Abstract
Advanced holographic visualization techniques are becoming increasingly important in clinical practice, not only for diagnostic purposes but also in the planning of interventional or surgical procedures. The traditional approach for visualizing anatomic structures is based on standard imaging modalities such as echocardiography, cardiac magnetic resonance (CMR) and cardiac CT scan (CCT) which, however, can only provide two-dimensional (2D) images thus limiting 3D perception. Many recent studies have shown that the use of 3D imaging modalities such as augmented reality, virtual reality, mixed reality and holography improve the short and long-term outcome of percutaneous or surgical procedures. In this article, we report our experience on the use of the hologram in different clinical scenarios and in the field of university education.
Keywords: Holography , cardiac interventions , cardiac surgery
Introduction
Visualization technology is very important for acquiring clinical information. The traditional approach for visualizing CHD anatomy is based on standard imaging modalities such as echocardiography, cardiac magnetic resonance (CMR) and cardiac CT scan (CCT) which, however, can only provide two-dimensional (2D) images thus limiting 3D perception and leaving the understanding of CHD lesions to individual spatial imagination [1].
Recent advances in 3D imaging modalities such as augmented reality, virtual reality, mixed reality and holography have granted the possibility of applying these technologies in the medical field and are nowadays used to generate accurate representations of anatomic structures [2, 3, 4].
Several studies have demonstrated that novel 3D imaging techniques provide a better depth and volume perception compared to standard imaging modalities [5] leading to increased diagnostic accuracy and facilitating surgical and interventional programming [6, 7, 8, 9].
3D holography is today common practice in the evaluation of complex anatomies in high-volume centres to offer a tailored patients approach [4].
Moreover, holographic techniques have been successfully exploited for educational purposes [10, 11, 12, 13].
At IRCCS Policlinico San Donato, augmented reality has been implemented multiple times for different clinical and educational purposes. The approval of the institutional ethics committee was obtained for each case presented. This article is a review of our activity.
Mixed reality for planning stent implantation in systemic venous baffle obstruction in TGA after atrial switch operation
Considerations
In the past decades transposition of great arteries (TGA) were corrected with the Mustard-Senning procedure, which involves implantation of a baffle to redirect caval blood flows to the left atrium [14].
TGA after Mustard-Senning procedure is a condition characterized by the presence of a morphological right ventricle (RV) and tricuspid valve (TV) on the systemic position which means that the RV is called upon to generate the systemic blood pressure [15].
The RV is, however, anatomically poorly suited to generate high pressure loads, so it may fail in the longer term leading to chronic heart failure [14].
A late complication of Mustard-Senning procedure is obstruction of the systemic venous baffle (SVB), usually presenting with exercise intolerance, signs of venous obstruction and heart failure [15, 16, 17].
SVB obstruction is defined as mean doppler/catheterization gradient >6mmHg or anatomic narrowing <10 mm (or less than 50% the diameter of the proximal systemic vein) [18].
SVB obstruction may be due to improper baffle geometry and suture line placement, baffle material, contraction of the pericardial or synthetic baffle material, scar tissue or adhesions involving the baffle and the excised margins of the atrial septum [16, 17, 19, 20].
In this setting, baffle stenting can lead to prompt symptom reversal [21, 22], where accurate evaluation of the extension of the obstruction is essential for correct stent sizing [18, 21, 23].
Practice
At IRCCS Policlinico San Donato this was made possible through the implementation of a holographic augmented reality prototype (ARTINESS, Milan, Italy) [24].
In fact, the prototype allowed us to inspect the 3D anatomy of a patient with SVB obstruction status post atrial switch operation and allowed the operator to perceive the severity of SVB stenosis together with its longitudinal extension [24].
This helped the congenital interventionalist to predict the feasibility of the procedure, particularly the location of stent implantation in order to avoid obstruction of the pulmonary venous baffle [24].
Subsequently, a multiplanar analysis of superior vena cava (SVC) cross-section was also exploited to support stent sizing and, basing on this, a 48-mm ANDRA stent was chosen (Andramed, Reutlingen, Germany) post-dilated with a balloon-in-balloon catheter 18x50mm (BiB Catheter, NUMED, Hopkinton, NY, USA). At the end of the procedure a normalization of the peak-to-peak gradient was documented. The results of the procedure were then detected via a 3D computed tomography reconstruction.
Holography in tracheoesophageal fistula treatment planning
Considerations
Tracheoesophageal fistulas (TOF) are abnormal connections between the oesophagus and the trachea [25] and may be congenital or acquired in aetiology [25, 26].
