Abstract
Characterization of immune responses induced by live attenuated simian immunodeficiency virus (SIV) strains may yield clues to the nature of protective immunity induced by this vaccine approach. We investigated the ability of CD8+ T lymphocytes from rhesus macaques immunized with the live, attenuated SIV strain SIVmac239Δnef or SIVmac239Δ3 to inhibit SIV replication. CD8+ T lymphocytes from immunized animals were able to potently suppress SIV replication in autologous SIV-infected CD4+ T cells. Suppression of SIV replication by unstimulated CD8+ T cells required direct contact and was major histocompatibility complex (MHC) restricted. However, CD3-stimulated CD8+ T cells produced soluble factors that inhibited SIV replication in an MHC-unrestricted fashion as much as 30-fold. Supernatants from stimulated CD8+ T cells were also able to inhibit replication of both CCR5- and CXCR4-dependent human immunodeficiency virus type 1 (HIV-1) strains. Stimulation of CD8+ cells with cognate cytotoxic T-lymphocyte epitopes also induced secretion of soluble factors able to inhibit SIV replication. Production of RANTES, macrophage inhibitory protein 1α (MIP-1α), or MIP-1β from stimulated CD8+ T cells of vaccinated animals was almost 10-fold higher than that from stimulated CD8+ T cells of control animals. However, addition of antibodies that neutralize these β-chemokines, either alone or in combination, only partly blocked inhibition of SIV and HIV replication by soluble factors produced by stimulated CD8+ T cells. Our results indicate that inhibition of SIV replication by CD8+ T cells from animals immunized with live attenuated SIV strains involves both MHC-restricted and -unrestricted mechanisms and that MHC-unrestricted inhibition of SIV replication is due principally to soluble factors other than RANTES, MIP-1α, and MIP-1β.
Efforts to develop an effective AIDS vaccine have been thwarted by a number of factors, including our incomplete understanding of the specific immune responses involved in protective immunity. Analysis of immune responses induced by vaccination of macaques with live attenuated simian immunodeficiency virus (SIV) strains, an approach that has yielded the most consistent protection to date in the SIV-macaque model (11), offers an opportunity to begin to identify humoral and cellular immune responses that may play a role in mediating protection against infection. Studies of animals vaccinated with live attenuated SIV strains have demonstrated the presence of SIV-specific cytotoxic T-lymphocyte (CTL) responses (22, 52), proliferative responses (13), and neutralizing antibodies (11, 51). However, the relative contribution of each responses in mediating protective immunity remains to be determined.
In human immunodeficiency virus type 1 (HIV-1)-infected subjects, CD8+ T cells are believed to play a major role in inhibiting viral replication (40, 54). Two types of CD8+ T-cell-mediated HIV-specific responses have been described: a cytotoxic mechanism mediated by CTL that lyse HIV-1-expressing target cells in a major histocompatibility complex (MHC) class I-restricted manner, and a noncytotoxic mechanism mediated by soluble suppressive factors secreted by T lymphocytes from HIV-infected subjects (5, 48–50). As initially demonstrated by Walker et al. (49) and subsequently confirmed by others (5, 23, 47), CD8+ T cells are capable of suppressing in vitro HIV replication in CD4+ T cells in a noncytolytic, MHC class I-unrestricted manner (for reviews, see references 32 and 50). The role of CD8+ T-cell-derived soluble factors in suppressing HIV infection in vivo is not known. However, detection of this activity in HIV-infected long-term nonprogressors (35, 37, 50) and in acutely infected subjects prior to the detection of neutralizing antibodies (38), and its decline in subjects with advanced disease (30), all suggest that soluble factors produced by CD8+ T cells may contribute to suppression of HIV in vivo.
Initial studies indicated that soluble factors able to inhibit HIV replication were not related to known cytokines, including alpha, beta, and gamma interferons, tumor necrosis factor alpha, interleukin-1 (IL-1), IL-2, IL-4, IL-6, or IL-12 (36). Recently, Cocchi et al. (10) reported that antiviral effects of supernatants from CD8+ T cells were primarily due to the chemoattractant cytokines (β-chemokines) RANTES (regulated on activation, normal T-cell expressed and secreted), macrophage inhibitory protein 1α (MIP-1α), and MIP-1β. Inhibition of HIV-1 replication by these β-chemokines appears to occur in part by their ability to interfere with viral entry via the CCR5 coreceptor (1, 7, 12, 14). However, the presence of RANTES, MIP-1α, and MIP-1β in CD8+ T-cell supernatants from HIV-specific CTL clones does not appear to correlate with the ability of these supernatants to inhibit HIV, and the addition of neutralizing antibodies to these chemokines only partially blocks antiviral activity (44). These observations suggested the presence of additional unidentified factors produced by stimulated T cells that are able to inhibit viral replication. Pal et al. (43) have recently identified a β-chemokine macrophage-derived chemokine secreted by CD8+ T lymphocytes from HIV-1-infected individuals that suppresses replication of HIV-1. A possible role of another chemotactic cytokine, IL-16, in controlling HIV replication has also been suggested (4), but again neither β-chemokines nor IL-16 seems to account in full for the CD8+ T-cell-mediated antiviral activity described previously (9, 32, 45).
CD8+ T lymphocytes from SIV-infected (47) and SIV Nef-vaccinated (19) macaques have also been shown to inhibit SIV replication in vitro. Production of β-chemokines by CD8+ T cells from vaccinated animals has been reported to correlate with protection against mucosal challenge (31). However, no information is currently available about the ability of CD8+ T cells from animals vaccinated with live attenuated SIV strains to inhibit SIV replication. In this study, we characterized the ability of CD8+ T lymphocytes from macaques immunized with SIVmac239Δnef or SIVmac239Δ3 to inhibit SIV replication. The potential role of β-chemokines RANTES, MIP-1α, and MIP-1β in mediating CD8+ T-lymphocyte antiviral activity in this model was also evaluated. CD8+ T cells from vaccinated animals were able to inhibit SIV replication when in direct contact with infected cells and to produce soluble factors able to inhibit viral replication. Although stimulated CD8+ T cells from animals immunized with live, attenuated SIV produced increased amounts of RANTES, MIP-1α, and MIP-1β, these β-chemokines did not appear to mediate the dominant effect of CD8+ T-cell-derived factors able to suppress SIV and HIV replication.
MATERIALS AND METHODS
Animals.
Rhesus macaques used in this study were housed at the New England Regional Primate Research Center. Three groups of SIV-infected macaques were studied: (i) five animals infected with the live attenuated SIV strain SIVmac239Δnef (26) 8 to 9 years prior to our studies; (ii) five animals infected with SIVmac239Δ3 (deficient in nef, vpr, and the negative regulatory elements of the long terminal repeat [LTR]) 5 to 6 years prior to our studies; and (iii) two animals infected with pathogenic strain SIVmac239 (25) or SIVmac251. At the time of the study, the two animals infected with a pathogenic SIV strain had relatively advanced disease, with CD4 counts of ≤300 mm3 and virus loads of 12 × 103 and 10.8 × 105 copies/ml, respectively. In addition, four other macaques from the conventional colony that were seronegative for SIV were used as control animals. All animals were maintained in accordance with the guidelines of the local institutional animal use committees and the federal government (47a). Three of the five rhesus macaques infected with SIVmac239Δnef were challenged 2 years later either with cloned pathogenic SIVmac239/nef-open (intact nef) (animals 353.91 and 397.88) or with pathogenic SIVmac251 (animal 71.88). Of macaques immunized with SIVmac239Δ3, two animals (358.91 and 437.91) were challenged 2 years later with uncloned pathogenic SIVmac251. All challenged macaques remained healthy without evidence for wild-type SIV infection at time of our study.
Cell lines.
C8166-45, CEMx174, and PM1 (34) cells were maintained in RPMI 1640 medium (Sigma, St. Louis, Mo.) supplemented with 10% heat-inactivated fetal calf serum (Sigma), 10 mM HEPES 2 mM l-glutamine, 50 U of penicillin per ml, and 50 μg of streptomycin per ml (R-10 medium). C8166-45-SEAP and CEMx174-SEAP cell lines expressing secreted alkaline phosphatase (SEAP) under the control of the SIV LTR have been previously described (41). Stimulator cells consisted of autologous or allogeneic herpesvirus papio-transformed B-cell lines (B-LCL).
Virus stocks.
The SIV isolates used in this study were SIVmac239, derived from the pathogenic molecular clone SIVmac239 (25), and SIVmac251, a pathogenic virus stock that has been passaged only in rhesus peripheral blood mononuclear cells (PBMC). In addition, three HIV-1 isolates were used: HIV-1Ba-L, a monocytotropic primary HIV-1 isolate; HIV-1JR-CSF, a molecularly cloned primary HIV-1 isolate (29) that demonstrates many of the characteristics of a primary HIV isolate and will not grow in most transformed T-cell lines (29); and HIV-1NL4-3, which is derived from a molecular clone that grows well in transformed T-cell lines.
