Abstract

Integrating multiple functional components into vertically stacked heterostructures offers a prospective approach to manipulating the physicochemical properties of materials. The synthesis of vertically stacked heterogeneous noble metal oxides remains a challenge. Herein, we report a surface segregation approach to create vertically stacked amorphous Ir/Ru/Ir oxide nanosheets (NSs). Cross-sectional high-angle annular darkfield scanning transmission electron microscopy images demonstrate a three-layer heterostructure in the amorphous Ir/Ru/Ir oxide NSs, with IrOx layers located on the upper and lower surfaces, and a layer of RuOx sandwiched between the two IrOx layers. The vertically stacked heterostructure is a result of the diffusion of Ir atoms from the amorphous IrRuOx solid solution to the surface. The obtained A-Ir/Ru/Ir oxide NSs display an ultralow overpotential of 191 mV at 10 mA cm–2 toward acid oxygen evolution reaction and demonstrate excellent performance in a proton exchange membrane water electrolyzer, which requires only 1.63 V to achieve 1 A cm–2 at 60 °C, with virtually no activity decay observed after a 1300 h test.
Keywords: vertically stacked heterostructures, amorphous nanosheets, surface segregation, acidic water splitting, PEM water electrolyzer
Anodic oxygen evolution reaction (OER) poses a critical bottleneck in the efficiency and durability of proton exchange membrane (PEM) electrolyzers.1,2 The harsh acidic and oxidative environment restricts the practical application of most OER electrocatalysts, with only Ir-based oxides exhibiting necessary corrosion resistance.3−6 Nevertheless, the scarcity and relatively low OER activity of Ir fall short of industrial demands.7,8 Incorporating Ru in Ir-based oxides is an effective strategy to achieve both high activity and long-term stability.9−12 For example, IrRuOx solid solution effectively modulates the electronic properties of active sites by the synergy between Ru and Ir, facilitating the adsorption of oxygen intermediates.13−16 However, the IrRuOx solid solution inevitably undergoes Ir surface enrichment with partial active Ru species leaching under electrochemically induced restructuring,17 which suggests that surface Ir enrichment is conducive to the stability enhancement of IrRu oxides and a better trade-off of the activity–stability relationship.
Two-dimensional (2D) vertically stacked heterostructures offer three- or multilayer structures with distinct interfaces, allowing diverse matching according to specific needs and showcasing remarkable tunability in surface-exposed atoms.18−21 2D vertically stacked heterostructures provide a favorable configuration for constructing an IrRu oxide with surfaces enriched Ir for the acidic OER. Mechanical stacking of exfoliated flakes and epitaxial growth are common methods for fabricating vertically stacked heterostructures.22,23 However, mechanical stacking is suitable for constructing vertically stacked heterostructures in layered nanomaterials, but not for nonlayered noble metal oxides.24−26 Moreover, achieving 2D vertically stacked heterostructures of noble metal oxides through epitaxial growth, in addition to strict restrictions on the crystallinity, lattice symmetry, and lattice constant of materials, precious control of nucleation and growth kinetics is also required to prevent island growth.27,28 Alternatively, amorphous vertically stacked heterostructures, which are not bound by lattice matching, thus provide more flexibility in the integration of multiple functional components. Meanwhile, compared with the periodic atomic arrangement of crystalline structure, disordered atomic packing in amorphous nanostructure endows them with lower volume density and more vacancies as well as isotropic diffusion channels.29,30 Additionally, atomic diffusion in amorphous structures shows lower activation energy31−33 and higher diffusion rates34,35 compared to its crystalline counterpart. These factors open up exciting prospects for creating vertically stacked heterostructures of noble metal oxides through atomic diffusion in amorphous nanomaterials.
Herein, we synthesized amorphous Ir/Ru/Ir oxide nanosheets (denoted as A-Ir/Ru/Ir oxide NSs) with a three-layer vertically stacked heterostructure through surface segregation. The synthesized A-Ir/Ru/Ir oxide NSs exhibit a 191 mV overpotential for achieving 10 mA cm–2 in acidic OER and sustained operation for 1300 h in a PEM water electrolyzer with negligible activity decay.
