Abstract
Transcriptional enhancers within the long terminal repeats of murine leukemia viruses are major determinants of the pathogenic properties of these viruses. Mutations were introduced into the adjacent binding sites for three transcription factors within the enhancer of the T-cell-lymphomagenic virus SL3-3. The sites that were tested were, in 5′-to-3′ order, a binding site for core binding factor (CBF) called core II, a binding site for c-Myb, a site that binds members of the Ets family of factors, and a second CBF binding site called core I. Mutation of each site individually reduced transcriptional activity in T lymphocytes. However, mutation of the Myb and core I binding sites had larger effects than mutation of the Ets or core II site. The relative effects on transcription in T cells paralleled the effects of the same mutations on viral lymphomagenicity, consistent with the idea that the role of these sequences in viral lymphomagenicity is indeed to regulate transcription in T cells. Mutations were also introduced simultaneously into multiple sites in the SL3-3 enhancer. The inhibitory effects of these mutations indicated that the transcription factor in T cells that recognizes the core I element of SL3-3, presumably CBF, needed to synergize with one or more factors bound at the upstream sites to function. This was tested further by generating a multimer construct that contained five tandem core I elements linked to a basal long terminal repeat promoter. This construct was inactive in T cells. However, transcriptional activity was detected with a multimer construct in which the transcription factor binding sites upstream of the core were also present. These results are consistent with the hypothesis that CBF requires heterologous transcription factors bound at nearby sites to function in T cells.
Transcriptional enhancers within the long terminal repeats (LTRs) of murine leukemia viruses (MuLVs) are major genetic determinants of tumorigenicity of these viruses (8, 12, 16, 25, 28, 29, 43, 51). These sequences are important determinants of the cell type specificity of viral transformation, the fraction of inoculated mice that develop disease, and the length of the latent period before tumors appear. SL3-3 (SL3) is a retrovirus that induces T-cell lymphomas in mice (28, 40). The LTR enhancer of this virus is composed of two tandem 72-bp repeat units. A fraction of each 72-bp unit in the SL3 enhancer was found to be crucial for the T-cell specificity of transcription and lymphomagenesis of this virus (31). This portion of each enhancer repeat contains binding sites for multiple transcription factors. In the 5′-to-3′ orientation on the coding strand, there are binding sites for core binding factor (CBF), c-Myb, and the Ets family of factors, as well as a second binding site for CBF (Fig. 1) (36, 49, 54, 55). CBF is also called AML1, PEBP2, and SEF1 (1, 26, 38, 46, 48). The CBF binding sites are called core elements (27, 28, 42, 53). SL3 has two core elements. The 3′ core element, which is adjacent to the Ets site, is called core I in SL3. This element is conserved in all members of the MuLV family of retroviruses, including feline leukemia viruses and gibbon ape leukemia viruses; hence, this site is called the core element of these viruses (19). Frequently there are single-base substitutions among the different isolates, and these differences have modest effects on CBF binding (19). The 5′ core element of SL3, which is adjacent to the Myb site, is called core II (49, 54, 55). This element is unique to SL3 among viruses in this family.
FIG. 1.
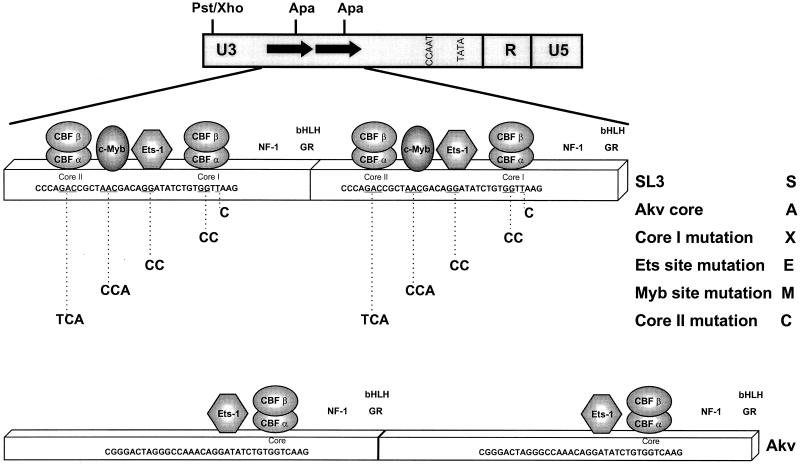
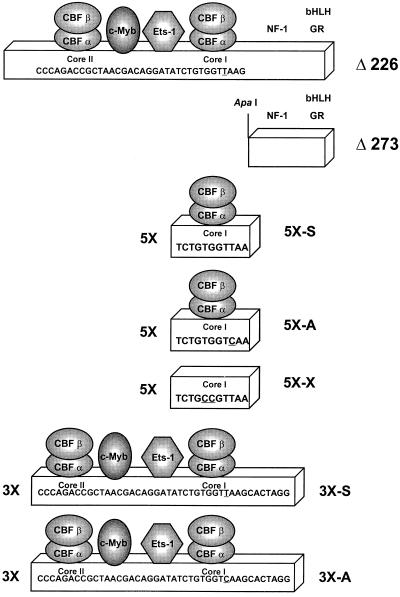
Structures of the SL3, Akv, and mutated enhancers. The top line shows the structure of the MuLV LTR. Arrows indicate the locations of the tandem repeat units of the viral LTR enhancer. Positions of restriction sites are shown. A PstI site in SL3 was changed to an XhoI site in the CAT plasmids by the attachment of a linker. The second line shows the structure of the SL3 enhancer. Positions of binding sites for various transcription factors are shown. The factors whose sites were mutated in these studies are indicated by geometric figures. The portion of the nucleotide sequence of SL3 that encompasses these binding sites is also shown. The underlined nucleotides are those that were mutated. Shown below the SL3 sequence are the sequences of the mutations introduced at each site. The identity of each mutation is indicated at the right, including the single-letter names that are used in the other figures. The single-letter names are used throughout the text to identify particular mutants. S, A, and X indicate the sequences of the core I elements of SL3, Akv, and a mutant of SL3 with a 2-bp substitution in the core I element, respectively. C, M, and E indicate mutations in the core II, Myb, and Ets sites, respectively. Thus, the designation ME-S indicates an LTR with mutated Myb and Ets sites and a core element of SL3 virus, while CME-A indicates an LTR with the core II, Myb, and Ets sites all mutated and the core sequence of Akv virus. Positions of binding sites for other factors (7, 10, 33–35, 42), nuclear factor 1 (NF-1), basic helix-loop-helix factors (bHLH), and the glucocorticoid receptor (GR) are also shown. The third line shows the structure of the tandem-repeat units of the Akv LTR and the sequence of the equivalent stretch of its enhancer. The fourth and fifth lines show the structures of the deletion mutants Δ226 and Δ273. The numbers refer to the number of nucleotides from the upstream portion of SL3 that were deleted in each construct. The sixth, seventh, and eighth lines show the sequence of one 11-bp unit in the 5× multimers. The last two lines show the sequence of the individual repeat units of the 3× multimers.
