Abstract
Background
In Australia, the incidence of hepatitis C virus (HCV) has declined among gay and bisexual men (GBM) with human immunodeficiency virus (HIV) since 2015 and is low among GBM using HIV preexposure prophylaxis (PrEP). However, ongoing HCV testing and treatment remains necessary to sustain this. To assess the potential utility of sexually transmissible infections (STIs) to inform HCV testing among GBM with HIV and GBM using PrEP, we examined the association between bacterial STI diagnoses and subsequent primary HCV infection.
Methods
Data were from a national network of 46 clinics participating in the Australian Collaboration for Coordinated Enhanced Sentinel Surveillance. GBM included had ≥1 HCV antibody negative test result and ≥1 subsequent HCV antibody and/or RNA test. Discrete time survival analysis was used to estimate the association between a positive syphilis, rectal chlamydia, and rectal gonorrhea diagnosis in the previous 2 years and a primary HCV diagnosis, defined as a positive HCV antibody or RNA test result.
Results
Among 6529 GBM with HIV, 92 (1.4%) had an incident HCV infection. A prior positive syphilis diagnosis was associated with an incident HCV diagnosis (adjusted hazard ratio, 1.99 [95% confidence interval, 1.11–3.58]). Among 13 061 GBM prescribed PrEP, 48 (0.4%) had an incident HCV diagnosis. Prior rectal chlamydia (adjusted hazard ratio, 2.75 [95% confidence interval, 1.42–5.32]) and rectal gonorrhea (2.54 [1.28–5.05]) diagnoses were associated with incident HCV.
Conclusions
Diagnoses of bacterial STIs in the past 2 years was associated with HCV incidence. These findings suggest that STIs might be useful for informing HCV testing decisions and guidelines for GBM with HIV and GBM using PrEP.
Keywords: chlamydia, gonorrhea, hepatitis C, HIV, syphilis
Sexually transmissible infections (STIs) are a marker of hepatitis C virus (HCV) risk among gay and bisexual men with human immunodeficiency virus (HIV) and those prescribed HIV preexposure prophylaxis in the context of declining HCV incidence. These STIs may be useful for guiding more tailored HCV testing.
Since the availability of hepatitis C virus (HCV) direct-acting antiviral (DAA) treatment, declines in the incidence of first HCV infection (hereafter primary incidence) among gay and bisexual men (GBM) with human immunodeficiency virus (HIV) have been reported in Australia [1], England [2], France [3], the Netherlands [4], and Switzerland [5]. While this is encouraging, there is still ongoing transmission, with a pooled primary incidence rate of 0.41/100 person-years (PY) reported among GBM with HIV in Australia and Western Europe in 2019 [6]. HCV reinfection has also been reported among GBM previously treated in many settings, with a pooled incidence rate of 3.9 per 100 PY [7]. Though somewhat lower, reinfection has also been reported from Australian cohort and treatment studies, with reinfection incidence rates of 1.05/100 PY and 2.5/100 PY, respectively [8, 9].
Although historically concentrated among GBM with HIV, there is evidence of primary HCV incidence among GBM using HIV preexposure prophylaxis (PrEP) [10]. However, the epidemiology of HCV among GBM using PrEP varies globally. Rates of primary incidence have been reported as 1.00/100 PY and 1.27/100 PY in France and the Netherlands, respectively [11, 12], but much lower in Australia (0.20/100PY) [1] and Canada (0.15/100 PY) [13]. In an Australian context, the intentional focus on the rapid upscale of HCV DAA access among people with HIV [9, 14], of whom the vast majority are GBM, has likely averted widespread transmission of HCV among GBM using PrEP. However, similar to findings in GBM with HIV, evidence indicates ongoing transmission also among GBM using PrEP in recent years [1].
In Australia, annual testing for HCV has long been recommended among GBM with HIV [15]. However, among GBM attending clinics for their HIV care in Melbourne, Australia, only 50%–60% were tested for HCV annually between 2012 and 2019 [9]. Likewise, in Canada and the United States, a substantial proportion of GBM with HIV are not tested annually [16, 17]. Strategies to increase HCV testing (accompanied by immediate treatment for anyone newly diagnosed) are likely to be needed to sustain effective HCV elimination efforts. Consistent with guidelines for GBM with HIV, Australian guidelines for GBM also recommend annual HCV testing for all GBM using PrEP and clinical guidelines for PrEP also suggest “more frequent testing if necessary” [18]. However, approximately 30% of GBM using PrEP in Australia have had only one HCV test since their first PrEP prescription [1].
Among GBM, there is a substantial body of evidence supporting sexual transmission of HCV with a number of sexual behaviors associated with an increased risk of HCV, including condomless anal sex, group sex, and fisting [19]. These behaviors are not mutually exclusive and sometimes occur together with substance use, including injecting drug use [20, 21]. While these behaviors could and should inform HCV testing, there is a limited understanding of whether and how GBM and clinicians discuss this array of behaviors with specific regard to HCV testing during routine clinical care. The best available evidence suggests that such discussion is likely to be limited, if occurring at all [22].
Incident HCV infections among GBM with HIV have been associated with prior sexually transmissible infections (STIs), particularly syphilis, in Australia, Canada, Switzerland, and Taiwan [23–26]. Given this relationship, in the absence of discussions on sexual and substance use behaviors, STIs may be useful for informing more tailored HCV testing among GBM. However, these studies were conducted predominantly in the context of increasing or stable HCV incidence and before the scaling up of HCV DAA treatment. Despite concerns about HCV and STIs among GBM prescribed PrEP, there remains a limited understanding of the relationship between STIs and HCV incidence among this group. In the context of declining HCV incidence among GBM with HIV and low incidence of HCV among GBM using PrEP in Australia, and to understand whether STIs are a marker of increased HCV risk, we examined the association between bacterial STIs and primary HCV infection among GBM after widespread availability of HCV DAAs from 2016 to 2020.
METHODS
Data Source
Data were drawn from the Australian Collaboration for Coordinated Enhanced Sentinel Surveillance of Sexually Transmissible Infections and Blood-borne Viruses (ACCESS) which has been described in detail elsewhere [27, 28]. ACCESS collates blood-borne virus and STI testing data and demographic data from clinics and laboratories, linking individuals’ data over time and across services participating in ACCESS.
