Abstract
Herpesvirus saimiri (HVS) transforms human T cells to stable growth in vitro. Since HVS codes for two different antiapoptotic proteins, growth transformation by HVS might be expected to confer resistance to apoptosis. We found that the expression of both viral antiapoptotic genes was restricted to cultures with viral replication and absent in growth-transformed human T cells. A comparative examination of HVS-transformed T-cell clones and their native parental clones revealed that the expression of Bcl-2, Bcl-XL, Bax, and members of the tumor necrosis factor receptor (TNF-R) superfamily with a death domain, namely, TNF-RI, CD95, and TRAMP, were not modulated by HVS. Expression of CD30 was induced in HVS-transformed T cells, and these cells also expressed the CD30 ligand. Uninfected and transformed T cells were sensitive to CD95 ligation but resistant to apoptosis mediated by TRAIL or soluble TNF-α. CD95 ligand was constitutively expressed on transformed but not uninfected parental T cells. Both cell types showed similar sensitivity to cell death induction or inhibition of T-cell activation mediated by irradiation, oxygen radicals, dexamethasone, cyclosporine, and prostaglandin E2. Altogether, this study strongly suggests that growth transformation by HVS is based not on resistance to apoptosis but, rather, on utilization of normal cellular activation pathways.
Escape from apoptosis is frequently an essential component of growth transformation and tumor development. For example, overexpression of the antiapoptotic Bcl-2 as a consequence of t(14;18) translocations leads to a follicular B-cell lymphoma. Bcl-2 may cooperate with cellular oncogenes like c-myc and viral oncogenes like E1A (15). In some systems, expression of Bcl-2 converts a lytic viral infection to a persistent one (32).
Herpesvirus saimiri (HVS), a lymphotropic and oncogenic herpesvirus, which is closely related to human herpesvirus 8 (HHV-8), induces leukemia and lymphoma in susceptible primates. Different strains of subgroup C of HVS transform in vitro T cells from New World monkeys, rhesus monkeys, and humans to stable growth (4, 18, 37). Human T cells transformed by HVS express the surface phenotype of mature activated T cells and retain a functionally intact T-cell receptor (reviewed in reference 38). In contrast to native T cells, they become activated via CD2 upon binding to its ligand CD58 (39). Human transformed T cells harbor HVS exclusively in an episomal state and do not release infectious virus (17).
HVS encodes two antiapoptotic proteins. The HVS-encoded FLICE inhibitory protein (HVS-FLIP) represents a novel class of viral effectors that inhibit apoptosis mediated by death receptors like CD95 (3, 27, 50). The second antiapoptotic protein of this virus is a member of the Bcl-2 family, HVS-Bcl-2 (40). A Bcl-2 homolog is also encoded by HHV-8 and by Epstein-Barr virus (EBV), while a viral FLIP is present in HHV-8 but missing in EBV. In the present study, we analyzed whether the stable growth of the HVS-transformed T cells is based on a resistance to apoptosis and examined how the regulation of proliferation and cell cycle progression is modified by HVS. To address these questions, three approaches were used. First, we analyzed the expression pattern of the antiapoptotic genes of HVS in transformed human T cells that lack viral replication, in transformed T cells from New World monkeys that release infectious virus, and in infected permissive owl monkey kidney cells. Second, we analyzed whether the transformation of T cells with HVS modulates the expression of cellular proteins involved in the regulation of apoptosis. Third, we examined whether transformed T cells and uninfected T cells have different sensitivities to apoptosis. Since native T cells are a heterogeneous population, these comparative examinations were done with uninfected T-cell clones and their HVS-transformed derivatives. The expression of cellular regulators of apoptosis belonging to the Bcl-2 family and the tumor necrosis factor receptor (TNF-R) superfamily was analyzed at the protein level. The TNF-R superfamily includes a set of molecules with a death domain through which apoptosis is mediated. This subfamily includes TNF-RI and CD95 (Apo-1 and Fas), as well as the recently identified death receptors TRAMP (DR3, WSL-1, Apo-3, and LARD) (6, 12, 29, 34, 45) and several receptors for TRAIL (41, 42, 44, 46). In addition to the members of the TNF-R superfamily that contain a death receptor, TNF-RII and CD30 can be involved in the induction of apoptosis (31, 53) and were included in our study.
Apoptosis can be triggered from outside the cell via engagement of death receptors of the TNF-R family or from inside the cell after DNA damage or after disturbance of the intracellular homeostasis, e.g., by oxygen radicals. Both main pathways to induce apoptosis were included in this study. Since regulation of apoptosis and proliferation can be intimately associated, we analyzed cell death development, proliferation, and cell cycle distribution after different treatments that may induce cell death in susceptible targets and interfere with T-cell activation. Cyclosporine (CsA), prostaglandin E2 (PGE2), dexamethasone, irradiation, and menadione were selected for these analyses. CsA inhibits the serine/threonine phosphatase calmodulin, blocks the expression of certain T-cell activation genes like the interleukin-2 (IL-2) gene, and arrests cell cycle progression at G1 (47). PGE-2 enhances the intracellular cyclic AMP level and reduces the proliferation of native T cells by inducing a G1 arrest (51). Glucocorticoids, such as dexamethasone, reduce the expression of IL-2 and of the IL-2 receptor of mature T cells (8) and induce apoptosis in immature thymocytes and T-cell hybridomas (13). Irradiation may lead to both cell cycle arrest in G1 or apoptosis depending on the cell type (13). Menadione gives rise to oxygen radicals that induce apoptosis. The sensitivity to this type of apoptosis is reduced in some cell lines by overexpression of Bcl-2 (25). For comparison, the human T-cell leukemia cell line Jurkat was included in the functional assays.
Our analysis of the expression of viral antiapoptotic genes and of the potential modulation of cellular apoptosis regulators and our functional comparisons of native uninfected T-cell clones with their HVS-transformed derivatives led to the same conclusion: growth transformation of human T cells by HVS is not associated with resistance to apoptosis but, rather, depends on the utilization of cellular activation pathways.
MATERIALS AND METHODS
Reagents.
CsA (Novartis, Nuremberg, Germany), PGE2 (Cascade, London, United Kingdom), dexamethasone, concanavalin A, menadione, phorbol-12-myristate-13-acetate (PMA) (all from Sigma), and human recombinant TNF-α (R&D, Heidelberg, Germany) were purchased. The monoclonal antibody (MAb) CH-11 (Immunotech, Marseilles, France) to CD95 has the immunoglobulin M (IgM) isotype and induces apoptosis. Recombinant Flag-tagged TRAIL (6) and an anti-Flag MAb (Integra, Fernwald, Germany) were used to study TRAIL-mediated cell death.
T-cell lines and proliferation assays.
The native CD4+ T-cell clones SS-BP8 and ES-BP8, which are specific for myelin basic protein, were infected with HVS. The transformed derivatives were termed SS-BP8T (52) and ES-BP8T (36). The HVS-transformed human T-cell line CB-15 (4) and the transformed T-cell lines P-1079 and P-1081 from Callithrix jacchus (17) have been described previously. The HVS-transformed T-cell line 93C488 was established from Saguinus fuscicollis. None of the transformed human T-cell lines analyzed produced infectious virus. The transformed T-cell line from tamarin marmosets had stopped producing infectious virus, while both transformed T-cell lines from common marmosets kept producing infectious virus, as was seen in coculture experiments with owl monkey kidney (OMK) cells.
