Abstract

Floating microplastics are susceptible to sunlight-driven photodegradation, which can convert plastic carbon to dissolved organic carbon (DOC) and can facilitate microplastic fragmentation by mechanical forces. To understand the photochemical fate of sub-millimeter buoyant plastics, ∼0.6 mm polypropylene microplastics were photodegraded while tracking plastic mass, carbon, and particle size distributions. Plastic mass loss and carbon loss followed linear kinetics. At most time points DOC accumulation accounted for under 50% of the total plastic carbon lost. DOC accumulation followed sigmoidal kinetics, not the exponential kinetics previously reported for shorter irradiations. Thus, we suggest that estimates of plastic lifespan based on exponential DOC accumulation are inaccurate. Instead, linear plastic-C mass and plastic mass loss kinetics should be used, and these methods result in longer estimates of photochemical lifetimes for plastics in surface waters. Scanning electron microscopy revealed that photoirradiation produced two distinct patterns of cracking on the particles. However, size distribution analyses indicated that fragmentation was minimal. Instead, the initial population of microplastics shrank in size during irradiations, indicating photoirradiation in tranquil waters (i.e., without mechanical forcing) dissolved sub-millimeter plastics without fragmentation.
Keywords: Microplastics, Photochemistry, Photodissolution, Polypropylene, Fragmentation, Dissolved organic carbon, Size distribution
Short abstract
Photochemical losses of sub-millimeter microplastics occurred without production of fragments, indicating photochemistry alone does not fragment polypropylene microplastics. Photochemical losses of microplastic mass followed linear kinetics resulting in longer estimates of plastic lifetimes at sea.
1. Introduction
Since the beginning of widespread plastic manufacturing and use in the 1950s, the mass of plastic-carbon (e.g., mass of carbon contained in synthetic polymers, plastic-C) has grown to rival biogeochemically relevant natural carbon stocks.1 Much of this plastic is used in the production of one-use or short-lifespan commercial items; of all plastics produced since 1950 only an estimated 30% is still in use.2 In 2010 mismanaged waste resulted in an estimated 4.8 to 12.7 million metric tons of plastics entering the ocean, a number estimated to have increased over the subsequent five years.3,4 Floating plastic debris in the ocean is considered extremely long-lived, with evidence of persisting at the ocean surface for decades,5 and experiencing embrittlement and fragmentation by weathering and wave action.6−8 The estimate of buoyant plastic pieces floating on the ocean surface is in the trillions, but may not account for all buoyant plastics believed to have entered the ocean.9,10 In the past decade evidence has grown that some ocean plastics are undergoing transport or removal that is not fully understood,11 although recently updated models suggest undercounting of large plastic debris may be responsible for the apparent mass imbalance.12
Many routes of plastic removal from surface waters have been studied including biofouling, ingestion, fragmentation, nanoparticle production, and photodegradation. Fragmentation is the process of generating multiple smaller particles from a single larger particle. In a system experiencing mechanical forces that induce fragmentation, particle count increases as particle size decreases in a power relationship.18 Power function fragmentation models have been used to predict and describe the size distribution of surface ocean plastics, and surveys of aquatic plastic pollution support this model for microplastics larger than ∼5 mm.19−28 Production of large numbers of nanoparticles or other fragments too small to capture by current sampling methods (e.g., most studies use tow nets with 200–300 μm screens1,11) has also been observed in laboratory experiments.13−16 Detection of nanoparticles in ocean samples has not been reported in many studies; however, nanoplastics of mixed polymer type were reported in the North Atlantic.17 While mixing into deeper waters,29 biofouling induced sinking,30−33 and ingestion34−36 offer routes of sub-millimeter plastic transport, fragmentation may result in production of undersampled microplastics or nanoplastics, and photodegradation is a compelling mechanism for sub-millimeter plastic removal.
In the presence of sunlight, water, and oxygen, plastics photodegrade, accumulating oxidative products on the plastic’s surface, releasing carbonaceous gases (e.g., carbon dioxide and carbon monoxide), volatiles, and dissolved organic carbon (DOC).37−39 The pure polymeric structure of common polyolefins, polyethylene (PE), and polypropylene (PP) does not absorb environmentally relevant wavelengths of ultraviolet light and therefore should not be inherently chemically reactive to sunlight, yet, these plastics photodegrade in seawater.37,40,41 This may be due to the presence of additives or structural abnormalities42 that can initiate radical reactions43 which then propagate along the polymer chains resulting in oxidation and chain scission.8,44 The accumulation of DOC as PP photodegrades has been reported to follow exponential kinetics (Zhu et al).41 Based on this finding, it was suggested that plastic mass loss may also follow exponential kinetics; however, in this earlier work only the initial and final plastic masses of a short irradiation were measured. A progressive time series tracking PP mass loss kinetics over long-term irradiation is needed to fully capture PP mass loss kinetics.
Plastic photodegradation increases oxidation and crystallinity and decreases molecular weight, primarily impacting the first ∼100 μm surface layer of plastics.45 Crack formation also begins on the particle surface, permeating on average 10 to 100 μm deep.46 Thus, we hypothesize that because photodegradation predominantly involves chemical alterations and product formation at the particle surface, over time the size of the initial plastic particle will decrease as plastic-C is converted to other forms such as DOC and carbon dioxide, serving as a nonfragmenting removal mechanism for small plastic particles at the ocean surface. While fragmentation of PP during irradiation has been reported, these experiments were conducted dry14 or in turbulent water.47 Whether photochemistry alone can fragment floating plastics into microplastics with the power-law size distribution, without concurrent mechanical force, is not well understood.
To improve understanding of the fate of microplastics in sunlit waters, including the kinetics of photochemical dissolution, the fate of plastic-C, and photofragmentation in the absence of mechanical forces, sub-millimeter PP microplastics were irradiated in a solar simulator for 364 days. PP was used as it is prevalent in surface ocean samples,48 is inherently buoyant due to a density lower than seawater (seawater ∼1.05 g cm–3, PP 0.83–0.85 g cm–3), and photodegrades faster than PE, the other most abundant, buoyant, marine microplastic.41 PP standards rather than postconsumer or field collected PP were used to capture the full progression of degradation beginning with unweathered, well characterized (i.e., a standard) plastic and to allow others to repeat or expand upon this study using the same polymer sample. Future work investigating other polymer types and formulations of PP will be needed. Irradiations were conducted in ultrapure water which lacks reactive ions present in aquatic ecosystems, as ultrapure water also lacks organic carbon sources found in natural waters, allowing better resolution of the DOC and any organic particles (i.e., nanoplastics) formed as photodegradation products.41
During the irradiation, time series data for plastic mass loss, plastic-C loss, DOC accumulation, and microplastic particle size distribution were collected. Scanning electron microscopy (SEM) provided additional insight into the physical breakdown of the microplastics. The data are presented and used to test the hypothesis that photochemistry without mechanical forces does not fragment plastics but instead leads to a gradual reduction in the size of the initial population of microplastics. Data were also used to assess the kinetics of both DOC accumulation and plastic losses (as total mass and organic carbon mass of all particulate matter), offering insight into the fate of PP derived carbon.
2. Materials and Methods
2.1. General Sample Handling
To avoid plastic and carbon contamination, all quartz and glassware was cleaned by soaking overnight in a pH 2 hydrochloric acid bath, rinsed five times with ultrapure water (Milli-Q, Millipore), dried overnight at 60 °C, and then combusted at 500 °C for 5 h to remove trace organic carbon. A glass vacuum filtration setup was used to filter particulates onto similarly precombusted 47 mm Whatman GF/F glass microfiber filters (nominal pore size 0.7 μm).
