Abstract
Purpose:
Investigate clinical and morphologic characteristics of serous retinal disturbances in patients taking extracellular signal-regulated kinase (ERK) inhibitors.
Participants:
Of 61 patients receiving ERK inhibitors for treatment of metastatic cancer, this study included 40 eyes of 20 patients with evidence of retinopathy confirmed by optical coherence tomography (OCT).
Design:
Single center, retrospective study of prospectively collected data
Methods:
Clinical exam, fundus photography and OCT were used to evaluate ERK inhibitor retinopathy. The morphology, distribution and location of fluid foci were serially evaluated for each eye. Visual acuity and choroidal thickness measurements were compared at baseline, fluid accumulation and resolution.
Main Outcome Measures:
Characteristcs of treatment-emergent choroid and retinal OCT abnormalities as compared to baseline OCT, and the impact of toxicity on visual acuity and final visual acuity.
Results:
Of 20 patients with retinopathy the majority of patients had fluid foci that were bilateral (100%), multifocal in each eye (75%) and at least one focus involving the fovea (95%). All subretinal fluid foci occurred between the interdigitation zone and an intact retinal pigment epithelium. There was no statistical difference in choroidal thickness at fluid accumulation and resolution compared to baseline. 45% eyes had evidence of concomitant intraretinal edema localized to the outer nuclear layer. At the time of fluid accumulation, 57.5% eyes had a decline in visual acuity (mainly by 1–2 lines from baseline). For all eyes with follow-up, the subretinal fluid and intraretinal edema was reversible and resolved without medical intervention; and best-corrected visual acuity at fluid resolution was not statistically different from baseline. Concomitant intraretinal fluid was not associated with worsening of visual acuity. No patient discontinued or decreased drug dose on account of their retinopathy.
Conclusion:
This study shows ERK inhibitors may cause subretinal fluid foci with unique clinical and morphologic characteristics. The observed foci are similar to MEK inhibitor associated retinopathy and distinct from central serous chorioretinopathy. However, unlike MEK inhibitors, there appears to be an increased occurrence of concomitant intraretinal fluid without significant additive visual impact. In this series, ERK inhibitors did not cause irreversible loss of vision or serious eye damage: retinopathy was self-limited and did not require medical intervention.
Precis
Extracellular signal-regulated kinase (ERK)-inhibitors are associated with self-limited serous detachments of the neuroretina and the morphologic and clinical characteristics are similar but also distinct from the retinopathy associated with Mitogen-activated protein kinase kinase (MEK)-inhibitors.
Introduction
The Mitogen-activated protein kinase (MAPK) pathway (also known as the Ras-Raf-MEK-ERK pathway) represents a cascade of proteins that transmits signals from cell surface receptors to the nucleus to alter DNA transcription. The signaling cascade controls many cell actions that impact cellular proliferation and survival. Many cancers are driven by dysregulation of MAPK, making them amenable to treatment with targeted agents that block this pathway, such as extracellular signal-regulated kinase (ERK) inhibitors or mitogen-activated protein kinase kinase (MEK) inhibitors1–4 5,6. As of April 2021, Federal Drug Administration (FDA) approved MEK inhibitors include trametinib, selumetinib, cobimetinib, binimetinib; while ERK inhibitor, ulixertinib, has Access been granted an Expanded Program by the FDA and others are in phase 1 or 2 clinical trials alone or in combination. Although spontaneous ERK mutations are uncommon in human cancers, oncogenic mutations involving other MAPK pathway genes (RAS, BRAF or MEK) are frequent and converge on ERK activation. ERK inhibition is therefore a rational strategy to block this aberrant malignancy-driving pathway6.
MEK inhibitors are associated with serous elevations of the retina initially thought to be akin to central serous choroidopathy but now recognized to be a unique ocular toxicity spectrum designated MEK inhibitor-associated retinopathy7–13. ERK is downstream of MEK in the MAPK pathway, suggesting ERK inhibitors may share a similar toxicity profile with MEK inhibitors. To this point, in phase I studies of ERK inhibitors, the reported ocular signs and symptoms are “halo vision”, “retinal detachment”, “retinopathy”, “chorioretinopathy”,6, 14 which are suggestive of retinopathy with fluid foci.
In an effort better understand this topic, this study systematically explored ERK inhibitor retinopathy observed in patients undergoing ERK inhibitor treatment within prospective clinical trials. There has been one case reported of ERK inhibitor retinopathy15 and we report 40 eyes of 20 subjects with this finding. In the present study, clinical and morphologic characteristics were evaluated along with the associated retinal, RPE and choroidal changes of the fluid foci. In the present study, clinical and morphologic characteristics were evaluated along with the associated retinal, RPE and choroidal changes of the fluid foci that were then compared with MEK inhibitor retinopathy.