Synthesis of A-Ir/Ru/Ir Oxide NSs
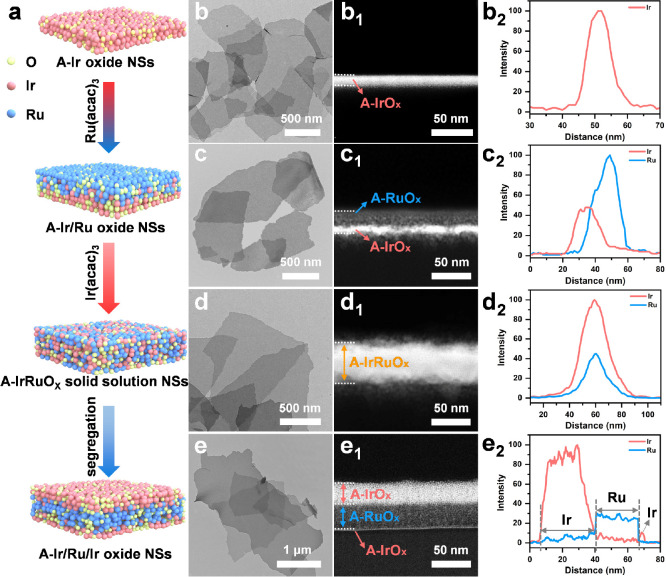
Figures 1a and S1 illustrate the formation process of A-Ir/Ru/Ir oxide NSs. Amorphous Ir oxide nanosheets (A-Ir oxide NSs) were synthesized by annealing a mixture of Ir(acac)3 and KNO3 in air. Subsequently, Ru(acac)3 is deposited on the A-Ir oxide NSs to obtain amorphous Ir/Ru oxide nanosheets (A–Ir/Ru oxide NSs). Finally, Ir(acac)3 and A-Ir/Ru oxide NSs were calcined at 285 °C. An intermediate state, characterized by amorphous IrRuOx solid solution nanosheets (A-IrRuOx solid solution NSs), were captured through rapid cooling in liquid nitrogen to preserve structural characteristics at 285 °C, while A-Ir/Ru/Ir oxide NSs were obtained through natural cooling. The X-ray diffraction (XRD) pattern affirms their amorphous nature, as there are no discernible diffraction peaks (Figure S2). Transmission electron microscopy (TEM) image (Figure 1b) and atomic force microscopy (AFM) image (Figure S3) reveal the micrometer-scale dimensions of A-Ir oxide NSs, with a thickness of 11 nm. Cross-sectional high-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) image (Figure 1b1), corresponding energy dispersive X-ray spectroscopy (EDS) line profiles (Figures 1b2 and S4a), and EDS elemental mappings (Figure S5) show homogeneous distribution of Ir throughout the A-Ir oxide NSs. For A-Ir/Ru oxide NSs, the TEM image (Figure 1c) and AFM image (Figure S6) clearly show an increase in its thickness to 35.5 nm. Cross-sectional HAADF-STEM image (Figure 1c1) clearly reveals a two-layer vertical structure of A-Ir/Ru oxide NSs, featuring a pronounced contrast difference at the interface. Moreover, cross-sectional EDS line profiles (Figures 1c2 and S4b) and EDS elemental mappings (Figure S7) confirm that the top dark layer corresponds to the RuOx layer, while the bottom bright layer corresponds to the IrOx layer. Meanwhile, the cross-sectional high-resolution transmission electron microscopy (HRTEM) image (Figure S8) clearly shows the vertical distribution trend of the Ir and Ru atoms at the interface. A-IrRuOx solid solution NSs, depicted in Figures 1d and S9, exhibit a micrometer size and a thickness of 51.8 nm. The random atomic arrangement of Ir and Ru depicted in the HRTEM image (Figure S10) indicates the amorphous structure of the A-IrRuOx solid solution NSs. Cross-sectional HAADF-STEM image (Figure 1d1), corresponding EDS line profiles (Figures 1d2 and S4c) and EDS elemental mappings (Figure S11) indicate a uniform distribution of Ir and Ru atoms within the A-IrRuOx solid solution NSs. Figure 1e displays A-Ir/Ru/Ir oxide NSs, maintaining a micrometer-scale transverse dimension. Elemental mappings (Figure S12) indicate a homogeneous distribution of Ir, Ru, and O in the planar. Cross-sectional HAADF-STEM image (Figure 1e1) and corresponding EDS line profiles (Figures 1e2 and S4d) provide a clear visualization of the three-layer vertical heterostructure of A-Ir/Ru/Ir oxide NSs. Among them, the bright regions observed on the upper and lower surfaces correspond to the IrOx layer, while the middle section corresponds to the RuOx layer. This distinction is clearly discernible due to the contrast disparity between Ir and Ru. We propose that the transition from IrRuOx solid solution to the three-layer vertical structure of A-Ir/Ru/Ir oxide NSs results from the rearrangement of atoms induced by surface segregation of Ir atoms, driven by the difference in surface energy between Ir and Ru.36,37
Figure 1.
(a) Schematic illustration of the synthesis process from A-Ir NSs to A-Ir/Ru/Ir oxide NSs. Note: the red balls represent Ir atoms, the blue balls represent Ru atoms, and yellow ones represent O atoms. (b–e) TEM images. (b1, c1, d1, e1) Cross-sectional HAADF-STEM images. (b2, c2, d2, e2) Corresponding cross-sectional EDS line profiles of A-Ir oxide NSs, A-Ir/Ru oxide NSs, A-IrRuOx solid solution NSs, and A-Ir/Ru/Ir oxide NSs, respectively.
