ABSTRACT
BACKGROUND:
This study aims to assess pediatric patients with Open Globe Injury (OGI) using the Modified Pediatric Ocular Trauma Score (MPOTS) and to investigate the correlation between Neutrophil-to-Lymphocyte ratio (NLR) and Platelet-to-Lymphocyte ratio (PLR) parameters with the prognosis determined by MPOTS.
METHODS:
In this retrospective study, we included pediatric patients with OGI. Recorded data encompassed demographic details, injury type, duration from injury to surgery, complete ophthalmological examinations, initial and final visual acuities, anterior segment and fundus findings, and NLR and PLR values. Patients were categorized into three groups based on their MPOTS scores: Grade I (≤30 points), indicating high risk; Grade II (35-65 points), moderate risk; and Grade III (≥70 points), low risk. Differences between categories were statistically evaluated.
RESULTS:
The study comprised 22 patients. In Category I, the duration from injury to surgery was longer (p=0.018). The most common injury type in this category was globe rupture, occurring in four (50%) patients, with a significant difference noted between the groups (p=0.041). Category I exhibited lower final visual acuity and higher NLR and PLR values compared to the other categories (p<0.050 for all values). Both NLR and PLR demonstrated significant negative correlations with MPOTS (respectively, r=-0.869, p<0.001; r=-0.809, p<0.001).
CONCLUSION:
The Modified Pediatric Ocular Trauma Score is an effective and practical method for assessing the prognosis of pediatric patients with Open Globe Injury (OGI). Furthermore, there is evidence indicating a negative correlation between MPOTS and the increase in NLR and PLR values that often follows OGI in this patient population.
Keywords: Modified pediatric ocular trauma score, open globe injury, pediatric ocular trauma, systemic inflammation
INTRODUCTION
Many patients admitted to hospitals for ocular trauma fall within the pediatric age group.[1] Open Globe Injuries (OGI), characterized by a breach in the integrity of the globe, can lead to severe visual loss. This is attributed both to the severe damage caused by the trauma itself and to the increased susceptibility of this age group to the development of amblyopia.[2]
Various trauma scoring systems have been developed to assess the extent of damage following ocular trauma and to predict prognosis.[3-6] Visual acuity (VA) and relative afferent pupil defect (RAPD) are parameters in the ocular trauma scoring system developed by Kuhn et al.[4] However, since VA and RAPD assessments require patient cooperation, they are not always feasible to perform in children. Consequently, Acar et al. developed a new pediatric ocular trauma scoring system that does not include VA and RAPD. Many studies have evaluated the effectiveness of these two scoring systems in pediatric populations.[5,7-10] Building on previous research, Cohen et al. formulated a new Modified Pediatric Ocular Trauma Scoring (MPOTS) system by omitting parameters that do not affect prognosis in pediatric age groups.[6] They concluded that this new method is easier to use and more effective.
In many studies, the Neutrophil-to-Lymphocyte Ratio (NLR) and Platelet-to-Lymphocyte Ratio (PLR) have been recognized as blood parameters indicative of systemic inflammation.[11-13] It has been observed that these ratios increase in various eye diseases and are correlated with the severity of the condition.[14-19] A study focusing on adult populations with OGI found that high baseline NLR and PLR values were associated with a worse prognosis.[20]
Our study aims to evaluate pediatric patients with OGI using the MPOTS and to investigate the impact of NLR and PLR parameters on prognosis and MPOTS scores.
MATERIALS AND METHODS
This retrospective study was conducted on pediatric patients who underwent primary surgical treatment for OGI between January 2020 and December 2022. The study received approval from the local ethics committee and was conducted in accordance with the Declaration of Helsinki.
Patients under 18 years of age with a minimum of six months follow-up were included in the study. Collected data included patient demographics, type of injury, time from injury to surgery, complete ophthalmological examination results, initial and final visual acuity, and anterior segment and fundus findings (such as hyphema, iris prolapse, traumatic cataract, vitreous hemorrhage, etc.). Patients were scored using the MPOTS as described by Cohen et al.[6] This scoring system considers initial visual acuity, wound location, and the presence of concomitant ocular pathologies (Table 1). In cases where visual acuity could not be determined in children, MPOTS was calculated as follows: double the points for the injury location, minus points for the corresponding pathologies. Patients were then divided into three groups based on their MPOTS scores: Grade I (≤30 points), indicating high risk; Grade II (35-65 points), moderate risk; and Grade III (≥70 points), low risk. The type of injury was classified according to the Birmingham Eye Trauma Terminology system.[21] The injury zone was determined based on the Ocular Trauma Classification System (Fig. 1).[22] Blood test results were recorded from routine preoperative tests. The NLR was calculated by dividing the number of neutrophils (NEU) by lymphocytes (LYM), and the PLR by dividing platelets (PTL) by LYM. Patients missing MPOTS evaluation criteria or blood parameter data were excluded from the study.
