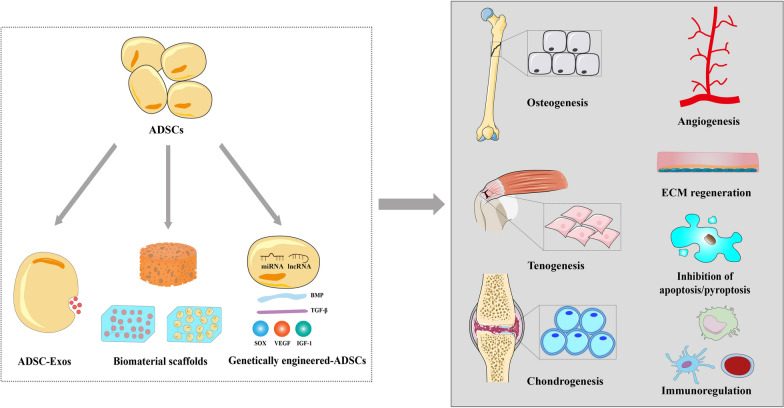
Abstract
Musculoskeletal disorders are the leading causes of physical disabilities worldwide. The poor self-repair capacity of musculoskeletal tissues and the absence of effective therapies have driven the development of novel bioengineering-based therapeutic approaches. Adipose-derived stem cell (ADSC)-based therapies are being explored as new regenerative strategies for the repair and regeneration of bone, cartilage, and tendon owing to the accessibility, multipotency, and active paracrine activity of ADSCs. In this review, recent advances in ADSCs and their optimization strategies, including ADSC-derived exosomes (ADSC-Exos), biomaterials, and genetic modifications, are summarized. Furthermore, the preclinical and clinical applications of ADSCs and ADSC-Exos, either alone or in combination with growth factors or biomaterials or in genetically modified forms, for bone, cartilage, and tendon regeneration are reviewed. ADSC-based optimization strategies hold promise for the management of multiple types of musculoskeletal injuries. The timely summary and highlights provided here could offer guidance for further investigations to accelerate the development and clinical application of ADSC-based therapies in musculoskeletal regeneration.
Graphical abstract
Keywords: Adipose-derived stem cells, Exosomes, Bone regeneration, Cartilage regeneration, Tendon regeneration, Musculoskeletal disorders, Optimization strategies
Background
Musculoskeletal disorders that affect bone, cartilage, tendons, ligaments, and skeletal muscles are characterized by tissue degeneration, debilitating pain, functional impairment, and disability. These disorders impact 1.7 billion people worldwide, with the burden doubling over the last few decades owing to factors such as aging, obesity, traumatic injuries, and genetic mutations [1]. Musculoskeletal tissues such as tendons and ligaments have a limited self-repair capacity, whereas articular cartilage exhibits poor intrinsic regenerative potential. Bones and skeletal muscles possess the ability to regenerate after minor injuries. However, larger or chronic injuries, including critical-sized bone defects and pathological fractures, can exceed the self-repair capacity of these tissues [2]. These characteristics contribute to the development and progression of musculoskeletal diseases.
Tissue engineering and regenerative medicine are the focus of research aimed at repairing or replacing defective tissues and restoring their biological functions, and such work can potentially provide solutions to the growing prevalence of musculoskeletal disorders. These approaches typically integrate stem cells, engineering materials, and bioactive molecules. Researchers have engineered and delivered cells with regenerative capacity to damaged tissues, which represents the basis for diverse bioengineering approaches for musculoskeletal regeneration [3].
Adipose tissue, derived from the embryonic mesoderm, is a valuable cell source for tissue engineering. Adipose-derived stem cells (ADSCs) were first characterized in 2001 [4]. As an important subpopulation of mesenchymal stem cells (MSCs), ADSCs have the potential for multidirectional differentiation, regeneration, and multiple cytokine secretions. Compared with MSCs derived from bone marrow (BM) or other sources, ADSCs have the advantages of accessibility, abundance, and reduced donor site morbidity [5]. Adipose tissue yields approximately 500 times more stem cells compared to an equivalent amount of BM, with approximately 5000 ADSCs obtained per 1 g of adipose tissue [6]. Hence, large amounts of ADSCs can be obtained from adipose tissue via minimally invasive procedures. Moreover, ADSCs exhibit a higher proliferation rate and better colony-forming potency than BM-MSCs under similar culture conditions [7]. Single-cell RNA sequencing analysis indicates that ADSCs exhibit lower transcriptomic heterogeneity, lower human leukocyte antigen (HLA) class I expression, and higher immunosuppressive capacity than BM-MSCs [8]. In addition, ADSCs have low immunogenicity and immune-privileged potential owing to the absence or low expression of HLA and co-stimulatory molecules [9, 10]. Moreover, unlike embryonic stem cells, there are no ethical issues surrounding the acquisition of ADSCs. All these properties indicate that ADSCs represent an interesting and feasible alternative to BM-MSCs or other types of MSCs. Therefore, they are considered prominent candidates for cellular bioengineering and hold promising prospects in tissue repair and regeneration.
In recent decades, ADSCs have been increasingly explored for their regenerative potential in various conditions, including wound healing, neurodegenerative diseases, cardiovascular diseases, and liver cirrhosis [11–16]. Furthermore, ADSC-based and related tissue engineering strategies are being investigated for bone, cartilage, and tendon repair and regeneration [3, 17–21]. Hence, in this study, we review ADSCs and novel optimization strategies for improving their regenerative potential, including ADSC-derived exosomes (ADSC-Exos), biomaterials, and genetically engineered modifications. Next, we discuss the preclinical and clinical applications of ADSCs and ADSC-Exos, either alone or in combination with growth factors or biomaterials or in a genetically modified form, in bone, cartilage, and tendon regeneration in various musculoskeletal disorders. With this review, we hope to provide further insights into the vital roles and applications of ADSCs in musculoskeletal tissue engineering and regenerative medicine. To achieve this, we searched PubMed, Web of Science, and EMBASE using “adipose-derived stem cells” or “ADSCs” and the three related topics mentioned above as keywords to retrieve the relevant orthopedic studies published over the past 5 years. We focused on studies involving cell and animal experiments or clinical trials, particularly original articles related to the topic.
ADSC characteristics
Apart from being an energy reservoir for the body, adipose tissue acts as an endocrine organ that regulates the body’s metabolism and immunity [22]. There are two types of adipose tissues, i.e., white and brown, and they differ in terms of their distribution, function, and metabolic activity. White adipose tissue is predominantly involved in lipid storage and serves as a source of ADSCs. Brown adipose tissue, a key regulator of thermogenesis, is predominantly present in fetuses and newborns and declines with age [23]. Stromal vascular fraction (SVF) is conventionally isolated from the enzymatic digestion of white adipose tissue and represents a heterogeneous cell group consisting of preadipocytes, vascular smooth muscle cells, endothelial cells, monocytes/macrophages, lymphocytes, fibroblasts, pericytes, and ADSCs. ADSCs account for 15–30% of the SVF [24]. Owing to their plastic adherence capacity, ADSCs can be easily harvested from the SVF in a culture system.
However, ADSCs are not completely homogeneous, and there is no single biomarker to explicitly characterize them. In 2013, the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT) described the phenotypic and functional criteria for ADSCs [24]. Phenotypic identification includes primary positive markers, such as cluster of differentiation (CD)13, CD29, CD44, CD73, CD90, and CD105, and primary negative markers, such as CD31, CD45, and CD235a. Additional secondary positive markers include CD10, CD26, CD36, CD49d, and CD49e, whereas secondary low or negative markers include CD3, CD11b, CD49f, CD106, and podocalyxin-like protein. CD34 is the primary unstable marker present at various levels in ADSCs. It is generally expressed during the early culture phase (within 8–12 population doublings after SVF culture); however, its expression decreases with continued cell division [24, 25]. Moreover, the IFATS and ISCT provided recommendations for the functional assessment of ADSCs. The fibroblastoid colony-forming unit assay is generally used to calculate the population-doubling capacity of ADSCs. Chondroblastic, osteoblastic, and adipocytic differentiation assays have been employed to assess the differentiation potential of ADSCs. Furthermore, quantitative evaluations of lineage-specific gene or protein biomarkers using biochemical methods (western blotting, enzyme-linked immunosorbent assay) or reverse transcription polymerase chain reaction have been proposed to characterize the differentiation potential of ADSCs. The following biomarkers have been proposed: aggrecan, collagen type II, and Sox9 for chondrogenesis; alkaline phosphatase, bone sialoprotein, osteocalcin, osterix, and Runx2 for osteogenesis; and collagen III, tenomodulin, and tenascin C for tenogenesis [24, 26–28]. Recent advances in single-cell technology provide new methods of identifying the multidirectional differentiation potential of ADSCs [29, 30].
ADSCs can differentiate in vitro and in vivo into osteogenic, chondrogenic, myogenic, epithelial, endothelial, and neuron-like cells in the presence of lineage-specific induction factors [31]. In addition, mechanical or electromagnetic stimulation, pharmaceuticals, and genetic reprogramming have been widely explored to promote the differentiation of ADSCs into specific cell lineages [32, 33]. However, the therapeutic potential of ADSCs is not limited to the specific differentiation and replacement of defective cells. The “secretome” theory is based on the ability of ADSCs to secrete multiple cytokines, growth factors, chemokines, and antioxidant factors [e.g., vascular endothelial growth factor (VEGF), insulin-like growth factor-1 (IGF-1), nerve growth factor (NGF), hepatocyte growth factor, and multiple interleukins (ILs)] alone or as cell-free extracellular vesicles (EVs) [34–36] (Fig. 1), while the “building block” or “host replacement” theories indicate that ADSCs can differentiate and replace damaged cells. Studies on the secretome profile have also indicated that the paracrine activity of the secretome may regulate various biological effects, including angiogenesis, apoptosis suppression, and immunoregulation [16, 17, 36–38]. Thus, the ADSC secretome may be an important therapeutic mediator necessary in tissue repair and regeneration.
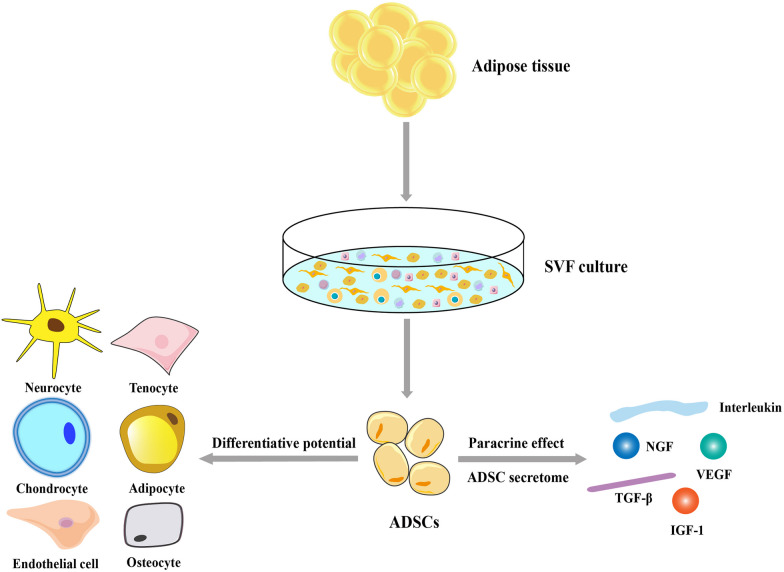
Fig. 1.
Differentiation potential and paracrine activity of ADSCs. ADSCs can be harvested as part of the SVF, which is traditionally isolated from the enzymatic digestion of the adipose tissues. ADSCs exhibit regenerative abilities and can differentiate into specific cell types in the presence of lineage-specific induction factors and secrete multiple bioactive molecules, which constitute the “secretome” profile. ADSCs: adipose-derived stem cells; IGF-1: insulin-like growth factor 1; NGF: nerve growth factor; SVF: stromal vascular fraction; TGF-β: transforming growth factor-β; VEGF: vascular endothelial growth factor
Optimization of ADSC-based therapeutic strategies
ADSC secretome
As mentioned above, ADSCs exert regenerative effects not only by direct cell replacement but also via the secretome, including EVs and other bioactive molecules. Considering the numerous limitations associated with the direct transplantation of ADSCs, such as the need for strict monitoring during production and storage and the potential risks of tumorigenicity and immune rejection, the “cell-based but cell-free” therapy approach utilizing the ADSC secretome has recently gained considerable attention as an alternative strategy. During cell culture, ADSCs typically release various bioactive molecules into the medium, which is known as the conditioned medium (CM). ADSC-CM consists of a complex cocktail of proteins, nucleic acids, and lipids released as active factors and/or conveyed to EVs, collectively constituting the entire ADSC secretome and contributing to the paracrine effects of ADSCs [39]. Studies have demonstrated the beneficial effects of ADSC-CM on wound healing [40], skin photoaging [41], and cardioprotection [16]. EVs are a heterogeneous group of cell-derived lipid bilayer membranes that can be categorized into various subclasses, including exosomes, microvesicles, and apoptotic bodies [42]. In recent years, ADSC-Exos extracted from ADSC-CM has emerged as the focus of ongoing investigations in regenerative medicine.
ADSC-Exos
Exosomes are the most abundant and well-characterized type of EVs and have diameters ranging between 40 and 160 nm [43]. They originate within endosomal compartments and encapsulate specific proteins, nucleic acids (mRNAs, miRNAs, and long noncoding RNAs [lncRNAs]), lipids, amino acids, and metabolites within their lipid bilayer membranes. Upon release into the extracellular space, they are transported to target cells and participate in intercellular communication [43]. ADSC-Exos, which are naturally secreted by ADSCs, can promote angiogenesis, regulate immune and inflammatory responses, affect collagen remodeling, inhibit cell apoptosis, and promote tissue repair and regeneration. These functions stem from the diverse proteins and genetic materials encapsulated within them [44–46]. Compared with ADSCs, ADSC-Exos are smaller and less complex than their parent cells, and they are also more stable and convenient to store. Additionally, their dosage can be easily controlled [47]. Functionally, ADSC-Exos have low immunogenicity and can reduce immune recognition to maintain cell membrane integrity [48].
Several methods are available for harvesting exosomes. Among them, ultracentrifugation is the most commonly used and considered the gold standard for isolation. Ultrafiltration can be used both as a standalone isolation approach and as a complement to ultracentrifugation. In addition to these traditional approaches, innovative technologies and new platforms, such as a magnetic bead-based adsorption strategy, have emerged for exosome isolation [49]. During the biosynthesis of ADSC-Exos, a range of proteins are enriched, among which tetraspanins (CD9, CD63, and CD81), vesicle-forming proteins (Alix and tumor suppressor gene 101 [TSG101]), and heat shock proteins (HSP70 and HSP90) are generally recognized as characteristic biomarkers [50] (Fig. 2). Furthermore, electron microscopy and nanoparticle tracking analysis can be used to identify ADSC-Exos. Multiomics has recently been used to identify specific marker proteins in exosomes [51, 52]. This advancement may provide a novel method to elucidate the functions of ADSC-Exos in the future. Thus far, the therapeutic effects of ADSC-Exos have been demonstrated in the field of regenerative medicine [53, 54]. ADSC-Exos combined with optimization strategies, including exosome incorporation into biomaterials [55] or genetic modification [56], represents a feasible strategy for further application of ADSC-Exos.