Each virus stock was prepared from the supernatant of either macaque PBMC (SIVmac251), CEMx174 cells (SIVmac239 and HIV-1NL4-3), or PM1 cells (HIV-1JR-CSF and HIV-1Ba-L). Briefly, cell-free virus was harvested on days 5 to 7 from acutely infected CEMx174 or concanavalin (ConA; Sigma)-stimulated rhesus macaque PBMC and titrated on 24-well plates by serial endpoint dilutions on uninfected CEMx174 cells or on ConA-stimulated macaque PBMC. The infectious dose of each virus stock was expressed as the 50% tissue culture infective dose (TCID50) per milliliter, using the method of Reed and Muench (44a) as described previously (15, 20, 28). Virus stocks were stored in aliquots at −80°C.
Separation of CD8+ and CD4+ T lymphocytes from rhesus macaques.
Rhesus macaque PBMC were isolated from fresh heparinized blood by centrifugation over a Ficoll-sodium diatrizoate (Ficoll 1077; Sigma) gradient, washed two times in phosphate-buffered saline, and resuspended at 2 × 106 cells/ml in R-10 medium. For lectin stimulation, PBMC were incubated with ConA at 5 μg/ml for 2 days, washed, and resuspended in R-10 medium supplemented with 10 to 20 U of recombinant human IL-2 (donated by M. Gately, Hoffman-La Roche). CD8+ and CD4+ T lymphocytes were isolated by negative selection of rhesus macaque PBMC. CD8+ T cells were separated from PBMC by direct depletion of CD4+ cells by using CD4-stimulated immunomagnetic beads (Dynal, Oslo, Norway) or indirectly by using an anti-CD4 monoclonal antibody (OKT4, at 20 μg/106 cells) and then by incubation with anti-mouse immunoglobulin G (IgG)-coated magnetic beads (PerSeptive Diagnostics, Framingham, Mass.) at a 50:1 bead-to-cell ratio for 30 min at 4°C. The supernatant enriched for CD8+ cells was collected by using a magnetic separation device (Dynal). Similarly, CD4+ lymphocytes were obtained by depleting CD8+ cells by using an anti-CD8 (51.1; American Type Culture Collection catalog no. HB230) antibody at 20 μg/106 cells and then incubating them with anti-IgG2a-coated magnetic beads (Dynal) at a 10:1 bead-to-cell ratio for 60 min at 4°C. The CD4+ cell-enriched supernatant was then collected by using a magnetic separation device (Dynal). Finally, CD4+ cells were stimulated in R-10 medium supplemented with ConA (5 μg/ml) overnight (37°C, 5% CO2) before infection with SIV. After cell separation, CD8+ cell populations were greater than 85% CD8+ and contained <5% CD4+ cells as assessed by flow cytometry, and CD4+ T lymphocytes were greater than 95% CD4+, with less than 1% residual CD8+ cells.
Transmembrane assay for viral inhibition.
CD4+ T cells were stimulated with ConA (5 μg/ml) for 24 h and then infected with SIVmac239 at a multiplicity of infection (MOI) of 0.01 TCID50/cell. A total of 1 × 106 to 1.5 × 106 CD4+ T cells per well were placed in a 24-well plate (lower well) and overlaid with a 0.4 μm-pore-size semipermeable membrane insert (Millipore, Bedford, Mass.). Within the insert (upper well) were placed 2 × 106 CD8+ T cells (MHC matched or mismatched) stimulated with 6 × 106 goat anti-mouse IgG-coated beads (PerSeptive Diagnostics) previously saturated with the mouse anti-rhesus CD3 antibody (6G12; hybridoma provided by J. Wong, Massachusetts General Hospital, Boston) (24). Lower and upper wells contained a total of 1.4 and 0.7 ml, respectively, of 10% IL-2-supplemented RPMI 1640. Controls included CD8+ T cells mixed directly with the infected CD4+ T cells in the lower well, CD8+ T cells not stimulated by CD3-specific beads in the upper well, and CD3-specific beads alone in the upper well. Plates were incubated at 37°C for 10 days. At day 4, 6, 8, and 10, half of the supernatant fluid was removed from the lower well, analyzed for p27 antigen (Ag) quantitation by standard quantitative SIV p27 Ag enzyme-linked immunosorbent assay (ELISA; Coulter), and cryopreserved at −80°C for later use. Cryopreserved supernatant fluids from stimulated or unstimulated CD8+ T cells were tested for concentrations of RANTES, MIP-1α, and MIP-1β by using human-specific ELISA kits known to cross-react with rhesus chemokines (R&D Systems, Minneapolis, Minn.).
Generation of soluble factors from CD8+ T lymphocytes activated by specific peptides.
Soluble factors were generated as described above, using the transmembrane assay. CD8+ T cells from the upper well were stimulated by specific peptides. Briefly, CD8+ T cells were cocultured at 2 × 106 cells/ml in the upper well with 106 autologous B-LCL labeled with specific epitope peptide. These B cells had been labeled with 100 μg of specific peptide per ml, washed twice, and gamma irradiated (10,000 rads). At day 4, 6, 8, and 10, supernatant fluid from the lower well was harvested and cryopreserved at −80°C for later use. Controls included cell-free supernatants from autologous B cells alone, autologous B cells labeled with unrelated peptide, and allogeneic B cells labeled with specific peptide. Peptides used for these experiments (10 μg/well) included the Mamu-A*01-restricted SIV Gag peptide 11C (TPYDINQML) (42), the SIV Gag peptide 7G (HQAAMQIIR) (19a), and the unrelated peptide 12K (NYSETDRWG) (16).
SEAP assay.
The SEAP assay has been described previously (41) and was performed as follows. Briefly, 100 μl of CEMx174-SEAP or C8166-45-SEAP cells was first plated in triplicate in 96-well plates at 0.4 × 106 cells/ml of R-10 medium. Each well received 50 μl of either control antibody, chemokine, or soluble factor fluid. SEAP cells were then infected by addition of 50 μl of virus (SIVmac239 or SIVmac251) at the indicated concentrations. In addition, the CEMx174-SEAP cell line was cultured under the same conditions to analyze the ability of either control antibody, chemokine, or soluble factor fluid to inhibit HIV-1NL4-3 replication. The plates were then incubated at 37°C for 72 to 96 h in a 5% CO2 incubator. SEAP activity was monitored at several time points postinfection by harvesting supernatant from each well and by using a Phospha-Light assay kit as described previously (41). Generally three types of controls were included on each plate: CEMx174 or C8166-45 cells as a background control; SIV-infected CEMx174 (or C8166-45)-SEAP cells for measurement of basal levels of SEAP production; and virus-infected CEMx174 (or C8166-45)-SEAP cells alone to measure SEAP expression in the absence of supernatant.
Assay for inhibition of HIV-1 replication by chemokines or supernatants from stimulated CD8+ T cells.
PM1 cells were acutely infected with various dilutions of HIV-1Ba-L or HIV-1JR-CSF, resuspended in RPMI 1640 supplemented with 20% fetal calf serum, and plated at 2.5 × 106 cells per well in a 24-well plate. CD8+ T-cell supernatants were then tested at a final dilution of 1:2. The chemokines RANTES, MIP-1α, and MIP-1β (PeproTech, Inc., Rocky Hill, N.J.) were also tested for antiviral activity alone or in combination at different concentrations. In some experiments, CD8+ T cells, supernatants, or chemokines were first incubated at room temperature for 30 min with polyclonal neutralizing antibodies to RANTES, MIP-1α, and MIP-1β (R&D Systems) (separately or in combination) and then added to acutely infected PM1 cells. Supernatant fluid (0.5 ml) was removed on day 5 and 7 for p24 measurement and replaced with fresh medium.
RESULTS
CD8+ T lymphocytes from macaques immunized with SIVmac239Δnef or SIVmac239Δ3 inhibit SIV replication.