Table 1.
The modified pediatric ocular trauma score variables and raw points
| Variables | Raw point |
|---|---|
| Initial visual acuity | |
| No light perception | 10 |
| Light perception/hand motion | 20 |
| Counting fingers | 30 |
| 0.1-0.5 | 40 |
| 0.6-1.0 | 50 |
| Wound location | |
| Zone I | 50 |
| Zone II/III | 30 |
| Concomitant eye pathologies | |
| Iris prolapse | -5 |
| Hyphema | -5 |
| Vitreous hemorrhage | -20 |
| Retinal detachment | -20 |
| Endophthalmitis | -30 |
| Sum of raw points | MPOTS category |
| ≤ 30 | I |
| 35–65 | II |
| ≥ 70 | III |
| Category | Risk for poor outcome |
| (VA≤20/200) | |
| I | |
| II | |
| III |
Figure 1.
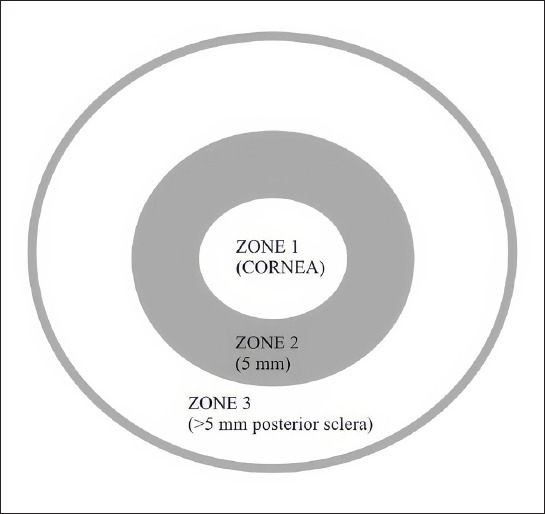
Injury zones.
Categorical data were expressed as numbers and percentages, and continuous variables as medians and interquartile ranges (IQR). The normality of data distribution was evaluated using the Shapiro-Wilk test. Categorical data were analyzed using the chi-square test, and intergroup variables were assessed with one-way Analysis of Variance (ANOVA) (post-hoc Tamhane’s T2 test). The Spearman correlation test was used to determine the correlation between parameters. Statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS version 21.0, SPSS Inc., Chicago, IL). A p-value of < 0.05 was considered statistically significant.
RESULTS
A total of 28 patients who underwent primary surgical treatment for OGI were included in the study. Four patients were excluded due to incomplete data; two had less than six months of follow-up. The remaining 22 patients were categorized according to the MPOTS into three risk groups: eight patients in Category 1 (high risk), six in Category 2 (moderate risk), and eight in Category 3 (low risk). The mean age of the 22 patients was 13.50 years (IQR: 3.75), with seven of them (31.8%) being female. There were no differences in age or gender between the groups (p=0.524, p=0.323, respectively). The mean follow-up duration was 10.50 months (IQR: 5.75), and the average time from injury to surgery was eight hours (IQR: 4.50). The time from injury to surgery was 11 hours (IQR: 5.25) in Category 1, 7 hours (IQR: 4) in Category 2, and 6.50 hours (IQR: 2.50) in Category 3. The difference between the three groups was significant (p=0.018). Of the injuries, 13 (59.1%) occurred indoors and nine (40.9%) outdoors, with no difference between the categories (p=0.797). Demographic data, accident mechanisms, and clinical characteristics of the patients are presented in Table 2.
Table 2.
