Abstract
BACKGROUND:
Recently shown to regulate cardiac development, the secreted axon guidance molecule SLIT3 maintains its expression in the postnatal heart. Despite its known expression in the cardiovascular system after birth, SLIT3’s relevance to cardiovascular function in the postnatal state remains unknown. As such, the objectives of this study were to determine the postnatal myocardial sources of SLIT3 and to evaluate its functional role in regulating the cardiac response to pressure overload stress.
METHODS:
We performed in vitro studies on cardiomyocytes and myocardial tissue samples from patients and performed in vivo investigation with SLIT3 and ROBO1 (roundabout homolog 1) mutant mice undergoing transverse aortic constriction to establish the role of SLIT3-ROBO1 in adverse cardiac remodeling.
RESULTS:
We first found that SLIT3 transcription was increased in myocardial tissue obtained from patients with congenital heart defects that caused ventricular pressure overload. Immunostaining of hearts from WT (wild-type) and reporter mice revealed that SLIT3 is secreted by cardiac stromal cells, namely fibroblasts and vascular mural cells, within the heart. Conditioned media from cardiac fibroblasts and vascular mural cells both stimulated cardiomyocyte hypertrophy in vitro, an effect that was partially inhibited by an anti-SLIT3 antibody. Also, the N-terminal, but not the C-terminal, fragment of SLIT3 and the forced overexpression of SLIT3 stimulated cardiomyocyte hypertrophy and the transcription of hypertrophy-related genes. We next determined that ROBO1 was the most highly expressed roundabout receptor in cardiomyocytes and that ROBO1 mediated SLIT3’s hypertrophic effects in vitro. In vivo, Tcf21+ fibroblast and Tbx18+ vascular mural cell-specific knockout of SLIT3 in mice resulted in decreased left ventricular hypertrophy and cardiac fibrosis after transverse aortic constriction. Furthermore, α-MHC+ cardiomyocyte-specific deletion of ROBO1 also preserved left ventricular function and abrogated hypertrophy, but not fibrosis, after transverse aortic constriction.
CONCLUSIONS:
Collectively, these results indicate a novel role for the SLIT3-ROBO1–signaling axis in regulating postnatal cardiomyocyte hypertrophy induced by pressure overload.
Keywords: axon guidance; fibroblasts; fibrosis; myocytes, cardiac; ROBO1; stromal cells; ventricular pressure
NOVELTY AND SIGNIFICANCE.
What Is Known?
SLIT3 is a secreted, axon guidance molecule that also regulates heart development.
SLIT3 is also expressed in the postnatal heart, but its cardiovascular function remains unclear.
What New Information Does This Article Contribute?
SLIT3 is produced by cardiac stromal cells, specifically cardiac fibroblasts, and vascular mural cells.
SLIT3 stimulates cardiac hypertrophy and cardiac fibrosis under conditions of pressure overload.
SLIT3 can stimulate cardiomyocyte hypertrophy via the ROBO1 receptor.
Prior work indicated the importance of the axon guidance molecule SLIT3 in cardiac development. The rationale for this study was to determine the postnatal role of SLIT3 in regulating adverse cardiac remodeling (cardiac hypertrophy and fibrosis) induced by pressure overload. We found that SLIT3, produced by cardiac stromal cells, that is, cardiac fibroblasts and vascular mural cells, can stimulate hypertrophy and fibrosis induced by pressure overload. We also found that SLIT3 can directly stimulate cardiomyocyte hypertrophy via the ROBO1 receptor. These findings indicate, for the first time, the role of SLIT3 in the postnatal heart in health and adverse cardiac remodeling. These results unveil a novel cardiac stromal cell-cardiomyocyte axis that may be targeted to reduce adverse cardiac remodeling.
Meet the First Author, see p 836
Chronic cardiac pressure overload is encountered in multiple conditions such as systemic arterial hypertension, congenital heart disease, ischemic heart disease, and pulmonary arterial hypertension.1–5 A universal myocardial response to chronic pressure overload is hypertrophy, or the thickening of the ventricular walls,6 and fibrosis, or the increased deposition of extracellular matrix such as fibrillar collagen.7 On a cellular level, cardiac hypertrophy derives from the addition of sarcomere units, leading to cardiomyocyte enlargement in the cross-sectional area.6 While cardiac hypertrophy is a physiological response to exercise and pregnancy,8–10 under chronic pressure overload conditions, this type of myocardial remodeling becomes pathological and is one of the most significant risk factors for the development of heart failure6,11–14 and lethal ventricular arrhythmias.15–17 Pathological cardiac hypertrophy precedes many forms of heart failure with systolic dysfunction, diastolic dysfunction, or a combination of both.6,11,12 Cardiac fibrosis that develops in response to pressure overload also contributes to diastolic dysfunction.7 Thus, preventing the development of adverse cardiac remodeling in the form of pathological cardiac hypertrophy and cardiac fibrosis would benefit many patients.18–20 However, the continued high morbidity and mortality seen with heart failure, a major public health concern that affects nearly 7 million patients in the United States21 is indicative of ineffective therapies as well as an incomplete understanding of the mechanisms that drive adverse cardiac remodeling.
In both contexts of cardiac health and disease, accumulating evidence has indicated that cross-talk between cardiac nonmyocytes (CNMs) and cardiomyocytes plays a pivotal role in myocardial remodeling under various loading conditions.22 Specifically, CNM-derived mediators may act in a paracrine fashion to stimulate cardiomyocyte hypertrophy and modulate their contractile function under increased afterload. Identification of these factors will provide insight into mechanisms of the cardiomyocyte hypertrophic response and may lead to the revelation of potential therapeutic targets for pathological cardiac hypertrophy.23
Recently, we observed that SLIT3, a large and secreted glycoprotein, is produced by fibrillar collagen-producing cells, and global and constitutive SLIT3 deficiency attenuated cardiac fibrosis under conditions of pressure overload.24 We also found that SLIT3 stimulated adult cardiac fibroblasts to proliferate, produce collagen, and become contractile in vitro. Intriguingly, global and constitutive SLIT3 deficiency, throughout development and the postnatal state, was also associated with decreased hypertrophy, preserved ventricular systolic function, and improved survival of mice after the transverse aortic constriction (TAC).24 These observations indicate that the developmental absence of SLIT3 modulates the hypertrophic response; however, the precise role played by SLIT3 in the adult heart remains unknown and is the focus of the current study.
SLIT3 belongs to the family of SLIT proteins, which was initially characterized as a repulsive guidance cue for neuronal axons.25 Subsequently, the highly conserved SLIT ligands have been shown to participate in diverse developmental processes and the regulation of several cellular functions.26–29 Full-length SLIT ligands are cleaved by an unknown protease into N- and C-terminal fragments, which can bind to the ROBO receptors26 and plexins,30 respectively. Slit3 is the predominant Slit transcribed in the embryonic mouse heart.31 Nevertheless, it is unknown whether SLIT3 modulates cardiac hypertrophy indirectly, such as via modulation of the secretion of hypertrophic factors by fibroblasts, directly by binding to a cardiomyocyte cell-surface receptor, or as a result of a developmental defect resulting from constitutive SLIT3 deficiency that could lead to a defective hypertrophic response in the postnatal period.
To fill these knowledge gaps, we utilized inducible and conditional knockout mice in the current study to investigate the postnatal and tissue-specific roles of SLIT3 in regulating cardiac hypertrophy. Here, we show that cardiac fibroblast and vascular mural cell–mediated SLIT3 directly regulates the hypertrophic response of postnatal cardiomyocytes in response to pressure overload, and this stimulatory effect occurs via the ROBO1 receptor.
METHODS
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request. Please see the Supplemental Material for a detailed description.
RESULTS
Inducible Global-SLIT3 Deficiency Inhibits Pressure Overload–Induced Adverse Cardiac Remodeling and Ventricular Dysfunction
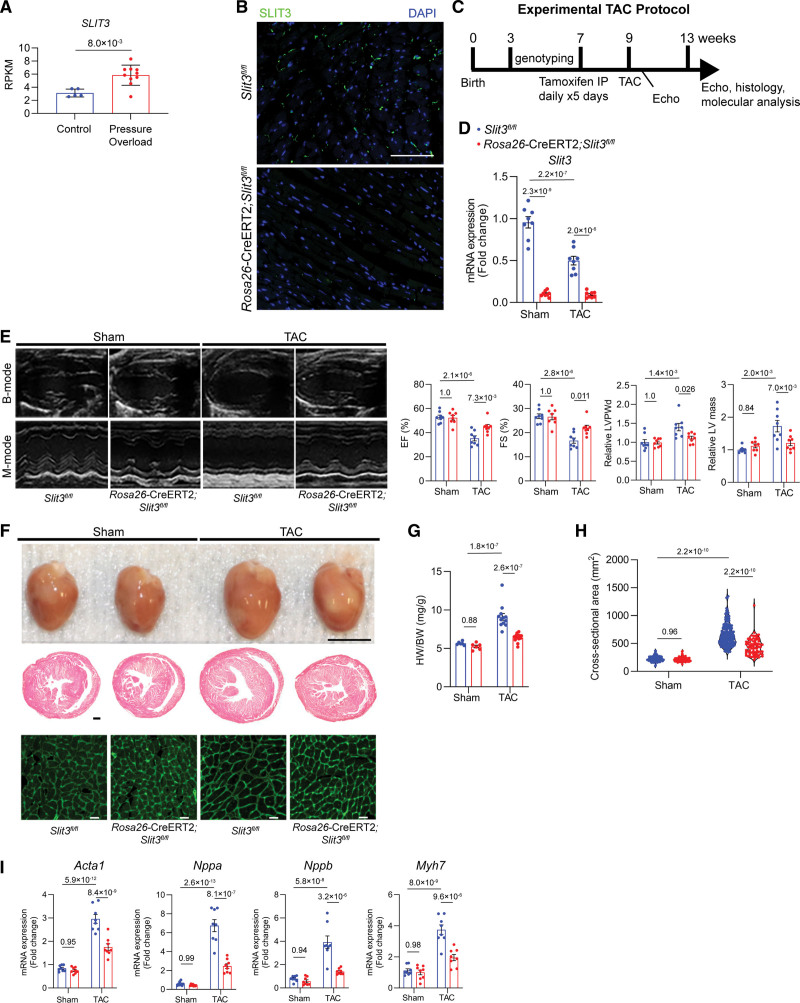
Consistent with our previous work in a TAC mouse model,24 Slit3 transcription in the adult wild-type (WT) mouse left ventricle (LV) was rapidly induced by pressure overload and reached its peak 2 weeks after TAC (Figure S1A), suggesting that the postnatal expression of this ligand may be induced by pressure overload.24 To determine whether this finding would extend to humans, we queried our biorepository (the UCLA CHD-BioCore) to determine the level of SLIT3 transcription in an RNA sequencing database of resected ventricular outflow tract tissue samples from patients with control (normal hearts declined for transplant) and pressure overload–causing congenital heart disease (subaortic membrane and tetralogy of Fallot). Significantly increased SLIT3 transcript levels were observed in ventricular tissue from patients with pressure overload–induced hypertrophy (Figure 1A), supporting the contention that SLIT3 plays a role in postnatal cardiac hypertrophy under pressure overload. We also analyzed a potential association between SLIT3 expression and cardiac fibrosis in myocardial samples obtained from normal controls, patients with tetralogy of Fallot, where cardiac fibrosis is anticipated from the pressure overload,32 and those with ventricular septal defect, where less cardiac fibrosis is anticipated from this volume overload defect.33 We found that SLIT3 transcript levels were significantly correlated with COL1A1 transcript levels when considering all patient and control samples (Figure S1B). Furthermore, higher SLIT3 and COL1A1 transcript levels were found in myocardial samples taken from pressure overload defect patients (Figure S1B).
