risser G.E., Spiller K.L. Biomaterial-Assisted Macrophage Cell Therapy for Regenerative Medicine. Adv. Drug Deliv. Rev. 2023;199:114979. doi: 10.1016/j.addr.2023.114979. [DOI] [PubMed] [Google Scholar]
- 17.Muñoz-González P.U., Flores-Moreno J.M., Quintero-Ortega I.A., Mantovani D., Mendoza-Novelo B., González-García G. Water-Dispersible Fluorescent Silicon Nanoparticles That Modulate Inflammatory Response in Macrophages. ACS Appl. Nano Mater. 2023;6:11187–11197. doi: 10.1021/acsanm.3c01210. [DOI] [Google Scholar]
- 18.Benoit A., Vogin G., Duhem C., Berchem G., Janji B. Lighting Up the Fire in the Microenvironment of Cold Tumors: A Major Challenge to Improve Cancer Immunotherapy. Cells. 2023;12:1787. doi: 10.3390/cells12131787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parikh S.D., Wang W., Nelson M.T., Sulentic C.E.W., Mukhopadhyay S.M. Bioinspired Hierarchical Carbon Structures as Potential Scaffolds for Wound Healing and Tissue Regeneration Applications. Nanomaterials. 2023;13:1791. doi: 10.3390/nano13111791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soni S.S., D’Elia A.M., Rodell C.B. Control of the Post-Infarct Immune Microenvironment through Biotherapeutic and Biomaterial-Based Approaches. Drug Deliv. Transl. Res. 2023;13:1983–2014. doi: 10.1007/s13346-023-01290-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mehryab F., Taghizadeh F., Goshtasbi N., Merati F., Rabbani S., Haeri A. Exosomes as Cutting-Edge Therapeutics in Various Biomedical Applications: An Update on Engineering, Delivery, and Preclinical Studies. Biochimie. 2023;213:139–167. doi: 10.1016/j.biochi.2023.05.010. [DOI] [PubMed] [Google Scholar]
- 22.Zhao W., Zhang H., Liu R., Cui R. Advances in Immunomodulatory Mechanisms of Mesenchymal Stem Cells-Derived Exosome on Immune Cells in Scar Formation. Int. J. Nanomed. 2023;18:3643–3662. doi: 10.2147/IJN.S412717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Desai N., Rana D., Salave S., Gupta R., Patel P., Karunakaran B., Sharma A., Giri J., Benival D., Kommineni N. Chitosan: A Potential Biopolymer in Drug Delivery and Biomedical Applications. Pharmaceutics. 2023;15:1313. doi: 10.3390/pharmaceutics15041313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pang Q., Jiang Z., Wu K., Hou R., Zhu Y. Nanomaterials-Based Wound Dressing for Advanced Management of Infected Wound. Antibiotics. 2023;12:351. doi: 10.3390/antibiotics12020351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou Y., Li M., Gao W., Li X., Long L., Hou X., Zhao J., Li S., Yuan X. Microstructure-United Heterogeneous Sodium Alginate Doped Injectable Hydrogel for Stable Hemostasis in Dynamic Mechanical Environments. Int. J. Biol. Macromol. 2023;248:125877. doi: 10.1016/j.ijbiomac.2023.125877. [DOI] [PubMed] [Google Scholar]
- 26.Tuli H.S., Joshi R., Kaur G., Garg V.K., Sak K., Varol M., Kaur J., Alharbi S.A., Alahmadi T.A., Aggarwal D., et al. Metal Nanoparticles in Cancer: From Synthesis and Metabolism to Cellular Interactions. J. Nanostructure Chem. 2023;13:321–348. doi: 10.1007/s40097-022-00504-2. [DOI] [Google Scholar]
- 27.Lu Z., Li J., Chen B., Feng J., Hu Q., Jin Y., Fu Z. Mitochondria Targeted Nanoparticles Potentiate Tumor Chemo-Phototherapy by Toxic Oxidative Stress Mediated Oxeiptosis. Macromol. Biosci. 2023;23:e2300151. doi: 10.1002/mabi.202300151. [DOI] [PubMed] [Google Scholar]
- 28.Lee J., Kang S., Park H., Sun J.G., Kim E.C., Shim G. Nanoparticles for Lymph Node-Directed Delivery. Pharmaceutics. 2023;15:565. doi: 10.3390/pharmaceutics15020565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abdel-Halim A. Strategies for Cancer Therapy: Targeting Tumor Microenvironment and Nanotechnology. Egypt. Pharm. J. 2023;22:165–176. doi: 10.4103/epj.epj_188_22. [DOI] [Google Scholar]
- 30.Choudhury H., Pandey M., Saravanan V., Mun A.T.Y., Bhattamisra S.K., Parikh A., Garg S., Gorain B. Recent Progress of Targeted Nanocarriers in Diagnostic, Therapeutic, and Theranostic Applications in Colorectal Cancer. Biomater. Adv. 2023;153:213556. doi: 10.1016/j.bioadv.2023.213556. [DOI] [PubMed] [Google Scholar]
- 31.Bao M., Wang K., Li J., Li Y., Zhu H., Lu M., Zhang Y., Fan Q., Han L., Wang K., et al. ROS Scavenging and Inflammation-Directed Polydopamine Nanoparticles Regulate Gut Immunity and Flora Therapy in Inflammatory Bowel Disease. Acta Biomater. 2023;161:250–264. doi: 10.1016/j.actbio.2023.02.026. [DOI] [PubMed] [Google Scholar]
- 32.Chen S., Xing Z., Geng M., Zhao R., Yang X., Zhu X., Anderson J.M., Zhang X. Macrophage Fusion Event as One Prerequisite for Inorganic Nanoparticle-Induced Antitumor Response. Sci. Adv. 2023;9:eadd9871. doi: 10.1126/sciadv.add9871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang J., Liu Y., Liu Y., Huang H., Roy S., Song Z., Guo B. Recent Advances in Nanomedicines for Imaging and Therapy of Myocardial Ischemia-Reperfusion Injury. J. Control. Release. 2023;353:563–590. doi: 10.1016/j.jconrel.2022.11.057. [DOI] [PubMed] [Google Scholar]
- 34.Tonelli A., Lumngwena E.N., Ntusi N.A.B. The Oral Microbiome in the Pathophysiology of Cardiovascular Disease. Nat. Rev. Cardiol. 2023;20:386–403. doi: 10.1038/s41569-022-00825-3. [DOI] [PubMed] [Google Scholar]
- 35.Zhang F., Xu Z., Jolly K.J. Myeloid Cell-Mediated Drug Delivery: From Nanomedicine to Cell Therapy. Adv. Drug Deliv. Rev. 2023;197:114827. doi: 10.1016/j.addr.2023.114827. [DOI] [PubMed] [Google Scholar]
- 36.Ribeiro A.M., Santos A.I., Veiga F., Figueiras A. Functional Nanocomposite Hydrogels: Synthesis, Characterization, and Biomedical Applications. Elsevier; Amsterdam, The Netherlands: 2023. Lignin Nanoparticle–Based Nanocomposite Hydrogels for Biomedical Applications. [DOI] [Google Scholar]
- 37.Kushwaha R., Kumar S., Das A., Sukriti, Verma M.L. Functional Nanocomposite Hydrogels: Synthesis, Characterization, and Biomedical Applications. Elsevier; Amsterdam, The Netherlands: 2023. Silver Nanoparticle-Based Nanocomposite Hydrogels for Biomedical Applications. [DOI] [Google Scholar]
- 38.Okoye C.O., Okeke E.S., Ezeorba T.P.C., Chukwudozie K.I., Chiejina C.O., Fomena Temgoua N.S. Microbes for Natural Food Additives. Springer; Singapore: 2023. Microbial and Bio-Based Preservatives: Recent Advances in Antimicrobial Compounds. [DOI] [Google Scholar]
