Abstract
The purpose of this study is to predict the mRNA expression of CSF1R in HGG non-invasively using MRI (magnetic resonance imaging) omics technology and to evaluate the correlation between the established radiomics model and prognosis. We investigated the predictive value of CSF1R in the Cancer Genome Atlas (TCGA) and The Cancer Imaging Archive (TCIA) database. The Support vector machine (SVM) and the Logistic regression (LR) algorithms were used to create a radiomics_score (Rad_score), respectively. The effectiveness and performance of the radiomics model was assessed in the training (n = 89) and tenfold cross-validation sets. We further analyzed the correlation between Rad_score and macrophage-related genes using Spearman correlation analysis. A radiomics nomogram combining the clinical factors and Rad_score was constructed to validate the radiomic signatures for individualized survival estimation and risk stratification. The results showed that CSF1R expression was markedly elevated in HGG tissues, which was related to worse prognosis. CSF1R expression was closely related to the abundance of infiltrating immune cells, such as macrophages. We identified nine features for establishing a radiomics model. The radiomics model predicting CSF1R achieved high AUC in training (0.768 in SVM and 0.792 in LR) and tenfold cross-validation sets (0.706 in SVM and 0.717 in LR). Rad_score was highly associated with tumor-related macrophage genes. A radiomics nomogram combining the Rad_score and clinical factors was constructed and revealed satisfactory performance. MRI-based Rad_score is a novel way to predict CSF1R expression and prognosis in high-grade glioma patients. The radiomics nomogram could optimize individualized survival estimation for HGG patients.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10278-023-00905-x.
Keywords: Radiomics, Machine learning, High-grade gliomas, Prognosis, Tumor immune infiltration
Introduction
Glioma is the most common primary central nervous system tumor that threatens human health seriously [1]. According to the standards of the World Health Organization (WHO), brain gliomas are categorized into grades I–IV, with grades I–II symbolizing low-grade gliomas with low malignancy and relatively good prognosis, while grade III–IV symbolizing high-grade gliomas (HGG), with high malignancy and poor prognosis [2, 3]. Current glioma treatments include surgery, radiotherapy, chemotherapy, and targeted therapy. However, patients with HGG still have poor prognosis and high mortality rate [4, 5]. The classic prognostic indicators of gliomas include WHO grading, molecular genotypes, such as isocitrate dehydrogenase (IDH) mutations and 1p/19q co-deletions, as well as characteristics of MRI based on subjective judgment [6]. These hinder molecular pathology from predicting the prognosis due to highly invasive procedures, expensive equipment, and tumoral heterogeneity [6–8]. Therefore, we need to seek new prognostic markers further or develop new predictive models to forecast the prognosis of HGG patients and carry out individualized treatment.
Colony-stimulating factor-1 receptor (CSF1R) is also known as CD115 and M-CSF1R-R. CSF1R belongs to the type III protein tyrosine kinase receptor family and is expressed on monocyte/macrophage, peritoneal exudate cells, plasma cell-like and conventional dendritic cells, and osteoclasts [9]. This gene product’s natural ligands include CSF1R1 and IL-34 [10]. CSF1R-mediated signal transduction is essential for the survival and differentiation of macrophages [11, 12]. Pexidartinib, an inhibitor of macrophage colony-stimulating factor-1 receptor (CSF1R), is currently undergoing clinical evaluation in glioblastoma patients [12, 13]. Pexidartinib can simulate the depletion of CSF1R-1 in preclinical mouse models, potentially limiting macrophage function in the tumor microenvironment and affecting the progression tumors in nerve tissue [14]. CSF1R inhibition by small molecule BLZ945 can alter macrophage polarization, preventing neuroglioblastoma progression in a mouse-proneural Glioblastoma multiforme (GBM) model [15]. Presently, molecular detection has many disadvantages, such as high invasion, long time-consuming, tumor heterogeneity, and expensive gene sequencing. Thus, alternative methods are needed to obtain CSF1R expression in HGG accurately.
MRI is commonly used for HGG diagnosis because it can obtain information of the whole glioma [16]. Radiomics is a procedure of converting digital medical images into high-dimension data that can be mined. Radiomics has been widely used in various disease diagnoses, biological behavior evaluations, and prognosis evaluations by extracting and analyzing the features of the gray histogram, morphological and other features that the naked eye cannot recognize in different imaging, displaying great potential in the preoperative non-invasive evaluation of tumor molecular typing [17, 18]. It can recognize invisible sub-visual texture features in images and has the potential to capture pathophysiological features of tumors and characterize heterogeneity within tumors non-invasively [19, 20]. According to previous studies, radiomics can be applied to early diagnosis and classification of HGG, estimation of tumor microenvironment, and heterogeneity [21, 22].
Radiogenomics is a new field to explore the relationship between radiomics and genomics [16, 23–25]. CSF1R is an immunotherapeutic target of HGG. Radiological methods have accurately predicted some vital genetic characteristics of gliomas, such as 1p/19q co-deletion and IDH mutation [26, 27], but no research has been conducted to predict CSF1R expression level using radiological features. This innovative study proposes MRI imaging omics technology to predict CSF1R mRNA expression in HGG tissues non-invasively. According to the above factors, the correlation between the established radiomics model and prognosis was evaluated. Simultaneously, we investigated the underlying molecular mechanism of CSF1R expression and its association with the immune microenvironment using bioinformatics.
Methods
Data and Image Sources
The transcriptome sequencing data and medical imaging downloaded from The Cancer Genome Atlas (TCGA) and The Cancer Imaging Archive (TCIA) databases were used to study the prognostic value of CSF1R and newly established radiomics model (TCIA-GBM data: https://www.cancerimagingarchive.net/collections/ and TCGA-GBM data: The Cancer Genome Atlas Glioblastoma Multiforme Collection (TCGA-GBM)—The Cancer Imaging Archive (TCIA) Public Access—Cancer Imaging Archive Wiki). All TCIA and TCGA data and images are anonymous and publicly available. As for data from TCGA, the inclusion criteria were HGG diagnosed according to histological evidence and primary solid tumor with sequencing data (n = 807). We excluded patients with WHO I–II glioma (n = 271), loss of pathological grade (n = 67), loss of survival data (n = 4), survival time less than one month (n = 35) or lack of clinical data (n = 132). Finally, 298 HGG patients with RNA-seq data and complete clinical data from TCGA database were included in this study. As for data from TCIA, the inclusion criteria were HGG diagnosed according to histological evidence with enhanced MRI image (n = 458). We excluded patients with poor image quality or only with postoperative image (n = 132). 326 HGG patients with high-quality image data from TCIA database were included in this study. However, cases that met the above two conditions at the same time were even more valuable, so there were only 89 cases selected finally (Fig. 1A). The TCIA and TCGA data intersection was completed using the “caret” R package. The use of public database conforms to the citation and data use policy published on the public portal of TCGA-TCIA website.
Fig. 1.
Schematic diagram of the predictive radiomics model construction and validation. A. 807 TCGA data with primary HGG were downloaded, and 298 cases were selected based on the exclusion criteria. Among the 458 cases from TCIA, 132 cases with poor image quality or only with postoperative image were excluded. TCGA data intersected with TCIA, and 89 cases were selected finally. B. The main phases of radiomics model construction and validation are image processing, feature extraction, feature selection, and radiomics model establishment. TCGA: the Cancer Genome Atlas; TCIA: The Cancer Imaging Archive; mRMR: minimal-redundancy-maximal-relevance; RFE: recursive feature elimination; SVM: Support vector machine; LR: Logistic regression
TCGA Analysis
We assessed the expression and function of CSF1R in HGG using TCGA analysis. We downloaded RNAseq data of TCGA and GTEx in TPM format from UCSC XENA (https://xenabrowser.net/datapages/), which has been processed uniformly by the Toil process [28]. Tissue data were collected from GBM/HGG of TCGA, while normal tissue data were collected from GTEx. After log2 transformation of RNAseq data in TPM format, CSF1R expressions between cancer and normal tissue were compared. We used Kaplan–Meier survival and Cox regression analyses to assess the predictive value of CSF1R for survival in HGG patients. Subgroup analysis was carried out to analyze the impact of CSF1R on patient prognosis in different subgroups. The likelihood ratio test and Spearman correlation analysis was employed to analyze the interaction between CSF1R expression and other clinical covariables.
Analysis of the Difference in Immune Cell Infiltration
We uploaded the gene expression matrix data of glioma tissue samples which were downloaded from TCGA to CIBERSORTx (CIBERSORTx (stanford.edu)). The infiltration of immune cells in each sample was calculated.
Gene Ontology
(GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) Enrichment Analysis
GO is an extensively used functional gene annotation tool, especially biological pathways (BP), cellular components (CC) and molecular functions (MF). KEGG is an extensively used database for depositing information about biological pathways, genomes, diseases and medications. The enrichment analysis of BP, CC and MF showed the first 10 significant enrichment pathways, while the enrichment analysis of KEGG showed the first 30 significant enrichment pathways.
Radiomics Feature Extraction
MR: N4 bias field correction was performed on MR images to reduce the uneven signal of MRI images caused by magnetic field changes, such as excessive brightness or darkness in some areas of the image, or brightness differences in the same tissue, resulting in bias field effects. The image was resampled to make it isotropic to reduce the variation caused by the difference in scanning equipment and scheme and the size of the focus of the patient. Image standardization was adopted to reduce image noise and standardize intensity and to reduce the difference in image signal strength collected by different machines.
