Abstract
With the escalating utilization of plastic products, global attention has been increasingly drawn to environmental pollution and recycling challenges stemming from plastic waste. Against this backdrop, biodegradable plastics have emerged as viable alternatives owing to their sustainability and capacity for biodegradation. Polylactic acid (PLA) presently commands the largest market share among biodegradable plastics, finding extensive application in products such as thin films, medical materials, and biodegradable straws. However, the widespread adoption of PLA is hindered by challenges such as high cost, low recycling rates, and complete degradation to H2O and CO2 in natural conditions. Therefore, it is imperative and time-sensitive to explore solutions for the depolymerization and re/upcycling of PLA waste plastics. This review comprehensively outlines the current landscape of PLA recycling methods, emphasizing the advantages and significance of chemical re/upcycling. The subsequent exploration encompasses recent breakthroughs and technical obstacles inherent in diverse chemical depolymerization methods. Ultimately, this review accentuates the impediments and forthcoming possibilities in the realm of PLA plastics, emphasizing the pursuit of closed-loop recycling and upcycling.
1. Introduction
The properties of plastics, such as their low densities, high strength, ease of production, and affordability, make them popular choices for everyday use in the automotive, aerospace, and other industries. The global production of plastic products in 2019 was nearly 460 million tons, and the cumulative global plastic products have exceeded 8 billion tons.1−3 Plastics can take the place of traditional materials such as metal and wood, but how to deal with used plastic waste is an urgent problem. In 2022 alone, the amount of plastic waste generated due to inadequate waste management and recycling technologies is estimated to be about 380 million tons, predominantly composed of commonly used plastics such as PET, PVC, and PE.4 However, the robust chemical stability of these plastics renders them resistant to natural degradation.5−7 In recent times, the manufacture and utilization of conventional nonbiodegradable plastics have been drastically curtailed or prohibited around the globe, and the invention of novel biodegradable plastics (hereinafter referred to as biodegradable plastics) has been promoted.10,11 Biodegradable plastics could undergo complete degradation into carbon dioxide, methane, water, and other mineralized substances in various natural or specific environments such as composting, anaerobic digestion, or aqueous cultures, due to the microbial activity found in nature.8,9,12,13 At present, widely available biodegradable plastics include poly(glycolic acid) (PGA), poly(lactic acid) (PLA), poly(3-hydroxybutyrate) (PHB), polycaprolactone (PCL), poly(butylene succinate) (PBS), and poly(butylene adipate-co-terephthalate) (PBAT). Among these, PLA stands out as one of the most esteemed biodegradable plastics globally, exhibiting consistent chemical characteristics, excellent biocompatibility, renewable capacity, and characteristics akin to conventional plastics, with a $2.1 billion market share in 2021 that is expected to grow to $4.1 billion by 2026.14 PLA has gained prominence across multifarious domains, notably as a material of choice for thin film applications, medical constructs, and textiles. In the realm of thin films, PLA’s utilization in agricultural film, food packaging, paper cups, and straws is motivated by its nontoxic composition, optical transparency, glossy surface, commendable mechanical strength, efficient gas barrier characteristics, and facile processability. In the medical arena, PLA-based polymers stand out due to their commendable mechanical robustness and physicochemical attributes. Significantly, these materials exhibit exemplary biocompatibility with human tissue, mitigating the immunogenic responses often associated with conventional metal medical materials. Furthermore, PLA extends its influence to the textile sector, where synthetic fibers derived from PLA manifest traits analogous to those of traditional nylon and polyester. This encompasses heightened comfort, breathability, and superior resistance to ultraviolet radiation and electrostatic forces. In summation, PLA emerges as a versatile material, offering a confluence of attributes that render it well-suited for diverse industrial applications, spanning from thin film technologies to medical advancements and textile innovations.
Even though PLA is a biodegradable plastic with excellent performance and great potential for growth, there are still obstacles that impede PLA’s ability to make large-scale manufacturing and industrial advancements. On the one hand, PLA synthesis typically involves polycondensation and ring-opening polymerization (ROP). The process of direct condensation with lactic acid monomer is straightforward and cost-effective, yet byproduct water is generated during the condensation process, making it difficult to remove the water from the reaction system, thus preventing the production of high molecular weight PLA. Accordingly, when lactic acid is dehydrated, the reaction can be reversed, and when the reaction reaches a certain point, even a small amount of water in the system can cause the condensation to stop, resulting in a decrease in the molecular weight of the end product.15 Currently, PLA with a large molecular weight is mainly from the ROP of lactide (Figure 1), a polymerization technique suggested by Carothers16 and others in 1932: initially by dehydrating the condensation of lactic acid to PLA oligomer, then by pyrolyzing the oligomer to cyclic diester (PLA), and finally by initiating the ROP for PLA with a large molecular weight. Nevertheless, the procedure is intricate and the intermediates encompass impurities like water, lactic acid, and its oligomer, necessitating purification prior to polymerization.
Figure 1.
ROP route of lactide to PLA and proposed recycling routes of PLA polyesters.
Simultaneously, there are financial difficulties, primarily comprised of two elements. Initially, the lactic acid of PLA is mainly produced from the fermentation of food crops such as corn. However, lactic acid and its raw materials are expensive.17 Subsequently, the procedure of ROP is intricate, requiring numerous steps to separate and purify the input, thus resulting in a higher input cost. As a result, PLA plastic is very expensive on the market; accordingly, the cost of 1 kg of PLA in 2021 stands at $1.91, in contrast to $1.16 for 1 kg of polyethylene terephthalate (PET), $1.34 for 1 kg of HDPE, $1.66 for 1 kg of polybutylene adipate terephthalate (PBAT), $1.8 for 1 kg of polypropylene carbonate (PPC), and $1.00 for 1 kg of polypropylene (PP).18 Additionally, despite PLA being a biodegradable plastic, it is not a polymer material that can be completely broken down in its natural environment. The environment in which PLA is present greatly impacts its degradation rates, with complete degradation taking place under present industrial compost (>50 °C) conditions, whereas in soil at 30 °C it requires a minimum of 1 year for complete degradation, and the PLA weight remains relatively stable throughout a year of immersion in icy depths.19−22 Consequently, it would be a big mistake to use PLA on a large scale and discard it into the environment to biodegrade. On the other hand, in comparison to polyolefins, PLA, synthesized through the ROP reaction, exhibits a smaller equilibrium constant in its polymerization reactions. This characteristic enhances the feasibility of disassembling PLA through the cleavage of ester bonds and recovering monomers and chemicals. To this end, the chemical re/upcycling of biodegradable PLA plastics not only demonstrates technical feasibility but also holds significant scientific and practical value.23 Recently, Ma et al. have demonstrated some interesting works on the topic, including converting waste PLA into alanine at 140 °C over Ru/TiO2 catalyst using ammonia solution,24 transforming waste PLA into methyl propionate over the α-MoC catalyst in methanol solution at first25 and further reacting with formaldehyde to produce methyl methacrylate, and a one-pot catalytic process with Bu4PBr and an ionic liquid for the direct conversion of PLA into acrylic acid.26 This paper will furnish a comprehensive survey of the advancements achieved in the catalytic depolymerization of PLA polyesters. Additionally, it will delineate the impediments encountered in realizing effective closed-loop recycling and upcycling processes. The insights garnered from this examination will be instrumental in advancing strategic approaches to address the chemical re/upcycling challenges associated with PCL, PGA, PHB, and other polyesters necessitating ring-opening polymerization in industrial production.
2. PLA Depolymerization and Re/Upcycling
Currently, plastics are largely used in a linear fashion, leading to large amounts of plastics being thrown away. In comparison, it is more beneficial from a scientific standpoint to make use of used plastics as a plentiful and cost-effective chemical source, break them down physically and chemically, recycle their chemical components and energy, and convert them into new chemicals using other techniques.17 The shift of discarded plastics from the end of the plastic value chain to the start of the industry chain will be a critical element in forming a circular economy of plastics, diminishing the industry’s dependence on petrochemical resources and reducing the amount of carbon dioxide released during the related activities.
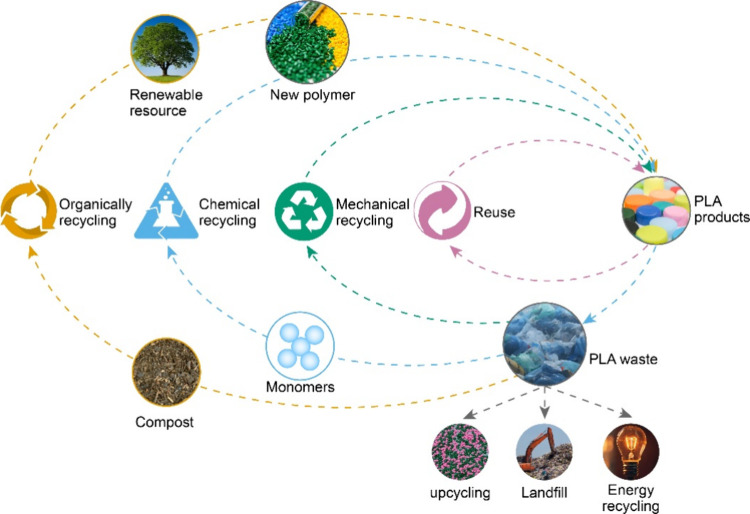
At present, PLA plastics undergo recycling through mechanical, energy, and chemical methods (Figure 2). Mechanical recycling is the act of transforming discarded plastic into a form that is similar to or equivalent to the original plastic. Mechanical recycling typically involves two steps, with the initial one being the gathering, sorting, grinding, cleaning, etc. and transformation of plastic waste into plastic particles or sheets. The next step is to liquefy the plastic following the initial stage prior to the resin-forming process, resulting in the production of the new plastic products.27 Typically, the focus is not on mechanical recovery costs or product quality. During the second phase of melting, plastics experience both thermal and mechanical deterioration, leading to the fracturing of their primary and secondary chains to different extents, thus causing a decrease in the mechanical strength, longevity, and steadiness of the plastics produced.28,29
Figure 2.
Existing PLA plastic degradation and recycling methods.