- 39.Rahim G., Qureshi R., Hazrat A., Ahmad B., Khan A.A., Aziz T., Alharbi M., Alshammari A. Phytochemical, Antimicrobial, Radical Scavenging and In-Vitro Biological Activities of Teucrium stocksianum Leaves. J. Chil. Chem. Soc. 2023;68:5748–5754. doi: 10.4067/S0717-97072023000105748. [DOI] [Google Scholar]
- 40.Gambhir K., Tyagi N., Verma Y.K. Fiber and Textile Engineering in Drug Delivery Systems. Woodhead Publishing; Sawston, UK: 2023. Next-Generation Bandages to Overcome Oxygen Limitation during Wound Healing/Tissue Repair. [DOI] [Google Scholar]
- 41.Musyuni P., Nagpal M., Singh M., Goyal R.K., Aggarwal G. Nanotechnology Enabled Solutions to Combat COVID-19: Prevention, Treatment, and Diagnosis. Curr. Pharm. Biotechnol. 2021;23:98–111. doi: 10.2174/1389201022666210122124311. [DOI] [PubMed] [Google Scholar]
- 42.Aydeger A., Aysit N., Baydas G., Cakici C., Erim U.C., Arpa M.D., Ozcicek I. Design of IKVAV Peptide/Gold Nanoparticle Decorated, Micro/Nano-Channeled PCL/PLGA Film Scaffolds for Neuronal Differentiation and Neurite Outgrowth. Biomater. Adv. 2023;152:213472. doi: 10.1016/j.bioadv.2023.213472. [DOI] [PubMed] [Google Scholar]
- 43.Londhe S., Haque S., Patra C.R. Gold and Silver Nanoparticles: Synthesis and Applications. Elsevier; Amsterdam, The Netherlands: 2023. Silver and Gold Nanoparticles: Potential Cancer Theranostic Applications, Recent Development, Challenges, and Future Perspectives. [DOI] [Google Scholar]
- 44.Dam P., Celik M., Ustun M., Saha S., Saha C., Kacar E.A., Kugu S., Karagulle E.N., Tasoglu S., Buyukserin F., et al. Wound Healing Strategies Based on Nanoparticles Incorporated in Hydrogel Wound Patches. RSC Adv. 2023;13:21345–21364. doi: 10.1039/D3RA03477A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alvandi H., Rajati H., Naseriyeh T., Rahmatabadi S.S., Hosseinzadeh L., Arkan E. Incorporation of Aloe vera and Green Synthesized ZnO Nanoparticles into the Chitosan/PVA Nanocomposite Hydrogel for Wound Dressing Application. Polym. Bull. 2023;2023:1–16. doi: 10.1007/s00289-023-04874-7. [DOI] [Google Scholar]
- 46.Xiang Y., Qi X., Cai E., Zhang C., Wang J., Lan Y., Deng H., Shen J., Hu R. Highly Efficient Bacteria-Infected Diabetic Wound Healing Employing a Melanin-Reinforced Biopolymer Hydrogel. Chem. Eng. J. 2023;460:141852. doi: 10.1016/j.cej.2023.141852. [DOI] [Google Scholar]
- 47.Tiwari N., Kumar D., Priyadarshani A., Jain G.K., Mittal G., Kesharwani P., Aggarwal G. Recent Progress in Polymeric Biomaterials and Their Potential Applications in Skin Regeneration and Wound Care Management. J. Drug Deliv. Sci. Technol. 2023;82:104319. doi: 10.1016/j.jddst.2023.104319. [DOI] [Google Scholar]
- 48.Latiyan S., Kumar T.S.S., Doble M., Kennedy J.F. Perspectives of Nanofibrous Wound Dressings Based on Glucans and Galactans-A Review. Int. J. Biol. Macromol. 2023;244:125358. doi: 10.1016/j.ijbiomac.2023.125358. [DOI] [PubMed] [Google Scholar]
- 49.Zhang Y., Zhu Y., Ma P., Wu H., Xiao D., Zhang Y., Sui X., Zhang L., Dong A. Functional Carbohydrate-Based Hydrogels for Diabetic Wound Therapy. Carbohydr. Polym. 2023;312:120823. doi: 10.1016/j.carbpol.2023.120823. [DOI] [PubMed] [Google Scholar]
- 50.Rezvani Ghomi E., Niazi M., Ramakrishna S. The Evolution of Wound Dressings: From Traditional to Smart Dressings. Polym. Adv. Technol. 2023;34:520–530. doi: 10.1002/pat.5929. [DOI] [Google Scholar]
- 51.Wang S., Wu W., Yeo J.C.C., Soo X.Y.D., Thitsartarn W., Liu S., Tan B.H., Suwardi A., Li Z., Zhu Q., et al. Responsive Hydrogel Dressings for Intelligent Wound Management. BMEMat. 2023;1:e12021. doi: 10.1002/bmm2.12021. [DOI] [Google Scholar]
- 52.Li Z.Y., Zhang X.J., Gao Y.M., Song Y., Sands M.X., Zhou S.B., Li Q.F., Zhang J. Photo-Responsive Hydrogel for Contactless Dressing Change to Attenuate Secondary Damage and Promote Diabetic Wound Healing. Adv. Health Mater. 2023;12:e2202770. doi: 10.1002/adhm.202202770. [DOI] [PubMed] [Google Scholar]
- 53.Huanbutta K., Sittikijyothin W., Sangnim T. Development of Topical Natural Based Film Forming System Loaded Propolis from Stingless Bees for Wound Healing Application. J. Pharm. Investig. 2020;50:625–634. doi: 10.1007/s40005-020-00493-w. [DOI] [Google Scholar]
- 54.Sangnim T., Meeboon P., Phongsewalak P., Prasongdee P., Sriamornsak P., Singh I., Manmuan S., Huanbutta K. Development and Evaluation of Liquid Plaster Loaded with Chromolaena Odorata Leaf Extract Endowed with Several Beneficial Properties to Wound Healing. Gels. 2022;8:72. doi: 10.3390/gels8020072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lu H., Shao W., Gao B., Zheng S., He B. Intestine-Inspired Wrinkled MXene Microneedle Dressings for Smart Wound Management. Acta Biomater. 2023;159:201–210. doi: 10.1016/j.actbio.2023.01.035. [DOI] [PubMed] [Google Scholar]
- 56.Zhuang X., Deng G., Wu X., Xie J., Li D., Peng S., Tang D., Zhou G. Recent Advances of Three-Dimensional Bioprinting Technology in Hepato-Pancreato-Biliary Cancer Models. Front. Oncol. 2023;13:1143600. doi: 10.3389/fonc.2023.1143600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Armstrong D.G., Edmonds M.E., Serena T.E. Point-of-Care Fluorescence Imaging Reveals Extent of Bacterial Load in Diabetic Foot Ulcers. Int. Wound J. 2023;20:554–566. doi: 10.1111/iwj.14080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Arshad R., Razlansari M., Maryam Hosseinikhah S., Tiwari Pandey A., Ajalli N., Ezra Manicum A.L., Thorat N., Rahdar A., Zhu Y., Tabish T.A. Antimicrobial and Anti-Biofilm Activities of Bio-Inspired Nanomaterials for Wound Healing Applications. Drug Discov. Today. 2023;28:103673. doi: 10.1016/j.drudis.2023.103673. [DOI] [PubMed] [Google Scholar]
- 59.Yaşayan G., Nejati O., Ceylan A.F., Karasu Ç., Kelicen Ugur P., Bal-Öztürk A., Zarepour A., Zarrabi A., Mostafavi E. Tackling Chronic Wound Healing Using Nanomaterials: Advancements, Challenges, and Future Perspectives. Appl. Mater. Today. 2023;32:101829. doi: 10.1016/j.apmt.2023.101829. [DOI] [Google Scholar]
- 60.Chandrasekaran R., Krishnan M., Bupesh G., Chacko S., Gawade O., Hasan S., George E., Vijayakumar T.S., Sundaram M., Sagadevan S. Prospective Features of Functional 2D Nanomaterial Graphene Oxide in the Wound Healing Process. J. Drug Deliv. Sci. Technol. 2023;82:104352. doi: 10.1016/j.jddst.2023.104352. [DOI] [Google Scholar]