We used 3D Slicer software to extract the Volume-of-interest (VOI). The VOI was manually outlined layer by layer by a radiologist (10 years of experience in the radiology department), who blind to the patient’s clinical data and diagnostic results. Another physician (10 years of experience in the radiology department) randomly selected 30 cases for secondary delineation by the "random number table method". These two physicians outlined the lesions of each image and extracted their imaging features based on VOI. The intraclass correlation coefficient (ICC) was employed to assess the uniformity of imaging characteristics extracted by two physicians. ICC ≥ 0.80 was considered highly consistent, 0.51 ~ 0.79 as medium, and < 0.50 as poor. The extracted radiomics features with ICC ≥ 0.8 are selected for the next step of feature screening. It was performed using the “Irr” R package (Fig. 1B).
Radiomics Feature Selection
The correlation and redundancy among imaging features will lead to over-fitting of the model, which will affect the generalization ability of the predictive CSF1R gene expression model. The minimal-redundancy-maximal-relevance (mRMR) algorithm was used for feature screening to eliminate the potential redundant features and avoid over-fitting the model. The algorithm considers the correlation between features and labels. It selects the feature subset with the largest mutual information (MI) to class labels and the least MI with other features for final modeling. Thirty features were screened using the mRMR algorithm, and then the best feature subset was screened using the RFE algorithm. mRMR was performed using the “mRMRe” R package.
Recursive feature elimination (RFE) feature screening: before modeling, the importance of features (contribution to the model) was sorted using an algorithm, and the less important features were deleted. The aim was to determine a subset of predictive values that can be employed to create accurate models. In the process of model training, after each training, characteristics of low importance were deleted; iterations were repeated many times until the number of remaining characteristics reached the required number of characteristics, and the most valuable feature was obtained subset. RFE was performed using the “caret” R package (Fig. 1B).
Support Vector Machine (SVM) Model Construction Establishment
The SVM algorithm utilizes support vectors to seek high-latitude hyperplanes as decision boundaries. The SVM algorithm was used to model the selected imaging features and predict the gene expression of CSF1R using the "caret" R package (Fig. 1B).
Logistic Regression (LR) Model Construction Establishment
The radiomics model was established using the LR algorithm. The essence of logical regression was derived from linear regression, a generalized regression algorithm used extensively for classification problems. The logical regression transformed the linear regression using the Sigmoid function to distribute the model’s output value between 0 and 1. The selected radiomics features were fitted using a logical regression algorithm, and a two-classification model for predicting CSF1R expression was established using the “stats” R package (Fig. 1B).
Radiomics Model Evaluation
We evaluated the effectiveness of the radiomics model in the training set and tenfold cross-validation set [29, 30]. We estimated the area under the ROC curve (AUC) using “pROC” R package. The X-axis of the Precision-Recall (PR) was Recall or the true positive rate, while the Y-axis was Precision. PR-AUC was the average accuracy calculated for each coverage threshold. We analyzed the PR and its AUC using the “ModEvA” R package. It is more appropriate to evaluate the model’s performance by focusing on positive samples due to the PR curve’s high sensitivity to positive samples. We assessed the calibration degree of the radiomics prediction model using the Hosmer–Lemeshow goodness-of-fit test and calibration curve. We plotted decision curve analysis (DCA) to exhibit the clinical advantage of the radiomics prediction model using the “rmda” R package (Fig. 1B).
Nomogram Establishment and Evaluation
We can draw a straight line at each time point, add up the scores of each factor on the score table, and locate it on the total score scale to obtain the estimated prognosis of each time point. Stepwise logistic regression was used to select clinical variables. The Akaike information criterion (AIC) was taken as the criterion to balance the model’s complexity and goodness of fit. The clinical variables were selected by selecting the minimum AIC, and the model was established in conjunction with Rad_score. The COX regression line chart of the 12-month, 36-month, and 60-month survival were established using the "regploy" R package. We plotted the time-dependent receiver ROC curve to show the prediction capability of the nomogram at different time points. The diagonal of the calibration curve indicates that the predictive probability corresponds to the realistic probability. The greater the deviation from the diagonal, the larger the prediction error. The DCA demonstrates the clinical practicability of the radiomics prediction model (Fig. 1B).
Data and Image Sources from REMBRANDT Databases
An external validation set is necessary for the evaluation of the model’s performance. So, we use the REMBRANDT database (https://wiki.cancerimagingarchive.net/display/Public/REMBRANDT)) as external validation to further verify the performance of our radiomics model. CSF1R gene expression data were downloaded from GEO (https://www.ncbi.nlm.nih.gov/geo/) database. The dataset number is GSE108474. The transcriptome sequencing data and MRI image downloaded from REMBRANDT databases were used to validate the prognostic value of CSF1R and LR radiomics model. A total of 550 patients with primary HGG having sequencing data were downloaded from the REMBRANDT database. 331 patients with grade WHO I-II gliomas were excluded. Finally, 219 patients with HGG were included in this study. As for T1enhancement image data from REMBRANDT database, there were 130 patients with HGG having enhanced MRI images. We excluded 11 patients with poor image quality or only with postoperative images. This study included 119 HGG patients with high quality imaging data. However, cases that met the above two conditions at the same time were very valuable, so there were only 37 cases selected finally. We standardize the REMBRANDT image data based on the mean and standard deviation of the TCGA training set. We evaluated the effectiveness of the LR radiomics model in the REMBRANDT database using “caret” and “pROC” R package.
Statistics and R Packages
All statistical analyses and plots were completed using R software (v.3.6.1, R Foundation for Statistical Computing, Austria). The “survminer” R package was used to calculate the cut-off value of CSF1R [31–33], dividing it into CSF1R high and low expression groups. The CSF1R expressions between cancer and normal tissue were compared and illustrated using the “ggplot2” R package. We assessed the relationship between CSF1R and clinical pathologic characteristics of HGG patients using Chi-square test and Spearman correlation analysis. The Spearman analysis was presented in a radar map using “stats” R package. The "survival" R package was employed to assess the value of CSF1R in predicting the survival of HGG patients. The "survminer" R package was employed for summary and visualization. The effects of CSF1R expression and other clinical features on survival were compared using "survival" and "forestplot" R package. When the HR value exceeds 1, the independent variable is a risk factor. Subgroup analysis and interaction tests were performed with the “cmprsk,” “survival,” and “forestplot” R package. The degrees of immune cells infiltration were compared between the high and low CSF1R groups using the "limma" R package. GO and KEGG enrichment analysis were conducted using “clusterProfiler” R package, with a filter condition of qvalueFilter < 0.05. The Radiomics_score (Rad_score) was compared between high and low CSF1R groups using Wilcoxon test. The Spearman correlation coefficient was employed to evaluate the correlation between the Rad_score and Macrophages M1- and M2-related genes using the “stats” R package. The difference was considered significant in all tests at *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Results
Patient Information Obtained from TCGA
The gene expression information and clinical characteristics of 298 HGG downloaded from TCGA were depicted in Table 1. The numbers of patients with Grade III and IV disease were 170 and 128, respectively. Most patients (73%, n = 219; 77%, n = 228) receive chemotherapy and radiotherapy, respectively. Patients were divided into CSF1R high (n = 194) and low (n = 104) expression groups, depending on the cut-off value = 4.53455 of CSF1R expression.
Table 1.
The relationship between CSF1R expression and the clinicopathological characteristics of HGG patients in TCGA
| Variables | Total (n = 298) |
Low expression of CSF1R (n = 104) |
High expression of CSF1R (n = 194) |
P value |
|---|---|---|---|---|
| Age, n (%) | > 0.99 | |||
| < 60 | 197 (66) | 69 (66) | 128 (66) | |
| > = 60 | 101 (34) | 35 (34) | 66 (34) | |
| Gender, n (%) | > 0.99 | |||
| Female | 120 (40) | 42 (40) | 78 (40) | |
| Male | 178 (60) | 62 (60) | 116 (60) | |
| Grade, n (%) | 0.045 | |||
| III | 170 (57) | 68 (65) | 102 (53) | |
| IV | 128 (43) | 36 (35) | 92 (47) | |
| IDH status, n (%) | 0.005 | |||
| Wildtype | 169 (57) | 47 (45) | 122 (63) | |
| Mutant | 129 (43) | 57 (55) | 72 (37) | |
| Chr_1p_19q_codeletion, n (%) | < 0.001 | |||
| Non-codel | 248 (83) | 62 (60) | 186 (96) | |
| Codel | 50 (17) | 42 (40) | 8 (4) | |
| MGMT promoter status, n (%) | 0.016 | |||
| Unmethylated/Unknown | 118 (40) | 31 (30) | 87 (45) | |
| Methylated | 180 (60) | 73 (70) | 107 (55) | |
| Chemotherapy, n (%) | 0.768 | |||
| NO | 79 (27) | 26 (25) | 53 (27) | |
| YES | 219 (73) | 78 (75) | 141 (73) | |
| Radiotherapy, n (%) | 0.082 | |||
| NO | 70 (23) | 31 (30) | 39 (20) | |
| YES | 228 (77) | 73 (70) | 155 (80) | |
CSF1R colony-stimulating factor-1 receptor, IDH Isocitrate dehydrogenase, MGMT O6-methylguanine DNA methyltransferase, TCGA the Cancer Genome Atlas
The CSF1R Up-Regulation is Related to Malignant Progression and Poor HGG Patient Prognosis
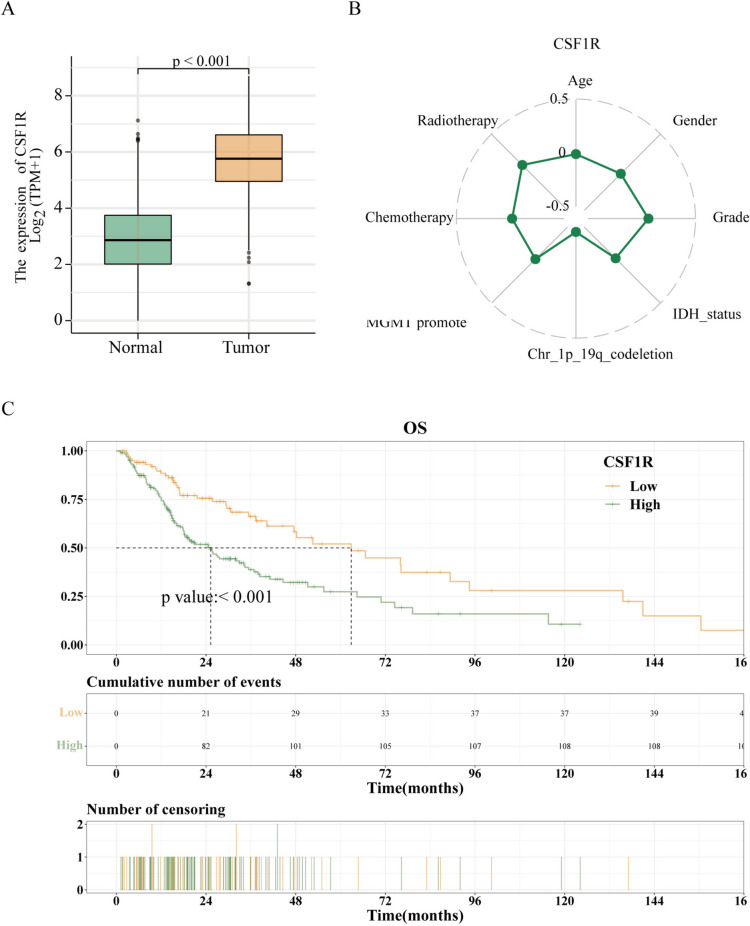
CSF1R is critical for developing and maintaining microglia, and CSF1R inhibitors have been tested in preclinical and clinical studies. Here, we compared CSF1R expression in HGG normal tissues from TCGA combined with GTEx database and cancer tissues from TCGA. The results indicated that the expression of CSF1R in HGG cancer tissue was much higher than that in normal tissue (Fig. 2A; P < 0.001).