The process of energy recovery involves the direct combustion of the discarded plastic to harness a portion of its energy in the form of thermal energy. Research has indicated that the direct burning of 1 kg of plastic waste generates approximately 46 000 kJ of energy, whereas the combustion of 1 kg of coal yields 20 000 kJ of energy.17 In contrast to mechanical recycling, direct incineration does not necessitate a significant amount of plastic. It does not necessitate more refined components and can be directly disposed of in the incinerator without undergoing any form of treatment. Despite the potential benefits of direct incineration, the emission of toxic gases such as dioxins, furans, polycyclic aromatic hydrocarbons (PAHs), hydrofluorocarbons (HFCs), and soot from plastic combustion can have a detrimental impact on the environment.30 Despite this, the majority of plastic waste is still disposed of through direct incineration.31 However, a significant quantity of plastic waste is not being utilized efficiently, leading to an increase in resource wastage and environmental contamination.
Chemical recycling is the process of breaking down plastic waste into chemicals through chemical reactions. As a result, in comparison with mechanical recycling and energy recovery, chemical recycling methods are more suitable for the smaller, pricier PLA plastics, for which the complete degradation under natural conditions is feasible. Despite the fact that mechanical recycling can provide a certain level of closed-loop utilization, the mechanical performance of PLA products downcycles as the number of recoveries rises and eventually ceases to be useful once PLA is no longer in closed-loop use. In the case of energy recovery, it does not allow for PLA to be recycled in a closed-loop manner, making it an unsustainable development. On the other hand, the decomposition of PLA plastic waste into lactic acid monomers or other high-value chemicals can result in a re/upcycling economy in PLA, as well as a decrease in resource waste and ecological damage caused by waste plastics.17 Furthermore, PLA synthesis is a step-by-step polymerization process initiated by ester-based functional groups with a low equilibrium constant of polymerization, which makes it simpler to break down into monomers than traditional polyolefin.32 Achieving chemical recovery in polymer processes necessitates meticulous selection of solvents, catalysts, and precise control of reaction conditions, particularly in depolymerizing polymers such as PLA. A critical challenge involves devising techniques to effectively disrupt the crystalline structure of the polymer, leading to the generation of a reactive material and eventual cleavage of ester bonds to produce oligomers and monomers of value. In the context of the inherent complexities associated with depolymerization and re/upcycling, discerning analyses have been proffered, addressing varied depolymerization methodologies.
2.1. Pyrolysis
The pyrolysis process, a thermochemical degradation method applied to both organic and inorganic compounds, assumes particular significance in PLA degradation. PLA pyrolysis necessitates elevated temperatures, inducing complex secondary reactions. The challenge lies in maintaining precise control over product distribution, hindering downstream applications. Despite complexities, pyrolysis remains a pragmatic approach for PLA polymerization, especially when lenient purity standards are tolerable. Prevailing scientific consensus posits that, at lower temperatures, the principal PLA pyrolytic reaction involves the intramolecular transesterification of terminal hydroxyl groups. Conversely, at elevated temperatures, the stochastic cleavage of ester bonds within the polymer’s main chain prevails.33,34 A nuanced understanding of these temperature-dependent reactions enhances our comprehension of PLA pyrolysis, facilitating optimization for diverse applications within polymer science and industrial processes.
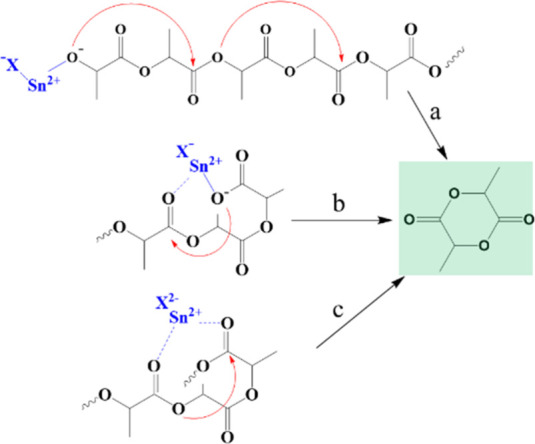
Metal catalysts are regularly employed to control the rate of pyrolysis and the distribution of the product during PLA pyrolysis. Chen et al.35 used metallic tin (Sn) as a catalyst to catalyze the pyrolysis of PLA and then experimentally altered the PLA terminal groups to investigate the part played by various terminal groups in the depolymerization reaction (Figure 3). It was determined that the depolymerization of PLA with active groups at the end was mainly due to Sn catalyzing intramolecular transesterification, resulting in the selective production of lactide. In contrast, when PLA has inactive groups at the end, Sn reacts with the PLA master chain to form catalytic intermediates, which are then selectively broken down to form lactide. Furthermore, Wang et al.36 used zinc oxide (ZnO) as a catalyst to catalyze PLA pyrolysis and found that the PLA did not degrade significantly until the temperature reached 300 °C without the catalyst; however, when ZnO was added, PLA was almost completely broken down into monomers when the temperature reached 300 °C. Coulembier et al.37 studied the impact of the organic catalyst diazacycloundecene (DBU) on PLA pyrolysis in addition to metal catalysts. Furthermore, Saeaung et al.38 found that the copyrolysis of wood powder was improved by combining 50% PLA and 50% wood powder, and the results showed that PLA increased the pyrolysis of the wood powder. The activation energy of wood powder pyrolysis decreased by 29%, the phenol content in the product increased, and the acetaldehyde content decreased. Despite this, the pyrolysis effect of PLA in this process is not particularly effective. To conclude, while pyrolysis is a more efficient PLA chemical depolymerization technique, the main drawbacks of pyrolysis are elevated reaction temperature, increased energy expenditure, intricate postpyrolysis output, and reduced monomer yield.
Figure 3.
Pyrolysis mechanisms with different terminal groups.35 Reproduced with permission from ref (35). Copyright 2018 Elsevier.
2.2. Hydrolysis
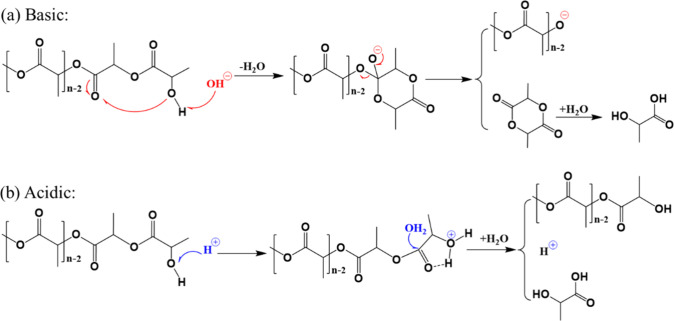
Hydrolysis, a pivotal process in polymer degradation, employs water as a cost-effective and environmentally friendly solvent. Polylactic acid (PLA), an aliphatic polyester, is synthesized through esterification of alcohol hydroxyl and carboxyl groups in monomers. The reversible esterification reaction, characterized by a low equilibrium constant, facilitates the breakdown of PLA’s ester bonds into constituent monomers. The most straightforward hydrolysis process is neutral hydrolysis in sub-supercritical water. However, under neutral conditions without a catalyst, hydrolysis necessitates elevated reaction temperatures (>200 °C) due to PLA’s pronounced hydrophobicity and rigid molecular structure. Although neutral hydrolysis demonstrates the capability to depolymerize certain polyesters into monomers without the need for acid/base reagents, the exigent conditions of high temperature and high pressure or vacuum are harsh, potentially inducing side reactions and diminishing monomer purity. To this end, careful optimization of these reaction parameters is imperative to strike a balance between achieving rapid hydrolysis and maintaining the desired purity of the resulting PLA monomers. Consequently, contemporary methods for PLA hydrolysis primarily rely on the catalytic influence of acids or alkalis, ensuring efficient and controlled degradation processes. Esteemed in the literature are acid catalysis, wherein a carbonyl group attacks an acid proton ester bond, and alkali catalysis, involving direct nucleophilic attack on an alkali molecule and carbonyl carbon. These mechanisms delineate the sophisticated nature of PLA hydrolysis, influencing its controlled degradation and environmental impact. De Jong al.39 elucidated the hydrolysis mechanism of PLA plastics (Figure 4). In alkaline conditions, the hydroxide negative ions in the solution initiate an attack on the hydroxyl group in PLA, leading to a nucleophilic reaction between the hydroxyl group and the carbonyl group in the adjacent ester bonds, resulting in the formation of a hexagonal ring. This ring then breaks off from the polymer chain to form a lactide intermediate, while the remaining short-chain PLA undergoes the same process to achieve depolymerization. When the PLA chain is acidic, the solution passes through proton hydroxyl groups, resulting in the formation of hydrogen bonds between molecules and the formation of a stable arrangement of five-element rings. Subsequently, the water molecules initiate an assault on the carbonyl groups of the ester bond, leading to the hydrolysis of ester bonds and the subsequent peeling off of the five-element rings, resulting in the formation of lactic acid and short-chain PLA.
Figure 4.
Hydrolysis mechanism of PLA under different pHs.39 Reproduced with permission from ref (39). Copyright 2001 Elsevier.
Furthermore, Lv et al.40 studied the hydrolysis process of PLA on a large scale and demonstrated that the dimensions of PLA plastics had a noteworthy effect on the hydrolysis process. The two degradation models of PLA during hydrolysis, bulk erosion and surface erosion, are illustrated in Figure 5, and the conversion of the two degradation models requires a minimum thicknesses of 2.0 and 7.4 mm. Essentially, PLA with a thickness of less than 2.0 mm is primarily eroded through bulk erosion in hydrolysis, PLA with a thickness of 7.4 mm or more is primarily eroded on the surface, and PLA with a thickness between 2 and 7.4 mm is depolymerized through self-catalyzed bulk erosion. Chemically, bulk erosion refers to the degradation of PLA by water and catalysts from within the PLA, while macroscopically PLA does not appear to change significantly during hydrolysis. Surface erosion refers to the gradual degradation of PLA from the surface by water and catalyst, and this can be seen in the shrinking of PLA when hydrolysis takes place. Furthermore, self-catalyzed bulk erosion is caused by the breakdown of ester bonds, which prevents carboxyl and hydroxyl groups from rapidly spreading from the inside to the outside of PLA. This, combined with the catalytic activity of these two groups in the depolymerization of PLA, significantly boosts PLA degradation rates.41 Beyond the PLA scale, various factors such as molecular weight, crystallinity, temperature, reaction duration, and additives influence PLA hydrolysis. Tsuji’s research indicates that higher crystallinity in PLA correlates with a decelerated hydrolysis rate.42 Concurrently, suboptimal temperatures reduce hydrolysis rates, while excessive heat induces plastic recrystallization, posing challenges to the degradation kinetics of PLA.43−48
Figure 5.