Human native T-cell clones were restimulated every 2 to 3 weeks with irradiated HLA-DR-compatible blood cells and bovine myelin basic protein (Sigma, Deisenhofen, Germany) in a medium that consisted of 45% cell growth medium (Vitromex, Selters, Germany), 45% RPMI 1640, and 10% fetal calf serum (Boehringer, Mannheim, Germany) supplemented with 2 mM glutamine and 50 μg of gentamicin (Gibco, Berlin, Germany) per ml. After 2 days, recombinant human IL-2 (Chiron, Ratingen, Germany) was added at 100 U/ml. The HVS-transformed T-cell clones were continuously kept in IL-2-containing medium. The human T-cell leukemia cell line Jurkat and the murine T-cell hybridoma cell line DO were kept in RPMI 1640 with 10% fetal bovine serum, 2 mM glutamine, and 50 μg of gentamicin per ml.
HVS-transformed T cells were seeded in 96-well flat-bottom plates at a density of 5 × 104 cells per well in 200 μl of culture medium without IL-2. Native T-cell clones were seeded at the same density along with 2 × 105 irradiated HLA-compatible irradiated peripheral blood mononuclear cells and 10 μg of myelin basic protein per ml. The Jurkat cells were seeded at 5 × 103 cells per well. These different cell concentrations were selected to obtain similar proliferation levels after 3 days and to compensate for the different growth rates of the HVS-transformed and native T cells on the one hand and the Jurkat cells on the other. Subsequently, the immunosuppressive substances were added. All experiments were done in triplicate. Two days later, 0.2 μCi of [3H]thymidine (Amersham, Braunschweig, Germany) was added for another 16 h. The cultures were harvested with the cell harvester Matrix TM96 (Packard, Frankfurt, Germany). The filters were dried, exposed overnight on a [3H]thymidine-sensitive screen, and analyzed with a BAS2000 imaging system (Fuji Raytest, Straubenhardt, Germany). Comparative measurements of the same filters with the direct β-counter (Packard) and the BAS2000 system showed that these two evaluation systems had a correlation of >0.95 and comparable sensitivities.
Determination of cell death.
To quantify cell death, cells were collected, washed once with phosphate-buffered saline (PBS), incubated for about 30 min in PBS containing 20 μg of propidium iodide (PI) per ml, and analyzed with a flow cytometer (FACStrak; Becton Dickinson, Heidelberg, Germany). Viable and dead cells were distinguished by both forward-scatter analysis and fluorescence caused by PI uptake. The specific cell death was calculated as 100 × (% experimental cell death − spontaneous cell death)/(100% − % spontaneous cell death). DNA fragmentation was detected essentially as described previously (24). Briefly, the cells to be analyzed were washed with PBS and incubated in a mild lysis buffer (1% Nonidet P-40, 100 mM EDTA, 50 mM Tris-HCl [pH 7.5]) to enrich for DNA from cells undergoing apoptosis. The supernatant was obtained after centrifugation at 1,600 × g, and the pellet was treated with the same lysis buffer again. After centrifugation, the supernatants were pooled and SDS was added to a final concentration of 1%. The material was then digested with RNase A and proteinase K (both from Boehringer). The DNA was precipitated, washed with 70% ethanol, loaded onto an agarose gel, and stained with ethidium bromide.
Flow cytometry.
The MAbs directed to CD30 (Ki-1; Immunotech), CD28 (C293; kindly provided by R. de Waal Malefyt [DNAX]), CD95 (DX2; Pharmingen, Hamburg, Germany), TNF-RI (16803.1; R&D), TNF-RII (1888-01; Genzyme), and CD95 ligand (Nok-2; Pharmingen) were used to detect the surface expression of these molecules. Binding was detected with a fluorescein isothiocyanate-labeled goat anti-mouse IgG F(ab′)2 fragment (Dianova, Hamburg, Germany) or a fluorescein isothiocyanate-labeled goat anti-rat IgG F(ab′)2 fragment (Dianova) for the MAb to TNF-RII. TRAMP expression was determined with a polyclonal rabbit Ab (6). The intracellular expression of members of the Bcl-2 family was analyzed on cells that were fixed with 2% formalin and permeabilized with saponin buffer (PBS, 0.5% saponin [Sigma], 5% fetal calf serum, 0.02% NaN3). The MAb to Bcl-2 (sc-509; Santa Cruz, Heidelberg, Germany) and the polyclonal rabbit antibodies to Bax (Santa Cruz) and Bcl-X (Dianova) were applied at 1 μg/ml. The Ab applied to Bcl-X is suitable for detection of both the Bcl-XL and the Bcl-XS isoforms of Bcl-X. Binding of these Abs was detected with phycoerythrin-labeled goat anti-mouse IgG F(ab′)2 fragment and a donkey anti-rabbit IgG F(ab′)2 fragment (Dianova), respectively. The stained cells were analyzed in a FACStrak flow cytometer. To determine the cell cycle distribution, the cells were fixed with 70% ethanol and resuspended, after being washed, in PBS containing 20 μg of PI per ml, 2 mg of RNase A per ml, and 0.1% glucose. The cell cycle distribution was measured with an Epics cytometer and evaluated with the program Multicycle (Coulter). The proportion of cells in the S phase was also determined with the bromodeoxyuridine detection kit (Boehringer Mannheim).
Western blot analysis.
The applied lysis buffer contained 50 mM Tris-HCl (pH 8.0), 1% Triton X-100, 150 mM NaCl, 2 mM EDTA, 5 mM NaF, and 10 μg each of aprotinin and leupeptin (Sigma) per ml. Cleared lysates were separated under reducing conditions on an SDS–15% polyacrylamide gel and electroblotted on Immobilon P membrane (Millipore, Eschborn, Germany). The blots were blocked with PBS containing 5% low-fat milk and 0.05% Tween 20 and incubated with 1 μg of the primary Abs per ml and then with a 1:1,000 dilution of peroxidase-conjugated goat anti-mouse F(ab′)2 or a peroxidase-conjugated donkey anti-rabbit F(ab′)2 fragment (Amersham). They were developed with the ECL Western blotting detection system (Amersham).
Transcript analysis and DNA sequencing.
OMK cells were infected with HVS C488 and collected for RNA preparation when a strong cytopathic effect was visible. T-cell lines were stimulated with 2 ng of PMA per ml for 6 h. RNA was prepared by the phenol-guanidinium thiocyanate method, and polyadenylated mRNA was obtained with oligo(dT)-coated Dynabeads (Dynal, Hamburg, Germany). RNA was separated in a formaldehyde-agarose gel and transferred to nylon membranes (Hybond N; Amersham). Purified DNA fragments were radiolabeled with [32P]dATP by the random-labeling method and used for hybridization under stringent conditions. The signals were analyzed with a BAS2000 imaging system. Virion DNA of HVS C488 was digested with HindIII and XbaI in parallel reactions. The resulting overlapping fragments were cloned into Bluescript KS+ vector (Stratagene, Heidelberg, Germany). Sequencing was done by the Dye Didesoxy Terminator method (ABI, Weiterstadt, Germany).
Nucleotide sequence accession number.
The HVS-flip sequence of HVS C488 is available from the EMBL nucleotide sequence database under accession no. Y13660.
RESULTS
Viral antiapoptotic genes.
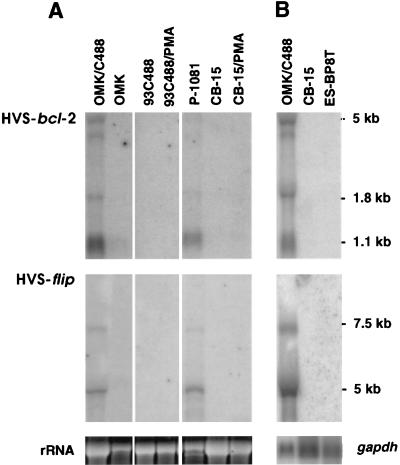
The expression of HVS-flip and of HVS-bcl-2 was analyzed in four different cellular systems: (i) infected permissive OMK cells, (ii) growth-transformed T cells from New World monkeys that produce infectious virus, (iii) growth-transformed T cells from New World monkeys that stopped releasing virus, and (iv) growth-transformed human T cells that showed a strictly episomal persistence of HVS. We found that the expression of both HVS-flip and HVS-bcl-2 was restricted to cultures with viral replication. Activation with PMA does not induce lytic replication of HVS in human transformed T cells but enhances the expression of some viral genes (17, 30). HVS-flip and HVS-bcl-2 transcripts were not detected by Northern blot hybridization in human transformed T cells, neither constitutively nor after activation with PMA (Fig. 1A). To increase the sensitivity of detection, we used polyadenylated RNA for analysis. Again, no transcripts of HVS-flip and HVS-bcl-2 were detected in human growth-transformed T cells, while strong expression was observed in lytically infected OMK cells (Fig. 1B).