Acquired tracheoesophageal fistulas may result from penetrating trauma, surgery and infectious diseases [25, 27, 28, 29, 30].
However, the two most common causes of tracheoesophageal fistulas are cancer and cuff-related tracheal injury in mechanically ventilated patients [25].
Malignant tracheoesophageal fistulas (mTOF) may occur both in the context of oesophageal carcinoma but also in lung, trachea, larynx and thyroid neoplasms [26].
mTOF are devastating complications for oncologic patients and usually present with increased secretions and evidence of aspiration of gastric contents possibly leading to life-threatening pneumonia [26].
Closure of the tract has been traditionally performed via open surgery, a procedure that bears, however, a poor prognosis [25, 26].
Recently, the use of cardiac septal occluders (CSO) has been proposed as an endoscopic option for the closure of different types of tracheoesophageal fistulas in patients at high surgical risk with satisfactory results [31, 32, 33, 34, 35].
Practice
Starting from this promising evidence we have opted to endoscopically close with a CSO a tracheoesophageal fistula in one of our patients who presented this condition several years after radiation therapy for oesophageal adenocarcinoma.
Pre-procedural planning was achieved through a holographic 3D reconstruction (Artiness, Milan, Italy) of the abnormal connection starting from pre-operative CT scans [36].
Microsoft Hololens were then used to visualize the anatomy of the fistulous path (Figure 1).
Figure 1.

Holographic representation of tracheoesophageal fistula
The 3D reconstruction was also used to support a multidisciplinary discussion involving oncologists, surgeons, endoscopists and cardiologists during which the endoscopic closure of the fistula with a Amplatzer device (AGA Medical, Plymouth, Minnesota, USA) was planned through a combined bronchoscopy, esophagoscopy and fluoroscopy approach.
The procedure was successfully performed under general anaesthesia [36] and an oral gastrografin swallow CT scan performed on post-operative day three confirmed the absence of leaks and complete closure of the fistula.
The implementation of 3D holography was essential to fully understand the spatial relationship between the trachea and the oesophagus, to choose the most appropriate Amplatzer device and to match the defect size thus improve the safety and efficacy of the procedure.
Holography in sinus venosus atrial septal defect interventional planning
Considerations
Atrial septal defects (ASDs) may run asymptomatic until advanced age [37] when they present with right heart enlargement and dysfunction following lifelong left-to-right shunt possibly leading to atrial arrhythmias, pulmonary artery hypertension and heart failure [38, 39, 40].
With the intention of preventing morbidity and mortality associated with late presentation of ASDs, current guidelines recommend closure of the defect in adults with right heart enlargement, with or without symptoms [41, 42, 43].
Atrial septal defects are classified as sinus venous (SV-ASD) when the defect develops near the entry of the superior or inferior venae cavae, these defects may be associated with partial anomalous pulmonary venous drainage [44].
Although current guidelines recommend surgical closure of SV-ASD [41, 42, 43], in recent years percutaneous techniques for SV-ASD closure have been developed with promising results [45, 46, 47].
Percutaneous treatment of SV-ASD implies the use of a covered stent that, when strategically positioned, leads to the separation between the superior vena cava (SVC) and the right atrium (RA) from the right upper pulmonary vein (RUPV) and the left atrium (LA) thus together sealing the defect and redirecting partial anomalous pulmonary drainage to the LA [48].
In this context, a profound understanding of the 3D anatomy of the defect is essential to avoid impairment in pulmonary venous return [45,49, 50].
Practice
One of our patients was diagnosed with right ventricular (RV) failure in the presence of ostium secundum atrial septal defect (OS-ASD) and SV-ASD with partial anomalous pulmonary venous return, consequently correction of the defects was deemed necessary.
In order to plan treatment and choose between minimally invasive percutaneous correction and surgical correction, we developed a holographic augmented reality prototype (ARTINESS, Milan,
Italy) starting from computed tomography (CT) scans. The holographic model allowed us to evaluate the anatomy using HoloLens (Microsoft) (Figure 2).
Figure 2.

Holographic representation of superior sinus-venosus ASD with partial anomalous venous return
This allowed us to directly investigate the feasibility of either approach on the instrumented model, predicting the post-operative anatomic relationship between the stent, the superior vena cava (SVC) and the anomalous pulmonary veins (Figure 3).
Figure 3.

Holography-guided pre-procedural planning of SV-ASD percutaneous closure. The balloon is used to predict the site of stent implantation
Basing on these analyses transcatheter treatment was preferred to open surgery.