- 61.Prakashan D., Roberts A., Gandhi S. Recent Advancement of Nanotherapeutics in Accelerating Chronic Wound Healing Process for Surgical Wounds and Diabetic Ulcers. Biotechnol. Genet. Eng. Rev. 2023;2023:1–29. doi: 10.1080/02648725.2023.2167432. [DOI] [PubMed] [Google Scholar]
- 62.Hou L., Zhang X., Du H. Advances in Mesenchymal Stromal Cells and Nanomaterials for Diabetic Wound Healing. Diabetes/Metab. Res. Rev. 2023;39:e3638. doi: 10.1002/dmrr.3638. [DOI] [PubMed] [Google Scholar]
- 63.Issaka E. State-of-the-Art of Synthesized Exosomes and NPs-Based Biomimetic Nanoparticles for Wound Rehabilitation: A Review. Biomed. Mater. Devices. 2023;2023:1–34. doi: 10.1007/s44174-023-00112-w. [DOI] [Google Scholar]
- 64.Liu T., Lu Y., Zhan R., Qian W., Luo G. Nanomaterials and Nanomaterials-Based Drug Delivery to Promote Cutaneous Wound Healing. Adv. Drug Deliv. Rev. 2023;193:114670. doi: 10.1016/j.addr.2022.114670. [DOI] [PubMed] [Google Scholar]
- 65.Kushwaha A., Goswami L., Kim B.S. Nanomaterial-Based Therapy for Wound Healing. Nanomaterials. 2022;12:618. doi: 10.3390/nano12040618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rauti R., Musto M., Bosi S., Prato M., Ballerini L. Properties and Behavior of Carbon Nanomaterials When Interfacing Neuronal Cells: How Far Have We Come? Carbon. 2019;143:430–446. doi: 10.1016/j.carbon.2018.11.026. [DOI] [Google Scholar]
- 67.Chen D., Dougherty C.A., Zhu K., Hong H. Theranostic Applications of Carbon Nanomaterials in Cancer: Focus on Imaging and Cargo Delivery. J. Control. Release. 2015;210:230–245. doi: 10.1016/j.jconrel.2015.04.021. [DOI] [PubMed] [Google Scholar]
- 68.Sajjadi M., Nasrollahzadeh M., Jaleh B., Soufi G.J., Iravani S. Carbon-Based Nanomaterials for Targeted Cancer Nanotherapy: Recent Trends and Future Prospects. J. Drug Target. 2021;29:716–741. doi: 10.1080/1061186X.2021.1886301. [DOI] [PubMed] [Google Scholar]
- 69.Tonelli F.M., Goulart V.A., Gomes K.N., Ladeira M.S., Santos A.K., Lorençon E., Ladeira L.O., Resende R.R. Graphene-Based Nanomaterials: Biological and Medical Applications and Toxicity. Nanomedicine. 2015;10:2423–2450. doi: 10.2217/nnm.15.65. [DOI] [PubMed] [Google Scholar]
- 70.Bhattacharya K., Mukherjee S.P., Gallud A., Burkert S.C., Bistarelli S., Bellucci S., Bottini M., Star A., Fadeel B. Biological Interactions of Carbon-Based Nanomaterials: From Coronation to Degradation. Nanomed. Nanotechnol. Biol. Med. 2016;12:333–351. doi: 10.1016/j.nano.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mohajeri M., Behnam B., Sahebkar A. Biomedical Applications of Carbon Nanomaterials: Drug and Gene Delivery Potentials. J. Cell. Physiol. 2018;234:298–319. doi: 10.1002/jcp.26899. [DOI] [PubMed] [Google Scholar]
- 72.Debnath S.K., Srivastava R. Drug Delivery with Carbon-Based Nanomaterials as Versatile Nanocarriers: Progress and Prospects. Front. Nanotechnol. 2021;3:644564. doi: 10.3389/fnano.2021.644564. [DOI] [Google Scholar]
- 73.Iijima S. Helical Microtubules of Graphitic Carbon. Nature. 1991;354:56–58. doi: 10.1038/354056a0. [DOI] [Google Scholar]
- 74.Ahmad M., Silva S.R.P. Low Temperature Growth of Carbon Nanotubes—A Review. Carbon. 2020;158:24–44. doi: 10.1016/j.carbon.2019.11.061. [DOI] [Google Scholar]
- 75.Gupta N., Gupta S.M., Sharma S.K. Carbon Nanotubes: Synthesis, Properties and Engineering Applications. Carbon Lett. 2019;29:419–447. doi: 10.1007/s42823-019-00068-2. [DOI] [Google Scholar]
- 76.Faraji S., Stano K.L., Yildiz O., Li A., Zhu Y., Bradford P.D. Ultralight Anisotropic Foams from Layered Aligned Carbon Nanotube Sheets. Nanoscale. 2015;7:17038–17047. doi: 10.1039/C5NR03899E. [DOI] [PubMed] [Google Scholar]
- 77.Lee E., Salgado R.A., Lee B., Sumant A.V., Rajh T., Johnson C., Balandin A.A., Shevchenko E.V. Design of Lithium Cobalt Oxide Electrodes with High Thermal Conductivity and Electrochemical Performance Using Carbon Nanotubes and Diamond Particles. Carbon N. Y. 2018;129:702–710. doi: 10.1016/j.carbon.2017.12.061. [DOI] [Google Scholar]
- 78.Marconnet A.M., Panzer M.A., Goodson K.E. Thermal Conduction Phenomena in Carbon Nanotubes and Related Nanostructured Materials. Rev. Mod. Phys. 2013;85:1295–1326. doi: 10.1103/RevModPhys.85.1295. [DOI] [Google Scholar]
- 79.Han Z., Fina A. Thermal Conductivity of Carbon Nanotubes and Their Polymer Nanocomposites: A Review. Prog. Polym. Sci. 2011;36:914–944. doi: 10.1016/j.progpolymsci.2010.11.004. [DOI] [Google Scholar]
- 80.Maruyama S. A Molecular Dynamics Simulation of Heat Conduction of a Finite Length Single-Walled Carbon Nanotube. Microscale Thermophys. Eng. 2003;7:41–50. doi: 10.1080/10893950390150467. [DOI] [Google Scholar]
- 81.Kumanek B., Janas D. Thermal Conductivity of Carbon Nanotube Networks: A Review. J. Mater. Sci. 2019;54:7397–7427. doi: 10.1007/s10853-019-03368-0. [DOI] [Google Scholar]
- 82.Berber S., Kwon Y.K., Tománek D. Unusually High Thermal Conductivity of Carbon Nanotubes. Phys. Rev. Lett. 2000;84:4613–4616. doi: 10.1103/PhysRevLett.84.4613. [DOI] [PubMed] [Google Scholar]
- 83.Osman M.A., Srivastava D. Temperature Dependence of the Thermal Conductivity of Single-Wall Carbon Nanotubes. Nanotechnology. 2001;12:21–24. doi: 10.1088/0957-4484/12/1/305. [DOI] [Google Scholar]
- 84.Patil T.V., Patel D.K., Dutta S.D., Ganguly K., Randhawa A., Lim K.T. Carbon Nanotubes-Based Hydrogels for Bacterial Eradiation and Wound-Healing Applications. Appl. Sci. 2021;11:9550. doi: 10.3390/app11209550. [DOI] [Google Scholar]
- 85.Khalid A., Madni A., Raza B., Islam M.U., Hassan A., Ahmad F., Ali H., Khan T., Wahid F. Multiwalled Carbon Nanotubes Functionalized Bacterial Cellulose as an Efficient Healing Material for Diabetic Wounds. Int. J. Biol. Macromol. 2022;203:256–267. doi: 10.1016/j.ijbiomac.2022.01.146. [DOI] [PubMed] [Google Scholar]
- 86.Chen G., Wu Y., Yu D., Li R., Luo W., Ma G., Zhang C. Isoniazid-Loaded Chitosan/Carbon Nanotubes Microspheres Promote Secondary Wound Healing of Bone Tuberculosis. J. Biomater. Appl. 2019;33:989–996. doi: 10.1177/0885328218814988. [DOI] [PubMed] [Google Scholar]
