Abstract
Introduction
We aim to study the utility of 6-minute walk distance (6MWD) and cardiopulmonary exercise testing (CPET) in patients with acute systolic heart failure (HF) in predicting post-discharge outcomes.
Methods
The ESCAPE trial data was utilized to examine the prognostic role of 6MWD and CPET in predicting 6-month all-cause mortality and rehospitalization in acute HF.
Results
The average 6MWD recorded in 271 and 292 patients on admission and discharge was 597 and 765 ft., respectively. Compared with non-survivors, survivors had significantly higher 6MWD on admission (624 vs. 463 ft., P = 0.006) and discharge (789 vs. 636 ft., P = 0.006). Admission and discharge 6MWD had an AUC of 0.629 (P = 0.0047) and 0.628 (P = 0.0093) in predicting mortality. The combination of optimal 6MWD cutoff values of >288 ft. on admission and > 320 ft. on discharge was associated with significantly lower mortality (11.1% vs. 28.3%, OR 0.316, P = 0.002). When dividing the sample into quartiles of increasing walking distance, patients in the 1st quartile had significantly higher mortality on admission (OR 3.59, 95% CI 1.396–9.282, P = 0.008) and discharge (OR 3.66, 95% CI 1.357–9.839, P = 0.01) compared with 4th quartile. P-value for the trend in mortality across quartiles of 6MWD on admission and discharge was 0.016 and 0.047, respectively. Cox proportional hazard analysis revealed that admission (HR 0.632, 95% CI 0.449–0.890, P = 0.009) and discharge 6MWD (HR 0.657, 95% CI 0.467–0.926, P = 0.016) were independent mortality determinants after adjustment for age, creatinine, sodium, systolic blood pressure and NYHA class, all on admission. CPET-derived variables did not predict either outcomes.
Conclusion
6MWD is an independent mortality determinant in advanced systolic HF.
Keywords: 6-minute walk test, Cardiopulmonary exercise test, Heart failure, Mortality, Rehospitalization
1. Introduction
Heart failure (HF) affects 2% of the population in developed countries and is associated with high rates of morbidity and mortality [1]. Identifying reliable prognostic markers is crucial to aid in predicting outcomes and identifying those in need of more advanced monitoring and therapy. Functional capacity carries important prognostic information as it is closely associated with outcomes in patients with HF [2]. Quantification of functional capacity is beneficial for objective staging of the clinical severity of HF [3]. The two main methods used to measure the functional capacity in HF is the 6-minute walk test (6MWT) and cardiopulmonary exercise testing (CPET) [4].
Since 1991, when Mancini and colleagues [4] demonstrated that peak exercise VO2 of <14 mL/min/kg in ambulatory patients with severe left ventricular dysfunction predicts mortality, CPET became widely considered the “gold standard” for assessing exercise capacity through direct measurement of oxygen consumption, and this cutoff is used to justify cardiac transplantation. Meanwhile, the 6MWT is more widely available, easy to perform, and can be a surrogate for the patient's quality of life because the physical intensity of the test mimics activities of daily living [5,6]. Approximately one third of the patients with HF may not be able to perform maximal symptom-limited exercise test [7] and only a submaximal exercise test may be feasible. In advanced HF, the ability of the 6MWT to predict outcomes was questioned [8,9], and prior studies have yielded conflicting results with regards to the comparison of the 6MWT versus CPET in predicting post-discharge outcomes. Moreover, the prognostic value of the 6MWT was more extensively examined in patients with chronic HF [8,[10], [11], [12], [13]] and mild to moderate HF [7] but not in patients with severe systolic HF with advanced symptoms.
In patients with severe systolic HF and advanced symptoms, it is expected that their daily level of activity is approximately closer to their maximal exercise capacity, and we were indeed able to prove in a previous analysis in the same subset of patients, the significant association between the 6-minute walk distance (6MWD) and several CPET-derived variables including peak oxygen uptake (peak VO2) [14]. To further support this, the 6MWT was previously shown to have best performance in predicting outcomes in patients walking ≤300 m (900 ft) and only in this subset of patients was the 6MWD significantly correlated with peak VO2 [12]. We therefore hypothesized that the 6MWT has valuable prognostic ability in predicting post-discharge outcomes in patients with severe left ventricular systolic dysfunction and advanced symptoms. In this analysis, we studied the utility of 6MWT and various CPET-derived variables in predicting post-discharge morbidity and mortality among patients with acute systolic HF.
