Abstract
Chemokine ligand 11 is a member of the CXC chemokine family and exerts its biological function mainly through binding to CXCR3 and CXCR7. The CXCL11 gene is ubiquitously overexpressed in various human malignant tumors; however, its specific mechanisms vary among different cancer types. Recent studies have found that CXCL11 is involved in the activation of multiple oncogenic signaling pathways and is closely related to tumorigenesis, progression, chemotherapy tolerance, immunotherapy efficacy, and poor prognosis. Depending on the specific expression of its receptor subtype, CXCL11 also has a complex 2-fold role in tumours; therefore, directly targeting the structure-function of CXCL11 and its receptors may be a challenging task. In this review, we summarize the biological functions of CXCL11 and its receptors and their roles in various types of malignant tumors and point out the directions for clinical applications.
Keywords: CXCL11, malignant tumor, chemokine, chemokine receptor, targeted therapy
Plain language summary
CXCL11 is found in many types of cancer and affects how cancer cells grow and respond to treatments. This paper delves into the intricate dance between CXCL11 and its receptors in various types of cancer. Like a versatile actor playing different roles on stage, CXCL11 can either promote or hinder cancer growth depending on its interaction with specific receptors. Understanding how CXCL11 works could help develop new treatments for cancer, but it's a complex challenge because CXCL11 can have different effects depending on the type of cancer and which receptors it binds to.
Introduction
With changes in global demographic characteristics and lifestyles, the risk of malignant tumors is increasingly becoming a serious burden on the lives and economies of people in all countries. According to estimates by the World Health Organization, in 2019, cancer was the leading or second leading cause of death in 183 countries worldwide. 1 And according to projections, these burdens are still expected to continue to increase, with 22 million new cancer cases and associated deaths with 13 million cancers expected by 2030. 2 A recent study found that CXCL11 of the chemokine family is significantly more expressed in most types of malignant tissues than in non-tumour tissues. 3
Unlike the earliest beliefs that it only participates in the intrinsic inflammatory response or functions as an immunosurveillance mediator, CXCL11 not only regulates the directional movement of cancer cells but also participates in the entry and exit of cancer cells into and out of blood vessels, immune evasion, proliferation, and angiogenesis. 4 Meanwhile, CXCL11 exhibits two sides in tumor development depending on the different receptors or receptor subtypes to which it binds; thus, chemokines are not only confounding with their receptors (multiple chemokines can share the same receptor), but also the same ligand itself is pleiotropic (binds to different receptors or receptor subtypes and thus mediates different effects), which poses an even greater challenge for its clinical application.
It is currently believed that CXCL11 overexpression in a variety of human malignant tumors leads to an imbalance between cell proliferation and apoptosis, and in particular, its effects on immune cells are involved in a variety of tumorigenesis and developmental processes. Although current studies have made some progress, the main mechanism of action of CXCL11 on malignant tumors of different tissue types is not the same. We have conducted a comprehensive review of CXCL11 and its receptors with a focus on their biological functions, relevance in various malignancies (Table 1), and clinical value.
Table 1.
Role of CXCL11 and its Receptors in Human Malignancies.
| Tumour type | Ligand - Receptors | Meaning | Mechanism of action | Results | Ref. |
|---|---|---|---|---|---|
| COADREAD | CXCL11 | Tumor promotor | Colon cancer cells overexpressing RBP-Jκ induce the expression of TGF-β1 in TAMs through the secretion of CXCL11, enhancing the infiltration of TAMs into the tumor environment | Promotes EMT of cancer and facilitates tumor metastasis | 5,6 |
| CXCL11–CXCR3 | Associates with tumor | CXCR3-A and CXCR3-B have antagonistic effects on each other, with CXCR3-B being more expressed in non-tumor tissues | Elevated expression of CXCR3-A signifies a poor prognosis, whereas CXCR3-B suggests the opposite | 7 | |
| STAD | CXCL11–CXCR3 | Tumour inhibitor | CXCL11 activates CXCR3 in GC cells, upregulating PD-L1 expression through STAT and PI3K-Akt pathways | Enhancing the effectiveness of immunotherapy | 8 |
| Tumour inhibitor | Mediates the infiltration of cytotoxic T lymphocytes and inhibits angiogenesis, which can be inhibited by H. pylori | Inhibition of tumor growth | 9,10 | ||
| CXCL11 | Tumour inhibitor | Activates Th1 and promotes M1 polarization in macrophages | Inhibition of tumor growth | 11 | |
| HCC | CXCL11–CXCR3 | Tumor promotor | α2δ1+ HCC TIC promotes tumor stem cells through a self-secretory pathway, activating the ERK1/2 pathway | Promotes tumor self-renewal and chemotherapy resistance | 12 |
| CXCL11 | Tumor promotor | Promotes HCC cell proliferation and migration through the LINC00152/miR-205-5p/CXCL11 axis | Promotes tumor growth and metastasis | 13 | |
| Promotes HCC cell migration through the circUBAP2/miR4756/IFIT1/3 axis | Promotes tumor metastasis | 13 | |||
| Associates with tumor | Elevated expression in viral hepatitis and liver cirrhosis | Elevated with the progression of liver inflammation or fibrosis | 14-16 | ||
| BLCA | CXCL11-CXCR3 | Tumour inhibitor | Highly active CD8 + cells in the tumor microenvironment express more CXCR3-alt | Improvement in chemotherapy sensitivity | 17 |
| CXCL11 | Tumour inhibitor | BCG treatment enhances the presentation of BCG-cancer cell conjugates to antitumor immune cells | Inhibition of tumor growth | 18 | |
| RC | CXCL11 | Tumor promotor | EP300/CBP modifies and enhances the expression of RBM15, stabilizes the level of CXCL11 mRNA, and ultimately leads to M2 polarization of macrophages | Promotes tumor proliferation and migration | 19 |
| CXCL11-CXCR3 | Tumor promotor | Highly expressed in the tumor vasculature | Promotes tumor angiogenesis | 20 | |
| CXCL11-CXCR3 | Tumour inhibitor | CXCR3-B has immunosuppressive tumor vasculature activity | Inhibition of tumor angiogenesis | 21 | |
| LUNG | CXCL11-CXCR3 | Tumour inhibitor | CXCL11-CXCR3 induces infiltration of CD8 + T cells and suppresses angiogenesis | Augmenting the efficacy of immunotherapy | 22 |
| CXCL11 | Tumour inhibitor | DOC upregulates the secretion of HMGB1/NF-Κb/CXCL11 via the ROS pathway, resulting in increased infiltration of CD8 + T cells | Enhances chemotherapy sensitivity | 23 | |
| Tumor inhibitor | Knocking out the RUNX3 gene leads to downregulation of CXCL11 mRNA expression and increases osteoclast activity | Counteracts the osteolytic activity of tumor cells | 24 | ||
| HNSCC | CXCL11-CXR3 | Tumor promotor | Tumorinflamed LEC overexpress MMP7 and activate the JAK-STAT and AKT pathways, inducing tumor cell EMT | Promotes lymph node metastasis of tumors | 25 |
| OC | CXCL11-CXCR3 | Tumor promotor | Markedly elevated OC expression, promoting intracellular D274 and IDO1 expression | Promotes tumor growth and immune escape | 26 |
| Significantly elevated expression in oral mucosal leukoplakia | Promotion of precancerous lesions | ||||
| CXCL11-CXCR7 | Tumor promotor | Expression gradually increases in premalignant oral lesions and advanced-stage oral cancer | Promotes early malignant transformation and tumor development | 27 | |
| SKCM | CXCL11 | Associates with tumor | Associations with immune infiltration and inverse correlation with prognosis | Suggests poor prognosis (Bioinformatics analysis) | 28,29 |
| CXCL11-CXCR3 | Tumor promotor | Induces cellular cytoskeletal remodeling | Promotes tumor metastasis | 30 | |
| CXCL11 | Tumor Inhibitor | Recruits immune cells, promotes marrow activation, and enhances antitumor immune responses | Inhibition of tumor growth | 31 | |
| BC | CXCL11 | Tumor Inhibitor | The IFI16-STING pathway promotes CD8 + T cell infiltration and enhances memory T cell abundance | Influence on the efficacy of targeted therapy | 32 |
| Associates with tumor | Serum CXCL11 levels are lower in HR+ breast cancer patients compared to HR- patients | Predicts treatment efficacy | 33 | ||
| Associates with tumor | Expression levels of I-TAC are downregulated in tissue and negatively correlate with staging of BC | Suggests tumor progression | 34 | ||
| CXCL11-CXCR3 | Tumor promotor | Aged endothelial cells release through the chemokine receptor axis, activating the ERK pathway | Enhances tumor invasion | 35 | |
| MM | CXCL11 | Tumor promotor | Activates tyrosine kinase, inducing secretion of MMP-2 and MMP-9 | Promotes tumor growth and metastasis | 36 |
| CXCL11-CXCR3 | Tumor inhibitor | Chemotaxis attracts immunoreactive cells | Inhibition of tumor growth | ||
| CLL | CXCL11 | Associates with tumor | GHV mutation significantly correlates with CXCL11 | Predicts prognosis | 37 |
| DLBCL | CXCL11 | Associates with tumor | Expresses higher levels in advanced-stage tumours; associates with tumor diagnosis | Diagnostic support; Assess the extent of tumor progression | 38 |
| OV | CXCL11-CXCR7 | Tumor promotor | ERα enhances the expression of the CXCL11–CXCR7 axis, promoting tumor cell EMT through a feedforward regulatory mechanism | Promotes tumor metastasis | 39 |
| CXCL11-CXCR3 | Tumor promotor | High expression of CXCR3-A in endometriosis inhibits cytotoxic T cells | Promotion of precancerous lesions | 40,41 | |
| PAAD | CXCL11 | Associations with tumor development | Elevated serum CXCL11 levels in patients | Diagnostic support | 42 |
| PRAD | CXCL11 | Associates with tumor | Elevated serum CXCL11 levels in patients | Diagnostic support | 43 |
| BCC | CXCL11-CXCR3 | Tumor promotor | Promotes the proliferation of human immortalized keratinocytes | Promotes tumor growth | 44 |
| THCA | CXCL11-CXCR7 | Tumor promotor | Promotes angiogenesis in metastatic THCA through an EGF-EGFR positive feedback loop | Promotes tumor angiogenesis | 45 |
| CXCL11 | Tumor inhibitor | Induction of CXCL11 release by IFN-γ or PPARγ agonists inhibits endothelial cell proliferation, suppresses basal cell migration, and induces apoptosis | Inhibition of tumor growth and migration | 46 |
Carcinoma abbreviations: COADREAD: colon adenocarcinoma and rectal adenocarcinoma, STAD: stomach adenocarcinoma, HCC: hepatocellular carcinoma, BLCA: bladder urothelial carcinoma, RC: renal carcinoma, LUNG: lung cancer, HNSCC: head and neck squamous cell carcinoma, OC: oral cancer, SKCM: skin cutaneous melanomas, BC: breast cancer, MM: multiple myeloma, CLL: chronic lymphocytic leukemia, DLBCL: diffuse large B-cell lymphoma, OV: ovarian cancer, PAAD: pancreatic adenocarcinoma, PRAD: prostate adenocarcinoma, BCC: basal cell carcinoma, THCA: thyroid carcinoma.