As with previous analyses [1], data were from GBM attending ACCESS clinics in 7 of 8 Australian states and territories, with no HCV testing data among GBM available from clinics in the Northern Territory. In ACCESS, GBM status among people recorded as male is based being recorded as gay or bisexual in patient management systems and/or reporting male sexual partners in behavioral surveys at sexual health clinics. In addition, people who are recorded as male who have ever had a rectal STI test for chlamydia or gonorrhea recorded in ACCESS are defined as GBM, and this has been validated to identify GBM in surveillance data [29]. GBM were defined as living with HIV based on having a recorded HIV diagnosis or quantifiable HIV viral load test at a date preceding their first included HCV test. Given that HCV testing is not recommended among HIV-negative GBM who are not using PrEP, HIV-negative GBM without PrEP prescription data were not included in these analyses. HIV-negative GBM with PrEP prescription data were defined as using PrEP from the date of their first PrEP prescription from 1 January 2016 onward. Data are from 2016 onward, as this was the first year of widespread availability of both HCV DAA treatment and HIV PrEP in Australia.
Inclusion Criteria
GBM were included in these analyses if they were HCV antibody negative at their first HCV antibody test recorded in ACCESS from 1 January 2016 onward and had ≥1 additional HCV antibody or HCV RNA test for assessing incident HCV during the observation period from 1 January 2016 to 31 December 2020.
Outcome
A primary incident HCV infection was defined as a positive HCV antibody test result and/or a positive HCV RNA test result following a negative antibody test result. No restrictions were applied to the time between tests.
Exposures
STI exposures were defined as positive rectal chlamydia, rectal gonorrhea, and infectious (primary, secondary, or early [<2 years] latent) syphilis test results in the 2 years preceding an HCV test. After 2 years, a person who was defined as having an STI exposure reverted to no exposure provided that they did not have another positive test result in the preceding 2 years (Supplementary Figure 1). People could be included at one time point but excluded at another depending on their STI testing relative to the HCV test event (Supplementary Figure 2). A positive STI test result obtained on the same day as an HCV test was not included, because the HCV infection may have preceded the STI. We chose a 2-year time period to provide temporal proximity between STI exposures and the HCV outcome. To examine how this methodological choice may affect our findings, we conducted 2 sensitivity analyses where we did not limit the exposure period and we reduced the exposure period to 12 months. Consistent with Australian guidelines, nucleic acid amplification testing was used for chlamydia and gonorrhea [15].
Confounders
Age at the time of an HCV test was included in multivariable analyses and categorized as <30, 30–50, or >50 years. As Australian guidelines recommend quarterly testing for bacterial STIs among both GBM with HIV and GBM using PrEP, regardless of symptoms, the number of tests for each STI recorded in the previous 2 years, regardless of the result, was included as a count variable; in the sensitivity analyses, this was the number of tests in the previous 12 months or during the entire observation period as applicable.
Statistical Analyses
Analyses were stratified a priori by GBM with HIV and GBM using PrEP. Because the outcome of HCV infection was interval censored—that is, not observed and occurring between the date of the last negative result and the date of diagnosis—test event–level discrete-time survival analyses were undertaken using generalized linear mixed modeling with a binomial distribution and a complementary log-log link function [30]. While people could potentially be tested at more than one clinic, we included the clinic a priori as a random intercept, as there may be clustering owing to localized variations in the epidemiology of HCV and STIs and difference between clinics in protocols or systems to guide STI and/or HCV testing. Models were offset for the natural log of days between HCV tests to account for individual-level variation in the time between HCV tests. As the rates of HCV incidence are unlikely to be constant, which is verified by our previous work [1], the cumulative number of HCV tests undertaken was also included to allow variation in the baseline hazard across tests (Supplementary Figure 1).
Follow-up commenced from the date of the first recorded HCV antibody test for each person during the observation period. Censoring occurred at the date of diagnosis for an incident HCV infection, the date of the last HCV (antibody or RNA) test for those who were not recorded as testing HCV positive, or at the end of the observation period, which was 31 December 2020. Models were adjusted for age and the number of each respective STI test undertaken in the previous 2 years, regardless of the result. Potential differences in HCV infection risk between GBM included and not included in the multivariable analyses based on their STI testing history was estimated using a χ2 test of independence.
Patient Consent Statement
These analyses were conducted under the auspices of ethical approval for ACCESS and the analysis of routinely collected and deidentified data. Ethical approval for ACCESS was received from the Alfred Hospital in Melbourne, Victoria, the University of Tasmania in Hobart, Tasmania, and Menzies School of Health Research in Darwin, Northern Territory. Ethical reviews were also undertaken by community organizations representing key populations including GBM and people living with HIV. The requirement for individual-level written consent was waived by all ethics committees, but patients could opt of out the system if requested.
RESULTS
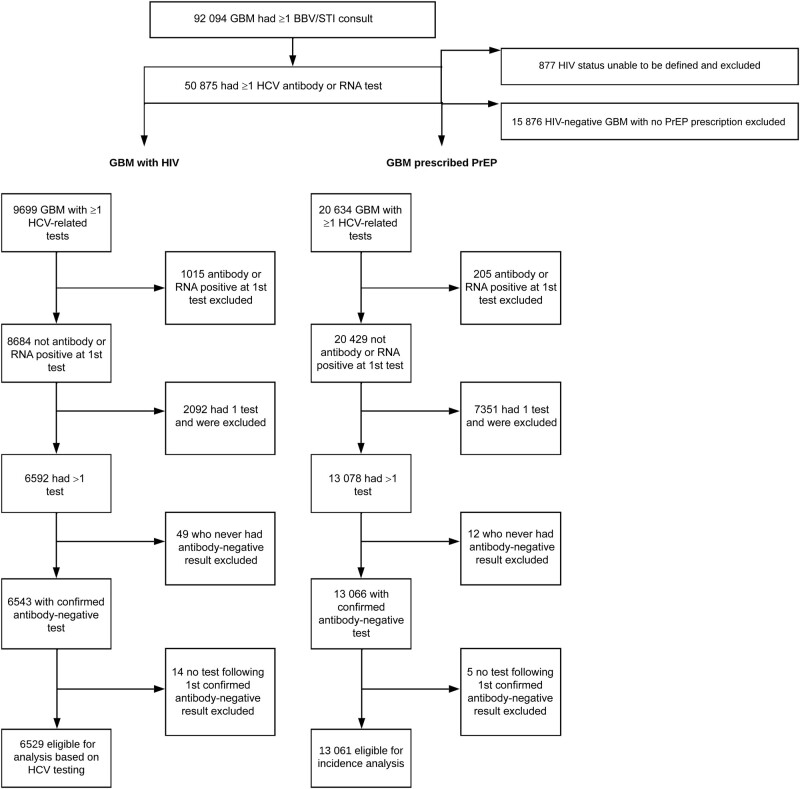
From 1 January 2016 to 31 December 2020, a total of 92 094 individual GBM had a recorded consultation, of whom 50 875 (55%) had ≥1 HCV antibody or RNA test. The HIV status of 877 people (1.7%) could not be determined, and they were excluded from further analyses (Figure 1). Of the remaining GBM, 9699 (19.4%) had a record of being HIV positive. Among the 40 299 defined as HIV negative, 24 423 (60.6%) had a record of PrEP prescription, and those who had no record of PrEP prescription were excluded from further analyses.
