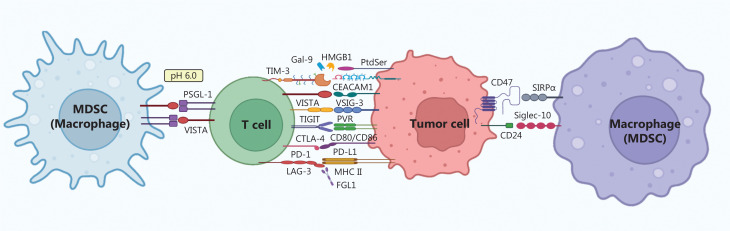
Cancer immunotherapy has emerged as a promising approach in cancer treatment and is considered a major advancement after surgical interventions, radiotherapy, chemotherapy, and targeted therapy. The clinical use of immunotherapeutic drugs, particularly antibody-based drugs that target immune checkpoints, has notably increased1. Unlike traditional antitumor drugs, which exclusively targeting tumor cells, these drugs have a unique mechanism of action, as they inhibit protein-protein interactions across multiple cell types, such as PD-1/PD-L1 blockade may function to interfere among T cell, tumor cell, macrophage, and dendritic cell (Figure 1). Nevertheless, timely withdrawal of antibody therapeutics in the presence of immune-mediated adverse reactions poses a substantial challenge, because of their high molecular weight and long half-life. The cellular uptake of most small molecule chemical drugs is generally feasible but may be accompanied by off-target effects. Peptide therapeutics, in contrast, occupy an intermediate position between monoclonal antibody therapeutics and small molecule chemical drugs in the spectrum of therapeutic agents. Peptides have notable advantages, including remarkable selectivity, particularly to drug targets on the cell surface, robust infiltration into solid tumors, and facile synthesis; therefore, they are critical contenders for immune checkpoint inhibition.
Figure 1.
Immune checkpoints as drug targets for cancer immunotherapy. The interaction of immune checkpoint receptors and ligands expressed on the surfaces of MDSCs, macrophages, T cells, and tumor cells are shown.
However, the clinical utility of peptide therapeutics is constrained by 2 primary obstacles: enzymatic degradation within the physiological milieu and suboptimal oral bioavailability. Various approaches have been implemented to avoid enzymatic degradation, including the use of non-natural amino acids, cyclization modification, and mirror-image phage display technology to develop peptides consisting of amino acids in all-D configuration2,3. The strategic development of suitable formulations can improve the oral characteristics and bioavailability of peptides. The primary objective of this review is to provide a brief overview of advancements in peptide immune checkpoint inhibitors. In addition, the potential applications and future prospects of these peptides in cancer immunotherapy and diagnosis are examined.
Peptide inhibitors that selectively target T cell immune checkpoints
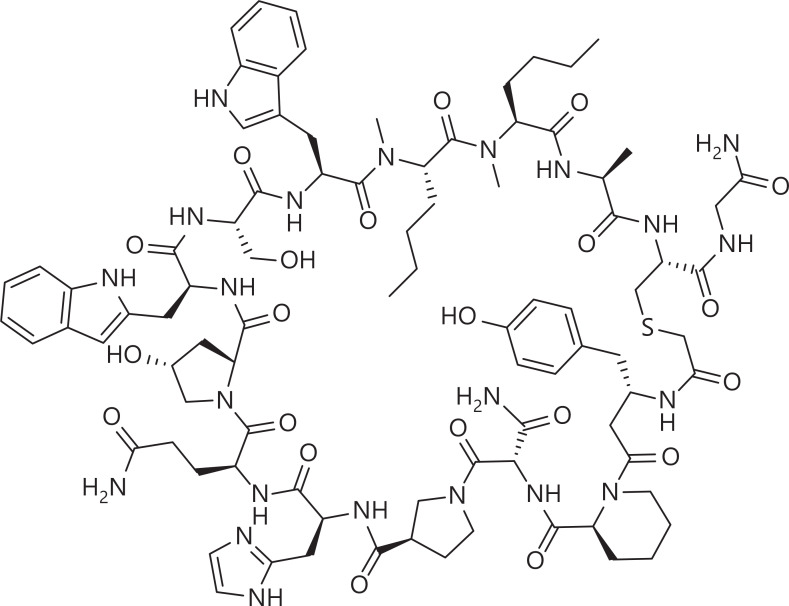