Consequently, a covered Cheatham-Platinum stent (NUMED, Hopkinton, New York) was used to close the SV-ASD and redirect the anomalous venous return to the left atrium. Successful closure of the defects was confirmed via 3D holographic reconstruction of post-operative CT scans (Figure 4).
Figure 4.
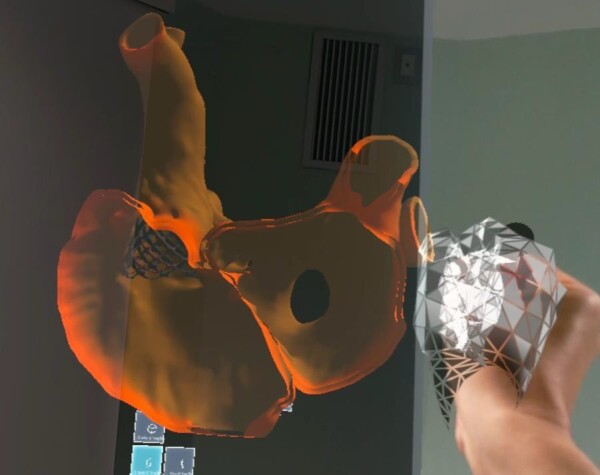
Holographic representation of the anatomical relationship between the superior vena cava, the pulmonary veins and the deployed stent.
Holography in cardiac fibroma surgical planning
Considerations
Cardiac fibromas are the second most common primary cardiac tumour in the paediatric population [51].
These are primarily composed of connective tissue and fibroblasts and although benign, can be symptomatic due to intracavitary obstruction, inflow and outflow tract obstruction, coronary artery compromise, thromboembolic events, conduction defects, and can also lead to sudden death [52, 53, 54].
A recent literature review showed that these tumours are usually located in the left ventricle (LV) and less commonly at other locations such as the ventricular septum and the left atrioventricular groove [52].
Anatomical understanding of tumour location, tumour involvement of heart wall layers and its vascularization is essential for appropriate surgical planning [52].
Practice
We developed a 3D holography model (Figure 5) to explore the anatomy of a cardiac fibroma in a twelve-year old patient who presented recurrent sustained ventricular tachycardias needing repeated electrical cardioversions and was therefore candidate for surgical excision of the tumour.
Figure 5.

Holographic representation of cardiac fibroma (T) embracing the left ventricle (LV) posterior myocardial wall (LVPM). RV=right ventricle
In this context, the three-dimensional anatomy of the tumour was reconstructed from short axis views CMR dynamic sequences and was visualized via HoloLens (Microsoft, Redmond, WA, USA) using a prototype software platform (ARTINESS SRL, Milan, Italy). This model helped us to understand fully the extension of the tumour and the involvement of myocardial layers so to plan with extreme precision resection margins. The same holographic 3D model wars displayed during the surgical procedure to guide incision margins and the surgical steps (Figure 6).
Figure 6.

Congenital cardiac surgeons visualizing the 3D anatomy of a cardiac fibroma via holography mounted on HoloLens
The procedure was safely performed under cardio-pulmonary bypass and the tumour was removed without entering the LV cavity as predicted by the hologram. The girl was discharged home on the 6th post-operative day with a normal LV ejection fraction (EF) and remained free of arrhythmic events at follow-up.
Holography in Teaching Congenital Heart Defects
Considerations
Holographic techniques have been successfully exploited for educational purposes, where they can provide interactive learning experiences to improve and facilitate tuition [10, 11, 12, 13].
In the context of CHD, holography can aid in visualizing their complex three-dimensional anatomy which may be particularly difficult to envision, especially for those who are nave to the subject [55].
In order to explore the efficacy of holography in CHD teaching we have developed an exploratory study in which we have compared holography to traditional tuition methods.
Practice
In pursuance of this, we selected fifty-nine third year medical students who had just completed the course of general cardiology.
The participants were randomly allocated into three groups (20, 20 and 19 people respectively). Every group received the same recorded lecture on the anatomy of sinus-venous atrial septal defect (SV- ASD) superior vena cava type with partial anomalous pulmonary venous return (PAPVR) and its related percutaneous treatment.
The lecture was performed with different iconographic materials. The 1st group (regular slideware group, RS) received a traditional lecture with slides showing SV-ASD anatomy projected onto a flat screen. The 2nd group (holographic videos group, HV) was shown slides incorporating videos of holographic anatomical models of SV-ASD with PAPVR, and the related transcatheter intervention planning, both displayed on a traditional projector screen. The 3rd group (augmented reality group, AR) interacted with holographic images of the CHD and SV-ASD anatomy, procedural planning and procedural results wearing Microsoft HoloLens.