FIG. 1.
Differential expression of HVS-bcl-2 and HVS-flip. (A) Total RNA of the indicated cell lines was loaded. OMK cells were infected with HVS C488 or left uninfected. The transformed T-cell line 93C488 from Saguinus fuscicollis had stopped releasing infectious virus and remained a nonproducer of infectious virus even after stimulation with PMA, while the HVS-transformed T-cell line P1081 from Callithrix jacchus produced infectious virus. Like all studied human T-cell lines, the CB-15 cell line did not produce infectious virus, either before or after activation with PMA. The top panel shows the transcripts of HVS-bcl-2, the middle panel shows the transcripts of HVS-flip, and the bottom panel shows the corresponding rRNA as detected by ethidium bromide staining. Three pieces of the same Northern blot are shown. (B) Poly(A)+ RNA of the two human transformed T-cell lines CB-15 and ES-BP8T and RNA of lytically infected OMK cells were analyzed. The bottom panel shows the hybridization with a gapdh probe. In the top panel of both A and B, the bands at 1.1, 1.8, and ca. 5 kb are specifically detected by the HVS-bcl-2 probe. In the middle panel of A and B, the bands at 5 and 7.5 kb are specific for HVS-flip.
The completely sequenced prototype strain A11 of HVS does not transform human T cells to stable growth. Therefore, we cloned and sequenced the gene coding for HVS-flip of strain C488 that transforms human T cells and found that the amino acid sequence identity of the FLIPs of strains A11 and C488 was 94%. Likewise, the sequence identity of the HVS-Bcl-2 of strains A11 and C488 is 92.5% (30). This suggests that strain-specific sequence differences of the two HVS antiapoptotic genes do not contribute to the different transforming capacities.
Cellular proteins regulating cell death.
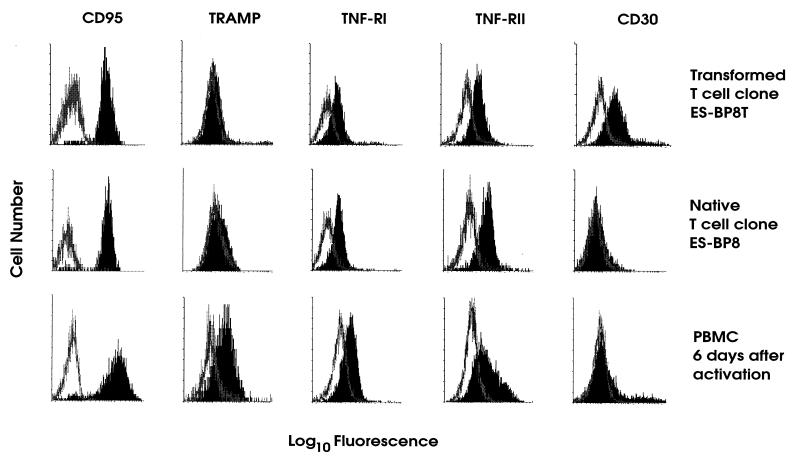
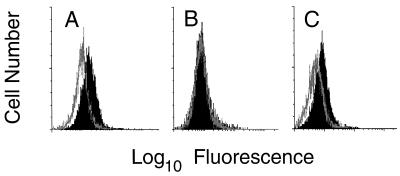
Molecules of the TNF-R superfamily containing a death domain were expressed at different levels. CD95 and TNF-RI continued to be expressed at high levels after transformation, while TRAMP was hardly detectable on the native and transformed T-cell clones but was expressed on freshly activated peripheral blood mononuclear cells (Fig. 2). TNF-RII was expressed to a similar extent before and after transformation. Remarkably, CD30 expression, which was absent on the parental clones, was clearly induced after transformation with HVS (Fig. 2). We followed this up and found that both HVS-transformed and native T cells expressed CD30 ligand as detected by reverse transcriptase PCR with published primer sequences (20) (data not shown). HVS-transformed T cells constitutively expressed CD95 ligand on the cell surface (Fig. 3). In contrast, CD95 ligand was not constitutively expressed by uninfected parental T cells, but additional activation was required to detect surface expression (Fig. 3). Constitutive expression of CD95 ligand by HVS-transformed T cells was confirmed by reverse transcriptase PCR (data not shown).
FIG. 2.
Expression of members of the TNF-R superfamily. The transformed T-cell clone ES-BP8T (top), the native T-cell clone ES-BP8 (middle), and peripheral blood mononuclear cells that had been activated 6 days before with concanavalin A (bottom) were stained for the expression of TNF-RI, CD95, TRAMP, TNF-RII, and CD30. The open graph represents the negative control, and the solid graph represents the specific staining.
FIG. 3.
Constitutive expression of CD95 ligand by HVS-transformed T cells. (A) HVS-transformed T cells were analyzed for expression of CD95 ligand. (B and C) Their nontransformed parental T cells were analyzed 2 weeks after the last restimulation (B) or 1 day after activation with PMA plus ionomycin (C). The open graph represents the negative control, and the solid graph represents the specific staining of CD95 ligand.
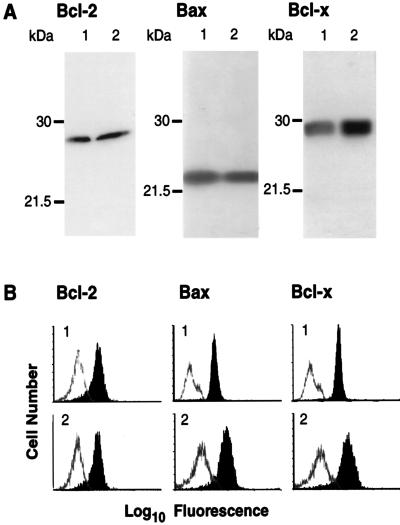
Bcl-2 family members were detected both by flow cytometry and by immunoblotting. The expression of Bcl-2, Bcl-X, and Bax was altogether unaltered by the transformation as shown with either readout system (Fig. 4). The Western blot analysis showed that both the transformed and parental clones exclusively expressed the Bcl-XL and not the Bcl-XS isoform of Bcl-X (Fig. 4). The costimulatory molecule CD28, which can mediate the prevention of apoptosis via induction of Bcl-XL (7), was not expressed in the parental or the transformed T cells (data not shown).
FIG. 4.
Expression of Bcl-2, Bcl-X, and Bax. The uninfected parental T-cell clone ES-BP8 (lanes 1) and its HVS-transformed derivative ES-BP8T (lanes 2) were analysed by Western blotting (A) and flow cytometry (B). (A) Aliquots containing 20 μg of total protein were used to detect Bcl-2 or Bcl-X, and 5 μg of total protein was loaded to detect Bax. The positions of prestained molecular mass markers are indicated in kilodaltons. (B) Staining of the native clone ES-BP8 is labeled 1, and staining of the transformed clone is labeled with 2. The open graph represents the negative control, and the solid graph represents the specific staining.
Sensitivity to apoptosis.
