Abstract
Background
Relationships between dyslipidaemia and leukocyte counts have been investigated in several studies, demonstrating limited evidence of associations in humans. As such, studying a diverse range of cohorts will ensure evidence is robust. This study focused on investigating cross-sectional and longitudinal relationships in three large-scale cohorts.
Methods
The cross-sectional analysis included a total of 27,566 participants with valid data on lipid measures and leukocyte counts from three study cohorts: National Health and Nutrition Survey (NHANES), Korean National Health and Nutrition Survey (KNHANES) and Treating to New Targets (TNT) trial. The longitudinal analysis included 9323 participants with valid data on lipid measures and leukocyte counts at baseline and one year with statin treatment. Associations between lipid levels and leukocyte counts were analysed by multivariable linear regression and adjusted for basic demographic and cardiovascular risk factors.
Results
Cross-sectional data from NHANES demonstrated the association of lower high-density lipoprotein (HDL) cholesterol and higher triglycerides with higher leukocyte count (0.9% lower and 0.3% higher count per 10 mg/dL increase in HDL cholesterol and triglycerides respectively, both p < 0.001). Similar trends were found in TNT trial (both p < 0.001), but not in KNHANES. In the TNT trial, 10 mg/dL increase in triglycerides over one year was also associated with a 0.09 × 103/μL increase in leukocyte count over the same period.
Conclusions
The findings of this study are consistent with those of previous human studies, supporting weak yet noteworthy associations between dyslipidaemia and leukocytosis.
Keywords: Inflammation, Lipids, Cholesterol, Leukocytes, Atherosclerosis
Highlights
-
•
High-density lipoprotein cholesterol is inversely associated with leukocyte count
-
•
Triglyceride levels are positively associated with leukocyte count
-
•
The relationships between lipid levels and leukocyte numbers in humans are distinct from dyslipidaemic animal models
1. Introduction
Inflammation and more specifically leukocytes are essential in the development of atherosclerosis [1]. The pathogenesis of atherosclerotic disease is the product of immune-mediated lipid deposition within the intima of large- and medium-sized vessels. As such, defining the relationship between circulating leukocyte counts and serum lipid levels would provide further insight into the development of this complex disease.
Several animal studies have supported an association of dyslipidaemia with leukocytosis. In rabbits a positive relationship between leukocytosis and serum cholesterol has been identified [2], while hyperlipemic diet has been proven to drive monocytopoiesis in swine [3]. Furthermore, deletion of the apolipoprotein E gene in mice is associated with monocytosis [4,5] and neutrophilia [6]. This is supported by Murphy et al. [7] who illustrated that in apoE−/− mice, reduced apolipoprotein-mediated cholesterol efflux leads to proliferation and expansion of hematopoietic stem and multipotential progenitor cells (HSPC), providing a mechanistic link between hypercholesterolemia and leukocytosis. Conversely, inhibition of HSPC proliferation has been described with elevated high-density lipoprotein (HDL) cholesterol [8].
Despite abundance of preclinical data, there is limited evidence that confirms such a robust relationship in humans. Moreover, there is only one study, to our knowledge, that has investigated the longitudinal relationship of leukocyte counts with lipid levels [9]. A recent study conducted on healthy participants of the Multi-Ethnic Study of Atherosclerosis (MESA) demonstrated that increased total cholesterol (TC) and low-density lipoprotein (LDL) cholesterol were associated with lower total leukocyte, monocyte and neutrophil counts [10]. However, there was an inverse relationship between HDL cholesterol and total leukocyte count, in agreement with animal studies. Additionally, higher triglyceride levels were associated with elevated total leukocyte and lymphocyte counts. Consistent with these findings, other studies have identified a positive correlation between triglyceride levels and total leukocyte, lymphocyte, neutrophil, monocyte and basophil counts [[11], [12], [13]], and a negative correlation between HDL cholesterol and total leukocyte as well as monocyte counts [11,14]. In contrast to the described inverse correlation of LDL cholesterol with total leukocyte, monocyte and neutrophil counts, LDL cholesterol may positively correlate with lymphocyte counts [15,16].
The results of studies in humans demonstrate a modest yet conflicting relationship when compared to animal studies, highlighting potential differences in biological processes between mice and humans that are not currently understood. Due to the central role of lipids and leukocytes in the development of atherosclerotic disease, it is important to delineate the relationship between these factors to better understand the basic pathobiology of atherosclerosis. By utilising a combination of two large-scale, population-based studies, and one clinical trial study, we sought to examine the relationship of serum lipid levels with total and differential leukocyte counts in humans.
2. Methods
This study assessed the relationship of lipid levels with leukocyte counts in three study cohorts (Fig. 1): National Health and Nutrition Survey (NHANES), Korean National Health and Nutrition Survey (KNHANES) and Treating to New Targets (TNT) trial.
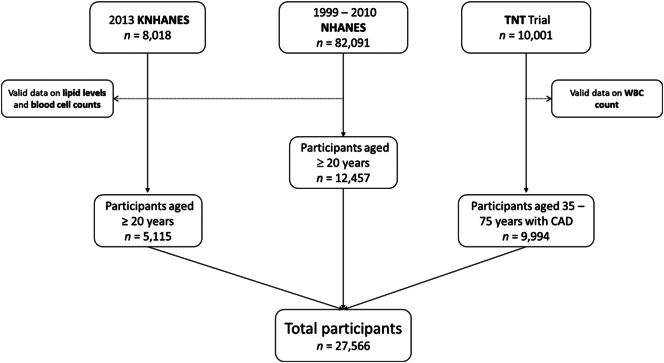
Fig. 1.
Participant flow in the three datasets. Three data registries were used in this study. In the NHANES (1999–2010) and KNHANES (2013) a total of 82,091 and 8018 participants were included, respectively. After exclusion of participants with missing data on lipid levels and blood cell counts 12,457 participants aged ≥20 years were included in the NHANES final analysis; 5115 participants aged >20 years were included in the KNHANES final analysis. In the TNT trial, a total of 10,001 participants aged 35–75 with known coronary artery disease (CAD) were recruited. Of these, 9994 participants had valid data on leukocyte count and were included in the final analysis.
