Abstract
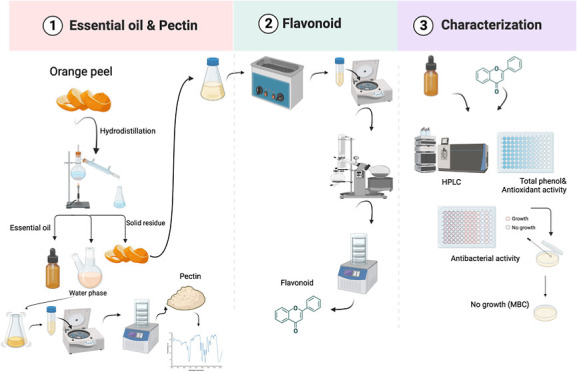
Orange is one of the primary fruits processed into juice and other products worldwide, leading to a vast amount of waste accumulation. Such waste has been considered as an attractive candidate for upcycling to obtain bioactive components remaining. The present study investigated the extraction of essential oil (EO), flavonoids, and pectin from industrial orange waste with a holistic approach. To maximize EO yield and d-limonene concentration, hydrodistillation (HD) conditions were selected to be 5.5 mL water/g solid for 180 min. Remaining solids were further used for flavonoid extraction where conventional solvent, sequential ultrasound + solvent, and ultrasound-assisted extraction (UE) were applied. UE applied for 50 min with 120 mL solvent/g solid yielded the highest total phenolic (TPCs) and total flavonoid contents (TFCs), antioxidant capacity, and hesperidin and neohesperidin concentrations. In terms of TPC, TFC, antioxidant capacity, and antibacterial activity, both EO and flavonoid fractions demonstrated moderate to high bioactivity. At the final step, ethanol precipitation was applied to obtain the pectin that was solubilized in hot water during HD and it was characterized by Fourier transform infrared, degree of esterification, and galacturonic acid content. Practical application: to ensure utilization in the food, pharmaceutical, and cosmetic industries, this study presents a combined method to obtain several value-added compounds from industrial orange waste. Bioactive EO and flavonoids obtained could have applications in functional food, supplements, or cosmetic formulations, whereas extracted pectin can be used in many formulated foods and drugs.
1. Introduction
Orange (Citrus sinensis) represents the major citrus fruit produced globally, accounting to 83.2 million tonnes in 2021, 175 million of which was produced in Turkey.1 Several processed food products, such as juice, jams, marmalade, etc., are produced from approximately 1/3 of oranges produced, leading to a vast amount of waste. It has been reported that orange waste accumulates at around half of the total weight of oranges,2 mostly composed of flavedo, albedo, pulp, and seeds. Solid waste management has been said to be “the most important concern in citrus-processing industries”.3 Orange waste is high in fermentable carbohydrates and moisture and has antibacterial properties, both of which are making the traditional landfilling treatment problematic.4 Additionally, the 2018/850 directive of the EU states that solid waste that has not been treated for energy or resource recovery cannot be diverted to landfill. Therefore, alternative approaches to handling orange waste need to be considered. A typical of those approaches involves upcycling of orange waste to be used as animal feed; however, it lacks an adequate amount of protein and is low in pH and has been suggested to require modification to improve its suitability for feedstock.5 On the other hand, value-added ingredients for food, pharmaceutical, and cosmetic applications, such as essential oil (EO), polyphenols, and pectin, have been produced from orange peel (OP) obtained from orange processing for decades, well before “upcycling” was defined.
Orange EO is mainly composed of monoterpenes that are produced as secondary metabolites, about 90% of which is d-limonene.6 Several bioactive functions of orange EO, including antibacterial,7 antifungal,8 antioxidant,7,9 antidiabetic,10 and anticarcinogenic effects,11 have been reported. It is stated that limonene and p-cymene, which are contained in the EOs obtained from OP, can be economically feasible with their high financial value.12 Orange-derived polyphenols, flavonoids in particular, are also of great interest due to several bioactive functions. Main components of flavonoid fraction obtained from OP are naringin, hesperidin, and rutin.13 This fraction, especially hesperidin, has been emphasized for having antimicrobial, antioxidant, anticarcinogenic, and antidiabetic activities, and it is the major flavonone in citrus fruits.4,14−16 Pectin is one of the most abundant components remaining in fruit and vegetable waste and has been traditionally produced from citrus peels and pomace to be used as a thickener and emulsifier in formulated foods, as well as in the pharmaceutical industry for the production of many drugs.17
The orange juice industry is a dynamic sector in the food industry that generates a lot of waste, as more than half of processed fruit is known to be discarded. Based on the reduce, reuse, recycle, recover, and restore (5R) principle of sustainable development, the circular economy concept describes the use of waste from one industry as a raw material to another, replacing the traditional linear model of the economy (make-use-throw) with a much more effective circular model.18 Furthermore, the concept of bioeconomy, which is explained by the utilization of renewable biological resources into economically valuable products and bioenergy, has emerged.3 Therefore, the current study suggests that orange juice industry leftovers could make excellent feedstock for the generation of bioactive components, fulfilling the goals and objectives of the circular economy. Additionally, the orange juice industry also is an ideal feedstock for the production of energy.19 From this point of view, several approaches exist for the valorization of citrus waste, and OP in particular, to extract the above-mentioned components for food, pharmaceutical, and cosmetic applications.3,20,21 Most of these attempts concentrate on obtaining a single fraction (EO, phenolic compounds or pectin) from such waste. However, the processing of industrial waste with a holistic approach that allows obtaining as much material as possible is important in terms of waste reduction and sustainable and economical production.22−24 However, some components included in the wastes limit their utilization. Since the limonene-rich EO contained in orange waste decreases its usage potential, EO must be extracted. Consequently, while a high added value component is obtained, it is possible to obtain more than one fraction by utilizing the waste separated after the extraction process.25 Existing literature on extraction of more than one bioactive component from citrus waste includes fermentation,26 microwave-assisted solvent-free27 or water extraction,28 steam-distillation followed by acidic extraction,29,30 and Ohmic heating-assisted hydrodistillation (HD).31
The present work focused on procedures that could be scaled up, such as conventional HD, ultrasound-assisted solvent extraction (UAE), and ethanol precipitation, to obtain EO, flavonoids, and pectin from OP. UAE is an environmentally friendly extraction technique that uses less energy and solvent, requires less time and money for extraction, and yields a greater product recovery rate than traditional techniques.32
Tamminen et al.33 highlighted that scaling up the sonochemical process to continuous or large-scale operations is a critical barrier for industrializing the UAE technology. However, there have been few published reports of industrial or experimental processing plants in the literature.33−36
The primary objective of the current study is to sequentially extract EO, flavonoid, and pectin from orange waste. However, various components from OP, as opposed to the literature, primarily focused on the extraction of single fraction from orange waste, such as pectin,1−4 flavonoids,38 and d-limonene.39 EO and flavonoid fractions were especially focused on due to their biological activity. Therefore, extraction routes for these two components were optimized to maximize the yield of d-limonene and selected flavonoids, and the bioactive functions (antimicrobial and antioxidant activities, total phenolic compounds, and flavonoid concentrations) were investigated. Finally, the structural characterization of extracted pectin was reported. Pectins are extensively utilized in the food and medical industries. The are primarily utilized as a gelling agent, thickening, emulsifier, and stabilizer in the food sector to enhance the quality of food items. Pectin can also serve as a material for developing edible and coated films due to its excellent biodegradability, biocompatibility, and diverse physicochemical features, apart from its role in food production.40
Hence, the main goal was to discover a practical application for the excess orange waste that is unsuitable for consumption and is generated in large quantities during the process of manufacturing orange juice. This waste would be utilized as a raw material for the production of EO, flavonoid, and pectin for different purposes including the food industry, cosmetic, and biomaterial preparation. Instead of the previous efforts in upcycling of such waste that focused on production of a single component, the present work enables the extraction of several components from industrial orange waste, thereby leading to an improved cost efficiency of several steps that must be implemented.
2. Materials and Methods
2.1. Materials
2.1.1. Plant Materials
Orange (C. sinensis) peel (OP) waste originated from Aydin (Aegean region) in 2022 was obtained from industrial fruit juice producers, Dimes Gıda Sanayi ve Ticaret A. Ş. and AEP Anadolu Etap Penkon Gıda ve Tarım Ürünleri Sanayi ve Tic. A. Ş. (Türkiye). OP was stored at −40 °C, and before the experiment, it was coarsely ground by a blender. Compositional analysis revealed that OP was composed of 75.4% moisture, 21.4% carbohydrates, 1.4% protein, 1% lipids, and 0.7% ash. OP was stored at −20 °C until used.
2.1.2. Chemicals
High-performance liquid chromatography (HPLC)-grade acetonitrile, formic acid, methanol, and Folin-Ciocalteu reagent were purchased from Merck (Darmstadt, Germany). HPLC-grade flavonoid standards, sodium carbonate, 1,1-diphenyl-2-picrylhydrazyl (DPPH), Trolox (6-hydroxy-2, 5, 7, 8-tetramethylchroman-2-carboxylic acid), and all of the chemicals and growth media used in the antimicrobial analysis were purchased from Sigma-Aldrich (St. Louis, MO, USA). All other chemicals used were of analytical grade.
2.2. Extraction and Characterization of EO
Seventy-five grams of OP were homogenized with distilled water and subjected to HD in a 1 L round-bottom flask connected to a Clevenger apparatus at 100 °C. The crude EO and a few condensation products were collected after extraction. Na2SO4 was used to dry the EO, totally removing any lingering condensation byproducts, after which both the EO and the solid residue of HD (OP-HD) were kept at −18 °C until further experiments.41
To achieve the highest EO yield and d-limonene concentration, the experimental conditions were optimized using a 32 factorial design. Water-to-solid ratio (4, 5, and 7.5, v/w) and HD time (120, 180, and 240 min) were chosen as the factors.
The yield of EO has also been calculated by the following formula42
d-limonene concentration was measured by the method of Park et al.,43 with slight modifications. HPLC (Agilent, 1100) equipped with a DAD detector and a C18 column (250 × 4.6 mm, 5 μm; Supelco, USA) was used. The column was held at 25 °C. Chromatographic analyses were performed using a 20 μL manual sample injector. The flow rate was 1 mL/min, and the wavelength of the DAD detector was 200 nm (d-limonene chromatogram is available at Figure S1). The mobile phase was composed of 95% methanol. Standard d-limonene at known concentrations was used to determine the compound yield in EO.
A factorial analysis of variance (ANOVA) was conducted to assume the main and interaction effects of the chosen factors on the dependent variables. According to this analysis, an HD duration of 180 min and a water/solid ratio of 5, which were statistically more effective, were selected for further experiments.
2.3. Extraction of Flavonoids
2.3.1. Comparison of Flavonoid Extraction Methods
Three different extraction methods, namely, conventional solvent extraction (SE), sequential ultrasound + solvent extraction (USE), and ultrasound-assisted extraction (UE), have been used to maximize the flavonoid concentration, total phenolic compound content, and antioxidant activity. Moreover, the flavonoid profile had also been taken into account. 70% ethanol was used in all approaches since it was reported to be the appropriate solvent to obtain the highest total phenolic and flavonoid content and antioxidant activity.44−46
2.3.1.1. Conventional SE
Two grams of lyophilized OP-HD were extracted with 40 mL of 70% ethanol. A mechanical stirrer was used to shake the mixture at 200 rpm in the dark for 30 min at room temperature. After that, flavonoids were obtained by centrifugation at 4 °C and 8000g for 10 min, and the supernatant was collected. The same procedure was repeated for a second time by using the remaining pellet. The supernatants were pooled and concentrated in a rotary vacuum evaporator at 40 °C.
