Abstract
Background
The prognostic significance of cardiac magnetic resonance (CMR)-based left atrial ejection fraction (LAEF) is not well defined in the ischemic cardiomyopathy (ICM) cohort.
Objectives
The authors sought to assess the prognostic impact of LAEF, when adjusted for left ventricular remodeling, myocardial infarct size (MIS), left atrial volume index, and functional mitral regurgitation (FMR), on outcomes in patients with advanced ICM.
Methods
ICM patients who underwent CMR were retrospectively evaluated (April 2001-December 2019). LAEF, left atrial volume index, MIS, left ventricular remodeling, and FMR were derived from CMR. The primary clinical endpoint was a composite of all-cause mortality and cardiac transplant. A baseline multivariable Cox proportional hazards regression model was constructed to assess prognostic power of LAEF.
Results
There were 718 patients (416 primary events) evaluated, with a median duration of follow-up of 1,763 days (4.8 years) and a mean LAEF of 36% ± 15%. On multivariable analysis, higher LAEF was independently associated with reduced risk (HR: 0.24, 95% CI: 0.12-0.48, P < 0.001), even after adjusting for FMR and MIS. The highest adjusted risk was observed in patients with an LAEF <20% and an MIS of >30% (HR: 3.20, 95% CI: 1.73-5.93). The lowest risk was in patients within the comparator group with an LAEF of >50% and a MIS of <15% (HR: 1.07, 95% CI: 0.81-1.42).
Conclusions
Reduced LAEF is independently associated with increased mortality in ICM. Risk associated with declining LAEF is continuous and incremental to other risk factors for adverse outcomes in patients with ICM even after adjusting for MIS and FMR severity.
Key words: ischemic cardiomyopathy, left atrial ejection fraction, patient factors and left atrial function, prognostic indices in advanced ICM
Central Illustration
Patients with ischemic cardiomyopathy (ICM) represent a complex and clinically challenging cohort with significant morbidity and mortality.1 Such poor outcomes underpin the importance of accurate risk stratification to allow earlier initiation of optimal guideline-derived medical therapy. Adverse left atrial (LA) remodeling, specifically increased LA size, is recognized as a risk factor for adverse outcomes across a range of cardiomyopathy phenotypes.2, 3, 4 In addition, indices of LA function such as left atrial ejection fraction (LAEF) have also been increasingly recognized as an important prognostic marker in patients with impaired cardiac function.5 Reduced LA function has also been demonstrated to be correlated with diminished functional capacity, greater rates of hospitalization, and increased mortality across the entire spectrum of left ventricular (LV) function.4, 5, 6, 7, 8, 9, 10, 11
Although a significant proportion of LAEF outcome data is derived from transthoracic echocardiography (TTE)-based measurements, obtaining accurate and reproducible echocardiographic measures of LA function can be challenging.6, 7, 8, 9 Optimal LA endocardial border definition is required to accurately quantify LAEF, which is often challenging due to a myriad of patient and sonographic operator-dependent factors.10 In addition, TTE-based measurements of both atrial and ventricular volumes systematically underestimate chamber size in comparison to cardiac magnetic resonance (CMR) imaging.11
CMR is the gold standard imaging modality for the assessment of cardiac chamber volume and function, as it affords superior spatial resolution, contrast between the blood pool and myocardium, and greater reproducibility in comparison to TTE.12,13 Consequently, CMR-based measurement of LA size and function have been described as important factors associated with adverse outcomes across various cardiomyopathy processes.14, 15, 16 The prognostic capacity of CMR-based LAEF and its interaction with more traditional risk factors for ICM outcomes have not been widely explored previously.
Accordingly, we retrospectively evaluated CMR-based LAEF and associations with adverse outcomes and explored its interaction with recognized risk factors. In addition, we sought to determine the prognostic impact of LAEF on outcomes after adjusting for baseline demographic variables, LV size/function, infarct, functional mitral regurgitation (FMR) severity, and subsequent therapeutic interventions in a large, single-center ICM cohort.
Methods
Study population
This was an observational, retrospective cohort study of consecutive patients who were referred for CMR imaging between April 2001 and March 2017 at the Cleveland Clinic for the evaluation of advanced ICM. The ethics approval of this study was obtained from the institution review board of our institution (IRB 11-083) with a patient consent waiver. For the purposes of this study, we defined ICM as ≥70% stenosis in ≥1 epicardial coronary vessels on angiography or history of myocardial infarction/classic ischemic scar pattern on CMR, or prior coronary revascularization (CRT) with an LV ejection fraction of ≤40% as quantified by CMR. Coronary artery disease severity is defined as the number of major coronary artery vessels with significant stenoses (>70% stenosis), and the score of 4 was assigned to patients with 3-vessel + left main coronary disease. Patients with no significant coronary artery disease on coronary angiography but history of prior MI with characteristic infarct pattern on CMR or prior percutaneous coronary intervention were assigned a score of 0. Exclusion criteria included prior mitral valve (MV) surgery, intrinsic MV pathology (prolapse/flail) concurrent mitral and other valvular stenosis, and patients with frequent atrial and/or ventricular dysrhythmia. Given the complexity of our patient cohort, we utilized a medical risk score capturing baseline clinical characteristics previously described in ICM patients to facilitate subsequent multivariable analyses, which include the following parameters: age, sex, body mass index, sex, diabetes, glomerular filtration rate, hypertension, dyslipidemia, medications (beta-blocker, angiotensin-converting enzyme inhibitor/receptor blocker), left or right bundle branch block, and QRS duration.17,18
Clinical outcomes and associations with LAEF
The primary clinical outcome is the composite endpoint of all-cause mortality or cardiac transplant. Death was determined using the EPIC electronic health record and an online obituary search if no record of death was found in the electronic health record.