Methods
The study adhered to the tenets of the Declaration of Helsinki and was approved by the Institutional Review Board of Memorial Sloan Kettering Cancer Center. A literature search of published reports on retinal toxicity related to ERK inhibitors was conducted through PubMed using the search terms “retina” and “ERK inhibitor” or “extracellular signal-regulated kinase inhibitor” or “ulixertinib” or “LY3214996” or “LTT462” or “BVD-523” or KO-947”. This retrospective, single-center study included 61 patients recruited from Memorial Sloan Kettering Cancer Center, New York between May 2014 and August 2020. Patients were enrolled in an ERK inhibitor (ulixertinib/BVD-5236, investigational ERK inhibitor and KO-947 (NCT03051035)) study for treatment of their metastatic cancer and, as per the primary therapeutic protocol, ophthalmic examinations were conducted at baseline and at required 6–12-week intervals or if symptomatic. All data were collected prospectively. Additional demographics and patient specific data were extracted retrospectively under an IRB approved protocol. Twenty patients exhibited subretinal fluid on optical coherence tomography in one or both eyes and were included in the present study. Ocular findings that fulfilled the criteria for an adverse event were defined and graded by the Common Terminology for Adverse Events version 4.03.
Examination
All enrolled patients received an ophthalmological examination complete with best-corrected visual acuity, automated refraction, intraocular pressure, dilated fundus examination and fundus photography. Enhanced Depth Imaging Optical Coherence Tomography (EDI-OCT) images were obtained with the Heidelberg Spectralis HRA+OCT (Heidelberg Engineering). A 9mm scan and a 32-line cross scan pattern (in the horizontal direction, each consisting of a maximum of 50 averaged scans) was used. Patients were examined at baseline, followed by exams either required by the study protocol, or if the patient was symptomatic.
Data collection
Demographic data were collected on each patient including gender, age and primary cancer diagnosis. Treatment data included the initial drug, dose, route, frequency, duration, number of cycles, concomitant drugs and any alterations in this plan over the treatment course. Comorbidities or other contributing factors were also recorded such as diabetes, hypertension, kidney disease, cortisol use, sildenafil use, gastroesophageal reflex disease. Clinical data included best-corrected visual acuity (in Snellen and logMAR) at baseline, fluid accumulation and fluid resolution and whether the patient was symptomatic at the time of fluid accumulation. Further data included time from medication start to initial subretinal fluid detection by OCT, the cycle number during which subretinal fluid was initially detected by OCT and time to resolution of subretinal fluid by OCT (evaluable in 22 eyes).
By OCT, the foci of fluid were carefully examined for each eye: details on the number of foci, laterality of foci, location within the fundus, location within OCT layers, configuration/morphology of fluid, caliber of the OCT layers and other chorioretinal abnormalities (intraretinal cysts, presence of pigment epithelial detachment, hyperreflective dots) were all recorded. Two independent observers performed grading of the fluid foci morphology.
Choroidal thickness was measured on EDI-OCT imaging with the caliper tool, as the vertical distance from the hyper-reflective line (corresponding to Bruch’s membrane) to the chorioscleral border. Choroidal thickness was compared from baseline to fluid accumulation (evaluable in 38 eyes), fluid accumulation to resolution (evaluable in 22 eyes) and baseline to fluid resolution (evaluable in 22 eyes). Eyes were deemed unevaluable if no images were available or if the OCT image was not enhanced-depth, which is preferred for assessment of choroidal thickness.
Statistical analysis
Choroidal thickness was expressed as mean ± standard error of the mean (SEM). Choroidal thickness was analyzed with a two-tailed paired t-test and confirmed with a 2-way analysis of variance (ANOVA). Two-tailed Fisher exact test was used to compare categorical variables. A p-value less than or equal to 0.05 was considered statistically significant. Statistical analysis was performed using on GraphPad Software, Inc. (La Jolla, CA).
Results
Of the 61 patients on ERK inhibition, 20 patients (40 eyes) exhibited ERK-inhibitor associated retinopathy and were included. For each ERK inhibitor, the proportion of patients with ERK inhibitor retinopathy was as follows: 13.5% (5 of 37 patients) on ulixertinib/BVD-523, 60% (6 of 10 patients) on investigational ERK inhibitor and 64% (9 of 14 patients) on KO-947. Details regarding patient characteristics and drug information are provided in Table 1. The main primary cancer diagnoses included colorectal (6) melanoma (3), lung (3) and prostate (2), etc. No patient was on concomitant drug therapy nor radiation for their cancer. Of the 20 patients with retinopathy, no patient was a diabetic nor on oral steroid, 3 patients had controlled hypertension and 3 patients were on pantoprazole. The mean patient age was 54 years (median 62 years, range 15–75 years). Twelve of 20 (60%) patients were female.