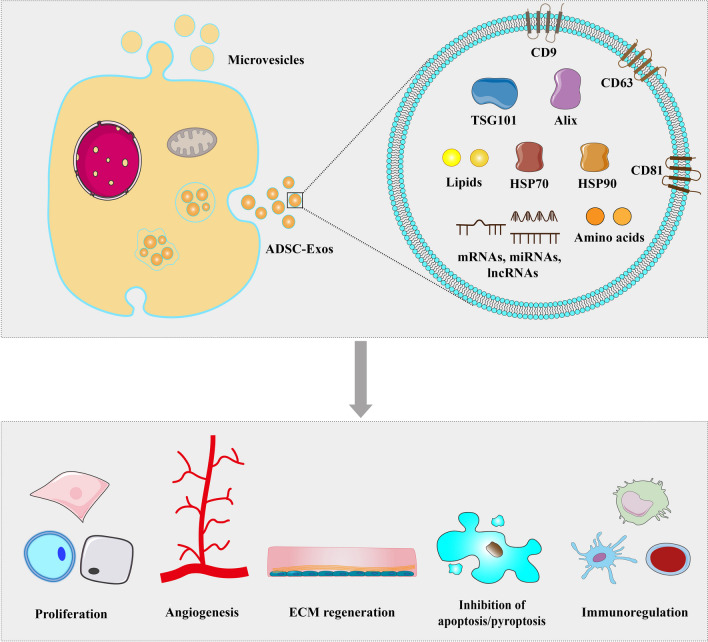
Fig. 2.
Characteristics and therapeutic potential of ADSC-Exos. ADSC-Exos contain tetraspanins (CD9, CD63, and CD81), vesicle-forming proteins (Alix and TSG101), heat shock proteins (HSP70 and HSP90), nucleic acids (mRNAs, miRNAs, and lncRNAs), lipids, and amino acids. ADSC-Exos exert their therapeutic potential by enhancing cell proliferation, angiogenesis, and ECM synthesis, participating in immunoregulation, and inhibiting apoptosis or pyroptosis. ADSC-Exos: adipose-derived stem cell-derived exosomes; ECM: extracellular matrix; HSP: heat shock protein; TSG: tumor suppressor gene
Biomaterial scaffolds
An ideal biomaterial scaffold can mimic the natural extracellular environment and provide mechanical support to facilitate the delivery, proliferation, migration, and differentiation of ADSCs [3]. Moreover, incorporating ADSC-Exos into suitable biomaterials can help control their release, distribution, and retention in vivo, thereby enhancing their therapeutic function [57]. Thus, the combination of ADSCs or exosomes with novel scaffold materials is a promising alternative administration method.
In musculoskeletal tissue engineering, scaffold materials include organic and inorganic sources, such as natural polymers (e.g., fibrin, hyaluronic acid, chitosan, collagen, alginate, and silk fibroin), inorganic materials (e.g., hydroxyapatite, tricalcium phosphate, glass ceramics, and titanium), and synthetic biodegradable polymers [e.g., polycaprolactone, polylactic acid (PLA), polyglycolic acid, and polylactic-co-glycolic acid (PLGA)] [58, 59]. Novel synthetic scaffolds can be fabricated from different biomaterials, such as natural and synthetic polymers or inorganic ceramics and polymers, thereby eliminating the disadvantages of conventional scaffolds while enhancing their properties, including mechanical strength, porosity, wettability, angiogenic potential, and cell-material interactions required for tissue regeneration [60, 61]. Decellularized extracellular matrix (ECM)-based biomaterials, such as injectable hydrogels and electrospun scaffolds, have also been developed to provide structural support, act as local reservoirs of growth factors, and provide biochemical cues to guide ADSC differentiation and regeneration [62, 63]. Three-dimensional (3D) bioprinting is an advanced strategy for replicating the functional organization of human tissues and simulating 3D micro-tissue environments. Through 3D bioprinting, various biomaterials can be built layer-by-layer to fabricate bioengineered constructs with predefined dimensions and spatial distributions. Combination of 3D-printed scaffolds with ADSCs or ADSC-Exos can be used to generate zonal distributions of cells/exosomes, matrix proteins, and bioactive cues; construct an optimal biomimetic environment; and promote the simultaneous regeneration of different musculoskeletal tissues [55, 64, 65] (Fig. 3).
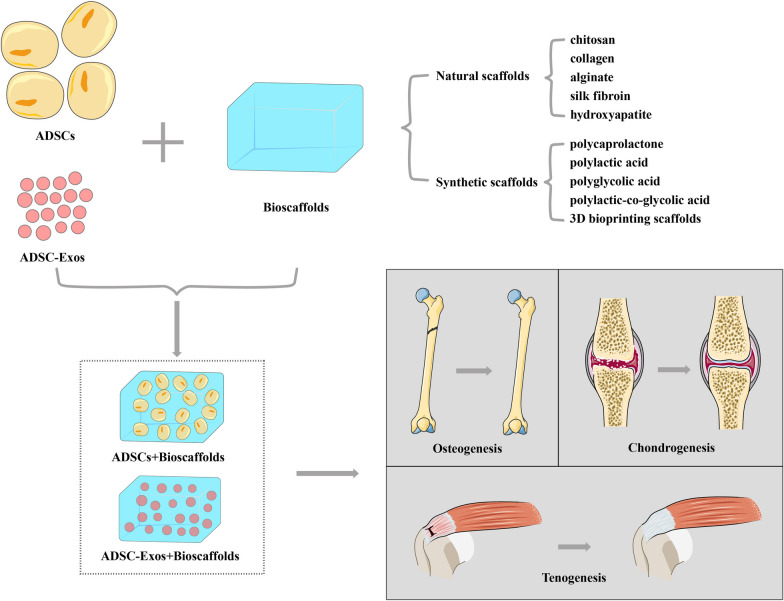
Fig. 3.
ADSCs or ADSC-Exos combined with biomaterial scaffolds for musculoskeletal regeneration. ADSCs: adipose-derived stem cells; ADSC-Exos: adipose-derived stem cell-derived exosomes
Genetically engineered ADSCs
Genetic modification of ADSCs can enable the sustained localized expression of specific genes, thereby avoiding the off-target effects associated with systemic delivery or burst release and subsequently enhancing their therapeutic potential, including proliferation, lineage-specific differentiation, or immunoregulation. Most studies using genetically modified ADSCs have involved osteoinductive bone morphogenetic proteins (BMPs), which are members of the transforming growth factor-β (TGF-β) family and represent the most effective factors in regulating bone induction, maintenance, and repair [66]. TGF-β [67], IGF-1 [68], and the transcription factor SOX [69] have been genetically engineered with ADSCs for the stimulation of chondrogenic differentiation and synthesis of cartilage-specific matrix components and VEGF for triggering angiogenesis [70]. Furthermore, genetically modified ADSCs can be embedded within biomaterial scaffolds to increase cell survival and retention at implantation sites [71].
In addition to genetic transformation to express specific protein-coding genes, epigenetic regulation plays an important role in the induction of ADSC differentiation. MicroRNAs (miRNAs) are a class of small noncoding RNAs (ncRNAs) that act as post-transcriptional regulators of gene expression, and they are associated with the regulation of several cellular processes, including proliferation, migration, and differentiation [72]. Thus, miRNA mimics, anti-/antago-miRNAs, or vectors that overexpress or suppress miRNA levels have been used to modulate ADSCs for osteochondral regeneration [73–75]. Moreover, another class of ncRNAs called lncRNAs has been explored as a mediator for inducing osteogenesis in ADSCs [76, 77].
In general, the abovementioned genetically engineered ADSC-based therapies represent promising approaches for enhancing the therapeutic potential of ADSCs. However, the consistent activation of specific genes may be a risk factor for stem cell-derived tumors. Hence, in clinical practice, the application of genetic modifications must be approached cautiously and needs further validation.
ADSCs and ADSC-based therapies for musculoskeletal regeneration
Based on the potential of ADSCs for multidirectional differentiation, regeneration, and paracrine activity, they can be applied in novel bioengineering approaches to address the requirement for musculoskeletal regeneration in various musculoskeletal disorders, including bone defects, non-union fractures, osteoarthritis, tendon injuries, and intervertebral disk degeneration (IDD).
Bone regeneration
Bone tissue has inherent repair and regeneration capacities. However, defective bone tissue cannot recover its integrity when extensive or chronic injuries occur, such as critical-sized bone defects and bone non-unions, which may be caused by heavy trauma, osteomyelitis, bone tumor resection, congenital malformations, and prosthesis revision, or when the regenerative process is impaired owing to factors such as age, comorbidities, and genetic factors [78]. The reconstruction of bone defects or bone non-unions usually requires invasive bone transfer or foreign body implants, which may result in secondary donor site morbidity and an increased risk of infection and extrusion. Bone tissue engineering can promote bone healing without encountering these disadvantages. Notably, ADSC-based therapies represent a promising alternative for bone regeneration (Table 1).
Table 1.
Recent preclinical studies on ADSC-based optimization strategies for bone regeneration
| ADSC-based optimization strategies | Models | Methods | Results | References | |
|---|---|---|---|---|---|
| Bioscaffolds | Heterogeneous deproteinized bone (HDB) | Rat radial defect model | ADSCs seeded onto the HDB were implanted into the defective area | ADSC-HDB composites exhibited a strong osteogenic ability | Liu et al. [89] |
| Modified hierarchical mesoporous bioactive glass (MBG) scaffold | Rat femoral defect model | Osteogenically induced ADSCs were seeded on the MBG scaffolds prevascularized by endothelial-induced ADSCs; the composite scaffolds were implanted into the defective area | Time-phased sequential application of ADSCs on the MBG scaffolds promoted better angiogenesis and mineral deposition | Du et al. [90] | |
| Genetic modification + Bioscaffolds |
Transduction of BMP-2; Biofabricated cryogel scaffolds |
Mouse atrophic non-union model | ADSCs were seeded onto biofabricated cryogel scaffolds after BMP-2 transduction and implanted into the non-union site | ADSC-seeded cryogels promoted osseous healing | Hixon et al. [94] |
|
miR-150-5p inhibition; Hydroxyapatite/tricalcium phosphate (HA/TCP) ceramic powder |
BALB/c nu/nu mice | miR-150-5p-modified ADSCs were loaded in HA/TCP ceramic powders and implanted into the dorsal surface of BALB/c nu/nu mice | Combination of ADSCs, miR-150-5p inhibition, and HA/TCP promoted bone damage repair and bone regeneration | Wang et al. [95] | |
| Engineered ADSC spheroids | Mouse calvarial defect model | ADSCs were assembled with PDGF and biomineral-coated fibers to form spheroids and implanted into the defective area | ADSC spheroids incorporating PDGF and biominerals exhibited greater endothelial lineage mRNA expression and vascularized bone regeneration | Lee et al. [91] | |
| Mouse calvarial defect model | ADSCs were assembled with adenosine and polydopamine-coated fibers to form spheroids and implanted into the defective area | ADSC spheroids impregnated with engineered fibers enabled adenosine delivery and promoted bone regeneration with enhanced osteogenic differentiation | Ahmad et al. [92] | ||
| ADSC-Exos + Bioscaffolds | Gelatin nanoparticles (GNPs) | Rat skull defect model | ADSC-Exos loaded within GNPs were implanted into the defective area | GNP-ADSC-Exos effectively regulated bone immune metabolism and promoted bone healing partly via the immune regulation of miR-451a | Li et al. [17] |
| Metal–organic framework (MOF) scaffolds | Rat calvarial defect model | ADSC-Exos were coated on the PLGA/Mg-GA MOF scaffold and implanted into the defective area | PLGA/Exo-Mg-GA MOF scaffolds promoted osteogenesis and satisfactory osseointegration | Kang et al. [57] | |
| PLGA/pDA scaffolds | Mouse calvarial defect model | ADSC-Exos were immobilized on PLGA/pDA scaffolds and then implanted into the defective area | Composite of ADSC-Exos and PLGA/pDA scaffolds enhanced bone regeneration, partially via their osteoinductive effects and by promoting stem cell migration and homing | Li et al. [96] | |
| Gelatin sponge/polydopamine (GS-PDA) scaffolds | Rat femoral defect model | ADSC-Exos-modified GS-PDA scaffolds (GS-PDA-Exos) were implanted into the defective area | GS-PDA-Exos promoted osteogenesis and bone repair | Li et al. [99] | |
| ADSC-Exos + Genetic modification + Bioscaffolds |
Exosomes derived from genetically modified ADSCs; Hydrogel comprising thiol‐modified hyaluronan, hydroxyapatite, and thiol‐modified heparin |
Rat calvarial defect model | Exosomes derived from miR-375-overexpressing ADSCs were embedded in hydrogels and implanted into the defective area | ADSC-Exos enriched with miR-375 could enhance bone regeneration | Chen et al. [56] |
ADSCs can differentiate into the osteogenic lineage when exposed to differentiation media containing BMPs [79], platelet-rich plasma [80], vitamin D3 [81], and human platelet lysates [82]. BMPs are the most effective osteoinductive factors. They interact with pathways such as the Smad, mitogen-activated protein kinase, Wnt, and Sonic Hedgehog pathways to regulate osteogenesis [83]. The abovementioned agents may serve as potential candidates to accelerate the osteogenic differentiation of ADSCs. The bone regenerative effects of ADSCs were explored in a murine model with large bony defects resulting from osteomyelitis, and ADSCs were locally administered to the damaged area after sufficient debridement of the infected bones. ADSCs promoted bone healing by increasing osteoblastogenesis and decreasing osteoclast and B cell numbers, thereby highlighting the bone regeneration potential of ADSCs in post-infectious inflammatory states [84].