Figure 1.
Exclusion and inclusion from analyses among gay and bisexual men (GBM) with human immunodeficiency virus (HIV) and GBM prescribed preexposure prophylaxis (PrEP) based on hepatitis C virus (HCV) testing.
GBM With HIV
Of the 9699 GBM with HIV with any HCV testing data, 6529 were eligible for inclusion based on being HCV antibody negative at their first test in the observation period and having ≥1 subsequent HCV antibody or RNA test. Approximately 10% (n = 1015) were excluded as they were HCV antibody and/or RNA positive at their first test. No subsequent test was recorded after the first HCV antibody negative for approximately 22% (n = 2092), who were therefore not eligible for this analysis.
Among the 6529 GBM with HIV who were eligible for inclusion, 92 (1.4%) had a primary incident HCV diagnosis recorded. The median time (interquartile range [IQR]) between HCV tests was 5.1 (3.8–6.9) months overall and 4 months (2.7–6.3) months among those with incident HCV detected. Of these 6529 GBM with HIV, 98.4% (n = 6428) had ≥1 syphilis test, 77.4% (n = 5053) had ≥1 rectal gonorrhea test, and 76.7% (n = 5006) had ≥1 rectal chlamydia test in the 2 years preceding an HCV test. Compared with GBM without an incident HCV infection, a positive diagnosis of each STI in the 2 years before an HCV test was more common among GBM with an incident HCV infection (Table 1).
Table 1.
Person-Level Sexually Transmissible Infection Testing and Diagnoses in the 2 Years Preceding a Hepatitis C Virus Test Among 6529 Gay and Bisexual Men With Human Immunodeficiency Virus in Australia, 2016–2020
| STI Result | GBM With HIV, No. (%) | |
|---|---|---|
| Incident HCVa (n = 92) | No Incident HCVb (n = 6437) | |
| Rectal chlamydia | ||
| ≥1 Positive resultc | 23 (25.0) | 1489 (23.1) |
| No positive result | 49 (53.3) | 3445 (53.5) |
| No test | 20 (21.7) | 1503 (23.3) |
| Rectal gonorrhea | ||
| ≥1 Positive resultc | 20 (21.7) | 1080 (16.8) |
| No positive result | 52 (56.5) | 3890 (60.4) |
| No test | 20 (21.7) | 1456 (22.6) |
| Syphilis | ||
| ≥1 Positive resultc | 24 (26.1) | 1137 (17.7) |
| No positive result | 66 (71.7) | 5190 (80.6) |
| No test | 2 (2.2) | 99 (1.5) |
Abbreviations: GBM, gay and bisexual men; HCV, hepatitis C virus; STI, sexually transmissible infection.
aEver classified as having an incident HCV infection during the observation period.
bNever classified as having an incident HCV infection during the observation period.
cAt any point from inclusion to censoring, not necessarily in the 2 years before an incident infection.
In the multivariable analysis, a positive syphilis diagnosis in the previous 2 years was associated with a 2-fold increased risk of incident HCV (adjusted hazard ratio [aHR], 1.99 [95% confidence interval (CI), 1.11–3.58]) (Table 2), while there was no evidence that rectal gonorrhea and chlamydia were associated with an incident HCV infection. There was modest intraclass correlation (25%) among the clinics in this analysis and there was no significant difference in the proportion of GBM defined as having incident HCV between those included in (n = 71 [1.45%]) and those excluded from (n = 21 [1.32%]) this analysis (χ2[df = 1] = 0.13; P = .72) on the basis of their STI testing.
Table 2.
Test Event–Level Discrete Time Analysis of Incident Hepatitis C Virus Infection Among Gay and Bisexual Men With Human Immunodeficiency Virus in Australia, 2016–2020 (n = 4925)
| Exposure | Incident HCVa | HR | 95% CI | P Value | aHR | 95% CI | P Value |
|---|---|---|---|---|---|---|---|
| Rectal chlamydiab | |||||||
| No | 50 | 1 | … | 1 | … | ||
| Yes | 17 | 1.26 | .72–2.20 | .41 | 0.92 | .50–1.70 | .78 |
| Rectal gonorrheab | |||||||
| No | 51 | 1 | … | 1 | … | .26 | |
| Yes | 16 | 1.79 | 1.01–3.16 | .05 | 1.47 | .79–2.76 | .26 |
| Syphilisb | |||||||
| No | 66 | 1 | … | 1 | … | .02 | |
| Yes | 21 | 2.86 | 1.73–4.72 | <.001 | 1.99 | 1.11–3.58 | .02 |
Abbreviations: aHR, adjusted hazard ratio; CI, confidence interval; HCV, hepatitis C virus; HR, hazard ratio.
aNot including incident HCV where there was no sexually transmissible infection testing in the preceding 2 years.
bTime-varying exposure in the preceding 2 years.
Results were similar when there was no limit to the STI exposure period—for syphilis (aHR, 1.81 [95% CI, 1.04–3.14]), rectal chlamydia (1.11 [.62–1.99]), and rectal gonorrhea (1.52 [.84–2.75]). When the STI exposure period was reduced to the 12 months before an HCV test, a stronger association was found between syphilis and primary HCV incidence (aHR, 2.99 [95% CI. 1.59–5.64]) and no evidence of an association with rectal chlamydia (0.63 [.29–1.34]) or rectal gonorrhea (1.36 [.65–2.83]).