Through high-throughput screening or rational design guided by the crystal structure of the PD-1/PD-L1 complex, peptide inhibitors have been designed to target PD-1/PD-L14–6. Nevertheless, these peptides, comprising L-amino acids, have shown suboptimal stability and have encountered challenges in achieving robust target blockade. Peptide cyclization has been demonstrated to enhance the enzymatic degradation stability and conformational stability of peptides7. BMS-986189 (Figure 2), a macrocyclic peptide developed by Bristol-Myers Squibb (BMS), has been designed to target PD-L1. This promising therapeutic candidate has progressed to a phase I clinical trial8. In an alternative approach, mirror-image phage display technology enables the screening of peptides with an all-D configuration. This innovative method effectively addresses concerns regarding enzymatic degradation stability and offers a comprehensive solution. We have successfully generated a novel class of anti-proteolytic peptides denoted DPPA-1 (nyskptdrqyhf), which target PD-L19 and comprise exclusively all-D amino acids. Moreover, another D-peptide targeting PD-L1, OPBP-1 (Gqsehhmrvysf), has been obtained and loaded with trimethyl chitosan hydrogel for oral administration. This peptide shows notably enhanced oral bioavailability and half-life in rats. Therefore, OPBP-1 is the first peptide for oral administration for immune checkpoint blockade10.
Figure 2.
Possible structure of the PD-L1 macrocyclic peptide inhibtor BMS-986189, which is undergoing a phase I clinical trial8.
Beyond the PD-1/PD-L1 axis, other pairs of immune checkpoints have major roles in the context of tumor-induced immunosuppression; these pairs include the T cell immunoreceptor with immunoglobulin and ITIM domains (TIGIT) and poliovirus receptor (PVR); lymphocyte activation gene 3 (LAG-3) with major histocompatibility complex class II (MHC-II); and V-domain Ig suppressor of T cell activation (VISTA) with P-selectin glycoprotein ligand 1 (PSGL-1). Concurrently inhibiting these targets is anticipated to increase the efficacy of PD-1/PD-L1 blockade.
Using mirror-image phage display, our team has successfully acquired DTBP-3 (GGytfhwhrlnp), the first D peptide inhibitor that blocks the TIGIT/PVR interaction. The remarkable efficacy of DTBP-3 has been observed in 2 distinct murine cancer models: the anti-PD-1 antibody-responsive CT26 colorectal cancer model and the anti-PD-1 antibody-resistant 4T1 breast cancer model11. By using phage display, we have discovered the initial cyclic peptide inhibitor C25 (CVPMTYRAC), which blocks the LAG-3/MHC-II interaction. The administration of C25 peptide notably increases both the quantity and efficacy of CD8+ T cells, but not CD4+ T cells, localized at tumor sites. Additionally, the relative abundance of Treg cells within tumor tissue decreases12. Given the presence of an acidic microenvironment within tumor tissues, we have identified peptide inhibitors that selectively target immune checkpoints within this acidic milieu. Pal-DVS3 (dpGWSFGKLHWPGS-Pal), a peptide inhibitor with incorporation of D-amino acids to enhance enzymatic stability and fatty acid modifications to prolong the in vivo half-life, has been specifically designed to target VISTA/PSGL-1. This peptide exhibits enduring anti-tumor effects by mitigating the immunosuppressive effects of myeloid-derived suppressor cells (MDSCs) on CD8+ T cells13.