2.3.1.2. Sequential USE
Two grams of lyophilized OP-HD in 40 mL of 70% ethanol were ultrasonicated in an ultrasonic bath (VWR USC900TH ultrasonic cleaner, VWR Int. Radnor, PA, USA) for 30 min and subsequently centrifuged at 4 °C and 8000g for 10 min, and the supernatant was collected. The remaining pellet was mixed with 40 mL of 70% ethanol and shaken by a mechanical stirrer at 200 rpm for 30 min at room temperature. After that, flavonoids were obtained by centrifugation and concentrated as above.
2.3.1.3. Ultrasound-Assisted Extraction
Two grams of lyophilized OP-HD were extracted with 40 mL of 70% ethanol in an ultrasonic bath for 30 min and subsequently centrifuged at 4 °C and 8000g for 10 min. The same procedure was repeated for a second time using the remaining pellet. After that, flavonoids were obtained by centrifuging and concentrated as mentioned above.
2.3.2. Optimization of Flavonoid Extraction Conditions
Depending on the results from Section 2.3.1, the UE was chosen for further experiments. In order to maximize both total phenolic and total flavonoid contents (TPCs and TFCs, respectively) as well as antioxidant activity, the experimental conditions were optimized using a 32 factorial design. Solvent-to-solid ratio (40, 80, and 120, v/w) and total extraction time (50, 60, and 70 min) were chosen as the factors. Table 3 shows the various combinations of these factors as well as the results for the dependent variables.
Table 3. Flavonoid Concentrations Obtained by Different Extraction Methodsa.
| extraction methods | mg/g citrus peel |
||
|---|---|---|---|
| rutin | hesperidin | neohesperidin | |
| SE | 0.31 ± 0.08c | 1.41 ± 0.18b | 2.37 ± 0.14b |
| UAE | 0.74 ± 0.09b | 3.58 ± 0.08a | 4.07 ± 0.46a |
| USE | 1.26 ± 0.28a | 0.83 ± 0.04c | 3.49 ± 0.34a |
Means with different letters at each column are significantly different (p < 0.05).
2.3.3. Quantification of Flavonoids
The contents of p-anisidine, rutin, hesperidin, neohesperidin, naringenin, and hesperetin were quantified with modifications of the method by Kim and Lim.47 Flavonoids were identified by the same HPLC instrument that is described in Section 2.2 at 270 nm. The mobile phases consisted of 0.05% formic acid in water (A) and 0.05% formic acid in acetonitrile (B). The solvent flow rate was 0.5 mL/min, the injection volume was 10 μL, and the column temperature was set to 30 °C using gradients for of B: 0 min 15%, 8 min 25%, 15 min 25%, and 35 min 65%. TPC and TFC procedures are given below.
2.4. Extraction and Characterization of Pectin
Pectin was extracted from the residual aqueous phase of HD by the combined and modified methods by Oliveira et al.48 and Saberian et al.49 The sample (pH: 1.5) was treated with 95% ethanol (1:1 v/v) at 4 °C for 1 h, and the resulting mixture was centrifuged at 4000 rpm for 7 min to collect the precipitated pectin. After lyophilization, the extraction yield of pectin was calculated as the percentage of pectin (g) to the initial OP (g).
To determine the galacturonic acid (GalA) content of pectin, the method of Blumenkrantz and Asboe-Hansen50 was applied with slight modifications. Pectin (5 mg) was mixed with 2 mL of 72% H2SO4 and 15 mL of distilled water. After mixing for 1 h at room temperature, 3 mL of concentrated sulfuric acid containing 12.5 mM sodium tetraborate was added to the 0.5 mL sample. The mixture was kept in boiling water for 5 min and cooled immediately in a water-ice bath. Then, 50 μL of 0.15% 3-phenylphenol reagent (weight of reagent to volume of 0.5% NaOH) was added, and the mixture was left for 10 min at room temperature. The absorbance was read at 520 nm, and the GalA content was determined according to the standard curve of GalA solutions at 100–600 nmol/mL.
The degree of esterification (DE) of extracted pectin was determined by the titrimetric method of Hosseini et al.51 with slight modification. Lyophilized pectin (75 mg) was dissolved in 100 mL of distilled water while stirring. After complete dissolving, five drops of phenolphthalein reagent were added, and the solution was titrated with 0.1 M NaOH (V1). Then, 20 mL of 0.5 M HCl was added to the sample and left for 15 min at room temperature. The sample was mixed with a magnetic stirrer until the pink color disappeared. After adding five drops of phenolphthalein reagent, the mixture was titrated with 0.1 M NaOH until a slight pink color was observed (V2). The DE (%) was calculated as
The extracted pectin powders’ spectra of Fourier transform infrared (FT-IR) spectroscopy (Bruker Tensor II, Massachusetts, USA) in the range of 400–4000 cm–1 were obtained and compared with the spectra of commercial pectin.
2.5. Bioactivity Analysis
EO diluted in ethanol (10 mg/mL) 1% dimethyl sulfoxide (DMSO) and extracts obtained from flavonoid extraction procedures were used for bioactivity analysis.52
2.5.1. Determination of TPC
The Folin-Ciocalteu method was used to determine the TPC.53,54 Briefly, 0.75 mL of 0.2 N Folin-Ciocalteu reagent (10%) was added to 100 μL of samples. After keeping the samples in the dark for 5 min, 750 μL of saturated Na2CO3 (6%) solution was added to this mixture. After the samples were vortexed and kept at room temperature in a dark environment for 90 min, absorbance measurements were made at 765 nm in a UV–vis spectrophotometer (BioTek Instruments, Winooski, Vermont, USA). Results were expressed as milligrams of gallic acid equivalent/100 g of the sample and gallic acid equivalent/L of EO.
2.5.2. Determination of TFC
TFC of the EO and flavonoid extracts have been determined by the method by Zhishen et al.55 Briefly, 0.25 mL of the sample was mixed with 1.25 mL of distilled water. After that, 75 μL of 5% NaNO2 was added. After 6 min, 150 μL of 10% AlCl3·6H2O was added, and the mixture was stored for 5 min. Finally, 0.5 mL of 1 M NaOH was added and the volume of the mixture was completed to 2.5 mL with distilled water. The mixture was shaken, and the absorbance of the mixture was determined at 510 nm by a UV–vis spectrophotometer. Results were expressed as milligrams of rutin equivalent/100 g extract and milligrams of rutin equivalent/L EO.
2.5.3. Determination of Total Antioxidant Capacity
DPPH and cupric-reducing antioxidant capacity (CUPRAC) methods were used to determine the total antioxidant activities of the EOs and flavonoids. The DPPH method was conducted according to the method by Kumaran and Joel Karunakaran.56 100 μL of the sample was added to 2 mL of a 0.1 mM DPPH solution prepared in 100% methanol. The samples were then kept in a dark room for 30 min. Absorbance of the samples was measured at 517 nm in a UV–vis spectrophotometer. In the CUPRAC analysis, 100 μL of samples was mixed with 1 mL of each of 10 mM CuCl2, 7.5 mM neocuprine, NH4Ac (pH: 7), and distilled water. After incubation for 30 min at room temperature, the absorbance of the samples was determined at 450 nm by a UV–vis spectrophotometer.57 Results were expressed as milligrams of Trolox equivalent/100 g of extract and milligrams of Trolox equivalent/L of EO.
2.5.4. Antimicrobial Activity
The antimicrobial activities of EO and flavonoids were tested against three Gram-positive (Staphylococcus aureus ATCC 6538, Streptococcus pyogenes ATCC 19615, and methicillin-resistant S. aureus ATCC 43300) and two Gram-negative (Pseudomonas aeruginosa ATCC 27853 and Escherichia coli ATCC 25922) bacteria. All microorganisms were cultured overnight at 37 °C in Mueller-Hinton broth (MHB) and diluted in sterile saline solution (0.85% w/v NaCl) to reach a final concentration of approximately 105 CFU/mL.
The minimum inhibitory concentrations (MICs) and minimum bactericidal concentrations (MBCs) of EO and flavonoids were determined with the broth microdilution technique in a 96-well plate according to modified methods of Chahbi et al.58 The stock solutions of EO (512 mg/mL) and flavonoids (128 mg/mL) were prepared in sterilized 10% DMSO including 2% Tween 80 and sterilized 10% DMSO, respectively. 2-Fold diluted EO (4–256 mg/mL) and flavonoid (64–0.0625 mg/mL) concentrations were prepared in MHB. Briefly, 180 μL of the diluted sample and 20 μL of bacterial suspension were transferred to wells. For the samples’ negative control, the mixture of 180 μL of the diluted sample and 20 μL of MHB was used, and wells without the diluted sample were used as the positive control. The MIC value was defined as the lowest sample concentration without turbidity at 600 nm after incubation at 37 °C for 24 h. Then, the mixture in the wells was inoculated on the Mueller-Hinton agar plates, and the concentration with no bacterial growth was defined as MBC. Finally, the results were confirmed by the resazurin assay based on color change principle specified by Foerster et al.59
2.6. Statistical Analysis
All experiments were reported as mean ± standard deviation of three independent replicates with three parallel measurements. The statistical significance of differences among groups was evaluated by factorial ANOVA, followed by Tukey test using Minitab (Ver. 18.0, USA), and significance was identified with a value of p < 0.05.
3. Results and Discussion
3.1. Extraction and Characterization of EO
EO was extracted from the OP residue in the orange juice industry. The effects of HD time and water-to-solid ratio were investigated on EO yield and d-limonene concentration. The results are shown in Table 1.
Table 1. Factorial Design with the Observed Results for the EO Yield and d-Limonene Concentration.
| HD time (min) | water-to-solid ratio (v/w) | EO yield (%) | d-limonene concentration (%) |
|---|---|---|---|
| 120 | 4 | 0.29 | 37.43 |
| 120 | 5.5 | 0.35 | 19.19 |
| 120 | 7 | 0.38 | 24.68 |
| 180 | 4 | 0.45 | 23.84 |
| 180 | 5.5 | 0.38 | 20.49 |
| 180 | 7 | 0.26 | 21.21 |
| 240 | 4 | 0.57 | 13.37 |
| 240 | 5.5 | 0.44 | 18.30 |
| 240 | 7 | 0.48 | 14.90 |
| 120 | 4 | 0.32 | 14.74 |
| 120 | 5.5 | 0.29 | 28.83 |
| 120 | 7 | 0.78 | 20.53 |
| 180 | 4 | 0.23 | 22.27 |
| 180 | 5.5 | 0.45 | 20.83 |
| 180 | 7 | 0.29 | 23.92 |
| 240 | 4 | 0.23 | 16.60 |
| 240 | 5.5 | 0.73 | 21.73 |
| 240 | 7 | 0.47 | 20.31 |
According to the findings, water-to-solid ratio and HD time significantly affected d-limonene concentration (p < 0.05) but not the EO yield, the latter of which was in contrast to previous studies.60 Extraction yield of EO was in the range of 0.29–0.76%; however, no significant differences were observed. Similar to our findings, Mohagheghniapour et al.,61 Bourgou et al.,62 and Visakh et al.63 reported citrus EO yield as 0.20, 0.35, 0.45, and 0.48%, respectively, while the yield of EO has also been reported as 1–3% by several researchers.60,64−66 In a recent study by Wei et al.,67Citrus medica L. var. arcodactylis EO yield was reported as 1.29 ± 0.03% with the HD method. These variations can be explained by the fact that the citrus peel waste used in the present study differed.67 Another explanation for varying EO yields could be the proper separation of hydrosols, which consists of a small portion of EO that is separated into distilled water during the HD process. This secondary product is formed as a result of hydrogen bonding between polar oil vapors and water during the extended distillation period, thereby leading to loss of some EO yield.21,68 The results of the chemical analysis of the EO were consistent with prior studies that d-limonene was the major constituent of orange EO.60,69 Orange EO in the present study contained lower d-limonene content than in previous studies9,60,70,71 and was accounted as 16.60–37.43%. Similar to our results, Heydari Koochi et al.10 reported that sweet orange and bitter OP waste EO contained 19.91 and 18.12% of d-limonene. Soil conditions, management, topography, and climate conditions, including temperature, light, wind, and rainfall, can all have an impact on the quantity and quality of citrus EO. Additionally, the method used to extract the EO impacts the quality.10 At 95% confidence level, HD time and water-to-solvent ratio as well as the interaction of these two independent variables (p = 0.003) (Figure 1) were found to have statistically significant effects on the d-limonene concentration.
