ABSTRACT
Acinetobacter baumannii, associated with nosocomial infections, is considered a significant mortality risk if not adequately addressed. A. baumannii infections typically occur after surgery or trauma. Our patient developed complicated A. baumannii meningitis with lateral ventriculitis and a lumbar abscess post-surgery following a fall. The patient was treated with a 21-day regimen of intrathecally administered colistin and polymyxin B. Following this therapeutic period, the patient’s condition improved, leading to successful recovery and subsequent discharge. This case report highlights the effectiveness of intrathecal administration of antibiotics, which normally have limited potential for crossing the blood-brain barrier, in improving survival outcomes in multi-drug-resistant nosocomial meningitis.
Keywords: Acinetobacter baumannii, meningitis, colistin, polymyxin B, intrathecal
INTRODUCTION
Acinetobacter baumannii, a gram-negative rod typically found in soil and water, has been linked to nosocomial infections that can be life threatening if not treated urgently, particularly in patients who develop these infections post-surgically. These infections can manifest as meningitis, with symptoms including fever, headache, stiff neck, altered mental status, and seizures. Nosocomial infections with A. baumannii account for 4% of nosocomial meningitis cases and are frequently multi-drug resistant, often due to widespread use of antibiotics. A study of 2,667 post-neurosurgical meningitis patients identified cerebrospinal fluid (CSF) positivity in 45 patients, with 8.9% attributed to Acinetobacter baumannii.[1] This report discusses a case of multi-drug resistant Acinetobacter baumannii and our treatment approach.
CASE REPORT
A 41-year-old male fell from a horse and sustained traumatic hemopneumothorax, left posterior rib fractures, and multiple lumbar vertebral fractures in Libya. He underwent tube thoracostomy for his left lung and posterior instrumentation operation at T12-L1-L2 levels, followed by a 6-day intensive care unit (ICU) stay. One week later, on November 18th, 2022, he arrived in Istanbul, Türkiye, intending to continue his physical therapy at Medipol University. However, he soon presented with clouded consciousness and was urgently taken to the emergency department. Physical examination revealed poor cooperation, disorientation, a Glasgow Coma Scale (GCS) of 11 (E4M5V2), and paraplegia of the lower extremities. The thoracostomy tube was removed the following day, but the patient’s GCS decreased to 10 (E3M5V2), accompanied by persistent impaired consciousness. Furthermore, confusion, elevated C-reactive protein (CRP) and procalcitonin levels were noted, albeit without fever. A lumbar puncture was considered appropriate, and cultures were taken, including blood cultures, urine cultures, and CSF cultures. The Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) test was negative. As of November 19th, intravenous meropenem (3x2 g) and vancomycin (3x900 mg) were initiated. On November 20th, Acinetobacter baumannii was identified in blood cultures, and CRP levels peaked at 301 mg/L. Following consultation with the infectious disease department, it was suggested to discontinue vancomycin and narrow the patient’s treatment to intravenous meropenem (3x2 g), intravenous polymyxin B (2x800,000 IU), intravenous ampicillin-sulbactam (3x6 g), and intravenous and intrathecal colistin (1x10 mg) via an interspinous catheter. The main goal was a treatment regimen of 10 mg daily of intravenous and intrathecal colistin for 21 days, due to the multi-drug resistance of the pathogen, as seen in Table 1. This is in accordance with the Infectious Diseases Society of America’s guidelines on multidrug-resistant gram-negative infections,[2] alongside a review of the literature.[3,4,5] Furthermore, as per the manufacturer’s suggestions, polymyxin B was administered at maximum doses of 25,000 IU/kg daily, divided into two doses.[6]
Table 1.
Antibiotic resistance testing of CSF and blood
| MIC | Sensitivity | |
|---|---|---|
| Colistin | <1 | S |
| Gentamicin | >4 | R |
| Ciprofloxacin | >1 | R |
| Levofloxacin | >8 | R |
| Cefepime | >16 | R |
| Imipenem | >8 | R |
| Amikacin | >32 | R |
| TMP-SMX | >8/152 | R |
| Tobramycin | >8 | R |
The patient still displayed confusion and neck stiffness, and possessed a GCS of 10 (E3, M5, V3). On the following day, November 21st, symptoms of agitation, impaired consciousness, confusion, and neck stiffness persisted, and A. baumannii was identified in CSF cultures as well. The CSF analysis showed increased total protein, low glucose, and a predominance of polymorphonuclear neutrophils (PMN) at 25,085 x 10³/μl, as seen in Table 2. Additionally, thoracolumbar magnetic resonance imaging (MRI) findings indicated signs of meningitis and lateral ventriculitis, leading to a diagnosis of complicated A. baumannii meningitis. A posterior subdural collection from T7 to L1, highly suspicious of an abscess, was also found (Figure 1).