Figure 1.
Inducible global-SLIT3 deficiency alleviates the development of cardiac hypertrophy and dysfunction after transverse aortic constriction (TAC). A, SLIT3 transcription as measured by RNA sequencing and displayed as reads per kilobase of transcript per million mapped reads (RPKM) in ventricular tissue samples from normal controls and patients with pressure overload induced by congenital heart disease. Each data point represents a unique subject. Comparison performed with Mann-Whitney U test. B, Immunofluorescence staining of myocardial sections Slit3fl/fl and Rosa26-CreERT2;Slit3fl/fl mice 4 wk after tamoxifen injection using anti-SLIT3 antibody (green) and DAPI (blue) demonstrated loss of SLIT3 in the Rosa26-CreERT2;Slit3fl/fl mice. Scale bar, 500 µm. C, TAC experimental design and timeline. D, Slit3 transcript levels in hearts from Slit3fl/fl and Rosa26-CreERT2;Slit3fl/fl mice after sham or TAC surgery. E, B-mode and M-mode echocardiography in Slit3fl/fl and Rosa26-CreERT2; Slit3fl/fl mice after sham or TAC surgery with analysis of ejection fraction (EF), shortening fraction (FS), relative left ventricle posterior wall thickness at diastole normalized to mice body weight (end-diastolic left ventricle posterior wall thickness [LVPWd]), relative left ventricle mass, and normalized to body weight. N=8 mice/group. F, Explanted hearts from Slit3fl/fl and Rosa26-CreERT2;Slit3fl/fl mice after sham or TAC surgery (top), representative images of heart sections stained with hematoxylin & eosin (H&E; scale bar, 500 µm; middle), and representative images of wheat germ agglutinin (WGA) staining of myocardial sections (scale bar, 20 µm, bottom). G, Heart weight to body weight (HW/BW) ratio in Slit3fl/fl or Rosa26-CreERT2;Slit3fl/fl mice after sham or TAC surgery. N=8 mice in each group. H, Quantification of myocyte cross-sectional area from WGA staining. N=400 cells from 6 to 8 mice in each group. I, Quantitative PCR (qPCR) analysis of transcript levels of hypertrophy-associated genes (Nppa, Nppb, Myh7, and Acta1, normalized to Gapdh transcript levels) in hearts from Slit3fl/fl and Rosa26-CreERT2;Slit3fl/fl mice after sham or TAC surgery. N=8 mice in each group. Two-way ANOVA with the Tukey multiple comparisons test used in (D through I). DAPI indicates 4’,6-diamidino-2-phenylindole.
To directly interrogate the presence of a postnatal role of SLIT3 in the heart and its response to pressure overload, we generated tamoxifen-inducible SLIT3 global knockout mice (Rosa26-CreERT2;Slit3fl/fl)34,35 with the non-Cre-expressing littermates (Slit3fl/fl) serving as controls. Immunofluorescence analysis confirmed a significant reduction of SLIT3 in hearts from Rosa26-CreERT2;Slit3fl/fl mice at 6 weeks after tamoxifen induction compared with that in Slit3fl/fl control mouse hearts (Figure 1B). Experimental and control mice were administered tamoxifen at 7 weeks of age and then, 2 weeks later, subjected to sham or TAC surgery, with the experiments terminated 4 weeks later (Figure 1C). Only TAC mice with a peak pressure gradient >35 mm Hg were included in downstream studies. The LV afterload in Rosa26-CreERT2 Slit3fl/fl mice and Slit3fl/fl control mice were not significantly different following TAC surgery (Figure S1C). We confirmed a significant and substantial reduction in Slit3 transcript levels in Rosa26-CreERT2;Slit3fl/fl mice (Figure 1D). After TAC, Slit3fl/fl control mice experienced decreased ejection fraction and fractional shortening, along with increased end-diastolic LV posterior wall thickness and LV mass compared with control mice undergoing sham surgery (Figure 1E). By contrast, Rosa26-CreERT2;Slit3fl/fl mice undergoing TAC manifested significantly improved ejection fraction and fractional shortening and reduced end-diastolic LV posterior wall thickness and LV mass after TAC surgery (Figure 1E).
In tandem with these stress-induced echocardiogram changes, Slit3fl/fl mice undergoing TAC had more hypertrophy, manifested by a larger heart size, higher ratios of heart weight to body weight, and increased cardiomyocyte cross-sectional area compared with mice subjected to sham surgery (Figure 1F through 1H). Importantly, these hypertrophic changes induced by TAC were markedly attenuated in Rosa26-CreERT2;Slit3fl/fl mice (Figure 1F through 1H). Moreover, transcript levels of several hypertrophy-related genes, including Acta1, Nppa, Nppb, and Myh7, were increased in Slit3fl/fl control mice after TAC, while these increases were blunted by SLIT3 deletion in Rosa26-CreERT2;Slit3fl/fl mice (Figure 1I).
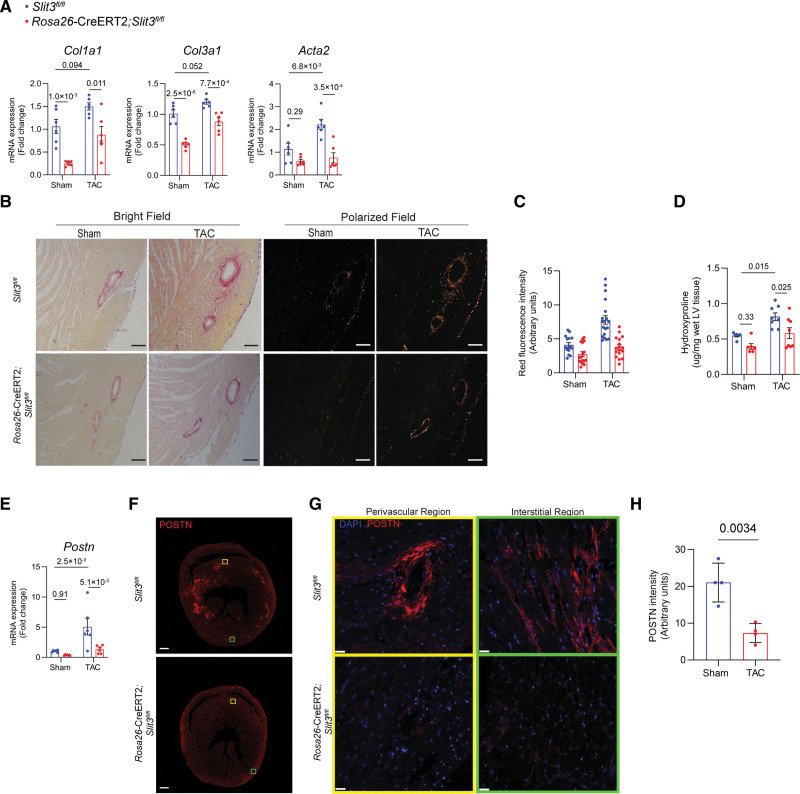
Fibrosis-related gene expression was also examined in the LV tissue of explanted hearts from Rosa26-CreERT2;Slit3fl/fl mice and compared with those of control mice 4 weeks after sham or TAC surgery (Figure 2A). The transcript levels of Col1a1 and Col3a1 were markedly decreased in both sham and TAC groups of Rosa26-CreERT2;Slit3fl/fl mice compared with Slit3fl/fl control mice. Acta2 transcription was not different between sham groups but significantly decreased in Rosa26-CreERT2;Slit3fl/fl mice under pressure overload conditions. To visualize the accumulation of fibrillar collagen in the ventricular myocardium, we performed picrosirius red staining (Figure 2B). The quantity of fibrillar collagen in the hearts of Rosa26-CreERT2;Slit3fl/fl mice was significantly less than in Slit3fl/fl control mice as determined by the amount of red fluorescence intensity under polarized light (Figure 2B and 2C). Furthermore, we confirmed this result by biochemically measuring the amount of cardiac collagen using the hydroxyproline assay (Figure 2D). As the differentiation of resting resident cardiac fibroblasts to activated myofibroblasts is key to the development of cardiac fibrosis,36,37 we further examined the expression of POSTN (periostin), a marker for myofibroblasts and fibroblast activation,37–39 in post-TAC mouse heart tissue. We found that the transcript levels of Postn were increased in control mice after TAC and that acute, global Slit3 deficiency abrogated this inductive response (Figure 2E). This corresponded to decreased myocardial POSTN expression after TAC in SLIT3 deficient animals (Figure 2F through 2H). Taken altogether, these data indicate that SLIT3 has a postnatal role in regulating cardiac hypertrophy and fibrosis in response to pressure overload.
Figure 2.
Inducible global-SLIT3 deficiency alleviates the development of cardiac fibrosis after transverse aortic constriction (TAC). A, Quantitative PCR (qPCR) analysis of transcript levels of fibrosis-associated genes (Col1a1, Col3a1, and Acta2, normalized to Gapdh transcript levels) in hearts from Slit3fl/fl and Rosa26-CreERT2;Slit3fl/fl mice after sham or TAC surgery. N=8 mice in each group. B, Picrosirius red staining of myocardial sections from Slit3fl/fl and Rosa26-CreERT2;Slit3fl/fl mice after sham or TAC surgery and visualized under brightfield microscopy and polarized light. Representative images are shown. Scale bar, 100 µm. C, Quantification of red channel signal under polarized light of picrosirius red–stained myocardial sections from (B). Data from N=4 to 5 animals/group with 3 to 4 high power fields analyzed per animal. D, Collagen content determination from left ventricle tissue using the hydroxyproline assay. Data from n=8 animals/group. E, Postn transcript levels in hearts from Slit3fl/fl and Rosa26-CreERT2;Slit3fl/fl mice after sham or TAC surgery. Data from N=8 animals/group. F and G, Representative images of post-TAC myocardial sections stained with an anti-POSTN (periostin) antibody (scale bar=100 µm in [F] and 25 µm in [G]). H, Quantification of POSTN stained myocardial sections in (F). N=4 mice in each group. Two-way ANOVA with the Tukey multiple comparisons test used in (A, D, and E). A linear regression model with cluster option was used to evaluate the data in (C), where the comparison of genotype (Slit3fl/fl vs Rosa26-CreERT2;Slit3fl/fl) and surgery (sham vs TAC) and the interaction between genotype and surgery were all found to be significant (P<1.0×10−4 for all comparisons and interaction). Two-tailed t test was used in (H), with data passing the Shapiro-Wilk normality test and assuming the application of the central limit theorem.