At the end of the lecture, each group compiled a multiple-choice questionnaire as a proxy to evaluate the efficacy of the lecture in terms of information acquired. The test was divided into two different parts the first part consisted of questions regarding the epidemiology, anatomy, and pathophysiology of the disease, the second part consisted of questions regarding the anatomical relationships between the SV-ASD and the surrounding structures and their implications in the transcatheter treatment of the device. When the test results were analysed, we have found out that students in the AR performed better than students from the other groups in the second part of the test thus implying that holography helped students to understand better the complicated anatomical relationships of the congenital defect (Figure 7).
Figure 7.

Test scores stratified per group (Blue group regular slideware, Green group holographic videos, Red group augmented reality
Discussions
Holographic visualization technology has existed for several years [56]; however, its application in medical imaging has been impeded by equipment that was unable to display high-quality holographic images.
Nowadays, the most modern equipment allows operators to interact with holograms through gestures to move the anatomical structures in space, resize them, cut digital images, and simulate surgical or percutaneous interventions and their results [57].
It provides a true perception of 3D depth-integrated into the real world and helps to create an interactive model between the virtual and the real world. This technology has already been applied in several areas such as neurosurgery, visceral, and orthopedic surgery [58].
In this article, we report our single-center experience on the complementary use of the hologram for surgical and percutaneous planning of different clinical conditions.
The first observation is the wide variability of the clinical cases presented, from the transposition of the large arteries to the tracheoesophageal fistula, which allows us to conclude that every clinical scenario susceptible to intervention can theoretically benefit from the use of the hologram.
In addition, each presented clinical case had an underlying anatomical complexity that could not be corrected with common surgical strategies, so the hologram in such contexts made possible the treatment in all cases with satisfactory results.
Therefore, the use of virtual reality allows us to increase a patient's eligibility for a complex surgical or percutaneous operation and to improve the immediate anatomical result.
Further studies are needed to analyze the use of the hologram in the operative planning of specific clinical conditions and therefore to support its use increasingly on a large scale, at least for the most complex anatomies.
An additional field of use of the hologram is now represented by university education, particularly in learning complex anatomies such as congenital heart disease.
It is often difficult to learn the complex anatomies resulting from alteration of the embryological development as in congenital heart disease. For teaching purposes, the use of the hologram will become increasingly widespread.
Conclusions
In conclusion, this initial experience suggests that holographic techniques will be increasingly used and implemented for educational, clinical, and therapeutic use and might be a new next-generation operation-supportive tool in terms of spatial awareness, sharing, and simplicity.
Conflict of interests
None to declare.
Acknowledgments
European Reference Network for Rare and Low Prevalence Complex Disease of the Heart (ERN GUARD-Heart), Milan, Italy
References