Table 2.
CSF laboratory findings corresponding to treatment day
| Treatment Day | Date | CSF Findings |
|---|---|---|
| 1 Day Before Treatment | 19/11/22 | - ↓ Glucose: <1.22 mg/dl |
| - ↑ Total protein: 1,471 mg/dl | ||
| - WBC: 25,085 x 10³/μl | ||
| - PMN: 91.7% | ||
| - CSF culture positive for A. baumannii | ||
| 12th Day of Treatment | 02/12/22 | - Normal glucose: 57.4 mg/dl |
| - ↑ Total protein: 71.6 mg/dl | ||
| - WBC: 0.016 x 10³/μl | ||
| - PMN: 24.9% | ||
| 13th Day of Treatment | 03/12/22 | - Normal glucose: 58.9 mg/dl |
| - ↑ Total protein: 49 mg/dl | ||
| - WBC: 0.016 x 10³/μl | ||
| - PMN: 24.9% | ||
| 17th Day of Treatment | 06/12/22 | |
| - WBC: 0.139 x 10³/μl | ||
| - PMN: 76.9% | ||
| - Culture negative | ||
| 20th Day of Treatment | 09/12/22 | - WBC: 0.005 x 10³/μl |
| - PMN: 0% | ||
| Final Day of Treatment | 11/12/22 | Culture negative |
| 3 Days After Final Treatment | 14/12/22 | Culture negative |
Figure 1.

Lumbar abscess.
Due to these findings, the intrathecal catheter was removed, and on November 30th, the patient underwent surgery for the removal of posterior instrumentation, drainage of the lumbar abscess, laminectomy at L1, and debridement of infected tissue. A new catheter was inserted into the lumbar spine by an experienced anesthesiologist familiar with the procedure. After the operation, the patient was admitted to the ICU, and the GCS improved to 14 (E4M6V4). Blood cultures taken on November 24th, November 25th, and January 12th showed no trace of the bacteria. On December 3rd, due to low hematocrit and a high CRP level of 174 mg/L, the patient underwent a Direct Coombs test, which returned positive. Hemolytic anemia, induced by ampicillin-sulbactam, was suspected to be the cause. Once the drug was discontinued, the patient’s hematocrit and CRP values improved and returned to normal limits.
The patient was then transferred to the internal medicine wards on December 2nd, and ampicillin-sulbactam and meropenem were discontinued that day. Intrathecal colistin and polymyxin B treatment continued, following the 21-day treatment plan advised by the infectious diseases department, concluding on January 11th. The patient was simultaneously monitored in the internal medicine ward for kidney functions. Creatinine levels gradually increased throughout the treatment plan, becoming significantly elevated from January 11th onwards, with peak levels reaching 2.71 mg/dL. Due to suspicion of drug-induced nephrotoxicity, intravenous N-acetylcysteine at 300 mg (3x2 ampules) was initiated on January 11th. The suspected causative agent, polymyxin B, was discontinued, and creatinine levels began to normalize. Subsequently, the patient recovered and was discharged from the hospital, as summarized in Figure 2.
Figure 2.
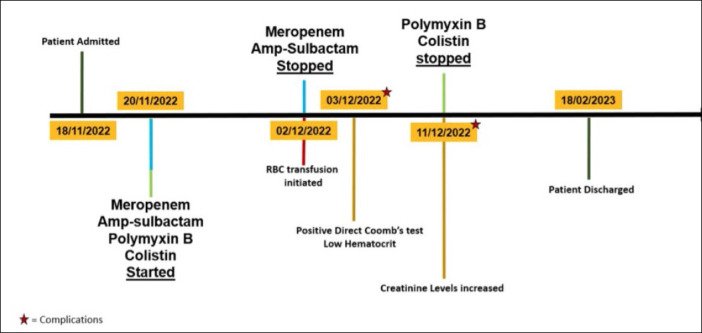
Major prognostic and diagnostic events timeline.