2.1. NHANES
2.1.1. Study population
The NHANES is a continuous cross-sectional survey of the health and nutritional status of United States residents, citizens and non-industrialised individuals [17]. The NHANES collects data on approximately 5000 individuals each year using a complex sampling procedure, as previously described [18]. In 1999–2010, 82,091 participants were surveyed in NHANES, and 17,736 of them underwent both interview and examination, with fasting samples for lipids and lipids and leukocyte count measurements. Of these, 12,457 participants were included in the adult cohort of this analysis which comprised of those who were aged ≥20 years and had valid data on TC, LDL cholesterol, triglycerides, HDL cholesterol, differential leukocyte counts, red blood cell (RBC) count and platelet count.
2.1.2. Lipids and leukocyte count measurements
Fasting blood samples were obtained from participants to measure lipid levels. TC, HDL cholesterol and triglycerides were measured enzymatically (Hitachi 704 Analyzer, Roche Diagnostics). LDL cholesterol was calculated using the Friedewald formula [19] in participants with triglycerides <400 mg/dL, and otherwise measured directly if triglycerides were > 400 mg/dL. The complete blood count was performed using a Beckman Coulter MAXM (Beckman Coulter) as per the manufactures guidelines [18].
2.2. KNHANES
2.2.1. Study population
KNHANES is an annual, cross-sectional survey composed of health interview, health examination and nutrition surveys [20]. Participants included in this study were non-institutionalised Korean citizens selected using a stratified, multistage, cluster sampling study design with proportional allocation based on geographical area, sex and age group using data from the National Census Registry [20]. Data were extracted from the sixth KNHANES conducted in 2013, which included a total of 8018 participants. Of these, 5115 participants aged ≥20 years with valid data on TC, HDL cholesterol, LDL cholesterol and triglycerides, total leukocyte count, RBC count and platelet count were included in the analysis.
2.2.2. Lipids and leukocyte count measurements
Fasting blood samples were obtained to measure TC, HDL cholesterol and triglycerides enzymatically using commercially available kits (Hitachi Automatic Analyzer 7600, Hitachi). LDL cholesterol was calculated using the Friedewald formula [19] for participants with triglycerides <400 mg/dL or measured directly in those with triglycerides >400 mg/dL. Leukocyte counts were quantified using an automated blood cell counter (ADVIA 120, Bayer).
2.3. TNT study
2.3.1. Study population
The design and results of the TNT study have been previously reported [21]. Briefly, the TNT cohort consisted of participants recruited from 14 countries, from 1998 to 1999. Men and women aged 35–75 years with stable coronary heart disease were recruited to the study, with exclusion criteria described in detail previously [21]. 10,001 patients with an LDL cholesterol level off-therapy of 130–250 mg/dL (3.4–6.5 mmol/L), decreasing to <130 mg/dL (<3.4 mmol/L) after an 8-week run-in period on atorvastatin 10 mg/day, were randomised to treatment with either atorvastatin 10 or 80 mg/day. Of the 10,001 participants included in the TNT trial 9994 participants with valid data on baseline leukocyte count were included in the final analysis of the present study.
2.3.2. Lipids and leukocyte cell count measurements
TC, HDL cholesterol and triglycerides were measured on fasting blood samples at baseline and at 12 months by standard methodologies, as previously described [22]. LDL cholesterol was calculated using the Friedewald formula [19] when triglyceride levels were < 174 mg/dL (<4.5 mmol/L), above this LDL cholesterol was measured directly by ultracentrifugation, as previously described [22]. Leukocyte counts were quantified using a Beckman Coulter GEN S (Beckman Coulter) automated blood cell counter.
2.4. Covariates of interest
Common definitions of covariates among the three cohorts included those of smoking status and body mass index (BMI). Smoking status was categorised as ‘non’, ‘former’ or ‘current’. Weight and height measured during clinical examination were used to calculate BMI. In the NHANES and KNHANES, diabetes was defined as a fasting glucose >125 mg/dL, measured using an automated analyzer and enzymatic assay (Hexokinase-mediated reaction Roche/Hitachi Modular P Chemistry Analyzer and Pureauto S GLU: Daiichi), or use of hypoglycaemic medication.
In the NHANES, data on demographics, smoking status, race/ethnicity, physical activity and medications was obtained from self-reported questionnaires. Ratio of family income to poverty was calculated by dividing family income by the poverty measure from Department of Health and Human Services' poverty guidelines [23]. History of cardiovascular disease (CVD) was defined as self-reported congestive heart failure, coronary artery disease, angina, myocardial infarction or stroke. Of three consecutive blood pressure readings, the average of the final two was used as the reported value. C-reactive protein (CRP) levels were analysed by latex-enhanced nephelometry with high sensitivity using a Dade Behring Nephelometer II Analyzer System (Dade Behring Diagnostics, Somerville, New Jersey).
With respect to the KNHANES, information on demographics, smoking status, alcohol consumption, education, medications and history of CVD was obtained using health interviews and clinical examinations. Regular alcohol consumption was defined as more than one glass of alcohol per month. Education was categorised as ‘<high school diploma’, ‘high school diploma’ and ‘≥college’. HbA1c was measured using high performance liquid chromatography by Tosoh G8 (Tosoh) using reagent of HLC-723G8 HbA1c (Tosoh).
In the TNT trial, participants provided written informed consent, sociodemographic information was obtained, vital signs measured, concomitant medications documented and a complete medical history recorded [21]. At the follow up point of 12 months, vital signs, clinical end points, adverse events and concurrent medication use were again documented.
2.5. Statistical analysis
To provide accurate variance estimates, data analysis for the NHANES and KNHANES utilised sample weights that accounted for the unequal probabilities of sampling selection, oversampling of some participants with certain characteristics, and nonresponse. Data from these studies were presented as mean or percent and standard error. Variables with skewed distribution are expressed as geometric mean (95% confidence interval). Data from the TNT trial are expressed as mean (standard deviation) or frequency (percentage) where appropriate. All data analyses were performed using SPSS 25 (IBM, Armonk, NY, USA) or STATA 14 (StataCorp).
