Abstract
Sleep difficulties may be one explanatory factor in the association between depression and insulin resistance; yet, explicit tests of this hypothesis are lacking. We determined if there was an indirect effect of depression symptoms on insulin resistance through sleep duration in adolescents at risk for excess weight gain. We also investigated whether dispositional mindfulness moderated the interconnections among depression, sleep, and insulin resistance. Ninety adolescents (14.2 ± 1.6y; 50% female) at risk for excess weight gain (body mass index [BMI, kg/m2] z score 1.6 ± 0.6) participated in the cross-sectional, baseline phase of a health behaviors study. Depression was assessed with the Center for Epidemiologic Studies–Depression Scale, sleep duration with the Sleep Habits Survey, and mindfulness with the Mindful Attention and Awareness Scale. Homeostatic model assessment of insulin resistance was determined from fasting insulin and glucose. The product-of-coefficients method was used to test the indirect effect of depression on insulin resistance through sleep duration, accounting for age, sex, BMIz, puberty, and socioeconomic status (SES). Dispositional mindfulness was tested as a moderator of the associations among depression, sleep, and insulin resistance. There was a significant indirect effect of depression on insulin resistance through sleep duration, controlling for age, sex, BMIz, puberty, and SES, 95%CI [0.001, 0.05]. Dispositional mindfulness moderated the association between sleep duration and insulin resistance, such that lower sleep duration related to greater insulin resistance only among adolescents with lower mindfulness (p < .001). Short sleep may be one explanatory factor in the depression-insulin resistance connection in adolescents at risk for excess weight gain. Adolescents with poorer mindfulness and short sleep are at highest risk for insulin resistance, whereas higher mindfulness may be protective.
Keywords: Adolescents, Depression, Sleep, Mindfulness, Insulin resistance
Introduction
Adolescents experience higher levels of depression symptoms and poorer sleep health relative to childhood (Maslowsky & Ozer, 2014). Developmental changes in depression and sleep have been attributed to the pubertal transition, in combination with a host of social and psychological stressors of the adolescent period (Maslowsky & Ozer, 2014). Depression and sleep are interconnected. Short sleep duration, < 8 hours per night in adolescence (Hirshkowitz et al., 2015), is one symptom of major depressive disorder (American Psychiatric Association, 2013), and even in the absence of a mood disorder, adolescents with elevated depression symptoms experience shorter sleep than those with no or low depression symptoms (Lovato & Gradisar, 2014). Depression symptoms (Hannon et al., 2014; Shomaker et al., 2011) and shorter sleep duration (Flint et al., 2007; Javaheri et al., 2011; Matthews et al., 2012; Simon et al., 2019) each have been related to higher insulin resistance and increases in insulin resistance over time, consequently heightening adolescents’ risk for developing type 2 diabetes.
One possible explanation is that depression symptoms worsen insulin resistance through their effect on sleep. From this framework, depression symptoms are hypothesized to impair adequate sleep duration, which in turn worsens insulin resistance (Holt et al., 2014). The sleep-wake cycle plays a role in modulating insulin resistance and secretion in complex ways. From a physiological perspective, short sleep duration alters the functioning of the hypothalamic-pituitary-adrenocortical (HPA) axis, the critical neuroendocrine system that, in conjunction with the autonomic nervous system, governs the body’s peripheral physiologic response to stress, resulting in elevated daily cortisol output (Dutil & Chaput, 2017; Reutrakul & Van Cauter, 2018). More elevated cortisol, in turn, has been associated with higher insulin resistance (Misra et al., 2008) and worsening of insulin resistance over time (Adam et al., 2010), even after adjusting for body mass index (BMI; kg/m2) or adiposity. Short sleep duration also has been proposed to amplify sympathetic nervous symptom activity, which becomes heightened in response to short sleep, and leads to dysregulation of appetitive hormones (e.g., leptin) and disturbance of appetite/satiety regulation, which can increase insulin resistance (Dutil & Chaput, 2017).
Existing literature provides support for the connection of depression and shorter sleep duration in adolescents (Lovato & Gradisar, 2014), and in separate studies, for the connection of shorter sleep duration and insulin resistance (Flint et al., 2007; Javaheri et al., 2011; Matthews et al., 2012; Simon et al., 2019). Yet, to our knowledge, no study has investigated the indirect effect of depression symptoms on insulin resistance through sleep duration in an integrated model. Understanding the interconnections of depression, sleep, and insulin resistance in adolescents is important, because it offers the potential to inform preventative approaches for type 2 diabetes. Thus, the first aim of the current project was to evaluate the extent to which sleep duration underlies the association between depression symptoms and insulin resistance in adolescents. We hypothesized that sleep duration would be a significant intervening variable explaining the association of adolescent depression symptoms with insulin resistance.
Further, we sought to characterize the potential moderating role of general, dispositional mindfulness in the interconnections among depression, sleep duration, and insulin resistance. Dispositional mindfulness refers to a propensity to purposefully pay attention to the present moment with an attitude of non-judgment or equanimity (Kabat-Zinn, 1991). From a biopsychosocial theoretical lens, enhanced monitoring of thoughts, emotions, and body sensations as they unfold moment-to-moment is theorized to protect or buffer individuals against stress exposure through biological, psychological, and behavioral mechanisms (Lazarus & Folkman, 1984). Higher dispositional mindfulness could be anticipated to decrease insulin resistance through its effect on lowering depression symptoms, but mindfulness also may directly improve awareness of and changes to health-related behaviors, like sleep, and underlying stress-related physiology (Turner & Hingle, 2017). Another possibility is that mindfulness acts directly on reducing stress and ameliorating stress physiology (Epel et al., 2009). Mindfulness can be trained and has been proposed to be most valuable for improved physical health among populations with high stress (Creswell et al., 2019). Interventions focused on increasing mindfulness have been efficacious for decreasing depression symptoms in adolescents in the short-term, as compared to active comparisons (Dunning et al., 2019). Also, pilot studies suggest that mindfulness-based interventions may decrease depression symptoms and ameliorate insulin resistance in adolescents at risk for type 2 diabetes (Shomaker et al., 2017, 2019). The mindfulness stress-buffering hypothesis purports that individuals with higher dispositional mindfulness have lower stress reactivity and cope more effectively in the face of stress (Creswell & Lindsay, 2014). However, there have been minimal tests of this concept in adolescents.
To address this gap, we tested dispositional mindfulness as a moderator of the associations of depression-insulin resistance, depression-sleep duration, and sleep durationinsulin resistance in order to delineate where exactly mindfulness may modify the proposed interconnections among these constructs. In line with biopsychosocial theory (Lazarus & Folkman, 1984) and the stress buffering hypothesis (Creswell & Lindsay, 2014), we predicted that each of these associations would be stronger at lower, or poorer, levels of dispositional mindfulness and conversely, that the associations would be weaker at higher, or more positive, levels of mindfulness.
