Abstract
Ocular position drifts during gaze fixation are significantly less well understood than microsaccades. We recently identified a short-latency ocular position drift response, of ∼1 min arc amplitude, that is triggered within <100 ms by visual onsets. This systematic eye movement response is feature-tuned and seems to be coordinated with a simultaneous resetting of the saccadic system by visual stimuli. However, much remains to be learned about the drift response, especially for designing better-informed neurophysiological experiments unraveling its mechanistic substrates. Here we systematically tested multiple new feature tuning properties of drift responses. Using highly precise eye tracking in three male rhesus macaque monkeys, we found that drift responses still occur for tiny foveal visual stimuli. Moreover, the responses exhibit size tuning, scaling their amplitude (both up and down) as a function of stimulus size, and they also possess a monotonically increasing contrast sensitivity curve. Importantly, short-latency drift responses still occur for small peripheral visual targets, which additionally introduce spatially directed modulations in drift trajectories toward the appearing peripheral stimuli. Drift responses also remain predominantly upward even for stimuli exclusively located in the lower visual field and even when starting gaze position is upward. When we checked the timing of drift responses, we found it was better synchronized to stimulus-induced saccadic inhibition than to stimulus onset. These results, along with a suppression of drift response amplitudes by peristimulus saccades, suggest that drift responses reflect the rapid impacts of short-latency and feature-tuned visual neural activity on final oculomotor control circuitry in the brain.
Keywords: contrast sensitivity, fixational eye movements, ocular position drifts, saccadic inhibition, saccadic suppression, stimulus size
Significance Statement
During gaze fixation, the eye drifts slowly in between microsaccades. While eye position drifts are not entirely random, they remain to be significantly more enigmatic than microsaccades, and they are typically mathematically modeled as random walk processes. Having recently found that these eye movements are systematically modulated with very short latencies by some stimulus onsets, here we characterized the feature tuning properties of such stimulus-driven drift responses. Our results suggest that drift eye movements reflect the impacts of short-latency sensory signals on the oculomotor system. These results also demonstrate that visual stimuli can impact drift eye movements in a manner similar to how such stimuli impact microsaccades.
Introduction
The eye is never completely still during gaze fixation (Barlow, 1952; Steinman et al., 1967, 1973), resulting in subtle, but continuous, alterations of the retinal image streams entering the visual system. Two primary components of fixational eye movements are microsaccades and slow ocular position drifts (Fig. 1A). While the neural control of microsaccades is relatively well established (Krauzlis et al., 2017; Hafed et al., 2021b), that of drifts is less understood. Moreover, the ways with which external sensory transients interact with these two types of eye movements are not fully investigated.
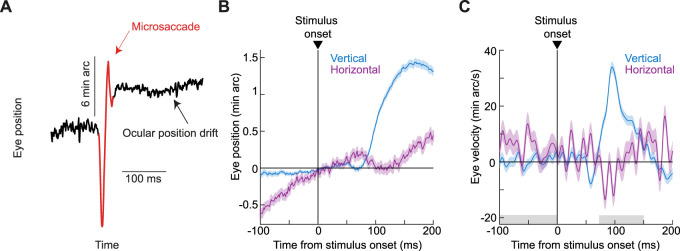
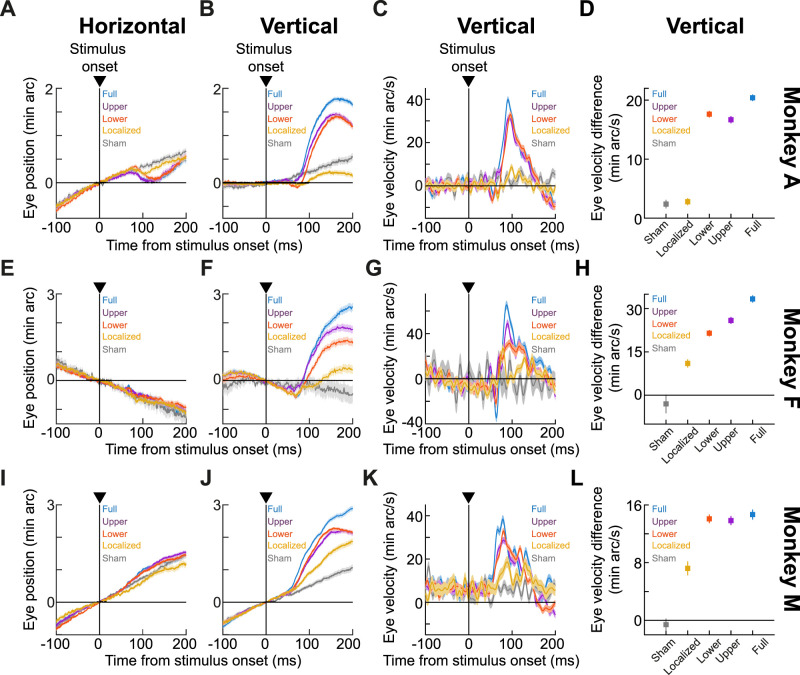
Figure 1.
Stimulus-driven ocular position drift responses. A, Accurate gaze fixation is characterized by two prominent features: (1) microsaccades occur occasionally to realign the line of sight (red); and (2) the eye drifts continuously with slow speeds otherwise (black). B, We recently found (Malevich et al., 2020) that large stimulus onsets result in a short-latency change in ocular position drift statistics, primarily marked by a small upward deviation (although an earlier, even smaller, downward movement component jumpstarts the whole response sequence). The figure shows average horizontal and vertical eye positions (surrounded by SEM ranges; n = 882 trials) from an example condition and an example monkey (A) from Experiment 1 of the current study. Positive deflections indicate rightward and upward eye position deviations, respectively, and the data across trials were first aligned to eye position at time zero before averaging (Malevich et al., 2020; Materials and Methods). The monkey exhibited rightward prestimulus drifts; after stimulus onset, there was a predominantly upward drift response, accompanied by a small leftward component. The upward drift response was also preceded by a much smaller and shorter-lived downward eye position deviation, although we primarily focus here on the overall upward nature of the whole response sequence. C, Horizontal and vertical eye velocity curves (surrounded by SEM ranges) from the same trials as in B. Shaded regions on the x-axis indicate our measurement intervals of baseline (prestimulus) and poststimulus eye velocities, for use in our summary statistics in the remainder of this article.
For microsaccades, visual transients rapidly reset the oculomotor rhythm, causing microsaccadic inhibition (Engbert and Kliegl, 2003; Hafed et al., 2021a; Buonocore and Hafed, 2023) and influencing subsequent perceptual performance and visual neural sensitivity (Hafed et al., 2015). Moreover, such inhibition is feature-tuned, altering its time course and strength as a function of the appearing visual patterns (Khademi et al., 2023). This likely reflects the tuning properties of visually sensitive neurons mediating microsaccadic inhibition (Buonocore and Hafed, 2023).
For drifts, and consistent with the idea that these eye movements are not entirely random (Nachmias, 1961; Murphy et al., 1975; Snodderly and Kurtz, 1985; Skinner et al., 2019; Intoy and Rucci, 2020), we recently found that certain visual stimuli robustly trigger a short-latency change in drift statistics, referred to here as the drift response (Malevich et al., 2020). This response is characterized by a small predominantly upward displacement, superseding the ongoing drift direction and being much slower than even the slowest microsaccades. For example, in Figure 1B, aligning all eye position epochs at the time of stimulus onset reveals a predominantly rightward prestimulus drift trajectory, which was momentarily transformed into a predominantly upward drift pulse within <100 ms after stimulus onset (Fig. 1B,C; Malevich et al., 2020).
Our previous work revealed that the drift response occurred when we presented relatively large stimuli (Malevich et al., 2020). We also found that this response, much like saccadic inhibition (Khademi et al., 2023), is feature-tuned: it was stronger for low spatial frequency patterns, as well as for certain grating orientations (Malevich et al., 2020). However, understanding the full mechanisms underlying the drift response requires much deeper characterization of this response's properties. For example, might such a drift response still occur for small visual stimuli just like microsaccades can be affected by small eccentric targets (Hafed and Clark, 2002; Engbert and Kliegl, 2003)? And, would the predominantly upward nature of the drift response change if we only presented lower visual field stimuli?
Here we answered these, and other, questions, and we laid down a rich foundation for testing the neurophysiological underpinnings of not only the drift response but also of the coordination between multiple types of fixational and targeting eye movements with external sensory events. We first found that the drift response is size-tuned and can still happen for tiny, foveal visual stimuli. We also characterized the contrast sensitivity of the drift response, as well as its modulation by small peripheral visual targets. Interestingly, and unlike our expectation (Malevich et al., 2020) that the drift response might reflect the preference of the superior colliculus (SC) for the upper visual field (Hafed and Chen, 2016; Fracasso et al., 2023), we found that the drift response is still predominantly upward even for stimuli below the horizon. Finally, we characterized the temporal coordination between microsaccades and the drift response, as well as the alteration of the drift response magnitude by the occurrence of peristimulus microsaccades, mimicking the classic phenomenon of saccadic suppression (Zuber and Stark, 1966; Beeler, 1967; Hafed and Krauzlis, 2010; Idrees et al., 2020).
Our results demonstrate that the “lens” through which the oculomotor system processes visual scenes may be similar for dictating the visual feature tuning properties of both saccadic inhibition (Khademi et al., 2023) and drift responses, and that these two ubiquitous eye movement phenomena likely arise from a common underlying source.
Materials and Methods
Experimental animals and ethical approvals
We collected data from three adult, male rhesus macaque monkeys (Macaca mulatta), referred to here as A, F, and M, respectively. The monkeys were aged 7–14 years, and they weighed 9.5–12.5 kg. All experiments were approved by the ethics committees at the regional governmental offices of the city of Tübingen.
Laboratory setup and animal procedures
Some experiments involved analysis of ocular position drifts from our recent study, which only focused on saccades (Khademi et al., 2023). Other experiments were run specifically for the purposes of the current study but in the same experimental setups as in Khademi et al. (2023). The reader is referred to our recent publication for details on our laboratory equipment (Khademi et al., 2023). Briefly, we used precise eye tracking, using the scleral search coil technique (Robinson, 1963; Fuchs and Robinson, 1966; Judge et al., 1980), and a real-time experimental control system based on PLDAPS (Eastman and Huk, 2012) and the Psychophysics Toolbox (Brainard, 1997; Pelli, 1997; Kleiner et al., 2007). The monkeys had their heads stabilized during the experiments, and they watched stimuli on a computer-controlled display in front of them. The display size was spanning ∼31° horizontally and 23° vertically, and the experimental room was otherwise dark. The display was placed 72 cm in front of the animals, and stimulus luminances are described in the relevant places below.
Experimental procedures
The experiments all involved gaze fixation, and we analyzed fixational eye movements. The experimental procedures were described in detail recently (Khademi et al., 2023). In particular, the monkeys fixated a small, stationary fixation spot presented over a gray background (of luminance 26.11 or 36.5 cd/m2). The fixation spot was white, and it spanned 10.8 by 10.8 min arc. A trial started by the appearance of the fixation spot at the center of the display. At a random time during fixation (500–1,200 ms), a single-frame flash (∼12 or ∼7–8 ms) was presented. Across trials and experiments, the flash could have different feature properties (e.g., full-screen flash or small, localized target, and so on). In what follows, we describe the experiment-specific details, explaining what image features the brief flashes had in the different experiments. After stimulus presentation, the monkeys were required to maintain fixation of the central fixation spot for an additional 900–1,000 ms, after which the screen went blank (still with a gray background), and the monkeys were rewarded. Whenever a stimulus was presented centered on the fixation spot, we did not display the fixation spot, but this was not a problem for the monkeys because the fixation spot was only vanished for a tiny fraction of a second.
Experiment 1: size tuning
The stimulus flash in this experiment consisted of a black circle of different radii across trials, and the circle was centered on the fixation spot. The range of sizes tested included stimuli approximately as small as the fixation spot (0.09° radius), stimuli approximately as large as the entire display (9.12° radius), and stimuli with sizes in between these two extremes. Moreover, the numbers of trials collected were the same as those reported in (Khademi et al., 2023). For the numbers of trials that were analyzed, these depended on whether we picked drift response trials (saccade-free) or trials with peristimulus microsaccades (see Data Analysis below for details). That is why we document the specific numbers of trials included in the analyses of each figure shown in Results separately.
Note that this experiment was the same as that used recently (Khademi et al., 2023). In that study, we analyzed the fixational saccades that took place around stimulus onset. In the current study, we analyzed ocular position drifts (in saccade-free epochs), as well as saccade–drift interactions, as we describe in more detail below.
Experiment 2: contrast sensitivity with full-screen stimuli
This experiment was again the same as that used recently (Khademi et al., 2023). Briefly, the stimulus onset could be a full-screen flash having one of five different Weber contrasts (5, 10, 20, 40, or 80%). Once again, we analyzed saccade-free drift response trials as well as trials having microsaccades within specific time intervals relative to stimulus onset (see Data Analysis below for more details). For each analysis, the numbers of trials included are documented individually in Results. Drift-only (saccade-free) trials were not analyzed previously in (Khademi et al., 2023).
