Abstract
The molecular subtypes of endometrial carcinoma (EC) were first described by The Cancer Genome Atlas (TCGA) a decade ago. Using surrogate approaches, the molecular classification has been demonstrated to be prognostic across EC patients and to have predictive implications. Starting in 2020, the molecular classification has been incorporated into multiple guidelines as part of the risk assessment and most recently into the International Federation of Gynecology and Obstetrics (FIGO) staging. This review article discusses the implementation of the EC molecular classification into clinical practice, the therapeutic implications, and the molecular and clinical heterogeneity of the EC molecular subtypes.
Introduction
The four molecular subtypes of endometrial carcinoma (EC), including POLE ultramutated, microsatellite instability hypermutated (MSI-H), copy number-low (CN-L), and copy number-high (CN-H), were first described by The Cancer Genome Atlas (TCGA) in a landmark study a decade ago.1 Subsequently, these molecular subtypes have been shown to drive prognosis and treatment decisions. As a result, the importance of elucidating these subtypes has been incorporated into multiple guidelines, including the World Health Organization Classification,2 National Comprehensive Cancer Network (NCCN) guidelines,3 the European Society of Gynaecological Oncology (ESGO), the European SocieTy for Radiotherapy and Oncology (ESTRO), and the European Society of Pathology (ESP) guidelines4 and the European Society of Medical Oncology (ESMO) guidelines.5 Most recently, also the International Federation of Gynecology and Obstetrics (FIGO) 2023 EC staging system has integrated molecular classification.6 Here, we discuss the identification of the EC molecular subtypes and their implementation into clinical practice, their prognostic and therapeutic implications, and the molecular and clinical heterogeneity within the EC molecular subtype classes.
Molecular subtypes of EC: the beginning and implementation into clinical practice
The TCGA classified ECs into four groups based on whole-exome sequencing analysis of 248 endometrioid, serous and mixed endometrioid/serous ECs using a combination of somatic mutations, CN alterations and MSI.1 Importantly, each molecular subtype category, as a group, was shown to be associated with distinct clinical outcomes with the POLE ECs exhibiting the best outcomes, CN-H the worst outcomes and the CN-L and MSI-H having intermediate outcomes.1 Over time, it was demonstrated that molecular classification adds another layer of relevant information, and there is a growing body of literature suggesting molecular data should be integrated with other well-known prognostic clinicopathologic features, including age, stage, histologic type, tumor grade, myometrial invasion, and lymphovascular space invasion (LVSI).6–10 Implementation of a whole-exome sequencing based classification into clinical practice is not practical, and surrogate marker approaches have been developed, employing mutational analysis of the exonuclease domain of POLE, immunohistochemical analysis (IHC) of the DNA mismatch repair (MMR) proteins MLH1, MSH2, MSH6 and PMS2 as a surrogate for MSI-H, and p53 IHC as a surrogate for CN-H to identify prognostic groups analogous to the TCGA classification (Figure 1).11,12 The feasibility and validity of this surrogate algorithm has been confirmed in numerous studies, including data from clinical trials, consistently demonstrating the prognostic association of the molecular subtypes with outcome.11,13–16 Importantly, it has been shown that the prognostic relevance of molecular classification extends to other histologic subtypes including clear cell carcinoma, carcinosarcoma and un/de-differentiated carcinoma, however that the prevalence of each molecular subtype varies across histologic types.14,17–22
Figure 1. Schematic of molecular subtype classification of endometrial carcinoma using surrogates based on immunohistochemistry and/or molecular data.