To further investigate A-Ir/Ru/Ir oxide NSs, we conducted a detailed characterization. As shown in Figure 2a, the AFM image indicates a thickness of 62 nm for A-Ir/Ru/Ir oxide NSs. Figure 2b is an enlargement of Figure 1e1, providing a clearer view of the vertical heterostructure of the A-Ir/Ru/Ir oxide NSs. The top IrOx layer is approximately 28 nm thick, the middle RuOx layer is about 32 nm thick, and the bottom IrOx layer is approximately 2 nm thick. Two distinct heterointerfaces, labeled interfaces I and II, are formed between the surface IrOx layer and the intermediate RuOx layer. Cross-sectional aberration-corrected HAADF-STEM image (Figure 2c) reveals the disordered atomic arrangement of the IrOx and RuOx layers at interface I. EDS elemental mapping (Figure 2d) clearly illustrates the distribution region of Ir and Ru, confirming the locations of the IrOx layer and RuOx layer at interface I. Figure 2e,f demonstrates the disordered atomic arrangement and the distribution region of Ir and Ru at interface II, providing further compelling evidence for the amorphous three-layer vertical structure of A-Ir/Ru/Ir oxide NSs.
Figure 2.

Characterization of A-Ir/Ru/Ir oxide NSs. (a) AFM image and (b) cross-sectional HAADF-STEM image of A-Ir/Ru/Ir oxide NSs. (c) Cross-sectional aberration-corrected HAADF-STEM image and (d) corresponding EDS elemental mapping of interface I in A-Ir/Ru/Ir oxide NSs. (e) Cross-sectional aberration-corrected HAADF-STEM image and (f) corresponding EDS elemental mapping of interface II in A-Ir/Ru/Ir oxide NSs.
Atomic and Electronic Structures of A-Ir/Ru/Ir Oxide NSs
X-ray photoelectron spectroscopy (XPS) measurements were carried out to determine the oxidation state of the Ru and Ir species. As shown in Figure 3a, A-Ir/Ru/Ir oxide NSs demonstrate a 0.2 eV positive shift in the Ru(III) and Ru(IV) peaks compared to A-Ir/Ru oxide NSs,38 confirming a higher valence state of Ru in A-Ir/Ru/Ir oxide NSs. Ir 4f XPS spectra (Figure 3b) of A-Ir/Ru/Ir oxide NSs be fitted with three pairs of peaks centered at 61.5 and 64.5 eV, 62.1 and 65.1 eV, and 63.2 and 66.2 eV, which can be attributed to Ir (0), Ir (III), and Ir (IV), respectively.39,40 In comparison with A-Ir oxide NSs and A-Ir/Ru oxide NSs, the Ir 4f XPS spectra of A-Ir/Ru/Ir oxide NSs show a significant negative shift, suggesting that Ir in A-Ir/Ru/Ir oxide NSs possesses the lowest oxidation states.
Figure 3.
Spectroscopic studies of A-Ir oxide NSs, A-Ir/Ru oxide NSs, and A-Ir/Ru/Ir oxide NSs. (a) Ru 3d XPS spectra. (b) Ir 4f XPS spectra. (c) Ru K-edge XANES spectra. (d) Ir L3-edge XANES spectra. (e) Fourier transforms of Ru K-edge EXAFS. (f) Fourier transforms of Ir L3-edge EXAFS of A-Ir oxide NSs, A-Ir/Ru oxide NSs, and A-Ir/Ru/Ir oxide NSs, respectively.
X-ray absorption near-edge structure (XANES) spectra were employed to investigate the electron transition behavior and electronic structure. The normalized Ru K-edge of A-Ir/Ru oxide NSs and A-Ir/Ru/Ir oxide NSs shows a similar spectral shape and edge position to the reference RuO2 (Figure 3c), which identified that the average valence state of Ru species in A-Ir/Ru oxide NSs and A-Ir/Ru/Ir oxide NSs is close to +4. A-Ir/Ru/Ir oxide NSs exhibit a slight positive shift compared to A-Ir/Ru oxide NSs, suggesting an increase in the Ru oxidation state. The Ir L3-edge (Figure 3d) shows the average valence states of Ir in the order of IrO2 > A-Ir/Ru oxide NSs > A-Ir oxide NSs > A-Ir/Ru/Ir oxide NSs > Ir foil. XANES results for the Ru K-edge and Ir L3-edge reveal an increase in Ru valence and a decrease in Ir valence in A-Ir/Ru/Ir oxide NSs compared to A-Ir/Ru oxide NSs, consistent with the XPS results. The converse shift of Ru and Ir valence states within the A-Ir/Ru/Ir oxide NSs could be attributed to changes in the heterogeneous interface, which triggers the charge redistribution of Ir and Ru atoms on the vertically stacked heterointerfaces, with a transfer of electrons from Ru to Ir, and thus an increase in the Ru valence and a decrease in the Ir valence.40 The Fourier transforms of the extended X-ray absorption fine structure (FT-EXAFS) spectra of A-Ir/Ru/Ir oxide NSs at the Ru K-edge (Figures 3e, S13, and Table S1) show a dominant peak at approximately 1.50 Å, corresponding to the Ru–O bond. According to the fitting results, the coordination numbers (CNs) of the Ru–O bond in A-Ir/Ru/Ir oxide are smaller than those in crystalline RuO2, which is attributed to the rich unsaturated coordination properties of the amorphous nanostructure. The FT-EXAFS spectra of Ir in R-space (Figures 3f, S14, and Table S2) demonstrate that IrOx in A-Ir/Ru/Ir oxide NSs exhibit nearly identical Ir–O bond lengths and similar coordination numbers as crystalline IrO2, revealing the typical coordination octahedra of IrO6 in A-Ir/Ru/Ir oxide NSs. Moreover, the presence of Ru–Ir/Ir-Ru bonds in the Ru K-edge and Ir L3-edge spectra indicate an interaction between Ru and Ir at the heterointerface of the A-Ir/Ru/Ir oxide NSs.13 The interactions in Ir–Ru local structures can prevent the formation of more soluble Ru/Ir high-valent complexes.14,41
Electrocatalytic Activity of A-Ir/Ru/Ir Oxide NSs Toward OER
OER performances for A-Ir/Ru/Ir oxide NSs, A-Ir/Ru oxide NSs, A-Ir oxide NSs, commercial IrO2 (Figure S15), and commercial RuO2 (Figure S16) were evaluated in O2-saturated 0.1 M HClO4 electrolyte. As shown in the linear sweep voltammetry (LSV) curves and corresponding Tafel slopes (Figure 4a,b), the A-Ir/Ru/Ir oxide NSs show the best OER catalytic performance. The lowest overpotential was 191 mV at a current density of 10 mA cm–2 and a Tafel slope of 41.6 mV dec–1, indicating that the A-Ir/Ru/Ir oxide NSs possess the fastest OER kinetics.