- 1.Lau I, Gupta A, S undefined. Z. Clinical Value of Virtual Reality versus 3D Printing in Congenital Heart Disease. Biomolecules. 2021;11(6):884–884. doi: 10.3390/biom11060884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Silva JNA, Southworth M, Raptis C, Silva J. Emerging Applications of Virtual Reality in Cardiovascular Medicine. J Am Coll Cardiol Basic Trans Science. 2018;3(3):420–430. doi: 10.1016/j.jacbts.2017.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Southworth MK, Silva JR, Silva JNA. Use of extended realities in cardiology. Trends Cardiovasc Med. 2020;30(3):143–148. doi: 10.1016/j.tcm.2019.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chessa M, Van De, Farooqi K, Valverde I, Jung C, Votta E, Sturla F, Diller GP, Brida M, Sun Z, Little SH, Gatzoulis MA. Three-dimensional printing, holograms, computational modelling, and artificial intelligence for adult congenital heart disease care: an exciting future. Eur Heart J. 2022;43(28):2672–2684. doi: 10.1093/eurheartj/ehac266. [DOI] [PubMed] [Google Scholar]
- 5.Brun H, Bugge RAB, Suther LKR, Birkeland S, Kumar R, Pelanis E, E undefined. OJ. Mixed reality holograms for heart surgery planning: first user experience in congenital heart disease. Eur Heart J Cardiovasc Imaging. 2019;20(8):883–888. doi: 10.1093/ehjci/jey184. [DOI] [PubMed] [Google Scholar]
- 6.Butera G, Sturla F, Pluchinotta FR, Caimi A, Carminati M. Holographic Augmented Reality and 3D Printing for Advanced Planning of Sinus Venosus ASD/Partial Anomalous Pulmonary Venous Return Percutaneous Management. JACC Cardiovasc Interv. 2019;12(14):1389–1391. doi: 10.1016/j.jcin.2019.03.020. [DOI] [PubMed] [Google Scholar]
- 7.Bruno RR, Lin Y, Wolff G, Polzin A, Veulemans V, Klein K, Westenfeld R, Zeus T, Kelm M, J undefined. C. Virtual reality-assisted conscious sedation during transcatheter aortic valve implantation: a randomised pilot study. EuroIntervention. 2020;16(12):1014–1020. doi: 10.4244/EIJ-D-20-00269. [DOI] [PubMed] [Google Scholar]
- 8.Moore RA, Riggs KW, Kourtidou S, Schneider K, Szugye N, Troja W, D’Souza G, Rattan M, Bryant R, Taylor MD, M undefined. D. Three-dimensional printing and virtual surgery for congenital heart procedural planning. Birth Defects Res. 2018;110(13):1082–1090. doi: 10.1002/bdr2.1370. [DOI] [PubMed] [Google Scholar]
- 9.Ye W, Zhang X, Li T, Luo C, Yang L. Mixed-reality hologram for diagnosis and surgical planning of double outlet of the right ventricle: a pilot study. Clin Radiol. 2021;76(3):237–237. doi: 10.1016/j.crad.2020.10.017. [DOI] [PubMed] [Google Scholar]
- 10.Moro C, Phelps C, Jones D, Stromberga Z. Using Holograms to Enhance Learning in Health Sciences and Medicine. Med Sci Educ. 2020;30(4):1351–1352. doi: 10.1007/s40670-020-01051-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mathew PS , Pillai AS . In: Virtual and Augmented Reality in Education, Art, and Museums . Guazzaroni G , Pillai AS , et al., editors. 2020 . Role of Immersive (XR) Technologies in Improving Healthcare Competencies ; pp. 23 – 46 . [Google Scholar]
- 12.González Izard, Juanes Méndez, Ruisoto Palomera, García-Peñalvo FJ. Applications of Virtual and Augmented Reality in Biomedical Imaging. J Med Syst. 2019;43(4):102–102. doi: 10.1007/s10916-019-1239-z. [DOI] [PubMed] [Google Scholar]
- 13.Miller undefined, M undefined. Use of computer-aided holographic models improves performance in a cadaver dissection-based course in gross anatomy. Clin Anat. 2016;29(7):917–924. doi: 10.1002/ca.22766. [DOI] [PubMed] [Google Scholar]
- 14.Spigel Z, Binsalamah ZM, Caldarone C. Congenitally Corrected Transposition of the Great Arteries: Anatomic, Physiologic Repair, and Palliation. Seminars in Thoracic and Cardiovascular Surgery: Pediatric Cardiac Surgery Annual. 2019;22:32–42. doi: 10.1053/j.pcsu.2019.02.008. [DOI] [PubMed] [Google Scholar]
- 15.Kutty S, Danford DA, Diller GP, Tutarel O. Contemporary management and outcomes in congenitally corrected transposition of the great arteries. Heart. 2018;104(14):1148–1155. doi: 10.1136/heartjnl-2016-311032. [DOI] [PubMed] [Google Scholar]