Fibroblast-Specific Deletion of SLIT3 Reduces Post-TAC Adverse Cardiac Remodeling and Ventricular Dysfunction
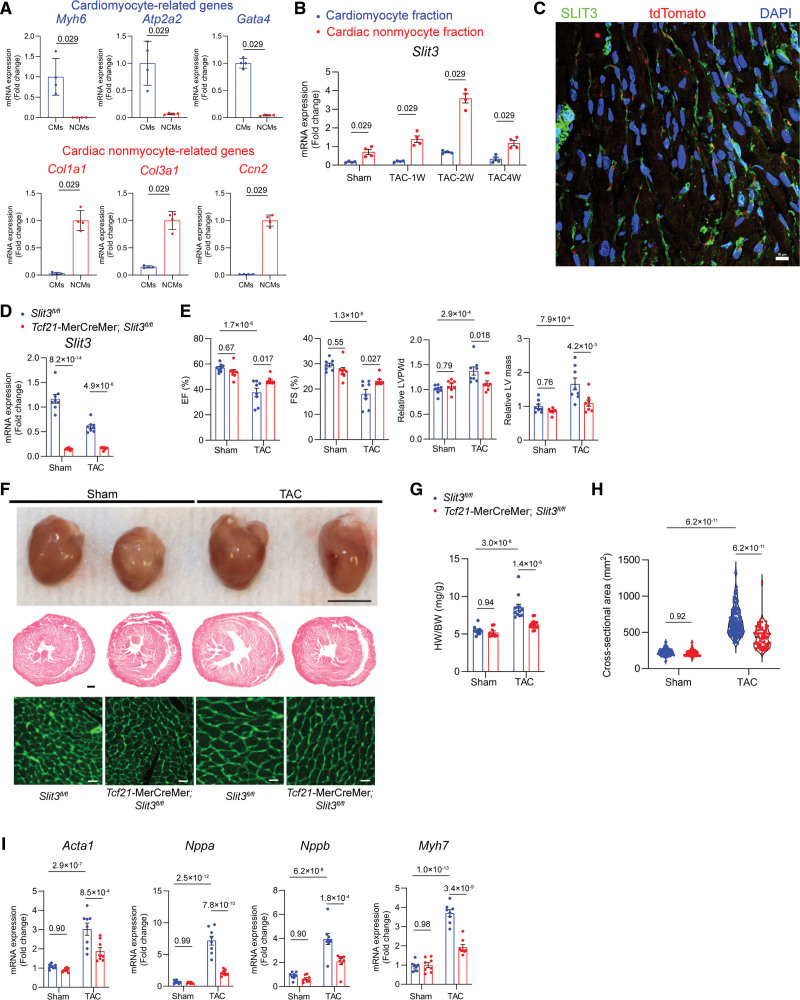
In light of the above findings and that SLIT3 is a secreted molecule, we hypothesized that the SLIT3 ligand serves a paracrine role in regulating cardiac hypertrophy and fibrosis. To define the cellular sources of cardiac SLIT3 in homeostatic and pressure overload conditions, we first separated cardiomyocytes from CNMs in isolated hearts of mice subjected to sham surgery or TAC. After confirming the purity of the isolated cell fractions (Figure 3A), we found that the CNM cell fraction exhibited several-fold higher levels of Slit3 transcripts compared with the cardiomyocyte fraction from WT mouse hearts after TAC (Figure 3B).
Figure 3.
Fibroblast-specific deletion of SLIT3 reduces cardiac hypertrophy and dysfunction after transverse aortic constriction (TAC). A, Isolation of cardiac nonmyocyte (NCM) and cardiomyocyte (CM) cellular fractions from adult wild-type (WT) mice was verified by measuring Col1a1, Col3a1, Ccn2, Myh7, Myh6, and Acta1 mRNA levels (normalized to Gapdh transcript levels). Cells from N=4 WT mice and comparisons made with the Mann-Whitney U test. B, Slit3 transcript levels in isolated NCM and CM fractions from adult mouse hearts subjected to sham or TAC surgery. N=4 mice/group and comparisons were made with the Mann-Whitney U test. C, Immunofluorescence staining of a heart section from Tcf21-CreERT2;Rosa26-tdTomato mice using anti-SLIT3 antibody (green), anti-tdTomato (red), and 4’,6-diamidino-2-phenylindole (DAPI; blue). Scale bar, 50 µm. D, Slit3 transcript levels determined by quantitative PCR (qPCR) in Slit3fl/fl and Tcf21-CreERT2;Slit3fl/fl mice after 13 wk after sham or TAC surgery. N=8 mice/group. E, Echocardiography data from Slit3fl/fl and Tcf21-CreERT2;Slit3fl/fl mice after sham or TAC surgery with analysis of ejection fraction, fractional shortening, relative end-diastolic left ventricle posterior wall thickness (LVPWd), and relative left ventricle mass. N=8 mice/group. F, Explanted hearts from Slit3fl/fl control or Tcf21-CreERT2;Slit3fl/fl mice after sham or TAC surgery (top); representative images of heart sections stained with hematoxylin & eosin (H&E; scale bar, 500 µm; middle); and representative images of wheat germ agglutinin (WGA) stained heart sections (scale bar, 20 µm; bottom). G, Heart weight to body weight (HW/BW) ratio. N=8 mice/group. H, Quantification of CM cross-sectional area from WGA-stained sections. N=400 cells from 6 to 7 mice in each group. I, qPCR analysis hypertrophy-related genes (Nppa, Nppb, Myh7, and Acta1, normalized to Gapdh mRNA levels) in hearts from Slit3fl/fl and Tcf21-CreERT2;Slit3fl/fl mice after sham or TAC surgery. N=8 mice/group. Two-way ANOVA with the Tukey multiple comparisons test used in (D, E, and G–I).
As cardiac fibroblasts and vascular mural cells are myocardial stromal cells that comprise a large fraction of the CNMs of the heart,40,41 we next evaluated the importance of each cell type as a myocardial SLIT3 source. Given that expression of Tcf21 is a quiescent cardiac fibroblast marker,42 we immunostained hearts recovered from Tcf21-MerCreMer;Rosa26-tdTomato reporter mice with an anti-SLIT3 antibody and confirmed SLIT3 expression associated with this cell population (Figure 3C), consistent with immunostaining of WT hearts (Figure S2A). The tdTomato+ cell population in these reporter mice also expressed PDGFRA (platelet-derived growth factor receptor A; Figure S2B). Next, we crossed Slit3fl/fl mice with Tcf21-MerCreMer transgenic mice to obtain tamoxifen-inducible and fibroblast-specific SLIT3 knockout mice. After tamoxifen administration at 7 weeks (Figure 1C), we subjected Tcf21-MerCreMer;Slit3fl/fl experimental and Slit3fl/fl control mice to sham surgery or TAC. There was no difference in the TAC gradient of the 2 groups as determined by echocardiography (Figure S2C), and Slit3 transcript levels were significantly decreased in experimental animals (Figure 3D), confirming Cre-mediated recombination. Under these conditions, echocardiograms performed 4 weeks after TAC demonstrated that ventricular hypertrophy (as measured by end-diastolic LV posterior wall thickness and LV mass) and LV dysfunction (as measured by ejection fraction and fractional shortening) were attenuated in cardiac fibroblast–targeted mice (Figure 3E). This finding was corroborated by a smaller heart, decreased heart weight to body weight ratio, and smaller cardiomyocyte cross-sectional area in Tcf21+ fibroblast-specific knockout of SLIT3 (Figure 3F through 3H) after TAC. In addition, Slit3 deletion restricted in fibroblasts in Tcf21-MerCreMer;Slit3fl/fl mice significantly reduced TAC-induced myocardial expression of hypertrophy-related and fibrosis-related genes compared with Slit3fl/fl control mice (Figure 3I). Taken together, these studies demonstrated that SLIT3 produced in cardiac fibroblasts plays an important role in the hypertrophic response to pressure overload.
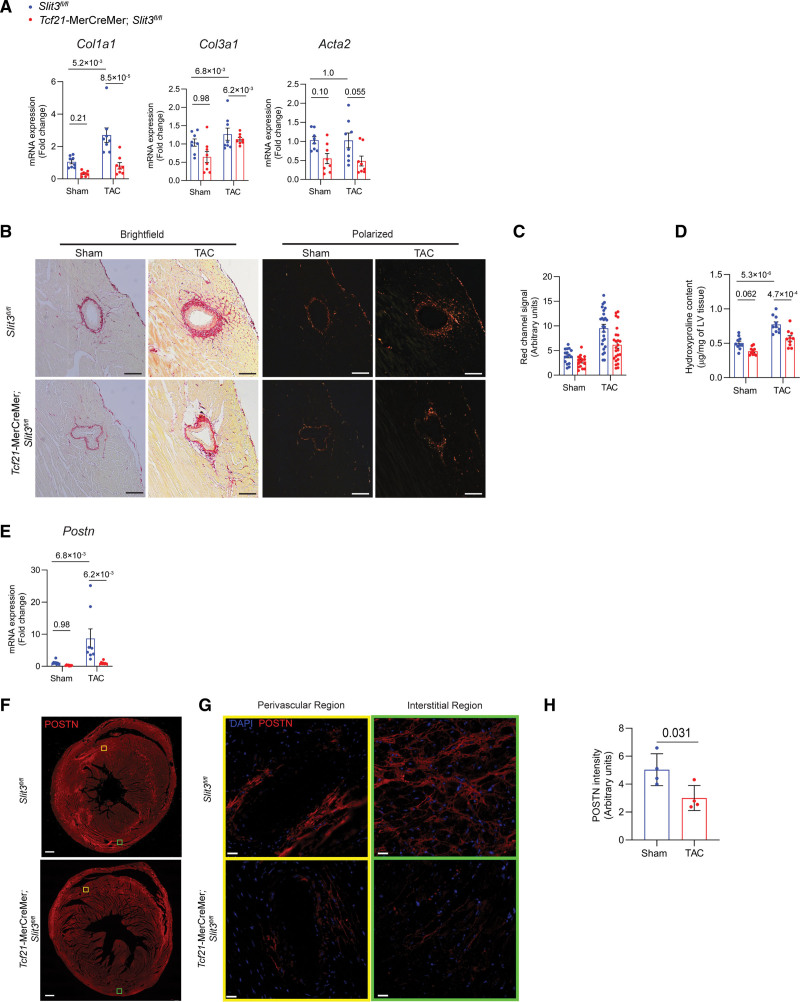
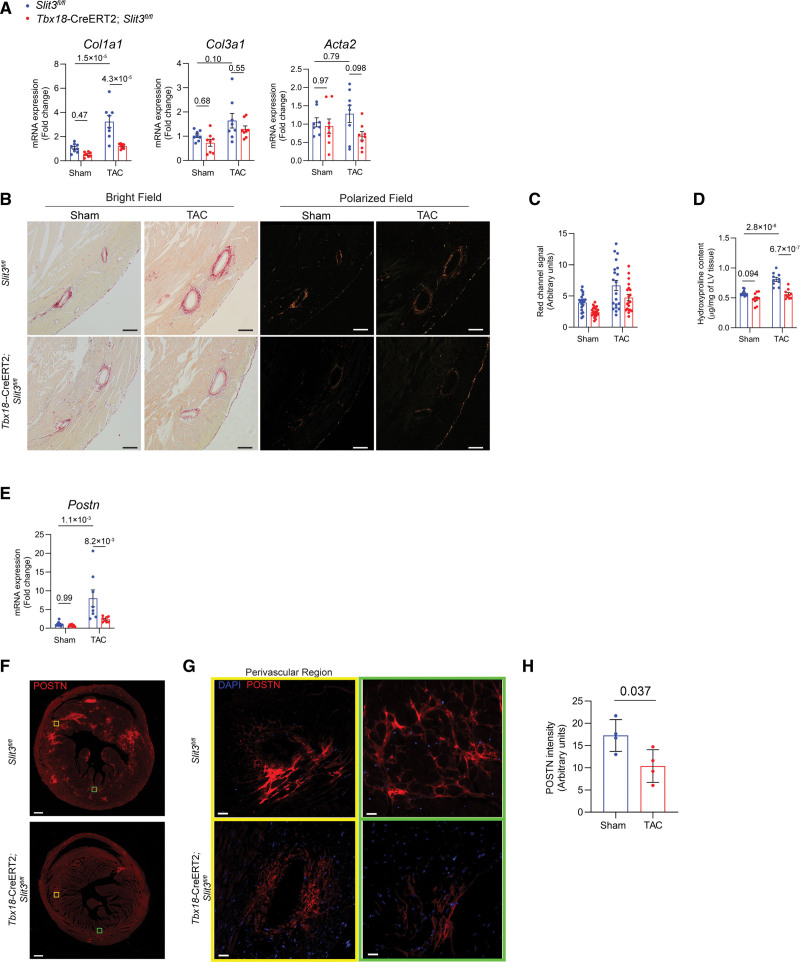
Moreover, Tcf21-MerCreMer;Slit3fl/fl mice in both Sham and TAC groups also exhibited reduced cardiac fibrosis. Transcription of fibrillar collagen genes Col1a1 and Col3a1 were decreased in Tcf21-MerCreMer; Slit3fl/fl mice that had undergone TAC (Figure 4A). Collagen content as determined by picrosirius red staining and polarized microscopy (Figure 4B and 4C) and LV hydroxyproline content (Figure 4D) was decreased in Tcf21-MerCreMer;Slit3fl/fl mice after TAC. Postn transcript levels were decreased in Tcf21-MerCreMer;Slit3fl/fl mice after TAC (Figure 4E). The amount of POSTN in the LV of Tcf21-MerCreMer;Slit3fl/fl mice as determined by immunofluorescence was decreased compared with Slit3f/f control mice after TAC (Figure 4F through 4H). These results indicate that cardiac fibroblast-mediated SLIT3 is important for the development of cardiac hypertrophy and fibrosis induced by pressure overload.