2. Methods
2.1. Study population and the ESCAPE trial
This study is a retrospective analysis of a limited access dataset from the Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness (ESCAPE) trial which was a National Heart, Lung, and Blood Institute-funded study. The ESCAPE trial enrolled 433 patients with acute HF and ejection fraction ≤30% and compared outcomes of patients managed with clinical assessment plus pulmonary artery catheterization (PAC) versus clinical assessment alone. All patients were admitted with New York Heart Association (NYHA) class IV symptoms and had a left ventricular ejection fraction (LVEF) ≤30%, 3 months of symptoms despite appropriate therapy, a systolic blood pressure < 125 mmHg and at least one sign and one symptom of congestion. The study showed that the PAC did not significantly improve or worsen outcomes as determined by the primary endpoint: number of days alive outside the hospital at 6-months following randomization. Design, rationale and results of the ESCAPE trial have been previously published [15].
2.2. 6MWT, CPET and study endpoints
The 6MWD was measured in feet at multiple points in the study including admission, discharge, at 3 months and 6 months. At the same time points, various CPET-derived variables were measured including peak oxygen uptake (peak VO2), ventilatory efficiency (VE max) and minute ventilation‑carbon dioxide production (VE/VCO2). The study endpoints were all-cause rehospitalization and mortality up to 6-months after randomization. We initially examined the prognostic effect of the 6MWT and CPET-derived variables on post-discharge outcomes, then we did the same analysis according to quartiles of admission and discharge 6MWD.
2.3. Statistical analysis
Primary analysis compared survivors versus non-survivors and rehospitalized versus non-rehospitalized patients with regards to 6MWD and CPET-derived variables. Continuous variables were tested for distribution normality using the Shapiro-Wilk test and were expressed as mean ± standard deviation or median and interquartile range (IQR) and compared using independent sample t-test for normally distributed variables, or Mann-Whitney test for non-normally distributed variables. Categorical variables were compared using the Chi-square test.
A receiver operating characteristics (ROC) analysis was implemented to detect the ability of 6MWD and CPET-derived variables to predict mortality and rehospitalization and to calculate the area under curve (AUC). Comparisons of time-to-event outcomes was performed using Kaplan-Meier estimates and log-rank tests. In order to study the incremental effect of reduction in 6MWD on rehospitalization and death, we have divided the cohort into 4 groups according to the 4 quartiles of the admission and discharge 6MWD, and the Cochran-Mantel-Haenszel common odds ratio (OR) test was used to evaluate the OR and P value for the risk of rehospitalization and death in the 1st, 2nd and 3rd quartiles using the 4th quartile as the comparator. Chi-square custom table function was utilized to detect whether the trend in outcomes across the four quartiles of admission and discharge 6MWD was significant or not.
Cox proportional hazards analysis was performed to identify whether admission and discharge 6MWD predicted mortality after adjusting for known risk factors of mortality in acute HF (age, baseline creatinine, baseline sodium, baseline systolic blood pressure and NYHA class). Admission and discharge 6MWD were examined in the models independently to avoid the effect of collinearity. Patient's age, creatinine, sodium and systolic blood pressure at baseline were included in the modeling in units of standard deviations which represent 13.9 years, 0.6 mg/dL, 4.4 meq/L, and 16.3 mmHg, respectively; thus, an odds ratio or hazard ratio estimate for age should be interpreted as per 13.9-year change (holding all other quantities fixed), and so forth. Comparison of the prognostic effect of admission and discharge 6MWD on mortality and rehospitalization was performed using the Hanley and McNeil method as implemented in the MedCalc software. A P-value <0.05 was considered statistically significant. All statistical significance was assessed using a 2-sided P values. Data were analyzed using IBM SPSS 21.0 statistical software (IBM SPSS Version 21.0. Armonk, NY).
3. Results
3.1. Patient characteristics
A total of 433 patients were enrolled in the ECAPE trial: mean age 56 years, 74% men, 60% white and 28% were African American race. The average LVEF of the study population was 20%, and their left ventricular end-diastolic dimension was 6.6 cm and left ventricular end-systolic dimension was 5.9 cm. 86% of the patients were classified as having NYHA class IV symptoms at baseline. Patients randomized to receive PAC had an average pulmonary capillary wedge pressure of 25 mmHg and right atrial pressure of 13 mmHg. Table 1 summarizes baseline demographic, clinical, laboratory, echocardiographic and central hemodynamic characteristics of the ESCAPE study population.
Table 1.