Methods
Participants
The current study represents a cross-sectional, secondary analysis of adolescent females and males ages 12–17 years taking part in the baseline phase of a study on health behaviors and attitudes in teenagers; a portion (60%) went on to participate in a pilot study of a group mindfulness-based intervention (ClinicalTrials.gov ID; Shomaker et al., 2019). Volunteers were recruited via letters to area families, newspaper and radio advertisements, community-based informational sessions, community emails, and school/physician office flyers. Inclusion criteria were: (1) age 12–17 years, (2) risk for adult obesity, determined as adolescent BMI ≥ 70th percentile for age/sex or two biological parents with BMI ≥ 30 kg/m2 (Field et al., 2005), and (3) good general health, as reported by parents. Exclusion criteria included: (1) a major medical condition such as type 1 or type 2 diabetes, (2) a psychiatric disorder that was likely to interfere with study compliance, as determined by semi-structured diagnostic psychological interview, (3) medication use such as anti-depressants, stimulants, or insulin sensitizers that could influence mood, weight, or eating behavior, and (4) pregnancy (females).
Procedures
After an initial phone screen to evaluate study eligibility, adolescents and their parent/guardian attended a laboratory screening visit to provide written informed consent/assent, determine eligibility, and collect assessments. Participants were instructed to fast starting at 10:00 pm the night prior to the visit. Adolescents were financially compensated for their time. All procedures were reviewed and approved by Institutional Review Board.
Measures
Body measurements
Height (cm) was measured three times without shoes to the nearest 0.1 cm using a stadiometer and averaged. A calibrated digital scale was used to measure weight (kg) to the nearest 0.1 kg in a fasted state and wearing light, indoor clothing. Height and weight were used to calculate BMI (kg/m2), BMI standard score (BMIz), and BMI percentile based on CDC 2000 growth charts (Kuczmarski et al., 2002).
Depression symptoms
The 20-item Center for Epidemiologic Studies-Depression Scale (CES-D) is a widely-used continuous measure of depression symptoms experienced over the past week (Radloff, 1977). Each item is scored on a Likert scale from “0” (rarely or none of the time; less than 1 day) to “3” (all of the time; 5–7 days). The total score is calculated as the sum of all items, with higher values indicative of greater depression symptomatology (Radloff, 1977). The CES-D total score is reliable and validated in adolescent samples (Phillips et al., 2006) and showed acceptable internal reliability (α = 0.72) in the current study.
Sleep duration
Sleep duration was derived from two items on the Sleep Habits Survey (SHS; Wolfson & Carskadon, 1998) to estimate weekday sleep duration. Adolescents reported on their weekday bedtime and weekday waketime. Sleep duration in hours was calculated as the difference between the self-recorded bedtime and waketime. The SHS has demonstrated reasonable correspondence with objective sleep measurements in adolescents (Wolfson et al., 2003).
Dispositional mindfulness
The Mindful Attention and Awareness Scale (MAAS) is a 15-item questionnaire of general, dispositional mindfulness (Brown & Ryan, 2003), referring to how one assesses their present-moment experiences in everyday activities. In a systematic review, the MAAS demonstrated reliability and validity in adolescent samples (Pallozzi et al., 2017). Items are rated on a Likert-scale from “1” (almost always) to “6” (almost never) with questions such as “I find it difficult to stay focused on what’s happening in the present.” The total score is the average of all items, with higher scores indicating relatively higher, or more positive, dispositional mindfulness. In the current study, the MAAS showed good internal reliability (α = 0.92).
Insulin resistance
A trained phlebotomist collected a fasting blood sample from an antecubital vein into a blood tube containing EDTA. A STAT 2300 Plus Glucose Lactate Analyzer (Yellow Springs Instruments, Yellow Springs, OH) was used to immediately analyze in duplicate the glucose concentrations in whole blood. The tubes were then centrifuged at 2500 rpm for 12 minutes, and plasma was removed and transferred to plastic bullet tubes which were frozen at −70 degrees Celsius until the insulin assays were completed. Radioimmunoassay was used to analyze plasma insulin (Millipore, Billerica, Massachusetts). Insulin resistance was estimated with the homeostatic model assessment of insulin resistance (HOMA-IR), calculated as (fasting insulin [μU/mL] X fasting glucose [mmol/L])/22.5, which shows reasonable correspondence with clamp-derived measures in adolescents who are lean and those with overweight/obesity (George et al., 2011; Gungor et al., 2004).
Puberty
Tanner stage was collected through adolescents’ self-report on a validated questionnaire (Morris & Udry, 1980). Participants were asked to define their physical development based on illustrations of their external characteristics, which corresponded to Tanner stages 1–5 of breast development for girls and Tanner stages 1–5 of pubic hair development for boys.
Socioeconomic status
Parents reported educational attainment, which was categorized as less than college degree or college degree or higher (Braveman et al., 2005). Self-reported educational attainment has been used as an indicator of socioeconomic status (SES), and has been associated with other area-based SES indicators (Berkowitz et al., 2015; Braveman et al., 2005).
Data analysis
Data were analyzed using SPSS for Windows, version 25 (IBM Corporation, Armonk, NY, USA). Multiple imputation with five imputed datasets and aggregated pooled estimates was used to handle systematically missing data (3% of all data). To address the first study objective, the indirect association of depression symptoms and insulin resistance through sleep duration was tested with the PROCESS SPSS macro by Hayes (v3.0; 2018), which uses a product-of-coefficients approach with 5,000 bias-corrected bootstrapped estimates to assess the significance of the indirect association or intervening variable (Preacher & Hayes, 2004). Sex, age, BMIz score, puberty, and SES were included as covariates, due to their known relationships with depression, sleep duration, and insulin resistance and to ensure that results were independent of these variables. A simple mediation model (PROCESS Model 4; Fig. 1) was used to examine the indirect association (ab-path), which represents the portion of the total association between depression symptoms and insulin resistance that occurs through sleep duration. As the indirect association (ab-path) is a product of the individual a- and b-paths, the indirect effect can still be significant even if one of the individual paths does not reach statistical significance (Hayes, 2018). Following standard procedures (Hayes, 2009), we also report the a-path, representing the association between depression symptoms and sleep duration; the b-path, reflecting the association between sleep duration and insulin resistance; the c’-path, representing the direct association between depression symptoms and insulin resistance, independent of the association that occurs through sleep duration; and the c-path, which is the overall, total association between depression symptoms and insulin resistance that combines the direct and indirect associations.
Fig. 1.