DISCUSSION
This report demonstrates the effectiveness of a combination therapy using intrathecal colistin alongside systemic colistin to treat A. baumannii. The patient received a regimen that included a 21-day course of 10 mg of both intravenous and intrathecal colistin, per guidelines published by the Infectious Diseases Society of America.[2] Following the initiation of this treatment course, A. baumannii was not detectable in blood cultures after four days and in CSF cultures 14 days post-treatment. These results are consistent with other reports suggesting the benefit of combining intravenous and intrathecal colistin in treating A. baumannii.[7,8] The literature provides no clear consensus on whether systemic or intrathecal administration of colistin is more effective. Some reports indicate that eradication can be achieved with intravenous colistin therapy alone,[9] while others advocate for the exclusive use of intrathecal administration to treat A. baumannii.[3,10,11,12,13]
Regardless of the causative agents, studies indicate that the intrathecal introduction of antibiotics, which have limited potential to cross the blood-brain barrier, such as colistin, in cases of multi-drug resistant nosocomial meningitis, improves survival outcomes.[14] Colistin (Polymyxin E) is typically available in vials containing one million units, corresponding to 80 mg of colistimethate. Colistin has been linked to multiple side effects, most notably nephrotoxicity and neurotoxicity.[15] The ability of colistin to penetrate into the CSF is controversial; thus, its exclusive use via intravenous administration in the treatment of A. baumannii meningitis is not recommended.[16] In a study examining 13 patients with nosocomial gram-negative meningitis between 2014 and 2018, Ayhan et al. found A. baumannii in eight out of 13 patients. Colistin was used in eight patients, and only two failed to achieve CSF negativity. This led the authors to recommend considering Intrathecal/Intraventricular (ITH/IVT) administration as a viable treatment option in gram-negative nosocomial meningitis, including cases caused by A. baumannii.[17] Moreover, studies have supported this by showing that patients treated with ITH/IVT displayed shorter hospital and ICU stays, as well as a trend towards lower mortality rates, particularly in severely ill patients.[18]
Polymyxin B, another antibiotic increasingly used as a last resort in treating multi-drug resistant gram-negative bacterial infections, was initially introduced in the 1950s but fell out of favor due to concerns over its potential to cause nephrotoxicity and neurotoxicity.[19] Similar to colistin, polymyxin B is most effective against A. baumannii, K. pneumoniae, and P. aeruginosa.[20,21,22] Polymyxin B has been linked to the safe and effective treatment of multidrug-resistant bacterial meningitis.[23] In the case of a 14-year-old male patient with post-surgical multidrug-resistant A. baumannii meningitis, Xing et al. successfully utilized a combination of intravenous (IV) and intrathecal (ITH) polymyxin B as a treatment. The report concluded that the combination of ITH and IV polymyxin B is an optimal therapeutic option against multidrug-resistant A. baumannii.
Irrespective of the treatment methodology and approach, it is paramount to consider the risk factors and prevention of nosocomial A. baumannii meningitis. Common risk factors include prolonged dural opening during surgical procedures, insertion techniques and location, duration of catheterization, presence of blood in CSF, CSF leak, and regular catheter sampling.[24] Furthermore, studies suggest that intravenous colistin therapy is more susceptible to nephrotoxic damage, and a combination with intrathecal administration can help mitigate that.[25] Our patient experienced elevations in creatinine levels that peaked at 2.71 mg/dL, possibly attributed to the toxicity of polymyxin B, which was therefore discontinued. Moreover, the patient received intravenous N-acetyl cysteine at 300 mg 3x2 ampules, which further helped normalize creatinine levels. Elevated creatinine levels could also be due to contrast nephropathy, as the patient had a contrast-enhanced computed tomography (CT) scan on January 10th, one day prior to the creatinine level elevations.
CONCLUSION
In conclusion, healthcare professionals should exercise the utmost care to prevent contamination and must utilize appropriate sterilization techniques to avoid potentially fatal, multidrug-resistant Acinetobacter baumannii infections.
Footnotes
Peer-review: Externally peer-reviewed.