Figure 4.
Electrochemical OER performance of A-Ir/Ru/Ir oxide NSs. (a) LSV curves. (b) Tafel plots. (c) Overpotential at 10 mA cm–2 (left axis) and TOF at η = 300 mV (right axis). (d) Mass activity of A-Ir/Ru/Ir oxide NSs, A-Ir/Ru oxide NSs, A-Ir oxide NSs, commercial RuO2 and IrO2, respectively. (e) Comparison of the OER performance between A-Ir/Ru/Ir oxide NS and A-Ir/Ru oxide NS. Light red represents A-Ir/Ru/Ir oxide NS, sky blue represents A-Ir/Ru oxide NS. (f) In situ ATR-SEIRAS of A-Ir/Ru/Ir oxide NSs at different potentials. (g) Polarization curves of the PEM electrolyzer using A-Ir/Ru/Ir oxide NSs as anodic catalysts coated on Nafion 115 membrane operated at 60 °C. (h) Chronopotentiometric curves of the PEM electrolyzer using A-Ir/Ru/Ir oxide NSs catalyst at a sequential current density of 0.2, 0.4, 0.6, 0.8, 1.0, 1.5, 2.0, and 3.0 A cm–2.
To compare the intrinsic catalytic activities of these catalysts, we calculated turnover frequency (TOF) at each noble metal site and mass activities according to the total loading mass of the noble metal (Table S3) in the catalysts. In Figure 4c, the TOF of A-Ir/Ru/Ir oxide NSs (0.855 s–1 at η = 300 mV) was about 4.4 and 4.5 times higher than that of A-Ir/Ru oxide NSs (0.194 s–1) and A-Ir oxide NSs (0.192 s–1), respectively. Additionally, the A-Ir/Ru/Ir oxide NSs reached the highest mass activity of 2377.1 A gIr+Ru–1 at η = 300 mV (Figure 4d), which was nearly 17.5 and 69 times higher than that of commercial RuO2 and commercial IrO2, respectively. Furthermore, the A-Ir/Ru/Ir oxide NSs exhibit extremely high mass activity compared with previously reported noble metal electrocatalysts (Table S4). Apart from outstanding OER activity, A-Ir/Ru/Ir oxide NSs demonstrate excellent long-term stability, maintaining approximately 92% of current density over a 100 h chronoamperometry stability test, whereas A-Ir/Ru oxide NSs exhibit rapid decay after 22 h (Figure S17). We also employed ICP-MS to measure the dissolved amounts of Ru and Ir ions in the electrolyte at various intervals during the stability test of A-Ir/Ru/Ir oxide NSs (Figure S18). The exceedingly low concentration of Ru ions in the electrolyte indicates the effective protection of RuOx by the IrOx protective skeleton layer. Besides, the OER activity of A-Ir/Ru/Ir oxide NSs demonstrated minimal degradation even after 10,000 cycles (Figure S19), highlighting their outstanding stability. The results indicate that IrOx protective skeleton layer of A-Ir/Ru/Ir oxide NSs can significantly reduce the leaching of Ru and improve the stability of the catalyst. Meanwhile, as illustrated in Figure 4e, we systemically compared the OER performance of A-Ir/Ru/Ir oxide NSs and A-Ir/Ru oxide NSs, including overpotential, mass activity, Tafel slope, TOF, and long-term stability. Compared to A-Ir/Ru oxide NSs, A-Ir/Ru/Ir oxide NSs demonstrate better performance in all of these metrics. The superior catalytic activity and overall performance of A-Ir/Ru/Ir oxide NSs surpass those of A-Ir/Ru oxide NSs, highlighting the significant performance advantage of the vertical heterostructure with RuOx sandwiched in the IrOx layer over the directly exposed RuOx surface, which underscores the importance of the vertical heterostructure with IrOx-enriched surfaces for enhancing performance. Moreover, the Nyquist plots (Figure S20) show that the A-Ir/Ru/Ir oxide NSs have the lowest charge-transfer resistance among all of the tested catalysts, verifying the faster charge transfer process for A-Ir/Ru/Ir oxide NSs. To confirm the mechanism of the OER on A-Ir/Ru/Ir oxide NSs, in situ attenuated total reflection surface enhanced infrared absorption spectroscopy (ATR-SEIRAS) was employed. The in situ ATR-SEIRAS spectra for A-Ir/Ru/Ir oxide NSs at different working potentials (Figure 4f) reveal a distinct absorption peak at 1148 cm–1 attributed to the O–O stretching of surface adsorbed *OOH,42,43 which is a characteristic intermediate of the adsorption evolution mechanism (AEM) pathway, indicating that A-Ir/Ru/Ir oxide NSs follow the AEM pathway.