Fig. 2.
CSF1R is elevated in high-grade gliomas and is associated with malignant progression. A. CSF1R expression was higher in HGG cancer tissues than in normal tissues in TCGA database. B. Spearman correlation analysis revealed the correlation between CSF1R and clinicopathological characteristics, such as age, gender, grade, IDH status, Chr 1p19q codeletion, MGMT promoter status, chemotherapy, and radiotherapy. C. High level of CSF1R expression was associated with shorter OS in HGG patients in the TCGA database. * P < 0.05, ** P < 0.01, or *** P < 0.001 were considered significant differences
In an effort to further reveal the effect of CSF1R in HGG, we evaluated the relevance between CSF1R expression and the clinical features of HGG patients. A high CSF1R expression was strongly related to the grade IV (P = 0.045), Chr_1p_19q_non-codeletion (P < 0.001), IDH wildtype status (P = 0.005), and Unmethylated MGMT (O6-methylguanine DNA methyltransferase) promoter status (P = 0.016), but not with age, sex, chemotherapy, and radiotherapy (all P > 0.05; Table 1). A Spearman correlation analysis revealed a negative correlation between CSF1R and 1p/19q co-deletion (spearman coefficient = -0.486, P < 0.001; Fig. 2B).
Then, the relevance between the prognosis of patients with HGG and CSF1R expression was appraised. The Kaplan–Meier survival analysis indicated that the higher expression of CSF1R was, the shorter overall survival (OS) would be, with a median OS of 62.87 months (95% CI: 47.53–135.6) vs. 25.27 months (95% CI: 18.27–34.43). (P < 0.001; Fig. 2C). These outcomes suggested that a higher CSF1R expression was related to poor HGG patient survival.
We also explored the risk factors affecting OS using univariate and multivariate Cox regression analyses (Table 2; Figs. 3A–B). High CSF1R expression (HR, 1.764; 95% CI, 1.167–2.666; P = 0.007), higher tumor grade (HR, 7.407; 95% CI, 4.642–11.82; P < 0.001), Wildtype IDH status (HR, 0.504; 95% CI, 0.289–0.879; P = 0.016), and without chemotherapy (HR, 0.474; 95% CI, 0.321–0.702; P < 0.001) were independent risk factors bound up with OS (Table 2; Fig. 3B). Thus, these data indicate that CSF1R level was associated with poor prognosis.
Table 2.
Univariate and Multivariate Cox regression analysis of correlation between OS and clinicopathological characteristics of HGG patients in TCGA
| Characteristics | HR (95% CI) Univariate analysis |
P value Univariate analysis |
HR (95% CI) Multivariate analysis |
P value Multivariate analysis |
|---|---|---|---|---|
| CSF1R: High vs. Low | 2.096(1.437–3.057) | < 0.001 | 1.764(1.167–2.666) | 0.007 |
| Age: > = 60 vs. < 60 | 2.614(1.881–3.631) | < 0.001 | 1.129(0.775–1.643) | 0.527 |
| Gender: Male vs. Female | 1.24(0.878–1.752) | 0.221 | 1.003(0.684–1.47) | 0.988 |
| Grade: IV vs. III | 8.772(5.97–12.888) | < 0.001 | 7.407(4.642–11.82) | < 0.001 |
| IDH_status: Mutant vs. Wildtype | 0.182(0.121–0.273) | < 0.001 | 0.504(0.289–0.879) | 0.016 |
| Chr_1p_19q_codeletion: Codel vs. Non-codel | 0.201(0.104–0.387) | < 0.001 | 0.767(0.331–1.778) | 0.537 |
| MGMT_promoter_status: Methylated vs. Unmethylated/Unknown | 0.362(0.26–0.505) | < 0.001 | 1.07(0.716–1.597) | 0.742 |
| Chemotherapy: YES vs. NO | 0.562(0.399–0.792) | < 0.001 | 0.474(0.321–0.702) | < 0.001 |
| Radiotherapy: YES vs. NO | 1.254(0.842–1.868) | 0.266 | 0.721(0.453–1.147) | 0.168 |
OS overall survival, TCGA the Cancer Genome Atlas
Fig. 3.
Uni- and multivariate Cox regression analyses of OS and clinicopathological characteristics in HGG patients. A. Univariate Cox regression analyses of OS and clinicopathological characteristics in HGG patients. B. Multivariate Cox regression analyses of OS and clinicopathological characteristics in HGG patients. * P < 0.05, ** P < 0.01, or *** P < 0.001 were considered significant differences
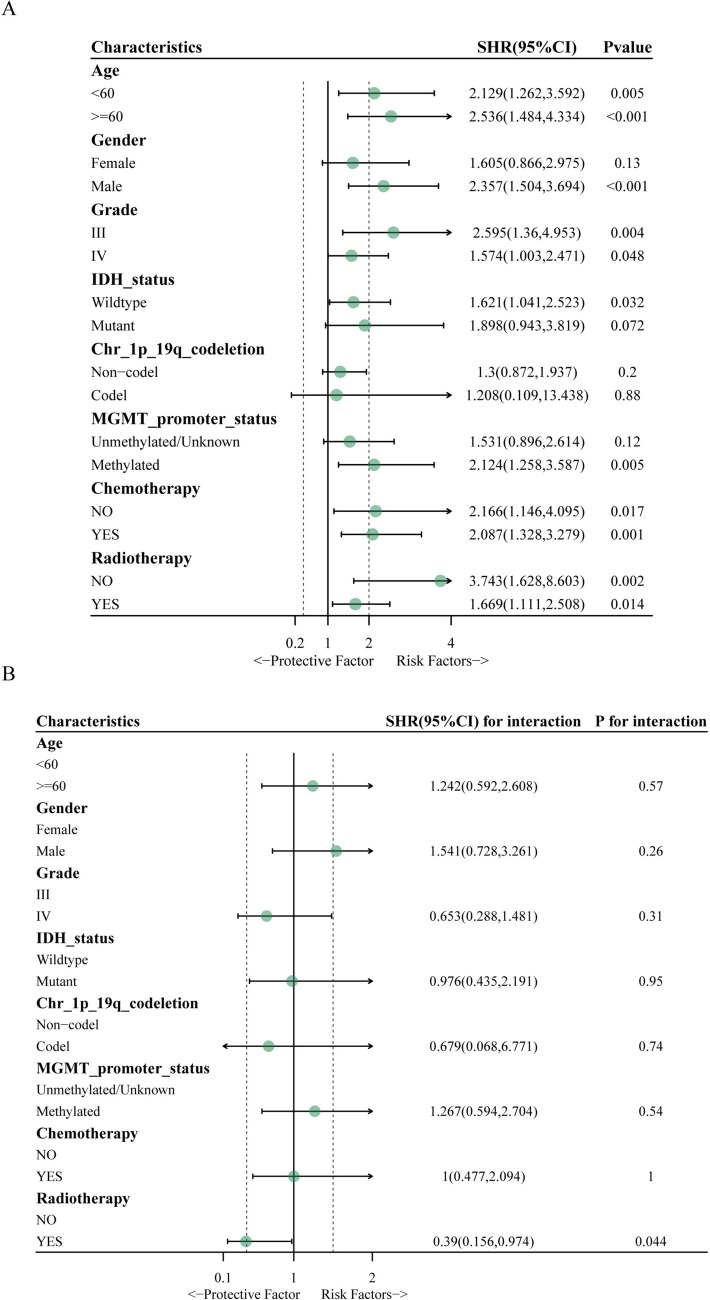
Then, we performed subgroup and interaction analysis between CSF1R and clinicopathological characteristics in HGG patients. In the age subgroup analysis, the increase in CSF1R was the risk factor for OS in age < 60 (HR, 2.129; 95% CI, 1.262–3.592; P = 0.005) and age ≥ 60 (HR, 2.536; 95% CI, 1.484–4.334; P < 0.001) groups. In the gender subgroup analysis, the increase in CSF1R was the risk factor of OS in the male group (HR, 2.357; 95% CI, 1.504–3.694; P < 0.001). In the tumor grade subgroup analysis, the increase in CSF1R was the risk factor of OS in Grade III (HR, 2.595; 95% CI, 1.36–4.953; P = 0.004) and Grade IV (HR, 1.574; 95% CI, 1.003–2.471; P = 0.048) groups. In the IDH status subgroup analysis, the increase in CSF1R was the risk factor of OS in the wildtype group (HR, 1.621; 95% CI, 1.041–2.523; P = 0.032). In the MGMT promoter_status subgroup analysis, the increase in CSF1R was the risk factor of OS in the Methylated group (HR, 2.124; 95% CI, 1.258–3.587; P = 0.005). In the chemotherapy subgroup analysis, the increase in CSF1R was the risk factor of OS in the non-chemotherapy (HR, 2.166; 95% CI, 1.146–4.095; P = 0.017) and chemotherapy (HR, 2.087; 95% CI, 1.328–3.279; P = 0.001) groups. In the radiotherapy subgroup analysis, the increase in CSF1R was the risk factor of OS in the non-radiotherapy (HR, 3.743; 95% CI, 1.628–8.603, P = 0.002) and radiotherapy (HR, 1.669; 95% CI, 1.111–2.508, P = 0.014; Fig. 4A) groups. The interplay between CSF1R and radiotherapy was notable (P = 0.044), indicating that the impact of CSF1R on OS differed significantly between patients with and without radiotherapy; no other characteristics exhibited significant interactions (Fig. 4B).