2.2. Plastic Preparation and Irradiation
Isotactic PP granules (average Mw = 340,000; average Mn = 97,000; Aldrich, 427861) were used to generate microplastics. Attenuated total reflectance Fourier transform infrared (FT-IR) spectroscopy characterization of this plastic was conducted by the manufacturer and is available online. The PP granules were submerged in liquid nitrogen for 30 s and then transferred to a spice grinder and ground for 1 min in 5 s pulses. The PP was not evaluated for thermal oxidation following the cryogen cooled grinding procedure and any chemical changes would have impacted all time points and the dark controls as particles were prepared as a single batch. After grinding, the PP was dry sieved with a sieve shaker plate (Gilson SS-3) to obtain a 300 to 600 μm size class. The freezing and grinding process was repeated for particles larger than 600 μm. Once prepared, the PP particles were wet sieved to remove plastic dust and other smaller particles and soaked overnight in Milli-Q ultrapure water. Particulates were collected in a 300 μm sieve, covered, and dried overnight in a 60 °C oven.
Irradiations were conducted in a solar simulator, approximating natural sunlight. The system consisted of ten UVA-340+ bulbs (Q-Lab) simulating sunlight in the region of 295 to 365 nm with peak emission at 340 nm. This wavelength range is responsible for the majority of photochemical reactions occurring with environmental plastics debris.49,50 Over a 24-h period, this solar simulator system, previously described,41 produces irradiation equivalent to one solar day in surface water at the subtropical ocean gyres. Plastics accumulation is significant in this region,9 which intercepts over half of global ultraviolet light that reaches the planet surface.51,52 Plastic samples were massed using an analytical balance with 0.001 g precision (Sartorius, SECURA324-1S) into 100 mL quartz round-bottom flasks with a target mass of 0.300 ± 0.002 g PP and exact mass recorded. A total of 50 mL of Milli-Q ultrapure water was added to each flask. Two dark controls were prepared as above in glass bottles and wrapped with heavy duty aluminum foil. Dark controls were stored in the solar simulator alongside irradiating samples and sampled after 364 days (i.e., at the end of the experiment). Light samples were continuously irradiated for 364 days (actual time), with two samples sacrificed per time point for analysis, one for size distribution measurements, and one for carbon accounting.
2.3. Size Distribution Analysis
Samples removed for size distribution analysis were analyzed with a Malvern Mastersizer 3000 particle sizer (Malvern Analytical) with an attached automated wet dispersion unit. To ensure an appropriate suspension and entrapment of PP particles, the dispersion unit was filled with a 52% methanol–water solution. The irradiated sample was diluted with methanol to reach 52% methanol in water prior to adding the sample to the dispersion unit without any prefiltering. The refractive index was set to 1.36. The measurement method described above was validated with a set of PP particles with sizes ranging from 125 to 710 μm. Based upon the instrument’s manual, the Mastersizer has a maximum measurement range of 0.01 to 3500 μm. Replicate measurements of each sample were taken (n ≥ 4) and averaged for analysis; any measurements below the obscuration threshold were excluded.
2.4. Carbon Accounting
Samples removed for carbon accounting were vacuum filtered through 0.7 μm pore size and 47 mm diameter Whatman GF/F glass microfiber filters. Filtrate was removed for DOC analysis prior to rinsing the irradiation flask with Milli-Q ultrapure water to attempt to collect all particulates. Filtered particulates were dried overnight at 60 °C and massed on an analytical balance with 0.001 g precision (Sartorius, SECURA324-1S). Plastic recovery had an error of mass of ±0.004 g, n = 10.
Elemental analysis was used to determine the percent carbon composition of PP particulates.41 Randomly selected particles of PP from each time point were selected by inserting a metal scoop into the particles and shaking off any excess. Approximately 200–300 μg of particles were packed into 3 × 5 mm tin capsules (Elemental Microanalysis, Marlton, NJ, USA). Samples were massed on an analytical balance with 0.002 mg precision (Sartorius, SECURA26-1S). Capsules were combusted in a Flash 2000 Elemental Analyzer (Thermo Scientific) coupled to a Delta V Plus isotope ratio mass spectrometer (Thermo Scientific) calibrated daily with chitin standards to correlate peak area with carbon mass. The average percentage carbon by mass of PP and the gravimetric mass of PP, with plastic-mass recovery determined using the preirradiation mass of each sample recorded at the beginning of the experiment, were used to calculate the mass of plastic-C at each time point.
To measure DOC, filtrate was acidified to pH 2 using hydrochloric acid and analyzed on a Shimadzu TOC-L total organic carbon analyzer.53 Certified DOC standard deep seawater reference material available from Consensus Reference Materials (University of Miami) were included in each run and deep sweater reference values verified against the consensus range of 43 to 45 μM. Routine minimum DOC detection limits for the calibration range and instrument configuration are 10 to 15 μM. High concentrations, time point 181 days and later, were diluted prior to DOC measurement.
Carbon accounting was conducted based upon the measurements of DOC and particulate organic carbon, with missing carbon assigned as purgeable carbon based on the following considerations. Particulate carbon above 0.7 μm was collected on GF/F and was plastics-only, given the lack of other carbon sources in the experiments. For DOC, the operational definition was the nonpurgeable organic carbon that passed the 0.7 μm GF/F filters. This may include potential nanoparticles smaller than 0.7 μm but is the community operational definition of DOC.53 Finally, all carbon unaccounted for as DOC or particulates was described as purgeable carbon, which includes any volatile organic carbon and inorganic carbon that passes the 0.7 μm filter but is removed by the process of sample acidification and purging.
Time point 53 day particulates were lost during the drying step; therefore, day 53 is not present in particulate or purgeable carbon data.
2.5. Assessment of Procedural Controls
Ten procedural controls equivalent to time = 0 days were processed following sampling protocols to determine procedural error. Procedural controls showed minimal error in particulate mass recovery (100.00 ± 0.47%), and the sample handling procedure was deemed suitable to separate POC and DOC. Samples consisting of Milli-Q ultrapure water were analyzed for DOC to evaluate background carbon from water during sample preparation, which was found negligible (∼10 μM).
2.6. Scanning Electron Microscopy (SEM) and Energy Dispersive Spectroscopy (EDS)
Randomly selected PP particles were deposited onto carbon adhesive by inverting a vial of particulates over the adhesive; then adhesives were fixed to an aluminum sample holder, and unattached particles were removed using compressed air. Samples were then sputter coated with platinum using a Cressington 108Auto instrument operated at 0.1 mbar vacuum with argon gas. Images were taken on a Hitachi S-4800 scanning electron microscope at 3.0 kV. Energy dispersive spectroscopy was conducted using an attached EDAX system to evaluate fiber contamination (see SI).
2.7. Statistical Analysis
Statistical analysis was conducted in JMP and Origin Pro.
3. Results and Discussion
3.1. Plastic Stability in the Dark
Plastics appeared stable in the dark with no significant difference between plastic masses recovered from the 364-day dark control versus those recovered at day 0 (i.e., the start of the experiment; Table 1). To evaluate the impact of photoirradiation on PP, the plastic-mass and plastic-C recovery of the unirradiated day 0 were treated as 100% and day 0 DOC treated as 0%. Compared to day 0, 99.3 ± 0.5% of the dark control was recovered on day 364, and DOC accumulation in the dark was 0.00 ± 0.00%. The PP particle size of the 364-day dark control was within error of the day 0 sample size, and no discernible structural differences were observed on the particle surface between the two samples in SEM images (see Sections 3.6 and 3.7 for more details). Finally, the percent carbon by mass of the plastic particles did not change in the dark (day 0, 90.4 ± 0.7; dark day 364, 90.1 ± 0.2; Table 1). These results are consistent with other studies reporting the stability of PP in the dark41,54 and the insolubility of PP in water.55 Thus, biorefractory plastics such as PP are expected to be stable in tranquil aphotic water at ambient temperature.