Multivariable linear regression analysis was performed with each lipid parameter (TC, HDL cholesterol, LDL cholesterol and triglycerides) as independent predictor variables and total or differential (neutrophil, monocyte, lymphocyte, basophil and eosinophil) leukocyte counts as the dependent variables. For the NHANES and KNHANES the regression coefficient was estimated as the ln-transformed leukocyte counts (×10 [3]/μL) per 10 mg/dL increase in lipid level. The regression coefficient was converted to a percentage change using the following formula: (EXP(B)-1) × 100; where EXP is the exponent and B is the regression coefficient. For the TNT trial, the regression coefficient was estimated as the leukocyte count (×10 [3]/μL) per 1 mg/dL increase in lipid level as a pilot analysis using diagnostic plot of residues did not suggest any improvement in model fitting after taking ln-transformation of leukocyte count. Follow-up data in the TNT trial facilitated longitudinal analysis, whereby the absolute change in leukocyte count and lipid levels were included in the final regression model as the dependent and independent variables respectively.
Across all three studies data were adjusted for basic demographics and cardiovascular risk factors. More specifically, data from all three datasets were adjusted for age, sex, BMI, smoking status, diabetes, LDL cholesterol (except for analysis of TC and LDL cholesterol), HDL cholesterol (except for analysis of HDL cholesterol) and triglycerides (except for analysis of triglycerides). Data in the NHANES and KNHANES were further adjusted for education, alcohol consumption, systolic blood pressure, use of anti-hypertensive medication, use of lipid-lowering medication, history of CVD, RBC count and platelet count. Beyond this the NHANES was further adjusted for race/ethnicity, ratio of family income to poverty, physical activity and ln-transformed CRP, and the KNHANES for HbA1c. Data in the TNT trial were further adjusted for race/ethnicity, hypertension and blood urea nitrogen. In the longitudinal analysis of the TNT trial, data were further adjusted for baseline leukocyte count and treatment allocation. Multiple testing correction was performed using false discovery rate with the study-wide false discovery rate at 0.05. A two-tailed p < 0.05 was considered statistically significant.
3. Results
3.1. NHANES
In the NHANES 70.9% of participants were non-Hispanic white, the mean age was 46.3 years, 52.3% were female and the mean BMI was 28.3 kg/m2. 51.8% were non-smokers while 74.2% were alcohol drinkers and 9.3% had diabetes (Table 1).
Table 1.
Baseline characteristics of NHANES study participants.
| Characteristics | n | Estimate |
|---|---|---|
| Age, years | 12,457 | 46.3 (0.3) |
| Women/girls, % | 12,457 | 52.3 (0.4) |
| Race/ethnicity, % | 12,457 | |
| Non-Hispanic White | 70.9 (1.3) | |
| Non-Hispanic Black | 10.9 (0.7) | |
| Mexican American | 7.8 (0.6) | |
| Others | 10.4 (0.8) | |
| Education, %a | 12,436 | |
| <High school | 19.3 (0.6) | |
| High school graduate | 25.0 (0.6) | |
| >High school | 55.7 (0.9) | |
| Ratio of family income to poverty, % | 12,355 | |
| ≤1.30 | 18.6 (0.7) | |
| 1.31–3.50 | 40.7 (0.8) | |
| >3.50 | 40.7 (1.0) | |
| BMI, kg/m2 | 12,242 | 28.3 (0.1) |
| Smoking, %a | 12,443 | |
| Non-smoker | 51.8 (0.8) | |
| Former smoker | 25.5 (0.7) | |
| Current smoker | 22.7 (0.6) | |
| Alcohol drinker, %a | 11,707 | 74.2 (0.9) |
| Engage in moderate/vigorous exercise, % | 12,452 | 59.9 (0.9) |
| Systolic blood pressure, mm Hg | 12,009 | 120.9 (0.2) |
| Hypertension, %a | 12,140 | 31.4 (0.7) |
| Anti-hypertensive medication, %a | 12,455 | 23.4 (0.6) |
| Cholesterol-lowering medication, %a | 12,457 | 12.8 (0.5) |
| Diabetes, % | 12,455 | 9.3 (0.3) |
| History of CVD, %a | 12,385 | 8.2 (0.4) |
| CRP, mg/dL | 12,454 | 1.9 (1.8, 1.9) |
| Lipid profile, mg/dL | ||
| Total cholesterol | 12,457 | 197.2 (0.5) |
| HDL cholesterol | 12,457 | 53.6 (0.2) |
| LDL cholesterol | 12,457 | 118.1 (0.5) |
| Triglycerides | 12,457 | 127.4 (0.9) |
| Blood cell counts | ||
| Total leukocyte count, ×103/μL | 12,457 | 6.515 (6.457, 6.574) |
| Basophils, ×103/μL | 12,457 | 1.033 (1.031, 1.034) |
| Eosinophils, ×103/μL | 12,457 | 1.194 (1.192, 1.197) |
| Lymphocytes, ×103/μL | 12,457 | 1.874 (1.859, 1.889) |
| Monocytes, ×103/μL | 12,457 | 1.525 (1.520, 1.531) |
| Neutrophils, ×103/μL | 12,457 | 3.747 (3.700, 3.794) |
| Red blood cells, ×106/μL | 12,457 | 4.74 (0.01) |
| Platelets, ×103/μL | 12,457 | 262.1 (0.9) |
Data are expressed as mean (SE) or % (SE). For variables with skewed distribution, data are expressed as geometric mean (95% CI).
Abbreviations: BMI, body mass index; TC, total cholesterol; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; TG, triglyceride; RBC, red blood cells.
Data not available in adolescent cohort.