Fig. 4.
Subgroup and interaction analysis between CSF1R and clinicopathological characteristics in patients with HGG. A subgroup analysis between CSF1R and clinicopathological characteristics in HGG patients. B. Interaction analysis between CSF1R and clinicopathological characteristics in HGG patients. * P < 0.05, ** P < 0.01, or *** P < 0.001 were considered significant differences
Relationship Between CSF1R and Immune Infiltration and Enrichment Analysis
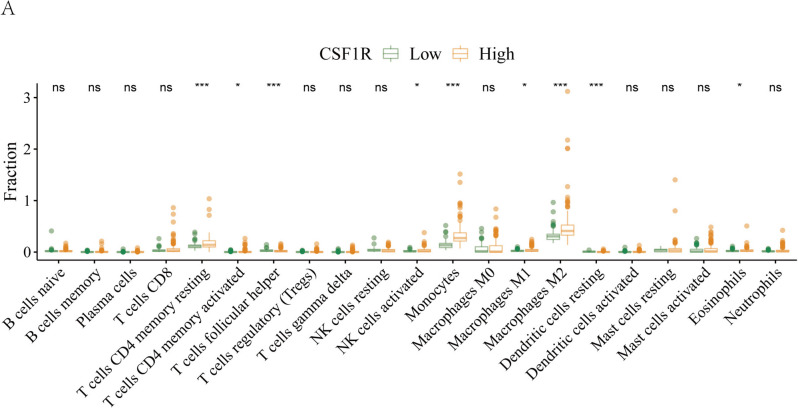
Then, we analyzed the distribution of immune cells in high-grade gliomas. Figure 5A displays that CSF1R expression correlates significantly with high infiltration of immune cells, including Monocytes, Macrophages M2, resting memory CD4 T (Trm) cells, and T follicular helper (Tfh) cells (all P < 0.001). Through the enrichment analysis of the differentially expressed genes (DEGs) between CSF1R high and low groups, the potential functional pathway and biological function of CSF1R were explored. According to GO, high CSF1R expression was enriched in DNA-binding transcription and signaling receptor activator activities in MF, cell-substrate junction and focal adhesion in CC, and response to xenobiotic stimulus and positive regulation of kinase activity in BP (Fig. 6A). According to KEGG, high CSF1R was enriched in neurodegeneration pathways, MAPK (mitogen-activated protein kinase), Focal adhesion, Wnt, mTOR (Mechanistic Target of Rapamycin) and Autophagy signaling pathways (Fig. 6B).
Fig. 5.
Relationship between CSF1R expression and immune infiltration. A. CSF1R expression was markedly associated with dominant immunocyte infiltration in the TCGA cohort. * P < 0.05, ** P < 0.01, or *** P < 0.001 were considered significant differences
Fig. 6.
Functional enrichment analysis of the differentially expressed genes (DEGs) between CSF1R high and low groups. A. The top 10 most enriched biological functions, including BP, CC, and MF, in the TCGA dataset were shown in a lollipop. B The top 30 most enriched KEGG pathways in the TCGA dataset were shown in a lollipop
Feature Extraction and Radiomics Model Construction for the Prediction of CSF1R Expression
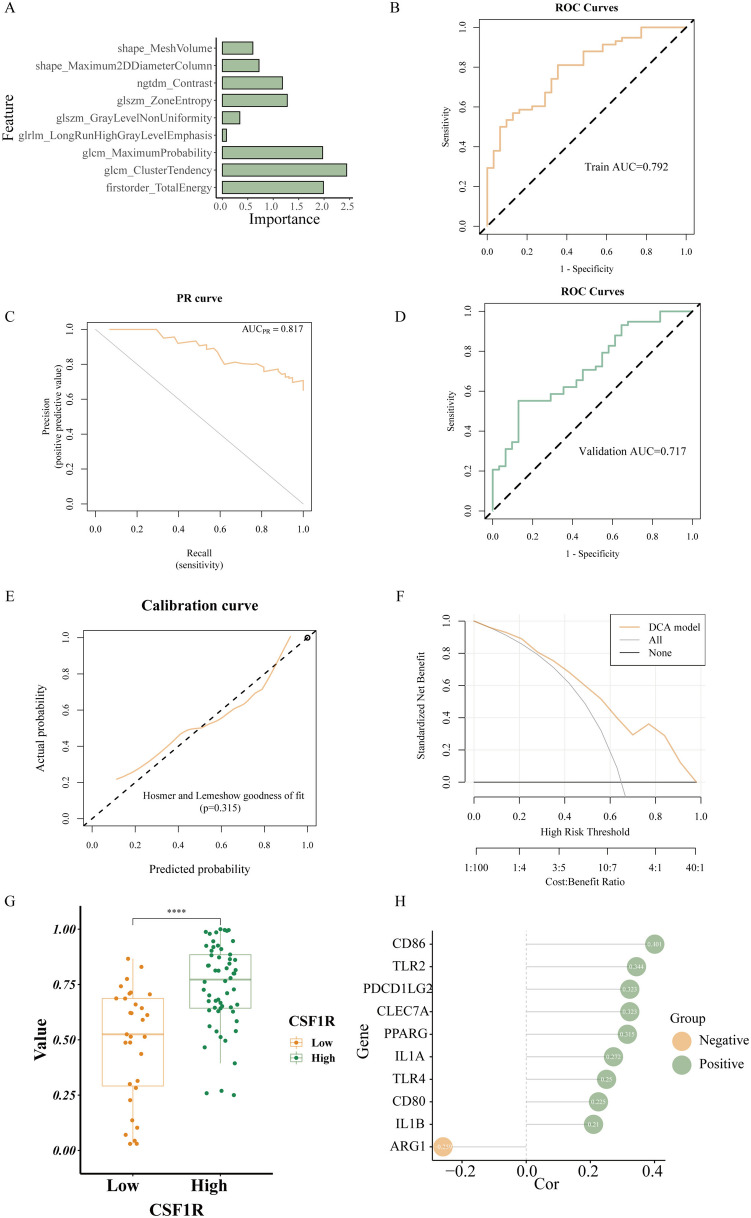
The median intraclass correlation coefficient (ICC) value of radiomics features is 0.933. There are 94 radiomics features (accounting for 87.9% of all features) with ICC value ≥ 0.80. These features revealed excellent inter-reader agreement and then entered the mRMR and RFE analysis (Table 3). Finally, nine optimal features were selected to establish the radiomics model (Fig. 7A). These nine selected the radiomics features were first modeled using SVM algorithm. Then, a radiomics prediction model was developed and used to predict CSF1R expression. Figure 7B depicts the feature importance histogram, where the ordinate represents the nine selected features, and the abscissa represents the importance of the feature. The ROC curves of the SVM model indicated that the AUC value in the training set was 0.768 (95% CI 0.663–0.873; Fig. 7C). The PR curves of the SVM model revealed that the AUC value in the training set was 0.839 (Fig. 7D). After tenfold cross-validation, the ROC curves of the SVM model implied the AUC value was 0.706 (95% CI 0.596–0.817; Fig. 7E). Additionally, the Hosmer–Lemeshow goodness-of-fit test and calibration curve demonstrated that the prediction probability of the SVM model was highly consistent with the true value (Fig. 7F). According to DCA, the radiomics prediction model had high clinical usefulness (Fig. 7G). In the SVM model, Radiomics_score (Rad_score) differed significantly between groups with high and low CSF1R (P < 0.01; Fig. 7H).
Table 3.
The consistency evaluation of imaging features extracted by two physicians
| ICC ≥ 0.8 | 0.5 ≤ ICC < 0.8 | ICC < 0.5 | ICC_Mean | ICC_Median | |
|---|---|---|---|---|---|
| Percentage | 0.879 | 0.056 | 0.065 | 0.871 | 0.933 |
| Number | 94 | 6 | 7 | NA | NA |
ICC intraclass correlation coefficient
Fig. 7.