One strategy to enhance the osteogenic potential of ADSCs involves the use of biomaterials and/or matrix proteins to recreate a precursor cell niche that promotes cell adhesion, proliferation, and differentiation [85, 86]. ADSCs have been successfully combined with biomaterials such as bioactive glass [87], tricalcium phosphate, fibronectin [88], and heterogeneous deproteinized bone [89] to improve bone regeneration in animal models. Du et al. designed a composite that utilized the multilineage differentiation capacity of ADSCs into osteogenic and vascular cells and seeded the endothelial-induced ADSCs into a mesoporous bioactive glass (MBG) scaffold [90]. This prevascularized MBG scaffold was combined with osteogenically induced ADSCs to repair critical-size bone defects in a rat model. The time-phased sequential application of ADSCs promoted better angiogenesis and mineral deposition compared to nonvascularized MBG-carrying osteogenically induced ADSCs, attributed to the rapid angiogenesis and improved survival of seeded ADSCs [90]. Recently, engineered stem cell spheroids have demonstrated great potential for bone regeneration. For example, Lee et al. assembled ADSCs with platelet-derived growth factor (PDGF) and biomineral-coated fibers to build ASDC spheroids that exhibited high mRNA expression associated with the endothelial lineage and vascularized bone regeneration [91]. Similarly, Ahmad et al. assembled ADSCs with adenosine and polydopamine-coated fibers to engineer ADSC spheroids, which enabled adenosine delivery and promoted bone regeneration by enhancing osteogenic differentiation [92]. In summary, engineered ADSC spheroids are promising alternatives for bone regeneration.
Genetic modifications of ADSCs to promote angiogenesis and osteogenic differentiation have been verified in a mouse model of femoral fracture [93]. Furthermore, genetically engineered ADSCs combined with bioscaffolds have been explored as an optimization strategy. Hixon et al. seeded murine ADSCs onto biofabricated cryogel scaffolds after the lentiviral transduction of BMP-2 and surgically implanted the seeded scaffolds around the non-union site in a 3.6Col1A1-tk (Col1-tk) mouse model. The ADSC-seeded cryogel scaffolds promoted bone formation while enhancing healing [94]. miRNAs play synergistic or antagonistic roles in regulating cell differentiation. Recently, Wang et al. demonstrated that miR-150-5p inhibition can increase ADSC osteogenesis by regulating Notch3 and that the combination of ADSCs, miR-150-5p inhibitors, and hydroxyapatite/tricalcium phosphate ceramic powders can enhance bone regeneration and bone damage repair [95].
The therapeutic effects of the ADSC secretome on bone repair and regeneration have been validated in several preclinical studies. In vitro, ADSC-Exos effectively antagonizes osteocyte apoptosis by promoting anti-apoptotic Bcl-2 expression and suppressing pro-apoptotic Bax expression [46]. The combination of ADSC-Exos with novel biomaterials can enhance bone regeneration. ADSC-Exos modified with efficient biocompatible carriers, such as polydopamine (pDA)-coated PLGA [96], alginate-cobalt ferrite [97], mineral-doped porous scaffolds constructed with PLA, calcium silicates, and dicalcium phosphate dihydrate [98], and gelatin sponge/polydopamine scaffolds [99], can improve osteogenesis and repair bone defects. Kang et al. synthesized a metal–organic framework (MOF) using human ADSC-Exos, PLGA, Mg2+, and gallic acid (PLGA/Exo-Mg-GA MOF) to create unique nanostructural interfaces that could enhance the osteogenic, angiogenic, and anti-inflammatory capabilities of ADSC-Exos [57]. Furthermore, the bone formation enhancement effect of ADSC-Exos loaded with effective osteogenic agents has been assessed. Lu et al. demonstrated that exosomes derived from tumor necrosis factor (TNF)-α-pre-conditioned ADSCs could enhance human osteoblastic proliferation and differentiation in vitro by activating Wnt signaling, achieved by increasing Wnt-3a levels within the exosomes [100]. Exosomes derived from miR-375-overexpressing human ADSCs embedded in hydrogels could deliver miRNAs to human BMSCs. This composite inhibited the expression of IGF-binding protein 3 and subsequently promoted bone regeneration [56]. Overall, these studies suggest that ADSC-Exos have considerable potential for use in bone tissue engineering.
Encouraging results from preclinical studies on ADSCs have prompted multiple clinical trials. The majority of these trials focused on treating craniomaxillofacial bone injuries or defects and achieved generally meaningful clinical effects. Defects were reconstructed using either bioactive glass or β-tricalcium phosphate (β-TCP) scaffolds seeded with ADSCs and, in some cases, combined with recombinant human BMP-2 [101]. The transplantation of autologous human ADSCs did not result in major adverse events, the focal bone formation was good, and a few patients achieved excellent clinical results. However, problems surrounding graft loosening, infection, and tumor recurrence were observed [102]. A 6-year follow-up study of cranioplasty based on ADSCs, β-TCP, and a supporting mesh illustrated unsatisfactory results, such as marked graft resorption, late infection (7.3 years postoperatively), or meningioma recurrence (2.2 years postoperatively). These observations indicate that ADSC-based treatment is not superior to conventional cranial repair methods [103]. Despite the potential of ADSCs in bone repair and regeneration, difficulties and barriers remain in clinical trials. Hence, the clinical application of ADSCs for bone defects requires further investigation.
Cartilage regeneration
Cartilage damage caused by acute trauma or chronic degeneration predominantly presents as joint lesions. Owing to the poor intrinsic regenerative potential of human cartilage tissue, these damages can progress to osteoarthritis or necessitate arthroplasty [104]. Current management of cartilage defects, particularly osteoarthritis, only provides symptom relief rather than a cure. Recently, cartilage tissue engineering, which integrates engineering and biological approaches to promote cartilage regeneration and damaged tissue replacement, has become an important research area. Stem cell therapies, including ADSCs and ADSC-based optimization strategies, provide promising new alternatives for treating cartilage defects (Table 2).
Table 2.
Recent preclinical studies on ADSC-based optimization strategies for cartilage regeneration
| ADSC-based optimization strategies | Models | Methods | Results | References | |
|---|---|---|---|---|---|
| ADSCs | Rat osteoarthritis model | ADSCs were injected into the articular cavity | ADSCs inhibited the expression of NLRP3, Caspase1, GSDMD, and TNFR1; improved the pathological changes in the joints; and delayed osteoarthritis development | Xu et al. [107] | |
| ADSC-EVs | Rat osteoarthritis model | ADSC-EVs were injected into the articular cavity | ADSC-EVs protected the cartilage from degeneration, inhibited the infiltration of M1 macrophages into the synovium, and attenuated osteoarthritis progression | Woo et al. [21] | |
| ADSC-Exos | Hypoxic pre-condition of ADSCs | Rat model of post-traumatic osteoarthritis | Intra-articular injection of hypoxia-cultured ADSC-secreted exosomes (hypoxia-ADSC-Exos) | Hypoxia-ADSC-Exos exhibited a chondroprotective effect and suppressed osteoarthritis progression | Chang et al. [114] |
| Tropoelastin pretreatment of ADSCs | Rat model of osteoarthritis induced by the anterior cruciate ligament transection (ACLT) | Intra-articular injection of exosomes from tropoelastin-pretreated ADSCs (TE-ADSCs-Exos) | TE-ADSCs-Exos enhanced matrix synthesis and promoted cartilage repair | Meng et al. [115] | |
| Bioscaffolds | Biomimetic injectable hydrogel using amnion membrane (AM) | Collagenase-induced osteoarthritis rat model | Intra-articular injections of AM hydrogels with ADSCs | AM hydrogels with or without ADSCs alleviated inflammation and cartilage degeneration | Bhattacharjee et al. [18] |
| Glycol chitosan/dibenzaldehyde- functionalized- polyethylene glycol (GCS/DF-PEG) hydrogel | Rat model of articular cartilage defect | ADSCs with GCS/DF-PEG hydrogel were injected into the defective area | ADSCs and GCS/DF-PEG hydrogel complexes exhibited obvious cartilage regeneration ability | Yang et al. [119] | |
| Coacervate-embedded gelatin-SH/PEGDA IPN hydrogels | Rabbit femoral trochlear osteochondral defect model | ADSCs and IGF-1 were transplanted with coacervate-embedded composite hydrogels | Dual delivery platform could induce chondrogenic differentiation of embedded ADSCs and promote osteochondral tissue regeneration | Cho et al. [123] | |
| Acellular cartilage extracellular matrix (ACECM) scaffolds | Pig osteochondral defect model | ADSCs combined with ACECM were transplanted into the defective area | ADSCs-ACECM composites successfully repaired the cartilage defect | Lu et al. [120] | |
| Genetic modification | Chemically modified mRNA (modRNA)-mediated IGF-1 gene transfer | Mice osteoarthritis model | IGF-1-ADSCs were injected into the articular cavity | IGF-1-ADSCs decreased the loss of cartilage ECM, promoted cartilage repair, and exerted a chondroprotective effect | Wu et al. [68] |
| ADSCs were transfected with miR-486-5p | Rat osteoarthritis model | Intra-articular injection of miR-486-5p-modified ADSC-Exos | miR-486-5p-modified ADSC-Exos attenuated chondrocyte apoptosis and alleviated osteoarthritis | Wang et al. [125] | |
| Genetic modification + Bioscaffolds | Genetically engineered ADSCs overexpressing TGF-β1 (T-ADSCs); An injectable extracellular matrix (ECM)-mimicking hydrogel | Rat osteoarthritis model | T-ADSCs and hydrogels were injected into the articular cavity | T-ADSC-loaded hydrogels markedly reduced cartilage degeneration, joint inflammation, and subchondral bone loss | Yu et al. [67] |
| ADSCs transfected with miR-99 b-3p-mimic; Hyaluronan-based hydrogel microparticles (HMPs) | Mice osteoarthritis model induced by surgical destabilization of the medial meniscus (DMM) | miR-99 b-3p-overexpressing ADSC-Exos were encapsulated in HMPs and injected into the articular cavity | miR-99 b-3p-modified ADSC-Exos combined with HMPs inhibited the degradation of the cartilage ECM | Yin et al. [126] | |
ADSCs have the potential for chondrogenic differentiation. The most characterized chondrogenic-inducing factors include TGF-β, fibroblast growth factor (FGF), IGF, and BMPs. These factors can be applied alone or in combination in the culture medium to promote the chondrogenesis of ADSCs [105, 106]. Moreover, SOX proteins are involved in chondrogenesis. The overexpression of SOX proteins enhances the chondrogenic differentiation of ADSCs and promotes cartilage healing [69]. In addition to chondrogenic differentiation, ADSCs contribute to cartilage regeneration via various mechanisms, including inhibiting chondrocyte pyroptosis [107], reducing chondrocyte reactive oxygen species production, suppressing inflammatory responses [108], and promoting ECM synthesis [21].
ADSCs exert cytoprotective, anti-inflammatory, and ECM synthetic effects on cartilage regeneration through paracrine mechanisms. The ADSC secretome can rectify abnormal osteoblast metabolism by suppressing the production of inflammatory mediators, such as IL-6 and prostaglandin E2 (PGE2) [109]. In an in vitro study, ADSC-EVs reduced the expression of pro-inflammatory cytokines and chemokines while inhibiting fibroblast-like synoviocyte-induced inflammation by interacting with the hyaluronan (HA) matrix and releasing miRNAs [110]. Another study confirmed that ADSCs-EVs inhibited chondrocyte matrix degradation and slowed osteoarthritis progression in a rat model by increasing and decreasing the expression of type II collagen and matrix metalloproteinases (MMPs), respectively [21]. Additionally, ADSC-derived microvesicles can suppress inflammation and modulate the metabolism of osteoarthritic synoviocytes [111]. In recent years, there has been a growing research interest in the use of ADSC-Exos for cartilage regeneration. Zhao et al. found that ADSC-Exos protected articular chondrocytes from H2O2-induced apoptosis and promoted chondrogenesis by upregulating miR-145 and miR-221 and downregulating pro-inflammatory cytokines, including IL-6 and TNF-α [53]. Another study demonstrated that ADSC-Exos could promote chondrocyte proliferation by miR-429 targeting FEZ2 and promoting autophagy [112]. Notably, hypoxic preconditioning of human ADSCs enhances their potential for chondrogenic differentiation [113]. Chang et al. demonstrated that hypoxia-cultured ADSC-secreted exosomes (hypoxia-ADSC-Exos) could enhance the synthesis of cartilaginous matrix and suppress the expression of inflammatory cytokines (TNF-α and IL-6) and degradation enzymes (MMP13 and ADAMT5) in vitro. Moreover, intra-articular treatment with hypoxia-ADSC-Exos exerted a chondroprotective effect by suppressing cartilage erosion, thereby slowing osteoarthritis progression [114]. Meng et al. harvested exosomes from tropoelastin-pretreated ADSCs and found that these exosomes effectively enhanced the matrix synthesis of chondrocytes in vitro and promoted cartilage repair in vivo [115]. These in vitro and in vivo experiments indicated that the ADSC secretome, particularly ADSC-Exos, is a promising option for treating cartilage lesions and osteoarthritis.
The combination of ADSCs with novel biomaterials or matrix proteins is being investigated as potential optimization strategies for cartilage regeneration. Biomaterials and matrix proteins can create a niche microenvironment that enhances the delivery, migration, proliferation, and differentiation of ADSCs [59]. As natural polymers, hyaluronic acid combined with allogeneic ADSCs efficiently promoted cartilage regeneration and prevented osteoarthritis progression in sheep [116, 117]. The amnion membrane (AM) from the human placenta was used to fabricate a minimally invasive injectable hydrogel as an ADSC carrier. Intra-articular injections of AM hydrogels with or without ADSCs alleviated inflammation and cartilage degeneration in a collagenase-induced osteoarthritis rat model, thus demonstrating the synergistic anti-inflammatory and chondroprotective effects of AM and ADSCs on cartilage tissues [18]. Najafi et al. fabricated a composite scaffold containing ECM powder and several layers of hydrogel and nanofibers to mimic the structure and characteristics of cartilage and subsequently encapsulated human ADSCs within this scaffold [118]. The composite scaffold improved cell proliferation and enhanced the chondrogenic-like differentiation of ADSCs. In addition, studies have revealed the beneficial effects of an injectable hydrogel consisting of glycol chitosan/dibenzaldehyde-terminated polyethylene glycol [119] or acellular cartilage ECM scaffolds [120] combined with ADSCs on cartilage tissue repair and regeneration.
Notably, severe cartilage lesions can also compromise the subchondral bone. Subsequently, osteochondral regeneration strategies must be employed to restore both the zonal articular cartilage and the subchondral bone using three elements: scaffolds, cells, and bioactive factors. These components are being employed for structural reconstruction and tissue activity remodeling [121]. Zhang et al. fabricated a composite scaffold consisting of hyaluronic acid, chitosan, and PLGA with two different sites: one designed to support hyaline chondrogenesis and the other designed to support osteogenesis by binding to BMP-2. These osteochondral scaffolds were seeded with ADSCs, resulting in the regeneration of rabbit osteochondral defects [122]. Cho et al. fabricated a coacervate-embedded gelatin-SH/PEGDA IPN hydrogel for the co-delivery of ADSCs and chondrogenic factor IGF-1 and demonstrated that this dual delivery system could induce chondrogenic differentiation of the embedded ADSCs and effectively augment osteochondral tissue regeneration [123]. Furthermore, 3D printing is advantageous for producing scaffolds with customized shapes and structures/composition gradients. Seeding multiphasic 3D-bioprinted scaffolds with human ADSCs could facilitate the site-specific chondrogenic and osteogenic differentiation of ADSCs in vitro [124]. However, this advanced bioengineering strategy for cartilage regeneration requires further in vivo validation.