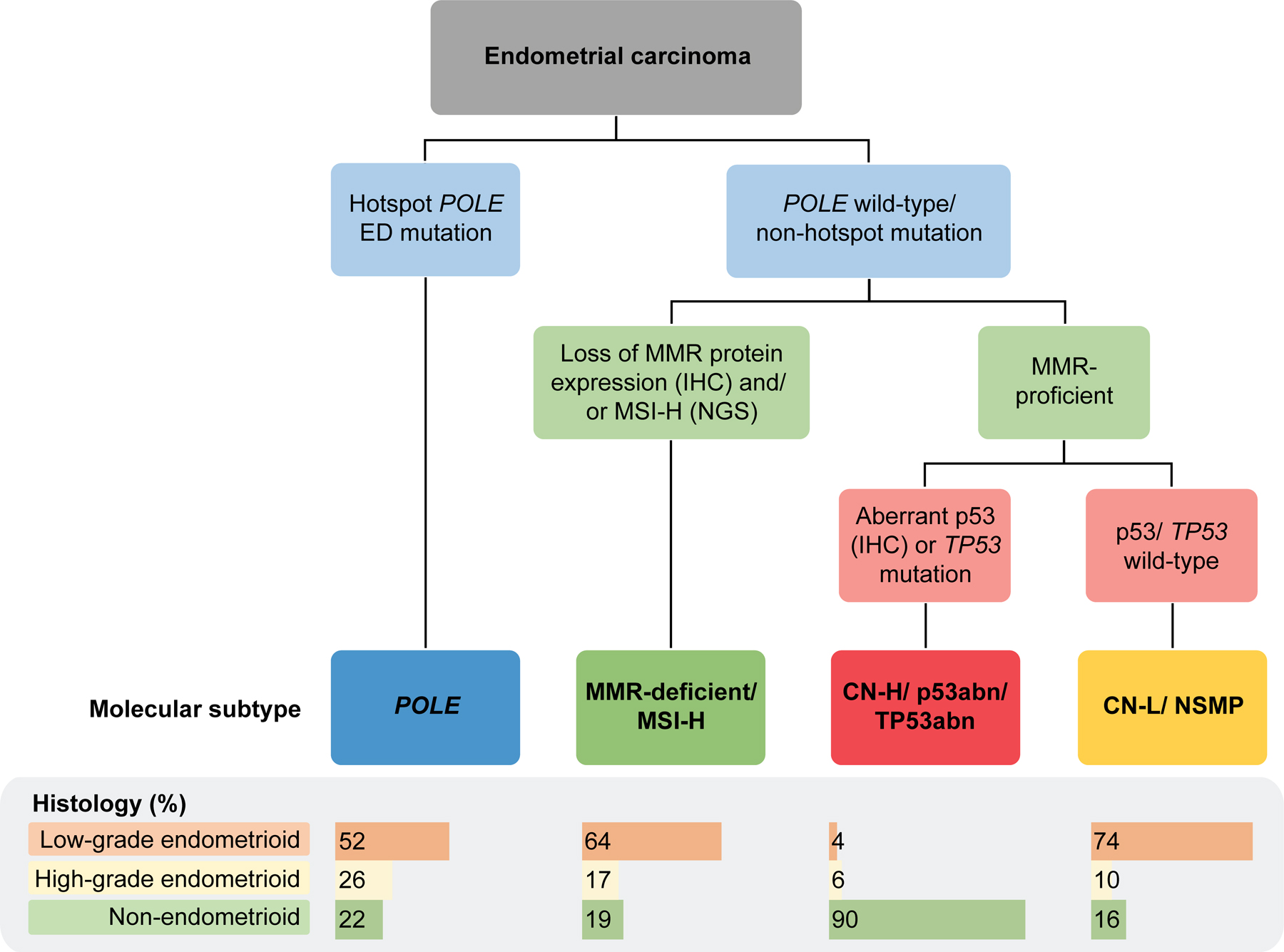
Abn, abnormal; CN-H, copy number-high; CN-L, copy number-low; ED, exonuclease domain; IHC, immunohistochemistry; MMR, mismatch repair; MSI-H, microsatellite instability-high; NGS, next generation sequencing; NSMP, no specific molecular profile; POLE, polymerase epsilon. The distribution of histological types by molecular subtype are obtained from Rios-Doria et al.14
With the introduction of clinical sequencing, our team has demonstrated that clinical tumor-normal sequencing data obtained from an FDA-authorized cancer gene panel (MSK-IMPACT)23 can be utilized for the molecular classification of EC across histologic types.14 The integration of molecular and IHC data (Figure 1), including POLE mutation status, sequencing-based MSIsensor score and/or MMR IHC for the MSI-H subtype classification and TP53 mutation and/or MDM2 amplification status for the CN-H/TP53abnormal (abn) molecular subtype, this integrated classification had a near perfect agreement with the conventional surrogate approach.14 Furthermore, it was demonstrated that the defining characteristics of the molecular subtypes can be obtained from next-generation sequencing (NGS) data and can be utilized for EC molecular classification, and that at the same time discrepancies between TP53 mutation and p53 IHC status can be overcome.14,24,25 Other groups have published on cost-effective and efficient approaches for POLE sequencing, which can be utilized in the molecular work-up of ECs.26–29 These data show that distinct approaches, technologies, and assays may be used for the molecular classification of ECs.25 This is of particular importance with the integration of molecular subtype into the 2023 FIGO staging classification; the latest iteration of staging for EC incorporates molecular, distinguishes aggressive versus non-aggressive histologic subtypes and extent of LVSI.6,10,30
Therapeutic implications of the EC molecular subtypes
While the molecular subtypes of EC have been shown repeatedly to provide independent prognostic information, the data for therapeutic implications of molecular subtypes are more limited to date, however there are developments of how the molecular classification can be used to guide both conventional and targeted therapy.9 Retrospective analysis of the Post-Operative Radiation Therapy in Endometrial Carcinoma (PORTEC-3) clinical trial data showed that the addition of chemotherapy to adjuvant radiotherapy significantly improved recurrence-free survival in patients with p53abn ECs compared to radiotherapy alone (59% vs 36%), however, this was not observed in any other molecular subtype.31 The MMR-deficient/MSI-H molecular subtype has been shown to be a predictive biomarker for immune checkpoint blockade (ICB) across all cancers.32 The PD-1 inhibitors pembrolizumab and dostarlimab are both approved in recurrent MMR-deficient/MSI-H EC. In March 2023, two large, randomized, placebo-controlled, phase III clinical trials (GY018 and RUBY) demonstrated that the addition of ICB to standard chemotherapy in EC patients with advanced or recurrent disease resulted in significantly improved progression-free survival, in particular in those with MMR-deficient/MSI-H tumors.33,34 In July 2023, based on the results of the RUBY trial, the FDA approved dostarlimab in combination with chemotherapy for the treatment of primary advanced or recurrent MMR-deficient EC including carcinosarcomas.34,35 In addition to MSI-H/MMR-deficiency, tumor mutational burden (TMB) is a marker of response to ICB. Pembrolizumab is approved for unresectable and metastatic solid tumors with TMB of ≥10 mutations per megabase,36 which could include subsets of MMR-deficient/MSI-H and POLE EC.