Figure 4.
Fibroblast-specific deletion of SLIT3 abrogates pressure overload–induced cardiac fibrosis. A, Quantitative PCR (qPCR) analysis of transcript levels of fibrosis-associated genes (Col1a1, Col3a1, and Acta2, normalized to Gapdh transcript levels) in hearts from Slit3fl/fl and Tcf21-MerCreMer;Slit3fl/fl mice after sham or transverse aortic constriction (TAC) surgery. N=8 mice in each group. B, Picrosirius red staining of myocardial sections from Slit3fl/fl and Tcf21-MerCreMer;Slit3fl/fl mice after sham or TAC surgery and visualized under brightfield microscopy and polarized light. Representative images are shown. Scale bar, 100 µm. C, Quantification of red channel signal under polarized light of picrosirius red–stained myocardial sections from (B). Data from N=4 to 5 animals/group with 4 high power fields analyzed per animal. D, Collagen content determination from left ventricle tissue using the hydroxyproline assay. Data from n=8 animals/group. E, Postn transcript levels in hearts from Slit3fl/fl and Tcf21-MerCreMer;Slit3fl/fl mice after sham or TAC surgery. Data from N=8 animals/group. F and G, Representative images of post-TAC myocardial sections stained with an anti-POSTN (periostin) antibody (scale bar=500 µm in [F] and 25 µm in [G]). H, Quantification of POSTN stained myocardial sections in (F). N=4 mice in each group. Two-way ANOVA with the Tukey multiple comparisons test used in (A, D, and E). A linear regression model with cluster option was used to evaluate the data in (C), where the comparison of genotype (Slit3fl/fl vs Tcf21-MerCreMer;Slit3fl/lf) and surgery (sham vs TAC) and the interaction between genotype and surgery were all found to be significant (P=2.4×10−3, P<1.0×10−4, and P=6.5×10−3, respectively). Two-tailed t test was used in (H), with data passing the Shapiro-Wilk normality test and assuming the application of the central limit theorem.
Vascular Mural Cell–Specific Deletion of SLIT3 Reduces Cardiac Hypertrophy and Dysfunction After TAC
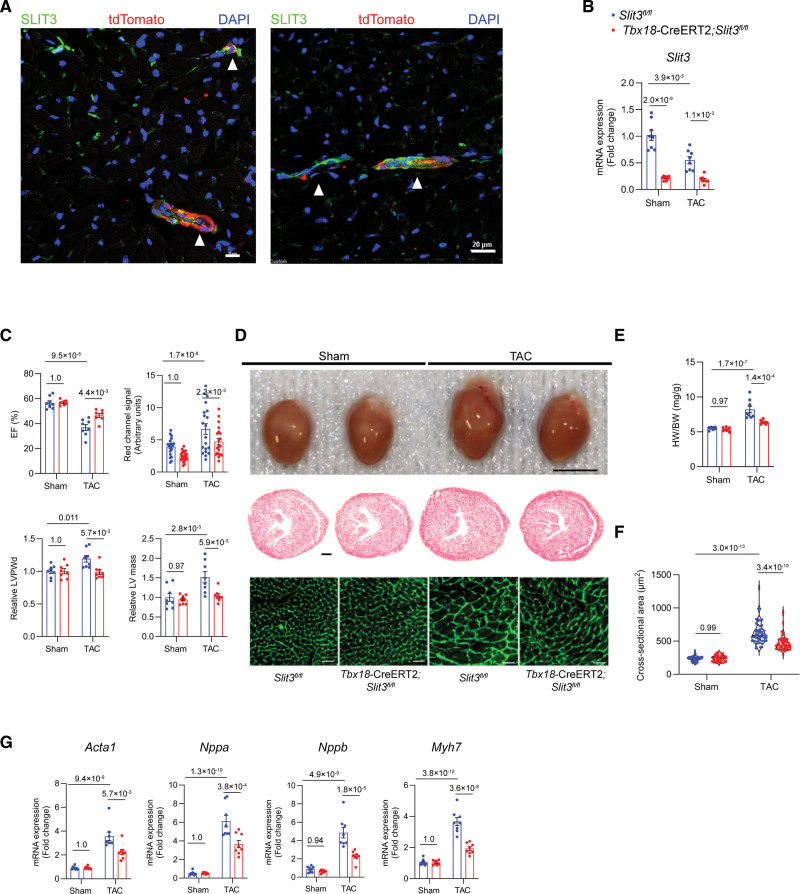
In our previous work, immunofluorescence staining demonstrated that SLIT3 was localized not only to the cardiac interstitium but also to the mural cells of myocardial blood vessels.24 Furthermore, we previously determined that human mesenchymal stem/stromal cells, which also reside in the perivascular region,43 expressed SLIT3.44 As such, we considered the possibility that SLIT3 derived from vascular mural cells in the heart may also play a role in regulating cardiac hypertrophy. To specifically target vascular mural cells, we utilized a Tbx18-CreERT2 mouse that has been previously generated and characterized extensively to be a vascular mural cell–specific Cre mouse line with minimal crossover into fibroblasts, endothelial cells, and cardiomyocytes.45 Using Tbx18-CreERT2;Rosa26-tdTomato reporter mice, we confirmed that the tdTomato+ cells were located in the periendothelial region of myocardial vessels. Furthermore, these tdTomato+ vascular mural cells also expressed SLIT3 (Figure 5A) and PDGFRB (platelet-derived growth factor receptor B and vascular smooth muscle cell marker46), NG2 (neural/glial antigen 2 and pericyte marker47), and MYH11 (vascular smooth muscle cell marker) by immunostaining48 (Figure S3A through 3C). We also confirmed that Tcf21-MerCreMer and Tbx18-CreERT2 reporter mice had different tdTomato expression patterns, with the Tbx18-CreERT2 mice having predominantly a vascular mural expression (Figure 5A; Figure 3A through S3C), while the Tcf21-MerCreMer had a more interstitial (Figure 3C; Figure S2B) and valvar staining pattern (Figure 4A).
Figure 5.
Vascular mural cell–specific deletion of SLIT3 reduces cardiac hypertrophy and dysfunction after transverse aortic constriction (TAC). A, Immunofluorescence staining of heart sections from Tbx18-CreERT2;Rosa26-Tdtomato mice using anti-SLIT3 antibody (green), anti-tdTomato antibody (red), and 4’,6-diamidino-2-phenylindole (DAPI; blue). White arrowheads denote the overlap of SLIT3 and tdTomato signals in the mural region of myocardial blood vessels in cross-section (left) and axially (right). Scale bar for (left), 10 µm. Scale bar for (right), 20 µm. B, Slit3 transcription determined by quantitative PCR (qPCR; normalized to Gapdh transcript levels) in Slit3fl/fl or Tbx18-CreERT2;Slit3fl/fl mice hearts after sham or TAC surgery. N=8 mice/group. C, Echocardiographic studies of Slit3fl/fl and Tbx18-CreERT2;Slit3fl/fl mice after sham or TAC surgery. N=8 mice/group. D, Appearance of hearts explanted from Slit3fl/fl control and Tbx18-CreERT2;Slit3fl/fl mice after sham or TAC surgery (top); representative images of heart sections stained with hematoxylin & eosin (H&E; scale bar, 500 µm; middle); and representative images of heart sections stained with wheat germ agglutinin (WGA; scale bar, 20 µm; bottom). E, Heart weight to body weight (HW/BW) ratio. N=8 mice/group. F, Quantification of cardiomyocyte cross-sectional area on WGA staining. N=600 cells from 6 mice in each group. G, qPCR analysis of hypertrophy-associated gene transcription (Nppa, Nppb, Myh7, and Acta1, normalized to Gapdh mRNA expression) in hearts from Slit3fl/fl control or Tbx18-CreERT2, Slit3fl/fl mice after sham or TAC surgery. N=8 mice/group. Two-way ANOVA with the Tukey multiple comparisons test was used in all analyses. EF indicates ejection fraction; LV, left ventricle; and LVPWd, end-diastolic left ventricle posterior wall thickness.
Next, we crossed Slit3fl/fl mice with Tbx18-CreERT2 mice to obtain Tbx18-CreERT2, Slit3fl/fl mice and their non-Cre expressing (Slit3fl/fl) littermates. As described above, mice were then administered vehicle or tamoxifen and then subjected to sham surgery or TAC (Figure 1C). For the TAC groups, only mice with adequate arch gradients as determined by echocardiogram were chosen for further study (Figure S4B). Having confirmed that Slit3 transcript levels were decreased in tamoxifen-treated Tbx18-CreERT2;Slit3fl/fl mice (Figure 5B), echocardiography at 4 weeks after sham surgery or TAC revealed improved cardiac function and reduced LV wall thickness and mass in mural cell-targeted mice after TAC (Figure 5C). Grossly, hearts from the non–Cre-containing animals that had undergone TAC were larger and had a larger heart weight to body weight ratio than those from Tbx18-CreERT2;Slit3fl/fl post-TAC mice (Figure 5D and 5E). Cardiomyocyte cross-sectional area was decreased in Tbx18-CreERT2;Slit3fl/fl post-TAC mice, also consistent with decreased hypertrophy (Figure 5D and 5F), as well as attenuated TAC-stimulated transcription of Acta1, Nppa, Nppb, and Myh7 (Figure 5G). Collectively, these results confirm that in addition to fibroblast-derived SLIT3, vascular mural cells also serve as a source of this ligand in regulating pressure overload–induced cardiac hypertrophy.
We also found that vascular mural cell–mediated SLIT3 affected the development of cardiac fibrosis induced by pressure overload. While the transcript levels of Col3a1 and Acta2 were not significantly different between control and Tbx18-CreERT2;Slit3fl/fl mice (Figure 6A), Col1a1 transcript levels were decreased in Tbx18-CreERT2;Slit3fl/fl mice compared with control mice after sham and TAC surgery (Figure 6A). Consistent with the changes in Col1a1 transcript levels, fibrillar collagen content in Tbx18-CreERT2;Slit3fl/fl hearts was markedly less as determined by picrosirius red staining followed by polarized light microscopy (Figure 6B and 6C) and the hydroxyproline assay of LV tissue from Tbx18-CreERT2;Slit3fl/fl mice in both sham and TAC groups (Figure 6D). The Postn transcript and POSTN protein levels in the LVs after TAC were also decreased in Tbx18-CreERT2;Slit3fl/fl mice, indicating that loss of SLIT3 in vascular mural cells inhibited the activation of cardiac fibroblasts induced by pressure overload (Figure 6E through 6H).