Composition and Role of the Chemokine Family
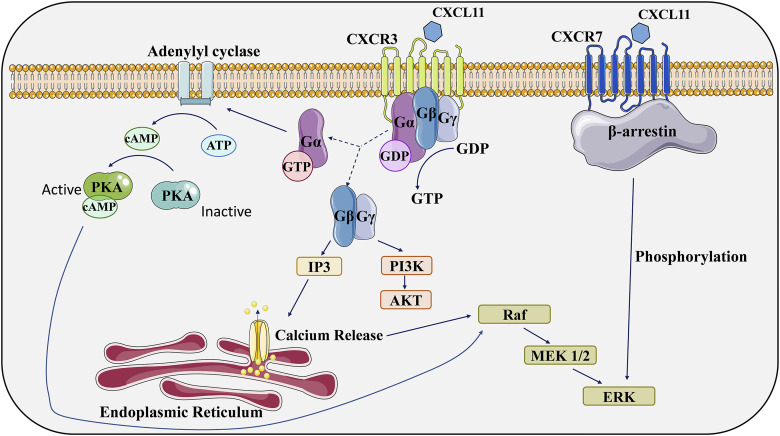
Chemokines refer to a group of small-molecule proteins that are secreted. To date, over 50 chemokines and 20 chemokine receptors have been characterized. These chemokines are categorized into four distinct subfamilies, namely CC, CXC, CX3C, and XC, based on the specific configuration of their two N-terminal cysteine residues.47,48 They mediate signaling events through seven-transmembrane structures, specifically G protein-coupled receptors (GPCRs), which are on the cellular surface. These receptors, known as chemokine receptors, have extracellular and cytoplasmic N termini. Their interaction with chemokine ligands initiates the activation of the G protein-coupled receptor (GPCR) signaling pathway. This activation typically starts with the disassociation of classical G protein subunits, specifically Gα, Gβ, and Gγ. Subsequently, this cascade eventuates in the generation of a secondary messenger, which in turn activates various effector enzymes (Figure 1). This intricate series of intracellular events encompasses calcium efflux and culminates in the orchestration of many biological effects. 49 Chemokine receptors are commonly expressed on tumor cells, vascular endothelial cells and leukocytes, which also secrete some chemokine ligands by autocrine or paracrine means. As a class of structurally related small molecules, they are approximately 20%–50% identical, meaning that they share genetic and amino acid sequences. Chemokines assist in this process called “chemotaxis” by regulating the transport of various types of leukocytes , 48 allowing cells to NF-κB rate to the source of the chemokine along with signals of increased chemokine concentration when the body is clearing invading pathogens or performing autoimmunity. However, the functions of chemokines in the growth and spread of a variety of tumors have also been confirmed in recent studies ,50,51and they are also closely associated with tumor chemoresistance and poor prognosis. These qualities have made chemokines increasingly the focus of clinical therapy, and understanding their mechanisms of action may lead to new therapies for various human malignancies.
Figure 1.
Receptors for CXCL11 act through multiple signaling pathways. (1). Typical chemokine receptors, such as CXCR3, function as G-protein-coupled receptors and exert their biological effects through multiple downstream signaling pathways. (2). CXCR7 was previously thought to be incapable of activating typical G-protein signaling pathways. However, recent studies have found that it can activate ERK through the β-arrestin pathway. The figure was partly generated using Servier Medical Art, provided by Servier, licensed under a Creative Commons Attribution 3.0 unported license. GTP, guanosine triphosphate; GDP, guanosine diphosphate; ATP, adenosine triphosphate; cAMP, cyclic adenosine monophosphate.
Structure and Biological Function of CXCL11 and its Receptor
CXCL11
The gene encoding C-X-C Motif Chemokine Ligand 11 is located on human chromosome 4q21.2 and contains four exons and at least three polyadenylation signals, IFN-γ, IFN-α and IFN-β are effective inducers of transcription of the gene, but the induction of IFN-α is relatively weak; therefore, CXCL11 is also known as an IFN-inducible T-cell α-chemoattractant. In the human body, it is secreted mainly by monocytes, endothelial cells, fibroblasts, and cancer cells in response to IFNs. The CXC chemokine family is subdivided into two subfamilies based on the presence or absence of the Glu-Leu-Arg (ELR) amino acid sequence before the first cysteine residue. In general, ELR + chemokines preferentially attract neutrophils and act as pro-angiogenic, whereas ELR chemokines act on lymphocytes but are vaso-inhibitory.50,52 Meanwhile, CXCL11 belongs to the latter. 53 The CXCL11 protein comprises 94 amino acids and has a molecular weight of approximately 10,365 Da. It has a typical chemokine sheeting structure, as determined by an NMR spectroscopy study, with a three-stranded antiparallel β-sheet opposite an α-helix, a long N-terminal end stabilized by two disulfide bonds, and a 310-helix just at the N-terminal end of the first β-sheet. 54 The classical receptor for CXC11 is predominantly CXCR3, and it also has the strongest affinity of the multiple chemokines that share this receptor. It has recently been shown to bind to another chemokine receptor, CXCR7. Given the undeniable role of CXCL11 in modulating the body’s immune response, extensive evaluations have been conducted regarding the potential of targeting CXCL11 in cancer immunotherapy. Immune checkpoint inhibitors (ICIs) play a crucial role in this context (Table 2). However, the role of CXCL11 in neoplastic diseases cannot yet be generalized, and although there is a large body of evidence that CXCL11 is associated with the development of a variety of tumours, some studies have demonstrated that CXCL11 can bind to some proteins to exert anti-tumour effects, 60 which makes it necessary to maintain a more cautious approach when intending to use it as a target for targeted tumour therapy. We will elaborate on its relationship with malignant diseases in subsequent chapters.
Table 2.
Associations Between ICIs and CXCL11 in Cancer Immunotherapy.
| Tumor Type | Association | ref |
|---|---|---|
| Gastric cancer | 1. The CXCL11–CXCR3 axis may upregulate the expression of PD-L1 in gastric cancer (GC) cells through the activation of STAT and PI3K– Akt signaling pathways | 8 |
| 2. A significant positive correlation was observed between the expression levels of PD-L1 and CXCR3 in gastric cancer tissues | ||
| Lung cancer | 1. Silencing CXCL11 and CXCR3 in tumor cells can suppress the antitumor and antiangiogenic effects of PD-L1 inhibitors. Upregulation of IFN-γ-stimulated CXCL11 expression is associated with sensitivity to PD-L1 receptor blockade | 22,55 |
| 2. Early elevation of peripheral blood levels of CXCL11 and IFN-γ may serve as indicators of increased immune-related adverse events | ||
| Head and neck squamous cell carcinoma | 1. The low expression of the immune-related lncRNA TRG-AS1 can induce the expression of CXCL11 in the tumor microenvironment and serve as a novel biomarker for predicting the efficacy of PD-1 treatment | 56,57 |
| 2. Patients with a history of smoking exhibit a lower number of PD-1+-positive cells, possibly because of a weakened ability of CXCL11 to recruit cytotoxic T cells and NK cells | ||
| Oral leukoplakia (precancerous lesions) | Knocking down CXCL11 inhibits the expression of intracellular immune checkpoints, primarily CD274 and IDO1 | 26 |
| Skin melanoma | 1. Early elevation of peripheral blood levels of CXCL11 and IFN-γ may serve as indicators of increased immune-related adverse events | 22,58 |
| 2. Tumor microenvironments rich in CD16 + macrophages can enhance the response to combined immune therapy targeting PD-1 and CTLA-4. Upregulation of CXCL11 gene expression is observed in this context | ||
| Breast cancer | 1. Exercise training can enhance CD8 + T cell infiltration in breast cancer, making it more responsive to ICI treatment through the CXCL11-CXCR3 signaling pathway | 59 |
CXCR3
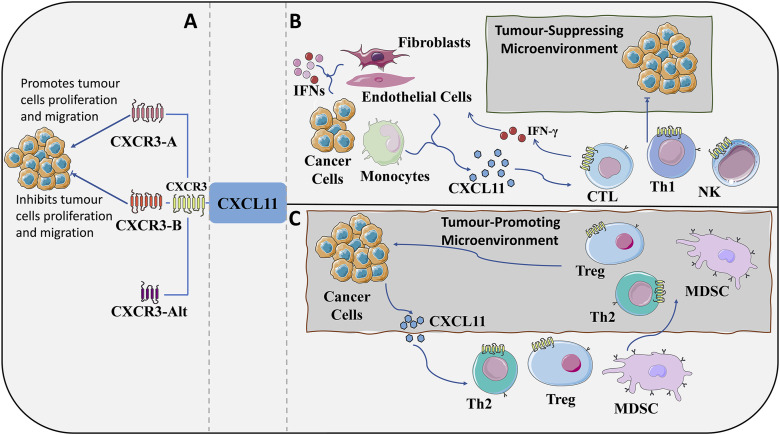
The gene encoding CXCR3 is located on human X chromosome q13.1. 61 In tumourous tumorous, CXCR3 and its ligands are expressed on tumor cells, stromal cells, blood vessels and recruited leukocytes. 62 CXCR3 has at least three variable splicesomes: CXCR3A,CXCR3B, and CXCR3-alt, 63 with CXCL11 having higher affinity potency for CACR3-A. CXCRA/B has opposing roles in tumor function due to mediating different signaling cascade pathways or affinity for different cell types.64,65 Usually, CXCR3-A, which is mainly expressed in T lymphocytes and NK cells, 66 transmits signals that promote cell proliferation and chemotaxis. CXCR3-B, which is mainly expressed in fibroblasts, endothelial cells, and epithelial cells, mainly inhibits cell proliferation and migration and induces apoptosis. CXCR3-B has a high structural similarity to CXCR3-A, which contains a longer NH2-terminal extracellular domain, 67 but the rest of the structure is highly overlapping.CXCR3-alt has a very different structure, which contains a truncated C-terminus and only four transmembrane helices, and has been found to bind only to CXCL11,63,68 and as with its most recent discovery, its function has not yet been thoroughly investigated. CXCL11 interacts with its receptor CXCR3 at two successive sites in its structure,69,70 with the receptor first being recognized and bound by the N-loop region of the protein, followed by activation of the CXCR3 receptor by the flexible N-terminal region of the chemokine, which presents a “two-step” mode of action . 54 The two main variants mediating CXCR3 may be expressed in the tumor microenvironment; therefore, it cannot be simply asserted that CXCR3 is an oncogenic or a manostatic factor (Figure 2).