- 87.Kittana N., Assali M., Abu-Rass H., Lutz S., Hindawi R., Ghannam L., Zakarneh M., Mousa A. Enhancement of Wound Healing by Single-Wall/Multi-Wall Carbon Nanotubes Complexed with Chitosan. Int. J. Nanomed. 2018;13:7195–7206. doi: 10.2147/IJN.S183342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kolahdouz M., Xu B., Nasiri A.F., Fathollahzadeh M., Manian M., Aghababa H., Wu Y., Radamson H.H. Carbon-Related Materials: Graphene and Carbon Nanotubes in Semiconductor Applications and Design. Micromachines. 2022;13:1257. doi: 10.3390/mi13081257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Beg S., Rahman M., Jain A., Saini S., Hasnain M.S., Swain S., Imam S., Kazmi I., Akhter S. Fullerenes, Graphenes and Nanotubes: A Pharmaceutical Approach. William Andrew Publishing; Norwich, NY, USA: 2018. Emergence in the Functionalized Carbon Nanotubes as Smart Nanocarriers for Drug Delivery Applications. [DOI] [Google Scholar]
- 90.Liang F., Chen B. A Review on Biomedical Applications of Single-Walled Carbon Nanotubes. Curr. Med. Chem. 2009;17:10–24. doi: 10.2174/092986710789957742. [DOI] [PubMed] [Google Scholar]
- 91.Vander Wal R.L., Berger G.M., Ticich T.M. Carbon Nanotube Synthesis in a Flame Using Laser Ablation for in Situ Catalyst Generation. Appl. Phys. A Mater. Sci. Process. 2003;77:885–889. doi: 10.1007/s00339-003-2196-3. [DOI] [Google Scholar]
- 92.Vashist A., Kaushik A., Vashist A., Sagar V., Ghosal A., Gupta Y.K., Ahmad S., Nair M. Advances in Carbon Nanotubes–Hydrogel Hybrids in Nanomedicine for Therapeutics. Adv. Healthc. Mater. 2018;7:e1701213. doi: 10.1002/adhm.201701213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Punbusayakul N., Talapatra S., Ajayan P.M., Surareungchai W. Label-Free as-Grown Double Wall Carbon Nanotubes Bundles for Salmonella Typhimurium Immunoassay. Chem. Cent. J. 2013;7:102. doi: 10.1186/1752-153X-7-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bhatt A., Jain A., Gurnany E., Jain R., Modi A., Jain A. Nanoarchitectonics for Smart Delivery and Drug Targeting. William Andrew Publishing; Norwich, NY, USA: 2016. Carbon Nanotubes: A Promising Carrier for Drug Delivery and Targeting. [DOI] [Google Scholar]
- 95.Matta-Domjan B., King A., Totti S., Matta C., Dover G., Martinez P., Zakhidov A., La Ragione R., Macedo H., Jurewicz I., et al. Biophysical Interactions between Pancreatic Cancer Cells and Pristine Carbon Nanotube Substrates: Potential Application for Pancreatic Cancer Tissue Engineering. J. Biomed. Mater. Res. B Appl. Biomater. 2018;106:1637–1644. doi: 10.1002/jbm.b.34012. [DOI] [PubMed] [Google Scholar]
- 96.Jeevanandam J., Barhoum A., Chan Y.S., Dufresne A., Danquah M.K. Review on Nanoparticles and Nanostructured Materials: History, Sources, Toxicity and Regulations. Beilstein J. Nanotechnol. 2018;9:1050–1074. doi: 10.3762/bjnano.9.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lee S.H., Jun B.H. Silver Nanoparticles: Synthesis and Application for Nanomedicine. Int. J. Mol. Sci. 2019;20:865. doi: 10.3390/ijms20040865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kordy M.G.M., Abdel-Gabbar M., Soliman H.A., Aljohani G., Binsabt M., Ahmed I.A., Shaban M. Phyto-Capped Ag Nanoparticles: Green Synthesis, Characterization, and Catalytic and Antioxidant Activities. Nanomaterials. 2022;12:373. doi: 10.3390/nano12030373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mitchell M.J., Billingsley M.M., Haley R.M., Wechsler M.E., Peppas N.A., Langer R. Engineering Precision Nanoparticles for Drug Delivery. Nat. Rev. Drug Discov. 2021;20:101–124. doi: 10.1038/s41573-020-0090-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Garg U., Chauhan S., Nagaich U., Jain N. Current Advances in Chitosan Nanoparticles Based Drug Delivery and Targeting. Adv. Pharm. Bull. 2019;9:195–204. doi: 10.15171/apb.2019.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yu Y.Q., Yang X., Wu X.F., Fan Y.B. Enhancing Permeation of Drug Molecules Across the Skin via Delivery in Nanocarriers: Novel Strategies for Effective Transdermal Applications. Front. Bioeng. Biotechnol. 2021;9:646554. doi: 10.3389/fbioe.2021.646554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Balaure P.C., Grumezescu A.M. Recent Advances in Surface Nanoengineering for Biofilm Prevention and Control. Part i: Molecular Basis of Biofilm Recalcitrance. Passive Anti-Biofouling Nanocoatings. Nanomaterials. 2020;10:1230. doi: 10.3390/nano10061230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nikiforov A., Ma C., Choukourov A., Palumbo F. Plasma Technology in Antimicrobial Surface Engineering. J. Appl. Phys. 2022;131:011102. doi: 10.1063/5.0066724. [DOI] [Google Scholar]
- 104.Galogahi F.M., Zhu Y., An H., Nguyen N.T. Core-Shell Microparticles: Generation Approaches and Applications. J. Sci. Adv. Mater. Devices. 2020;5:417–435. doi: 10.1016/j.jsamd.2020.09.001. [DOI] [Google Scholar]
- 105.Wieszczycka K., Staszak K., Woźniak-Budych M.J., Litowczenko J., Maciejewska B.M., Jurga S. Surface Functionalization–The Way for Advanced Applications of Smart Materials. Coord. Chem. Rev. 2021;436:213846. doi: 10.1016/j.ccr.2021.213846. [DOI] [Google Scholar]
- 106.Huanbutta K., Sittikijyothin W., Sangnim T. Development and Characterization of Bilayer Wound Healing Patch Nanofiber Fabricated by Electrospinning. J. Nano Res. 2019;59:46–56. doi: 10.4028/www.scientific.net/JNanoR.59.46. [DOI] [Google Scholar]
- 107.Doolan J.A., Williams G.T., Hilton K.L.F., Chaudhari R., Fossey J.S., Goult B.T., Hiscock J.R. Advancements in Antimicrobial Nanoscale Materials and Self-Assembling Systems. Chem. Soc. Rev. 2022;51:8696–8755. doi: 10.1039/D1CS00915J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nakhaei P., Margiana R., Bokov D.O., Abdelbasset W.K., Jadidi Kouhbanani M.A., Varma R.S., Marofi F., Jarahian M., Beheshtkhoo N. Liposomes: Structure, Biomedical Applications, and Stability Parameters with Emphasis on Cholesterol. Front. Bioeng. Biotechnol. 2021;9:705886. doi: 10.3389/fbioe.2021.705886. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 109.Ruseska I., Fresacher K., Petschacher C., Zimmer A. Use of Protamine in Nanopharmaceuticals—A Review. Nanomaterials. 2021;11:1508. doi: 10.3390/nano11061508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nsairat H., Khater D., Sayed U., Odeh F., Al Bawab A., Alshaer W. Liposomes: Structure, Composition, Types, and Clinical Applications. Heliyon. 2022;8:e09394. doi: 10.1016/j.heliyon.2022.e09394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Brayden D.J., Hill T.A., Fairlie D.P., Maher S., Mrsny R.J. Systemic Delivery of Peptides by the Oral Route: Formulation and Medicinal Chemistry Approaches. Adv. Drug Deliv. Rev. 2020;157:2–36. doi: 10.1016/j.addr.2020.05.007. [DOI] [PubMed] [Google Scholar]