Figure 1.
Effect of interaction of water-to-solid ratio (●, 4; ■, 5.5; and ⧫, 7) and time on d-limonene concentration.
The highest d-limonene concentration was found at 3 h and a water-to-solid ratio of 5.5. Results in Figure 1 show that d-limonene extraction progressively increased up to 3 h, and decreased afterward, at the specified water-to-solid ratio. Fick’s second law of diffusion could be used to explain this as mass transfer of the solute from biomass to solvent phase can only occur until the system reaches equilibrium.72 Additionally, because of the characteristics of d-limonene, prolonged extraction times may have caused this unsaturated compound to degrade under the influence of light, heat, and air.73
One of the crucial factors influencing HD is the ratio of water-to-solid materials, which is defined as the weight of the material dissolved in a certain volume of the solvent such as water. The effect of the water-to-solid ratio was investigated at 4, 5.5, and 7 g/mL. The low water amount would not be sufficient to diffuse into the material. Additionally, a larger sample amount might enable the extraction of a higher percentage of EO, but it leads to a concentrated sample or poor chemical solubilization because there is only so much solvent available. As a result, the ratio between water and solids should permit the equilibrium concentration to stay below the solute’s saturation concentration.71,74 According to Table 1 and Figure 1, the EO yield was unaffected, whereas the concentration of d-limonene declined at higher water-to-solid ratios and was lowest at the lowest level used. Similar to our results, Dao et al.74 reported optimum water-to-solid ratio as 3 mL/g when 2, 3, and 4 mL/g were investigated by HD of citrus leaves. The same phenomenon was also explained by several researchers.31,71,74,75
In light of results regarding both dependent variables, HD conditions were selected to be 5.5 mL of water/g of solid for 180 min. At these conditions, EO with 0.42 ± 0.05% yield and 20.66 ± 0.24% d-limonene was obtained.
3.2. Comparison of Flavonoid Extraction Methods
The residue of OP from EO extraction (OP-HD) was used to extract flavonoids with several methods including conventional SE, UE, and sequential USE extraction. Table 2 shows the TPC, TFC, and antioxidant activities of the flavonoids obtained from different extraction techniques, where the extraction method had a significant impact. It was obvious that flavonoids extracted by the UE approach had significantly higher TFC and antioxidant capacity (measured by the DPPH assay) than SE and USE methods (p < 0.05), as a result of extended UAE durations, which encourage more breakdown of the solid vacuole and cell wall, allowing solvent penetration and biocompound diffusion.76 USE yielded significantly lower TPC, TFC, and antioxidant activity when compared to the other two methods. This is believed to be a result of increased temperature due to ultrasound treatment, which was reported to be as high as 48 °C when cooling is not implemented.77 In a study conducted by Zimare et al.,32 phenolics from Lobelia nicotianifolia leaves were extracted with the assistance of the UAE; however, at higher temperatures and longer extraction times, a decline in TPC and TFC was observed.32 Improved tissue disruption overcoming the negative effects of temperature increase is thought to be the reason for UE performing significantly better than USE. Hesperidin, naringin, narirutin, and neohesperidin are the most prevalent citrus flavonoids.78 Hesperidin has a wide range of biological properties, including anti-inflammatory, antibacterial, antioxidant, and angiogenic activity. Additionally, hesperidin has been extensively used to treat inflammation, allergies, liver diseases, and acceleration of wound healing. From this point of view, the hesperidin content is taken into account for the selection of the extraction method. Similar results have been reported by several researchers.37,79−83 The positive effect of ultrasound on bioactive compounds was linked to improved penetration of solvent into the plant matrix, plus improved diffusion of the compound of interest into the solvent, both of which are due to jet impacts of cavitation bubbles when collapsed, as the main driving force for UE, as well as due to possible pore enlargement of the plant tissue.84
Table 2. TPC, TFC, Antioxidant Activity by DPPH, and CUPRAC Assays of Flavonoids Obtained by Different Extraction Methodsa.
| extraction method | TPC (mg GAE/g OP-HD) | TFC (mg RE/g OP-HD) | antioxidant activity by DPPH assay (mg TEAC/g OP-HD) | antioxidant activity by CUPRAC assay (mg TEAC/g OP-HD) |
|---|---|---|---|---|
| SE | 4.77 ± 0.65a | 1.87 ± 0.24b | 3.16 ± 0.09b | 7.31 ± 0.59a |
| UE | 4.67 ± 0.18a | 2.50 ± 0.01a | 3.70 ± 0.07a | 6.93 ± 1.14a |
| USE | 1.29 ± 0.17b | 1.07 ± 0.34c | 2.07 ± 0.19c | 5.68 ± 0.18a |
GAE: gallic acid equivalent; RE: rutin equivalent antioxidant capacity; and TEAC: Trolox equivalent antioxidant capacity. Means with different letters at each column are significantly different (p < 0.05).
To summarize, because of cavitation and the quick creation and collapse of air bubbles that rupture the cell wall and release the phenolic compounds, ultrasonication-based extraction yielded a greater recovery of the phenolic compounds.
Hesperidin and neohesperidin were the most prevalent flavonoids in the obtained extracts (Table 3). Rutin contents were significantly improved by UE and USE methods (p < 0.05). Flavonoids were higher in OP extracts from UE than SE. Wang et al.37 also reported that flavonoid concentrations and phenolic fractions were generally higher in UE and enzyme-assisted extraction than conventional SE from brocade orange (C. sinensis) peels. There were no significant differences between UE and USE on neohesperidin content, while hesperidin content was significantly higher by UE compared to other methods, similar to previous results,37,85,86 probably due to improved extraction as discussed above. The reason why higher concentrations of flavonoids are obtained as a result of UE treatments is that the interactions such as covalent and hydrogen bonds that keep the phenolic components bound to the food matrix are destructed.83
After the selection of the appropriate method as UE, optimal extraction conditions were investigated based on TPC, TFC, and antioxidant activity, as well as individual concentrations of rutin, hesperidin, and neohesperidin (Table 4). Previous studies showed that flavonoid extraction yield varied by the fruit type. About 8.23–15.56 g/100 g, 3.26–8.93 g/100 g, 8.66–13.56 g/100 g, and 7.43–12.73 g/100 g were extracted from sweet orange, lemon, tangerine, and grapefruit peels, respectively.7 Additionally, Selahvarzi et al.87 reported that the extraction yield of OP was 11%, and our extraction yield was 20.89%, higher than the study. In another study conducted by Chen et al.,64 five different OP source extraction yield ranged from 27.3 to 41.4% (w/w) of dried OP, with hot alkaline water extraction. Flavanone yield from orange (C. sinensis L.) peel by ethanol extraction was 6.27–10.03%.80 Dalmau et al.89 observed that orange byproduct extraction yields were 22, 19, and 13% at 5, 15, and 25 °C with ultrasound extraction, respectively. These differences should be explained with the differentiation of extraction methods and solvents.
Table 4. Factorial Design with the Observed TPC, TFC, Antioxidant Capacity, and Individual Flavonoid Concentrationsa.
| time (min) | solvent-to-solid ratio (v/w) | TPC (mg GAE/g OP-HD) | TFC (mg rutin/g OP-HD) | antioxidant activity by DPPH assay (mg Trolox/g OP-HD) | antioxidant activity by CUPRAC assay (mg Trolox/g OP-HD) | rutin (mg/g OP-HD) | hesperidin (mg/g OP-HD) | neohesperidin (mg/g OP-HD) |
|---|---|---|---|---|---|---|---|---|
| 50 | 40 | 2.84 ± 0.53b | 8.14 ± 3.03b | 2.03 ± 0.64c | 5.87 ± 0.73c | 0.69 ± 0.76 | 2.51 ± 2.21 | 3.15 ± 2.25 |
| 80 | 6.29 ± 1.06a | 11.7 ± 1.71ab | 5.76 ± 1.39b | 9.8 ± 2.24b | 6.02 ± 3.73 | 10.81 ± 6.45 | 5.20 ± 2.16 | |
| 120 | 7.32 ± 0.65a | 16.2 ± 1.04a | 8.23 ± 2.46a | 13.41 ± 3.53a | 5.13 ± 1.08 | 14.41 ± 3.53 | 5.36 ± 2.7 | |
| 60 | 40 | 2.97 ± 0.36b | 10.01 ± 0.98b | 2.59 ± 1.15c | 4.92 ± 0.58c | 3.16 ± 1.66 | 8.49 ± 2.97 | 2.65 ± 0.11 |
| 80 | 5.32 ± 0.32a | 11.03 ± 1.02ab | 4.43 ± 1.48b | 8.35 ± 1.62b | 6.92 ± 1.42 | 14.02 ± 2.73 | 4.38 ± 0.55 | |
| 120 | 6.4 ± 0.43a | 14.78 ± 2.36a | 6.94 ± 2.09a | 10.96 ± 1.74a | 5.67 ± 0.34 | 3.31 ± 1.73 | 14.88 ± 2.5 | |
| 70 | 40 | 3.57 ± 0.45b | 6.67 ± 3.42b | 2.13 ± 0.34c | 6.74 ± 1.44c | 2.37 ± 1.87 | 3.05 ± 1.9 | 3.76 ± 1.71 |
| 80 | 6.28 ± 2.74a | 10.29 ± 0.65ab | 4.91 ± 1.61b | 9.42 ± 1.71b | 0.47 ± 0.32 | 3.02 ± 1.27 | 3.22 ± 1.06 | |
| 120 | 7.15 ± 0.91a | 13.04 ± 3.06a | 6.31 ± 1.61a | 9.27 ± 2.31a | 2.94 ± 1.8 | 9.35 ± 3.38 | 5.56 ± 1.48 |
Means with different letters at each column are significantly different (p < 0.05).
Statistical analysis revealed that solvent-to-solid ratio had a significant effect on all dependent variables (p < 0.05). When solvent proportion was increased, TPC, TFC, and antioxidant capacity of flavonoids increased as well; several researchers reported similar patterns in their study.37,87,90 The reason behind this effect was thought to be similar to our previous discussion regarding the effect of water amount for EO extraction, which was the improved diffusion of compounds into the solvent due to a larger concentration gradient at higher solvent-to-solid ratios.