Table 1. Summary of Polypropylene Microplastic Photochemical Degradation, Carbon Tracking, and Chemical Changes Initial, Final, and Percent Recovery of Plastic Mass, as well as Carbon Accounting of Plastic C as Plastic, DOC, or Purgeable Carbona.
| Plastic
mass recovery |
Carbon
accounting |
|||||||
|---|---|---|---|---|---|---|---|---|
| Irradiation time (days) | Starting PP mass (g) | Recovered PP mass (g) | % of initial PP mass recovered | PP % C by mass | % of initial plastic-C recovered | DOC accumulation (mg DOC g plastic-C–1) | % of initial plastic-C as DOC | % of initial plastic-C as purgeable-C |
| 0 | 0.300 ± 0.001 | 0.296 ± 0.001 | 100.0 ± 0.5 | 90.4 ± 0.7 | 100.0 ± 1.0 | 0.114 ± 0.022 | 0.00 ± 0.00 | 0.00 ± 0.96 |
| 26 | 0.302 ± 0.001 | 0.292 ± 0.001 | 98.0 ± 0.5 | 88.4 ± 1.7 | 95.6 ± 1.8 | 0.89 ± 0.18 | 0.08 ± 0.02 | 3.98 ± 1.84 |
| 53 | 0.299 ± 0.001 | N/A | N/A | N/A | N/A | 8.36 ± 0.18 | 0.82 ± 0.02 | N/A |
| 181 | 0.299 ± 0.001 | 0.253 ± 0.001 | 85.9 ± 0.4 | 86.8 ± 0.5 | 82.6 ± 0.2 | 82.09 ± 0.32 | 8.20 ± 0.03 | 9.17 ± 0.7 |
| 279 | 0.299 ± 0.001 | 0.233 ± 0.001 | 79.3 ± 0.4 | 85.8 ± 1.4 | 75.3 ± 1.3 | 123.0 ± 6.7 | 12.3 ± 0.67 | 12.4 ± 1.5 |
| 364 | 0.302 ± 0.001 | 0.215 ± 0.001 | 72.5 ± 0.4 | 85.9 ± 0.8 | 69.0 ± 0.8 | 172.9 ± 2.2 | 17.3 ± 0.22 | 13.7 ± 0.8 |
| Dark 364 | 0.301 ± 0.001 | 0.295 ± 0.001 | 99.3 ± 0.5 | 90.1 ± 0.2 | 99.1 ± 0.6 | 0.129 ± 0.022 | 0.00 ± 0.00 | 0.92 ± 0.57 |
Plastic carbon content and DOC accumulation values included for carbon tracking. Missing data for day 56 is due to sample loss during analysis.
3.2. Photochemical Loss of Plastic-Mass and Plastic-C
In contrast to the results in the dark, PP microplastics were degraded in light by all metrics (Table 1). Plastic gravimetric mass decreased linearly during irradiation (R2 = 0.9999) losing 0.0752 ± 0.0009% per day with 72.5 ± 0.6% recovered after 364 days (Figure 1). Plastic-C mass also decreased linearly (R2 = 0.9998, Figure 1). The loss rate of plastic-C was 0.0837 ± 0.0034% per day, and 69.0 ± 0.8% of plastic-C was recovered as particulates on day 364. If linear kinetics are maintained, the rates suggest it would take between 1195 (by plastic-C) and 1330 (by mass) days to completely dissolve the ∼0.5 mm PP microplastics under the conditions of this study (aphotic, ultrapure water, with constant irradiation), equivalent to a lifespan of 3.3 to 3.6 years. Effects of diurnal sunlight exposure were not investigated in this study. We previously reported a comparable rate of 0.0638% mass loss per day for irradiated PP of larger size when floating on seawater (3.45% over 54 days for particles initially ∼3 mm in size) and higher rates of carbon loss than mass loss.41
Figure 1.

Percent recovery of polypropylene microplastics recovered on 0.7 um GF/F filters after floating on ultrapure water during one year of irradiation, shown as plastic-mass (linear, R2 = 0.9999, Table S1) and plastic-C (linear, R2 = 0.9998, Table S1). Tjhe shaded region represents 95% confidence interval.
Lifespan estimates of plastics photodegraded under simulated conditions vary widely; Zhu et al. reported a lifespan of 4.3 years for ∼3 mm PP under near identical conditions41 and Delre et al. predicted a lifespan of several decades for larger, 6 mm PP disks 1 mm thick.40 Variation in lifespans calculated from plastic mass loss may be due to the multitude of factors that influence the rate of photodegradation of a given plastic sample, including surface area to volume ratio (related to particle size and shape), composition of additives, and potential copolymer formulations. Experimental or environmental conditions will also impact the rate of degradation,56−58 which is further discussed in Section 3.4.
3.3. Kinetics of DOC Photoaccumulation
Although plastic-C loss was linear (Figure 2), DOC accumulation was nonlinear and was lower throughout the 364 days than that of the loss of plastic-C (Figure 2). An exponential increase in DOC accumulation was observed through the first 181 days of irradiation and has been previously reported during irradiations of less than one year of PP and other plastics.41,59 If our experiments had stopped after half a year, data would also have been best fit by an exponential increase in DOC. However, irradiating samples for one year revealed that plastic-derived DOC accumulation slowed in the second half of the year and that longer term kinetics were best described using a sigmoidal fit (Figure 2; see Table S2 for a comparison of linear, exponential, and sigmoidal fits to the data).
Figure 2.

Loss of polypropylene carbon mass (plastic C loss) and dissolved organic carbon (DOC) accumulation, during one year of constant irradiation of polypropylene microplastics floating on ultrapure water displayed as percent of initial plastic C mass (left axis) and as mg-C per g of initial C (right axis). Lines of fit and 95% confidence intervals (shaded regions) are included for plastic C loss (linear, R2 = 0.9965, Table S1) and DOC accumulation (sigmoidal, R2 = 0.9997, Table S2).
In earlier work, we suggested that the exponential kinetics of DOC accumulation may be used to estimate plastic photodissolution rates.41 However, the new longer-term irradiation data presented here indicate that exponential kinetics do not apply to DOC accumulation over longer irradiation times (Figure 2). Using exponentially increasing rates of plastic photoloss based on DOC accumulation results in much shorter lifetimes of plastics in surface waters than when linear rates of plastic loss are applied. For instance, we previously reported photochemical lifetimes for PP ranging from approximately three months based on exponential DOC production kinetics versus 4.3 years based on linear extrapolation of a single measurement of PP mass loss after 54-days of irradiation.41 Similar ranges were also reported for PE (6 months using exponential kinetics of DOC production; 33 to 49 years using linear kinetics of mass loss) and expanded polystyrene (∼3 months using exponential kinetics of DOC production; 2.7 years using linear kinetics of mass loss).41 As the results presented in this study suggest that linear plastic-mass and plastic-C kinetics should be used, the photochemical lifetimes of microplastics at the sea surface are likely at the longer end of the estimates provided by Zhu et al. (2020).
3.4. A Full Accounting of Plastic-C Fate during Photodegradation
To do a full accounting of the apparent fate of plastic-C during irradiation, the percentage DOC accumulation and plastic-C losses were summed. The sum of these two measured terms did not account for all of the plastic-C added at the start of the experiments, even when corrected for procedural controls (i.e., the DOC blanks and the plastic sampling error). Plastic-C was measured as solids captured by a GF/F filter (0.7 μm) and DOC as the nonpurgeable organic carbon that passed through that same filter. Thus, the unaccounted for carbon can be best described as purgeable carbon and could include dissolved inorganic carbon (i.e., dissolved carbon dioxide or carbonates),60 and other short chain hydrocarbons, carbonyls, and low molecular weight volatile organic products8,61 that can be formed during photodegradation of plastics.