Figure 2.
Role of the CXCL11–CXCR3 axis in the tumor microenvironment. (A): CXCR3 composition. According to the difference in the fragment of the amino terminus of the receptor protein, CXCR3 can be classified into three isoforms: CXCR3-A, CXCR3-B, and the truncated variant CXCR3-alt. CXCR3A promotes the proliferation and migration of cancer cells, whereas CXCR3B inhibits the proliferation and migration of cells, and the function of CXCR3-alt has not yet been fully clarified. (B&C): CXCL11-CXCR3 axis: The CXCL11–CXCR3 axis acts in two main directions: the direction of paracrine signaling for the promotion of immune responses and the direction of autocrine signaling for the proliferation and metastasis of cancer cells. (B): Paracrine secretion of CXCR3. Mediated by IFNs, CXCL 11 is secreted mainly by monocytes, endothelial cells, fibroblasts, and cancer cells. The main function of this axis is to stimulate the migration, differentiation, and activation of immune cells. It acts as a tumor suppressor by recruiting CTL, NK cells and macrophages. Simultaneously secreted CXCL11 recruits CD8+ CTLs to local tissues, and IFN-γ produced by CD8+ CTLs further stimulate tissue cells to produce more CXCL11. Increased release of chemokines and enhanced inflammatory response leads to further accumulation of anti-tumor immune cells expressing CXCR3, forming a tumor-suppressive microenvironment. (C): Autocrine secretion of CXCR3. In the direction of autocrine signaling, CXCR3 (mainly CXCR3A) can make cancer cells susceptible and further grow and metastasize. Tumour-derived CXCL11 is also responsible for the recruitment of Th cells, Tregs, and MDSCs, which play a role in creating a tumour-friendly microenvironment. The figure was partly generated using Servier Medical Art, provided by Servier, licensed under a Creative Commons Attribution 3.0 unported license. CTL, cytotoxic lymphocytes; NK, natural killer cells; Th1, T help1; Th2, T help2; Tregs, regulatory T cells; MDSC, myeloid-derived suppressor cells.
CXCR7
CXCR7 was first cloned from a dog thyroid cDNA library by Liber et al. in 1989. 71 However, its biological function was not thoroughly understood for a long time. Initially, it was believed to be the receptor for vasoactive intestinal peptide,72,73 but this hypothesis was not supported by subsequent research. Until 2005, CXCR7 was found to be a high-affinity receptor for CXCL12. 74 Prior to this, CXCL12 was thought to only interact with CXCR4. Subsequently, Burns demonstrated that CXCR7 also has a certain affinity for CXCL11. 75 The human CXCR7 gene is at position 2q37.3 on the chromosome and contains 7 exons, but only the last exon is responsible for translation. 76 Unlike other classical chemokine receptors, CXCR7 has been shown to not induce calcium flux in various cell lines; hence, it may not signal through the traditional G protein-mediated pathway. Currently, one possible mechanism by which CXCR7 exerts its effects is through the β-arrestin pathway. 77 It has also been renamed as atypical chemokine receptor 3 (ACKR3) by the Nomenclature and Standards Committee of the International Union of Basic and Clinical Pharmacology. 78 In multiple malignant tumor diseases, CXCR7 has been found to be associated with cancer cell growth, metastasis, or angiogenesis, such as colorectal cancer, 79 breast cancer, 80 prostate cancer, 81 melanoma, 82 etc.
Role of CXCL11 in Human Malignant Tumors
CXCL11 in Colon Adenocarcinoma and Rectal Adenocarcinoma (COADREAD)
RBP-Jκ is a protein that can bind to a DNA fragment carrying the immunoglobulin Jκ recombination signal sequence. In addition, it plays a pivotal role as a core factor in the classical Notch signaling pathway. 83 Overexpression of RBP-Jκ in colorectal cancer can lead to the secretion of CXCL11 by cancer cells, enhancing the metastatic potential of colorectal cancer. CXCL11 can induce a high degree of infiltration of tumor-associated macrophages (TAMs) in colorectal tumors and stimulate these macrophages to secrete TGF-β1. This ultimately promotes the epithelial– mesenchymal transition (EMT), migration, and invasion of colorectal cancer cells. 5 Neuroendocrine differentiation in colorectal cancer is a significant factor leading to poor prognosis in patients. Studies have shown that CXCL11 is also a key chemokine in neuroendocrine-like cells. Similarly, it can recruit tumorassociated macrophages to infiltrate tumor tissues, leading to more malignant biological behavior. 6 Similarly, as with its role in other parts of the tumor, the receptor of CXCL11, CXCR3, exhibits a dual role of tumor suppression or promotion in colorectal cancer because of its different splice variants. Rupertus found that colorectal cancer cells locally exposed to CXCL11 significantly increased their invasiveness, but there was no obvious angiogenic effect. Furthermore, if the blockage of CXL11 and CXCL12 is combined, a decrease in tumor growth and invasive growth characteristics of the tumor can be observed. 84 Moreover, due to the independent regulatory role of CXCL12 on tumor angiogenesis mediated by CXCR7, 85 it is possible that the CXCL11–CXCR7 axis plays a significant role in the angiogenesis of colorectal tumors rather than the CXCL11–CXCR3 axis. Given the potential for competitive expression between CXCR3-B and CXCR3-A, the interplay within the CXCL11–CXCR3 axis in colorectal cancer and its implications for tumor progression in patients are rendered more intricate. Presently, research has revealed a marked upregulation of CXCR3-A within tumor tissues as compared to their adjacent counterparts, whereas CXCR3-B exhibits a contrasting pattern of expression. 7 Concurrently, analyses have indicated that upregulation of CXCL11 gene expression is associated with a favorable prognosis in patients with colon adenocarcinoma. This phenomenon may be attributed to a higher proportion of anticancer immune cells in the tumor microenvironment (TME). 86 Across different datasets, it becomes clear that the levels of CXCL11 expression defy simplistic categorization as merely “good” or “bad”. In summary, the impact of CXCL11 on colorectal cancer is multifaceted, involving diverse pathways and bidirectional effects. The potential avenue for future drug development may be the exploration of CXCL11 as a combinatorial inhibitor with CXCL12.
CXCL11 in Stomach Adenocarcinoma (STAD)
In gastric cancer, adenocarcinoma constitutes 90% of all cases. The most prominent risk factor is Helicobacter pylori infection. This pathogen induces recurrent inflammation of the gastric mucosa, subsequently progressing to atrophic gastritis. The IFN-γ-inducible ELR− CXCR3 ligands are considered to exert an influence on tumor growth by mediating the infiltration of cytotoxic T lymphocytes and suppressing angiogenesis. 9 In contrast, in vitro experiments have revealed that Helicobacter pylori can suppress the induction of IFN-γ. 10 The CXCL11–CXCR3 axis may upregulate PD-L1 expression in gastric cancer (GC) cells by activating STAT and PI3K– Akt signaling pathways. This could aid the application of checkpoint inhibitors for patients with advanced gastric cancer. Furthermore, a significant positive correlation was observed between PD-L1 and CXCR3 expression levels in gastric cancer tissues. 8 Moreover, in a study that combined data array analysis and expression detection, CXCL11 was identified as an independent prognostic marker for gastric cancer. Lower expression of CXCL11 was associated with poor prognosis. This could be attributed to a reduction in Th1 activation and alterations in macrophage polarization. 11 Hence, CXCL11 appears to play a more proactive role in gastric cancer. Further research into this topic could potentially enhance our understanding of clinical strategies for gastric cancer.
CXCL11 in Liver Cancer (LC)
Similar to gastric cancer, the onset of liver cancer also has distinct risk factors, including alcohol and hepatitis virus infection. Both can cause chronic hepatitis, leading to cirrhosis and ultimately to malignant liver tumors, a progression often referred to as the “trilogy of liver cancer”. In a controlled trial involving patients with chronic hepatitis caused by various etiologies, the serum concentration of CXCL11 was found to be higher than that in the healthy control group and was also found to be significantly elevated in the late stages of cirrhosis. 14 In addition, Chalin, Helbig, and others have found that the expression of CXCL11 is elevated in hepatitis C (CHC).15,87 Most liver cancers are hepatocellular carcinoma (HCC). Berres et al. further discovered that the level of CXCL11 increases with the severity of liver fibrosis 16 CXCL11 exhibits the capability to engage the CXCR3 receptor located on α2δ1+ Hepatocellular Carcinoma Tumor Initiating Cells (α2δ1+ HCC TICs) through an autocrine secretion mechanism. This instigates the activation of the ERK1/2 signaling pathway, exerting regulatory control over tumor stem cell populations and ultimately sustaining the self-renewal, tumorigenic potential and chemoresistance traits of HCC TICs. 12 In hepatocellular carcinoma cancer-associated fibroblasts (HCC CAFs), CXCL11 secreted by CAFs can promote HCC cell proliferation and migration through the LINC00152-miR-205-5p-CXCL11 axis. In vitro experiments and mouse models showed that LINC00152 knockdown reduced the expression of CXCL11, thereby inhibiting HCC cell vitality, colony formation, and migration ability. In addition, miR-205-5p can bind to the 3′UTR of LINC00152 and CXCL11 RNA, inhibiting the expression of CXCL11. In this context, LINC00152 may act as a ceRNA to counteract the miR-205-5p-mediated inhibition of CXCL11. 13 Coincidentally, research has found that CXCL11 can also activate the circUBAP2-miR4756-IFIT1/3 axis in tumor cells to promote HCC migration and metastasis. 13 This suggests that CXCL11 could serve as key mediator integrating CAF cells to influence the invasive ability of HCC. In summary, the role of CXCL11 in the pathogenesis and progression of liver cancer suggests that it could serve as a potential drug target to contribute to patient disease control.