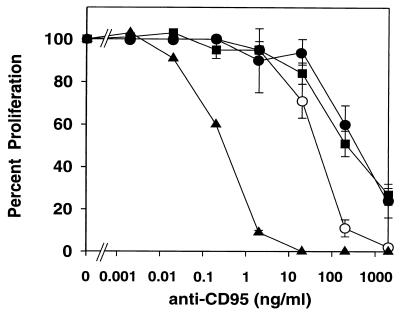
Ligation of CD95 induced apoptosis in HVS-transformed T cells, as seen by DNA fragmentation (Fig. 5). To quantify the effects of CD95 ligation, both specific cell death and proliferation were measured, and almost identical dose-response curves were obtained. HVS-transformed cells showed a sensitivity to CD95 ligation similar to that of the parental clones 1 day after activation (Fig. 6). The native T cells that had been activated 6 days before the CD95 ligation were slightly more sensitive. Jurkat cells, however, were far more sensitive to this kind of cell death induction (Fig. 6). TNF-α did not induce cell death at concentrations up to 300 ng/ml in either HVS-transformed or native T-cell clones, although both TNF-RI and TNF-RII were expressed. To analyze the sensitivity to TRAIL-mediated apoptosis, the different cell types were treated with recombinant Flag-TRAIL that was cross-linked by an anti-Flag MAb. Such a treatment with recombinant TRAIL did not induce cell death in HVS-transformed or native T-cell clones up to a concentration of 1 μg of Flag-TRAIL per ml. By contrast, a specific cell death of about 50% was induced by 0.1 μg of Flag-TRAIL per ml in Jurkat cells.
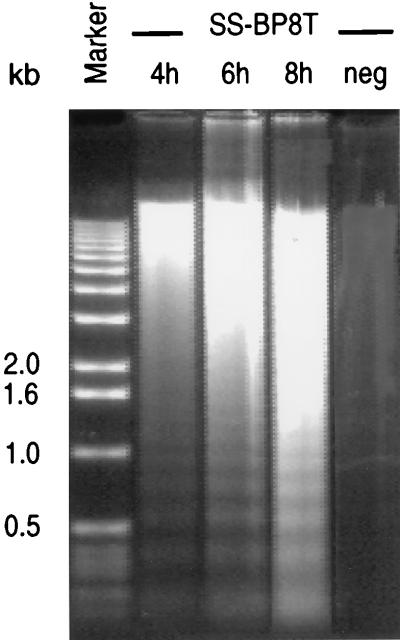
FIG. 5.
Induction of apoptosis in HVS-transformed T cells by CD95 ligation. The HVS-transformed T-cell clone SS-BP8T was treated with 2 μg of MAb CH-11 per ml, and 107 T cells were collected at each indicated time after the addition of CH-11. The method used to obtain fragmented DNA includes a mild detergent lysis that leads to an enrichment of apoptotic DNA. The DNA in the right lane was obtained from untreated SS-BP8T (neg). The DNA fragmentation after treatment with MAbs to CD95 was visualized on an agarose gel. The DNA size marker is on the left; sizes are given in kilobases.
FIG. 6.
Quantification of the effects of CD95 ligation. HVS-transformed T-cell clones ES-BP8T and SS-BP8T, their native parental T-cell clones, and Jurkat cells were compared. The native T-cell clones have been activated with irradiated HLA-compatible PBMC and myelin basic protein. One portion of the native T-cell clones received MAb CH-11 to CD95 1 day after restimulation, and another portion of the native T cells was treated with this MAb 6 days after activation. Spontaneous autocrine proliferation was assessed for the HVS-transformed T cells and the Jurkat cells. About 24 h after addition of the MAbs, the cultures were labeled with [3H]thymidine. The results obtained with the two native T-cell clones treated 1 day after activation (solid circles) or 6 days after activation (open circles), the two HVS-transformed T-cell clones (squares), and Jurkat cells (triangles) were combined. The proliferation in the absence of anti-CD95 was set as 100%, and the inhibition (± standard error of the mean [SEM]) was calculated. Three to six independent experiments were performed per cell line.
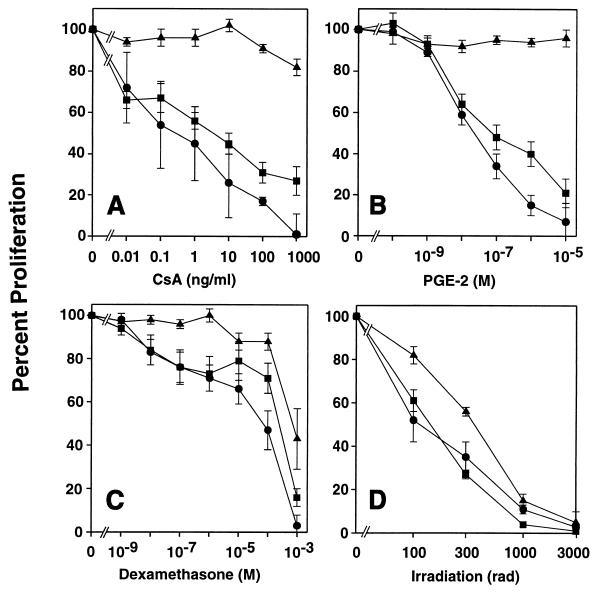
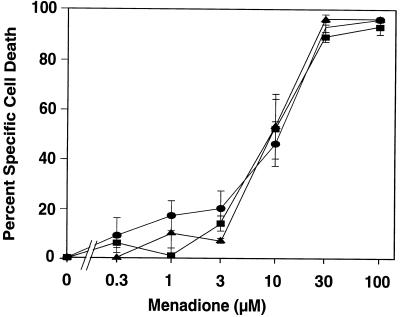
HVS-transformed T cells, native T-cell clones, and Jurkat cells showed a similar sensitivity to irradiation (Fig. 7) or oxygen radicals (Fig. 8). We noted that about one-third of the HVS-transformed T cells were dead in the absence of any cell death-inducing treatment. This level of spontaneous cell death was seen constantly throughout our study and distinguishes HVS-transformed T cells from other transformed lymphocytic cell lines (data not shown).
FIG. 7.
Sensitivity to CsA (A), PGE2 (B), dexamethasone (C), and irradiation (D). HVS-transformed T-cell clones ES-BP8T and SS-BP8T, their native parental clones, and Jurkat cells were compared. To induce proliferation, native T-cell clones were activated with irradiated HLA-compatible peripheral blood mononuclear cells and myelin basic protein. Spontaneous autocrine proliferation was assessed for HVS-transformed T cells and Jurkat cells. T cells were irradiated shortly before seeding, and immunosuppressive reagents were added at the beginning of the culture. Two days later, [3H]thymidine was added, the cells were harvested and another 16 h later. The results obtained with the two native clones (circles), the two transformed T-cell clones (squares), and the Jurkat cells (triangles) were combined. Proliferation in the absence of inhibitor was set as 100%, and the inhibition (± SEM) of three to four independent experiments was calculated.
FIG. 8.
Sensitivity to menadione-induced cell death. The HVS-transformed T-cell clones ES-BP8T and SS-BP8T, their native parental clones, and Jurkat cells were cultured in the presence of different concentrations of menadione, and after 48 h PI uptake was measured and the specific cell death was determined. The native T-cell clones were treated 6 or 20 days after their last restimulation. Since no difference in the sensitivity to menadione was detected at these time points after restimulation, the results of the 10 experiments with the native T-cell clones were combined (triangles). The results of four experiments per cell line with the two transformed T-cell clones (squares) and the Jurkat cells (circles) were also combined, and the mean values (± SEM) are shown.
Regulation of proliferation and cell cycle progression.