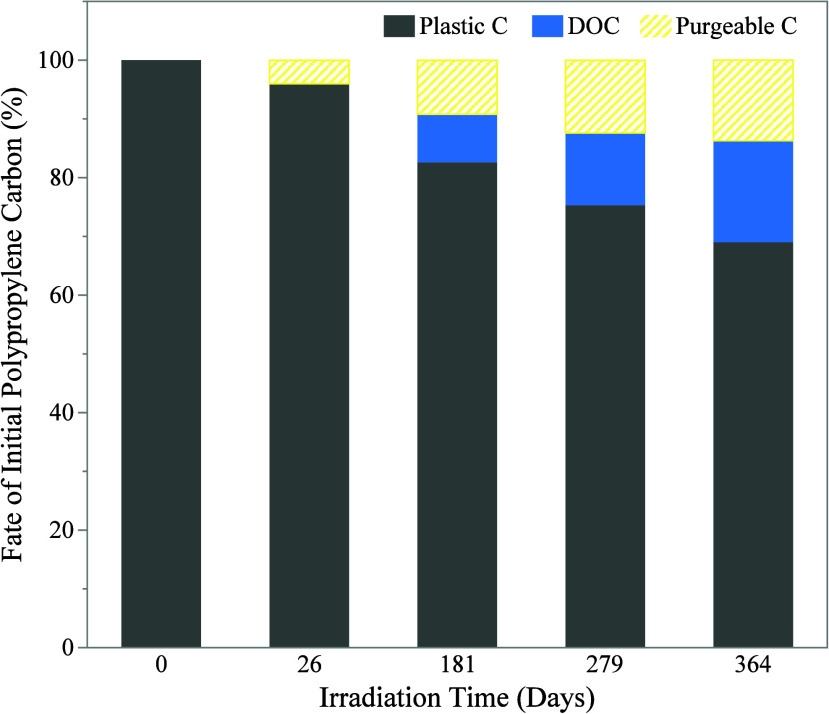
At the start of the experiment, when DOC accumulation was slowest, the majority of carbon lost from the microplastics was purgeable carbon (Figure 3). As DOC accumulation accelerated, the proportion of lost plastic-C accumulating as DOC increased, but the percentage of carbon accumulating as DOC only exceeded purgeable carbon (i.e., accounted for more than 50% of plastic-C losses) on day 364 (Table 1). The existence of a purgeable fraction of C that accounts for the majority of plastic-C losses at most time points also indicates that DOC accumulation will underestimate the plastic particle lifespan. As discussed above (Section 3.3), DOC accumulation had a sigmoidal trend that did not correlate with linear plastic-C loss. Thus, the initial loss of plastic-C may be due mainly to direct production of purgeable forms of carbon (e.g., low molecular weight organics and carbonaceous gases). Alternatively, there may also be initial production of photochemically reactive DOC that is rapidly converted to purgeable carbon in the optically thin water (high UV permeation). In this hypothesis, the gradual accumulation of UV-absorbing compounds in the water may result in increased shielding62 and greater DOC accumulation at later time points. The impact of UV-shielding may also be increased due to the experimental design, in which there is no circulation of the water that could diffuse shielding compounds, nor any microbial activity that could remove shielding compounds and is known to consume the DOC formed as plastics photodegrade.41 Thus, extrapolation of this hypothesis to natural waters is difficult. While other variables will likely contribute to the trend, over the course of the experiment it can be generally stated that DOC accumulation is affected by the competing factors of DOC production and loss via conversion to purgeable carbon.63−65 Toward the end of the one year irradiation, the rate of DOC accumulation slows or halts, likely due to similar rates of DOC production and photochemical losses of DOC. Increased rates of photochemical losses may be conceptualized with simple kinetics whereby the general pool of DOC (in actuality a diverse mix of compounds; Dittmar and Stubbins 2014) is the sole kinetically relevant reactant for the conversion of DOC to purgeable carbon and increased DOC concentration results in increased rates of photochemical losses. Presumably if the experiment were run until all plastic-C is lost, DOC concentrations would decrease beyond the asymptote of the one year kinetics, to a second, final asymptote representing the photoresistant fraction of DOC formed as the plastics photodissolve.
Figure 3.
Fate of polypropylene microplastic carbon during one year of constant irradiation floating on ultrapure water under a solar simulator. Data presented as the percentage of the initial plastic carbon recovered as (a) plastic particulates larger than 0.7 μm (Plastic C), (b) dissolved organic carbon smaller than 0.7 μm (DOC), and (c) carbon not accounted for in either the plastic C or DOC pools (Purgeable C). Error bars omitted for clarity. Standard deviations available in Table 1. Data compared to day 0 recovery.
Other kinetic scenarios may also explain the initial slow rates of DOC accumulation and subsequent increase in DOC accumulation rate (autoacceleration), including autocatalysis or autoinduction mechanisms.66 Linear polyolefins (i.e., PE and PP) display autocatalytic kinetics during formation of volatiles in thermal degradation through chain scission,67 suggesting autocatalytic kinetics may also appear during photodegradation of nanoplastics or short polymer chains in the DOC fraction, as chain scission is also a dominant reaction mechanism.8 Autoacceleration has also been observed in production of oxidative products of PP during photoirradiation.60 To fully understand the photodegradation of plastic debris in water, more work is needed to deconvolute the kinetics and mechanisms of plastic-C conversion to DOC and plastic-derived DOC photodegradation.
Irradiation in Milli-Q water, which contains only trace levels of ions and nonplastic derived organic carbon, versus in environmental matrices such as seawater, is also expected to impact the mechanisms and rates of photodegradation, complicating comparisons between studies. Dissolved compounds, such as inorganic ions and DOC, are known to impact rates and mechanisms of photodegradation. For example, chlorine ions have been reported to inhibit oxidative aging of PP particles due to quenching of reactive oxygen containing radicals by chlorine radicals.54 Conversely, DOC can facilitate indirect photochemistry that increases the overall rate of plastic degradation.57 Interactions between ions and dissolved organic matter can also affect the degradation rates. Increased salinity has been observed to improve adsorption of humic acid, a common dissolved organic compound, onto plastics where humic acid can then produce hydroxide radicals and increase photoaging rates.68 Humic acid can also quench chlorine radicals thereby reducing chlorine inhibition of plastic degradation.69 The role of ions and other dissolved compounds in determining the plastic photodegradation mechanisms and rate is undeniably important. However, in our previous work irradiating larger (3 mm) PP microplastic under the same light conditions but floating in natural seawater, we determined near-identical rates of plastic mass loss (i.e., 0.0638% per day in Zhu et al. versus 0.0752 ± 0.0009% per day here for 579 ± 12 μm PP microplastics; Figure 1). Thus, although changes in the salt content of natural waters can impact rates and mechanisms of microplastic photodegradation, the effects appear modest.
3.5. Photochemical Changes in the Carbon Content of Microplastics
The carbon content of the PP microplastics decreased from 90.4 ± 0.7% C by mass on day 0 to 85.9 ± 0.8% C by mass on day 364 (Table 1). Most of the decrease in percent carbon content by mass occurred within the first 26 days of the experiment, with slight but insignificant reductions occurring from day 26 through day 364 (one-way ANOVA, p = 0.3609, α = 0.05). Photochemical degradation of plastics is known to introduce oxygen containing functional groups such as carbonyls, lactones, peroxides, and esters.8,70 Studies show a rapid increase in oxygenation of PP can begin in as few as 2 days of irradiation71 with evidence of autoacceleration during the first 25 days of irradiation.60 Oxygen content as measured by indicators of oxygen-containing functional groups (carbonyl index, chemiluminescence of hydroperoxides, etc.) gradually stabilizes during long irradiations (i.e., beyond 25 days).60,71 These trends in oxygen uptake may explain the initial decrease in carbon content during the first 26 days of this study and following stabilization of carbon content from day 26 onward.
3.6. Photochemical Changes to Microplastic Surface Structure
The surfaces of unirradiated (day 0), irradiated, and dark control PP microplastics were examined using SEM (Figure 4). At the beginning of the experiment, the microplastics had irregular shapes with both smooth and angled edges, attributed to the production of these particles by cryomilling larger plastic granules (see Section 2.2). Their surfaces were free of pitting and cracking, appearing smooth at low magnification (8.3 mm ×30). At higher magnification (8.2 mm ×150 and ×300), a rough surface was visible with the appearance of directionally organized, scale-like layers. The dark day 364 control was similar to the day 0 sample, with no identifiable differences in particle appearance and surface structure. At high magnification fibers were visible on the surface of some particles. Using energy dispersive spectroscopy (Figure S1), these fibers were identified as glass microfibers that were contaminated from the GF/F filters used to collect the microplastics for SEM imaging.
Figure 4.