Baseline demographic, clinical, laboratory, echocardiographic and central hemodynamic characteristics of the ESCAPE study population.
| Mean ± SD or n (%) | |
|---|---|
| Age (years) | 56.13 ± 13.92 |
| Male sex | 321 (74.1%) |
| White race | 258 (59.6%) |
| Black race | 119 (27.5%) |
| BMI (Kg/m2) | 29.01 ± 6.79 |
| NYHA class IV | 368 (85.6%) |
| Rales | 225 (52.3%) |
| JVD > 12 cm | 225 (53.7%) |
| S3 gallop | 283 (65.8%) |
| HJR | 333 (79.9%) |
| LE edema at least 2+ | 155 (36%) |
| Hepatomegaly | 249 (58.2%) |
| Laboratory values | |
| BNP (pg/mL) | 1006 ± 1314 |
| BUN (mg/dL) | 34.89 ± 22.67 |
| Creatinine (mg/dL) | 1.51 ± 0.62 |
| Total bilirubin (mg/dL) | 0.86 ± 0.53 |
| Na (meq/L) | 136.66 ± 4.44 |
| Hb (gm/dL) | 12.69 ± 3.82 |
| Echocardiography | |
| EF (%) | 20.02 ± 9.55 |
| LVEDD (cm) | 6.63 ± 1.1 |
| LVESD (cm) | 5.88 ± 1.15 |
| IVC inspiration (cm) | 1.59 ± 0.72 |
| IVC expiration (cm) | 2.19 ± 0.62 |
| IVC Collapsibility index (%) | 30.11 ± 20.75 |
| PAC variables | |
| RAP (mmHg) | 12.97 ± 7.08 |
| PCWP (mmHg) | 24.72 ± 9.16 |
| COP (L/min, m ± SD) | 3.94 ± 1.37 |
| CI (L/min/m2, m ± SD) | 2 ± 0.62 |
| 6MWT | |
| Baseline 6MWD (feet) | 597 ± 374 |
| Improvement in 6MWD (feet) | 205 ± 160 |
| CPET | |
| Peak VO2 (mL/kg/min) | 10.05 ± 3.39 |
| VE max (L/min) | 41.98 ± 14.69 |
| VE/VCO2 | 41.42 ± 39.17 |
BMI: body mass index, NYHA: New York Heart Association, JVD: jugular venous distension, HJR: hepatojugular reflux, LE: lower extremity, 6MWD: 6-minute walk distance, BNP: B-type natriuretic peptide, EF: ejection fraction, LVEDD: left ventricular end-diastolic dimension, LVESD: left ventricular end-systolic dimension, IVC: inferior vena cava, RAP: right atrial pressure, PCWP: pulmonary capillary wedge pressure, COP: cardiac output, CI: cardiac index.
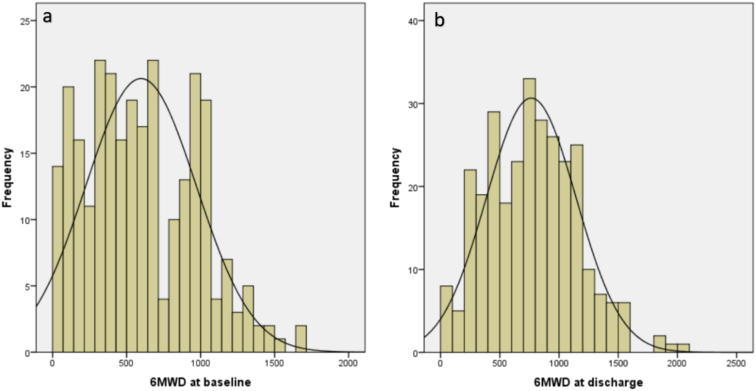
All patients who had either admission or discharge 6MWT were included in our analysis. The average 6MWD in the 271 (63%) patients who had the test on admission was 597 ± 374 f. and in the 292 (67%) patients who had the test on discharge was 765 ± 380 f. with an average increase of 205 ± 160 from admission to discharge. Peak VO2, VE max and VE/VCO2 was measured in 126, 118 and 111 patients on hospital admission, with average values of 10 mL/kg/min, 42 L/min and 41, respectively. Fig. 1 illustrates the distribution of 6MWD on admission and discharge.
Fig. 1.
Distribution of 6-minute walk distance on admission (n = 271) and discharge (n = 292) among ESCAPE trial patients hospitalized with acute decompensated heart failure.