Irradiation of HVS-transformed T cells induced both cell death and an arrest of the cell cycle at G1 (Table 1). We followed this up and studied the effects of CsA, PGE2, and dexamethasone in addition to irradiation. The spontaneous proliferation of HVS-transformed T-cell clones and the activation-induced proliferation of native T-cell clones were inhibited to similar extents (Fig. 7). By contrast, the autonomous proliferation of Jurkat cells was not reduced by PGE2 or CsA. Cell cycle analysis showed that PGE2, CsA, and dexamethasone shifted the cell cycle distribution toward G1. Remarkably, PGE2 stopped the proliferation almost completely without inducing significant cell death, indicating that proliferation and cell death are regulated independently. The sensitivity of the HVS-transformed T cells to dexamethasone was similar to the sensitivity of the native T-cell clones (Fig. 7) but different from the reactivity of murine T-cell hybridomas. Murine T-cell hybridoma cells all died in response to 10−7 M of dexamethasone (data not shown), whereas at the high concentration of 10−3 M dexamethasone, only 25% of the HVS-transformed T cells died within 48 h (Table 1).
TABLE 1.
Effects of CsA, PGE2 dexamethasone, irradiation, and CD95 ligation on HVS-transformed T cell clones
| Treatment | Inhibition of proliferation (%)a | Specific cell death (%)b | Cell cycle distribution (%)c
|
||
|---|---|---|---|---|---|
| G1 | S | G2 | |||
| None (Control) | 0 | 76 ± 2 | 16 ± 2 | 8 ± 1 | |
| Irradiation (3,000 rads) | 99 ± 0 | 34 ± 4 | 91 ± 2 | 6 ± 2 | 3 ± 1 |
| CsA (1 μg/ml) | 73 ± 7 | 11 ± 3 | 85 ± 1 | 10 ± 1 | 5 ± 1 |
| Dexamethasone (10−3 M) | 84 ± 4 | 33 ± 7 | 84 ± 0 | 12 ± 0 | 4 ± 0 |
| PGE2 (10−4 M) | 100 ± 0 | 4 ± 3 | 87 ± 2 | 8 ± 3 | 5 ± 1 |
| Anti-CD95 (2 μg/ml) | 70 ± 5 | 74 ± 5 | NDd | ND | ND |
To measure proliferation, [3H]thymidine was added 24 h (anti-CD95) or 48 h (all other treatments) after initiation of the treatments for another 16 h. Values are means and standard errors of the mean (SEM) for 6 to 12 experiments.
The specific cell death was determined as described in Materials and Methods 24 h (anti-CD95) or 48 h (all other treatments) after initiation of the treatments. Values are means and SEM for 6 to 12 experiments.
The cell cycle distribution of the viable cells was quantified 48 h after treatment by measuring the PI uptake by the fixed and permeabilized cells as described in Materials and Methods. Values are means and SEM for three experiments.
ND, not determined.
DISCUSSION
T-cell regulation after transformation with HVS.
The comparative analysis of HVS-transformed T-cell clones and their native parental T-cell clones revealed two major points. First, the transformation with HVS was not associated with an altered sensitivity to apoptosis, as elaborated in three different approaches. Second, inhibitors of T-cell activation blocked the spontaneous proliferation of the transformed T cells and the T-cell receptor-mediated proliferation of native T cells to a similar extent.
Key mediators in the regulation of cell death are members of the TNF-R superfamily, in particular, those that contain a death domain, namely, TNF-RI, CD95, TRAMP, and receptors for TRAIL. Our analysis revealed differences in the expression and in the functional consequences of ligation of these four death receptors in HVS-transformed T cells. TNF-RI continued to be expressed after transformation, but both the transformed and the native T-cell clones were resistant to TNF-α-mediated cell death. These cell types were also resistant to TRAIL-mediated apoptosis but sensitive to CD95-mediated cell death. HVS-transformed T cells and native T-cell clones were equally reactive to CD95-mediated apoptosis 1 day after restimulation, whereas native T-cell clones were more sensitive 6 days after activation. This is consistent with the phenotype of HVS-transformed T cells and the observation that activation of native T cells reduces their sensitivity to CD95-mediated cell death (43). The recently identified death receptor TRAMP was hardly expressed by either native or transformed T-cell clones but was found on activated peripheral blood mononuclear cells. This suggests that both native and transformed T cells downregulate TRAMP during long-term culture. Our studies of these four death receptors led to the same conclusion: HVS does not modulate their expression. Importantly, the sensitivity to cell death induction by these death receptors was not altered through transformation. This might be surprising at first, since the HVS-encoded FLIP prevents apoptosis induced by death receptors (50). This study, however, shows that the expression of HVS-flip as well as HVS-bcl-2 is restricted to cultures with viral replication. Transcripts of these genes were not detected in transformed human T cells that lack viral replication.
Further analysis of the TNF-R superfamily revealed that TNF-RII continued to be expressed at the same level whereas CD30 was induced by HVS in transformed T cells. CD30 was originally described as a marker for Hodgkin’s lymphoma, but it is also found on some non-Hodgkin’s lymphoma cell lines and on a subpopulation of activated native T cells (20). Interaction of CD30 with its ligand may result in proliferation, differentiation, or cell death. Direct comparison of HVS-transformed T-cell clones with their native progenitors revealed that CD30 was induced after transformation by HVS. CD30 has been found to interact with TRAF-1 and TRAF-2 (31), signal-transducing molecules that also interact with LMP1, the EBV-encoded oncoprotein (16). Since HVS-transformed T cells expressed both CD30 and CD30 ligand, a mutual activation of HVS-transformed T cells via this receptor-ligand pair seems possible. Further studies are required to evaluate a potential role of CD30-mediated signaling in the stable growth of HVS-transformed T cells.
HVS-transformed T cells, but not their uninfected parental clones, constitutively expressed CD95 ligand on their surface. CD95 ligand-expressing tumor cells can kill attacking cytotoxic T cells (22). In vitro, the simultaneous expression of CD95 ligand and of its receptor CD95 by HVS-transformed T cells might contribute to the spontaneous cell death we observed. In vivo, the expression of CD95 ligand by HVS-transformed T cells may protect these transformed T cells from attack by HVS-specific cytotoxic T cells, which could facilitate spread of the virus and development of lymphoma. The amount of CD95 ligand expression on HVS-transformed T cells that we detected was similar to that found on malignant cells of patients with large granular lymphocyte leukemia (49).
Native and HVS-transformed T cells showed a similar sensitivity to apoptosis mediated by oxygen radicals and to the effects of irradiation and dexamethasone. Dexamethasone induced a shift of the cell cycle distribution toward G1 and a minor degree of cell death only at very high concentrations. These experiments demonstrated that the reactivity of HVS-transformed T cells to glucocorticoids resembles the reactivity of mature T cells but is different from the reactivity of immature thymocytes or murine T-cell hybridomas, which are highly sensitive to glucocorticoid-induced apoptosis.
To further evaluate a potential modification of the T-cell regulation after transformation with HVS, the effects of CsA and PGE2 on proliferation, cell cycle distribution, and development of cell death were analyzed. Both substances reduced the spontaneous proliferation of HVS-transformed T-cell clones by inducing a cell cycle block at the G1 phase without causing significant cell death. This indicates that proliferation and cell death are regulated independently in HVS-transformed T cells and suggests that the corresponding regulatory pathways remain functional. The T-cell leukemia cell line Jurkat showed a different reactivity; the autocrine growth of Jurkat cells was not inhibited by CsA or PGE2. Importantly, the spontaneous growth of HVS-transformed T cells and the antigen-driven proliferation of native T-cell clones showed a similar sensitivity to reagents that interfere with T-cell activation. This supports the hypothesis that HVS transforms human T cells by using normal cellular activation pathways. This concept is also supported by the observation that HVS-transformed T cells activate each other via CD2-CD58 interactions and continue to express a functionally intact T-cell receptor (11, 52). The interactions of the viral proteins saimiri transformation-associated protein of C strains (Stp-C) and tyrosine kinase-interacting protein (Tip) with the signal transducing molecules ras (28) and p56lck (5, 28) also support the concept that HVS uses cellular activation pathways for transformation. Our conclusion that the transformation by HVS does not induce resistance to apoptosis is also supported by the observed spontaneous cell death of HVS-transformed T cells and the finding that HVS-transformed T cells readily undergo cell death upon ligation of their T-cell receptor (10).