When individual concentrations of selected flavonoids were examined (Table 4), both of the independent variables as well as their interactions were statistically significant (p < 0.05). Extending the treatment period caused a decrease in hesperidin and rutin concentrations. Prolonged exposure to ultrasonication results in structural damage to the solute and degradation of bioactive chemicals. It was reported that at higher temperatures and/or for longer periods of time, the contents of hesperidin decreased to varying degrees.91 Hesperidin contents were reported in the range of 8.19–20.17 mg/g dw in C. sinensis pulp.90 Hesperetin is converted into the tasteless hesperidin and its bitter isomer neohesperidin by the actions of 1,6-Rhat or 1,2-Rhat, respectively, both of which are widely distributed in OP, despite the fact that typically only one isomer exists at a predominate level. For instance, Citrus grandis peels from Wendun contained a high amount of neohesperidin,92 while in sweet oranges (C. sinensis)64 and brocade orange (C. sinensis L. Osbeck), Citrus reticulata(88) was the most abundant.86 Similar patterns are also shown in Table 4.
Considering data in both Tables 3 and 4, the selected conditions for the flavonoid extraction were determined as the solvent-to-solid ratio of 120 mL/g and an extraction time of 50 min. Rutin, hesperidin, and neohesperidin contents of flavonoids at selected conditions were 2.31 ± 0.58, 14.25 ± 3.39, and 4.02 ± 0.87 mg/g OP-HD, respectively.
It is also important to discuss the feasibility of extraction methods in addition to knowing that the added value of bioactive compounds is high.93,94 The current study showed that the ultrasound-assisted approach resulted in a bioactive component with higher bioactivity. The equipment cost of the UAE method is lower compared to suggested novel methods in the literature, and it also uses less energy during short-term extraction. In addition, the automated system has the benefit of requiring fewer workers than conventional techniques.95 Apart from its economic aspect, the UAE method is notable due to its support for sustainable food system and being environmentally friendly.96 In spite of the fact that disposing of one ton of solid waste in Europe costs around between 28 and 60 US $,97 since the payback period of the UAE system is less than a year, it is understood that UAE method offers a significant economic advantage in addition to the higher biological activities of bioactive compounds.98 UAE, which is stated to be applied in orange juice byproducts, has the potential to have a significant place in the evaluation of the these wastes, which is around 15 million tons per year, and the related sustainability issue.99
3.3. Bioactivity of EO and Flavonoids under the Selected Extraction Conditions
Total phenolic and flavonoid contents, antioxidant capacity with DPPH and ABTS assays, and antimicrobial activity of EO and flavonoids extracted under selected conditions were evaluated (Table 5). In accordance with our results, Değirmenci and Erkurt100 determined TPC of EO as 1.54 ± 0.08 mg GAE/g EO. Raspo et al.101 investigated the orange EO antioxidant activity with several methods, including DPPH, CUPRAC, FRAP, and ABTS assay. Nearly, 8 mg of Trolox/mL of EO and 2.5–4.3 mg of Trolox/mL of EO capacity with DPPH and CUPRAC assay were reported, similar to our results. DPPH radicals can be deactivated through two methods: by either hydrogen atom-transfer or single-electron-transfer (SET) mechanisms. The antioxidant activity of the CUPRAC test relies on the reduction of copper(II) to copper(I) facilitated by the SET mechanism.102 These results show that the antioxidant activities of flavonoids were consistently higher than those detected by the DPPH assay (Table 4). It means that EO and flavonoids in this study exhibit higher antioxidant qualities in reduction capacity compared to free-radical scavenging activity.
Table 5. Bioactivity of EO and Flavonoids Obtained under Selected Extraction Conditionsa.
| TPC | TFC | antioxidant activity by DPPH assay | antioxidant activity by CUPRAC assay | |
|---|---|---|---|---|
| EO | 3.07 ± 0.19 (mg GAE/mL EO) | 29.48 ± 2.48 (mg RE/mL EO) | 3.82 ± 0.09 (mg TEAC/mL EO) | 5.50 ± 1.37 (mg TEAC/mL EO) |
| flavonoids | 8.35 ± 1.55 (mg GAE/g OP-HD) | 6.48 ± 3.03 (mg RE/g OP-HD) | 10.61 ± 0.32 (mg TEAC/g OP-HD) | 10.61 ± 3.15 (mg TEAC/g OP-HD) |
Extraction conditions for EO: 5.5 mL water/g OP and HD time of 180 min. Extraction conditions for flavonoids: 50 min of UE with 120 mL solvent/g OP-HD.
Additionally, Manzur et al.103 determined antioxidant capacity and TPC of orange EO as 10.53 ± 1.20 μg GAE/mL EO and 0.52 ± 0.006 mg TEAC/mL, respectively, which were lower than our results. The antioxidant activities of various species of citrus peel EOs vary. The percentage variation of the main chemical compounds found in the extracted plant EO was most likely the cause of this difference.63 The results in different cultivars of oranges were also different, such as bitter orange (IC50 = 19.6–200 μg/mL),104 navel orange (IC50 = 9.45 μg/mL),105 sweet peel orange (375–643 mg Trolox/g EO),10 and bitter OP (5.23 mg GAE/mL EO).106 Numerous antioxidant assays were used due to antioxidants’ complexity and reactivity. It is not surprising that the relative activities with these antioxidant tests will vary, given the complexity of these mixtures and the various test principles.
MIC and MBC values of orange EO and flavonoids obtained under selected conditions against five different microorganisms are shown in Table 6. While the MIC and MBC values of EO were higher than those of flavonoids, the lowest-resistant microorganism was noted as S. pyogenes ATCC 19615 against both bioactive fractions. In general, it was reported that the effectiveness of EO against Gram-positive microorganisms should be greater than Gram-negative microorganisms due to their difference in the cell walls’ structure which explains the sensitivity of S. pyogenes ATCC 19615.101 However, on the contrary, EO (MIC: 0.39–3.13 μL/mL) was found to be more effective against Gram-negative microorganisms.70
Table 6. MIC and MBC Values of EO and Flavonoids Obtained under Selected Extraction Conditionsa.
| microorganism | EO |
flavonoids |
||
|---|---|---|---|---|
| MIC (mg/mL) | MBC (mg/mL) | MIC (mg/mL) | MBC (mg/mL) | |
| E. coli ATCC 25922 | 256 | 256 | 64 | 64 |
| P. aeruginosa ATCC 27853 | 256 | 256 | 64 | 64 |
| S. aureus ATCC 6538 | 128 | 256 | 16 | 16 |
| S. aureus ATCC 43300 | 256 | 256 | 16 | 16 |
| S. pyogenes ATCC 19615 | 32 | 32 | 8 | 8 |
In other studies, while the MIC value of sweet orange EO was determined between 4.66 and 18.75 μL/mL,107 Torres-Alvarez et al.108 determined MIC and MBC values in the range of 0.5–2 mg/mL and 2 → 2.5 mg/mL, respectively. These variations in antibacterial activity might be due to the method used for extraction, the type of orange, or the part of the orange used. For example, Geraci et al.109 exposed Listeria monocytogenes to EO obtained from the Sanguinello Paternò (SPP) and Moro Solarino (MSP) orange peels. They concluded that the inhibitory effect of the MSP source EO (92 mg/mL) was approximately 6 times lower than that of SPP (15 mg/mL).109 Besides, MIC values against two strains of S. aureus and P. aeruginosa were indicated as higher than 100 mg/mL in accordance with the current study.
Flavonoids, which have been shown by various studies to be in the composition of OP,88,106 have various biological roles, one of which is their antimicrobial effect.110 It is known that they present this effect with different mechanisms,111,112 and rutin and hesperidin-neohesperidin, which are included in the extracted flavonoid content, have been shown to have effects such as membrane-disrupting and inhibition of biofilm formation, respectively.113 While studies generally focus on the antimicrobial effect of a specific component in the flavonoid composition, MIC values against different bacteria were shown as >1000 μg/mL for neohesperidin, ≤1000 μg/mL for hesperetin,114 and 500–1000 μg/mL for rutin.115 The inhibitory concentration may be higher as it is known that a wide variety of components are included in the extracted flavonoid composition.
Besides, antimicrobial differences result from variations in the antimicrobial components present in the resultant bioactive agent’s composition. The amount of d-limonene in orange EO, which is known to have antibacterial properties,116 varies depending on the orange variety,109 and this circumstance has a significant impact on the effectiveness against pathogens as seen in flavonoid.
3.4. Extraction and Characterization of Pectin
Pectin is a valuable component with a wide range of uses including food, cosmetic, and medicine.28 Therefore, pectin that was solubilized in hot water during HD was extracted with a yield of 3.19 ± 0.03 g/g OP. On the other hand, the yield of pectin directly extracted from orange juice waste was around 13–14 g/100 g dry matter.49
While the DE of extracted pectin was found as 54.25% analogous with the study of Saberian et al.,49 the quality of pectin was evaluated as high with a DE greater than 50%. On the other hand, Fidalgo et al.’s28 work resulted in the DE of orange pectin as 35–36% which is lower than the current study. Since the DE impacts the gelling ability of pectin, high methoxyl pectin (DE > 50%) is preferred in the food industry.23 Thus, extracted pectin has the potential to be highly evaluated in various prospective studies. Besides the DE, pectin should include a certain amount of GalA, and the GalA content present in the pectin was 22.06 ± 1.72%. For the polymer to be considered commercial pectin, it must contain at least 65% GalA.23 Although the result obtained is different than expected, it is stated that the GalA content is also affected by various extraction conditions.48 Also, while the DE of OP was between 1.7 and 37.5%, which is lower than the current study, the GalA content was 71.0% under optimum conditions, with a DE of 1.5%. From these results, it can be understood that DE was not related with the extraction yield.51
In Figure 2, the FT-IR spectra of produced orange pectin and commercial pectin are shown. FTIR analysis was used to assess the structural characteristics of produced pectin. Pectin can be classified into high-methoxylated pectins (HMPs, DM > 50%) and low-methoxylated pectins (LMPs, DM < 50%). The wavelength range of 950–1200 cm– 1, referred to as the carbohydrate fingerprint zone, offers important information about the existence of key functional groups in polysaccharides.117 The results show that this study effectively extract pectins from OP powder, when compared with commercial pectin FTIR, due to similar spectra. The only difference is the intensity of the peaks, similar to Du et al.38 studies. The characteristic peaks of pectin at 1748 and 1630 cm–1 can also be used to distinguish whether the pectin is HMP or LMP. The pectin obtained in this experiment was weakly absorbed at 1748 cm–1 and strongly absorbed at 1630 cm–1, so the produced pectin was specified as LMP, which was also proved by the methoxylated determination of pectin by titration, similar to the study conducted by Qi et al.118 that investigated the citrus pectin. The GalA methyl esters’ CH, CH2, and CH3 lengths are responsible for the absorbance at about 2900 cm–1. The peak at 1740 cm–1 is the C=O stretch observed in the ester and derived from the acetyl (COCH3) group. The bands at 1380–1445 cm–1 denote the existence of CH3 groups, while the peak at 1630 cm–1 is associated with the OH tensile vibration band. The bands at 1015–1100 cm–1 belong to C–O bending or stretching.119 It has been observed that while the produced OP pectin has characteristic 1630 and 1748 cm–1 peaks, it also includes GalA methyl ester bonds. The peaks between 1200 and 900 cm–1 represent prominent and distinctive bands for glucose, fructose, and sucrose120 which are also seen in the OP pectin. Eventually, it was concluded that the peaks were found compatible with the range in the literature to compare with other produced citrus pectin.24,49,118
Figure 2.
FT-IR spectra of orange and commercial pectin.
The data confirm that orange pectin is comparable to commercial pectin. Pectin is particularly ideal for usage in the gelly and candy sector due to its high degree of methylation.