Table 1.
Patient Characteristics and Drug Information for Study Patients
| Patient No. | Gender | Age (yrs) | Primary Malignancy | Drug | Dose (mg) | Route | Cycle Duration (days) | Cycle Interval | Schedule | Cycle No. When Subretinal Fluid First Detected | Duration to Subretinal Fluid Detection (days) | Symptoms* | Dead or Alive |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 65 | Endometrial | Inv. ERKi | 200 | Oral | 7 | Every 4 wks | Twice daily | Cycle 1, day 5 | 5 | Yes* | Alive |
| 2 | M | 63 | Prostate | Ulixertinib | 600 | Oral | 7 | Every 1 wk | Twice daily | Cycle 1, day 15 | 15 | No | Dead |
| 3 | F | 20 | Glioma | KO-947 | 420 | IV | 1 | Every 1 wk | Daily | Cycle 5, day 23 | 143 | No | Alive |
| 4 | F | 62 | Glioblastoma | Ulixertinib | 600 | Oral | 7 | Every 1 wk | Twice daily | Cycle 1, day 11 | 11 | Yes* | Dead |
| 5 | F | 62 | Colorectal | KO-947 | 460 | IV | 1 | Every 1 wk | Daily | Cycle 1, day 2 | 2 | Yes† | Dead |
| 6 | F | 67 | Colorectal | KO-947 | 260 | IV | 1 | Every 1 wk | Daily | Cycle 1, day 28 | 28 | No | Dead |
| 7 | F | 54 | Colorectal | Inv. ERKi | 400 | Oral | 7 | Every 4 wks | Daily | Cycle 3, day 27 | 87 | Yes* | Dead |
| 8 | F | 75 | Melanoma | Inv. ERKi | 300 | Oral | 7 | Every 4 wks | Daily | Cycle 1, day 15 | 15 | Yes† | Dead |
| 9 | M | 22 | Melanoma | Ulixertinib | 600 | Oral | 7 | Every 1 wk | Twice daily | Cycle 1, day 8 | 8 | No | Dead |
| 10 | F | 72 | Lung | Ulixertinib | 600 | Oral | 7 | Every 1 wk | Twice daily | Cycle 1, day 8 | 8 | No | Dead |
| 11 | M | 62 | Prostate | Ulixertinib | 600 | Oral | 7 | Every 1 wk | Twice daily | Cycle 1, day 8 | 8 | No | Alive |
| 12 | M | 68 | Nasopharyngeal SCC | KO-947 | 420 | IV | 1 | Every 1 wk | Daily | Cycle 1, day 2 | 2 | No | Dead |
| 13 | M | 50 | Cholangiocarcinoma | KO-947 | 650 | IV | 1 | Every 1 wk | Daily | Cycle 1, day 2 | 2 | Yes* | Alive |
| 14 | F | 38 | Colorectal | Inv. ERKi | 600 | Oral | 7 | 4 wks | Daily | Cycle 1, day 7 | 7 | No | Dead |
| 15 | M | 53 | Colorectal | KO-947 | 750 | IV | 1 | Every 1 wk | Daily | Cycle 1. day 2 | 2 | Yes* | Dead |
| 16 | M | 16 | Melanoma | Inv. ERKi | 300 | Oral | 7 | Every 4 wks | Daily | Cycle 5, day 1 | 121 | No | Alive |
| 17 | F | 70 | Lung | Inv. ERKi | 200 | Oral | 7 | Every 4 wks | Daily | Cycle 1, day 4 | 4 | No | Alive |
| 18 | M | 63 | Lung | KO-947 | 380 | IV | 1 | Every 1 wk | Daily | Cycle 5, day 1 | 121 | Yes* | Dead |
| 19 | F | 44 | Cervical | KO-947 | 370 | IV | 1 | Every 1 wk | Daily | Cycle 1, day 2 | 2 | No | Alive |
| 20 | F | 48 | Colorectal | KO-947 | 1150 | IV | 1 | Every 1 wk | Daily | Cycle 1, day 3 | 3 | Yes* | Alive |
F = female; Inv. ERKi = investigational extracellular singal-regulated kinase inhibitor, IV = intravenous; M = male; SCC = squamous cell carcinoma; SRF = suberinal fluid.
Blurry vision.
Metamorphopsia.