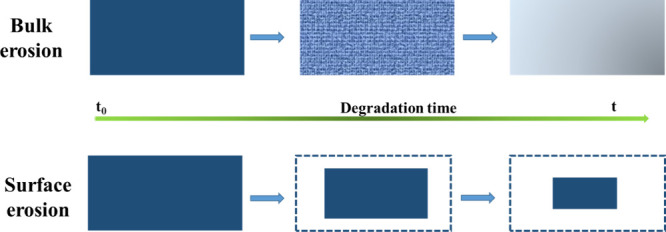
Two erosion models in PLA degradation process.41 Reproduced with permission from ref (41). Copyright 2022 Wiley-VCH GmbH.
Regarding hydrolytic catalysts, contemporary choices predominantly encompass inorganic acids and bases such as sulfonic acid, hydrochloric acid, sodium hydroxide, and potassium hydroxide. Notably, alkaline hydrolysis surpasses acid hydrolysis in speed due to the heightened nucleophilicity of alkaline molecules. Chauliac et al.49 examined the hydrolysis of PLA in various scenarios, indicating that surface erosion can make it easier to reduce PLA size and expand the surface area of the hydrolyzed substrate. Despite PLA’s high crystallinity, hydrophobicity, and hydrolysis activation, its hydrolysis usually necessitates high reaction temperatures or more extreme pH conditions. As a result, the use of this method is not viable due to the increased energy consumption caused by high temperatures, as well as the numerous corrosion and environmental pollution issues that come with strong acid and alkali conditions, which in turn necessitate higher cost inputs for reprocessing. In addition, Achilias et al.50 have employed phase transfer catalysts (2-dimethylamine ethyl), triphenylbrominated phosphine (2DME-TPPB), hexadecyl trimethylammonium bromide (HTMBC), and hexadecyl trimethylammonium chloride (HTMAC) to enhance the alkaline hydrolysis of PLA. Furthermore, Liu et al.51 have discovered the efficiency of ionic liquids in PLA hydrolysis, such as [HSO3-pmim][HSO4] and [Bmim][OAc], with [HSO3-pmim][HSO4] yielding 85% lactic acid at 130 °C and [Bmim][OAc] yielding 93% lactic acid under optimal conditions. Furthermore, Auras et al.52 report that the incorporation of cosolvents into the hydrolysis process had a noteworthy influence on the hydrolysis of PLA. The authors demonstrate that ethanol, when used as a reaction solvent with a 50% ethanol–water mixture, can effectively dissolve and expand PLA plastic, causing water molecules to move inward to PLA, thus increasing the contact surface and making hydrolysis easier. The ethanol–aqueous system appears to be more conducive to PLA hydrolysis than PLA hydrolysis activation energy in pure water, as evidenced by the fact that PLA hydrolysis activation energy in 50% ethanol–aqueous solvents is lower. Furthermore, Palkovits et al.53 documented the synthesis of lactic acid through PLA hydrolysis with Ru/CeO2 as a solid catalyst at a reaction temperature of 200 °C and a hydrogen condition of 1 MPa (resulting in a 94% yield of lactic acid in the product). However, the solid reaction system lacks sufficient contact between catalyst and PLA, and it is also easy to produce byproducts via dehydration reactions of alcohols at elevated temperatures.
In conclusion, while hydrolysis stands out as an environmentally friendly method for PLA plastics polymerization, the prevalent use of uniform inorganic acids and bases as catalysts poses challenges, leading to difficulties in separation, equipment corrosion, and environmental contamination. Additionally, PLA’s high polymerization and hydrophobicity hinder its solubility in water, making solid catalysts less effective compared to alkali catalysts in binding to and absorbing PLA. The high activation energy in PLA hydrolysis necessitates supplementary technologies like elevated reaction temperatures, cosolvents with increased solubility, and external microwaves for effective polymerization. Consequently, developing a catalytic system with a moderate hydrolysis temperature, high activity, and excellent stability for PLA hydrolysis remains a critical challenge.
2.3. Alcoholysis
PLA alcoholysis involves breaking ester bonds for polyester polymerization using alcohol molecules as solvents. Common alcoholysis reagents include methanol, ethanol, and ethylene glycol. Methanol, due to its molecular compactness and potent nucleophilic properties, is widely utilized. Methanolysis of PLA yields methyl lactate, serving as both a foundational material for PLA polymerization and a significant chemical precursor.54−56 Metallic organic complexes, organic bases, and ionic liquids are primary catalysts in PLA alcoholysis, as detailed in Table 1, highlighting various catalysts and their catalytic effects.
Table 1. Alcoholysis Catalysts and Their Catalytic Effectsa.
| catalyst | solvent | t (h) | T (°C) | conv (%) | yield (%) | ref |
|---|---|---|---|---|---|---|
| Zn(OAc)2 | MeOH | 15 | 64.7 | – | 70 | (65) |
| Zn(IMP)2 | MeOH | 0.5 | 130 | 97 | – | (58) |
| Zn(HMDS)2 | MeOH | 2 | 25 | – | 99 | (58) |
| Mg(OEt)2 | EtOH | 1 | 200 | 89 | 86 | (58) |
| bismuth catalyst | MeOH | 0.17 | 160 | – | 99 | (59) |
| TBD | EtOH/CH2Cl2 | 0.03 | 25 | 100 | 95 | (60) |
| TMAF | MeOH | 1 | 100 | 100 | 100 | (61) |
| FeCl3 | MeOH | 4 | 130 | 96 | 87.2 | (61) |
| [Bmim]FeCl4 | MeOH | 3 | 120 | 99.3 | 94.6 | (61) |
| [Bmim][Ac] | MeOH | 3 | 115 | 97 | 92 | (62) |
| [HDBU][2-MeIm] | MeOH | 1 | 70 | 100 | 87 | (63) |
| [HDBU][AA] | MeOH | 5 | 100 | 100 | 91 | (64) |
MeOH, methanol; EtOH, ethanol; t, reaction time; T, reaction temperature.
ZnCl2 was first used as a catalyst for the methanolysis of PLA in the production of methyl lactate.56 Under reflux conditions, Alberti et al.65 reported that zinc acetate (Zn(OAc)2) catalyzed PLA alcoholysis, yielding 70% methyl lactate and 21% ethyl lactate after 15 h in methanol or ethanol solvents. Furthermore, by utilizing Zn(OAc)2 as a catalyst, Enthaler et al.57 realized the full depolymerization of several PLA products, such as transparent glass, coffee cup lids, and disposable forks, at 160 °C with microwave radiation, within yielding 99% methyl lactate in the products. In addition, they reported on the selective depolymerization of PLA to lactide, indicating that when Zn(OAc)2 was used at 200 °C for 6 h the lactide yield was 98% with 88% selectivity to l-lactide.58 It is worth mentioning that the industrial production of PLA is the ROP of lactide, which necessitates prepolymerization and pyrolysis to obtain lactic acid (ester) for the synthesis of lactide. Consequently, the utilization of the direct depolymerization of PLA to synthesize lactide is highly beneficial for PLA closed-loop cycling. Furthermore, Enthaler et al.59 discovered that bismuth salicylate was an effective catalyst for PLA alcoholysis, producing 99% methyl lactate in all genuine PLA products when exposed to 160 °C and microwave radiation for 10 min. Furthermore, when the amino monophenol zinc complex (Zn(IMP)2) was used, it was found that full PLA depolymerization was accomplished at 130 °C.58 Magnesium ethoxide was also employed as the catalyst, where PLA was able to be effectively depolymerized at 200 °C, yielding 86% ethyl lactate in the product.58 Enthaler et al.58 discovered that Zn(HMDS)2 obtained effective depolymerization of PLA at 25 °C for 2 h, yielding 99% lactate in the final product. Naturally, an organometallic salt encounters numerous obstacles when used as a catalyst. At the outset, it is difficult to distinguish when it acts as a homogeneous catalyst. Additionally, it can cause extreme hydrolysis under higher temperatures, thus hindering the progress and utilization of catalysts.
Polyester alcoholysis is also facilitated by alkaline organic small molecules, which possess a strong nuclear affinity and minimal spatial resistance, thereby exhibiting a favorable catalytic effect. Leibfarth et al.60 obtained 95% methyl lactate yield at ambient temperature through PLA depolymerization by trichloromethane dichlorodecene (TBD) in CH2Cl2. Furthermore, Alberti et al.65 also reported 4-dimethylamine (DMAP), triethylene dimethylamine (DABCO), and diazoxycyclohexene (DBU) for PLA alcoholysis; however, due to the fact that the solvent is only methanol, the reaction necessitates 180 °C conditions for full polymerization. Research has demonstrated that the remarkable reactivity of cosolvents such as dichloromethane and toluene in PLA alcohols is a result of the cosolvents’ capacity to completely dissolve PLA and transform heterogeneous catalysis into homogeneous catalysis, which significantly expedites the depolymerization process. Moreover, Lin et al.61 investigated the catalytic effects of ammonium tetramethyl fluoride (TMAF), ammonium tetraethyl bromide (TMABr), ammonium tetraethyl chloride (TMACl), ammonium tetraethyl chloride (TEACl), ammonium tetraethyl iodide (TMAI), and ammonium tetraethiodide (TEAI) at 100 °C. The result demonstrated that F serves as the primary role in catalysis in comparison to the ammonium root (NH4+), in which the formation of hydrogen bonds between F and hydrogen atoms in methanol solution will increase the nucleophilic properties of methanol, allowing it to attack the carbonyl carbon and thus enhance the alcoholysis process. Currently, the primary obstacles encountered by organic alkali catalysts encompass the challenge of recovering from the reaction system following a homogeneous catalyst reaction, as well as the thermal stability of the organic alkali.