Moreover, high methoxy pectin is primarily used to stabilize specific types of sour milk products. From the point of view, produced pectin may be used on confectionery and dairy-based food products and various citrus peel pectins have been studied for their role as components in food-packaging films.117,121
4. Conclusions
The effects of extraction conditions for EO (water-to-solid ratio and HD time) as well as for flavonoids (solvent-to-solid ratio and extraction time) were thoroughly investigated to maximize yield and d-limonene concentration for the former and higher bioactivity of selected flavonoids for the latter. Ultrasonication improved both bioactivity and flavonoid concentrations when applied in the extraction of flavonoids on the remaining solids after EO extraction (OP-HD). UAE is recognized as a quick, cost-effective, environmentally friendly, and efficient way to extract flavonoid fractions from orange juice waste. Both the EO and flavonoid fractions had moderate to high bioactivity. Finally, pectin that was solubilized in hot water during HD was extracted by ethanol precipitation and characterized. Therefore, this study suggests that orange juice waste is a promising candidate for achieving the recovery of value-added d-limonene, natural antioxidants, and pectin.
Overall, this work reports an environmentally friendly approach for valorization of industrial orange waste to produce several components that would be of interest for food, pharmaceutical, and cosmetic industries. Considering the extraction process of orange juice waste from a different angle, it will be interesting to look into factors such as temperature, frequency of sonication, and solvent mixture that are important on an industrial scale and need additional study and development. Future studies might focus on investigating the application of these bioactive compounds to produce biomaterials for the drug, food, and cosmetic industry. Moreover, the safety of the compounds based on orange waste should be investigated. Through the reduction and utilization of fruit waste byproducts that are now being placed in landfills, the sequential extraction of these compounds could help in the transformation of the agricultural industry from a linear economy to a circular economy.
Acknowledgments
This work was supported by Istanbul Technical University Scientific Research Projects Coordination Department (MDA-2022-43603). The graphical abstract was created by BIORENDER (biorender.com).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.4c00112.
HPLC chromatogram of d-limonene at 200 nm (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- FAOSTAT . Crops and livestock products. https://www.fao.org/faostat/en/#data/QCL/visualize (accessed February 8, 2023).
- Ben Hsouna A.; Sadaka C.; Generalić Mekinić I.; Garzoli S.; Švarc-Gajić J.; Rodrigues F.; Morais S.; Moreira M. M.; Ferreira E.; Spigno G.; Brezo-Borjan T.; Akacha B. B.; Saad R. B.; Delerue-Matos C.; Mnif W. The Chemical Variability, Nutraceutical Value, and Food-Industry and Cosmetic Applications of Citrus Plants: A Critical Review. Antioxidants 2023, 12 (2), 481. 10.3390/antiox12020481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satari B.; Karimi K. Citrus Processing Wastes: Environmental Impacts, Recent Advances, and Future Perspectives in Total Valorization. Resour., Conserv. Recycl. 2018, 129, 153–167. 10.1016/j.resconrec.2017.10.032. [DOI] [Google Scholar]
- Sharma K.; Mahato N.; Lee Y. R. Extraction, Characterization and Biological Activity of Citrus Flavonoids. Rev. Chem. Eng. 2019, 35 (2), 265–284. 10.1515/revce-2017-0027. [DOI] [Google Scholar]
- Andrianou C.; Passadis K.; Malamis D.; Moustakas K.; Mai S.; Barampouti E. M. Upcycled Animal Feed: Sustainable Solution to Orange Peels Waste. Sustainability 2023, 15 (3), 2033. 10.3390/su15032033. [DOI] [Google Scholar]
- Bora H.; Kamle M.; Mahato D. K.; Tiwari P.; Kumar P. Citrus Essential Oils (CEOs) and Their Applications in Food: An Overview. Plants 2020, 9 (3), 357. 10.3390/plants9030357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shehata M. G.; Awad T. S.; Asker D.; El Sohaimy S. A.; Abd El-Aziz N. M.; Youssef M. M. Antioxidant and Antimicrobial Activities and UPLC-ESI-MS/MS Polyphenolic Profile of Sweet Orange Peel Extracts. Curr. Res. Food Sci. 2021, 4, 326–335. 10.1016/j.crfs.2021.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh P. X.; Hong Tran N. T.; Thuy Ly D. T.; Nguyen T. N.; Dang Le T. Physiochemical Properties, Antibacterial, Antifungal, and Antioxidant Activities of Essential Oils from Orange (Citrus Nobilis) Peel. Emir. J. Food Agric. 2022, 34, 289–296. 10.9755/ejfa.2022.v34.i4.2843. [DOI] [Google Scholar]
- Radünz M.; Mota Camargo T.; Santos Hackbart H. C. D.; Inchauspe Correa Alves P.; Radünz A. L.; Avila Gandra E.; Da Rosa Zavareze E. Chemical Composition and in Vitro Antioxidant and Antihyperglycemic Activities of Clove, Thyme, Oregano, and Sweet Orange Essential Oils. LWT 2021, 138, 110632. 10.1016/j.lwt.2020.110632. [DOI] [Google Scholar]
- Heydari Koochi Z.; Jahromi K. G.; Kavoosi G.; Babaei S. Citrus Peel Waste Essential Oil: Chemical Composition along with Anti-amylase and Anti-glucosidase Potential. Int. J. Food Sci. Technol. 2022, 57 (10), 6795–6804. 10.1111/ijfs.16031. [DOI] [Google Scholar]
- Yu X.; Lin H.; Wang Y.; Lv W.; Zhang S.; Qian Y.; Deng X.; Feng N.; Yu H.; Qian B. D-Limonene Exhibits Antitumor Activity by Inducing Autophagy and Apoptosis in Lung Cancer. OncoTargets Ther. 2018, 11, 1833–1847. 10.2147/OTT.S155716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Torre I.; Martin-Dominguez V.; Acedos M. G.; Esteban J.; Santos V. E.; Ladero M. Utilisation/Upgrading of Orange Peel Waste from a Biological Biorefinery Perspective. Appl. Microbiol. Biotechnol. 2019, 103 (15), 5975–5991. 10.1007/s00253-019-09929-2. [DOI] [PubMed] [Google Scholar]
- Puri M.; Verma M. L.; Mahale K.. Processing of Citrus Peel for the Extraction of Flavonoids for Biotechnological Applications. Handbook on Flavonoids: Dietary Sources, Properties and Health Benefits; Nova Science Publishers: Hauppauge, NY, 2012, pp 443–459. [Google Scholar]
- Montenegro-Landívar M. F.; Tapia-Quirós P.; Vecino X.; Reig M.; Valderrama C.; Granados M.; Cortina J. L.; Saurina J. Recovery of Added-Value Compounds from Orange and Spinach Processing Residues: Green Extraction of Phenolic Compounds and Evaluation of Antioxidant Activity. Antioxidants 2021, 10 (11), 1800. 10.3390/antiox10111800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samota M. K.; Kaur M.; Sharma M.; Sarita; Krishnan V.; Thakur J.; Rawat M.; Phogat B.; Guru P. N. Hesperidin from Citrus Peel Waste: Extraction and Its Health Implications. Qual. Assur. Saf. Crops Foods 2023, 15 (2), 71–99. 10.15586/qas.v15i2.1256. [DOI] [Google Scholar]
- Tripoli E.; Guardia M. L.; Giammanco S.; Majo D. D.; Giammanco M. Citrus Flavonoids: Molecular Structure, Biological Activity and Nutritional Properties: A Review. Food Chem. 2007, 104 (2), 466–479. 10.1016/j.foodchem.2006.11.054. [DOI] [Google Scholar]
- Kumar S.; Konwar J.; Purkayastha M. D.; Kalita S.; Mukherjee A.; Dutta J. Current Progress in Valorization of Food Processing Waste and By-Products for Pectin Extraction. Int. J. Biol. Macromol. 2023, 239, 124332. 10.1016/j.ijbiomac.2023.124332. [DOI] [PubMed] [Google Scholar]
- Rojas L. F.; Zapata P.; Ruiz-Tirado L. Agro-Industrial Waste Enzymes: Perspectives in Circular Economy. Curr. Opin. Green Sustainable Chem. 2022, 34, 100585. 10.1016/j.cogsc.2021.100585. [DOI] [Google Scholar]
- Christofi A.; Tsipiras D.; Malamis D.; Moustakas K.; Mai S.; Barampouti E. M. Biofuels Production from Orange Juice Industrial Waste within a Circular Economy Vision. J. Water Process Eng. 2022, 49, 103028. 10.1016/j.jwpe.2022.103028. [DOI] [Google Scholar]
- Anticona M.; Blesa J.; Frigola A.; Esteve M. J. High Biological Value Compounds Extraction from Citrus Waste with Non-Conventional Methods. Foods 2020, 9 (6), 811. 10.3390/foods9060811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panwar D.; Panesar P. S.; Chopra H. K. Recent Trends on the Valorization Strategies for the Management of Citrus By-Products. Food Rev. Int. 2021, 37 (1), 91–120. 10.1080/87559129.2019.1695834. [DOI] [Google Scholar]
- de Souza C. G.; Rodrigues T. H.; e Silva L. M.; Ribeiro P. R.; de Brito E. S. Sequential Extraction of Flavonoids and Pectin from Yellow Passion Fruit Rind Using Pressurized Solvent or Ultrasound. J. Sci. Food Agric. 2018, 98 (4), 1362–1368. 10.1002/jsfa.8601. [DOI] [PubMed] [Google Scholar]
- Guandalini B. B. V.; Rodrigues N. P.; Marczak L. D. F. Sequential Extraction of Phenolics and Pectin from Mango Peel Assisted by Ultrasound. Food Res. Int. 2019, 119, 455–461. 10.1016/j.foodres.2018.12.011. [DOI] [PubMed] [Google Scholar]
- Hosseini S.; Parastouei K.; Khodaiyan F. Simultaneous Extraction Optimization and Characterization of Pectin and Phenolics from Sour Cherry Pomace. Int. J. Biol. Macromol. 2020, 158, 911–921. 10.1016/j.ijbiomac.2020.04.241. [DOI] [PubMed] [Google Scholar]
- Teigiserova D. A.; Tiruta-Barna L.; Ahmadi A.; Hamelin L.; Thomsen M. A Step Closer to Circular Bioeconomy for Citrus Peel Waste: A Review of Yields and Technologies for Sustainable Management of Essential Oils. J. Environ. Manage. 2021, 280, 111832. 10.1016/j.jenvman.2020.111832. [DOI] [PubMed] [Google Scholar]