There are currently several phase II/III clinical trials ongoing, focusing on specific molecular subtypes. The RAINBO trial (NCT05255653) is a large umbrella study that elects treatment for patients with EC based on molecular subtype and includes four arms.37 1) the RED trial compares adjuvant chemoradiation with/ without the PARP inhibitor Olaparib in p53abn EC; 2) the GREEN trial compares adjuvant radiotherapy with/without durvalumab in MMR-deficient EC; 3) the ORANGE trial assesses in specific subgroups CN-L/ no specific molecular profile (NSMP) EC treatment de-escalation (ER+ stage II EC with LVSI) and compares radiotherapy followed by progestin and adjuvant chemoradiation; 4) the BLUE trial assesses the safety of de-escalation of adjuvant therapy in stage I–III POLE EC. Other trials looking at treatment modification based on molecular classification include, amongst others, PORTEC-4a,38 KEYNOTE-C93/GOG-3064/ENGOT-en15 (NCT05173987), NRG-GY020 (NCT04214067), TAPER (NCT04705649), ECMC-GART(NCT05524389), DOMENICA (NCT05201547), 2022-54 (NCT05454358), and CAN-STAMP (NCT04159155). The results of these prospective trials are eagerly awaited as these will shed more light into the association of the molecular subtypes with therapy response.
Heterogeneity within the EC molecular subtypes
In addition, there is heterogeneity within molecular subtypes (Figure 2). Our team and others have shown that the clinicopathologic features of MMR-deficient/MSI-H ECs differ according to the mechanism underpinning MSI-instability.39–41 MLH1-hypermethylated ECs were shown to be older, more obese, and had more advanced disease at diagnosis compared to those with germline or somatic MMR gene mutations.39 Furthermore, there is evidence to suggest that at least a subset of MLH1 hypermethylated EC patients have worse oncologic outcomes and response to ICB when compared to their gene-mutated counterparts (germline/somatic).39–41
Figure 2. Prognostic implication and heterogeneity within endometrial cancer molecular subtypes.
