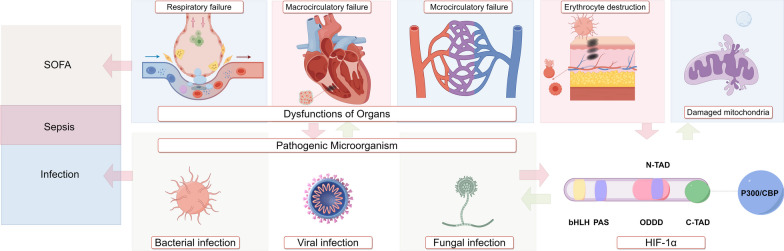
Abstract
Sepsis is characterized by organ dysfunction resulting from a dysregulated inflammatory response triggered by infection, involving multifactorial and intricate molecular mechanisms. Hypoxia-inducible factor-1α (HIF-1α), a notable transcription factor, assumes a pivotal role in the onset and progression of sepsis. This review aims to furnish a comprehensive overview of HIF-1α's mechanism of action in sepsis, scrutinizing its involvement in inflammatory regulation, hypoxia adaptation, immune response, and organ dysfunction. The review encompasses an analysis of the structural features, regulatory activation, and downstream signaling pathways of HIF-1α, alongside its mechanism of action in the pathophysiological processes of sepsis. Furthermore, it will delve into the roles of HIF-1α in modulating the inflammatory response, including its association with inflammatory mediators, immune cell activation, and vasodilation. Additionally, attention will be directed toward the regulatory function of HIF-1α in hypoxic environments and its linkage with intracellular signaling, oxidative stress, and mitochondrial damage. Finally, the potential therapeutic value of HIF-1α as a targeted therapy and its significance in the clinical management of sepsis will be discussed, aiming to serve as a significant reference for an in-depth understanding of sepsis pathogenesis and potential therapeutic targets, as well as to establish a theoretical foundation for clinical applications.
Graphical Abstract
Keywords: Sepsis, Critical care, HIF-1α, Hypoxia, Molecular medicine
Background
Sepsis is a complex and multifaceted condition triggered by infection, leading to a series of pathological, physiological, and molecular alterations in the body [1]. As both basic and clinical research on sepsis advance, the understanding and characterization of this condition undergo continuous refinement and expansion. The evolution of the sepsis definition can be traced from its initial description in Sepsis 1.0, which defined sepsis as systemic inflammatory response syndrome (SIRS) resulting from infection [2]. This progressed in Sepsis 2.0, which integrated clinical symptoms and signs into the assessment of Sepsis 1.0[3]. Subsequently, Sepsis 3.0 redefined sepsis as a life-threatening condition arising from an uncontrolled inflammatory response to infection, coupled with life-threatening organ dysfunction caused by the same [4]. The evolution of the concept of sepsis mirrors an advancement in the understanding of the disease, shifting from the examination of external signs and symptoms to the exploration of molecular-level abnormalities inherent to sepsis.
Hypoxia is a prevalent pathophysiological alteration observed in sepsis. Heightened inflammatory responses increase vascular permeability, resulting in acute pulmonary edema and subsequent development of Acute Respiratory Distress Syndrome (ARDS) [5]. Moreover, organ dysfunction in septic patients arises from ineffective perfusion to organ tissues due to vascular endothelial damage, cellular dysfunction, and activation of the coagulation system, leading to intravascular microthrombosis and subsequent macrocirculatory and microcirculatory failure [6]. Furthermore, inadequate perfusion exacerbates hypoxia and compromises cellular oxygen utilization [7]. Hypoxia acts as the link connecting the pathophysiological changes in sepsis to the molecular alterations of hypoxia-inducible factor-1α (HIF-1α). Specifically, HIF-1α levels are intricately regulated by oxygen, undergoing degradation under normoxic conditions and accumulation in hypoxic environments [8]. HIF-1α is a heterodimeric protein complex that serves as a critical regulator of the cellular response to physiological hypoxia and infection, exerting diverse pathophysiological effects at the cellular, tissue, and organismal levels [9–11]. The aim of this review is to elucidate the role of HIF-1α and its related mechanisms in the initiation, progression, and immune response of sepsis, as well as to evaluate its potential therapeutic implications.
Molecular biology of HIF-1α
Basic concepts of HIF-1α
Hypoxia-inducible factor (HIF) is a heterodimeric protein complex consisting of a constitutively expressed subunit β and an oxygen-dependent subunit α [11]. In mammals, the α-subunit is found in three isoforms: HIF-1α, HIF-2α, and HIF-3α [12]. HIF-1α is typically linked to acute hypoxia and is accountable for activating glycolytic genes, reducing oxygen consumption, and alleviating reactive oxygen species (ROS) production [8].
HIF-1α belongs to the basic Helix-Loop-Helix-Periodicity-Aryl Hydrocarbon Receptor Nuclear Translocator-Single-Minded (bHLH-PAS) family, which includes bHLH and PAS protein structural domains [13]. The bHLH-PAS motifs enable HIF-1α and HIF-1β to form a dimer, facilitating their binding of HIF to hypoxia response elements (HRE) on target genes [12, 13]. Additionally, these motifs aid in promoting the binding of HIF to HREs on target genes and consist of two transactivation domains (TAD): the NH2-terminal domains (N-TAD) and the COOH-terminal domains (C-TAD) [13]. The N-TAD stabilizes HIF-1α and prevents degradation [13]. Cyclic adenosine monophosphate-response binding protein binding protein (CBP) and p300 are two closely related histone acetyltransferase (HAT) enzymes capable of binding to the C-TAD, acting as binding proteins to regulate HIF-1α transcription under hypoxic conditions [14].
HIF-2α primarily functions in chronic hypoxia [15]. The stability of HIF-2α mRNA levels exceeds that of HIF-1α mRNA [16]. HIF-2α enhances erythropoietin (EPO) synthesis, manages iron metabolism, regulates fatty acid synthesis and uptake, and significantly influences chronic inflammation, fibrosis, and tumorigenesis [8]. Although less explored than HIF-1α and HIF-2α, HIF-3α also holds significance in the hypoxia response. HIF-3α possesses a transcriptional regulatory function and competes with HIF-1α and HIF-2α for binding to the transcriptional elements of target genes during hypoxia, thus exerting a negative regulatory influence on the expression of genes associated with the HIF pathway [17].
Oxygen-dependent regulatory pathway of HIF-1α
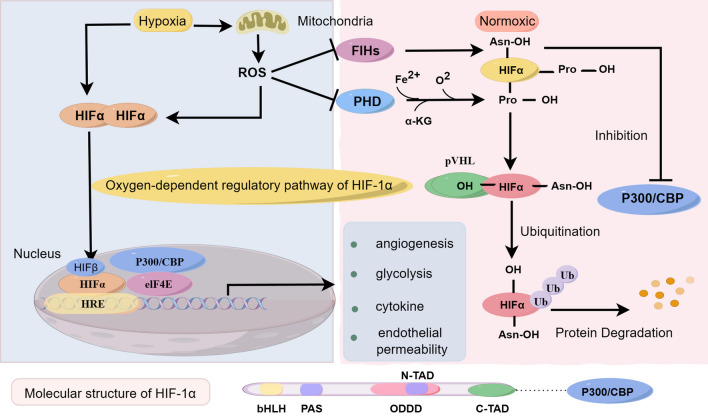
Although HIF-1α is widely expressed in cells, it undergoes rapid degradation in vivo under normoxia (21% oxygen) [13, 18, 19]. On HIF-1α, three hydroxylation sites exist: an oxygen-dependent degradation domain (ODDD) that overlaps with the N-TAD and encompasses two proline residues, as well as one asparaginyl residue in the C-TAD [12]. In a cellular environment rich in oxygen, HIF-1α undergoes oxygen-dependent prolyl-4-hydroxylase (PHD)-mediated hydroxylation of proline residues [13]. PHD, also known as prolyl hydroxylase, consists of three isoforms: PHD1, PHD2, and PHD3, serving as crucial enzymes in the cell by identifying and hydroxylating proline residues in proteins for modification. This hydroxylation modification of proline enables its interaction with von Hippel–Lindau protein (pVHL), an E3 ubiquitin ligase capable of selectively degrading HIF-1α [12].
Furthermore, under oxygen-sufficient conditions, HIF-α is suppressed by factor-inhibiting HIF-1 (FIH) through FIH-mediated hydroxylation modification of asparaginyl residues on HIF-α, preventing it from binding to the co-activating protein p300/CBP [12, 20]. Hypoxia can inhibit both hydroxylation modes of HIF-1α, leading to HIF-1α accumulation. Generation of ROS under hypoxic conditions can inhibit PHD via cysteine (Cys) oxidation and promote stabilization of HIF-1α levels [21]. Accumulated HIF-1α translocates to the nucleus, heterodimerizes with HIF-1β and binds to the HRE in the promoter region of HIF target genes [12, 22]. Figure 1 illustrates the transcriptional regulation of HIF-1α under hypoxia and normoxia, respectively.
Fig. 1.
Oxygen-dependent regulatory pathway of HIF-1α. (1) The oxygen-dependent regulatory pathway of HIF-1α encompasses two mechanisms: Under normal oxygen levels, the stability of HIF-1α is controlled by intracellular prolyl hydroxylase (PHD), which modifies specific proline residues on HIF-1α through hydroxylation when oxygen levels are sufficient. The VHL protein (Von Hippel–Lindau protein) is also involved in oxygen-dependent regulation, leading to the ubiquitination and subsequent degradation of HIF-1α. However, under hypoxic conditions or reduced oxygen levels, decreased PHD activity diminishes the degradation of HIF-1α, resulting in increased protein stability and the capacity to enter the nucleus and activate HIF-1α-dependent gene transcription. (2) Factor-inhibiting HIF (FIH) protein is another crucial oxygen-dependent regulatory protein. Under normoxic conditions, FIH restricts the transcriptional activity of HIF-1α by hydroxylating aspartic acid residues on HIF-1α, thereby impeding its binding to the transcriptional cofactor p300/CBP. Conversely, under hypoxic conditions, decreased activity of FIH allows HIF-1α to enhance its transcriptional activity by binding to p300/CBP. EIF4E, HIF-1α, HIF-1β, and the coactivators p300/CBP form a complex to activate downstream target genes as part of the hypoxia response. Under low oxygen conditions, HIF-1α stability is enhanced, enabling it to translocate to the cell nucleus, where it dimerizes with HIF-1β to form the HIF-1 complex. This complex binds to hypoxia response elements in the promoter regions of target genes. Concurrently, p300/CBP coactivators interact with the HIF-1 complex, enhancing its transcriptional activity. EIF4E, a key post-transcriptional regulatory factor, interacts with the HIF-1/p300/CBP complex, modulating the translation of specific mRNAs, and resulting in enhanced protein synthesis. This collective action serves to activate downstream target genes involved in the cellular response to low oxygen levels and related biological processes
Non-oxygen-dependent regulatory pathway of HIF-1α
HIF-1α is involved in several non-oxygen-dependent regulatory pathways, including the nuclear factor-κB (NF-κB) signaling pathway [23]. This pathway controls hypoxia-responsive inflammatory gene expression and can directly trigger HIF-1α transcription [24]. Inhibiting NF-κB using pyrrolidine dithiocarbamate, a selective inhibitor, halts the production of HIF-1 protein. Furthermore, HIF-1α exhibits negative feedback regulation of NF-κB, as seen in the inhibition of NF-κB-dependent genes in a mouse model of periapical lesions [25]. M2-type pyruvate kinase isozyme type M2 (PKM2) relocates to the nucleus and interacts with NF-κB, aiding the transcriptional activation of hypoxia-inducible factors [26].