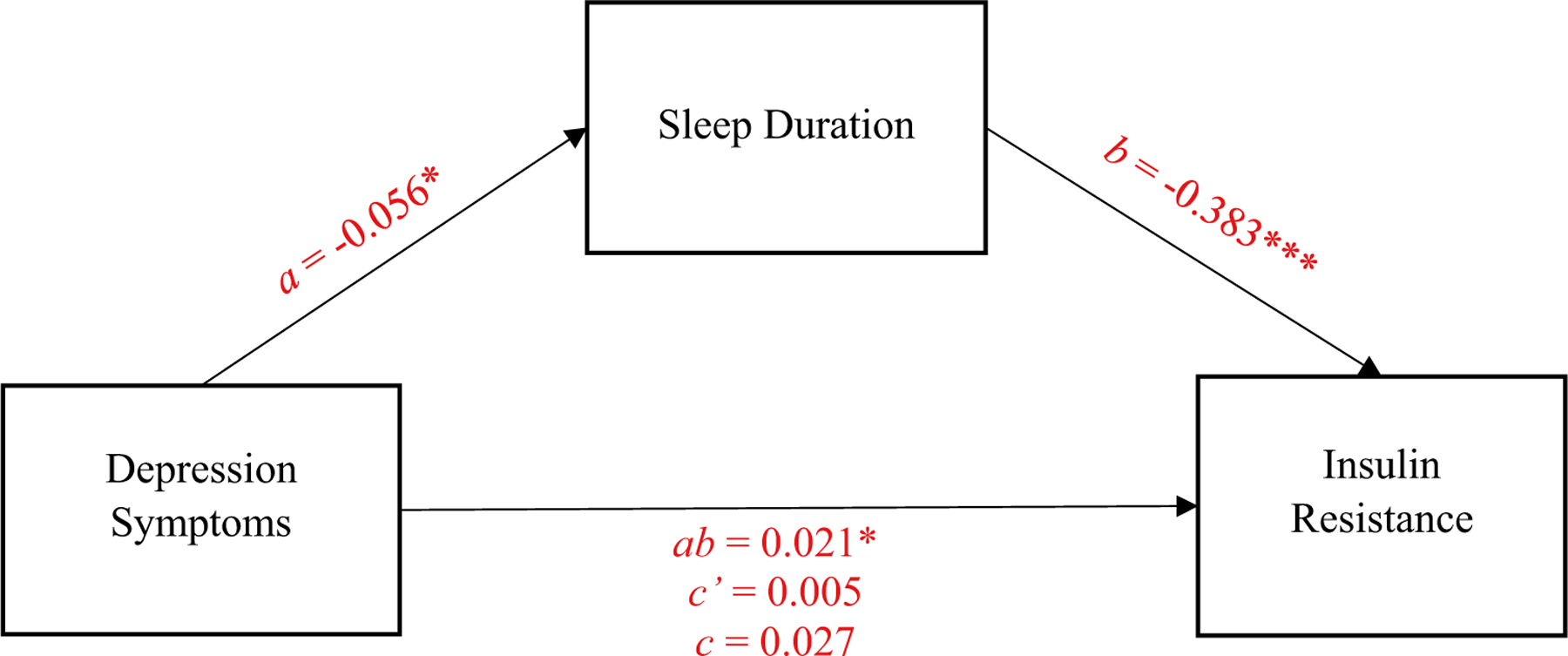
Path model of the mediating role of sleep duration in the relationship between depression insulin resistance. Values presented are unstandardized beta coefficients. ***p < 0.001. *p < 0.05.
To address the second study objective, moderation models (PROCESS Model 1; Fig. 2) were used to test dispositional mindfulness as a moderator of each of the associations modeled in the first aim. We examined moderation for these associations separately, as opposed to using a single moderated indirect effect model, in order to pinpoint which associations were affected by mindfulness level and due to sample size constraints of modeling indirect effects and multiple moderation simultaneously. We tested whether dispositional mindfulness moderated the association between depression symptoms and sleep duration (the “a-path” in the original simple mediation model), the association between sleep duration and insulin resistance (“b-path”), and finally, the association of depression symptoms and insulin resistance (“c-path”). Following standard procedures, in order to interpret the meaning of any significant moderation findings, we examined conditional associations at low (−1 SD below the mean), average (mean), and high (+ 1 SD above the mean) values of dispositional mindfulness (Preacher et al., 2007).
Fig. 2.
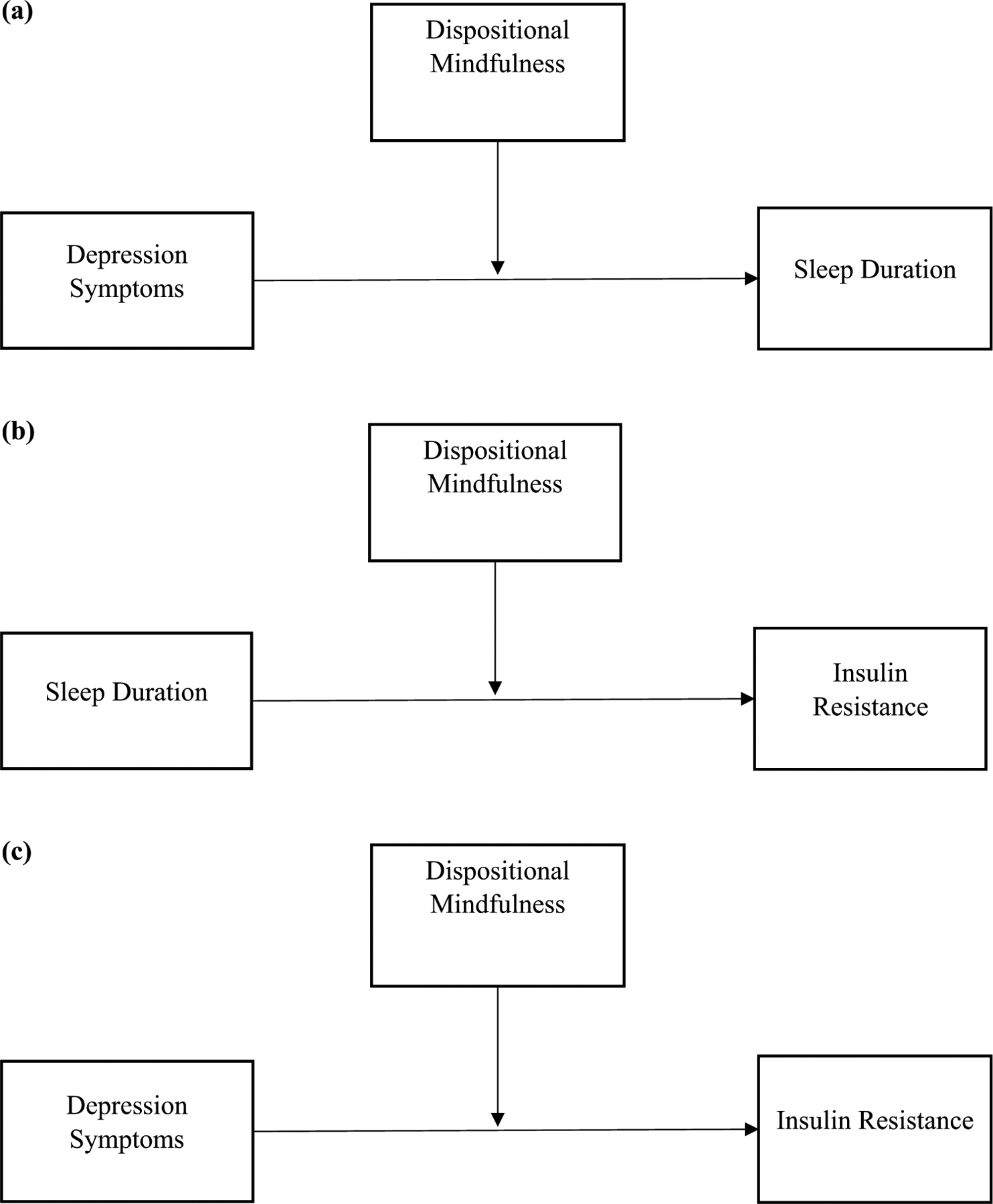
Path model of the moderating role of dispositional mindfulness on (a): depression symptoms-sleep duration, (b): sleep duration-insulin resistance, and (c): depression symptoms-insulin resistance
Results
Descriptive information
A total of N = 90 adolescents (50% female; M ± SD age 14.2 ± 1.6 years) participated in the study. Descriptive information on socio-demographic characteristics and key variables is provided in Table 1. Eighteen percent of the sample endorsed elevated depression symptoms (CES-D total score ≥ 21; Stockings et al., 2015) and 28% reported insufficient weekday sleep (< 8 hours; Hirshkowitz et al., 2015).