Authorship Contributions: Concept: C.E., B.B; Design: C.E., D.K.; Supervision: C.E., B.B., İ.S.; Resource: B.B., D.K.; Materials: C.E., B.B.; Data collection and/or processing: C.E., D.K.; Analysis and/or interpretation: C.E., I.S.; Literature search: C.E., İ.S., D.K.; Writing: C.E., B.B.Ç.; Critical review: C.E., D.K.
Conflict of Interest: None declared.
Financial Disclosure: The author declared that this study has received no financial support.
REFERENCES
- 1.Zeinalizadeh M, Yazdani R, Feizabadi MM, Shadkam M, Seifi A, Dehghan Manshadi SA, et al. Post-neurosurgical meningitis;gram negative bacilli vs. gram positive cocci. Caspian J Intern Med. 2022;13:469–74. doi: 10.22088/cjim.13.3.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tunkel AR, Hartman BJ, Kaplan SL, Kaufman BA, Roos KL, Scheld WM, et al. Practice guidelines for the management of bacterial meningitis. Clin Infect Dis. 2004;39:1267–84. doi: 10.1086/425368. [DOI] [PubMed] [Google Scholar]
- 3.Vasen W, Desmery P, Ilutovich S, Di Martino A. Intrathecal use of colistin. J Clin Microbiol. 2000;38:3523. doi: 10.1128/jcm.38.9.3523-3523.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Motaouakkil S, Charra B, Hachimi A, Nejmi H, Benslama A, Elmdaghri N, et al. Colistin and rifampicin in the treatment of nosocomial infections from multiresistant Acinetobacter baumannii. J Infect. 2006;53:274–8. doi: 10.1016/j.jinf.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 5.Ng J, Gosbell IB, Kelly JA, Boyle MJ, Ferguson JK. Cure of multiresistant Acinetobacter baumannii central nervous system infections with intraventricular or intrathecal colistin:case series and literature review. J Antimicrob Chemother. 2006;58:1078–81. doi: 10.1093/jac/dkl347. [DOI] [PubMed] [Google Scholar]
- 6.Kwa AL, Tam VH, Falagas ME. Polymyxins:a review of the current status including recent developments. Ann Acad Med Singap. 2008;37:870–83. [PubMed] [Google Scholar]
- 7.Dalgic N, Ceylan Y, Sancar M, Telhan L, Kafadar I, Cavusoglu H, et al. Successful treatment of multidrug-resistant Acinetobacter baumannii ventriculitis with intravenous and intraventricular colistin. Ann Trop Paediatr. 2009;29:141–7. doi: 10.1179/146532809X440761. [DOI] [PubMed] [Google Scholar]
- 8.Fotakopoulos G, Makris D, Chatzi M, Tsimitrea E, Zakynthinos E, Fountas K. Outcomes in meningitis/ventriculitis treated with intravenous or intraventricular plus intravenous colistin. Acta Neurochir (Wien) 2016;158:603–10. doi: 10.1007/s00701-016-2702-y. discussion 610. [DOI] [PubMed] [Google Scholar]
- 9.Kasiakou SK, Michalopoulos A, Soteriades ES, Samonis G, Sermaides GJ, Falagas ME. Combination therapy with intravenous colistin for management of infections due to multidrug-resistant Gram-negative bacteria in patients without cystic fibrosis. Antimicrob Agents Chemother. 2005;49:3136–46. doi: 10.1128/AAC.49.8.3136-3146.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fernandez-Viladrich P, Corbella X, Corral L, Tubau F, Mateu A. Successful treatment of ventriculitis due to carbapenem-resistant Acinetobacter baumannii with intraventricular colistin sulfomethate sodium. Clin Infect Dis. 1999;28:916–7. doi: 10.1086/517243. [DOI] [PubMed] [Google Scholar]
- 11.Kaplan SL, Patrick CC. Cefotaxime and aminoglycoside treatment of meningitis caused by gram-negative enteric organisms. Pediatr Infect Dis J. 1990;9:810–4. doi: 10.1097/00006454-199011000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Berlana D, Llop JM, Fort E, Badia MB, Jódar R. Use of colistin in the treatment of multiple-drug-resistant gram-negative infections. Am J Health Syst Pharm. 2005;621:39–47. doi: 10.1093/ajhp/62.1.39. [DOI] [PubMed] [Google Scholar]