Moreover, the mitogen-activated protein kinase (MAPK) pathway plays a significant role in regulating HIF-1α. MAPK can phosphorylate HIF-1α, facilitating the binding and degradation of phosphorylated HIF-1α to pVHL, leading to increased HIF-1α accumulation [27]. Additionally, MAPK signaling influences HIF activity by promoting the formation of the HIF-p300/CBP complex and regulating the trans-activating activity of p300/CBP[28].
The phosphatidylinositol 3-kinase/protein kinase B (PI3K/Akt) signaling pathway is activated by hypoxia in a cell type-specific manner [29]. This pathway regulates protein synthesis and drives HIF-1α synthesis through components such as mammalian Target of Rapamycin (mTOR) and signal transducer and activator of transcription 3 (STAT3) [30].
Additionally, the Mousedouble minute 2 (Mdm2) pathway regulates HIF-1α. Under normoxic conditions, HIF-1α binds to p53 and promotes Mdm2-mediated ubiquitination and subsequent proteasomal degradation of HIF-1α [13, 31]. This pathway contributes to elevated levels of HIF-1α protein in hypoxic cells, thereby positively regulating the transcriptional activation of HIF-1 target genes and vascular endothelial growth factor (VEGF) in tumor cells during hypoxia [32]. Upon the accumulation of HIF-1α, it forms a heterodimer with HIF-1β, binding to the HRE in the promoter region of target genes, thereby activating downstream gene transcription.
Hypoxia-induced modulation of HIF-1α in sepsis
Respiratory failure and HIF-1α
ARDS is a complex syndrome characterized by heightened permeability of pulmonary capillary endothelial and alveolar epithelial cells, often leading to severe respiratory failure [33]. Bioinformatics research has pinpointed HIF-1α messenger ribonucleic acid (mRNA) as a potential autophagy-related gene associated with sepsis-associated ARDS [34]. Within endothelial cells, HIF-1α plays a pivotal role in supporting vascular repair and regression of inflammation in ARDS through the Forkhead Box Protein M1 (FOXM1) signaling pathway [35, 36]. Mice exhibiting suppressed HIF-1α demonstrate compromised vascular repair, persistent inflammatory response, and elevated mortality rates [35]. Furthermore, in a mouse model of sepsis-induced lung injury, the PHD inhibitor roxadustat showed promise in alleviating sepsis-induced acute lung injury [37].
Circulatory failure and HIF-1α
In sepsis, patients often encounter both macrocirculatory and microcirculatory failures, resulting in impaired local tissue oxygenation. On the macro-level, HIF-1α plays a pivotal role in enhancing the myocardial tissue's tolerance to ischemic injury [8]. Diabetic mice deficient in HIF-1α exhibit significant cardiac contractile dysfunction, increased cardiac sympathetic innervation, and subsequent myocardial structural remodeling [38]. The expression of HIF-1α in myeloid cells provides protection during ischemia and reperfusion injury in the heart [39]. Moreover, HIF-1α alleviates myocardial inflammatory injury induced by coronary microembolization and enhances cardiac function by inhibiting the activation of the TLR4/MyD88/NF-κΒ signaling pathway [40]. Upregulation of Inducible Nitric Oxide Synthase (iNOS) may have a significant role [41]. HIF-1α can upregulate iNOS levels, thereby attenuating myocardial ischemia–reperfusion injury [8]. Additionally, treating rats with myocardial ischemia using recombinant adeno-associated virus expressing HIF-1α improves cardiac function and enhances cardiac capillary density [42]. While initially, high expression of HIF-1α protects cardiac function, prolonged elevation may negatively impact the heart. Long-term elevation of HIF-1α levels results in lipid accumulation, myocardial fibrosis, remodeling, and ultimately heart failure in mouse cardiomyocytes [43]. HIF-1α also influences the microcirculatory system. HIF-1α signaling increases nitric oxide (NO) production through the modulation of iNOS, whereas HIF-2α inhibits NO production [44]. Since NO acts as a vasodilator and vascular tone modulator, it can lead to a decrease in blood pressure. Additionally, elevated expression levels of both HIF-1α and HIF-2α are linked to increased microthrombosis in the lungs of mice [45].
Cytopathic hypoxia
Studies have shown that pro-inflammatory cytokines can activate the oxygen-linked pathway through ROS-related mechanisms [46, 47]. In a mouse model of endotoxemia, lipopolysaccharide (LPS) exacerbated mechanical ventilation-induced diaphragmatic dysfunction and mitochondrial damage, partially through the HIF-1α signaling pathway [48]. Mitochondrial dysfunction was observed to reduce HIF-1α protein synthesis in HepG2 cells [49]. This finding may elucidate the escalation of mitochondrial dysfunction and reduction in HIF-1α levels during the middle and late stages of sepsis.
Sepsis-induced anemia can result in tissue hypoxia
Sepsis-related anemia often results from factors such as iatrogenic blood loss, reduced plasma iron levels, diminished EPO production, shortened erythrocyte lifespan, and malnutrition [50]. In sepsis-associated anemia, the reduction in hemoglobin volume and oxygen-carrying capacity leads to lowered oxygen levels and inadequate oxygen delivery to tissues and cells. Furthermore, impaired erythrocyte deformability in sepsis patients can exacerbate microcirculatory blood flow disturbances [50]. The presence of anemia can trigger HIF-1α signaling through hypoxic and neuronal nitric oxide synthase (nNOS)-dependent mechanisms [51]. Given that HIF-1α stimulates EPO production, considering HIF-1α signaling as a potential therapeutic target in renal anemia is plausible. Clinical trials have explored the application of various hypoxia-inducible factor stabilizers for treating anemia in chronic kidney disease [52].
Infection-induced modulation of HIF-1α in sepsis
The role of HIF-1α in the context of bacterial sepsis
Elevated HIF-1α levels manifest in bacterial sepsis, with the immune response to diverse bacterial pathogens such as Streptococcus pyogenes, Staphylococcus aureus, and Pseudomonas aeruginosa serves as a stimulant for the augmentation of HIF-1α levels [9, 53]. Various potential mechanisms underlie the elevation of HIF-1α in response to infection. Firstly, tissue inflammation induces local tissue hypoxia, attributed to heightened cellular oxygen consumption resulting from bacterial infection, as well as the migration and proliferation of immune cells at the infection site—a phenomenon known as inflammatory hypoxia [54, 55]. Secondly, distinct bacterial components, such as outer membrane proteins, Baltonsomal Adhesin A, or LPS from Escherichia coli, have been found to contribute to the upregulation of HIF-1α levels [56]. Particularly, LPS from Escherichia coli has been associated with the stability of HIF-1α, increasing its levels in macrophages through the activity of mitogen-activated protein kinase (MAPK) and NF-κB signaling pathways [57–59]. Moreover, cytokines released by immune cells post-infection, including IL (interleukin) -6, -4, -12, -1, and tumor necrosis factor alpha (TNF-α), can contribute to the increased expression of HIF-1α. Table 1 demonstrates the role of HIF-1α in major pathogenic microbial infections. Figure 2 illustrates the process of infection of endothelial cells by different pathogenic microorganisms.
Table 1.
Role of HIF-1α in different pathogenic microbial infections
| Pathogenic microorganism | Model | The role of HIF-1α in infections | References |
|---|---|---|---|
| Bacteria | |||
| Escherichia coli | UTI model | Promotes the production of NO and antimicrobial peptides | [71] |
| Pseudomonas aeruginosa | Keratitis model | Enhances the activation of inflammatory cells, production of antimicrobial peptides, and ability to kill bacteria | [72] |
| Klebsiella pneumoniae | Pneumonia model | HIF-1α is a susceptibility factor for bacterial invasion during pneumonia | [73] |
| Clostridium difficile | Ileal loop model | Protects the intestinal mucosa from C difficile toxins | [74] |
| Staphylococcus aureus | Kidney abscesses model | Participation in abscess formation | [75] |
| Streptococcus pneumoniae | Pneumonia model | No significant impact | [76] |
| Salmonella Typhimurium | Salmonella infection model | No significant impact | [77] |
| Viruses | |||
| BKV | Kidney tissue samples | bind the BKV promoter and enhance BKV replication | [78] |
| RSV | Primary human small alveolar epithelial cells | RSV replication and the glycolytic pathway | [79] |
| DENV | Primary monocytes | Enhance antibody‐dependent DENV infection in monocytic cells | [80] |
| HBV | Liver-derived cell | Increases HBV RNA transcript levels, core protein levels, cytoplasmic localization of core protein, and replication of the HBV | [81] |
| VACV | HEK293T cell | Involved in virus-induced hypoxic responses | [61] |
| SARS-CoV-2 | PBMCs | Virus replication and monocyte cytokine production | [82] |
| Fungi | |||
| Aspergillus fumigatus | A549 cells and mouse airway cells | Upregulation induces pro-inflammatory factors | [70] |
| Candida albicans | CA-colonized mice | Inhibits Candida albicans colonization | [69] |
BKV BK polyomavirus, CA Candida albicans, DENV Dengue virus, HBV hepatitis B virus, NO nitric oxide, PBMCs Peripheral blood mononuclear cells, RSV respiratory syncytial virus, UTI urinary tract infections, VACV Vaccinia Virus
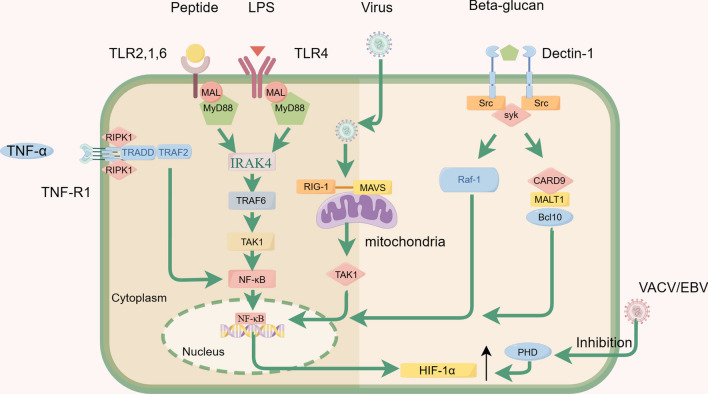
Fig. 2.
The infection of endothelial cells by pathogenic microorganisms triggers the activation of the NF-κB pathway, resulting in increased levels of HIF-1α. The figure mainly illustrates the pathways through which bacteria, viruses, fungi, and cytokines activate the NF-κB pathway: (1) The TLR activation by bacterial infection recruits the adaptor protein MyD88 (myeloid differentiation primary response 88) to propagate downstream signals. MyD88 subsequently activates a series of kinases, leading to the activation and nuclear translocation of nuclear factor-κB (NF-κB), a transcription factor that regulates the expression of several pro-inflammatory genes. (2) RIG-I plays a crucial role in initiating the innate antiviral immune response by serving as a key pattern recognition receptor for host recognition of viruses. RIG-I recognizes the RNA component of viruses and transmits signals by interacting with the downstream signaling molecule MAVS through its own CARD. This process activates the cellular transcription factors IRF-3 and NF-κB, allowing them to enter the nucleus. (3) Recognition of β-1–3-glucan in the fungal cell wall by dectin-1 enables the sensing of fungal pathogens and initiates host immune responses. Dectin-1 triggers the downstream signaling pathways of Syk and Raf1, which subsequently modulate the activation of both classical and non-classical NF-κB signaling pathways. (4) Upon activation by TNF-α, the TNFR1 triggers the formation of a signaling complex that induces a cellular response. In the assembly of Complex I, the activated TNFR1 binds to TRADD (TNFRSF1A-associated via death domain), followed by the interaction with a variety of components, such as receptor-interacting protein kinase 1 (RIPK1). This signaling pathway activates NF-κB and MAPK
The role of HIF-1α in the context of viral sepsis
In the context of viral sepsis, elevated levels of HIF-1α are evident in response to viral infections. Several factors contribute to the increased levels of HIF-1α. Firstly, specific viruses can accumulate HIF-1α during infection through inducing the degradation of prolyl hydroxylase (PHD) [60]. For example, the vaccinia virus (VACV) can inhibit the PHD2-dependent hydroxylation pathway of HIF-1α by binding to PHD2 via the C16 protein post-organismal infection, expediting the rapid accumulation of HIF-1α [61]. A similar mechanism has been observed in the Epstein–Barr virus (EBV), where the Latent Membrane Protein 1 (LMP1) of EBV induces the degradation of PHD1 and PHD3, leading to an upregulation of HIF-1α [62]. Secondly, activated inflammatory pathways during the inflammatory response can induce increased levels of HIF-1α. Pattern recognition receptors (PRRs) activate nonspecific innate immunity by recognizing specific endogenous or exogenous ligands, recruiting inflammatory cell aggregates, activating inflammatory factor pathways, and releasing inflammatory factors [63]. Finally, some respiratory viral infections can directly damage lung tissue, leading to hypoxia.