CXCL11 in Bladder Urothelial Carcinoma (BLCA)
Bladder cancer, a high-risk tumor, has an incidence rate in men that is approximately four times that in women. The inner wall of the bladder is mainly composed of epithelial cells, known as urothelial cells. It has been reported that BLCA is one of the most immunogenic tumors. Currently, BCG has become one of the most important biological therapies for treating BLCA. tumor cells may bind and internalize BCG and present BCG and tumor antigens to immune cells through the secretion of chemokines. In this treatment, the CXCL11 gene was significantly upregulated, leading to Th1 chemotaxis. This process ultimately exerts an anti-tumour effect by inducing tumour-suppressive cells (CTLs, NKs, etc.). 18 In muscle invasive bladder cancer (MIBC), high expression of CXCL11-CXCR3alt is associated with high efficacy of neoadjuvant chemotherapy. This may be because highly active CD8+ T cells in the tumor microenvironment (especially TSCM) express more CXCR3-alt. 17 The intratumoural CD3 content can predict the abundance of CXCL11, suggesting a potential role of immunogenic cell death (ICD). 88 Besides, a survival analysis of the GEO and TCGA databases indicated that high levels of CXCL11 mRNA expression are significantly associated with poorer patient overall survival (OS). 89 These studies could aid in the development of more precise immunotherapeutic strategies for bladder cancer patients in the future, such as T cell adoptive therapy and immune checkpoint inhibitors.
CXCL11 in Renal Carcinoma (RC)
The value of chemokines in targeted therapy for renal cell carcinoma has been confirmed by multiple studies.90,91 We have also conducted a comprehensive evaluation of the diagnostic, therapeutic, and prognostic capabilities of CXCL11 and its receptors in renal malignancies. Zeng et al. found that EP300/CBP, as a histone acetyltransferase, can modify the promoter of RNA-binding motif protein 15 (RBM15), leading to enhanced expression of RBM15. This process stabilizes the mRNA levels of CXCL11. The secretion of CXCL11 further induces macrophage infiltration and M2 polarization, ultimately promoting the proliferation and migration of clear cell renal cell carcinoma (ccRCC). 19 Research has also found that CXCL11 is highly expressed in the vascular system of human renal cell carcinoma. Given the high affinity of CXCL11 for CXCR3, the CXCL11–CXCR3 axis plays a significant physiological and pathological role in the occurrence and development of renal cell carcinoma. In fact, RT-PCR has also shown significant upregulation of CXC11 and CXCR3 mRNA in RCC samples. 20 As another example, a system evaluation model based on ICL scores indicates that overexpression of CXCL11 predicts adverse clinical outcomes in ccRCC patients. 92 This is consistent with the examples mentioned earlier. A variant splice receptor of CXCL11, CXCR3-B, has been found to have immunovascular inhibitory activity in non-metastatic human renal cell carcinoma. 21 Therefore, we believe that CXCL11 and its receptors are valuable for the prognostic analysis and targeted therapy of renal cell carcinoma.
CXCL11 in Lung Cancer (LUNG)
Over the past several decades, the concept of early diagnosis and the advent of many novel biopharmaceuticals have greatly improved the survival rate of lung cancer patients. However, lung cancer remains a major health hazard for humans, with an estimated 2 million new cases and 1.76 million deaths annually. 93 Immune checkpoint inhibitors (ICIs) play a pivotal role for treating lung cancer. In an animal experiment involving ICI-sensitive cell lines, CXCL11 was upregulated by PD-L1 blockade. Silencing CXCL11 in tumor cells can inhibit the antitumor and vascular inhibitory effects of PD-L1 inhibitors. 22 The researchers also used anti-CXCR3 antibodies in conjunction with PD-L1 antibodies and found that blocking CXCR3 can yield similar results. This may be due to the fact that although early treatment with ICIs requires the antitumor ability of CD8+ T cell infiltration induced by CXCL11-CXCR3, the antiangiogenic ability of CXCL11 dominates in the late stage. In vitro experiments corroborated this result, with the upregulation of CXCL11 expression stimulated by IFN-γ being associated with sensitivity to PD-L1 receptor blockade. At the same time, serum CXCL11 levels in lung cancer patients may also be a potential biomarker for clinical treatment with ICIs. 22 In 2023, through a prospective multicenter study, researchers identified that the early elevation of peripheral blood levels of CXCL11 and IFN-g may serve as indicators for the increased occurrence of immune-related adverse events (irAEs). 55 This conclusion is primarily drawn from the clinical data of patients with non-small-cell lung cancer (NSCLC) and cutaneous melanoma who underwent ICI treatment. In another comprehensive study on NSCLC, docetaxel increased the release of HMGB1 in a ROS-dependent manner. HMGB1 further stimulated the secretion of CXCL11 by activating NF-κB, ultimately leading to an increase in CD8+ T cell infiltration. 23 In a control study that included various types of malignant effusions, CXCL11 was found to be present in higher concentrations in malignant effusions than in non-malignant effusions. 94 CXCL11 also participates in the malignant biological behavior of lung cancer. The human runt-related transcription factor (RUNX) protein is instrumental in both normal development and tumor progression, among which RUNX3 is a tumor suppressor. The receptor activator of nuclear factor-κB ligand (RANKL) serves as an important pathway to mediate the differentiation of osteoclasts, thereby stimulating bone resorption. 95 This process requires the binding of RANKL to the receptor activator of nuclear factor-κB (RANK) to initiate, whereas osteoprotegerin (OPG) can prevent this process. Kim et al. found that in non-small-cell lung cancer, knocking out RUNX3 can downregulate CXCL11 mRNA expression. This affects the relative ratio of RANKL/OPG in osteoclasts, increasing the metastasis of tumors to bone tissue. 24 This suggests that CXCL11 can also counteract the osteolytic effects of NSCLC. In summary, CXCL11 is closely related to the prognosis of patients with lung cancer, the efficacy of immunotherapy, chemotherapy-induced immune cell infiltration, and anti-malignant biological behavior.
CXCL11 in Head and Neck Squamous Cell Carcinoma (HNSCC) (excluding Oral Carcinoma)
General head and neck tumors encompass three major parts: neck tumors otolaryngological tumours and oral and maxillofacial tumors. Most head and neck tumors are squamous cell carcinomas. Surgery and concurrent chemoradiotherapy are the main treatment methods for HNSCC. However, the era of immuno-oncology also highlights the limitations of traditional preclinical HNSCC models. Lymphatic endothelial cells (LECs) play a crucial role in lymph node metastasis of tumors. Inflammatory LECs in tumors can produce more CXCL11, promoting the expression of various carcinogenic factors such as MMP7. The overexpressed CXCR3 receptor in HNSCC ‘responds’ to these stimuli, activating the JAK-STAT and AKT pathways, further inducing EMT and chemotaxis toward LECs. 25 In fact, CXCL11 is widely expressed in head and neck tumor cell lines. Cao et al 56 found that low expression of an immune-related lncRNA, TRG-AS1, can cause the expression of CXCL11 in the tumor environment, which may be a new marker for predicting the efficacy of PD-1 treatment.In another control experiment on the impact of smoking on HNSCC, tobacco inhibited the expression of CXCR3 ligands in the immune microenvironment. Patients with a history of smoking have a lower number of PD-1+ positive cells, which may be due to the weakened ability of CXCL11 to recruit cytotoxic T cells and NK cells. 57 In summary, the CXCL11–CXCR3 axis has great potential for treating head and neck squamous cell carcinoma, and the specific inhibition of its expression in tumour cells without affecting the recruitment of immune cells should be a key research objective.
CXCL11 in oral cancer (OC)
Oral squamous cell carcinoma (OSCC) is a common pathological type of oral cancer. In OSCC, the expression of CXCL11 in tumor tissues is increased compared with that in normal tissues, and the content of its receptor subtype CXCR3-A, rather than CXCR3-B or CXCR-alt, is significantly increased. Moreover, the same trend is also observed in important precancerous lesions, oral leukoplakia (OLK). Simultaneously, knocking down CXCL11 inhibits the expression of intracellular immune checkpoints (mainly CD274 and IDO1). 26 This could be a potential mechanism causing immune escape in OSCC. Similarly, another receptor of CXCL11, CXCR7, has also been found to have increased expression in OSCC and OLK tissues. The CXCL11–CXCR7 axis is significantly correlated with the developmental stages of oral cancer and may start to play a role from the early stage of malignant transformation in the oral cavity. 27 Furthermore, the autoimmune regulator (AIRE) can interact with the oncogene ETS1 to promote the expression of CXCL11 in OSCC, but the mechanism of this mixed transcriptional activation is not yet fully understood. 96
CXCL11 in Skin Melanoma (SKCM)
Melanoma, the most malignant type of skin cancer, has always been the focus of attention for the development of treatment methods. Melanoma is one of the most sensitive malignant tumors to immune regulation. Although immunotherapy is currently widely used for treating unresectable or metastatic melanoma, its high cost and immune resistance of tumors limit its further clinical promotion. In a comprehensive study combining qRT–PCR and TCGA data analysis, CXCL11 was identified as one of the differentially expressed genes (DEGs) related to immune cell infiltration, and it has a negative correlation with the prognosis of melanoma. 28 In a similar fashion, Zhou et al. identified that SKCM patients with a lower transcription level of CXCL11 exhibited a more favorable prognosis in a bioinformatics analysis. 29 In an in vitro study using the M24Met melanoma cell line, it was discerned that the receptor CXCR3, a known target of CXCL11, could potentiate the migratory and invasive phenotypes of tumor cells. 30 However, researchers have also found that CXCL11 may enhance the immune response to melanoma by recruiting immune cells (CD8+ T cells) or serving as a marker for bone marrow activation (MA).31,97 CD13, a myeloid cell marker and metalloprotease, truncates CXCL11. This truncation not only results in the loss of chemotaxis of lymphocytes such as NK cells but also promotes local angiogenesis in the environment. 98 Additionally, current research has confirmed that a tumor microenvironment enriched with CD16+ macrophages enhances the response to combined PD-1 and CTLA-4 immune therapies. In melanomas with a higher density of CD16+ macrophages, an upregulation of CXCL11 gene expression is observed. 58 This indicates the functional role of CXCL11 in recruiting immune cells. Jacquelot et al. reported that the loss of CXCR3 on circulating T cell subsets was associated with skin or lymph node metastases. 99 The complexity of CXCL11 function in tumors may be related to the expression location of its receptor CXCR3. The expression of CXCR3 on locally infiltrating immune cells usually inhibits the development of melanoma, but the expression of this receptor on melanoma cells themselves can lead to metastatic invasion. These findings reveal new molecular strategies for the impact of impact on tumors and are expected to further promote the development of immunotherapy in melanoma.