Genetic modifications have been used to optimize ADSC-based therapies for cartilage regeneration. Wu et al. transfected ADSCs with IGF-1-modified mRNA (termed IGF-1-ADSCs) to enhance chondrogenesis and found that IGF-1-ADSCs increased anabolic marker expression in chondrocytes in vitro, decreased cartilage ECM loss, and promoted cartilage repair in vivo [68]. Additionally, Yu et al. applied genetically engineered ADSCs overexpressing TGF-β1 (termed T-ADSCs) to exert anti-inflammatory and pro-anabolic effects on chondrocytes and confirmed that T-ADSC-loaded hydrogels markedly reduced cartilage degeneration, joint inflammation, and subchondral bone loss in vivo [67]. In another study, miR-486-5p-modified ADSC-Exos modulated endoplasmic reticulum stress, attenuated chondrocyte apoptosis in vitro, and alleviated osteoarthritis in vivo [125]. The overexpression of mir-99 b-3p in exosomes extracted from subcutaneous adipose tissue-derived stem cells (Sc-ADSCs-Exos) could enhance the therapeutic effect of inhibiting cartilage ECM degradation and promoting cartilage repair. Moreover, the encapsulation of these genetically engineered Sc-ADSCs-Exos in an HA-based hydrogel microparticle scaffold provided for sustained local drug release and simultaneously strengthened the chondroprotective effects [126].
The efficacy and safety of intra-articular ADSC injection for patients with knee osteoarthritis have been confirmed clinically, with this treatment leading to functional improvement, pain relief, cartilage restoration, and retardation of disease progression, as measured via magnetic resonance imaging (MRI), with no serious adverse events [127–132]. In addition, the intra-articular injection of autologous adipose-derived SVFs (ADSVFs) in patients with knee osteoarthritis has yielded good results [133–136]. Although the evidence is limited, the conclusions of these studies suggest that ADSCs or ADSVFs, which are rich in ADSCs, exert promising effects and are considered safe as an alternative treatment option for osteoarthritis. However, further large-scale randomized controlled trials and long-term follow-up studies are warranted to validate their clinical use.
Unlike articular cartilage, which is a smooth elastic tissue covered with hyaline cartilage on the joint surface, the intervertebral disk (IVD) is a fibrocartilaginous tissue that consists of inner soft nucleus pulposus (NP) cells, the surrounding annulus fibrosus, and cartilaginous endplates. IDD is the most common musculoskeletal disorder in the elderly and involves several endogenous molecular processes, including ECM degeneration, senescence, apoptosis, oxidative stress, inflammation, and reduced autophagy, which is similar to the effects of osteoarthritis on the facet joint of the spine [137]. Current evidence indicates that ADSC-based strategies have promising effects on IDD. Yu et al. used a genipin-crosslinked decellularized NP hydrogel (GDH) as a novel system to load ADSCs and observed that GDH could promote the differentiation of ADSCs into NP cells while enhancing the intervertebral height, MRI index, and histological grading scores in vivo [138]. In addition, Xing et al. demonstrated that the injection of ECM acellular biological scaffolds loaded with ADSC-Exos could regulate IVD microenvironment homeostasis and ameliorate IDD [139]. However, additional studies are warranted to explore and verify the effects of ADSC-based strategies on human IDD.
Tendon regeneration
The tendon is a well-organized, dense connective tissue that connects the muscle to the bone and transmits force, thereby enabling motion and posture maintenance. Tendon injuries are the most common injuries of the musculoskeletal system, and they are frequently caused by overloading and characterized by tendon swelling, local pain, and disability. No current therapeutic approach for tendon injuries provides a successful, long-term solution. Furthermore, owing to the inferior regenerative capacity of tendons, repaired tendons cannot restore their pre-injury strength and function, leading to degenerative changes and a high risk of re-injury. Tendon tissue engineering involving ADSCs is expected to promote tendon regeneration and reconstruction (Table 3).
Table 3.
Recent preclinical studies on ADSC-based optimization strategies for tendon regeneration
| ADSC-based optimization strategies | Models | Methods | Results | References | |
|---|---|---|---|---|---|
| ADSCs | ADSCs preconditioned with GDF-6 and PDGF-BB; collagen/alginate gel | Rat model of Achilles tendon excision defect | Tenogenically differentiated ADSCs with the hydrogel were injected into the defective area | Tenogenically differentiated ADSCs enhanced the collagen fiber dispersion range closest to the normal tendon | Norelli et al. [147] |
| ADSCs induced by GDF-5 and PDGF; collagen/alginate gel | Rat model of Achilles tendon defect | ADSCs pre-treated with hydrogel were injected into the tendon defect area | GDF5/PDGF-induced ADSCs promoted tendon repair by improving cellular proliferation, tenogenesis, and vascular infiltration | Fitzgerald et al. [142] | |
| Engineered ADSCs | ADSC sheets | Rat model of chronic rotator cuff tear | ADSC sheets were transplanted into the rotator cuff tear area | ADSC sheets significantly enhanced the biomechanical properties of the repaired rotator cuff | Shin et al. [148] |
| Engineered ADSCs + Bioscaffolds | ADSC sheets (P(LLA-CL)/Silk fibroin nanoyarn scaffolds | Rabbit model of patellar tendon defect | GDF-5-induced ADSC sheets were seeded on nanoyarn scaffolds and implanted into the patellar tendon defect area | GDF-5-induced ADSC sheets stimulated higher expression of tenogenesis-related markers and promoted functional tendon regeneration | Chen et al. [28] |
| Bioscaffolds | Fibrin or gelatin methacrylate [GelMA] | Rat model of massive rotator cuff tears | ADSCs seeded in fibrin or GelMA hydrogel were implanted into the repair site | ADSCs combined with fibrin or GelMA hydrogel could decrease bone loss and augment the efficacy of surgical repair | Rothrauff et al. [150] |
| Novel injectable porous gelatin microcryogels (GMs) | Rat model of acute Achilles tendon rupture | GMs loaded with ADSCs were injected into the gap | ADSCs with GMs could effectively improve the macroscopic appearance, histological morphology, and biomechanical properties of the repair tissue | Yang et al. [151] | |
| ADSC-Exos | Rat model of a massive rotator cuff tear | ADSC-Exos were injected into the damaged site | ADSC-Exos treatment could prevent atrophy, fatty infiltration, and inflammation and promote myofiber regeneration and the biomechanical properties of the injured rotator cuff | Wang et al. [155] | |
| Rabbit model of chronic rotator cuff tear | ADSC-Exos were injected into the repair site | ADSC-Exos could prevent fatty infiltration, improve biomechanical properties, and promote tendon–bone healing after surgical repair | Wang et al. [54] | ||
| ADSC-Exos + Bioscaffolds | Hydrogel | Rat model of rotator cuff injury | ADSC-Exos-hydrogel complex was injected in the shoulder after surgical repair | ADSC-Exos-hydrogel promoted rotator cuff repair by mediating the differentiation of the tendon-derived stem cells | Fu et al. [156] |
| In situ-forming fibrin gel | Rabbit model of partial-thickness rotator cuff tears | Local administration of in situ-forming fibrin gel containing ADSC-Exos (ADSC-Exos/fibrin) | ADSC-Exos/fibrin significantly prevented tear progression, enhanced the biomechanical properties of the injured tendon, and promoted high-quality tendon healing | Wang et al. [157] | |
ADSCs can differentiate into tenocytes to repair the injured tendon. Several growth factors have been utilized to differentiate stem cells into tendon-like cells in vitro, including IGF-1, TGF-β, FGF, PDGF, growth differentiation factor (GDF)-5, and connective tissue growth factor [140–142]. Hypoxic preconditioning can enhance the expression of tendon-related markers and has been proposed to stimulate the tenogenic differentiation of ADSCs [143]. In addition, ECM-based approaches can be utilized for tendon regeneration. Rao et al. prepared a soluble, DNA-free, bovine tendon-derived ECM using a urea-based method, and it showed a strong pro-tenogenic capacity on human ADSCs [20].
Behfar et al. confirmed that the intratendinous injection of ADSVFs enriched with ADSCs improved the mechanical properties of the injured tendon, thereby increasing yield loads and energy absorption [144]. Local injection of ADSCs improved muscle function and tendon healing and decreased fatty infiltration in a chronic rotator cuff tear rabbit model [145], and it also increased the expression of collagen I and collagen III following Achilles tendon rupture in rabbits [146]. Norelli et al. explored the efficacy of ADSCs with or without tenogenic differentiation induced by GDFs and PDGF-BB in vivo and found that ADSCs improved the biomechanical properties of repaired tendons, and tenogenically differentiated ADSCs could enhance the mean histological score and collagen fiber dispersion range closest to the normal tendon [147]. Another study demonstrated that pre-conditioned ADSCs with GDF-5 and PDGF increased the expression of the protenogenesis gene SOX9 and promoted cell–cell connections and cellular proliferation, thereby accelerating the remodeling process of the injured tendon [142].
Cell sheet technology is an innovative tool for tissue regeneration that preserves intact cell–cell connections and the ECM. Shin et al. confirmed the efficacy of ADSC sheets as a viable cell delivery tool for tendon reconstruction [148]. In their study, ADSC sheets were fabricated using a temperature-responsive dish and transplanted into the defective area during repair procedures in a rat rotator cuff tear model. The ADSC sheets significantly enhanced the biomechanical properties of the repaired rotator cuff. In another study, Chen et al. observed that GDF-5-induced ADSC sheets combined with nanoyarn scaffolds could stimulate higher expression of tenogenesis-related markers and promote functional tendon regeneration [28]. These findings suggest that engineered ADSC sheets can be used in tendon repair and regeneration.
Novel scaffold materials could facilitate the engraftment and differentiation of ADSCs while promoting tendon healing. Chiou et al. confirmed that supplementing a biocompatible tendon hydrogel with platelet-rich plasma and ADSCs could promote early mechanical strength and functional restoration, thereby accelerating injured tendon repair [149]. Rothrauff et al. reported that fibrin- or gelatin methacrylate-seeded ADSCs could decrease bone loss and promote surgical repair efficacy in rats with massive rotator cuff tears [150]. Injectable porous gelatin microcryogels (GMs) promoted ADSC proliferation and facilitated ECM secretion, and ADSC-GM matrices exhibited regeneration and repair potential for Achilles tendon ruptures in rats [151]. Guo et al. used the small intestinal submucosa (SIS) as a scaffold to seed ADSCs and repair Achilles tendon defects in a rat model. Engineered tendon grafts created by seeding ADSCs on SIS could significantly improve ECM production and collagen fiber compactness, thus allowing for a higher peak tensile load of the restored Achilles tendon [143]. Recently, a functionally graded 3D scaffold was fabricated by incorporating platelet lysate within an electrospun fiber core. These 3D functional scaffolds featuring gradients in composition and topography promoted the proliferation and differentiation of ADSCs and induced the regeneration of different elements, which exhibited the hierarchical structure of the tendon-bone interface [152]. However, in vivo studies using clinical tendon injury models are warranted to evaluate the regenerative performance of these 3D functional gradient scaffolds.
Exosomes, as a kind of cell-free therapeutic strategy, may provide new perspectives for tendon-bone healing [153]. ADSC-Exos could decrease the expression of pro-inflammatory cytokines, maintain metabolic homeostasis, and improve the histological properties of injured tendons in vitro [154]. Wang et al. [155] demonstrated that ADSC-Exos effectively decreased atrophy and degeneration while improving the myofiber regeneration and biomechanical properties of torn rotator cuff muscles. The ADSC-Exos could also prevent fatty infiltration, promote tendon-bone healing, and improve biomechanical properties in a rabbit model of chronic rotator cuff tears [54]. Hydrogels can provide mechanical support and promote the sustained release of ADSC-Exos. Fu et al. injected an ADSC-Exos-hydrogel complex into the shoulders of rats in a rotator cuff injury model and observed enhanced rotator cuff repair and tendon-bone healing attributed to ADSC-Exos promoting the differentiation of tendon-derived stem cells [156]. Wang et al. [157] demonstrated that the local administration of a fibrin gel containing ADSC-Exos could significantly prevent tear progression, enhance the biomechanical properties of the injured tendon, and promote high-quality tendon healing in a rabbit model of partial-thickness rotator cuff tears.
To date, only a few clinical trials have been conducted using ADSCs to treat tendon injuries. One study confirmed the beneficial effect of injecting ADSCs loaded with fibrin glue during arthroscopic rotator cuff repair on structural integrity, as assessed by MRI [158]. Although no clinical differences were observed during the 28-month follow-up period, the results indicated that ADSCs provided an adequate biological environment around the tendon repair site. Furthermore, Jo et al. demonstrated that the intra-tendinous injection of autologous ADSCs in patients with rotator cuff injuries could significantly reduce shoulder pain, increase muscle strength, improve shoulder function, and reduce bursal-sided defect volumes without treatment-related adverse events at a minimum follow-up of 2 years [159, 160]. In addition, Hurd et al. observed that fresh, uncultured, unmodified, autologous adipose-derived regenerative cells isolated from lipoaspirate were more effective than corticosteroid injection and could improve shoulder function without adverse effects [161]. Recently, Randelli et al. applied autologous microfragmented lipoaspirate tissue containing ADSCs to treat patients with degenerative posterosuperior rotator cuff tears and observed that the intraoperative injection of autologous microfragmented adipose tissue could effectively improve the function of rotator cuff repair [162]. Collectively, the abovementioned clinical findings indicate the beneficial effects of ADSCs on rotator cuff tears. However, larger samples and long-term follow-up studies are required to validate these findings.
Conclusions and perspectives
ADSCs and ADSC-based optimization strategies represent promising therapeutic tools in the fields of tissue engineering and regenerative medicine. Preclinical studies have revealed the therapeutic function of ADSC-based strategies in bone, cartilage, and tendon regeneration. Furthermore, other properties of ADSCs, such as pro-angiogenic, anti-inflammatory, anti-apoptotic, and pro-ECM synthesis, have been demonstrated both in vitro and in vivo. Finally, the findings of preliminary clinical trials indicate the beneficial effects of ADSCs in promoting musculoskeletal tissue repair and reconstruction (Table 4).
Table 4.