- Patsalou M.; Chrysargyris A.; Tzortzakis N.; Koutinas M. A Biorefinery for Conversion of Citrus Peel Waste into Essential Oils, Pectin, Fertilizer and Succinic Acid via Different Fermentation Strategies. Waste Manage. 2020, 113, 469–477. 10.1016/j.wasman.2020.06.020. [DOI] [PubMed] [Google Scholar]
- Boukroufa M.; Boutekedjiret C.; Petigny L.; Rakotomanomana N.; Chemat F. Bio-Refinery of Orange Peels Waste: A New Concept Based on Integrated Green and Solvent Free Extraction Processes Using Ultrasound and Microwave Techniques to Obtain Essential Oil, Polyphenols and Pectin. Ultrason. Sonochem. 2015, 24, 72–79. 10.1016/j.ultsonch.2014.11.015. [DOI] [PubMed] [Google Scholar]
- Fidalgo A.; Ciriminna R.; Carnaroglio D.; Tamburino A.; Cravotto G.; Grillo G.; Ilharco L. M.; Pagliaro M. Eco-Friendly Extraction of Pectin and Essential Oils from Orange and Lemon Peels. ACS Sustain. Chem. Eng. 2016, 4 (4), 2243–2251. 10.1021/acssuschemeng.5b01716. [DOI] [Google Scholar]
- Ortiz-Sanchez M.; Solarte-Toro J. C.; Orrego-Alzate C. E.; Acosta-Medina C. D.; Cardona-Alzate C. A. Integral Use of Orange Peel Waste through the Biorefinery Concept: An Experimental, Technical, Energy, and Economic Assessment. Biomass Convers. Biorefin. 2021, 11 (2), 645–659. 10.1007/s13399-020-00627-y. [DOI] [Google Scholar]
- Tsouko E.; Maina S.; Ladakis D.; Kookos I. K.; Koutinas A. Integrated Biorefinery Development for the Extraction of Value-Added Components and Bacterial Cellulose Production from Orange Peel Waste Streams. Renewable Energy 2020, 160, 944–954. 10.1016/j.renene.2020.05.108. [DOI] [Google Scholar]
- Tunç M. T.; Odabaş H. İ. Single-Step Recovery of Pectin and Essential Oil from Lemon Waste by Ohmic Heating Assisted Extraction/Hydrodistillation: A Multi-Response Optimization Study. Innovative Food Sci. Emerging Technol. 2021, 74, 102850. 10.1016/j.ifset.2021.102850. [DOI] [Google Scholar]
- Zimare S. B.; Mankar G. D.; Barmukh R. B. Optimization of Ultrasound-Assisted Extraction of Total Phenolics and Flavonoids from the Leaves of Lobelia Nicotianifolia and Their Radical Scavenging Potential. Curr. Res. Green Sustainable Chem. 2021, 4, 100109. 10.1016/j.crgsc.2021.100109. [DOI] [Google Scholar]
- Tamminen J.; Holappa J.; Vladimirovich Gradov D.; Koiranen T. Scaling up Continuous Ultrasound-Assisted Extractor for Plant Extracts by Using Spinach Leaves as a Test Material. Ultrason. Sonochem. 2022, 90, 106171. 10.1016/j.ultsonch.2022.106171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petigny L.; Périno-Issartier S.; Wajsman J.; Chemat F. Batch and Continuous Ultrasound Assisted Extraction of Boldo Leaves (Peumus Boldus Mol.). Int. J. Mol. Sci. 2013, 14 (3), 5750–5764. 10.3390/ijms14035750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clodoveo M. L.; Moramarco V.; Paduano A.; Sacchi R.; Di Palmo T.; Crupi P.; Corbo F.; Pesce V.; Distaso E.; Tamburrano P.; Amirante R. Engineering Design and Prototype Development of a Full Scale Ultrasound System for Virgin Olive Oil by Means of Numerical and Experimental Analysis. Ultrason. Sonochem. 2017, 37, 169–181. 10.1016/j.ultsonch.2017.01.004. [DOI] [PubMed] [Google Scholar]
- Khavari M.; Priyadarshi A.; Subroto T.; Beckwith C.; Pericleous K.; Eskin D. G.; Tzanakis I. Scale up Design Study on Process Vessel Dimensions for Ultrasonic Processing of Water and Liquid Aluminium. Ultrason. Sonochem. 2021, 76, 105647. 10.1016/j.ultsonch.2021.105647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z.; Mei X.; Chen X.; Rao S.; Ju T.; Li J.; Yang Z. Extraction and Recovery of Bioactive Soluble Phenolic Compounds from Brocade Orange (Citrus Sinensis) Peels: Effect of Different Extraction Methods Thereon. LWT 2023, 173, 114337. 10.1016/j.lwt.2022.114337. [DOI] [Google Scholar]
- Du Y.; Zhang S.; Waterhouse G. I.; Zhou T.; Xu F.; Wang R.; Sun-Waterhouse D.; Wu P. High-Intensity Pulsed Electric Field-Assisted Acidic Extraction of Pectin from Citrus Peel: Physicochemical Characteristics and Emulsifying Properties. Food Hydrocolloids 2024, 146, 109291. 10.1016/j.foodhyd.2023.109291. [DOI] [Google Scholar]
- Khandare R. D.; Tomke P. D.; Rathod V. K. Kinetic Modeling and Process Intensification of Ultrasound-Assisted Extraction of d-Limonene Using Citrus Industry Waste. Chem. Eng. Process. 2021, 159, 108181. 10.1016/j.cep.2020.108181. [DOI] [Google Scholar]
- Yue Y.; Wang B.; Xi W.; Liu X.; Tang S.; Tan X.; Li G.; Huang L.; Liu Y.; Bai J. Modification Methods, Biological Activities and Applications of Pectin: A Review. Int. J. Biol. Macromol. 2023, 253, 127523. 10.1016/j.ijbiomac.2023.127523. [DOI] [PubMed] [Google Scholar]
- Le X. D.; Pham Thi N. M.; Cam T. I.; Do H. N.; Nguyen Thi H. V.; Thang T. D.; Thao L. P. P.; Do T. S.; Nguyen T. D.; Pham Q. L.; Dao T. P.; Pham T. N.; Tran Q. T. Optimization of the Essential Oil Extraction Process from Dong Van Marjoram (E. Winitiana Var. Dongvanensis Phuong.) by Using Microwave Assisted Hydrodistillation, and the Bioactivities of the Oil Against Some Cancer Cell Lines and Bacteria. Nat. Prod. Commun. 2021, 16 (10), 1934578X2110542. 10.1177/1934578X211054235. [DOI] [Google Scholar]
- Teshale F.; Narendiran K.; Beyan S. M.; Srinivasan N. R. Extraction of Essential Oil from Rosemary Leaves: Optimization by Response Surface Methodology and Mathematical Modeling. Appl. Food Res. 2022, 2 (2), 100133. 10.1016/j.afres.2022.100133. [DOI] [Google Scholar]
- Park S. M.; Ko K. Y.; Kim I. H. Optimization of d-limonene Extraction from Tangerine Peel in Various Solvents by Using Soxhlet Extractor. Korean Chem. Eng. Res. 2015, 53 (6), 717–722. 10.9713/KCER.2015.53.6.717. [DOI] [Google Scholar]
- Sembiring E. N.; Elya B.; Sauriasari R. Phytochemical Screening, Total Flavonoid and Total Phenolic Content and Antioxidant Activity of Different Parts of Caesalpinia Bonduc (L.) Roxb. Pharmacogn. J. 2017, 10 (1), 123–127. 10.5530/pj.2018.1.22. [DOI] [Google Scholar]
- Sirichan T.; Kijpatanasilp I.; Asadatorn N.; Assatarakul K. Optimization of Ultrasound Extraction of Functional Compound from Makiang Seed by Response Surface Methodology and Antimicrobial Activity of Optimized Extract with Its Application in Orange Juice. Ultrason. Sonochem. 2022, 83, 105916. 10.1016/j.ultsonch.2022.105916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stabrauskiene J.; Marksa M.; Ivanauskas L.; Bernatoniene J. Optimization of Naringin and Naringenin Extraction from Citrus × paradisi L. Using Hydrolysis and Excipients as Adsorbent. Pharmaceutics 2022, 14 (5), 890. 10.3390/pharmaceutics14050890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D.-S.; Lim S.-B. Extraction of Flavanones from Immature Citrus Unshiu Pomace: Process Optimization and Antioxidant Evaluation. Sci. Rep. 2020, 10 (1), 19950. 10.1038/s41598-020-76965-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira T. Í. S.; Rosa M. F.; Cavalcante F. L.; Pereira P. H. F.; Moates G. K.; Wellner N.; Mazzetto S. E.; Waldron K. W.; Azeredo H. M. C. Optimization of Pectin Extraction from Banana Peels with Citric Acid by Using Response Surface Methodology. Food Chem. 2016, 198, 113–118. 10.1016/j.foodchem.2015.08.080. [DOI] [PubMed] [Google Scholar]
- Saberian H.; Hamidi-Esfahani Z.; Ahmadi Gavlighi H.; Barzegar M. Optimization of Pectin Extraction from Orange Juice Waste Assisted by Ohmic Heating. Chem. Eng. Process. 2017, 117, 154–161. 10.1016/j.cep.2017.03.025. [DOI] [Google Scholar]
- Blumenkrantz N.; Asboe-Hansen G. New Method for Quantitative Determination of Uronic Acids. Anal. Biochem. 1973, 54 (2), 484–489. 10.1016/0003-2697(73)90377-1. [DOI] [PubMed] [Google Scholar]
- Hosseini S. S.; Khodaiyan F.; Yarmand M. S. Optimization of Microwave Assisted Extraction of Pectin from Sour Orange Peel and Its Physicochemical Properties. Carbohydr. Polym. 2016, 140, 59–65. 10.1016/j.carbpol.2015.12.051. [DOI] [PubMed] [Google Scholar]
- Mutlu-Ingok A.; Catalkaya G.; Capanoglu E.; Karbancioglu-Guler F. Antioxidant and Antimicrobial Activities of Fennel, Ginger, Oregano and Thyme Essential Oils. Food Front. 2021, 2 (4), 508–518. 10.1002/fft2.77. [DOI] [Google Scholar]
- Spanos G. A.; Wrolstad R. E. Influence of Processing and Storage on the Phenolic Composition of Thompson Seedless Grape Juice. J. Agric. Food Chem. 1990, 38 (7), 1565–1571. 10.1021/jf00097a030. [DOI] [Google Scholar]
- Singleton V. L.; Rossi J. A. Jr. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16 (3), 144–158. 10.5344/ajev.1965.16.3.144. [DOI] [Google Scholar]
- Zhishen J.; Mengcheng T.; Jianming W. The Determination of Flavonoid Contents in Mulberry and Their Scavenging Effects on Superoxide Radicals. Food Chem. 1999, 64, 555–559. 10.1016/S0308-8146(98)00102-2. [DOI] [Google Scholar]
- Kumaran A.; Joel Karunakaran R. Antioxidant and Free Radical Scavenging Activity of an Aqueous Extract of Coleus Aromaticus. Food Chem. 2006, 97 (1), 109–114. 10.1016/j.foodchem.2005.03.032. [DOI] [Google Scholar]
- Apak R.; Güçlü K.; Özyürek M.; Karademir S. E. Novel Total Antioxidant Capacity Index for Dietary Polyphenols and Vitamins C and E, Using Their Cupric Ion Reducing Capability in the Presence of Neocuproine: CUPRAC Method. J. Agric. Food Chem. 2004, 52 (26), 7970–7981. 10.1021/jf048741x. [DOI] [PubMed] [Google Scholar]