3.2. Mortality
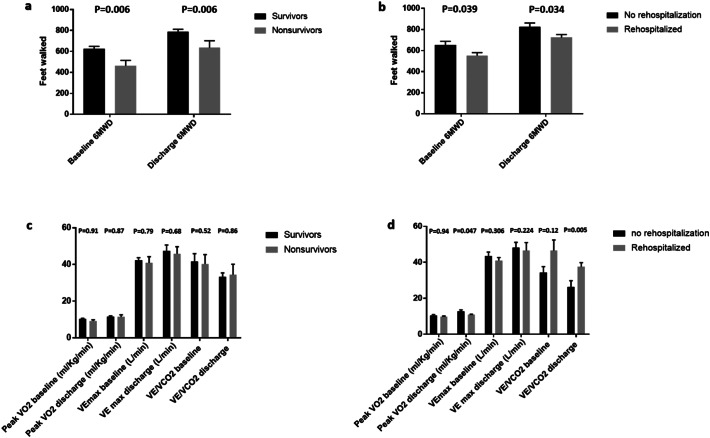
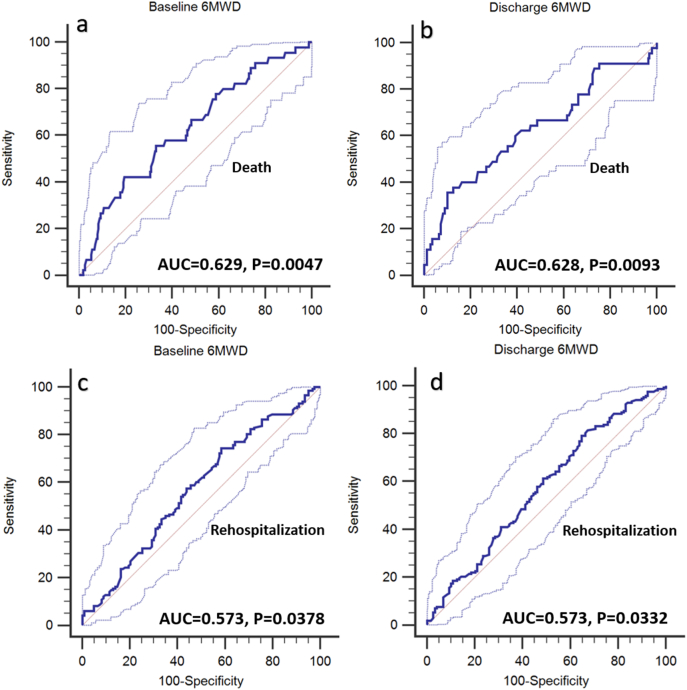
At 6-months, 19.2% (83/433) of the patients participating in the study died. Compared with non-survivors, survivors had significantly higher 6MWD on admission (624 vs. 463 ft, P = 0.006) and discharge (789 vs. 636 ft, P = 0.006) [Fig. 2, panel a]. ROC curve analysis showed that admission 6MWD had an AUC of 0.629, 95% CI 0.569–0.687, P = 0.0047, and an optimum criterion ≤288 f. had a 42.2% sensitivity and 80.5% specificity in predicting mortality (Fig. 3, panel a). Discharge 6MWD had an AUC of 0.628, 95% CI 0.570–0.684, P = 0.0093 and an optimum cutoff criterion of ≤320 f. had a 35.6% sensitivity and 89.9% specificity in predicting mortality (Fig. 3, panel b). The combination of optimal cutoff values of admission 6MWD (>288 ft) and discharge 6MWD (>320 ft) was associated with significantly lower 6-month all-cause mortality (11.1% vs. 28.3%, univariate OR 0.316, 95% CI 0.150–0.666, P = 0.002) and Kaplan Meier survival curves revealed a significant intergroup difference in mortality (Chi square 11.632, Log-rank P = 0.001) [Fig. 4]. There were no differences between survivors and non-survivors with regards to CPET- derived variables such as peak VO2, VE max and VE/VCO2 on admission or discharge (Fig. 2, panels c).
Fig. 2.
Mean and standard error of admission 6MWD and discharge 6MWD in survivors and non-survivors (panel a) and those who rehospitalized or non-rehospitalized at 6 months (panel b). Panels c and d represent mean and standard error of various cardiopulmonary exercise test variables to study both endpoints.
Fig. 3.
ROC curves showing the ability of admission 6MWD and discharge 6MWD to predict mortality (panels a and b, respectively) and rehospitalization (panels c and d, respectively). Each panel shows AUC and respective P value.
Fig. 4.
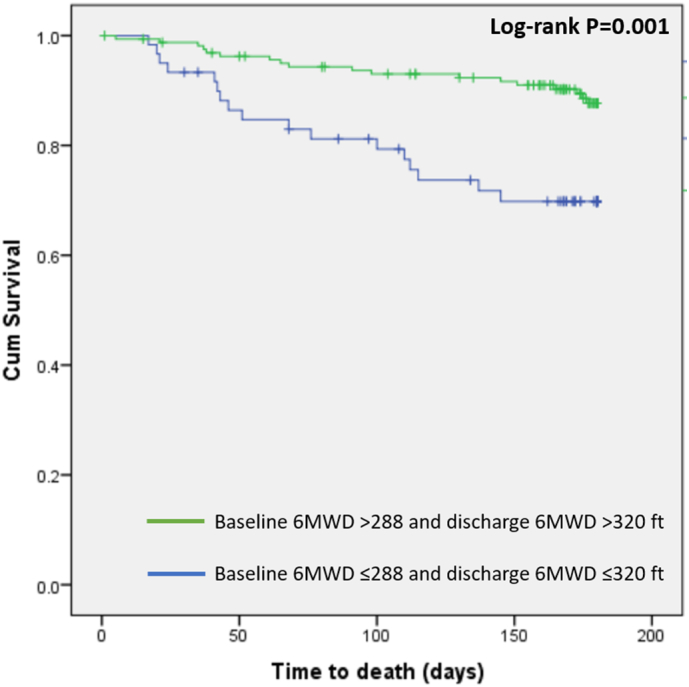
Kaplan Meier curves showing a significant difference in survival between patients enrolled in the ESCAPE trial with advanced systolic heart failure whose admission and discharge 6-minute walk distance are either >288 and >320 f. or ≤288 and ≤320 ft, respectively.