CXCL11 in Other Malignant Tumors
We have also found that I-TAC plays a role in other types of tumors through various pathways. Anti-HER2 monoclonal antibody therapy has an important role in the comprehensive treatment of breast cancer (BC). In HER2 + BC tumourstumors low expression of the IFI16–CXCL11 axis can result in higher drug resistance. Suppression of the STING pathway mediated by Interferon-γ inducible protein 16 (IFI16) can lead to insufficient expression of CXCL11, thereby reducing the infiltration of CD8+ T cells and memory T cells. 32 If it is possible to ‘reverse’ its abnormal regulation through molecular means, this may be a new direction for future targeted drug research. The serum CXCL11 levels in HR + breast cancer patients are lower than those in HR patients, suggesting that CXCL11 may exhibit HR- dependent regulatory expression in breast cancer. 33 Chu et al. observed in their animal studies that the expression level of I-TAC in breast cancer tissues was downregulated, exhibiting a negative correlation with breast cancer staging. Furthermore, the injection of cells overexpressing I-TAC into juvenile Balb/c mouse tumors ultimately led to tumor regression. 34 However, another study found that senescent endothelial cells could enhance the invasiveness of breast cancer cells through the release of the CXCL11–CXCR3 axis. This process is achieved through activation of the ERK pathway. 35 Furthermore, intriguing findings suggest that exercise training can enhance the infiltration of CD8+ T cells into the tumor microenvironment (TME) through the CXCL11–CXCR3 axis. This augmentation contributes to an increased responsiveness of breast cancer to ICI treatment. 59 I-TAC can influence the progression of breast cancer through various molecular signaling pathways, and the above evidence provides a new direction for the treatment of breast cancer. In the hematological system, CXCL11 has also been studied in diffuse large B-cell lymphoma (DLBCL), chronic lymphocytic leukemia (CLL), and multiple myeloma (MM). I-TAC can activate tyrosine kinase and induce the secretion of MMP-2 and MMP-9 in MM cells, which contributes to the progression and metastasis of MM. However, if the surrounding immune cells express more CXCR3, then CXCL11 can induce immune-active cells to enter the tumor site and exert anticancer effects. 36 The mutation status of the immunoglobulin heavy chain variable region (IGHV-mutated) is a prognostic biomarker related to CLL, and CXCL11 is significantly associated with CLL. 37 CXCL11 can also serve as a diagnostic and progression prediction factor for diffuse large B-cell lymphoma. Zhou et al. found that CXCL11 is expressed more in patients with later-stage DLBCL and has a high diagnostic prediction ability (AUC = 1.000). 38 Additionally, an animal study demonstrated that CXCL11 can exert an anti-lymphoma effect through the recruitment of CD8+ cells. 100 Postmenopausal estrogen replacement therapy is an important risk factor for women to develop ovarian cancer (OV). Estrogen receptor α (ERα) can enhance the expression of the CXCL11–CXCR7 axis and promote EMT and invasion of OV cells in a feed-forward regulatory manner. 39 Simultaneously, it has been discovered that CXCL11–CXCR3 is pathologically elevated in OV with pre-lesions of endometriosis. Among them, the CXCR3A subtype is predominantly expressed in cancer cells and infiltrating lymphocytes, which may be related to the suppression of cytotoxic T cells in the local inflammatory environment.40,41 In pancreatic cancer (PAAD), which can be considered one of the most lethal malignant tumors, serum CXCL11 levels have been found to serve as an effective diagnostic indicator. 42 Similar findings have been observed in prostate adenocarcinoma (PRAD), where the serum CXCL11 content in patients with advanced prostate cancer is higher than that in healthy controls. 43 Basal cell carcinoma is the most common malignant skin tumor. In BCC (Basal Cell Carcinoma) cells, there is a significant upregulation of CXCL11 and all subtypes of its receptor CXCR3. At the same time, CXCL11 has been found in in vitro cell experiments to promote the proliferation of human immortalized keratinocytes. This indicates that the CXCL11–CXCR3 axis is an important signal transduction mechanism in BCC. 44 The positive feedback loop of CXCL11-EGF-EGFR promotes angiogenesis in anaplastic thyroid carcinoma (THCA), with the receptor CXCR7 involved. This provides a new approach for alotinib-targeted therapy for thyroid cancer. 45 In papillary thyroid carcinoma (PTC), the release of CXCL11 induced by IFN-γ inhibits tumor proliferation and spread, and PPARγ agonists can also cause similar effects. 46 However, the role of CXCL11 and its receptors in the aforementioned tumors requires further study.
Discussions
As an important mediator of leukocyte attraction in the body, CXCL11 undoubtedly plays an indispensable role in the tumor microenvironment. In addition to their chemotactic attraction, chemokines can guide the biological functions of immune cells that have receptors. In fact, as an IFN-induced chemokine, it often promotes or inhibits the occurrence and development of tumors by affecting lymphocyte infiltration. Li et al. found that CXCL11 is positively correlated with almost all immune-related genes in almost all cancers. 3 The application of immune checkpoint inhibitors such as PD-L1 has been associated with CXCL11 in various tumors. It can be imagined that if a method is found to enhance the release of CXCL11 in the local environment, immune checkpoint inhibitors can play a greater role for treating malignant tumors.
When we are looking for new ‘druggable’ targets in tumor treatment, we are often looking for proteins that can be regulated. Currently, antitumor treatments targeting chemokines and their receptors have been studied in tumors such as breast cancer, 101 kidney cancer, 90 acute lymphoblastic leukemia 102 and ovarian cancer. 103 At the outset, research on highly targeted therapeutic interventions for CXCL11 did not show significant advancement. However, as investigations progressed, the preclinical challenges associated with CXCL11 and its receptors garnered sustained attention. CXCR3 gained early clinical attention, and in 2009, a randomized double-blind pharmacokinetic study involving 9 volunteers was conducted for the oral administration of AMG 487 (a selective bioavailable CXCR3 antagonist). The study also observed dose- and time-dependent kinetics, particularly at high doses. 104 Melanoma is a highly immunogenic cutaneous malignancy. In addition, melanoma vaccines play a crucial role for treating late-stage patients. A clinical study aimed at evaluating intratumoral injection of IFN-γ during vaccination in patients with metastatic melanoma (Identifier: NCT00977145, https://ClinicalTrials.gov) indicates that IFN-γ can induce the expression on tumor antigen-specific T cells. In subsequent investigations, researchers reiterated that the administration of the peptide vaccine in montanide adjuvant induces peripheral CD8+ T cells, with a predominant positive expression of CXCR3. 105 This outcome suggests that enhancing the induction of circulating CXCR3 + immune cells represents a viable avenue for controlling malignant tumors. In studies constructing relevant biological indicators, CXCL11 and its receptors receive comparatively more comprehensive evaluation. A clinical trial measuring the expression levels and prognostic value of CXCR3 in renal cell carcinoma (Identifier: NCT01339975, https://ClinicalTrials.gov) has completed recruitment. They posit that a low CXCR3 tumor expression level is associated with unfavorable prognostic factors in RCC; however, further evidence is required. At present, clinical studies primarily consider CXCL11 as an accompanying indicator for evaluating treatments in metastatic NSCLC, peritoneal surface malignancies, and non-melanoma skin cancer (Identifier: NCT03168464; NCT03458117; NCT02151448, https://ClinicalTrials.gov). Researchers incorporate the levels in peripheral blood or local tissues as crucial indicators for observation. The expression levels of CXCL11 and its receptors may reflect the biological behavior of malignant tumors, providing predictive information on treatment outcomes. In glioblastoma, Wang et al 106 experimentally equipped oncolytic adenoviruses with the chemokine CXCL11 to enhance CAR-T cell infiltration and modify the immunosuppressive TME.These advancements are expected to prompt increased attention from clinical research toward the application of CXCL11 and its receptors in the field of oncology, thereby driving a deeper understanding of chemokine.
The main receptor of CXCL11, CXCR3, has different subtypes. We found that CXCR3-A often promotes the occurrence and migration of tumors through the autocrine axis, whereas CXCR3-B does the opposite. CXCR3 can recruit CTL, NK cells, macrophages and other anti-tumour immune cells to increase the inflammatory response. Simultaneously, CXCR3-B can also inhibit tumor angiogenesis. This process may be mediated by activation of the MAPK pathway. 107 The onset and progression of neoplastic diseases are often associated with dysregulation of the body’s immune functions. In the early tumorigenic microenvironment, the activity of immune cells may be compromised, leading to insufficient immune responses against tumors. In fact, the occurrence of tumors is often accompanied by a change in the ratio of CXCR3-A/CXCR3-B from low to high. 62 Changes in the tumor microenvironment, such as the degree of inflammation and immune cell infiltration, may influence the expression and activity of CXCR3 subtypes. Dynamically monitoring changes in CXCR3 subtypes can aid in predicting tumor development and behavior, particularly in the significant prediction of tumor migration and metastasis. Furthermore, a comprehensive understanding of CXCR3 subtypes can provide a basis for designing personalized treatment strategies and enhance the predictability of antitumor treatment outcomes. However, as mentioned earlier in this article, the realization of such concepts may pose challenges because of the high structural similarity between CXCR3-A and CXCR3-B. Returning to the discussion, considering the distinct cellular localization of CXCR3 expression, it is hypothesized that the regulation of CXCL11 to selectively bind with a specific receptor holds promising therapeutic potential. We acknowledge that one of the core goals of precision medicine is to avoid unnecessary treatment side effects. By understanding the molecular interaction between CXCL11 and CXCR3 subtypes, it may be possible to circumvent treatment approaches that may not be suitable for certain patients, thus reducing treatment risks. Shifting the topic to CXCR7, as a seven-transmembrane receptor (7TMR), they are the most common targets in medical treatment. 108 Contrary to the long-held belief that CXCR7 could not signal through the traditional heterotrimeric G protein, thereby diminishing its pharmacological significance, this receptor exhibits unique value in malignant tumors such as ovarian cancer or thyroid cancer.39,45 Initially identified as negative regulators of G protein signaling, β-arrestings were later found to positively regulate cellular signaling. In our evaluation of the medicalization or diaphanization of CXCR7, we may have only focused on the measurement of G protein-mediated effects. In the future, observing the biological characteristics of both G proteins and β-arrestins will help us gain a more comprehensive understanding of CXCR7 pharmacology.