The secondary aim of this study was to determine the presence of significant interactions between LAEF and LA volume (LAV) with end systolic volume index, FMR severity by CMR, myocardial infarct size (MIS), and subsequent therapeutic interventions. In addition, factors that were associated with LAEF in this study cohort were analyzed.
CMR protocol
CMR examinations were performed on 1.5-T magnetic resonance imaging scanners (Sonata and Avanto, Siemens Medical Solutions, for imaging between 2002 and 2006, and Philips Achieva XR, for imaging between 2007 and 2017), using 40 to 45 mT/m maximum gradient strength and 200 T/m per second maximum slew rate with electrocardiographic gating. Ventricular chamber assessment on CMR was performed on short-axis cine images in accordance with guidelines.19 Phase contrast imaging was acquired to assess aortic forward flow at the mid-ascending aorta with a VENC of 200 cm/s. Approximately 15 to 20 minutes following injection of 0.1 to 0.2 mmol/kg gadolinium dimeglumine, late gadolinium enhancement (LGE) images were acquired in long- and short-axis orientations.
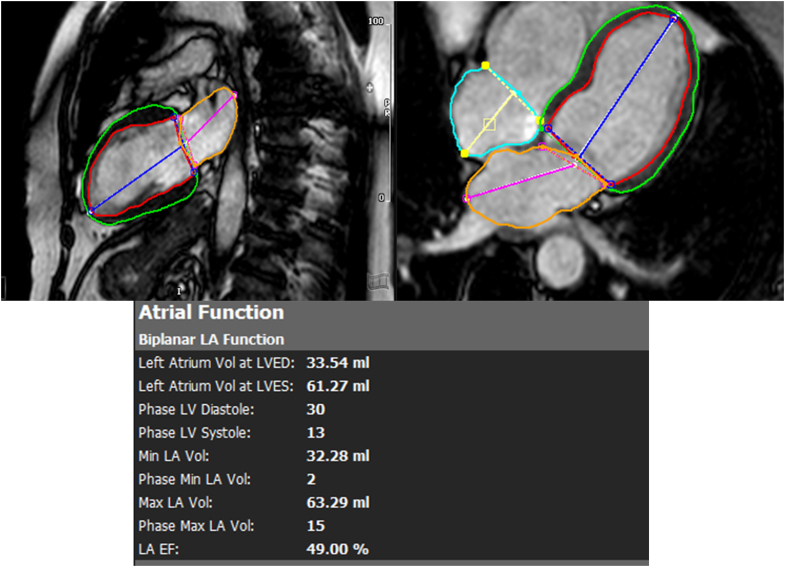
LA endocardial borders were manually traced in apical 2- and 4-chamber orientation on CMR just after MV closure and immediately before MV opening to estimate minimal and maximal left atrial volumes (LAV), respectively, using the biplane area-length method (Figure 1).20 Measurements were indexed to body surface area. Emptying fractions of the left atrium (LAEF) were calculated as: (maximal LAV − minimal LAV)/maximal LAV × 100%. Mitral regurgitation (MR) quantification on CMR was based on regurgitant fraction (RF) (using the formula [LV stroke volume-aortic phase-contrast forward flow]/LV stroke volume).21
Figure 1.
Cardiac Magnetic Resonance Evaluation of LA Volume Index
EF = ejection fraction; LA = left atrial; LV = left ventricular; LVED = left ventricular end diastolic; LVES = left ventricular end systolic.
Left and right ventricular volumes, EF, and mass were calculated from the short-axis cine stack excluding papillary muscles and trabeculations.7 Myocardial scar was quantified on late gadolinium-enhanced images. LGE was classified as having a greater intensity than user-specified viable myocardium by >2 SDs, as previously described.17,18,22 Scar burden was automatically determined by the cvi 42 software as the percentage of total myocardium (infarct size/mass divided by total LV volume/mass). SA commercially available software was used for image analysis on CMR (cvi42, Circle Cardiovascular Imaging Inc).
Statistical methods
Data is presented as mean ± SD for continuous variables and as a count (percentage) for categorical variables. Factors associated with LAEF were determined on multivariable regression analysis. A baseline multivariable Cox proportional hazards regression model was first constructed, as previously published.23 This baseline multivariable model included the following covariates: medical risk score,18 implantable cardioverter-defibrillator (ICD) (binary variable), CRT (binary), surgical MV intervention (binary), pre-CMR coronary artery bypass graft or percutaneous coronary intervention (binary), incomplete revascularization (binary), LV end-systolic volume index (LVESVi), scar (<15, 15-29, ≥30), MR fraction (<35%, ≥35%),22 and the interaction of scar and MR fraction. Due to collinearity, LVESVi was included in the model in lieu of LV ejection fraction.