Peptide inhibitors that selectively target immune checkpoints on other immune cell subsets
The intriguing phenomenon of “cold tumors”, distinguished by a lack of T cell infiltration within specific tumor tissues, is a formidable obstacle in immunotherapy. Nevertheless, most solid tumors have substantial presence of macrophages—immune cells with crucial roles in the tumor microenvironment. Interestingly, certain immune checkpoints, such as CD47/SIRPα and CD24/Siglec10, and even PD-1/PD-L1, have been identified as key players in regulating the “don’t eat me” signal. These checkpoints effectively hinder macrophage phagocytosis, thereby impeding the elimination of tumor cells by macrophages.
Our group has used phage display technology to develop peptide inhibitors targeting CD47/SIRPα. Notably, we have successfully identified pep-20, along with its terminal D-amino acid substituted analogue, pep-20-D12 (awsATWSNYwrh), as potential inhibitors. Our findings suggest that peptides without Fc-mediated ADCC effects may be superior alternatives to CD47 antibodies because these peptides can specifically activate macrophages in the tumor microenvironment rather than inducing the phagocytosis of peripheral red blood cells, thus avoiding anemia14. Recently, using phage display and subsequent retro-inverse strategies, we have successfully developed a D-peptide CSBP (ldvflyse) that targets both CD24/Siglec10 and PD-1/PD-L1. These peptides increase the phagocytic activity of both macrophages and M-MDSCs, thus fortifying the innate immune response. Moreover, they increase the functionality of CD8+ T cells and consequently foster adaptive anti-tumor immune responses. Particularly when used in conjunction with radiotherapy, these peptides have robust synergistic anti-tumor effects, owing to substantial infiltration of macrophages and MDSCs into tumor tissues induced by radiotherapy15.
Other immune cells also express immune checkpoints, such as TIPE216 and KIR2DL417 on NK cells; TIM-118 on B cells; and gp49B19 on neutrophils, NK cells, monocytes, etc. Although these immune checkpoints and various immune cells play a crucial immune modulatory role in the anti-tumor immune response, studies on their peptide inhibitors remain limited. Future research will greatly benefit from the development of peptides targeting these immune checkpoints.
Dual-function peptides uniquely exhibiting distinct biological functions
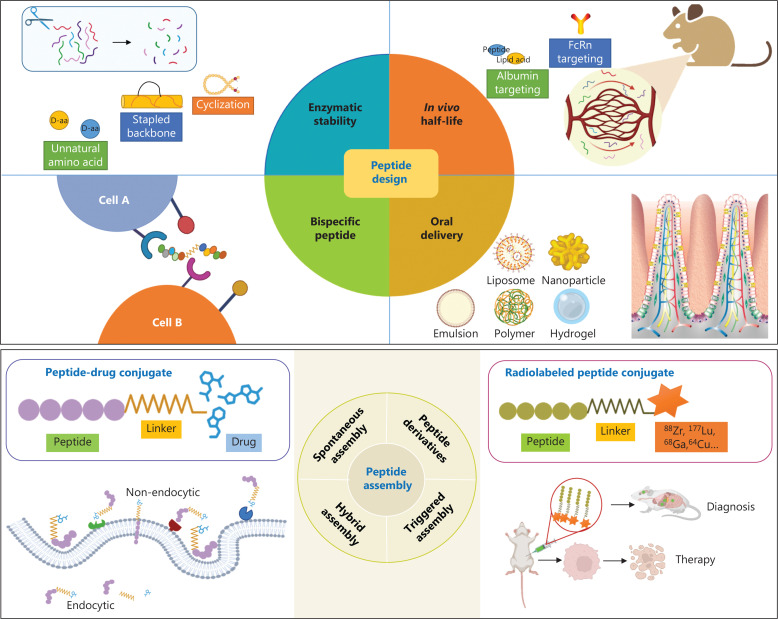
Dual-function peptides, akin to bispecific antibodies, are peptide entities that have been conjugated to effectively target 2 functionally related or complementary entities. This unique characteristic enables them to concurrently regulate distinct signaling pathways, thereby exhibiting multifaceted modulatory effects (Figure 3). In contrast to bispecific antibodies, dual-function peptides have relatively streamlined, less complex design and synthesis processes.