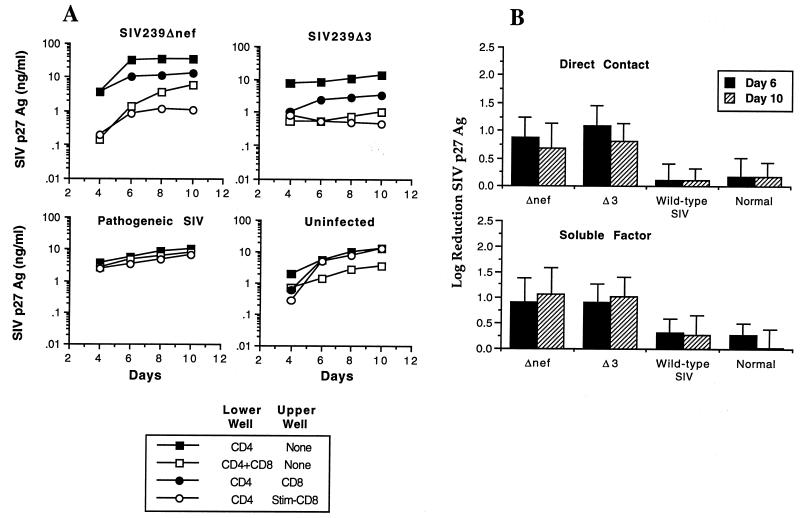
In initial experiments, we investigated the ability of CD8+ T lymphocytes isolated from macaques infected with either an attenuated SIV strain (SIVmac239Δnef or SIVmac239Δ3) or a pathogenic strain (SIVmac251 or SIVmac239) to inhibit SIV replication. Inhibition of SIV replication was assessed by a transmembrane assay where CD4+ T lymphocytes were infected in vitro with SIVmac239 (MOI of 0.01 TCID50 per cell) and then cultured either in direct contact with autologous CD8+ T lymphocytes or separated from them by a 0.4-μm-pore-size membrane. CD8+ T cells not in direct contact with CD4+ T cells were either unstimulated or activated by CD3-specific antibodies bound to paramagnetic beads. SIV replication was then assessed by SIV p27 Ag ELISA at multiple time points during a 10-day period. The results presented in Fig. 1 are representative of experiments using cells from five SIVmac239Δnef-infected animals, five SIVmac239Δ3-infected animals, two wild-type SIV-infected animals, and four uninfected animals. CD4+ T lymphocytes from animals infected with attenuated SIV strains were readily infected with SIV in vitro, resulting in peak levels of viral replication that did not differ significantly from those obtained for CD4+ T cells from normal controls (Fig. 1A and data not shown). When in direct contact with infected CD4+ T cells, CD8+ T cells from macaques immunized with SIVmac239Δnef or SIVmac239Δ3 were able to potently inhibit SIV replication (Fig. 1A). In addition, CD3-stimulated CD8+ T lymphocytes from immunized animals were able to secrete soluble factors that inhibited viral replication by 1 log or more in autologous SIV-infected CD4+ T cells (Fig. 1A). The addition of CD3-specific immunomagnetic beads alone to the upper well had no effect on SIV replication (data not shown). Although we occasionally observed a low level inhibition of replication in PBMC from uninfected animals, comparison of results from multiple animals showed that the level of inhibition of SIV replication by CD8+ T cells from macaques infected with live attenuated SIV strains was 30-fold greater than that for normal controls (Fig. 1B) for cells in direct contact with infected CD4+ T cells and 20-fold greater for soluble factors produced by activated T cells (Fig. 1B) (P < 0.01). Thus, immunization of macaques with live attenuated SIV strains induces a specific CD8+ T-cell response that is able to inhibit SIV replication and was significantly greater than that found in animals infected with wild-type SIV or normal controls.
FIG. 1.
Inhibition of SIV replication by CD8+ T lymphocytes from macaques immunized with SIVmac239Δnef or SIVmac239Δ3. (A) Transmembrane experiments using PBMC from macaques infected with either wild-type SIV or live attenuated SIV and from uninfected controls. PBMC were separated into CD4+ and CD8+ populations by using immunomagnetic beads. CD4+ T cells were ConA stimulated overnight and acutely infected with SIVmac239 at an MOI of 0.01 TCID50/cell. Infected CD4+ T cells were then cultured in the lower well either alone, in direct contact with CD8+ T cells, or with stimulated or unstimulated CD8+ T cells placed in the upper well. Inhibition of SIV replication by CD8+ T cells was assessed by measuring the production of SIV p27 Ag over a period of 10 days. Representative assays are shown for individual animals infected with the indicated SIV strain and for an uninfected control. (B) Quantitative analysis of suppression of SIV replication by CD8+ T lymphocytes from uninfected animals and animals infected with pathogenic or live attenuated strains of SIV. Experiments were carried out as described above, and log reduction of SIV replication (90% inhibition = 1 log, 99% = 2 logs, etc.) was analyzed on days 6 and 10. The data represent the means ± standard deviations for a total of 16 animals: 5 SIVmac239Δnef-, 5 SIVmac239Δ3-, and 2 wild-type (SIVmac239 and SIVmac251)-infected animals and 4 uninfected animals. Suppression of SIV replication by CD8+ T cells from animals infected with either SIVmac239Δnef or SIVmac239Δ3 was statistically significant compared with inhibition by either wild-type SIV-infected animals or normal controls (P < 0.01), for both cells in direct contact and for soluble factors. The difference between wild-type SIV-infected animals and normal controls was not significant.
Suppression of SIV replication by unstimulated CD8+ T lymphocytes is MHC restricted.
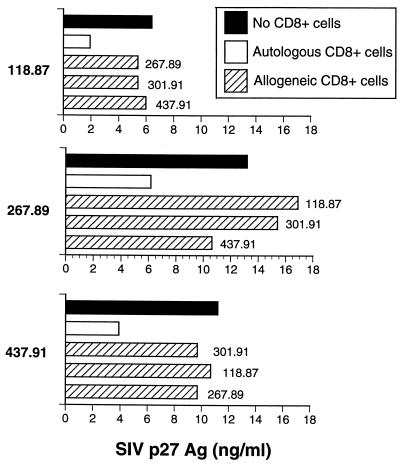
In the next series of experiments, we examined whether inhibition of SIV replication by CD8+ T cells from vaccinated animals in direct contact with CD4+ T cells was MHC restricted. Acutely infected CD4+ T cells from SIVmac239Δnef- or SIVmac239Δ3-immunized animals were incubated in direct contact with either autologous or allogeneic CD8+ T cells. Allogeneic donors were prescreened by isoelectric focusing of MHC class I alleles so as to minimize class I homology (22). As shown in Fig. 2, allogeneic CD8+ T lymphocytes (MHC mismatched) from each of three different immunized macaques were not effective in suppressing SIV replication in CD4+ T lymphocytes, whereas autologous CD8+ T cells efficiently suppressed viral replication in each instance. Therefore, suppression of SIV replication by CD8+ T lymphocytes in direct contact with CD4+ cells was MHC restricted.
FIG. 2.
Suppression of SIV replication by unstimulated CD8+ T lymphocytes is MHC restricted. Acutely SIV infected CD4+ T cells were cocultured in direct contact with MHC class I-mismatched allogeneic CD8+ T lymphocytes isolated from uninfected animals or animals infected with pathogenic or live attenuated strains of SIV. Inhibition of SIV replication by allogeneic CD8+ T cells was assessed by measuring the production of SIV p27 Ag for 10 days.
Suppression of SIV replication by soluble factors from CD3-stimulated CD8+ T cells is not MHC restricted.
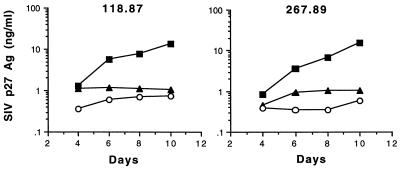
We next investigated if inhibition of SIV replication by soluble factors secreted by CD3-activated CD8+ T cells was MHC restricted. Acutely infected CD4+ T cells from SIVmac239Δnef-immunized macaques were incubated (lower well) with allogeneic CD8+ T cells from SIVmac239Δnef-immunized macaques (mismatched animals). All CD8+ T cells were activated with CD3-stimulated immunomagnetic beads (upper well). Autologous and allogeneic CD3-specific CD8+ T lymphocytes were able to inhibit SIV replication to similar degrees (Fig. 3). Therefore, inhibition of SIV replication by soluble factors produced by activated CD8+ T cells was not MHC restricted.
FIG. 3.
Suppression of SIV replication by soluble factors secreted from CD3-activated CD8+ T lymphocytes is not MHC restricted. CD4+ T cells from animals (118.87 and 267.89) immunized with SIVmac239Δnef were infected with SIVmac239 and then cultured (lower well) with autologous (○) and allogeneic (▴) CD8+ T cells (mismatched; 118.87 and 267.89) stimulated with CD3-specific beads. Similar data were obtained in two independent experiments. ▪, no CD8+ cells.
Stimulation with cognate CTL epitopes triggers CD8+ T lymphocytes to produce soluble factors that inhibit SIV replication.