GBM Prescribed HIV PrEP
Of 20 634 GBM prescribed PrEP with ≥1 HCV related test, 13 061 were eligible for inclusion based on their HCV test results (Figure 1). The predominant reason for exclusion was the lack of a second HCV test, which resulted in 7351 (35.6%) being excluded. Among the 13 061 GBM eligible for inclusion in the analysis, 48 (0.37%) had an incident HCV infection. The median number of tests (IQR) 4 (2–6) among all GBM was and 3 (2–5) among those with incident HCV. The median time (IQR) between tests was 5.2 months (3.3–9.7) overall and 4.1 months (3.0–6.9) among those with incident HCV.
Approximately 95% of GBM prescribed PrEP had ≥1 test for each STI in the 2 years preceding an HCV test (Table 3). Rectal chlamydia and rectal gonorrhea were more common among GBM with incident HCV than in those who did not have incident HCV. Conversely, syphilis was less common among GBM with an incident HCV infection, and none had syphilis infection detected in the 2 years preceding an incident HCV diagnosis; as such, syphilis was not included in the multivariable analysis.
Table 3.
Person-Level Sexually Transmissible Infection Testing and Diagnoses in the 2 Years Before a Hepatitis C Virus Test Among 13 061 Gay and Bisexual Men Prescribed Preexposure Prophylaxis in Australia, 2016–2020
| STI Result | GBM, No. (%) | |
|---|---|---|
| Incident HCVa (n = 48) | No Incident HCVb (n = 13 013) | |
| Rectal chlamydia | ||
| ≥1 Positive resultc | 20 (41.7) | 3638 (28.0) |
| No positive result | 23 (47.9) | 8775 (67.4) |
| No test | 5 (5.4) | 600 (4.6) |
| Rectal gonorrhea | ||
| ≥1 Positive resultc | 15 (31.3) | 2479 (19.0) |
| No positive result | 28 (58.3) | 9990 (76.8) |
| No test | 5 (5.4) | 544 (4.2) |
| Syphilis | ||
| ≥1 Positive resultc | 2 (4.2) | 1252 (9.6) |
| No positive result | 46 (95.8) | 11 681 (89.8) |
| No test | 0 | 73 (0.6) |
Abbreviations: GBM, gay and bisexual men; HCV, hepatitis C virus; STI, STI, sexually transmissible infection.
aEver classified as having incident HCV during the observation period.
bNever classified as having incident HCV during the observation period.
cAt any point from inclusion to censoring, not necessarily in the 2 years before an incident infection.
In the multivariable analysis among GBM prescribed PrEP, rectal chlamydia (aHR, 2.74, [95% CI, 1.42–5.32]) and rectal gonorrhea (2.54 [1.28–5.05]) in the preceding 2 years were associated with an increased risk of incident HCV (Table 4). There was limited intraclass correlation (5.8%) between the clinics in this analysis and no significant difference in the proportion of incident HCV among GBM included in (0.35% [n = 43]) and excluded from (0.77% [n = 5]) this analysis (χ 2[df = 1] = 3.01; P = .08).
Table 4.
Test Event–Level Discrete Time Analysis of Incident Hepatitis C Virus Infection Among 12 404 Gay and Bisexual Men Prescribed Preexposure Prophylaxis in Australia, 2016–2020
| Exposure | Incident HCVa | HR | 95% CI | P Value | aHR | 95% CI | P Value |
|---|---|---|---|---|---|---|---|
| Rectal chlamydiab | |||||||
| No | 23 | 1 | … | 1 | … | ||
| Yes | 20 | 2.62 | 1.43–4.82 | .002 | 2.75 | 1.42–5.32 | .003 |
| Rectal gonorrheab | |||||||
| No | 28 | 1 | … | 1 | … | .008 | |
| Yes | 15 | 2.66 | 1.41–5.02 | .003 | 2.54 | 1.28–5.05 | .008 |
| Syphilisb | |||||||
| No | 48 | … | … | … | … | ||
| Yes | 0 | NA | NA | NA | NA | NA | NA |
Abbreviations: aHR, adjusted hazard ratio; CI, confidence interval; HCV, hepatitis C virus; HR, hazard ratio; NA, not applicable.
aNot including incident HCV where there was no sexually transmissible infection testing in the preceding 2 years.
bTime-varying exposure in the preceding 2 years.
Results were similar when there was no limit on the exposure period for both rectal chlamydia (aHR, 2.48 [95% CI, 1.27–4.83]) and rectal gonorrhea (2.37 [1.18–4.73]). When the STI exposure period was reduced to the 12 months before an HCV test, the associations found were stronger for both rectal chlamydia (aHR. 3.41 [95% CI, 1.77–6.59]) and rectal gonorrhea (3.08 [1.54–6.11]).
DISCUSSION
In our analyses of STIs as a marker of incident HCV risk among 6529 GBM with HIV across Australia from 2016 to 2020, we found that a syphilis diagnosis within the past 2 years was associated with an increased risk of HCV. Among 13 061 GBM using PrEP, rectal chlamydia and rectal gonorrhea within the past 2 years were independently associated with an increased risk of HCV. Taken together, these findings suggest that bacterial STIs in the previous 2 years could be used as a marker of increased HCV risk in the context of declining HCV incidence among GBM with HIV and low incidence among GBM using PrEP [1] to help guide targeted HCV testing.
To the best of our knowledge, no other studies have examined the relationship between STIs and primary HCV incidence among GBM with HIV with data exclusively from the HCV DAA treatment era. Our findings among GBM with HIV are consistent with studies before 2016 and before broad DAA availability in Australia. At a single sexual health service in Melbourne, Australia, syphilis, but not rectal chlamydia, was associated with an almost 5-fold increase in the risk of HCV; rectal gonorrhea was not included [23]. A notable methodological difference to our study was the “peri-incidence” definition used, which included syphilis in the 6 months before, at the same time as, or after an HCV test. In Canadian and Swiss cohort studies, a history of syphilis (ie, including only syphilis before HCV infection) was associated with a 2–3-fold increased risk of HCV [24, 25], more closely reflecting our findings.