Scanning electron microscopy images of polypropylene particles (left to right: dark control for day 364, unirradiated day 0, irradiated day 181, irradiated day 364). Full scale bars top to bottom: 1.00 mm, 300 μm, and 100 μm; a white line above each scale bar in the right column has been added for visibility. Each tick represents 1/10th of full-scale length. Additional time points available in the SI.
Day 26 irradiated samples were not visually distinguishable from either day 0 or day 364 dark controls. However, from day 181 of irradiation onward, distinct surface cracking was observed on all particles. A visual trend was observed with the width and length of cracks increasing with irradiation time. Two distinct cracking patterns were observed: (1) regions of parallel, predominantly linear cracks with little branching, and (2) regions of unorganized cracking with shorter cracks that may be linear or curved and frequently branch (Figure 4). Some single particles had both cracking patterns. However, each face of a particle displayed only one pattern. Of particle faces visible in the imaging, unorganized cracking appears to predominate. The patterns may indicate regions of direct vs indirect irradiation based on particle orientation at the water surface during irradiation or may be related to regions of differing crystallinity or crystal orientation, which are known to impact physical properties of PP.72,73 Alternatively, the cracking may have resulted from the grinding process used to prepare the PP samples and was later exacerbated by irradiation. However, similar cracking as both the organized and unorganized patterns have been reported for polyolefins fragments collected on beaches74 suggesting these cracks are not likely an artifact of the sample preparation process. A small number of particles smaller than approximately 150 μm were distinguishable from the starting ∼600 μm PP particles on day 181 (Figure 5), indicating that cracking produced occasional fragments. In agreement with the size distribution data below, the smaller fragments visible via SEM did not constitute a power law increase in counts with decreasing size, nor even a majority of particles in any sample. Care was taken with the samples to avoid all mechanical stress on the particles prior to measurements to avoid fragments from any cause other than irradiation, but it is possible that the small number of fragments visible in images resulted from mechanical forces inherent in sample handling and preparation (e.g., physical manipulation, air pressure, or vacuum).
Figure 5.
Size distribution of polypropylene microplastics during one year of constant irradiation floating on an ultrapure water under a solar simulator. (A) Size distribution of particles exposed to simulated sunlight for 0 to 364 days. (B) Particle size distribution mode with standard deviation of replicate measurements (n ≥ 4 for all time points; mode describes the larger, primary population for time points 181 and 279 with bimodal distribution).
3.7. Impact of Photochemistry on Microplastics Size Distributions
The population of microplastics in the unirradiated day 0 sample was symmetrically unimodal in size with a mode of 579 ± 12 μm (Figure 5). The size distribution of PP microplastics did not change following 364 days of incubation while floating on Milli-Q water in the dark (Table 1). In the light, the size distributions of microplastics remained dominated by a symmetrical unimodal population throughout. However, the mode of this unimodal population decreased throughout the irradiation, reaching 409 ± 22 μm on day 364 (Figure 5). Although most particles fell within the unimodal population, a small second population of particles centered around 67 μm emerged on day 181 when it represented 7% of the particle counts. This second population was also visible on day 279, though at a lower abundance (6% of counts) and a greater span than on day 181. This secondary population disappeared for the remaining irradiation time points, and its cause is unknown. The distribution of particle sizes in the samples was evaluated through peak span (describing the range of particle sizes) and calculated using the 10, 50, and 90 percentiles of particle counts. Peak spans ranged from 0.75 to 1.02 with no statistically significant correlation between span and irradiation time (Table S3).
When considering the above results, it is important to remember the analytical fractions that are being addressed. The Mastersizer used to count particles was calibrated with PP down to 125 μm and had a minimum detection limit of 0.01 μm. Thus, if nanoplastics were produced below 0.01 μm, they would not be identified. Photoproduction of nanoplastics in the sub-0.5 μm range have been reported during the photoirradiation of polystyrene75 but fall within the typical category of DOC operationally defined as submicron (typically <0.7 μm or <0.45 μm).76 In this work, any sub-0.7 μm nanoparticles produced would be captured in the DOC fraction when accounting for carbon in the system, and consideration of fragmentation is limited to microplastics.
Mechanical fragmentation results in increasing particle abundance with decreasing particle size via a power relationship.18,77 Field studies of microplastics in natural waters have reported that particle abundance follows a power function (i.e., mechanical fragmentation pattern) with increasing particle numbers as size decreases.19−28 Based on our results, photodegradation of PP microplastics while floating on tranquil Milli-Q water did not result in size distributions consistent with photofragmentation being a dominant process; instead, results suggest that photochemical losses occurred from the surface of microplastics, indicated by plastic-mass loss and DOC accumulation, was the main driver of changing size distributions. In this case, particle size distributions depended on the initial size and abundance of particles added, and particle count should remain constant throughout the irradiation with the particles shrinking in size as they lose mass and carbon.
Multiple studies have demonstrated a link between photoirradiation and increased brittleness.78,79 The extensive cracking observed via SEM imaging at the surface of the plastic in this and other studies likely contributes to changes in tensile strength in plastics after irradiation.7,80,81 Cracking and brittleness can also explain the link between photoirradiation and subsequent formation of a greater number of nano- and microscale fragments in response to mechanical forces, such as stirring or abrasion with sand.15,16,82,83 The presence of water may also be critical in determining which mechanism of degradation will predominate during photoirradiation, as a recent study by Song et al. demonstrated particles, including large numbers of nanoparticles, were generated from PP irradiated under dry conditions without mechanical force.14 Our results complement these findings, demonstrating that sunlight exposure without mechanical force will predominantly result in carbon conversion to DOC and/or purgeable forms of C without significant particle production when PP is irradiated floating in water. When irradiation is combined with mechanical forces, these past studies have shown that fragments are produced in the expected power-law relationship with increased abundance as size decreases. Thus, photoirradiation likely enhances fragmentation of plastic debris in surface water environments experiencing mechanical forces like wave action, abrasion with sand, or impacts, resulting in a power-law relationship between size and abundance as reported in field studies.15,16,22−24,82 However, models suggest that particles in the low μm range will not experience sufficient stress from wave action to promote fragmentation and in these cases dissolution may predominate.84
The results of this study show that for sub-millimeter PP sunlight exposure induced photodissolution without significant fragmentation over one year of irradiation. Instead of fragmenting into a greater number of smaller microplastics, the initial population of microplastics shrank in size. The conversion of plastic-C to DOC or purgeable carbon due to photoirradiation is a powerful route of sub-millimeter plastic removal. In this work, we demonstrate that photodissolution of PP floating in calm water can occur without production of secondary fragments.
Acknowledgments
The authors thank Bill Fowel for assistance with the SEM imaging. This work was funded by NSF EAGER 2127669 and NSF CBET 1910621 and 1911257. Manuscript preparation was supported by the Albert Seabag PhD ’02 Graduate Fellowship.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.est.3c07161.