Univariable Cox proportional hazards regression models were constructed for LA function and LAV index. These variables were then separately added to the baseline model. A significance level of 0.05 was used for testing these 3 variables, with Holm’s method applied to control the type I error rate. Harrell’s concordance statistic was reported for each model.
In the final multivariable survival model, 4 prespecified interactions of LA function with LVESVi, surgical revascularization, scar, and MR fraction were tested, each at a significance level of 0.05.
Results
Of the 782 patients who were included in the study, LA functional data was measurable in 718 patients and included in the analyses. The total number of patients screened and the reasons for exclusion are outlined in Figure 2. LAEF could not be measured in 64 patients (8%) due to suboptimal image quality or incompletely visualized LA. Patients were followed for a median of 1,763 days (or 4.8 years), with last follow-up occurring in March 2019. There were 416 primary outcomes for analyses, including 399 all-cause deaths, and 17 patients underwent heart transplantation after extensive evaluation by an expert multidisciplinary team and approval by the Ohio Solid Organ Transplant Consortium. The baseline characteristics are described in Table 1. The means and standard deviations for LAEF and left atrial volume index (LAVi) 36% ± 15% and 54.4 ± 20.0 mL/m2, respectively. Procedures during follow-up after CMR include 65 (9.1%) having CRT, 220 (30.6%) having ICD, 346 (48.2%) having coronary artery bypass graft, 32 (4.5%) having percutaneous coronary intervention, and 132 (18.4%) having MV surgery.
Figure 2.
Flowchart for Study Patient Enrollment
Table 1.
Baseline Characteristics (N = 718)
| Clinical parameters | |
| Age (y) | 61.9 ± 11.2 |
| Female | 151 (23.4%) |
| Body mass index (kg/m2) | 28.8 ± 5.4 |
| Body surface area (m2) | 1.99 ± 20.4 |
| NYHA functional class | |
| 1 | 198 (25.3%) |
| 2 | 223 (28.5%) |
| 3 | 276 (35.3%) |
| 4 | 85 (10.9%) |
| Systolic blood pressure | 118 ± 19 |
| Diastolic blood pressure | 69 ± 11 |
| Hypertension | 398 (55.4%) |
| Diabetes | 269 (37.5%) |
| Dyslipidemia | 387 (53.9%) |
| Atrial fibrillation | 44 (6.1%) |
| Estimated glomerular filtration rate (mL/min/1.73 m2) | 82.9 ± 37 |
| Coronary artery disease severity | |
| 0 | 84 (12%) |
| 1 | 135 (19.3%) |
| 2 | 226 (32.4%) |
| 3 | 236 (33.8%) |
| 4 | 17 (2.4%) |
| Medications | |
| Angiotensin-converting enzyme inhibitor/angiotensin receptor blocker use | 509 (78.8%) |
| Beta-blocker | 531 (74%) |
| Statin | 556 (77.4%) |
| Aspirin | 407 (56.7%) |
| Diuretics | 266 (37%) |
| Mineralocorticoid antagonist | 246 (34.2%) |
| Magnetic resonance imaging parameters | |
| Left ventricular ejection fraction (%) | 25.6 ± 10.4 |
| Left ventricular end-diastolic volume index | 136.9 ± 44.4 |
| Left ventricular end-systolic volume index | 104.7 ± 44.8 |
| Left ventricular mass index | 92.0 ± 39.5 |
| Left atrial volume index | 54.4 ± 20.0 |
| Left atrial ejection fraction | 36 ± 15 |
| Mitral regurgitation volume | 17.4 ± 17.2 |
| Late gadolinium enhancement | 12 ± 11 |
| Right ventricular ejection fraction | 42.9 ± 13.6 |
Values are mean ± SD or n (%).
Multivariable analysis for factors associated with LAEF
The multivariable regression analysis for LAEF in the cohort is listed in Table 2 and illustrated in Figure 3. Key factors associated with LAEF include renal function, diabetes, atrial fibrillation, indexed LAV, and left ventricular ejection fraction, P < 0.001. Of interest, the presence of diabetes and MR fraction were also associated with LAEF, P = 0.001, as well as body mass index, P = 0.009. However, age (P = 0.52), sex (P = 0.48), hypertension (P = 0.66), and the presence of LGE (P = 0.073) were not associated with LAEF.
Table 2.
Multivariable Regression Analysis to Determine Factors Associated With Left Atrial Ejection Fraction
| Beta | 95% CI | P Value | |
|---|---|---|---|
| Age | 0.0003 | −0.0006 to 0.0012 | 0.52 |
| Female | −0.008 | −0.029 to 0.014 | 0.48 |
| Body mass index | −0.002 | −0.004 to −0.001 | 0.009 |
| Glomerular filtration rate | 0.0006 | 0.0003 to 0.0009 | <0.001 |
| Hypertension | −0.004 | −0.023 to 0.014 | 0.66 |
| Diabetes | −0.035 | −0.055 to −0.016 | 0.001 |
| Atrial fibrillation | −0.080 | −0.111 to −0.048 | <0.001 |
| Left atrial volume index | −0.0027 | −0.0032 to −0.0022 | <0.001 |
| Left ventricular ejection fraction | 0.0059 | 0.0048 to 0.0070 | <0.001 |
| Mitral regurgitant fraction | −0.0017 | −0.0028 to −0.0007 | 0.001 |
| Late gadolinium enhancement | −0.0008 | −0.0016 to 0.0001 | 0.073 |
| Mitral regurgitant fraction × late gadolinium enhancement | 0.000003 | −0.00003 to 0.00004 | 0.85 |
BoldP values are significant and <0.05.