Most of the research on CXCL11 in oncology is based on correlation studies at the molecular expression level, bioinformatics analysis data, and in vitro experiments. There is still a lack of direct evidence to promote its other practical applications. Given the complexity of chemokine regulatory mechanisms, CXCL11 often has a bidirectional effect on tumor development (and often may be the same receptor). Therefore, its clinical application has been in a relatively limited situation for a long time. As mentioned earlier in this article, it is worth exploring agonists or neutralizing antibodies against CXCL11, and even high-targeting antagonists against different receptor subtypes. At the same time, gradually available scientific models are being built. Some clinical studies have used it as an indicator of biological effects. This progress has a positive effect on the ability to establish tumor risk stratification, predict treatment responses and assess patient prognosis. At the same time, the relationship between CXCL11 and chemotherapy resistance sensitivity should not be ignored, and the study of I-TAC’s auxiliary role in existing therapies is also a direction worth exploring. Several prospective studies have clearly indicated that CXCL11 plays an important role in various diseases. However, to deeply understand its specific molecular biological mechanisms and related signaling pathways, further research is required.
Conclusions and Prospects
Tumors are not merely composed of a single type of abnormal cells; rather, they resemble a multidimensional community that brings together members of various cell types. In particular, growing research in recent years has demonstrated that CXCL11 influences tumor cell functions through various downstream signaling pathways. Their aberrant differential expression in malignant tumors results in the recruitment of leukocytes, angiogenesis, epithelial– mesenchymal transition, proliferation, and metastasis of cancer cells, but can also evoke anti-tumor effects. It is worth emphasizing that the role of chemokines is no longer limited to a single molecular regulatory pathway. They can not only serve as independent predictors for diagnosis but also can become new targets for tumor treatment. An in-depth understanding of the molecular mechanisms of CXCL11 in various tumor types will contribute to the design of personalized therapeutic strategies for cancer.
Appendix.
Abbreviation Full Name or Explanation
- AKT
Protein kinase B
- BCG
Bacillus Calmette-Guérin
- ccRCC
Clear cell renal cell carcinoma
- ceRNA
Competing endogenous RNA
- CXCL11
C-X-C motif chemokine ligand 11
- CXCR3
C-X-C motif chemokine receptor 3
- CXCR7
C-X-C motif chemokine receptor 7
- ELR
The Glu–Leu– Arg amino acid sequence potentially present at the terminus of the CXC chemokine protein
- EMT
Epithelial-mesenchymal transition
- EP300/CBP
E1A Binding Protein P300/CREB Binding Protein
- ICIs
Immune checkpoint inhibitors
- ICL
IFN-inducible CXCR3 ligands (such as CXCL9, CXCL10 and CXCL11)
- ICL score
Prespecified scoring system based on the expression levels of CXCR3 ligands
- IDO1
Indoleamine 2, 3-dioxygenase 1
- IFI16
Interferon-γ inducible protein 16
- IFIT
Interferon-induced proteins with tetratricopeptide repeats
- IFN
Interferon (mainly comprising α, β, and γ types)
- I-TAC
IFN-inducible T cell α chemoattractant
- NF-κB
Nuclear Factor Kappa Light Chain Enhancer of Activated B Cells
- NMR
Nuclear magnetic resonance
- NSCLC
Non-small cell lung cancer
- OLK
Oral leukoplakia (a precancerous lesion)
- PD-L1
Programmed Death-Ligand 1
- PI3K
Phosphoinositide 3-kinase
- PPARγ
Peroxisome proliferator-activated receptor gamma
- RBP-Jκ
Recombination Signal Binding Protein for the Immunoglobulin Kappa J Region
- STAT
Signal transducers and activators of transcription
- STING
Stimulator of Interferon Genes, a protein encoded by TMEM173, serves as a crucial component of the STING pathway, participating in the cellular innate immune response
- TAMs
Tumour-associated macrophages
- TGF
Transforming growth factor
- TSCM
T memory stem cells
Footnotes
Author Contributions: JW: As the first author, responsible for overall paper writing and organization. XO: Involved in literature review and data collection. Made significant contributions to the content modification and revision of the paper. WZ: Involved in data collection and drew the tables. QY: Designed and drew the figures. Corresponding Author (JZ): Responsible for the research design and supervision, actively participated in paper writing and revision, and took responsibility for the paper’s modifications and submission process and the access to funding. All authors have read and agreed to the published version of the manuscript.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was funded by the National Natural Science Foundation of China (grant number 82260604) and the Natural Science Foundation of Jiangxi Province (grant number 20192BAB205053).
Ethical Statement
Ethical Approval
Our study did not require an ethical board approval because it did not contain human or animal trials.
ORCID iD
Jiaqi Wang https://orcid.org/0009-0000-6057-0687
References
- 1.WHO . Global Health Estimates 2020: Deaths by Cause, Age, Sex, by Country and by Region. Geneva, Switzerland: WHO; 2023. [Google Scholar]
- 2.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359-E386. [DOI] [PubMed] [Google Scholar]
- 3.Li Y, Han S, Wu B, et al. CXCL11 correlates with immune infiltration and impacts patient immunotherapy efficacy: a pan-cancer analysis. Front Immunol. 2022;13:951247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balkwill F. Cancer and the chemokine network. Nat Rev Cancer. 2004;4(7):540-550. [DOI] [PubMed] [Google Scholar]
- 5.Liu M, Fu X, Jiang L, et al. Colon cancer cells secreted CXCL11 via RBP-Jκ to facilitated tumour-associated macrophage-induced cancer metastasis. J Cell Mol Med. 2021;25(22):10575-10590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zeng YJ, Lai W, Wu H, et al. Neuroendocrine-like cells -derived CXCL10 and CXCL11 induce the infiltration of tumor-associated macrophage leading to the poor prognosis of colorectal cancer. Oncotarget. 2016;7(19):27394-27407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li H, Rong S, Chen C, et al. Disparate roles of CXCR3A and CXCR3B in regulating progressive properties of colorectal cancer cells. Mol Carcinog. 2019;58(2):171-184. [DOI] [PubMed] [Google Scholar]
- 8.Zhang C, Li Z, Xu L, et al. CXCL9/10/11, a regulator of PD-L1 expression in gastric cancer. BMC Cancer. 2018;18(1):462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Verbeke H, Geboes K, Van Damme J, Struyf S. The role of CXC chemokines in the transition of chronic inflammation to esophageal and gastric cancer. Biochim Biophys Acta. 2012;1825(1):117-129. [DOI] [PubMed] [Google Scholar]
- 10.Kraft M, Riedel S, Maaser C, et al. IFN-gamma synergizes with TNF-alpha but not with viable H. pylori in up-regulating CXC chemokine secretion in gastric epithelial cells. Clin Exp Immunol. 2001;126(3):474-481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pasini FS, Zilberstein B, Snitcovsky I, et al. A gene expression profile related to immune dampening in the tumor microenvironment is associated with poor prognosis in gastric adenocarcinoma. J Gastroenterol. 2014;49(11):1453-1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Y, Zhao W, Li S, et al. CXCL11 promotes self-renewal and tumorigenicity of α2δ1(+) liver tumor-initiating cells through CXCR3/ERK1/2 signaling. Cancer Lett. 2019;449:163-171. [DOI] [PubMed] [Google Scholar]
- 13.Liu G, Yang ZF, Sun J, et al. The LINC00152/miR-205-5p/CXCL11 axis in hepatocellular carcinoma cancer-associated fibroblasts affects cancer cell phenotypes and tumor growth. Cell Oncol (Dordr). 2022;45(6):1435-1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tacke F, Zimmermann HW, Berres ML, Trautwein C, Wasmuth HE. Serum chemokine receptor CXCR3 ligands are associated with progression, organ dysfunction and complications of chronic liver diseases. Liver Int. 2011;31(6):840-849. [DOI] [PubMed] [Google Scholar]
- 15.Chalin A, Lefevre B, Devisme C, et al. Circulating levels of CXCL11 and CXCL12 are biomarkers of cirrhosis in patients with chronic hepatitis C infection. Cytokine. 2019;117:72-78. [DOI] [PubMed] [Google Scholar]
- 16.Berres ML, Lehmann J, Jansen C, et al. Chemokine (C-X-C motif) ligand 11 levels predict survival in cirrhotic patients with transjugular intrahepatic portosystemic shunt. Liver Int. 2016;36(3):386-394. [DOI] [PubMed] [Google Scholar]
- 17.Vollmer T, Schlickeiser S, Amini L, et al. The intratumoral CXCR3 chemokine system is predictive of chemotherapy response in human bladder cancer. Sci Transl Med. 2021;13(576). [DOI] [PubMed] [Google Scholar]
- 18.Nazari A, Ahmadi Z, Hassanshahi G, et al. Effective treatments for bladder cancer affecting CXCL9/CXCL10/CXCL11/CXCR3 Axis: a review. Oman Med J. 2020;35(2):e103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zeng X, Chen K, Li L, et al. Epigenetic activation of RBM15 promotes clear cell renal cell carcinoma growth, metastasis and macrophage infiltration by regulating the m6A modification of CXCL11. Free Radic Biol Med. 2022;184:135-147. [DOI] [PubMed] [Google Scholar]
- 20.Suyama T, Furuya M, Nishiyama M, et al. Up-regulation of the interferon gamma (IFN-gamma)-inducible chemokines IFN-inducible T-cell alpha chemoattractant and monokine induced by IFN-gamma and of their receptor CXC receptor 3 in human renal cell carcinoma. Cancer. 2005;103(2):258-267. [DOI] [PubMed] [Google Scholar]