Recent clinical trials on ADSC-based therapies for musculoskeletal regeneration
| Application | Participants | Methods | Follow-up time | Results | References |
|---|---|---|---|---|---|
| Bone regeneration | 13 patients with craniomaxillofacial hard-tissue defects | Autologous ADSCs were seeded onto either bioactive glass or β-tricalcium phosphate scaffolds and transplanted into the bone defect area | 12–52 months | In 10 of 13 cases, the construct was successfully integrated into the surrounding skeleton | Sándor et al. [101] |
| 5 cranial defect patients | Patients received cranioplasties using autologous ADSCs, beta-tricalcium phosphate granules, and supporting meshes | 6 years | Long-term clinical results were not satisfactory, partially owing to graft resorption, tumor recurrence, or late infection | Thesleff et al. [103] | |
| Cartilage regeneration | 12 patients with knee osteoarthritis | Autologous ADSCs were intra-articularly administered | 6 months | ADSC injection provided significant functional improvement and pain relief | Lee et al. [127] |
| 53 patients with symptomatic knee osteoarthritis | Autologous adipose-derived mesenchymal progenitor cells (haMPCs) expanded in vitro were intra-articularly injected | 12 months | Significant improvements in joint function, pain, quality of life, and cartilage regeneration with good tolerance | Lu et al. [128] | |
| 30 patients with symptomatic knee osteoarthritis | Intra-articular ADSC therapy | 12 months | Autologous ADSC therapy is safe and effective and can prevent disease progression | Freitag et al. [129] | |
| 18 patients with knee osteoarthritis | Autologous ADSCs were intra-articularly injected | 96 weeks | Autologous ADSCs improved the pain, function, and cartilage volume of the knee joint | Song et al. [131] | |
| Tendon regeneration | 70 patients with full-thickness rotator cuff tear | 35 patients underwent arthroscopic rotator cuff repair with autologous ADSC injections, whereas 35 patients underwent only repair surgery | At least 12 months | ADSC injection during rotator cuff repair significantly decreased the retear rate; furthermore, the function of the repaired tissue was similarly ameliorated in both groups | Kim et al. [158] |
| 18 patients with partial-thickness rotator cuff tear | Intratendinous injection of autologous ADSCs | 2 years | Autologous ADSC injection improved shoulder function, relieved pain, and exhibited continued safety and efficacy | Jo et al. [160] | |
| 11 patients with partial-thickness rotator cuff tears | Autologous adipose-derived regenerative cells were injected into the shoulder with a single injection | 12 months | Autologous adipose-derived regenerative cell injection improved shoulder function without adverse effects | Hurd et al. [161] | |
| 44 patients with degenerative posterosuperior rotator cuff tear | 22 patients underwent arthroscopic rotator cuff repair augmentation with autologous microfragmented lipoaspirate tissue, whereas 22 patients underwent only repair surgery | 24 months | Injection of autologous microfragmented adipose tissue effectively promoted functional rotator cuff repair | Randelli et al. [162] |
However, concerns such as limited cell survival, senescence-induced genetic instability, functional inactivation, the possibility of unfavorable ADSC differentiation, and the relatively low purity and yield of ADSC-Exos must be addressed before applying ADSCs and their cell-free derivatives. The intrinsic characteristics of donors (e.g., age, sex, and obesity), differences in adipose tissue sources, and differences in the isolation procedures can affect the properties of ADSCs or ADSC-Exos. These limitations indicate the need for quality control of ADSCs and ADSC-Exos. In addition, further exploration of appropriate scaffolds, potent bioactive factors, and genetically modified approaches is required to provide more optimized conditions for the proliferation and differentiation of ADSCs and verify their efficacy and safety in humans. Considering the differences between preclinical studies and clinical trials, the oncogenicity of ADSC differentiation should be further investigated. ADSC-Exos can avoid many of the shortcomings associated with administering ADSCs, including potential tumorigenicity and storage problems, and thus may represent an effective regenerative agent for musculoskeletal regeneration. However, limited cellular and animal experiments have been performed. Therefore, large-scale clinical trials are urgently warranted.
Despite the current challenges, the significant progress achieved in this field suggests that ADSC-based therapies will play increasingly crucial roles in tissue regeneration. Technological improvements and additional investigations may help gradually tackle these problems. Researchers can harvest suitable ADSC subpopulations using standardized isolation procedures and then enhance the therapeutic potential of these cells by preconditioning them with bioactive factors or combining them with biomaterials before transplantation. Nevertheless, larger prospective, blinded, randomized clinical trials are warranted to further establish the long-term effectiveness and safety of ADSC-based therapies in humans. Such work may result in a paradigm shift in the treatment of musculoskeletal disorders.
Acknowledgements
Not applicable.
Abbreviations
- ADSCs
Adipose-derived stem cells
- ADSC-Exos
ADSC-derived exosomes
- ADSC-CM
ADSC-conditioned medium
- AM
Amnion membrane
- BM
Bone marrow
- BMP
Bone morphogenetic protein
- CD
Cluster of differentiation
- 3D
Three-dimensional
- ECM
Extracellular matrix
- EVs
Extracellular vesicles
- GA
Gallic acid
- GDF
Growth differentiation factor
- GDH
Genipin-crosslinked decellularized nucleus pulposus hydrogel
- GMs
Gelatin microcryogels
- HA
Hyaluronan
- HLA
Human leukocyte antigen
- HSP
Heat shock proteins
- IDD
Intervertebral disk degeneration
- IFATS
International Federation for Adipose Therapeutics and Science
- IGF-1
Insulin-like growth factor 1
- IL
Interleukin
- ISCT
International Society for Cellular Therapy
- IVD
Intervertebral disk
- MBG
Mesoporous bioactive glass
- miRNAs
MicroRNAs
- MMP
Matrix metalloproteinases
- MOF
Metal–organic framework
- MRI
Magnetic resonance imaging
- MSCs
Mesenchymal stem cells
- ncRNAs
Noncoding RNAs
- NP
Nucleus pulposus
- PGE2
Prostaglandin E2
- PLA
Polylactic acid
- PLGA
Polylactic-co-glycolic acid
- PLGA/pDA
Polydopamine-coating poly lactic-co-glycolic acid
- SIS
Small intestinal submucosa
- SOX
SRY (sex-determining region Y) box containing gene
- SVF
Stromal vascular fraction
- TCP
Tricalcium phosphate
- β-TCP
β-Tricalcium phosphate
- TGF-β
Transforming growth factor-β
- TNF
Tumor necrosis factor
- TSG
Tumor suppressor gene
- VEGF
Vascular endothelial growth factor
Author contributions
CY searched the literature, collected data, wrote the manuscript, and drafted the figures. WS, XJ, and YW conceived the project and revised the manuscript. CL and WY edited the manuscript. YH designed the outline and revised the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (82072401, 82172515). The funding body played no role in the study design, collection, analysis, data interpretation, and manuscript drafting.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cieza A, Causey K, Kamenov K, Hanson SW, Chatterji S, Vos T. Global estimates of the need for rehabilitation based on the Global Burden of Disease study 2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2021;396(10267):2006–2017. doi: 10.1016/S0140-6736(20)32340-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khodabukus A, Guyer T, Moore AC, Stevens MM, Guldberg RE, Bursac N. Translating musculoskeletal bioengineering into tissue regeneration therapies. Sci Transl Med. 2022;14(666):eabn9074. doi: 10.1126/scitranslmed.abn9074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tevlin R, des Jardins-Park H, Huber J, DiIorio SE, Longaker MT, Wan DC. Musculoskeletal tissue engineering: adipose derived stromal cell implementation for the treatment of osteoarthritis. Biomaterials. 2022;286:121544. doi: 10.1016/j.biomaterials.2022.121544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7(2):211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 5.Mazini L, Rochette L, Amine M, Malka G. Regenerative capacity of adipose derived stem cells (ADSCs), comparison with mesenchymal stem cells (MSCs) Int J Mol Sci. 2019;20(10):2523. doi: 10.3390/ijms20102523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoang DM, Pham PT, Bach TQ, Ngo ATL, Nguyen QT, Phan TTK, et al. Stem cell-based therapy for human diseases. Signal Transduct Target Ther. 2022;7(1):272. doi: 10.1038/s41392-022-01134-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoang VT, Trinh QM, Phuong DTM, Bui HTH, Hang LM, Ngan NTH, et al. Standardized xeno- and serum-free culture platform enables large-scale expansion of high-quality mesenchymal stem/stromal cells from perinatal and adult tissue sources. Cytotherapy. 2021;23(1):88–99. doi: 10.1016/j.jcyt.2020.09.004. [DOI] [PubMed] [Google Scholar]
- 8.Zhou W, Lin J, Zhao K, Jin K, He Q, Hu Y, et al. Single-cell profiles and clinically useful properties of human mesenchymal stem cells of adipose and bone marrow origin. Am J Sports Med. 2019;47(7):1722–1733. doi: 10.1177/0363546519848678. [DOI] [PubMed] [Google Scholar]
- 9.DelaRosa O, Sánchez-Correa B, Morgado S, Ramírez C, del Río B, Menta R, et al. Human adipose-derived stem cells impair natural killer cell function and exhibit low susceptibility to natural killer-mediated lysis. Stem Cells Dev. 2012;21(8):1333–1343. doi: 10.1089/scd.2011.0139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sabol RA, Bowles AC, Côté A, Wise R, Pashos N, Bunnell BA. Therapeutic potential of adipose stem cells. Adv Exp Med Biol. 2021;1341:15–25. doi: 10.1007/5584_2018_248. [DOI] [PubMed] [Google Scholar]
- 11.Xiao S, Xiao C, Miao Y, Wang J, Chen R, Fan Z, et al. Human acellular amniotic membrane incorporating exosomes from adipose-derived mesenchymal stem cells promotes diabetic wound healing. Stem Cell Res Ther. 2021;12(1):255. doi: 10.1186/s13287-021-02333-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou J, Wei T, He Z. ADSCs enhance VEGFR3-mediated lymphangiogenesis via METTL3-mediated VEGF-C m(6)A modification to improve wound healing of diabetic foot ulcers. Mol Med. 2021;27(1):146. doi: 10.1186/s10020-021-00406-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Z, Shang J, Yang Q, Dai Z, Liang Y, Lai C, et al. Exosomes derived from human adipose mesenchymal stem cells ameliorate hepatic fibrosis by inhibiting PI3K/Akt/mTOR pathway and remodeling choline metabolism. J Nanobiotechnol. 2023;21(1):29. doi: 10.1186/s12951-023-01788-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yan Y, Ma T, Gong K, Ao Q, Zhang X, Gong Y. Adipose-derived mesenchymal stem cell transplantation promotes adult neurogenesis in the brains of Alzheimer’s disease mice. Neural Regen Res. 2014;9(8):798–805. doi: 10.4103/1673-5374.131596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park H, Chang KA. Therapeutic potential of repeated intravenous transplantation of human adipose-derived stem cells in subchronic MPTP-induced Parkinson’s disease mouse model. Int J Mol Sci. 2020;21(21):8129. doi: 10.3390/ijms21218129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee TL, Lai TC, Lin SR, Lin SW, Chen YC, Pu CM, et al. Conditioned medium from adipose-derived stem cells attenuates ischemia/reperfusion-induced cardiac injury through the microRNA-221/222/PUMA/ETS-1 pathway. Theranostics. 2021;11(7):3131–3149. doi: 10.7150/thno.52677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li R, Li D, Wang H, Chen K, Wang S, Xu J, et al. Exosomes from adipose-derived stem cells regulate M1/M2 macrophage phenotypic polarization to promote bone healing via miR-451a/MIF. Stem Cell Res Ther. 2022;13(1):149. doi: 10.1186/s13287-022-02823-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bhattacharjee M, Escobar Ivirico JL, Kan HM, Shah S, Otsuka T, Bordett R, et al. Injectable amnion hydrogel-mediated delivery of adipose-derived stem cells for osteoarthritis treatment. Proc Natl Acad Sci U S A. 2022;119(4):e2120968119. doi: 10.1073/pnas.2120968119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao H, Jiang H, Zhang H, Sun Z, Lin Q, Wang T, et al. Adipose-derived mesenchymal stem cell-derived extracellular vesicles rescue tendon injury in rat via the miR-19 a/IGFBP3 axis. Stem Cells Int. 2022;2022:4197473. doi: 10.1155/2022/4197473. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20.Rao Y, Zhu C, Suen HC, Huang S, Liao J, Ker DFE, et al. Tenogenic induction of human adipose-derived stem cells by soluble tendon extracellular matrix: composition and transcriptomic analyses. Stem Cell Res Ther. 2022;13(1):380. doi: 10.1186/s13287-022-03038-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Woo CH, Kim HK, Jung GY, Jung YJ, Lee KS, Yun YE, et al. Small extracellular vesicles from human adipose-derived stem cells attenuate cartilage degeneration. J Extracell Vesicles. 2020;9(1):1735249. doi: 10.1080/20013078.2020.1735249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89(6):2548–2556. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- 23.Saely CH, Geiger K, Drexel H. Brown versus white adipose tissue: a mini-review. Gerontology. 2012;58(1):15–23. doi: 10.1159/000321319. [DOI] [PubMed] [Google Scholar]