Figure 6.
Vascular mural cell–specific deletion of SLIT3 abrogates pressure overload–induced cardiac fibrosis. A, Quantitative PCR (qPCR) analysis of transcript levels of fibrosis-associated genes (Col1a1, Col3a1, and Acta2, normalized to Gapdh transcript levels) in hearts from Slit3fl/fl and Tbx18-CreERT2;Slit3fl/fl mice after sham or transverse aortic constriction (TAC) surgery. N=8 mice in each group. B, Picrosirius red staining of myocardial sections from Slit3fl/fl and Tbx18-CreERT2;Slit3fl/fl mice after sham or TAC surgery and visualized under brightfield microscopy and polarized light. Representative images are shown. Scale bar, 100 µm. C, Quantification of red channel signal under polarized light of picrosirius red–stained myocardial sections from (B). Data from N=4 animals/group with 4 high power fields analyzed per animal. D, Collagen content determination from left ventricle tissue using the hydroxyproline assay. Data from n=8 animals/group. E, Postn transcript levels in hearts from Slit3fl/fl and Tbx18-CreERT2;Slit3fl/fl mice after sham or TAC surgery. Data from N=8 animals/group. F and G, Representative images of post-TAC myocardial sections stained with an anti-POSTN (periostin) antibody (scale bar=500 µm in [F] and 25 µm in [G]). H, Quantification of POSTN stained myocardial sections in (F). N=4 mice in each group. Two-way ANOVA with the Tukey multiple comparisons test used in (A, D, and E). A linear regression model with cluster option was used to evaluate the data in (C), where the comparison of genotype (Slit3fl/fl vs Tbx18-CreER2;Slit3fl/lf) and surgery (sham vs TAC) were different (P<1.0×10−4 and P<1.0×10−4, respectively). The interaction between genotype and surgery was not significant (P=0.53). Two-tailed t test was used in (H), with data passing the Shapiro-Wilk normality test and assuming the application of the central limit theorem.
Our in vivo studies demonstrated that global, fibroblast-specific, and vascular mural cell–specific deletion of SLIT3 appeared to decrease TAC-induced hypertrophy to the same degree (Figures 1, 3, and 5). However, we observed subtle differences in cardiac fibrosis in these different groups of mutant mice. Acute, global deletion of SLIT3 in Rosa26-CreERT2;Slit3fl/fl mice resulted in the greatest decrease in Col1a1, Col3a1, and Acta2 transcription in sham and TAC animals (Figure 2A). However, fibroblast-specific deletion of SLIT3 in Tcf21-MerCreMer;Slit3fl/fl mice resulted in a significant decrease in Col1a1 and Col3a1 transcription only after TAC, but not sham, surgery (Figure 4A). Vascular mural cell–specific deletion of SLIT3 in Tbx18-CreERT2;Slit3fl/fl mice appeared to have the least impact and resulted only in a decrease in Col1a1 transcription after TAC surgery (Figure 6A). This differential impact on cardiac fibrosis genes paralleled the amount of reduction in LV Slit3 transcripts in Rosa26-CreERT2;Slit3fl/fl (Figure 1D), Tcf21-MerCreMer;Slit3fl/fl (Figure 3D), and Tbx18-CreERT2;Slit3fl/fl mice (Figure 5B). Thus, our in vivo results suggest that the adverse cardiac remodeling response to pressure overload is sensitive to the amount and source of SLIT3.
Fibroblast and Vascular Mural Cell–Derived SLIT3 Can Directly Induce Hypertrophy in Cardiomyocytes In Vitro
To further evaluate the ability of CNM-derived SLIT3 to directly trigger hypertrophic responses of cardiomyocytes, we conducted in vitro experiments by exposing neonatal rat cardiomyocytes (NRCMs) to conditioned media from cardiac fibroblasts and vascular mural cells isolated from SLIT3 global knockout or Slit3fl/fl adult mice in vitro. Relative to conditioned media from WT (Slit3+/+) adult mouse cardiac fibroblasts that stimulated cardiomyocyte hypertrophy, conditioned media from Slit3 knockout (Slit3−/−) cardiac fibroblasts did not stimulate hypertrophy or Nppa and Nppb transcription (Figure S5A through S5C). Likewise, when Slit3fl/fl cardiac fibroblasts were transduced with a Cre-recombinase adenoviral vector (AdCre), Slit3 expression was abolished (Figure S5D), and the conditioned media no longer induced a hypertrophic response relative to cells transduced with a control GFP adenoviral vector (AdGFP; Figure S5E and S5F). By contrast, when Slit3fl/fl cardiac fibroblasts were transduced with a SLIT3-expressing adenoviral construct (AdSLIT3), Slit3 expression levels were increased and conditioned media from these cells increased NRCM size relative to controls in tandem with higher transcript level of Nppa and Nppb (Figure S5E through S5G). Furthermore, the addition of an anti-SLIT3 antibody inhibited the prohypertrophic effect of conditioned media from SLIT3 overexpressing adult mouse cardiac fibroblasts on NRCMs, as reflected in the downregulated expression of Nppa and Nppb, as well as smaller cell size compared with an isotype control IgG (Figure S5H through S5J). Furthermore, similar effects were observed using mural cell populations engineered to silence or increase SLIT3 expression (Figure 6A through 6F).
These in vitro data suggested that fibroblast and vascular mural cell–mediated SLIT3 can stimulate cardiomyocyte hypertrophy. As SLITs can be cleaved into N- and C-terminal fragments in vivo,49 we next investigated which portion of SLIT3 was responsible for stimulating cardiomyocyte hypertrophy because these fragments have been determined to bind to different receptors.26,30 We cultured NRCMs with recombinant N- and C-terminal fragments of SLIT3 (SLIT3-NT and SLIT3-CT, respectively) and observed that only SLIT3-NT could promote the transcription of Nppa and Nppb and stimulate NRCMs hypertrophy (Figure S5K through S5M). Of note, SLIT3-NT also stimulated hypertrophic responses of human-induced pluripotent stem cell–derived cardiomyocytes (Figure S5N through S5P). Finally, forced expression of SLIT3 in NRCMs strongly induced hypertrophy (Figure S5G and S5H). Taken together, these results indicate that SLIT3, normally expressed by fibroblasts and vascular mural cells in the heart, can stimulate hypertrophy in cardiomyocytes.
The In Vitro Hypertrophic Effects of SLIT3 on Cardiomyocytes Are ROBO1-Dependent
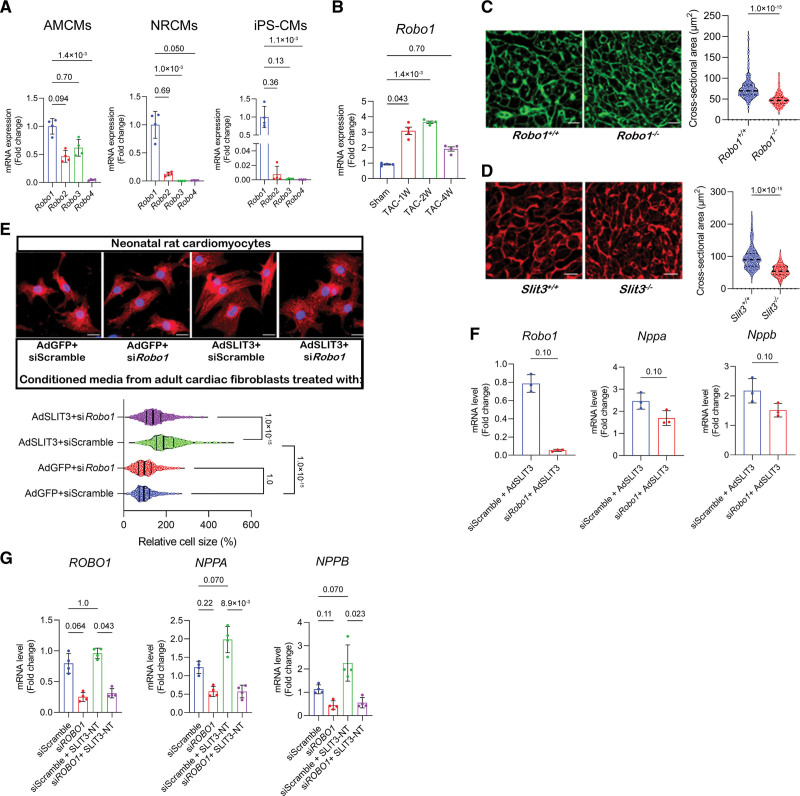
The N-terminal fragment of SLIT proteins mediates their functions by binding to cognate transmembrane receptors belonging to the roundabout receptor family.26 There are 4 mammalian ROBOs (ROBO1-4), each containing 5 immunoglobulin-like domains and FN (fibronectin) III motifs in the extracellular domain with the exception of ROBO4, which has only 2 immunoglobulin domains and the Fn III motifs.50 To determine the identity of the ROBO receptor that mediates SLIT3 effects on cardiomyocytes, we first measured the transcript levels of Robo1, Robo2, Robo3, and Robo4 in adult mouse cardiomyocytes, NRCMs, and human-induced pluripotent stem cell–derived cardiomyocytes. We found that Robo1 transcript levels were dominant in each of the cardiomyocyte populations (Figure 7A). Furthermore, TAC surgery stimulated Robo1 transcription in the heart up to 4 weeks after TAC (Figure 7B). Interestingly, ROBO1 global knockout mice (Robo1−/−) exhibited smaller cardiomyocytes compared with their WT control mice (Robo1+/+) at the age of 7 days, phenocopying our observations in comparatively aged Slit3 knockout mice (Figure 7C and 7D). Taken together, these findings suggested ROBO1 as a possible receptor on cardiomyocytes for SLIT3.
Figure 7.
The in vitro hypertrophic effect of SLIT3 on cardiomyocytes is ROBO1-dependent. A, Transcript levels of Robo1, Robo2, Robo3, and Robo4 in cardiomyocytes of different origins. Robo3 transcripts were not detectable in neonatal rat cardiomyocytes (NRCMs). N=3 independent experiments. B, Transcript levels of Robo1 in mice heart tissue subjected to sham or transverse aortic constriction (TAC) surgery. N=4 mice/group. C, Representative images of wheat germ agglutinin (WGA)–stained heart sections and quantification of cardiomyocyte cross-sectional area from 7-d-old Robo1 knockout mice (Robo1−/−) or wild-type (WT) littermates (Robo1+/+). Scale bar, 10 µm. N=400 cells from 3 mice/group. D, Representative images of WGA-stained heart sections and quantification of cardiomyocyte cross-sectional area from 7-d-old Slit3−/− or WT littermates (Slit3+/+). Scale bar, 10 µm. N=400 cells from 3 mice/group. E, Immunofluorescence with anti-α-actinin antibody and 4’,6-diamidino-2-phenylindole (DAPI) counterstain of NRCMs that were treated with conditioned media from WT adult cardiac fibroblasts transduced with AdGFP or AdSLIT3 and transfected with either siScramble or siRobo1. Cell size quantification was performed by analyzing N=500 cells per group. F, Corresponding Robo1, Nppa, and Nppb transcript levels in NRCMs measured by quantitative PCR (qPCR) after treatment with conditioned media for 48 h. N=3 independent experiments. G, Human iPS-derived cardiomyocytes were transfected with siScramble or siROBO1 for 48 h and then treated with recombinant SLIT3-NT for 8 d. Transcript levels of ROBO1, NPPA, and NPPB were assessed by qPCR. N=3 independent experiment. The Kruskal-Wallis test with the Dunn multiple comparisons test was used in (A, B, E, and G). The Mann-Whitney U test was used in (C, D, and F). AMCM indicates adult mouse cardiomyocyte; and iPS-CM, induced pluripotent stem cell–derived cardiomyocyte.