Tables describing fit equations for all figures in the main text; DOC accumulation fits and tabulated size distribution peak spans; energy dispersive spectroscopy of glass microfiber contamination in SEM images; and additional time points of SEM images not included in the main text for clarity (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Stubbins A.; Law K. L.; Muñoz S. E.; Bianchi T. S.; Zhu L. Plastics in the Earth system. Science 2021, 373 (6550), 51–55. 10.1126/science.abb0354. [DOI] [PubMed] [Google Scholar]
- Geyer R.; Jambeck J. R.; Law K. L. Production, use, and fate of all plastics ever made. Science advances 2017, 3 (7), e1700782 10.1126/sciadv.1700782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrelle S. B.; Ringma J.; Law K. L.; Monnahan C. C.; Lebreton L.; McGivern A.; Murphy E.; Jambeck J.; Leonard G. H.; Hilleary M. A.; et al. Predicted growth in plastic waste exceeds efforts to mitigate plastic pollution. Science 2020, 369 (6510), 1515–1518. 10.1126/science.aba3656. [DOI] [PubMed] [Google Scholar]
- Jambeck J. R.; Geyer R.; Wilcox C.; Siegler T. R.; Perryman M.; Andrady A.; Narayan R.; Law K. L. Plastic waste inputs from land into the ocean. Science 2015, 347 (6223), 768–771. 10.1126/science.1260352. [DOI] [PubMed] [Google Scholar]
- Lebreton L.; Egger M.; Slat B. A global mass budget for positively buoyant macroplastic debris in the ocean. Sci. Rep. 2019, 9 (1), 12922. 10.1038/s41598-019-49413-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes D. K.; Galgani F.; Thompson R. C.; Barlaz M. Accumulation and fragmentation of plastic debris in global environments. Philosophical transactions of the royal society B: biological sciences 2009, 364 (1526), 1985–1998. 10.1098/rstb.2008.0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrady A. L. Microplastics in the marine environment. Marine pollution bulletin 2011, 62 (8), 1596–1605. 10.1016/j.marpolbul.2011.05.030. [DOI] [PubMed] [Google Scholar]
- Chamas A.; Moon H.; Zheng J.; Qiu Y.; Tabassum T.; Jang J. H.; Abu-Omar M.; Scott S. L.; Suh S. Degradation rates of plastics in the environment. ACS Sustainable Chem. Eng. 2020, 8 (9), 3494–3511. 10.1021/acssuschemeng.9b06635. [DOI] [Google Scholar]
- Van Sebille E.; Wilcox C.; Lebreton L.; Maximenko N.; Hardesty B. D.; Van Franeker J. A.; Eriksen M.; Siegel D.; Galgani F.; Law K. L. A global inventory of small floating plastic debris. Environmental Research Letters 2015, 10 (12), 124006 10.1088/1748-9326/10/12/124006. [DOI] [Google Scholar]
- Isobe A.; Iwasaki S. The fate of missing ocean plastics: Are they just a marine environmental problem?. Sci. Total Environ. 2022, 825, 153935 10.1016/j.scitotenv.2022.153935. [DOI] [PubMed] [Google Scholar]
- Law K. L.; Morét-Ferguson S.; Maximenko N. A.; Proskurowski G.; Peacock E. E.; Hafner J.; Reddy C. M. Plastic accumulation in the North Atlantic subtropical gyre. Science 2010, 329 (5996), 1185–1188. 10.1126/science.1192321. [DOI] [PubMed] [Google Scholar]
- Kaandorp M. L.; Lobelle D.; Kehl C.; Dijkstra H. A.; van Sebille E. Global mass of buoyant marine plastics dominated by large long-lived debris. Nature Geoscience 2023, 16, 689. 10.1038/s41561-023-01216-0. [DOI] [Google Scholar]
- Mattsson K.; Björkroth F.; Karlsson T.; Hassellöv M. Nanofragmentation of expanded polystyrene under simulated environmental weathering (thermooxidative degradation and hydrodynamic turbulence). Frontiers in Marine Science 2021, 7, 578178 10.3389/fmars.2020.578178. [DOI] [Google Scholar]
- Song Y. K.; Hong S. H.; Eo S.; Shim W. J. The fragmentation of nano-and microplastic particles from thermoplastics accelerated by simulated-sunlight-mediated photooxidation. Environ. Pollut. 2022, 311, 119847 10.1016/j.envpol.2022.119847. [DOI] [PubMed] [Google Scholar]
- Sorasan C.; Edo C.; González-Pleiter M.; Fernández-Piñas F.; Leganés F.; Rodríguez A.; Rosal R. Generation of nanoplastics during the photoageing of low-density polyethylene. Environ. Pollut. 2021, 289, 117919 10.1016/j.envpol.2021.117919. [DOI] [PubMed] [Google Scholar]
- Sorasan C.; Edo C.; González-Pleiter M.; Fernández-Piñas F.; Leganés F.; Rodríguez A.; Rosal R. Ageing and fragmentation of marine microplastics. Science of The Total Environment 2022, 827, 154438 10.1016/j.scitotenv.2022.154438. [DOI] [PubMed] [Google Scholar]
- Ter Halle A.; Jeanneau L.; Martignac M.; Jardé E.; Pedrono B.; Brach L.; Gigault J. Nanoplastic in the North Atlantic subtropical gyre. Environ. Sci. Technol. 2017, 51 (23), 13689–13697. 10.1021/acs.est.7b03667. [DOI] [PubMed] [Google Scholar]
- Turcotte D. L. Fractals and fragmentation. Journal of Geophysical Research: Solid Earth 1986, 91 (B2), 1921–1926. 10.1029/JB091iB02p01921. [DOI] [Google Scholar]
- Cózar A.; Echevarría F.; González-Gordillo J. I.; Irigoien X.; Úbeda B.; Hernández-León S.; Palma Á. T.; Navarro S.; García-de-Lomas J.; Ruiz A.; et al. Plastic debris in the open ocean. Proc. Natl. Acad. Sci. U. S. A. 2014, 111 (28), 10239–10244. 10.1073/pnas.1314705111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cózar A.; Sanz-Martín M.; Martí E.; González-Gordillo J. I.; Ubeda B.; Gálvez J. Á.; Irigoien X.; Duarte C. M. Plastic accumulation in the Mediterranean Sea. PloS one 2015, 10 (4), e0121762 10.1371/journal.pone.0121762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksen M.; Maximenko N.; Thiel M.; Cummins A.; Lattin G.; Wilson S.; Hafner J.; Zellers A.; Rifman S. Plastic pollution in the South Pacific subtropical gyre. Marine pollution bulletin 2013, 68 (1–2), 71–76. 10.1016/j.marpolbul.2012.12.021. [DOI] [PubMed] [Google Scholar]
- Isobe A.; Kubo K.; Tamura Y.; Kako S.; Nakashima E.; Fujii N. Selective transport of microplastics and mesoplastics by drifting in coastal waters. Marine pollution bulletin 2014, 89 (1–2), 324–330. 10.1016/j.marpolbul.2014.09.041. [DOI] [PubMed] [Google Scholar]
- Isobe A.; Uchida K.; Tokai T.; Iwasaki S. East Asian seas: a hot spot of pelagic microplastics. Marine pollution bulletin 2015, 101 (2), 618–623. 10.1016/j.marpolbul.2015.10.042. [DOI] [PubMed] [Google Scholar]
- Ruiz-Orejón L. F.; Sardá R.; Ramis-Pujol J. Now, you see me: high concentrations of floating plastic debris in the coastal waters of the Balearic Islands (Spain). Marine pollution bulletin 2018, 133, 636–646. 10.1016/j.marpolbul.2018.06.010. [DOI] [PubMed] [Google Scholar]
- Kooi M.; Primpke S.; Mintenig S. M.; Lorenz C.; Gerdts G.; Koelmans A. A. Characterizing the multidimensionality of microplastics across environmental compartments. Water Res. 2021, 202, 117429 10.1016/j.watres.2021.117429. [DOI] [PubMed] [Google Scholar]
- Leusch F. D.; Lu H.-C.; Perera K.; Neale P. A.; Ziajahromi S. Analysis of the literature shows a remarkably consistent relationship between size and abundance of microplastics across different environmental matrices. Environ. Pollut. 2023, 319, 120984 10.1016/j.envpol.2022.120984. [DOI] [PubMed] [Google Scholar]