Table 1.
Descriptive information for the study sample
| Variable | Mean | SD | % |
|---|---|---|---|
| Age, years | 14.25 | 1.65 | – |
| BMI, kg/m2 | 27.63 | 4.88 | – |
| BMIz, standard score for age/sex | 1.64 | 0.57 | – |
| BMI, percentile for age/sex | 91.50 | 12.18 | – |
| Weight status | |||
| Lean (5–84th percentile) | – | – | 18 |
| Overweight (85–94th percentile) | – | – | 22 |
| Obesity (≥ 95th percentile) | – | – | 60 |
| Sex | |||
| Male | – | – | 50 |
| Female | – | – | 50 |
| Race/ethnicity | |||
| Non-Hispanic White | – | – | 67 |
| Hispanic | – | – | 30 |
| Asian | – | – | 2 |
| American Indian or Alaskan Native | – | – | 1 |
| Depression symptoms (CES-D total) | 12.58 | 7.36 | – |
| No or low depression symptoms | – | – | 82 |
| Elevated depression symptoms | – | – | 18 |
| Weekday sleep duration (hours) | 8.45 | 1.78 | – |
| < 8 hours | 28 | ||
| 8–10 hours | 67 | ||
| > 10 hours | 5 | ||
| Dispositional mindfulness (MAAS) | 4.15 | 1.05 | – |
| HOMA-IR | 2.44 | 1.73 | – |
N = 90; BMI is body mass index. CES-D is the 20-item Center for Epidemiologic Studies-Depression Scale; the total sum score has a possible range of 0 to 60, with higher values representing greater depression symptomatology (more negative valence) and elevated depressive symptoms referring to a total score ≥ 21. MAAS is the Mindful Attention Awareness Scale with possible scores of 1 to 6, with higher scores indicating more mindfulness (more positive valence). Weekday sleep duration is self-reported by adolescents and calculated from bedtime/waketime. HOMA-IR is the homeostatic model assessment of insulin resistance, with higher values representing greater insulin resistance (more negative valence).
Indirect effect of depression symptoms on insulin resistance through sleep duration
In the simple intervening variable model, the indirect association (ab-path) was significant, meaning that depression symptoms were related to insulin resistance, indirectly, through sleep duration (ab-path: B = 0.02; SE = 0.01; biascorrected bootstrapped 95% CI = [0.001, 0.05]), controlling for sex, age, BMIz score, puberty, and SES. Depression symptoms were inversely related to sleep duration (a-path: B = −0.06, SE = 0.02, 95% CI [−0.10, −0.01]), such that higher depression symptoms were associated with lower sleep duration, even after accounting for covariates. Sleep duration, in turn, was inversely related to insulin resistance (b-path: B = −0.38, SE = 0.10, 95% CI [−0.58, −0.19]), such that longer sleep duration was associated with lower insulin resistance, even after accounting for covariates. The direct association (c’-path) and the total association (c-path) were not significant.
Dispositional mindfulness as a moderator
A summary of results with dispositional mindfulness as a moderator is provided in Table 2. Dispositional mindfulness did not moderate the association between depression symptoms and sleep duration (the “a-path” in the original model). However, dispositional mindfulness did significantly moderate the association between sleep duration and insulin resistance (“b-path”; B = 0.19, SE = 0.08, p = 0.03), accounting for sex, age, BMIz, puberty, and SES. To probe the meaning of this interaction, we examined the conditional relationship between sleep duration and insulin resistance at different levels of dispositional mindfulness (Fig. 3). Sleep duration was inversely related to insulin resistance at relatively lower levels of mindfulness (conditional relationship at −1 SD; B = −0.41, SE = 0.10, 95% CI [−0.61, −0.21], p < 0.001). However, sleep duration was not related to insulin resistance at average levels of mindfulness (conditional relationship at M; B = −0.21, SE = 0.11, 95% CI [−0.44, 0.01], p = 0.06) nor at relatively higher levels of mindfulness (conditional relationship at + 1 SD; B = −0.01, SE = 0.18, 95% CI [−0.37, 0.34], p = 0.94). Dispositional mindfulness did not moderate the association between depression and insulin resistance (the “c-path” in the original model).
Table 2.
Conditional effects of mindfulness as a moderator
| Predictor | Outcome | B | SE | p | LL CI | UL CI |
|---|---|---|---|---|---|---|
| Depression | Sleep Duration | |||||
| − 1 SD | − 0.04 | 0.04 | .30 | − .11 | .04 | |
| M | − 0.03 | 0.03 | .41 | − .09 | .04 | |
| + 1 SD | − 0.01 | 0.04 | .78 | − .10 | .07 | |
| Sleep Duration | Insulin Resistance | |||||
| − 1 SD | − 0.41 | 0.10 | < .001 | − .61 | − .21 | |
| M | − 0.21 | 0.11 | .06 | − .44 | .01 | |
| + 1 SD | − 0.01 | 0.18 | .94 | − .37 | .34 | |
| Depression | Insulin Resistance | |||||
| − 1 SD | 0.01 | 0.04 | .88 | − .07 | .08 | |
| M | − 0.01 | 0.04 | .67 | − .07 | .05 | |
| + 1 SD | − 0.03 | 0.05 | .47 | − .11 | .05 |
N = 90 participants. LL CI = lower limit 95% confidence interval; UL CI = upper limit 95% confidence interval. Sex, age, BMIz (body mass index [BMI; k/gm2] standardized for age/sex), puberty (coded 0 = early/mid puberty/Tanner 1–4, 1 = late puberty/Tanner 5), and socioeconomic status (SES; coded 0 = parental educational attainment of less than college degree, 1 = college degree and above) were controlled for in the analyses.