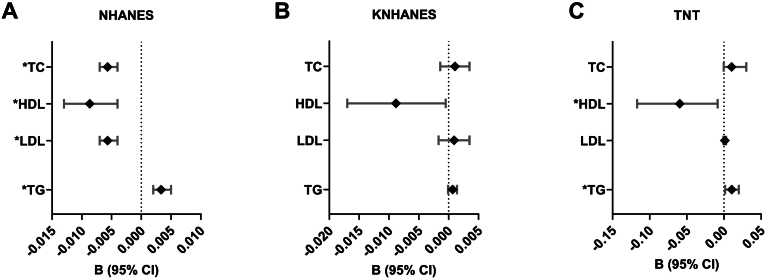
Each 10 mg/dL increment in total, HDL and LDL cholesterol was associated with a 0.6% (95% CI -0.7%, −0.4%; p < 0.001), 0.9% (95% CI -1.3%, −0.4%; p < 0.001) and 0.6% (95% CI -0.7%, −0.4%; p < 0.001) lower total leukocyte count, respectively (Fig. 2A; Supplementary Table 1). Conversely, each 10 mg/dL increment in triglycerides was associated with a 0.3% (95% CI 0.2%, 0.5%; p < 0.001) higher total leukocyte count.
Fig. 2.
Association of lipid levels with total leukocyte count in the NHANES, KNHANES and TNT trial. For (A) NHANES and (B) KNHANES, regression coefficient B (95% CI) is expressed as ln-transformed cell counts (×103/μL) per 10 mg/dL increase in lipid measures. In the (C) TNT study, a pilot analysis using diagnostic plot of residues does not suggest any improvement in model fitting after taking ln-transformation of leukocyte count. Therefore, the regression coefficient B (95% CI) is expressed as leukocyte count (×103/μL) per 10 mg/dL increase in lipid measures. Data from all three registries were adjusted for age, gender, BMI, smoking status, diabetes, LDL cholesterol (except for analysis of total cholesterol and LDL cholesterol), HDL cholesterol (except for analysis of HDL cholesterol) and triglycerides (except for analysis of triglycerides). Data in the NHANES and KNHANES were further adjusted for education, alcohol consumption, systolic blood pressure, use of anti-hypertensive medication, use of lipid-lowering medication, history of CVD, RBC count and platelet count. Beyond this the NHANES was further adjusted for race/ethnicity, ratio of family income to poverty, physical activity and ln-transformed CRP, and the KNHANES was further adjusted for HbA1c. Data in the TNT study were further adjusted for race/ethnicity, hypertension and blood urea nitrogen.
Abbreviations: B, regression coefficient; HDL, high-density lipoprotein; LDL, low-density lipoprotein; TC, total cholesterol; TG, triglycerides.
*Remained significant after adjusting for multiple comparisons using a false discovery rate of <0.05.
To further delineate this relationship, the association of lipid levels with differential leukocyte counts was explored (Fig. 3; Supplementary Table 2). After adjusting for all covariates, each 10 mg/dL increment in TC, LDL cholesterol and triglycerides was associated with a 0.4% (95% CI [0.3%, 0.6%], [0.3%, 0.6%], [0.3%, 0.5%] respectively; p < 0.001) higher lymphocyte count, a 0.1% (CI 95% [−0.2%, 0.0%], [−0.2%, 0.0%], [−0.1%, 0.0%]; p = 0.002, p = 0.003, p < 0.001) lower monocyte count and 0.1% (CI 95% [−0.2%, 0.0%], [−0.2%, 0.0%], [−0.2%, −0.1%]; p = 0.027, p = 0.026, p < 0.001) lower neutrophil count. Further, each 10 mg/dL increment in HDL cholesterol was associated with a 0.2% (CI 95% -0.4%, 0.0%; p = 0.017) lower eosinophil count. None of the lipid parameters in this study were associated with basophil count.
Fig. 3.
Association of lipid levels with differential cell counts in the NHANES study. The regression coefficient B (95% CI) are expressed as ln-transformed cell counts (×103/μL) per 10 mg/dL increase in lipid measures. Data were adjusted for ln-transformed total leukocyte count (except for analysis of total leukocyte count), age, sex, race/ethnicity, education, ratio of family income to poverty, BMI, smoking, alcohol consumption, exercise, systolic blood pressure, use of hypertensive medication, use of cholesterol lowering medication, diabetes, history of CVD, ln-transformed CRP, LDL cholesterol (except for analysis of total and LDL cholesterol), HDL cholesterol (except for analysis of HDL cholesterol), triglycerides (except for analysis of triglycerides), RBC count and platelet count.
Abbreviations: B, regression coefficient; HDL, high-density lipoprotein; LDL, low-density lipoprotein; TC, total cholesterol; TG, triglycerides.
*Remained significant after adjusting for multiple comparisons using a false discovery rate of <0.05.
In a separate analysis, exclusion of 1788 participants taking any lipid-lowering medication showed similar results. In addition, the association of triglycerides with eosinophil count (B = -0.001; CI 95% -0.001, 0.000; p = 0.029) and the association of HDL cholesterol with lymphocyte count (B = -0.005; CI 95% -0.010, 0.000; p = 0.032) became significant (Supplementary Table 3).
3.2. KNHANES
In the KNHANES, the mean age of study participants was 45.5 years, 49.1% were female and the average BMI was 23.8 kg/m2. 55.7% participants were non-smokers and 10.1% had diabetes (Table 2).
Table 2.
Baseline characteristics of KNAHNES study participants.
| Characteristics | n | Estimate |
|---|---|---|
| Age (years) | 5115 | 45.5 (0.4) |
| Females, % | 5115 | 49.1 (0.6) |
| BMI, kg/m2 | 5108 | 23.9 (0.1) |
| Household income, % | 5076 | |
| Quartile 1 | 926 | 14.8 (1.0) |
| Quartile 2 | 1340 | 25.9 (1.1) |
| Quartile 3 | 1338 | 28.5 (1.0) |
| Quartile 4 | 1472 | 30.8 (1.5) |
| Education, % | 4822 | |
| <High school | 1533 | 25.3 (1.1) |
| High school | 1707 | 38.7 (1.1) |
| >High school | 1582 | 36.0 (1.2) |
| Smoking, % | 4808 | |
| Non-smoker | 2886 | 55.7 (0.8) |
| Former smoker | 928 | 19.5 (0.6) |
| Current smoker | 994 | 24.8 (0.9) |
| Regular drinker, % | 4813 | 59.5 (0.9) |
| Hypertension, % | 4871 | 25.6 (0.9) |
| Cholesterol-lowering medication, % | 4827 | 6.0 (0.4) |
| HbA1c | 5115 | 5.9 (0.02) |
| Diabetes, % | 4854 | 10.1 (0.6) |
| Stroke, % | 4827 | 1.8 (0.2) |
| Myocardial Infarction, % | 4827 | 0.6 (0.1) |
| Lipid levels | ||
| TC (mg/dL) | 5115 | 187.7 (0.7) |
| HDL-C (mg/dL) | 5115 | 50.8 (0.2) |
| LDL-C (mg/dL) | 5115 | 112.0 (0.5) |
| TG (mg/dL) | 5115 | 138.7 (2.0) |
| Blood cell counts | ||
| RBC (×106/μL) | 5115 | 4.63 (0.01) |
| Leukocyte count (×103/μL) | 5115 | 5.920 (5.857, 5.983) |
| Platelets (×103/μL) | 5115 | 253.3 (1.1) |
Data are expressed as mean (SE) or % (SE). For variables with skewed distribution, data are expressed as geometric mean (95% CI).