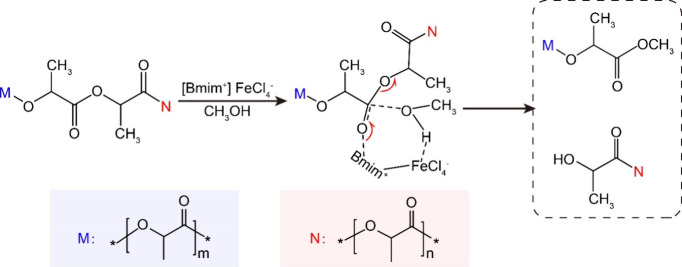
Ionic liquids were also found to have good catalytic activities. The research conducted by Liu64,66−69 revealed that the utilization of FeCl3 as a Lewis acid yielded superior catalytic results in PLA alcoholysis. Subsequently, it was observed that Lewis acid ionic liquids incorporating FeCl3 also exhibited greater catalytic activity, with the addition of 1-butyl 3-methylimidazole ferrite ([Bmim]FeCl4], resulting in a remarkable 94.6% yield of methyl lactate in PLA alcoholysis at 120 °C.63 A series of ionic liquids derived from 1-butyl trimethimidazole (1-Bmin) were also found with good catalytic activity in PLA depolymerization, including [Bmim][Ac], [Bmim]2[CoCl4], and [Bmim]HSO4.62,63 Furthermore, Liu et al. discovered that the amalgamation of [Bmim]Ac and Zn(OAc)2 exhibited greater catalytic activity compared to a solitary application, yielding 92% methyl lactate at 110 °C in 2 h, without any notable decrease in 5 repetitions.70 Compared to imidazole cationic ionic liquids, alkaline ionic liquids based on 2-methylimidazole anions ([2-MeIm]) also have excellent catalytic activities, such as HDBU[2-MeIm], which catalyzes PLA alcoholysis with 87% methyl lactate yield.81,82 The catalytic process of the ionic liquids mentioned above for PLA alcoholysis is similar. The interaction between cationic ions and ester bonded carbonyl group enhanced the electrophilicity of the carbonyl group while the interaction between anion and methanol hydroxyl group increased the nuclear affinity of methanol. These interactions lead to the formation of a hexagonal ring transition state, and eventually the ester bond breaks when the proton in methanol’s hydroxyl group is transferred to the hydroxyl group in the monomer (see Figure 6).71
Figure 6.
Possible alcoholysis mechanism of PLA (“M” and “N” refer to different oligomers).71 Reproduced with permission from ref (71). Copyright 2023 Elsevier.
In contrast to the above homogeneous catalysts, heterogeneous catalysts in PLA alcoholysis offer facile separation and recovery from the reaction system. This characteristic has garnered considerable attention, emphasizing the utility of heterogeneous catalysts in the field. Ma et al. report a two-step catalytic process to convert PLA waste into methyl methacrylate, in which PLA is first transformed into methyl propionate with near-quantitative yields (conversion >99%, selectivity 98%) over an α-MoC catalyst in methanol solution, followed by reaction with formaldehyde to produce methyl methacrylate with a conversion of 81% and a selectivity of 90%.25
In PLA alcoholysis, utilizing alcohol reagents such as methanol and ethanol, and catalysts like Zn(OAc)2, bismuth salicylate, and FeCl3-based ionic liquids, methyl lactate is produced, serving as a valuable precursor. Challenges include recovering homogeneous catalysts and ensuring their thermal stability. Future efforts should prioritize improving catalyst activity and stability, addressing homogeneous catalyst recovery, and advancing sustainable PLA closed-loop cycling. Success in these areas will contribute to efficient and environmentally conscious PLA depolymerization.
2.4. Enzymatic Hydrolysis
The essence of biodegradation is the degradation and mineralization of plastics through microorganisms in nature, which eventually break down into environmentally sound biomass, carbon dioxide, and water. Although PLA is widely recognized as biodegradable plastic, it takes a long time for it to fully degrade under natural conditions. Currently, PLA biodegrades under the conditions of industrial aerobic composting. Typical composting standards include ASTM D5338-15(2021), EN 13432:2000, ISO 14855-1:2012, and GB/T 19277.1-2011. Accordingly, the degradation process typically requires a composting temperature of around 60 °C and 50% humidity.72−74 In addition, the biodegradation process of PLA is influenced by a number of factors, including PLA properties (molecular weight, crystallinity, impurities, etc.), microbial factors (strain type and number and their ability to produce enzymes, etc.), and environmental factors (temperature, humidity, pH, oxygen content, etc.).75 As for the biodegradation mechanism of PLA, it is generally accepted that microbes adhere to the surface of PLA, secrete extracellular degradation enzymes that make long-chain PLA lysates into small molecular weight secondary PLA, assimilate them into cells, and then degrade the secondary PLA into tiny fragments that organisms can convert into carbon sources for microbial growth and metabolism and eventually metabolize out of water cells in the form of carbon dioxide, water, and methane (see Figure 7).75
Figure 7.
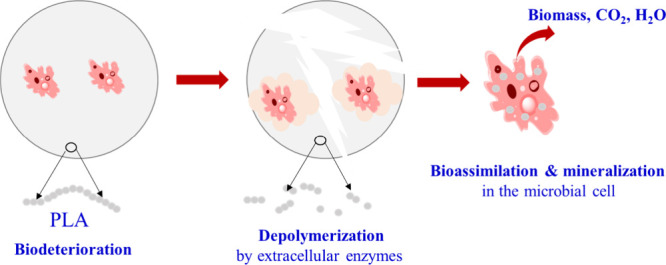
Demonstration of PLA enzymatic hydrolysis.
Research has demonstrated that PLA primarily breaks down by bacteria and actinomycetes, while fungi have a lower activity for degradation.76 Generally, the degradation of PLA is largely dependent on the physical and chemical activities of microorganisms that produce lysozymes (such as proteases, esterases, lipases, keratins, etc.). Protease K stands out as one of the most captivating degradation enzymes in PLA biodegradation. Research has demonstrated that degradation enzymes typically have nucleophilic groups (e.g., serine), catalytic acid groups (e.g., aspartic acid or glutamate), and alkaline groups (e.g., histidine) as their active sites, which are usually found in the particular spatial arrangement of degradation enzymes. When hydrophobic polyester is exposed to degradation enzymes, the active sites interact with the substrate and create a chemical reaction. Hegyesi et al.76 conducted research on cellulose nanocrystals and PLA composites. The experiment revealed that enzymes such as lipase from Candida rugosa and protease K from Tritirachium album exhibited enhanced catalytic activities. It was also found that the pH of the solution had a significant effect on the enzymatic process in addition. When the concentration of lactic acid produced by enzymatic lysis reached 27 mmol/L, the pH of the solution reached a critical value of 6.5. However, when the buffer failed, the enzyme degeneration was severe and the catalytic effect plummeted. The cellulose–PLA complex was compared for various proportions (0–15 wt %), and it was observed that the enzymatic rate increased when the cellulose–PLA content was increased, with pure PLA degradation taking 97.6 h and 15 wt % cellulose–PLA composites requiring 41.4 h. The study revealed that cellulose has a lower natural enzymatic capacity than PLA, so cellulose is enzymatic initially, leading to a large number of pores, expanding the material’s specific surface area and stimulating PLA enzymatic hydrolysis.76,77 Recently, researchers, led by Yakunin et al.78 unveiled a groundbreaking enzymatic approach for polylactic acid (PLA) degradation. Two microbial carboxyl esterases, ABO2449 and RPA1511, were identified, showcasing a remarkable ability to break down PLA into lactic acid derivatives. This novel process diverges from conventional CO2 end-product pathways, presenting a more sustainable and resource-efficient method for PLA disposal. Complementing this, Myburgh et al.79 employed genetically modified Saccharomyces cerevisiae equipped with the CLE1 enzyme, achieving effective catalysis of PLA materials and yielding 9.44 g/L lactic acid from PLA films. These findings represent significant strides in enzymatic plastic degradation, offering eco-friendly alternatives for managing PLA waste. The prospect of converting PLA into valuable compounds, such as lactic acid, not only diversifies the toolkit for plastic degradation but also aligns with the global pursuit of sustainable waste management solutions, contributing to a circular and environmentally conscious approach to PLA waste.
2.5. Other Methods
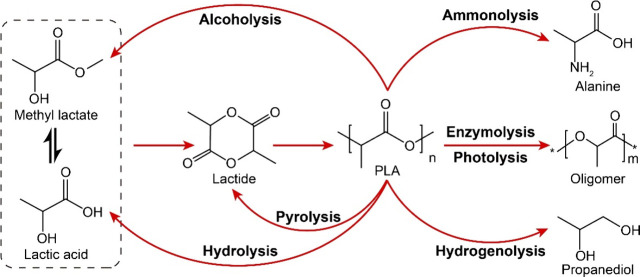
In addition to the aforementioned pyrolysis, hydrolysis, alcoholysis, and enzymolysis, methods such as aminolysis, reductive/oxidative depolymerization, photolysis, and electrolysis have also been the focus of much research in recent years as novel approaches to PLA polymerization.56 Among these, ammonolysis/aminolysis yields amines, while hydrogenolysis yields saturated alcohols or hydrocarbons and oxidative depolymerization obtains carboxylic acids. Despite the challenge of reverting their products to the monomeric forms of the raw materials, they can still be utilized as the base materials for other polymer monomers or other chemical products. Although photolysis and electrolysis have made great strides in recent years, the fear surrounding the methods has not been addressed until now.
Ammonia is primarily utilized in aminolysis to cleave ester bonds within polyesters, facilitating the depolymerization of PLA. The depolymerization of ester bonds in PLA is accomplished through the nucleophilic reaction of carbonyl groups. In aqueous and alcoholic environments, amino groups exhibit greater alkalinity and negative electrical charge compared to hydroxyl groups, rendering small amino molecules more susceptible to nucleophilic attacks on polyester. In 2021, Ma et al.80 utilized ammonia solutions to break down PLA into amino acids and discovered that the Ru/TiO2 catalyst was capable of effectively breaking down PLA at 140 °C, with a 94% alanine selectivity in the product. The reaction mechanism is indirect ammonia, as shown by isotopic mechanism studies. PLA is first dissolved in lactate, then hydrolyzed into ammonium lactate, and finally aminated on the surface of the catalyst to form alanine. Presently, a paucity of research exists on PLA ammonolysis and aminolysis, a circumstance potentially attributed to their hazardous nature and the associated risk of environmental contamination.