3.3. Rehospitalization
The rate of all-cause rehospitalization at 6 months in the study cohort was 57% (247/433). Patients who were not rehospitalizaed had significantly higher 6MWD on admission (653 vs. 551 ft, P = 0.039) and discharge (825 vs. 724 ft, P = 0.034) compared with rehospitalized patients (Fig. 2, panel b). ROC curve analysis showed that admission 6MWD had an AUC of 0.573, 95% CI 0.511–0.632, P = 0.0378, and an optimum criterion ≤741 f. had a 74.3% sensitivity and 41.5% specificity in predicting rehospitalization (Fig. 3, panel c). Discharge 6MWD had an AUC of 0.573, 95% CI 0.514–0.631, P = 0.0332 and an optimum cutoff criterion of ≤1000 f. had a 79.2% sensitivity and 35.3% specificity in predicting rehospitalizaiton (Fig. 3, panel d). Patients who were not rehospitalized did not have better CPET- derived variables such as peak VO2, VE max and VE/VCO2 compared with those who were rehospitalized (Fig. 2, panel d).
3.4. Mortality and rehospitalization according to 6MWD quartiles
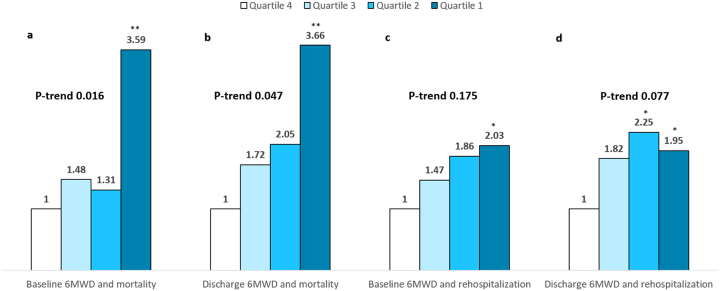
To study post-discharge outcomes according to quartiles of 6MWD, the study cohort was divided into quartiles according to admission 6MWD as follows: 1st quartile (n = 65, 6MWD <300 ft), 2nd quartile (n = 69, 6MWD 300–549 ft), 3rd quartile (n = 69, 6MWD 550–904 ft) and 4th quartile (n = 68, 6MWD ≥905 ft) then into quartiles according to discharge 6MWD as follows: 1st quartile (n = 73, 6MWD <446 ft), 2nd quartile (n = 71, 6MWD 446–760 ft), 3rd quartile (n = 75, 6MWD 760–1049 ft) and 4th quartile (n = 73, 6MWD ≥1050 ft). With regards to mortality, patients in the 1st quartile of admission 6MWD had significantly higher mortality compared with patient in the 4th quartile (univariate OR 3.59, 95% CI 1.396–9.282, P = 0.008). Also, patients in the 1st quartile of discharge 6MWD had significantly higher mortality compared with patients in the 4th quartile (univariate OR 3.66, 95% CI 1.357–9.839, P = 0.01). P value for the trend in mortality across quartiles of 6MWD on admission and discharge was 0.016 and 0.047, respectively [Fig. 5, panels a, b].
Fig. 5.
Unadjusted comparison of quartile of admission and discharge 6-minute walk distance and all-cause mortality (panels a, b) and all-cause rehospitalization (panels c, d) at 6-months among patients hospitalized with systolic heart failure enrolled in the ESCAPE trial. *P < 0.05, **P ≤ 0.01 vs. 4th quartile of 6-minute walk distance.
With regards to rehospitalization, patients in the 1st quartile of 6MWD had significantly higher rehospitalization according to admission 6MWD (univariate OR 2.03, 95% CI 1.02–4.05, P = 0.045) and discharge 6MWD (univariate OR 1.95, 95% CI 1.01–3.79, P = 0.047) compared with patients in the 4th quartile [Fig. 5, panels c, d]. Nonetheless, P value for the trend in mortality across quartiles of 6MWD on admission and discharge was 0.175 and 0.077, respectively. Fig. 5 shows the unadjusted OR of mortality and rehospitalization according to admission and discharge 6MWD quartiles.