Abbreviations: BMI, body mass index; HbA1c, glycated haemoglobin; TC, total cholesterol; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; TG, triglyceride; RBC, red blood cells.
After adjusting for sociodemographic information and cardiovascular risk factors, TC, LDL cholesterol and triglycerides were not associated with total leukocyte count (Fig. 2B, Supplementary Table 1). In contrast, a 10 mg/dL increment in HDL cholesterol was associated with a 1% lower total leukocyte count (CI 95% -1.8%, −0.1%; p = 0.027) although this relationship was not significant after adjusting for multiple comparisons. In a separate analysis (Supplementary Table 4), exclusion of participants taking a lipid-lowering medication (n = 372) did not dramatically influence the aforementioned relationships.
3.3. TNT
The mean age of TNT participants was 61.0 years, with 94.1% white population, 19% females and an average BMI of 28.5 kg/m2. 23.4% were non-smokers and 15% had diabetes (Table 3).
Table 3.
Characteristics of TNT trial participants at baseline and at one year.
| Characteristic | n | Total | n | 10 mg | n | 80 mg |
|---|---|---|---|---|---|---|
| Age, years | 9994 | 61.0 (8.8) | 5004 | 60.9 (8.9) | 4990 | 61.2 (8.8) |
| Female, n (%) | 9994 | 1902 (19.0) | 5004 | 961 (19.2) | 4990 | 941 (18.9) |
| Race/ethnicity, n (%) | 9994 | 5004 | 4990 | |||
| White | 9403 (94.1) | 4709 (94.1) | 4694 (94.1) | |||
| Black | 289 (2.9) | 154 (3.1) | 135 (2.7) | |||
| Asian | 102 (1.0) | 54 (1.1) | 48 (1.0) | |||
| Other | 200 (2.0) | 87 (1.7) | 113 (2.3) | |||
| BMI, kg/m2 | 9960 | 28.5 (4.6) | 4993 | 28.64, 4.66 | 4967 | 28.41, 4.46 |
| Smoking status, n (%) | 9994 | 5004 | 4990 | |||
| Non-smoker | 2336 (23.4) | 1167 (23.3) | 1169 (23.4) | |||
| Former smoker | 6318 (63.2) | 3165 (63.2) | 3153 (63.2) | |||
| Current smoker | 1340 (13.4) | 672 (13.4) | 668 (13.4) | |||
| Diabetes, n (%) | 9994 | 1500 (15.0) | 5004 | 752 (15.0) | 4990 | 748 (15.0) |
| Systolic blood pressure, mmHg | 9990 | 130.7 (16.8) | 5002 | 130.9 (16.8) | 4988 | 130.5 (16.8) |
| Blood Urea Nitrogen, mg/dL | 9994 | 16.9 (5.0) | 5004 | 16.8 (4.9) | 4990 | 16.9 (5.0) |
| Baseline valuesa | ||||||
| Lipid levels (mg/dL) | ||||||
| Total cholesterol | 9987 | 174.7 (23.9) | 5002 | 174.7 (24.0) | 4985 | 174.8 (23.8) |
| HDL cholesterol | 9987 | 47.3 (11.0) | 5002 | 47.1 (10.8) | 4985 | 47.5 (11.1) |
| LDL cholesterol | 9986 | 97.5 (17.6) | 5001 | 97.7 (17.6) | 4985 | 97.3 (17.6) |
| Triglycerides | 9987 | 150.6 (70.9) | 5002 | 150.5 (71.7) | 4985 | 150.6 (69.8) |
| Leukocyte count (×103/μL) | 9994 | 6.3 (1.6) | 5004 | 6.3 (1.7) | 4990 | 6.3 (1.6) |
| Follow up values | ||||||
| Lipid levels (mg/dL) | ||||||
| Total cholesterol | 9441 | 162.3 (31.5) | 4737 | 177.4 (27.2) | 4704 | 147.0 (28.0) |
| HDL cholesterol | 9440 | 46.1 (11.0) | 4736 | 46.1 (10.8) | 4704 | 46.1 (11.1) |
| LDL cholesterol | 9431 | 87.7 (24.5) | 4730 | 100.3 (20.8) | 4701 | 75.0 (21.1) |
| Triglycerides | 9441 | 143.3 (78.3) | 4737 | 155.9 (83.3) | 4704 | 130.5 (70.7) |
| Leukocyte count (×103/μL) | 9323 | 6.5 (1.8) | 4685 | 6.4 (1.8) | 4638 | 6.5 (1.8) |
Data are expressed as mean (SD) or frequency (%) where appropriate.
Abbreviations: BMI, body mass index; n, valid number of participants.
At baseline all patients had all been on atorvastatin 10 mg for 8 weeks.
In the cross-sectional analysis at baseline, after adjustment for all covariates, total and LDL cholesterol were not associated with leukocyte count (Fig. 2C; Supplementary Table 1). In contrast, each 10 mg/dL increment in HDL cholesterol was associated with a decrease in leukocyte count of 0.09 × 103 cells/μL (CI 95% -0.117, −0.053; p < 0.001). Whereas each 10 mg/dL increment in triglycerides was associated with an increase in leukocyte count of 0.02 × 103 cells/μL (CI 95% 0.010, 0.020; p < 0.001).