Experiment 3: upper and lower visual field stimuli
This experiment was collected specifically for this study (as well as related ongoing neurophysiological experiments). The general trial sequence was the same as that in the above two experiments. Specifically, the monkeys fixated a central spot. After a random time, one of five different events took place, depending on the trial type. The first trial type was just a sham condition: no stimulus display update occurred at all, but we just used the sham event in the data file to study baseline drift trajectories and compare them to trajectories with a real stimulus. The second trial type had the stimulus being a 1° × 1° black square that was flashed for a single display frame. The location of the flash was somewhere in the periphery relative to the central fixation spot (∼3.5–11°), but this location was constant within a given session. This location was typically dictated by the locations of receptive fields of neurons that we were recording simultaneously for other purposes, since this task was typically run while we recorded SC and/or primary visual cortex activity. The third trial type was a 100% black full-screen flash (again with a duration of a single frame). Here, the stimulus was basically similar to the stimuli used in Experiment 2 above. And, finally, the fourth and fifth trial types were half-screen flashes. Specifically, we split the screen in half along the vertical dimension. In one condition, the flash was only in the upper half of the screen (above the midline defined by the vertical position of the fixation spot), and in another condition, the flash was only in the lower half of the screen.
We typically ran this task in daily blocks of ∼100–500 trials per session, and we collected a total of 7,524, 7,521, and 7,495 trials in monkeys A, F, and M, respectively. This resulted in 72–1,208 trials per condition per animal for the saccade-free drift response analyses (like in Fig. 1B,C).
Experiment 4: small, localized stimuli across different visual field directions
Because the locations of the small stimuli used in Experiment 3 were dictated by other experimental constraints (such as receptive field locations), we ran an additional experiment in which we sampled eccentric locations more evenly. Specifically, the experiment consisted of the transient flash being a 1° × 1° black square at a 7.9° eccentricity from the display center. The square could appear in one of eight equally spaced directions, thus covering both right and left as well as up and down visual field locations. The flash location was randomly interleaved across trials, and the timing of events in the task was otherwise the same as described above.
We typically ran this task in daily blocks of 310–900 trials per session, and we collected a total of 5,961, 4,357, and 6,048 trials in monkeys A, F, and M, respectively. This resulted in 65–383 analyzed trials per location per animal for the basic saccade-free drift response analyses. We typically pooled multiple locations for a given analysis, as we describe below, in order to increase statistical confidence in the results. Once again, all numbers of trials are documented in appropriate sections of Results.
Experiment 5: gaze position
This task was the same as that in Experiment 2 above, with only one difference. Across sessions, the fixation spot could be at 4° to the right, left, up, and down relative to the display center. This task, therefore, allowed us to test whether the drift response (Fig. 1B,C) was substantially different if the starting gaze position of the eye was different.
We ran four sessions of this task in monkey A, collecting a total of 2,206 trials. This resulted in 500–602 analyzed trials per eye position for the basic saccade-free drift response analyses.
Data analysis
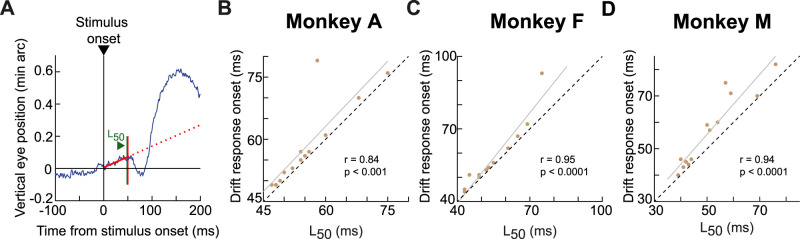
All saccades, which were typically in the microsaccade amplitude range because of fixation spot size, were analyzed as described recently (Khademi et al., 2023). Briefly, we detected saccades of all sizes using our established methods (Chen and Hafed, 2013; Bellet et al., 2019), and we included all detected saccades that took place around stimulus onset. This allowed us to estimate saccadic inhibition latency using the L50 parameter (Reingold and Stampe, 2002, 2004; Rolfs et al., 2008; Khademi et al., 2023). Simply put, this parameter describes when the saccade rate curve drops by 50% of the dynamic range between prestimulus (baseline) saccade rate and the minimum saccade rate during saccadic inhibition (Khademi et al., 2023). That is, we first estimated saccade rate: we calculated saccade onset likelihood within 50 ms moving windows that were stepped in time by 1 ms steps, and we did this on a per-trial basis; across-trial average rates were then obtained and aligned to stimulus onset in order to calculate L50 from the global saccade rate. This calculation involved first measuring baseline saccade rate by averaging this rate in the interval from −100 to 0 ms before stimulus onset in a given condition. Then, we searched for the minimum saccade rate during saccadic inhibition. L50 was defined as the time in which half of the dynamic range (from baseline to minimum rate) was crossed. While we acknowledge that there might be other means to estimate the latency of saccadic inhibition (Bompas et al., 2023), we used L50 because of its consistent use in other studies (Reingold and Stampe, 2002, 2004; Rolfs et al., 2008; Khademi et al., 2023), and also because it does a good job in capturing the drop in saccade likelihood across conditions (see Fig. 7 later in Results).
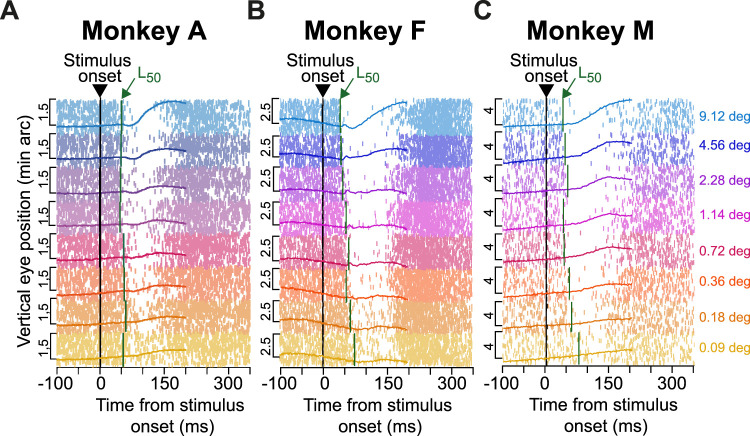
Figure 7.
Coincidence between drift response onset and saccadic inhibition timing. A, In the size tuning experiment, the timing of saccadic inhibition depends on stimulus size (Khademi et al., 2023). This is indicated here, for monkey A, by the raw saccade onset times (tick marks) and a measure (vertical dark green lines marked with L50) of saccadic inhibition timing (Materials and Methods; Khademi et al., 2023). Each row of tick marks represents a single trial, and each tick mark represents a saccade onset. The L50 line in each condition (dark green color) indicates our estimate of the saccadic inhibition timing (Khademi et al., 2023), and all trials of a given stimulus size are grouped together according to the color legend. Within each group, we also plotted the drift response (on trials without saccades; Materials and Methods) by showing vertical eye position aligned on stimulus onset (scale bars are shown on the left of each curve). Despite the variable saccadic inhibition timing, the drift response was synchronized with such timing. That is, both the timing of the drift response (on trials without saccades) and the timing of saccadic inhibition (on trials with saccades) depended on the stimulus properties (also see Figs. 8, 9). B, Similar observations from monkey F. C, Similar observations from monkey M. The saccade data in B were directly replotted, with permission, from Khademi et al. (2023). Numbers of trials in the saccade data can be inferred from the rasters and from Khademi et al. (2023); numbers of trials in the smooth drift data were reported in Figure 2.
To visualize drift responses, we averaged the horizontal and vertical eye position traces of a given animal and condition across-trial repetitions. Before such averaging, we realigned each trace to the position of the eye at the time of stimulus onset (Malevich et al., 2020). This allowed us to isolate visualization of the drift statistics despite variations in absolute eye position at the time of stimulus onset, due to continuous fixational eye movements. We also visualized drift responses by plotting vertical eye velocity traces (Fig. 1C). We obtained these traces using a smooth differentiating filter (Chen and Hafed, 2013; Malevich et al., 2020) applied to vertical eye position on a trial-by-trial basis. We then averaged the individual trial velocity traces.
For all analyses characterizing the drift response, we only picked trials without any saccades in the interval from −100 to 200 ms relative to stimulus onset. This was done for two reasons: to avoid masking the slow drift responses by large velocity pulses associated with saccades and to avoid potential perisaccadic modulations in the drift response strength. In some analyses, we specifically wanted to study such perisaccadic modulations, as well as drift–saccade interactions in general. In that case, we replaced all velocity samples that were part of a saccade with not-a-number (NaN) labels before averaging the eye velocity traces across trials.
For summary statistics, we estimated the size of the drift response by calculating average vertical eye velocity in a poststimulus response interval (70–150 ms; second gray interval on the x-axis in Fig. 1C) and subtracting from it the baseline vertical eye velocity in a prestimulus interval (first gray interval on the x-axis in Fig. 1C). We did this on a trial-by-trial basis, and we then averaged the difference measures across trials for population statistics. Note that this velocity difference measure could quantitatively be negative, especially in the cases with weak or nonexistent drift responses (Malevich et al., 2020). Note also that we picked the poststimulus response interval (70–150 ms) by inspecting drift responses across many different trials, conditions, and animals. While this interval was fixed for all analyses, it was long enough to avoid biasing our results in the cases in which the drift response was rendered a bit earlier or a bit later by specific visual feature dimensions.
We also estimated the latency of the drift response, especially for relating it to the time of saccadic inhibition. To do so, we exploited our observation here and in Malevich et al. (2020) that the drift response always started when saccades were inhibited. Therefore, for each monkey and stimulus condition, we estimated the direction of the baseline prestimulus drift of eye positions by fitting a straight line to average vertical eye position in the final 50 ms before L50. We then extrapolated this linear fit forward in time. If the stimulus onset did not alter ocular position drifts, then the average vertical eye position should have remained close to this extrapolation for at least some time after L50, but this was not the case for the drift response. As a result, we estimated the onset of the drift response as the first time point resulting in a sustained deviation of average vertical eye position away from the extrapolation for at least 15 ms. We then checked how the onset latency varied with different stimulus conditions.
For analyzing the impacts of peristimulus saccades on the drift response, we calculated the drift response strength measure described above but now only for trials in which saccade onsets occurred within a specific time window relative to stimulus onset. This time window was defined by the purposes of the specific analysis (see Results). For example, for some analyses, we only considered trials with saccades happening in the final 100 ms before stimulus onset, and in others, we considered only trials with later saccades (e.g., 175–275 ms). The latter trials allowed us to observe a recovery from possible saccadic suppression of the drift response in the former analysis interval. Note also that if an analysis involved trials with saccades in a given interval (e.g., 175–275 ms from stimulus onset), we additionally ensured that no other saccades could happen at any other time within our standard saccade-free interval for analyzing the drift response (−100 to 200 ms). This meant that no other nearby saccades, which were outside of our specific interval of choice, could influence the results.
For specifically the trials with saccades occurring from −100 to 0 ms from stimulus onset (which were associated with suppressed drift response magnitudes; see Results), we repeated the analyses one more time but now exploring the impact of saccade radial amplitude on drift response suppression. To do so, we performed a median split on all saccade amplitudes in each monkey and condition. We then recalculated the drift response magnitude when saccades smaller than or larger than the median occurred within −100 to 0 ms from stimulus onset. If saccade size mattered for the suppression effect, then there should have been different drift response strengths in the two groups of trials.
In a related analysis on interactions with saccades, we also explored whether a motor competition between the drift response and subsequent saccade generation might exist. This was motivated by prior work demonstrating how microsaccade generation can delay subsequent large saccade generation (Rolfs et al., 2006; Hafed and Krauzlis, 2010; Chen and Hafed, 2017). That is, there might be a potential tradeoff between the generation of the drift response and the generation of subsequent microsaccades. If so, one might ask whether a stronger drift response is associated with later postdrift saccades (i.e., whether the drift response competes with saccades for behavioral expression). To test this, we picked a condition with a substantial drift response magnitude (largest stimulus radius from the size tuning experiment). Then, in each monkey, we found the time of the first saccade to occur after a time of L50 +100 ms. This time was dictated by the period of saccadic inhibition that we observed from this condition (Fig. 7 in Results), and it ensured that we were isolating the first saccade to occur after the drift response. We also ensured that we obtained a robust drift response by additionally excluding any trials with saccades occurring from −100 ms up to the first postdrift saccade. We then performed a median split on the first postdrift saccade latency, and we analyzed the drift response for trials with saccades earlier or later than the median. If there was motor competition, stronger drift responses should have been expected to be observed with later postdrift saccades.
Finally, for analyzing effects of localized flash locations on drift responses, we sometimes also measured eye position rather than eye velocity. In this case, we grouped trials according to whether a flash was in the right or left visual field (independent of its vertical position), and we took the difference in eye position (after aligning all traces at time zero like above) between the two groups of trials in a given poststimulus interval. Similarly, we also grouped trials according to whether a flash was in the upper or lower visual field (independent of its horizontal position), and we took the difference in eye position between the two groups of trials (again, after all traces were aligned at the time of stimulus onset, like described above). Using eye position instead of eye velocity in these particular analyses allowed us to directly test whether there were spatially directed modulations in drift statistics that were caused by eccentric stimulus onsets (see Results), similar to how eccentric stimulus onsets can bias microsaccade directions (Hafed and Clark, 2002; Engbert and Kliegl, 2003).
Experimental design and statistical analyses
We always replicated all of our results in three monkeys (except for Experiment 5; see justification below). Moreover, within each animal, we typically had hundreds to thousands of trial repetitions per condition (Fig. 1). This increased our confidence in our population measures. Our choice of trial numbers to collect was guided by calculating power estimates before and during the experimental phases of the study. We also randomly interleaved all conditions in a given experiment, except when we were constrained by the experimental setup. For example, in Experiment 3, the location of the small, localized flashes was constant within a given session, and this was dictated by other factors external to the study (like receptive field locations). However, given the reflexive nature of our drift responses (see Results and Discussion), this should not have affected our interpretations in any substantial manner. More importantly, we also designed Experiment 4 with randomly interleaved target locations exactly to compensate for the nonrandom nature of localized flash locations in Experiment 3.