80% (16 of 20 patients) of ERK inhibitor retinopathy was detected during the first cycle of drug. The median time from medication start to initial subretinal fluid detection by OCT was 8 days (mean 28.0 days, range 2–143). The median time to resolution of the subretinal fluid by OCT was 23 days (n=28, mean 35.5 days, range 6–132 days). 50% were treated at the drugs maximum tolerated dose and 50% were treated at lower doses. Dose of drug was not significantly associated with the detection of subretinal fluid (p-value = 0.1) nor intraretinal fluid (p-value = 0.6). Retinopathy occurred at the same time for both eyes. No patient decreased dose nor discontinued drug temporarily or permanently solely on account of their retinopathy. In all eyes with follow-up, the fluid was self-limiting and did not require medical intervention.
Clinical characteristics of subretinal and intraretinal fluid
Details on the location and number of fluid foci per eye are schematically outlined in Figure 1. The foci of fluid appeared as grey-yellow elevations of the fundus (yellow in 4 eyes, grey in 36 eyes), either in a circular shape or in the configuration of non-gravitational globules without inferior fluid tracking (Figure 2). All patients (100%) had subretinal fluid in both eyes.
Figure 1:
Schematic diagram of 40 eyes of 20 patients showing the location, size and configuration of each fluid focus: blue = dome, green = caterpillar, red = wavy, yellow = splitting, grey = multifoil. Number represents patient number and circle designates those patients with visual symptoms. X at fovea designates the presence of intraretinal edema. Note the predominantly bilateral, multifocal involvement of the foci and relative symmetry between each eye.
Figure 2:
A representative case (patient 8) demonstrating fundus findings at time of fluid accumulation. Left column = right eye, right column = left eye. (A) Color fundus photograph showing placoid vitelliform lesions along the arcades and in the fovea corresponding to fluid foci. (B) These foci demonstrate hyper-autofluorescence. (C) Optical coherence tomography of macula demonstrating subretinal dome fluid with intraretinal edema in the outer nuclear layer. Note the absence of pigment epithelial detachments and hyperreflectile dots.
There were multiple foci of subretinal fluid in 35 of 40 eyes (75%). All eyes but two eyes had at least one foci of subretinal fluid involving the fovea. The mean number of fluid foci per eye was 5.6 (range 1 to 14). Note that the location and number of foci was relatively symmetric between both eyes. Eighteen of 40 (45%) of eyes had concomitant intraretinal cystic edema, which resolved in all cases.
Visual Acuity
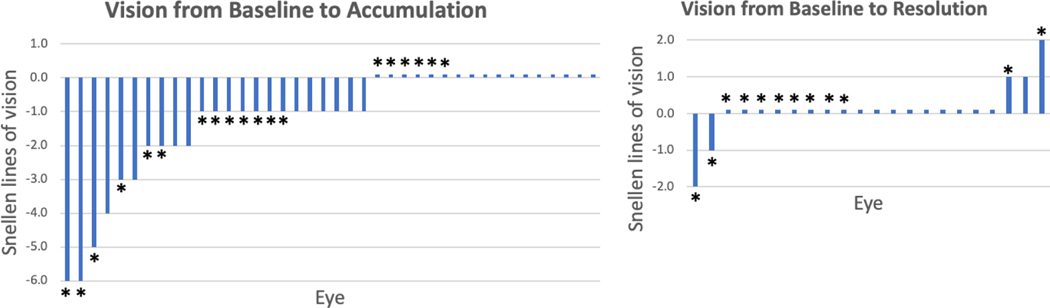
Nine patients (45%) reported symptoms at the time of fluid accumulation. In all evaluable eyes, the BCVA ranged: at baseline between 20/20 and NLP, at fluid accumulation 20/20 to NLP and at fluid resolution 20/20 to NLP. The change in number of lines of best-corrected Snellen visual acuity (BCVA) for all eyes from baseline to fluid accumulation and baseline to fluid resolution are depicted in Figure 3. At the time of fluid accumulation, 57.5% eyes had a decline in visual acuity: by no more than 6 lines of Snellen. 74% of eyes lost no more than 2 Snellen lines from baseline. At time of fluid resolution, no eye was less than two Snellen line from its baseline vision. The mean and median logMAR BCVA was: at baseline 0.07 and 0, at fluid accumulation 0.18 and 0.1, and at fluid resolution 0.06 and 0. The logMAR BCVA at fluid accumulation was significantly worse than baseline (p<0.001), but logMAR BCVA at fluid resolution was not significantly different from baseline (p=0.72). The presence of intraretinal cystic edema was not significantly associated with worse vision (p-value = 0.46).