Radiomics model construction based on the SVM algorithm and validation. A. After mRMR and RFE analysis, nine features were selected for the radiomics model construction. B. The importance of nine features in the SVM model. C. ROC curves of the SVM model for predicting CSF1R expression in the training set. D. PR curves of the SVM model for predicting CSF1R expression in the training set. E. ROC curves of SVM model for predicting CSF1R expression in the validation set. F. The calibration curves of the SVM model demonstrated that prediction probability was in good agreement with the true value. G. DCA for the SVM model had high clinical practicability. H. Wilcoxon test of RAD_score in CSF1R low- and high-expression groups in SVM model. * P < 0.05, ** P < 0.01, *** P < 0.001, or **** P < 0.0001 were considered significant differences
We used the same nine features to construct a radiomics model based on the LR algorithm. Figure 8A depicts the importance of nine features in the LR model. The ROC curves of the LR model revealed that the AUC value in the training set was 0.792 (95% CI 0.698–0.886; Fig. 8B). The PR curves of the LR model revealed that the AUC value in the training set was 0.817 (Fig. 8C). After tenfold cross-validation, LR model ROC curves revealed that the AUC value was 0.717 (95% CI 0.607–0.826; Fig. 8D). Furthermore, the Hosmer–Lemeshow goodness-of-fit test and calibration curve demonstrate that the prediction probability of the LR model is highly consistent with the true value (Figs. 8E). In the light of DCA, the radiomics prediction model had high clinical usefulness (Figs. 8F).
Fig. 8.
Radiomics model construction based on LR algorithm and validation. A. The importance of nine features in the LR model. B. ROC curves of LR model for predicting CSF1R expression in the training set. C. PR curves of the LR model for predicting CSF1R expression in the training set. D. ROC curves of LR model for predicting CSF1R expression in the validation set. E. The calibration curves of the LR model demonstrated that prediction probability was in good agreement with the true value. F. DCA for the LR model had high clinical practicability. G. Wilcoxon test of RAD_score in CSF1R low- and high-expression groups in LR model. H. Spearman correlation analysis of the correlation between Rad_scores and macrophage-related genes in the LR model. * P < 0.05, ** P < 0.01, *** P < 0.001, or **** P < 0.0001 were considered significant differences
Although by comparing the performance of different machine learning algorithms (SVM and LR) for CSF1R prediction, we discovered that the AUC value of LR model is slightly higher than that of SVM model. There was no statistically remarkable difference between the AUC values of the SVM and LR models depending on the Delong test (P = 0.354), and both models have good prediction efficiency. Rad_score also differed significantly between groups with high and low CSF1R in the LR model (Fig. 8G; P < 0.001).
The Relationship Between Rad_Score and Macrophage-Related Gene
Figure 5A indicates that M2 Macrophages were enriched in CSF1R high-expression gliomas based on TCGA cohort. Therefore, we analyzed the association between Rad_scores and Macrophages-related genes using Spearman correlation analysis in the LR model (Fig. 8H). Rad_score was positively correlated with M2 macrophages-related genes, such as CD86 (r = 0.401, P < 0.001), TLR2 (r = 0.343, P < 0.01), and TLR4 (r = 0.250, P < 0.05), but negatively related to M1 macrophage-related gene ARG1 (r = -0.259, P < 0.05).
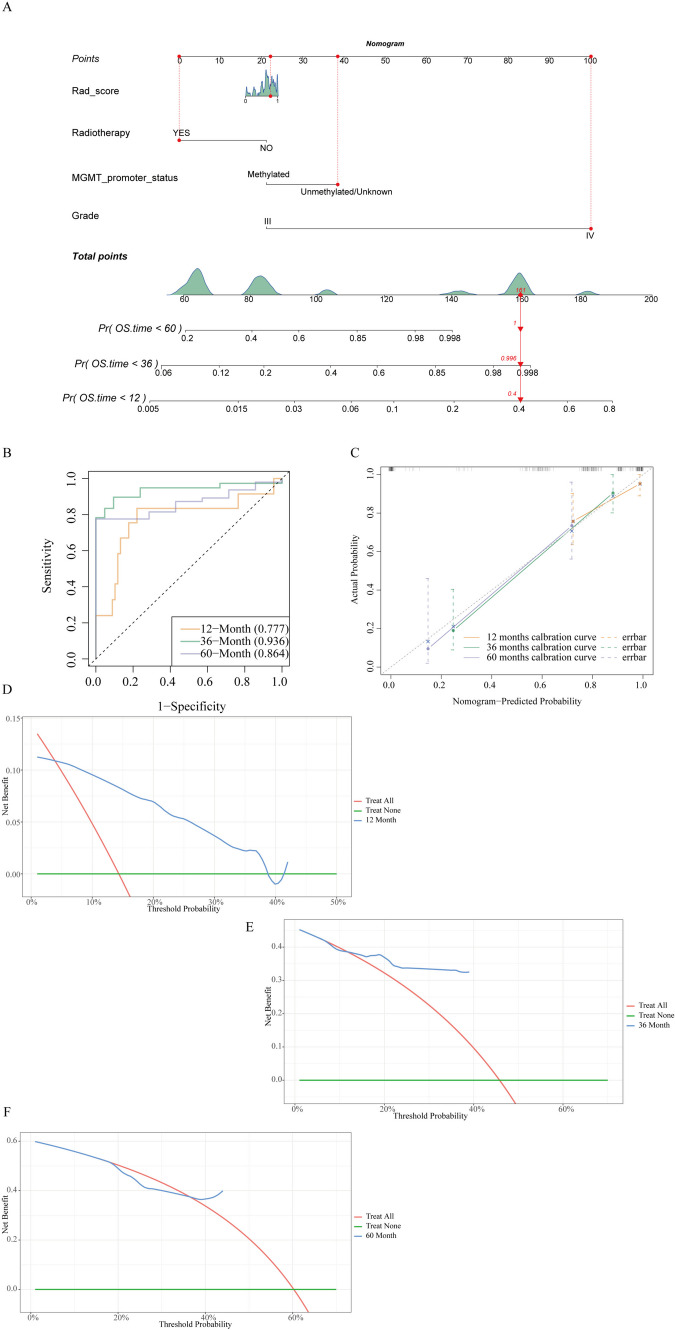
Construction and Validation of a Prediction Nomogram
Multivariate logistic regression analysis of Rad_score and clinical factors (Radiotherapy, MGMT_promoter_status, and Tumor Grade) was used to build a predictive nomogram (Fig. 9A). Figure 9B illustrates that the capability of the nomogram to predict the survival of patients changes with time. The AUC values under the curve of ROC at 12-month, 36-month, and 60-month were 0.77 (95% CI, 0.602–0.951), 0.936 (95% CI, 0.869–1.000), and 0.864 (95% CI, 0.747–0.98) severally, with the highest value at 36-months. The calibration curve revealed that the 12-month, 36-month, and 60-month curves were adjacent to the diagonal of 45°, illustrating that the prediction of survival rates by nomograph was in good agreement with the actual probabilities (Fig. 9C). The DCA manifested that the nomogram possessed high clinical practicability with the threshold of 0.08–0.38 (12-month) and 0.12–0.4 (36-month) (Figs. 9D–F).
Fig. 9.
The predictive nomogram construction and validation. A. A nomogram was constructed based on Rad_score, radiotherapy, MGMT promoter status, and grade. B. ROC curves of 12-month, 36-month, and 60-month for predicting the OS. C. The calibration curves of the nomogram demonstrated satisfactory agreement between the predicted and actual observation of the 12-month, 36-month, and 60-month survival. D–F. DCA for the radiomics nomogram of 12-month, 36-month, and 60-month
LR Radiomics Model External Validation
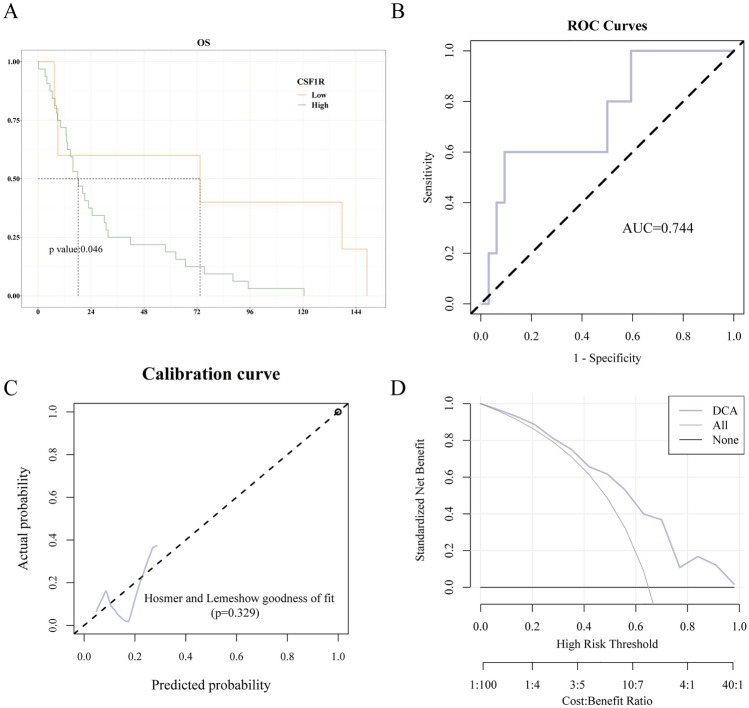
The gene expression information and clinical characteristics of 37 HGG downloaded from REMBRANDT database were depicted in Supplementary Table 1. We further evaluated the correlation between CSF1R expression and the prognosis of patients with HGG. The Kaplan–Meier survival analysis showed that the higher expression of CSF1R was, the shorter overall survival (OS) would be, with a median OS of 18.15 months vs. 73.4 months (P = 0.046; Fig. 10A). These results suggested that a higher CSF1R expression was associated with poor survival of patients with HGG in REMBRANDT database.