The role of HIF-1α in the context of fungal sepsis
Hypoxia assumes a pivotal role in shaping the host microenvironment during fungal infections [64]. Research has documented hypoxic conditions in infected tissues in mouse models of both Candida albicans and Aspergillus infections [65, 66]. The emergence of infection foci or biofilms by host cells and fungi at the site of infection leads to the emergence of hypoxia [67]. This local hypoxia is exacerbated by structural damage to the vascular system, resulting in reduced oxygen delivery. The restricted availability of oxygen induces a hypoxic response in the fungi, ultimately contributing to increased levels of HIF-1α [68]. HIF-1α plays a protective role in fungal infections by effectively reducing Candida albicans colonization in the gastrointestinal tract, as demonstrated in research [69]. Furthermore, the upregulation of HIF-1α has been shown to alleviate airway inflammation in a mouse model exposed to Aspergillus fumigatus [70].
Immune-induced modulation of HIF-1α in sepsis
Inflammatory mediator-induced modulation of HIF-1α in sepsis
Immunologically, sepsis is initiated by the simultaneous recognition of various infection-derived microbial products and endogenous danger signals by the complement system and specific cell-surface receptors, closely linked to inflammatory dysregulation [63]. Pathogen-associated molecular patterns (PAMPs) released by invading pathogenic microorganisms trigger the immune response and prompt immune cells to release a spectrum of inflammatory factors, leading to a cytokine storm and activation of the innate immune system [1]. Furthermore, injuries such as sepsis, trauma, and burns result in the release of endogenous pattern recognition receptor agonists, known as damage-associated molecular patterns (DAMPs), which in turn induce an inflammatory response [83]. These interlocking positive feedback loops between PAMPs, DAMPs, and their receptors can serve as the molecular basis for the systemic inflammatory response initiated by infection, as well as damaged tissues or non-specific stressors [41]. Indeed, multiple inflammatory factors could modulate the levels of HIF-1α. This modulation of HIF-1α levels by inflammatory factors plays a significant role in various physiological and pathological processes. Table 2 details the effects of common inflammatory factors on HIF-1α levels.
Table 2.
Major inflammatory factors that modulate HIF-1α
| Inflammatory factors | Model | Molecular mechanism/signaling pathway | Regulation of HIF-1α | References |
|---|---|---|---|---|
| IL-1β | In vitro | NF-κΒ/COX-2 | Up | [90] |
| IL-2 | In vitro | PI3K/AKT | Up | [91] |
| IL-4 | In vitro | PI3K/AKT | Up | [92] |
| IL-6 | In vitro | STAT3 | Up | [93] |
| IL-8 | Mouse fatty liver model | PI3K/AKT | Up | [94] |
| IL-10 | Rat hypoxia model | Not given | None | [95] |
| IL-13 | In vitro | Induce hypoxia signaling pathway genes | Up | [96] |
| IL-15 | In vitro | STAT3 | Up | [97] |
| IL-17 | MTB Mouse model | Not given | Down | [98] |
| IL-18 | Bioinformatics analysis | NF-κB | Up | [99] |
| IL-27 | In vitro | STAT1 | Down | [100] |
| IL-33 | Murine model of HPH | IL-33/ST2/HIF-1α | Up | [101] |
| IL-34 | Clinical research | Not given | Up | [102] |
| IL-37 | PDAC | Inhibition of STAT3 | Down | [103] |
| IL-38 | CIA rats | SIRT1/HIF-1α | Down | [104] |
| TNF-α | In vitro | IKKβ | Up | [105] |
| IFN | In vitro | PI3K/AKT | Up | [106] |
| NO | In vitro | PI3K/AKT | Up | [107] |
| ROS | In vitro | Inactivate PHD | Up | [108] |
CIA collagen-induced arthritis, HPH hypoxia-induced pulmonary hypertension, IKKβ I kappa B kinase beta, MTB Mycobacterium tuberculosis, PDAC pancreatic ductal adenocarcinoma, Ref reference, ROS Reactive Oxygen Species
Immune cell-induced modulation of HIF-1α in sepsis
HIF-1α plays a pivotal role in regulating the innate immune system. The suppression of the HIF gene in myeloid cells reduces ATP production, leading to a notable decrease in inflammatory responses [9]. Consequently, macrophages exhibit reduced invasiveness and motility, coupled with impaired bacterial clearance within macrophages [9]. Additionally, HIF-1α enhances cellular antimicrobial activity by promoting the formation of extracellular traps in mast cells [84]. Moreover, HIF-1α modulates the survival, function, and activity of dendritic cells. Under hypoxic conditions, increased HIF-1α levels in immature dendritic cells promote apoptosis, while in mature dendritic cells, it alleviates hypoxia-induced cell death [85].
A complex interplay exists between HIF-1α and the adaptive immune system, with both activating and inhibitory effects on immune cell regulation influenced by the cellular milieu and specific conditions [86]. Notably, HIF-1α exerts an inhibitory influence on T lymphocytes, as demonstrated by research indicating that enhanced activation of the HIF pathway effectively suppresses T cell proliferation in myeloid/T cell co-cultures [87]. This suppression may contribute to the reduced proliferation of lymphocytes observed with increased HIF-1α levels in early sepsis. Studies have revealed an accumulation of B lymphocytes in mice with specific deficiencies in HIF-1α expression [88]. However, HIF-1α also plays a critical role in immune cell activation. Genetic abnormalities in the HIF-1α gene lead to disruptions in glycolysis and energy metabolism in B cells, resulting in altered cell differentiation and autoimmunity [89].
Regulation of HIF-1α by iron metabolism in sepsis
The hydroxylation of HIF-1α is facilitated by prolyl hydroxylase (PHD) in the presence of oxygen, divalent iron, 2-oxoglutarate (2-OG), and ascorbic acid, targeting the proline residues of HIF-1α [23]. In septic patients, pathogenic microorganisms competitively acquire iron within the host by processes such as elemental metal import, removal of metal by iron carriers from extracellular sites, and acquisition from host proteins [108]. The sequestration of iron by pathogenic microorganisms impacts the PHD-mediated hydroxylation of HIF-1α, leading to the accumulation of HIF-1α. This process may contribute to the elevated levels of HIF-1α observed in sepsis.
Potential targeted drugs
Given its involvement in critical aspects of sepsis, HIF-1α has emerged as a potential therapeutic target for treating sepsis in humans. The pharmacological effects of HIF-1α include the stimulation of erythropoiesis, modulating inflammatory factors, reprogramming cell metabolism during hypoxia, and influencing the body's adaptive response to ischemia and inflammation [19].
Targeted therapies for HIF-1α upregulation
The medications that stimulate HIF-1α upregulation can be broadly classified into direct HIF-1α inducers and PHD inhibitors [36, 37, 74, 109–113]. Acetate, a significant short-chain fatty acid produced by gut microbes, enhances HIF-1α levels by triggering increased glycolysis, thereby improving macrophage killing through the HIF-1α/IL-1β axis [109]. Moreover, the HIF-1α agonist mimosine enhances phagocytosis, increases bactericidal capacity, and reduces lesion severity in a murine model of Staphylococcus aureus skin infection [111]. Established pharmaceuticals, known for their clinical efficacy, also exhibit modulation of HIF-1α. For instance, rabeprazole acts as a potent HIF-1α inducer, facilitating vascular repair and reducing sepsis-induced lung inflammation through the endothelial HIF-1α/FoxM1 signaling pathway [36].
Several PHD inhibitors have demonstrated symptomatic and prognostic benefits in animal models of sepsis or infection. Roxadustat (FG-4592), a transient small-molecule PHD inhibitor, increases HIF-1α expression in the lungs through the HIF-1α/heme oxygenase-1 (HO-1) signaling pathway, ameliorating LPS-induced lung injury and inflammation [37]. Dimethyloxaloylglycine (DMOG), another PHD inhibitor, creates a hypoxic microenvironment by inhibiting PHD enzyme activity, stabilizing HIF-1α, and impacting various intracellular signaling pathways. DMOG has been found to alleviate toxin-induced intestinal inflammation, maintain epithelial barrier function, and protect against C. difficile toxin-induced intestinal injury [74]. Additionally, AKB-4924, a PHD inhibitor with no direct antibacterial activity, elevates HIF-1α levels and inhibits the proliferation of S. aureus, reducing lesion formation in a murine skin abscess model [110]. The potential therapeutic application of AKB-4924 is promising due to the reduced risk of developing bacterial resistance. Table 3 details the primary medications and compounds that upregulated the expression of HIF-1α.
Table 3.
Medications for treating sepsis and its complications by increasing HIF-1α expression
| Drug Names | The role of HIF-1α | Modulation of HIF-1α | Model | References |
|---|---|---|---|---|
| Rabeprazole | Facilitates vascular repair and resolution of lung injury | Up (directly HIF-1α pathway) | LPS-induced sepsis mouse | [36] |
| Roxadustat | Mitigates sepsis-induced acute lung injury | Up (PHD inhibitor) | I/R-induced AKI mouse mice | [37] |
| Acetate | Improved killing of S. pneumoniae by alveolar macrophages | Up (directly HIF-1α pathway) | S. pneumoniae infection mouse model | [109] |
| DMOG | Reducing Clostridium difficile toxin-induced intestinal damage | Up (PHD inhibitor) | Mouse ileal loop model | [74] |
| AKB-4924 | Enhances the cutaneous innate defenses against bacterial infections | Up (PHD inhibitor) | Mouse skin abscess model | [110] |
| Mimosine | Enhancement of the bactericidal capacity of phagocytes | Up (directly HIF-1α pathway) | S. aureus skin infection model | [111] |
| Edaravone | Exerts cardioprotective effects | Up (directlyHIF-1 α/HO-1 pathway) | CLP-induced sepsis rats | [112] |
| Phlorizin | Improve sepsis-induced cardiomyocyte injury | Up (induces the oxygen deprivation) | SIMD mouse model | [113] |
AKI acute kidney injury, CLP cecal ligation and puncture, DMOG Dimethyloxaloylglycine, HCP Houttuynia cordata polysaccharide, I/R ischemia/reperfusion, Ref. reference, S. aureus Staphylococcus aureus, S. pneumoniae Streptococcus pneumoniae, SIMD Sepsis-induced myocardial dysfunction
Targeted therapies for HIF-1α downregulation
HIF-1α activity inhibitors can be categorized into distinct groups based on their mechanisms of action [114]: (1) those affecting the degradation of HIF-1α; (2) those inhibiting the DNA transcription and expression of HIF-1α; (3) those blocking mRNA translation; (4) those preventing the binding of HIF-1α and Hypoxia-Response Element (HRE); and (5) those disrupting the formation of HIF-1α transcriptional complexes, among others. In the context of sepsis, medications that reduce HIF-1α activity typically function by inhibiting the DNA transcription and expression of HIF-1α.