- 24.Bourin P, Bunnell BA, Casteilla L, Dominici M, Katz AJ, March KL, et al. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT) Cytotherapy. 2013;15(6):641–648. doi: 10.1016/j.jcyt.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maumus M, Peyrafitte JA, D’Angelo R, Fournier-Wirth C, Bouloumié A, Casteilla L, et al. Native human adipose stromal cells: localization, morphology and phenotype. Int J Obes (Lond) 2011;35(9):1141–1153. doi: 10.1038/ijo.2010.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu G, Wu X, Dietrich MA, Polk P, Scott LK, Ptitsyn AA, et al. Yield and characterization of subcutaneous human adipose-derived stem cells by flow cytometric and adipogenic mRNA analyzes. Cytotherapy. 2010;12(4):538–546. doi: 10.3109/14653241003649528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seda Tigli R, Ghosh S, Laha MM, Shevde NK, Daheron L, Gimble J, et al. Comparative chondrogenesis of human cell sources in 3D scaffolds. J Tissue Eng Regen Med. 2009;3(5):348–360. doi: 10.1002/term.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen S, Wang J, Chen Y, Mo X, Fan C. Tenogenic adipose-derived stem cell sheets with nanoyarn scaffolds for tendon regeneration. Mater Sci Eng C Mater Biol Appl. 2021;119:111506. doi: 10.1016/j.msec.2020.111506. [DOI] [PubMed] [Google Scholar]
- 29.Canepa DD, Casanova EA, Arvaniti E, Tosevski V, Märsmann S, Eggerschwiler B, et al. Identification of ALP+/CD73+ defining markers for enhanced osteogenic potential in human adipose-derived mesenchymal stromal cells by mass cytometry. Stem Cell Res Ther. 2021;12(1):7. doi: 10.1186/s13287-020-02044-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin K, Yang Y, Cao Y, Liang J, Qian J, Wang X, et al. Combining single-cell transcriptomics and Cell Tagging to identify differentiation trajectories of human adipose-derived mesenchymal stem cells. Stem Cell Res Ther. 2023;14(1):14. doi: 10.1186/s13287-023-03237-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qin Y, Ge G, Yang P, Wang L, Qiao Y, Pan G, et al. An update on adipose-derived stem cells for regenerative medicine: where challenge meets opportunity. Adv Sci (Weinh) 2023;10(20):e2207334. doi: 10.1002/advs.202207334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Calabrese EJ. Hormesis and adult adipose-derived stem cells. Pharmacol Res. 2021;172:105803. doi: 10.1016/j.phrs.2021.105803. [DOI] [PubMed] [Google Scholar]
- 33.Chen CH, Lin YS, Fu YC, Wang CK, Wu SC, Wang GJ, et al. Electromagnetic fields enhance chondrogenesis of human adipose-derived stem cells in a chondrogenic microenvironment in vitro. J Appl Physiol (1985) 2013;114(5):647–655. doi: 10.1152/japplphysiol.01216.2012. [DOI] [PubMed] [Google Scholar]
- 34.Lombardi F, Palumbo P, Augello FR, Cifone MG, Cinque B, Giuliani M. Secretome of adipose tissue-derived stem cells (ASCs) as a novel trend in chronic non-healing wounds: an overview of experimental in vitro and in vivo studies and methodological variables. Int J Mol Sci. 2019;20(15):3721. doi: 10.3390/ijms20153721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Giannasi C, Niada S, Magagnotti C, Ragni E, Andolfo A, Brini AT. Comparison of two ASC-derived therapeutics in an in vitro OA model: secretome versus extracellular vesicles. Stem Cell Res Ther. 2020;11(1):521. doi: 10.1186/s13287-020-02035-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ceccarelli S, Pontecorvi P, Anastasiadou E, Napoli C, Marchese C. Immunomodulatory effect of adipose-derived stem cells: the cutting edge of clinical application. Front Cell Dev Biol. 2020;8:236. doi: 10.3389/fcell.2020.00236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nan K, Zhang Y, Zhang X, Li D, Zhao Y, Jing Z, et al. Exosomes from miRNA-378-modified adipose-derived stem cells prevent glucocorticoid-induced osteonecrosis of the femoral head by enhancing angiogenesis and osteogenesis via targeting miR-378 negatively regulated suppressor of fused (Sufu) Stem Cell Res Ther. 2021;12(1):331. doi: 10.1186/s13287-021-02390-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liang ZH, Pan NF, Lin SS, Qiu ZY, Liang P, Wang J, et al. Exosomes from mmu_circ_0001052-modified adipose-derived stem cells promote angiogenesis of DFU via miR-106a-5p and FGF4/p38MAPK pathway. Stem Cell Res Ther. 2022;13(1):336. doi: 10.1186/s13287-022-03015-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mitchell R, Mellows B, Sheard J, Antonioli M, Kretz O, Chambers D, et al. Secretome of adipose-derived mesenchymal stem cells promotes skeletal muscle regeneration through synergistic action of extracellular vesicle cargo and soluble proteins. Stem Cell Res Ther. 2019;10(1):116. doi: 10.1186/s13287-019-1213-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guo X, Schaudinn C, Blume-Peytavi U, Vogt A, Rancan F. Effects of adipose-derived stem cells and their conditioned medium in a human ex vivo wound model. Cells. 2022;11(7):1198. doi: 10.3390/cells11071198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li L, Ngo HTT, Hwang E, Wei X, Liu Y, Liu J, et al. Conditioned medium from human adipose-derived mesenchymal stem cell culture prevents UVB-induced skin aging in human keratinocytes and dermal fibroblasts. Int J Mol Sci. 2019;21(1):49. doi: 10.3390/ijms21010049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Niel G, D’Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19(4):213–228. doi: 10.1038/nrm.2017.125. [DOI] [PubMed] [Google Scholar]
- 43.Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367(6478):eaau6977. doi: 10.1126/science.aau6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shen K, Jia Y, Wang X, Zhang J, Liu K, Wang J, et al. Exosomes from adipose-derived stem cells alleviate the inflammation and oxidative stress via regulating Nrf2/HO-1 axis in macrophages. Free Radic Biol Med. 2021;165:54–66. doi: 10.1016/j.freeradbiomed.2021.01.023. [DOI] [PubMed] [Google Scholar]
- 45.Xu F, Xiang Q, Huang J, Chen Q, Yu N, Long X, et al. Exosomal miR-423-5p mediates the proangiogenic activity of human adipose-derived stem cells by targeting Sufu. Stem Cell Res Ther. 2019;10(1):106. doi: 10.1186/s13287-019-1196-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ren L, Song ZJ, Cai QW, Chen RX, Zou Y, Fu Q, et al. Adipose mesenchymal stem cell-derived exosomes ameliorate hypoxia/serum deprivation-induced osteocyte apoptosis and osteocyte-mediated osteoclastogenesis in vitro. Biochem Biophys Res Commun. 2019;508(1):138–144. doi: 10.1016/j.bbrc.2018.11.109. [DOI] [PubMed] [Google Scholar]
- 47.An Y, Lin S, Tan X, Zhu S, Nie F, Zhen Y, et al. Exosomes from adipose-derived stem cells and application to skin wound healing. Cell Prolif. 2021;54(3):e12993. doi: 10.1111/cpr.12993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Choi EW, Seo MK, Woo EY, Kim SH, Park EJ, Kim S. Exosomes from human adipose-derived stem cells promote proliferation and migration of skin fibroblasts. Exp Dermatol. 2018;27(10):1170–1172. doi: 10.1111/exd.13451. [DOI] [PubMed] [Google Scholar]
- 49.Jiawei S, Zhi C, Kewei T, Xiaoping L. Magnetic bead-based adsorption strategy for exosome isolation. Front Bioeng Biotechnol. 2022;10:942077. doi: 10.3389/fbioe.2022.942077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhao R, Zhao T, He Z, Cai R, Pang W. Composition, isolation, identification and function of adipose tissue-derived exosomes. Adipocyte. 2021;10(1):587–604. doi: 10.1080/21623945.2021.1983242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Luo HT, Zheng YY, Tang J, Shao LJ, Mao YH, Yang W, et al. Dissecting the multi-omics atlas of the exosomes released by human lung adenocarcinoma stem-like cells. NPJ Genom Med. 2021;6(1):48. doi: 10.1038/s41525-021-00217-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang C, Zhang J, Wu T, Zhao K, Wu X, Shi J, et al. Multi-omics analysis to examine gene expression and metabolites from multisite adipose-derived mesenchymal stem cells. Front Genet. 2021;12:627347. doi: 10.3389/fgene.2021.627347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhao C, Chen JY, Peng WM, Yuan B, Bi Q, Xu YJ. Exosomes from adipose-derived stem cells promote chondrogenesis and suppress inflammation by upregulating miR-145 and miR-221. Mol Med Rep. 2020;21(4):1881–1889. doi: 10.3892/mmr.2020.10982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang C, Hu Q, Song W, Yu W, He Y. Adipose stem cell-derived exosomes decrease fatty infiltration and enhance rotator cuff healing in a rabbit model of chronic tears. Am J Sports Med. 2020;48(6):1456–1464. doi: 10.1177/0363546520908847. [DOI] [PubMed] [Google Scholar]
- 55.Kang Y, Xu J, Meng L, Su Y, Fang H, Liu J, et al. 3D bioprinting of dECM/Gel/QCS/nHAp hybrid scaffolds laden with mesenchymal stem cell-derived exosomes to improve angiogenesis and osteogenesis. Biofabrication. 2023;15(2):024103. doi: 10.1088/1758-5090/acb6b8. [DOI] [PubMed] [Google Scholar]
- 56.Chen S, Tang Y, Liu Y, Zhang P, Lv L, Zhang X, et al. Exosomes derived from miR-375-overexpressing human adipose mesenchymal stem cells promote bone regeneration. Cell Prolif. 2019;52(5):e12669. doi: 10.1111/cpr.12669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kang Y, Xu C, Meng L, Dong X, Qi M, Jiang D. Exosome-functionalized magnesium-organic framework-based scaffolds with osteogenic, angiogenic and anti-inflammatory properties for accelerated bone regeneration. Bioact Mater. 2022;18:26–41. doi: 10.1016/j.bioactmat.2022.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu N, Liu J, Ma W, Dong X, Wang F, Yang D, et al. Degradable calcium deficient hydroxyapatite/poly(lactic-glycolic acid copolymer) bilayer scaffold through integral molding 3D printing for bone defect repair. Biofabrication. 2021;13(2):025005. doi: 10.1088/1758-5090/abcb48. [DOI] [PubMed] [Google Scholar]
- 59.Rahman G, Frazier TP, Gimble JM, Mohiuddin OA. The emerging use of ASC/scaffold composites for the regeneration of osteochondral defects. Front Bioeng Biotechnol. 2022;10:893992. doi: 10.3389/fbioe.2022.893992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sawadkar P, Mohanakrishnan J, Rajasekar P, Rahmani B, Kohli N, Bozec L, et al. A synergistic relationship between polycaprolactone and natural polymers enhances the physical properties and biological activity of scaffolds. ACS Appl Mater Interfaces. 2020;12(12):13587–13597. doi: 10.1021/acsami.9b19715. [DOI] [PubMed] [Google Scholar]
- 61.Reis CHB, Buchaim RL, Pomini KT, Hamzé AL, Zattiti IV, Duarte MAH, et al. Effects of a biocomplex formed by two scaffold biomaterials, hydroxyapatite/tricalcium phosphate ceramic and fibrin biopolymer, with photobiomodulation, on bone repair. Polymers (Basel). 2022;14(10):2075. doi: 10.3390/polym14102075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brown M, Li J, Moraes C, Tabrizian M, Li-Jessen NYK. Decellularized extracellular matrix: new promising and challenging biomaterials for regenerative medicine. Biomaterials. 2022;289:121786. doi: 10.1016/j.biomaterials.2022.121786. [DOI] [PubMed] [Google Scholar]
- 63.Huang Q, Zou Y, Arno MC, Chen S, Wang T, Gao J, et al. Hydrogel scaffolds for differentiation of adipose-derived stem cells. Chem Soc Rev. 2017;46(20):6255–6275. doi: 10.1039/C6CS00052E. [DOI] [PubMed] [Google Scholar]
- 64.Stanco D, Boffito M, Bogni A, Puricelli L, Barrero J, Soldati G, et al. 3D bioprinting of human adipose-derived stem cells and their tenogenic differentiation in clinical-grade medium. Int J Mol Sci. 2020;21(22):8694. doi: 10.3390/ijms21228694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xu J, Ji J, Jiao J, Zheng L, Hong Q, Tang H, et al. 3D printing for bone-cartilage interface regeneration. Front Bioeng Biotechnol. 2022;10:828921. doi: 10.3389/fbioe.2022.828921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bougioukli S, Sugiyama O, Pannell W, Ortega B, Tan MH, Tang AH, et al. Gene therapy for bone repair using human cells: superior osteogenic potential of bone morphogenetic protein 2-transduced mesenchymal stem cells derived from adipose tissue compared to bone marrow. Hum Gene Ther. 2018;29(4):507–519. doi: 10.1089/hum.2017.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yu W, Hu B, Boakye-Yiadom KO, Ho W, Chen Q, Xu X, et al. Injectable hydrogel mediated delivery of gene-engineered adipose-derived stem cells for enhanced osteoarthritis treatment. Biomater Sci. 2021;9(22):7603–7616. doi: 10.1039/D1BM01122G. [DOI] [PubMed] [Google Scholar]
- 68.Wu H, Peng Z, Xu Y, Sheng Z, Liu Y, Liao Y, et al. Engineered adipose-derived stem cells with IGF-1-modified mRNA ameliorates osteoarthritis development. Stem Cell Res Ther. 2022;13(1):19. doi: 10.1186/s13287-021-02695-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lee JM, Im GI. SOX trio-co-transduced adipose stem cells in fibrin gel to enhance cartilage repair and delay the progression of osteoarthritis in the rat. Biomaterials. 2012;33(7):2016–2024. doi: 10.1016/j.biomaterials.2011.11.050. [DOI] [PubMed] [Google Scholar]
- 70.Nauta A, Seidel C, Deveza L, Montoro D, Grova M, Ko SH, et al. Adipose-derived stromal cells overexpressing vascular endothelial growth factor accelerate mouse excisional wound healing. Mol Ther. 2013;21(2):445–455. doi: 10.1038/mt.2012.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Park S, Heo HA, Lee KB, Kim HG, Pyo SW. Improved bone regeneration with multiporous PLGA scaffold and BMP-2-transduced human adipose-derived stem cells by cell-permeable peptide. Implant Dent. 2017;26(1):4–11. doi: 10.1097/ID.0000000000000523. [DOI] [PubMed] [Google Scholar]
- 72.Freitas J, Santos SG, Gonçalves RM, Teixeira JH, Barbosa MA, Almeida MI. Genetically engineered-MSC therapies for non-unions, delayed unions and critical-size bone defects. Int J Mol Sci. 2019;20(14):3430. doi: 10.3390/ijms20143430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wan S, Wu Q, Ji Y, Fu X, Wang Y. Promotion of the immunomodulatory properties and osteogenic differentiation of adipose-derived mesenchymal stem cells in vitro by lentivirus-mediated mir-146a sponge expression. J Tissue Eng Regen Med. 2020;14(11):1581–1591. doi: 10.1002/term.3113. [DOI] [PubMed] [Google Scholar]