Previous reports demonstrated that a ROBO1-Fc fusion protein lacking its FN motifs can bind SLIT family member proteins in a soluble receptor ligand pull-down assay.51,52 Hence, we examined the impact of ROBO1-Fc on the ability of conditioned media from SLIT3 overexpressing adult mouse cardiac fibroblasts to stimulate NRCM hypertrophy in vitro. Under these conditions, ROBO1-Fc inhibited the hypertrophic effects of the conditioned media from SLIT3 overexpressing adult mouse cardiac fibroblasts relative to isotype IgG controls (Figure S7). Next, NRCMs were transfected with siScramble or siRNA specifically targeting Robo1 (siRobo1) before AdGFP or AdSLIT3 transduction. Inhibition of Robo1 transcription by siRobo1 abrogated the hypertrophic effects of AdSLIT3 on NRCMs (Figure 7E and 7F). We confirmed that these findings in SLIT3-NT stimulated human-induced pluripotent stem cell–derived cardiomyocytes where siROBO1 also decreased the transcription of NPPA and NPPB (Figure 7G). Likewise, siROBO1 inhibited baseline levels of NPPA and NPPB in iPSC-CMs as these cells can also express SLIT3 (data not shown), which, thus, may act in an autocrine effect.
ROBO1 Regulates the Development of Pathological Cardiac Hypertrophy After TAC In Vivo
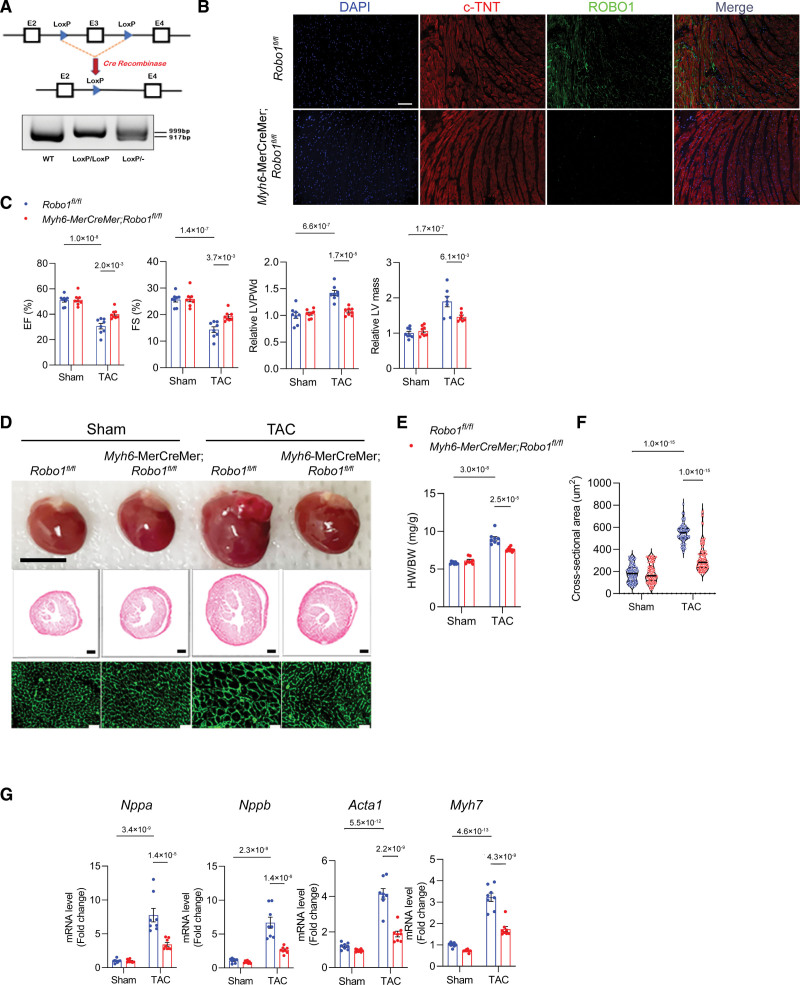
To directly investigate the function of ROBO1 in vivo, we generated Robo1fl/fl mice, in which the exon3 of the Robo1 gene was flanked by Loxp (Figure 8A). Then, inducible and cardiomyocyte-specific knockout of Robo1 mice was generated by crossing Robo1fl/fl mice with Myh6-MerCreMer mice. After tamoxifen administration, the knockout efficiency of ROBO1 in cardiomyocytes was confirmed by immunofluorescence staining of heart tissue. ROBO1 expression was predominant on the cell membrane of cardiomyocytes in Robo1fl/fl control mice, and this signal was significantly abrogated in heart tissue from the Myh6-MerCreMer;Robo1fl/fl mice (Figure 8B). We also confirmed by quantitative PCR (qPCR) that Robo1 transcription was effectively reduced in cardiomyocytes from Myh6-MerCreMer;Robo1fl/fl mice (Figure S8A). Sham or TAC surgery was conducted (Figure 1C), and only mice with an adequate TAC gradient were included in the downstream studies (Figure S8B). Robo1 transcript levels were significantly decreased in hearts from Myh6-MerCreMer;Robo1fl/fl mice 1 month after TAC (Figure S8C). Mice with cardiomyocyte-specific deletion of ROBO1 exhibited on echocardiogram improved cardiac function and reduced LV wall thickness and mass after TAC (Figure 8C), as well as a smaller heart weight to body weight ratio, heart size, and cross-sectional area after TAC compared with hearts from Robo1fl/fl littermate controls (Figure 8D through 8F). This was accompanied by downregulation of cardiac hypertrophy–associated genes such as Nppa, Nppb, Myh7, and Acta1 (Figure 8G). Finally, cardiomyocyte-specific deletion of ROBO1 had no significant impact on pressure overload–induced cardiac fibrosis (Figure S8D and S8E). Taken together, these results indicate that SLIT3-ROBO1 regulates cardiac hypertrophy and dysfunction induced by pressure overload (Figure S9).
Figure 8.
Cardiomyocyte-specific deletion of ROBO1 reduces cardiac hypertrophy and dysfunction after transverse aortic constriction (TAC). A, Schematic of Robo1fl/fl mouse model and PCR analysis for quantitation of loxp-flanked Robo1 gene region in wild-type (WT), heterozygous (loxp/-), or homozygous (loxp/loxp) mice. B, Immunofluorescence staining using anti-cardiac troponin (c-TNT) antibody (red), anti-ROBO1 antibody (green), and 4’,6-diamidino-2-phenylindole (DAPI; blue) of heart sections from Robo1fl/fl control or Myh6-CreERT2;Robo1fl/fl mice. C, B-mode and M-mode echocardiographies of Robo1fl/fl or Myh6-CreERT2;Robo1fl/fl mice after sham or TAC surgery with analysis of ejection fraction (EF), fractional shortening (FS), relative end-diastolic left ventricle posterior wall thickness (LVPWd), and relative left ventricle mass. N=8 mice/group. D, Explanted hearts from Robo1fl/fl and Myh6-MerCreMer;Robo1fl/fl mice after sham or TAC surgery (top); representative images of heart sections stained with hematoxylin & eosin (H&E; scale bar, 500 µm; middle); and representative images of wheat germ agglutinin (WGA)–stained heart sections (scale bar, 20 µm; bottom). E, Heart weight to body weight (HW/BW) ratio in Robo1fl/fl and Myh6-MerCreMer mice after sham or TAC surgery. N=8 mice/group. F, Quantification of myocyte cross-sectional area on WGA staining. G, Quantitative PCR (qPCR) analysis of transcription of hypertrophy-related genes (Nppa, Nppb, Myh7, and Acta1, normalized to Gapdh mRNA levels) in hearts from Robo1fl/fl or Myh6-MerCreMer;Robo1fl/fl mice after sham or TAC surgery. Two-way ANOVA and the Tukey multiple comparisons test were used for all comparisons.
DISCUSSION
While SLIT3 is known to partake in the embryonic development of the heart and other organs and is expressed in the adult state, the function of SLIT3 in the postnatal cardiovascular system has up to now remained unknown. Our previous study demonstrated that SLIT3 global knockout mice manifested a blunted fibrotic and hypertrophic response to pressure overload,24 a phenotype that could have been the result of faulty developmental processes. In the present study, we provide several lines of evidence in humans and mice to demonstrate SLIT3’s essential role in regulating the hypertrophic and fibrotic response of the adult myocardium. To exclude the effects of SLIT3 deficiency on cardiovascular development in our postnatal studies, we generated inducible and stromal cell–specific SLIT3 knockout mice. Conditional and inducible deletion of SLIT3 in fibroblasts or vascular mural cells alleviated the development of cardiac hypertrophy, fibrosis, and dysfunction after TAC, phenocopying the response in SLIT3 global knockout mice and confirming the stromal cell origin of the SLIT3 ligand in the adult heart.
Factors expressed by CNMs are known to regulate cardiac function during healthy and disease states,53–55 and our findings indicate that SLIT3 is a critical cardiac stromal cell-derived paracrine factor that regulates the cardiomyocyte response to pressure overload. Our in vitro results reveal that SLIT3 secreted by cardiac fibroblasts and vascular mural cells can directly stimulate cardiomyocyte hypertrophy, an effect that could be blunted by an anti-SLIT3 neutralizing antibody. While cardiac fibroblasts are known to modulate the hypertrophic response,56–58 the influence of vascular mural cells such as cardiac pericytes, a prevalent type of cardiac stromal cell,59 on cardiac homeostasis and hypertrophy is poorly understood.60 We provide the first evidence that vascular mural cells regulate the cardiomyocyte hypertrophic response to pressure overload via the secretion of SLIT3. This finding expands the known functional repertoire of cardiac vascular mural cells beyond that of vascular support61 and control of vascular tone.61,62
The cardiovascular phenotype that resulted from SLIT3 deletion was dependent on the cellular sources that were targeted. Interestingly, global and stromal cell–specific deletion of SLIT3 inhibited pressure overload–induced cardiac hypertrophy to similar degrees. The absence of a linear and directly proportional effect on cardiac hypertrophy in the context of tissue-specific SLIT3 knockouts indicates a more complex relationship. Several alternative explanations could account for our findings. For instance, deleting SLIT3 in one cell type (eg, cardiac fibroblasts) might alter the biomechanical properties of the myocardium, thereby influencing SLIT3 transcription in another cell population (eg, vascular mural cells). Conversely, removing SLIT3 in a different cell population (eg, vascular mural cells) could trigger the release of an unknown factor that then regulates SLIT3 transcription in another cell population (eg, cardiac fibroblasts). Another possibility is that a threshold amount of SLIT3 is needed to regulate hypertrophy. This on/off response has been previously documented for Myh6 and Myh7 transcription in response to TAC.63 Addressing these hypotheses necessitates further investigation into the factors governing SLIT3 transcription in these specific cell types, a significant and intricate topic that extends well beyond the scope of our current study.