Various drugs demonstrate protective effects against sepsis-induced damage in different target organs by modulating HIF-1α activity [115–139]. For instance, lidocaine mitigates the inflammatory cascade induced by HIF-1α by inhibiting the NF-κB signaling pathway, effectively downregulating HIF-1α transcription and expression [115]. Houttuynia cordata polysaccharide (HCP) also displays inhibitory effects on HIF-1α DNA transcription and expression, offering beneficial effects in H1N1-induced intestinal injury by modulating the TLR4 pathway, reducing HIF-1α expression, and preserving tight junction proteins such as zonula occludens-1 (ZO-1) [116]. Cynaroside suppresses hepatic inflammation by inhibiting PKM2/HIF-1α interactions, resulting in decreased activation of HIF-1α target genes and facilitating the transition of M1 macrophages to M2 macrophages [118]. Norisoboldine hinders the translocation of PKM2 from the cytoplasm to the nucleus, reducing HIF-1α expression and mitigating sepsis-induced acute lung injury [119]. Additionally, resveratrol enhances endothelial nitric oxide synthase (eNOS) expression and lowers HIF-1α levels to enhance vasodilatory function in a septic shock model [125]. Table 4 outlines the primary medications and compounds that downregulate the expression of HIF-1α.
Table 4.
Drugs targeting sepsis and complications by down-regulation of HIF-1α
| Drug Names | The role of HIF-1α | Modulation of HIF-1α | Model | References |
|---|---|---|---|---|
| Lidocaine | Inhibiting glycolysis to attenuate inflammatory response | Down (indirectly through NF-κB pathway) | LPS-induced sepsis mouse | [115] |
| HCP | Reduce intestinal damage in H1N1 virus-infected individuals | Down (indirectly through TLR4 pathway) | H1N1 virus infected mouse mice | [116] |
| XJDHT | Participation in aerobic glycolysis in sepsis | Down (indirectly through TLR4 pathway) | CLP-induced sepsis rats | [117] |
| Cynaroside | Attenuates liver injury | Down (indirectly through PKM2 pathway) | CLP-induced sepsis mouse | [118] |
| Norisoboldine | Mitigates sepsis-induced acute lung injury | Down (indirectly through PKM2 pathway) | LPS-induced sepsis mouse | [119] |
| LBP | Altering glycolysis and the M1 differentiation of macrophages | Down (indirectly through PKM2 pathway) | LPS-induced macrophages model | [120] |
| Cya | Affects B cell Migration | Down (stimulate PHD activity) | Human and mouse B cell | [121] |
| AV | Promoting inflammatory responses | Down (stimulate PHD2 activity) | CLP-induced sepsis mouse | [122] |
| TIIA | Promoting inflammatory responses | Down (indirectly through PI3K and MAPK pathway) | LPS-induced lung injury mouse | [123] |
| Propofol | Promoting inflammatory responses | Down (indirectly through MAPK pathway) | Mouse model of endotoxemia | [124] |
| Resveratrol | Improvement of vasodilatory function in a septic shock model | Down (not given) | CLP-induced septic shock rats | [125] |
| Chicoric acid | Mediated glycolysis and mitochondrial oxidative burst | Down (reducing ROS production) | LPS-induced sepsis mouse | [126] |
| TASE | Leading to endotoxin tolerance in sepsis monocytes | Down (indirectly through IRAK-M pathway) | Monocytes in patients with sepsis | [127] |
| AmB | Regulation of EPO expression | Down (reinforcing FIH-mediated repression) | Hypoxia and anemia rats | [128] |
| Rosmarinic acid | Regulation of LPS-induced microglial M1 polarization | Down (indirectly through RACK1 pathway) | CLP-induced sepsis mouse | [129] |
| 2ME2 | Promoting inflammatory responses | Down (reduced HIF-1α activity) | CLP- and LPS-induced sepsis mouse | [130] |
| Emodin | Modulates intestinal barrier injury | Down (inhibits the expression of HIF-1α) | LPS-induced intestinal epithelial cells model | [131] |
| N5P | Promoting inflammatory responses | Down (inhibits the expression of HIF-1α) | ALI rat model | [132] |
| Valproic acid | Participation in burn-induced gut barrier dysfunction | Down (inhibits HIF-1α accumulation) | Rat burn model | [133] |
| DHMF | Promoting inflammatory responses | Down (inhibits HIF-1α accumulation) | LPS-induced lung injury mouse | [134] |
| Eriocitrin | Regulation of glycolysis in sepsis | Down (inhibition of HIF-1α mRNA) | LPS-induced sepsis-associated ALI mouse | [135] |
| Metformin | Promoting inflammatory responses | Down (inhibition of HIF-1α mRNA) | LPS-induced sepsis-associated liver injury mouse | [136] |
| Landiolol | Involved in sepsis-related AKI | Down (inhibition of HIF-1α mRNA) | Mouse model of endotoxaemia | [137] |
| Enoxaparin | Causes diaphragm damage | Down (inhibition of HIF-1α mRNA and Protein) | LPS-induced sepsis mouse | [138] |
| Dexmedetomidine | Participation in aerobic glycolysis in sepsis | Down (inhibition of HIF-1α mRNA and Protein) | LPS-treated macrophages | [139] |
AKI acute kidney injury, ALI acute liver injury, AmB Amphotericin B, AV Adhatoda Vasica, CLP Cecal ligation and puncture, Cya Cyclosporine A, DHMF 5,7-dihydroxy-8-methoxyflavone, LBP Lycium barbarum polysaccharide, LPS Lipopolysaccharide, N5P N-phenethyl-5-phenylpicolinamide, Ref. reference, ROS reactive oxygen species, TASE Thiosulfinate-enriched Allium sativum extract, TIIA tanshinone IIA, XJDHT Xijiao Dihuang decoction, 2ME2 2-Methoxyestradiol
Clinical value of HIF-1α
Recent clinical studies have shifted focus toward translating HIF-1α research findings from basic science to clinical applications, emphasizing its relevance in post-diagnostic and prognostic aspects of sepsis. In a prospective study comparing HIF-1α Mrna levels in the blood of healthy volunteers and patients in shock, significantly elevated levels of HIF-1α Mrna were observed in shock patients compared to healthy volunteers [140]. Similarly, serum HIF-1α levels in intensive care patients exhibited diagnostic potential in sepsis, with significantly higher concentrations detected in patients with septic shock, septic non-shock, and infection groups than in those undergoing elective surgery (160.39 ± 19.68 vs 135.24 ± 20.34 vs 114.34 ± 15.50 vs 113.37 ± 15.50 pg/Ml, respectively, P < 0.01) [141]. Further research highlighted the use of HIF-1α, combined with other clinical parameters, as a tool for sepsis diagnosis, demonstrating high diagnostic accuracy (AUC 0.926, 95% CI 0.885–0.968) and revealing a U-shaped relationship between HIF-1α levels and ICU mortality [15].
In contrast, some studies have presented conflicting results. A prospective study reported a significant decrease in HIF-1α expression levels in septic patients compared to healthy volunteers [140]. This discrepancy may be attributed to LPS tolerance, where repetitive inflammatory or hypoxic stimuli initially upregulate the expression of inflammatory genes, followed by subsequent suppression of their expression levels [15]. Additionally, experiments with LPS-stimulated neutrophils indicated an initial rise in HIF-1α protein levels after 4 h of LPS stimulation, followed by a gradual and significant decline [142].
Conclusion and prospect
HIF-1α plays a crucial role in sepsis, and its activation is closely tied not only to intracellular hypoxia but also to the inflammatory process and immune regulation. During sepsis, the activation of HIF-1α governs the host’s adaptive response to hypoxia and influences the release of inflammatory mediators, as well as the balance between anti-inflammatory and immune tolerance states. Furthermore, HIF-1α activation is implicated in regulating a spectrum of pathophysiological processes, including mitochondrial function and apoptosis. Future studies can explore the molecular mechanisms and pathways of HIF-1α in sepsis, with the potential to reveal new targets and strategies for the early diagnosis and treatment of sepsis.
Acknowledgements
The figures were drawn using the Figdraw2.0 tool. The author has obtained authorization to use the image (OPRUU117b7).
Abbreviations
- 2-OG
2-Oxoglutarate
- 2ME2
2-Methoxyestradiol
- α-KG
Alpha-ketoglutarate
- AKI
Acute kidney injury
- ALI
Acute liver injury
- AmB
Amphotericin B
- ATP
Adenosine triphosphate
- AV
Adhatoda vasica
- Bhlh-PAS
Basic Helix-Loop-Helix-Periodicity-Aryl Hydrocarbon Receptor Nuclear Translocator-Single-Minded
- BKV
BK polyomavirus
- CA
Candida albicans
- CBP
Cyclic adenosine monophosphate-response binding protein binding protein
- CIA
Collagen-induced arthritis
- CLP
Cecal ligation and puncture
- Cys
Cysteine
- Cya
Cyclosporine A
- DAMPs
Damage-associated molecular patterns
- DNA
Deoxyribonucleic acid
- DENV
Dengue virus
- DHMF
5,7-Dihydroxy-8-methoxyflavone
- EBV
Epstein–Barr virus
- Enos
Endothelial nitric oxide synthase
- EPO
Erythropoietin
- FOXM1
Forkhead Box Protein M1
- FIH
Factor-inhibiting HIF-1
- HAT
Histone acetyltransferase
- HIF
Hypoxia-inducible factor
- HIF-1α
Hypoxia-inducible factor-1α
- HPH
Hypoxia-induced pulmonary hypertension
- IKKβ
I kappa B kinase beta
- IAV
Influenza A virus
- I/R
Ischemia/reperfusion
- Inos
Inducible nitric oxide synthase
- JAKs
Janus Kinases
- LBP
Lycium barbarum polysaccharide
- LPS
Lipopolysaccharide
- mTOR
Mammalian Target of Rapamycin
- MAPK
Mitogen-activated protein kinase
- Mdm2
Mousedouble minute 2
- mRNA
Messenger ribonucleic acid
- MTB
Mycobacterium tuberculosis
- N5P
N-phenethyl-5-phenylpicolinamide
- NF-κB
Nuclear factor-κB
- NH2-terminal domains
N-TAD
- NK
Natural killer
- NO
Nitric oxide
- ODDD
Oxygen-dependent degradation domain
- PDAC
Pancreatic ductal adenocarcinoma
- PAMPs
Pathogen-associated molecular patterns
- PBMCs
Peripheral blood mononuclear cells
- PHD
Prolyl hydroxylase
- PHD
Prolyl hydroxylases domain
- PI3K/Akt
Phosphatidylinositol 3-kinase/protein kinase B
- PRR
Pattern recognition receptors
- PKM2
Pyruvate kinase isozyme type M2
- PSM
Propensity score matching
- ROS
Reactive oxygen species
- RSV
Respiratory syncytial virus
- Ref
Reference
- SIMD
Sepsis-induced myocardial dysfunction
- S. aureus
Staphylococcus aureus
- S. pneumoniae
Streptococcus pneumoniae
- STAT3
Signal transducer and activator of transcription 3
- STATs
Signal transducers and activators of transcription
- TAD
Transactivation domains
- TASE
Thiosulfinate-enriched Allium sativum extract
- TIIA
Tanshinone IIA
- UTI
Urinary tract infection
- VACV
Vaccinia virus
- VEGF
Vascular endothelial growth factor
- XJDHT
Xijiao Dihuang decoction
- ZO-1
Zonula occludens-1
Author contributions
HR was responsible for statistical analyses and the initial draft of the manuscript. XR and S‒SL conducted data cleaning and contributed to the study design, data analysis and interpretation. QZ and Y-PZ contributed to revising the manuscript critically for intellectual content and approved the final version for publication. All authors reviewed the manuscript critically for intellectual content and have read and approved the final manuscript.