We finally assembled a single cell using A-Ir/Ru/Ir oxide NSs as the anode to assess its performance in a real proton exchange membrane (PEM) electrolyzer device (Figure S21). The A-Ir/Ru/Ir oxide NS electrolyzer (at 60 °C) required only 1.63 V to reach a current density of 1.0 A cm–2 (Figure 4g), surpassing the performance of commercial RuO2 (1.72 V) and IrO2 (1.80 V), as well as outperforming other previously reported advanced Ir/Ru-based catalysts (Table S5). Impressively, no significant activity decay was observed during the 1300 h test at gradient increasing current density (Figure 4h). Specifically, the cell voltage remained stable (with a fluctuation within 20 mV) for 600 h at a current density of 1.0 A cm–2, and the voltage fluctuations were small when the current density was increased to 2.0 and 3.0 A cm–2. Additionally, the polarization curve indicates a slight increase in the cell voltage after the 1300 h test (Figure S22). PEM electrolyzer test results demonstrate the good stability of the A-Ir/Ru/Ir oxide NSs and its excellent applicability under varying current densities. In addition, as shown in Figure S23, the TEM image and XRD pattern of A-Ir/Ru/Ir oxide NSs after the long-term durability test indicate negligible changes in morphology during the OER process, and the structural amorphous features remain unchanged.
In summary, we synthesized vertically stacked A–Ir–Ru-Ir oxide NSs. The prepared A-Ir/Ru/Ir oxide NSs exhibit a three-layer vertical heterostructure, with amorphous IrOx layers on the upper and lower surfaces, and a layer of amorphous RuOx sandwiched between the two IrOx layers. Impressively, the as-prepared A-Ir/Ru/Ir oxide NSs exhibit an ultralow overpotential (191 mV@10 mA cm–2) and high mass activity (2377.1 A gIr+Ru–1@300 mV) in acidic OER. The significant enhanced catalytic performance of A-Ir/Ru/Ir oxide NSs could be attributed to rich heterointerfaces, enabling substantially increased catalytically active sites and accelerated charge transfer kinetics. Additionally, A-Ir/Ru/Ir oxide NSs exhibited outstanding durability, sustaining performance for 1300 h in the PEM electrolyzer with virtually no activity decay. Moreover, A-Ir/Ru/Ir oxide NSs exhibited stable operation even at high current densities of 2.0 and 3.0 A cm–2. This work opens a new horizon for rationally exploiting surface segregation to deliberately synthesize and design electrocatalysts.
Acknowledgments
This work was supported by the National Key R&D Program of China (2018YFA0702001), National Natural Science Foundation of China (22371268, 22301287), Fundamental Research Funds for the Central Universities (WK2060000016), Anhui Province for Outstanding Youth (2208085J09), Collaborative Innovation Program of Hefei Science Center, CAS (2022HSC–CIP020), Anhui Development and Reform Commission (AHZDCYCX-2SDT2023-07), Youth Innovation Promotion Association of the Chinese Academy of Science (2018494), and USTC Tang Scholar. We thank the USTC Center for Micro and Nanoscale Research and Fabrication and the BL14W1 in Shanghai Synchrotron Radiation Facility (SSRF) and for help in characterizations.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacsau.4c00085.