Comparison of human lymphocytes transformed by HVS, EBV, and HTLV-1.
HVS, EBV, and human T-cell leukemia virus type 1 (HTLV-1) have different effects on the regulation of proliferation and apoptosis. HTLV-1 infected T cells become resistant to CsA-mediated inhibition (26). By contrast, the spontaneous growth of HVS-transformed T cell clones is as sensitive to CsA as the antigen-induced proliferation of the native parental T-cell clones. Protection from apoptosis by Tax, the transforming gene of HTLV-1, has been suggested to favor the survival of HTLV-1-infected cells (14). Tax reduces the expression of Bax and might thereby suppress apoptosis in HTLV-1-infected cells (9). The present study demonstrates that human HVS-transformed T cells continue to express high levels of the pro-apoptotic protein Bax.
When looking at the effects of EBV on cellular regulation, both differences from and similarities to HVS become obvious. The EBV-encoded proteins EBNA-2 and LMP1 induce the expression of Bcl-2 after transfection into EBV-negative Burkitt’s lymphoma cells (19, 23). Fractionated B cells from peripheral blood constitutively express Bcl-2. EBV infection does not upregulate Bcl-2, neither in these cells nor in their immortalized progeny (35). Induction of EBV latent genes in Burkitt’s lymphoma cells renders these B cells resistant to some stimuli that induce apoptosis (21). Our data argue that transformation with HVS does not induce resistance to apoptosis and does not modulate the expression level of Bcl-2 or Bcl-XL. Both HVS and EBV code for a bcl-2 homolog, whereas only HVS contains a flip gene. The EBV-encoded Bcl-2 homolog is expressed predominantly during lytic infection, but expression in latently infected B lymphocytes has also been reported (2). Deletion of this gene does not affect the ability of EBV to transform B cells (33). Expression of HVS-bcl-2 is restricted to cultures with lytic replication. Transcripts of HVS-bcl-2 could not be detected in growth-transformed human T cells. A reduced glucocorticoid-mediated signal transduction was described in EBV-positive Burkitt’s lymphomas (48). The present study indicates that HVS does not alter the sensitivity to the glucocorticoid dexamethasone. Both EBV-transformed B cells and HVS-transformed T cells are very sensitive to DNA damage. Upon DNA damage by cisplatin, EBV-transformed B cells die by apoptosis whereas normal activated B cells undergo growth arrest (1). Upon irradiation, HVS-transformed T cells arrest in the G1 phase of the cell cycle and some of them die.
In summary, we used three experimental approaches to study effects of HVS on apoptosis in T-cell transformation. First, analysis of virus encoded antiapoptotic genes showed that expression of HVS-bcl-2 and HVS-flip was confined to cultures with viral replication and was not associated with T-cell transformation. Second, analysis of cellular regulators of apoptosis demonstrated that HVS neither upregulated cellular antiapoptotic effectors nor downregulated proapoptotic molecules. Constitutive surface expression of CD95 ligand by HVS-transformed T cells may contribute to immune evasion in primate species that are susceptible to HVS-induced lymphomas. Third, HVS-transformed T-cell clones were compared with their uninfected parental T-cell clones. Both cell types were equally sensitive to apoptosis mediated by death receptors or by disturbances of intracellular homoestasis. The spontaneous growth of HVS-transformed T cells and the T-cell receptor-mediated proliferation of native T-cell clones showed a similar sensitivity to inhibitors of T-cell activation, while the T-cell leukemia cell line Jurkat showed a different response. These findings indicate that HVS transforms human T cells to stable growth without inducing resistance to apoptosis but, rather, by using normal cellular activation pathways.
ACKNOWLEDGMENTS
We thank S. Wittmann for technical assistance and P. Rohwer for help with the cell cycle analysis. We are grateful to B. Biesinger and B. Bröker for valuable discussions.
This work was supported by the Deutsche Forschungsgemeinschaft (SFB 466), the Bayerische Forschungsstiftung, the Bundesministerium für Bildung und Forschung, and the EU-Biomed 2 Program (Immunoregulatory aspects of T cell autoimmunity in multiple sclerosis).
REFERENCES
- 1.Allday M J, Sinclair A, Parker G, Crawford D H, Farrell P J. Epstein-Barr virus efficiently immortalizes human B cells without neutralizing the function of p53. EMBO J. 1995;14:1382–1391. doi: 10.1002/j.1460-2075.1995.tb07124.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Austin P J, Flemington E, Yandava C N, Strominger J L, Speck S H. Complex transcription of the Epstein-Barr virus BamHI fragment H rightward open reading frame 1 (BHRF1) in latently and lytically infected B lymphocytes. Proc Natl Acad Sci USA. 1988;85:3678–3682. doi: 10.1073/pnas.85.11.3678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bertin J, Armstrong R C, Ottilie S, Martin D A, Wang Y, Banks S, Wang G H, Senkevich T G, Alnemri E S, Moss B, Lenardo M J, Tomaselli K J, Cohen J I. Death effector domain-containing herpesvirus and poxvirus proteins inhibit both Fas- and TNFR1-induced apoptosis. Proc Natl Acad Sci USA. 1997;94:1172–1176. doi: 10.1073/pnas.94.4.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biesinger B, Müller Fleckenstein I, Simmer B, Lang G, Wittmann S, Platzer E, Desrosiers R C, Fleckenstein B. Stable growth transformation of human T lymphocytes by herpesvirus saimiri. Proc Natl Acad Sci USA. 1992;89:3116–3119. doi: 10.1073/pnas.89.7.3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biesinger B, Tsygankov A Y, Fickenscher H, Emmrich F, Fleckenstein B, Bolen J B, Bröker B M. The product of the herpesvirus saimiri open reading frame 1 (tip) interacts with T cell-specific kinase p56lck in transformed cells. J Biol Chem. 1995;270:4729–4734. doi: 10.1074/jbc.270.9.4729. [DOI] [PubMed] [Google Scholar]
- 6.Bodmer J L, Burns K, Schneider P, Hofmann K, Steiner V, Thome M, Bornand T, Hahne M, Schroter M, Becker K, Wilson A, French L E, Browning J L, MacDonald H R, Tschopp J. TRAMP, a novel apoptosis-mediating receptor with sequence homology to tumor necrosis factor receptor 1 and Fas(Apo-1/CD95) Immunity. 1997;6:79–88. doi: 10.1016/s1074-7613(00)80244-7. [DOI] [PubMed] [Google Scholar]
- 7.Boise L H, Minn A J, Noel P J, June C H, Accavitti M A, Lindsten T, Thompson C B. CD28 costimulation can promote T cell survival by enhancing the expression of Bcl-XL. Immunity. 1995;3:87–98. doi: 10.1016/1074-7613(95)90161-2. [DOI] [PubMed] [Google Scholar]