For Experiment 5, we only ran it in one monkey. However, the results were virtually identical, in a qualitative sense, to everything else that we had tested with the other two animals in other experiments. As a result, we decided that our conclusions from this experiment were already convincing. Similarly, we blocked gaze position in this experiment, meaning that we tested each gaze position condition in a block of contiguous trials (as opposed to randomly changing gaze position from trial to trial). Again, this provided a stronger support for our conclusions that the drift response remains to be predominantly upward independent of gaze position (see Results).
All statistical tests and outcomes, as well as trial repetition counts, are detailed in Results. We also performed statistical tests for each animal separately.
Results
We recently found that ocular position drifts can be quite sensitive to visual stimulus onsets, exhibiting short-latency, brief responses (Fig. 1; Malevich et al., 2020). Here, we performed extensive additional experiments characterizing the feature tuning properties of such stimulus-driven drift responses.
We used three rhesus macaque monkeys as our experimental subjects, and we did so for at least four reasons. First, we employed highly precise eye tracking in these animals, using the scleral search coil technique (Robinson, 1963; Fuchs and Robinson, 1966; Judge et al., 1980), to increase our confidence in the measurements. Commercial video-based eye trackers commonly used with human subjects would make measuring these tiny drift responses very challenging (Wyatt, 2010; Kimmel et al., 2012; Chen and Hafed, 2013; Choe et al., 2016; Malevich et al., 2020). Second, we could collect several experimental sessions per animal per condition, resulting in many trial repetitions and statistically robust results across all of our experimental conditions (Materials and Methods). Third, these animals were already used in our characterization of the closely related phenomenon of saccadic inhibition (Khademi et al., 2023), and we often used the very same data for characterizing drift responses here. Fourth, and most importantly, these animals are part of the ongoing efforts in our laboratory to explore the neurophysiological underpinnings of drift responses, which we hope to document in the near future.
The drift response exhibits size tuning
In our first experiment, we asked whether the ocular position drift response is parametrically tuned to the size of the appearing visual stimulus. In our initial characterization of the drift response (Malevich et al., 2020), we mostly used large visual stimuli. This raises the question of how small the visual target needs to be for the drift response to disappear. We instructed our monkeys to maintain fixation on a central fixation spot, and we presented a brief flash of a black circle centered on the fixation spot (Materials and Methods). The flash could be approximately as small as the fixation spot or as large as the entire display, with intermediate radii in between, and we analyzed data from the same experiments in which we recently characterized saccadic inhibition as a function of stimulus size (Khademi et al., 2023). The difference in the current study is that we specifically focused here on trials in which there were no microsaccades occurring within the interval between −100 and 200 ms from stimulus onset (Materials and Methods; also see later for our separate analyses investigating interactions between microsaccades and the drift response).
The smallest foveal visual stimulus could still evoke a clear drift response. Figure 2, A and B (yellow), shows average horizontal (Fig. 2A) and vertical (Fig. 2B) eye position from monkey A when the smallest visual flash occurred. In each panel, we always aligned all eye position traces across trials to the eye position at time zero (stimulus onset), in order to isolate the impact of the stimulus event on drift statistics (despite variable eye positions during gaze fixation; Materials and Methods; Malevich et al., 2020). As can be seen, this monkey had a systematic rightward drift trajectory before stimulus onset (Fig. 2A, yellow); that is, the horizontal eye position curve in Figure 2A was steadily shifting upward in the plot (meaning a rightward displacement) during the prestimulus interval; the vertical eye position curve in Figure 2B was more or less steady. After stimulus onset, Figure 2B shows that there was still a small upward drift response that occurred (not unlike that seen in Fig. 1B,C), despite the vanishingly small stimulus size relative to the size of the fixation spot. Such a small upward drift response was also clearly visible in the vertical eye velocity (Fig. 2C), and in monkey F (Fig. 2E–G, yellow curves), even though this monkey had a different prestimulus drift trajectory (which was now predominantly leftward and downward). In monkey M, the smallest visual stimulus barely modified the ongoing drift statistics (Fig. 2I–K, yellow curves), but this monkey also had the fastest prestimulus drift speeds from among all three animals (compare the rates of change in eye positions during the prestimulus epochs across all panels). This faster baseline drift speed might have masked any potential impacts of the smallest stimulus size on drift eye movements in this monkey. Nonetheless, and as we describe next, drift responses were still clearly visible in this animal for the slightly larger stimulus radii of only 0.18 or 0.36°. Thus, in all three animals, even the smallest, foveal stimuli could still evoke a reliable, predominantly upward, drift response.
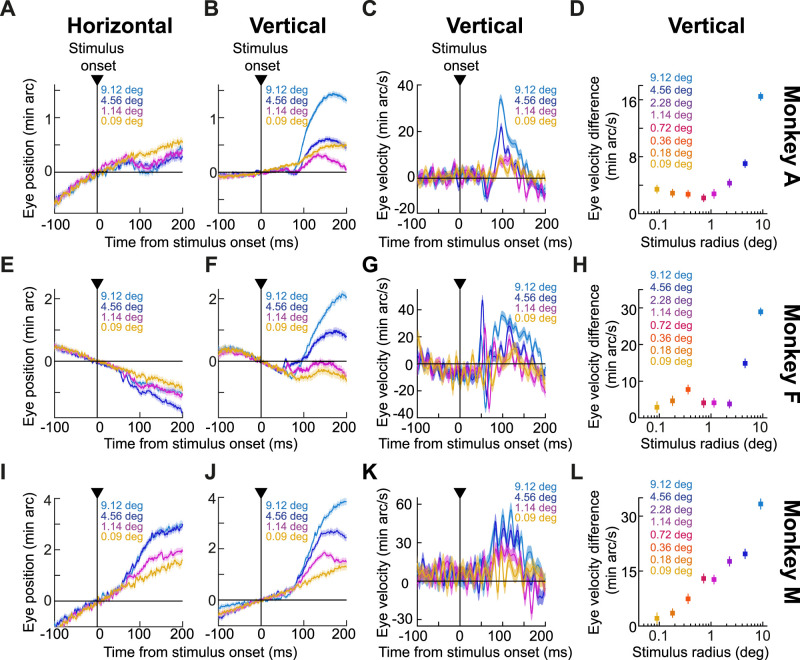
Figure 2.
Size tuning of ocular position drift responses. A, Average horizontal eye position from monkey A for four example stimulus sizes (0.09°, 1.14°, 4.56°, and 9.12°). Error bars denote SEM, and the numbers of trials were 827, 804, 927, and 882, respectively. Upward deflections denote rightward eye position deviations. B, Average vertical eye position from the same trials as in A; error bars again denote SEM, and upward deflections denote upward eye position deviations. A clear dependence of the ocular position drift response on stimulus size can be seen. Note also how the smallest tested stimulus (0.09°) still caused a vertical drift response, but its initial smaller downward component was missing. C, Same as B but for average vertical eye velocity. D, Our measure of the drift response magnitude (average baseline-corrected vertical eye velocity in the interval 70–150 ms after stimulus onset; Fig. 1C; Materials and Methods) for all tested stimulus sizes in monkey A (n = 827, 729, 872, 868, 804, 885, 927, and 882 trials from the smallest to the largest stimulus size). Error bars denote SEM. E–H, Similar results for monkey F (n = 223, 219, 235, 266, 308, 339, 350, and 399 trials). Note how this monkey also showed small transient oscillations in both horizontal and vertical eye positions at the very initial phases of the drift response. I–L, Similar results for monkey M (n = 327, 369, 397, 423, 456, 420, 416, and 405 trials).
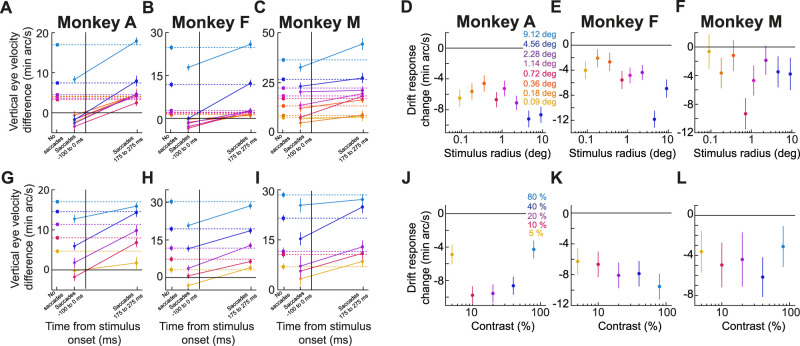
The drift response not only occurred for small, foveal stimuli, but its magnitude also systematically depended on stimulus size. Specifically, the remaining curves of Figure 2A–C,E–G,I–K show eye position and velocity traces from three additional stimulus sizes that we used in our experiments, covering stimulus radii larger than ∼1°. In all cases, the drift response was rendered larger with larger stimuli. When we now considered all tested stimulus sizes, we found evidence of tuning. This latter observation can be better appreciated from Figure 2D,H,L, summarizing the relationship between drift response magnitude and stimulus size. In these panels, and for each animal, we measured the drift response magnitude like we did in our earlier study (Malevich et al., 2020). Specifically, we took the difference in vertical eye velocity between two measurement intervals, a stimulus response epoch and a prestimulus baseline epoch (Fig. 1C, gray shaded regions; Materials and Methods). There was clear size tuning of the drift response magnitude in each animal: monkey A showed a decreasing relationship of drift response magnitude versus stimulus radius up to ∼1–2°, and this was followed by a rise in response magnitude for larger stimuli; monkey F showed a floor effect up to ∼1–2°, before a monotonically rising relationship; and monkey M (generally having significantly faster baseline drift speeds) exhibited a monotonic increase with stimulus size, even for stimuli smaller than 1° in radius.
We confirmed the above interpretations statistically. We performed, within each animal's data, a one-way ANOVA relating drift response magnitude to stimulus size. In all three monkeys, there was a significant main effect of stimulus size (p < 0.0001 for monkeys A, F, and M; F(7,6,856) = 63.23, F(7,2,331) = 57.78, and F(7,3,205) = 50.71 for monkeys A, F, and M, respectively). Therefore, besides still occurring for tiny foveal stimuli, the drift response also exhibits size tuning, which we will later link to the size tuning of saccadic inhibition that we recently characterized in the same experiments (Khademi et al., 2023).
It is also interesting to note that in all three animals, larger stimulus sizes also increased the likelihood of observing a small transient modulation of eye position right at the very beginning of the overall drift response. For example, for the largest flashes, all three monkeys exhibited a small, but short-lived, downward change in eye position before the upward drift pulse (Fig. 2B,F,J, largest stimulus size), and this is similar to the downward transient that is evident in Figure 1B. We frequently observed this small transient in our earlier study as well (Malevich et al., 2020). Monkey F additionally showed transient small oscillations in eye position at the beginning of the drift response for different sizes (Fig. 2G).
The larger stimuli in the current experiment additionally increased the likelihood that the upward drift response had a horizontal component to it. For example, monkey A's upward drift response for large stimuli was accompanied by a slight leftward trajectory (Fig. 2A), and monkey M's upward drift response for large stimuli was accompanied by a rightward trajectory (Fig. 2I). Once again, we observed such horizontal deviations accompanying the upward drift response in our earlier experiments as well (Malevich et al., 2020).
Therefore, our results so far demonstrate that the stimulus-driven ocular position drift response (Malevich et al., 2020) can still happen for tiny foveal visual transients.
The drift response is stronger for high contrast stimuli
We next studied the contrast sensitivity curve of the drift response. We had the three monkeys view brief, transient full-screen flashes while they fixated their gaze at the center of the display. Across trials, the flashes (which were all darker than the background) could have a different Weber contrast (Materials and Methods). In all three animals, the drift response magnitude monotonically increased with stimulus contrast, increasing quasi-linearly as a function of log-contrast. These results can be seen in Figure 3, which is organized similarly to Figure 2. The lowest tested contrast (5%; yellow curves) still showed a reliable drift response in all three monkeys. Moreover, the drift response magnitude increased with increasing contrast.
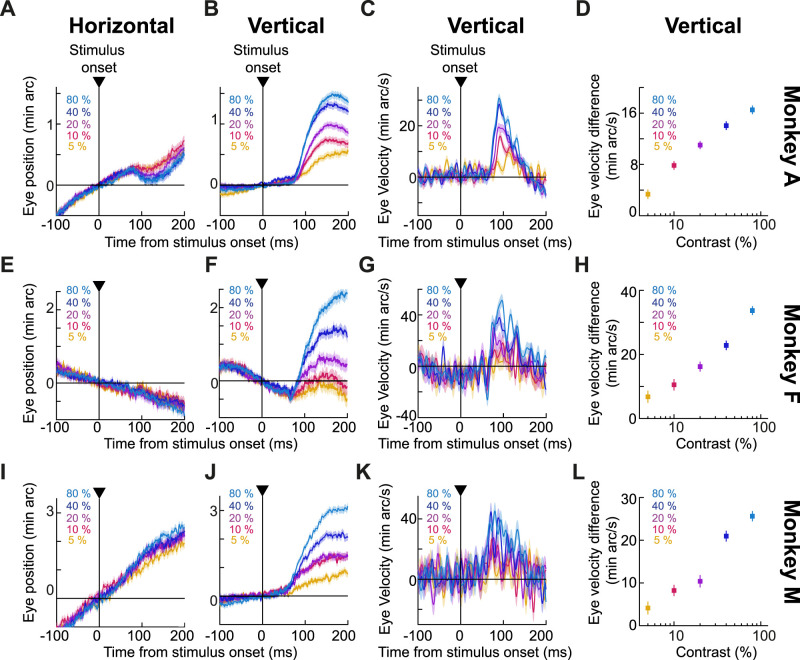
Figure 3.