Studies examining the association between STIs and HCV among GBM using PrEP are limited. Our findings that rectal chlamydia and rectal gonorrhea are associated with an increased risk of HCV among GBM using PrEP is consistent with findings from the Netherlands [12] that identified a 2-fold increased risk for both rectal chlamydia and gonorrhea in GBM enrolled in an early PrEP demonstration study among 350 GBM. Prior STIs have also been associated with prevalent HCV among GBM commencing PrEP in British Columbia, Canada, though there were not enough incident HCV infections to examine factors associated with incidence [13].
The different associations between STIs and HCV that we found between the 2 groups warrants some consideration. Among GBM with HIV included in our analyses, ≥ 1 rectal chlamydia infection in the 2 years before an HCV test was just as common among GBM with or without incident HCV. Likewise, there was little difference with rectal gonorrhea. However, a considerable proportion did not have rectal STI testing in the 2 years before an HCV test. This may be because HIV itself is not a marker of recent and/or ongoing behavior and rectal STI testing requires swab testing, whereas HCV testing can be done from blood obtained for routine HIV viral load monitoring. Conversely, rectal STI testing was more common among GBM using PrEP, with 95% having a rectal STI test in the 2 years before an HCV test.
Positive rectal STI tests were also markedly more common among GBM using PrEP in whom HCV was subsequently diagnosed than in those in whom it was not. It is well established that STIs, particularly rectal STIs, are highly concentrated among subgroups of GBM using PrEP [31]. As such, it is possible that these GBM are also engaging in behaviors that increase their risk of HCV and/or were members of sexual networks with other GBM who were yet to be treated for HCV. While syphilis was diagnosed among many GBM using PrEP who were included in our analyses, HCV was subsequently diagnosed in only 2, neither of whom had syphilis in the 2 years before their incident HCV diagnosis. In the Netherlands, syphilis was also not associated with increased HCV risk among GBM using PrEP, likely also because of the relatively low numbers [12]. It remains an open question whether an association would have been seen between syphilis and incident HCV for GBM using PrEP if the number of incident HCV infections among those with syphilis had been larger during the observation period.
Determining what proportion of the association between STIs and increased HCV risk is driven by biological factors and what is due to behaviors that are common to each remains challenging. There may be some biological plausibility that STIs increase the risk of HCV, particularly syphilis owing to the chancre associated with this infection. However, chancres usually spontaneously resolve within <3 months [32]. This points to reduced biological plausibility in our analyses and others, where syphilis has been measured over an extended period of time. It is more likely that the associations we found are due to sexual and substance use behaviors that often overlap and are associated with both HCV and STIs. For example, in Australia, gonorrhea incidence has been shown to be associated with inconsistent condom use during anal sex, with having ≥20 sexual partners, and with injection drug use [33].
Another Australian study reported associations between methamphetamine use and a higher number of condomless receptive anal sex partners and each of rectal chlamydia, rectal gonorrhea, and syphilis [34]. STIs have also been associated with condomless anal sex, a higher number of sexual partners, and group sex among GBM using PrEP [31, 35]. A Dutch study, specific to GBM with HIV, reported an association between syphilis infection and fisting and an association between chlamydia or gonorrhea and drug use during sex [36]. While we found an association between bacterial STIs and an increased risk of incident HCV, it is important to recognize that only 1.4% of GBM with HIV and 0.37% of GBM using PrEP had incident HCV infection diagnosed. Conversely, bacterial STIs were far more common. This suggests an HCV treatment as prevention effect among GBM with HIV and GBM using PrEP rather than as a result of changes in behavior, as has been postulated at an ecological level among GBM with HIV in the Netherlands [37].
Given that there is evidence of ongoing HCV transmission, guidelines from Australia [38], Europe [39], and the United States [40] all recommend immediate HCV treatment, including for acute infections. Treatment requires testing to begin with, and there are indications that 40%–50% of GBM with HIV are not tested annually [1, 9, 16, 17], despite most guidelines globally recommending at least annual testing. Similarly, these analyses and our previous work [1] show that 35% of GBM using PrEP only ever had a single test. As such, while at least annual HCV testing is still warranted among all GBM with HIV or using PrEP to achieve and sustain elimination, we suggest that a review and offer of HCV testing is particularly warranted among GBM with diagnosed STIs.
In addition to annual testing, some guidelines recommend more frequent testing. For example, US guidelines state that “Depending on sexual or drug use risk practices, more frequent testing may be warranted” for both GBM with HIV and those using PrEP [41]. Likewise, Australian PrEP clinical guidelines suggest HCV testing “at least every 12 months or more frequently if necessary” [18]. While work is needed to examine the potential impact and cost-effectiveness, more frequent testing may be warranted among some GBM [42]. The rationale for this is that the longer there is between tests, the more chances there are for onward transmission, including to people who have previously been treated, which could potentially offset progress toward HCV elimination among GBM. STIs may provide an objective marker of more frequent testing in the absence of discussions on the array of behaviors that are associated with HCV among GBM [22].
Our analyses have some limitations. Our data were drawn from a large national surveillance system, which does not systematically collect behavioral data across all clinics. As such, we did not undertake analyses adjusting for these to examine whether STIs are an independent risk factor for HCV. Rather, we suggest that STIs may serve as a marker of these behaviors in the absence of discussions regarding them. It is important to acknowledge that in 2020 coronavirus disease 2019 and related lockdowns/restrictions of movement likely had some impact on both HCV and STI testing, as well as on behaviors associated with an increased risk of both HCV and STIs. All clinics participating in ACCESS were selected owing to their high caseload of key population groups. Our data do not capture STIs, or HCV, diagnosed outside these clinics. Similarly, most clinics are located in inner urban areas and therefore our findings may not be generalizable to GBM attending clinics in outer suburban or regional areas; work is ongoing to expand coverage. Although the HCV testing frequency among the GBM included in our analyses was often within the recommended annual time frame, these analyses by definition are limited to GBM who test at least once and have ≥1 additional test. Further work is needed to understand HCV testing in general among GBM, as these analyses and our previous analyses [1] indicate that a considerable proportion of GBM with HIV, and now GBM using PrEP, do not test for HCV at the annual frequency recommended in guidelines or have only ever had one test.