- 21.Gacci M, Serni S, Lapini A, et al. CXCR3-B expression correlates with tumor necrosis extension in renal cell carcinoma. J Urol. 2009;181(2):843-848. [DOI] [PubMed] [Google Scholar]
- 22.Mitsuhashi A, Kondoh K, Horikawa K, et al. Programmed death (PD)-1/PD-ligand 1 blockade mediates antiangiogenic effects by tumor-derived CXCL10/11 as a potential predictive biomarker. Cancer Sci. 2021;112(12):4853-4866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao Q, Wang S, Chen X, et al. Cancer-cell-secreted CXCL11 promoted CD8(+) T cells infiltration through docetaxel-induced-release of HMGB1 in NSCLC. J Immunother Cancer. 2019;7(1):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim HJ, Park J, Lee SK, et al. Loss of RUNX3 expression promotes cancer-associated bone destruction by regulating CCL5, CCL19 and CXCL11 in non-small cell lung cancer. J Pathol. 2015;237(4):520-531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumaravel S, Singh S, Roy S, et al. CXCL11-CXCR3 Axis mediates tumor lymphatic cross talk and inflammation-induced tumor, promoting pathways in head and neck cancers. Am J Pathol. 2020;190(4):900-915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang X, Zhang J, Zhou G. The CXCL11-CXCR3A axis influences the infiltration of CD274 and Ido1 in oral squamous cell carcinoma. J Oral Pathol Med. 2021;50(4):362-370. [DOI] [PubMed] [Google Scholar]
- 27.Xia J, Wang J, Chen N, et al. Expressions of CXCR7/ligands may be involved in oral carcinogenesis. J Mol Histol. 2011;42(2):175-180. [DOI] [PubMed] [Google Scholar]
- 28.Tímár J, Ladányi A. Molecular pathology of skin melanoma: epidemiology, differential diagnostics, prognosis and therapy prediction. Int J Mol Sci. 2022;23(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou X, Peng M, He Y, et al. CXC chemokines as therapeutic targets and prognostic biomarkers in skin cutaneous melanoma microenvironment. Front Oncol. 2021;11:619003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kawada K, Sonoshita M, Sakashita H, et al. Pivotal role of CXCR3 in melanoma cell metastasis to lymph nodes. Cancer Res. 2004;64(11):4010-4017. [DOI] [PubMed] [Google Scholar]
- 31.Harlin H, Meng Y, Peterson AC, et al. Chemokine expression in melanoma metastases associated with CD8+ T-cell recruitment. Cancer Res. 2009;69(7):3077-3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ong LT, Lee WC, Ma S, et al. IFI16-dependent STING signaling is a crucial regulator of anti-HER2 immune response in HER2+ breast cancer. Proc Natl Acad Sci U S A. 2022;119(31):e2201376119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ejaeidi AA, Craft BS, Puneky LV, Lewis RE, Cruse JM. Hormone receptor-independent CXCL10 production is associated with the regulation of cellular factors linked to breast cancer progression and metastasis. Exp Mol Pathol. 2015;99(1):163-172. [DOI] [PubMed] [Google Scholar]
- 34.Chu Y, Yang X, Xu W, et al. In situ expression of IFN-gamma-inducible T cell alpha chemoattractant in breast cancer mounts an enhanced specific anti-tumor immunity which leads to tumor regression. Cancer Immunol Immunother. 2007;56(10):1539-1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hwang HJ, Lee YR, Kang D, et al. Endothelial cells under therapy-induced senescence secrete CXCL11, which increases aggressiveness of breast cancer cells. Cancer Lett. 2020;490:100-110. [DOI] [PubMed] [Google Scholar]
- 36.Pellegrino A, Antonaci F, Russo F, et al. CXCR3-binding chemokines in multiple myeloma. Cancer Lett. 2004;207(2):221-227. [DOI] [PubMed] [Google Scholar]
- 37.Landeira-Viñuela A, Arias-Hidalgo C, Juanes-Velasco P, et al. Unravelling soluble immune checkpoints in chronic lymphocytic leukemia: physiological immunomodulators or immune dysfunction. Front Immunol. 2022;13:965905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou X, Guo S, Shi Y. Comprehensive analysis of the expression and significance of CXCLs in human diffuse large B-cell lymphoma. Sci Rep. 2022;12(1):2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Benhadjeba S, Edjekouane L, Sauvé K, Carmona E, Tremblay A. Feedback control of the CXCR7/CXCL11 chemokine axis by estrogen receptor α in ovarian cancer. Mol Oncol. 2018;12(10):1689-1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Furuya M, Suyama T, Usui H, et al. Up-regulation of CXC chemokines and their receptors: implications for proinflammatory microenvironments of ovarian carcinomas and endometriosis. Hum Pathol. 2007;38(11):1676-1687. [DOI] [PubMed] [Google Scholar]
- 41.Furuya M, Yoneyama T, Miyagi E, et al. Differential expression patterns of CXCR3 variants and corresponding CXC chemokines in clear cell ovarian cancers and endometriosis. Gynecol Oncol. 2011;122(3):648-655. [DOI] [PubMed] [Google Scholar]
- 42.Torres C, Perales S, Alejandre MJ, et al. Serum cytokine profile in patients with pancreatic cancer. Pancreas. 2014;43(7):1042-1049. [DOI] [PubMed] [Google Scholar]
- 43.Klee EW, Bondar OP, Goodmanson MK, et al. Candidate serum biomarkers for prostate adenocarcinoma identified by mRNA differences in prostate tissue and verified with protein measurements in tissue and blood. Clin Chem. 2012;58(3):599-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lo BK, Yu M, Zloty D, et al. CXCR3/ligands are significantly involved in the tumorigenesis of basal cell carcinomas. Am J Pathol. 2010;176(5):2435-2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liang J, Jin Z, Kuang J, et al. The role of anlotinib-mediated EGFR blockade in a positive feedback loop of CXCL11-EGF-EGFR signalling in anaplastic thyroid cancer angiogenesis. Br J Cancer. 2021;125(3):390-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fallahi P, Ferrari SM, Piaggi S, et al. The paramount role of cytokines and chemokines in papillary thyroid cancer: a review and experimental results. Immunol Res. 2018;66(6):710-722. [DOI] [PubMed] [Google Scholar]
- 47.Hughes CE, Nibbs R. A guide to chemokines and their receptors. FEBS J. 2018;285(16):2944-2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000;12(2):121-127. [DOI] [PubMed] [Google Scholar]
- 49.Horuk R. Chemokine receptors. Cytokine Growth Factor Rev. 2001;12(4):313-335. [DOI] [PubMed] [Google Scholar]
- 50.Vandercappellen J, Van Damme J, Struyf S. The role of CXC chemokines and their receptors in cancer. Cancer Lett. 2008;267(2):226-244. [DOI] [PubMed] [Google Scholar]
- 51.Arya M, Patel HR. Expanding role of chemokines and their receptors in cancer. Expert Rev Anticancer Ther. 2003;3(6):749-752. [DOI] [PubMed] [Google Scholar]
- 52.Keeley EC, Mehrad B, Strieter RM. CXC chemokines in cancer angiogenesis and metastases. Adv Cancer Res. 2010;106:91-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Müller M, Carter S, Hofer MJ, Campbell IL. Review: the chemokine receptor CXCR3 and its ligands CXCL9, CXCL10 and CXCL11 in neuroimmunity--a tale of conflict and conundrum. Neuropathol Appl Neurobiol. 2010;36(5):368-387. [DOI] [PubMed] [Google Scholar]
- 54.Booth V, Clark-Lewis I, Sykes BD. NMR structure of CXCR3 binding chemokine CXCL11 (ITAC). Protein Sci. 2004;13(8):2022-2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nuñez NG, Berner F, Friebel E, et al. Immune signatures predict development of autoimmune toxicity in patients with cancer treated with immune checkpoint inhibitors. Méd. 2023;4(2):113-129.e7. [DOI] [PubMed] [Google Scholar]
- 56.Cao R, Cui L, Zhang J, et al. Immune-related lncRNA classification of head and neck squamous cell carcinoma. Cancer Cell Int. 2022;22(1):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.de la Iglesia JV, Slebos R, Martin-Gomez L, et al. Effects of tobacco smoking on the tumor immune microenvironment in head and neck squamous cell carcinoma. Clin Cancer Res. 2020;26(6):1474-1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee H, Ferguson AL, Quek C, et al. Intratumoral CD16+ macrophages are associated with clinical outcomes of patients with metastatic melanoma treated with combination anti-PD-1 and anti-CTLA-4 therapy. Clin Cancer Res. 2023;29(13):2513-2524. [DOI] [PubMed] [Google Scholar]
- 59.Gomes-Santos IL, Amoozgar Z, Kumar AS, et al. Exercise training improves tumor control by increasing CD8(+) T-cell infiltration via CXCR3 signaling and sensitizes breast cancer to immune checkpoint blockade. Cancer Immunol Res. 2021;9(7):765-778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Koch C, Fischer NC, Puchert M, Engele J. Interactions of the chemokines CXCL11 and CXCL12 in human tumor cells. BMC Cancer. 2022;22(1):1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Loetscher M, Loetscher P, Brass N, Meese E, Moser B. Lymphocyte-specific chemokine receptor CXCR3: regulation, chemokine binding and gene localization. Eur J Immunol. 1998;28(11):3696-3705. [DOI] [PubMed] [Google Scholar]
- 62.Ma B, Khazali A, Wells A. CXCR3 in carcinoma progression. Histol Histopathol. 2015;30(7):781-792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ehlert JE, Addison CA, Burdick MD, Kunkel SL, Strieter RM. Identification and partial characterization of a variant of human CXCR3 generated by posttranscriptional exon skipping. J Immunol. 2004;173(10):6234-6240. [DOI] [PubMed] [Google Scholar]
- 64.Datta D, Flaxenburg JA, Laxmanan S, et al. Ras-induced modulation of CXCL10 and its receptor splice variant CXCR3-B in MDA-MB-435 and MCF-7 cells: relevance for the development of human breast cancer. Cancer Res. 2006;66(19):9509-9518. [DOI] [PubMed] [Google Scholar]