Multiple elements within the SL3 enhancer are important for viral lymphomagenicity. Mutation of the c-Myb binding site eliminated most of the pathogenicity of SL3 (36). Mutation of the core I element also strongly inhibited the lymphomagenicity of SL3 (21, 32). In particular, a single base pair difference between the core I element of SL3 and the core of the weakly leukemogenic retrovirus Akv was found to be important (32). Mutation of the Ets site had a small, though statistically significant, effect on the lymphomagenicity of SL3 (36). Mutation of the core II site by itself had little effect on pathogenicity, although in the presence of a core I mutation it did significantly reduce the potency of the virus (15, 21).
Transcriptional studies have suggested a correlation between the relative importance of an element for the transcriptional activity of MuLV enhancers in T cells and viral lymphomagenicity. In the case of SL3, only T lymphocytes can distinguish the single-base-pair difference between the core elements of SL3 and Akv in transcription assays (3, 32). For Moloney MuLV (Mo-MuLV), another T-cell-lymphomagenic virus, mutation of the enhancer core element resulted in the largest inhibition of transcription in T cells and the most profound effects on lymphomagenicity of any site in the viral enhancer (43, 44). These results suggest that mutations that alter the transcriptional activity of the viral enhancers in T cells inhibit the ability of the virus to perform one or more steps in the process of lymphomagenesis, including replication in the target cells for disease, formation and propagation of recombinant mink cell focus-forming viruses, and activation of cellular proto-oncogenes (5, 13, 32, 41).
In this study, we systematically compared the effects of mutation of several sites in the SL3 enhancer on transcriptional activity of the SL3 LTR. In particular, we asked whether mutation of the sites upstream of core I affected transcription to an extent comparable to mutations in core I itself. This allowed us to compare the relative importance of individual binding sites in transcriptional activity with previous observations of their importance for viral lymphomagenicity. We also measured the effects of simultaneous mutation of multiple binding sites on transcriptional activity and addressed whether intact binding sites for additional transcription factors were necessary for T lymphocytes to recognize core I.
MATERIALS AND METHODS
Cell lines.
The mouse T-cell lines SL3B and SL3H and the human T-cell line Jurkat were maintained in RPMI 1640 supplemented with 10% fetal bovine serum, 100 U of penicillin per ml, 10 μg of streptomycin per ml, and 2 mM glutamine. The mouse T-cell line L691-6 was maintained in Dulbecco’s modified Eagle’s medium with the same supplements. All cells were maintained at 37°C in 100% humidity and 7.5% CO2.
LTR mutants.
Nucleotide substitution mutations were introduced into the various transcription factor binding sites in the SL3 LTR enhancer (Fig. 1) by a PCR-based strategy that was previously described in detail (36, 54, 55). Mutations of the indicated nucleotides were all previously shown to block the binding of the cognate factor to the site (20, 30, 49, 52, 54, 55), except for the 1-bp substitution of the Akv sequence in core I, which was previously shown to result in only a twofold reduction in CBF binding (55). All nucleotide substitution mutations in this study were introduced simultaneously into the identical sites in both of the tandem 72-bp repeats of the SL3 enhancer. LTRs were sequenced after mutagenesis to confirm the introduction of the mutations and to verify that no additional changes were introduced elsewhere in the LTRs.
Two 5′ deletion mutants of the SL3 LTR were used in these studies. In Δ226, the upstream repeat unit and all sequences upstream of the repeats were removed (Fig. 1). In Δ273, the deletion extended further 3′ through the promoter-proximal core I element to an ApaI site (Fig. 1). Construction of these deletions was previously described (31).
Two different types of multimer constructs were generated. The constructs named 5×-S, 5×-A, and 5×-× contained five 11-bp tandem binding sites for CBF, comprising the SL3 core I, Akv core, and a mutated SL3 core I site, respectively (Fig. 1). These were generated by using complementary synthetic oligonucleotides containing overhanging ends that were compatible with the XhoI and ApaI sites in the SL3 chloramphenicol acetyltransferase (CAT) plasmids (Fig. 1). The second set of multimer constructs contained three tandem copies of the stretch of the SL3 enhancer containing the core II, Myb, Ets, and core I sites (Fig. 1). 3×-S contained the SL3 core I, while 3×-A had the 1-bp substitution of the Akv core (Fig. 1). The synthetic oligonucleotides containing these sequences were inserted into the XhoI and ApaI sites in the SL3 CAT plasmids (Fig. 1).
CAT assays.
Transfections were performed by an approach described previously (32, 36, 39, 54, 55). Briefly, DNA was introduced into the cells by the DEAE-dextran method. A Rous sarcoma virus-luciferase plasmid was used as an internal standard. Cell extracts were prepared 48 h later. CAT activity was measured by thin-layer chromatography using 14C-labeled chloramphenicol. The fraction of chloramphenicol that was acetylated was quantified by PhosphorImager analysis. Data were normalized to those of the internal standard. Each experiment was performed in duplicate on at least two separate occasions. Data are presented as means of the multiple trials ± standard deviations.
EMSAs.
Electrophoretic mobility shift assays (EMSAs) were performed with a radiolabeled 31-bp probe that spanned the SL3 core I sequence, as previously described (54). Nuclear extract from the T-cell line WEHI7.1 was used as previously described (3). The same double-stranded, unlabeled oligonucleotides that were used for the generation of the 5× multimers were also used for competition experiments.
RESULTS
Relative importance of the core II, Myb, Ets, and core I sites for transcriptional activity of the SL3 enhancer.
Lymphomagenicity studies indicated that the Myb and core I sites in the SL3 enhancer had greater importance for viral lymphomagenicity than the Ets and core II sites (36). We hypothesized that the effects of the mutations on viral lymphomagenicity were in fact due to inhibition of viral LTR enhancer activity in T cells. Therefore, we tested systematically whether mutation of these binding sites had similar relative effects on transcriptional activity of the viral enhancer. Mutations were introduced into the core II, Myb, Ets, and core I sites of the SL3 enhancer (Fig. 1). A PCR-based strategy (36, 54, 55) was used to introduce the mutations simultaneously into identical sites in each of the 72-bp repeat units. Mutations of the indicated nucleotides in the core II, Myb, and Ets sites (Fig. 1) were all previously shown to block the binding of the corresponding factor to that site (20, 30, 49, 52, 54). The mutations at the core II, Myb, and Ets sites were designated C, M, and E, respectively (Fig. 1). Two different mutations were introduced at the core I site. One, designated X (Fig. 1), was previously shown to eliminate binding of CBF (20, 54, 55). The other, designated A (Fig. 1), changed the sequence to that of the Akv core element. This mutation was previously shown to result in about a twofold reduction in CBF binding (55). The effects of the mutations on the transcriptional activity of the SL3 LTR were tested by transfection of plasmids containing the LTR linked to the CAT reporter gene into cultured T cells. T cells were used because they are the target for lymphomagenesis by SL3 and because it was previously shown that only T cells can distinguish the SL3 and Akv core elements in transcription assays (3, 32).