Demographic and Clinical Features of the Patients
| Age, years (median, Q25-Q75) | 13.50 (6.25-15) |
|---|---|
| Female gender (n,%) | 7 (31.8) |
| Laterality, left eye (n,%) | 13 (59.1) |
| Follow-up time (months), (median, Q25-Q75) | 10.50 (8-14.25) |
| Accident-surgery interval (hours), (median, Q25-Q75) | 8.00 (6-11) |
| Place of Injury (indoor) (n,%) | 13 (56.5) |
| Mechanism of Injury (n,%) | |
| Sharp object | 12 (54.5) |
| Blunt object | 6 (27.3) |
| Explosive/Gunshot | 2 (9.1) |
| Other | 2 (9,1) |
| Wound location (n,%) | |
| Zone 1 | 9 (40.9) |
| Zone 2-3 | 13 (59.1) |
| Type of Injury (n,%) | |
| Penetration | 16 (72.7) |
| IOFB | 2 (9.1) |
| Globe rupture | 4 (18.2) |
| Perforation | - |
| Concomitant eye pathologies (n,%) | |
| Iris prolapse | 5 (22.7) |
| Hyphema | 9 (40.1) |
| Vitreous hemorrhage | 6 (27.2) |
| Retinal detachment | - |
| Endophthalmitis | 1 (4.5) |
| Initial BCVA (logMAR) (median, Q25-Q75) n=16 | 1.20 (1-1.40) |
| Final BCVA (logMAR) (median, Q25-Q75) n=19 | 0.45 (0-1) |
| NLR (median, Q25-Q75) | 4.32 (2.22-6.25) |
| PLR (median, Q25-Q75) | 129.45 (112.42-143.87) |
IOFB, intraocular foreign body; BCVA, best corrected visual acuity; NLR, neutrophil to lymphocyte ratio; PLR, platelet to lymphocyte ratio.
The final visual acuity in Category 1 was significantly lower than in Categories 2 and 3 (p=0.020 and p=0.032, respectively). Blunt trauma was present in four (50%) patients in Category 1, compared to one patient in the other categories (16.7% and 12.5%, respectively) (p=0.192). The most common type of injury in Category 1 was globe rupture, occurring in four (50%) patients. All patients in Category 2 had penetrating injuries. In Category 3, seven (87.5%) patients had penetrating injuries, and one (12.5%) had an intraocular foreign body (IOFB) within the lens. There was a significant difference between the categories regarding the type of injury (p=0.041). The most common injury zone was Zone 2 in Category 1 (four patients, 50%) and Zone 1 in Categories 2 and 3 (four patients, 66.7%, and four patients, 50%, respectively), with no significant difference in terms of injury zone (p=0.084). One patient in Category 1 developed endophthalmitis. Traumatic cataract was observed in four (50%) patients in Category 1, three (50%) in Category 2, and three (37.5%) in Category 3 (p=0.852).
Final visual acuity, NLR, and PLR values by MPOTS categories are detailed in Table 3. These parameters differ significantly among the categories (p<0.050 for all). Figure 2 illustrates a significant negative correlation between NLR and MPOTS (r=-0.869, p<0.001). Figure 3 demonstrates a significant negative correlation between PLR and MPOTS (r=-0.809, p<0.001).
Table 3.
Blood parameters and final visual acuity among groups
| Category 1 | Category 2 | Category 3 | p value | |
|---|---|---|---|---|
| Final BCVA (logMAR) (median, Q25-Q75) | 1 (0.6-1) | 0.30 (0-0.40) | 0.00 (0-0.70) | pa=0.02 |
| pb=0.032 | ||||
| pc=0.964 | ||||
| NLR (median, Q25-Q75) | 7.15 (6.62-8.75) | 3.45 (2.91-4.26) | 2.00 (1.42-2.25) | pa<0.001 |
| pb<0.001 | ||||
| pc=0.477 | ||||
| PLR (median, Q25-Q75) | 150.45 (135.75-161) | 121.80 (120.45-131.75) | 107.30 (104.52-122.02) | pa=0.002 |
| pb=0.001 | ||||
| pc=0.190 |
BCVA, best corrected visual acuity; NLR, neutrophil to lymphocyte ratio; PLR, platelet to lymphocyte ratio. pa= comparison between category 1 and category 2; pb= comparison between category 1 and category 3; pc= comparison between category 2 and category 3.
Figure 2.