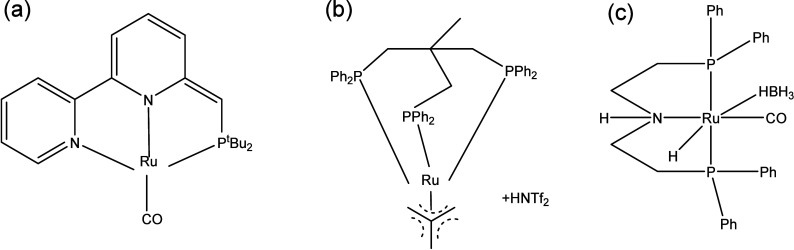
Reductive/oxidative depolymerization involves the utilization of decomposition techniques in conjunction with redox reaction to attain optimal depolymerization and re/upcycling. Among these, hydrogenolysis uses hydrogen as a reactant agent to break down ester bonds in PLA, resulting in saturated hydrocarbons and alcohols. Krall et al.81 employed ruthenium-based complexes (Figure 8a) to catalyze the hydrogenation of PLA, resulting in the formation of 1,2-propanediol. It was demonstrated that PLA can be completely broken down at 160 °C with a H2 pressure of 5.4 MPa in a combination of benzene ether and tetrahydrofuran solvents. Furthermore, Westhues et al.82 employed ruthenium-based complexes (Figure 8b) as catalysts and demonstrated that PLA could be completely broken down at 140 °C in 16 h. Enthaler et al.83 also utilized a commercially accessible Ru-MACHO-BH catalyst (Figure 8c) and discovered that achieving full polyester polymerization was possible at a temperature of 140 °C. Apart from the ruthenium catalyst, Monsigny et al.20 discovered that an organometallic Ir complex exhibited remarkable efficacy in PLA hydrogenolysis. The authors demonstrated that PLA could be fully broken down with 62% selectivity of silane propanediol. Currently, the major limitations to PLA hydrogenolysis are the expensive chemicals; the expensive and unstable precious metal complex catalysts are difficult to distinguish from the homogeneous catalyst reaction. Oxidative decomposition denotes a process wherein depolymerization and transformative reactions yield carboxylic acids or syngas, facilitated by the introduction of oxidants. This oxidative regime encompasses thermal oxidation, dehydrogenation, and gasification mechanisms. Despite its potential significance, limited documentation currently exists on this subject. Consequently, there exists a pronounced imperative to allocate increased scholarly attention to the domain of oxidation depolymerization. This necessitates a more comprehensive exploration of the mechanistic intricacies and optimization strategies inherent in oxidative depolymerization pathways for a nuanced understanding of its potential applications and implications.
Figure 8.
Ruthenium-based catalysts in PLA hydrogenolysis.
Furthermore, PLA photodegradation has been the subject of considerable interest and investigation in recent years.84−87 PLA photodegradation largely relies on its master chain to take in ultraviolet radiation from the sun, resulting in the production of free radicals that then cause a breakdown of molecules. The photodegradation performance of PLA materials with calcium oxides was studied by Loyo, who discovered that calcium oxide additives play a role in the PLA photolysis process.84 The surface of calcium oxide nanoparticles can absorb active hydroxyl groups, which would greatly contribute to the free radical reaction and, as a result, accelerate PLA photolysis. Likewise, Luo85 and colleagues discovered that nanotubes composed of titanium dioxide, possessing an exceptionally high specific surface area, have the ability to adsorb active groups like hydroxyl, carboxyl, and oxygen-negative groups, thereby expediting the disintegration and breakdown of chemical bonds within polyesters. Salač et al.86 discovered that whey acid functions as a photosensitizer, leading to a substantial enhancement in the breaking rate and photodegradation efficiency of PLA chains. Hou et al.88 suggest the incorporation of photodegradation agents into PLA materials in order to enhance the inherent degradation of utilized PLA materials. PLA photodegradation is a relatively straightforward process, particularly when multiple photodegradation agents are incorporated. Despite the fact that photodegradation is a free radical reaction that is hard to precisely control, it is hard to utilize the technology for the reuse of PLA plastics.
The process of the electrocatalytic degradation of PLA plastics involves the utilization of an electrocatalytic approach to break down PLA plastics. In 2022, Chen et al.89 used CuCo2O4/Ni as anode catalysts for the selective electrocatalytic degradation and recycling of PET, PBT, PLA, and other polyesters. At present, electrocatalytic recycling of plastics is still an emerging research field, and little research has been reported on it.
In addition, PLA plastics are also employed as a carbon source in the fabrication of high-value carbon-based materials. Among these, Ladewig et al.90 reported on the fabrication of single-chiral MOFs using PLA plastic depolymerization and the initiation of condensation. Zhang et al.91 synthesized monomers of 3D-printed materials through the combination of aminolysis and directed condensation. Wang et al.92 have successfully utilized PLA alcoholysis products to repolymerize a new PLA material, resulting in the chemical recovery of PLA.
In summary, PLA depolymerization, a pivotal process in closed-loop recycling and upcycling strategies, is intricately governed by the judicious selection of solvent and nucleophilic reagents or catalysts. From a chemical perspective, achieving selective depolymerization hinges on the precise manipulation of ester bonds through nucleophilic substitutions of acyl groups, involving the exchange of nucleophilic reagents. The cornerstone of depolymerization lies in breaking ester bonds, a process vital for both closed-loop recycling and upcycling. The focus on nucleophilic reagents underscores the need for a meticulous selection process, as showcased in Figure 9, which classifies these reagents by element: oxygen (O), nitrogen (N), carbon (C), and sulfur (S). Among these, oxygen-based reagents, comprising water, alcohols, glycols, and carboxylic acids, partake in nucleophilic attacks on the carbonyl carbon, forming new C–O bonds. The reversible nature of these processes necessitates catalysts and controlled conditions to modulate reaction equilibria effectively. Nucleophilic reagents based on nitrogen (N)—ammonia, primary amines, and secondary amines—preferentially form C–N bonds through N atom nucleophilic attacks on the carbonyl carbon. Structural stability conferred by resulting amides facilitates facile reactions, making them a promising avenue for depolymerization. C-based nucleophilic reagents, including hydrogen cyanide, alkynes, and Grignard reagents, predominantly form C–C bonds through nucleophilic attack on the carbonyl carbon, providing an alternative pathway for depolymerization. S-based nucleophilic reagents, encompassing hydrogen sulfide, thiol, and sodium bisulfite, predominantly form S–C bonds via S atom nucleophilic attacks on the carbonyl carbon. The small energy difference in the resulting S–C and C–O bonds renders these processes reversible and adds a layer of complexity to depolymerization involving sulfur-containing reagents. In the pursuit of closed-loop recycling, the strategic use of O atom nucleophilic reagents proves indispensable. In contrast, upcycling necessitates a nuanced consideration of alternative atom nucleophilic reagents, such as N-based and C-based, contingent upon the desired outcome. This intricate interplay of solvent, nucleophilic reagents, catalysis, and controlled conditions delineates a sophisticated landscape for advancing sustainable polyester depolymerization and recycling strategies.
Figure 9.
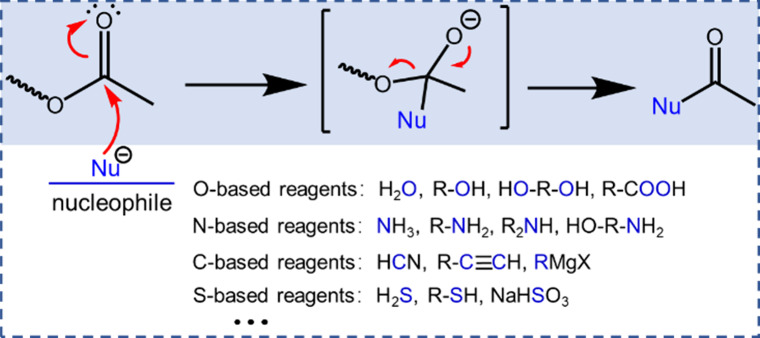
Nucleophilic substitutions to the acyl group of PLA polyester.
3. Conclusion
In pursuit of a circular plastics economy, diverse strategies have been implemented to counter the unsustainable generation and disposal of millions of metric tons of plastic waste annually. The utilization of renewable resources as feedstock, while advantageous, does not comprehensively address the overarching issue of plastic waste. Biodegradable polymers, designed for biological recycling, represent a partial solution but are limited in their ability to recover valuable building block chemicals. Chemical re/upcycling emerges as a promising solution to the end-of-use challenges posed by PLA polyester, a material characterized by high renewability, degradability, and recyclability. PLA polyester constitutes a significant market share outside of PET, and notably, its depolymerization processes generally exhibit lower reaction barriers compared to polyolefins. Consequently, the chemical depolymerization and re/upcycling of PLA not only prove feasible but also confer distinct advantages. Despite the attractive aspect of utilizing cost-effective raw materials, widespread adoption of these methods has been hindered by low efficiency and the expensive nature of degradation and transformation processes. The closely intertwined processes of depolymerization and re/upcycling highlight the pivotal importance of recovering high monomer selectivity and achieving effective separation, forming the foundational prerequisites for their practical application. This paper aims to elucidate the fundamental elements and obstacles intrinsic to the logical progression of catalytic depolymerization of PLA polyester, specifically within the context of closed-loop recycling and upcycling methodologies. By shedding light on these essential aspects, it contributes to a comprehensive understanding of the challenges and opportunities inherent in advancing sustainable practices for PLA polyester within the broader landscape of circular plastics economy initiatives.
3.1. Closed-Loop Recycling
The direct polycondensation of lactic acid for obtaining high-molecular-weight PLA presents significant challenges. Industrial synthesis of PLA typically involves ring-opening polymerization of lactide derived from the pyrolysis of oligomers. This method allows PLA to retain the characteristics of monomer functional groups without releasing small molecules, resulting in a more controlled polymerization process and higher molecular weight. While the current depolymerization products can be repurposed to create new products, the overall procedure is intricate and costly. A more cost-effective and practical solution could be achieved by breaking down plastic waste into key lactide intermediates, facilitating recycling within a closed-loop system. Hydrolysis and alcoholysis stand out as the two most successful techniques for closed-loop recycling of PLA through chemical depolymerization. Hydrolysis employs water as a green solvent, yet it faces challenges with lower hydrolysis activity and higher reaction temperatures. Methanolysis is a prevalent method in alcoholysis techniques due to its superior depolymerization capabilities. However, it is prone to etherification side reactions when exposed to elevated temperatures in an acidic environment. In addition, enzymatic PLA depolymerization is eco-friendly but is hindered by costly pretreatment and efficiency constraints; however, designing enzyme mimetic catalysts offers an intriguing solution for chemical depolymerization. Balancing the efficiency and selectivity of these depolymerization methods is crucial for achieving a sustainable and economically viable closed-loop recycling system for PLA.