Fig. 10.
LR radiomics model external validation. A. High level of CSF1R expression was associated with shorter OS in HGG patients in the REMBRANDT database. B. ROC curves of radiomics model for predicting CSF1R expression in the external validation set. C. The calibration curves of the radiomics model demonstrated that prediction probability was in good agreement with the true value. D. DCA for the radiomics model had high clinical practicability. * P < 0.05, ** P < 0.01, or *** P < 0.001 were considered significant differences
The performance of radiomics model was externally validated in REMBRANDT database. The ROC curves of the radiomics model indicated that the AUC value was 0.744 (95% CI 0.492–0.996; Fig. 10B). Additionally, the Hosmer–Lemeshow goodness-of-fit test and calibration curve demonstrated that the prediction probability of the radiomics model was highly consistent with the true value (Fig. 10C). According to DCA, the radiomics prediction model had high clinical usefulness (Fig. 10D).
Discussion
This study aimed to predict CSF1R expression and HGG patient prognosis by analyzing MRI images. In the TCGA cohort, the CSF1R up-regulation is related to worse prognosis, malignant progression and increased immune infiltration in patients with HGG patients. Based on TCGA and TCIA cohorts, we verified that Rad_score between CSF1R high and low groups was significantly different and built a predictive radiomics prediction model of CSF1R expression. The AUC of the ROC curve was 0.768 (95% CI 0.663–0.873) and 0.792 (95% CI 0.698–0.886) in SVM and LR models, severally. Rad_score correlated positively with tumor-related macrophage genes. A radiomics nomogram which combined the Rad_score with clinical factors was constructed and demonstrated satisfactory performance. Our research indicated that MRI-based machine learning radiomics can obtain molecular information of CSF1R, which may provide a worthy implement for clinical practice and stratification of high-grade glioma patients.
The more prognostic indicators about glioma we can obtain, the better the early clinical decision we can make. However, the traditional biopsy method is invasive and expensive, making it difficult to reflect comprehensive information on the pathological heterogeneity of the whole tumor, even if the information is obtained from multiple parts of the tumor [7, 8]. In contrast, MRI-based machine learning radiomics can be used to evaluate the nature of the whole tumor non-invasively with strong repeatability [17, 18]. Recent multiple studies have attempted to correlate radiomics features with cancer patients, including glioma patients [34–36]. It was showed that a radiomics nomogram combined with EPI, age and a radiomic characteristics was reliable for classifying glioma patient OS [37]. IDH mutation is a critical biomarker for glioma patients [38], and it has been demonstrated that the radiomics prediction model could predict IDH mutation status non-invasively [39, 40]. CSF1R is an immunotherapy of HGG, but CSF1R is rarely involved in radiomics studies. Therefore, it is necessary to use radiomics methods to obtain CSF1R expression in HGG accurately.
After TCGA analysis, we detected that the increased expression of CSF1R was connected with malignant progression and worse outcome in patients with HGG. CSF1R and tumor grade were independent risk factors associated with shorter OS in multivariate Cox regression analysis. Subgroup analysis indicated that the increased expression of CSF1R was a risk factor for OS in age, tumor grade, Chr_1p_19q codeletion, chemotherapy, and radiotherapy subgroups. Only the interaction between CSF1R and radiotherapy was significantly different, illustrating that the effect of CSF1R on OS differed significantly between patients who received radiotherapy and those who did not. Although radiotherapy can improve care for patients with HGG, the median survival is still limited [41]. Thus, CSF1R could be used as a screening tool to identify patients with a poor outcome and inferior response to radiotherapy, so as to optimize the targeted treatment of patients with HGG. A recent study reported that CSF1R inhibition could enhance the effect of radiotherapy by blocking microglia and macrophage activation [42]. Another analysis revealed that inhibiting CSF1R (BLZ-945) in the in situ immunocompetent mouse model improved the efficacy of radiotherapy in treating GBM and significantly improved the survival rate compared to radiotherapy alone [43]. Considering the important role of CSF1R in HGG, we believe that non-invasive prediction of CSF1R expression level is helpful for personalized clinical decision-making, such as screening patients with the poor radiotherapy-induced antitumor immune response for CSF1R inhibitor.
The glioma microenvironment is heavily infiltrated by non-myeloid immune cells, leading to tumor progression and immunotherapy resistance, especially by M2-polarized macrophages [44]. We also discovered that monocytes, macrophages, CD4+ T cells, helper T cells, activated NK cells, and eosinophils were significantly elevated in CSF1R high expression group. The most up-regulated cells were macrophages M2, Monocytes, Trm cells, and Tfh cells. Immunocytes in the tumor microenvironment (TME) are key factors in tumor evolution. Increasing evidence supports the significance of immune cell distribution, composition, density, and functional status in forecasting tumor patient prognosis [45–47]. Tumor-associated macrophages was instrumental in tumor initiation, growth and distant metastasis, and are considered the primary component of the TME. Most solid cancers are associated with a poor prognosis when macrophage density is high [48]. The GO and KEGG analysis were conducted to estimate the biological implications of differentially expressed genes. Our results revealed that the DEGs were prominently enriched in DNA transcriptional activation and receptor-ligand activity. The identified pathways, such as Focal adhesion, MAPK, Wnt, and mTOR signaling pathways, have proven to be important in glioma [49–51].
Radiomics analysis has become a hopeful tool for the diagnosis, curative effect, and survival prediction of different cancers [20, 52, 53]. Our study attempted to explore radiomics features related to CSF1R in HGG patients. Ultimately, we identified nine features for establishing the radiomics model. The radiomics model predicting CSF1R was constructed based on SVM and LR algorithms, achieving high AUC values (0.786 and 0.706 in SVM; 0.792 and 0.717 in LR, respectively) and clinical practicability in training and validation sets. In both SVM and LR models, the Rad_score between the CSF1R high and low groups showed dramatic differences. Our study suggested that the model based on radiomic signatures and clinical characteristics may be a promising approach to predicting CSF1R expression. A previous study of low-grade gliomas revealed that radiomics features, such as GLSZM (Gray Level Size Zone Matrix), shape and GLCM (Gray-Level Co-occurrence Matrix), were promising for forecasting the TERT promoter status and tumor outcome [54]. Another study developed radiological features to predict MGMT promoter methylation in GBM, which may help to predict the molecular pathology of tumor targets [55]. Other studies constructed novel models based on the combination of radiomics and deep characteristics of MRI that can better predict high-grade glioma prognosis [56, 57]. We are the first to report the use of radiological features to predict CSF1R expression levels in tumors.
Our study revealed a positive correlation between CSF1R and macrophage infiltration in HGG in TCGA. Therefore, we analyzed the association between the Rad_score and macrophage-related genes in LR model. Notably, our results indicated that Rad_score was positively correlated with M2 macrophage-related genes, such as CD86, TLR2, and TLR4, while negatively correlated with M1 macrophage-related gene ARG1. These results further validated that this prediction model has good performance in predicting CSF1R, and Rad_score was positively associated with tumor-related macrophage genes. CSF1R was essential to the differentiation and maturation of macrophages [58]. CSF1R signals regulate macrophages' proliferation, differentiation, and survival in most vertebrate species [59]. The combination of CSF1 and CSF1R promotes macrophage maturation and upregulates gene expression leading to the M2 macrophage phenotype [58]. CSF1R inhibitors can reverse M2 polarization, reduce M2 macrophage markers, and impair tumor-promoting function [15]. Therefore, our results were identical with those reported in the literature. The predicted Rad_score is likely to affect the HGG progression through macrophage, which is helpful to tailor personalized treatment and has clinical translational potential.
The interactions between multiple risk factors might be thoroughly considered to better reflect the complex nature of tumor [60]. A recent study integrating KPS, MGMT promoter methylation, and tumor location successfully predicted the glioma patient OS [61]. In this study, the radiomics nomogram produced was derived from Rad_score and three clinical variables, namely, radiotherapy, MGMT promoter status, and tumor grade. Our nomogram demonstrated the incremental value of combining multiple variables for OS prediction in glioma patients, presenting satisfactory performance. Only a few studies predicted the molecular subtypes and OS of gliomas using the radiomics approach. The nomogram composed of age, choline/creatine (Cho/Cr), lactate peak of magnetic resonance spectroscopy (MRS), necrotic volume percentages of core (CNV), and Rad_score was used to predict TERT promoter mutation in HGG with high precision [62]. It was demonstrated that a radiomics nomogram combined with age, a radiomic characteristic and EPI was reliable for classifying glioma patient OS [37]. The combination of radiological features, IDH, and age nomogram improves the performance of OS prediction, which may be a new supplement to HGG treatment guidelines [63]. Although there are few studies on the radiomics nomogram of HGG, our research and literature have indicated that the construction of nomogram by Rad_score and clinical factors can better predict patient prognosis with gliomas.
Tumor treating fields (TTfields) is a non-invasive, low-intensity, intermediate-frequency (200 kHz) alternating electric field [64]. The inhibitory effect of TTfields on cell growth is mainly achieved by the interference of mitotic cell cycle [65]. The combination of TTFields and temozolomide maintenance therapy has been shown to significantly prolong survival and have good tolerance for newly diagnosed adult GBM [66]. As the fourth modality in cancer treatment, TTFields can be delivered locally to minimize the risk of systemic adverse effects. When combined with other anticancer treatments, it can achieve greater efficacy without increasing toxicity [67]. Based on the results of several trials, TTFields therapy was approved for adults with recurrent and newly diagnosed GBM [68]. But its application in other solid tumors needs to be further studied.