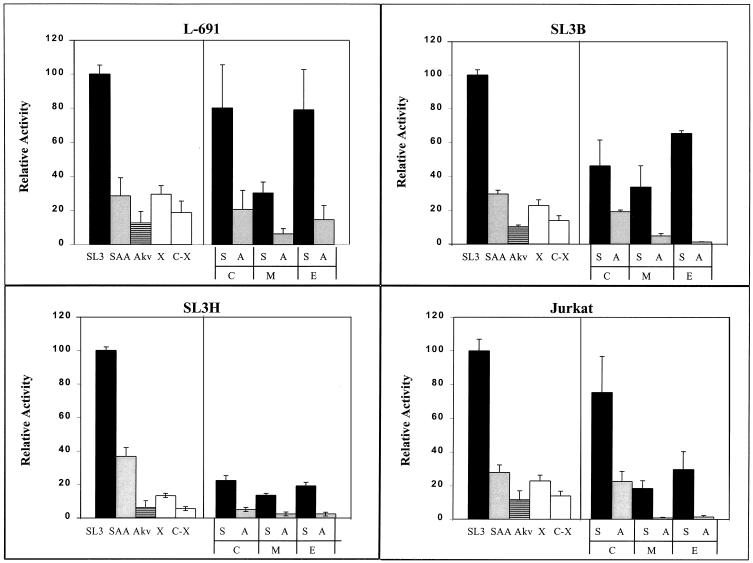
Mutation of individual elements in the SL3 enhancer had differing effects on transcriptional activity (Fig. 2). As previously reported (3, 11, 32), the SL3 LTR was about 10 times more active than the Akv LTR in various T-cell lines. Changing the SL3 core I element into the Akv core (SAA in Fig. 2) reduced the activity of the SL3 enhancer three- to fourfold, resulting in a level intermediate between those of SL3 and Akv. A different mutation in core I that blocked CBF binding (X in Fig. 2) also resulted in transcriptional activity that was intermediate between those of SL3 and Akv. In one of the four T-cell lines tested (SL3H), this mutation reduced enhancer activity to about one-half the level of SAA (compare X and SAA in Fig. 2). In the other three lines, it did not result in a clear reduction of activity below the level of SAA. Thus, changing the SL3 core I sequence to the Akv core sequence had effects on transcription similar to those of introducing the mutation that eliminated detectable CBF binding.
FIG. 2.
Transcriptional activity of SL3 enhancer mutants in four T-cell lines. Abbreviations indicate the mutations, as described in the legend to Fig. 1. Thus, S, A, and X indicate a core I element sequence while C, M, and E indicate mutations in the core II, Myb, and Ets sites, respectively. Activity is shown compared to that of the SL3 LTR, which was set at 100%, in each cell line. Error bars indicate 1 standard deviation. Black bars indicate enhancers with the SL3 core I element. Gray bars indicate enhancers in which the Akv core was substituted for the SL3 core I element. White bars indicate enhancers in which the 2-bp mutation (X) that blocks binding of CBF was introduced into the SL3 core I element. The horizontally lined bars indicate the Akv LTR.
Mutation of the elements in the SL3 enhancer upstream of core I had effects on transcriptional activity that varied in their extent among the different T-cell lines (Fig. 2). However, in each of the four cell lines, mutation of the Myb site gave the highest degree of inhibition of transcription (Fig. 2). The inhibition due to mutation of the Myb site was quantitatively similar to that due to mutation of the core I element. The effects of mutation of the core II or Ets site were variable, ranging from little or no effect in L691 cells to about a fivefold effect in Jurkat cells. In summary, these results show that multiple sites contribute to the activity of the SL3 enhancer. The quantitative effect of mutation of a particular site varied among the different T-cell lines. However, the relative effects of mutation of various sites were generally consistent in all the lines. Mutation of the Myb and core I sites had the largest inhibitory effects. The inhibitory effect of mutation of the Ets or core II site was evident but less extensive than that of the Myb or core I site. Thus, the Myb and core I sites are the most important for both transcriptional activity of the SL3 LTR in T cells and viral lymphomagenicity.
Effects of upstream transcription factor binding sites on activity of the SL3 core I element.
We tested whether the sites upstream of core I affected the ability of T cells to distinguish the SL3 and Akv cores (Fig. 2). When the core II element was mutated, the relative activities of the SL3 and Akv cores were similar to those in the native LTRs. Curiously, when the Myb or Ets site was mutated, the difference between the SL3 and Akv LTRs was often greater than that seen with the wild-type LTRs. Thus, if either the Myb or the Ets site was mutated, T cells were often more sensitive to the single base pair difference between the two LTRs in the core element.
Mutation of both core elements of SL3 resulted in a virus that was less lymphomagenic than SL3 with a mutation in only core I. Therefore, we also tested the transcriptional effects of mutating both core elements simultaneously. Blocking the binding of CBF to both core I and core II by simultaneous mutation of both sites resulted in a slight but reproducible decrease in transcriptional activity in all four T-cell lines over that seen with mutation of core I alone (compare C-X and C with the Akv core in Fig. 2). The effect of simultaneous mutation of the core I and core II elements was the same whether the core I sequence was changed to that of Akv or it was changed to the sequence that eliminated measurable CBF binding (compare C-X and C-A in Fig. 2). This again supports the hypothesis that the 1-bp mutation in the Akv core that reduces CBF binding about twofold has the same effect on transcription as the mutation that eliminates binding. It is also worth noting that even when CBF binding to core I was inhibited, the SL3 enhancer still had residual activity that was severalfold higher than that of an LTR in which the enhancer region was more substantially mutated (see Fig. 3 and 4). Presumably, the remaining elements in the SL3 enhancer endow it with activity even when CBF is not bound to core I.
FIG. 3.