- 74.Liao YH, Chang YH, Sung LY, Li KC, Yeh CL, Yen TC, et al. Osteogenic differentiation of adipose-derived stem cells and calvarial defect repair using baculovirus-mediated co-expression of BMP-2 and miR-148b. Biomaterials. 2014;35(18):4901–4910. doi: 10.1016/j.biomaterials.2014.02.055. [DOI] [PubMed] [Google Scholar]
- 75.Xie Q, Wang Z, Zhou H, Yu Z, Huang Y, Sun H, et al. The role of miR-135-modified adipose-derived mesenchymal stem cells in bone regeneration. Biomaterials. 2016;75:279–294. doi: 10.1016/j.biomaterials.2015.10.042. [DOI] [PubMed] [Google Scholar]
- 76.Sun J, Zhang F, Luo X, Shi G, Li F, Zheng B, et al. Long noncoding RNA AC092155 facilitates osteogenic differentiation of adipose-derived stem cells through the miR-143-3p/STMN1 axis. J Gene Med. 2021;23(8):e3363. doi: 10.1002/jgm.3363. [DOI] [PubMed] [Google Scholar]
- 77.Shi ZL, Zhang H, Fan ZY, Ma W, Song YZ, Li M, et al. Long noncoding RNA LINC00314 facilitates osteogenic differentiation of adipose-derived stem cells through the hsa-miR-129-5p/GRM5 axis via the Wnt signaling pathway. Stem Cell Res Ther. 2020;11(1):240. doi: 10.1186/s13287-020-01754-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ho-Shui-Ling A, Bolander J, Rustom LE, Johnson AW, Luyten FP, Picart C. Bone regeneration strategies: engineered scaffolds, bioactive molecules and stem cells current stage and future perspectives. Biomaterials. 2018;180:143–162. doi: 10.1016/j.biomaterials.2018.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yuan C, Gou X, Deng J, Dong Z, Ye P, Hu Z. FAK and BMP-9 synergistically trigger osteogenic differentiation and bone formation of adipose derived stem cells through enhancing Wnt-β-catenin signaling. Biomed Pharmacother. 2018;105:753–757. doi: 10.1016/j.biopha.2018.04.185. [DOI] [PubMed] [Google Scholar]
- 80.Gersch RP, Glahn J, Tecce MG, Wilson AJ, Percec I. Platelet rich plasma augments adipose-derived stem cell growth and differentiation. Aesthet Surg J. 2017;37(6):723–729. doi: 10.1093/asj/sjw235. [DOI] [PubMed] [Google Scholar]
- 81.Sattary M, Rafienia M, Kazemi M, Salehi H, Mahmoudzadeh M. Promoting effect of nano hydroxyapatite and vitamin D3 on the osteogenic differentiation of human adipose-derived stem cells in polycaprolactone/gelatin scaffold for bone tissue engineering. Mater Sci Eng C Mater Biol Appl. 2019;97:141–155. doi: 10.1016/j.msec.2018.12.030. [DOI] [PubMed] [Google Scholar]
- 82.Gao Y, Ku NJ, Sung TC, Higuchi A, Hung CS, Lee HH, et al. The effect of human platelet lysate on the differentiation ability of human adipose-derived stem cells cultured on ECM-coated surfaces. J Mater Chem B. 2019;7(45):7110–7119. doi: 10.1039/C9TB01764J. [DOI] [PubMed] [Google Scholar]
- 83.Zhang X, Guo J, Zhou Y, Wu G. The roles of bone morphogenetic proteins and their signaling in the osteogenesis of adipose-derived stem cells. Tissue Eng Part B Rev. 2014;20(1):84–92. doi: 10.1089/ten.teb.2013.0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wagner JM, Reinkemeier F, Wallner C, Dadras M, Huber J, Schmidt SV, et al. Adipose-derived stromal cells are capable of restoring bone regeneration after post-traumatic osteomyelitis and modulate B-cell response. Stem Cells Transl Med. 2019;8(10):1084–1091. doi: 10.1002/sctm.18-0266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nii M, Lai JH, Keeney M, Han LH, Behn A, Imanbayev G, et al. The effects of interactive mechanical and biochemical niche signaling on osteogenic differentiation of adipose-derived stem cells using combinatorial hydrogels. Acta Biomater. 2013;9(3):5475–5483. doi: 10.1016/j.actbio.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 86.Minardi S, Corradetti B, Taraballi F, Sandri M, Van Eps J, Cabrera FJ, et al. Evaluation of the osteoinductive potential of a bio-inspired scaffold mimicking the osteogenic niche for bone augmentation. Biomaterials. 2015;62:128–137. doi: 10.1016/j.biomaterials.2015.05.011. [DOI] [PubMed] [Google Scholar]
- 87.Saçak B, Certel F, Akdeniz ZD, Karademir B, Ercan F, Özkan N, et al. Repair of critical size defects using bioactive glass seeded with adipose-derived mesenchymal stem cells. J Biomed Mater Res B Appl Biomater. 2017;105(5):1002–1008. doi: 10.1002/jbm.b.33634. [DOI] [PubMed] [Google Scholar]
- 88.Alvira-González J, Sánchez-Garcés M, Cairó JR, Del Pozo MR, Sánchez CM, Gay-Escoda C. Assessment of bone regeneration using adipose-derived stem cells in critical-size alveolar ridge defects: an experimental study in a dog model. Int J Oral Maxillofac Implants. 2016;31(1):196–203. doi: 10.11607/jomi.4190. [DOI] [PubMed] [Google Scholar]
- 89.Liu J, Zhou P, Long Y, Huang C, Chen D. Repair of bone defects in rat radii with a composite of allogeneic adipose-derived stem cells and heterogeneous deproteinized bone. Stem Cell Res Ther. 2018;9(1):79. doi: 10.1186/s13287-018-0817-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Du J, Xie P, Lin S, Wu Y, Zeng D, Li Y, et al. Time-phase sequential utilization of adipose-derived mesenchymal stem cells on mesoporous bioactive glass for restoration of critical size bone defects. ACS Appl Mater Interfaces. 2018;10(34):28340–28350. doi: 10.1021/acsami.8b08563. [DOI] [PubMed] [Google Scholar]
- 91.Lee J, Lee S, Ahmad T, Madhurakkat Perikamana SK, Lee J, Kim EM, et al. Human adipose-derived stem cell spheroids incorporating platelet-derived growth factor (PDGF) and bio-minerals for vascularized bone tissue engineering. Biomaterials. 2020;255:120192. doi: 10.1016/j.biomaterials.2020.120192. [DOI] [PubMed] [Google Scholar]
- 92.Ahmad T, Byun H, Lee J, Madhurakat Perikamana SK, Shin YM, Kim EM, et al. Stem cell spheroids incorporating fibers coated with adenosine and polydopamine as a modular building blocks for bone tissue engineering. Biomaterials. 2020;230:119652. doi: 10.1016/j.biomaterials.2019.119652. [DOI] [PubMed] [Google Scholar]
- 93.Zhang H, Kot A, Lay YE, Fierro FA, Chen H, Lane NE, et al. Acceleration of fracture healing by overexpression of basic fibroblast growth factor in the mesenchymal stromal cells. Stem Cells Transl Med. 2017;6(10):1880–1893. doi: 10.1002/sctm.17-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hixon KR, Katz DB, McKenzie JA, Miller AN, Guilak F, Silva MJ. Cryogel scaffold-mediated delivery of adipose-derived stem cells promotes healing in murine model of atrophic non-union. Front Bioeng Biotechnol. 2022;10:851904. doi: 10.3389/fbioe.2022.851904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang F, Wang Q, Zhao Y, Tian Z, Chang S, Tong H, et al. Adipose-derived stem cells with miR-150-5p inhibition laden in hydroxyapatite/tricalcium phosphate ceramic powders promote osteogenesis via regulating Notch3 and activating FAK/ERK and RhoA. Acta Biomater. 2023;155:644–653. doi: 10.1016/j.actbio.2022.09.070. [DOI] [PubMed] [Google Scholar]
- 96.Li W, Liu Y, Zhang P, Tang Y, Zhou M, Jiang W, et al. Tissue-engineered bone immobilized with human adipose stem cells-derived exosomes promotes bone regeneration. ACS Appl Mater Interfaces. 2018;10(6):5240–5254. doi: 10.1021/acsami.7b17620. [DOI] [PubMed] [Google Scholar]
- 97.Sadeghian-Nodoushan F, Nikukar H, Soleimani M, Jalali-Jahromi A, Hosseinzadeh S, Khojasteh A. A smart magnetic hydrogel containing exosome promotes osteogenic commitment of human adipose-derived mesenchymal stem cells. Iran J Basic Med Sci. 2022;25(9):1123–1131. doi: 10.22038/IJBMS.2022.64682.14237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gandolfi MG, Gardin C, Zamparini F, Ferroni L, Esposti MD, Parchi G, et al. Mineral-doped poly(L-lactide) acid scaffolds enriched with exosomes improve osteogenic commitment of human adipose-derived mesenchymal stem Cells. Nanomater. (Basel). 2020;10(3):432. doi: 10.3390/nano10030432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Li G, Zhang Y, Wu J, Yang R, Sun Q, Xu Y, et al. Adipose stem cells-derived exosomes modified gelatin sponge promotes bone regeneration. Front Bioeng Biotechnol. 2023;11:1096390. doi: 10.3389/fbioe.2023.1096390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lu Z, Chen Y, Dunstan C, Roohani-Esfahani S, Zreiqat H. Priming adipose stem cells with tumor necrosis factor-alpha preconditioning potentiates their exosome efficacy for bone regeneration. Tissue Eng Part A. 2017;23(21–22):1212–1220. doi: 10.1089/ten.tea.2016.0548. [DOI] [PubMed] [Google Scholar]
- 101.Sándor GK, Numminen J, Wolff J, Thesleff T, Miettinen A, Tuovinen VJ, et al. Adipose stem cells used to reconstruct 13 cases with cranio-maxillofacial hard-tissue defects. Stem Cells Transl Med. 2014;3(4):530–540. doi: 10.5966/sctm.2013-0173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhang Z, Yang X, Cao X, Qin A, Zhao J. Current applications of adipose-derived mesenchymal stem cells in bone repair and regeneration: a review of cell experiments, animal models, and clinical trials. Front Bioeng Biotechnol. 2022;10:942128. doi: 10.3389/fbioe.2022.942128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Thesleff T, Lehtimäki K, Niskakangas T, Huovinen S, Mannerström B, Miettinen S, et al. Cranioplasty with adipose-derived stem cells, beta-tricalcium phosphate granules and supporting mesh: six-year clinical follow-up results. Stem Cells Transl Med. 2017;6(7):1576–1582. doi: 10.1002/sctm.16-0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Eccleston A. Cartilage regeneration for osteoarthritis. Nat Rev Drug Discov. 2023;22(2):96. doi: 10.1038/d41573-022-00215-x. [DOI] [PubMed] [Google Scholar]
- 105.López-Ruiz E, Jiménez G, García M, Antich C, Boulaiz H, Marchal JA, et al. Polymers, scaffolds and bioactive molecules with therapeutic properties in osteochondral pathologies: what's new? Expert Opin Ther Pat. 2016;26(8):877–890. doi: 10.1080/13543776.2016.1203903. [DOI] [PubMed] [Google Scholar]
- 106.Huang L, Yi L, Zhang C, He Y, Zhou L, Liu Y, et al. Synergistic effects of FGF-18 and TGF-β3 on the chondrogenesis of human adipose-derived mesenchymal stem cells in the pellet culture. Stem Cells Int. 2018;2018:7139485. doi: 10.1155/2018/7139485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Xu L, Zhang F, Cheng G, Yuan X, Wu Y, Wu H, et al. Attenuation of experimental osteoarthritis with human adipose-derived mesenchymal stem cell therapy: inhibition of the pyroptosis in chondrocytes. Inflamm Res. 2023;72(1):89–105. doi: 10.1007/s00011-022-01655-2. [DOI] [PubMed] [Google Scholar]
- 108.Liu X, Liu Y, He H, Xiang W, He C. Human adipose and synovial mesenchymal stem cells improve osteoarthritis in rats by reducing chondrocyte reactive oxygen species and inhibiting inflammatory response. J Clin Lab Anal. 2022;36(5):e24353. doi: 10.1002/jcla.24353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tofiño-Vian M, Guillén MI, Pérez Del Caz MD, Castejón MA, Alcaraz MJ. Extracellular vesicles from adipose-derived mesenchymal stem cells downregulate senescence features in osteoarthritic osteoblasts. Oxid Med Cell Longev. 2017;2017:7197598. doi: 10.1155/2017/7197598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ragni E, Perucca Orfei C, De Luca P, Lugano G, Viganò M, Colombini A, et al. Interaction with hyaluronan matrix and miRNA cargo as contributors for in vitro potential of mesenchymal stem cell-derived extracellular vesicles in a model of human osteoarthritic synoviocytes. Stem Cell Res Ther. 2019;10(1):109. doi: 10.1186/s13287-019-1215-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tofiño-Vian M, Guillén MI, Pérez Del Caz MD, Silvestre A, Alcaraz MJ. Microvesicles from human adipose tissue-derived mesenchymal stem cells as a new protective strategy in osteoarthritic chondrocytes. Cell Physiol Biochem. 2018;47(1):11–25. doi: 10.1159/000489739. [DOI] [PubMed] [Google Scholar]
- 112.Meng C, Na Y, Han C, Ren Y, Liu M, Ma P, et al. Exosomal miR-429 derived from adipose-derived stem cells ameliorated chondral injury in osteoarthritis via autophagy by targeting FEZ2. Int Immunopharmacol. 2023;120:110315. doi: 10.1016/j.intimp.2023.110315. [DOI] [PubMed] [Google Scholar]
- 113.Hwang OK, Noh YW, Hong JT, Lee JW. Hypoxia pretreatment promotes chondrocyte differentiation of human adipose-derived stem cells via vascular endothelial growth factor. Tissue Eng Regen Med. 2020;17(3):335–350. doi: 10.1007/s13770-020-00265-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chang LH, Wu SC, Chen CH, Chen JW, Huang WC, Wu CW, et al. Exosomes derived from hypoxia-cultured human adipose stem cells alleviate articular chondrocyte inflammaging and post-traumatic osteoarthritis progression. Int J Mol Sci. 2023;24(17):13414. doi: 10.3390/ijms241713414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Meng S, Tang C, Deng M, Yuan J, Fan Y, Gao S, et al. Tropoelastin-pretreated exosomes from adipose-derived stem cells improve the synthesis of cartilage matrix and alleviate osteoarthritis. J Funct Biomater. 2023;14(4):203. doi: 10.3390/jfb14040203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Feng C, Luo X, He N, Xia H, Lv X, Zhang X, et al. Efficacy and persistence of allogeneic adipose-derived mesenchymal stem cells combined with hyaluronic acid in osteoarthritis after intra-articular injection in a sheep model. Tissue Eng Part A. 2018;24(3–4):219–233. doi: 10.1089/ten.tea.2017.0039. [DOI] [PubMed] [Google Scholar]
- 117.Lv X, He J, Zhang X, Luo X, He N, Sun Z, et al. Comparative efficacy of autologous stromal vascular fraction and autologous adipose-derived mesenchymal stem cells combined with hyaluronic acid for the treatment of sheep osteoarthritis. Cell Transplant. 2018;27(7):1111–1125. doi: 10.1177/0963689718773333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Najafi R, Chahsetareh H, Pezeshki-Modaress M, Aleemardani M, Simorgh S, Davachi SM, et al. Alginate sulfate/ECM composite hydrogel containing electrospun nanofiber with encapsulated human adipose-derived stem cells for cartilage tissue engineering. Int J Biol Macromol. 2023;238:124098. doi: 10.1016/j.ijbiomac.2023.124098. [DOI] [PubMed] [Google Scholar]