3.5. Comparison of admission and discharge 6MWD in predicting outcomes
There was no significant difference between AUC of admission and discharge 6MWD in predicting both endpoints. With regards to mortality outcome, comparing the ROC curves showed that the difference in AUC between admission and discharge 6MWD is 0.00346 (P = 0.939). With regards to rehospitalization, that the difference in AUC between admission and discharge 6MWD is 0.00251 (P = 0.937).
3.6. Comparison of outcomes according to whether 6MWT was performed or not
A total of 28% (120/428) patient were too ill to measure 6MWD at baseline. Patients who were too ill to walk had a mortality of 21.7% (26/120) vs. 18.2% (56/308), P = 0.411, and a rehospitalization rate of 58.3% and 56.5%, P = 0.730, compared with those who performed the 6MWT.
3.7. Cox proportional hazard analysis
Among the 271 and 292 patients who had 6MWT performed on admission and discharge, the mortality rate was 16.6% (45/271) and 15.4% (45/292), respectively. We found that for every standard deviation increase in 6MWD at baseline, there was a 27% reduction in mortality after adjustment for age, creatinine, sodium, systolic blood pressure and NYHA class, all on admission (estimated hazard ratio 0.632, 95% CI 0.449–0.890, P = 0.009). When 6MWD was replaced by discharge 6MWD in the same model, we found that for every standard deviation increase in 6MWD at discharge, there was a 34% reduction in mortality after adjustment for the same aforementioned risk factors (estimated hazard ratio 0.657, 95% CI 0.467–0.926, P = 0.016). Table 2 lists the variables included in the multivariate analysis according to whether admission or discharge 6MWD was included in the model.
Table 2.
Cox proportional hazard analysis for predictors of 6-month mortality.
| HR; 95% CI; P-value | |
|---|---|
| Admission 6MWD | |
| Age/SD | 1.059 (0.751, 1.493) 0.745 |
| Admission Na/SD | 0.980 (0.722, 1.330) 0.898 |
| Admission Creatinine/SD | 1.308 (0.975, 1.753) 0.073 |
| Admission SBP/SD | 0.697 (0.479, 1.013) 0.058 |
| Baseline NYHA class | 0.743 (0.326, 1.690) 0.478 |
| 6MWD on admission/SD | 0.632 (0.449, 0.890) 0.009 |
| Discharge 6MWD | |
| Age/SD | 1.027 (0.716, 1.473) 0.886 |
| Admission Na/SD | 0.863 (0.635, 1.172) 0.346 |
| Admission Creatinine/SD | 1.071 (0.799, 1.434) 0.647 |
| Admission SBP/SD | 0.641 (0.443, 0.928) 0.018 |
| Baseline NYHA class | 0.619 (0.270, 1.419) 0.257 |
| 6MWD on discharge/SD | 0.657 (0.467, 0.926) 0.016 |
6MWD: 6-minute walk distance, SBP: systolic blood pressure, NYHA: New York Heart Association, SD: standard deviation.
4. Discussion
In this analysis, we examined the value of the 6MWD and various CPET variables -not just peak VO2- in predicting mortality in patients hospitalized with acute HF who have severe systolic dysfunction and advanced symptoms. The major finding was that the 6MWT performed on admission and discharge predicted intermediate term mortality. This prognostic effect remained unaltered despite multivariable analysis adjusting for known mortality determinants among ESCAPE study subjects. We have also noted that the prognostic effect of the admission and discharge 6MWD on mortality was more powerful in patients included in the first quartile of 6MWD. When examining mortality according to quartiles of the 6MWD on admission and discharge using the 4th quartile as a reference (6MWD ≥ 905 f. and ≥ 1050 f. on admission and discharge, respectively), only the 1st quartile had significantly higher mortality, but not the 2nd and 3rd quartile. This suggests that the best performance for the 6MWT is obtained in patients with HF walking lower distances and its value decreases as the walking distance increase. We therefore propose that the 6MWT is an important mortality determinant in those with systolic HF whose 6MWD is significantly reduced on admission (<300 ft) or discharge (<446 ft).
Although we found no difference in the ability of admission versus discharge 6MWD to predict post-discharge outcomes, the combination of admission 6MWD >288 f. and discharge 6MWD >320 f. was associated with almost 2/3 lower risk of death at 6-months. Therefore, patients with walking distance equivalent to 100 m on admission or discharge should be considered high risk and may benefit from closer monitoring, more aggressive therapy or possibly faster listing for ventricular assist device or cardiac transplantation.