- 112.Lombardo D., Kiselev M.A. Methods of Liposomes Preparation: Formation and Control Factors of Versatile Nanocarriers for Biomedical and Nanomedicine Application. Pharmaceutics. 2022;14:543. doi: 10.3390/pharmaceutics14030543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Eid H.M., Ali A.A., Ali A.M.A., Eissa E.M., Hassan R.M., El-Ela F.I.A., Hassan A.H. Potential Use of Tailored Citicoline Chitosan-Coated Liposomes for Effective Wound Healing in Diabetic Rat Model. Int. J. Nanomed. 2022;17:555–575. doi: 10.2147/IJN.S342504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cardoso-Daodu I.M., Ilomuanya M.O., Azubuike C.P. Development of Curcumin-Loaded Liposomes in Lysine–Collagen Hydrogel for Surgical Wound Healing. Beni Suef Univ. J. Basic Appl. Sci. 2022;11:100. doi: 10.1186/s43088-022-00284-2. [DOI] [Google Scholar]
- 115.Hemmingsen L.M., Panchai P., Julin K., Basnet P., Nystad M., Johannessen M., Škalko-Basnet N. Chitosan-Based Delivery System Enhances Antimicrobial Activity of Chlorhexidine. Front. Microbiol. 2022;13:1023083. doi: 10.3389/fmicb.2022.1023083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Shu G., Xu D., Zhang W., Zhao X., Li H., Xu F., Yin L., Peng X., Fu H., Chang L.J., et al. Preparation of Shikonin Liposome and Evaluation of Its in Vitro Antibacterial and in Vivo Infected Wound Healing Activity. Phytomedicine. 2022;99:154035. doi: 10.1016/j.phymed.2022.154035. [DOI] [PubMed] [Google Scholar]
- 117.Mengoni T., Adrian M., Pereira S., Santos-Carballal B., Kaiser M., Goycoolea F.M. A Chitosan-Based Liposome Formulation Enhances the in Vitro Wound Healing Efficacy of Substance P Neuropeptide. Pharmaceutics. 2017;9:56. doi: 10.3390/pharmaceutics9040056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ternullo S., Werning L.V.S., Holsæter A.M., Škalko-Basnet N. Curcumin-in-Deformable Liposomes-in-Chitosan-Hydrogel as a Novel Wound Dressing. Pharmaceutics. 2020;12:8. doi: 10.3390/pharmaceutics12010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Liu Y., Song S., Liu S., Zhu X., Wang P. Application of Nanomaterial in Hydrogels Related to Wound Healing. J. Nanomater. 2022;2022:4656037. doi: 10.1155/2022/4656037. [DOI] [Google Scholar]
- 120.Liang Y., He J., Guo B. Functional Hydrogels as Wound Dressing to Enhance Wound Healing. ACS Nano. 2021;15:12687–12722. doi: 10.1021/acsnano.1c04206. [DOI] [PubMed] [Google Scholar]
- 121.Luneva O., Olekhnovich R., Uspenskaya M. Bilayer Hydrogels for Wound Dressing and Tissue Engineering. Polymers. 2022;14:3135. doi: 10.3390/polym14153135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Divyashri G., Badhe R.V., Sadanandan B., Vijayalakshmi V., Kumari M., Ashrit P., Bijukumar D., Mathew M.T., Shetty K., Raghu A.V. Applications of Hydrogel-Based Delivery Systems in Wound Care and Treatment: An up-to-Date Review. Polym. Adv. Technol. 2022;33:2025–2043. doi: 10.1002/pat.5661. [DOI] [Google Scholar]
- 123.Ding Y.W., Wang Z.Y., Ren Z.W., Zhang X.W., Wei D.X. Advances in Modified Hyaluronic Acid-Based Hydrogels for Skin Wound Healing. Biomater. Sci. 2022;10:3393–3409. doi: 10.1039/D2BM00397J. [DOI] [PubMed] [Google Scholar]
- 124.Sun A., Hu D., He X., Ji X., Li T., Wei X., Qian Z. Mussel-Inspired Hydrogel with Injectable Self-Healing and Antibacterial Properties Promotes Wound Healing in Burn Wound Infection. NPG Asia Mater. 2022;14:86. doi: 10.1038/s41427-022-00434-z. [DOI] [Google Scholar]
- 125.Shang K., Tao L., Jiang S., Yan J., Hu S., Yang G., Ma C., Cheng S., Wang X., Yin J. Highly Flexible Hydrogel Dressing with Efficient Antibacterial, Antioxidative, and Wound Healing Performances. Biomater. Sci. 2022;10:1373–1383. doi: 10.1039/D1BM02010B. [DOI] [PubMed] [Google Scholar]
- 126.Uddin T.M., Chakraborty A.J., Khusro A., Zidan B.R.M., Mitra S., Emran T.B., Dhama K., Ripon M.K.H., Gajdács M., Sahibzada M.U.K., et al. Antibiotic Resistance in Microbes: History, Mechanisms, Therapeutic Strategies and Future Prospects. J. Infect. Public Health. 2021;14:1750–1766. doi: 10.1016/j.jiph.2021.10.020. [DOI] [PubMed] [Google Scholar]
- 127.Duan C., Yu M., Xu J., Li B.Y., Zhao Y., Kankala R.K. Overcoming Cancer Multi-Drug Resistance (MDR): Reasons, Mechanisms, Nanotherapeutic Solutions, and Challenges. Biomed. Pharmacother. 2023;162:114643. doi: 10.1016/j.biopha.2023.114643. [DOI] [PubMed] [Google Scholar]
- 128.Murugaiyan J., Kumar P.A., Rao G.S., Iskandar K., Hawser S., Hays J.P., Mohsen Y., Adukkadukkam S., Awuah W.A., Jose R.A.M., et al. Progress in Alternative Strategies to Combat Antimicrobial Resistance: Focus on Antibiotics. Antibiotics. 2022;11:200. doi: 10.3390/antibiotics11020200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Pasika S.R., Bulusu R., Rao B.V.K., Kommineni N., Bolla P.K., Kala S.G., Godugu C. Nanomaterials: Advances and Applications. Springer; Singapore: 2023. Nanotechnology for Biomedical Applications. [DOI] [Google Scholar]
- 130.Altun E., Aydogdu M.O., Chung E., Ren G., Homer-Vanniasinkam S., Edirisinghe M. Metal-Based Nanoparticles for Combating Antibiotic Resistance. Appl. Phys. Rev. 2021;8:041303. doi: 10.1063/5.0060299. [DOI] [Google Scholar]
- 131.Makvandi P., Wang C.Y., Zare E.N., Borzacchiello A., Niu L.N., Tay F.R. Metal-Based Nanomaterials in Biomedical Applications: Antimicrobial Activity and Cytotoxicity Aspects. Adv. Funct. Mater. 2020;30:1910021. doi: 10.1002/adfm.201910021. [DOI] [Google Scholar]
- 132.Altun E., Aydogdu M.O., Crabbe-Mann M., Ahmed J., Brako F., Karademir B., Aksu B., Sennaroglu M., Eroglu M.S., Ren G., et al. Co-Culture of Keratinocyte-Staphylococcus Aureus on Cu-Ag-Zn/CuO and Cu-Ag-W Nanoparticle Loaded Bacterial Cellulose:PMMA Bandages. Macromol. Mater. Eng. 2019;304:1800537. doi: 10.1002/mame.201800537. [DOI] [Google Scholar]