- 119.Yang J, Jing X, Wang Z, Liu X, Zhu X, Lei T, et al. In vitro and in vivo study on an injectable glycol chitosan/dibenzaldehyde-terminated polyethylene glycol hydrogel in repairing articular cartilage defects. Front Bioeng Biotechnol. 2021;9:607709. doi: 10.3389/fbioe.2021.607709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lu L, Shang X, Liu B, Chen W, Zhang Y, Liu S, et al. Repair of articular cartilage defect using adipose-derived stem cell-loaded scaffold derived from native cartilage extracellular matrix. J Cell Physiol. 2021;236(6):4244–4257. doi: 10.1002/jcp.30020. [DOI] [PubMed] [Google Scholar]
- 121.Wang Z, Le H, Wang Y, Liu H, Li Z, Yang X, et al. Instructive cartilage regeneration modalities with advanced therapeutic implantations under abnormal conditions. Bioact Mater. 2022;11:317–338. doi: 10.1016/j.bioactmat.2021.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhang K, He S, Yan S, Li G, Zhang D, Cui L, et al. Regeneration of hyaline-like cartilage and subchondral bone simultaneously by poly(l-glutamic acid) based osteochondral scaffolds with induced autologous adipose derived stem cells. J Mater Chem B. 2016;4(15):2628–2645. doi: 10.1039/C5TB02113H. [DOI] [PubMed] [Google Scholar]
- 123.Cho H, Kim J, Kim S, Jung YC, Wang Y, Kang BJ, et al. Dual delivery of stem cells and insulin-like growth factor-1 in coacervate-embedded composite hydrogels for enhanced cartilage regeneration in osteochondral defects. J Control Release. 2020;327:284–295. doi: 10.1016/j.jconrel.2020.08.002. [DOI] [PubMed] [Google Scholar]
- 124.Mellor LF, Nordberg RC, Huebner P, Mohiti-Asli M, Taylor MA, Efird W, et al. Investigation of multiphasic 3D-bioplotted scaffolds for site-specific chondrogenic and osteogenic differentiation of human adipose-derived stem cells for osteochondral tissue engineering applications. J Biomed Mater Res B Appl Biomater. 2020;108(5):2017–2030. doi: 10.1002/jbm.b.34542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wang Y, Fan A, Lu L, Pan Z, Ma M, Luo S, et al. Exosome modification to better alleviates endoplasmic reticulum stress induced chondrocyte apoptosis and osteoarthritis. Biochem Pharmacol. 2022;206:115343. doi: 10.1016/j.bcp.2022.115343. [DOI] [PubMed] [Google Scholar]
- 126.Yin Z, Qin C, Pan S, Shi C, Wu G, Feng Y, et al. Injectable hyperbranched PEG crosslinked hyaluronan hydrogel microparticles containing mir-99a-3p modified subcutaneous ADSCs-derived exosomes was beneficial for long-term treatment of osteoarthritis. Mater Today Biol. 2023;23:100813. doi: 10.1016/j.mtbio.2023.100813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lee WS, Kim HJ, Kim KI, Kim GB, Jin W. Intra-articular injection of autologous adipose tissue-derived mesenchymal stem cells for the treatment of knee osteoarthritis: a phase IIb, randomized, placebo-controlled clinical trial. Stem Cells Transl Med. 2019;8(6):504–511. doi: 10.1002/sctm.18-0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lu L, Dai C, Zhang Z, Du H, Li S, Ye P, et al. Treatment of knee osteoarthritis with intra-articular injection of autologous adipose-derived mesenchymal progenitor cells: a prospective, randomized, double-blind, active-controlled, phase IIb clinical trial. Stem Cell Res Ther. 2019;10(1):143. doi: 10.1186/s13287-019-1248-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Freitag J, Bates D, Wickham J, Shah K, Huguenin L, Tenen A, et al. Adipose-derived mesenchymal stem cell therapy in the treatment of knee osteoarthritis: a randomized controlled trial. Regen Med. 2019;14(3):213–230. doi: 10.2217/rme-2018-0161. [DOI] [PubMed] [Google Scholar]
- 130.Spasovski D, Spasovski V, Baščarević Z, Stojiljković M, Vreća M, Anđelković M, et al. Intra-articular injection of autologous adipose-derived mesenchymal stem cells in the treatment of knee osteoarthritis. J Gene Med. 2018;20(1):e3002. doi: 10.1002/jgm.3002. [DOI] [PubMed] [Google Scholar]
- 131.Song Y, Du H, Dai C, Zhang L, Li S, Hunter DJ, et al. Human adipose-derived mesenchymal stem cells for osteoarthritis: a pilot study with long-term follow-up and repeated injections. Regen Med. 2018;13(3):295–307. doi: 10.2217/rme-2017-0152. [DOI] [PubMed] [Google Scholar]
- 132.Kim KI, Kim MS, Kim JH. Intra-articular injection of autologous adipose-derived stem cells or stromal vascular fractions: are they effective for patients with knee osteoarthritis? A systematic review with meta-analysis of randomized controlled trials. Am J Sports Med. 2023;51(3):837–848. doi: 10.1177/03635465211053893. [DOI] [PubMed] [Google Scholar]
- 133.Tran TDX, Wu CM, Dubey NK, Deng YH, Su CW, Pham TT, et al. Time- and Kellgren–Lawrence grade-dependent changes in intra-articularly transplanted stromal vascular fraction in osteoarthritic patients. Cells. 2019;8(4):308. doi: 10.3390/cells8040308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hong Z, Chen J, Zhang S, Zhao C, Bi M, Chen X, et al. Intra-articular injection of autologous adipose-derived stromal vascular fractions for knee osteoarthritis: a double-blind randomized self-controlled trial. Int Orthop. 2019;43(5):1123–1134. doi: 10.1007/s00264-018-4099-0. [DOI] [PubMed] [Google Scholar]
- 135.Tsubosaka M, Matsumoto T, Sobajima S, Matsushita T, Iwaguro H, Kuroda R. The influence of adipose-derived stromal vascular fraction cells on the treatment of knee osteoarthritis. BMC Musculoskelet Disord. 2020;21(1):207. doi: 10.1186/s12891-020-03231-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Aletto C, Giordano L, Quaranta M, Zara A, Notarfrancesco D, Maffulli N. Short-term results of intra-articular injections of stromal vascular fraction for early knee osteoarthritis. J Orthop Surg Res. 2022;17(1):310. doi: 10.1186/s13018-022-03196-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Fine N, Lively S, Séguin CA, Perruccio AV, Kapoor M, Rampersaud R. Intervertebral disc degeneration and osteoarthritis: a common molecular disease spectrum. Nat Rev Rheumatol. 2023;19(3):136–152. doi: 10.1038/s41584-022-00888-z. [DOI] [PubMed] [Google Scholar]
- 138.Yu L, Liu Y, Wu J, Wang S, Yu J, Wang W, et al. Genipin cross-linked decellularized nucleus pulposus hydrogel-like cell delivery system induces differentiation of ADSCs and retards intervertebral disc degeneration. Front Bioeng Biotechnol. 2021;9:807883. doi: 10.3389/fbioe.2021.807883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Xing H, Zhang Z, Mao Q, Wang C, Zhou Y, Zhou X, et al. Injectable exosome-functionalized extracellular matrix hydrogel for metabolism balance and pyroptosis regulation in intervertebral disc degeneration. J Nanobiotechnol. 2021;19(1):264. doi: 10.1186/s12951-021-00991-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Molloy T, Wang Y, Murrell G. The roles of growth factors in tendon and ligament healing. Sports Med. 2003;33(5):381–394. doi: 10.2165/00007256-200333050-00004. [DOI] [PubMed] [Google Scholar]
- 141.Li X, Pongkitwitoon S, Lu H, Lee C, Gelberman R, Thomopoulos S. CTGF induces tenogenic differentiation and proliferation of adipose-derived stromal cells. J Orthop Res. 2019;37(3):574–582. doi: 10.1002/jor.24248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Fitzgerald MJ, Mustapich T, Liang H, Larsen CG, Nellans KW, Grande DA. Tendon transection healing can be improved with adipose-derived stem cells cultured with growth differentiation factor 5 and platelet-derived growth factor. Hand (N Y) 2023;18(3):436–445. doi: 10.1177/15589447211028929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Guo X, Lv H, Fan Z, Duan K, Liang J, Zou L, et al. Effects of hypoxia on Achilles tendon repair using adipose tissue-derived mesenchymal stem cells seeded small intestinal submucosa. J Orthop Surg Res. 2021;16(1):570. doi: 10.1186/s13018-021-02713-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Behfar M, Sarrafzadeh-Rezaei F, Hobbenaghi R, Delirezh N, Dalir-Naghadeh B. Enhanced mechanical properties of rabbit flexor tendons in response to intratendinous injection of adipose derived stromal vascular fraction. Curr Stem Cell Res Ther. 2012;7(3):173–178. doi: 10.2174/157488812799859874. [DOI] [PubMed] [Google Scholar]
- 145.Oh JH, Chung SW, Kim SH, Chung JY, Kim JY. 2013 Neer Award: effect of the adipose-derived stem cell for the improvement of fatty degeneration and rotator cuff healing in rabbit model. J Shoulder Elbow Surg. 2014;23(4):445–455. doi: 10.1016/j.jse.2013.07.054. [DOI] [PubMed] [Google Scholar]
- 146.Arnaud-Franco Á, Lara-Arias J, Marino-Martínez IA, Cienfuegos-Jiménez O, Barbosa-Quintana Á, Peña-Martínez VM. Effect of adipose-derived mesenchymal stem cells (ADMSCs) application in achilles-tendon injury in an animal model. Curr Issues Mol Biol. 2022;44(12):5827–5838. doi: 10.3390/cimb44120396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Norelli JB, Plaza DP, Stal DN, Varghese AM, Liang H, Grande DA. Tenogenically differentiated adipose-derived stem cells are effective in Achilles tendon repair in vivo. J Tissue Eng. 2018;9:2041731418811183. doi: 10.1177/2041731418811183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Shin MJ, Shim IK, Kim DM, Choi JH, Lee YN, Jeon IH, et al. Engineered cell sheets for the effective delivery of adipose-derived stem cells for tendon-to-bone healing. Am J Sports Med. 2020;48(13):3347–3358. doi: 10.1177/0363546520964445. [DOI] [PubMed] [Google Scholar]
- 149.Chiou GJ, Crowe C, McGoldrick R, Hui K, Pham H, Chang J. Optimization of an injectable tendon hydrogel: the effects of platelet-rich plasma and adipose-derived stem cells on tendon healing in vivo. Tissue Eng Part A. 2015;21(9–10):1579–1586. doi: 10.1089/ten.tea.2014.0490. [DOI] [PubMed] [Google Scholar]
- 150.Rothrauff BB, Smith CA, Ferrer GA, Novaretti JV, Pauyo T, Chao T, et al. The effect of adipose-derived stem cells on enthesis healing after repair of acute and chronic massive rotator cuff tears in rats. J Shoulder Elbow Surg. 2019;28(4):654–664. doi: 10.1016/j.jse.2018.08.044. [DOI] [PubMed] [Google Scholar]
- 151.Yang X, Meng H, Peng J, Xu L, Wang Y, Sun X, et al. Construction of microunits by adipose-derived mesenchymal stem cells laden with porous microcryogels for repairing an acute Achilles tendon rupture in a rat model. Int J Nanomed. 2020;15:7155–7171. doi: 10.2147/IJN.S238399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Calejo I, Reis RL, Domingues RMA, Gomes ME. Texturing hierarchical tissues by gradient assembling of microengineered platelet-lysates activated fibers. Adv Healthc Mater. 2022;11(8):e2102076. doi: 10.1002/adhm.202102076. [DOI] [PubMed] [Google Scholar]
- 153.Zou J, Yang W, Cui W, Li C, Ma C, Ji X, et al. Therapeutic potential and mechanisms of mesenchymal stem cell-derived exosomes as bioactive materials in tendon-bone healing. J Nanobiotechnol. 2023;21(1):14. doi: 10.1186/s12951-023-01778-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zhang X, Cai Z, Wu M, Huangfu X, Li J, Liu X. Adipose stem cell-derived exosomes recover impaired matrix metabolism of torn human rotator cuff tendons by maintaining tissue homeostasis. Am J Sports Med. 2021;49(4):899–908. doi: 10.1177/0363546521992469. [DOI] [PubMed] [Google Scholar]
- 155.Wang C, Song W, Chen B, Liu X, He Y. Exosomes isolated from adipose-derived stem cells: a new cell-free approach to prevent the muscle degeneration associated with torn rotator cuffs. Am J Sports Med. 2019;47(13):3247–3255. doi: 10.1177/0363546519876323. [DOI] [PubMed] [Google Scholar]
- 156.Fu G, Lu L, Pan Z, Fan A, Yin F. Adipose-derived stem cell exosomes facilitate rotator cuff repair by mediating tendon-derived stem cells. Regen Med. 2021;16(4):359–372. doi: 10.2217/rme-2021-0004. [DOI] [PubMed] [Google Scholar]
- 157.Wang C, Tan J, Zhang Y, Chen D, He Y. In situ-forming fibrin gel encapsulation of MSC-exosomes for partial-thickness rotator cuff tears in a rabbit model: effectiveness shown in preventing tear progression and promoting healing. J Bone Joint Surg Am. 2022;104(16):1492–1502. doi: 10.2106/JBJS.21.01157. [DOI] [PubMed] [Google Scholar]
- 158.Kim YS, Sung CH, Chung SH, Kwak SJ, Koh YG. Does an injection of adipose-derived mesenchymal stem cells loaded in fibrin glue influence rotator cuff repair outcomes? A clinical and magnetic resonance imaging study. Am J Sports Med. 2017;45(9):2010–2018. doi: 10.1177/0363546517702863. [DOI] [PubMed] [Google Scholar]
- 159.Jo CH, Chai JW, Jeong EC, Oh S, Kim PS, Yoon JY, et al. Intratendinous injection of autologous adipose tissue-derived mesenchymal stem cells for the treatment of rotator cuff disease: a first-in-human trial. Stem Cells. 2018;36(9):1441–1450. doi: 10.1002/stem.2855. [DOI] [PubMed] [Google Scholar]
- 160.Jo CH, Chai JW, Jeong EC, Oh S, Yoon KS. Intratendinous injection of mesenchymal stem cells for the treatment of rotator cuff disease: a 2-year follow-up study. Arthroscopy. 2020;36(4):971–980. doi: 10.1016/j.arthro.2019.11.120. [DOI] [PubMed] [Google Scholar]
- 161.Hurd JL, Facile TR, Weiss J, Hayes M, Hayes M, Furia JP, et al. Safety and efficacy of treating symptomatic, partial-thickness rotator cuff tears with fresh, uncultured, unmodified, autologous adipose-derived regenerative cells (UA-ADRCs) isolated at the point of care: a prospective, randomized, controlled first-in-human pilot study. J Orthop Surg Res. 2020;15(1):122. doi: 10.1186/s13018-020-01631-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Randelli PS, Cucchi D, Fossati C, Boerci L, Nocerino E, Ambrogi F, et al. Arthroscopic rotator cuff repair augmentation with autologous microfragmented lipoaspirate tissue is safe and effectively improves short-term clinical and functional results: a prospective randomized controlled trial with 24-month follow-up. Am J Sports Med. 2022;50(5):1344–1357. doi: 10.1177/03635465221083324. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.