- 16.Stark J, Silove ED, Taylor JF, Graham GR. Obstruction to systemic venous return following the Mustard operation for transposition of the great arteries. J Thorac Cardiovasc Surg. 1974;68(5):742–749. [PubMed] [Google Scholar]
- 17.Abbruzzese PA, Issenberg H, Cobanoglu A, Garcia C, Nunley D, Starr A. Superior vena cava obstruction after Mustard repair of d-transposition of the great arteries. Scand J Thorac Cardiovasc Surg. 1984;18(1):5–7. doi: 10.3109/14017438409099374. [DOI] [PubMed] [Google Scholar]
- 18.Poterucha JT, Taggart NW, Johnson JN, Cannon BC, Cabalka AK, Hagler DJ, Dearani JA, Cetta F. Intravascular and hybrid intraoperative stent placement for baffle obstruction in transposition of the great arteries after atrial switch. Catheter Cardiovasc Interv. 2017;89(2):306–314. doi: 10.1002/ccd.26783. [DOI] [PubMed] [Google Scholar]
- 19.Clarkson PM, Neutze JM, Barratt-Boyles BG, Brandt PW. Late Postoperative Hemodynamic Results and Cineangiocardiographic Findings after Mustard Atrial Baffle Repair for Transposition of the Great Arteries. Circulation. 1976;53(3):525–532. doi: 10.1161/01.cir.53.3.525. [DOI] [PubMed] [Google Scholar]
- 20.Graham TP. Hemodynamic residua and sequelae following intraatrial repair of transposition of the great arteries: a review. Pediatr Cardiol. 1982;2(3):203–213. doi: 10.1007/BF02332111. [DOI] [PubMed] [Google Scholar]
- 21.Bradley EA, Cai A, Cheatham SL, Chisolm J, Sisk T, Daniels CJ, Cheatham JP. Mustard baffle obstruction and leak-How successful are percutaneous interventions in adults. Prog Pediatr Cardiol. 2015;39(2 Pt B):157–163. doi: 10.1016/j.ppedcard.2015.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Santoro G, Ballerini L, Bialkowski J, Bermùdez-Canete R. Stent implantation for post-Mustard systemic venous obstruction. Eur J Cardiothorac Surg. 1998;14(3):332–334. doi: 10.1016/s1010-7940(98)00174-2. [DOI] [PubMed] [Google Scholar]
- 23.Brown SC, Eyskens B, Mertens L, Stockx L, Dumoulin M, Gewillig M. Self-expandable stents for relief of venous baffle obstruction after the Mustard operation. Heart. 1998;79(3):230–233. doi: 10.1136/hrt.79.3.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pasqualin G, Sturla F, D'Aiello AF, Chessa M. Mixed reality navigation of a systemic venous baffle obstruction: unravelling the percutaneous approach in atrial switch operation. Eur Heart J. 2021;42(41):4284–4284. doi: 10.1093/eurheartj/ehaa961. [DOI] [PubMed] [Google Scholar]
- 25.Reed MF, Mathisen DJ. Tracheoesophageal fistula. Chest Surg Clin N Am. 2003;13(2):271–289. doi: 10.1016/s1052-3359(03)00030-9. [DOI] [PubMed] [Google Scholar]
- 26.Burt M, Diehl W, Martini N, Bains MS, Ginsberg RJ, McCormack PM, Rusch VW. Malignant esophagorespiratory fistula: management options and survival. Ann Thorac Surg. 1991;52(6):1222–1228. doi: 10.1016/0003-4975(91)90005-b. [DOI] [PubMed] [Google Scholar]
- 27.Antkowiak JG. Tracheoesophageal fistula following blunt trauma. Arch Surg. 1974;109(4):529–531. doi: 10.1001/archsurg.1974.01360040047012. [DOI] [PubMed] [Google Scholar]
- 28.Kelly JP, Watts R. Webb, Peter V. Moulder, Nicholas M. Moustouakas, Mitchell Lirtzman. Management of airway trauma. II: Combined injuries of the trachea and esophagus. Ann Thorac Surg. 1987;2(43):160–163. doi: 10.1016/s0003-4975(10)60387-6. [DOI] [PubMed] [Google Scholar]
- 29.Temes RT, Wong RS, Davis M, Kessler RM, Wernly JA. Esophago-airway fistula in AIDS. Ann Thorac Surg. 1995;60(2):440–442. doi: 10.1016/0003-4975(95)00004-5. [DOI] [PubMed] [Google Scholar]
- 30.Macchiarini P, Delamare N, Beuzeboc P, Labussière AS, Cerrina J, Dulmet E, Chapelier A, Dartevelle P. Tracheoesophageal fistula caused by mycobacterial tuberculosis adenopathy. Ann Thorac Surg. 1993;55(6):1561–1563. doi: 10.1016/0003-4975(93)91111-y. [DOI] [PubMed] [Google Scholar]
- 31.Kumbhari V, Azola A, Okolo PI, Hughes A, Saxena P, Bapat V, Storm AC, Yung R, Khashab MA. Closure of a chronic tracheoesophageal fistula by use of a cardiac septal occluder. Gastrointest Endosc. 2014;80(2):332–332. doi: 10.1016/j.gie.2014.05.335. [DOI] [PubMed] [Google Scholar]