Over the follow up period of 12 months, participants in the atorvastatin 10 mg cohort had an average increase in TC, LDL cholesterol and triglycerides of 2.9, 2.8 and 6.0 mg/dL respectively (all P < 0.001) (Table 3). HDL cholesterol decreased by 1.1 mg/dL on average (P < 0.001). In the atorvastatin 80 mg cohort, there was an average decrease in TC, HDL cholesterol, LDL cholesterol and triglycerides of 27.5, 1.4, 22.2 and 19.5 mg/dL respectively (all P < 0.001). Over the same period leukocyte count increased by 0.1 × 103 and 0.3 × 103 cells/μL in the atorvastatin 10 mg and 80 mg cohorts, respectively (both P < 0.001). In the longitudinal analysis (Fig. 4; Supplementary Table 5), increases in TC (0.002; CI 95% 0.001, 0.004; p < 0.001), HDL cholesterol (0.007; CI 95% 0.003, 0.011; p = 0.002) and triglycerides (0.001; CI 95% 0.000, 0.001; p < 0.001) over one year were associated with an increase in leukocyte count over the same period. A significant interaction with treatment allocation was detected for the association of change in total, HDL, and LDL cholesterol with change in leukocyte count (all p < 0.01). Stratification by treatment allocation revealed that these relationships were significant only in the atorvastatin 10 mg cohort (all p < 0.001).
Fig. 4.
Association of change in lipid levels with change in leukocyte count in TNT trial cohort over 12 months. This data reflects all participants (n = 9318), including those who took 10 mg atorvastatin (n = 4683) and 80 mg atorvastatin (n = 4635). The regression coefficient (B) and 95% confidence intervals (CI) were expressed as increase in cell counts (×103/μL) over one year per one mg/dL increase in lipid levels over the same period.
Data were adjusted for age, sex, Caucasian race, BMI, smoking status, hypertension, diabetes, LDL cholesterol (except for analysis of total cholesterol and LDL cholesterol), HDL cholesterol (except for analysis of HDL cholesterol), triglycerides (except for analysis of triglycerides), blood urea nitrogen, leukocyte count at baseline and treatment allocation.
4. Discussion
Dyslipidaemia and inflammation are two of the hallmarks of CVD. By utilising data from three large cohorts, the present study analyses the relationship of lipid levels with leukocyte counts in a total of 27,566 individuals. Data of NHANES and TNT demonstrate an inverse association of HDL cholesterol with leukocyte count – a finding supported by previous cross-sectional studies [10,11,14]. Collectively, cross-sectional as well as longitudinal data of NHANES and TNT support a positive correlation between triglycerides and leukocyte count, in accordance with other cross-sectional studies in humans [[10], [11], [12], [13],15,16]. Due to heterogeneity among cohorts and differences in available variables, such as differential cell counts, a meta-analysis of cross-sectional results was not performed.
Elevated triglyceride levels [24,25] and reduced HDL cholesterol levels [26,27] are known to be associated with an increased risk of CVD. Although dyslipidaemia is commonly known to be a combination of hypertriglyceridemia, elevated LDL cholesterol and low HDL cholesterol, these factors do not always occur simultaneously, as supported by NHANES and longitudinal TNT results. Mechanistic links between these lipid parameters and atherosclerosis remain poorly understood [28,29]. CANTOS showed that Canakinumab suppresses CRP and IL-6 levels in patients with elevated inflammatory markers and atherosclerotic disease, and potentially reduces risk of recurrent myocardial infarction, supporting a role for inflammation in the pathobiology of atherosclerosis [30,31]. Correspondingly, leukocytosis is known to be associated with incidence of coronary artery disease and CVD mortality [32,33], with strong evidence that leukocytes directly augment atherosclerosis and thrombosis [34]. Whilst the inflammatory action of LDL on leukocyte activity is well described, this study, together with our previous work [9,10] indicates that higher LDL levels are not correlated with increased leukocyte count, despite this phenomenon being well described in mice.
An association between metabolic syndrome and leukocytosis has been described [35] and therefore a possible explanation is that visceral adipose tissue produces pro-inflammatory cytokines which stimulate leukocyte production, supporting an indirect link between dyslipidaemia and leukocytosis as release of fatty acids stored in visceral adipose tissue results in concomitant hypertriglyceridaemia and low HDL cholesterol levels. A causal relationship is supported by animal studies whereby HDL cholesterol inhibits proliferation of HSPC [8]. Although this mechanistic link has not been identified in human studies, our cross-sectional data would be consistent with similar phenomena.
As CVD develops over decades, assessment of longitudinal relationships provides useful information regarding interaction of lipids and leukocytes over time [36]. Based on evidence to date, it is plausible that hypercholesterolaemia as well as hypertriglyceridemia directly and indirectly enhance leukocytosis, by stimulating proliferation of HSPC [7] and associated pro-inflammatory cytokine production by adipose tissue [35]. Positive temporal associations identified in TNT data support this. Notably however, elevated HDL cholesterol was found to be temporally associated with leukocytosis. This could reflect progression of atherosclerotic disease over 12 months despite improvement in HDL cholesterol while on lipid-lowering therapy throughout the trial.
Higher dose of statin appears to weaken positive temporal associations of TC, HDL cholesterol and LDL cholesterol with leukocyte count, as these are only significant in those taking only 10 mg atorvastatin following stratification by treatment. This suggests that aggressive management of hypercholesterolaemia may in fact lessen the extent to which proliferation and expansion of HSPC is augmented, even if dyslipidaemia remains. However, sensitivity analyses of cross-sectional NHANES data suggest that these relationships are independent of treatment with lipid-lowering medication. Furthermore, this study did not account for a history of familial and non-familial hypercholesterolaemia in participants. This would be a potential confounder, particularly if not managed by lipid-regulating medications. The absolute temporal increase in leukocyte count while on statin treatment is interesting yet challenging to interpret in terms of whether it reflects disease progression, as while leukocyte recruitment and adhesion to atherosclerotic lesions has been investigated in detail, information regarding leukocyte egress is lacking [37,38].