- Chahbi A.; Nassik S.; El Amri H.; Douaik A.; El Maadoudi E. H.; Boukharta M.; El Hadrami E. M. Chemical Composition and Antimicrobial Activity of the Essential Oils of Two Aromatic Plants Cultivated in Morocco (Cinnamomum Cassia and Origanum Compactum). J. Chem. 2020, 2020, 1–10. 10.1155/2020/1628710. [DOI] [Google Scholar]
- Foerster S.; Desilvestro V.; Hathaway L. J.; Althaus C. L.; Unemo M. A New Rapid Resazurin-Based Microdilution Assay for Antimicrobial Susceptibility Testing of Neisseria Gonorrhoeae. J. Antimicrob. Chemother. 2017, 72 (7), 1961–1968. 10.1093/jac/dkx113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou H.-S.; Bonku E. M.; Zhai R.; Zeng R.; Hou Y.-L.; Yang Z.-H.; Quan C. Extraction of Essential Oil from Citrus Reticulate Blanco Peel and Its Antibacterial Activity against Cutibacterium Acnes (Formerly Propionibacterium Acnes). Heliyon 2019, 5 (12), e02947 10.1016/j.heliyon.2019.e02947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohagheghniapour A.; Saharkhiz M. J.; Golmakani M. T.; Niakousari M. Variations in Chemical Compositions of Essential Oil from Sour Orange (Citrus Aurantium L.) Blossoms by Different Isolation Methods. Sustainable Chem. Pharm. 2018, 10, 118–124. 10.1016/j.scp.2018.10.008. [DOI] [Google Scholar]
- Bourgou S.; Rahali F. Z.; Ourghemmi I.; Saïdani Tounsi M. Changes of Peel Essential Oil Composition of Four Tunisian Citrus during Fruit Maturation. Sci. World J. 2012, 2012, 1–10. 10.1100/2012/528593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visakh N. U.; Pathrose B.; Narayanankutty A.; Alfarhan A.; Ramesh V. Utilization of Pomelo (Citrus Maxima) Peel Waste into Bioactive Essential Oils: Chemical Composition and Insecticidal Properties. Insects 2022, 13 (5), 480. 10.3390/insects13050480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y.; Wu J.; Xu Y.; Fu M.; Xiao G. Effect of Second Cooling on the Chemical Components of Essential Oils from Orange Peel (Citrus Sinensis). J. Agric. Food Chem. 2014, 62 (35), 8786–8790. 10.1021/jf501079r. [DOI] [PubMed] [Google Scholar]
- González-Rivera J.; Spepi A.; Ferrari C.; Duce C.; Longo I.; Falconieri D.; Piras A.; Tinè M. R. Novel Configurations for a Citrus Waste Based Biorefinery: From Solventless to Simultaneous Ultrasound and Microwave Assisted Extraction. Green Chem. 2016, 18 (24), 6482–6492. 10.1039/C6GC02200F. [DOI] [Google Scholar]
- Lemes R. S.; Alves C. C. F.; Estevam E. B. B.; Santiago M. B.; Martins C. H. G.; Santos T. C. D.; Crotti A. E. M.; Miranda M. L. D. Chemical Composition and Antibacterial Activity of Essential Oils from Citrus Aurantifolia Leaves and Fruit Peel against Oral Pathogenic Bacteria. An. Acad. Bras. Ciênc. 2018, 90 (2), 1285–1292. 10.1590/0001-3765201820170847. [DOI] [PubMed] [Google Scholar]
- Wei L.; Yu X.; Li H.; Zhu M.; Pu D.; Lu Q.; Bao Y.; Zu Y. Optimization of Solvent-Free Microwave Extraction of Essential Oil from the Fresh Peel of Citrus Medica L. Var. Arcodactylis Swingle by Response Surface Methodology, Chemical Composition and Activity Characterization. Sci. Hortic. 2023, 309, 111663. 10.1016/j.scienta.2022.111663. [DOI] [Google Scholar]
- D’Amato S.; Serio A.; López C. C.; Paparella A. Hydrosols: Biological Activity and Potential as Antimicrobials for Food Applications. Food Control 2018, 86, 126–137. 10.1016/j.foodcont.2017.10.030. [DOI] [Google Scholar]
- de Araújo J. S. F.; De Souza E. L.; Oliveira J. R.; Gomes A. C. A.; Kotzebue L. R. V.; Da Silva Agostini D. L.; De Oliveira D. L. V.; Mazzetto S. E.; Da Silva A. L.; Cavalcanti M. T. Microencapsulation of Sweet Orange Essential Oil (Citrus Aurantium Var. Dulcis) by Liophylization Using Maltodextrin and Maltodextrin/Gelatin Mixtures: Preparation, Characterization, Antimicrobial and Antioxidant Activities. Int. J. Biol. Macromol. 2020, 143, 991–999. 10.1016/j.ijbiomac.2019.09.160. [DOI] [PubMed] [Google Scholar]
- Guo Q.; Liu K.; Deng W.; Zhong B.; Yang W.; Chun J. Chemical Composition and Antimicrobial Activity of Gannan Navel Orange (Citrus Sinensis Osbeck Cv. Newhall) Peel Essential Oils. Food Sci. Nutr. 2018, 6 (6), 1431–1437. 10.1002/fsn3.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H.; Lou Z.; Chen X.; Cui Y.; Wang H.; Kou X.; Ma C. Effect of Simultaneous Ultrasonic and Microwave Assisted Hydrodistillation on the Yield, Composition, Antibacterial and Antibiofilm Activity of Essential Oils from Citrus Medica L. Var. Sarcodactylis. J. Food Eng. 2019, 244, 126–135. 10.1016/j.jfoodeng.2018.09.014. [DOI] [Google Scholar]
- Durling N.; Catchpole O.; Grey J.; Webby R.; Mitchell K.; Foo L.; Perry N. Extraction of Phenolics and Essential Oil from Dried Sage (Salvia Officinalis) Using Ethanol-Water Mixtures. Food Chem. 2007, 101 (4), 1417–1424. 10.1016/j.foodchem.2006.03.050. [DOI] [Google Scholar]
- Ozturk B.; Winterburn J.; Gonzalez-Miquel M. Orange Peel Waste Valorisation through Limonene Extraction Using Bio-Based Solvents. Biochem. Eng. J. 2019, 151, 107298. 10.1016/j.bej.2019.107298. [DOI] [Google Scholar]
- Dao T. P.; Tran N. Q.; Tran T. T.; Lam V. T. Assessing the Kinetic Model on Extraction of Essential Oil and Chemical Composition from Lemon Peels (Citrus Aurantifolia) by Hydro-Distillation Process. Mater. Today: Proc. 2022, 51, 172–177. 10.1016/j.matpr.2021.05.069. [DOI] [Google Scholar]
- Halim N. A. A.; Abidin Z. Z.; Siajam S. I.; Hean C. G.; Harun M. R. Optimization Studies and Compositional Analysis of Subcritical Water Extraction of Essential Oil from Citrus Hystrix DC. Leaves. J. Supercrit. Fluids 2021, 178, 105384. 10.1016/j.supflu.2021.105384. [DOI] [Google Scholar]
- Santos L. G.; Martins V. G. Optimization of the Green Extraction of Polyphenols from the Edible Flower Clitoria Ternatea by High-Power Ultrasound: A Comparative Study with Conventional Extraction Techniques. J. Appl. Res. Med. Aromat. Plants 2023, 34, 100458. 10.1016/j.jarmap.2023.100458. [DOI] [Google Scholar]
- Golmohamadi A.; Möller G.; Powers J.; Nindo C. Effect of Ultrasound Frequency on Antioxidant Activity, Total Phenolic and Anthocyanin Content of Red Raspberry Puree. Ultrason. Sonochem. 2013, 20 (5), 1316–1323. 10.1016/j.ultsonch.2013.01.020. [DOI] [PubMed] [Google Scholar]
- Cheigh C.-I.; Chung E.-Y.; Chung M.-S. Enhanced Extraction of Flavanones Hesperidin and Narirutin from Citrus Unshiu Peel Using Subcritical Water. J. Food Eng. 2012, 110 (3), 472–477. 10.1016/j.jfoodeng.2011.12.019. [DOI] [Google Scholar]
- Garcia-Castello E. M.; Rodriguez-Lopez A. D.; Mayor L.; Ballesteros R.; Conidi C.; Cassano A. Optimization of Conventional and Ultrasound Assisted Extraction of Flavonoids from Grapefruit (Citrus Paradisi L.) Solid Wastes. LWT—Food Sci. Technol. 2015, 64 (2), 1114–1122. 10.1016/j.lwt.2015.07.024. [DOI] [Google Scholar]
- Khan M. K.; Abert-Vian M.; Fabiano-Tixier A.-S.; Dangles O.; Chemat F. Ultrasound-Assisted Extraction of Polyphenols (Flavanone Glycosides) from Orange (Citrus Sinensis L.) Peel. Food Chem. 2010, 119 (2), 851–858. 10.1016/j.foodchem.2009.08.046. [DOI] [Google Scholar]
- Meregalli M. M.; Puton B. M. S.; Camera F. D.; Amaral A. U.; Zeni J.; Cansian R. L.; Mignoni M. L.; Backes G. T. Conventional and Ultrasound-Assisted Methods for Extraction of Bioactive Compounds from Red Araçá Peel (Psidium Cattleianum Sabine). Arabian J. Chem. 2020, 13 (6), 5800–5809. 10.1016/j.arabjc.2020.04.017. [DOI] [Google Scholar]
- Nayak B.; Dahmoune F.; Moussi K.; Remini H.; Dairi S.; Aoun O.; Khodir M. Comparison of Microwave, Ultrasound and Accelerated-Assisted Solvent Extraction for Recovery of Polyphenols from Citrus Sinensis Peels. Food Chem. 2015, 187, 507–516. 10.1016/j.foodchem.2015.04.081. [DOI] [PubMed] [Google Scholar]
- Nishad J.; Saha S.; Dubey A. K.; Varghese E.; Kaur C. Optimization and Comparison of Non-Conventional Extraction Technologies for Citrus Paradisi L. Peels: A Valorization Approach. J. Food Sci. Technol. 2019, 56 (3), 1221–1233. 10.1007/s13197-019-03585-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinatoru M.; Mason T. J.; Calinescu I. Ultrasonically Assisted Extraction (UAE) and Microwave Assisted Extraction (MAE) of Functional Compounds from Plant Materials. TrAC, Trends Anal. Chem. 2017, 97, 159–178. 10.1016/j.trac.2017.09.002. [DOI] [Google Scholar]
- M’hiri N.; Ioannou I.; Mihoubi Boudhrioua N.; Ghoul M. Effect of Different Operating Conditions on the Extraction of Phenolic Compounds in Orange Peel. Food Bioprod. Process. 2015, 96, 161–170. 10.1016/j.fbp.2015.07.010. [DOI] [Google Scholar]
- Wang Z.; Chen X.; Guo Z.; Feng X.; Huang P.; Du M.; Zalán Z.; Kan J. Distribution and Natural Variation of Free, Esterified, Glycosylated, and Insoluble-Bound Phenolic Compounds in Brocade Orange (Citrus Sinensis L. Osbeck) Peel. Food Res. Int. 2022, 153, 110958. 10.1016/j.foodres.2022.110958. [DOI] [PubMed] [Google Scholar]
- Selahvarzi A.; Ramezan Y.; Sanjabi M. R.; Namdar B.; Akbarmivehie M.; Mirsaeedghazi H.; Azarikia F. Optimization of Ultrasonic-Assisted Extraction of Phenolic Compounds from Pomegranate and Orange Peels and Their Antioxidant Activity in a Functional Drink. Food Biosci. 2022, 49, 101918. 10.1016/j.fbio.2022.101918. [DOI] [Google Scholar]
- Chen X.-M.; Tait A. R.; Kitts D. D. Flavonoid Composition of Orange Peel and Its Association with Antioxidant and Anti-Inflammatory Activities. Food Chem. 2017, 218, 15–21. 10.1016/j.foodchem.2016.09.016. [DOI] [PubMed] [Google Scholar]