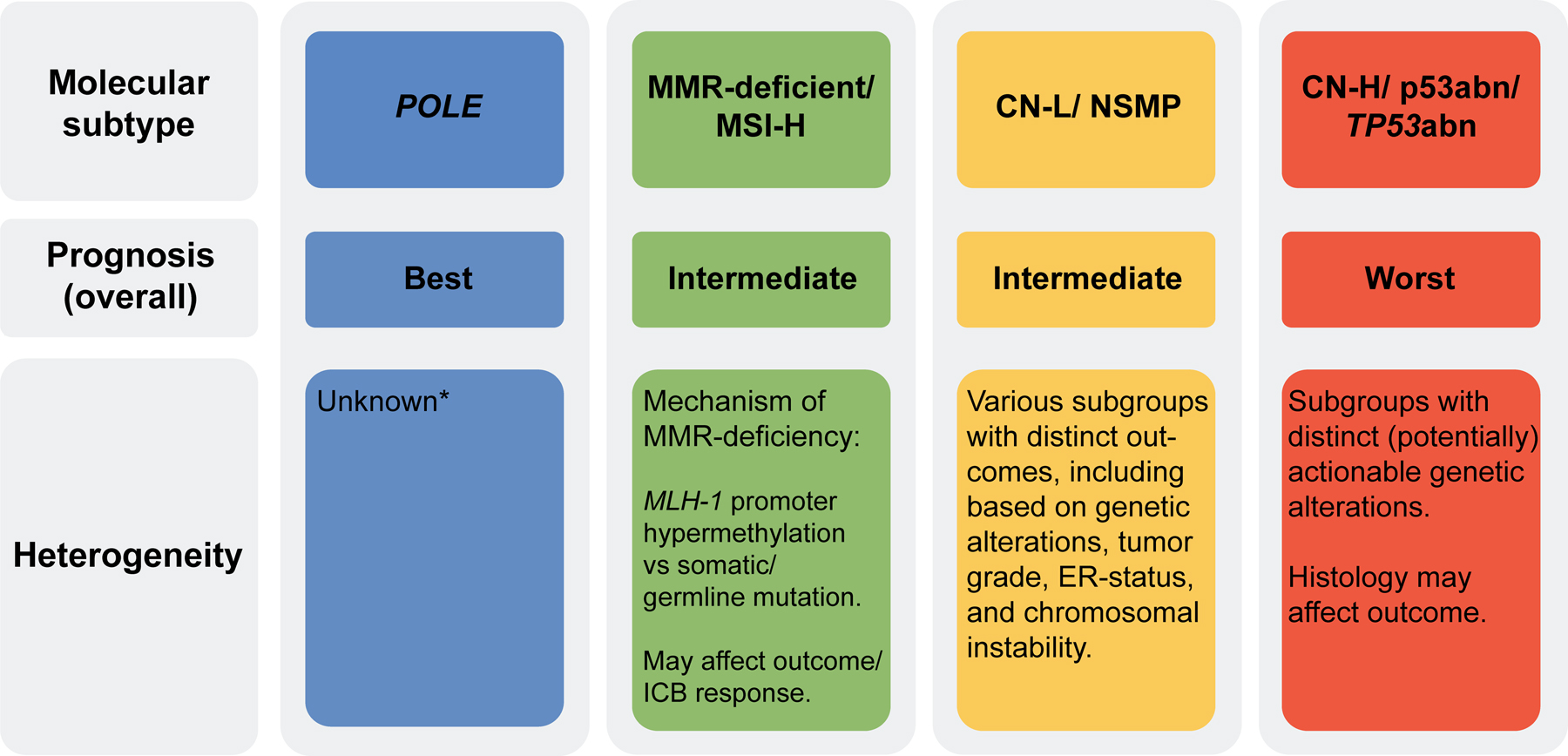
*Due to small numbers to date; abn, abnormal; CN-H, copy number-high; CN-L, copy number-low; ER, estrogen receptor; ICB, immune checkpoint blockade; MMR, mismatch repair; MSI-H, microsatellite instability-high; NSMP, no specific molecular profile; POLE, polymerase epsilon.
CN-low ECs, also referred to as of no specific molecular subtype (NSMP), is a heterogeneous group of ECs classified by exclusion and encompassing tumors that do not display any of the other 3 defining molecular subtype features (i.e., POLE mutation, MSI, p53/TP53 alteration).8,42 Several studies have focused on this NSMP group and showed that based on PTEN/ PIK3CA mutation status, histology/grade, chromosome 1q gain/amplification, estrogen receptor (ER) status or chromosomal instability subgroups with distinct outcomes could be identified.14,43–47
Finally, the CN-H/p53abn/TP53abn ECs, associated with the worst outcome among the four molecular subtypes groups, are genetically heterogeneous and several subgroups harboring potentially targetable alterations have been identified across histologic types.1,19,24,48 An area of active interest is the ERBB2/HER2-altered subgroup. Since Fader and colleagues showed that the addition of trastuzumab to carboplatin and paclitaxel in patients with stage III-IV or recurrent HER2-positive EC is associated with an improvement in progression-free survival,49,50 trastuzumab is endorsed by the NCCN guidelines for the treatment of advanced stage HER2-positive EC in combination with carboplatin/paclitaxel.51 Following the promising results of the antibody drug conjugate trastuzumab deruxtecan in breast and lung cancer,52–54 the NCCH1615/STATICE and DESTINY-PanTumor02 studies reported on trastuzumab deruxtecan for the treatment of HER2-positive advanced or recurrent ECs and carcinosarcomas with encouraging results.51,55 Several treatment strategies are currently being investigated in this CN-H/p53abn/TP53abn subgroup, including ICB, DNA damage response (DDR), and cell cycle regulator modulation/ inhibition, such as the WEE1 inhibitor adavosertib in combination with DNA-damaging chemotherapy (NCT03345784), the MDM2 inhibitor milademetan, or even chemotherapy optimization, contrasting trabectedin with standard chemotherapy regimens (MITO-END-3 trial, NCT03059485).