Figure 3.
Factors Associated With Left Atrial Ejection Fraction in Generalized Linear Model
Baseline multivariable survival model
A baseline multivariable survival model was developed before adding LAVi and LAEF, which is presented in Table 3. A higher medical risk score and incomplete revascularization were the strongest covariates associated with adverse outcomes (HR: 2.11, 95% CI: 1.72-2.59 and HR: 1.63, 95% CI: 1.25-2.13) respectively; P < 0.001 (Table 2). A significant interaction between MIS and MR fraction demonstrated that risk associated with MIS increased, with the highest risk in patients with MIS of >30% and MR fraction of >35% (HR: 3.55, 95% CI: 1.78-3.52, P = 0.030) (Table 3).
Table 3.
Baseline Multivariable Model for Primary Clinical Endpoint
| Estimated HR (95% CI) | P Value | |
|---|---|---|
| Medical risk score | 2.11 (1.72-2.59) | <0.001 |
| Left ventricular end-systolic volume index | 1.00 (1.00-1.01) | 0.46 |
| Pre-CMR revascularization | 1.12 (0.87-1.43) | 0.39 |
| Revascularization during follow-up | 1.24 (0.94-1.66) | 0.13 |
| Implantable cardiac defibrillator during follow-up | 1.33 (1.00-1.75) | 0.048 |
| Mitral valve surgery during follow-up | 1.35 (0.98-1.87) | 0.066 |
| Incomplete revascularization | 1.63 (1.25-2.13) | <0.001 |
| 2-way interactions (MIS: myocardial infarct size) | 0.030 | |
| MIS 15-29% vs MIS <15% at MR fraction <35% | 1.23 (0.87-1.74) | |
| MIS ≥30% vs MIS <15% at MR fraction <35% | 1.30 (0.93-1.81) | |
| MIS ≥30% vs MIS 15-29% at MR fraction <35% | 1.06 (0.77-1.45) | |
| MIS 15-29% vs MIS <15% at MR fraction ≥35% | 1.93 (0.93-4.01) | |
| MIS ≥30% vs MIS 15-29% at MR fraction ≥35% | 1.84 (0.96-3.52) | |
| MIS ≥30% vs MIS <15% at MR fraction ≥35% | 3.55 (1.78-3.52) |
CMR = cardiac magnetic resonance; MIS = myocardial infarct size; MR = mitral regurgitant.
Prognostic impact of LAEF and LAVi
On univariable analysis, LAEF and LAVi were associated with adverse outcomes (HR: 0.12, 95% CI: 0.06-0.25, P < 0.001, and HR: 1.008, 95% CI: 1.003-1.014, P = 0.003, respectively). However, after adjusting for the other important predictors, only LAEF emerged as independently associated with the primary endpoint when added to the baseline multivariable survival model (Table 4). In this multivariable model, hazard significantly decreased as LAEF increased (HR: 0.24, 95% CI: 0.12-0.48, P < 0.001). LAVi had a HR: 1.00 (95% CI: 0.99-1.01, P = 0.12). The estimated HR for LAEF, after adjusting for other risk factors, is illustrated in Figure 4, demonstrating increased risk as LAEF decreases. Risk associated with LAEF is continuous without a clear sharp demarcation of increasing risk at a certain threshold. Figure 4 compares the HR for subjects with LAEF >0.6 to various categorizations of LAEF (0.50-0.59, 0.40-0.49, and so on), which demonstrates the continuous effect of LAEF on risk. For every 1% increase in LAEF, there is a 0.24 reduction in the risk of death. Alternatively, for every 1% decrease in LAEF, there is a 4.2 increase in the risk of death. The final model has a Harrell’s concordance index of 0.676 (SE = 0.015).
Table 4.