- 133.Saleh A., Akkuş-Daǧdeviren Z.B., Haddadzadegan S., Wibel R., Bernkop-Schnürch A. Peptide Antibiotic-Polyphosphate Nanoparticles: A Promising Strategy to Overcome the Enzymatic and Mucus Barrier of the Intestine. Biomacromolecules. 2023;24:2587–2595. doi: 10.1021/acs.biomac.3c00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Singh C., Mehata A.K., Priya V., Malik A.K., Setia A., Suseela M.N.L., Vikas M.N.L., Gokul P., Samridhi P., Singh S.K., et al. Bimetallic Au–Ag Nanoparticles: Advanced Nanotechnology for Tackling Antimicrobial Resistance. Molecules. 2022;27:7059. doi: 10.3390/molecules27207059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Meng Y., Chen L., Chen Y., Shi J., Zhang Z., Wang Y., Wu F., Jiang X., Yang W., Zhang L., et al. Reactive Metal Boride Nanoparticles Trap Lipopolysaccharide and Peptidoglycan for Bacteria-Infected Wound Healing. Nat. Commun. 2022;13:7353. doi: 10.1038/s41467-022-35050-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Soares C., Pereira R., Fidalgo F. Phytotoxicity of Nanoparticles. Springer; Cham, Switzerland: 2018. Metal-Based Nanomaterials and Oxidative Stress in Plants: Current Aspects and Overview. [DOI] [Google Scholar]
- 137.Bhatt S., Upadhyay T., Patil C., Pai K.S.R., Chellappan D.K., Dua K. Role of Oxidative Stress in Pathophysiological Progression of Schizophrenia. Curr. Psychiatry Res. Rev. 2023;19:11–27. doi: 10.2174/2666082218666220822154558. [DOI] [Google Scholar]
- 138.Parmar S., Kaur H., Singh J., Matharu A.S., Ramakrishna S., Bechelany M. Recent Advances in Green Synthesis of Ag NPs for Extenuating Antimicrobial Resistance. Nanomaterials. 2022;12:1115. doi: 10.3390/nano12071115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Liu Z., Yi Y., Wang S., Dou H., Fan Y., Tian L., Zhao J., Ren L. Bio-Inspired Self-Adaptive Nanocomposite Array: From Non-Antibiotic Antibacterial Actions to Cell Proliferation. ACS Nano. 2022;16:16549–16562. doi: 10.1021/acsnano.2c05980. [DOI] [PubMed] [Google Scholar]
- 140.Zhang F., Hu Q., Wei Y., Meng W., Wang R., Liu J., Nie Y., Luo R., Wang Y., Shen B. Surface Modification of Titanium Implants by PH-Responsive Coating Designed for Self-Adaptive Antibacterial and Promoted Osseointegration. Chem. Eng. J. 2022;435:134802. doi: 10.1016/j.cej.2022.134802. [DOI] [Google Scholar]
- 141.Pemmada R., Shrivastava A., Dash M., Cui K., Kumar P., Ramakrishna S., Zhou Y., Thomas V., Nanda H.S. Science-Based Strategies of Antibacterial Coatings with Bactericidal Properties for Biomedical and Healthcare Settings. Curr. Opin. Biomed. Eng. 2023;25:100442. doi: 10.1016/j.cobme.2022.100442. [DOI] [Google Scholar]
- 142.Li Y., Zhang P., Li M., Shakoor N., Adeel M., Zhou P., Guo M., Jiang Y., Zhao W., Lou B.Z., et al. Application and Mechanisms of Metal-Based Nanoparticles in the Control of Bacterial and Fungal Crop Diseases. Pest Manag. Sci. 2023;79:21–36. doi: 10.1002/ps.7218. [DOI] [PubMed] [Google Scholar]
- 143.Franco D., Calabrese G., Guglielmino S.P.P., Conoci S. Metal-Based Nanoparticles: Antibacterial Mechanisms and Biomedical Application. Microorganisms. 2022;10:1778. doi: 10.3390/microorganisms10091778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Rybka M., Mazurek Ł., Konop M. Beneficial Effect of Wound Dressings Containing Silver and Silver Nanoparticles in Wound Healing—From Experimental Studies to Clinical Practice. Life. 2023;13:69. doi: 10.3390/life13010069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Toczek J., Sadłocha M., Major K., Stojko R. Benefit of Silver and Gold Nanoparticles in Wound Healing Process after Endometrial Cancer Protocol. Biomedicines. 2022;10:679. doi: 10.3390/biomedicines10030679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sabarees G., Velmurugan V., Tamilarasi G.P., Alagarsamy V., Raja Solomon V. Recent Advances in Silver Nanoparticles Containing Nanofibers for Chronic Wound Management. Polymers. 2022;14:3994. doi: 10.3390/polym14193994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Banerjee K., Madhyastha R., Nakajima Y., Maruyama M., Madhyastha H. Nanoceutical Adjuvants as Wound Healing Material: Precepts and Prospects. Int. J. Mol. Sci. 2021;22:4748. doi: 10.3390/ijms22094748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Siddique M.H., Aslam B., Imran M., Ashraf A., Nadeem H., Hayat S., Khurshid M., Afzal M., Malik I.R., Shahzad M., et al. Effect of Silver Nanoparticles on Biofilm Formation and EPS Production of Multidrug-Resistant Klebsiella Pneumoniae. BioMed Res. Int. 2020;2020:6398165. doi: 10.1155/2020/6398165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Miškovská A., Rabochová M., Michailidu J., Masák J., Čejková A., Lorinčík J., Maťátková O. Antibiofilm Activity of Silver Nanoparticles Biosynthesized Using Viticultural Waste. PLoS ONE. 2022;17:e0272844. doi: 10.1371/journal.pone.0272844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Krishnan P.D., Banas D., Durai R.D., Kabanov D., Hosnedlova B., Kepinska M., Fernandez C., Ruttkay-Nedecky B., Nguyen H.V., Farid A., et al. Silver Nanomaterials for Wound Dressing Applications. Pharmaceutics. 2020;12:821. doi: 10.3390/pharmaceutics12090821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Nguyen T.T.T., Tran N.T.K., Le T.Q., Nguyen T.T.A., Nguyen L.T.M., Van Tran T. Passion Fruit Peel Pectin/Chitosan Based Antibacterial Films Incorporated with Biosynthesized Silver Nanoparticles for Wound Healing Application. Alex. Eng. J. 2023;69:419–430. doi: 10.1016/j.aej.2023.01.066. [DOI] [Google Scholar]
- 152.Rather A.H., Khan R.S., Wani T.U., Rafiq M., Jadhav A.H., Srinivasappa P.M., Abdal-hay A., Sultan P., Rather S.U., Macossay J., et al. Polyurethane and Cellulose Acetate Micro-Nanofibers Containing Rosemary Essential Oil, and Decorated with Silver Nanoparticles for Wound Healing Application. Int. J. Biol. Macromol. 2023;226:690–705. doi: 10.1016/j.ijbiomac.2022.12.048. [DOI] [PubMed] [Google Scholar]
- 153.Salem H.F., Nafady M.M., Ewees M.G.E.D., Hassan H., Khallaf R.A. Rosuvastatin Calcium-Based Novel Nanocubic Vesicles Capped with Silver Nanoparticles-Loaded Hydrogel for Wound Healing Management: Optimization Employing Box–Behnken Design: In Vitro and in Vivo Assessment. J. Liposome Res. 2022;32:45–61. doi: 10.1080/08982104.2020.1867166. [DOI] [PubMed] [Google Scholar]
- 154.Massey S., Iqbal F., Rehman A.U., Iqbal M.S., Iram F. Preparation, Characterization and Biological Evaluation of Silver Nanoparticles and Drug Loaded Composites for Wound Dressings Formed from Lallemantia Royleana Seeds’ Mucilage. J. Biomater. Sci. Polym. Ed. 2022;33:481–498. doi: 10.1080/09205063.2021.1992590. [DOI] [PubMed] [Google Scholar]