- 65.Kouroumalis A, Nibbs RJ, Aptel H, et al. The chemokines CXCL9, CXCL10, and CXCL11 differentially stimulate G alpha i-independent signaling and actin responses in human intestinal myofibroblasts. J Immunol. 2005;175(8):5403-5411. [DOI] [PubMed] [Google Scholar]
- 66.Ali A, Canaday LM, Feldman HA, et al. Natural killer cell immunosuppressive function requires CXCR3-dependent redistribution within lymphoid tissues. J Clin Invest. 2021;131(18). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wu Z, Zhang Y, Chen X, et al. Characterization of the prognostic values of the CXCR1-7 in clear cell renal cell carcinoma (ccRCC) microenvironment. Front Mol Biosci. 2020;7:601206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Andrews SP, Cox RJ. Small molecule CXCR3 antagonists. J Med Chem. 2016;59(7):2894-2917. [DOI] [PubMed] [Google Scholar]
- 69.Clark-Lewis I, Kim KS, Rajarathnam K, et al. Structure-activity relationships of chemokines. J Leukoc Biol. 1995;57(5):703-711. [DOI] [PubMed] [Google Scholar]
- 70.Fernandez EJ, Lolis E. Structure, function, and inhibition of chemokines. Annu Rev Pharmacol Toxicol. 2002;42:469-499. [DOI] [PubMed] [Google Scholar]
- 71.Libert F, Parmentier M, Lefort A, et al. Selective amplification and cloning of four new members of the G protein-coupled receptor family. Science. 1989;244(4904):569-572. [DOI] [PubMed] [Google Scholar]
- 72.Sreedharan SP, Robichon A, Peterson KE, Goetzl EJ. Cloning and expression of the human vasoactive intestinal peptide receptor. Proc Natl Acad Sci U S A. 1991;88(11):4986-4990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Libert F, Passage E, Parmentier M, et al. Chromosomal mapping of A1 and A2 adenosine receptors, VIP receptor, and a new subtype of serotonin receptor. Genomics. 1991;11(1):225-227. [DOI] [PubMed] [Google Scholar]
- 74.Balabanian K, Lagane B, Infantino S, et al. The chemokine SDF-1/CXCL12 binds to and signals through the orphan receptor RDC1 in T lymphocytes. J Biol Chem. 2005;280(42):35760-35766. [DOI] [PubMed] [Google Scholar]
- 75.Burns JM, Summers BC, Wang Y, et al. A novel chemokine receptor for SDF-1 and I-TAC involved in cell survival, cell adhesion, and tumor development. J Exp Med. 2006;203(9):2201-2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Broberg K, Zhang M, Strömbeck B, et al. Fusion of RDC1 with HMGA2 in lipomas as the result of chromosome aberrations involving 2q35-37 and 12q13-15. Int J Oncol. 2002;21(2):321-326. [PubMed] [Google Scholar]
- 77.Rajagopal S, Kim J, Ahn S, et al. Beta-arrestin- but not G protein-mediated signaling by the “decoy” receptor CXCR7. Proc Natl Acad Sci U S A. 2010;107(2):628-632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bachelerie F, Ben-Baruch A, Burkhardt AM, et al. International Union of Basic and Clinical Pharmacology. [corrected]. LXXXIX. Update on the extended family of chemokine receptors and introducing a new nomenclature for atypical chemokine receptors. Pharmacol Rev. 2014;66(1):1-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Khare T, Bissonnette M, Khare S. CXCL12-CXCR4/CXCR7 Axis in colorectal cancer: therapeutic target in preclinical and clinical studies. Int J Mol Sci. 2021;22(14). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yu Y, Li H, Xue B, et al. SDF-1/CXCR7 axis enhances ovarian cancer cell invasion by MMP-9 expression through p38 MAPK pathway. DNA Cell Biol. 2014;33(8):543-549. [DOI] [PubMed] [Google Scholar]
- 81.Gritsina G, Yu J. CXCR7 as a novel therapeutic target for advanced prostate cancer. Oncogene. 2023;42(11):785-792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Xu S, Tang J, Wang C, et al. CXCR7 promotes melanoma tumorigenesis via Src kinase signaling. Cell Death Dis. 2019;10(3):191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tyagi A, Sharma AK, Damodaran C. A review on Notch signaling and colorectal cancer. Cells. 2020;9(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rupertus K, Sinistra J, Scheuer C, et al. Interaction of the chemokines I-TAC (CXCL11) and SDF-1 (CXCL12) in the regulation of tumor angiogenesis of colorectal cancer. Clin Exp Metastasis. 2014;31(4):447-459. [DOI] [PubMed] [Google Scholar]
- 85.Kollmar O, Rupertus K, Scheuer C, et al. CXCR4 and CXCR7 regulate angiogenesis and CT26.WT tumor growth independent from SDF-1. Int J Cancer. 2010;126(6):1302-1315. [DOI] [PubMed] [Google Scholar]
- 86.Cao Y, Jiao N, Sun T, et al. CXCL11 correlates with antitumor immunity and an improved prognosis in colon cancer. Front Cell Dev Biol. 2021;9:646252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Helbig KJ, Ruszkiewicz A, Semendric L, et al. Expression of the CXCR3 ligand I-TAC by hepatocytes in chronic hepatitis C and its correlation with hepatic inflammation. Hepatology. 2004;39(5):1220-1229. [DOI] [PubMed] [Google Scholar]
- 88.Chenard S, Robert SD, Koti M. The CXCR3alt-CXCL11 axis in bladder cancer: potential for prediction of neoadjuvant chemotherapy response. Cell Mol Immunol. 2021;18(7):1631-1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sun X, Chen Q, Zhang L, Chen J, Zhang X. Exploration of prognostic biomarkers and therapeutic targets in the microenvironment of bladder cancer based on CXC chemokines. Math Biosci Eng. 2021;18(5):6262-6287. [DOI] [PubMed] [Google Scholar]
- 90.Berlato C, Khan MN, Schioppa T, et al. A CCR4 antagonist reverses the tumor-promoting microenvironment of renal cancer. J Clin Invest. 2017;127(3):801-813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dai S, Zeng H, Liu Z, et al. Intratumoral CXCL13(+)CD8(+)T cell infiltration determines poor clinical outcomes and immunoevasive contexture in patients with clear cell renal cell carcinoma. J Immunother Cancer. 2021;9(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Liu W, Liu Y, Fu Q, et al. Elevated expression of IFN-inducible CXCR3 ligands predicts poor prognosis in patients with non-metastatic clear-cell renal cell carcinoma. Oncotarget. 2016;7(12):13976-13983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Thai AA, Solomon BJ, Sequist LV, Gainor JF, Heist RS. Lung cancer. Lancet. 2021;398(10299):535-554. [DOI] [PubMed] [Google Scholar]
- 94.Atanackovic D, Cao Y, Kim JW, et al. The local cytokine and chemokine milieu within malignant effusions. Tumour Biol. 2008;29(2):93-104. [DOI] [PubMed] [Google Scholar]
- 95.Hsu YL, Hung JY, Ko YC, et al. Phospholipase D signaling pathway is involved in lung cancer-derived IL-8 increased osteoclastogenesis. Carcinogenesis. 2010;31(4):587-596. [DOI] [PubMed] [Google Scholar]
- 96.Nguyen C, Sawangarun W, Mandasari M, et al. AIRE is induced in oral squamous cell carcinoma and promotes cancer gene expression. PLoS One. 2020;15(2):e0222689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kremenovic M, Rombini N, Chan AA, et al. Characterization of a myeloid activation signature that correlates with survival in melanoma patients. Cancers. 2020;12(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Proost P, Mortier A, Loos T, et al. Proteolytic processing of CXCL11 by CD13/aminopeptidase N impairs CXCR3 and CXCR7 binding and signaling and reduces lymphocyte and endothelial cell migration. Blood. 2007;110(1):37-44. [DOI] [PubMed] [Google Scholar]
- 99.Jacquelot N, Enot DP, Flament C, et al. Chemokine receptor patterns in lymphocytes mirror metastatic spreading in melanoma. J Clin Invest. 2016;126(3):921-937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hensbergen PJ, Wijnands PG, Schreurs MW, et al. The CXCR3 targeting chemokine CXCL11 has potent antitumor activity in vivo involving attraction of CD8+ T lymphocytes but not inhibition of angiogenesis. J Immunother. 2005;28(4):343-351. [DOI] [PubMed] [Google Scholar]
- 101.Schott AF, Goldstein LJ, Cristofanilli M, et al. Phase ib pilot study to evaluate reparixin in combination with weekly paclitaxel in patients with HER-2-negative metastatic breast cancer. Clin Cancer Res. 2017;23(18):5358-5365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hong Z, Wei Z, Xie T, et al. Targeting chemokines for acute lymphoblastic leukemia therapy. J Hematol Oncol. 2021;14(1):48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Xue J, Li R, Gao D, Chen F, Xie H. CXCL12/CXCR4 axis-targeted dual-functional nano-drug delivery system against ovarian cancer. Int J Nanomed. 2020;15:5701-5718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tonn GR, Wong SG, Wong SC, et al. An inhibitory metabolite leads to dose- and time-dependent pharmacokinetics of (R)-N-{1-[3-(4-ethoxy-phenyl)-4-oxo-3,4-dihydro-pyrido[2,3-d]pyrimidin-2-yl]-ethyl}-N-pyridin-3-yl-methyl-2-(4-trifluoromethoxy-phenyl)-acetamide (AMG 487) in human subjects after multiple dosing. Drug Metab Dispos. 2009;37(3):502-513. [DOI] [PubMed] [Google Scholar]
- 105.Clancy-Thompson E, King LK, Nunnley LD, Mullins IM, Slingluff CL, Mullins DW. Peptide vaccination in Montanide adjuvant induces and GM-CSF increases CXCR3 and cutaneous lymphocyte antigen expression by tumor antigen-specific CD8 T cells. Cancer Immunol Res. 2013;1(5):332-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wang G, Zhang Z, Zhong K, et al. CXCL11-armed oncolytic adenoviruses enhance CAR-T cell therapeutic efficacy and reprogram tumor microenvironment in glioblastoma. Mol Ther. 2023;31(1):134-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Petrai I, Rombouts K, Lasagni L, et al. Activation of p38(MAPK) mediates the angiostatic effect of the chemokine receptor CXCR3-B. Int J Biochem Cell Biol. 2008;40(9):1764-1774. [DOI] [PubMed] [Google Scholar]
- 108.Ma P, Zemmel R. Value of novelty? Nat Rev Drug Discov. 2002;1(8):571-572. [DOI] [PubMed] [Google Scholar]