- 13.Benifla M, Zucker G, Cohen A, Alkan M. Successful treatment of Acinetobacter meningitis with intrathecal polymyxin E. J Antimicrob Chemother. 2004;54:290–2. doi: 10.1093/jac/dkh289. [DOI] [PubMed] [Google Scholar]
- 14.Kizilates F, Keskin AS, Onder KD. Clinical features of post-operative nosocomial meningitis in adults and evaluation of efficiency of intrathecal treatment. Surg Infect (Larchmt) 2021;22:1059–63. doi: 10.1089/sur.2021.024. [DOI] [PubMed] [Google Scholar]
- 15.Cheng CY, Sheng WH, Wang JT, Chen YC, Chang SC. Safety and efficacy of intravenous colistin (colistin methanesulphonate) for severe multidrug-resistant Gram-negative bacterial infections. Int J Antimicrob Agents. 2010;35:297–300. doi: 10.1016/j.ijantimicag.2009.11.016. [DOI] [PubMed] [Google Scholar]
- 16.Kim BN, Peleg AY, Lodise TP, Lipman J, Li J, Nation R, et al. Management of meningitis due to antibiotic-resistant Acinetobacter species. Lancet Infect Dis. 2009;9:245–55. doi: 10.1016/S1473-3099(09)70055-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ayhan M, Kalem AK, Hasanoglu I, Kayaaslan B, Ozates MO, Izdes S, et al. Intrathecal and intraventricular administration of antibiotics in gram-negative nosocomial meningitis in a research hospital in Turkey. Turk Neurosurg. 2021;31:348–54. doi: 10.5137/1019-5149.JTN.29844-20.2. [DOI] [PubMed] [Google Scholar]
- 18.Chusri S, Sakarunchai I, Kositpantawong N, Panthuwong S, Santimaleeworagun W, Pattharachayakul S Y, et al. Outcomes of adjunctive therapy with intrathecal or intraventricular administration of colistin for post-neurosurgical meningitis and ventriculitis due to carbapenem-resistant acinetobacter baumannii. Int J Antimicrob Agents. 2018;51:646–50. doi: 10.1016/j.ijantimicag.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 19.Koch-Weser J, Sidel VW, Federman EB, Kanarek P, Finer DC, Eaton AE. Adverse effects of sodium colistimethate. Manifestations and specific reaction rates during 317 courses of therapy. Ann Intern Med. 1970;72:857–68. doi: 10.7326/0003-4819-72-6-857. [DOI] [PubMed] [Google Scholar]
- 20.Tam VH, Schilling AN, Vo G, Kabbara S, Kwa AL, Wiederhold NP, et al. Pharmacodynamics of polymyxin B against Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2005;49:3624–30. doi: 10.1128/AAC.49.9.3624-3630.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abdul Rahim N, Cheah SE, Johnson MD, Yu H, Sidjabat HE, Boyce J, et al. Synergistic killing of NDM-producing MDR Klebsiella pneumoniae by two 'old'antibiotics-polymyxin B and chloramphenicol. J Antimicrob Chemother. 2015;70:2589–97. doi: 10.1093/jac/dkv135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tran TB, Cheah SE, Yu HH, Bergen PJ, Nation RL, Creek DJ, et al. Anthelmintic closantel enhances bacterial killing of polymyxin B against multidrug-resistant Acinetobacter baumannii. J Antibiot (Tokyo) 2016;69:415–21. doi: 10.1038/ja.2015.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ye J, Tan LH, Shen ZP, Yu YS, Lai DM, Fan J, et al. Polymyxin for the treatment of intracranial infections of extensively drug-resistant bacteria in children after neurosurgical operation. World J Pediatr. 2020;16:528–32. doi: 10.1007/s12519-020-00350-8. [DOI] [PubMed] [Google Scholar]
- 24.Karvouniaris M, Brotis A, Tsiakos K, Palli E, Koulenti D. Current perspectives on the diagnosis and management of healthcare-associated ventriculitis and meningitis. Infect Drug Resist. 2022;15:697–721. doi: 10.2147/IDR.S326456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Bonis P, Lofrese G, Scoppettuolo G, Spanu T, Cultrera R, Labonia M, et al. Intraventricular versus intravenous colistin for the treatment of extensively drug resistant Acinetobacter baumannii meningitis. Eur J Neurol. 2016;23:68–75. doi: 10.1111/ene.12789. [DOI] [PubMed] [Google Scholar]