A more stoichiometric relationship was observed between SLIT3 and cardiac collagen. Only acute, global deletion of SLIT3 resulted in decreased fibrosis-related gene transcription under both basal and pressure overload conditions. Fibroblast-specific deletion of SLIT3 reduced Col1a1 and Col3a1 transcription induced by LV pressure overload, while vascular mural cell–specific deletion of SLIT3 only reduced Col1a1 transcription after TAC. On the other hand, interfering with SLIT3 signaling by ROBO1 deletion in cardiomyocytes only reduced hypertrophy while not impacting cardiac fibrosis. Our results provide important insight into how SLIT3, a cardiac stromal cell-derived factor, can stimulate both components of adverse cardiac remodeling: cardiac fibrosis and cardiac hypertrophy.
Determining the cell-surface receptor for SLIT3 is necessary to understand how this secreted ligand imparts its hypertrophic effects on cardiomyocytes. Full-length SLIT proteins can be cleaved into 2 bioactive N- and C-terminal fragments.64 SLIT fragments have different cell association characteristics in cell culture, suggesting that they may also have different extents of diffusion and binding properties and, hence, different functional activities in vivo.64 ROBO-mediated signaling is initiated by the N-terminal fragment of SLIT ligands, while the C-terminal fragment of SLITs has been shown to bind PlexinA1.30 Our initial results demonstrated that SLIT3-NT is responsible for the hypertrophic effects of SLIT3 on cardiomyocytes, suggesting the involvement of a roundabout receptor. We found that ROBO1 was predominantly expressed in cardiomyocytes compared with the other roundabout receptors, and the ROBO1 expression was stimulated in hearts after TAC, paralleling the induction of SLIT3 expression. Importantly, the deletion of ROBO1 in cardiomyocytes recapitulated the phenotypes exhibited in SLIT3 global, conditional, and tissue-specific knockout mice. Thus, we have provided several lines of evidence to indicate that ROBO1 mediates the regulatory effects of SLIT3 in cardiomyocytes. As such, the current study is the first to describe the role of SLIT3-ROBO1 signaling in postnatal cardiac function and disease. We did not observe any other clinical phenotypes in other organ systems at homeostasis or under pressure overload conditions although we did not perform a detailed microscopic or molecular investigation into other extracardiac tissues and organs in SLIT3 deficient mice.
In addition to those highlighted above, there are also other important, unanswered questions about the role of SLIT3-ROBO1 in postnatal cardiac function. Cardiac fibroblasts and vascular mural cells are heterogeneous populations of cells, and the subpopulations expressing SLIT3 have yet to be identified. The signaling pathways downstream of SLIT3-ROBO1 leading to the hypertrophic response in cardiomyocytes remain undefined. The importance of SLIT3 in regulating physiological hypertrophy of exercise or pregnancy is also unknown and will need a different animal model to evaluate this. The details of how SLIT3 modulates adult cardiac fibroblast function are also unknown and are currently under active investigation in our laboratory. Finally, the mechanisms by which pathological stimuli promote SLIT3 secretion from stromal cells and the roles of SLIT3 in other types of cardiovascular conditions, such as coronary artery and valvular heart disease, are unknown.
In conclusion, the secreted axon guidance molecule SLIT3 controls pressure overload–induced cardiac fibrosis and cardiomyocyte hypertrophy, with the latter via the ROBO1 receptor. These findings highlight SLIT3 as a novel mediator of pathological cardiac hypertrophy and fibrosis in the postnatal period, expanding the functional repertoire of this conserved glycoprotein ligand.
ARTICLE INFORMATION
Acknowledgments
The authors acknowledge the University of Michigan Transgenic Animal Model Core for the production of the Robo1fl/fl mice. The authors also acknowledge the statistical consultation services from the Advanced Research Computing Group, University of California, Los Angeles.
Sources of Funding
The research reported in this publication was supported by the National Heart Lung and Blood Institute of the National Institutes of Health (NIH) under award numbers K08HL146351 and R01HL160730-01 (to M.S. Si) and R01HL153853-01 (M. Touma), a grant from the Children’s Health Foundation (to M.S. Si), a University of Michigan Frankel Cardiovascular Center McKay Award (to M.S. Si), the Scheutz and Peace families, the University of Michigan Department of Cardiac Surgery, and the Department of Surgery, University of California, Los Angeles.
Disclosures
None.
Supplementary Material
Nonstandard Abbreviations and Acronyms
- CNM
- cardiac nonmyocyte
- Fn
- fibronectin
- LV
- left ventricle
- NG2
- neural/glial antigen 2
- NRCM
- neonatal rat cardiomyocyte
- PDGFRA
- platelet-derived growth factor receptor A
- PDGFRB
- platelet-derived growth factor receptor B
- POSTN
- periostin
- TAC
- transverse aortic constriction
- WT
- wild type
X. Liu and B. Li share the first authorship.
For Sources of Funding and Disclosures, see page 929.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/CIRCRESAHA.122.321292.
REFERENCES
- 1.Frangogiannis NG. The extracellular matrix in ischemic and nonischemic heart failure. Circ Res. 2019;125:117–146. doi: 10.1161/CIRCRESAHA.119.311148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Boer RA, De Keulenaer G, Bauersachs J, Brutsaert D, Cleland JG, Diez J, Du XJ, Ford P, Heinzel FR, Lipson KE, et al. Towards better definition, quantification and treatment of fibrosis in heart failure. A scientific roadmap by the committee of translational research of the Heart Failure Association (HFA) of the European Society of Cardiology. Eur J Heart Fail. 2019;21:272–285. doi: 10.1002/ejhf.1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meagher P, Adam M, Civitarese R, Bugyei-Twum A, Connelly KA. Heart failure with preserved ejection fraction in diabetes: mechanisms and management. Can J Cardiol. 2018;34:632–643. doi: 10.1016/j.cjca.2018.02.026 [DOI] [PubMed] [Google Scholar]
- 4.Sharma K, Kass DA. Heart failure with preserved ejection fraction: mechanisms, clinical features, and therapies. Circ Res. 2014;115:79–96. doi: 10.1161/CIRCRESAHA.115.302922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ytrehus K, Hulot JS, Perrino C, Schiattarella GG, Madonna R. Perivascular fibrosis and the microvasculature of the heart. Still hidden secrets of pathophysiology? Vascul Pharmacol. 2018;107:78–83. doi: 10.1016/j.vph.2018.04.007 [DOI] [PubMed] [Google Scholar]
- 6.Nakamura M, Sadoshima J. Mechanisms of physiological and pathological cardiac hypertrophy. Nat Rev Cardiol. 2018;15:387–407. doi: 10.1038/s41569-018-0007-y [DOI] [PubMed] [Google Scholar]
- 7.Frangogiannis NG. Cardiac fibrosis. Cardiovasc Res. 2021;117:1450–1488. doi: 10.1093/cvr/cvaa324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weeks KL, McMullen JR. The athlete’s heart vs. the failing heart: can signaling explain the two distinct outcomes? Physiology (Bethesda). 2011;26:97–105. doi: 10.1152/physiol.00043.2010 [DOI] [PubMed] [Google Scholar]
- 9.Wang H, Xie Y, Guan L, Elkin K, Xiao J. Targets identified from exercised heart: killing multiple birds with one stone. NPJ Regen Med. 2021;6:23. doi: 10.1038/s41536-021-00128-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li J, Umar S, Amjedi M, Iorga A, Sharma S, Nadadur RD, Regitz-Zagrosek V, Eghbali M. New frontiers in heart hypertrophy during pregnancy. Am J Cardiovasc Dis. 2012;2:192–207. [PMC free article] [PubMed] [Google Scholar]
- 11.Oldfield CJ, Duhamel TA, Dhalla NS. Mechanisms for the transition from physiological to pathological cardiac hypertrophy. Can J Physiol Pharmacol. 2020;98:74–84. doi: 10.1139/cjpp-2019-0566 [DOI] [PubMed] [Google Scholar]
- 12.Bernardo BC, Weeks KL, Pretorius L, McMullen JR. Molecular distinction between physiological and pathological cardiac hypertrophy: experimental findings and therapeutic strategies. Pharmacol Ther. 2010;128:191–227. doi: 10.1016/j.pharmthera.2010.04.005 [DOI] [PubMed] [Google Scholar]
- 13.Diez J. Arterial hypertension in patients with heart failure. Heart Fail Clin. 2014;10:233–242. doi: 10.1016/j.hfc.2013.12.004 [DOI] [PubMed] [Google Scholar]
- 14.Frohlich ED, Gonzalez A, Diez J. Hypertensive left ventricular hypertrophy risk: beyond adaptive cardiomyocytic hypertrophy. J Hypertens. 2011;29:17–26. doi: 10.1097/HJH.0b013e328340d787 [DOI] [PubMed] [Google Scholar]
- 15.Levy D, Anderson KM, Savage DD, Balkus SA, Kannel WB, Castelli WP. Risk of ventricular arrhythmias in left ventricular hypertrophy: the Framingham Heart Study. Am J Cardiol. 1987;60:560–565. doi: 10.1016/0002-9149(87)90305-5 [DOI] [PubMed] [Google Scholar]
- 16.Ghali JK, Kadakia S, Cooper RS, Liao YL. Impact of left ventricular hypertrophy on ventricular arrhythmias in the absence of coronary artery disease. J Am Coll Cardiol. 1991;17:1277–1282. doi: 10.1016/s0735-1097(10)80135-4 [DOI] [PubMed] [Google Scholar]
- 17.Siegel D, Cheitlin MD, Black DM, Seeley D, Hearst N, Hulley SB. Risk of ventricular arrhythmias in hypertensive men with left ventricular hypertrophy. Am J Cardiol. 1990;65:742–747. doi: 10.1016/0002-9149(90)91381-f [DOI] [PubMed] [Google Scholar]
- 18.Schiattarella GG, Hill JA. Inhibition of hypertrophy is a good therapeutic strategy in ventricular pressure overload. Circulation. 2015;131:1435–1447. doi: 10.1161/CIRCULATIONAHA.115.013894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xie M, Burchfield JS, Hill JA. Pathological ventricular remodeling: therapies: part 2 of 2. Circulation. 2013;128:1021–1030. doi: 10.1161/CIRCULATIONAHA.113.001879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bisping E, Wakula P, Poteser M, Heinzel FR. Targeting cardiac hypertrophy: toward a causal heart failure therapy. J Cardiovasc Pharmacol. 2014;64:293–305. doi: 10.1097/FJC.0000000000000126 [DOI] [PubMed] [Google Scholar]