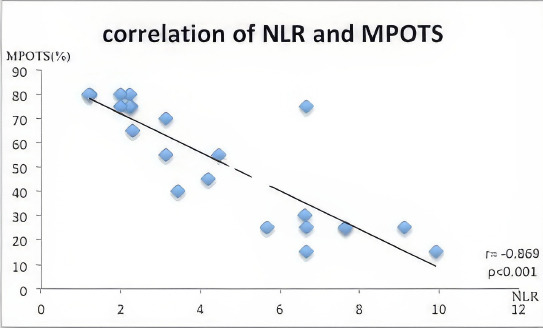
Correlation between Neutrophil-to-Lymphocyte Ratio (NLR) and Modified Pediatric Ocular Trauma Score MPOTS).
Figure 3.
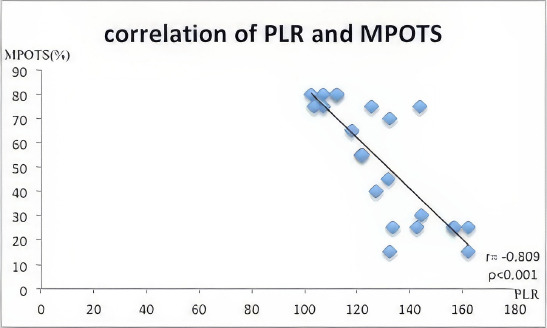
Correlation between Platelet-to-Lymphocyte Ratio (PLR) and Modified Pediatric Ocular Trauma Score (MPOTS).
DISCUSSION
In the present study, OGI in pediatric patients was classified according to MPOTS. According to MPOTS, blunt trauma and globe rupture were more common in Category 1. In this category, final visual acuity was lower, and both NLR and PLR were higher.
In our study, no significant differences in age and gender were observed among the groups of patients divided according to risk categories. Some studies suggest that OGI is more common in children younger than 7 years, while others indicate a higher prevalence in the age group of 7-12 years.[7-10] In the study by Cohen et al., age was excluded from MPOTS staging because it was not predictive,[6] aligning with our findings. While the literature shows that ocular trauma in the pediatric age group is more common in males, there are also studies suggesting no gender difference, especially in the preschool age group.[1-6] In the present study, although OGI was more frequent in males, this difference was not statistically significant.
Our study revealed that patients in Category 1 took longer to undergo surgery, possibly due to more extensive systematic screening required because of the injury’s severity. The literature does not show a significant relationship between delay in surgical time and final visual acuity.[23,24] Furthermore, our study found no statistically significant difference between home versus outdoor injuries. Previous studies vary, with some indicating a higher incidence of in-home injuries, while others report the opposite.[2,7-10]
Most injuries in our study were penetrating. Two patients had an IOFB, and four had globe rupture. One patient developed endophthalmitis, and all cases of globe rupture were in Category 1. The rate of blunt trauma was also high in this group. The literature reports that both the area and type of injury significantly impact prognosis.[3-6] OGI, particularly with blunt trauma, may lead to retinal and vitreous injuries, irregular incision lines, and an increased risk of endophthalmitis. In our study, there was no significant difference between groups in terms of the injury zone. However, the most common injury zone was Zone 2 in Category 1, while in other categories, it was in Zone 1. In our study, there was also no significant difference between the patient groups regarding the frequency of traumatic cataract development. Although this may affect initial visual acuity, it has been reported that it does not significantly impact final visual acuity.[6,9]
Other significant findings of our study include higher NLR and PLR in Category 1. Furthermore, NLR and PLR demonstrated a significant inverse correlation with MPOTS. In their study on adult OGI patients, Mohamed-Noriega et al. determined that NLR and PLR were elevated in patient groups with visual acuity of 20/200 or below, showing a positive correlation with injury severity.[20] The breach of the eye’s natural protective barriers due to OGI can trigger a local and systemic immune response. Numerous studies have indicated that NLR and PLR are markers of increased systemic inflammation.[11-19] One study associated a high NLR with a poor prognosis in trauma patients, particularly those with traumatic brain injury.[25] High NLR and PLR have also been linked to poor outcomes in various diseases. According to our study’s results, high NLR and PLR are associated with poor prognosis.
The main limitation of our study is its retrospective design and the small patient sample. This is mainly because the study population represents a specific age group. Nevertheless, the follow-up of patients at a single center with more standardized treatments is crucial. To our knowledge, this is the first study evaluating inflammatory blood parameters in pediatric OGI patients.
CONCLUSION
We found that MPOTS is an effective and practical method for assessing the prognosis of pediatric OGI patients. Our study concluded that both NLR and PLR increase following OGI in pediatric patients, and these increases are associated with the prognosis.