There are some limitations in this study. First, retrospective data from two public databases were utilized, with relatively small sample sizes. We should conduct some multi-center studies to confirm the generalizability of the model in the future. Second, this is a retrospective study, and there are inevitably confounding factors. Third, post-operative MR images were unavailable in most of the patients. The changes in the Rad_score may correlate better with the clinicopathological data and provide the model with additional information. Finally, manually delineate VOI, which may lead to observer bias. Although manual VOI delineation is the most accurate of the current tumor segmentation methods, it is also affected by subjective factors to some extent; semi-automatic or automatic target delineation may be more objective and less time-consuming.
Conclusions
This study proved that MRI-based radiomic analysis is a novel and non-invasively convenient approach for predicting CSF1R expression and prognosis in high-grade glioma patients. The radiomics nomogram could optimize individualized survival estimation and risk stratification for HGG patients.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Dr. Di Zhou for providing financial support.
Author Contributions
M.L.W. designed the study; M.L.W., Y.L.L, Y.Y.W. and X.Y.C. performed the research; M.L.W., Y.L.L. and Y.Y.W analyzed the data; M.L.W., Y.L.L. wrote the paper. M.L.W., G.X.Z. and W.C.G revised the paper.
Funding
This study was supported by the National Natural Science Foundation of China (No.82002538 and No. 82372502). Science and Technology Commission of Shanghai Municipality-Medical Innovation Research Special Project (23Y11902300); Zhongshan Hospital Clinical Research Special Funding (ZSLCYJ202346); Natural Science Foundation of Shanghai Municipal Commission of Science and Technology (No. 23ZR1411400).
Data Availability
If the requirement is reasonable, any original data of this study can be obtained from the author.
Declarations
Ethics Approval
This is an observational study. The Zhongshan hospital Research Ethics Committee has confirmed that no ethical approval is required.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors claim that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yuling Lai, Yiyang Wu and Xiangyuan Chen these authors have contributed equally to this work and share first authorship.
Contributor Information
Wenchao Gu, Email: sunferreero@gmail.com.
Guoxia Zhou, Email: zgx6742997@163.com.
Meilin Weng, Email: 15111230032@fudan.edu.cn.
References
- 1.Sung H, et al.: Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: a cancer journal for clinicians 71:209–249, 2021 [DOI] [PubMed]
- 2.Gupta A, Dwivedi T. A Simplified Overview of World Health Organization Classification Update of Central Nervous System Tumors 2016. Journal of neurosciences in rural practice. 2017;8:629–641. doi: 10.4103/jnrp.jnrp_168_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Larsen J, et al. 'Low grade glioma': an update for radiologists. The British journal of radiology. 2017;90:20160600. doi: 10.1259/bjr.20160600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brat DJ, et al. Comprehensive, Integrative Genomic Analysis of Diffuse Lower-Grade Gliomas. The New England journal of medicine. 2015;372:2481–2498. doi: 10.1056/NEJMoa1402121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Omuro A, DeAngelis LM. Glioblastoma and other malignant gliomas: a clinical review. Jama. 2013;310:1842–1850. doi: 10.1001/jama.2013.280319. [DOI] [PubMed] [Google Scholar]
- 6.Louis DN, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta neuropathologica. 2016;131:803–820. doi: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- 7.Ostrom QT, et al. CBTRUS Statistical Report: Primary brain and other central nervous system tumors diagnosed in the United States in 2010–2014. Neuro-oncology. 2017;19:v1–v88. doi: 10.1093/neuonc/nox158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aum DJ, Kim DH, Beaumont TL, Leuthardt EC, Dunn GP, Kim AH. Molecular and cellular heterogeneity: the hallmark of glioblastoma. Neurosurgical focus. 2014;37:E11. doi: 10.3171/2014.9.FOCUS14521. [DOI] [PubMed] [Google Scholar]
- 9.Hume DA. The mononuclear phagocyte system. Current opinion in immunology. 2006;18:49–53. doi: 10.1016/j.coi.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 10.Chitu V, Biundo F, Stanley ER. Colony stimulating factors in the nervous system. Seminars in immunology. 2021;54:101511. doi: 10.1016/j.smim.2021.101511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wiehagen KR, et al. Combination of CD40 Agonism and CSF-1R Blockade Reconditions Tumor-Associated Macrophages and Drives Potent Antitumor Immunity. Cancer immunology research. 2017;5:1109–1121. doi: 10.1158/2326-6066.CIR-17-0258. [DOI] [PubMed] [Google Scholar]
- 12.Quail DF, et al.: The tumor microenvironment underlies acquired resistance to CSF-1R inhibition in gliomas. Science 352:aad3018, 2016 [DOI] [PMC free article] [PubMed]
- 13.Butowski N, et al. Orally administered colony stimulating factor 1 receptor inhibitor PLX3397 in recurrent glioblastoma: an Ivy Foundation Early Phase Clinical Trials Consortium phase II study. Neuro-oncology. 2016;18:557–564. doi: 10.1093/neuonc/nov245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patwardhan PP, et al. Sustained inhibition of receptor tyrosine kinases and macrophage depletion by PLX3397 and rapamycin as a potential new approach for the treatment of MPNSTs. Clinical cancer research : an official journal of the American Association for Cancer Research. 2014;20:3146–3158. doi: 10.1158/1078-0432.CCR-13-2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pyonteck SM, et al. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nature medicine. 2013;19:1264–1272. doi: 10.1038/nm.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zonari P, Baraldi P, Crisi G. Multimodal MRI in the characterization of glial neoplasms: the combined role of single-voxel MR spectroscopy, diffusion imaging and echo-planar perfusion imaging. Neuroradiology. 2007;49:795–803. doi: 10.1007/s00234-007-0253-x. [DOI] [PubMed] [Google Scholar]
- 17.Lu CF, et al. Machine Learning-Based Radiomics for Molecular Subtyping of Gliomas. Clinical cancer research : an official journal of the American Association for Cancer Research. 2018;24:4429–4436. doi: 10.1158/1078-0432.CCR-17-3445. [DOI] [PubMed] [Google Scholar]
- 18.Zhou H, et al. MRI features predict survival and molecular markers in diffuse lower-grade gliomas. Neuro-oncology. 2017;19:862–870. doi: 10.1093/neuonc/now256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Artzi M, Bressler I, Ben Bashat D. Differentiation between glioblastoma, brain metastasis and subtypes using radiomics analysis. Journal of magnetic resonance imaging : JMRI. 2019;50:519–528. doi: 10.1002/jmri.26643. [DOI] [PubMed] [Google Scholar]
- 20.Lambin P, et al. Radiomics: the bridge between medical imaging and personalized medicine. Nature reviews Clinical oncology. 2017;14:749–762. doi: 10.1038/nrclinonc.2017.141. [DOI] [PubMed] [Google Scholar]
- 21.Leu K, et al. Perfusion and diffusion MRI signatures in histologic and genetic subtypes of WHO grade II-III diffuse gliomas. Journal of neuro-oncology. 2017;134:177–188. doi: 10.1007/s11060-017-2506-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reza SMS, Samad MD, Shboul ZA, Jones KA, Iftekharuddin KM: Glioma grading using structural magnetic resonance imaging and molecular data. Journal of medical imaging (Bellingham, Wash) 6:024501, 2019 [DOI] [PMC free article] [PubMed]
- 23.Zeng WJ, et al. Integrative Analysis of DNA Methylation and Gene Expression Identify a Three-Gene Signature for Predicting Prognosis in Lower-Grade Gliomas. Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry, and pharmacology. 2018;47:428–439. doi: 10.1159/000489954. [DOI] [PubMed] [Google Scholar]
- 24.Beig N, et al. Radiogenomic-Based Survival Risk Stratification of Tumor Habitat on Gd-T1w MRI Is Associated with Biological Processes in Glioblastoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2020;26:1866–1876. doi: 10.1158/1078-0432.CCR-19-2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bodalal Z, Trebeschi S, Nguyen-Kim TDL, Schats W, Beets-Tan R. Radiogenomics: bridging imaging and genomics. Abdominal radiology (New York) 2019;44:1960–1984. doi: 10.1007/s00261-019-02028-w. [DOI] [PubMed] [Google Scholar]
- 26.Cohen AL, Holmen SL, Colman H. IDH1 and IDH2 mutations in gliomas. Current neurology and neuroscience reports. 2013;13:345. doi: 10.1007/s11910-013-0345-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boots-Sprenger SH, et al.: Significance of complete 1p/19q co-deletion, IDH1 mutation and MGMT promoter methylation in gliomas: use with caution. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc 26:922–929, 2013 [DOI] [PubMed]
- 28.Vivian J, et al. Toil enables reproducible, open source, big biomedical data analyses. Nature biotechnology. 2017;35:314–316. doi: 10.1038/nbt.3772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim JY, et al. Incorporating diffusion- and perfusion-weighted MRI into a radiomics model improves diagnostic performance for pseudoprogression in glioblastoma patients. Neuro-oncology. 2019;21:404–414. doi: 10.1093/neuonc/noy133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hong JH, et al. Development and Validation of a Radiomics Model for Differentiating Bone Islands and Osteoblastic Bone Metastases at Abdominal CT. Radiology. 2021;299:626–632. doi: 10.1148/radiol.2021203783. [DOI] [PubMed] [Google Scholar]