Contrast sensitivity of ocular position drift responses. A, Average horizontal eye position from monkey A for all tested contrasts. Error bars denote SEM, and the numbers of trials were 689, 699, 739, 754, and 750 for 5%, 10%, 20%, 40%, and 80% contrast, respectively. B, Average vertical eye position from the same trials as in A (error bars again denote SEM). A clear dependence of the ocular position drift response on contrast can be seen. C, Same as B but for average vertical eye velocity. D, Our measure of the drift response magnitude for all tested stimulus contrasts in monkey A. Error bars denote SEM. E–H, Similar results for monkey F (n = 135, 165, 179, 223, and 262 trials from the lowest to the highest contrast). I–L, Similar results for monkey M (n = 384, 412, 443, 433, and 475 trials).
To summarize these results, we again calculated the drift response size as described above (difference in vertical eye velocity between a response and a baseline epoch; Materials and Methods), and we plotted it as a function of stimulus contrast for each animal. These plots are shown in Figure 3D,H,L, and they demonstrate the contrast sensitivity curve of the drift response. Statistically, there was a clear effect of contrast on drift response magnitude in each animal (p < 0.0001 across all animals; one-way ANOVA on drift response magnitude as a function of contrast; F(4,3,626) = 56.65, F(4,959) = 46.71, and F(4,2,142) = 45.43 for monkey A, F, and M, respectively). These results are directly complementary to similar dependencies of saccadic inhibition on stimulus contrast (Bonneh et al., 2015; Scholes et al., 2015; White and Rolfs, 2016; Khademi et al., 2023), and they further motivate our analyses of saccade–drift interactions, which we detail later below.
Therefore, to the extent that stimulus-driven neural responses somewhere in the visual/oculomotor system might mediate short-latency ocular position drift responses (Malevich et al., 2020), these visual responses are expected to monotonically depend on stimulus contrast. Given the short time interval between stimulus onset and the actual eye movement modulations, we hypothesize (Buonocore and Hafed, 2023; Khademi et al., 2023) that these visual responses that are relevant for the drift response can be observed late in the oculomotor control circuitry, perhaps even in the brainstem premotor network.
The drift response is predominantly upward even for lower visual field stimuli
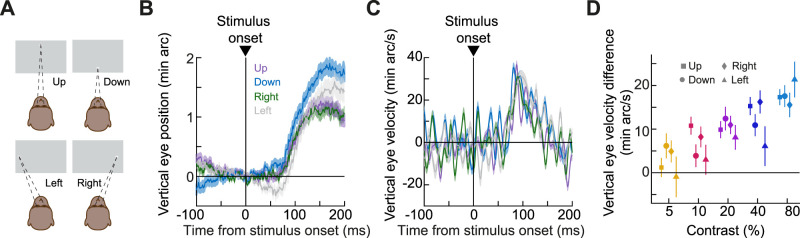
Speaking of oculomotor control circuitry, a candidate brain structure possessing short-latency visual responses and having direct access to the oculomotor system is the SC, and it is also a structure that can contribute to smooth eye movements (Krauzlis et al., 1997, 2000; Basso et al., 2000; Hafed et al., 2008; Hafed and Krauzlis, 2008). Because the SC has stronger visual sensitivity for the upper visual field (Hafed and Chen, 2016; Fracasso et al., 2023) and seems to also magnify its representation for the upper visual field (Hafed and Chen, 2016), we hypothesized earlier that the predominantly upward nature of the drift response (for stimuli spanning both the upper and lower visual fields) might be mediated, at least partially, by SC visual activity (Malevich et al., 2020). If so, then presenting stimuli exclusively in the lower visual field (below the line of sight) should make the drift response downward instead, since it now shifts the balance of SC visual activity in favor of the lower visual field. We, therefore, next tested how the drift response was affected by presenting a half-screen brief flash either only in the upper half of the entire display or in the lower half (Materials and Methods). We also interleaved sham trials (without any flashes) as well as trials with small, localized flashes in the periphery (Materials and Methods).
The drift response was still predominantly upward even for lower visual field half-screen stimuli. Figure 4 shows the eye position and velocity measures from this experiment in a manner similar to how we presented data in the earlier figures (Figs. 2, 3). The critical comparison here is between the upper and lower visual field stimulus conditions (Fig. 4, red and purple). In these conditions, the brief flash could consist of a black rectangle covering either exactly the top or bottom half of the display. In each monkey, the drift response was still predominantly upward for lower visual field flashes (Fig. 4B,C,F,G,J,K), which is inconsistent with the hypothesis that SC visual responses dictate the upward direction of the drift response. Moreover, across the animals, there was no systematic relationship between the strength of the upward drift response and the visual field location of the stimulus. For example, in monkeys A and M, the overall drift response magnitude was similar for the upper and lower visual field stimuli (Fig. 4B,C for monkey A and Fig. 4J,K for monkey M). On the other hand, for monkey F, upper visual field stimuli did indeed cause a stronger upward component of the drift response than lower visual field stimuli (Fig. 4F,G). Statistical tests between the velocity difference measures of the two conditions confirmed these observations (Fig. 4D,H,L). In monkey A, there was no significant difference between upper and lower visual field flashes in Figure 4D (p = 0.26; t test; t = −1,241). For monkey F, the drift response magnitude was significantly stronger for the upper visual field stimuli (p = 0.0079; t test; t = 2.6642; Fig. 4H). And, for monkey M, there was again no reliable difference between the upper and lower visual field stimuli (p = 0.77; t test; t = −0.2818; Fig. 4L).
Figure 4.
Predominantly upward ocular position drift responses even with lower visual field stimuli. A, Average horizontal eye position from monkey A in the visual field experiment. Gray indicates sham stimulus onsets (n = 899 trials), yellow a small localized flash eccentric from the fixation spot (Materials and Methods; n = 833 trials), red a stimulus onset in the lower half of the display (n = 890 trials), purple a stimulus onset in the upper half of the display (n = 848 trials), and blue a full-screen flash (n = 474 trials). Error bars denote SEM. B, Average vertical eye position from the same trials (error bars again denote SEM). The drift response was predominantly upward even for lower visual field stimulus onsets (red). Note also how the initial downward component of the global drift response was weaker for the upper visual field stimulus onsets. C, Same as B but for vertical eye velocity. D, Our measure of the drift response magnitude for all conditions. Sham and localized stimulus onsets had weak drift responses (also see Figs. 5, 6); upper and lower visual field stimulus onsets had generally similar drift response magnitudes (and were both globally upward); and full-screen stimuli had stronger drift response magnitudes (consistent with the size tuning effects of Fig. 2). E–H, Similar results for monkey F (n = 401, 341, 372, 415, and 72 trials for the shown conditions: sham, localized, lower visual field, upper visual field, and full-screen flashes, respectively). I–L, Similar results for monkey M (n = 835, 439, 1,208, 1,143, and 553 trials).
Therefore, the drift response remains to be predominantly upward even with lower visual field stimuli, and the strength of this drift response may or may not reflect the presence of lower or upper visual field stimulus energy (also see later for further tests of this idea with small, localized flashes).
The other conditions shown in Figure 4 were also informative in the broader context of this study. For example, in all animals, the drift response was always the strongest for the largest stimulus flashes (full-screen stimuli; Fig. 4, blue). This is consistent with our observations in Figure 2. Interestingly, in the present experiments, we also interleaved trials with a 1° × 1° localized stimulus flash in the periphery relative to the fixation spot location (Materials and Methods; this is complementary to the small, foveal flashes of Fig. 2). Remarkably, there was still a small upward drift response in this case (Fig. 4, all yellow curves). This prompted us to investigate the influences of small, localized eccentric (rather than foveal) flashes on ocular position drifts in much more detail, as we describe next.
Small, localized stimuli additionally cause spatially directed drift modulations
Our results so far demonstrate that the upward drift response occurs under a large variety of stimulus conditions, which hints that this response may be a reflexive movement of some kind. Indeed, the drift response remains predominantly upward even for lower visual field flashes (Fig. 4), and it also occurs for small foveal (Fig. 2) and eccentric (Fig. 4) targets. However, whether the drift response is a reflex or not, it is still likely the outcome of readout of stimulus-driven neural activity in the oculomotor control network. For small, localized targets, such activity can be highly spatially localized, especially in topographically organized structures like the SC. Might it then be the case that spatially localized visual bursts somewhere in the oculomotor system may play a modulatory role on ocular position drifts during fixation? Indeed, we recently found that at the time of saccade readout, spatially localized SC spiking systematically altered saccade metrics and kinematics even when such spiking was not part of the movements’ motor bursts (Buonocore et al., 2021), and the question now becomes whether a similar effect can be seen in ocular position drifts as well. A related idea to this hypothesis also came up in an earlier academic exchange (between Rolfs and the authors) on potential neural mechanisms for drift eye movements (see the eLetter section of Hafed et al., 2009), which was in the context of the SC's documented role in microsaccade generation (Hafed et al., 2009).
In previous work with peripheral cueing, we uncovered evidence that peripheral stimulus onsets can indeed give rise to spatially directed drift trajectories (Tian et al., 2018), but our localized stimulus experiments in the drift response study of Malevich et al. (2020) did not exhaustively study spatially directed effects. Moreover, the stimulus locations for the localized targets in Figure 4, and in Tian et al. (2018), were not distributed enough to explore different spatially directed modulations (Materials and Methods). Therefore, we explicitly ran an additional experiment with localized stimulus flashes, this time systematically sampling different directions relative to the line of sight.
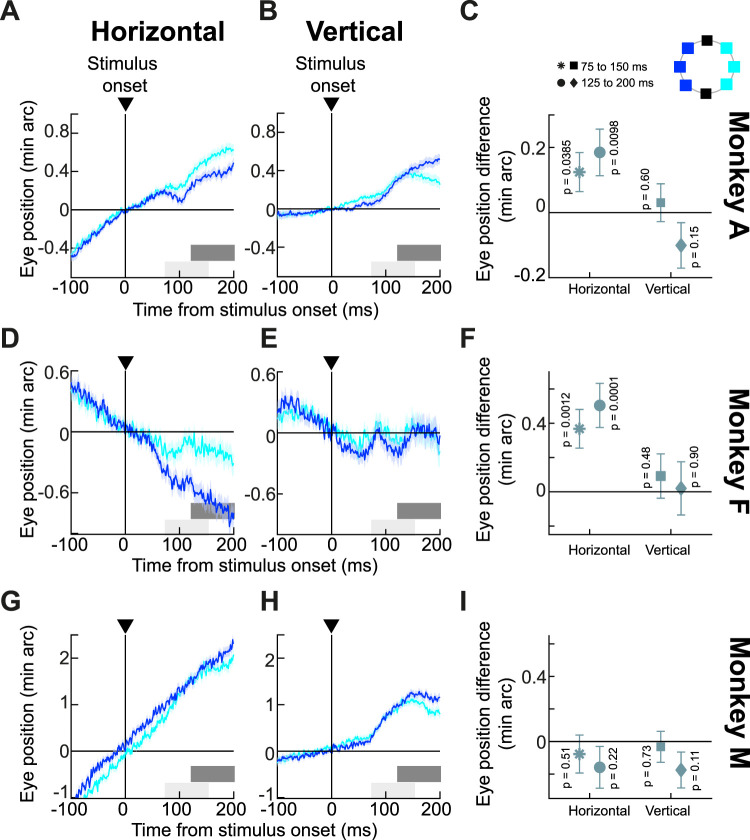
The experiment consisted of the monkeys fixating a central spot, and a brief black flash of 1° × 1° size occurred at an eccentricity of 7.9°. The flash could occur at one of eight equally spaced directions relative to the fixation spot (see inset schematic in Fig. 5C). To robustly infer (from a statistical perspective) potential spatially directed drift modulations, we first grouped all target locations along the horizontal direction. That is, any localized flash that was in the right visual field was grouped into the rightward target group, and any localized flash that was in the left visual field was grouped into the leftward target group (see the two different colors in the schematic inset of Fig. 5C). We then analyzed the eye positions of the three animals in the two groups of trials. We focused, here, on eye positions rather than eye velocities (like we did in earlier analyses) because we wanted to directly assess the potential spatial biasing that was caused by the stimulus onsets.
Figure 5.
Spatially directed drift modulations with localized stimuli along the horizontal direction. A, Average horizontal eye position from monkey A when localized flashes (1 × 1° squares; 7.9° eccentricity) appeared in the right (cyan) or left (blue) visual field (see inset schematic in C). Error bars denote SEM (n = 1,120 and 999 trials for right and left stimulus locations, respectively). Drift trajectory was affected by stimulus location, and the effect increased with time. The two gray bars near the x-axis indicate measurement intervals for comparing eye positions between the two groups of flash locations. B, Vertical eye position from the same trials as in A. There was a general upward drift component, which was similar for rightward or leftward flashes. C, We measured the difference between the cyan and blue curves in A and B for the two measurement intervals. Positive values mean rightward or upward differences between the cyan and blue curves. Horizontal eye position reflected the spatial layout of the flashes, and this difference increased with time. Vertical eye position did not. D–F, Similar observations for monkey F (n = 349 and 398 trials for the right and left stimulus locations, respectively). This monkey showed an even clearer drift response modulation by stimulus location, also consistent with the same monkey's performance in earlier experiments (Tian et al., 2018). G–I, Similar analyses for monkey M (n = 649 and 1,091 trials for the right and left stimulus locations, respectively). This monkey did not show horizontal modulation of drifts by stimulus location, but this monkey also had significantly faster baseline drift speed. As with the other two monkeys, there was still an upward stimulus-triggered drift response component (H). P values indicate results of t tests comparing eye positions within a given measurement interval.