Footnotes
Ethics Committee Approval: This study was approved by the Balıkesir University, Faculty of Medicine Ethics Committee (Date: 04.01.2023, Decision No: 2023/11).
Peer-review: Externally peer-reviewed.
Authorship Contributions: Concept: M.M.U., Ö.Ö.Ç.; Design: M.M.U., Ö.Ö.Ç.; Supervision: E.K.; Resource: M.M.U., Ö.Ö.Ç.; Materials: M.M.U., E.K.; Data collection and/or processing: Ö.Ö.Ç., M.M.U.; Analysis and/or interpretation: M.M.U.; Literature search: Ö.Ö.Ç.; Writing: Ö.Ö.Ç., M.M.U; Critical review: E.K.
Conflict of Interest: None declared.
Financial Disclosure: The author declared that this study has received no financial support.
REFERENCES
- 1.Thompson CG, Kumar N, Billson FA, Martin F. The etiology of perforating ocular injuries in children. Br J Ophthalmol. 2002;86:920–2. doi: 10.1136/bjo.86.8.920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.TGB M, Valbuena M. Epidemiology and visual outcomes of pediatric ocular trauma cases in a tertiary hospital. Philipp J Ophthalmol. 2014;39:27–31. [Google Scholar]
- 3.Sii F, Barry RJ, Blanch RJ, Abbott J, MacEwen CJ, Shah P. The UK Paediatric Ocular Trauma Study 1 (POTS1):development of a global standardized protocol for prospective data collection in pediatric ocular trauma. Clin Ophthalmol. 2017;11:449–52. doi: 10.2147/OPTH.S125160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuhn F, Maisiak R, Mann L, Mester V, Morris R, Witherspoon CD. The ocular trauma score (OTS) Ophthalmol Clin N Am. 2002;15:163–5. doi: 10.1016/s0896-1549(02)00007-x. [DOI] [PubMed] [Google Scholar]
- 5.Acar U, Tok OY, Acar DE, Burcu A, Ornek F. A new ocular trauma score in pediatric penetrating eye injuries. Eye. 2011;25:370–4. doi: 10.1038/eye.2010.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen E, Antman G, Katzburg E, Cohen N, Varssano D, Glatz MM, et al. A modified pediatric ocular trauma score for predicting visual outcome post open globe injury. Graefes Arch Clin Exp Ophthalmol. 2022;260:3711–8. doi: 10.1007/s00417-022-05723-5. [DOI] [PubMed] [Google Scholar]
- 7.El-Sebaity DM, Soliman W, Soliman A, Fathalla AM. Pediatric eye injuries in upper Egypt. Clin Ophthalmol. 2011;5:1417–23. doi: 10.2147/OPTH.S24679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee CH, Su WY, Lee L, Yang ML. Pediatric ocular trauma in Taiwan. Chang Gung Med J. 2008;31:59–65. [PubMed] [Google Scholar]
- 9.Puodžiuvienė E, Jokūbauskienė G, Vieversytė M, Asselineau K. A five-year retrospective study of the epidemiological characteristics and visual outcomes of pediatric ocular trauma. BMC Ophthalmol. 2018;18:10. doi: 10.1186/s12886-018-0676-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shoja MR, Miratashi AM. Pediatric ocular trauma. Acta Medica Iranica. 2006;44:125–30. [Google Scholar]
- 11.Bhat T, Teli S, Rijal J, Bhat H, Raza M, Khoueiry G, et al. Neutrophil to lymphocyte ratio and cardiovascular diseases:a review. Expert Rev Cardiovasc Ther. 2013;11:55–9. doi: 10.1586/erc.12.159. [DOI] [PubMed] [Google Scholar]
- 12.Erre GL, Paliogiannis P, Castagna F, Mangoni AA, Carru C, Passiu G, et al. Meta-analysis of neutrophil-to-lymphocyte and platelet-tolymphocyte ratio in rheumatoid arthritis. Eur J Clin Invest. 2019;49:e13037. doi: 10.1111/eci.13037. [DOI] [PubMed] [Google Scholar]
- 13.Qin B, Ma N, Tang Q, Wei T, Yang M, Fu H, et al. Neutrophil to lymphocyte ratio (NLR) and platelet to lymphocyte ratio (PLR) were useful markers in the assessment of inflammatory response and disease activity in SLE patients. Mod Rheumatol. 2016;26:372–6. doi: 10.3109/14397595.2015.1091136. [DOI] [PubMed] [Google Scholar]