3.2. Upcycling
The transformation of plastic waste into chemicals and materials with increased value is known as upcycling. In the case of PLA polyester, distinct from polyolefins, the presence of active functional groups enables precise deconstruction and subsequent reconstruction into novel chemicals or materials. This renders PLA waste upcycling an innovative open-loop strategy, integrating advanced depolymerization chemistry and catalyst science breakthroughs. Several intriguing methods for PLA waste upcycling include ammonolysis/aminolysis, reductive/oxidative upcycling, and photolysis/electrocatalysis. These approaches leverage targeted deconstruction and reconstruction processes, showcasing the versatility of PLA in the upcycling realm. However, the utilization of expensive and hazardous chemicals in these methods poses challenges, rendering them impractical for large-scale industrial production. To address this limitation, it is proposed to delve deeper into emerging technologies that can optimize the PLA plastic upcycling process. The focus should be on reducing costs, enhancing overall effectiveness, and ensuring the safety of the reaction system. This strategic exploration into new technologies aims to pave the way for scalable and industrial-friendly PLA upcycling methods, aligning with sustainability goals and economic viability.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China Youth program (22208085) and Henan Provincial Outstanding Foreign Scientists Studio (GZS2020012). The authors thank Prof. Haichao Liu of Peking University.
The authors declare no competing financial interest.
References
- Adyel T. M. Accumulation of plastic waste during COVID-19. Science 2020, 369 (6059), 1314–1315. 10.1126/science.abd9925. [DOI] [PubMed] [Google Scholar]
- Samantaray P. K.; Little A.; Haddleton D. M.; McNally T.; Tan B.; Sun Z.; Huang W.; Ji Y.; Wan C. Poly(glycolic acid) (PGA), a versatile building block expanding high performance and sustainable Bioplastic applications. Green Chem. 2020, 22 (13), 4055–4081. 10.1039/D0GC01394C. [DOI] [Google Scholar]
- Borrelle S. B.; Ringma J.; Law K. L.; Monnahan C. C.; Lebreton L.; McGivern A.; Murphy E.; Jambeck J.; Leonard G. H.; Hilleary M. A.; Eriksen M.; Possingham H. P.; De Frond H.; Gerber L. R.; Polidoro B.; Tahir A.; Bernard M.; Mallos N.; Barnes M.; Rochman C. M. Predicted growth in plastic waste exceeds efforts to mitigate plastic pollution. Science 2020, 369, 1515–1518. 10.1126/science.aba3656. [DOI] [PubMed] [Google Scholar]
- Geyer R.; Jambeck J. R.; Law K. L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3 (7), e1700782 10.1126/sciadv.1700782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubbins A.; Law K. L.; Muñoz S. E.; Bianchi T. S.; Zhu L. Plastics in the Earth system. Science 2021, 373, 51–55. 10.1126/science.abb0354. [DOI] [PubMed] [Google Scholar]
- Santos R. G.; Machovsky-Capuska G. E.; Andrades R. Plastic ingestion as an evolutionary trap: Toward a holistic understanding. Science 2021, 373, 56–60. 10.1126/science.abh0945. [DOI] [PubMed] [Google Scholar]
- Lau W. W. Y.; Shiran Y.; Bailey R. M.; Cook E.; Stuchtey M. R.; Koskella J.; Velis C. A.; Godfrey L.; Boucher J.; Murphy M. B.; Thompson R. C.; Jankowska E.; Castillo Castillo A.; Pilditch T. D.; Dixon B.; Koerselman L.; Kosior E.; Favoino E.; Gutberlet J.; Baulch S.; Atreya M. E.; Fischer D.; He K. K.; Petit M. M.; Sumaila U. R.; Neil E.; Bernhofen M. V.; Lawrence K.; Palardy J. E. Evaluating scenarios toward zero plastic pollution. Science 2020, 369, 1455–1461. 10.1126/science.aba9475. [DOI] [PubMed] [Google Scholar]
- Sen Gupta R.; Samantaray P. K.; Bose S. Going beyond Cellulose and Chitosan: Synthetic Biodegradable Membranes for Drinking Water, Wastewater, and Oil-Water Remediation. ACS Omega 2023, 8, 24695–24717. 10.1021/acsomega.3c01699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samantaray P. K.; Little A.; Wemyss M.; Iacovidou E.; Wan C. Design and Control of Compostability in Synthetic Biopolyesters. ACS Sustain. Chem. Eng. 2021, 9, 9151–9164. 10.1021/acssuschemeng.1c01424. [DOI] [Google Scholar]
- Ellis L. D.; Rorrer N. A.; Sullivan K. P.; Beckham G. T.; Otto M.; Wierckx N.; Mcgeehan J. E.; Roman-Leshkov Y. Chemical and biological catalysis for plastics recycling and upcycling. Nat. Catal. 2021, 4 (7), 539–556. 10.1038/s41929-021-00648-4. [DOI] [Google Scholar]
- Iwata T. Biodegradable and Bio-Based Polymers: Future Prospects of Eco-Friendly Plastics. Angew. Chem., Int. Ed. 2015, 54 (11), 3210–3215. 10.1002/anie.201410770. [DOI] [PubMed] [Google Scholar]
- Haider T. P.; Voelker C.; Kramm J.; Landfester K.; Wurm F. R. Plastics of the Future? The Impact of Biodegradable Polymers on the Environment and on Societ. Angew. Chem., Int. Ed. 2019, 58 (1), 50–62. 10.1002/anie.201805766. [DOI] [PubMed] [Google Scholar]
- Narancic T.; Verstichel S.; Reddy Chaganti S.; Morales-Gamez L.; Kenny S. T.; De Wilde B.; Babu Padamati R.; O’connor K. E. Biodegradable Plastic Blends Create New Possibilities for End-of-Life Management of Plastics but They Are Not a Panacea for Plastic Pollution. Environ. Sci. Technol. 2018, 52 (18), 10441–10452. 10.1021/acs.est.8b02963. [DOI] [PubMed] [Google Scholar]
- Mckeown P.; Jones M. D. The Chemical Recycling of PLA: A Review. Sustain. Chem. 2020, 1 (1), 1–22. 10.3390/suschem1010001. [DOI] [Google Scholar]
- Montané X.; Montornes J. M.; Nogalska A.; Olkiewicz M.; Giamberini M.; Garcia-Valls R.; Badia-Fabregat M.; Jubany I.; Tylkowski B. Synthesis and synthetic mechanism of Polylactic acid. Phys. Sci. Rev. 2020, 5 (12), 20190102. 10.1515/psr-2019-0102. [DOI] [Google Scholar]
- Carothers W. H.; Dorough G. L.; Natta F. J. V. Studies of Polymerization and Ring Formation. X. The Reversibile Polymerization of Six-Membered Cyclic Esters. J. Am. Chem. Soc. 1932, 54 (2), 761–772. 10.1021/ja01341a046. [DOI] [Google Scholar]
- Tsuji H.; Daimon H.; Fujie K. A New Strategy for Recycling and Preparation of Poly(L-lactic acid): Hydrolysis in the Melt. Biomacromolecules 2003, 4 (3), 835–840. 10.1021/bm034060j. [DOI] [PubMed] [Google Scholar]
- Karan H.; Funk C.; Grabert M.; Oey M.; Hankamer B. Green Bioplastics as Part of a Circular Bioeconomy. J. Trends Plant Sci. 2019, 24 (3), 237–249. 10.1016/j.tplants.2018.11.010. [DOI] [PubMed] [Google Scholar]
- Mishra S.; Goje A. S.; Zope V. S. Chemical Recycling, Kinetics, and Thermodynamics of Hydrolysis of Poly(Ethylene Terephthalate) (PET) Waste in Sulfuric Acid in Presence of Phosphoric Acid. Polym. Plast Technol. Eng. 2003, 42 (4), 581–603. 10.1081/PPT-120023097. [DOI] [Google Scholar]
- Monsigny L.; Berthet J.-C.; Cantat T. Depolymerization of Waste Plastics to Monomers and Chemicals Using a Hydrosilylation Strategy Facilitated by Brookhart’s Iridium(III) Catalyst. ACS. Sustain. Chem. Eng. 2018, 6 (8), 10481–10488. 10.1021/acssuschemeng.8b01842. [DOI] [Google Scholar]
- Song X.; Wang H.; Liu F.; Yu S. Kinetics and mechanism of monomeric product from methanolysis of poly (3-hydroxybutyrate) catalyzed by acidic functionalized ionic liquids. Polym. Degrad. Stab. 2016, 130, 22–29. 10.1016/j.polymdegradstab.2016.05.023. [DOI] [Google Scholar]
- Song X.; Wang H.; Wang C.; Liu F.; Yu S.; Liu S.; Song Z. Chemical Recycling of Bio-based Poly(3-hydroxybutyrate) Wastes Under Methanolysis Condition Catalyzed by Fe-Containing Magnetic Ionic Liquid. J. Polym. Environ 2019, 27 (4), 862–870. 10.1007/s10924-018-1347-8. [DOI] [Google Scholar]
- Weng Y.; Hong C.-B.; Zhang Y.; Liu H. Catalytic depolymerization of polyester plastics toward closed-loop recycling and upcycling. Green Chem. 2024, 26, 571. 10.1039/D3GC04174C. [DOI] [Google Scholar]
- Tian S.; Jiao Y.; Gao Z.; Xu Y.; Fu L.; Fu H.; Zhou W.; Hu C.; Liu G.; Wang M.; Ma D. Catalytic Amination of Polylactic Acid to Alanine. J. Am. Chem. Soc. 2021, 143 (40), 16358–16363. 10.1021/jacs.1c08159. [DOI] [PubMed] [Google Scholar]
- Sun B.; Zhang J.; Wang M.; Yu S.; Xu Y.; Tian S.; Gao Z.; Xiao D.; Liu G.; Zhou W.; Wang M.; Ma D. Valorization of waste biodegradable polyester for methyl methacrylate production. Nat. Sustain 2023, 6, 712–719. 10.1038/s41893-023-01082-z. [DOI] [Google Scholar]
- Jiao Y.; Wang M.; Ma D. Catalytic Cracking of Polylactic Acid to Acrylic Acid. Chin. J. Chem. 2023, 41 (17), 2071–2076. 10.1002/cjoc.202200752. [DOI] [Google Scholar]