Our team has reported on the prevalence of molecular subtypes across different races/ ancestries using data from clinically sequenced ECs.56 Almost 70% of ECs in patients who self-identified as Black were of CN-H/TP53abn molecular subtype compared with just 35% of tumors among patients who self-identified as White. On the other hand, high TMB (≥ 10 mut/Mb) molecular subtypes such as MMR-deficient/MSI-H or POLE were less common in Black as compared with White patients with EC, and less likely to have actionable alterations including high TMB/MSI, which would preclude use of breakthrough treatments such as ICB.56 Additionally, the MMR-deficient/MSI-H molecular subtype is associated with Lynch Syndrome, an inherited cancer predisposition syndrome caused by germline pathogenic variants (gPV) in the mismatch repair genes, and ancestry-based variations in gPV and Lynch Syndrome have been observed.57 These ancestry-based tumor molecular differences may influence the racial/ethnic disparities in EC outcomes and are important to address in clinical trial accrual and efforts to improve health equity.
Conclusions
The modern era of molecular classification of EC has profoundly influenced the landscape of diagnosis, prognosis, and therapeutic interventions. The translation of the molecular subtypes into clinical practice, through both surrogates and clinical sequencing approaches, has allowed for a nuanced application of personalized medicine. The agreement between these methodologies ensures that molecular subtyping remains accessible and practical across various healthcare settings. While clinical outcomes diverge significantly among the molecular subtypes overall, with implications for prognosis and therapeutic choices, it is important to acknowledge the molecular and clinical heterogeneity within EC molecular subtypes with potential benefits of emerging therapies.
Synopsis:
The molecular classification of endometrial cancer, first described by The Cancer Genome Atlas a decade ago, has recently been incorporated into several international guidelines for risk assessment. We review the implementation of the endometrial cancer molecular classification into clinical practice, the prognostic and therapeutic implications, and the molecular and clinical heterogeneity within the molecular subtype classes.
Acknowledgments
This publication was supported in part by a Cancer Center Support Grant of the NIH/NCI (Grant No. P30CA008748). B. Weigelt is funded in part by Breast Cancer Research Foundation and Cycle for Survival grants.
Footnotes
Conflicts of interest
YLL receives research funding from AstraZeneca, GSK, and Repare Therapeutics unrelated to this work. BW reports research funding from Repare Therapeutics, outside of this work. The remaining authors have no conflicts of interest to disclose.
References:
- 1.Cancer Genome Atlas Research Network, Kandoth C, Schultz N, Cherniack AD, Akbani R, Liu Y, et al. Integrated genomic characterization of endometrial carcinoma. Nature 497, 67–73 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCluggage WG, Singh N, Gilks CB. Key changes to the World Health Organization (WHO) classification of female genital tumours introduced in the 5th edition (2020). Histopathology 80, 762–78 (2022). [DOI] [PubMed] [Google Scholar]
- 3.Abu-Rustum N, Yashar C, Arend R, Barber E, Bradley K, Brooks R, et al. Uterine Neoplasms, Version 1.2023, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 21, 181–209 (2023). [DOI] [PubMed] [Google Scholar]
- 4.Concin N, Matias-Guiu X, Vergote I, Cibula D, Mirza MR, Marnitz S, et al. ESGO/ESTRO/ESP guidelines for the management of patients with endometrial carcinoma. International Journal of Gynecologic Cancer 31, 12–39 (2021). [DOI] [PubMed] [Google Scholar]
- 5.Oaknin A, Bosse TJ, Creutzberg CL, Giornelli G, Harter P, Joly F, et al. Endometrial cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol 33, 860–77 (2022). [DOI] [PubMed] [Google Scholar]
- 6.Berek JS, Matias-Guiu X, Creutzberg C, Fotopoulou C, Gaffney D, Kehoe S, et al. FIGO staging of endometrial cancer: 2023. Int J Gynaecol Obstet 162, 383–94 (2023). [DOI] [PubMed] [Google Scholar]
- 7.Murali R, Soslow RA, Weigelt B. Classification of endometrial carcinoma: more than two types. Lancet Oncol 15, e268–78 (2014). [DOI] [PubMed] [Google Scholar]