Detailed experimental procedures, synthesis, physical characterizations, computational details, electrochemical measurements, and performance comparison (PDF)
Author Contributions
# J.L., G.W., and Z.H. contributed equally to this work. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Xu J.; Jin H.; Lu T.; Li J.; Liu Y.; Davey K.; Zheng Y.; Qiao S.-Z. IrOx·nH2O with lattice water–assisted oxygen exchange for high-performance proton exchange membrane water electrolyzers. Sci. Adv. 2023, 9 (25), eadh1718 10.1126/sciadv.adh1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Y.; Hung S.-F.; Zeng W.-J.; Wang Y.; Zhang C.; Kuo C.-H.; Wang L.; Zhao S.; Zhang Y.; Chen H.-Y.; et al. Switching the Oxygen Evolution Mechanism on Atomically Dispersed Ru for Enhanced Acidic Reaction Kinetics. J. Am. Chem. Soc. 2023, 145 (43), 23659–23669. 10.1021/jacs.3c07777. [DOI] [PubMed] [Google Scholar]
- Zu L.; Qian X.; Zhao S.; Liang Q.; Chen Y. E.; Liu M.; Su B.-J.; Wu K.-H.; Qu L.; Duan L.; et al. Self-Assembly of Ir-Based Nanosheets with Ordered Interlayer Space for Enhanced Electrocatalytic Water Oxidation. J. Am. Chem. Soc. 2022, 144 (5), 2208–2217. 10.1021/jacs.1c11241. [DOI] [PubMed] [Google Scholar]
- Wu G.; Zheng X.; Cui P.; Jiang H.; Wang X.; Qu Y.; Chen W.; Lin Y.; Li H.; Han X.; et al. A general synthesis approach for amorphous noble metal nanosheets. Nat. Commun. 2019, 10, 4855. 10.1038/s41467-019-12859-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H.; Liu X.; An P.; Tang C.; Yu H.; Zhang Q.; Peng H.-J.; Gu L.; Zheng Y.; Song T.; et al. Dynamic rhenium dopant boosts ruthenium oxide for durable oxygen evolution. Nat. Commun. 2023, 14, 354. 10.1038/s41467-023-35913-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan R.-Y.; Zhang Y.-S.; Lv J.-Y.; Han G.-Q.; Chai Y.-M.; Dong B. The Promising Seesaw Relationship Between Activity and Stability of Ru-Based Electrocatalysts for Acid Oxygen Evolution and Proton Exchange Membrane Water Electrolysis. Small 2023, 20, 2304636. 10.1002/smll.202304636. [DOI] [PubMed] [Google Scholar]
- Danilovic N.; Subbaraman R.; Chang K. C.; Chang S. H.; Kang Y. J.; Snyder J.; Paulikas A. P.; Strmcnik D.; Kim Y. T.; Myers D.; et al. Activity–Stability Trends for the Oxygen Evolution Reaction on Monometallic Oxides in Acidic Environments. J. Phys. Chem. Lett. 2014, 5 (14), 2474–2478. 10.1021/jz501061n. [DOI] [PubMed] [Google Scholar]
- Wohlfahrt-Mehrens M.; Heitbaum J. Oxygen evolution on Ru and RuO2 electrodes studied using isotope labelling and on-line mass spectrometry. J. Electroanal. Chem. Interface Electrochem. 1987, 237 (2), 251–260. 10.1016/0022-0728(87)85237-3. [DOI] [Google Scholar]
- Kötz R.; Stucki S. Stabilization of RuO2 by IrO2 for anodic oxygen evolution in acid media. Electrochim. Acta 1986, 31 (10), 1311–1316. 10.1016/0013-4686(86)80153-0. [DOI] [Google Scholar]
- Danilovic N.; Subbaraman R.; Chang K. C.; Chang S. H.; Kang Y.; Snyder J.; Paulikas A. P.; Strmcnik D.; Kim Y. T.; Myers D.; et al. Using Surface Segregation To Design Stable Ru-Ir Oxides for the Oxygen Evolution Reaction in Acidic Environments. Angew. Chem., Int. Ed. 2014, 53 (51), 14016–14021. 10.1002/anie.201406455. [DOI] [PubMed] [Google Scholar]
- Xia T.; Liu C.; Lu Y.; Jiang W.; Li H.; Ma Y.; Wu Y.; Che G. Regulating Ru-based double perovskite against lattice oxygen oxidation by incorporating Ir for efficient and stable acidic oxygen evolution reaction. Appl. Surf. Sci. 2022, 605, 154727. 10.1016/j.apsusc.2022.154727. [DOI] [Google Scholar]
- Wang L.; Saveleva V. A.; Zafeiratos S.; Savinova E. R.; Lettenmeier P.; Gazdzicki P.; Gago A. S.; Friedrich K. A. Highly active anode electrocatalysts derived from electrochemical leaching of Ru from metallic Ir0.7Ru0.3 for proton exchange membrane electrolyzers. Nano Energy 2017, 34, 385–391. 10.1016/j.nanoen.2017.02.045. [DOI] [Google Scholar]