- 8.Boumpas D T, Anastassiou E D, Older S A, Tsokos G C, Nelson D L, Balow J E. Dexamethasone inhibits human interleukin 2 but not interleukin 2 receptor gene expression in vitro at the level of nuclear transcription. J Clin Invest. 1991;87:1739–1747. doi: 10.1172/JCI115192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brauweiler A, Garrus J E, Reed J C, Nyborg J K. Repression of Bax gene expression by the HTLV-I Tax protein: implications for suppression of apoptosis in virally infected cells. Virology. 1997;231:135–140. doi: 10.1006/viro.1997.8509. [DOI] [PubMed] [Google Scholar]
- 10.Bröker B M, Kraft M S, Klauenberg U, Le Deist F, de Villartay J-P, Fleckenstein B, Fleischer B, Meinl E. Activation induces apoptosis in herpesvirus saimiri-transformed T cells independent of CD95 (Fas, APO-1) Eur J Immunol. 1997;27:2774–2780. doi: 10.1002/eji.1830271105. [DOI] [PubMed] [Google Scholar]
- 11.Bröker B M, Tsygankov A Y, Müller Fleckenstein I, Guse A H, Chitaev N A, Biesinger B, Fleckenstein B, Emmrich F. Immortalization of human T cell clones by herpesvirus saimiri. Signal transduction analysis reveals functional CD3, CD4, and IL-2 receptors. J Immunol. 1993;151:1184–1192. [PubMed] [Google Scholar]
- 12.Chinnaiyan A M, Tepper C G, Seldin M F, O’Rourke K, Kischkel F C, Hellbardt S, Krammer P H, Peter M E, Dixit V M. FADD/MORT1 is a common mediator of CD95 (Fas/APO-1) and tumor necrosis factor receptor-induced apoptosis. J Biol Chem. 1996;271:4961–4965. doi: 10.1074/jbc.271.9.4961. [DOI] [PubMed] [Google Scholar]
- 13.Cohen J J, Duke R C, Fadok V A, Sellins K S. Apoptosis and programmed cell death in immunity. Annu Rev Immunol. 1992;10:267–293. doi: 10.1146/annurev.iy.10.040192.001411. [DOI] [PubMed] [Google Scholar]
- 14.Copeland K F, Haaksma A G, Goudsmit J, Krammer P H, Heeney J L. Inhibition of apoptosis in T cells expressing human T cell leukemia virus type I Tax. AIDS Res Hum Retroviruses. 1994;10:1259–1268. doi: 10.1089/aid.1994.10.1259. [DOI] [PubMed] [Google Scholar]
- 15.Cory S. Regulation of lymphocyte survival by the bcl-2 gene family. Annu Rev Immunol. 1995;13:513–543. doi: 10.1146/annurev.iy.13.040195.002501. [DOI] [PubMed] [Google Scholar]
- 16.Devergne O, Hatzivassiliou E, Izumi K M, Kaye K M, Kleijnen M F, Kieff E, Mosialos G. Association of TRAF1, TRAF2, and TRAF3 with an Epstein-Barr virus LMP1 domain important for B-lymphocyte transformation: role in NF-κB activation. Mol Cell Biol. 1996;16:7098–7108. doi: 10.1128/mcb.16.12.7098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fickenscher H, Biesinger B, Knappe A, Wittmann S, Fleckenstein B. Regulation of the herpesvirus saimiri oncogene stpC, similar to that of T-cell activation genes, in growth-transformed human T lymphocytes. J Virol. 1996;70:6012–6019. doi: 10.1128/jvi.70.9.6012-6019.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fickenscher H, Bökel C, Knappe A, Biesinger B, Meinl E, Fleischer B, Fleckenstein B, Bröker B M. Functional phenotype of transformed human alphabeta and gammadelta T cells determined by different subgroup C strains of herpesvirus saimiri. J Virol. 1997;71:2252–2263. doi: 10.1128/jvi.71.3.2252-2263.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Finke J, Fritzen R, Ternes P, Trivedi P, Bross K J, Lange W, Mertelsmann R, Dolken G. Expression of bcl-2 in Burkitt’s lymphoma cell lines: induction by latent Epstein-Barr virus genes. Blood. 1992;80:459–469. [PubMed] [Google Scholar]
- 20.Gattei V, Degan M, Gloghini A, De Iuliis A, Improta S, Rossi F M, Aldinucci D, Perin V, Serraino D, Babare R, Zagonel V, Gruss H J, Carbone A, Pinto A. CD30 ligand is frequently expressed in human hematopoietic malignancies of myeloid and lymphoid origin. Blood. 1997;89:2048–2059. [PubMed] [Google Scholar]
- 21.Gregory C D, Dive C, Henderson S, Smith C A, Williams G T, Gordon J, Rickinson A B. Activation of Epstein-Barr virus latent genes protects human B cells from death by apoptosis. Nature. 1991;349:612–614. doi: 10.1038/349612a0. [DOI] [PubMed] [Google Scholar]
- 22.Hahne M, Rimoldi D, Schroter M, Romero P, Schreier M, French L E, Schneider P, Bornand T, Fontana A, Lienard D, Cerottini J, Tschopp J. Melanoma cell expression of Fas(Apo-1/CD95) ligand: implications for tumor immune escape. Science. 1996;274:1363–1366. doi: 10.1126/science.274.5291.1363. [DOI] [PubMed] [Google Scholar]
- 23.Henderson S, Rowe M, Gregory C, Croom Carter D, Wang F, Longnecker R, Kieff E, Rickinson A. Induction of bcl-2 expression by Epstein-Barr virus latent membrane protein 1 protects infected B cells from programmed cell death. Cell. 1991;65:1107–1115. doi: 10.1016/0092-8674(91)90007-l. [DOI] [PubMed] [Google Scholar]
- 24.Herrmann M, Lorenz H M, Voll R, Grunke M, Woith W, Kalden J R. A rapid and simple method for the isolation of apoptotic DNA fragments. Nucleic Acids Res. 1994;22:5506–5507. doi: 10.1093/nar/22.24.5506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hockenbery D M, Oltvai Z N, Yin X M, Milliman C L, Korsmeyer S J. Bcl-2 functions in an antioxidant pathway to prevent apoptosis. Cell. 1993;75:241–251. doi: 10.1016/0092-8674(93)80066-n. [DOI] [PubMed] [Google Scholar]
- 26.Hollsberg P, Wucherpfennig K W, Ausubel L J, Calvo V, Bierer B E, Hafler D A. Characterization of HTLV-I in vivo infected T cell clones. IL-2-independent growth of nontransformed T cells. J Immunol. 1992;148:3256–3263. [PubMed] [Google Scholar]
- 27.Hu, S., C. Vincenz, M. Buller, and V. M. Dixit. 1997. A novel family of viral death effector domain-containing molecules that inhibit both CD-95- and tumor necrosis factor-1-induced apoptosis. J. Biol. Chem. 9621–9624. [DOI] [PubMed]
- 28.Jung J U, Lang S M, Friedrich U, Jun T, Roberts T M, Desrosiers R C, Biesinger B. Identification of Lck-binding elements in tip of herpesvirus saimiri. J Biol Chem. 1995;270:20660–20667. doi: 10.1074/jbc.270.35.20660. [DOI] [PubMed] [Google Scholar]
- 29.Kitson J, Raven T, Jiang Y P, Goeddel D V, Giles K M, Pun K T, Grinham C J, Brown R, Farrow S N. A death-domain-containing receptor that mediates apoptosis. Nature. 1996;384:372–375. doi: 10.1038/384372a0. [DOI] [PubMed] [Google Scholar]