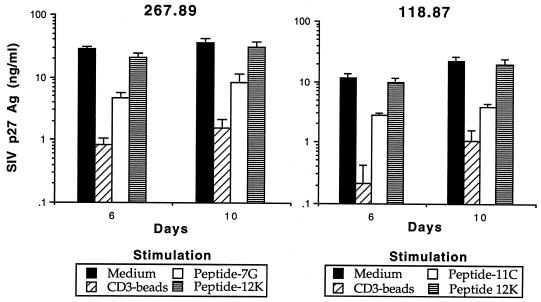
Based on the above findings and on previous studies of inhibition of HIV replication by HIV-specific CTL clones (53), we hypothesized that inhibition of SIV replication by CD8+ T cells was mediated, at least in part, by CTL that required MHC-restricted Ag presentation in order to be activated. Previous analysis of SIVmac239Δnef-immunized macaques has demonstrated that these animals develop a strong CTL response against gag- and env-expressing target cells (22). Recent mapping of CTL epitopes recognized by these animals has identified several distinct epitopes (23a), including a SIV Gag peptide previously shown to be presented by the Mamu-A*01 MHC class I allele (42). To determine whether peptide-specific stimulation of CTL could elicit production of soluble factors able to inhibit SIV replication, CD8+ T lymphocytes from SIVmac239Δnef-immunized macaques (animals 267.89 and 118.87) were stimulated with autologous B-LCL sensitized with either an immunodominant CTL epitope (7G for animal 267.89; 11C for animal 118.87) or, as a control, a previously identified SIV envelope CTL epitope (16). Following stimulation with the cognate CTL epitope but not the irrelevant control peptide 12K, CD8+ T cells from animals immunized with SIVmac239Δnef were able to secrete soluble factors that inhibited SIV replication 3- to 10-fold (Fig. 4) (3). Since these experiments were performed, Allen et al. (3) have revised the optimal Gag epitope presented by Mamu-A*01 to include one additional NH2-terminal amino acid (CTPYDINQML instead of TPYNINQML). However, since the concentration of peptide used for these experiments (10 μg/ml) results in maximal levels of target cell lysis when bulk effector cells are used, we feel it unlikely that use of the optimal peptide would alter these results. In subsequent experiments, autologous CD8+ T cells from either SIVmac239Δnef- or SIVmac239Δ3-immunized macaques were stimulated with autologous B-LCL infected with a recombinant vaccinia virus expressing gag, pol, and env and then inactivated with UV-psoralen (22). Following stimulation with B-LCL expressing SIV Ag, CD8+ T cells from both animals were also able to inhibit SIV replication three- to fivefold compared with cells stimulated with control vaccinia virus-infected B-LCL (data not shown). Thus, SIV-specific CTL are able to produce soluble factors which inhibit SIV replication.
FIG. 4.
Stimulation of CD8+ T cells with cognate CTL epitopes results in production of soluble factors that suppress SIV replication. Autologous CD4+ T cells were acutely infected with SIVmac239 (lower well) and cocultured with stimulated CD8+ T cells from animals (267.89 and 118.87) immunized with SIVmac239Δnef (upper well). Stimulation of CD8+ T cells included either CD3-specific immunomagnetic beads or specific peptides (7G for animal 267.89; 11C for animal 118.87) previously shown to represent CTL epitopes for these animals. A previously reported SIV CTL epitope (12K) not recognized by these animals was used as a negative control. All assays were performed in triplicate.
Stimulated CD8+ T cells from macaques immunized with live attenuated SIV strains produce increased concentrations of RANTES, MIP-1α, and MIP-1β.
The recent demonstration that chemokine receptors serve as coreceptors for entry of HIV and SIV (6, 12, 14, 17, 18, 33, 39) has helped to elucidate the role of chemokines in inhibiting viral replication. In particular, the chemokines RANTES, MIP-1α, and MIP-1β have been identified as being in part responsible for the inhibition of CCR5-dependent HIV strains by soluble factors produced by CD8+ T cells (10). We therefore assessed whether CD8+ T lymphocytes from macaques immunized with live attenuated SIV strains were able to produce these soluble factors after CD3 stimulation. The levels of RANTES, MIP-1α, and MIP-1β were measured in supernatants from unstimulated and CD3-stimulated CD8+ T cells from normal and immunized macaques (Table 1). Four to six days after stimulation, peak levels of the three β-chemokines were six- to ninefold higher in CD8+ T cells from animals vaccinated with SIVmac239Δnef or SIVmac239Δ3 compared with normal controls (Table 1). Similar increase of β-chemokines were observed at days 8 and 10 after stimulation (data not shown).
TABLE 1.
Production of RANTES, MIP-1α and MIP-1β by CD8+ T cellsa
| Cells | RANTES (ng/ml)
|
MIP-1α (ng/ml)
|
MIP-1β (ng/ml)
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Unstimulated | Stimulated
|
Unstimulated | Stimulated
|
Unstimulated | Stimulated
|
||||
| D4 | D6 | D4 | D6 | D4 | D6 | ||||
| Uninfected | 0.5 ± 0.05 | 1.8 ± 0.1 | 2.3 ± 0.2 | 0.7 ± 0.05 | 2.7 ± 0.4 | 3.2 ± 0.4 | 0.9 ± 0.05 | 2.7 ± 0.4 | 3.8 ± 0.6 |
| Infected with: | |||||||||
| SIVmac239Δnef | 1.0 ± 0.2 | 15.3 ± 0.5 | 16.4 ± 0.4 | 1.3 ± 0.2 | 18.7 ± 0.5 | 22.3 ± 0.6 | 1.6 ± 0.2 | 19.7 ± 0.5 | 28.9 ± 0.4 |
| SIVmac239Δ3 | 1.2 ± 0.3 | 16.8 ± 0.3 | 18.2 ± 0.5 | 1.4 ± 0.4 | 19.8 ± 0.3 | 26.2 ± 0.7 | 1.4 ± 0.3 | 21.8 ± 0.4 | 33.3 ± 0.5 |
CD8+ T lymphocytes at 2.5 × 106 to 3 × 106 cells/well were activated by CD3 cross-linking. Supernatants from stimulated or unstimulated CD8+ cells were harvested after 4 and 6 days (D4 and D6) for ELISA quantitation of RANTES, MIP-1α, and MIP-1β. Supernatants from unstimulated were pooled. Results are expressed as the means ± standard deviations of triplicate wells.
RANTES, MIP-1α, and MIP-1β mediate only low-level inhibition of SIV replication in the C8166 and CEMx174 cell lines compared to soluble factors produced by CD8+ T cells from SIVmac239Δnef- and SIVmac239Δ3-infected macaques.
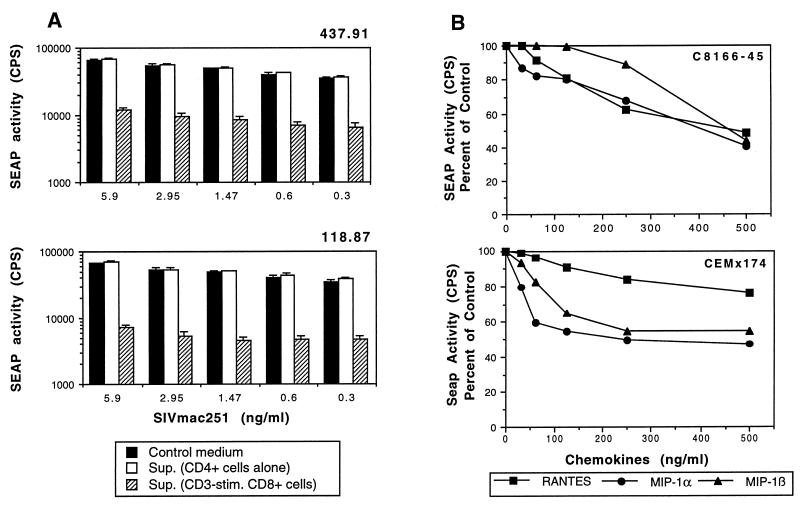
To facilitate characterization of soluble factors responsible for inhibition of virus replication, we wanted to establish a reproducible and quantitative system that provided a relatively rapid assessment of inhibition of viral replication. To accomplish this, we used two cell lines (C8166-45 and CEMx174) that stably express SEAP under the control of the SIV LTR (41). Infection of these cell lines with SIV results in a dose-dependent release of SEAP over a 2- to 3-log range, allowing rapid quantitation of the level of SIV infection 72 to 96 h after infection (41). We first evaluated the ability of soluble factors from CD3-stimulated CD8+ T cells from macaques immunized with SIVmac239Δnef or SIVmac239Δ3 to inhibit replication in this system. C8166-45-SEAP cells were preincubated for 1 h at 37°C with soluble factors secreted by CD3-stimulated CD8+ T lymphocytes from either SIVmac239Δnef- or SIVmac239Δ3-immunized macaques. SIV replication, as assessed by SEAP release, was inhibited up to 10-fold by the addition of soluble factors secreted by activated CD8+ T lymphocytes from macaques immunized with SIVmac239Δnef or SIVmac239Δ3 (Fig. 5A).
FIG. 5.