In conclusion, in these analyses of primary HCV and bacterial STIs among GBM attending clinics across Australia, we found that syphilis was associated with primary HCV among GBM with HIV. Rectal chlamydia and rectal gonorrhea were associated with primary HCV among GBM using PrEP. This suggests that STIs remain a marker of increased HCV risk, even in the context of declining incidence among GBM with HIV and low incidence among GBM using PrEP, and that STIs might be useful for informing HCV testing decisions and guidelines among these key population groups for HCV elimination.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Acknowledgments
We acknowledge foremost that these data and the resulting research would not be possible without the invaluable contribution of people attending the clinics participating in ACCESS. We acknowledge the contribution of the ACCESS research team and advisory committee members who are not coauthors of this article, including the following: Htein Linn Aung, Kirby Institute, University of New South Wales (UNSW) Sydney; Greta Baillie, Kirby Institute, UNSW Sydney; Lisa Bastian, WA Health; Deborah Bateson, Family Planning NSW; Mark Boyd, University of Adelaide; Allison Carter, Kirby Institute, UNSW Sydney; Aaron Cogle, National Association of People with HIV Australia; Jane Costello, Positive Life NSW; Wayne Dimech, NRL; Carol El-Hayek, Burnet Institute; Jeanne Ellard, Australian Federation of AIDS Organisations; Lucinda Franklin, Victorian Department of Health; Jane Hocking, University of Melbourne; Jules Kim, Scarlet Alliance; Long Nguyen, Burnet Institute; Thi Nguyen, Burnet Institute; David Nolan, Royal Perth Hospital; Catherine O’Connor, Kirby Institute, UNSW Sydney; Prital Patel, Kirby Institute, UNSW Sydney; Stella Pendle, Australian Clinical Laboratories; Victoria Polkinghorne, Burnet Institute; Philip Reed, Kirkton Road Centre; Nathan Ryder, NSW Sexual Health Service Directors; Christine Selvey, NSW Ministry of Health; and Melanie Walker, Australian Injecting and Illicit Drug Users League, Nyssa Watson, Burnet Institute.
Author contributions. Conceptualization: B. L. H., R. S. D., M. E. H., and J. S. D. Methodology: B. L. H., R. S. D., P. A., D. K. v. S., and A. L. W. Data analysis: B. L. H. Data interpretation: B. L. H., R. S. D., M. E. H., and J. S. D. Data collection: C. K. F., N. R., and M. B. Data curation: M. W. T. and J. A. Funding acquisition: B. D., R. G., M. S., and M. E. H. Writing of the original draft: B. L. H. Review and editing of the manuscript: All authors.
Disclaimer. The funders played no role in the conception, analyses, or interpretation of these data, nor in the decision to submit this work for publication.
Financial support. This work was supported by the National Health and Medical Research Council (support to ACCESS, a partnership between Burnet Institute, Kirby Institute, and the National Reference Laboratory); the National Health and Medical Research Council (support to B. L. H., R. S. D., B. D., M.S., M. E. H., and J. S. D.); and the Victorian Government Operational Infrastructure Fund (support to Burnet Institute).
Potential conflicts of interest. D. K. v. S. reports payment to her institution (Public Health Service of Amsterdam) for liver debate, sponsored by Gilead, AbbVie, and Norgine. M. W. T. has received speaker's honoraria and support to attend the 2019 Hitos en Investigación Básica y Clínica en VIH/SIDA conference from Gilead Sciences. G. V. M. has received grants from Gilead Sciences and AbbVie, paid to her institution, and payment from Janssen for chairing a meeting. M. B. reports grants paid to his institution for clinical research from Gilead Sciences, ViiV Healthcare, MSD, AbbVie, Eli Lilly, Novartis, and Pfizer; has received consulting fees for attendance at medical advisory boards from Gilead Sciences, ViiV Healthcare, and AbbVie; has received payment or honoraria from presentations/lectures from Gilead Sciences and AbbVie; and has received payments to attend scientific meetings or advisory boards from Gilead Sciences, ViiV Healthcare, and GSK. M. S. served as an advisory board member for and received honoraria from Gilead Sciences and has received investigator-initiated funding from Gilead Sciences, AbbVie, and Bristol-Myers Squibb (BMS). M. E. H. has received investigator-initiated funding from Gilead Sciences, Merck, AbbVie, and BMS. J. S. D. has received investigator-initiated funding from AbbVie, BMS, Gilead Sciences, and Merck; consultancies from Gilead Sciences and AbbVie; and support for attending meetings and/or travel from Gilead Sciences. All other authors report no potential conflicts.
Contributor Information
Brendan L Harney, Disease Elimination Program, Burnet Institute, Melbourne, Victoria, Australia; School of Public Health and Preventive Medicine, Monash University, Melbourne, Victoria, Australia; Department of Infectious Disease, Alfred Health & Monash University, Melbourne, Victoria Australia.
Rachel Sacks-Davis, Disease Elimination Program, Burnet Institute, Melbourne, Victoria, Australia; School of Public Health and Preventive Medicine, Monash University, Melbourne, Victoria, Australia.
Paul Agius, Disease Elimination Program, Burnet Institute, Melbourne, Victoria, Australia; School of Public Health and Preventive Medicine, Monash University, Melbourne, Victoria, Australia.
Daniela K van Santen, Disease Elimination Program, Burnet Institute, Melbourne, Victoria, Australia; School of Public Health and Preventive Medicine, Monash University, Melbourne, Victoria, Australia; Department of Infectious Diseases, Public Health Service of Amsterdam, Amsterdam, the Netherlands.
Michael W Traeger, Disease Elimination Program, Burnet Institute, Melbourne, Victoria, Australia; School of Public Health and Preventive Medicine, Monash University, Melbourne, Victoria, Australia; Department of Population Medicine, Harvard Pilgrim Health Care Institute and Harvard Medical School, Harvard University, Boston, Massachusetts, USA.
Anna L Wilkinson, Disease Elimination Program, Burnet Institute, Melbourne, Victoria, Australia; School of Public Health and Preventive Medicine, Monash University, Melbourne, Victoria, Australia.
Jason Asselin, Disease Elimination Program, Burnet Institute, Melbourne, Victoria, Australia.
Christopher K Fairley, Alfred Health, Melbourne Sexual Health Centre, Melbourne, Victoria, Australia; Central Clinical School, Monash University, Melbourne, Victoria, Australia.