- 31.Yu Y, et al. Development and Validation of a Preoperative Magnetic Resonance Imaging Radiomics-Based Signature to Predict Axillary Lymph Node Metastasis and Disease-Free Survival in Patients With Early-Stage Breast Cancer. JAMA network open. 2020;3:e2028086. doi: 10.1001/jamanetworkopen.2020.28086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lv L, et al. Radiomic analysis for predicting prognosis of colorectal cancer from preoperative (18)F-FDG PET/CT. J Transl Med. 2022;20:66. doi: 10.1186/s12967-022-03262-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fang Q, Chen H: The significance of m6A RNA methylation regulators in predicting the prognosis and clinical course of HBV-related hepatocellular carcinoma. Molecular medicine (Cambridge, Mass) 26:60, 2020 [DOI] [PMC free article] [PubMed]
- 34.McKenney AS, et al.: Radiomic Analysis to Predict Histopathologically Confirmed Pseudoprogression in Glioblastoma Patients. Advances in Radiation Oncology:100916, 2022 [DOI] [PMC free article] [PubMed]
- 35.Patel M, et al. Machine learning-based radiomic evaluation of treatment response prediction in glioblastoma. Clinical Radiology. 2021;76:628.e617–628.e627. doi: 10.1016/j.crad.2021.03.019. [DOI] [PubMed] [Google Scholar]
- 36.Saltybaeva N, et al. Robustness of radiomic features in magnetic resonance imaging for patients with glioblastoma: Multi-center study. Physics and Imaging in Radiation Oncology. 2022;22:131–136. doi: 10.1016/j.phro.2022.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu Y, He X, Li Y, Pang P, Shu Z, Gong X. The Nomogram of MRI-based Radiomics with Complementary Visual Features by Machine Learning Improves Stratification of Glioblastoma Patients: A Multicenter Study. J Magn Reson Imaging. 2021;54:571–583. doi: 10.1002/jmri.27536. [DOI] [PubMed] [Google Scholar]
- 38.Eckel-Passow JE, et al. Glioma Groups Based on 1p/19q, IDH, and TERT Promoter Mutations in Tumors. The New England journal of medicine. 2015;372:2499–2508. doi: 10.1056/NEJMoa1407279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Patel SH, et al. T2-FLAIR Mismatch, an Imaging Biomarker for IDH and 1p/19q Status in Lower-grade Gliomas: A TCGA/TCIA Project. Clinical cancer research : an official journal of the American Association for Cancer Research. 2017;23:6078–6085. doi: 10.1158/1078-0432.CCR-17-0560. [DOI] [PubMed] [Google Scholar]
- 40.Su X, et al. A radiomics-clinical nomogram for preoperative prediction of IDH1 mutation in primary glioblastoma multiforme. Clin Radiol. 2020;75:963.e967–963.e915. doi: 10.1016/j.crad.2020.07.036. [DOI] [PubMed] [Google Scholar]
- 41.Prestwich RJ, Sivapalasunrtharam A, Johnston C, Evans K, Gerrard GE: Survival in high-grade glioma: a study of survival in patients unfit for or declining radiotherapy. Clinical oncology (Royal College of Radiologists (Great Britain)) 17:133–137, 2005 [DOI] [PubMed]
- 42.Akkari L, et al.: Dynamic changes in glioma macrophage populations after radiotherapy reveal CSF-1R inhibition as a strategy to overcome resistance. Sci Transl Med 12, 2020 [DOI] [PubMed]
- 43.Almahariq MF, Quinn TJ, Kesarwani P, Kant S, Miller CR, Chinnaiyan P. Inhibition of Colony-Stimulating Factor-1 Receptor Enhances the Efficacy of Radiotherapy and Reduces Immune Suppression in Glioblastoma. In vivo (Athens, Greece) 2021;35:119–129. doi: 10.21873/invivo.12239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Domingues P, et al. Tumor infiltrating immune cells in gliomas and meningiomas. Brain, behavior, and immunity. 2016;53:1–15. doi: 10.1016/j.bbi.2015.07.019. [DOI] [PubMed] [Google Scholar]
- 45.Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nature immunology. 2013;14:1014–1022. doi: 10.1038/ni.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mao X, et al. Crosstalk between cancer-associated fibroblasts and immune cells in the tumor microenvironment: new findings and future perspectives. Mol Cancer. 2021;20:131. doi: 10.1186/s12943-021-01428-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vitale I, Manic G, Coussens LM, Kroemer G, Galluzzi L. Macrophages and Metabolism in the Tumor Microenvironment. Cell metabolism. 2019;30:36–50. doi: 10.1016/j.cmet.2019.06.001. [DOI] [PubMed] [Google Scholar]
- 48.Chanmee T, Ontong P, Konno K, Itano N. Tumor-associated macrophages as major players in the tumor microenvironment. Cancers. 2014;6:1670–1690. doi: 10.3390/cancers6031670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Perreault S, et al. A phase 2 study of trametinib for patients with pediatric glioma or plexiform neurofibroma with refractory tumor and activation of the MAPK/ERK pathway: TRAM-01. BMC Cancer. 2019;19:1250. doi: 10.1186/s12885-019-6442-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.He L, Zhou H, Zeng Z, Yao H, Jiang W, Qu H. Wnt/β-catenin signaling cascade: A promising target for glioma therapy. Journal of cellular physiology. 2019;234:2217–2228. doi: 10.1002/jcp.27186. [DOI] [PubMed] [Google Scholar]
- 51.Le Rhun E, et al. Molecular targeted therapy of glioblastoma. Cancer Treat Rev. 2019;80:101896. doi: 10.1016/j.ctrv.2019.101896. [DOI] [PubMed] [Google Scholar]
- 52.Fathi Kazerooni A, et al.: Applications of Radiomics and Radiogenomics in High-Grade Gliomas in the Era of Precision Medicine. Cancers 13, 2021 [DOI] [PMC free article] [PubMed]
- 53.Gillies RJ, Schabath MB. Radiomics Improves Cancer Screening and Early Detection. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2020;29:2556–2567. doi: 10.1158/1055-9965.EPI-20-0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lu J, Li X, Li H: A radiomics feature-based nomogram to predict telomerase reverse transcriptase promoter mutation status and the prognosis of lower-grade gliomas. Clin Radiol, 2022 [DOI] [PubMed]
- 55.Xi YB, et al. Radiomics signature: A potential biomarker for the prediction of MGMT promoter methylation in glioblastoma. Journal of magnetic resonance imaging : JMRI. 2018;47:1380–1387. doi: 10.1002/jmri.25860. [DOI] [PubMed] [Google Scholar]
- 56.Wang Y, Shao Q, Luo S, Fu R. Development of a nomograph integrating radiomics and deep features based on MRI to predict the prognosis of high grade Gliomas. Mathematical biosciences and engineering : MBE. 2021;18:8084–8095. doi: 10.3934/mbe.2021401. [DOI] [PubMed] [Google Scholar]
- 57.Ding J, et al. Developing and validating a deep learning and radiomic model for glioma grading using multiplanar reconstructed magnetic resonance contrast-enhanced T1-weighted imaging: a robust, multi-institutional study. Quantitative imaging in medicine and surgery. 2022;12:1517–1528. doi: 10.21037/qims-21-722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jeannin P, Paolini L, Adam C, Delneste Y. The roles of CSFs on the functional polarization of tumor-associated macrophages. The FEBS journal. 2018;285:680–699. doi: 10.1111/febs.14343. [DOI] [PubMed] [Google Scholar]
- 59.Lei F, Cui N, Zhou C, Chodosh J, Vavvas DG, Paschalis EI. CSF1R inhibition by a small-molecule inhibitor is not microglia specific; affecting hematopoiesis and the function of macrophages. Proc Natl Acad Sci U S A. 2020;117:23336–23338. doi: 10.1073/pnas.1922788117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Collins GS, Reitsma JB, Altman DG, Moons KG. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. Bmj. 2015;350:g7594. doi: 10.1136/bmj.g7594. [DOI] [PubMed] [Google Scholar]
- 61.Patil N, et al. Independently validated sex-specific nomograms for predicting survival in patients with newly diagnosed glioblastoma: NRG Oncology RTOG 0525 and 0825. Journal of neuro-oncology. 2021;155:363–372. doi: 10.1007/s11060-021-03886-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tian H, Wu H, Wu G, Xu G. Noninvasive Prediction of TERT Promoter Mutations in High-Grade Glioma by Radiomics Analysis Based on Multiparameter MRI. BioMed research international. 2020;2020:3872314. doi: 10.1155/2020/3872314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tan Y, Mu W, Wang XC, Yang GQ, Gillies RJ, Zhang H. Improving survival prediction of high-grade glioma via machine learning techniques based on MRI radiomic, genetic and clinical risk factors. European journal of radiology. 2019;120:108609. doi: 10.1016/j.ejrad.2019.07.010. [DOI] [PubMed] [Google Scholar]
- 64.Mun EJ, Babiker HM, Weinberg U, Kirson ED, Von Hoff DD. Tumor-Treating Fields: A Fourth Modality in Cancer Treatment. Clinical cancer research : an official journal of the American Association for Cancer Research. 2018;24:266–275. doi: 10.1158/1078-0432.CCR-17-1117. [DOI] [PubMed] [Google Scholar]
- 65.Kirson ED, et al. Alternating electric fields arrest cell proliferation in animal tumor models and human brain tumors. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:10152–10157. doi: 10.1073/pnas.0702916104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stupp R, et al. Maintenance Therapy With Tumor-Treating Fields Plus Temozolomide vs Temozolomide Alone for Glioblastoma: A Randomized Clinical Trial. Jama. 2015;314:2535–2543. doi: 10.1001/jama.2015.16669. [DOI] [PubMed] [Google Scholar]
- 67.Ghiaseddin AP, Shin D, Melnick K, Tran DD. Tumor Treating Fields in the Management of Patients with Malignant Gliomas. Current treatment options in oncology. 2020;21:76. doi: 10.1007/s11864-020-00773-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Guo X, et al.: Tumor-Treating Fields in Glioblastomas: Past, Present, and Future. Cancers 14, 2022 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
If the requirement is reasonable, any original data of this study can be obtained from the author.