Figure 3.
Strategies for the design and modification of peptides. The stability of peptides can be enhanced by cyclization, stapling, or the use of non-natural amino acids. FcRn or albumin targeting designs or lipid acid modifications can extend the in vivo half-life of peptides. Rational conjugation designs can endow peptides with dual functions. Nanomaterials can be used for oral peptide delivery. Peptides can be self-assembled, coupled to create PDCs, or conjugated with radiolabels for tumor diagnosis or treatment.
In our recent study, we designed a novel peptide inhibitor known as Pal-DMPOP (Pal-PEG4-WSMTWWNYWrvysf), by combining the minimal active fragment of the PD-1/PD-L1 blocking peptide OPBP-1 with the minimal variant of the CD47/SIRPα peptide inhibitor pep-20. Pal-DMPOP exerts its immunomodulatory effects by selectively engaging and modulating the signaling pathways associated with both T cell and macrophage checkpoints, thus leading to the activation and enhancement of anti-tumor immune responses20. In another study, we have conjugated the minimal active fragment of OPBP-1 with the anti-angiogenesis peptide DA7R, and subsequently coupled this bispecific peptide with the albumin-binding peptide DSP, thus yielding a long-acting dual-functional peptide termed DSPOGS. The DA7R peptide has notable anti-tumor effects through impeding neovascularization within tumor tissues. Unfortunately, these anti-angiogenic effects impede the infiltration of immune cells into tumor tissue. However, DSPOGS effectively addresses this concern and has demonstrated synergistic anti-tumor effects21.
Peptide-drug conjugates (PDCs) and self-assembly nanoparticles
In contrast to antibody-drug conjugates (ADCs), peptides can be more easily conjugated with small molecule drugs, thereby facilitating straightforward synthesis. Amphiphilic PDCs have been engineered by conjugating the small molecule radiosensitizers with the PD-1/PD-L1 blocking peptide DPPA-1. The self-assembly of these molecules results in the formation of nanoparticles, which effectively encapsulate TLR7/8 agonists. This intricate process has remarkable synergistic outcomes, wherein radiotherapy sensitization, activation of innate immunity, and enhancement of adaptive immune responses are achieved concurrently22. In another study, the DPPA-1 peptide has been conjugated with the chemotherapeutic agent doxorubicin by using a substrate sequence specifically designed for matrix metalloproteinase 2 (MMP2). This strategic approach has led to the development of nanoparticles that are responsive to the enzymatic activity of MMP2. The nanoparticles have a dual mechanism of action, thus effectively inducing both the cytotoxic effects of chemotherapeutics and T cell-mediated immune killing functions23. PDCs comprising peptides targeting non-endocytic receptors and non-toxic drugs targeting immunotherapeutic targets hold immense future promise, because their mechanisms of action can deviate from those of conventional ADCs, thereby avoiding unforeseen toxicity associated with toxin molecules in ADCs.
Radiolabeled peptides for tumor diagnosis and therapy
The efficacy of immune checkpoint inhibitors is intricately associated with the expression levels of immune checkpoint molecules. The dynamic nature of immune checkpoint molecule expression is evident throughout tumor progression and treatment. Hence, non-invasive in vivo detection of immune checkpoint expression with high sensitivity is imperative. The tissue penetration and long half-life of radiolabeled immune checkpoint antibodies poses several limitations, thus potentially resulting in extended clearance from the body and subsequent unnecessary toxicity. Radiolabeled peptides, serving as diagnostic probes, have remarkable attributes, including robust tumor infiltration, negligible toxicity, and favorable half-life characteristics.