- 155.Pino P., Bosco F., Mollea C., Onida B. Antimicrobial Nano-Zinc Oxide Biocomposites for Wound Healing Applications: A Review. Pharmaceutics. 2023;15:970. doi: 10.3390/pharmaceutics15030970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Mandal A.K., Katuwal S., Tettey F., Gupta A., Bhattarai S., Jaisi S., Bhandari D.P., Shah A.K., Bhattarai N., Parajuli N. Current Research on Zinc Oxide Nanoparticles: Synthesis, Characterization, and Biomedical Applications. Nanomaterials. 2022;12:3066. doi: 10.3390/nano12173066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Rosman N.F., Nurfazianawatie M.Z., Omar H., Malek N.S.A., Hajar N., Buniyamin I., Abdullah S., Rusop M., Asli N.A. Fungal Growth Physicochemical Properties Inhibition by Novel Zinc Oxide/Glutinous Tapioca Starch Composite. J. Adv. Res. Appl. Sci. Eng. Technol. 2022;29:76–89. doi: 10.37934/araset.29.1.7689. [DOI] [Google Scholar]
- 158.Seisenbaeva G.A., Fromell K., Vinogradov V.V., Terekhov A.N., Pakhomov A.V., Nilsson B., Ekdahl K.N., Vinogradov V.V., Kessler V.G. Dispersion of TiO2 Nanoparticles Improves Burn Wound Healing and Tissue Regeneration through Specific Interaction with Blood Serum Proteins. Sci. Rep. 2017;7:15448. doi: 10.1038/s41598-017-15792-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.De Moura F.B.R., Ferreira B.A., Muniz E.H., Santos R.A., Gomide J.A.L., Justino A.B., Silva A.C.A., Dantas N.O., Ribeiro D.L., Araújo F.A., et al. TiO2 Nanocrystals and Annona Crassiflora Polyphenols Used Alone or Mixed Impact Differently on Wound Repair. Acad. Bras. Cienc. 2022;94:e20210230. doi: 10.1590/0001-3765202220210230. [DOI] [PubMed] [Google Scholar]
- 160.Aleem A.R., Shahzadi L., Nasir M., Hajivand P., Alvi F., Akhtar A., Zehra M., Mehmood A., Yar M. Developing Sulfur-Doped Titanium Oxide Nanoparticles Loaded Chitosan/Cellulose-Based Proangiogenic Dressings for Chronic Ulcer and Burn Wounds Healing. J. Biomed. Mater. Res. B Appl. Biomater. 2022;110:1069–1081. doi: 10.1002/jbm.b.34981. [DOI] [PubMed] [Google Scholar]
- 161.Ismail N.A., Amin K.A.M., Majid F.A.A., Razali M.H. Gellan Gum Incorporating Titanium Dioxide Nanoparticles Biofilm as Wound Dressing: Physicochemical, Mechanical, Antibacterial Properties and Wound Healing Studies. Mater. Sci. Eng. C. 2019;103:109770. doi: 10.1016/j.msec.2019.109770. [DOI] [PubMed] [Google Scholar]
- 162.Mahdavi B., Paydarfard S., Zangeneh M.M., Goorani S., Seydi N., Zangeneh A. Assessment of Antioxidant, Cytotoxicity, Antibacterial, Antifungal, and Cutaneous Wound Healing Activities of Green Synthesized Manganese Nanoparticles Using Ziziphora Clinopodioides Lam Leaves under in Vitro and in Vivo Condition. Appl. Organomet. Chem. 2020;34:e5248. doi: 10.1002/aoc.5248. [DOI] [Google Scholar]
- 163.Chia C.H., Lau K.S., Chin S.X., Rosli N.H., Vincent J., Chowdhury M.S. Carbon Nanotubes for Biomedical Applications and Healthcare. Apple Academic Press; Burlington, ON, Canada: 2024. Carbon Nanotubes for Biomedical Applications and Health Care: New Horizons; pp. 255–331. [Google Scholar]
- 164.Nanda S.S., Wang T., Hossain M.I., Yoon H.Y., Selvan S.T., Kim K., Yi D.K. Gold-Nanorod-Based Scaffolds for Wound-Healing Applications. ACS Appl. Nano Mater. 2022;5:8640–8648. doi: 10.1021/acsanm.2c02230. [DOI] [Google Scholar]
- 165.Naraginti S., Kumari P.L., Das R.K., Sivakumar A., Patil S.H., Andhalkar V.V. Amelioration of Excision Wounds by Topical Application of Green Synthesized, Formulated Silver and Gold Nanoparticles in Albino Wistar Rats. Mater. Sci. Eng. C. 2016;62:293–300. doi: 10.1016/j.msec.2016.01.069. [DOI] [PubMed] [Google Scholar]
- 166.Shalaby M.A., Anwar M.M., Saeed H. Nanomaterials for Application in Wound Healing: Current State-of-the-Art and Future Perspectives. J. Polym. Res. 2022;29:91. doi: 10.1007/s10965-021-02870-x. [DOI] [Google Scholar]
- 167.He X., Dai L., Ye L., Sun X., Enoch O., Hu R., Zan X., Lin F., Shen J. A Vehicle-Free Antimicrobial Polymer Hybrid Gold Nanoparticle as Synergistically Therapeutic Platforms for Staphylococcus Aureus Infected Wound Healing. Adv. Sci. 2022;9:e2105223. doi: 10.1002/advs.202105223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Batool Z., Muhammad G., Iqbal M.M., Aslam M.S., Raza M.A., Sajjad N., Abdullah M., Akhtar N., Syed A., Elgorban A.M., et al. Hydrogel Assisted Synthesis of Gold Nanoparticles with Enhanced Microbicidal and in Vivo Wound Healing Potential. Sci. Rep. 2022;12:6575. doi: 10.1038/s41598-022-10495-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Rath G., Hussain T., Chauhan G., Garg T., Goyal A.K. Collagen nanofiber containing silver nanoparticles for improved wound-healing applications. J. Drug Target. 2016;24:520–529. doi: 10.3109/1061186X.2015.1095922. [DOI] [PubMed] [Google Scholar]
- 170.Cui C., Sun S., Wu S., Chen S., Ma J., Zhou F. Electrospun chitosan nanofibers for wound healing application. Eng. Regen. 2021;2:82–90. doi: 10.1016/j.engreg.2021.08.001. [DOI] [Google Scholar]
- 171.Mendes C., Thirupathi A., Corrêa M.E.A.B., Gu Y., Silveira P.C.L. The Use of Metallic Nanoparticles in Wound Healing: New Perspectives. Int. J. Mol. Sci. 2022;23:15376. doi: 10.3390/ijms232315376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Wang H., Xu Z., Li Q., Wu J. Application of Metal-Based Biomaterials in Wound Repair. Eng. Regen. 2021;2:137–153. doi: 10.1016/j.engreg.2021.09.005. [DOI] [Google Scholar]
- 173.Chen C.Y., Yin H., Chen X., Chen T.H., Liu H.M., Rao S.S., Tan Y.J., Qian Y.X., Liu Y.W., Hu X.K., et al. Ångstrom-Scale Silver Particle-Embedded Carbomer Gel Promotes Wound Healing by Inhibiting Bacterial Colonization and Inflammation. Sci. Adv. 2020;6:eaba0942. doi: 10.1126/sciadv.aba0942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Singh V., Marimuthu T., Makatini M.M., Choonara Y.E. Biopolymer-Based Wound Dressings with Biochemical Cues for Cell-Instructive Wound Repair. Polymers. 2022;14:5371. doi: 10.3390/polym14245371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Gaharwar A.K., Singh I., Khademhosseini A. Engineered Biomaterials for in Situ Tissue Regeneration. Nat. Rev. Mater. 2020;5:686–705. doi: 10.1038/s41578-020-0209-x. [DOI] [Google Scholar]