Although patients who were rehospitalized up to 6 months had significantly lower 6MWD on admission and discharge on univariate comparison, we could not find a significant trend of increase in rehospitalization with decreasing quartiles of 6MWD on admission (P for the trend 0.175) and discharge (P for the trend 0.077). These results suggest that the prognostic effect of the admission and discharge 6MWD was stronger on mortality compared with rehospitalization outcomes. Nonetheless a potential explanation for this is the death of 45 patients (~one sixth of the study population with available 6MWT data) in the first 6-month after randomization, thereby reducing the available subjects to study the outcome. On the other hand, survivors and those who were not rehospitalized did not have better CPET-derived variables specifically peak VO2, VE max and VE/VCO2 compared with non-survivors and those who were rehospitalized. Also, the generated C-statistics shows that various CPET variables did not predict post-discharge outcomes.
Our results further extend the literature on the value of the 6MWT as an important mortality determinant in acute HF in addition to the repeatedly cited logistic values including its simplicity, feasibility and negligible cost. It is considered as a surrogate to activities of daily living even though oxygen uptake measured during a standard 6MWT using a validated portable instrument showed that 19/26 examined patients exercised on a predominantly anerobic metabolism, suggesting that the energy expenditure is close to maximal [16,17]. The authors found that in the 26 examined patients with mild to severe heart failure (NYHA class II, 10 patients; III, 10 patients; IV, 6 patients; left ventricular ejection fraction: 22 +/− 6%), VO2 during 6MWT is only 15% lower than peak VO2. It is therefore reasonable to expect when examining a subset of patients in the ESCAPE trial with severe systolic HF with a majority being NYHA class IV who were in the 1st quartile of walking distance on admission and discharge, that their energy expenditure will be maximal. This explains why in multiple prior studies including ours [14], the 6MWD and peak VO2 were linearly correlated [8,9,11,12,18].
Our finding are in line with results concluded by Ingle and colleagues who reported the limited utility of the 6MWT in patients with mild left ventricular dysfunction, but it was a mortality predictor in those with severe heart disease [19]. While most studies used a cutoff limit of ≤300 m as discriminatory between survivors and non-survivors [7,8,12,20], we found in our analysis a cutoff value of ~100 m on admission and ~ 150 m on discharge as the discriminatory cutoff value. Because the value of CPET is explained by its ability to measure cardiopulmonary reserve at maximal exercise, it makes sense that 6MWT has most predictive significance in sickest patients, when such a trivial effort as walking at their own pace approaches maximal possible effort. We found that in advanced HF the inability to walk >100 m in 6 min has such a high prediction of mortality that CPET may be unnecessary.
4.1. Study limitations
Limitations of the study include those inherent to non-randomized trials. The analysis was retrospective and the sample size is moderate. Approximately only 40–45% of patients who had 6MWT performed on admission or discharge performed CPET and therefor the sample size used to examine the value of CPET in predicting outcomes is much smaller. Therefore, the value of CPET needs to be examined in a bigger sample. Our results are applicable only to patients with severe left ventricular systolic dysfunction and advanced symptoms but cannot be generalized to patients with chronic HF or those with mild to moderate HF. Among the limitations, is the relative youth of the sample (56 years), which is younger than the typical patient admitted with acute HF in the current era. Another limitation is that this post-hoc analysis is performed nearly 20 years after ESCAPE trial was conducted, with current HF treatment being different from two decades ago. There may have been other variables untested in the multivariate model that was not accounted for and may have confounded the results.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgement
The ESCAPE trial was conducted and supported by the NHLBI in collaboration with the ESCAPE Study Investigators. This article was prepared using a limited access dataset obtained from the NHLBI and does not necessarily reflect the opinions or views of the ESCAPE trial investigators or the NHLBI.
Footnotes
There are no conflicts of interest. Both authors contributed to manuscript.