SIV replication is inhibited by soluble factors from activated CD8+ T cells from immunized macaques and by β-chemokines RANTES, MIP-1α, and MIP-1β. (A) Inhibition of SIV replication in SIV-infected C8166-45 cells (SEAP activity) by soluble factors secreted by CD8+ T cells from macaques immunized with either SIVmac239Δnef (118.87) or SIVmac239Δ3 (437.91). C8166-45-SEAP cells were acutely infected with SIVmac251 at decreasing concentrations (from 5.9 to 0.3 ng/ml) and then incubated in the presence of soluble factors (1:2 dilution) secreted by CD3-activated CD8+ T cells from macaques immunized with either SIVmac239Δnef (118.87) or SIVmac239Δ3 (437.91). SEAP activity in cell-free supernatants (Sup.) was measured on day 4 after infection. (B) Inhibition of SIV replication (SEAP activity) in infected C8166-45 and CEMx174 cells by β-chemokines. C8166-45-SEAP and CEMx174-SEAP cell lines were acutely infected with SIVmac251 (0.3 ng/ml) and SIVmac239 (1.47 ng/ml), respectively. Infected cells were then incubated with decreasing concentrations (fivefold dilutions starting from 500 ng/ml) of RANTES, MIP-1α, or MIP-1β. SEAP activity was measured from cell-free medium collected on day 4. All SEAP assays were performed in quadruplicate.
We next examined the ability of RANTES, MIP-1α, and MIP-1β to inhibit SIV replication in infected C8166-45 and CEMx174 cells. The C8166-45 and CEMx174 cell lines were cultured in the presence of RANTES, MIP-α, or MIP-β (each at 500 ng/ml) for 1 h at 37°C and then infected with the SIVmac251 and SIVmac239, respectively. Even at low viral inocula, no more that twofold inhibition of SIV replication was observed, even when RANTES, MIP-α, and MIP-β were combined together (data not shown). We then evaluated the ability of a range of chemokine concentrations to inhibit viral replication following a relative low viral inoculum. SIV replication was inhibited in a dose-dependent manner in both cell lines by addition of RANTES, MIP-1α, or MIP-1β (Fig. 5B). However, in contrast to the 10-fold inhibition observed with soluble factors produced by activated CD8+ T cells, recombinant chemokines inhibited viral replication only twofold even at the highest chemokine concentration tested (500 ng/ml).
Soluble factors from stimulated CD8+ T cells mediate potent suppression of SIV replication in C8166 and CEMx174 cell lines that is not neutralized by antibodies to β-chemokines.
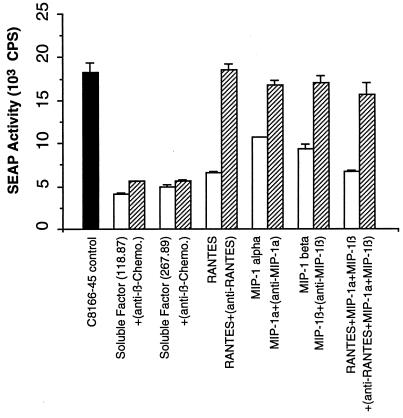
We next examined if inhibition of SIV replication by soluble factors from activated CD8+ T cells from macaques immunized with either SIVmac239Δnef or SIVmac239Δ3 could be blocked by antibodies to β-chemokines. Neutralizing antibodies to RANTES, MIP-1α, and MIP-1β were added to the CD8+ T-cell supernatants in concentrations sufficient to neutralize at least 100 ng of each chemokine per ml. These anti-β-chemokine antibodies consisted of goat polyclonal neutralizing antibodies used at a final concentration sufficient to neutralize at least 100 ng of each chemokine per ml. As controls, neutralizing antibodies were also added to known concentrations of recombinant RANTES, MIP-1α, and MIP-1β (each at 100 ng/ml) either alone or in combination. Both mixtures were incubated for 30 min at room temperature before addition of SIVmac251-infected C8166-45-SEAP cells. SEAP activity was measured in culture supernatants on day 4 after virus inoculation.
As shown in Fig. 6, the ability of supernatants from stimulated CD8+ T cells to inhibit SIVmac251 replication in the C8166-45-SEAP cell line was not neutralized by adding these antibodies individually or in combination. In contrast, when C8166-45-SEAP cells were incubated with the mixture of recombinant RANTES, MIP-1α, and MIP-1β 1 h prior to SIVmac251 infection, SIV replication was inhibited 2- to 2.5-fold. However, when neutralizing antibodies to β-chemokines were added to the mixture, the inhibition of viral replication was reversed (Fig. 6). These results indicate that β-chemokines do not account for the majority of anti-SIV activity observed with CD8+ T-cell supernatants.
FIG. 6.
Neutralizing antibodies to β-chemokines do not block the ability of soluble factors from CD8+ T cells to suppress SIV replication. C8166-45-SEAP cells were infected with SIVmac251 (0.3 ng/ml) and cultured either with supernatants (1:2 dilution) from CD3-activated CD8+ T cells from a macaque immunized with SIVmac239Δnef or with RANTES, MIP-1α, or MIP-1β (500 ng/ml) added individually or in combination. In addition, in some cases, neutralizing antisera to human RANTES, MIP-1α, and MIP-1β were preincubated at 200, 80, and 100 μg/ml, respectively, with β-chemokines and then were added to the indicated cultures (cross-hatched bars). Similar data were obtained in two independent experiments.
Soluble suppressive factors secreted by CD3-stimulated CD8+ T cells inhibit HIV-1 replication.
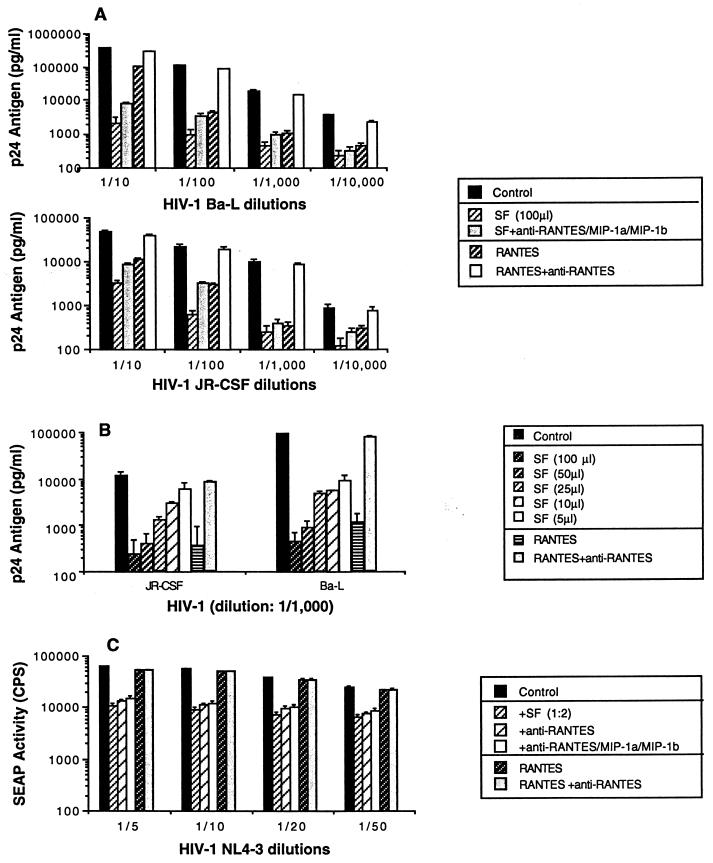
We next evaluated the ability of soluble factors produced from macaque CD8+ T cells to inhibit HIV-1 replication. The PM1 cell line described by Cocchi et al. (10), which expresses both CD4 and CCR5, was acutely infected with either HIV-1Ba-L or HIV-1JR-CSF and then cultured in the presence of either soluble factors (1:2 dilution) or RANTES (500 ng/ml). Both CD8+ T-cell supernatant and RANTES significantly inhibited HIV-1 replication (Fig. 7A). The ability of soluble factors to inhibit HIV-1 replication was dose dependent (Fig. 7B). In addition, HIV-1Ba-L and HIV-1JR-CSF were both suppressed by soluble factors produced by activated CD8+ T cells, even in the presence of a combination of neutralizing antibodies to the three chemokines (RANTES, MIP-1α, and MIP-1β) (Fig. 7A). The small reversion of suppressing activity observed after treatment with the β-chemokine antibodies suggests that elevated concentrations of β-chemokines present in the supernatant contribute to some suppression of HIV-1 replication but do not constitute the major factors involved in the viral inhibition seen.
FIG. 7.
Soluble factors from CD3-activated CD8+ T cells from SIVmac239Δnef-infected macaques inhibit HIV-1 replication. (A) Replication of both HIV-1Ba-L and HIV-1JR-CSF in PM1 cells is inhibited by SIVmac239Δnef-infected CD8+ T-cell-mediated soluble factors. PM1 cells were acutely infected with various dilutions of primary isolates HIV-1Ba-L and HIV-1JR-CSF and then incubated in the presence of either soluble factors (1:2 dilution) or RANTES (500 ng/ml). In addition, in some cases, neutralizing antisera to human RANTES, MIP-1α, and MIP-1β were preincubated at 200, 80, and 100 μg/ml, respectively, with soluble factors or RANTES and then added to the indicated cultures. (B) Soluble factors inhibited HIV-1 replication in a dose-dependent manner. In the same conditions, PM1 cells were acutely infected with primary isolates HIV-1Ba-L and HIV-1JR-CSF (dilution of 1/1,000) and then incubated in the presence of soluble factors at various concentrations. RANTES (500 ng/ml) was used as a control. (C) HIV-1NL4-3 replication in CEMx174-SEAP cells is inhibited by SIVmac239Δnef-infected CD8+ T-cell-mediated soluble factors. CEMx-174-SEAP cells were infected with various dilutions of HIV-1NL4-3 and cultured either with supernatants (1:2 dilution) from CD3-activated CD8+ T cells from a macaque immunized with SIVmac239Δnef or with RANTES (500 ng/ml). In addition, in some cases, neutralizing antisera to human RANTES, MIP-1α, and MIP-1β were preincubated at 200, 80, and 100 μg/ml, respectively, either alone or in combination with soluble factors or RANTES, and then were added to the indicated cultures. Similar data were obtained in two independent experiments.