Final Multivariable Model for Primary Clinical Endpoint Including Left Atrial Ejection Fraction
| Estimated HR (95% CI) | P Value | |
|---|---|---|
| Left atrial ejection fraction | 0.24 (0.12-0.48) | <0.001 |
| Medical risk score | 1.79 (1.52-2.11) | <0.001 |
| Left ventricular end-systolic volume index | 1.00 (0.99-1.01) | 0.53 |
| Pre-CMR revascularization | 1.15 (0.94-1.41) | 0.17 |
| Revascularization during follow-up | 1.22 (0.97-1.54) | 0.085 |
| Implantable cardiac defibrillator during follow-up | 0.67 (0.54-0.84) | <0.001 |
| Mitral valve surgery during follow-up | 0.94 (0.71-1.24) | 0.65 |
| Incomplete revascularization | 1.47 (1.18-1.82) | 0.001 |
| 2-way interaction of MIS and MR fraction (MIS: myocardial infarct size, MR: mitral regurgitation) | 0.009 | |
| MIS 15-29% vs MIS <15% at MR fraction <35% | 1.07 (0.81-1.42) | |
| MIS ≥30% vs MIS <15% at MR fraction <35% | 1.30 (1.00-1.68) | |
| MIS ≥30% vs MIS 15-29% at MR fraction <35% | 1.21 (0.93-1.57) | |
| MIS 15-29% vs MIS <15% at MR fraction ≥35% | 1.28 (0.66-2.50) | |
| MIS ≥30% vs MIS <15% at MR fraction ≥35% | 3.22 (1.73-5.93) | |
| MIS ≥30% vs MIS 15-29% at MR fraction ≥35% | 2.50 (1.37-4.57) |
CMR = cardiac magnetic resonance; MIS = myocardial infarct size; MR = mitral regurgitant.
Figure 4.
Estimated HR of the Primary Endpoint by Left Atrial Ejection Fraction as a Function of its Magnitude, Adjusting for Other Covariates in Multivariable Analyses of Table 4
Prognostic impact of LAEF in relation to MIS and FMR
The final multivariable model demonstrates that higher medical risk scores, absence of an ICD, incomplete revascularization, the combination of high MIS and MR RF ≥35%, and lower LAEF were associated with greater risk of death or heart transplant (Table 4). To further illustrate the relationship with LAEF and infarct size, estimated risk was derived from our study cohort and is illustrated in Figure 5. In Figure 5, risks associated with different LAEF and infarct sizes are illustrated in 4 potential subjects, while other risk variables are held at their most common (average) values, based on risk modeling from our study data. LAEF of 20% and 50% were chosen to represent normal and significantly decreased LAEF, yet were not at the extremes of our observed sample (ie, 20% is ∼20th percentile and 50% is ∼80th percentile). This figure demonstrates that patients with increasing LAEF and infarct size are at the highest risk, and LAEF provides further risk stratification. Similarly, the prognostic impact of LAEF in relation to the risk associated with MIS and MR fraction was assessed, and it was demonstrated that the highest risk was observed in patients with an MIS of >30% and an MR fraction of >35%, with a HR of 3.2 (95% CI: 1.73-5.93). The lowest risk was in patients with an MIS of 15 to 29% and an MR fraction of <35%. Additionally, patients with scar <15%, RF<35%, and high LAEF (eg, 50%) demonstrated significantly improved survival compared with patients with MIS>30%, MR RF>35%, and low LAEF (eg, 20%) (Central Illustration).
Figure 5.
Model-Based Estimated Freedom From the Primary Endpoint of 4 Individuals With Specific LAEF and Infarct Size, Based on the Final Multivariable Fitted Model for Ischemic Cardiomyopathy
LAEF = left atrial ejection fraction.
Central Illustration.
Impact of Cardiac Magnetic Resonance-Derived Left Atrial Ejection Fraction on Outcomes of Ischemic Cardiomyopathy Patients
The following interactions were not statistically significant: LAEF and FMR severity (P = 0.38), LAEF with revascularization of MV intervention (P = 0.88), LAEF with MIS (P = 0.89), and LAEF with LVESVi (P = 0.21).
Discussion
Our large single-center cohort study demonstrates that CMR based assessment of LAEF is an important and powerful risk prognosticator in patients with advanced ICM. LAEF is additive to MIS and FMR severity as factors associated with adverse outcomes in this cohort. In addition, our findings demonstrate the presence of a significant continuous effect of LAEF on adverse outcomes in this cohort.
Our multivariable analyses of 718 patients with ICM demonstrate that reduced LAEF portends a significantly higher hazard of adverse outcomes when compared to their counterparts with higher LAEF, even after adjusting for infarct size and MR severity. The current available literature highlighting the prognostic role of LAEF in patients with ICM is based on significantly smaller studies. Kuhl et al24 in a study of 384 patients post-non-ST-segment elevation myocardial infarction demonstrated that LAEF measured by multidetector computed tomography was associated with both future adverse cardiovascular events and mortality. A separate study by Lonborg et al,25 demonstrated that CMR-derived LAEF was independently associated with death, risk of reinfarction, stroke, and heart failure hospitalization while providing incremental prognostic information to LAV in 199 patients post-ST-segment elevation myocardial infarction. Contrary to these studies, Liu et al26 found that both LAV and LAEF were not associated with adverse outcomes in 164 patients with non-ST-segment elevation acute coronary syndromes.26 Of interest, this study utilized TTE for the assessment of LAV and LAEF, potentially highlighting the importance of adequate spatial resolution and endocardial border definition afforded by CMR in the evaluation of indices of LA size and function. Added advantages of CMR-based LAEF over LA strain analysis are its reproducibility, user-friendliness, and current capabilities for automated segmentation to provide highly efficient evaluation of LAEF with currently available postprocessing software. Strain analysis may also be cumbersome and time-consuming, requiring a requisite threshold of competence for accurate evaluation.