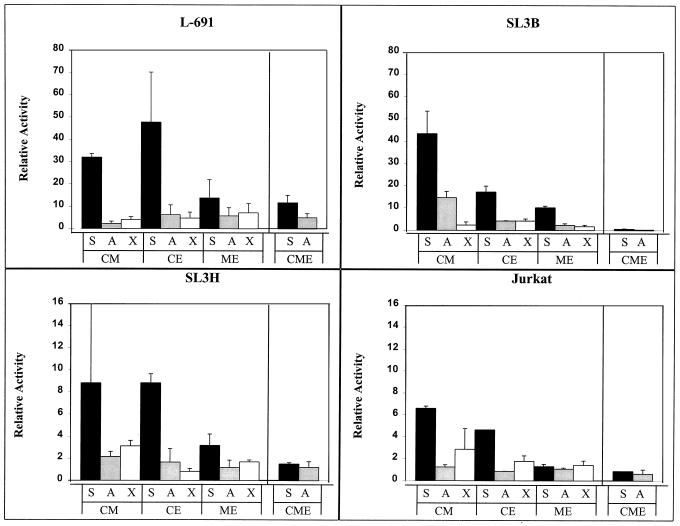
Transcriptional activity of the SL3 enhancer double and triple mutants in four T-cell lines. Abbreviations indicate the mutations, as described in the legend to Fig. 1. Thus, S, A, and X indicate a core I element sequence while C, M, and E indicate mutations in the core II, Myb, and Ets sites, respectively. The shading scheme used is described in the legend to Fig. 2. Activity is shown compared to that of the SL3 LTR, which was set at 100%, in each cell line.
FIG. 4.
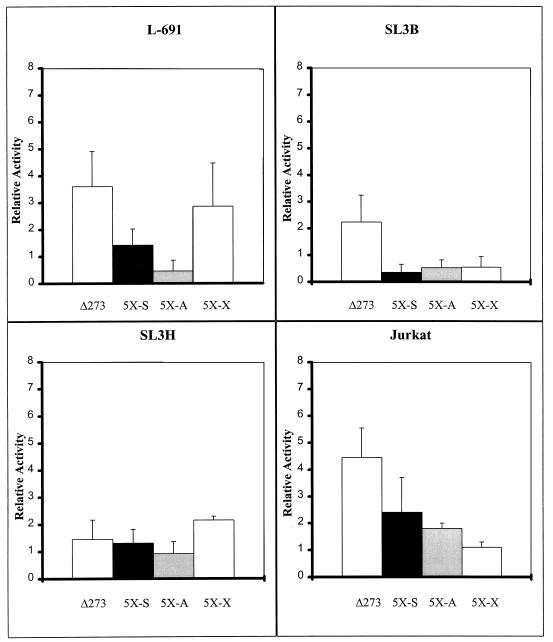
Transcriptional activity of the deletion mutant Δ273 and 5× multimers in four T-cell lines. Abbreviations and color schemes are described in the legend to Fig. 2. Activity is shown compared to that of the SL3 LTR, which was set at 100%, in each cell line.
Mutation of multiple sites upstream of the core I element.
We further tested the hypothesis that multiple sites are important for the activity of the SL3 enhancer by simultaneously mutating all three sites upstream of core I. This triple mutation, designated CME, was tested with both the SL3 core I and Akv core sequences (CME-S and CME-A, respectively, in Fig. 3). The CME mutations reduced the activity of the SL3 LTR to less than 2% of the activity of the intact LTR in three of the four cell lines tested, whether the core element I was from SL3 or Akv. Thus, mutation of all three sites upstream of the core I element eliminated most of the activity of the enhancer.
When the core I sequence was from SL3, the triple mutation inhibited activity to a greater extent than the multiplicative effect of the three individual mutations. For example, in the SL3B cell line, individual mutation of the core II, Myb, and Ets sites resulted in the LTR having, respectively, about 40, 30, and 60% of the level of activity of the intact SL3 LTR (Fig. 2). The CME-S triple mutant had about 1% of the level of activity of the intact LTR. Thus, the actual level of activity of the CME-S triple mutant was severalfold lower than the multiplicative effect of the three individual effects (0.4 × 0.3 × 0.6 = 0.07). Similar observations were evident for the experiments in L691 and Jurkat cells.
However, this was not the case when the core I element was changed to that of Akv. In that case, mutation of individual sites, particularly the Myb and Ets sites, had larger inhibitory effects and reduced the level of activity of the LTR to levels approaching that of the CME-A triple mutant (Fig. 2). Thus, when the core element had the Akv sequence, the remaining activity of the enhancer was more dependent on the Myb and Ets binding sites than when the core had the SL3 sequence.
To assess more precisely the importance of each of the sites upstream of the core I element in the SL3 enhancer, series of double mutations were made in which two of the three upstream elements were mutated (Fig. 3). These were generated in a reporter plasmid containing the SL3 core I (S). In addition, parallel constructs were made with the same mutations linked to the Akv core (A) or the core I mutation that blocked CBF binding (X). These were tested in transcription assays in the four T-cell lines. In almost every instance, the double mutations were more inhibitory than either of the corresponding single mutations. The only exception was that the core II-Myb site double mutation was not more inhibitory than the Myb site mutation alone in L691 and SL3B cells (Fig. 2 and 3). These results were again consistent with the idea that multiple sites contribute to the activity of the SL3 enhancer.
Of the three SL3 mutants with two disrupted sites situated upstream of the core I element, the one with mutations in the Myb and Ets sites was the most inhibited (Fig. 3). In L691, SL3B, and Jurkat cells, the inhibitory effect of this double mutation was as great or greater than the multiplicative effect of the corresponding single mutations. Interestingly, this was the only one of the double mutants for which this was the case. This result stresses the relative importance of these two sites for the activity of the SL3 enhancer.
In most of the experiments, the double mutants retained some ability to distinguish the SL3 and Akv cores (Fig. 3). As was observed with the intact SL3 enhancer, the core I mutation that strongly inhibited CBF binding (X) was generally no more inhibitory than the Akv core element (A). These results indicate that the transcription factor in T cells that distinguishes the SL3 and Akv cores, presumably CBF, can do so when any one of the three upstream sites is intact. However, the difference between S and A was generally greater when either the Myb or Ets site was intact than when just core II was intact.
The core element requires one or more additional sites to function in T cells.