- Wen Y.; Chen P.; Wang L.; Li S.; Wang Z.; Abed J.; Mao X.; Min Y.; Dinh C. T.; Luna P. D.; et al. Stabilizing Highly Active Ru Sites by Suppressing Lattice Oxygen Participation in Acidic Water Oxidation. J. Am. Chem. Soc. 2021, 143 (17), 6482–6490. 10.1021/jacs.1c00384. [DOI] [PubMed] [Google Scholar]
- Zhu W.; Song X.; Liao F.; Huang H.; Shao Q.; Feng K.; Zhou Y.; Ma M.; Wu J.; Yang H.; et al. Stable and oxidative charged Ru enhance the acidic oxygen evolution reaction activity in two-dimensional ruthenium-iridium oxide. Nat. Commun. 2023, 14, 5365. 10.1038/s41467-023-41036-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang Z.; Wang Y.; Xu C.-Q.; Liu S.; Chen C.; Peng Q.; Zhuang Z.; Xiao H.; Pan Y.; Lu S.; et al. Three-dimensional open nano-netcage electrocatalysts for efficient pH-universal overall water splitting. Nat. Commun. 2019, 10, 4875. 10.1038/s41467-019-12885-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S.; Tan H.; Huang Y.-C.; Zhang Q.; Lin H.; Li L.; Hu Z.; Huang W.-H.; Pao C.-W.; Lee J.-F.; et al. Structurally-Distorted RuIr-Based Nanoframes for Long-Duration Oxygen Evolution Catalysis. Adv. Mater. 2023, 35 (42), 2305659. 10.1002/adma.202305659. [DOI] [PubMed] [Google Scholar]
- Escalera-López D.; Czioska S.; Geppert J.; Boubnov A.; Röse P.; Saraçi E.; Krewer U.; Grunwaldt J.-D.; Cherevko S. Phase- and Surface Composition-Dependent Electrochemical Stability of Ir-Ru Nanoparticles during Oxygen Evolution Reaction. ACS Catal. 2021, 11 (15), 9300–9316. 10.1021/acscatal.1c02968. [DOI] [Google Scholar]
- Liu X.; Gao M.; Yang H.; Zhong X.; Yu Y. 2D sandwich-like nanosheets of ultrafine Sb nanoparticles anchored to graphene for high-efficiency sodium storage. Nano Res. 2017, 10 (12), 4360–4367. 10.1007/s12274-017-1627-y. [DOI] [Google Scholar]
- Sun J.; Yang D.; Lowe S.; Zhang L.; Wang Y.; Zhao S.; Liu P.; Wang Y.; Tang Z.; Zhao H.; et al. Sandwich-Like Reduced Graphene Oxide/Carbon Black/Amorphous Cobalt Borate Nanocomposites as Bifunctional Cathode Electrocatalyst in Rechargeable Zinc-Air Batteries. Adv. Energy Mater. 2018, 8 (27), 1801495. 10.1002/aenm.201801495. [DOI] [Google Scholar]
- Saleem F.; Zhang Z.; Cui X.; Gong Y.; Chen B.; Lai Z.; Yun Q.; Gu L.; Zhang H. Elemental Segregation in Multimetallic Core–Shell Nanoplates. J. Am. Chem. Soc. 2019, 141 (37), 14496–14500. 10.1021/jacs.9b05197. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Song J.; Wong W.-Y. Constructing 2D Sandwich-like MOF/MXene Heterostructures for Durable and Fast Aqueous Zinc-Ion Batteries. Angew. Chem., Int. Ed. 2023, 135 (8), e202218343 10.1002/ange.202218343. [DOI] [PubMed] [Google Scholar]
- Zhou Z.; Hou F.; Huang X.; Wang G.; Fu Z.; Liu W.; Yuan G.; Xi X.; Xu J.; Lin J.; et al. Stack growth of wafer-scale van der Waals superconductor heterostructures. Nature 2023, 621 (7979), 499–505. 10.1038/s41586-023-06404-x. [DOI] [PubMed] [Google Scholar]
- Lin Z.; Yin A.; Mao J.; Xia Y.; Kempf N.; He Q.; Wang Y.; Chen C.-Y.; Zhang Y.; Ozolins V.; et al. Scalable solution-phase epitaxial growth of symmetry-mismatched heterostructures on two-dimensional crystal soft template. Sci. Adv. 2016, 2 (10), e1600993 10.1126/sciadv.1600993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang K.; Lee K.-H.; Han Y.; Gao H.; Xie S.; Muller D. A.; Park J. Layer-by-layer assembly of two-dimensional materials into wafer-scale heterostructures. Nature 2017, 550, 229–233. 10.1038/nature23905. [DOI] [PubMed] [Google Scholar]
- Lotsch B. V. Vertical 2D Heterostructures. Annu. Rev. Mater. Res. 2015, 45 (1), 85–109. 10.1146/annurev-matsci-070214-020934. [DOI] [Google Scholar]
- Chen Y.; Lai Z.; Zhang X.; Fan Z.; He Q.; Tan C.; Zhang H. Phase engineering of nanomaterials. Nat. Rev. Chem. 2020, 4 (5), 243–256. 10.1038/s41570-020-0173-4. [DOI] [PubMed] [Google Scholar]
- Lu Q.; Wang A.-L.; Gong Y.; Hao W.; Cheng H.; Chen J.; Li B.; Yang N.; Niu W.; Wang J.; et al. Crystal phase-based epitaxial growth of hybrid noble metal nanostructures on 4H/fcc Au nanowires. Nat. Chem. 2018, 10 (4), 456–461. 10.1038/s41557-018-0012-0. [DOI] [PubMed] [Google Scholar]