Fig. 3.
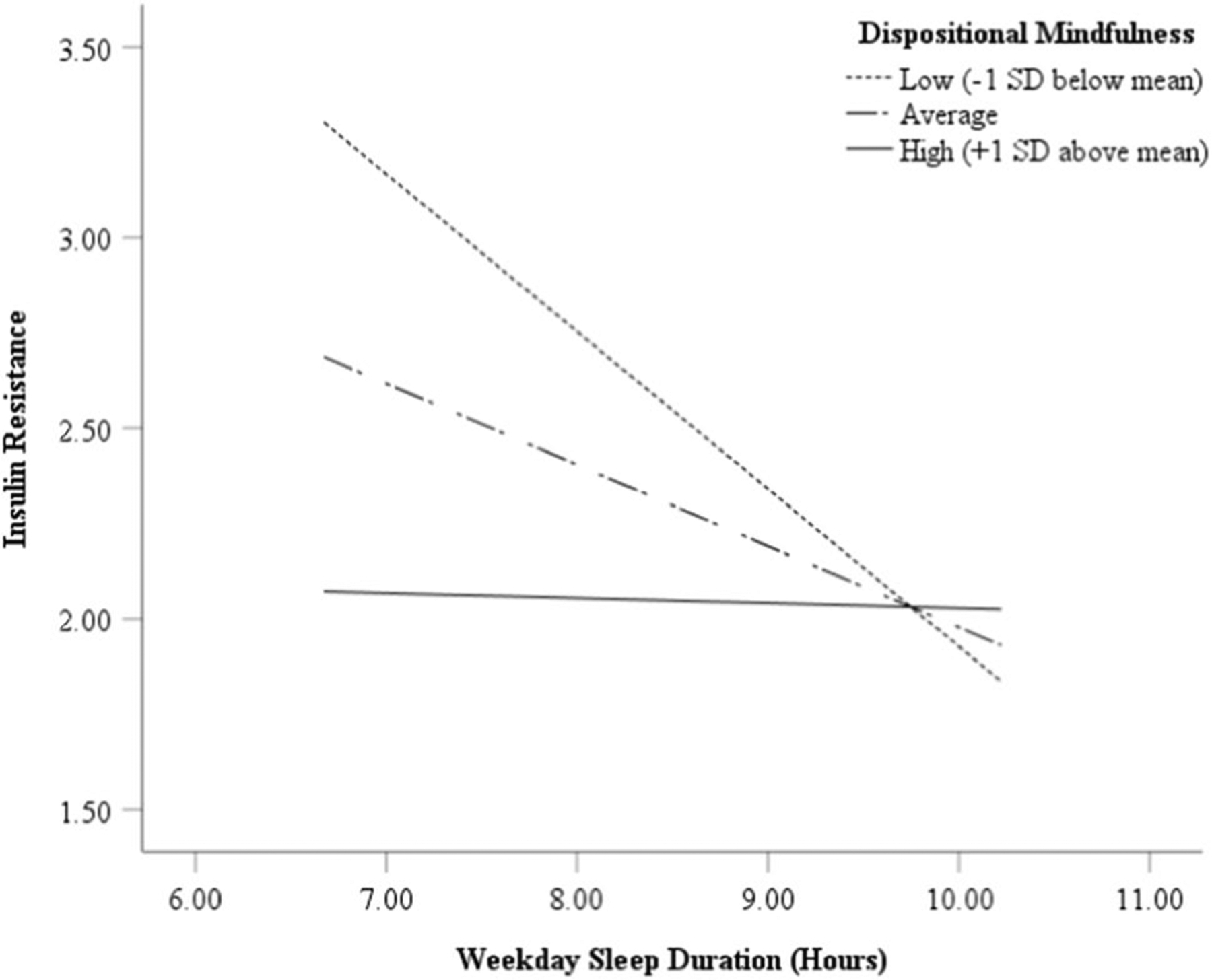
The moderating role of dispositional mindfulness in the association of adolescent self-reported weekday sleep duration with homeostasis model assessment of insulin resistance
Discussion
The results of the present study suggest that sleep duration may be an intervening variable in the relation of adolescent depression symptoms to insulin resistance. Further, adolescents’ dispositional mindfulness moderated the link between sleep and insulin resistance, such that lower sleep duration related to higher insulin resistance only among adolescents with lower mindfulness. In contrast, sleep duration was not related to insulin resistance for adolescents with average or higher mindfulness.
The current data add to the existing literature that has shown a significant association of depression with insulin resistance and heightened risk for type 2 diabetes in adults (Kan et al., 2013). These findings further add to a smaller body of literature suggesting a connection of depression symptoms with insulin resistance and type 2 diabetes in adolescents and young adults, independent of BMI or body composition (Hannon et al., 2014; Shomaker et al., 2011; Suglia et al., 2018). The mechanisms underlying the depression-insulin resistance connection are likely to be complex. Data from the current study are correlational and cross-sectional. Yet, these findings are suggestive that sleep duration could be one explanatory factor accounting for the association of depression with insulin resistance in adolescents. In the current study, the direct relationship between depression symptoms and sleep duration reached statistical significance, which is consistent with past literature (Lovato & Gradisar, 2014). For example, meta-analytic data suggest that adolescents with elevated depression symptoms experience shorter sleep duration and more sleep disturbances, including more wakefulness in bed, lighter sleep, longer sleep onset, more waking after sleep onset, and lower sleep efficiency, than adolescents with no or low depression symptoms (Lovato & Gradisar, 2014).
The current results also are in alignment with a growing body of literature indicating that shorter sleep duration is associated with higher insulin resistance in children and adolescents (Flint et al., 2007; Javaheri et al., 2011; Matthews et al., 2012; Simon et al., 2019). The association between sleep duration and insulin resistance could potentially be explained through stress-related hormones that influence insulin resistance, such as cortisol (Dutil & Chaput, 2017; Reutrakul & Van Cauter, 2018). Sleep affects a multitude of stress-related physiological factors, including the sympathetic nervous system, that affect appetite regulation (e.g., leptin) and metabolism, which also may be explanatory (Dutil & Chaput, 2017; Reutrakul & Van Cauter, 2018). For instance, children and adolescents with short sleep consume more carbohydrates from a laboratory test meal than those with adequate sleep, which may have downstream effects on insulin resistance (Mi et al., 2019). The interplay of adolescent sleep and eating behavior in relation to insulin resistance merits consideration in future research.