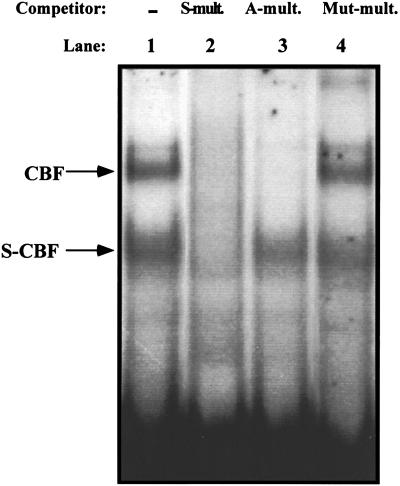
Mutation of all three sites upstream of core I strongly inhibited the activity of the SL3 enhancer and eliminated most or all of the ability of T cells to distinguish the SL3 and Akv cores (Fig. 3). These observations were consistent with the possibility that the SL3 core I element requires one or more of the upstream sites to function. This hypothesis was tested further by measuring the activities of multimers of the SL3 core I element. Many transcription factors are able to activate reporter genes that contain multiple tandem binding sites for the factor upstream of a promoter (4, 9, 50). A reporter plasmid that contained five tandem copies of the SL3 core I element upstream of the viral LTR promoter was generated (Fig. 1). Equivalent constructs containing the Akv core (A) or the mutation (X) that blocked CBF binding were generated (Fig. 1). Upon transfection into T cells, these constructs had no more activity than plasmids in which almost the entire enhancer was deleted (Fig. 4). Thus, multimers of the SL3 core I element lack activity in T cells. To confirm that the multimers of the SL3 and Akv core elements could indeed bind CBF, EMSAs were performed (Fig. 5). Both the SL3 and Akv core multimers bound CBF, while the mutated core (X) did not. These results provided additional evidence that the SL3 core element requires binding sites for one or more heterologous transcription factors to function.
FIG. 5.
Competition EMSAs with the 5× core multimers. An SL3 core I probe was tested in a nuclear extract from the T-cell line WEHI7.1 for binding of nuclear factors CBF and SL3-core binding factor (S-CBF). Competitors were present at a 50-fold molar excess where indicated. −, no competitor used; S-mult., multimer of SL3; A-mult., multimer of Akv; Mut-mult., multimer of SL3 containing the X mutation.
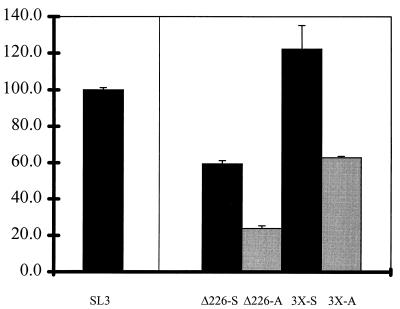
To test this hypothesis further, constructs in which the SL3 core element was linked to the three upstream sites were generated (Fig. 1). Multimers containing three tandem copies of the core II-Myb-Ets-core I segment linked to the enhancer-deleted LTR were generated (3×-S and 3×-A [Fig. 1]). The activities of these multimers were compared to those of 5′ SL3 LTR deletion mutants in which sequences upstream of the promoter-proximal 72-bp repeat unit were deleted (Δ226-S and Δ226-A [Fig. 1]). All of these constructs exhibited substantial levels of activity (Fig. 6). The constructs with single units had activities that were substantially higher than those with the enhancer-deleted LTR (compare Δ226-S and Δ226-A with Δ273 in Fig. 4). The constructs with three repeat units had even higher levels of activity (Fig. 6). The 3×-S construct exhibited activity similar to that of the intact SL3 LTR (Fig. 6). The 3×-A construct also had activity, although the level was lower than that of 3×-S (Fig. 6). Thus, enhancers containing core I were active in T cells provided that the upstream sites were also present. This strongly argues that the upstream elements are required for the transcription factors in T cells to be active on the SL3 core.
FIG. 6.
Transcriptional activity of the 3× multimers compared to that of Δ226, the 5′ deletion construct that contains a single 72-bp repeat unit. Two versions of Δ226 were tested, one with the SL3 core I element and the other with a substitution of the Akv core. Abbreviations and color schemes are described in the legend to Fig. 2. Activity is shown compared to that of the SL3 LTR, which was set at 100%.
DISCUSSION
These studies show that the Myb, Ets, core I, and core II sites contribute to the transcriptional activity of the SL3 LTR in T cells. They also show that the effects of the individual mutations on transcription in T cells parallel the effects of the same mutations on viral lymphomagenicity. Mutation of the Myb binding site and core I had the largest effects on both transcription and lymphomagenicity (21, 32, 36). Conversely, mutation of core II and the Ets binding site had smaller effects on transcription and viral lymphomagenicity (21, 36). This is consistent with the idea that the main function of the LTR enhancer sequences in the process of lymphomagenesis is to regulate transcription.
Studies with Mo-MuLV also showed a correlation between the importance of specific factor binding sites for transcription in T cells and viral lymphomagenicity (43, 44). However, the factor binding sites important for Mo-MuLV were somewhat different from those that were important for SL3, even though both viruses cause similar diseases. Thus, the Mo-MuLV core element, which is positioned equivalently to core I in SL3, was also important for that virus. However, the Ets (LVb) site in Mo-MuLV appeared to be more important for both transcription and lymphomagenicity of Mo-MuLV than was the case with SL3 (43, 44). Also, Mo-MuLV lacks the Myb and core II sites found in SL3 (36). Thus, the transcription factor binding sites that are important for T-cell transcription and lymphomagenicity of Mo-MuLV and SL3 are somewhat different. Nonetheless, for both viruses, the mutations that had the largest effects on transcriptional activity in T lymphocytes also had the largest effects on lymphomagenicity.
Additional insight regarding the significance of individual transcription factor binding sites for the activity of the SL3 enhancer can also be obtained by considering the effects of adding individual sites to an enhancer that lacks all four. The mutant CME-A had all four binding sites disrupted (Fig. 1) and had the lowest level of activity of all the nucleotide substitution mutants (Fig. 3). Changing the core I element to that of SL3 did not stimulate activity significantly (CME-S [Fig. 3]). Addition of any of the three upstream binding sites to an enhancer with Akv cores had little stimulatory activity (CM-A, CE-A, and ME-A [Fig. 3]). Thus, in the context of this core I element, the factors that bind to the upstream sites had little stimulatory effect. However, addition of the Myb site or the Ets site to an enhancer with the SL3 core did lead to increases in activity (CM-S and CE-S [Fig. 3]). The stimulatory effect required that both the Myb (or Ets) site and the core I site be intact, since they are in the native SL3 virus. These results suggest that there is synergistic stimulation of transcription by the factor bound to the SL3 core I element in T cells, presumably CBF, and the factors in T cells that are bound to the Myb or Ets site, presumably c-Myb or a member of the Ets family, respectively. This result is consistent with previous reports indicating cooperativity among these factors in stimulation of transcription of various viral and cellular promoters. We previously reported that c-Myb and CBF synergistically stimulate transcription from the SL3 LTR in cotransfection experiments in embryonal carcinoma (EC) cells (54). These studies utilized the form of the DNA-binding α subunit of CBF encoded by the cbfa2 gene (also called the AML1 gene). CBF acted through core I to give up to a five- to sixfold stimulation of activity. By itself, c-Myb had no activity on the SL3 LTR in these cells. However, c-Myb could act through the Myb site in the viral enhancer to stimulate transcription severalfold beyond that induced by CBF alone. Synergy between c-Myb and CBF has also been suggested to occur on the T-cell receptor δ enhancer, the myeloperoxidase promoter, and the neutrophil elastase promoter (17, 23, 37).