Horizontal eye position drifts systematically reflected the peripheral hemifield locations of the brief, localized flashes, confirming our earlier observations that ocular position drifts can be spatially directed (Tian et al., 2018). For example, Figure 5A shows the horizontal eye position of monkey A for the two groups of stimulus locations (Fig. 5C, inset). We aligned eye positions at time zero to better appreciate the stimulus-driven changes in drift statistics. Shortly after stimulus onset, the monkey's horizontal eye position deviated more rightward for the rightward flashes than for the leftward flashes, and the eye position deviation between the two stimulus groups increased with time. This modulation was riding on top of the upward drift response that we described above, as can also be seen from Figure 5B. Here, the vertical eye position of the same animal and in the same trials showed an upward drift pulse, which (unlike horizontal eye position) was largely not differentiating between stimulus locations (especially in the early phases of the response). Thus, small, localized eccentric targets along the horizontal direction were associated with both an upward drift pulse as well as horizontal modulation of ocular position drifts reflecting the horizontal locations of the targets.
We summarized these observations by measuring the eye position difference between the two curves of Figure 5A,B at two different poststimulus times (Fig. 5A,B, shaded gray bars near the x-axes). This difference was significant for horizontal eye position but not for vertical eye position (Fig. 5C). Moreover, the horizontal difference in eye position was larger for the later time interval. These observations were virtually identical in monkey F (Fig. 5D–F), despite the monkey's different baseline (prestimulus) drift trajectory. Thus, there can indeed be spatially directed drift modulations in addition to the upward drift pulse.
For monkey M, there was no clear evidence of spatially directed drift modulations in the horizontal direction, but this monkey did exhibit a clear upward drift pulse (Fig. 5G–I). As mentioned earlier, this monkey had the fastest baseline drift speeds from among the three animals, rendering a weak modulation by spatially localized peripheral activity harder to see. This is similar to our observations of the size tuning experiments described above (Fig. 2).
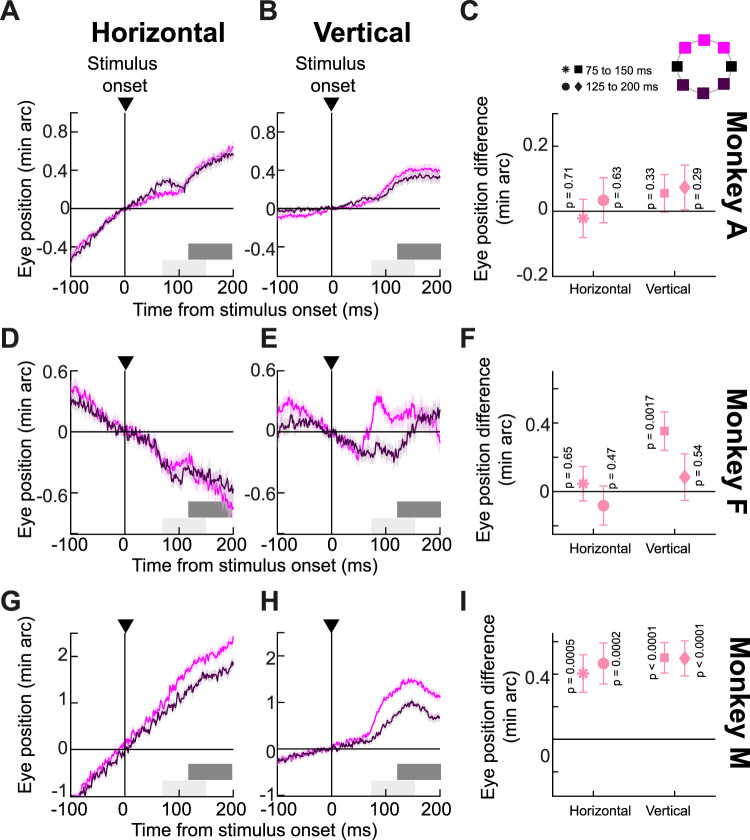
The results of Figure 5 confirm that ocular position drifts are not always random or stochastic (Kowler and Steinman, 1979a,b; Ahissar et al., 2016; Tian et al., 2018; Skinner et al., 2019; Intoy and Rucci, 2020; Bowers et al., 2021; Reiniger et al., 2021; Clark et al., 2022; Nghiem et al., 2022). Having said that, true dependence of ocular position drifts on localized stimulus locations should include evidence of spatially directed drift trajectories for the vertical dimension as well. Thus, we next regrouped our trials according to the vertical locations of the localized flashes (Fig. 6C, inset). In this case, all three monkeys showed evidence that vertical eye position deviated more upward for upper visual field target locations than for lower visual field target locations (Fig. 6); the effect was weakest in monkey A, but the trend was still clearly there. Moreover, in all cases except for monkey M, horizontal eye position deviations were similar to each other for the upper and lower visual field targets, exactly complementary to the results of Figure 5. Thus, in Figure 5, it was horizontal eye position that was most affected by horizontal target locations, and in Figure 6, it was vertical eye position instead that was most affected by vertical target locations. Such a complementary nature of the results of Figures 5 and 6 is consistent with the interpretation that spatially directed drift responses can indeed occur. Once again, these spatially directed effects were occurring in addition to a global upward drift response, which was similar to what we saw in all of our earlier analyses with other stimulus types.
Figure 6.
Spatially directed drift modulations with localized stimuli along the vertical direction. This figure is like Figure 5, except that we now grouped the trials according to whether the flashes were in the upper or lower visual field (see inset schematic in C for the color codes). All monkeys showed a vertical drift response. On top of that, the stimulus locations now modulated the vertical component of eye positions more than the horizontal component, consistent with the idea that localized stimuli can have a modulatory effect on ocular position drifts (compare the eye position traces to those in Fig. 5). Also note that the vertical position difference measurements in the later time interval did not increase relative to those in the earlier time interval. This is likely because the spatially driven modulation was riding on a drift response that was already predominantly vertical. A–C, n = 1,100 and 1,006 trials for upper and lower visual field stimulus locations, respectively. D–F, n = 303 and 312 trials for upper and lower visual field stimulus locations, respectively. G–I, n = 984 and 881 trials for upper and lower visual field stimulus locations, respectively.
Therefore, ocular position drifts exhibit a stimulus-driven upward response for a large range of stimulus types (including small foveal and peripheral targets; Figs. 1–4), and they also undergo spatially directed modulations by spatially localized flashes (Figs. 5, 6). These spatially directed modulations likely reflect localized visual bursts in oculomotor control circuits, such as the SC, that have an impact on eye movement generation in the brain. It would be interesting in the future to understand why large (nonspatially specific flashes) in the upper and lower visual field (Fig. 4) did not systematically modulate the drift response in the vertical eye position direction across all three animals even though small targets did (compare the vertical eye position results of Figs. 4, 6). One possibility could be that surround interactions substantially weaken SC visual responses with very large stimuli.
The drift response is synchronized with saccadic inhibition
Our analyses so far focused on trials in which there were no saccades in the interval from −100 to 200 ms relative to stimulus onset. This was important to allow us to best observe the drift response. However, the drift response might not be completely independent of saccade-related processes, especially because it occurs near the time of saccadic inhibition (Malevich et al., 2020). If this is indeed the case, then the timing of the drift response (not just its magnitude) should also depend on the stimulus features in our experiments. This is because saccadic inhibition timing does vary with stimulus features (Khademi et al., 2023), and revealing this for drift response latency would imply that the drift response and saccadic inhibition may be generated by common neural circuitry.
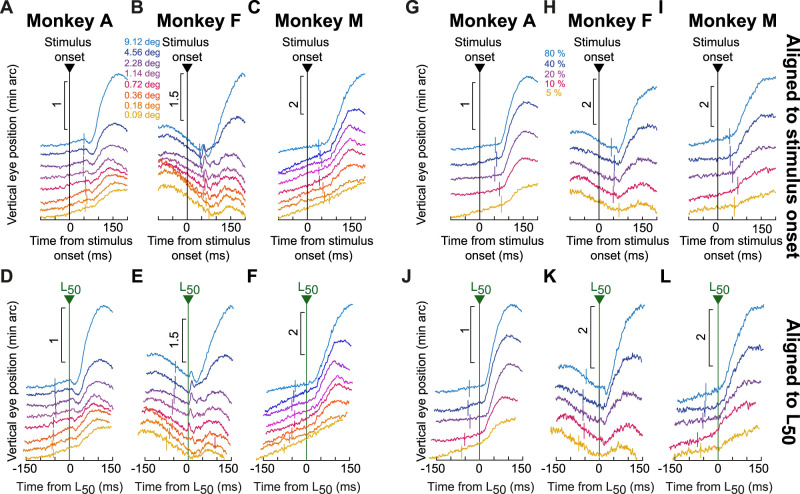
We first plotted drift responses and saccades together in the same graphs, and we checked whether drift response timing covaried with saccadic inhibition timing. Figure 7 illustrates this for the size tuning experiment. For each monkey, the individual rasters indicate individual saccade times across trials, grouped by stimulus size (different colors). These rasters were reproduced from our earlier study (Khademi et al., 2023), since we analyzed drift responses from the same set of experiments. Superimposed on the rasters, we additionally plotted average vertical eye positions for each stimulus size (similar to the example vertical eye position plots in Fig. 2). Each eye position curve was scaled to fit within the similar-colored group of saccade rasters, and position scale bars for each curve are included (on the left side of the curve) for reference. As can be seen, the drift response latency appeared synchronized with the latency of saccadic inhibition, as estimated by the L50 parameter (dark green vertical lines; Materials and Methods). This parameter is routinely used to characterize the latency of saccadic inhibition (Reingold and Stampe, 2002, 2004; Rolfs et al., 2008; Khademi et al., 2023), and Figure 7 shows that when L50 was late, so was the onset of the drift response, and vice versa.
We next checked this synchrony idea further by asking whether our drift response curves across stimulus sizes were better aligned to stimulus onset or to the onset of saccadic inhibition. For each animal, we plotted in Figure 8A–C the average vertical eye position traces for all stimulus sizes (the curves were displaced vertically from each other for easier viewing), and the small vertical tick marks indicate the time of saccadic inhibition (L50; calculated as described in Materials and Methods). In Figure 8D–F, the same traces were now aligned to the time of L50, with the small vertical tick marks now indicating stimulus onset time. In all three monkeys, the drift response curves were better synchronized with L50 than with stimulus onset. That is, the curves across the different stimulus sizes were less jittered in time relative to each other when they were referenced to L50 than to stimulus onset time. This was also the case for the contrast sensitivity experiment (Fig. 8G–L), in which lower contrasts were generally associated with later saccadic inhibition (Khademi et al., 2023). Thus, there seems to be an obligatory timing relationship between saccadic inhibition and drift response latency, irrespective of the stimulus type.
Figure 8.
Alignment of the drift response onset to saccadic inhibition timing. A–C, Average vertical eye position in each condition of the size tuning experiment from each monkey. Each curve in each panel was slightly offset vertically from the others for easier viewing. The vertical tick mark in each curve indicates the time of saccadic inhibition for the condition, as estimated by the parameter L50 (Materials and Methods; Khademi et al., 2023). Consistent with Figure 7, saccadic inhibition time varied with stimulus size (Khademi et al., 2023), and the drift response followed this relationship. D–F, This is better seen when aligning the drift response curves of A–C to the time of L50 rather than to the time of stimulus onset. Here, all the curves were better aligned in time. The vertical tick marks now indicate stimulus onset time. G–L, Similar results from the contrast sensitivity experiment. In all cases, the drift response was well synchronized with the timing of saccadic inhibition, potentially suggesting a common mechanism underlying both phenomena. The numbers of trials underlying each curve were reported in Figures 2, 3.
To explore this relationship further, we then explicitly estimated the onset latency of the drift response in each condition of the two tasks of Figure 8. To do so, we found the first time point at which there was a sustained deviation in average vertical eye position from the steady-state prestimulus drift direction of the monkey (Fig. 9A; Materials and Methods). We searched for this deviation in eye position after the time of L50 given the results of Figure 8 and Malevich et al. (2020). Then, for each monkey, we correlated the estimated drift response latency of a given condition to the time of saccadic inhibition of the same condition (Fig. 9B–D). There was clearly a tight relationship between these two measures. Therefore, across multiple tasks associated with multiple different times of saccadic inhibition (Khademi et al., 2023), we found that the drift response was synchronized with the reflexive interruption of saccade generation rhythms caused by visual onsets in the environment.
Figure 9.
Alignment of the drift response onset to saccadic inhibition timing. A, For each condition from both experiments of Figure 8, we estimated the baseline drift trajectory of a given monkey by fitting a line (solid red) to average vertical eye position in a baseline interval ending at the time of L50; this latter time was calculated from the saccade analyses (Materials and Methods). We then extrapolated this line forward in time (dotted red line), and we defined the latency of the drift response as the point of first sustained deviation of average vertical eye position from this extrapolation. B, Irrespective of condition (five contrasts and eight stimulus sizes from the data of Fig. 8), the drift response latency in monkey A was strongly correlated with the time of saccadic inhibition (as indicated by L50). The gray line shows the linear regression fit of the data. The dotted black line shows the unity slope line. C, D, Similar results from the two other monkeys. Thus, there was a tight relationship between drift response onset and the timing of saccadic inhibition.