- 8.McAlpine J, Leon-Castillo A, Bosse T. The rise of a novel classification system for endometrial carcinoma; integration of molecular subclasses. J Pathol 244, 538–49 (2018). [DOI] [PubMed] [Google Scholar]
- 9.Jamieson A, Bosse T, McAlpine JN. The emerging role of molecular pathology in directing the systemic treatment of endometrial cancer. Ther Adv Med Oncol 13, 17588359211035959 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bosse T, Peters EE, Creutzberg CL, Jurgenliemk-Schulz IM, Jobsen JJ, Mens JW, et al. Substantial lymph-vascular space invasion (LVSI) is a significant risk factor for recurrence in endometrial cancer--A pooled analysis of PORTEC 1 and 2 trials. Eur J Cancer 51, 1742–50 (2015). [DOI] [PubMed] [Google Scholar]
- 11.Stelloo E, Nout RA, Osse EM, Jurgenliemk-Schulz IJ, Jobsen JJ, Lutgens LC, et al. Improved Risk Assessment by Integrating Molecular and Clinicopathological Factors in Early-stage Endometrial Cancer-Combined Analysis of the PORTEC Cohorts. Clin Cancer Res 22, 4215–24 (2016). [DOI] [PubMed] [Google Scholar]
- 12.Talhouk A, McConechy MK, Leung S, Yang W, Lum A, Senz J, et al. Confirmation of ProMisE: A simple, genomics-based clinical classifier for endometrial cancer. Cancer 123, 802–13 (2017). [DOI] [PubMed] [Google Scholar]
- 13.Kommoss S, McConechy MK, Kommoss F, Leung S, Bunz A, Magrill J, et al. Final validation of the ProMisE molecular classifier for endometrial carcinoma in a large population-based case series. Ann Oncol 29, 1180–88 (2018). [DOI] [PubMed] [Google Scholar]
- 14.Rios-Doria E, Momeni-Boroujeni A, Friedman CF, Selenica P, Zhou Q, Wu M, et al. Integration of clinical sequencing and immunohistochemistry for the molecular classification of endometrial carcinoma. Gynecol Oncol 174, 262–72 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Asami Y, Kobayashi Kato M, Hiranuma K, Matsuda M, Shimada Y, Ishikawa M, et al. Utility of molecular subtypes and genetic alterations for evaluating clinical outcomes in 1029 patients with endometrial cancer. Br J Cancer 128, 1582–91 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stelloo E, Bosse T, Nout RA, MacKay HJ, Church DN, Nijman HW, et al. Refining prognosis and identifying targetable pathways for high-risk endometrial cancer; a TransPORTEC initiative. Mod Pathol 28, 836–44 (2015). [DOI] [PubMed] [Google Scholar]
- 17.Rosa-Rosa JM, Leskela S, Cristobal-Lana E, Santon A, Lopez-Garcia MA, Munoz G, et al. Molecular genetic heterogeneity in undifferentiated endometrial carcinomas. Mod Pathol 29, 1390–98 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DeLair DF, Burke KA, Selenica P, Lim RS, Scott SN, Middha S, et al. The genetic landscape of endometrial clear cell carcinomas. J Pathol 243, 230–41 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Da Cruz Paula A, DeLair DF, Ferrando L, Fix DJ, Soslow RA, Park KJ, et al. Genetic and molecular subtype heterogeneity in newly diagnosed early- and advanced-stage endometrial cancer. Gynecol Oncol 161, 535–44 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bogani G, Ray-Coquard I, Concin N, Ngoi NYL, Morice P, Enomoto T, et al. Clear cell carcinoma of the endometrium. Gynecol Oncol 164, 658–66 (2022). [DOI] [PubMed] [Google Scholar]
- 21.Kim SR, Cloutier BT, Leung S, Cochrane D, Britton H, Pina A, et al. Molecular subtypes of clear cell carcinoma of the endometrium: Opportunities for prognostic and predictive stratification. Gynecol Oncol 158, 3–11 (2020). [DOI] [PubMed] [Google Scholar]
- 22.McCluggage WG. Progress in the pathological arena of gynecological cancers. Int J Gynaecol Obstet 155 Suppl 1, 107–14 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng DT, Mitchell TN, Zehir A, Shah RH, Benayed R, Syed A, et al. Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT). The Journal of Molecular Diagnostics 17, 251–64 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Momeni-Boroujeni A, Dahoud W, Vanderbilt CM, Chiang S, Murali R, Rios-Doria EV, et al. Clinicopathologic and Genomic Analysis of TP53-Mutated Endometrial Carcinomas. Clin Cancer Res 27, 2613–23 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weigelt B, Mueller JJ, Ellenson LH, Abu-Rustum NR, Aghajanian C. Response to Letter to the Editor, Gilks et al. Gynecol Oncol 176, 181–82 (2023). [DOI] [PubMed] [Google Scholar]