This study is unique in that it includes a cross-sectional and longitudinal analysis of data from a large, diverse population. Particular associations identified in NHANES were not supported by KNHANES and TNT data, perhaps due to smaller sample size and inconsistent study designs, with clinically differing cohorts. Moreover, we could not exclude the possibility of the ethnic difference in such association, since the KNHANES cohort was comprised of South Koreans (Asians), whereas NHANES was comprised of mainly non-Hispanic Whites, African Americans and Mexican Americans. Overall, our study supports meaningful cross-sectional and longitudinal relationships between lipid levels and leukocyte counts. In general, triglycerides and HDL cholesterol are robust predictors of leukocyte count across the three cohorts analysed in this study. In NHANES, data were adjusted for the well-known inflammatory marker, CRP. However, data on CRP was not available in the full cohort of KNHANES and TNT, which limits further study on the role of inflammation in the relationship of these lipid species with leukocyte. Although it is difficult to draw conclusions on causation based on this observational study, together with the evidence from previous studies this data suggests that triglycerides and HDL cholesterol may be directly involved in leukogenesis, although the precise mechanism of this relationship and direction of causation is currently ill-defined.
Overall, by utilising a large, comprehensive dataset this study highlights significant cross-sectional and longitudinal relationships of lipid and lipoprotein parameters with leukocyte counts. However, unlike in murine models the majority of these relationships are modest, with the most robust association being between triglycerides and differential leukocyte counts.
This study provides further evidence that major differences exist in the relationships between lipids and leukocyte numbers in mediating atherosclerosis in mice and humans. The impact of lipoproteins on the atherogenic nature of immune cells in humans is likely due to modification of cellular function, rather than alteration of leukogenesis and resulting changes in cell number.
CRediT authorship contribution statement
Sonia Sawant: Validation, Writing – original draft. Bradley Tucker: Formal analysis, Writing – original draft. Praween Senanayake: Formal analysis. David D. Waters: Validation, Resources. Sanjay Patel: Supervision, Resources. Kerry-Anne Rye: Supervision, Resources. Kwok Leung Ong: Validation, Supervision, Writing – reviewing & editing. Blake J Cochran: Conceptualisation, Supervision, Writing – reviewing & editing.
Grant support
KLO is supported by National Health and Medical Research Council of Australia Career Development Fellowship (1122854). KAR and BJC are supported by National Health and Medical Research Council of Australia (1148468 and 2004064). KAR and SP are supported by a New South Wales Health Cardiovascular Research Capacity Program grants.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This research was completed using data from the NHANES, KNHANES and TNT trial. We thank Rana Fayyad for assistance with the TNT dataset. The authors acknowledge all study participants and personnel that made these resources possible.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ahjo.2021.100024.
Appendix A. Supplementary data
Supplementary tables
References
- 1.Libby P. Inflammation in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2012;32:2045–2051. doi: 10.1161/ATVBAHA.108.179705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feldman D.L., Mogelesky T.C., Liptak B.F., Gerrity R.G. Leukocytosis in rabbits with diet-induced atherosclerosis. Arterioscler. Thromb. 1991;11:985–994. doi: 10.1161/01.atv.11.4.985. [DOI] [PubMed] [Google Scholar]
- 3.Averill L.E., Meagher R.C., Gerrity R.G. Enhanced monocyte progenitor cell proliferation in bone marrow of hyperlipemic swine. Am. J. Pathol. 1989;135:369–377. [PMC free article] [PubMed] [Google Scholar]
- 4.Swirski F.K., et al. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J. Clin. Investig. 2007;117:195–205. doi: 10.1172/JCI29950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tacke F., et al. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J. Clin. Investig. 2007;117:185–194. doi: 10.1172/JCI28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drechsler M., Megens R.T., van Zandvoort M., Weber C., Soehnlein O. vol. 1. 2010. Hyperlipidemia-Triggered Neutrophilia Promotes Early Atherosclerosis; pp. 1837–1845. [DOI] [PubMed] [Google Scholar]
- 7.Murphy A.J., et al. ApoE regulates hematopoietic stem cell proliferation, monocytosis, and monocyte accumulation in atherosclerotic lesions in mice. J. Clin. Investig. 2011;121:4138–4149. doi: 10.1172/JCI57559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yvan-Charvet L., et al. ATP-binding cassette transporters and HDL suppress hematopoietic stem cell proliferation. Science. 2010;328:1689–1693. doi: 10.1126/science.1189731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tucker B., et al. The association of serum lipid and lipoprotein levels with total and differential leukocyte counts: results of a cross-sectional and longitudinal analysis of the UK Biobank. Atherosclerosis. 2021;319:1–9. doi: 10.1016/j.atherosclerosis.2020.12.016. [DOI] [PubMed] [Google Scholar]
- 10.Lai Y.C., et al. The association of plasma lipids with white blood cell counts: results from the multi-ethnic study of atherosclerosis. J. Clin. Lipidol. 2019;13:812–820. doi: 10.1016/j.jacl.2019.07.003. [DOI] [PubMed] [Google Scholar]
- 11.Bovill E.G., et al. White blood cell counts in persons aged 65 years or more from the cardiovascular health study. Correlations with baseline clinical and demographic characteristics. Am. J. Epidemiol. 1996;143:1107–1115. doi: 10.1093/oxfordjournals.aje.a008687. [DOI] [PubMed] [Google Scholar]