Our study further expands on the understanding of the importance of LA function by highlighting the additive importance of LAEF to more established CMR markers of adverse outcomes in the ICM cohort, specifically infarct size and severity of MR, which have not previously been described. While LA function has been shown to be closely related to LV systolic and diastolic function across cardiomyopathic processes, LAEF provides independent and important mechanical contributions to cardiac output. The LA modulates LV filling by acting as a compliant reservoir, a passive conduit, and an active booster pump.27 The LA also serves as a modulator between the systemic and pulmonary circulation, buffering pressure between the systemic and pulmonary circulation.23 Decreased atrial compliance and consequently elevated LA pressures may lead to increased pulmonary venous congestion and elevation in pulmonary artery pressures, further highlighting the co-contributory yet independent role that LA dysfunction may play as a pathophysiologic entity, in addition to LV systolic and diastolic dysfunction in patients with ICM. This is further evidenced by the incremental and continuous effect of LAEF on outcomes in patients with ICM, as demonstrated in our analyses.
While prior studies have demonstrated the correlation between LA and ventricular size, left ventricular ejection fraction, and atrial fibrillation with LAEF, our study provides greater insight into both clinical and structural factors associated with LA function.27, 23, 28, 29, 30 Of interest, patient factors such as the presence of diabetes, renal impairment, elevated body mass index, and MR were associated with impaired LAEF in our cohort (Table 2). The presence of impaired LAEF has been previously described in patients with diabetes and elevated body mass index, independent of the effects of hypertension.28,29 Our study further informs the limited available literature on the impact of metabolic risk factors on indices of LA function, independent of its effect on LV function and patient outcomes.
The concept of proportionate vs disproportionate MR has been proposed as a plausible explanation for discrepant outcomes in patients with FMR and LV dysfunction who are selected for transcatheter edge-to-edge mitral valve repair (TEER) with varying degrees of acceptance.28 Changes in LA function pre- and post-TEER have been demonstrated to be independently associated with adverse outcomes and are influenced by the success of the procedure.31,32 Furthermore, while numerous previous studies have demonstrated the powerful prognostic importance of LVESVi, our current study suggests that LAEF is a more powerful risk factor than LVESVi in patients with advanced ICM.
Given the independent prognostic impact of LAEF, even after adjusting for infarct size and MR severity, LAEF may be an important additional imaging feature that may help identify patients with beneficial treatment response and improvement in outcomes following TEER. Further studies are needed to determine if more descriptive imaging features can allow development of more refined selection criteria and if such optimized criteria can improve patient selection and outcomes following valvular repair techniques.
Study Limitations
This was a retrospective, nonrandomized, single-center observational study with its inherent biases; however, this is the largest CMR study for the evaluation of LAEF in the ICM cohort. Because significant foreshortening of the LA on standard long-axis CMR views can occasionally occur and because some patients were in atrial fibrillation at the time of CMR image acquisition, 8% of our study cohort were excluded from the analysis due to inability to acquire reliable LAEF measurements. NT-proBNP/BNP was not available on all patients. Patients with CRT-D or ICDs are likely to not be reed for CMR, and therefore, selection bias may impact our study findings. Referral for CMR was at the discretion of the referring cardiologist with heterogeneous indications and CMR findings may have impacted treatment decisions. However, LA function measurements were not reported in the clinical reports and would not have impacted treatment decisions. Furthermore, multivariable survival analysis adjusted for revascularization and ICD implantations to address the impact of treatment on survival.
Conclusions
Our study establishes the important prognostic impact of CMR-based assessment of LAEF in patients with ICM and uniquely highlights the complex interplay and additive role that atrial mechanics may play in risk prognostication for patients with LV dysfunction due to ICM. CMR-based evaluation of LAEF is independently associated with adverse outcomes in patients with advanced ICM, even after adjusting for infarct size and severity of FMR. A reduced LAEF was highly significant and additive in prognostication in patients with increased scar burden across the spectrum of severity of MR.
Funding support and author disclosures
Dr Kwon is funded by the National Heart, Lung, and Blood Institute of the National Institutes of Health under 1R01HL170090-01; and has had a research agreement with Circle cvi42. Dr Tang is a consultant for Sequana Medical, Cardiol Therapeutics, Genomics plc, Zehna Therapeutics, Renovacor, Boston Scientific, WhiteSwell, Kiniksa Pharmaceuticals, CardiaTec Biosciences, and Intellia Therapeutics, Bristol-Myers Squibb, Alleviant Medical; and has received honoraria from Springer Nature and the American Board of Internal Medicine. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
PERSPECTIVES.
COMPETENCY IN MEDICAL KNOWLEDGE: This study, being the largest CMR cohort of patients with advanced ICM, establishes the importance and prognostic impact of CMR-based assessment of LAEF in patients with advanced ICM. The importance of LAEF as a key risk factor in patients with advanced ICM is demonstrated in this cohort, even after adjusting for more intuitive factors associated with adverse outcomes such as infarct size and severity of FMR.