The 64Cu-labeled DPPA-1 peptide has notable efficacy in rapid and precise visualization of PD-L1 expression within tumor tissues. This peptide has potential as a radiotherapeutic agent24. Furthermore, 68Ga-GP12 (DTBP-3), as a positron emission tomography (PET) tracer, has been reported to specifically target TIGIT in patients diagnosed with advanced non-small cell lung cancer. The imaging findings obtained with 68Ga-GP12 PET/CT are similar to those obtained with the widely used 18F-FDG PET/CT, but confer particular advantages in the identification of lymph node metastases25.
Discussion
In cancer immunotherapy, in addition to the immune checkpoints present on T cells and macrophages, newly identified cell subsets and targets in the tumor microenvironment have been continually explored. These discoveries have been instrumental in advancing peptide-based therapeutics. In long-acting peptide design, cutting-edge methods, such as “click chemistry”, and intricate peptide modification techniques, such as stapled peptides, alongside approaches aimed at prolonging in vivo half-life with albumin-binding properties, will undoubtedly have critical roles2. As PDCs continue to be extensively investigated, the fundamental strategy remains rooted in the paradigm of antibody-drug conjugates. This approach entails the transport of toxin molecules into the intracellular milieu of tumor cells via endocytosis, which cannot fully harness the potential benefits of peptides. Unlike the component of ADCs, the peptide mass is similar to that of the small molecule payload in PDCs. Therefore, novel methods and strategies must be explored to increase therapeutic efficacy and avoid unwanted adverse effects. The advent of 177Lu-octreotate has propelled scientific investigation. Furthermore, given their in vivo stability and half-life of in tumor diagnosis, peptides have substantial research potential that might surpass that of therapeutic agents.
The oral route of administration has consistently posed a major hurdle in peptide therapeutics. The investigation of orally administered drugs for cancer immunotherapy has significance in the field, by enabling the flexible adaptation of dosing frequency in accordance with a patient’s immune status. The gastrointestinal tract is the primary site of absorption for peptide drugs administered orally. Importantly, the intestinal mucosal immune system, which encompasses the gut-associated lymphoid tissue, is the largest lymphoid organ in the human body26. Hence, deeper investigation of the mechanisms underlying the specific targeting of intestinal cells is of considerable scientific interest, to enhance the oral bioavailability of peptide-based therapeutics and stimulate these cells to elicit robust anti-tumor immune responses.
Beyond serving as immune checkpoint inhibitors, peptides may be used as vaccines or vaccine delivery carriers, thus playing critical roles in cancer immunotherapy. Hundreds of peptide vaccines, including long peptide vaccines, short peptide vaccines, and other forms, have entered clinical research. The combination of immune checkpoint blockade and vaccines has been widely recognized to achieve a synergistic antitumor response.
Funding Statement
This work was supported by grants from the National Natural Science Foundation of China (Grant No. U20A20369) and GuangDong Basic and Applied Basic Research Foundation (Grant No. 2022B1515120085).
Conflict of interest statement
No potential conflicts of interest are disclosed.
Author contributions
Conceived and designed the analysis: Xinghua Sui, Xiaoshuang Niu, Xiuman Zhou, Yanfeng Gao.
Wrote the paper: Xinghua Sui, Yanfeng Gao.