Funding
This study was supported by funding from the National Natural Science Foundation of China (Grant No. 82271358), the Scientific Research Foundation for Returned Overseas Chinese Scholars of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, and the Talent Project of Public Health in Hubei Province (Grant No. 2022SCZ048).
Availability of data and materials
Not applicable.
Declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Not applicable.
Competing interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Hang Ruan and Qin Zhang have contributed equally to this work.
Contributor Information
Shu-sheng Li, Email: Shushengli16@sina.com.
Xiao Ran, Email: ranxiao1001@tjh.tjmu.edu.cn.
References
- 1.Huang M, Cai S, Su J. The Pathogenesis of sepsis and potential therapeutic targets. Int J Mol Sci. 2019;20(21):5376. doi: 10.3390/ijms20215376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Critical care medicine 1992, 20(6):864–874. [PubMed]
- 3.Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent JL, Ramsay G, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS international sepsis definitions conference. Crit Care Med. 2003;31(4):1250–1256. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 4.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche J-D, Coopersmith CM, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3) Jama-J Am Med Assoc. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hu Q, Hao C, Tang S. From sepsis to acute respiratory distress syndrome (ARDS): emerging preventive strategies based on molecular and genetic researches. Biosci Rep. 2020;40:BSR20200830. doi: 10.1042/BSR20200830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hirota K. Involvement of hypoxia-inducible factors in the dysregulation of oxygen homeostasis in sepsis. Cardiovasc Hematol Disord Drug Targets. 2015;15(1):29–40. doi: 10.2174/1871529X15666150108115553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gyawali B, Ramakrishna K, Dhamoon AS. Sepsis: the evolution in definition, pathophysiology, and management. Sage Open Med. 2019;7:2050312119835043. doi: 10.1177/2050312119835043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao Y, Xiong W, Li C, Zhao R, Lu H, Song S, Zhou Y, Hu Y, Shi B, Ge J. Hypoxia-induced signaling in the cardiovascular system: pathogenesis and therapeutic targets. Signal Transduct Target Ther. 2023;8(1):431. doi: 10.1038/s41392-023-01652-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kiani AA, Elyasi H, Ghoreyshi S, Nouri N, Safarzadeh A, Nafari A. Study on hypoxia-inducible factor and its roles in immune system. Immunol Med. 2021;44(4):223–236. doi: 10.1080/25785826.2021.1910187. [DOI] [PubMed] [Google Scholar]
- 10.Knight M, Stanley S. HIF-1α as a central mediator of cellular resistance to intracellular pathogens. Curr Opin Immunol. 2019;60:111–116. doi: 10.1016/j.coi.2019.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Majmundar AJ, Wong WJ, Simon MC. Hypoxia-inducible factors and the response to hypoxic stress. Mol Cell. 2010;40(2):294–309. doi: 10.1016/j.molcel.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weidemann A, Johnson RS. Biology of HIF-1α. Cell Death Differ. 2008;15(4):621–627. doi: 10.1038/cdd.2008.12. [DOI] [PubMed] [Google Scholar]
- 13.Masoud GN, Li W. HIF-1α pathway: role, regulation and intervention for cancer therapy. Acta Pharmaceutica Sinica B. 2015;5(5):378–389. doi: 10.1016/j.apsb.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xiong Y, Zhang M, Li Y. Recent advances in the development of CBP/p300 bromodomain inhibitors. Curr Med Chem. 2020;27(33):5583–5598. doi: 10.2174/0929867326666190731141055. [DOI] [PubMed] [Google Scholar]
- 15.Ruan H, Li Y-Z, Zhang Q, Wang B-R, Wu R, Li S-S, Ran X. Identification and clinical validation of hypoxia-inducible factor 1a protein as the potential biomarker in patients with sepsis. Shock. 2023;59(6):855–863. doi: 10.1097/SHK.0000000000002122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jaskiewicz M, Moszynska A, Kroliczewski J, Cabaj A, Bartoszewska S, Charzynska A, Gebert M, Dabrowski M, Collawn JF, Bartoszewski R. The transition from HIF-1 to HIF-2 during prolonged hypoxia results from reactivation of PHDs and HIF1A mRNA instability. Cell Mol Biol Lett. 2022;27(1):109. doi: 10.1186/s11658-022-00408-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang S-L, Wu C, Xiong Z-F, Fang X. Progress on hypoxia-inducible factor-3: Its structure, gene regulation and biological function (Review) Mol Med Rep. 2015;12(2):2411–2416. doi: 10.3892/mmr.2015.3689. [DOI] [PubMed] [Google Scholar]
- 18.Levy MM, Artigas A, Phillips GS, Rhodes A, Beale R, Osborn T, Vincent J-L, Townsend S, Lemeshow S, Dellinger RP. Outcomes of the surviving sepsis campaign in intensive care units in the USA and Europe: a prospective cohort study. Lancet Infect Dis. 2012;12(12):919–924. doi: 10.1016/S1473-3099(12)70239-6. [DOI] [PubMed] [Google Scholar]
- 19.Yuan X, Ruan W, Bobrow B, Carmeliet P, Eltzschig HK. Targeting hypoxia-inducible factors: therapeutic opportunities and challenges. Nat Rev Drug Discov. 2023;23(3):175–200. doi: 10.1038/s41573-023-00848-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Strowitzki MJ, Cummins EP, Taylor CT. Protein hydroxylation by hypoxia-inducible factor (HIF) hydroxylases: unique or ubiquitous? Cells. 2019;8(5):384. doi: 10.3390/cells8050384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee P, Chandel NS, Simon MC. Cellular adaptation to hypoxia through hypoxia inducible factors and beyond. Nat Rev Mol Cell Biol. 2020;21(5):268–283. doi: 10.1038/s41580-020-0227-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heikkila M, Pasanen A, Kivirikko KI, Myllyharju J. Roles of the human hypoxia-inducible factor (HIF)-3α variants in the hypoxia response. Cell Mol Life Sci. 2011;68(23):3885–3901. doi: 10.1007/s00018-011-0679-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Du D, Zhang Y, Zhu C, Chen H, Sun J. Metabolic regulation of hypoxia-inducible factors in hypothalamus. Front Endocrinol. 2021;12:650284. doi: 10.3389/fendo.2021.650284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tacchini L, De Ponti C, Matteucci E, Follis R, Desiderio MA. Hepatocyte growth factor-activated NF-κB regulates HIF-1 activity and ODC expression, implicated in survival, differently in different carcinoma cell lines. Carcinogenesis. 2004;25(11):2089–2100. doi: 10.1093/carcin/bgh227. [DOI] [PubMed] [Google Scholar]
- 25.Hirai K, Furusho H, Hirota K, Sasaki H. Activation of hypoxia-inducible factor 1 attenuates periapical inflammation and bone loss. Int J Oral Sci. 2018;10(2):12. doi: 10.1038/s41368-018-0015-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Azoitei N, Becher A, Steinestel K, Rouhi A, Diepold K, Genze F, Simmet T, Seufferlein T. PKM2 promotes tumor angiogenesis by regulating HIF-1α through NF-κB activation. Mol Cancer. 2016;15(1):3. doi: 10.1186/s12943-015-0490-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kwon SJ, Song JJ, Lee YJ. Signal pathway of hypoxia-inducible factor-1α phosphorylation and its interaction with von Hippel-Lindau tumor suppressor protein during ischemia in MiaPaCa-2 pancreatic cancer cells. Clin Cancer Res. 2005;11(21):7607–7613. doi: 10.1158/1078-0432.CCR-05-0981. [DOI] [PubMed] [Google Scholar]
- 28.Sang NL, Stiehl DP, Bohensky J, Leshchinsky I, Srinivas V, Caro J. MAPK signaling up-regulates the activity of hypoxia-inducible factors by its effects on p300. J Biol Chem. 2003;278(16):14013–14019. doi: 10.1074/jbc.M209702200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alvarez-Tejado M, Alfranca A, Aragonés J, Vara A, Landázuri MO, del Peso L. Lack of evidence for the involvement of the phosphoinositide 3-kinase/Akt pathway in the activation of hypoxia-inducible factors by low oxygen tension. J Biol Chem. 2002;277(16):13508–13517. doi: 10.1074/jbc.M200017200. [DOI] [PubMed] [Google Scholar]
- 30.Dodd KM, Yang J, Shen MH, Sampson JR, Tee AR. mTORC1 drives HIF-1α and VEGF-a signalling via multiple mechanisms involving 4E-BP1, S6K1 and STAT3. Oncogene. 2015;34(17):2239–2250. doi: 10.1038/onc.2014.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ravi R, Mookerjee B, Bhujwalla ZM, Sutter CH, Artemov D, Zeng QW, Dillehay LE, Madan A, Semenza GL, Bedi A. Regulation of tumor angiogenesis by p53-induced degradation of hypoxia-inducible factor 1α. Genes Dev. 2000;14(1):34–44. doi: 10.1101/gad.14.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nieminen AL, Qanungo S, Schneider EA, Jiang BH, Agani FH. Mdm2 and HIF-1α interaction in tumor cells during hypoxia. J Cell Physiol. 2005;204(2):364–369. doi: 10.1002/jcp.20406. [DOI] [PubMed] [Google Scholar]
- 33.Kim W-Y, Hong S-B. Sepsis and acute respiratory distress syndrome: recent update. Tuberc Respir Dis. 2016;79(2):53–57. doi: 10.4046/trd.2016.79.2.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xie Y, Hu W, Chen X, Ren P, Ye C, Wang Y, Luo J, Li X. Identification and validation of autophagy-related genes in exogenous sepsis-induced acute respiratory distress syndrome. Immun Inflam Dis. 2022;10(10):e691. doi: 10.1002/iid3.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang X, Zhang X, Zhao DX, Yin J, Hu G, Evans CE, Zhao Y-Y. Endothelial hypoxia-inducible factor-1α is required for vascular repair and resolution of inflammatory lung injury through forkhead box protein M1. Am J Pathol. 2019;189(8):1664–1679. doi: 10.1016/j.ajpath.2019.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Evans CE, Peng Y, Zhu MM, Dai Z, Zhang X, Zhao Y-Y. Rabeprazole promotes vascular repair and resolution of sepsis-induced inflammatory lung injury through HIF-1α. Cells. 2022;11(9):1425. doi: 10.3390/cells11091425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Han F, Wu G, Han S, Li Z, Jia Y, Bai L, Li X, Wang K, Yang F, Zhang J, et al. Hypoxia-inducible factor prolyl-hydroxylase inhibitor roxadustat (FG-4592) alleviates sepsis-induced acute lung injury. Respir Physiol Neurobiol. 2020;281:103506. doi: 10.1016/j.resp.2020.103506. [DOI] [PubMed] [Google Scholar]