The drift response is independent of subsequent saccade timing
Our analyses of Figures 7–9 also prompted investigating another possible interaction between the drift response and saccade generation. In particular, in Figure 7, the conditions containing periods of longer saccadic inhibition (e.g., with larger stimulus radii) were typically associated with stronger drift responses. Might this then suggest that a larger drift response (for a single stimulus type) impedes the generation of subsequent postdrift saccades and delays them? We think that this is unlikely given our earlier results, in which we found similar drift response strengths for trials containing saccades at different times after the drift response (Malevich et al., 2020). Moreover, saccadic inhibition duration might be a function of the cortical decision to reinitiate oculomotor programming after the inhibition has started (Peel et al., 2016), which should be an independent process from the drift response. Nonetheless, we checked for this possibility in our data. For the largest stimulus radius in the size tuning experiment (eliciting a strong drift response), we split trials according to the median latency of the first postdrift saccade (Materials and Methods). We found the drift response to be virtually identical for the trials with longer or shorter than median postdrift saccade latencies (i.e., there were no statistically significant differences). Thus, whether and when saccades happen after the drift response is not fully explained by a potential competition between the drift response and subsequent saccade generation.
The drift response occurs with different starting eye positions
We also suggested in our earlier work that the drift response occurs independently of starting eye position (Malevich et al., 2020). However, in that study, we only used the natural variability of eye positions during fixation to test whether the drift response still occurred when the eye was momentarily fixating below or above some central value (such as the median eye position across trials). This left open the question of whether the drift response might depend on significantly larger eye position deviations from the primary position. To answer this, we performed a new version of our contrast sensitivity experiment, in which we now explicitly required gaze fixation away from the display center. Specifically, in each block of trials, we placed the fixation spot at 4° eccentricity from the center of the display, either to the right of it, to the left of it, above it, or below it (Fig. 10A).
Figure 10.
Independence of the drift response from starting eye position. A, We performed the contrast sensitivity experiment, but now requiring gaze fixation at 4° eccentricity from display center. B, Average vertical eye position from the four fixation positions and the highest contrast stimulus (error bars denote SEM, and n = 62, 71, 58, and 55 trials for the up, down, right, and left gaze positions, respectively). The upward drift response always occurred, even when the eye was already gazing up. Note that the prestimulus drift direction showed some dependence on gaze position. For example, downward gaze position was associated with more upward prestimulus drift, whereas upward gaze position was associated with more downward prestimulus drift (compare the blue and purple curves). However, in all cases, the stimulus-driven response was still upward. C, Same as B but for eye velocity. D, Our measure of the drift response magnitude as a function of stimulus contrast and fixation position. The drift response was stronger with higher contrasts. However, there was no systematic dependence on gaze position—a two-way ANOVA revealed a significant main effect of stimulus contrast (F(4,1,184) = 16.42; p < 0.0001) but not starting eye position (F(3,1,184) = 1.36; p = 0.25). The numbers of trials per condition were as follows: 63, 66, 44, and 49 for up, down, left, and right, respectively (5% contrast); 60, 73, 45, and 59 for up, down, left, and right, respectively (10% contrast); 61, 71, 52, and 60 for up, down, left, and right, respectively (20% contrast); 61, 78, 55, and 49 for up, down, left, and right, respectively (40% contrast); 62, 71, 58, and 55 for up, down, left, and right, respectively (80% contrast).
In all cases, the drift response still occurred, and it was largely independent of the starting eye position. Figure 10, B and C, shows vertical eye position and velocity traces for the highest contrast stimulus from each gaze position condition. Of course, and as with all of our earlier analyses, we aligned all traces to the eye position at stimulus onset, and that is why all curves are aligned to zero eye position on the y-axis despite the different starting gaze position conditions. As can be seen, the upward drift response always happened, irrespective of starting eye position. Interestingly, the prestimulus drift trajectory did depend on gaze position. For example, when gaze was up (Fig. 10B, purple curve), prestimulus drift in vertical eye position was downward, and when gaze was down (blue curve), prestimulus drift in vertical eye position was upward. Nonetheless, there was still an upward drift response in both cases.
Across all stimulus contrasts, we replicated the contrast sensitivity curve of Figure 3 for each gaze position condition (Fig. 10D). Indeed, there was no effect of gaze position on drift response magnitude, but there was a clear effect of stimulus contrast; statistical results are presented in the legend of Figure 10. Therefore, even with substantial deviations of gaze positions, the drift response still occurs, and it is still predominantly upward. Moreover, prestimulus drift trajectories can depend on gaze position, likely reflecting a pulling force (whether biomechanical or neural) to return the eye back to the primary position.
The drift response magnitude is affected by the occurrence of peristimulus saccades
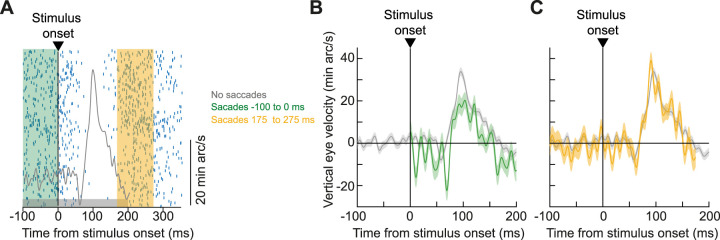
Finally, and still on the theme of interactions with saccades (Figs. 7–9) and gaze positions (Fig. 10), we next explored modulations in the drift response magnitude by the occurrence of peristimulus saccades. In our earlier work (Malevich et al., 2020), a coarse analysis suggested minimal (or even potentially no) interaction with peristimulus saccades. However, due to data sparsity, the analysis that we conducted at the time was not specific enough in its time course resolution. With our current experiments, we had an opportunity to explore such transient changes in more detail. Indeed, because suppression of both visual sensitivity and perception by peristimulus saccades is jumpstarted already in the retina (Idrees et al., 2020, 2022), it would be remarkable if the drift response magnitude was completely unaffected by saccades. This would suggest that whatever visual response is mediating, the drift response would be immune to perisaccadic suppression. This question, therefore, warranted more detailed analysis here.
We binned our data for investigations of potential “saccadic suppression” as we usually do for analyzing visual neural sensitivity (Hafed and Krauzlis, 2010; Chen and Hafed, 2017; Fracasso et al., 2023) or perception (Idrees et al., 2020; Baumann et al., 2021). For example, for a given stimulus condition, we took all trials in which there was a saccade onset occurring within the interval between −100 and 0 ms relative to stimulus onset (Fig. 11A, green shaded region). These trials would be expected to exhibit suppressed visual sensitivity if saccadic suppression does take place. We also took trials in which there was a saccade onset 175–275 ms after stimulus onset (Fig. 11A, yellow shaded region). These trials, instead, would be expected to not experience saccadic suppression (since the saccades occurred far away in time from stimulus onset). Critically, for both intervals, we ensured that there were no other saccades occurring around the drift response (Materials and Methods). Finally, we took trials in which there were no saccades at all in the interval from −100 to 200 ms relative to stimulus onset (Fig. 11A, shaded gray region), and these trials constituted our “standard” drift response trials (like in our other analyses above).
Figure 11.
Saccadic suppression of drift responses. A, Example saccade raster plot and drift response (shown by vertical eye velocity) from one monkey (A) and one condition (9.12° radius in the size tuning experiment). For each shaded colored bar, we picked only trials having saccade onsets occurring within the bar's time window. The shaded gray bar, on the other hand, indicates our standard analysis of no-saccade–drift responses. Note that we did not sample all peristimulus saccade times with high resolution; this was done to increase robustness of our observations, especially given how noisy velocity measures can be. Nonetheless, we had sufficient data to check whether stimulus onsets immediately after nearby saccades (green) had altered drift responses. B, Vertical eye velocity for stimulus onsets right after saccades (green) and without saccades (gray). Error bars denote SEM (n = 168 and 879 for the green and gray curves, respectively), and no eye velocity data are shown in the green curve in the interval from −100 to 0 ms because saccades were occurring. As with the case of saccadic suppression (Hafed and Krauzlis, 2010; Chen and Hafed, 2017), the drift response was suppressed, suggesting that it might depend on circuits in which visual responses experience saccadic suppression; note that this observation was also categorically different from postsaccadic enhancement, in which drift speeds increase rather than decrease (Chen and Hafed, 2013). C, For trials with a saccade occurring 175–275 ms after stimulus onset, the drift response was recovered. Error bars denote SEM (n = 171 and 879 for the colored and gray curves, respectively). Also see Figure 12 for summary data of suppression and recovery across other conditions and tasks.
The drift response magnitude was suppressed by the presence of peristimulus saccades. In Figure 11B, for an example monkey and condition, we compared the standard drift response (Fig. 11A,B, gray curves) to the response when the stimulus occurred right after microsaccades during prestimulus fixation (green). As can be seen, the upward stimulus-evoked velocity pulse was smaller in peak amplitude when the microsaccades occurred than when they did not occur. On the other hand, for microsaccades distant in time from stimulus onset (Fig. 11, yellow), the drift response was recovered (Fig. 11C). Thus, for a brief moment in time when stimulus onset occurred near saccade onset, the subsequent stimulus-driven drift response was systematically suppressed. This is qualitatively similar to the classic phenomenon of saccadic suppression.
This observation was consistent across all monkeys and in all conditions that we checked. For example, for each stimulus condition in both the size tuning (eight stimulus conditions) and contrast sensitivity (five stimulus conditions) tasks, we measured the drift response magnitude (as we did earlier; Figs. 2–4, 10) and plotted it as a function of which time window of Figure 11A the particular trials came from. For trials with saccades −100–0 ms from stimulus onset, the drift response magnitude was always smaller than the drift response magnitude in the absence of peristimulus saccades (Fig. 12A–C,G–I; compare the response for the trials with no saccades to that in the trials with saccades from −100 to 0 ms from stimulus onset). Moreover, for trials with saccades 175–275 ms from stimulus onset, the drift response magnitude was recovered and much closer to the standard drift response magnitude in the absence of peristimulus saccades (indicated by the horizontal dashed lines). We also confirmed these observations statistically. For example, a two-way ANOVA in the contrast sensitivity task revealed a main effect of both stimulus contrast (p < 0.0001 in monkeys A, F, and M) and saccade time relative to stimulus onset (p < 0.0001 in monkeys A, F, and M). There was also a significant interaction between saccade time and stimulus contrast in monkey A (F(4,1,343) = 3.76; p = 0.0048) but not in either monkey F (F(4,1,744) = 0.54; p = 0.70) or monkey M (F(4,1,162) = 0.89; p = 0.47). Similarly, a two-way ANOVA in the size tuning task revealed a main effect of both stimulus radius (p < 0.0001 in monkeys A, F, and M) and saccade time (p < 0.0001 in monkeys A, F, and M) in all three monkeys. However, once again there were no consistent interaction effects. Monkey A showed no significant interaction between stimulus radius and saccade time (F(7,2,633) = 1.38; p = 0.21), monkey F showed a significant interaction (F(7,4,118) = 5.17; p < 0.0001), and monkey M showed no significant interaction (F(7,1,542) = 1.7; p = 0.11).
Figure 12.
Suppression of the drift response strength by peristimulus saccades. A–C, Summary plots of saccadic suppression of the drift response strength for each monkey in the size tuning experiment. The x-axis shows the time bin in which saccades occurred (Fig. 11A), and the y-axis shows our measure of the drift response strength (Materials and Methods). The floating data points (and associated horizontal dashed lines) show the no-saccade–drift response strength for a given condition (Fig. 11, gray curves). Each color shows one tested size, and error bars denote SEM. As can be seen, the drift response magnitude was suppressed for saccades occurring near stimulus onset (saccades from −100 to 0 ms) and recovered for farther saccades (n ≥ 112, 182, or 50 trials in monkeys A, F, and M, respectively, across all conditions of the experiment). D–F, For each stimulus size, we took the difference in the drift response strength between no-saccade trials and trials with saccades from −100 to 0 ms. For each monkey, there was an increasing dependence of suppression strength on stimulus radius (one-way ANOVA; p < 1.6 × 10−9 in each monkey). This is reminiscent of a gain modulation of saccadic suppression strength by stimulus properties in visual neural sensitivity (Chen et al., 2015). G–I, Similar results to A–C but for the contrast sensitivity experiment (n ≥ 97, 149, 105 trials across all conditions in monkeys A, F, and M, respectively). There were always suppressed drift responses for stimulus onsets immediately after saccades. J–L, Similar results to D–F but for the contrast sensitivity experiment. Only monkey A showed a significant effect of contrast on suppression strength (one-way ANOVA; p < 3 × 10−28).
Therefore, evoked visual responses mediating the drift response are likely suppressed by the presence of peristimulus saccades, much like visual responses in some oculomotor areas including the SC (Hafed and Krauzlis, 2010; Chen and Hafed, 2017; Fracasso et al., 2023). Consistent with this, the amount of perisaccadic suppression of the drift response (difference in response magnitude between no-saccade trials and trials with saccades from −100 to 0 ms from stimulus onset) did exhibit some stimulus dependence in our data (Fig. 12D–F,J–L), which is reminiscent of stimulus-dependent saccadic suppression of visual neural sensitivity, for example, in the SC (Chen et al., 2015; Chen and Hafed, 2017).
We also checked whether the radial amplitude of peristimulus saccades modulated the strength of drift response suppression seen in Figures 11 and 12. For each monkey and condition, we performed a median split on saccade radial amplitude for trials with saccades occurring from −100 to 0 ms from stimulus onset. We then compared the drift response strength on trials with saccades smaller or larger than the median amplitude. In both the size tuning and contrast sensitivity experiments, there was always stronger suppression of the drift response for larger saccades in monkeys F and M (p < 0.005 for a main effect of saccade amplitude; two-way ANOVA with factors stimulus contrast or stimulus size and saccade amplitude). In monkey A, there was no statistically significant effect of saccade amplitude in both tasks, which could reflect a generally weaker drift response in this monkey for trials with peristimulus saccades.
Therefore, these results suggest that visual responses impacting the oculomotor system must exhibit saccadic suppression, and it would be interesting to identify in the near future which of these visual responses mediate the drift response.