The results presented here showed that mutation of either the Myb site or core I inhibited activity of the SL3 enhancer to approximately equal levels in every T-cell line tested (Fig. 2). In addition, when both sites were mutated, a further reduction was seen (Fig. 3). This suggests that c-Myb has activity on the SL3 enhancer even when core I is mutated. This is different from what was observed in the cotransfection studies in EC cells, in which c-Myb had no activity unless CBF was bound to core I. One possible explanation for this is that the T cells contain additional factors other than those present in EC cells, perhaps members of the Ets family, that can synergize with c-Myb even when CBF cannot bind to core I. This possibility is consistent with cotransfection studies in which c-Myb and Ets-2 were found to stimulate transcription from the mim-1 promoter cooperatively (14). T lymphocytes express several members of the Ets family of factors (2, 45, 47). It is uncertain which of these is responsible for the effects of the Ets site on the SL3 enhancer or whether other transcription factors that bind to the same site are important.
Several observations indicate that CBF is the transcription factor in T cells that recognizes the core elements of MuLVs. CBF is the only transcription factor in extracts of T cells that binds to the core elements of all MuLVs in EMSAs (3, 42, 48). Cotransfection studies showed that CBF could stimulate transcription of MuLV LTR-driven reporter genes in a manner that required intact core elements (45, 54, 55). CBF also binds to similar sequences in the transcriptional regulatory elements of many cellular genes that are expressed in T cells (6, 18, 22, 24).
One of the important questions about CBF that remains unanswered is how it distinguishes the single base pair difference between the core elements of SL3 and Akv. The form of CBFα encoded by the cbfa2 (AML1) gene binds to the SL3 core about twofold more strongly than the Akv core (55). However, it did not distinguish the two cores in cotransfection assays with viral LTR reporter constructs (55). In contrast, there was a significant difference in the activities of otherwise isogenic constructs that contain the SL3 or Akv core sequences in T lymphocytes in this study. In particular, the Akv core mutant had activity equivalent to that of a mutant in which measurable CBF binding was abolished (Fig. 2 and 3). An unexpected observation in the current study was that mutation of either the Myb or Ets site resulted in an even greater difference in the activities of the two core elements than was seen with the intact enhancer (Fig. 2). The reasons for the substantially lower activity of the Akv core are unclear. One possible explanation is that the combination of factors and coactivators that is present in T cells causes a substantially greater difference in CBF binding to the SL3 and Akv cores than was detected with the isolated factor or that occurs in EC cells. Another possibility is that CBFα subunits encoded by the cbfa1 and cbfa3 genes are better able to distinguish the single base pair difference between the two core elements. A third possibility is that the Akv core is recognized by a negatively acting factor that is specific to T cells and somehow interferes with the activity of CBF. Whatever the reason, a full understanding of the function of CBF requires an explanation of the ability of T cells to distinguish the SL3 and Akv core elements.
We and others previously reported that the activity of CBF in cotransfection assays in EC cells required the presence of a second, heterologous transcription factor. One such factor was c-Myb (54). Evidence suggested that factors in EC cells that could bind to the Ets sites of MuLV enhancers could also cooperate with CBF (45, 54). In particular, we previously showed that the multimer containing five tandem copies of the SL3 core I site could not be transactivated by cotransfection of CBFα in EC cells (54). The results presented here showed that a similar effect occurs in T lymphocytes, the normal target cells for transformation by SL3 and Mo-MuLV. Five tandem copies of core I of SL3 had no activity in T cells. However, transcriptional activity was detected if binding sites in addition to the core were present. These results indicate that CBF requires one or more additional factors to function in T cells.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grants CA44822 and CA57337 to J.L. A.L.Z. was supported by Public Health Service training grant GM7288. J.L. was the recipient of a Hirschl-Caulier Career Scientist Award. Core facilities for synthesis of oligonucleotides, automated DNA sequencing, and PhosphorImager analysis were supported by Public Health Service Cancer Center grant CA13330 to the Albert Einstein College of Medicine.
We thank Joseph LoSardo for helpful conversations and assistance with some of the experiments.
REFERENCES
- 1.Bae S C, Yamaguchi-Iwai Y, Ogawa E, Maruyama M, Inuzuka M, Kagoshima H, Shigesada K, Satake M, Ito Y. Isolation of PEBP2αB cDNA representing the mouse homolog of human acute myeloid leukemia gene, AML1. Oncogene. 1993;8:809–814. [PubMed] [Google Scholar]
- 2.Bhat N K, Komschlies K L, Fujiwara S, Fisher R J, Mathieson B J, Gregorio T A, Young H A, Kasik J W, Ozato K, Papas T S. Expression of ets genes in mouse thymocyte subsets and T cells. J Immunol. 1989;142:672–678. [PubMed] [Google Scholar]
- 3.Boral A L, Okenquist S A, Lenz J. Identification of the SL3-3 virus enhancer core as a T-lymphoma cell-specific element. J Virol. 1989;63:76–84. doi: 10.1128/jvi.63.1.76-84.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bradshaw M S, Tsai S Y, Leng X, Dobson A D W, Conneely O M, O’Malley B W, Tsai M. Studies on the mechanism of functional cooperativity between progesterone and estrogen receptors. J Biol Chem. 1991;266:16684–16690. [PubMed] [Google Scholar]
- 5.Brightman B K, Rein A, Trepp D J, Fan H. An enhancer variant of Moloney murine leukemia virus defective in leukemogenesis does not generate mink cell focus-inducing virus in vivo. Proc Natl Acad Sci USA. 1991;88:2264–2268. doi: 10.1073/pnas.88.6.2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruhn L, Munnerlyn A, Grosschedl R. ALY, a context-dependent coactivator of LEF-1 and AML-1, is required for TCRα enhancer function. Genes Dev. 1997;11:640–653. doi: 10.1101/gad.11.5.640. [DOI] [PubMed] [Google Scholar]