- 38.Hrabalova P, Bohuslavova R, Matejkova K, Papousek F, Sedmera D, Abaffy P, Kolar F, Pavlinkova G. Dysregulation of hypoxia-inducible factor 1α in the sympathetic nervous system accelerates diabetic cardiomyopathy. Cardiovasc Diabetol. 2023;22(1):88. doi: 10.1186/s12933-023-01824-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heck-Swain KL, Li J, Ruan W, Yuan X, Wang Y, Koeppen M, Eltzschig HK. Myeloid hypoxia-inducible factor HIF1A provides cardio-protection during ischemia and reperfusion via induction of netrin-1. Front Cardiovasc Med. 2022;9:970415. doi: 10.3389/fcvm.2022.970415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen Q-F, Wang W, Huang Z, Huang D-L. Hypoxia-inducible factor-1α attenuates myocardial inflammatory injury in rats induced by coronary microembolization. Anais Da Academia Brasileira De Ciencias. 2020;92(1):e20191004. doi: 10.1590/0001-3765202020190658. [DOI] [PubMed] [Google Scholar]
- 41.Hotchkiss RS, Moldawer LL, Opal SM, Reinhart K, Turnbull IR, Vincent J-L. Sepsis and septic shock. Nat Rev Dis Primers. 2016;2(1):1–21. doi: 10.1038/nrdp.2016.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peng J, Zhang P, Fan X, Yuan Z, Zhou M, Guo Y. Expression of hypoxia-inducible factor 1 alpha ameliorate myocardial ischemia in rat. Biochem Biophys Res Commun. 2015;465(4):691–695. doi: 10.1016/j.bbrc.2015.08.046. [DOI] [PubMed] [Google Scholar]
- 43.Lei L, Mason S, Liu D, Huang Y, Marks C, Hickey R, Jovin IS, Pypaert M, Johnson RS, Giordano FJ. Hypoxia-inducible factor-dependent degeneration, failure, and malignant transformation of the heart in the absence of the von Hippel-Lindau protein. Mol Cell Biol. 2008;28(11):3790–3803. doi: 10.1128/MCB.01580-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sato T, Takeda N. The roles of HIF-1α signaling in cardiovascular diseases. J Cardiol. 2023;81(2):202–208. doi: 10.1016/j.jjcc.2022.09.002. [DOI] [PubMed] [Google Scholar]
- 45.Evans CE, Palazon A, Sim J, Tyrakis PA, Prodger A, Lu X, Chan S, Bendahl P-O, Belting M, Von Euler L, et al. Modelling pulmonary microthrombosis coupled to metastasis: distinct effects of thrombogenesis on tumorigenesis. Biology Open. 2017;6(5):688–697. doi: 10.1242/bio.024653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morinet F, Casetti L, Francois J-H, Capron C, Pillet S. Oxygen tension level and human viral infections. Virology. 2013;444(1–2):31–36. doi: 10.1016/j.virol.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 47.Jang Y, Jeong SH, Park Y-H, Bae HC, Lee H, Ryu W-I, Park GH, Son SW. UVB induces HIF-1α-dependent TSLP expression via the JNK and ERK pathways. J Investig Dermatol. 2013;133(11):2601–2608. doi: 10.1038/jid.2013.203. [DOI] [PubMed] [Google Scholar]
- 48.Li L-F, Yu C-C, Wu H-P, Chu C-M, Huang C-Y, Liu P-C, Liu Y-Y. Reduction in ventilation-induced diaphragmatic mitochondrial injury through hypoxia-inducible factor 1α in a murine endotoxemia model. Int J Mol Sci. 2022;23(3):1083. doi: 10.3390/ijms23031083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hsu C-C, Wang C-H, Wu L-C, Hsia C-Y, Chi C-W, Yin P-H, Chang C-J, Sung M-T, Wei Y-H, Lu S-H, et al. Mitochondrial dysfunction represses HIF-1α protein synthesis through AMPK activation in human hepatoma HepG2 cells. BBA-Gen Subjects. 2013;1830(10):4743–4751. doi: 10.1016/j.bbagen.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 50.Baskurt OK, Gelmont D, Meiselman HJ. Red blood cell deformability in sepsis. Am J Respir Crit Care Med. 1998;157(2):421–427. doi: 10.1164/ajrccm.157.2.9611103. [DOI] [PubMed] [Google Scholar]
- 51.Tsui AKY, Marsden PA, Mazer CD, Adamson SL, Henkelman RM, Ho JJD, Wilson DF, Heximer SP, Connelly KA, Bolz S-S, et al. Priming of hypoxia-inducible factor by neuronal nitric oxide synthase is essential for adaptive responses to severe anemia. Proc Natl Acad Sci USA. 2011;108(42):17544–17549. doi: 10.1073/pnas.1114026108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hasegawa S, Tanaka T, Nangaku M. Hypoxia-inducible factor stabilizers for treating anemia of chronic kidney disease. Curr Opin Nephrol Hypertens. 2018;27(5):331–338. doi: 10.1097/MNH.0000000000000431. [DOI] [PubMed] [Google Scholar]
- 53.Zinkernagel AS, Johnson RS, Nizet V. Hypoxia inducible factor (HIF) function in innate immunity and infection. J Mol Med-Jmm. 2007;85(12):1339–1346. doi: 10.1007/s00109-007-0282-2. [DOI] [PubMed] [Google Scholar]
- 54.Devraj G, Beerlage C, Bruene B, Kempf VAJ. Hypoxia and HIF-1 activation in bacterial infections. Microbes Infect. 2017;19(3):144–156. doi: 10.1016/j.micinf.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 55.Watts ER, Walmsley SR. Inflammation and Hypoxia: HIF and PHD Isoform Selectivity. Trends Mol Med. 2019;25(1):33–46. doi: 10.1016/j.molmed.2018.10.006. [DOI] [PubMed] [Google Scholar]
- 56.dos Santos SA, de Andrade Junior DR. HIF-1alpha and infectious diseases: a new frontier for the development of new therapies. Rev Inst Med Trop Sao Paulo. 2017;59:e92. doi: 10.1590/s1678-9946201759092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Peyssonnaux C, Cejudo-Martin P, Doedens A, Zinkernagel AS, Johnson RS, Nizet V. Cutting edge: essential role of hypoxia inducible factor-1α in development of lipopolysaccharide-induced sepsis. J Immunol. 2007;178(12):7516–7519. doi: 10.4049/jimmunol.178.12.7516. [DOI] [PubMed] [Google Scholar]
- 58.Rius J, Guma M, Schachtrup C, Akassoglou K, Zinkernagel AS, Nizet V, Johnson RS, Haddad GG, Karin M. NF-κB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1α. Nature. 2008;453(7196):807–U809. doi: 10.1038/nature06905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nizet V, Johnson RS. Interdependence of hypoxic and innate immune responses. Nat Rev Immunol. 2009;9(9):609–617. doi: 10.1038/nri2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Reyes A, Corrales N, Galvez NMS, Bueno SM, Kalergis AM, Gonzalez PA. Contribution of hypoxia inducible factor-1 during viral infections. Virulence. 2020;11(1):1482–1500. doi: 10.1080/21505594.2020.1836904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mazzon M, Peters NE, Loenarz C, Krysztofinska EM, Ember SWJ, Ferguson BJ, Smith GL. A mechanism for induction of a hypoxic response by vaccinia virus. Proc Natl Acad Sci USA. 2013;110(30):12444–12449. doi: 10.1073/pnas.1302140110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kondo S, Seo SY, Yoshizaki T, Wakisaka N, Furukawa M, Joab I, Jang KL, Pagano JS. EBV latent membrane protein 1 up-regulates hypoxia-inducible factor 1α through Siah1-mediated down-regulation of prolyl hydroxylases 1 and 3 in nasopharyngeal epithelial cells. Can Res. 2006;66(20):9870–9877. doi: 10.1158/0008-5472.CAN-06-1679. [DOI] [PubMed] [Google Scholar]
- 63.Chen J, Wei H. Immune intervention in sepsis. Front Pharmacol. 2021;12:718089. doi: 10.3389/fphar.2021.718089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wezensky SJ, Cramer RA. Implications of hypoxic microenvironments during invasive aspergillosis. Med Mycol. 2011;49:S120–S124. doi: 10.3109/13693786.2010.495139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lopes JP, Urban CF. Visualizing hypoxia in a murine Model of Candida albicans infection using in vivo biofluorencence. Bio-Protoc. 2019;9(15):e33326. doi: 10.21769/BioProtoc.3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Grahl N, Dinamarco TM, Willger SD, Goldman GH, Cramer RA. Aspergillus fumigatus mitochondrial electron transport chain mediates oxidative stress homeostasis, hypoxia responses and fungal pathogenesis. Mol Microbiol. 2012;84(2):383–399. doi: 10.1111/j.1365-2958.2012.08034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.André AC, Laborde M, Marteyn BS. The battle for oxygen during bacterial and fungal infections. Trends Microbiol. 2022;30(7):643–653. doi: 10.1016/j.tim.2022.01.002. [DOI] [PubMed] [Google Scholar]
- 68.Puerner C, Vellanki S, Strauch JL, Cramer RA. Recent advances in understanding the human fungal pathogen hypoxia response in disease progression. Annu Rev Microbiol. 2023;77:403–425. doi: 10.1146/annurev-micro-032521-021745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fan D, Coughlin LA, Neubauer MM, Kim J, Kim MS, Zhan X, Simms-Waldrip TR, Xie Y, Hooper LV, Koh AY. Activation of HIF-1α and LL-37 by commensal bacteria inhibits Candida albicans colonization. Nat Med. 2015;21(7):808. doi: 10.1038/nm.3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Su H, Yi J, Tsui CKM, Li C, Zhu J, Li L, Zhang Q, Zhu Y, Xu J, Zhu M, et al. HIF1-a upregulation induces proinflammatory factors to boost host killing capacity after Aspergillus fumigatus exposure. Future Microbiol. 2023;18(1):27–41. doi: 10.2217/fmb-2022-0050. [DOI] [PubMed] [Google Scholar]
- 71.Lin AE, Beasley FC, Olson J, Keller N, Shalwitz RA, Hannan TJ, Hultgren SJ, Nizet V. Role of hypoxia inducible factor-1α (HIF-1α) in innate defense against uropathogenic Escherichia coli Infection. PLoS Pathog. 2015;11(4):1004818. doi: 10.1371/journal.ppat.1004818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Berger EA, McClellan SA, Vistisen KS, Hazlett LD. HIF-1α Is essential for effective pmn bacterial killing, antimicrobial peptide production and apoptosis in pseudomonas aeruginosa keratitis. PLoS Pathog. 2013;9(7):1003457. doi: 10.1371/journal.ppat.1003457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Holden VI, Breen P, Houle S, Dozois CM, Bachman MA. Klebsiella pneumoniae Siderophores Induce Inflammation, Bacterial Dissemination, and HIF-1α Stabilization during Pneumonia. MBio. 2016;7(5):10–128. doi: 10.1128/mBio.01397-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hirota SA, Fines K, Ng J, Traboulsi D, Lee J, Ihara E, Li Y, Willmore WG, Chung D, Scully MM, et al. Hypoxia-inducible factor signaling provides protection in clostridium difficile-induced intestinal injury. Gastroenterology. 2010;139(1):259–U378. doi: 10.1053/j.gastro.2010.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Beerlage C, Greb J, Kretschmer D, Assaggaf M, Trackman PC, Hansmann M-L, Bonin M, Eble JA, Peschel A, Bruene B, et al. Hypoxia-inducible factor 1-regulated lysyl oxidase is involved in staphylococcus aureus abscess formation. Infect Immun. 2013;81(7):2562–2573. doi: 10.1128/IAI.00302-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pereverzeva L, Otto NA, Peters-Sengers H, Roelofs JJTH, de Vos AF, van der Poll T. Role of Hypoxia-inducible factor 1α in host defense during pneumococcal pneumonia. Pathogens and Disease. 2023;81:047. doi: 10.1093/femspd/ftac047. [DOI] [PubMed] [Google Scholar]