- Perera K.; Ziajahromi S.; Bengtson Nash S.; Manage P. M.; Leusch F. D. Airborne microplastics in indoor and outdoor environments of a developing country in south asia: abundance, distribution, morphology, and possible sources. Environ. Sci. Technol. 2022, 56 (23), 16676–16685. 10.1021/acs.est.2c05885. [DOI] [PubMed] [Google Scholar]
- George M.; Nallet F.; Fabre P. A threshold model of plastic waste fragmentation: new insights into the distribution of microplastics in the ocean and its evolution over time. Mar. Pollut. Bull. 2024, 199, 116012 10.1016/j.marpolbul.2023.116012. [DOI] [PubMed] [Google Scholar]
- Enders K.; Lenz R.; Stedmon C. A.; Nielsen T. G. Abundance, size and polymer composition of marine microplastics≥ 10 μm in the Atlantic Ocean and their modelled vertical distribution. Marine pollution bulletin 2015, 100 (1), 70–81. 10.1016/j.marpolbul.2015.09.027. [DOI] [PubMed] [Google Scholar]
- Fazey F. M.; Ryan P. G. Biofouling on buoyant marine plastics: An experimental study into the effect of size on surface longevity. Environmental pollution 2016, 210, 354–360. 10.1016/j.envpol.2016.01.026. [DOI] [PubMed] [Google Scholar]
- Kooi M.; Nes E. H. v.; Scheffer M.; Koelmans A. A. Ups and downs in the ocean: effects of biofouling on vertical transport of microplastics. Environ. Sci. Technol. 2017, 51 (14), 7963–7971. 10.1021/acs.est.6b04702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral-Zettler L. A.; Zettler E. R.; Mincer T. J.; Klaassen M. A.; Gallager S. M. Biofouling impacts on polyethylene density and sinking in coastal waters: A macro/micro tipping point?. Water Res. 2021, 201, 117289 10.1016/j.watres.2021.117289. [DOI] [PubMed] [Google Scholar]
- Kaiser D.; Kowalski N.; Waniek J. J. Effects of biofouling on the sinking behavior of microplastics. Environmental research letters 2017, 12 (12), 124003 10.1088/1748-9326/aa8e8b. [DOI] [Google Scholar]
- Jâms I. B.; Windsor F. M.; Poudevigne-Durance T.; Ormerod S. J.; Durance I. Estimating the size distribution of plastics ingested by animals. Nat. Commun. 2020, 11 (1), 1594. 10.1038/s41467-020-15406-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foekema E. M.; De Gruijter C.; Mergia M. T.; van Franeker J. A.; Murk A. J.; Koelmans A. A. Plastic in north sea fish. Environ. Sci. Technol. 2013, 47 (15), 8818–8824. 10.1021/es400931b. [DOI] [PubMed] [Google Scholar]
- Lusher A. L.; Mchugh M.; Thompson R. C. Occurrence of microplastics in the gastrointestinal tract of pelagic and demersal fish from the English Channel. Marine pollution bulletin 2013, 67 (1–2), 94–99. 10.1016/j.marpolbul.2012.11.028. [DOI] [PubMed] [Google Scholar]
- Gewert B.; Plassmann M. M.; MacLeod M. Pathways for degradation of plastic polymers floating in the marine environment. Environmental science: processes & impacts 2015, 17 (9), 1513–1521. 10.1039/C5EM00207A. [DOI] [PubMed] [Google Scholar]
- Ranby B.; Lucki J. New aspects of photodegradation and photooxidation of polystyrene. Pure Appl. Chem. 1980, 52 (2), 295–303. 10.1351/pac198052020295. [DOI] [Google Scholar]
- Romera-Castillo C.; Pinto M.; Langer T. M.; Álvarez-Salgado X. A.; Herndl G. J. Dissolved organic carbon leaching from plastics stimulates microbial activity in the ocean. Nat. Commun. 2018, 9 (1), 1430. 10.1038/s41467-018-03798-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delre A.; Goudriaan M.; Morales V. H.; Vaksmaa A.; Ndhlovu R. T.; Baas M.; Keijzer E.; de Groot T.; Zeghal E.; Egger M.; et al. Plastic photodegradation under simulated marine conditions. Mar. Pollut. Bull. 2023, 187, 114544 10.1016/j.marpolbul.2022.114544. [DOI] [PubMed] [Google Scholar]
- Zhu L.; Zhao S.; Bittar T. B.; Stubbins A.; Li D. Photochemical dissolution of buoyant microplastics to dissolved organic carbon: rates and microbial impacts. Journal of hazardous materials 2020, 383, 121065 10.1016/j.jhazmat.2019.121065. [DOI] [PubMed] [Google Scholar]
- Corcoran P. L.Degradation of microplastics in the environment. In Handbook of Microplastics in the Environment; Springer: 2022; pp 531–542. [Google Scholar]
- Zhu K.; Jia H.; Zhao S.; Xia T.; Guo X.; Wang T.; Zhu L. Formation of environmentally persistent free radicals on microplastics under light irradiation. Environ. Sci. Technol. 2019, 53 (14), 8177–8186. 10.1021/acs.est.9b01474. [DOI] [PubMed] [Google Scholar]
- Gijsman P.; Meijers G.; Vitarelli G. Comparison of the UV-degradation chemistry of polypropylene, polyethylene, polyamide 6 and polybutylene terephthalate. Polym. Degrad. Stab. 1999, 65 (3), 433–441. 10.1016/S0141-3910(99)00033-6. [DOI] [Google Scholar]
- Rowenczyk L.; Dazzi A.; Deniset-Besseau A.; Beltran V.; Goudounèche D.; Wong-Wah-Chung P.; Boyron O.; George M.; Fabre P.; Roux C.; et al. Microstructure characterization of oceanic polyethylene debris. Environ. Sci. Technol. 2020, 54 (7), 4102–4109. 10.1021/acs.est.9b07061. [DOI] [PubMed] [Google Scholar]
- Takahashi Y.; Tanaka K.; Kajiwara T.; Suzuki G.; Osako M.; Kuramochi H. Cross-sectional microstructural analysis to evaluate the crack growth pattern of weathered marine plastics. Chemosphere 2023, 331, 138794 10.1016/j.chemosphere.2023.138794. [DOI] [PubMed] [Google Scholar]
- Hebner T. S.; Maurer-Jones M. A. Characterizing microplastic size and morphology of photodegraded polymers placed in simulated moving water conditions. Environmental Science: Processes & Impacts 2020, 22 (2), 398–407. 10.1039/C9EM00475K. [DOI] [PubMed] [Google Scholar]
- Erni-Cassola G.; Zadjelovic V.; Gibson M. I.; Christie-Oleza J. A. Distribution of plastic polymer types in the marine environment; A meta-analysis. Journal of hazardous materials 2019, 369, 691–698. 10.1016/j.jhazmat.2019.02.067. [DOI] [PubMed] [Google Scholar]
- Andrady A.; Pegram J.; Searle N. Wavelength sensitivity of enhanced photodegradable polyethylenes, ECO, and LDPE/MX. J. Appl. Polym. Sci. 1996, 62 (9), 1457–1463. . [DOI] [Google Scholar]
- Zhenfeng Z.; Xingzhou H.; Zubo L. Wavelength sensitivity of photooxidation of polypropylene. Polym. Degrad. Stab. 1996, 51 (1), 93–97. 10.1016/0141-3910(95)00210-3. [DOI] [Google Scholar]
- Powers L. C.; Brandes J. A.; Stubbins A.; Miller W. L. MoDIE: Moderate dissolved inorganic carbon (DI13C) isotope enrichment for improved evaluation of DIC photochemical production in natural waters. Marine chemistry 2017, 194, 1–9. 10.1016/j.marchem.2017.03.007. [DOI] [Google Scholar]
- Ruggaber A.; Dlugi R.; Nakajima T. Modelling radiation quantities and photolysis frequencies in the troposphere. Journal of Atmospheric Chemistry 1994, 18, 171–210. 10.1007/BF00696813. [DOI] [Google Scholar]
- Stubbins A.; Dittmar T. Low volume quantification of dissolved organic carbon and dissolved nitrogen. Limnology and Oceanography: Methods 2012, 10 (5), 347–352. 10.4319/lom.2012.10.347. [DOI] [Google Scholar]
- Wu X.; Liu P.; Shi H.; Wang H.; Huang H.; Shi Y.; Gao S. Photo aging and fragmentation of polypropylene food packaging materials in artificial seawater. Water Res. 2021, 188, 116456 10.1016/j.watres.2020.116456. [DOI] [PubMed] [Google Scholar]
- Barton A. F.Handbook of polymer-liquid interaction parameters and solubility parameters; CRC Press: 1990. [Google Scholar]