The indirect association of depression and insulin resistance through sleep duration adds value to our understanding of potentially modifiable risk factors for type 2 diabetes among at-risk adolescents. In future research, it will be important to both objectively characterize naturalistic sleep (e.g. actigraphy as opposed to relying on self-report) and to investigate additional dimensions of sleep behavior (e.g., sleep quality; Rawat et al., 2019), sleep physiology (e.g., sleep-disordered breathing), and circadian rhythms (e.g., misalignment between sleep time relative to the social clock; Leproult et al., 2014) that might be related to insulin resistance. Although sleep duration has been studied most extensively in relation to metabolic health, disentangling the unique associations of these various sleep health characteristics in relation to insulin resistance in adolescents would be valuable.
Additionally, our results indicated that the association of sleep duration and insulin resistance was moderated by dispositional mindfulness. Sleep duration was inversely related to insulin resistance when adolescents had relatively lower mindfulness. Conversely, consistent with the stress-buffering hypothesis (Creswell & Lindsay, 2014), among adolescents with average or relatively higher dispositional mindfulness, sleep duration did not relate to insulin resistance. One possible explanation is that if adolescents have average or greater dispositional mindfulness, compared to lower levels of mindfulness, they may be able to cope better with stressors, including the stress generated from insufficient sleep. In turn, more effective coping could mitigate the negative implications of insufficient sleep for insulin resistance (Dutil & Chaput, 2017; Matthews et al., 2016). In line with this explanation, adolescents with greater dispositional mindfulness have been shown to more frequently use effective coping skills (Lucas-Thompson et al., 2019; Metz et al., 2013) and to less frequently use maladaptive coping strategies such as emotional eating or overeating foods high in carbohydrate and fat, which promote insulin resistance (Pivarunas et al., 2015). In addition, greater dispositional mindfulness could directly translate into a more adaptive or positive physiological responding to stress (Lucas-Thompson et al., 2019), which also offers the potential to explain the protective associations with insulin resistance. The current data are consistent with studies demonstrating an inverse association of dispositional mindfulness with insulin resistance in adults (Loucks et al., 2016) and with better glycemic control in young adults with type 1 diabetes (Nagel et al., 2020).
The results of the current study may have potential implications for informing targeted, preventative interventions for worsening insulin resistance in adolescents. Cognitive-behavioral and mindfulness-based group interventions tailored to improve sleep in adolescents with elevated depression or anxiety symptoms have resulted in improved subjective sleep quality (Blake et al., 2017), and studies in young adults suggest that mindfulness-based interventions improve sleep quality and reduce pre-sleep arousal and daytime sleepiness (Bogusch et al., 2016; Caldwell et al., 2010; Howell et al., 2010). Therefore, it may be valuable to investigate the benefits of including sleep health intervention components within mindfulness-based interventions for insulin resistance, particularly in adolescents with elevated depression symptoms and/or sleep disturbance.
It is important to note that the current study was a secondary analysis with several limitations. The sample size was relatively small, which could have contributed to type II error. Although reflective of the demographics of the geographic location for recruitment, there was limited representation of adolescents from historically disadvantaged racial/ethnic groups who are most at risk for adult obesity and obesity-related comorbidities such as type 2 diabetes. Insulin resistance was estimated from fasting samples as opposed to repeated time sampling; repeated time sampling may yield more accurate estimation. Sleep duration was derived from adolescents’ self-reports rather than actigraphy, necessitating replication of the current results with objective sleep assessments. As such, our measurement of sleep duration might be better conceived as “sleep opportunity” or “time in bed.” Furthermore, as this was a cross-sectional study, we cannot determine causality nor rule out the influence of third variable effects. Future research using longitudinal data is needed to characterize the pathways among changes in depression symptoms, sleep, and insulin resistance, which would help us to better understand the complexity of these interconnected variables during adolescence. Moreover, the construct of mindfulness involves multiple components, including increased attention to thoughts, emotions, and behaviors, as well as self-acceptance or self-compassion. Understanding which facets of mindfulness may be most beneficial to possibly mitigating poor metabolic health outcomes will be important in future studies.
Taken together, the current results underscore the inter-connectedness of depression, sleep, and insulin resistance, and suggest a role for mindfulness in the association of sleep and insulin resistance in adolescents at risk for excess weight gain. Experimental studies are needed to determine if mindfulness-based training actually improves sleep and its downstream effects on risk for type 2 diabetes.
Funding for this work was provided by the Colorado Clinical and Translational Sciences Institute [NIH/NCATS Colorado CTSA Grant Number UL1TR002535] and the Colorado Agricultural Experiment Station [NIFA/USDA Grant Number COLO0724].
Funding
This work was supported by the Colorado Clinical and Translational Sciences Institute [NIH/NCATS Colorado CTSA Grant Number UL1 TR002535] and the Colorado Agricultural Experiment Station [NIFA/USDA Grant Number COLO0724]; efforts of MC and NS on this project were supported by graduate research assistantships from the Colorado School of Public Health.
Footnotes
Conflict of interest No author has a conflict of interest to disclose.
Ethical approval This study was approved by the Institutional Review Board (IRB) of Colorado State University. All study procedures were followed in accordance with the ethical standards of the Colorado State University IRB, and all parents/guardians and participants in the study provided written consent and assent, respectively.