- 77.Robrahn L, Dupont A, Jumpertz S, Zhang K, Holland CH, Guillaume J, Rappold S, Roth J, Cerovic V, Saez-Rodriguez J, et al. Stabilization but no functional influence of hif-1α expression in the intestinal epithelium during salmonella typhimurium infection. Infect Immun. 2022;90(2):e00222. doi: 10.1128/iai.00222-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Signorini L, Croci M, Boldorini R, Varella RB, Elia F, Carluccio S, Villani S, Bella R, Ferrante P, Delbue S. Interaction between human polyomavirus BK and hypoxia inducible factor-1 alpha. J Cell Physiol. 2016;231(6):1343–1349. doi: 10.1002/jcp.25238. [DOI] [PubMed] [Google Scholar]
- 79.Morris DR, Qu Y, Agrawal A, Garofalo RP, Casola A. HIF-1α modulates core metabolism and virus replication in primary airway epithelial cells infected with respiratory syncytial virus. Viruses-Basel. 2020;12(10):1088. doi: 10.3390/v12101088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gan ES, Cheong WF, Chan KR, Ong EZ, Chai X, Tan HC, Ghosh S, Wenk MR, Ooi EE. Hypoxia enhances antibody-dependent dengue virus infection. EMBO J. 2017;36(10):1348–1363. doi: 10.15252/embj.201695642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Duchemin NJ, Loonawat R, Yeakle K, Rosenkranz A, Bouchard MJ. Hypoxia-inducible factor affects hepatitis B virus transcripts and genome levels as well as the expression and subcellular location of the hepatitis B virus core protein. Virology. 2023;586:76–90. doi: 10.1016/j.virol.2023.06.013. [DOI] [PubMed] [Google Scholar]
- 82.Codo AC, Davanzo GG, Monteiro LdB, de Souza GF, Muraro SP, Virgilio-da-Silva JV, Prodonoff JS, Carregari VC, de Biagi O, Junior CA, Crunfli F, et al. Elevated glucose levels favor SARS-CoV-2 infection and monocyte response through a HIF-1α/glycolysis-dependent axis. Cell Metabol. 2020;32(3):437. doi: 10.1016/j.cmet.2020.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Denstaedt SJ, Singer BH, Standiford TJ. Sepsis and nosocomial infection: patient characteristics, mechanisms, and modulation. Front Immunol. 2018;9:417693. doi: 10.3389/fimmu.2018.02446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Branitzki-Heinemann K, Okumura CY, Voellger L, Kawakami Y, Kawakami T, Naim HY, Nizet V, Von Koeckritz-Blickwede M. A novel role for the transcription factor HIF-1α in the formation of mast cell extracellular traps. Biochem J. 2012;446:159–163. doi: 10.1042/BJ20120658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Palazon A, Goldrath AW, Nizet V, Johnson RS. HIF transcription factors, inflammation, and immunity. Immunity. 2014;41(4):518–528. doi: 10.1016/j.immuni.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tran CW, Gold MJ, Garcia-Batres C, Tai K, Elford AR, Himmel ME, Elia AJ, Ohashi PS. Hypoxia-inducible factor 1 alpha limits dendritic cell stimulation of CD8 T cell immunity. PLoS ONE. 2020;15(12):e0244366. doi: 10.1371/journal.pone.0244366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gojkovic M, Cunha PP, Darmasaputra GS, Barbieri L, Rundqvist H, Velica P, Johnson RS. Oxygen-mediated suppression of CD8+T cell proliferation by macrophages: role of pharmacological inhibitors of HIF degradation. Front Immunol. 2021;12:633586. doi: 10.3389/fimmu.2021.633586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lee KE, Spata M, Maduka R, Vonderheide RH, Simon MC. Hif1α deletion limits tissue regeneration via aberrant B cell accumulation in experimental pancreatitis. Cell Rep. 2018;23(12):3457–3464. doi: 10.1016/j.celrep.2018.05.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kojima H, Kobayashi A, Sakurai D, Kanno Y, Hase H, Takahashi R, Totsuka Y, Semenza GL, Sitkovsky MV, Kobata T. Differentiation stage-specific requirement in hypoxia-inducible factor-1α-regulated glycolytic pathway during murine B cell development in bone marrow. J Immunol. 2010;184(1):154–163. doi: 10.4049/jimmunol.0800167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jung YJ, Isaacs JS, Lee SM, Trepel J, Neckers L. IL-1β mediated up-regulation of HIF-1α via an NFkB/COX-2 pathway identifies HIF-1 as a critical link between inflammation and oncogenesis. Faseb J. 2003;17(12):2115. doi: 10.1096/fj.03-0329fje. [DOI] [PubMed] [Google Scholar]
- 91.Cluff E, Magdaleno CC, Fernandez E, House T, Swaminathan S, Varadaraj A, Rajasekaran N. Hypoxia-inducible factor-1 alpha expression is induced by IL-2 via the PI3K/mTOR pathway in hypoxic NK cells and supports effector functions in NKL cells and ex vivo expanded NK cells. Cancer Immunol Immunother. 2022;71(8):1989–2005. doi: 10.1007/s00262-021-03126-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Scharte M, Jurk K, Kehrel B, Zarbock A, Van Aken H, Singbartl K. IL-4 enhances hypoxia induced HIF-1α protein levels in human transformed intestinal cells. FEBS Lett. 2006;580(27):6399–6404. doi: 10.1016/j.febslet.2006.10.053. [DOI] [PubMed] [Google Scholar]
- 93.Xu S, Yu C, Ma X, Li Y, Shen Y, Chen Y, Huang S, Zhang T, Deng W, Wang Y. IL-6 promotes nuclear translocation of HIF-1α to aggravate chemoresistance of ovarian cancer cells. Eur J Pharmacol. 2021;894:173817. doi: 10.1016/j.ejphar.2020.173817. [DOI] [PubMed] [Google Scholar]
- 94.Wang Z, Li B, Jiang H, Ma Y, Bao Y, Zhu X, Xia H, Jin Y. IL-8 exacerbates alcohol-induced fatty liver disease via the Akt/HIF-1α pathway in human IL-8-expressing mice. Cytokine. 2021;138:155402. doi: 10.1016/j.cyto.2020.155402. [DOI] [PubMed] [Google Scholar]
- 95.Levin SG, Sirota NP, Nenov MN, Savina TA, Godukhin OV. Interleukin-10 and PD150606 modulate expression of AMPA receptor GluA1 and GluA2 subunits under hypoxic conditions. NeuroReport. 2018;29(2):84–91. doi: 10.1097/WNR.0000000000000928. [DOI] [PubMed] [Google Scholar]
- 96.Khalil SM, Bernstein I, Kulaga H, Gour N, Rowan N, Lajoie S, Lane AP. Interleukin 13 (IL-13) alters hypoxia-associated genes and upregulates CD73. Int Forum Allergy Rhinol. 2020;10(9):1096–1102. doi: 10.1002/alr.22630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Coulibaly A, Velasquez SY, Kassner N, Schulte J, Barbarossa MV, Lindner HA. STAT3 governs the HIF-1α response in IL-15 primed human NK cells. Sci Rep. 2021;11(1):7023. doi: 10.1038/s41598-021-84916-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Domingo-Gonzalez R, Das S, Griffiths KL, Ahmed M, Bambouskova M, Gopal R, Gondi S, Munoz-Torrico M, Salazar-Lezama MA, Cruz-Lagunas A, et al. Interleukin-17 limits hypoxia-inducible factor 1α and development of hypoxic granulomas during tuberculosis. Jci Insight. 2017;2(19):e92973. doi: 10.1172/jci.insight.92973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhang L, Li M, Wang Z, Sun P, Wei S, Zhang C, Wu H, Bai H. Cardiovascular risk After SARS-CoV-2 infection is mediated by IL18/IL18R1/HIF-1 signaling pathway axis. Front Immunol. 2022;12:780804. doi: 10.3389/fimmu.2021.780804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhang Q, da Cunha AP, Li S, Hao Q, Kainz V, Huang Q, Wu HY. IL-27 regulates HIF-1α-mediated VEGFA response in macrophages of diabetic retinopathy patients and healthy individuals. Cytokine. 2019;113:238–247. doi: 10.1016/j.cyto.2018.07.011. [DOI] [PubMed] [Google Scholar]
- 101.Liu J, Wang W, Wang L, Chen S, Tian B, Huang K, Corrigan CJ, Ying S, Wang W, Wang C. IL-33 initiates vascular remodelling in hypoxic pulmonary hypertension by up-regulating HIF-1α and VEGF expression in vascular endothelial cells. EBioMedicine. 2018;33:196–210. doi: 10.1016/j.ebiom.2018.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ding LL, Li X, Lei YM, Xia LP, Lu J, Shen H. Effect of interleukin-34 on secretion of angiogenesis cytokines by peripheral blood mononuclear cells of rheumatoid arthritis. Immunol Invest. 2020;49(1–2):81–87. doi: 10.1080/08820139.2019.1649281. [DOI] [PubMed] [Google Scholar]
- 103.Zhao T, Jin F, Xiao D, Wang H, Huang C, Wang X, Gao S, Liu J, Yang S, Hao J. IL-37/STAT3/HIF-1α negative feedback signaling drives gemcitabine resistance in pancreatic cancer. Theranostics. 2020;10(9):4088–4100. doi: 10.7150/thno.42416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pei B, Chen K, Zhou S, Min D, Xiao W. IL-38 restrains inflammatory response of collagen-induced arthritis in rats via SIRT1/HIF-1α signaling pathway. Biosci Rep. 2020;40:BSR20182431.. doi: 10.1042/BSR20182431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kuo H-P, Lee D-F, Xia W, Wei Y, Hung M-C. TNFα induces HIF-1α expression through activation of IKKβ. Biochem Biophys Res Commun. 2009;389(4):640–644. doi: 10.1016/j.bbrc.2009.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sandau KB, Zhou J, Kietzmann T, Brüne B. Regulation of the hypoxia-inducible factor 1α by the inflammatory mediators nitric oxide and tumor necrosis factor-α in contrast to desferroxamine and phenylarsine oxide. J Biol Chem. 2001;276(43):39805–39811. doi: 10.1074/jbc.M107689200. [DOI] [PubMed] [Google Scholar]
- 107.Comito G, Calvani M, Giannoni E, Bianchini F, Calorini L, Torre E, Migliore C, Giordano S, Chiarugi P. HIF-1α stabilization by mitochondrial ROS promotes Met-dependent invasive growth and vasculogenic mimicry in melanoma cells. Free Radical Biol Med. 2011;51(4):893–904. doi: 10.1016/j.freeradbiomed.2011.05.042. [DOI] [PubMed] [Google Scholar]
- 108.Weiss G, Carver PL. Role of divalent metals in infectious disease susceptibility and outcome. Clin Microbiol Infect. 2018;24(1):16–23. doi: 10.1016/j.cmi.2017.01.018. [DOI] [PubMed] [Google Scholar]