- Yin S.; Tuladhar R.; Shi F.; Shanks R. A.; Combe M.; Collister T. Mechanical reprocessing of polyolefin waste: A review. Polym. Eng. Sci. 2015, 55 (12), 2899–2909. 10.1002/pen.24182. [DOI] [Google Scholar]
- Sintim H. Y.; Bary A. I.; Hayes D. G.; English M. E.; Schaeffer S. M.; Miles C. A.; Zelenyuk A.; Suski K.; Flury M. Release of micro- and nanoparticles from biodegradable plastic during in situ composting. Sci. Total Environ. 2019, 675, 686–693. 10.1016/j.scitotenv.2019.04.179. [DOI] [PubMed] [Google Scholar]
- Manfra L.; Marengo V.; Libralato G.; Costantini M.; De Falco F.; Cocca M. Biodegradable polymers: A real opportunity to solve marine plastic pollution?. J. Hazard. Mater. 2021, 416, 125763. 10.1016/j.jhazmat.2021.125763. [DOI] [PubMed] [Google Scholar]
- Kumar S.; Panda A. K.; Singh R. K. A review on tertiary recycling of high-density polyethylene to fuel. Resour Conserv Recycl 2011, 55 (11), 893–910. 10.1016/j.resconrec.2011.05.005. [DOI] [Google Scholar]
- Hu K.; Yang Y.; Wang Y.; Duan X.; Wang S. Catalytic carbon and hydrogen cycles in plastics chemistry. Chem. Catalysis 2022, 2 (4), 724–761. 10.1016/j.checat.2022.02.003. [DOI] [Google Scholar]
- Piemonte V.; Sabatini S.; Gironi F. Chemical Recycling of PLA: A Great Opportunity Towards the Sustainable Development?. J. Polym. Environ 2013, 21 (3), 640–647. 10.1007/s10924-013-0608-9. [DOI] [Google Scholar]
- Gupta M. C.; Deshmukh V. G. Thermal oxidative degradation of poly-lactic acid Part I: Activation energy of thermal degradation in air. Colloid Polym. Sci. 1982, 260, 308–311. 10.1007/BF01447969. [DOI] [Google Scholar]
- Gupta M. C.; Deshmukh V. G. Thermal oxidative degradation of poly-lactic acid Part II: Molecular weight and electronic spectra during isothermal heating. Colloid Polym. Sci. 1982, 260 (5), 514–517. 10.1007/BF01452999. [DOI] [Google Scholar]
- Feng L.; Feng S.; Bian X.; Li G.; Chen X. Pyrolysis mechanism of Poly(lactic acid) for giving lactide under the catalysis of tin. Polym. Degrad. Stab. 2018, 157, 212–223. 10.1016/j.polymdegradstab.2018.10.008. [DOI] [Google Scholar]
- Wang X.-J.; Huang Z.; Wei M.-Y.; Lu T.; Nong D.-D.; Zhao J.-X.; Gao X.-Y.; Teng L.-J. Catalytic effect of nanosized ZnO and TiO2 on thermal degradation of poly(lactic acid) and isoconversional kinetic analysis. Thermochim. Acta 2019, 672, 14–24. 10.1016/j.tca.2018.12.008. [DOI] [Google Scholar]
- Coulembier O.; Moins S.; Raquez J.-M.; Meyer F.; Mespouille L.; Duquesne E.; Dubois P. Thermal degradation of poly(l-lactide), Accelerating effect of residual DBU-based organic catalysts. Polym. Degrad. Stab. 2011, 96 (5), 739–744. 10.1016/j.polymdegradstab.2011.02.014. [DOI] [Google Scholar]
- Saeaung K.; Phusunti N.; Phetwarotai W.; Assabumrungrat S.; Cheirsilp B. Catalytic pyrolysis of petroleum-based and biodegradable plastic waste to obtain high-value chemicals. Waste Manage. 2021, 127, 101–111. 10.1016/j.wasman.2021.04.024. [DOI] [PubMed] [Google Scholar]
- De Jong S. J.; Arias E. R.; Rijkers D. T. S.; van Nostrum C. F.; Kettenes-van den Bosch J. J.; Hennink W. E. New insights into the hydrolytic degradation of poly(lactic acid): participation of the alcohol terminus. Polymer. 2001, 42 (7), 2795–2802. 10.1016/S0032-3861(00)00646-7. [DOI] [Google Scholar]
- Lv S.; Gu J.; Tan H.; Zhang Y. Biodegradation behavior and modelling of soil burial effect on degradation rate of PLA blended with starch and wood flour. Colloids Surf., B 2017, 159, 800–808. 10.1016/j.colsurfb.2017.08.056. [DOI] [PubMed] [Google Scholar]
- Veskova J.; Sbordone F.; Frisch H. Trends in Polymer Degradation Across All Scales. Macromol. Chem. Phys. 2022, 223 (13), 2100472. 10.1002/macp.202100472. [DOI] [Google Scholar]
- Tsuji H.; Ikada Y. Properties and morphology of poly(L-lactide), II. Hydrolysis in alkaline solution. Polym. Chem. 1998, 36 (1), 59–66. . [DOI] [Google Scholar]
- Karst D.; Yang Y. Molecular modeling study of the resistance of PLA to hydrolysis based on the blending of PLLA and PDLA. Polymer 2006, 47 (13), 4845–4850. 10.1016/j.polymer.2006.05.002. [DOI] [Google Scholar]
- Saha S. K.; Tsuji H. Effects of rapid crystallization on hydrolytic degradation and mechanical properties of poly(l-lactide-co-ε-caprolactone). React. Funct Polym. 2006, 66 (11), 1362–1372. 10.1016/j.reactfunctpolym.2006.03.020. [DOI] [Google Scholar]
- Tsuji H. Autocatalytic hydrolysis of amorphous-made polylactides: effects of l-lactide content, tacticity, and enantiomeric polymer blending. Polymer 2002, 43 (6), 1789–1796. 10.1016/S0032-3861(01)00752-2. [DOI] [Google Scholar]
- Tsuji H.; Eto T.; Sakamoto Y. Synthesis and Hydrolytic Degradation of Substituted Poly(DL-Lactic Acid)s. Materials 2011, 4 (8), 1384–1398. 10.3390/ma4081384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji H.; Ikada Y. Blends of crystalline and amorphous poly(lactide), III. Hydrolysis of solution-cast blend films. J. Appl. Polym. Sci. 1997, 63 (7), 855–863. . [DOI] [Google Scholar]
- Tsuji H. Poly(lactide) Stereocomplexes: Formation, Structure, Properties, Degradation, and Applications. Macromol. Biosci 2005, 5 (7), 569–597. 10.1002/mabi.200500062. [DOI] [PubMed] [Google Scholar]
- Chauliac D.; Pullammanappallil P. C.; Ingram L. O.; Shanmugam K. T. A Combined Thermochemical and Microbial Process for Recycling Polylactic Acid Polymer to Optically Pure l-Lactic Acid for Reuse. J. Polym. Environ 2020, 28 (5), 1503–1512. 10.1007/s10924-020-01710-1. [DOI] [Google Scholar]
- Siddiqui M. N.; Kolokotsiou L.; Vouvoudi E.; Redhwi H. H.; Al-Arfaj A. A.; Achilias D. S. Depolymerization of PLA by Phase Transfer Catalysed Alkaline Hydrolysis in a Microwave Reactor. J. Polym. Environ 2020, 28 (6), 1664–1672. 10.1007/s10924-020-01716-9. [DOI] [Google Scholar]
- Song X.; Wang H.; Yang X.; Liu F.; Yu S.; Liu S. Hydrolysis of poly(lactic acid) into calcium lactate using ionic liquid [Bmim][OAc] for chemical recycling. Polym. Degrad. Stab. 2014, 110, 65–70. 10.1016/j.polymdegradstab.2014.08.020. [DOI] [Google Scholar]
- Iñiguez-Franco F.; Auras R.; Dolan K.; Selke S.; Holmes D.; Rubino M.; Soto-Valdez H. Chemical recycling of poly(lactic acid) by water-ethanol solutions. Polym. Degrad. Stab. 2018, 149, 28–38. 10.1016/j.polymdegradstab.2018.01.016. [DOI] [Google Scholar]
- Lehnertz M. S.; Mensah J. B.; Palkovits R. Chemical recycling of polyhydroxybutyrate and polylactic acid over supported Ru catalysts. Green Chem. 2022, 24 (10), 3957–3963. 10.1039/D2GC00216G. [DOI] [Google Scholar]
- Medina-Gonzalez Y.; Aimar P.; Lahitte J. F.; Remigy J. C. Towards green membranes: preparation of cellulose acetate ultrafiltration membranes using methyl lactate as a biosolvent. Int. J. Sustain. Eng. 2011, 4 (1), 75–83. 10.1080/19397038.2010.497230. [DOI] [Google Scholar]
- Rasool M. A.; Van Goethem C.; Vankelecom I. F. J. Green preparation process using methyl lactate for cellulose-acetate-based nanofiltration membranes. Sep. Purif. Technol. 2020, 232, 115903. 10.1016/j.seppur.2019.115903. [DOI] [Google Scholar]
- Filachione E. M.; Lengel J. H.; Fisher C. H. Preparation of Methyl Lactate. Ind. Eng. Chem. 1945, 37 (4), 388–390. 10.1021/ie50424a024. [DOI] [Google Scholar]
- Cheung E.; Alberti C.; Enthaler S. Chemical Recycling of End-of-Life Poly(lactide) via Zinc-Catalyzed Depolymerization and Polymerization. Chemistryopen 2020, 9 (12), 1224–1228. 10.1002/open.202000243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberti C.; Enthaler S. Depolymerization of End-of-Life Poly(lactide) to Lactide via Zinc-Catalysis. ChemistrySelect 2020, 5 (46), 14759–14763. 10.1002/slct.202003979. [DOI] [Google Scholar]
- Alberti C.; Kricheldorf H. R.; Enthaler S. Application of Bismuth Catalysts for the Methanolysis of End-of-Life Poly(lactide). ChemistrySelect 2020, 5 (39), 12313–12316. 10.1002/slct.202003389. [DOI] [Google Scholar]
- Leibfarth F. A.; Moreno N.; Hawker A. P.; Shand J. D. Transforming polylactide into value-added materials. J. Polym. Sci. A Polym. Chem. 2012, 50 (23), 4814–4822. 10.1002/pola.26303. [DOI] [Google Scholar]
- Xie S.; Sun Z.; Liu T.; Zhang J.; Li T.; Ouyang X.; Qiu X.; Luo S.; Fan W.; Lin H. Beyond biodegradation: Chemical upcycling of poly(lactic acid) plastic waste to methyl lactate catalyzed by quaternary ammonium fluoride. J. Catal. 2021, 402, 61–71. 10.1016/j.jcat.2021.08.032. [DOI] [Google Scholar]