In addition, the ability of soluble factors produced from macaque CD8+ T cells to inhibit replication of the CXCR4-dependent strain HIV-1NL4-3 was assessed by using the SEAP assay system. CEMx174-SEAP cells were cultured in the presence of either soluble factors (1:2 dilution) or RANTES (500 ng/ml). Addition of supernatant from stimulated CD8+ T cells resulted in a significant inhibition of HIV-1NL4-3 replication. As expected, recombinant RANTES (500 ng/ml) had no effect on replication of HIV-1NL4-3 and no reversal of inhibition was observed when supernatants from CD8+ T cells were incubated in the presence of a combination of neutralizing antibodies to the three chemokines (RANTES, MIP-α, and MIP-1β) (Fig. 7C). Thus, CD8+ T cells from immunized animals produced factors distinct from RANTES, MIP-1α, and MIP-1β that are able to inhibit replication of both CXCR4- and CCR5-dependent HIV-1 strains.
DISCUSSION
In this study, we analyzed the ability of CD8+ T lymphocytes from rhesus macaques immunized with live attenuated strains of SIV to suppress SIV infection and performed an initial evaluation of the soluble factors produced by these cells. Our data demonstrate that CD8+ T lymphocytes from macaques immunized with live attenuated SIV strains are able to inhibit SIV replication and suggest the existence of both MHC-restricted and -unrestricted mechanisms of inhibition. Although a subset of these animals had been challenged with wild-type SIV prior to these studies, no difference in CD8+ T-cell-mediated antiviral activity between challenged and unchallenged animals was observed (data not shown). In addition, there has been no evidence of wild-type SIV infection of these animals more than 3 years after challenge. It is therefore likely that the antiviral CD8+ T-cell activity was induced by infection with live attenuated SIV and not the challenge. In contrast to a previous report (47), we did not observe any significant inhibition of SIV replication by CD8+ T lymphocytes from animals infected with pathogenic SIV when these cells were cultured in direct contact with infected CD4+ T lymphocytes. Our failure to observe antiviral activity in wild-type-infected animals may reflect the fact that these animals had relatively advanced disease with high virus load, low CD4+ T-cell counts (≤300 mm3), and poor CTL responses (data not shown). Alternatively, our assay for analyzing the ability of CD8+ T cells to suppress SIV replication may be less sensitive than that used by Tsubota et al. (47), a finding that could be due to technical differences between our assays. For instance, we did not stimulate CD8+ T cells prior to their incubation in direct contact with CD4+ T cells, whereas the previous report used ConA-stimulated CD8+ cells (47). In addition, we superinfected CD4+ T cells with SIV.
Since the initial description of the ability of CD8+ T lymphocytes to suppress HIV replication (49), there has been considerable debate over the relative contribution of cytolytic and noncytolytic mechanisms of suppression. Early reports suggested that this inhibition was primarily mediated by noncytolytic mechanisms involving the production of soluble factors (35, 38, 49). However, a recent report has demonstrated that HIV-specific CTL clones can inhibit viral replication and, for cells in direct contact, that MHC-restricted mechanisms account for most of the observed inhibition of replication (53). Our results are comparable with those of Yang et al. (53) and suggest that SIV-specific CTL account, at least in part, for the ability of CD8+ T cells from immunized animals to inhibit viral replication. This conclusion is supported by the observation that for unstimulated CD8+ T cells, optimal inhibition was MHC restricted and required direct contact. Further demonstration of the ability of SIV-specific CTL to release soluble factors that inhibit SIV replication comes from our experiments in which stimulation of CD8+ T lymphocytes from immunized animals with the cognate CTL epitope results in production of soluble factors able to inhibit SIV replication. These observations suggest that inhibition of SIV replication is likely to be mediated by CTL that require an MHC-restricted antigen presentation in order to be activated. However, once activated, these CD8+ T cells can release soluble factors that inhibit SIV replication in an MHC-unrestricted fashion. At present, we cannot exclude the existence of noncytolytic cells that produce soluble factors able to inhibit viral replication. Although inhibition of HIV replication by soluble factors produced by CD8+ T cells has been well documented, the identity of these factors remains controversial. The initial report from Cocchi et al. suggested that RANTES, MIP-1α, and MIP-1β were largely responsible for the ability of supernatants from CD8+ T cells to inhibit replication of CCR5-dependent HIV-1 strains (10). However, subsequent reports have suggested that CD8+ T cells are likely to produce other soluble factors able to inhibit HIV-1 replication (32, 43, 54). We found that both SIVmac239 and SIVmac251 were inhibited by RANTES, MIP-1α, and MIP-1β, a finding consistent with the identification of CCR5 as a coreceptor for several SIV strains, including SIVmac251 (6). In addition, we observed that production of these β-chemokines by CD3-stimulated CD8+ T cells from animals vaccinated with live attenuated SIV strains is 8- to 10-fold greater than in uninfected controls. A similar finding was reported by Lehner et al., who analyzed production of RANTES, MIP-1α, and MIP-1β by stimulated CD8+ T cells from animals vaccinated with a subunit SIV vaccine and demonstrated greater production of β-chemokines in protected than in unprotected vaccinees (31).
However, our results suggest that the β-chemokines RANTES, MIP-1α, and MIP-1β are not the dominant soluble mediators of suppression of SIV replication by CD8+ T cells from animals vaccinated with live, attenuated SIV strains. First, we observed no significant blocking of the ability of supernatant from activated CD8+ T cells from SIV239Δnef-infected animals to inhibit SIV replication following the addition of a combination of polyclonal antibodies to RANTES, MIP-1α, and MIP-1β. Although neutralizing antibodies specific for rhesus macaque chemokines are not available, the amino acid sequences of rhesus RANTES and MIP-1α are 100% conserved with their human homologs, and MIP-1β is 95% identical (47b). Therefore, these human-specific antisera are likely to neutralize the appropriate rhesus molecules. Second, we observed a significantly greater inhibition of SIV replication by soluble factors produced from stimulated CD8+ T cells than by recombinant β-chemokines. We demonstrated a 10- to 30-fold inhibition of SIV replication by soluble factors from stimulated CD8+ T cells, whereas only 2- to 3-fold inhibition was observed with the recombinant β-chemokines RANTES, MIP-α, and MIP--β, even using concentrations as high as 500 ng/ml and combinations of these molecules. Measured levels of β-chemokines in these supernatants were generally 20 to 30 ng/ml, a level at which we observed only minimal inhibition of SIV replication in assays using recombinant chemokines. Third, soluble factors from stimulated CD8+ T cells were able to induce up to 30-fold inhibition of SIV replication in the CEMx174 cell line, which does not express CCR5 (27). Finally, soluble factors from activated CD8+ T cells were also able to inhibit replication of CXCR4-dependent HIV-1 strains. Thus, our data suggest that CD8+ T lymphocytes from macaques vaccinated with live attenuated SIV strains produce factors other than RANTES, MIP-1α, and MIP-1β that are able to inhibit SIV and HIV replication and that these alternative factors are likely to play a dominant role in mediating suppression of SIV replication. However, these findings do not exclude a potential role for the β-chemokines in suppressing SIV replication. Identification of the molecules responsible for inhibition of SIV replication may facilitate identification of ligands for the recently described additional SIV coreceptors BOB (Gpr15) (8, 12) and Bonzo (STRL33) (2, 33) and to a better understanding of the role of these coreceptors in infection.
Since macaques immunized with SIV239Δnef or SIV239Δ3 are known to be protected against challenge with pathogenic SIVmac251 (11, 51), characterization of soluble factors able to inhibit SIV replication produced by CD8+ T lymphocytes from these animals may prove relevant to defining the correlates of immune protection. Future studies will be necessary to define the role of soluble factors in protective immunity and whether this property is independent of SIV-specific CTL activity. Our data suggest that the ability of CD8+ T cells to suppress SIV replication is the result of a specific immune response rather than the result of viral interference. CD4+ T cells from animals immunized with SIVmac239Δnef or SIVmac239Δ3 were easily infected with SIV in vitro, resulting in levels of viral replication similar to those observed in CD4+ T cells from uninfected controls. Although we cannot exclude the possibility of viral interference in a reservoir other than CD4+ T lymphocytes, since CD4+ T lymphocytes are the dominant reservoir for HIV-1 replication in vivo (21, 46) and the frequency of SIV-infected cells in animals infected with live attenuated SIV strains is relatively low (26, 51), this possibility appears unlikely. Investigation of the ability of CD8+ T lymphocytes to inhibit SIV replication in vitro, and the identification of components involved in the protective immunity in vivo, may lead to a better understanding of AIDS pathogenesis and to new preventative strategies.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grants RR 00168 and AI35365.