- 12.Andersen C.J., Vance T.M. Gender dictates the relationship between serum lipids and leukocyte counts in the National Health and nutrition examination survey 1999–2004. J. Clin. Med. 2019;8(15) doi: 10.3390/jcm8030365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bernelot Moens S.J., et al. Remnant cholesterol elicits arterial wall inflammation and a multilevel cellular immune response in humans. Arterioscler. Thromb. Vasc. Biol. 2017;37:969–975. doi: 10.1161/ATVBAHA.116.308834. [DOI] [PubMed] [Google Scholar]
- 14.Tolani S., et al. Hypercholesterolemia and reduced HDL-C promote hematopoietic stem cell proliferation and monocytosis: studies in mice and FH children. Atherosclerosis. 2013;229:79–85. doi: 10.1016/j.atherosclerosis.2013.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oda E. Longitudinal associations between lymphocyte count and LDL cholesterol in a health screening population. J. Clin. Transl. Endocrinol. 2014;1:49–53. doi: 10.1016/j.jcte.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oda E., Kawai R., Aizawa Y. Lymphocyte count was significantly associated with hyper-LDL cholesterolemia independently of high-sensitivity C-reactive protein in apparently healthy Japanese. Heart Vessel. 2012;27:377–383. doi: 10.1007/s00380-011-0157-x. [DOI] [PubMed] [Google Scholar]
- 17.Ong K.L., et al. Trends in C-reactive protein levels in US adults from 1999 to 2010. Am. J. Epidemiol. 2013;177:1430–1442. doi: 10.1093/aje/kws443. [DOI] [PubMed] [Google Scholar]
- 18.National Center for Health Statistics . 2020. National Health and Nutrition Examination Survey. (June 30) [Google Scholar]
- 19.Rijks L.G. vol. 1. 1995. Friedewald Formula; p. 761. [PubMed] [Google Scholar]
- 20.Kim Y. The Korea National Health and nutrition examination survey (KNHANES): current status and challenges. Epidemiol. Health. 2014;36:e2014002. doi: 10.4178/epih/e2014002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Waters D.D., et al. Treating to new targets (TNT) study: does lowering low-density lipoprotein cholesterol levels below currently recommended guidelines yield incremental clinical benefit? Am. J. Cardiol. 2004;93:154–158. doi: 10.1016/j.amjcard.2003.09.031. [DOI] [PubMed] [Google Scholar]
- 22.van den Bogaard B., et al. On-treatment lipoprotein components and risk of cerebrovascular events in the treating to new targets study. Eur. J. Clin. Investig. 2011;41:134–142. doi: 10.1111/j.1365-2362.2010.02387.x. [DOI] [PubMed] [Google Scholar]
- 23.Okosun I.S., Annor F.B., Seale J.P., Eriksen M.P. Abdominal adiposity and family income-to-poverty ratio in American women. Obes. Res. Clin. Pract. 2014;8:e201–e211. doi: 10.1016/j.orcp.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 24.Nordestgaard B.G., Varbo A. Triglycerides and cardiovascular disease. Lancet. 2014;384:626–635. doi: 10.1016/S0140-6736(14)61177-6. [DOI] [PubMed] [Google Scholar]
- 25.Sarwar N., et al. Triglycerides and the risk of coronary heart disease: 10,158 incident cases among 262,525 participants in 29 Western prospective studies. Circulation. 2007;115:450–458. doi: 10.1161/CIRCULATIONAHA.106.637793. [DOI] [PubMed] [Google Scholar]
- 26.Nicholls S.J., Nelson A.J. HDL and cardiovascular disease. Pathology. 2019;51:142–147. doi: 10.1016/j.pathol.2018.10.017. [DOI] [PubMed] [Google Scholar]
- 27.Rosenson R.S., et al. HDL and atherosclerotic cardiovascular disease: genetic insights into complex biology. Nat. Rev. Cardiol. 2018;15:9–19. doi: 10.1038/nrcardio.2017.115. [DOI] [PubMed] [Google Scholar]
- 28.Linton M.R.F., et al. MDText. 2000. The role of lipids and lipoproteins in atherosclerosis. [PubMed] [Google Scholar]
- 29.Shapiro M.D., Fazio S. From lipids to inflammation: new approaches to reducing atherosclerotic risk. Circ. Res. 2016;118:732–749. doi: 10.1161/CIRCRESAHA.115.306471. [DOI] [PubMed] [Google Scholar]
- 30.Baylis R.A., Gomez D., Mallat Z., Pasterkamp G., Owens G.K. The CANTOS trial: one important step for clinical cardiology but a giant leap for vascular biology. Arterioscler. Thromb. Vasc. Biol. 2017;37:e174–e177. doi: 10.1161/ATVBAHA.117.310097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weber C., von Hundelshausen P. CANTOS trial validates the inflammatory pathogenesis of atherosclerosis: setting the stage for a new chapter in therapeutic targeting. Circ. Res. 2017;121:1119–1121. doi: 10.1161/CIRCRESAHA.117.311984. [DOI] [PubMed] [Google Scholar]
- 32.Haim M., Boyko V., Goldbourt U., Battler A., Behar S. Predictive value of elevated white blood cell count in patients with preexisting coronary heart disease: the Bezafibrate infarction prevention study. Arch. Intern. Med. 2004;164:433–439. doi: 10.1001/archinte.164.4.433. [DOI] [PubMed] [Google Scholar]
- 33.Lee C.D., et al. White blood cell count and incidence of coronary heart disease and ischemic stroke and mortality from cardiovascular disease in African-American and white men and women: atherosclerosis risk in communities study. Am. J. Epidemiol. 2001;154:758–764. doi: 10.1093/aje/154.8.758. [DOI] [PubMed] [Google Scholar]
- 34.Coller B.S. Leukocytosis and ischemic vascular disease morbidity and mortality: is it time to intervene? Arterioscler. Thromb. Vasc. Biol. 2005;25:658–670. doi: 10.1161/01.ATV.0000156877.94472.a5. [DOI] [PubMed] [Google Scholar]
- 35.Desai M.Y., et al. Association of body mass index, metabolic syndrome, and leukocyte count. Am. J. Cardiol. 2006;97:835–838. doi: 10.1016/j.amjcard.2005.10.021. [DOI] [PubMed] [Google Scholar]
- 36.Caruana E.J., Roman M., Hernandez-Sanchez J., Solli P. Longitudinal studies. J. Thorac. Dis. 2015;7:E537–E540. doi: 10.3978/j.issn.2072-1439.2015.10.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Swirski F.K., Nahrendorf M. Leukocyte behavior in atherosclerosis, myocardial infarction, and heart failure. Science. 2013;339:161–166. doi: 10.1126/science.1230719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schumski A., Winter C., Doring Y., Soehnlein O. The ins and outs of myeloid cells in atherosclerosis. J. Innate Immun. 2018;10:479–486. doi: 10.1159/000488091. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary tables