- 32.Li J, Gao X, Chen J, Lao M, Wang S, Zeng G. Endoscopic closure of acquired oesophagorespiratory fistulas with cardiac septal defect occluders or vascular plugs. Respir Med. 2015;109(8):1069–1078. doi: 10.1016/j.rmed.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 33.Coppola undefined, F undefined, Boccuzzi G, Rossi G, Gaia S, Cosimato M, Recchia S. Cardiac septal umbrella for closure of a tracheoesophageal fistula. Endoscopy. 2010;42(Suppl 2):318–319. doi: 10.1055/s-0030-1255822. [DOI] [PubMed] [Google Scholar]
- 34.Belle A, Lorut C, Lefebvre A, Ali EA, Hallit R, Leblanc S, Bordacahar B, Coriat R, Roche N, Chaussade S, Barret M. Amplatzer occluders for refractory esophago-respiratory fistulas: a case series. Endosc Int Open. 2021;9(9):1350–1354. doi: 10.1055/a-1490-9001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cohen-Atsmoni S, Tamir A, Avni Y, Priel IE, Roth Y. Endoscopic Occlusion of Tracheoesophageal Fistula in Ventilated Patients Using an Amplatzer Septal Occluder. Indian J Otolaryngol Head Neck Surg. 2015;67(2):196–199. doi: 10.1007/s12070-015-0842-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Siboni S, D’Aiello AF, Chessa M, Bonavina L. Tailored endoscopic treatment of tracheo-oesophageal fistula using preoperative holographic assessment and a cardiac septal occluder. BMJ Case Rep. 2022;15(3):248981–248981. doi: 10.1136/bcr-2022-248981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brida M, Chessa M, Celermajer D, Li W, Geva T, Khairy P, Griselli M, Baumgartner H, Gatzoulis MA. Atrial septal defect in adulthood: a new paradigm for congenital heart disease. Eur Heart J. 2022;43(28):2660–2671. doi: 10.1093/eurheartj/ehab646. [DOI] [PubMed] [Google Scholar]
- 38.Vecht JA, Saso S, Rao C, Dimopoulos K, Grapsa J, Terracciano CM, Peters NS, Nihoyannopoulos P, Holmes E, Gatzoulis MA, Athanasiou T. Atrial septal defect closure is associated with a reduced prevalence of atrial tachyarrhythmia in the short to medium term: a systematic review and meta-analysis. Heart. 2010;96(22):1789–1797. doi: 10.1136/hrt.2010.204933. [DOI] [PubMed] [Google Scholar]
- 39.Akseer S, Horlick E, Vishwanath V, Hobbes B, Huszti E, Mak S, Lee DS, Abrahamyan L. Prevalence and outcomes of pulmonary hypertension after percutaneous closure of atrial septal defect: a systematic review and meta-analysis. European Respiratory Review. 2020;29(158):200099–200099. doi: 10.1183/16000617.0099-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Himelfarb JD, Shulman H, Olesovsky CJ, Rumman RK, Oliva L, Friedland J, Farrell A, Huszti E, Horlick E, Abrahamyan L. Atrial fibrillation following transcatheter atrial septal defect closure: a systematic review and meta-analysis. Heart. 2022;108(15):1216–1224. doi: 10.1136/heartjnl-2021-319794. [DOI] [PubMed] [Google Scholar]
- 41.Baumgartner H, De Backer, Babu-Narayan SV, Budts W, Chessa M, Diller GP, Lung B, Kluin J, Lang IM, Meijboom F, Moons P, Mulder BJM, Oechslin E, Roos-Hesselink JW, Schwerzmann M, Sondergaard L, Zeppenfeld K; ESC Guidelines for the management of adult congenital heart disease. European Heart Journal vol. 2021;42(6):563–645. doi: 10.1093/eurheartj/ehaa554. [DOI] [PubMed] [Google Scholar]
- 42.Stout KK, Daniels CJ, Aboulhosn JA, Bozkurt B, Broberg CS, Colman JM, Crumb SR, Dearani JA, Fuller S, Gurvitz M, Khairy P, Landzberg MJ, Saidi A, Valente AM, Van Hare. AHA/ACC Guideline for the Management of Adults with Congenital Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73(12):81–192. doi: 10.1016/j.jacc.2018.08.1029. [DOI] [PubMed] [Google Scholar]
- 43.Oster M, Bhatt AB, Zaragoza-Macias E, Dendukuri N, Marelli A. Interventional Therapy Versus Medical Therapy for Secundum Atrial Septal Defect: A Systematic Review (Part 2) for the 2018 AHA/ACC Guideline for the Management of Adults with Congenital Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73(12):1579–1595. doi: 10.1016/j.jacc.2018.08.1032. [DOI] [PubMed] [Google Scholar]