We thank Ronald C. Desrosiers for many helpful discussions and providing samples from SIV-infected animals, Bruce Walker for encouragement and support, Ronald C. Desrosiers and Otto Yang for review of the manuscript, Otto Yang for providing the PM1 cell line and the HIV-1Ba-L and HIV-1JR-CSF isolates, Andrew Luster for providing recombinant chemokines, and Michael Rosenzweig and MaryAnn DeMaria for assistance with flow cytometry.
REFERENCES
- 1.Alkhatib G, Combadiere C, Broder C C, Feng Y, Kennedy P E, Murphy P M, Berger E A. CC CKR5: a RANTES, MIP-1α, MIP-1β receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 2.Alkhatib G, Liao F, Peden K W C, Berger E A, Farber J M. A new SIV co-receptor, STRL33. Nature. 1997;388:238. doi: 10.1038/40789. [DOI] [PubMed] [Google Scholar]
- 3.Allen, T. M., J. Sidney, M.-F. Del Guercio, R. Glickman, G. Lensmeyer, D. Wiebe, C. Pauza, R. P. Johnson, A. Sette, and D. I. Watkins. Characterization of the peptide binding motif of a rhesus MHC Class I molecule (Mamu-A*01) that binds an immunodominant CTL epitope from SIV. J. Immunol., in press. [PubMed]
- 4.Baier M, Werner A, Bannert N, Metzner K, Kurth R. HIV suppression by interleukin-16. Nature. 1995;378:563. doi: 10.1038/378563a0. [DOI] [PubMed] [Google Scholar]
- 5.Brinchmann J E, Gaudernack G, Vartdal F. CD8+ T cells inhibit HIV replication in naturally infected CD4+ T cells. Evidence for a soluble inhibitor. J Immunol. 1990;144:2961–2966. [PubMed] [Google Scholar]
- 6.Chen Z, Zhou P, Ho D D, Landau N R, Marx P A. Genetically divergent strains of simian immunodeficiency virus use CCR5 as a coreceptor for entry. J Virol. 1997;71:2705–2714. doi: 10.1128/jvi.71.4.2705-2714.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choe H, Farzan M, Sun Y, Sullivan N, Rollins B, Wu P P D, L, Mackay C R, LaRosa G, Newman W, Gerard N, Gerard C, Sodroski J. The beta-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell. 1996;85:1135–1148. doi: 10.1016/s0092-8674(00)81313-6. [DOI] [PubMed] [Google Scholar]
- 8.Clapham P R, Weiss R A. Spoilt for choice of co-receptors. Nature. 1997;388:230–231. doi: 10.1038/40758. [DOI] [PubMed] [Google Scholar]
- 9.Clerici M, Balotta C, Galli M. Soluble HIV suppressive factors: more than one Holy Grail? Immunol Today. 1996;17:297. doi: 10.1016/0167-5699(96)80790-0. [DOI] [PubMed] [Google Scholar]
- 10.Cocchi F, DeVico A L, Garzino-Demo A, Arya S K, Gallo R C, Lusso P. Identification of RANTES, MIP-1α, and MIP-1β as the major HIV-suppressive factors produced by CD8+ T cells. Science. 1995;270:1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 11.Daniel M D, Kirchhoff F, Czajak S C, Sehgal P K, Desrosiers R C. Protective effects of a live attenuated SIV vaccine with a deletion in the nef gene. Science. 1992;258:1938–1941. doi: 10.1126/science.1470917. [DOI] [PubMed] [Google Scholar]
- 12.Deng H, Unutmaz D, KewalRamani V N, Littman D R. Expression cloning of new receptors used by simian and human immunodeficiency viruses. Nature. 1997;388:296–300. doi: 10.1038/40894. [DOI] [PubMed] [Google Scholar]
- 13.Dittmer U, Nisslein T, Bodemer W, Petry H, Sauermann U, Stahl H C, Hunsmann G. Cellular immune response of rhesus monkeys infected with a partially attenuated nef deletion mutant of the simian immunodeficiency virus. Virology. 1995;212:392–397. doi: 10.1006/viro.1995.1496. [DOI] [PubMed] [Google Scholar]
- 14.Dragic T, Litwin V, Allaway G P, Martin S R, Huang Y, Nagashima K S, Cayanan C, Maddon P J, Koup R A, Moore J P, Paxon W A. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature. 1996;381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 15.Dulbecco R. Virology. Philadelphia, Pa: J. P. Lippincott; 1988. pp. 22–25. [Google Scholar]
- 16.Erickson A L, Walker C M. An epitope in the V1 domain of the simian immunodeficiency virus (SIV) gp120 protein is recognized by CD8+ cytotoxic T lymphocytes from an SIV-infected rhesus macaque. J Virol. 1994;68:2756–2759. doi: 10.1128/jvi.68.4.2756-2759.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Farzan M, Choe H, Martin K, Marcon L, Hofmann W, Karlsson G, Sun Y, Barrett P, Marchand N, Sullivan N, Gerard N, Gerard G, Sodroski J. Two orphan seven-transmembrane segment receptors which are expressed in CD4-positive cells support simian immunodeficiency virus infection. J Exp Med. 1997;186:405–411. doi: 10.1084/jem.186.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feng Y, Broder C C, Kennedy P E, Berger E A. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G-protein-coupled receptor. Science (Washington, DC) 1996;272:872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 19.Gallimore A, Cranage M, Cook N, Almond N, Bootman J, Rud E, Silvera P, Dennis M, Corcoran T, Stott J, McMichael A, Gotch F. Early suppression of SIV replication by CD8+nef-specific cytotoxic T cells in vaccinated macaques. Nat Med. 1995;1:1167–1173. doi: 10.1038/nm1195-1167. [DOI] [PubMed] [Google Scholar]
- 19a.Glickman, R., and P. Johnson. Unpublished data.
- 20.Ho D D, Moudgil T, Alam M. Quantitation of human immunodeficiency virus type 1 in the blood of infected persons. N Engl J Med. 1989;321:1621–1625. doi: 10.1056/NEJM198912143212401. [DOI] [PubMed] [Google Scholar]
- 21.Ho D D, Neumann A U, Perelson A S, Chen W, Leonard J M, Markowitz M. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature. 1995;373:123–126. doi: 10.1038/373123a0. [DOI] [PubMed] [Google Scholar]
- 22.Johnson R P, Glickman R L, Yang J Q, Kaur A, Dion J T, Mulligan M J, Desrosiers R C. Induction of vigorous cytotoxic T-lymphocyte responses by live attenuated simian immunodeficiency virus. J Virol. 1997;71:7711–7718. doi: 10.1128/jvi.71.10.7711-7718.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kannagi M, Masuda T, Hattori T, Kanoh T, Nasu K, Yamamoto N, Harada S. Interference with human immunodeficiency virus (HIV) replication by CD8+ T cells in peripheral blood leukocytes of asymptomatic HIV carriers in vitro. J Virol. 1990;64:3399–3406. doi: 10.1128/jvi.64.7.3399-3406.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23a.Kaur, A., R. Glickman, and R. P. Johnson. Unpublished data.