- 26.Van den Heerik A, Ter Haar NT, Vermij L, Jobsen JJ, Brinkhuis M, Roothaan SM, et al. QPOLE: A Quick, Simple, and Cheap Alternative for POLE Sequencing in Endometrial Cancer by Multiplex Genotyping Quantitative Polymerase Chain Reaction. JCO Glob Oncol 9, e2200384 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Devereaux KA, Steiner DF, Ho C, Gomez AJ, Gilks B, Longacre TA, et al. A Multiplex SNaPshot Assay is a Rapid and Cost-Effective Method for Detecting POLE Exonuclease Domain Mutations in Endometrial Carcinoma. Int J Gynecol Pathol 41, 541–51 (2022). [DOI] [PubMed] [Google Scholar]
- 28.Laczmanska I, Michalowska D, Jedryka M, Blomka D, Semeniuk M, Czykalko E, et al. Fast and reliable Sanger POLE sequencing protocol in FFPE tissues of endometrial cancer. Pathol Res Pract 242, 154315 (2023). [DOI] [PubMed] [Google Scholar]
- 29.Dorca E, Velasco A, Varela M, Gatius S, Villatoro S, Fullana N, et al. Validation of Modaplex POLE mutation assay in endometrial carcinoma. Virchows Arch 10.1007/s00428-023-03636-0, (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tortorella L, Restaino S, Zannoni GF, Vizzielli G, Chiantera V, Cappuccio S, et al. Substantial lymph-vascular space invasion (LVSI) as predictor of distant relapse and poor prognosis in low-risk early-stage endometrial cancer. J Gynecol Oncol 32, e11 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leon-Castillo A, de Boer SM, Powell ME, Mileshkin LR, Mackay HJ, Leary A, et al. Molecular Classification of the PORTEC-3 Trial for High-Risk Endometrial Cancer: Impact on Prognosis and Benefit From Adjuvant Therapy. J Clin Oncol 38, 3388–97 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee V, Murphy A, Le DT, Diaz LA Jr. Mismatch Repair Deficiency and Response to Immune Checkpoint Blockade. Oncologist 21, 1200–11 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eskander RN, Sill MW, Beffa L, Moore RG, Hope JM, Musa FB, et al. Pembrolizumab plus Chemotherapy in Advanced Endometrial Cancer. N Engl J Med 388, 2159–70 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mirza MR, Chase DM, Slomovitz BM, dePont Christensen R, Novak Z, Black D, et al. Dostarlimab for Primary Advanced or Recurrent Endometrial Cancer. N Engl J Med 388, 2145–58 (2023). [DOI] [PubMed] [Google Scholar]
- 35.Oaknin A, Tinker AV, Gilbert L, Samouelian V, Mathews C, Brown J, et al. Clinical Activity and Safety of the Anti-Programmed Death 1 Monoclonal Antibody Dostarlimab for Patients With Recurrent or Advanced Mismatch Repair-Deficient Endometrial Cancer: A Nonrandomized Phase 1 Clinical Trial. JAMA Oncol 6, 1766–72 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Casak SJ, Marcus L, Fashoyin-Aje L, Mushti SL, Cheng J, Shen YL, et al. FDA Approval Summary: Pembrolizumab for the First-line Treatment of Patients with MSI-H/dMMR Advanced Unresectable or Metastatic Colorectal Carcinoma. Clin Cancer Res 10.1158/1078-0432.CCR-21-0557, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rainbo Research Consortium. Refining adjuvant treatment in endometrial cancer based on molecular features: the RAINBO clinical trial program. Int J Gynecol Cancer 33, 109–17 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van den Heerik A, Horeweg N, Nout RA, Lutgens L, van der Steen-Banasik EM, Westerveld GH, et al. PORTEC-4a: international randomized trial of molecular profile-based adjuvant treatment for women with high-intermediate risk endometrial cancer. Int J Gynecol Cancer 30, 2002–07 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Manning-Geist BL, Liu YL, Devereaux KA, Paula ADC, Zhou QC, Ma W, et al. Microsatellite Instability-High Endometrial Cancers with MLH1 Promoter Hypermethylation Have Distinct Molecular and Clinical Profiles. Clin Cancer Res 28, 4302–11 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Toboni MD, Wu S, Farrell A, Xiu J, Ribeiro JR, Oberley MJ, et al. Differential outcomes and immune checkpoint inhibitor response among endometrial cancer patients with MLH1 hypermethylation versus MLH1 “Lynch-like” mismatch repair gene mutation. Gynecol Oncol 177, 132–41 (2023). [DOI] [PubMed] [Google Scholar]