Figure 3:
Visual acuity changes at fluid accumulation and resolution. A) Waterfall plot demonstrating change in lines of Snellen visual acuity lines from baseline to fluid accumulation in 40 eyes. B) Waterfall plot demonstrating change in lines of Snellen visual acuity from baseline to fluid resolution in 22 eyes. No statistically significant difference was observed between baseline and at fluid resolution. * = eyes with intraretinal edema
OCT characteristics of subretinal and intraretinal fluid
The OCT morphology of the subretinal fluid could be divided into four types as depicted in figures 1 and 3 and as previously described 16. These fluid morphologies occurred with the following frequency: The 225 fluid foci were classified into four morphologies as follows: 109 (48.4%) dome, 37 (16.4%) caterpillar, 52 (23.1%) wavy and 18 (8%) splitting. Another fluid configuration was observed in the subfoveal foci of 9 eyes (4%) and was termed “multifoil” because the neurosensory detachment simulated the undulating shape of a multifoil arch (Figure 4).
Figure 4:
Two representative cases (patient 14 on right, patient 3 on left) demonstrating the “multifoil” configuration of subretinal fluid. Note the multiple undulations of the detached neurosensory retina simulating a multifoil arch.
In all foci, the accumulation of the fluid occurred between the retinal pigment epithelium and the interdigitation zone (Figure 5). There were neither pigment epithelial detachments nor intraretinal or choroidal hyperreflective dots detected. The intraretinal cystic edema noted in 9 eyes occurred in the outer nuclear layer. In 28 of 40 eyes (70%), the subfoveal dome-shaped fluid foci exhibited elongation of the interdigitation zone. In all eyes, the IZ could be distinguished from the RPE and EZ at the time of fluid accumulation; the IZ could not be distinguished from the RPE at baseline, but became apparent with the accumulation of sub-IZ fluid. In all eyes, the RPE, IZ and ellipsoid zone layers remained hyper-reflectile, both at the time of fluid accumulation and its resolution. None of the fluid foci were associated with retinal pigment epithelial changes at fluid resolution.
Figure 5:

A representative case (patient 15) demonstrating optical coherence tomography findings at baseline, fluid accumulation and its resolution. Left column = right eye, right column = left eye. (A) Baseline optical coherence tomography showing normal retinal, retinal pigment epithelium (RPE) and choroidal structures. Note the difficulty in fully distinguishing the interdigitation zone (IZ) from the RPE and ellipsoid zone (EZ). (B) One day following first weekly dose of ERK-inhibitor fluid foci are detected in both eyes in dome configuration, fluid between RPE and IZ (“splitting”) with intraretinal edema in outer nuclear layer (ONL). (C) One week following first weekly dose, the fluid foci and intraretinal edema are resolving in both eyes. (D) One day after fourth weekly dose, the fluid foci and intraretinal edema have recurred. The IZ is elongated and both the IZ and EZ remain distinguishable and hyperreflectile. (E) One week following fourth weekly dose, the fluid foci and intraretinal edema are resolving in both eyes. (F) Two weeks after final sixth weekly dose, the retinal layers resume their normal appearance.
Choroidal thickness
There was no statistical difference between the mean choroidal thickness at baseline (246.0 ± 67.1μm) and during fluid accumulation (244.1 ± 67.3μm), (p=0.9, n= 38 eyes). In addition, there was no statistical difference between the mean choroidal thickness during fluid accumulation and at fluid resolution (243.5 ± 65.7μm), (p=0.97, n=22 eyes). Finally, there was no statistical difference between the mean choroidal thickness at baseline and at fluid resolution (p=0.89, n=22 eyes).
Other imaging
Autofluoresence was available in 18 eyes: the fluid foci had no abnormal autofluoresence in 10 eyes and demonstrated hyper-autofluorescence corresponding to the fluid foci in 8 eyes (Figure 2).
Discussion
Dysregulation of the MAPK pathway is common in cancer and can be susceptible to treatment with targeted drugs that block this pathway, such as ERK and MEK inhibitors. ERK is an attractive target because it is the most downstream kinase in the pathway. As the efficacy of ERK inhibition is gaining interest, so too are the toxic effects of this drug class, including the impact on the eye. The retinopathy that is associated with MEK inhibitors is well established. Given that ERK inhibitors work downstream in the same pathway, it follows that the toxicity profile may be analogous, as phase I study data has suggested.6,14
As would be expected, ERK inhibitor retinopathy shares many similarities to MEK inhibitor retinopathy. Both have clinical findings of bilateral, multifocal serous elevations typically involving the fovea. Extrafoveal foci appear to accumulate near the arcades and with relative symmetry between both eyes. On fundoscopy these fluid foci can appear as grey or sometimes yellow elevations. By OCT, the fluid accumulates between the RPE and IZ (which has been referred to as the vitelliform space), and the IZ may display elongation. The RPE, IZ and ellipsoid zone remain hyperreflective and clearly distinguishable at the time of fluid accumulation and reabsorption.