- 24.Kawai T, Wong J, MacLean J, Cosimi A B, Wee S. Characterization of a monoclonal antibody (6G12) recognizing the cynomolgus monkey CD3 antigen. Transplant Proc. 1994;26:1845–1846. [PubMed] [Google Scholar]
- 25.Kestler H, Kodama T, Ringler D, Marthas M, Pedersen N, Lackner A, Regier D, Sehgal P, Daniel M, King N, Desrosiers R. Induction of AIDS in rhesus monkeys by molecularly cloned simian immunodeficiency virus. Science. 1990;248:1109–1112. doi: 10.1126/science.2160735. [DOI] [PubMed] [Google Scholar]
- 26.Kestler H W, Ringler D J, Mori K, Panicali D L, Sehgal P D, Daniel M D, Desrosiers R C. Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell. 1991;65:651–662. doi: 10.1016/0092-8674(91)90097-i. [DOI] [PubMed] [Google Scholar]
- 27.Kirchhoff F, Pohlmann S, Hamacher M, Means R E, Kraus T, Uberla K, Marzio P D. Simian immunodeficiency virus variants with differential T-cell and macrophage tropism use CCR5 and an unidentified cofactor expressed in CEMx174 cells for efficient entry. J Virol. 1997;71:6509–6516. doi: 10.1128/jvi.71.9.6509-6516.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koup R A, Ho D D. Quantitative culture assay for HIV-1 in peripheral blood. In: Aldovini A, Walker B D, editors. Techniques in HIV research. New York, N.Y: Stockton Press; 1990. pp. 107–112. [Google Scholar]
- 29.Koyanagi Y, Miles S, Mitsuyasu R T, Merrill J E, Vinters H V, Chen I S. Dual infection of the central nervous system by AIDS viruses with distinct cellular tropisms. Science. 1987;236:819–822. doi: 10.1126/science.3646751. [DOI] [PubMed] [Google Scholar]
- 30.Landay A L, Mackewicz C E, Levy J A. An activated CD8+ T cell phenotype correlates with anti-HIV activity and asymptomatic clinical status. Clin Immunol Immunopathol. 1993;69:106–116. doi: 10.1006/clin.1993.1157. [DOI] [PubMed] [Google Scholar]
- 31.Lehner T, Wang Y, Cranage M, Bergmeier L A, Mitchell E, Tao L, Hall G, Dennis M, Cook N, Brookes R, Klavinskis L, Jones I, Doyle C, Ward R. Protective mucosal immunity elicited by targeted iliac lymph node immunization with subunit SIV envelope and core vaccine in macaques. Nat Med. 1996;2:767–775. doi: 10.1038/nm0796-767. [DOI] [PubMed] [Google Scholar]
- 32.Levy J A, Mackewicz C E, Barker E. Controlling HIV pathogenesis: the role of noncytotoxic anti-HIV activity of CD8+ T cells. Immunol Today. 1996;17:217–224. doi: 10.1016/0167-5699(96)10011-6. [DOI] [PubMed] [Google Scholar]
- 33.Liao F, Alkhatib G, Peden K W C, Sharma G, Berger E A, Farber J M. STRL33, a novel chemokine receptor-like protein, functions as a fusion cofactor for both macrophage-tropic and T cell line-tropic HIV-1. J Exp Med. 1997;185:2015–2023. doi: 10.1084/jem.185.11.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lusso P, Cocchi F, Balotta C, Markham P D, Louie A, Farci P, Pal R, Gallo R C, Reitz M S. Growth of macrophage-tropic and primary human immunodeficiency virus type 1 (HIV-1) isolates in a unique CD4+ T-cell clone (PM1): failure to downregulate CD4 and to interfere with cell-line-tropic HIV-1. J Virol. 1995;69:3712–3720. doi: 10.1128/jvi.69.6.3712-3720.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mackewicz C, Levy J A. CD8+ cell anti-HIV activity: nonlytic suppression of virus replication. AIDS Res Hum Retroviruses. 1992;8:1039–1050. doi: 10.1089/aid.1992.8.1039. [DOI] [PubMed] [Google Scholar]
- 36.Mackewicz C E, Ortega H, Levy J A. Effect of cytokines on HIV replication in CD4+ T lymphocytes: lack of identity with the CD8+ cell antiviral factor. Cell Immunol. 1994;153:329–343. doi: 10.1006/cimm.1994.1032. [DOI] [PubMed] [Google Scholar]
- 37.Mackewicz C E, Ortega H W, Levy J A. CD8+ cell anti-HIV activity correlates with the clinical state of the infected individual. J Clin Invest. 1991;87:1462–1466. doi: 10.1172/JCI115153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mackewicz C E, Yang L C, Lifson J D, Levy J A. Non-cytolytic CD8+ T cells anti-HIV responses in primary HIV-1 infection. Lancet. 1994;344:1671–1673. doi: 10.1016/s0140-6736(94)90459-6. [DOI] [PubMed] [Google Scholar]
- 39.Marcon L, Choe H, Martin K A, Farzan M, Ponath P D, Wu L, Newman W, Gerard N, Gerard C, Sodroski J. Utilization of C-C chemokine receptor 5 by the envelope glycoproteins of a pathogenic simian immunodeficiency virus, SIVmac239. J Virol. 1997;71:2522–2527. doi: 10.1128/jvi.71.3.2522-2527.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McMichael A J, Walker B D. Cytotoxic T lymphocyte epitopes: implications for HIV vaccines. AIDS. 1994;8:S155–S173. [Google Scholar]
- 41.Means R E, Greenough T, Desrosiers R C. Neutralization sensitivity of cell culture-passaged simian immunodeficiency virus. J Virol. 1997;71:7895–7902. doi: 10.1128/jvi.71.10.7895-7902.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miller M D, Lord C I, Stallard V, Mazzara G P, Letvin N L. The gag-specific cytotoxic T lymphocytes in rhesus monkeys infected with the simian immunodeficiency virus of macaques. J Immunol. 1990;144:122–128. [PubMed] [Google Scholar]
- 43.Pal R, Garzino-Demo A, Markham P D, Burns J, Brown M, Gallo R C, DeVico A L. Inhibition of HIV-1 infection by the beta-chemokine MDC. Science. 1997;278:695–698. doi: 10.1126/science.278.5338.695. [DOI] [PubMed] [Google Scholar]
- 44.Paliard X, Lee A Y, Walker C M. RANTES, MIP-1α and MIP-1β are not involved in the inhibition of HIV-1SF33 replication mediated by CD8+ T-cell clones. AIDS. 1996;10:1317–1321. doi: 10.1097/00002030-199610000-00002. [DOI] [PubMed] [Google Scholar]
- 44a.Reed L J, Muench H. A simple method of estimating fifty per cent endpoints. Am J Hyg. 1938;27:493–497. [Google Scholar]
- 45.Scala E, D’Offizi G, Rosso R, Turriziana O, Ferrara R, Mazzone A M, Antonelli G, Aiuti F, Paganelli R. C-C chemokines, IL-16, and soluble antiviral factor activity are increased in cloned T cells from subjects with long-term nonprogressive HIV infection. J Immunol. 1997;158:4485–4492. [PubMed] [Google Scholar]
- 46.Schnittman S M, Psallidopoulos M C, Lane H C, Thompson T, Baseler M, Massari F, Fox C H, Salzman N P, Fauci A S. The reservoir for HIV-1 in human peripheral blood is a T cell that maintains expression of CD4. Science. 1989;245:305–308. doi: 10.1126/science.2665081. [DOI] [PubMed] [Google Scholar]
- 47.Tsubota H, Lord C I, Watkins D I, Morimoto C, Letvin N L. A cytotoxic T lymphocyte inhibits AIDS virus replication in peripheral blood lymphocytes. J Exp Med. 1989;169:1421–1434. doi: 10.1084/jem.169.4.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47a.U.S. Department of Health and Human Services. Guide for the care and use of laboratory animals. DHHS publication no. (NIH) 85-23. U.S. Washington, D.C: Government Printing Office; 1985. [Google Scholar]
- 47b.Villinger, F. Personal communication.
- 48.Walker C M, Erickson A L, Hsueh F C, Levy J A. Inhibition of human immunodeficiency virus replication in acutely infected CD4+ cells by CD8+ cells involves a noncytolytic mechanism. J Virol. 1991;65:5921–5927. doi: 10.1128/jvi.65.11.5921-5927.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Walker C M, Moody D J, Stites D P, Levy J A. CD8+ lymphocytes can control HIV replication in vitro by suppressing virus replication. Science. 1986;234:1563–1566. doi: 10.1126/science.2431484. [DOI] [PubMed] [Google Scholar]
- 50.Walker M C, Levy J A. A diffusible lymphokine produced by CD8+ T lymphocytes suppresses HIV replication. Immunology. 1989;66:62–630. [PMC free article] [PubMed] [Google Scholar]
- 51.Wyand M S, Manson K H, Garcia-Moll M, Montefiori D, Desrosiers R C. Vaccine protection by a triple deletion mutant of simian immunodeficiency virus. J Virol. 1996;70:3724–3733. doi: 10.1128/jvi.70.6.3724-3733.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xu X-N, Screaton G R, Gotch F M, Dong T, Tan R, Almond N, Walker B, Stebbings R, Kent K, Nagata S, Stott J E, McMichael A J. Evasion of CTL responses by nef-dependent induction of fas ligand (CD95L) expression on simian immunodeficiency virus-infected cells. J Exp Med. 1997;186:7–16. doi: 10.1084/jem.186.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang O O, Kalams S A, Trocha A, Cao H, Luster A, Johnson R P, Walker B D. Suppression of human immunodeficiency virus type 1 replication by CD8+ cells: evidence for HLA class I-restricted triggering of cytolytic and noncytolytic mechanisms. J Virol. 1997;71:3120–3128. doi: 10.1128/jvi.71.4.3120-3128.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang O O, Walker B D. CD8+ cells in human immunodeficiency virus type 1 pathogenesis: cytolytic and noncytolytic inhibition of viral replication. Adv Immunol. 1997;66:273–311. doi: 10.1016/s0065-2776(08)60600-8. [DOI] [PubMed] [Google Scholar]