- Song X.; Zhang X.; Wang H.; Liu F.; Yu S.; Liu S. Methanolysis of poly(lactic acid) (PLA) catalyzed by ionic liquids. Polym. Degrad. Stab. 2013, 98 (12), 2760–2764. 10.1016/j.polymdegradstab.2013.10.012. [DOI] [Google Scholar]
- Liu M.; Guo J.; Gu Y.; Gao J.; Liu F. Versatile Imidazole-Anion-Derived Ionic Liquids with Unparalleled Activity for Alcoholysis of Polyester Wastes under Mild and Green Conditions. ACS. Sustain. Chem. Eng. 2018, 6 (11), 15127–15134. 10.1021/acssuschemeng.8b03591. [DOI] [Google Scholar]
- Liu F.; Guo J.; Zhao P.; Gu Y.; Gao J.; Liu M. Facile synthesis of DBU-based protic ionic liquid for efficient alcoholysis of waste poly(lactic acid) to lactate esters. Polym. Degrad. Stab. 2019, 167, 124–129. 10.1016/j.polymdegradstab.2019.06.028. [DOI] [Google Scholar]
- Alberti C.; Damps N.; Meißner R. R. R.; Enthaler S. Depolymerization of End-of-Life Poly(lactide) via 4-Dimethylaminopyridine-Catalyzed Methanolysis. Chemistryselect 2019, 4 (23), 6845–6848. 10.1002/slct.201901316. [DOI] [Google Scholar]
- Song X.; Zhang X.; Wang H.; Liu F.; Yu S.; Liu S. Methanolysis of poly(lactic acid) (PLA) catalyzed by ionic liquids. Polym. Degrad. Stab. 2013, 98 (12), 2760–2764. 10.1016/j.polymdegradstab.2013.10.012. [DOI] [Google Scholar]
- Song X.; Wang H.; Zheng X.; Liu F.; Yu S. Methanolysis of poly(lactic acid) using acidic functionalized ionic liquids as catalysts. J. Appl. Polym. Sci. 2014, 131 (19), 40817. 10.1002/app.40817. [DOI] [Google Scholar]
- Song X.; Bian Z.; Hui Y.; Wang H.; Liu F.; Yu S. Zn-Acetate-Containing ionic liquid as highly active catalyst for fast and mild methanolysis of Poly(lactic acid). Polym. Degrad. Stab. 2019, 168, 108937. 10.1016/j.polymdegradstab.2019.108937. [DOI] [Google Scholar]
- Liu H.; Zhao R.; Song X.; Liu F.; Yu S.; Liu S.; Ge X. Lewis Acidic Ionic Liquid [Bmim]FeCl4 as a High Efficient Catalyst for Methanolysis of Poly (lactic acid). Catal. Lett. 2017, 147 (9), 2298–2305. 10.1007/s10562-017-2138-x. [DOI] [Google Scholar]
- Song X.; Bian Z.; Hui Y.; Wang H.; Liu F.; Yu S. Zn-Acetate-Containing ionic liquid as highly active catalyst for fast and mild methanolysis of Poly(lactic acid). Polym. Degrad. Stab. 2019, 168, 108937. 10.1016/j.polymdegradstab.2019.108937. [DOI] [Google Scholar]
- Pang W.; Li B.; Wu Y.; Tian S.; Zhang Yu.; Yang J. Optimization of degradation behavior and conditions for the protease K of polylactic acid films by simulation. Int. J. Biol. Macromol. 2023, 253 (7), 127496. 10.1016/j.ijbiomac.2023.127496. [DOI] [PubMed] [Google Scholar]
- Hajighasemi M.; Nocek B. P.; Tchigvintsev A.; Brown G.; Flick R.; Xu X.; Cui H.; Hai T.; Joachimiak A.; Golyshin P. N.; Savchenko A.; Edwards E. A.; Yakunin A. F. Biochemical and Structural Insights into Enzymatic Depolymerization of Polylactic Acid and Other Polyesters by Microbial Carboxylesterases. Biomacromolecules 2016, 17 (6), 2027–2039. 10.1021/acs.biomac.6b00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai H.; Dave V.; Gross R. A.; Mccarthy S. P. Effects of physical aging, crystallinity, and orientation on the enzymatic degradation of poly(lactic acid). J. Polym. Sci. B Polym. Phys. 1996, 34 (16), 2701–2708. . [DOI] [Google Scholar]
- De Jong S. J.; Arias E. R.; Rijkers D. T. S.; Van Nostrum C. F.; Kettenes-Van Den Bosch J. J.; Hennink W. E. New insights into the hydrolytic degradation of poly(lactic acid), participation of the alcohol terminus. Polymer 2001, 42 (7), 2795–2802. 10.1016/S0032-3861(00)00646-7. [DOI] [Google Scholar]
- Oda Y.; Yonetsu A.; Urakami T.; Tonomura K. Degradation of Polylactide by Commercial Proteases. J. Polym. Environ 2000, 8 (1), 29–32. 10.1023/A:1010120128048. [DOI] [Google Scholar]
- Hegyesi N.; Zhang Y.; Kohári A.; Polyák P.; Sui X.; Pukánszky B. Enzymatic degradation of PLA/cellulose nanocrystal composites. Ind. Crops Prod 2019, 141, 111799. 10.1016/j.indcrop.2019.111799. [DOI] [Google Scholar]
- Donate R.; Monzón M.; Alemán-Domínguez M. E.; Ortega Z. Enzymatic degradation study of PLA-based composite scaffolds. Rev. Adv. Mater. Sci. 2020, 59 (1), 170–175. 10.1515/rams-2020-0005. [DOI] [Google Scholar]
- Hajighasemi M.; Nocek B. P.; Tchigvintsev A.; Brown G.; Flick R.; Xu X.; Cui H.; Hai T.; Joachimiak A.; Golyshin P. N.; Savchenko A.; Edwards E. A.; Yakunin A. F. Biochemical and structural insights into enzymatic depolymerization of polylactic acid and other polyesters by microbial carboxylesterases. Biomacromolecules. 2016, 17 (6), 2027–39. 10.1021/acs.biomac.6b00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myburgh M. W.; Favaro L.; van Zyl W. H.; Viljoen-Bloom M. Engineered yeast for the efficient hydrolysis of polylactic acid. Bioresour. Technol. 2023, 378, 129008. 10.1016/j.biortech.2023.129008. [DOI] [PubMed] [Google Scholar]
- Tian S.; Jiao Y.; Gao Z.; Xu Y.; Fu L.; Fu H.; Zhou W.; Hu C.; Liu G.; Wang M.; Ma D. Catalytic Amination of Polylactic Acid to Alanine. J. Am. Chem. Soc. 2021, 143 (40), 16358–16363. 10.1021/jacs.1c08159. [DOI] [PubMed] [Google Scholar]
- Krall E. M.; Klein T. W.; Andersen R. J.; Nett A. J.; Glasgow R. W.; Reader D. S.; Dauphinais B. C.; McIlrath S. P.; Fischer A. A.; Carney M. J.; Hudson D. J.; Robertson N. J. Controlled hydrogenative depolymerization of polyesters and polycarbonates catalyzed by ruthenium(II) PNN pincer complexes. Chem. Commun. 2014, 50 (38), 4884–4887. 10.1039/c4cc00541d. [DOI] [PubMed] [Google Scholar]
- Westhues S.; Idel J.; Klankermayer J. Molecular catalyst systems as key enablers for tailored polyesters and polycarbonate recycling concepts. Sci. Adv. 2018, 4 (8), eaat9669 10.1126/sciadv.aat9669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindler T.-O.; Alberti C.; Fedorenko E.; Santangelo N.; Enthaler S. Ruthenium-Catalyzed Hydrogenative Degradation of End-of-Life Poly(lactide) to Produce 1,2-Propanediol as Platform Chemical. Chemistryopen 2020, 9 (4), 401–404. 10.1002/open.202000050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loyo C.; Moreno-Serna V.; Fuentes J.; Amigo N. A.; Sepúlveda F.; Ortiz J. A.; Rivas L. M.; Ulloa M. T.; Benavente R.; Zapata P. A. PLA/CaO nanocomposites with antimicrobial and photodegradation properties. Polym. Degrad. Stab. 2022, 197, 109865. 10.1016/j.polymdegradstab.2022.109865. [DOI] [Google Scholar]
- Luo Y.; Cao Y.; Guo G. Effects of TiO2 nanoparticles on the photodegradation of poly(lactic acid). J. Appl. Polym. Sci. 2018, 135 (30), 46509. 10.1002/app.46509. [DOI] [Google Scholar]
- Salač J.; Šerá J.; Jurča M.; Verney V.; Marek A. A.; Koutný M. Photodegradation and Biodegradation of Poly(Lactic) Acid Containing Orotic Acid as a Nucleation Agent. Materials 2019, 12 (3), 481. 10.3390/ma12030481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X.; Xia M.; Su X.; Yuan P.; Li X.; Zhou C.; Wan Z.; Zou W. Photolytic degradation elevated the toxicity of polylactic acid microplastics to developing zebrafish by triggering mitochondrial dysfunction and apoptosis. J. Hazard. Mater. 2021, 413, 125321. 10.1016/j.jhazmat.2021.125321. [DOI] [PubMed] [Google Scholar]
- Hou Q.; Zhen M.; Qian H.; Nie Y.; Bai X.; Xia T.; Laiq Ur Rehman M.; Li Q.; Ju M. Upcycling and catalytic degradation of plastic wastes. Cell Rep. Phys. Sci. 2021, 2 (8), 100514. 10.1016/j.xcrp.2021.100514. [DOI] [Google Scholar]
- Liu F.; Gao X.; Shi R.; Tse E. C. M.; Chen Y. A general electrochemical strategy for upcycling polyester plastics into added-value chemicals by a CuCo2O4 catalyst. Green Chem. 2022, 24 (17), 6571–6577. 10.1039/D2GC02049A. [DOI] [Google Scholar]
- Slater B.; Wong S.-O.; Duckworth A.; White A. J. P.; Hill M. R.; Ladewig B. P. Upcycling a plastic cup: one-pot synthesis of lactate containing metal organic frameworks from polylactic acid. ChemComm 2019, 55 (51), 7319–7322. 10.1039/C9CC02861G. [DOI] [PubMed] [Google Scholar]
- Shao L.; Chang Y.-C.; Hao C.; Fei M.-E.; Zhao B.; Bliss B. J.; Zhang J. A chemical approach for the future of PLA upcycling: from plastic wastes to new 3D printing materials. Green Chem. 2022, 24 (22), 8716–8724. 10.1039/D2GC01745H. [DOI] [Google Scholar]
- Yang R.; Xu G.; Dong B.; Hou H.; Wang Q. A “Polymer to Polymer” Chemical Recycling of PLA Plastics by the “DE-RE Polymerization” Strategy. Macromolecules 2022, 55 (5), 1726–1735. 10.1021/acs.macromol.1c02085. [DOI] [Google Scholar]