- 44.Naqvi N, McCarthy KP, Ho SY. Anatomy of the atrial septum and interatrial communications. J Thorac Dis. 2018;10(Suppl 24):2837–2847. doi: 10.21037/jtd.2018.02.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thakkar AN, Chinnadurai P, Breinholt JP, Lin CH. Transcatheter closure of a sinus venosus atrial septal defect using 3D printing and image fusion guidance. Catheterization and Cardiovascular Interventions. 2018;92(2):353–357. doi: 10.1002/ccd.27645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Garg G, Tyagi H, Radha AS. Transcatheter closure of sinus venosus atrial septal defect with anomalous drainage of right upper pulmonary vein into superior vena cava-An innovative technique. Catheter Cradiovasc Interv. 2014;84(3):473–477. doi: 10.1002/ccd.25502. [DOI] [PubMed] [Google Scholar]
- 47.Abdullah HAM, Alsalkhi HA, Khalid KA. Transcatheter closure of sinus venosus atrial septal defect with anomalous pulmonary venous drainage: Innovative technique with long‐term follow‐up. Catheter Cradiovasc Interv. 2020;95(4):743–747. doi: 10.1002/ccd.28364. [DOI] [PubMed] [Google Scholar]
- 48. Butera G , Chessa M , Eicken A , Thomson JD . Atlas of Cardiac Catheterization for Congenital Heart Disease . Springer International Publishing ; 2019 . [Google Scholar]
- 49.Benson L, Horlick E, Osten M. Percutaneous Repair of the Sinus Venosus Atrial Defect. J Am Coll Cardiol. 2020;75(11):1279–1280. doi: 10.1016/j.jacc.2020.01.024. [DOI] [PubMed] [Google Scholar]
- 50.Butera G, Sturla F, Pluchinotta FR, Caimi A, Carminati M. Holographic Augmented Reality and 3D Printing for Advanced Planning of Sinus Venosus ASD/Partial Anomalous Pulmonary Venous Return Percutaneous Management. JACC Cardiovasc Interv. 2019;12(14):1389–1391. doi: 10.1016/j.jcin.2019.03.020. [DOI] [PubMed] [Google Scholar]
- 51. Rajput FA , Bishop MA , Limaiem F . Cardiac Fibroma . StatPearls ; 2023 . [PubMed] [Google Scholar]
- 52.Covington MK, Young PM, Bois MC, Maleszewski JJ, Anand V, Dearani JA, Klarich KW. Clinical Impact of Cardiac Fibromas. American Journal of Cardiology. 2022;182:95–103. doi: 10.1016/j.amjcard.2022.06.062. [DOI] [PubMed] [Google Scholar]
- 53.Miyake CY, Del Nido, Alexander ME, Cecchin F, Berul CI, Triedman JK, Geva T, Walsh EP. Cardiac tumors and associated arrhythmias in pediatric patients, with observations on surgical therapy for ventricular tachycardia. J Am Coll Cardiol. 2011;58(18):1903–1909. doi: 10.1016/j.jacc.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 54.Holley DG, Martin GR, Brenner JI, Fyfe DA, Huhta JC, Kleinman CS, Ritter SB, Silverman NH. Diagnosis and management of fetal cardiac tumors: a multicenter experience and review of published reports. J Am Coll Cardiol. 1995;26(2):516–520. doi: 10.1016/0735-1097(95)80031-b. [DOI] [PubMed] [Google Scholar]
- 55. Ji Y , Zhang X , Tang H , Luo H , Shengwei Z , Qiu Z , Qinghua Z , Wang K , Diao L . Education Platform of Congenital Heart Disease Based on Mixed Reality Technology. NA. 2019:313–334. [Google Scholar]
- 56.Redman JD, Wolton WP, Shuttleworth E. Use of holography to make truly three-dimensional x-ray images. Nature. 1968;220(5162):58–60. doi: 10.1038/220058a0. [DOI] [PubMed] [Google Scholar]
- 57.Karmonik C, Boone TB, Khavari R. Workflow for Visualization of Neuroimaging Data with an Augmented Reality Device. J Digit Imaging. 2018;31(1):26–31. doi: 10.1007/s10278-017-9991-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pratt P, Ives M, Lawton G, Simmons J, Radev N, Spyropoulou L, Amiras D. Through the HoloLens™ looking glass: augmented reality for extremity reconstruction surgery using 3D vascular models with perforating vessels. Eur Radiol Exp. 2018;2(1):2–2. doi: 10.1186/s41747-017-0033-2. [DOI] [PMC free article] [PubMed] [Google Scholar]