- 7.Celander D, Haseltine W A. Glucocorticoid regulation of murine leukemia virus transcription elements is specified by determinants within the viral enhancer region. J Virol. 1987;61:269–275. doi: 10.1128/jvi.61.2.269-275.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chatis P A, Holland C A, Silver J E, Frederickson T N, Hopkins N, Hartley J W. A 3′ end fragment encompassing the transcriptional enhancers of nondefective Friend virus confers erythroleukemogenicity on Moloney leukemia virus. J Virol. 1984;52:248–254. doi: 10.1128/jvi.52.1.248-254.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chi T, Lieberman P, Ellwood K, Carey M. A general mechanism for transcriptional synergy by eukaryotic activators. Nature. 1995;377:254–257. doi: 10.1038/377254a0. [DOI] [PubMed] [Google Scholar]
- 10.Corneliussen B, Thornell A, Hallberg B, Grundström T. Helix-loop-helix transcriptional activators bind to a sequence in glucocorticoid response elements of retrovirus enhancers. J Virol. 1991;65:6084–6093. doi: 10.1128/jvi.65.11.6084-6093.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dai H Y, Etzerodt M, Baekgaard A J, Lovmand S, Jorgensen P, Kjeldgaard N O, Pedersen F S. Multiple sequence elements in the U3 region of the leukemogenic murine retrovirus SL3-2 contribute to cell-dependent gene expression. Virology. 1990;175:581–585. doi: 10.1016/0042-6822(90)90445-w. [DOI] [PubMed] [Google Scholar]
- 12.DesGroseillers L, Jolicoeur P. The tandem direct repeats within the long terminal repeat of murine leukemia viruses are the primary determinant of their leukemogenic potential. J Virol. 1984;52:945–952. doi: 10.1128/jvi.52.3.945-952.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DesGroseillers L, Rassart E, Jolicoeur P. Thymotropism of murine leukemia virus is conferred by its long terminal repeat. Proc Natl Acad Sci USA. 1983;80:4203–4207. doi: 10.1073/pnas.80.14.4203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dudek H, Tantravahi R V, Rao V N, Reddy S P, Reddy E P. Myb and Ets proteins cooperate in transcriptional activation of the mim-1 promoter. Proc Natl Acad Sci USA. 1992;89:1291–1295. doi: 10.1073/pnas.89.4.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ethelberg S, Hallberg B, Lovmand J, Schmidt J, Luz A, Grundström T, Pedersen F S. Second-site proviral enhancer alterations in lymphomas induced by enhancer mutants of SL3-3 murine leukemia virus: negative effect of nuclear factor 1 binding site. J Virol. 1997;71:1196–1206. doi: 10.1128/jvi.71.2.1196-1206.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Evans L H, Morrey J D. Tissue-specific replication of Friend and Moloney murine leukemia viruses in infected mice. J Virol. 1987;61:1350–1357. doi: 10.1128/jvi.61.5.1350-1357.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Friedman A D. Regulation of immature myeloid cell differentiation by PEBP2/CBF, Myb, C/EBP and Ets family members. Curr Top Microbiol Immunol. 1996;211:149–157. doi: 10.1007/978-3-642-85232-9_15. [DOI] [PubMed] [Google Scholar]
- 18.Giese K, Kingsley C, Kirshner J R, Grosschedl R. Assembly and function of a TCRα enhancer complex is dependent on LEF-1-induced DNA bending and multiple protein-protein interactions. Genes Dev. 1995;9:995–1008. doi: 10.1101/gad.9.8.995. [DOI] [PubMed] [Google Scholar]
- 19.Golemis E A, Speck N A, Hopkins N. Alignment of U3 sequences of mammalian type C viruses: identification of highly conserved motifs and implications for enhancer design. J Virol. 1990;64:534–542. doi: 10.1128/jvi.64.2.534-542.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gunther C V, Graves B J. Identification of ETS domain proteins in murine T lymphocytes that interact with the Moloney murine leukemia virus enhancer. Mol Cell Biol. 1994;14:7569–7580. doi: 10.1128/mcb.14.11.7569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hallberg B, Schmidt J, Luz A, Pedersen F S, Grundström T. SL3-3 enhancer factor 1 transcriptional activators are required for tumor formation by SL3-3 murine leukemia virus. J Virol. 1991;65:4177–4181. doi: 10.1128/jvi.65.8.4177-4181.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hernandez-Munain C, Krangel M S. Regulation of the T-cell receptor δ enhancer by functional cooperation between c-Myb and core-binding factors. Mol Cell Biol. 1994;14:473–483. doi: 10.1128/mcb.14.1.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hernandez-Munain C, Krangel M S. c-Myb and core-binding factor/PEBP2 display functional synergy but bind independently to adjacent sites in the T-cell receptor δ enhancer. Mol Cell Biol. 1995;15:3090–3099. doi: 10.1128/mcb.15.6.3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hsiang Y H, Spencer D, Wang S, Speck N A, Raulet D H. The role of viral enhancer “core” motif-related sequences in regulating T cell receptor-γ and -δ gene expression. J Immunol. 1993;150:3905–3916. [PubMed] [Google Scholar]
- 25.Ishimoto A, Takimoto M, Adachi A, Kakuyama M, Kato S, Kakimi K, Fukuoka K, Ogiu T, Matsuyama M. Sequences responsible for erythroid and lymphoid leukemia in the long terminal repeats of Friend-mink cell focus-forming and Moloney murine leukemia viruses. J Virol. 1987;61:1861–1866. doi: 10.1128/jvi.61.6.1861-1866.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kamachi Y, Ogawa E, Asano M, Ishida S, Murakami Y, Satake M, Ito Y, Shigesada K. Purification of a mouse nuclear factor that binds to both the A and B cores of the polyomavirus enhancer. J Virol. 1990;64:4808–4819. doi: 10.1128/jvi.64.10.4808-4819.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khoury G, Gruss P. Enhancer elements. Cell. 1983;33:313–314. doi: 10.1016/0092-8674(83)90410-5. [DOI] [PubMed] [Google Scholar]
- 28.Lenz J, Celander D, Crowther R L, Patarca R, Perkins D W, Haseltine W A. Determination of the leukemogenicity of a murine retrovirus by sequences within the long terminal repeat. Nature. 1984;308:467–470. doi: 10.1038/308467a0. [DOI] [PubMed] [Google Scholar]
- 29.Li Y, Golemis E, Hartley J W, Hopkins N. Disease specificity of nondefective Friend and Moloney murine leukemia viruses is controlled by a small number of nucleotides. J Virol. 1987;61:693–700. doi: 10.1128/jvi.61.3.693-700.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.LoSardo, J., A. Nieves, and J. Lenz. Unpublished data.