- 176.Fadilah N.I.M., Phang S.J., Kamaruzaman N., Salleh A., Zawani M., Sanyal A., Maarof M., Fauzi M.B. Antioxidant Biomaterials in Cutaneous Wound Healing and Tissue Regeneration: A Critical Review. Antioxidants. 2023;12:787. doi: 10.3390/antiox12040787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Augustine R., Hasan A., Patan N.K., Augustine A., Dalvi Y.B., Varghese R., Unni R.N., Kalarikkal N., Al Moustafa A.E., Thomas S. Titanium Nanorods Loaded PCL Meshes with Enhanced Blood Vessel Formation and Cell Migration for Wound Dressing Applications. Macromol. Biosci. 2019;19:e1900058. doi: 10.1002/mabi.201900058. [DOI] [PubMed] [Google Scholar]
- 178.Begum S., Hassan Z., Bräse S., Wöll C., Tsotsalas M. Metal-Organic Framework-Templated Biomaterials: Recent Progress in Synthesis, Functionalization, and Applications. Acc. Chem. Res. 2019;52:1598–1610. doi: 10.1021/acs.accounts.9b00039. [DOI] [PubMed] [Google Scholar]
- 179.Nowak M., Barańska-Rybak W. Nanomaterials as a Successor of Antibiotics in Antibiotic-Resistant, Biofilm Infected Wounds? Antibiotics. 2021;10:941. doi: 10.3390/antibiotics10080941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Zhao F., Su Y., Wang J., Romanova S., DiMaio D.J., Xie J., Zhao S. A Highly Efficacious Electrical Biofilm Treatment System for Combating Chronic Wound Bacterial Infections. Adv. Mater. 2023;35:e2208069. doi: 10.1002/adma.202208069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Huanbutta K., Burapapadh K., Sriamornsak P., Sangnim T. Practical Application of 3D Printing for Pharmaceuticals in Hospitals and Pharmacies. Pharmaceutics. 2023;15:1877. doi: 10.3390/pharmaceutics15071877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Antezana P.E., Municoy S., Álvarez-Echazú M.I., Santo-Orihuela P.L., Catalano P.N., Al-Tel T.H., Kadumudi F.B., Dolatshahi-Pirouz A., Orive G., Desimone M.F. The 3D Bioprinted Scaffolds for Wound Healing. Pharmaceutics. 2022;14:464. doi: 10.3390/pharmaceutics14020464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Hooshmand S., Mollazadeh S., Akrami N., Ghanad M., El-Fiqi A., Baino F., Nazarnezhad S., Kargozar S. Mesoporous Silica Nanoparticles and Mesoporous Bioactive Glasses for Wound Management: From Skin Regeneration to Cancer Therapy. Materials. 2021;14:3337. doi: 10.3390/ma14123337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kim N., Lee H., Han G., Kang M., Park S., Kim D.E., Lee M., Kim M., Na Y., Oh S., et al. 3D-Printed Functional Hydrogel by DNA-Induced Biomineralization for Accelerated Diabetic Wound Healing. Adv. Sci. 2023;10:e2300816. doi: 10.1002/advs.202300816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Hu H., Tang Y., Pang L., Lin C., Huang W., Wang D., Jia W. Angiogenesis and Full-Thickness Wound Healing Efficiency of a Copper-Doped Borate Bioactive Glass/Poly(Lactic-Co-Glycolic Acid) Dressing Loaded with Vitamin E in Vivo and in Vitro. ACS Appl. Mater. Interfaces. 2018;10:22939–22950. doi: 10.1021/acsami.8b04903. [DOI] [PubMed] [Google Scholar]
- 186.Bao F., Pei G., Wu Z., Zhuang H., Zhang Z., Huan Z., Wu C., Chang J. Bioactive Self-Pumping Composite Wound Dressings with Micropore Array Modified Janus Membrane for Enhanced Diabetic Wound Healing. Adv. Funct. Mater. 2020;30:2005422. doi: 10.1002/adfm.202005422. [DOI] [Google Scholar]
- 187.Xiong P., Huang X., Ye N., Lu Q., Zhang G., Peng S., Wang H., Liu Y. Cytotoxicity of Metal-Based Nanoparticles: From Mechanisms and Methods of Evaluation to Pathological Manifestations. Adv. Sci. 2022;9:e2106049. doi: 10.1002/advs.202106049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Zhang N., Xiong G., Liu Z. Toxicity of Metal-Based Nanoparticles: Challenges in the Nano Era. Front. Bioeng. Biotechnol. 2022;10:1001572. doi: 10.3389/fbioe.2022.1001572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Tomé I., Francisco V., Fernandes H., Ferreira L. High-Throughput Screening of Nanoparticles in Drug Delivery. APL Bioeng. 2021;5:031511. doi: 10.1063/5.0057204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Suthar J.K., Vaidya A., Ravindran S. Toxic Implications of Silver Nanoparticles on the Central Nervous System: A Systematic Literature Review. J. Appl. Toxicol. 2023;43:4–21. doi: 10.1002/jat.4317. [DOI] [PubMed] [Google Scholar]
- 191.Tian L., Lin B., Wu L., Li K., Liu H., Yan J., Liu X., Xi Z. Neurotoxicity Induced by Zinc Oxide Nanoparticles: Age-Related Differences and Interaction. Sci. Rep. 2015;5:16117. doi: 10.1038/srep16117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Boraschi D., Canesi L., Drobne D., Kemmerling B., Pinsino A., Prochazkova P. Interaction between Nanomaterials and the Innate Immune System across Evolution. Biol. Rev. 2023;98:747–774. doi: 10.1111/brv.12928. [DOI] [PubMed] [Google Scholar]
- 193.Chaudhary V., Sonu, Chowdhury R., Thukral P., Pathania D., Saklani S., Lucky, Rustagi S., Gautam A., Mishra Y.K., et al. Biogenic Green Metal Nano Systems as Efficient Anti-Cancer Agents. Environ. Res. 2023;229:115933. doi: 10.1016/j.envres.2023.115933. [DOI] [PubMed] [Google Scholar]
- 194.Shukla R.K., Badiye A., Vajpayee K., Kapoor N. Genotoxic Potential of Nanoparticles: Structural and Functional Modifications in DNA. Front. Genet. 2021;12:728250. doi: 10.3389/fgene.2021.728250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Aldossary A.M., Tawfik E.A., Alomary M.N., Alsudir S.A., Alfahad A.J., Alshehri A.A., Almughem F.A., Mohammed R.Y., Alzaydi M.M. Recent Advances in Mitochondrial Diseases: From Molecular Insights to Therapeutic Perspectives. Saudi Pharm. J. 2022;30:1065–1078. doi: 10.1016/j.jsps.2022.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Abdelhalim M., Al-Ayed M.S., Moussa S.A. The effects of intraperitoneal administration of gold nanoparticles size and exposure duration on oxidative and antioxidants levels in various rat organs. Pak. J. Pharm. Sci. 2015;28:705–712. [PubMed] [Google Scholar]
- 197.Muller A.P., Ferreira G.K., Pires A.J., de Bem Silveira G., de Souza D.L., de Abreu Brandolfi J., de Souza C.T., Paula M.M., Silveira P.C.L. Gold nanoparticles prevent cognitive deficits, oxidative stress and inflammation in a rat model of sporadic dementia of Alzheimer’s type. Mater. Sci. Eng. C. 2017;77:476–483. doi: 10.1016/j.msec.2017.03.283. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.