- Cai L.; Wang J.; Peng J.; Wu Z.; Tan X. Observation of the degradation of three types of plastic pellets exposed to UV irradiation in three different environments. Sci. Total Environ. 2018, 628, 740–747. 10.1016/j.scitotenv.2018.02.079. [DOI] [PubMed] [Google Scholar]
- Mundhenke T. F.; Li S. C.; Maurer-Jones M. A. Photodegradation of polyolefin thin films in simulated freshwater conditions. Environmental Science: Processes & Impacts 2022, 24 (12), 2284–2293. 10.1039/D2EM00359G. [DOI] [PubMed] [Google Scholar]
- Ranjan V. P.; Goel S. Degradation of low-density polyethylene film exposed to UV radiation in four environments. Journal of Hazardous, Toxic, and Radioactive Waste 2019, 23 (4), 04019015 10.1061/(ASCE)HZ.2153-5515.0000453. [DOI] [Google Scholar]
- Wu M.; Ma Y.; Xie H.; Ji R. Photodissolution of submillimeter-sized microplastics and its dependences on temperature and light composition. Science of The Total Environment 2022, 848, 157714 10.1016/j.scitotenv.2022.157714. [DOI] [PubMed] [Google Scholar]
- Fernando S. S.; Christensen P. A.; Egerton T. A.; White J. R. Carbon dioxide evolution and carbonyl group development during photodegradation of polyethylene and polypropylene. Polym. Degrad. Stab. 2007, 92 (12), 2163–2172. 10.1016/j.polymdegradstab.2007.01.032. [DOI] [Google Scholar]
- Wayman C.; Niemann H. The fate of plastic in the ocean environment–a minireview. Environmental Science: Processes & Impacts 2021, 23 (2), 198–212. 10.1039/D0EM00446D. [DOI] [PubMed] [Google Scholar]
- Morris D. P.; Hargreaves B. R. The role of photochemical degradation of dissolved organic carbon in regulating the UV transparency of three lakes on the Pocono Plateau. Limnology and Oceanography 1997, 42 (2), 239–249. 10.4319/lo.1997.42.2.0239. [DOI] [Google Scholar]
- Granéli W.; Lindell M.; Tranvik L. Photo-oxidative production of dissolved inorganic carbon in lakes of different humic content. Limnology and oceanography 1996, 41 (4), 698–706. 10.4319/lo.1996.41.4.0698. [DOI] [Google Scholar]
- Porcal P.; Dillon P. J.; Molot L. A. Temperature dependence of photodegradation of dissolved organic matter to dissolved inorganic carbon and particulate organic carbon. PLoS One 2015, 10 (6), e0128884 10.1371/journal.pone.0128884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller W. L.; Zepp R. G. Photochemical production of dissolved inorganic carbon from terrestrial organic matter: Significance to the oceanic organic carbon cycle. Geophys. Res. Lett. 1995, 22 (4), 417–420. 10.1029/94GL03344. [DOI] [Google Scholar]
- Mower M. P.; Blackmond D. G. Mechanistic Rationalization of Unusual Sigmoidal Kinetic Profiles in the Machetti–De Sarlo Cycloaddition Reaction. J. Am. Chem. Soc. 2015, 137 (6), 2386–2391. 10.1021/ja512753v. [DOI] [PubMed] [Google Scholar]
- Wright M. R.Introduction to chemical kinetics; John Wiley & Sons: 2005. [Google Scholar]
- Wen Q.; Liu N.; Qu R.; Ge F. High salinity promotes the photoaging of polystyrene microplastics with humic acid in seawater. Science of The Total Environment 2023, 901, 165741 10.1016/j.scitotenv.2023.165741. [DOI] [PubMed] [Google Scholar]
- Lei Y.; Lei X.; Westerhoff P.; Zhang X.; Yang X. Reactivity of chlorine radicals (Cl• and Cl2•−) with dissolved organic matter and the formation of chlorinated byproducts. Environ. Sci. Technol. 2021, 55 (1), 689–699. 10.1021/acs.est.0c05596. [DOI] [PubMed] [Google Scholar]
- Carlsson D.; Wiles D. The photodegradation of polypropylene films. III. Photolysis of polypropylene hydroperoxides. Macromolecules 1969, 2 (6), 597–606. 10.1021/ma60012a007. [DOI] [Google Scholar]
- Rouillon C.; Bussiere P.-O.; Desnoux E.; Collin S.; Vial C.; Therias S.; Gardette J.-L. Is carbonyl index a quantitative probe to monitor polypropylene photodegradation?. Polymer degradation and stability 2016, 128, 200–208. 10.1016/j.polymdegradstab.2015.12.011. [DOI] [Google Scholar]
- Tabatabaei S. H.; Carreau P. J.; Ajji A. Effect of processing on the crystalline orientation, morphology, and mechanical properties of polypropylene cast films and microporous membrane formation. Polymer 2009, 50 (17), 4228–4240. 10.1016/j.polymer.2009.06.071. [DOI] [Google Scholar]
- Chang A.; Tau L.; Hiltner A.; Baer E. Structure of blown film from blends of polyethylene and high melt strength polypropylene. Polymer 2002, 43 (18), 4923–4933. 10.1016/S0032-3861(02)00304-X. [DOI] [Google Scholar]
- Deng H.; Su L.; Zheng Y.; Du F.; Liu Q.-X.; Zheng J.; Zhou Z.; Shi H. Crack patterns of environmental plastic fragments. Environ. Sci. Technol. 2022, 56 (10), 6399–6414. 10.1021/acs.est.1c08100. [DOI] [PubMed] [Google Scholar]
- Lambert S.; Wagner M. Characterisation of nanoplastics during the degradation of polystyrene. Chemosphere 2016, 145, 265–268. 10.1016/j.chemosphere.2015.11.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittmar T.; Stubbins A. 12.6-Dissolved organic matter in aquatic systems. Treatise on geochemistry 2014, 2, 125. 10.1016/B978-0-08-095975-7.01010-X. [DOI] [Google Scholar]
- Kaandorp M. L.; Dijkstra H. A.; Van Sebille E. Modelling size distributions of marine plastics under the influence of continuous cascading fragmentation. Environmental Research Letters 2021, 16 (5), 054075 10.1088/1748-9326/abe9ea. [DOI] [Google Scholar]
- Jabarin S. A.; Lofgren E. A. Photooxidative effects on properties and structure of high-density polyethylene. J. Appl. Polym. Sci. 1994, 53 (4), 411–423. 10.1002/app.1994.070530404. [DOI] [Google Scholar]
- Brandon J.; Goldstein M.; Ohman M. D. Long-term aging and degradation of microplastic particles: comparing in situ oceanic and experimental weathering patterns. Marine pollution bulletin 2016, 110 (1), 299–308. 10.1016/j.marpolbul.2016.06.048. [DOI] [PubMed] [Google Scholar]
- Qayyum M.; White J. Effect of stabilizers on failure mechanisms in weathered polypropylene. Polymer degradation and stability 1993, 41 (2), 163–172. 10.1016/0141-3910(93)90039-L. [DOI] [Google Scholar]
- Yakimets I.; Lai D.; Guigon M. Effect of photo-oxidation cracks on behaviour of thick polypropylene samples. Polym. Degrad. Stab. 2004, 86 (1), 59–67. 10.1016/j.polymdegradstab.2004.01.013. [DOI] [Google Scholar]
- Song Y. K.; Hong S. H.; Jang M.; Han G. M.; Jung S. W.; Shim W. J. Combined effects of UV exposure duration and mechanical abrasion on microplastic fragmentation by polymer type. Environ. Sci. Technol. 2017, 51 (8), 4368–4376. 10.1021/acs.est.6b06155. [DOI] [PubMed] [Google Scholar]
- Meides N.; Mauel A.; Menzel T.; Altstädt V.; Ruckdäschel H.; Senker J.; Strohriegl P. Quantifying the fragmentation of polypropylene upon exposure to accelerated weathering. Microplastics and Nanoplastics 2022, 2 (1), 23. 10.1186/s43591-022-00042-2. [DOI] [Google Scholar]
- Brouzet C.; Guiné R.; Dalbe M.-J.; Favier B.; Vandenberghe N.; Villermaux E.; Verhille G. Laboratory model for plastic fragmentation in the turbulent ocean. Physical Review Fluids 2021, 6 (2), 024601 10.1103/PhysRevFluids.6.024601. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.