References
- 1.Kiyotani K, Toyoshima Y, Nakamura Y. Personalized immunotherapy in cancer precision medicine. Cancer Biol Med. 2021;18:955–65. doi: 10.20892/j.issn.2095-3941.2021.0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Muttenthaler M, King GF, Adams DJ, Alewood PF. Trends in peptide drug discovery. Nat Rev Drug Discov. 2021;20:309–25. doi: 10.1038/s41573-020-00135-8. [DOI] [PubMed] [Google Scholar]
- 3.Lyu P, Kwok HF. High-throughput strategy accelerates the progress of marine anticancer peptide drug development. Recent Pat Anticancer Drug Discov. 2019;14:2–4. doi: 10.2174/1574892813999181114152127. [DOI] [PubMed] [Google Scholar]
- 4.Liu H, Zhao Z, Zhang L, Li Y, Jain A, Barve A, et al. Discovery of low-molecular weight anti-PD-L1 peptides for cancer immunotherapy. J Immunother Cancer. 2019;7:270. doi: 10.1186/s40425-019-0705-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li C, Zhang N, Zhou J, Ding C, Jin Y, Cui X, et al. Peptide blocking of PD-1/PD-L1 interaction for cancer immunotherapy. Cancer Immunol Res. 2018;6:178–88. doi: 10.1158/2326-6066.CIR-17-0035. [DOI] [PubMed] [Google Scholar]
- 6.Yin H, Zhou X, Huang YH, King GJ, Collins BM, Gao Y, et al. Rational design of potent peptide inhibitors of the PD-1:PD-L1 interaction for cancer immunotherapy. J Am Chem Soc. 2021;143:18536–47. doi: 10.1021/jacs.1c08132. [DOI] [PubMed] [Google Scholar]
- 7.Zhai W, Zhou X, Zhai M, Li W, Ran Y, Sun Y, et al. Blocking of the PD-1/PD-L1 interaction by a novel cyclic peptide inhibitor for cancer immunotherapy. Sci China Life Sci. 2021;64:548–62. doi: 10.1007/s11427-020-1740-8. [DOI] [PubMed] [Google Scholar]
- 8.Pan C, Yang H, Lu Y, Hu S, Wu Y, He Q, et al. Recent advance of peptide-based molecules and nonpeptidic small-molecules modulating PD-1/PD-L1 protein-protein interaction or targeting PD-L1 protein degradation. Eur J Med Chem. 2021;213:113170. doi: 10.1016/j.ejmech.2021.113170. [DOI] [PubMed] [Google Scholar]
- 9.Chang HN, Liu BY, Qi YK, Zhou Y, Chen YP, Pan KM, et al. Blocking of the PD-1/PD-L1 interaction by a D-peptide antagonist for cancer immunotherapy. Angew Chem Int Ed Engl. 2015;54:11760–4. doi: 10.1002/anie.201506225. [DOI] [PubMed] [Google Scholar]
- 10.Li W, Zhu X, Zhou X, Wang X, Zhai W, Li B, et al. An orally available PD-1/PD-L1 blocking peptide OPBP-1-loaded trimethyl chitosan hydrogel for cancer immunotherapy. J Control Release. 2021;334:376–88. doi: 10.1016/j.jconrel.2021.04.036. [DOI] [PubMed] [Google Scholar]
- 11.Zhou X, Zuo C, Li W, Shi W, Zhou X, Wang H, et al. A novel D-peptide identified by mirror-image phage display blocks TIGIT/PVR for cancer immunotherapy. Angew Chem Int Ed Engl. 2020;59:15114–8. doi: 10.1002/anie.202002783. [DOI] [PubMed] [Google Scholar]
- 12.Zhai W, Zhou X, Wang H, Li W, Chen G, Sui X, et al. A novel cyclic peptide targeting LAG-3 for cancer immunotherapy by activating antigen-specific CD8(+) T cell responses. Acta Pharm Sin B. 2020;10:1047–60. doi: 10.1016/j.apsb.2020.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Niu X, Wu M, Li G, Zhou X, Cao W, Zhai W, et al. Identification and optimization of peptide inhibitors to block VISTA/PSGL-1 interaction for cancer immunotherapy. Acta Pharm Sin B. 2023;13:4341–666. doi: 10.1016/j.apsb.2023.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang H, Sun Y, Zhou X, Chen C, Jiao L, Li W, et al. CD47/Sirpα blocking peptide identification and synergistic effect with irradiation for cancer immunotherapy. J Immunother Cancer. 