- 21.Heidenreich PA, Albert NM, Allen LA, Bluemke DA, Butler J, Fonarow GC, Ikonomidis JS, Khavjou O, Konstam MA, Maddox TM, et al. ; American Heart Association Advocacy Coordinating Committee. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6:606–619. doi: 10.1161/HHF.0b013e318291329a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nguyen BY, Azam T, Wang X. Cellular signaling cross-talk between different cardiac cell populations: an insight into the role of exosomes in the heart diseases and therapy. Am J Physiol Heart Circ Physiol. 2021;320:H1213–H1234. doi: 10.1152/ajpheart.00718.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tirziu D, Giordano FJ, Simons M. Cell communications in the heart. Circulation. 2010;122:928–937. doi: 10.1161/CIRCULATIONAHA.108.847731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gong L, Wang S, Shen L, Liu C, Shenouda M, Li B, Liu X, Shaw JA, Wineman AL, Yang Y, et al. SLIT3 deficiency attenuates pressure overload-induced cardiac fibrosis and remodeling. JCI Insight. 2020;5:e136852. doi: 10.1172/jci.insight.136852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kidd T, Bland KS, Goodman CS. Slit is the midline repellent for the robo receptor in Drosophila. Cell. 1999;96:785–794. doi: 10.1016/s0092-8674(00)80589-9 [DOI] [PubMed] [Google Scholar]
- 26.Blockus H, Chedotal A. Slit-Robo signaling. Development. 2016;143:3037–3044. doi: 10.1242/dev.132829 [DOI] [PubMed] [Google Scholar]
- 27.Mommersteeg MT, Yeh ML, Parnavelas JG, Andrews WD. Disrupted Slit-Robo signalling results in membranous ventricular septum defects and bicuspid aortic valves. Cardiovasc Res. 2015;106:55–66. doi: 10.1093/cvr/cvv040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mommersteeg MT, Andrews WD, Ypsilanti AR, Zelina P, Yeh ML, Norden J, Kispert A, Chedotal A, Christoffels VM, Parnavelas JG. Slit-roundabout signaling regulates the development of the cardiac systemic venous return and pericardium. Circ Res. 2013;112:465–475. doi: 10.1161/CIRCRESAHA.112.277426 [DOI] [PubMed] [Google Scholar]
- 29.Liu J, Zhang L, Wang D, Shen H, Jiang M, Mei P, Hayden PS, Sedor JR, Hu H. Congenital diaphragmatic hernia, kidney agenesis and cardiac defects associated with Slit3-deficiency in mice. Mech Dev. 2003;120:1059–1070. doi: 10.1016/s0925-4773(03)00161-8 [DOI] [PubMed] [Google Scholar]
- 30.Delloye-Bourgeois C, Jacquier A, Charoy C, Reynaud F, Nawabi H, Thoinet K, Kindbeiter K, Yoshida Y, Zagar Y, Kong Y, et al. PlexinA1 is a new Slit receptor and mediates axon guidance function of Slit C-terminal fragments. Nat Neurosci. 2015;18:36–45. doi: 10.1038/nn.3893 [DOI] [PubMed] [Google Scholar]
- 31.Medioni C, Bertrand N, Mesbah K, Hudry B, Dupays L, Wolstein O, Washkowitz AJ, Papaioannou VE, Mohun TJ, Harvey RP, et al. Expression of Slit and Robo genes in the developing mouse heart. Dev Dyn. 2010;239:3303–3311. doi: 10.1002/dvdy.22449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chowdhury UK, Jha A, Ray R, Kalaivani M, Hasija S, Kumari L, Chauhan A. Histopathology of the right ventricular outflow tract and the relation to hemodynamics in patients with repaired tetralogy of Fallot. J Thorac Cardiovasc Surg. 2019;158:1173–1183.e5. doi: 10.1016/j.jtcvs.2019.03.118 [DOI] [PubMed] [Google Scholar]
- 33.Hagdorn QAJ, Kurakula K, Koop AC, Bossers GPL, Mavrogiannis E, van Leusden T, van der Feen DE, de Boer RA, Goumans MTH, Berger RMF. Volume load-induced right ventricular failure in rats is not associated with myocardial fibrosis. Front Physiol. 2021;12:557514. doi: 10.3389/fphys.2021.557514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ventura A, Kirsch DG, McLaughlin ME, Tuveson DA, Grimm J, Lintault L, Newman J, Reczek EE, Weissleder R, Jacks T. Restoration of p53 function leads to tumour regression in vivo. Nature. 2007;445:661–665. doi: 10.1038/nature05541 [DOI] [PubMed] [Google Scholar]
- 35.Kim BJ, Lee YS, Lee SY, Baek WY, Choi YJ, Moon SA, Lee SH, Kim JE, Chang EJ, Kim EY, et al. Osteoclast-secreted SLIT3 coordinates bone resorption and formation. J Clin Invest. 2018;128:1429–1441. doi: 10.1172/JCI91086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ivey MJ, Kuwabara JT, Pai JT, Moore RE, Sun Z, Tallquist MD. Resident fibroblast expansion during cardiac growth and remodeling. J Mol Cell Cardiol. 2018;114:161–174. doi: 10.1016/j.yjmcc.2017.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kanisicak O, Khalil H, Ivey MJ, Karch J, Maliken BD, Correll RN, Brody MJ, Lin SCJ, Aronow BJ, Tallquist MD, et al. Genetic lineage tracing defines myofibroblast origin and function in the injured heart. Nat Commun. 2016;7:12260. doi: 10.1038/ncomms12260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Conway SJ, Molkentin JD. Periostin as a heterofunctional regulator of cardiac development and disease. Curr Genomics. 2008;9:548–555. doi: 10.2174/138920208786847917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Venugopal H, Hanna A, Humeres C, Frangogiannis NG. Properties and functions of fibroblasts and myofibroblasts in myocardial infarction. Cells. 2022;11:1386. doi: 10.3390/cells11091386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kamo T, Akazawa H, Komuro I. Cardiac nonmyocytes in the hub of cardiac hypertrophy. Circ Res. 2015;117:89–98. doi: 10.1161/CIRCRESAHA.117.305349 [DOI] [PubMed] [Google Scholar]
- 41.Skelly DA, Squiers GT, McLellan MA, Bolisetty MT, Robson P, Rosenthal NA, Pinto AR. Single-cell transcriptional profiling reveals cellular diversity and intercommunication in the mouse heart. Cell Rep. 2018;22:600–610. doi: 10.1016/j.celrep.2017.12.072 [DOI] [PubMed] [Google Scholar]
- 42.Tallquist MD, Molkentin JD. Redefining the identity of cardiac fibroblasts. Nat Rev Cardiol. 2017;14:484–491. doi: 10.1038/nrcardio.2017.57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003 [DOI] [PubMed] [Google Scholar]
- 44.Wang S, Huang S, Johnson S, Rosin V, Lee J, Colomb E, Witt R, Jaworski A, Weiss SJ, Si MS. Tissue-specific angiogenic and invasive properties of human neonatal thymus and bone MSCs: role of SLIT3-ROBO1. Stem Cells Transl Med. 2020;9:1102–1113. doi: 10.1002/sctm.19-0448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guimaraes-Camboa N, Cattaneo P, Sun Y, Moore-Morris T, Gu Y, Dalton ND, Rockenstein E, Masliah E, Peterson KL, Stallcup WB, et al. Pericytes of multiple organs do not behave as mesenchymal stem cells in vivo. Cell Stem Cell. 2017;20:345–359.e5. doi: 10.1016/j.stem.2016.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Foo SS, Turner CJ, Adams S, Compagni A, Aubyn D, Kogata N, Lindblom P, Shani M, Zicha D, Adams RH. Ephrin-B2 controls cell motility and adhesion during blood-vessel-wall assembly. Cell. 2006;124:161–173. doi: 10.1016/j.cell.2005.10.034 [DOI] [PubMed] [Google Scholar]
- 47.Ozerdem U, Grako KA, Dahlin-Huppe K, Monosov E, Stallcup WB. NG2 proteoglycan is expressed exclusively by mural cells during vascular morphogenesis. Dev Dyn. 2001;222:218–227. doi: 10.1002/dvdy.1200 [DOI] [PubMed] [Google Scholar]
- 48.Miano JM, Cserjesi P, Ligon KL, Periasamy M, Olson EN. Smooth muscle myosin heavy chain exclusively marks the smooth muscle lineage during mouse embryogenesis. Circ Res. 1994;75:803–812. doi: 10.1161/01.res.75.5.803 [DOI] [PubMed] [Google Scholar]
- 49.Patel K, Nash JA, Itoh A, Liu Z, Sundaresan V, Pini A. Slit proteins are not dominant chemorepellents for olfactory tract and spinal motor axons. Development. 2001;128:5031–5037. doi: 10.1242/dev.128.24.5031 [DOI] [PubMed] [Google Scholar]
- 50.Gara RK, Kumari S, Ganju A, Yallapu MM, Jaggi M, Chauhan SC. Slit/ROBO pathway: a promising therapeutic target for cancer. Drug Discov Today. 2015;20:156–164. doi: 10.1016/j.drudis.2014.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu Z, Patel K, Schmidt H, Andrews W, Pini A, Sundaresan V. Extracellular Ig domains 1 and 2 of ROBO are important for ligand (Slit) binding. Mol Cell Neurosci. 2004;26:232–240. doi: 10.1016/j.mcn.2004.01.002 [DOI] [PubMed] [Google Scholar]
- 52.Chen JH, Wen L, Dupuis S, Wu JY, Rao Y. The N-terminal leucine-rich regions in Slit are sufficient to repel olfactory bulb axons and subventricular zone neurons. J Neurosci. 2001;21:1548–1556. doi: 10.1523/JNEUROSCI.21-05-01548.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Howard CM, Baudino TA. Dynamic cell-cell and cell-ECM interactions in the heart. J Mol Cell Cardiol. 2014;70:19–26. doi: 10.1016/j.yjmcc.2013.10.006 [DOI] [PubMed] [Google Scholar]
- 54.Davis J, Molkentin JD. Myofibroblasts: trust your heart and let fate decide. J Mol Cell Cardiol. 2014;70:9–18. doi: 10.1016/j.yjmcc.2013.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fountoulaki K, Dagres N, Iliodromitis EK. Cellular communications in the heart. Card Fail Rev. 2015;1:64–68. doi: 10.15420/cfr.2015.1.2.64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xiang FL, Fang M, Yutzey KE. Loss of beta-catenin in resident cardiac fibroblasts attenuates fibrosis induced by pressure overload in mice. Nat Commun. 2017;8:712. doi: 10.1038/s41467-017-00840-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Takeda N, Manabe I, Uchino Y, Eguchi K, Matsumoto S, Nishimura S, Shindo T, Sano M, Otsu K, Snider P, et al. Cardiac fibroblasts are essential for the adaptive response of the murine heart to pressure overload. J Clin Invest. 2010;120:254–265. doi: 10.1172/JCI40295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moore-Morris T, Guimaraes-Camboa N, Banerjee I, Zambon AC, Kisseleva T, Velayoudon A, Stallcup WB, Gu Y, Dalton ND, Cedenilla M, et al. Resident fibroblast lineages mediate pressure overload-induced cardiac fibrosis. J Clin Invest. 2014;124:2921–2934. doi: 10.1172/JCI74783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nees S, Weiss DR, Senftl A, Knott M, Forch S, Schnurr M, Weyrich P, Juchem G. Isolation, bulk cultivation, and characterization of coronary microvascular pericytes: the second most frequent myocardial cell type in vitro. Am J Physiol Heart Circ Physiol. 2012;302:H69–H84. doi: 10.1152/ajpheart.00359.2011 [DOI] [PubMed] [Google Scholar]
- 60.Alex L, Frangogiannis NG. Pericytes in the infarcted heart. Vasc Biol. 2019;1:H23–H31. doi: 10.1530/VB-19-0007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sweeney M, Foldes G. It takes two: endothelial-perivascular cell cross-talk in vascular development and disease. Front Cardiovasc Med. 2018;5:154. doi: 10.3389/fcvm.2018.00154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.O’Farrell FM, Mastitskaya S, Hammond-Haley M, Freitas F, Wah WR, Attwell D. Capillary pericytes mediate coronary no-reflow after myocardial ischaemia. Elife. 2017;6:e29280. doi: 10.7554/eLife.29280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pandya K, Cowhig J, Brackhan J, Kim HS, Hagaman J, Rojas M, Carter CW, Jr, Mao L, Rockman HA, Maeda N, et al. Discordant on/off switching of gene expression in myocytes during cardiac hypertrophy in vivo. Proc Natl Acad Sci U S A. 2008;105:13063–13068. doi: 10.1073/pnas.0805120105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chedotal A. Slits and their receptors. Adv Exp Med Biol. 2007;621:65–80. doi: 10.1007/978-0-387-76715-4_5 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request. Please see the Supplemental Material for a detailed description.