- Liu Y.; Huang Y.; Duan X. Van der Waals integration before and beyond two-dimensional materials. Nature 2019, 567, 323–333. 10.1038/s41586-019-1013-x. [DOI] [PubMed] [Google Scholar]
- Deng S.; Yuan Z.; Tie Z.; Wang C.; Song L.; Niu Z. Electrochemically Induced Metal–Organic-Framework-Derived Amorphous V 2 O 5 for Superior Rate Aqueous Zinc-Ion Batteries. Angew. Chem., Int. Ed. 2020, 59 (49), 22002–22006. 10.1002/anie.202010287. [DOI] [PubMed] [Google Scholar]
- Wei Z.; Wang D.; Yang X.; Wang C.; Chen G.; Du F. From Crystalline to Amorphous: An Effective Avenue to Engineer High-Performance Electrode Materials for Sodium-Ion Batteries. Adv. Mater. Interface 2018, 5 (19), 1800639. 10.1002/admi.201800639. [DOI] [Google Scholar]
- Strauß F.; Dörrer L.; Geue T.; Stahn J.; Koutsioubas A.; Mattauch S.; Schmidt H. Self-Diffusion in Amorphous Silicon. Phys. Rev. Lett. 2016, 116 (2), 025901. 10.1103/PhysRevLett.116.025901. [DOI] [PubMed] [Google Scholar]
- Zhang W.; Huang J.; Yu X.; Liu G.; Yanqin H.; Fan D. Atomic-level diffusion at the amorphous Zr50Cu50/crystalline Cu interface: A molecular dynamics study. J. Adv. Join. Process. 2022, 6, 100120. 10.1016/j.jajp.2022.100120. [DOI] [Google Scholar]
- Posselt M.; Bracht H.; Ghorbani-Asl M.; Radić D. Atomic mechanisms of self-diffusion in amorphous silicon. AIP Adv. 2022, 12 (11), 115325. 10.1063/5.0111037. [DOI] [Google Scholar]
- Annamareddy A.; Voyles P. M.; Perepezko J.; Morgan D. Mechanisms of bulk and surface diffusion in metallic glasses determined from molecular dynamics simulations. Acta Mater. 2021, 209, 116794. 10.1016/j.actamat.2021.116794. [DOI] [Google Scholar]
- Wang Y.-J.; Du J.-P.; Shinzato S.; Dai L.-H.; Ogata S. A free energy landscape perspective on the nature of collective diffusion in amorphous solids. Acta Mater. 2018, 157, 165–173. 10.1016/j.actamat.2018.07.029. [DOI] [Google Scholar]
- Ruban A. V.; Skriver H. L.; No̷rskov J. K. Surface segregation energies in transition-metal alloys. Phys. Rev. B 1999, 59 (24), 15990–16000. 10.1103/PhysRevB.59.15990. [DOI] [Google Scholar]
- Farsi L.; Deskins N. A. First principles analysis of surface dependent segregation in bimetallic alloys. Phys. Chem. Chem. Phys. 2019, 21 (42), 23626–23637. 10.1039/C9CP03984H. [DOI] [PubMed] [Google Scholar]
- Cao D.; Wang J.; Xu H.; Cheng D. Growth of Highly Active Amorphous RuCu Nanosheets on Cu Nanotubes for the Hydrogen Evolution Reaction in Wide pH Values. Small 2020, 16 (37), 2000924. 10.1002/smll.202000924. [DOI] [PubMed] [Google Scholar]
- He J.; Zhou X.; Xu P.; Sun J. Regulating Electron Redistribution of Intermetallic Iridium Oxide by Incorporating Ru for Efficient Acidic Water Oxidation. Adv. Energy Mater. 2021, 11 (48), 2102883. 10.1002/aenm.202102883. [DOI] [Google Scholar]
- Shan J.; Guo C.; Zhu Y.; Chen S.; Song L.; Jaroniec M.; Zheng Y.; Qiao S.-Z. Charge-Redistribution-Enhanced Nanocrystalline Ru@IrOx Electrocatalysts for Oxygen Evolution in Acidic Media. Chem 2019, 5 (2), 445–459. 10.1016/j.chempr.2018.11.010. [DOI] [Google Scholar]
- Escudero-Escribano M.; Pedersen A. F.; Paoli E. A.; Frydendal R.; Friebel D.; Malacrida P.; Rossmeisl J.; Stephens I. E. L.; Chorkendorff I. Importance of Surface IrOx in Stabilizing RuO2 for Oxygen Evolution. J. Phys. Chem. B 2018, 122 (2), 947–955. 10.1021/acs.jpcb.7b07047. [DOI] [PubMed] [Google Scholar]
- Du K.; Zhang L.; Shan J.; Guo J.; Mao J.; Yang C.-C.; Wang C.-H.; Hu Z.; Ling T. Interface engineering breaks both stability and activity limits of RuO2 for sustainable water oxidation. Nat. Commun. 2022, 13, 5448. 10.1038/s41467-022-33150-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang C.; Li J.; Yang K. R.; Wang Y.; He D.; Thorne J. E.; Croslow S.; Dong Q.; Zhao Y.; Prostko G.; et al. Observation of a potential-dependent switch of water-oxidation mechanism on Co-oxide-based catalysts. Chem 2021, 7 (8), 2101–2117. 10.1016/j.chempr.2021.03.015. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.