- Dalmau E.; Rosselló C.; Eim V.; Ratti C.; Simal S. Ultrasound-Assisted Aqueous Extraction of Biocompounds from Orange Byproduct: Experimental Kinetics and Modeling. Antioxidants 2020, 9 (4), 352. 10.3390/antiox9040352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesias-Carres L.; Mas-Capdevila A.; Bravo F. I.; Aragonès G.; Muguerza B.; Arola-Arnal A. Optimization of a Polyphenol Extraction Method for Sweet Orange Pulp (Citrus Sinensis L.) to Identify Phenolic Compounds Consumed from Sweet Oranges. PLoS One 2019, 14 (1), e0211267 10.1371/journal.pone.0211267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agcam E.; Akyıldız A.; Akdemir Evrendilek G. Comparison of Phenolic Compounds of Orange Juice Processed by Pulsed Electric Fields (PEF) and Conventional Thermal Pasteurisation. Food Chem. 2014, 143, 354–361. 10.1016/j.foodchem.2013.07.115. [DOI] [PubMed] [Google Scholar]
- Wang Y.-C.; Chuang Y.-C.; Hsu H.-W. The Flavonoid, Carotenoid and Pectin Content in Peels of Citrus Cultivated in Taiwan. Food Chem. 2008, 106 (1), 277–284. 10.1016/j.foodchem.2007.05.086. [DOI] [Google Scholar]
- Chatzimitakos T.; Athanasiadis V.; Mantiniotou M.; Kalompatsios D.; Bozinou E.; Giovanoudis I.; Lalas S. I. Exploring the Feasibility of Cloud-Point Extraction for Bioactive Compound Recovery from Food Byproducts: A Review. Biomass 2023, 3 (3), 306–322. 10.3390/biomass3030019. [DOI] [Google Scholar]
- Restrepo-Serna D. L.; Cardona Alzate C. A. Economic Pre-Feasibility of Supercritical Fluid Extraction of Antioxidants from Fruit Residues. Sustainable Chem. Pharm. 2022, 25, 100600. 10.1016/j.scp.2022.100600. [DOI] [Google Scholar]
- Prado J. M.; Veggi P. C.; Meireles M. A. A.. Scale-Up Issues and Cost of Manufacturing Bioactive Compounds by Supercritical Fluid Extraction and Ultrasound Assisted Extraction. In Global Food Security and Wellness; Barbosa-Cánovas G. V., María Pastore G., Candoğan K., Medina Meza I. G., Caetano da Silva Lannes S., Buckle K., Yada R. Y., Rosenthal A., Eds.; Springer: New York, NY, 2017; pp 377–433. [Google Scholar]
- Usman I.; Hussain M.; Imran A.; Afzaal M.; Saeed F.; Javed M.; Afzal A.; Ashfaq I.; Al Jbawi E.; A. Saewan S. Traditional and Innovative Approaches for the Extraction of Bioactive Compounds. Int. J. Food Prop. 2022, 25 (1), 1215–1233. 10.1080/10942912.2022.2074030. [DOI] [Google Scholar]
- Caballero E.; Soto C.. Valorization of Agro-Industrial Waste into Bioactive Compounds: Techno-Economic Considerations. In Biorefinery: Integrated Sustainable Processes for Biomass Conversion to Biomaterials, Biofuels, and Fertilizers; Bastidas-Oyanedel J.-R., Schmidt J. E., Eds.; Springer International Publishing: Cham, 2019; pp 235–252. [Google Scholar]
- Patist A.; Bates D.. Industrial Applications of High Power Ultrasonics. In Ultrasound Technologies for Food and Bioprocessing; Feng H., Barbosa-Canovas G., Weiss J., Eds.; Food Engineering Series; Springer: New York, NY, 2011; pp 599–616. [Google Scholar]
- Panzella L.; Moccia F.; Nasti R.; Marzorati S.; Verotta L.; Napolitano A. Bioactive Phenolic Compounds From Agri-Food Wastes: An Update on Green and Sustainable Extraction Methodologies. Front. Nutr. 2020, 7, 60. 10.3389/fnut.2020.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Değirmenci H.; Erkurt H. Relationship between Volatile Components, Antimicrobial and Antioxidant Properties of the Essential Oil, Hydrosol and Extracts of Citrus Aurantium L. Flowers. J. Infect. Public Health 2020, 13 (1), 58–67. 10.1016/j.jiph.2019.06.017. [DOI] [PubMed] [Google Scholar]
- Raspo M. A.; Vignola M. B.; Andreatta A. E.; Juliani H. R. Antioxidant and Antimicrobial Activities of Citrus Essential Oils from Argentina and the United States. Food Biosci. 2020, 36, 100651. 10.1016/j.fbio.2020.100651. [DOI] [Google Scholar]
- Tunnisa F.; Nur Faridah D.; Afriyanti A.; Rosalina D.; Ana Syabana M.; Darmawan N.; Dewi Yuliana N. Antioxidant and Antidiabetic Compounds Identification in Several Indonesian Underutilized Zingiberaceae Spices Using SPME-GC/MS-Based Volatilomics and in Silico Methods. Food Chem.: X 2022, 14, 100285. 10.1016/j.fochx.2022.100285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzur M.; Luciardi M. C.; Blázquez M. A.; Alberto M. R.; Cartagena E.; Arena M. E. Citrus Sinensis Essential Oils an Innovative Antioxidant and Antipathogenic Dual Strategy in Food Preservation against Spoliage Bacteria. Antioxidants 2023, 12 (2), 246. 10.3390/antiox12020246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tundis R.; Loizzo M. R.; Bonesi M.; Menichini F.; Mastellone V.; Colica C.; Menichini F. Comparative Study on the Antioxidant Capacity and Cholinesterase Inhibitory Activity of Citrus Aurantifolia Swingle, C. Aurantium L., and C. Bergamia Risso and Poit. Peel Essential Oils. J. Food Sci. 2012, 77 (1), H40–H46. 10.1111/j.1750-3841.2011.02511.x. [DOI] [PubMed] [Google Scholar]
- Yang C.; Chen H.; Chen H.; Zhong B.; Luo X.; Chun J. Antioxidant and Anticancer Activities of Essential Oil from Gannan Navel Orange Peel. Molecules 2017, 22 (8), 1391. 10.3390/molecules22081391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabri Karoui I.; Marzouk B. Characterization of Bioactive Compounds in Tunisian Bitter Orange (Citrus Aurantium L.) Peel and Juice and Determination of Their Antioxidant Activities. BioMed Res. Int. 2013, 2013, 1–12. 10.1155/2013/345415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao N.; Liu Y.; Zhang M. Chemical Composition and Antimicrobial Activities of Essential Oil from the Peel of Bingtang Sweet Orange (Citrus Sinensis Osbeck). Int. J. Food Sci. Technol. 2009, 44 (7), 1281–1285. 10.1111/j.1365-2621.2009.01947.x. [DOI] [Google Scholar]
- Torres-Alvarez C.; Núñez González A.; Rodríguez J.; Castillo S.; Leos-Rivas C.; Báez-González J. G. Chemical Composition, Antimicrobial, and Antioxidant Activities of Orange Essential Oil and Its Concentrated Oils. CyTA—J. Food 2017, 15 (1), 129–135. 10.1080/19476337.2016.1220021. [DOI] [Google Scholar]
- Geraci A.; Di Stefano V.; Di Martino E.; Schillaci D.; Schicchi R. Essential Oil Components of Orange Peels and Antimicrobial Activity. Nat. Prod. Res. 2017, 31 (6), 653–659. 10.1080/14786419.2016.1219860. [DOI] [PubMed] [Google Scholar]
- Oikeh E. I.; Oviasogie F. E.; Omoregie E. S. Quantitative Phytochemical Analysis and Antimicrobial Activities of Fresh and Dry Ethanol Extracts of Citrus Sinensis (L.) Osbeck (Sweet Orange) Peels. Clin. Phytosci. 2020, 6 (1), 46. 10.1186/s40816-020-00193-w. [DOI] [Google Scholar]
- Górniak I.; Bartoszewski R.; Króliczewski J. Comprehensive Review of Antimicrobial Activities of Plant Flavonoids. Phytochem. Rev. 2019, 18 (1), 241–272. 10.1007/s11101-018-9591-z. [DOI] [Google Scholar]
- Halevas E.; Matsia S.; Hatzidimitriou A.; Geromichalou E.; Papadopoulos T. A.; Katsipis G.; Pantazaki A.; Litsardakis G.; Salifoglou A. A Unique Ternary Ce(III)-Quercetin-Phenanthroline Assembly with Antioxidant and Anti-Inflammatory Properties. J. Inorg. Biochem. 2022, 235, 111947. 10.1016/j.jinorgbio.2022.111947. [DOI] [PubMed] [Google Scholar]
- Biharee A.; Sharma A.; Kumar A.; Jaitak V. Antimicrobial Flavonoids as a Potential Substitute for Overcoming Antimicrobial Resistance. Fitoterapia 2020, 146, 104720. 10.1016/j.fitote.2020.104720. [DOI] [PubMed] [Google Scholar]
- Mandalari G.; Bennett R. N.; Bisignano G.; Trombetta D.; Saija A.; Faulds C. B.; Gasson M. J.; Narbad A. Antimicrobial Activity of Flavonoids Extracted from Bergamot (Citrus Bergamia Risso) Peel, a Byproduct of the Essential Oil Industry. J. Appl. Microbiol. 2007, 103 (6), 2056–2064. 10.1111/j.1365-2672.2007.03456.x. [DOI] [PubMed] [Google Scholar]
- Adamczak A.; Ożarowski M.; Karpiński T. M. Antibacterial Activity of Some Flavonoids and Organic Acids Widely Distributed in Plants. J. Clin. Med. 2019, 9 (1), 109. 10.3390/jcm9010109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y.; Li T.; Bai J.; Nong L.; Ning Z.; Hu Z.; Xu A.; Xu C.-P. Chemical Composition and Antibacterial Activity of the Essential Oil of Citrus Maxima (Burm.) Merr. Cv. Shatian Yu. J. Biol. Act. Prod. Nat. 2018, 8 (4), 228–233. 10.1080/22311866.2018.1509730. [DOI] [Google Scholar]
- Said N. S.; Olawuyi I. F.; Cho H.-S.; Lee W.-Y. Novel Edible Films Fabricated with HG-Type Pectin Extracted from Different Types of Hybrid Citrus Peels: Effects of Pectin Composition on Film Properties. Int. J. Biol. Macromol. 2023, 253, 127238. 10.1016/j.ijbiomac.2023.127238. [DOI] [PubMed] [Google Scholar]
- Qi T.; Ren J.; Li X.; An Q.; Zhang N.; Jia X.; Pan S.; Fan G.; Zhang Z.; Wu K. Structural Characteristics and Gel Properties of Pectin from Citrus Physiological Premature Fruit Drop. Carbohydr. Polym. 2023, 309, 120682. 10.1016/j.carbpol.2023.120682. [DOI] [PubMed] [Google Scholar]
- Güzel M.; Akpınar O. Valorisation of Fruit By-Products: Production Characterization of Pectins from Fruit Peels. Food Bioprod. Process. 2019, 115, 126–133. 10.1016/j.fbp.2019.03.009. [DOI] [Google Scholar]
- Florinela F.; Abdelmoumen T.; Carmen S. Application Of FTIR Spectroscopy For A Rapid Determination Of Some Hydrolytic Enzymes Activity On Sea Buckthorn Substrate. Rom. Biotechnol. Lett. 2010, 15 (6), 5738–5744. [Google Scholar]
- Singhal S.; Swami Hulle N. R. Citrus Pectins: Structural Properties, Extraction Methods, Modifications and Applications in Food Systems - A Review. Appl. Food Res. 2022, 2 (2), 100215. 10.1016/j.afres.2022.100215. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.