Norman Roth, Prahran Market Clinic, Melbourne, Victoria, Australia.
Mark Bloch, Holdsworth House Medical Practice, Sydney, New South Wales, Australia.
Gail V Matthews, Kirby Institute, University of New South Wales, Sydney, New South Wales, Australia; St Vincent's Hospital, Sydney, New South Wales, Australia.
Basil Donovan, Kirby Institute, University of New South Wales, Sydney, New South Wales, Australia.
Rebecca Guy, Kirby Institute, University of New South Wales, Sydney, New South Wales, Australia.
Mark Stoové, Disease Elimination Program, Burnet Institute, Melbourne, Victoria, Australia; School of Public Health and Preventive Medicine, Monash University, Melbourne, Victoria, Australia; Australian Research Centre in Sex, Health and Society, La Trobe University, Melbourne, Victoria, Australia.
Margaret E Hellard, Disease Elimination Program, Burnet Institute, Melbourne, Victoria, Australia; School of Public Health and Preventive Medicine, Monash University, Melbourne, Victoria, Australia; Department of Infectious Disease, Alfred Health & Monash University, Melbourne, Victoria Australia; Doherty Institute and School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia.
Joseph S Doyle, Disease Elimination Program, Burnet Institute, Melbourne, Victoria, Australia; School of Public Health and Preventive Medicine, Monash University, Melbourne, Victoria, Australia; Department of Infectious Disease, Alfred Health & Monash University, Melbourne, Victoria Australia.
References
- 1. Harney BL, Sacks-Davis R, van Santen DK, et al. The incidence of hepatitis C among gay, bisexual, and other men who have sex with men in Australia, 2009–2019. Clin Infect Dis 2022; 74:1804–11. [DOI] [PubMed] [Google Scholar]
- 2. Garvey LJ, Cooke GS, Smith C, et al. Decline in hepatitis C virus (HCV) incidence in men who have sex with men living with human immunodeficiency virus: progress to HCV microelimination in the United Kingdom? Clin Infect Dis 2021; 72:233–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Castry M, Cousien A, Bellet J, et al. Hepatitis C virus (HCV) incidence among men who have sex with men (MSM) living with HIV: results from the French hospital database on HIV (ANRS CO4-FHDH) cohort study, 2014 to 2017. Euro Surveill 2021; 26:2001321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Smit C, Boyd A, Rijnders BJA, et al. HCV micro-elimination in individuals with HIV in the Netherlands 4 years after universal access to direct-acting antivirals: a retrospective cohort study. Lancet HIV 2021; 8:e96–105. [DOI] [PubMed] [Google Scholar]
- 5. Braun DL, Hampel B, Ledergerber B, et al. A treatment as prevention trial to eliminate hepatitis C among men who have sex with men living with HIV in the Swiss HIV cohort study. Clin Infect Dis 2021; 73:e2194–202. [DOI] [PubMed] [Google Scholar]
- 6. van Santen DK, Sacks-Davis R, Stewart A, et al. Treatment as prevention effect of direct-acting antivirals on primary hepatitis C virus incidence: findings from a multinational cohort between 2010 and 2019. eClinicalMedicine 2023; 56:101810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hosseini-Hooshyar S, Hajarizadeh B, Bajis S, et al. Risk of hepatitis C reinfection following successful therapy among people living with HIV: a global systematic review, meta-analysis, and meta-regression. Lancet HIV 2022; 9:e414–27. [DOI] [PubMed] [Google Scholar]
- 8. Hosseini-Hooshyar S, Martinello M, Yee J, et al. Low hepatitis C virus reinfection rate despite ongoing risk following universal access to direct-acting antiviral therapy among people living with HIV. AIDS 2020; 34:1347–58. [DOI] [PubMed] [Google Scholar]
- 9. Doyle JS, van Santen DK, Iser D, et al. Microelimination of hepatitis C among people with human immunodeficiency virus coinfection: declining incidence and prevalence accompanying a multicenter treatment scale-up trial. Clin Infect Dis 2021; 73:e2164–72. [DOI] [PubMed] [Google Scholar]
- 10. Traeger MW, Harney BL, Sacks-Davis R, et al. Incidence and prevalence of hepatitis C virus among HIV-negative gay and bisexual men using HIV pre-exposure prophylaxis (PrEP): a systematic review and meta-analysis. Open Forum Infect Dis 2023; 10:ofad401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cotte L, Cua E, Reynes J, et al. Hepatitis C virus incidence in HIV-infected and in preexposure prophylaxis (PrEP)-using men having sex with men. Liver Int 2018; 38:1736–40. [DOI] [PubMed] [Google Scholar]
- 12. Hoornenborg E, Coyer L, Boyd A, et al. High incidence of HCV in HIV-negative men who have sex with men using pre-exposure prophylaxis. J Hepatol 2020; 72:855–64. [DOI] [PubMed] [Google Scholar]
- 13. Thompson KA, Blank G, Toy J, et al. Prevalence and incidence of hepatitis C infection amongst men who have sex with men in a population-based pre-exposure prophylaxis program in British Columbia, Canada. Liver Int 2022; 42:1528–35. [DOI] [PubMed] [Google Scholar]
- 14. Martinello M, Yee J, Bartlett SR, et al. Moving towards hepatitis C microelimination among people living with human immunodeficiency virus in Australia: the CEASE study. Clin Infect Dis 2020; 71:1502–10. [DOI] [PubMed] [Google Scholar]
- 15.Australian sexually transmitted infection & HIV testing guidelines 2019. Available at: https://stipu.nsw.gov.au/wp-content/uploads/STIGMA_Guidelines2019_Final-1.pdf. Accessed 10 April 2023.