Discussion
Ocular position drift eye movements have interested and intrigued neuroscientists for many decades (Ratliff and Riggs, 1950; Barlow, 1952; Nachmias, 1959, 1961; Kowler and Steinman, 1979a,b). The interactions between these eye movements and exogenous sensory events have, however, garnered significantly less attention. We recently observed a robust stimulus-driven ocular position drift response for some visual stimuli (Malevich et al., 2020), and our goal in the present study was to investigate its functional properties much more deeply. Such investigation provides an important foundation for pinpointing the neurophysiological mechanisms giving rise to this drift response, which is itself an important endeavor given how little knowledge we currently have about the neural control of ocular position drifts in general.
Our investigation revealed several properties of the drift response, most notable of which is its robustness even for small foveal and peripheral visual stimuli. There was always a subtle, predominantly upward deviation in ocular position drift trajectories with such stimuli. Given that this deviation alters the spatiotemporal patterns of images impinging on the retina (Kuang et al., 2012; Rucci and Victor, 2015; Ahissar et al., 2016), this suggests that visual onsets in a variety of neuroscientific and cognitive experiments can have sensory representational changes embedded within them, which are directly mediated by stimulus-driven ocular position drifts (in addition to whatever other experimental variables that were being considered by the experimenters). This idea has an interesting parallel in the field of microsaccades; in that field, it has been suggested that these tiny eye movements can have a significant impact on interpreting various perceptual and cognitive phenomena (Hafed, 2013; Chen et al., 2015; Hafed et al., 2015; Tian et al., 2016).
The ubiquitous nature of the upward velocity pulse that we observed under a variety of conditions might suggest that it is a reflexive eye movement. However, it seems to be too small to be related to a potential dorsal light reflex in lower animals (Brodsky, 1999), and it is also binocular (Malevich et al., 2020) and occurring under binocular visual stimulation conditions. The drift response is also not a general gaze position response to darkness (Malevich et al., 2020). Nonetheless, in the same general theme of linking ancient reflexes to effects in primate vision (Brodsky, 1999), the drift response might help us to learn about low-level, evolutionarily old components of the oculomotor control network, which are still present and active in the primate brain. In fact, given the discrepancy between the results of Figure 4 and our original hypothesis about the SC mediating the drift response (Malevich et al., 2020), we now ponder the possibility that visual responses downstream of the SC might be more important for observing this response. This might explain why the drift response happens so ubiquitously across many different stimulus types, since visual responses downstream of the SC are bound to influence eye movements, if ever so subtly (by mere proximity to the final oculomotor muscle drive).
Having said that, the drift response is not the only ocular position drift phenomenon that takes place after the onset of small, localized visual stimuli. Indeed, our results from Figures 4–6 clearly show that there can be spatially directed drift modulations reflecting the location of a peripheral visual stimulus. This is consistent with our earlier observations about ocular position drifts in peripheral Posner-like cueing tasks (Tian et al., 2018). An important implication of this is that ocular position drifts are not entirely random movements, consistent with both older studies on slow oculomotor control (Nachmias, 1961; Murphy et al., 1975; Kowler and Steinman, 1979a,b; Snodderly and Kurtz, 1985; Epelboim and Kowler, 1993) as well as more recent investigations of adaptive drift movements (Ahissar et al., 2016; Tian et al., 2018; Skinner et al., 2019; Intoy and Rucci, 2020; Bowers et al., 2021; Reiniger et al., 2021; Clark et al., 2022; Nghiem et al., 2022). Indeed, even volitional cognitive control can modulate ocular position drift statistics (Watanabe et al., 2019). All of this evidence again has parallels in the field of microsaccades, which were thought to be random until two decades ago (Hafed and Clark, 2002; Engbert and Kliegl, 2003).
Mechanistically, spatially directed drift modulations can emerge from readout of topographically organized visual–motor maps, like in the SC (Robinson, 1972; Ottes et al., 1986; Chen et al., 2019). For example, we recently found that at the time of saccade triggering, even spontaneous spiking in movement-unrelated locations of the SC map can be instantaneously readout by the oculomotor system to modify saccadic flight trajectory (Buonocore et al., 2021). In a similar light, spatial readout of the entire landscape of SC activity can dictate the smooth position deviations during gaze fixation, and such landscape will have clear spatial biases when some SC neurons discharge visual bursts after localized, peripheral stimulus onsets. Spatially directed drift effects would then reflect these biases. Such a mechanism would be consistent with how the SC contributes to the much faster smooth pursuit eye movements, like when tracking an invisible moving goal represented in a spatially broad manner across the SC map (Hafed and Krauzlis, 2008). Such a mechanism would also be consistent with the idea that the upward drift pulse that accompanies spatially directed drift modulations can be mediated by some other circuit operations (potentially even downstream of the SC); indeed, large stimuli (causing large upward drift responses) might be expected to trigger weak SC visual responses due to surround interactions.
Returning to the more reflex-like component, as we said, it is likely dissociated from SC activity because it remains predominantly upward even when SC neurons representing the lower visual field are expected to be bursting (even if weakly) after lower visual field stimulus onsets (Fig. 4). This idea can and should be explicitly tested by recording SC activity from the same task. We also think that other evidence could point to a dissociation from SC activity. Specifically, we often observed a transient eye position modulation right before the upward velocity pulse, a clear example of which is seen in Figure 1B,C. Such a transient modulation jumpstarts the whole drift response sequence, and it seems to also be feature-tuned. That is, it was modulated in strength and timing as a function of some stimulus properties, like size and contrast (Figs. 2, 3). This could suggest that visual bursts mediating the drift response (wherever they may actually be in the end) could initially cause such transients and that the subsequent upward drift pulse could reflect various time constants of the oculomotor control network and oculomotor plant (Robinson, 1964). For example, using a systems control perspective, imagine a negative feedback control loop driving an eye plant, and now drive the whole circuit with a temporal impulse function. Part of the resulting response would reflect the time constants of not only the control loop but also the eye plant. If that is the case, then future experiments need to understand why driving the oculomotor control network with a temporal impulse function (a brief visual burst) would eventually lead to a predominantly upward eye movement, as opposed to downward or horizontal or in some random direction, after the initial transient modulation.
Regardless of the mechanism, all of the above suggests that the drift response falls in a class of eye movement phenomena that may be evoked directly by visual bursts in the oculomotor system, as we recently discussed (Buonocore and Hafed, 2023; Khademi et al., 2023). These phenomena also include express saccades (Fischer and Boch, 1983; Edelman and Keller, 1996; Marino et al., 2015; Hall and Colby, 2016) and saccadic inhibition (Reingold and Stampe, 1999, 2002, 2004; Edelman and Xu, 2009; Khademi et al., 2023). In fact, we think that saccadic inhibition and the drift response are likely mediated by the same structures (Figs. 7–9), further emphasizing the idea that the drift response might be reflexive. If so, one might make some neurophysiological predictions. Specifically, if the hypothesis (Hafed et al., 2021a; Buonocore and Hafed, 2023) holds that omnipause neurons in the brainstem have visual pattern responses explaining the feature tuning properties of saccadic inhibition, and if drift responses are also triggered by these neural bursts, then one prediction is that visual bursts in these omnipause neurons might act as the “temporal impulse function,” which we alluded to above. If so, this would implicate omnipause neurons in more than just the interruption of saccades (Keller and Edelman, 1994; Kaneko, 1996; Keller et al., 1996; Gandhi and Keller, 1999), and the next question will be why brief burst impulses in omnipause neuron activity could cause a small, but smooth, eye position deviations (in addition to inhibiting saccade generation).
Finally, regardless of whether these ideas are experimentally validated or not, it is also important to consider our observation that the drift response was suppressed by the occurrence of peristimulus saccades (Figs. 11, 12). Some smooth eye movement phenomena are actually enhanced when stimuli occur right after microsaccades (Chen and Hafed, 2013), but these phenomena typically involve ocular following of moving stimuli (Chen and Hafed, 2013). In our case, the drift response was not to follow a moving target or pattern. Moreover, the saccades occurred near stimulus onset and well before when the actual drift response happened, unlike in postmicrosaccadic enhancement (Chen and Hafed, 2013). The suppression that we observed, thus, predicts that visual bursts mediating the drift response (wherever they may be) must be suppressed by peristimulus saccades. It would be interesting to also test for this idea neurophysiologically.
References
- Ahissar E, Arieli A, Fried M, Bonneh Y (2016) On the possible roles of microsaccades and drifts in visual perception. Vis Res 118:25–30. 10.1016/j.visres.2014.12.004 [DOI] [PubMed] [Google Scholar]
- Barlow HB (1952) Eye movements during fixation. J Physiol 116:290–306. 10.1113/jphysiol.1952.sp004706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso MA, Krauzlis RJ, Wurtz RH (2000) Activation and inactivation of rostral superior colliculus neurons during smooth-pursuit eye movements in monkeys. J Neurophysiol 84:892–908. 10.1152/jn.2000.84.2.892 [DOI] [PubMed] [Google Scholar]
- Baumann MP, Idrees S, Munch TA, Hafed ZM (2021) Dependence of perceptual saccadic suppression on peri-saccadic image flow properties and luminance contrast polarity. J Vis 21:15. 10.1167/jov.21.5.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeler GW Jr (1967) Visual threshold changes resulting from spontaneous saccadic eye movements. Vis Res 7:769–775. 10.1016/0042-6989(67)90039-9 [DOI] [PubMed] [Google Scholar]
- Bellet ME, Bellet J, Nienborg H, Hafed ZM, Berens P (2019) Human-level saccade detection performance using deep neural networks. J Neurophysiol 121:646–661. 10.1152/jn.00601.2018 [DOI] [PubMed] [Google Scholar]
- Bompas A, Sumner P, Hedge C (2023) Non-decision time: the Higg’s boson of decision. BioRxiv.