The clinical course of retinopathy from ERK inhibitors parallels that from MEK inhibitors. The vast majority of patients will have detectable retinopathy during the first cycle of drug. Approximately half the patients are asymptomatic despite fluid beneath the fovea of both eyes (patients were seen as part of a protocol, allowing even the asymptomatic eyes to be detected). The asymptomatic fluid can be explained by the intact outer retinal components and patient accommodation through the hyperopic shift created by the fluid elevation. The retinopathy self-resolves in all patients with follow-up and without medical intervention; and without drug dose-reduction nor discontinuation in any patient on account of their retinopathy. With both drug classes, the vision has the potential to decline during fluid accumulation, but returns to baseline with fluid resolution. We did note that ERK inhibitor retinopathy eyes lost more vision at the time of fluid accumulation (up to six Snellen lines), compared to MEK inhibitor retinopathy eyes (no more than 2 Snellen lines)16 (Figure 3). However, both have similar potential for vision reversal with fluid resolution. Both the subretinal and intraretinal findings, would suggest that ERK (and MEK) are important in maintaining retinal homeostasis. The mechanism for this is unknown but it has been shown that both the neuroretina and retinal pigment epithelium express ERK; and it has been suggested that reversible suppression of ERK may lead to disturbances in the neuroretina-RPE interaction, thereby resulting in fluid accumulation17.
In early trials, MEK inhibitor retinopathy was inaccurately described as being similar to central serous chorioretinopathy (CSC); however, expanded literature has revealed these disease entities are distinct. Following suit with MEK inhibitor retinopathy, ERK inhibitor retinopathy is also dissimilar to CSC. The bilateral, multifocal non-gravitational rounded globules do not display the fluid tracking or gutter that can be characteristics of CSC. In contrast to CSC, ERK inhibitor retinopathy exclusively accumulates in the sub-IZ space: often revealing this space in eyes with an IZ indistinguishable from the RPE at baseline. Unique from CSC, the RPE, IZ and EZ remain intact and hyperreflectile during fluid accumulation and undisturbed at resolution. Other OCT characteristics commonly found in CSC (pigment epithelial detachments, intraretinal, pachychoroid and choroidal hyperreflective dots) are not detected with ERK inhibitor retinopathy.
There are a few features that appear to be unique to ERK inhibitor retinopathy, compared to MEK inhibitor retinopathy. One if these is the higher propensity of concomitant intraretinal edema at the level of the ONL. In our cohort of MEK inhibitor retinopathy, only a single eye had an intraretinal cyst 16, compared to 45% of eyes receiving ERK inhibition (p-value < 0.001). We label this abnormality as “edema” but also recognize that it may represent an alteration in cell-to-cell adhesion, deposition of material, schisis or perhaps delamination. The presence of intraretinal edema is not associated with the drug dose nor the route of drug administration (intravenous versus oral). Furthermore, the intraretinal edema does not appear to have clinical significance: it self-resolves in a similar manner to the subretinal fluid and was not significantly associated with worse vision. Specifically, within the present ERK cohort, vision was not significantly different between eyes with and without intraretinal edema; but intraretinal edema may explain why this ERK inhibitor retinopathy cohort has worse vision at fluid accumulation compared to the MEK inhibitor retinopathy cohort. Another feature unique to ERK inhibitor retinopathy is the multifoil fluid configuration (Figure 4), which was not observed in the MEK inhibitor retinopathy eyes. Interestingly, all eyes with a multifoil configuration also exhibited intraretinal edema. One speculation is perhaps edema in the outer nuclear layer mechanistically generates the multifoil configuration through distorting/delaminating the outer retinal layers. It is intriguing to speculate whether the downstream activity of ERK impacts the retina differently from MEK inhibition and explains the occurrence of intraretinal edema and the multifoil configuration.
At some point in their course, ERK inhibitor retinopathy (and MEK inhibitor retinopathy) foci can be yellow in appearance and mimic vitelliform detachments; which are a shared feature with CSC and other retinal diseases including Acute Exudative Polymorphous Vitelliform Maculopathy (AEPVM) 18 (Figure 3). Given AEPVM can also occur in cancer patients (characteristically as a paraneoplastic process in metastatic melanoma), it is important to keep both differential diagnoses in mind. Like AEPVM, ERK inhibitor retinopathy vitelliform detachments can demonstrate hyperautofluoresence. However, unlike some cases of AEPVM, ERK inhibitor retinopathy does not demonstrate layering within the vitelliform foci and resolves relatively promptly.
