Dear Editor,
We are writing in response to a recent article published by Mahmoud et al., which found that enteral nimodipine formulations are not equivalent across multiple endpoints, such as the incidence of diarrhea and need for dosage reduction. 1 We would like to share our experience of successfully implementing in-house compounding of nimodipine oral syringes, given the favorability found in the endpoints studied and associated cost savings.
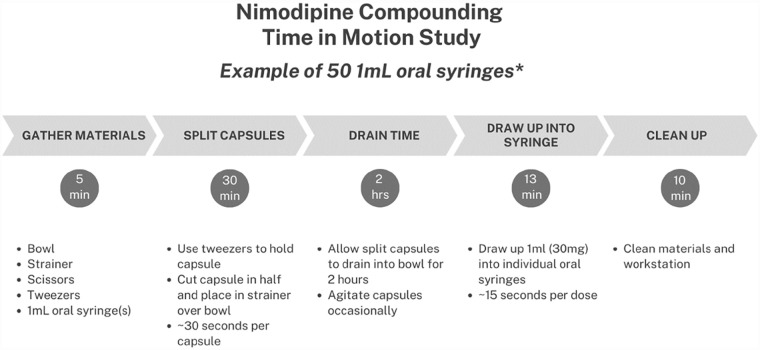
Pre-made nimodipine 6 mg/mL oral solution is commonly used in aneurysmal subarachnoid hemorrhage (aSAH) patients who require enteral administration of medications. While convenient, its high cost can be burdensome for hospitals and healthcare systems. Our facilities have been compounding nimodipine oral syringes in-house using commercially available capsules, which are more cost effective that the pre-made oral solution. The process and timing of each step are outlined in Figure 1. This approach not only circumvents the risk of inadvertent intravenous administration if performed at bedside and the associated black-box warning, but it also eliminates the need for time-consuming and potentially hazardous needle aspirations. Additionally, pharmacy-compounded syringes result in a more accurate yield compared to bedside extraction. 2
Figure 1.
Nimodipine compounding process and time in motion study.
*Note. Due to viscosity of medication there will be loss that must be accounted for in the compounding process. We noted a 15% volume loss in the product we used. Therefore, 50 doses would require 59 capsules to be compounded.
To implement this process at other hospitals, we recommend the following operational steps:
Confirm the nimodipine capsule gel concentration with the manufacturer.
Estimate the time and resources required for compounding based on the hospital’s specific needs (Figure 1).
Develop clear and standardized compounding processes (oral nimodipine syringes are stable for 31 days when stored in light-protected bags). 3
Obtain Pharmacy and Therapeutics approval and make necessary adjustments to ordering pathway in the electronic medical record.
By adopting this approach, other hospitals can achieve significant cost savings and avoid medication misadventures while maintaining the same level of quality care for aSAH patients. We encourage institutions to explore this option and adapt it to their specific needs and capabilities.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Keaton S. Smetana  https://orcid.org/0000-0002-4680-1116
https://orcid.org/0000-0002-4680-1116
References
- 1. Mahmoud SH, Hefny FR, Panos NG, et al. Comparison of nimodipine formulations and administration techniques via enteral feeding tubes in patients with aneurysmal subarachnoid hemorrhage: a multicenter retrospective cohort study. Pharmacotherapy. 2023;43(4):279-290. doi: 10.1002/phar.2791 [DOI] [PubMed] [Google Scholar]
- 2. Oyler DR, Stump SE, Cook AM. Accuracy of nimodipine gel extraction. Neurocrit Care. 2015;22(1):89-92. doi: 10.1007/s12028-014-0054-0 [DOI] [PubMed] [Google Scholar]
- 3. Green AE, Banks S, Jay M, Hatton J. Stability of nimodipine solution in oral syringes. Am J Health Syst Pharm. 2004;61(14):1493-1496. doi: 10.1093/ajhp/61.14.1493 [DOI] [PubMed] [Google Scholar]