- Bonneh YS, Adini Y, Polat U (2015) Contrast sensitivity revealed by microsaccades. J Vis 15:11. 10.1167/15.9.11 [DOI] [PubMed] [Google Scholar]
- Bowers NR, Gautier J, Lin S, Roorda A (2021) Fixational eye movements in passive versus active sustained fixation tasks. J Vis 21:16. 10.1167/jov.21.11.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard DH (1997) The Psychophysics Toolbox. Spat Vis 10:433–436. 10.1163/156856897X00357 [DOI] [PubMed] [Google Scholar]
- Brodsky MC (1999) Dissociated vertical divergence: a righting reflex gone wrong. Arch Ophthalmol 117:1216–1222. 10.1001/archopht.117.9.1216 [DOI] [PubMed] [Google Scholar]
- Buonocore A, Hafed ZM (2023) The inevitability of visual interruption. J Neurophysiol. 130:225–237. 10.1152/jn.00441.2022 [DOI] [PubMed] [Google Scholar]
- Buonocore A, Tian X, Khademi F, Hafed ZM (2021) Instantaneous movement-unrelated midbrain activity modifies ongoing eye movements. eLife 10:e64150. 10.7554/eLife.64150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CY, Hafed ZM (2013) Postmicrosaccadic enhancement of slow eye movements. J Neurosci 33:5375–5386. 10.1523/JNEUROSCI.3703-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CY, Hafed ZM (2017) A neural locus for spatial-frequency specific saccadic suppression in visual-motor neurons of the primate superior colliculus. J Neurophysiol 117:1657–1673. 10.1152/jn.00911.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CY, Hoffmann KP, Distler C, Hafed ZM (2019) The foveal visual representation of the primate superior colliculus. Curr Biol 29:2109–2119.e7. 10.1016/j.cub.2019.05.040 [DOI] [PubMed] [Google Scholar]
- Chen CY, Ignashchenkova A, Thier P, Hafed ZM (2015) Neuronal response gain enhancement prior to microsaccades. Curr Biol 25:2065–2074. 10.1016/j.cub.2015.06.022 [DOI] [PubMed] [Google Scholar]
- Choe KW, Blake R, Lee SH (2016) Pupil size dynamics during fixation impact the accuracy and precision of video-based gaze estimation. Vis Res 118:48–59. 10.1016/j.visres.2014.12.018 [DOI] [PubMed] [Google Scholar]
- Clark AM, Intoy J, Rucci M, Poletti M (2022) Eye drift during fixation predicts visual acuity. Proc Natl Acad Sci U S A 119:e2200256119. 10.1073/pnas.2200256119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastman KM, Huk AC (2012) PLDAPS: a hardware architecture and software toolbox for neurophysiology requiring complex visual stimuli and online behavioral control. Front Neuroinform 6:1. 10.3389/fninf.2012.00001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman JA, Keller EL (1996) Activity of visuomotor burst neurons in the superior colliculus accompanying express saccades. J Neurophysiol 76:908–926. 10.1152/jn.1996.76.2.908 [DOI] [PubMed] [Google Scholar]
- Edelman JA, Xu KZ (2009) Inhibition of voluntary saccadic eye movement commands by abrupt visual onsets. J Neurophysiol 101:1222–1234. 10.1152/jn.90708.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engbert R, Kliegl R (2003) Microsaccades uncover the orientation of covert attention. Vis Res 43:1035–1045. 10.1016/S0042-6989(03)00084-1 [DOI] [PubMed] [Google Scholar]
- Epelboim J, Kowler E (1993) Slow control with eccentric targets: evidence against a position-corrective model. Vis Res 33:361–380. 10.1016/0042-6989(93)90092-B [DOI] [PubMed] [Google Scholar]
- Fischer B, Boch R (1983) Saccadic eye movements after extremely short reaction times in the monkey. Brain Res 260:21–26. 10.1016/0006-8993(83)90760-6 [DOI] [PubMed] [Google Scholar]
- Fracasso A, Buonocore A, Hafed ZM (2023) Peri-saccadic orientation identification performance and visual neural sensitivity are higher in the upper visual field. J Neurosci. 43:6884–6897. 10.1523/JNEUROSCI.1740-22.2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs AF, Robinson DA (1966) A method for measuring horizontal and vertical eye movement chronically in the monkey. J Appl Physiol 21:1068–1070. 10.1152/jappl.1966.21.3.1068 [DOI] [PubMed] [Google Scholar]
- Gandhi NJ, Keller EL (1999) Comparison of saccades perturbed by stimulation of the rostral superior colliculus, the caudal superior colliculus, and the omnipause neuron region. J Neurophysiol 82:3236–3253. 10.1152/jn.1999.82.6.3236 [DOI] [PubMed] [Google Scholar]
- Hafed ZM (2013) Alteration of visual perception prior to microsaccades. Neuron 77:775–786. 10.1016/j.neuron.2012.12.014 [DOI] [PubMed] [Google Scholar]
- Hafed ZM, Chen CY (2016) Sharper, stronger, faster upper visual field representation in primate superior colliculus. Curr Biol 26:1647–1658. 10.1016/j.cub.2016.04.059 [DOI] [PubMed] [Google Scholar]
- Hafed ZM, Chen C-Y, Tian X (2015) Vision, perception, and attention through the lens of microsaccades: mechanisms and implications. Front Syst Neurosci 9:167. 10.3389/fnsys.2015.00167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafed ZM, Chen CY, Tian X, Baumann M, Zhang T (2021b) Active vision at the foveal scale in the primate superior colliculus. J Neurophysiol. 125:1121–1138. 10.1152/jn.00724.2020 [DOI] [PubMed] [Google Scholar]
- Hafed ZM, Clark JJ (2002) Microsaccades as an overt measure of covert attention shifts. Vis Res 42:2533–2545. 10.1016/S0042-6989(02)00263-8 [DOI] [PubMed] [Google Scholar]
- Hafed ZM, Goffart L, Krauzlis RJ (2008) Superior colliculus inactivation causes stable offsets in eye position during tracking. J Neurosci 28:8124–8137. 10.1523/JNEUROSCI.1317-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafed ZM, Goffart L, Krauzlis RJ (2009) A neural mechanism for microsaccade generation in the primate superior colliculus. Science 323:940–943. 10.1126/science.1166112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafed ZM, Krauzlis RJ (2008) Goal representations dominate superior colliculus activity during extrafoveal tracking. J Neurosci 28:9426–9439. 10.1523/JNEUROSCI.1313-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafed ZM, Krauzlis RJ (2010) Microsaccadic suppression of visual bursts in the primate superior colliculus. J Neurosci 30:9542–9547. 10.1523/JNEUROSCI.1137-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafed ZM, Yoshida M, Tian X, Buonocore A, Malevich T (2021a) Dissociable cortical and subcortical mechanisms for mediating the influences of visual cues on microsaccadic eye movements. Front Neural Circuits 15:638429. 10.3389/fncir.2021.638429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall NJ, Colby CL (2016) Express saccades and superior colliculus responses are sensitive to short-wavelength cone contrast. Proc Natl Acad Sci U S A 113:6743–6748. 10.1073/pnas.1600095113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idrees S, Baumann MP, Franke F, Munch TA, Hafed ZM (2020) Perceptual saccadic suppression starts in the retina. Nat Commun 11:1977. 10.1038/s41467-020-15890-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idrees S, Baumann MP, Korympidou MM, Schubert T, Kling A, Franke K, Hafed ZM, Franke F, Munch TA (2022) Suppression without inhibition: how retinal computation contributes to saccadic suppression. Commun Biol 5:692. 10.1038/s42003-022-03526-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Intoy J, Rucci M (2020) Finely tuned eye movements enhance visual acuity. Nat Commun 11:795. 10.1038/s41467-020-14616-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judge SJ, Richmond BJ, Chu FC (1980) Implantation of magnetic search coils for measurement of eye position: an improved method. Vis Res 20:535–538. 10.1016/0042-6989(80)90128-5 [DOI] [PubMed] [Google Scholar]
- Kaneko CR (1996) Effect of ibotenic acid lesions of the omnipause neurons on saccadic eye movements in rhesus macaques. J Neurophysiol 75:2229–2242. 10.1152/jn.1996.75.6.2229 [DOI] [PubMed] [Google Scholar]
- Keller EL, Edelman JA (1994) Use of interrupted saccade paradigm to study spatial and temporal dynamics of saccadic burst cells in superior colliculus in monkey. J Neurophysiol 72:2754–2770. 10.1152/jn.1994.72.6.2754 [DOI] [PubMed] [Google Scholar]
- Keller EL, Gandhi NJ, Shieh JM (1996) Endpoint accuracy in saccades interrupted by stimulation in the omnipause region in monkey. Vis Neurosci 13:1059–1067. 10.1017/S0952523800007719 [DOI] [PubMed] [Google Scholar]
- Khademi F, Zhang T, Baumann MP, Buonocore A, Malevich T, Yu Y, Hafed ZM (2023) Visual feature tuning properties of stimulus-driven saccadic inhibition in macaque monkeys. J Neurophysiol 130:1282–1302. 10.1152/jn.00289.2023 [DOI] [PubMed] [Google Scholar]
- Kimmel DL, Mammo D, Newsome WT (2012) Tracking the eye non-invasively: simultaneous comparison of the scleral search coil and optical tracking techniques in the macaque monkey. Front Behav Neurosci 6:49. 10.3389/fnbeh.2012.00049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiner M, Brainard D, Pelli DG (2007) What’s new in psychtoolbox-3? Perception 36:14. 10.1177/03010066070360S101 [DOI] [Google Scholar]
- Kowler E, Steinman RM (1979a) The effect of expectations on slow oculomotor control. I. Periodic target steps. Vis Res 19:619–632. 10.1016/0042-6989(79)90238-4 [DOI] [PubMed] [Google Scholar]
- Kowler E, Steinman RM (1979b) The effect of expectations on slow oculomotor control. II. Single target displacements. Vis Res 19:633–646. 10.1016/0042-6989(79)90239-6 [DOI] [PubMed] [Google Scholar]
- Krauzlis RJ, Basso MA, Wurtz RH (1997) Shared motor error for multiple eye movements. Science 276:1693–1695. 10.1126/science.276.5319.1693 [DOI] [PubMed] [Google Scholar]
- Krauzlis RJ, Basso MA, Wurtz RH (2000) Discharge properties of neurons in the rostral superior colliculus of the monkey during smooth-pursuit eye movements. J Neurophysiol 84:876–891. 10.1152/jn.2000.84.2.876 [DOI] [PubMed] [Google Scholar]
- Krauzlis RJ, Goffart L, Hafed ZM (2017) Neuronal control of fixation and fixational eye movements. Philos Trans R Soc Lond B Biol Sci 372:20160205. 10.1098/rstb.2016.0205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang X, Poletti M, Victor JD, Rucci M (2012) Temporal encoding of spatial information during active visual fixation. Curr Biol 22:510–514. 10.1016/j.cub.2012.01.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malevich T, Buonocore A, Hafed ZM (2020) Rapid stimulus-driven modulation of slow ocular position drifts. eLife 9:e57595. 10.7554/eLife.57595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino RA, Levy R, Munoz DP (2015) Linking express saccade occurance to stimulus properties and sensorimotor integration in the superior colliculus. J Neurophysiol 114:879–892. 10.1152/jn.00047.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy BJ, Kowler E, Steinman RM (1975) Slow oculomotor control in the presence of moving backgrounds. Vis Res 15:1263–1268. 10.1016/0042-6989(75)90172-8 [DOI] [PubMed] [Google Scholar]
- Nachmias J (1959) Two-dimensional motion of the retinal image during monocular fixation. J Opt Soc Am 49:901–908. 10.1364/JOSA.49.000901 [DOI] [PubMed] [Google Scholar]
- Nachmias J (1961) Determiners of the drift of the eye during monocular fixation. J Opt Soc Am 51:761–766. 10.1364/JOSA.51.000761 [DOI] [PubMed] [Google Scholar]
- Nghiem TE, Dufour O, Reiniger JL, Harmening WM, Azeredo da Silveira R (2022) Fixational eye movements as active sensation for high visual acuity. BioRxiv.
- Ottes FP, Van Gisbergen JA, Eggermont JJ (1986) Visuomotor fields of the superior colliculus: a quantitative model. Vis Res 26:857–873. 10.1016/0042-6989(86)90144-6 [DOI] [PubMed] [Google Scholar]
- Peel TR, Hafed ZM, Dash S, Lomber SG, Corneil BD (2016) A causal role for the cortical frontal eye fields in microsaccade deployment. PLoS Biol 14:e1002531. 10.1371/journal.pbio.1002531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelli DG (1997) The VideoToolbox software for visual psychophysics: transforming numbers into movies. Spat Vis 10:437–442. 10.1163/156856897X00366 [DOI] [PubMed] [Google Scholar]
- Ratliff F, Riggs LA (1950) Involuntary motions of the eye during monocular fixation. J Exp Psychol 40:687–701. 10.1037/h0057754 [DOI] [PubMed] [Google Scholar]
- Reingold EM, Stampe DM (1999) Saccadic inhibition in complex visual tasks. In: Current oculomotor research (Becker W, Deubel H, Mergner T, eds), pp 249–255. Boston: Springer. [Google Scholar]
- Reingold EM, Stampe DM (2002) Saccadic inhibition in voluntary and reflexive saccades. J Cogn Neurosci 14:371–388. 10.1162/089892902317361903 [DOI] [PubMed] [Google Scholar]
- Reingold EM, Stampe DM (2004) Saccadic inhibition in reading. J Exp Psychol Hum Percept Perform 30:194–211. 10.1037/0096-1523.30.1.194 [DOI] [PubMed] [Google Scholar]
- Reiniger JL, Domdei N, Holz FG, Harmening WM (2021) Human gaze is systematically offset from the center of cone topography. Curr Biol 31:4188–4193.e3. 10.1016/j.cub.2021.07.005 [DOI] [PubMed] [Google Scholar]
- Robinson DA (1963) A method of measuring eye movement using a scleral search coil in a magnetic field. IEEE Trans Biomed Eng 10:137–145. 10.1109/TBMEL.1963.4322822 [DOI] [PubMed] [Google Scholar]
- Robinson DA (1964) The mechanics of human saccadic eye movement. J Physiol 174:245–264. 10.1113/jphysiol.1964.sp007485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DA (1972) Eye movements evoked by collicular stimulation in the alert monkey. Vis Res 12:1795–1808. 10.1016/0042-6989(72)90070-3 [DOI] [PubMed] [Google Scholar]
- Rolfs M, Kliegl R, Engbert R (2008) Toward a model of microsaccade generation: the case of microsaccadic inhibition. J Vis 8:5.1–23. 10.1167/8.11.5 [DOI] [PubMed] [Google Scholar]
- Rolfs M, Laubrock J, Kliegl R (2006) Shortening and prolongation of saccade latencies following microsaccades. Exp Brain Res 169:369–376. 10.1007/s00221-005-0148-1 [DOI] [PubMed] [Google Scholar]
- Rucci M, Victor JD (2015) The unsteady eye: an information-processing stage, not a bug. Trends Neurosci. 38:195–206. 10.1016/j.tins.2015.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholes C, McGraw PV, Nystrom M, Roach NW (2015) Fixational eye movements predict visual sensitivity. Proc Biol Sci 282:20151568. 10.1098/rspb.2015.1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner J, Buonocore A, Hafed ZM (2019) Transfer function of the rhesus macaque oculomotor system for small-amplitude slow motion trajectories. J Neurophysiol 121:513–529. 10.1152/jn.00437.2018 [DOI] [PubMed] [Google Scholar]
- Snodderly DM, Kurtz D (1985) Eye position during fixation tasks: comparison of macaque and human. Vis Res 25:83–98. 10.1016/0042-6989(85)90083-5 [DOI] [PubMed] [Google Scholar]
- Steinman RM, Cunitz RJ, Timberlake GT, Herman M (1967) Voluntary control of microsaccades during maintained monocular fixation. Science 155:1577–1579. 10.1126/science.155.3769.1577 [DOI] [PubMed] [Google Scholar]
- Steinman RM, Haddad GM, Skavenski AA, Wyman D (1973) Miniature eye movement. Science 181:810–819. 10.1126/science.181.4102.810 [DOI] [PubMed] [Google Scholar]
- Tian X, Yoshida M, Hafed ZM (2016) A microsaccadic account of attentional capture and inhibition of return in Posner cueing. Front Syst Neurosci 10:23. 10.3389/fnsys.2016.00023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian X, Yoshida M, Hafed ZM (2018) Dynamics of fixational eye position and microsaccades during spatial cueing: the case of express microsaccades. J Neurophysiol 119:1962–1980. 10.1152/jn.00752.2017 [DOI] [PubMed] [Google Scholar]
- Watanabe M, Okada KI, Hamasaki Y, Funamoto M, Kobayashi Y, MacAskill M, Anderson T (2019) Ocular drift reflects volitional action preparation. Eur J Neurosci 50:1892–1910. 10.1111/ejn.14365 [DOI] [PubMed] [Google Scholar]
- White AL, Rolfs M (2016) Oculomotor inhibition covaries with conscious detection. J Neurophysiol 116:1507–1521. 10.1152/jn.00268.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt HJ (2010) The human pupil and the use of video-based eyetrackers. Vis Res 50:1982–1988. 10.1016/j.visres.2010.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuber BL, Stark L (1966) Saccadic suppression: elevation of visual threshold associated with saccadic eye movements. Exp Neurol 16:65–79. 10.1016/0014-4886(66)90087-2 [DOI] [PubMed] [Google Scholar]