References
- Adam TC, Hasson RE, Ventura EE, et al. (2010). Cortisol is negatively associated with insulin sensitivity in overweight Latino youth. Journal of Clinical Endocrinology and Metabolism, 95(10), 4729–4735. 10.1210/jc.2010-0322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). https://doi.org/ 10.1176/appi.books.9780890425596 [DOI] [Google Scholar]
- Berkowitz SA, Traore CY, Singer DE et al. (2015). Evaluating area-based socioeconomic status indicators for monitoring disparities within health care systems: Results from a primary care network. Health Services Research, 50(2), 398–417. 10.1111/1475-6773.12229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake MJ, Snoep L, Raniti M et al. (2017). A cognitive-behavioral and mindfulness-based group sleep intervention improves behavior problems in at-risk adolescents by improving perceived sleep quality. Behavior Research and Therapy, 99, 147–156. 10.1016/j.brat.2017.10.006 [DOI] [PubMed] [Google Scholar]
- Bogusch LM, Fekete EM, & Skinta MD (2016). Anxiety and depressive symptoms as mediators of trait mindfulness and sleep quality in emerging adults. Mindfulness, 7(4), 962–970. 10.1007/s12671-016-0535-7 [DOI] [Google Scholar]
- Braveman PA, Cubbin C, Egerter S et al. (2005). Socioeconomic status in health research: One size does not fit all. JAMA, 294(22), 2879–2888. 10.1001/jama.294.22.2879 [DOI] [PubMed] [Google Scholar]
- Brown KW, & Ryan RM (2003). The benefits of being present: Mindfulness and its role in psychological well-being. Journal of Personality and Social Psychology, 84(4), 822–848. 10.1037/0022-3514.84.4.822 [DOI] [PubMed] [Google Scholar]
- Caldwell K, Harrison M, Adams M et al. (2010). Developing mindfulness in college students through movement-based courses: Effects on self-regulatory self-efficacy, mood, stress, and sleep quality. Journal of American College Health, 58(5), 433–442. 10.1080/07448480903540481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creswell JD, & Lindsay EK (2014). How does mindfulness training affect health? A mindfulness stress buffering account. Current Directions in Psychological Science, 23(6), 401–407. 10.1177/0963721414547415 [DOI] [Google Scholar]
- Creswell JD, Lindsay EK, Villalba DK et al. (2019). Mindfulness training and physical health: Mechanisms and outcomes. Psychosomatic Medicine, 81(3), 224–232. 10.1097/PSY.0000000000000675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunning DL, Griffiths K, Kuyken W et al. (2019). Research review: The effects of mindfulness-based interventions on cognition and mental health in children and adolescents–a meta-analysis of randomized controlled trials. Journal of Child Psychology and Psychiatry and Allied Disciplines, 60(3), 244–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutil C, & Chaput JP (2017). Inadequate sleep as a contributor to type 2 diabetes in children and adolescents. Nutrition & Diabetes, 7(5), e266. 10.1038/nutd.2017.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel E, Daubenmier J, Moskowitz JT et al. (2009). Can meditation slow rate of cellular aging? Cognitive stress, mindfulness, and telomeres. Annals of the New York Academy of Sciences, 1172, 34–53. 10.1111/j.1749-6632.2009.04414.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field AE, Cook NR, & Gillman MW (2005). Weight status in childhood as a predictor of becoming overweight or hypertensive in early adulthood. Obesity Research, 13(1), 163–169. 10.1038/oby.2005.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint J, Kothare SV, Zihlif M et al. (2007). Association between inadequate sleep and insulin resistance in obese children. Journal of Pediatrics, 150(4), 364–369. 10.1016/j.jpeds.2006.08.063 [DOI] [PubMed] [Google Scholar]
- George L, Bacha F, Lee S et al. (2011). Surrogate estimates of insulin sensitivity in obese youth along the spectrum of glucose tolerance from normal to prediabetes to diabetes. Journal of Clinical Endocrinology and Metabolism, 96(7), 2136–2145. 10.1210/jc.2010-2813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gungor N, Saad R, Janosky J et al. (2004). Validation of surrogate estimates of insulin sensitivity and insulin secretion in children and adolescents. Journal of Pediatrics, 144(1), 47–55. 10.1016/j.jpeds.2003.09.045 [DOI] [PubMed] [Google Scholar]
- Hannon TS, Li Z, Tu W et al. (2014). Depressive symptoms are associated with fasting insulin resistance in obese youth. Pediatric Obesity, 9(5), e103–107. 10.1111/ijpo.237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AF (2009). Beyond Baron and Kenny: Statistical mediation analysis in the new millennium. Communication Monographs, 76(4), 408–420. 10.1080/03637750903310360 [DOI] [Google Scholar]
- Hayes AF (2018). Introduction to mediation, moderation, and conditional process analysis: A regression-based approach (2nd ed.). (New York: Guilford Press; ). [Google Scholar]
- Hirshkowitz M, Whiton K, Albert SM et al. (2015). National Sleep Foundation’s sleep time duration recommendations: methodology and results summary. Sleep Health, 1(1), 40–43. 10.1016/j.sleh.2014.12.010 [DOI] [PubMed] [Google Scholar]
- Holt RI, de Groot M, & Golden SH (2014). Diabetes and depression. Current Diabetes Reports, 14(6), 491. 10.1007/s11892-014-0491-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell AJ, Digdon NL, & Buro K (2010). Mindfulness predicts sleep-related self-regulation and well-being. Personality and Individual Differences, 48(4), 419–424. 10.1016/j.paid.2009.11.009 [DOI] [Google Scholar]
- Javaheri S, Storfer-Isser A, Rosen CL et al. (2011). Association of short and long sleep durations with insulin sensitivity in adolescents. Journal of Pediatrics, 158(4), 617–623. 10.1016/j.jpeds.2010.09.080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabat-Zinn J (1991). Full catastrophe living: Using the wisdom of your body and mind to face stress, pain, and illness. Dell Publishing. [Google Scholar]
- Kan C, Silva N, Golden SH et al. (2013). A systematic review and meta-analysis of the association between depression and insulin resistance. Diabetes Care, 36(2), 480–489. 10.2337/dc12-1442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuczmarski RJ, Ogden CL, Guo SS et al. (2002). 2000 CDC growth charts for the United States: methods and development. Vital Health Stat 11(246), 1–190. https://www.ncbi.nlm.nih.gov/pubmed/12043359 [PubMed] [Google Scholar]
- Lazarus RS, & Folkman S (1984). Coping and adaptations. In Gentry W (Ed.), The handbook of behavioral medicine (pp. 282–325). [Google Scholar]
- Leproult R, Holmbäck U, & Van Cauter E (2014). Circadian misalignment augments markers of insulin resistance and inflammation, independently of sleep loss. Diabetes, 63(6), 1860–1869. 10.2337/db13-1546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loucks EB, Gilman SE, Britton WB et al. (2016). Associations of mindfulness with glucose regulation and diabetes. American Journal of Health Behavior, 40(2), 258–267. 10.5993/AJHB.40.2.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovato N, & Gradisar M (2014). A meta-analysis and model of the relationship between sleep and depression in adolescents: Recommendations for future research and clinical practice. Sleep Medicine Reviews, 18(6), 521–529. 10.1016/j.smrv.2014.03.006 [DOI] [PubMed] [Google Scholar]