No funding has been received for this manuscript
References
- 1.Dickstein K., et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the diagnosis and treatment of acute and chronic heart failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM) Eur J Heart Fail. 2008;10(10):933–989. doi: 10.1016/j.ejheart.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 2.Schalcher C., et al. Prolonged oxygen uptake kinetics during low-intensity exercise are related to poor prognosis in patients with mild-to-moderate congestive heart failure. Chest. 2003;124(2):580–586. doi: 10.1378/chest.124.2.580. [DOI] [PubMed] [Google Scholar]
- 3.Witte K.K., Clark A.L. Why does chronic heart failure cause breathlessness and fatigue? Prog Cardiovasc Dis. 2007;49(5):366–384. doi: 10.1016/j.pcad.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 4.Mancini D.M., et al. Value of peak exercise oxygen consumption for optimal timing of cardiac transplantation in ambulatory patients with heart failure. Circulation. 1991;83(3):778–786. doi: 10.1161/01.cir.83.3.778. [DOI] [PubMed] [Google Scholar]
- 5.Ingle L., et al. Cardiorespiratory requirements of the 6-min walk test in older patients with left ventricular systolic dysfunction and no major structural heart disease. Int. J. Sports Med. 2007;28(8):678–684. doi: 10.1055/s-2007-964886. [DOI] [PubMed] [Google Scholar]
- 6.Fleg J.L., et al. Assessment of functional capacity in clinical and research applications: An advisory From the Committee on Exercise, Rehabilitation, and Prevention, Council on Clinical Cardiology, American Heart Association. Circulation. 2000;vol. 102(13):1591–1597. doi: 10.1161/01.cir.102.13.1591. [DOI] [PubMed] [Google Scholar]
- 7.Rostagno C., et al. Prognostic value of 6-minute walk corridor test in patients with mild to moderate heart failure: comparison with other methods of functional evaluation. Eur. J. Heart Fail. 2003;5(3):247–252. doi: 10.1016/s1388-9842(02)00244-1. [DOI] [PubMed] [Google Scholar]
- 8.Lucas C., et al. The 6-min walk and peak oxygen consumption in advanced heart failure: aerobic capacity and survival. Am. Heart J. 1999;138(4 Pt 1):618–624. doi: 10.1016/s0002-8703(99)70174-2. [DOI] [PubMed] [Google Scholar]
- 9.Opasich C., et al. Six-minute walking performance in patients with moderate-to-severe heart failure; is it a useful indicator in clinical practice? Eur. Heart J. 2001;22(6):488–496. doi: 10.1053/euhj.2000.2310. [DOI] [PubMed] [Google Scholar]
- 10.Bittner V., et al. Prediction of mortality and morbidity with a 6-minute walk test in patients with left ventricular dysfunction. SOLVD Investigators. JAMA. 1993;270(14):1702–1707. [PubMed] [Google Scholar]
- 11.Cahalin L.P., et al. The six-minute walk test predicts peak oxygen uptake and survival in patients with advanced heart failure. Chest. 1996;110(2):325–332. doi: 10.1378/chest.110.2.325. [DOI] [PubMed] [Google Scholar]
- 12.Roul G., Germain P., Bareiss P. Does the 6-minute walk test predict the prognosis in patients with NYHA class II or III chronic heart failure? Am. Heart J. 1998;136(3):449–457. doi: 10.1016/s0002-8703(98)70219-4. [DOI] [PubMed] [Google Scholar]
- 13.Forman D.E., et al. 6-min walk test provides prognostic utility comparable to cardiopulmonary exercise testing in ambulatory outpatients with systolic heart failure. J. Am. Coll. Cardiol. 2012;60(25):2653–2661. doi: 10.1016/j.jacc.2012.08.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Omar H.R., Guglin M. The longitudinal relationship between six-minute walk test and cardiopulmonary exercise testing, and association with symptoms in systolic heart failure: analysis from the ESCAPE trial. Eur. J. Intern. Med. 2017;40:e26–e28. doi: 10.1016/j.ejim.2016.12.017. [DOI] [PubMed] [Google Scholar]
- 15.Binanay C., et al. Evaluation study of congestive heart failure and pulmonary artery catheterization effectiveness: the ESCAPE trial. JAMA. 2005;294(13):1625–1633. doi: 10.1001/jama.294.13.1625. [DOI] [PubMed] [Google Scholar]
- 16.Faggiano P., et al. Assessment of oxygen uptake during the 6-minute walking test in patients with heart failure: preliminary experience with a portable device. Am. Heart J. 1997;134(2 Pt 1):203–206. doi: 10.1016/s0002-8703(97)70125-x. [DOI] [PubMed] [Google Scholar]
- 17.Kervio G., et al. Cardiorespiratory adaptations during the six-minute walk test in chronic heart failure patients. Eur J Cardiovasc Prev Rehabil. 2004;11(2):171–177. doi: 10.1097/01.hjr.0000119964.42813.98. [DOI] [PubMed] [Google Scholar]
- 18.Zugck C., et al. Is the 6-minute walk test a reliable substitute for peak oxygen uptake in patients with dilated cardiomyopathy? Eur. Heart J. 2000;21(7):540–549. doi: 10.1053/euhj.1999.1861. [DOI] [PubMed] [Google Scholar]
- 19.Ingle L., et al. Prognostic value of the 6 min walk test and self-perceived symptom severity in older patients with chronic heart failure. Eur. Heart J. 2007;28(5):560–568. doi: 10.1093/eurheartj/ehl527. [DOI] [PubMed] [Google Scholar]
- 20.Arslan S., et al. Prognostic value of 6-minute walk test in stable outpatients with heart failure. Tex. Heart Inst. J. 2007;34(2):166–169. [PMC free article] [PubMed] [Google Scholar]