TRANSLATIONAL OUTLOOK: Our data highlights the complex interplay and additive role that atrial mechanics may play in outcomes in patients with LV dysfunction due to ICM. Of interest, a reduced LAEF was highly significant and additive in being associated with adverse outcomes in patients with increased scar burden across the spectrum of severity of MR.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Nowbar A.N., Gitto M., Howard J.P., Francis D.P., Al-Lamee R. Mortality from ischemic heart disease. Circ Cardiovasc Qual Outcomes. 2019;12 doi: 10.1161/CIRCOUTCOMES.118.005375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abhayaratna W.P., Seward J.B., Appleton C.P., et al. Left atrial size: physiologic determinants and clinical applications. J Am Coll Cardiol. 2006;47:2357–2363. doi: 10.1016/j.jacc.2006.02.048. [DOI] [PubMed] [Google Scholar]
- 3.Quiñones M.A., Greenberg B.H., Kopelen H.A., et al. Echocardiographic predictors of clinical outcome in patients with left ventricular dysfunction enrolled in the SOLVD registry and trials: significance of left ventricular hypertrophy. Studies of Left Ventricular Dysfunction. J Am Coll Cardiol. 2000;35:1237–1244. doi: 10.1016/s0735-1097(00)00511-8. [DOI] [PubMed] [Google Scholar]
- 4.Tsang T.S., Abhayaratna W.P., Barnes M.E., et al. Prediction of cardiovascular outcomes with left atrial size: is volume superior to area or diameter? J Am Coll Cardiol. 2006;47:1018–1023. doi: 10.1016/j.jacc.2005.08.077. [DOI] [PubMed] [Google Scholar]
- 5.Pellicori P., Zhang J., Lukaschuk E., et al. Left atrial function measured by cardiac magnetic resonance imaging in patients with heart failure: clinical associations and prognostic value. Eur Heart J. 2015;36:733–742. doi: 10.1093/eurheartj/ehu405. [DOI] [PubMed] [Google Scholar]
- 6.de Groote P., Dagorn J., Soudan B., Lamblin N., McFadden E., Bauters C. B-type natriuretic peptide and peak exercise oxygen consumption provide independent information for risk stratification in patients with stable congestive heart failure. J Am Coll Cardiol. 2004;43:1584–1589. doi: 10.1016/j.jacc.2003.11.059. [DOI] [PubMed] [Google Scholar]
- 7.Lim T.K., Dwivedi G., Hayat S., Majumdar S., Senior R. Independent value of left atrial volume index for the prediction of mortality in patients with suspected heart failure referred from the community. Heart. 2009;95:1172–1178. doi: 10.1136/hrt.2008.151043. [DOI] [PubMed] [Google Scholar]
- 8.Rossi A., Cicoira M., Bonapace S., et al. Left atrial volume provides independent and incremental information compared with exercise tolerance parameters in patients with heart failure and left ventricular systolic dysfunction. Heart. 2007;93:1420–1425. doi: 10.1136/hrt.2006.101261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rossi A., Cicoira M., Zanolla L., et al. Determinants and prognostic value of left atrial volume in patients with dilated cardiomyopathy. J Am Coll Cardiol. 2002;40:1425. doi: 10.1016/s0735-1097(02)02305-7. [DOI] [PubMed] [Google Scholar]
- 10.Badano L.P., Miglioranza M.H., Mihăilă S., et al. Left atrial volumes and function by three-dimensional echocardiography. Circ Cardiovasc Imaging. 2016;9 doi: 10.1161/CIRCIMAGING.115.004229. [DOI] [PubMed] [Google Scholar]
- 11.Prakken N.H., Teske A.J., Cramer M.J., et al. Head-to-head comparison between echocardiography and cardiac MRI in the evaluation of the athlete's heart. Br J Sports Med. 2012;46:348–354. doi: 10.1136/bjsm.2010.077669. [DOI] [PubMed] [Google Scholar]
- 12.Hudsmith L.E., Petersen S.E., Francis J.M., Robson M.D., Neubauer S. Normal human left and right ventricular and left atrial dimensions using steady state free precession magnetic resonance imaging. J Cardiovasc Magn Reson. 2005;7:775–782. doi: 10.1080/10976640500295516. [DOI] [PubMed] [Google Scholar]
- 13.Kühl J.T., Lønborg J., Fuchs A., et al. Assessment of left atrial volume and function: a comparative study between echocardiography, magnetic resonance imaging and multi slice computed tomography. Int J Cardiovasc Imaging. 2012;28:1061–1071. doi: 10.1007/s10554-011-9930-2. [DOI] [PubMed] [Google Scholar]
- 14.Gulati A., Ismail T.F., Jabbour A., et al. Clinical utility and prognostic value of left atrial volume assessment by cardiovascular magnetic resonance in non-ischaemic dilated cardiomyopathy. Eur J Heart Fail. 2013;15:660–670. doi: 10.1093/eurjhf/hft019. [DOI] [PubMed] [Google Scholar]
- 15.Kanagala P., Arnold J.R., Cheng A.S.H., et al. Left atrial ejection fraction and outcomes in heart failure with preserved ejection fraction. Int J Cardiovasc Imaging. 2020;36:101–110. doi: 10.1007/s10554-019-01684-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schuster A., Backhaus S.J., Stiermaier T., et al. Left atrial function with MRI enables prediction of cardiovascular events after myocardial infarction: insights from the AIDA STEMI and TATORT NSTEMI trials. Radiology. 2019;293:292–302. doi: 10.1148/radiol.2019190559. [DOI] [PubMed] [Google Scholar]