- 14.Sengul EA, Artunay O, Kockar A, Afacan C, Rasier R, Gun P, et al. Correlation of neutrophil/lymphocyte and platelet/lymphocyte ratio with visual acuity and macular thickness in age-related macular degeneration. Int J Ophthalmol. 2017;10:754–9. doi: 10.18240/ijo.2017.05.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ozgonul C, Sertoglu E, Ayyildiz O, Mumcuoglu T, Kucukevcilioglu M, Gokce G, et al. Novel biomarkers for patients with idiopathic acute anterior uveitis:neutrophil to lymphocyte ratio and platelet to lymphocyte ratio. Int J Ophthalmol. 2017;10:262–6. doi: 10.18240/ijo.2017.02.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ulu SM, Dogan M, Ahsen A, Altug A, Demir K, Acartürk G, et al. Neutrophil to- lymphocyte ratio as a quick and reliable predictive marker to diagnose the severity of diabetic retinopathy. Diabetes Technol Ther. 2013;15:942–7. doi: 10.1089/dia.2013.0097. [DOI] [PubMed] [Google Scholar]
- 17.Karaca EE, Özmen MC, Ekici F, Yüksel E, Türkoglu Z. Neutrophil-to lymphocyte ratio may predict progression in patients with keratoconus. Cornea. 2014;33:1168–73. doi: 10.1097/ICO.0000000000000260. [DOI] [PubMed] [Google Scholar]
- 18.Celik T. Assessment of neutrophil-to-lymphocyte ratio and platelet-to lymphocyte ratio in patients with dry eye disease. Ocul Immunol Inflamm. 2018;26:1219–22. doi: 10.1080/09273948.2017.1340486. [DOI] [PubMed] [Google Scholar]
- 19.Ozgonul C, Sertoglu E, Mumcuoglu T, Kucukevcilioglu M. Neutrophil to- lymphocyte ratio and platelet-to-lymphocyte ratio as novel biomarkers of primary open-angle glaucoma. J Glaucoma. 2016;25:e815–20. doi: 10.1097/IJG.0000000000000392. [DOI] [PubMed] [Google Scholar]
- 20.Mohamed-Noriega K, Treviño-Herrera AB, Mohamed-Noriega J, Velasco-Sepúlveda BH, Martínez-Pacheco VA, Guevara-Villarreal DA, et al. Relationship of neutrophil-to-lymphocyte and platelet-to-lymphocyte ratio with visual acuity after surgical repair of open globe injury. Front Med (Lausanne) 2021;8:697585. doi: 10.3389/fmed.2021.697585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuhn F, Morris R, Witherspoon CD, Heimann K, Jeffers JB, Treister G. A standardized classification of ocular trauma. Graefes Arch Clin Exp Ophthalmol. 1996;234:399–403. doi: 10.1007/BF00190717. [DOI] [PubMed] [Google Scholar]
- 22.Pieramici DJ, Sternberg P, Aaberg TM, Bridges WZ, Capone A, Cardillo JA. A system for classifying mechanical injuries of the eye (globe). The ocular trauma classification group. Am J Ophthalmol. 1997;123:820–31. doi: 10.1016/s0002-9394(14)71132-8. [DOI] [PubMed] [Google Scholar]
- 23.Cohen N, Cohen E, Anafy A, Margaliot A, Kaganov K, Gomel N, et al. Predictors of traumatic eye injuries at high-risk for ophthalmic complications in children. Eur J Pediatr. 2021;180:177–85. doi: 10.1007/s00431-020-03734-0. [DOI] [PubMed] [Google Scholar]
- 24.Liu X, Liu Z, Liu Y, Zhao L, Xu S, Su G, et al. Determination of visual prognosis in children with open globe injuries. Eye (Basingstoke) 2014;28:852–6. doi: 10.1038/eye.2014.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu J, Li S, Lui KY, Song X, Hu X, Cao L, et al. The neutrophil-to-lymphocyte ratio:A potential predictor of poor prognosis in adult patients with trauma and traumatic brain injury. Front Surg. 2022;9:917172. doi: 10.3389/fsurg.2022.917172. [DOI] [PMC free article] [PubMed] [Google Scholar]