- 109.Machado MG, Patente TA, Rouille Y, Heumel S, Melo EM, Deruyter L, Pourcet B, Sencio V, Teixeira MM, Trottein F. Acetate Improves the killing of Streptococcus pneumoniae by alveolar macrophages via NLRP3 inflammasome and glycolysis-HIF-1α Axis. Front Immunol. 2022;13:773261. doi: 10.3389/fimmu.2022.773261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Okumura CYM, Hollands A, Tran DN, Olson J, Dahesh S, von Koeckritz-Blickwede M, Thienphrapa W, Corle C, Jeung SN, Kotsakis A, et al. A new pharmacological agent (AKB-4924) stabilizes hypoxia inducible factor-1 (HIF-1) and increases skin innate defenses against bacterial infection. J Mol Med-Jmm. 2012;90(9):1079–1089. doi: 10.1007/s00109-012-0882-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zinkernagel AS, Peyssonnaux C, Johnson RS, Nizet V. Pharmacologic augmentation of hypoxia-inducible factor-1α with mimosine boosts the bactericidal capacity of phagocytes. J Infect Dis. 2008;197(2):214–217. doi: 10.1086/524843. [DOI] [PubMed] [Google Scholar]
- 112.He C, Zhang W, Li SB, Ruan W, Xu JM, Xiao F. Edaravone improves septic cardiac function by inducing an HIF-1α/HO-1 pathway. Oxid Med Cell Long. 2018 doi: 10.1155/2018/5216383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yu YW, Chen X, Yan JY, Hu J, Huang KY, Ji KT, Cai HL. Phlorizin, a novel caloric restriction mimetic, stimulates hypoxia and protects cardiomyocytes through activating autophagy via modulating the Hif-1α/Bnip3 axis in sepsis-induced myocardial dysfunction. Int Immunopharmacol. 2024;126:11241. doi: 10.1016/j.intimp.2023.111241. [DOI] [PubMed] [Google Scholar]
- 114.Xu R, Wang F, Yang H, Wang Z. Action sites and clinical application of HIF-1α inhibitors. Molecules. 2022;27(11):3426. doi: 10.3390/molecules27113426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lin S, Jin P, Shao C, Lu W, Xiang Q, Jiang Z, Zhang Y, Bian J. Lidocaine attenuates lipopolysaccharide-induced inflammatory responses and protects against endotoxemia in mice by suppressing HIF1α-induced glycolysi. Int Immunopharmacol. 2020;80:106150. doi: 10.1016/j.intimp.2019.106150. [DOI] [PubMed] [Google Scholar]
- 116.Chen MY, Li H, Lu XX, Ling LJ, Weng HB, Sun W, Chen DF, Zhang YY. Houttuynia cordata polysaccharide alleviated intestinal injury and modulated intestinal microbiota in H1N1 virus infected. Chin J Nat Med. 2019;17(3):187–197. doi: 10.1016/S1875-5364(19)30021-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lu J, Zhang LY, Cheng L, He SY, Zhang Y, Yan J, Zhou J. Xijiao Dihuang decoction improves prognosis of sepsis via inhibition of aerobic glycolysis. Biomed Pharmacother. 2020;129:110501. doi: 10.1016/j.biopha.2020.110501. [DOI] [PubMed] [Google Scholar]
- 118.Pei L, Le Y, Chen H, Feng J, Liu Z, Zhu J, Wang C, Chen L, Dou X, Lu D. Cynaroside prevents macrophage polarization into pro-inflammatory phenotype and alleviates cecal ligation and puncture-induced liver injury by targeting PKM2/HIF-1α axis. Fitoterapia. 2021;152:104922. doi: 10.1016/j.fitote.2021.104922. [DOI] [PubMed] [Google Scholar]
- 119.Chen Q, Shao X, He Y, Lu E, Zhu L, Tang W. Norisoboldine attenuates sepsis-induced acute lung injury by modulating macrophage polarization via PKM2/HIF-1α/PGC-1α pathway. Biol Pharm Bull. 2021;44(10):1536–1547. doi: 10.1248/bpb.b21-00457. [DOI] [PubMed] [Google Scholar]
- 120.Ding H, Wang JJ, Zhang XY, Yin L, Feng T. Lycium barbarum polysaccharide antagonizes lps-induced inflammation by altering the glycolysis and differentiation of macrophages by triggering the degradation of PKM2. Biol Pharm Bull. 2021;44(3):379–388. doi: 10.1248/bpb.b20-00752. [DOI] [PubMed] [Google Scholar]
- 121.Hilchey SP, Palshikar MG, Emo JA, Li D, Garigen J, Wang J, Mendelson ES, Cipolla V, Thakar J, Zand MS. Cyclosporine a directly affects human and mouse b cell migration in vitro by disrupting a hIF-1α dependent, o2 sensing, molecular switch. Bmc Immunol. 2020;21(1):1–8. doi: 10.1186/s12865-020-0342-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gheware A, Dholakia D, Kannan S, Panda L, Rani R, Pattnaik BR, Jain V, Parekh Y, Enayathullah MG, Bokara KK, et al. Adhatoda Vasica attenuates inflammatory and hypoxic responses in preclinical mouse models: potential for repurposing in COVID-19-like conditions. Respir Res. 2021;22(1):1–15. doi: 10.1186/s12931-021-01698-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Xu M, Cao FL, Liu LL, Zhang B, Wang YX, Dong HY, Cui Y, Dong MQ, Xu DQ, Liu Y, et al. Tanshinone IIA-induced attenuation of lung injury in endotoxemic mice is associated with reduction of hypoxia-inducible factor 1α expression. Am J Respir Cell Mol Biol. 2011;45(5):1028–1035. doi: 10.1165/rcmb.2011-0113OC. [DOI] [PubMed] [Google Scholar]
- 124.Yeh CH, Cho W, So EC, Chu CC, Lin MC, Wang JJ, Hsing CH. Propofol inhibits lipopolysaccharide-induced lung epithelial cell injury by reducing hypoxia-inducible factor-1α expression. Br J Anaesth. 2011;106(4):590–599. doi: 10.1093/bja/aer005. [DOI] [PubMed] [Google Scholar]
- 125.Zhang Z-S, Zhao H-L, Yang G-M, Zang J-T, Zheng D-Y, Duan C-Y, Kuang L, Zhu Y, Wu Y, Li T, et al. Role of resveratrol in protecting vasodilatation function in septic shock rats and its mechanism. J Trauma Acute Care Surg. 2019;87(6):1336–1345. doi: 10.1097/TA.0000000000002466. [DOI] [PubMed] [Google Scholar]
- 126.Sun HJ, Zheng GL, Wang ZC, Liu Y, Bao N, Xiao PX, Lu QB, Zhang JR. Chicoric acid ameliorates sepsis-induced cardiomyopathy via regulating macrophage metabolism reprogramming. Phytomedicine. 2024;123:155715. doi: 10.1016/j.phymed.2023.155175. [DOI] [PubMed] [Google Scholar]
- 127.Avendaño-Ortiz J, Redondo-Calvo FJ, Lozano-Rodríguez R, Terrón-Arcos V, Bergón-Gutiérrez M, Rodríguez-Jiménez C, Rodríguez JF, del Campo R, Gómez LA, Bejarano-Ramírez N, et al. Thiosulfinate-enriched allium sativum extract exhibits differential effects between healthy and sepsis patients: the implication of HIF-1α. Int J Mol Sci. 2023;24(7):6234. doi: 10.3390/ijms24076234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yeo EJ, Ryu JH, Cho YS, Chun YS, Huang LE, Kim MS, Park JW. Amphotericin B blunts erythropoietin response to hypoxia by reinforcing FIH-mediated repression of HIF-1. Blood. 2006;107(3):916–923. doi: 10.1182/blood-2005-06-2564. [DOI] [PubMed] [Google Scholar]
- 129.Liu DY, Wu Y, Feng ZQ, Yu Y, Cai HW, Liao SP, Zeng T, Zhu L, Wang X, Wan LH. Rosmarinic acid against cognitive impairment via RACK1/HIF-1α regulated microglial polarization in sepsis-surviving mice. Chemico-Biol Interact. 2024;388:110830. doi: 10.1016/j.cbi.2023.110830. [DOI] [PubMed] [Google Scholar]
- 130.Yeh CH, Chou W, Chu CC, So EC, Chang HC, Wang JJ, Hsing CH. Anticancer agent 2-methoxyestradiol improves survival in septic mice by reducing the production of cytokines and nitric oxide. Shock. 2011;36(5):510–516. doi: 10.1097/SHK.0b013e318231866f. [DOI] [PubMed] [Google Scholar]
- 131.Qi L, Fu Q, Du C, Wu D, Zhang GQ, Yuan B, Yan LN. Amelioration of hypoxia and LPS-induced intestinal epithelial barrier dysfunction by emodin through the suppression of the NF-κB and HIF-1α signaling pathways. Int J Mol Med. 2014;34(6):1629–1639. doi: 10.3892/ijmm.2014.1965. [DOI] [PubMed] [Google Scholar]
- 132.Du N, Lin HM, Zhang AQ, Cao C, Hu XJ, Zhang J, Wang LL, Pan XS, Zhu YQ, Qian FY, et al. N-phenethyl-5-phenylpicolinamide alleviates inflammation in acute lung injury by inhibiting HIF-1?/glycolysis/ASIC1a pathway. Life Sci. 2022;309:120987. doi: 10.1016/j.lfs.2022.120987. [DOI] [PubMed] [Google Scholar]
- 133.Luo HM, Du MH, Lin ZL, Zhang L, Ma L, Wang H, Yu W, Lv Y, Lu JY, Pi YL, et al. Valproic acid treatment inhibits hypoxia-inducible factor 1α accumulation and protects against burn-induced gut barrier dysfunction in a rodent model. PLoS ONE. 2013;8(10):e77523. doi: 10.1371/journal.pone.0077523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sun HL, Peng ML, Lee SS, Chen CJ, Chen WY, Yang ML, Kuan YH. Endotoxin-induced acute lung injury in mice is protected by 5,7-dihydroxy-8-methoxyflavone via inhibition of oxidative stress and HIF-1α. Environ Toxicol. 2016;31(12):1700–1709. doi: 10.1002/tox.22172. [DOI] [PubMed] [Google Scholar]
- 135.Li DH, Yang L, Wang W, Song CK, Xiong R, Pan SZ, Li N, Geng Q. Eriocitrin attenuates sepsis-induced acute lung injury in mice by regulating MKP1/MAPK pathway mediated-glycolysis. Int Immunopharmacol. 2023;118:110021. doi: 10.1016/j.intimp.2023.110021. [DOI] [PubMed] [Google Scholar]
- 136.Song H, Zhang XJ, Zhai RQ, Liang HY, Song GF, Yuan YY, Xu YA, Yan Y, Qiu LX, Sun TW. Metformin attenuated sepsis-associated liver injury and inflammatory response in aged mice. Bioengineered. 2022;13(2):4598–4609. doi: 10.1080/21655979.2022.2036305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ogura Y, Jesmin S, Yamaguchi N, Oki M, Shimojo N, Islam MM, Khatun T, Kamiyama J, Sakuramoto H, Hagiya K, et al. Potential amelioration of upregulated renal HIF-1alpha-endothelin-1 system by landiolol hydrochloride in a rat model of endotoxemia. Life Sci. 2014;118(2):347–356. doi: 10.1016/j.lfs.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 138.Li LF, Yu CC, Huang HY, Wu HP, Chu CM, Huang CY, Liu PC, Liu YY. Suppression of hypoxia-inducible factor 1α by low-molecular-weight heparin mitigates ventilation-induced diaphragm dysfunction in a murine endotoxemia model. Int J Mol Sci. 2021;22(4):1702. doi: 10.3390/ijms22041702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Meng QY, Guo PH, Jiang ZY, Bo LL, Bian JJ. Dexmedetomidine inhibits LPS-induced proinflammatory responses via suppressing HIF1α-dependent glycolysis in macrophages. Aging-Us. 2020;12(10):9534–9548. doi: 10.18632/aging.103226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Textoris J, Beaufils N, Quintana G, Ben Lassoued A, Zieleskiewicz L, Wiramus S, Blasco V, Lesavre N, Martin C, Gabert J, et al. Hypoxia-inducible factor HIF1α gene expression in human shock states. Crit Care. 2012;16(4):1–6. doi: 10.1186/cc11414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Tong ZW, Wang GJ, Huang W, Zhang HM, Xie F, Wang XT. Hypoxia-inducible factor-1α is a biomarker for predicting patients with sepsis. J Int Med Res. 2023;51(9):03000605231202139.. doi: 10.1177/03000605231202139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Pan T, Sun S, Chen Y, Tian R, Chen E, Tan R, Wang X, Liu Z, Liu J, Qu H. Immune effects of PI3K/Akt/HIF-1α-regulated glycolysis in polymorphonuclear neutrophils during sepsis. Crit Care. 2022;26(1):29. doi: 10.1186/s13054-022-03893-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.