- 17.Kwon D.H., Obuchowski N.A., Marwick T.H., et al. Jeopardized myocardium defined by late gadolinium enhancement magnetic resonance imaging predicts survival in patients with ischemic cardiomyopathy: impact of revascularization. J Am Heart Assoc. 2018;7 doi: 10.1161/JAHA.118.009394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cavalcante J.L., Kusunose K., Obuchowski N.A., et al. Prognostic impact of ischemic mitral regurgitation severity and myocardial infarct quantification by cardiovascular magnetic resonance. J Am Coll Cardiol Img. 2020;13:1489–1501. doi: 10.1016/j.jcmg.2019.11.008. [DOI] [PubMed] [Google Scholar]
- 19.Schulz-Menger J., Bluemke D.A., Bremerich J., et al. Standardized image interpretation and post-processing in cardiovascular magnetic resonance - 2020 update. J Cardiovasc Magn Reson. 2020;22:19. doi: 10.1186/s12968-020-00610-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rijnierse M.T., Kamali Sadeghian M., Schuurmans Stekhoven S., et al. Usefulness of left atrial emptying fraction to predict ventricular arrhythmias in patients with implantable cardioverter defibrillators. Am J Cardiol. 2017;120:243–250. doi: 10.1016/j.amjcard.2017.04.015. [DOI] [PubMed] [Google Scholar]
- 21.Garg P., Swift A.J., Zhong L., et al. Assessment of mitral valve regurgitation by cardiovascular magnetic resonance imaging. Nat Rev Cardiol. 2020;17:298–312. doi: 10.1038/s41569-019-0305-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kwon D.H., Kusunose K., Obuchowski N.A., et al. Predictors and prognostic impact of progressive ischemic mitral regurgitation in patients with advanced ischemic cardiomyopathy: a multimodality study. Circ Cardiovasc Imaging. 2016;9 doi: 10.1161/CIRCIMAGING.115.004577. [DOI] [PubMed] [Google Scholar]
- 23.Melenovsky V., Hwang S.-J., Redfield M.M., Zakeri R., Lin G., Borlaug B.A. Left atrial remodeling and function in advanced heart failure with preserved or reduced ejection fraction. Circ Heart Fail. 2015;8:295–303. doi: 10.1161/CIRCHEARTFAILURE.114.001667. [DOI] [PubMed] [Google Scholar]
- 24.Kühl J.T., Møller J.E., Kristensen T.S., Kelbæk H., Kofoed K.F. Left atrial function and mortality in patients with NSTEMI an MDCT study. J Am Coll Cardiol Img. 2011;4:1080–1087. doi: 10.1016/j.jcmg.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 25.Lønborg J.T., Engstrøm T., Møller J.E., et al. Left atrial volume and function in patients following ST elevation myocardial infarction and the association with clinical outcome: a cardiovascular magnetic resonance study. Eur Heart J Cardiovasc Imaging. 2013;14:118–127. doi: 10.1093/ehjci/jes118. [DOI] [PubMed] [Google Scholar]
- 26.Liu Y.T., Li R.J., Fang F., et al. Left atrial function assessed by tissue Doppler imaging as a new predictor of cardiac events after non-ST-elevation acute coronary syndrome. Echocardiography. 2012;29:785–792. doi: 10.1111/j.1540-8175.2012.01696.x. [DOI] [PubMed] [Google Scholar]
- 27.Thomas L., Muraru D., Popescu B.A., et al. Evaluation of left atrial size and function: relevance for clinical practice. J Am Soc Echocardiogr. 2020;33:934–952. doi: 10.1016/j.echo.2020.03.021. [DOI] [PubMed] [Google Scholar]
- 28.Gulmez O., Parildar H., Cigerli O., Demirağ N. Assessment of left atrial function in patients with type 2 diabetes mellitus with a disease duration of six months. Cardiovasc J Afr. 2018;29:82–87. doi: 10.5830/CVJA-2017-048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang G., Zhang L., Xie M., Fu M., Huang J., Lv Q. Assessment of left atrial function in diabetes mellitus by left atrial volume tracking method. J Huazhong Univ Sci Technolog Med Sci. 2010;30:819–823. doi: 10.1007/s11596-010-0665-4. [DOI] [PubMed] [Google Scholar]
- 30.de Simone G., Devereux R.B., Roman M.J., et al. Does cardiovascular phenotype explain the association between diabetes and incident heart failure? The Strong Heart Study. Nutr Metab Cardiovasc Dis. 2013;23:285–291. doi: 10.1016/j.numecd.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Öztürk C., Fasell T., Sinning J.M., et al. Left atrial global function in chronic heart failure patients with functional mitral regurgitation after MitraClip. Catheter Cardiovasc Interv. 2020;96:678–684. doi: 10.1002/ccd.28775. [DOI] [PubMed] [Google Scholar]
- 32.Ledwoch J., Leidgschwendner K., Fellner C., et al. Prognostic impact of left atrial function following transcatheter mitral valve repair. J Am Heart Assoc. 2019;8 doi: 10.1161/JAHA.118.011727. [DOI] [PMC free article] [PubMed] [Google Scholar]