2020;8:e000905. doi: 10.1136/jitc-2020-000905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shen W, Shi P, Dong Q, Zhou X, Chen C, Sui X, et al. Discovery of a novel dual-targeting D-peptide to block CD24/Siglec-10 and PD-1/PD-L1 interaction and synergize with radiotherapy for cancer immunotherapy. J Immunother Cancer. 2023;11:e007068. doi: 10.1136/jitc-2023-007068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bi J, Cheng C, Zheng C, Huang C, Zheng X, Wan X, et al. TIPE2 is a checkpoint of natural killer cell maturation and antitumor immunity. Sci Adv. 2021;7:eabi6515. doi: 10.1126/sciadv.abi6515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zheng G, Jia L, Yang AG. Roles of HLA-G/KIR2DL4 in breast cancer immune microenvironment. Front Immunol. 2022;13:791975. doi: 10.3389/fimmu.2022.791975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bod L, Kye YC, Shi J, Torlai Triglia E, Schnell A, Fessler J, et al. B-cell-specific checkpoint molecules that regulate anti-tumour immunity. Nature. 2023;619:348–56. doi: 10.1038/s41586-023-06231-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee HN, Manangeeswaran M, Lewkowicz AP, Engel K, Chowdhury M, Garige M, et al. NK cells require immune checkpoint receptor LILRB4/gp49B to control neurotropic Zika virus infections in mice. JCI Insight. 2022;7:e151420. doi: 10.1172/jci.insight.151420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu Z, Li W, Chen S, Chen D, Xu R, Zheng D, et al. Design of a novel chimeric peptide via dual blockade of CD47/Sirpα and PD-1/PD-L1 for cancer immunotherapy. Sci China Life Sci. 2023;66:2310–28. doi: 10.1007/s11427-022-2285-6. [DOI] [PubMed] [Google Scholar]
- 21.Jiao L, Dong Q, Zhai W, Zhao W, Shi P, Wu Y, et al. A PD-L1 and VEGFR2 dual targeted peptide and its combination with irradiation for cancer immunotherapy. Pharmacol Res. 2022;182:106343. doi: 10.1016/j.phrs.2022.106343. [DOI] [PubMed] [Google Scholar]
- 22.Zhu X, Wang X, Li B, Zhang Y, Chen Y, Zhang W, et al. A three-in-one assembled nanoparticle containing peptide-radio-sensitizer conjugate and TLR7/8 agonist can initiate the cancer-immunity cycle to trigger antitumor immune response. Small. 2022;18:e2107001. doi: 10.1002/smll.202107001. [DOI] [PubMed] [Google Scholar]
- 23.Zhu X, Li C, Lu Y, Liu Y, Wan D, Zhu D, et al. Tumor microenvironment-activated therapeutic peptide-conjugated prodrug nanoparticles for enhanced tumor penetration and local T cell activation in the tumor microenvironment. Acta Biomater. 2021;119:337–48. doi: 10.1016/j.actbio.2020.11.008. [DOI] [PubMed] [Google Scholar]
- 24.Hu K, Wu W, Xie L, Geng H, Zhang Y, Hanyu M, et al. Whole-body pet tracking of a D-dodecapeptide and its radiotheranostic potential for PD-L1 overexpressing tumors. Acta Pharm Sin B. 2022;12:1363–76. doi: 10.1016/j.apsb.2021.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang X, Zhou M, Chen B, Liu H, Fang J, Xiang S, et al. Preclinical and exploratory human studies of novel 68Ga-labeled D-peptide antagonist for PET imaging of TIGIT expression in cancers. Eur J Nucl Med Mol Imaging. 2022;49:2584–94. doi: 10.1007/s00259-021-05672-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen G, Kang W, Li W, Chen S, Gao Y. Oral delivery of protein and peptide drugs: From non-specific formulation approaches to intestinal cell targeting strategies. Theranostics. 2022;12:1419–39. doi: 10.7150/thno.61747. [DOI] [PMC free article] [PubMed] [Google Scholar]