In this small cohort, we note that the proportion of patients who develop detectable ERK inhibitor retinopathy appears to differ between drugs and is likely related to each drug’s pharmacokinetics. The significance of this is unclear and would benefit from further investigation.
The subretinal fluid foci associated with ERK inhibitors have unique clinical and morphologic characteristics similar to MEK inhibitor associated retinopathy; and distinct from CSC. In contrast to MEK-inhibitors, ERK-inhibitors result in higher occurrence of concomitant intraretinal fluid: fortunately, this does not significantly impact vision and is reversible. This current analysis describes findings on specialized ophthalmic imaging studies and, in over half the cases, in asymptomatic patients. Many of these events would not have met definition of adverse event, and thus would not have resulted in drug interruption. Given our findings, the suggested surveillance of retinopathy includes ophthalmic assessment with OCT at baseline, during the first drug cycle or at new ophthalmic symptoms (to also distinguish ERK inhibitor retinopathy from retinal vein occlusion). The clinical findings presented in this manuscript suggest that ERK inhibitor retinopathy is self-limited, does not require medical intervention and does not cause irreversible loss of vision or serious eye damage.. Expansion of this cohort would be helpful to confirm these findings.
Acknowledgments:
This study was supported by The Fund for Ophthalmic Knowledge, The New York Community Trust, Research to Prevent Blindness, and Cancer Center Support Grant (P30 CA008748). The sponsor or funding organization had no role in the design or conduct of this research. Special thanks to Melissa Robbins MPH, Research Project Associate who helped identify these patients.
Footnotes
COI: JHF- none, DHL-none, JC-none, JJH: has received research support from Bristol Myers Squibb and consulting fees from Bristol Myers Squibb, Eli Lilly, Exelexis, Eisai, QED, CytomX, Adaptimmune, Zymeworks, Imvax, and Merck, EIL- discloses editorial support from Pfizer Inc, outside the submitted work. BTL- grants from BioMedValley Discoveries, grants from National Institutes of Health, during the conduct of the study; grants from Genentech Roche, grants and personal fees from Guardant Health, grants and personal fees from Hengrui Therapeutics, grants from Lilly, grants from AstraZeneca, grants from Daiichi Sankyo, grants from Illumina, grants from GRAIL, grants and non-financial support from MORE Health, non-financial support from Resolution Bioscience, non-financial support from Jiangsu Hengrui Medicine, grants from Boehringer Ingelheim, grants from Bolt Biotherapeutics, grants from Amgen, outside the submitted work; In addition, Dr. Li has a patent US62/685,057 issued, and a patent US62/514,661 issued., IG- grants from Novartis, during the conduct of the study; grants and personal fees from Mirati Therapeutics, grants and personal fees from Janssen, personal fees from Basilea Therapeutica, grants from Bayer, grants from DeBioPharm, grants from Seagen, Inc, outside the submitted work, AD-grants from National Institutes of Health/National Cancer Institute, during the conduct of the study; personal fees from Ignyta/Genentech/Roche, personal fees from Loxo/Bayer/Lilly, personal fees from Takeda/Ariad/Millenium, personal fees from TP Therapeutics, personal fees from AstraZeneca, personal fees from Blueprint Medicines, personal fees from Helsinn, personal fees from Beigene, personal fees from BergenBio, personal fees from Hengrui Therapeutics, personal fees from Exelixis, personal fees from Tyra Biosciences, personal fees from Verastem, personal fees from MORE Health, personal fees from Abbvie, personal fees from 14ner/Elevation Oncology, personal fees from Remedica Ltd., personal fees from ArcherDX, personal fees from Monopteros, personal fees from Novartis, personal fees from EMD Serono, personal fees from Melendi, personal fees from Repare RX, personal fees from Pfizer, personal fees from Liberum, outside the submitted work; and Associated research paid to the institution from Pfizer, Exelixis, GlaxoSmithKline, Teva, Taiho, PharmaMar; research from Foundation Medicine; royalties from Wolters Kluwer; other support from Merck, Puma, Merus, Boehringer Ingelheim;and CME honoraria from Medscape, OncLive, PeerVoice, Physicians Education Resources, Targeted Oncology, Research to Practice, Axis,Peerview