- 41.Chow RD, Michaels T, Bellone S, Hartwich TMP, Bonazzoli E, Iwasaki A, et al. Distinct Mechanisms of Mismatch-Repair Deficiency Delineate Two Modes of Response to Anti-PD-1 Immunotherapy in Endometrial Carcinoma. Cancer Discov 13, 312–31 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bosse T, Nout RA, McAlpine JN, McConechy MK, Britton H, Hussein YR, et al. Molecular Classification of Grade 3 Endometrioid Endometrial Cancers Identifies Distinct Prognostic Subgroups. Am J Surg Pathol 42, 561–68 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Depreeuw J, Stelloo E, Osse EM, Creutzberg CL, Nout RA, Moisse M, et al. Amplification of 1q32.1 Refines the Molecular Classification of Endometrial Carcinoma. Clin Cancer Res 23, 7232–41 (2017). [DOI] [PubMed] [Google Scholar]
- 44.Vermij L, Jobsen JJ, Leon-Castillo A, Brinkhuis M, Roothaan S, Powell ME, et al. Prognostic refinement of NSMP high-risk endometrial cancers using oestrogen receptor immunohistochemistry. Br J Cancer 128, 1360–68 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Momeni-Boroujeni A, Nguyen B, Vanderbilt CM, Ladanyi M, Abu-Rustum NR, Aghajanian C, et al. Genomic landscape of endometrial carcinomas of no specific molecular profile. Modern Pathology 35, 1269–78 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Arciuolo D, Travaglino A, Raffone A, Raimondo D, Santoro A, Russo D, et al. TCGA Molecular Prognostic Groups of Endometrial Carcinoma: Current Knowledge and Future Perspectives. Int J Mol Sci 23, (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jamieson A, Huvila J, Chiu D, Thompson EF, Scott S, Salvador S, et al. Grade and Estrogen Receptor Expression Identify a Subset of No Specific Molecular Profile Endometrial Carcinomas at a Very Low Risk of Disease-Specific Death. Mod Pathol 36, 100085 (2023). [DOI] [PubMed] [Google Scholar]
- 48.Vermij L, Horeweg N, Leon-Castillo A, Rutten TA, Mileshkin LR, Mackay HJ, et al. HER2 Status in High-Risk Endometrial Cancers (PORTEC-3): Relationship with Histotype, Molecular Classification, and Clinical Outcomes. Cancers (Basel) 13, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fader AN, Roque DM, Siegel E, Buza N, Hui P, Abdelghany O, et al. Randomized Phase II Trial of Carboplatin-Paclitaxel Versus Carboplatin-Paclitaxel-Trastuzumab in Uterine Serous Carcinomas That Overexpress Human Epidermal Growth Factor Receptor 2/neu. J Clin Oncol 36, 2044–51 (2018). [DOI] [PubMed] [Google Scholar]
- 50.Fader AN, Roque DM, Siegel E, Buza N, Hui P, Abdelghany O, et al. Randomized Phase II Trial of Carboplatin-Paclitaxel Compared with Carboplatin-Paclitaxel-Trastuzumab in Advanced (Stage III-IV) or Recurrent Uterine Serous Carcinomas that Overexpress Her2/Neu (NCT01367002): Updated Overall Survival Analysis. Clin Cancer Res 26, 3928–35 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Meric-Bernstam F, Makker V, Oaknin A, Oh D-Y, Banerjee SN, Gonzalez Martin A, et al. Efficacy and safety of trastuzumab deruxtecan (T-DXd) in patients (pts) with HER2-expressing solid tumors: DESTINY-PanTumor02 (DP-02) interim results. Journal of Clinical Oncology 41, LBA3000–LBA00 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cortes J, Kim SB, Chung WP, Im SA, Park YH, Hegg R, et al. Trastuzumab Deruxtecan versus Trastuzumab Emtansine for Breast Cancer. N Engl J Med 386, 1143–54 (2022). [DOI] [PubMed] [Google Scholar]
- 53.Modi S, Jacot W, Yamashita T, Sohn J, Vidal M, Tokunaga E, et al. Trastuzumab Deruxtecan in Previously Treated HER2-Low Advanced Breast Cancer. N Engl J Med 387, 9–20 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li BT, Smit EF, Goto Y, Nakagawa K, Udagawa H, Mazieres J, et al. Trastuzumab Deruxtecan in HER2-Mutant Non-Small-Cell Lung Cancer. N Engl J Med 386, 241–51 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nishikawa T, Hasegawa K, Matsumoto K, Mori M, Hirashima Y, Takehara K, et al. Trastuzumab Deruxtecan for Human Epidermal Growth Factor Receptor 2-Expressing Advanced or Recurrent Uterine Carcinosarcoma (NCCH1615): The STATICE Trial. J Clin Oncol 41, 2789–99 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Weigelt B, Marra A, Selenica P, Rios-Doria E, Momeni-Boroujeni A, Berger MF, et al. Molecular Characterization of Endometrial Carcinomas in Black and White Patients Reveals Disparate Drivers with Therapeutic Implications. Cancer Discov 13, 2356–69 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu YL, Gordhandas S, Arora K, Rios-Doria E, Cadoo KA, Catchings A, et al. Pathogenic germline variants in patients with endometrial cancer of diverse ancestry. Cancer 10.1002/cncr.35071, (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]