- 16. Moqueet N, Grewal R, Mazzulli T, et al. Hepatitis C virus testing in a clinical HIV cohort in Ontario, Canada, 2000 to 2015. Health Sci Rep 2021; 4:e358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li J, Armon C, Palella FJ, et al. Hepatitis C virus testing among men with human immunodeficiency virus who have sex with men: temporal trends and racial/ethnic disparities. Open Forum Infect Dis 2021; 8:ofaa645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wright E, Grulich A, Roy K, et al. Australasian society for HIV, viral hepatitis and sexual health medicine HIV pre-exposure prophylaxis: clinical guidelines. Update April 2018. J Virus Erad 2018; 4:143–59. [PMC free article] [PubMed] [Google Scholar]
- 19. Nijmeijer BM, Koopsen J, Schinkel J, Prins M, Geijtenbeek TBH. Sexually transmitted hepatitis C virus infections: current trends, and recent advances in understanding the spread in men who have sex with men. J Int AIDS Soc 2019; 22(suppl 6):e25348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Künzler-Heule P, Engberg S, Battegay M, et al. Screening HIV-positive men who have sex with men for hepatitis C re-infection risk: is a single question on condom-use enough? A sensitivity analysis. BMC Infect Dis 2019; 19:821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brener L, Murphy DA, Cama E, Murray J, Fraser N, Ellard J. HCV knowledge, disclosure practices, and risk perceptions among gay and bisexual men who do and do not engage in group sex while using drugs. AIDS Care 2020; 32:1258–61. [DOI] [PubMed] [Google Scholar]
- 22. Marshall AD, Martinello M, Treloar C, Matthews GV. Perceptions of hepatitis C treatment and reinfection risk among HIV-positive men who have sex with men and engage in high risk behaviours for hepatitis C transmission: the CEASE qualitative study. Int J Drug Pol 2022; 109:103828. [DOI] [PubMed] [Google Scholar]
- 23. Medland NA, Chow EP, Bradshaw CS, Read TH, Sasadeusz JJ, Fairley CK. Predictors and incidence of sexually transmitted hepatitis C virus infection in HIV positive men who have sex with men. BMC Infect Dis 2017; 17:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Burchell AN, Gardner SL, Mazzulli T, et al. Hepatitis C virus seroconversion among HIV-positive men who have sex with men with no history of injection drug use: results from a clinical HIV cohort. Can J Infect Dis Med Microbiol 2015; 26:17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wandeler G, Gsponer T, Bregenzer A, et al. Hepatitis C virus infections in the Swiss HIV cohort study: a rapidly evolving epidemic. Clin Infect Dis 2012; 55:1408–16. [DOI] [PubMed] [Google Scholar]
- 26. Ho S-Y, Su LH, Sun HY, et al. Trends of recent hepatitis C virus infection among HIV-positive men who have sex with men in Taiwan, 2011–2018. eClinicalMedicine 2020; 24:100441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Callander D, Moreira C, El-Hayek C, et al. Monitoring the control of sexually transmissible infections and blood-borne viruses: protocol for the Australian Collaboration for Coordinated Enhanced Sentinel Surveillance (ACCESS). JMIR Res Protoc 2018; 7:e11028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nguyen L, Stoové M, Boyle D, et al. Privacy-preserving record linkage of deidentified records within a public health surveillance system: evaluation study. J Med Internet Res 2020; 22:e16757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ampt FH, El Hayek C, Agius PA, et al. Anorectal swabs as a marker of male-to-male sexual exposure in STI surveillance systems. Epidemiol Infect 2017; 145:2530–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Singer JD, Willett JB, eds. Extending the Discrete-Time Hazard Model. In: Applied Longitudinal Data Analysis: Modeling Change and Event Occurrence. England: Oxford University Press, 2003. [Google Scholar]
- 31. Traeger MW, Cornelisse VJ, Asselin J, et al. Association of HIV preexposure prophylaxis with incidence of sexually transmitted infections among individuals at high risk of HIV infection. JAMA 2019; 321:1380–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lafond RE, Lukehart SA. Biological basis for syphilis. Clin Microbiol Rev 2006; 19:29–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Callander D, Guy R, Fairley CK, et al. Gonorrhoea gone wild: rising incidence of gonorrhoea and associated risk factors among gay and bisexual men attending Australian sexual health clinics. Sex Health 2019; 16:457–63. [DOI] [PubMed] [Google Scholar]
- 34. Goddard SL, Poynten IM, Petoumenous K, et al. Prevalence, incidence and predictors of anal Chlamydia trachomatis, anal Neisseria gonorrhoeae and syphilis among older gay and bisexual men in the longitudinal study for the prevention of anal cancer (SPANC). Sex Transm Infect 2019; 95:477–83. [DOI] [PubMed] [Google Scholar]
- 35. Hart TA, Noor SW, Berlin GW, et al. Pre-exposure prophylaxis and bacterial sexually transmitted infections (STIs) among gay and bisexual men. Sex Transm Infect 2023; 99:167–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Heiligenberg M, Rijnders B, Schim van der Loeff MF, et al. High prevalence of sexually transmitted infections in HIV-infected men during routine outpatient visits in the Netherlands. Sex Transm Dis 2012; 39:8–15. [DOI] [PubMed] [Google Scholar]
- 37. Boerekamps A, van den Berk GE, Lauw FN, et al. Declining hepatitis C virus (HCV) incidence in Dutch human immunodeficiency virus-positive men who have sex with men after unrestricted access to HCV therapy. Clin Infect Dis 2018; 66:1360–5. [DOI] [PubMed] [Google Scholar]
- 38. Gastroenterological Society of Australia . Australian recommendations for the management of hepatitis C virus infection: a consensus statement (2022). Melbourne, Australia: Gastroenterological Society of Australia, 2022. Available at: https://www.hepcguidelines.org.au/. Accessed 1 May 2023. [Google Scholar]
- 39. Pawlotsky JM, Negro F, Aghemo A, et al. EASL recommendations on treatment of hepatitis C: final update of the series. J Hepatol 2020; 73:1170–218. [DOI] [PubMed] [Google Scholar]
- 40. Ghany MG, Morgan TR; AASLD-IDSA Hepatitis C Guidance Panel . Hepatitis C guidance 2019 update: American Association for the Study of Liver Diseases—Infectious Diseases Society of America recommendations for testing, managing, and treating hepatitis C virus infection. Hepatology 2020; 71:686–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. American Association for the Study of Liver Diseases and Infectious Diseases Society of America . HCV in key populations: men who have sex with men. 2022. Available at: https://www.hcvguidelines.org/unique-populations/msm. Accessed 1 May 2023.
- 42. Sun HY, Liou BH, Chen TC, et al. Optimal frequency of hepatitis C virus (HCV) RNA testing for detection of acute HCV infection among at-risk people with human immunodeficiency virus: a multicenter study. Open Forum Infect Dis 2023; 10:ofad307. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.