- 30.Knappe A, Hiller C, Thurau M, Wittmann S, Hofmann H, Fleckenstein B, Fickenscher H. The superantigen-homologous viral immediate-early gene ie14/vsag in herpesvirus saimiri-transformed human T cells. J Virol. 1997;71:9124–9133. doi: 10.1128/jvi.71.12.9124-9133.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee S Y, Park C G, Choi Y. T cell receptor-dependent cell death of T cell hybridomas mediated by the CD30 cytoplasmic domain in association with tumor necrosis factor receptor-associated factors. J Exp Med. 1996;183:669–674. doi: 10.1084/jem.183.2.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levine B, Huang Q, Isaacs J T, Reed J C, Griffin D E, Hardwick J M. Conversion of lytic to persistent alphavirus infection by the bcl-2 cellular oncogene. Nature. 1993;361:739–742. doi: 10.1038/361739a0. [DOI] [PubMed] [Google Scholar]
- 33.Marchini A, Tomkinson B, Cohen J I, Kieff E. BHRF1, the Epstein-Barr virus gene with homology to Bc12, is dispensable for B-lymphocyte transformation and virus replication. J Virol. 1991;65:5991–6000. doi: 10.1128/jvi.65.11.5991-6000.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marsters S A, Sheridan J P, Donahue C J, Pitti R M, Gray C L, Goddard A D, Bauer K D, Ashkenazi A. Apo-3, a new member of the tumor necrosis factor receptor family, contains a death domain and activates apoptosis and NF-kappa B. Curr Biol. 1996;6:1669–1676. doi: 10.1016/s0960-9822(02)70791-4. [DOI] [PubMed] [Google Scholar]
- 35.Martin J M, Veis D, Korsmeyer S J, Sugden B. Latent membrane protein of Epstein-Barr virus induces cellular phenotypes independently of expression of Bcl-2. J Virol. 1993;67:5269–5278. doi: 10.1128/jvi.67.9.5269-5278.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meinl E, ’t Hart B A, Bontrop R E, Hoch R M, Iglesias A, de Waal Malefyt R, Fickenscher H, Müller Fleckenstein I, Fleckenstein B, Wekerle H, Hohlfeld R, Jonker M. Activation of a myelin basic protein-specific human T cell clone by antigen-presenting cells from rhesus monkeys. Int Immunol. 1995;7:1489–1495. doi: 10.1093/intimm/7.9.1489. [DOI] [PubMed] [Google Scholar]
- 37.Meinl E, Fickenscher H, Hoch R M, de Waal Malefyt R, ’t Hart B A, Wekerle H, Hohlfeld R, Fleckenstein B. Growth transformation of antigen-specific T cell lines from rhesus monkeys by herpesvirus saimiri. Virology. 1997;229:175–182. doi: 10.1006/viro.1996.8427. [DOI] [PubMed] [Google Scholar]
- 38.Meinl E, Hohlfeld R, Wekerle H, Fleckenstein B. Immortalization of human T cells by herpesvirus saimiri. Immunol Today. 1995;16:55–58. doi: 10.1016/0167-5699(95)80087-5. [DOI] [PubMed] [Google Scholar]
- 39.Mittrücker H W, Müller Fleckenstein I, Fleckenstein B, Fleischer B. CD2-mediated autocrine growth of herpes virus saimiri-transformed human T lymphocytes. J Exp Med. 1992;176:909–913. doi: 10.1084/jem.176.3.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nava V E, Cheng E H-Y, Veliuona M, Zou S, Clem R J, Mayer M L, Hardwick J M. Herpesvirus saimiri encodes a functional homolog of the human bcl-2 oncogene. J Virol. 1997;71:4118–4122. doi: 10.1128/jvi.71.5.4118-4122.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pan G, Ni J, Wei Y-F, Yu G-L, Gentz R, Dixit V M. An antagonist decoy receptor and a death domain-containing receptor for TRAIL. Science. 1997;277:815–818. doi: 10.1126/science.277.5327.815. [DOI] [PubMed] [Google Scholar]
- 42.Pan G, O’Rourke K, Chinnaiyan A M, Gentz R, Ebner R, Ni J, Dixit V M. The receptor for the cytotoxic ligand TRAIL. Science. 1997;276:111–113. doi: 10.1126/science.276.5309.111. [DOI] [PubMed] [Google Scholar]
- 43.Peter M E, Kischkel F C, Scheuerpflug C G, Medema J P, Debatin K M, Krammer P H. Resistance of cultured peripheral T cells towards activation-induced cell death involves a lack of recruitment of FLICE (MACH/caspase 8) to the CD95 death-inducing signaling complex. Eur J Immunol. 1997;27:1207–1212. doi: 10.1002/eji.1830270523. [DOI] [PubMed] [Google Scholar]
- 44.Schneider P, Bodmer J L, Thome M, Hofmann K, Holler N, Tschopp J. Characterization of two receptors for TRAIL. FEBS Lett. 1997;416:329–334. doi: 10.1016/s0014-5793(97)01231-3. [DOI] [PubMed] [Google Scholar]
- 45.Screaton G R, Xu X-N, Olsen A L, Cowper A E, Tan R, McMichael A J, Bell J I. LARD: a new lymphoid-specific death domain containing receptor regulated by alternative pre-mRNA splicing. Proc Natl Acad Sci USA. 1997;94:4615–4619. doi: 10.1073/pnas.94.9.4615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sheridan J P, Marsters S A, Pitti R M, Gurney A, Skubatch M, Baldwin D, Ramakrishnan L, Gray C L, Baker K, Wood W I, Goddard A D, Godowski P, Ashkenazi A. Control of TRAIL-induced apoptosis by a family of signaling and decoy receptors. Science. 1997;277:818–821. doi: 10.1126/science.277.5327.818. [DOI] [PubMed] [Google Scholar]
- 47.Sigal N H, Dumont F J. Cyclosporin A, FK-506, and rapamycin: pharmacologic probes of lymphocyte signal transduction. Annu Rev Immunol. 1992;10:519–560. doi: 10.1146/annurev.iy.10.040192.002511. [DOI] [PubMed] [Google Scholar]
- 48.Sinclair A J, Jacquemin M G, Brooks L, Shanahan F, Brimmell M, Rowe M, Farrell P J. Reduced signal transduction through glucocorticoid receptor in Burkitt’s lymphoma cell lines. Virology. 1994;199:339–353. doi: 10.1006/viro.1994.1132. [DOI] [PubMed] [Google Scholar]
- 49.Tanaka M, Suda T, Haze K, Nakamura N, Sato K, Kimura F, Motoyoshi K, Mizuki M, Tagawa S, Ohga S, Hatake K, Drummond A H, Nagata S. Fas ligand in human serum. Nat Med. 1996;2:317–322. doi: 10.1038/nm0396-317. [DOI] [PubMed] [Google Scholar]
- 50.Thome M, Schneider P, Hofmann K, Fickenscher H, Meinl E, Neipel F, Mattmann C, Burns K, Bodmer J-L, Schröter M, Scaffidi C, Krammer P H, Peter M E, Tschopp J. Viral FLICE-inhibitory proteins (FLIPs) prevent apoptosis induced by death receptors. Nature. 1997;386:517–521. doi: 10.1038/386517a0. [DOI] [PubMed] [Google Scholar]
- 51.Vercammen C, Ceuppens J L. Prostaglandin E2 inhibits human T-cell proliferation after crosslinking of the CD3-Ti complex by directly affecting T cells at an early step of the activation process. Cell Immunol. 1987;104:24–36. doi: 10.1016/0008-8749(87)90003-7. [DOI] [PubMed] [Google Scholar]
- 52.Weber F, Meinl E, Drexler K, Czlonkowska A, Huber S, Fickenscher H, Müller Fleckenstein I, Fleckenstein B, Wekerle H, Hohlfeld R. Transformation of human T-cell clones by herpesvirus saimiri: intact antigen recognition by autonomously growing myelin basic protein-specific T cells. Proc Natl Acad Sci USA. 1993;90:11049–11053. doi: 10.1073/pnas.90.23.11049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zheng L, Fisher G, Miller R E, Peschon J, Lynch D H, Lenardo M J. Induction of apoptosis in mature T cells by tumour necrosis factor. Nature. 1995;377:348–351. doi: 10.1038/377348a0. [DOI] [PubMed] [Google Scholar]