- Lucas-Thompson RG, Miller RL, Seiter NS et al. (2019). Dispositional mindfulness predicts cortisol, cardiovascular, and psychological stress responses in adolescence. Psychoneuroendocrinology, 110, 104405. 10.1016/j.psyneuen.2019.104405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maslowsky J, & Ozer EJ (2014). Developmental trends in sleep duration in adolescence and young adulthood: Evidence from a national United States sample. Journal of Adolescent Health, 54(6), 691–697. 10.1016/j.jadohealth.2013.10.201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews KA, Dahl RE, Owens JF et al. (2012). Sleep duration and insulin resistance in healthy black and white adolescents. Sleep, 35(10), 1353–1358. 10.5665/sleep.2112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews KA, Hall MH, Cousins J et al. (2016). Getting a good night’s sleep in adolescence: Do strategies for coping with stress matter? Behavior Sleep Medicine, 14(4), 367–377. 10.1080/15402002.2015.1007994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz SM, Frank JL, Reibel D et al. (2013). The effectiveness of the Learning to BREATHE program on adolescent emotion regulation. Research in Human Development, 10(3), 252–272. 10.1080/15427609.2013.818488 [DOI] [Google Scholar]
- Mi SJ, Kelly NR, Brychta RJ et al. (2019). Associations of sleep patterns with metabolic syndrome indices, body composition, and energy intake in children and adolescents. Pediatric Obesity, 14(6), e12507. 10.1111/ijpo.12507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra M, Bredella MA, Tsai P et al. (2008). Lower growth hormone and higher cortisol are associated with greater visceral adiposity, intramyocellular lipids, and insulin resistance in overweight girls. American Journal of Physiology: Endocrinology and Metabolism, 295(2), E385–392. 10.1152/ajpendo.00052.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris NM, & Udry JR (1980). Validation of a self-administered instrument to assess stage of adolescent development. Journal of Youth and Adolescence, 9(3), 271–280. 10.1007/BF02088471 [DOI] [PubMed] [Google Scholar]
- Nagel KE, Dearth-Wesley T, Herman AN et al. (2020). The association between dispositional mindfulness and glycemic control in type 1 diabetes during early adulthood: Differences by age and adverse childhood experiences. Pediatric Diabetes, 21(4), 681–691. 10.1111/pedi.13000 [DOI] [PubMed] [Google Scholar]
- Pallozzi R, Wertheim E, Paxton S et al. (2017). Trait mindfulness measures for use with adolescents: A systematic review. Mindfulness, 8(1), 110–125. 10.1007/s12671-016-0567-z [DOI] [Google Scholar]
- Phillips GA, Shadish WR, Murray DM et al. (2006). The Center for Epidemiologic Studies Depression Scale with a young adolescent population: A confirmatory factor analysis. Multivariate Behavior Research, 41(2), 147–163. 10.1207/s15327906mbr4102_3 [DOI] [PubMed] [Google Scholar]
- Pivarunas B, Kelly NR, Pickworth CK, Cassidy O, Radin RM, Shank LM, Vannucci A, Courville AB, Chen KY, Tanofsky-Kraff M, Yanovski JA, & Shomaker LB (2015). Mindfulness and eating behavior in adolescent girls at risk for type 2 diabetes. International Journal of Eating Disorders, 48(6), 563–569. 10.1002/eat.22435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preacher KJ, & Hayes AF (2004). SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behavior Research Methods Instruments and Computers, 36(4), 717–731. 10.3758/BF03206553 [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Rucker DD, & Hayes AF (2007). Addressing moderated mediation hypotheses: Theory, methods, and prescriptions. Multivariate Behavior Research, 42(1), 185–227. 10.1080/00273170701341316 [DOI] [PubMed] [Google Scholar]
- Radloff LS (1977). The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement, 1(3), 385–401. 10.1177/014662167700100306 [DOI] [Google Scholar]
- Rawat A, Gangwar AK, Tiwari S, Kant S, Garg RK, & Singh PK (2019). Sleep quality and insulin resistance in adolescent subjects with different circadian preference: A cross-sectional study. Journal of Family Medicine in Primary Care, 8(7), 2502–2505. 10.4103/jfmpc.jfmpc_400_19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reutrakul S, & Van Cauter E (2018). Sleep influences on obesity, insulin resistance, and risk of type 2 diabetes. Metabolism, 84, 56–66. 10.1016/j.metabol.2018.02.010 [DOI] [PubMed] [Google Scholar]
- Shomaker LB, Bruggink S, Pivarunas B, Skoranski A, Foss J, Chaffin E, Dalager S, Annameier S, Quaglia J, Brown KW, Broderick P, & Bell C (2017). Pilot randomized controlled trial of a mindfulness-based group intervention in adolescent girls at risk for type 2 diabetes with depressive symptoms. Complementary Therapies in Medicine, 32, 66–74. 10.1016/j.ctim.2017.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shomaker LB, Berman Z, Burke M, Annameier SK, Pivarunas B, Sanchez N, Smith AD, Hendrich S, Riggs NR, Legget KT, Cornier M, Melby C, Johnson SA, & Lucas-Thompson RG (2019). Mindfulness-based group intervention in adolescents at-risk for excess weight gain: A randomized controlled pilot study. Appetite 140, 213–222. 10.1016/j.appet.2019.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shomaker LB, Pivarunas B, Annameier SK, Gulley L, Quaglia J, Brown KW, Broderick P, & Bell C (2019). One-year follow-up of a randomized controlled trial piloting a mindfulness-based group intervention for adolescent insulin resistance. Frontiers in Psychology, 10, 1040. 10.3389/fpsyg.2019.01040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shomaker LB, Tanofsky-Kraff M, Stern EA, Miller R, Zocca JM, Field SE, Yanovski SZ, Hubbard VS, & Yanovski JA (2011). Longitudinal study of depressive symptoms and progression of insulin resistance in youth at risk for adult obesity. Diabetes Care, 34(11), 2458–2463. 10.2337/dc11-1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon SL, Behn CD, Cree-Green M, Kaar JL, Pyle L, Hawkins SMM, Rahat H, Garcia-Reyes Y, Wright KP Jr., & Nadeau KJ (2019). Too late and not enough: School year sleep duration, timing, and circadian misalignment are associated with reduced insulin sensitivity in adolescents with overweight/obesity. Journal of Pediatrics, 205(257–264), e251. 10.1016/j.jpeds.2018.10.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockings E, Degenhardt L, Lee YY, Mihalopoulos C, Liu A, Hobbs M, & Patton G (2015). Symptom screening scales for detecting major depressive disorder in children and adolescents: A systematic review and meta-analysis of reliability, validity and diagnostic utility. Journal of Affective Disorders, 174, 447–463. 10.1016/j.jad.2014.11.061 [DOI] [PubMed] [Google Scholar]
- Suglia SF, Koenen KC, Boynton-Jarrett R, Chan PS, Clark CJ, Danese A, Faith MS, Goldstein BI, Hayman LL, Isasi CR, Pratt CA, Slopen N, Sumner JA, Turer A, Turer CB, & Zachariah JP (2018). Childhood and adolescent adversity and cardiometabolic outcomes: A scientific statement from the American Heart Association. Circulation, 137(5), e15–e28. 10.1161/CIR.0000000000000536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner T, & Hingle M (2017). Evaluation of a mindfulness-based mobile app aimed at promoting awareness of weight-related behaviors in adolescents: A pilot study. JMIR Research Protocols, 6(4), e67. 10.2196/resprot.6695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfson AR, & Carskadon MA (1998). Sleep schedules and daytime functioning in adolescents. Child Development, 69(4), 875–887. 10.2307/1132351 [DOI] [PubMed] [Google Scholar]
- Wolfson AR, Carskadon MA, Acebo C, Seifer R, Fallone G, Labyak SE, & Martin JL (2003). Evidence for the validity of a sleep habits survey for adolescents. Sleep, 26(2), 213–216. 10.1093/sleep/26.2.213 [DOI] [PubMed] [Google Scholar]