Institute, Paradigm Medical Communications, WebMD, MJH Life Sciences,Med Learning, Imedex,Answers in CME, Medscape, Clinical Care Options., AS- reports Funding to her institution for Clinical Trials: Merus Recipient, Kura Oncology Recipient, Surface Oncology Recipient, AstraZeneca Recipient, Lilly Recipient, Northern Biologics Recipient, Pfizer Recipient, Black Diamond Therapeutics Recipient, BeiGene Recipient, Relay Therapeutics Recipient, DHA-none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Akinleye A, Furqan M, Mukhi N, et al. MEK and the inhibitors: from bench to bedside. J Hematol Oncol 2013;6:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fouladi M, Stewart CF, Blaney SM, et al. A molecular biology and phase II trial of lapatinib in children with refractory CNS malignancies: a pediatric brain tumor consortium study. J Neurooncol 2013;114:173–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Turner MC, Rossfeld K, Salama AKS, et al. Can binimetinib, encorafenib and masitinib be more efficacious than currently available mutation-based targeted therapies for melanoma treatment? Expert Opin Pharmacother 2017;18:487–495. [DOI] [PubMed] [Google Scholar]
- 4.Dummer R, Schadendorf D, Ascierto PA, et al. Binimetinib versus dacarbazine in patients with advanced NRAS-mutant melanoma (NEMO): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol 2017;18:435–445. [DOI] [PubMed] [Google Scholar]
- 5.Suresh PS, Jairam RK, Chandrasekhar DV, et al. Prediction of Human Pharmacokinetics of Ulixertinib, a Novel ERK1/2 Inhibitor from Mice, Rats, and Dogs Pharmacokinetics. Eur J Drug Metab Pharmacokinet 2018;43:453–460. [DOI] [PubMed] [Google Scholar]
- 6.Sullivan RJ, Infante JR, Janku F, et al. First-in-Class ERK1/2 Inhibitor Ulixertinib (BVD-523) in Patients with MAPK Mutant Advanced Solid Tumors: Results of a Phase I Dose-Escalation and Expansion Study. Cancer Discov 2018;8:184–195. [DOI] [PubMed] [Google Scholar]
- 7.Urner-Bloch U, Urner M, Jaberg-Bentele N, et al. MEK inhibitor-associated retinopathy (MEKAR) in metastatic melanoma: Long-term ophthalmic effects. Eur J Cancer 2016;65:130–138. [DOI] [PubMed] [Google Scholar]
- 8.Urner-Bloch U, Urner M, Stieger P, et al. Transient MEK inhibitor-associated retinopathy in metastatic melanoma. Ann Oncol 2014;25:1437–1441. [DOI] [PubMed] [Google Scholar]
- 9.Duncan KE, Chang LY, Patronas M. MEK inhibitors: a new class of chemotherapeutic agents with ocular toxicity. Eye (Lond) 2015;29:1003–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCannel TA, Chmielowski B, Finn RS, et al. Bilateral subfoveal neurosensory retinal detachment associated with MEK inhibitor use for metastatic cancer. JAMA Ophthalmol 2014;132:1005–1009. [DOI] [PubMed] [Google Scholar]
- 11.Weber ML, Liang MC, Flaherty KT, Heier JS. Subretinal Fluid Associated With MEK Inhibitor Use in the Treatment of Systemic Cancer. JAMA Ophthalmol 2016;134:855–862. [DOI] [PubMed] [Google Scholar]
- 12.Schoenberger SD, Kim SJ. Bilateral Multifocal Central Serous-Like Chorioretinopathy due to MEK Inhibition for Metastatic Cutaneous Melanoma. Case Rep Ophthalmol Med 2013;2013:673796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Niro A, Strippoli S, Alessio G, et al. Ocular Toxicity in Metastatic Melanoma Patients Treated With Mitogen-Activated Protein Kinase Kinase Inhibitors: A Case Series. Am J Ophthalmol 2015;160:959–967.e1. [DOI] [PubMed] [Google Scholar]
- 14.https://clinicaltrials.gov/ct2/show/results/NCT02711345.httpsclinicaltrialsgovctshowresultsNCT. Available at: [Accessed March 15, 2021].
- 15.Sioufi K, Das S, Say EAT. A Case of Extracellular Signal-Regulated Kinase Inhibitor-Associated Retinopathy. JAMA Ophthalmol 2020;138:1002–1004. [DOI] [PubMed] [Google Scholar]
- 16.Francis JH, Habib LA, Abramson DH, et al. Clinical and Morphologic Characteristics of MEK Inhibitor-Associated Retinopathy: Differences from Central Serous Chorioretinopathy. Ophthalmology 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Dijk EHC, Duits DEM, Versluis M, et al. Loss of MAPK Pathway Activation in Post-Mitotic Retinal Cells as Mechanism in MEK Inhibition-Related Retinopathy in Cancer Patients. Medicine (Baltimore) 2016;95:e3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barbazetto I, Dansingani KK, Dolz-Marco R, et al. Idiopathic Acute Exudative Polymorphous Vitelliform Maculopathy: Clinical Spectrum and Multimodal Imaging Characteristics. Ophthalmology 2018;125:75–88. [DOI] [PubMed] [Google Scholar]