- 31.LoSardo J E, Boral A L, Lenz J. Relative importance of elements within the SL3-3 virus enhancer for T-cell specificity. J Virol. 1990;64:1756–1763. doi: 10.1128/jvi.64.4.1756-1763.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morrison H L, Soni B, Lenz J. Long terminal repeat enhancer core sequences in proviruses adjacent to c-myc in T-cell lymphomas induced by a murine retrovirus. J Virol. 1995;69:446–455. doi: 10.1128/jvi.69.1.446-455.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nielsen A L, Norby P L, Pedersen F S, Jorgensen P. E-box sequence and context-dependent TAL1/SCL modulation of basic helix-loop-helix protein-mediated transcriptional activation. J Biol Chem. 1996;271:31463–31469. doi: 10.1074/jbc.271.49.31463. [DOI] [PubMed] [Google Scholar]
- 34.Nielsen A L, Nørby P L, Pedersen F S, Jørgensen P. Various modes of basic helix-loop-helix protein-mediated regulation of murine leukemia virus transcription in lymphoid cell lines. J Virol. 1996;70:5893–5901. doi: 10.1128/jvi.70.9.5893-5901.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nielsen A L, Pallisgaard N, Pedersen F S, Jøorgensen P. Basic helix-loop-helix proteins in murine type C retrovirus transcriptional regulation. J Virol. 1994;68:5638–5647. doi: 10.1128/jvi.68.9.5638-5647.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nieves A, Levy L S, Lenz J. Importance of a c-Myb binding site for lymphomagenesis by the retrovirus SL3-3. J Virol. 1997;71:1213–1219. doi: 10.1128/jvi.71.2.1213-1219.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nuchprayoon I, Meyers S, Scott L M, Suzow J, Hiebert S, Friedman A D. PEBP2/CBF, the murine homolog of the human myeloid AML1 and PEBP2β/CBFβ proto-oncoproteins, regulates the murine myeloperoxidase and neutrophil elastase genes in immature myeloid cells. Mol Cell Biol. 1994;14:5558–5568. doi: 10.1128/mcb.14.8.5558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ogawa E, Maruyama M, Kagoshima H, Inuzuka M, Lu J, Satake M, Shigesada K, Ito Y. PEBP2/PEA2 represents a family of transcription factors homologous to the products of the Drosophila runt gene and the human AML1 gene. Proc Natl Acad Sci USA. 1993;90:6859–6863. doi: 10.1073/pnas.90.14.6859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pantginis J, Beaty R M, Levy L S, Lenz J. The Feline leukemia virus long terminal repeat contains a potent determinant of T-cell lymphomagenicity. J Virol. 1997;71:9786–9791. doi: 10.1128/jvi.71.12.9786-9791.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pedersen F S, Crowther R L, Tenney D L, Reimhold A M, Haseltine W A. Novel leukaemogenic retroviruses isolated from cell line derived from spontaneous AKR tumour. Nature. 1981;292:167–170. doi: 10.1038/292167a0. [DOI] [PubMed] [Google Scholar]
- 41.Rosen C A, Haseltine W A, Lenz J, Ruprecht R, Cloyd M W. Tissue selectivity of murine leukemia virus infection is determined by long terminal repeat sequences. J Virol. 1985;55:862–866. doi: 10.1128/jvi.55.3.862-866.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Speck N A, Baltimore D. Six distinct nuclear factors interact with the 75-base-pair repeat of the Moloney murine leukemia virus enhancer. Mol Cell Biol. 1987;7:1101–1110. doi: 10.1128/mcb.7.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Speck N A, Renjifo B, Golemis E, Fredrickson T, Hartley J, Hopkins N. Mutation of the core or adjacent LVb elements of the Moloney murine leukemia virus enhancer alters disease specificity. Genes Dev. 1990;4:233–242. doi: 10.1101/gad.4.2.233. [DOI] [PubMed] [Google Scholar]
- 44.Speck N A, Renjifo B, Hopkins N. Point mutations in the Moloney murine leukemia virus enhancer identify a lymphoid-specific viral core motif and 1,3-phorbol myristate acetate-inducible element. J Virol. 1990;64:543–550. doi: 10.1128/jvi.64.2.543-550.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sun W, Graves B J, Speck N A. Transactivation of the Moloney murine leukemia virus and T-cell receptor β-chain enhancers by cbf and ets requires intact binding sites for both proteins. J Virol. 1995;69:4941–4949. doi: 10.1128/jvi.69.8.4941-4949.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sun W, O’Connell M, Speck N A. Characterization of a protein that binds multiple sequences in mammalian type C retrovirus enhancers. J Virol. 1993;67:1976–1986. doi: 10.1128/jvi.67.4.1976-1986.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thompson C B, Wang C-Y, Ho I, Bohjanen P R, Petryniak B, June C H, Miesfeldt S, Zhang L, Nabel G J, Karpinski B, Leiden J M. cis-acting sequences required for inducible interleukin-2 enhancer function bind a novel Ets-related protein, Elf-1. Mol Cell Biol. 1992;12:1043–1053. doi: 10.1128/mcb.12.3.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thornell A, Hallberg B, Grundström T. Differential protein binding in lymphocytes to a sequence in the enhancer of the mouse retrovirus SL3-3. Mol Cell Biol. 1988;8:1625–1637. doi: 10.1128/mcb.8.4.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thornell A, Hallberg B, Grundström T. Binding of SL3-3 enhancer factor 1 transcriptional activators to viral and chromosomal enhancer sequences. J Virol. 1991;65:42–50. doi: 10.1128/jvi.65.1.42-50.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tsai S Y, Tsai M, O’Malley B W. Cooperative binding of steroid hormone receptors contributes to transcriptional synergism at the target enhancer elements. Cell. 1989;57:443–448. doi: 10.1016/0092-8674(89)90919-7. [DOI] [PubMed] [Google Scholar]
- 51.Vogt M, Haggblom C, Swift S, Haas M. Envelope gene and long terminal repeat determine the different biological properties of Rauscher, Friend, and Moloney mink cell focus-inducing viruses. J Virol. 1985;55:184–192. doi: 10.1128/jvi.55.1.184-192.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang S, Speck N A. Purification of core-binding factor, a protein that binds the conserved core site in murine leukemia virus enhancers. Mol Cell Biol. 1992;12:89–102. doi: 10.1128/mcb.12.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weiher H, König M, Gruss P. Multiple point mutations affecting the simian virus 40 enhancer. Science. 1983;219:626–631. doi: 10.1126/science.6297005. [DOI] [PubMed] [Google Scholar]
- 54.Zaiman A L, Lenz J. Transcriptional activation of a retrovirus enhancer by CBF (AML1) requires a second factor: evidence for cooperativity with c-Myb. J Virol. 1996;70:5618–5629. doi: 10.1128/jvi.70.8.5618-5629.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zaiman A L, Lewis A F, Crute B E, Speck N A, Lenz J. Transcriptional activity of core binding factor α (AML1) and β subunits on murine leukemia virus enhancer cores. J Virol. 1995;69:2898–2906. doi: 10.1128/jvi.69.5.2898-2906.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]