Abstract
The serotonin transporter (SERT) readily takes up serotonin (5-HT), thereby regulating the availability of 5-HT within the intestine. In the absence of SERT, 5-HT remains in the interstitial space and has the potential to aberrantly activate the many 5-HT receptors distributed on the epithelium, immune cells and enteric neurons. Perturbation of SERT is common in many gastrointestinal disorders as well as mouse models of colitis. Select commensal microbes regulate intestinal SERT levels, but the mechanism of this regulation is poorly understood. Additionally, ethanol upregulates SERT in the brain and dendritic cells, but its effects in the intestine have never been examined. We report that the intestinal commensal microbe Limosilactobacillus (previously classified as Lactobacillus) reuteri ATCC PTA 6475 secretes 83.4 mM ethanol. Consistent with the activity of L. reuteri alcohol dehydrogenases, we found that L. reuteri tolerated various levels of ethanol. Application of L. reuteri conditioned media or exogenous ethanol to human colonic T84 cells was found to upregulate SERT at the level of mRNA. A 4-(4-(dimethylamino) phenyl)-1-methylpyridinium (APP+) uptake assay confirmed the functional activity of SERT. These findings were mirrored in mouse colonic organoids, where L. reuteri metabolites and ethanol were found to upregulate SERT at the apical membrane. Finally, in a trinitrobenzene sulphonic acid model of acute colitis, we observed that mice treated with L. reuteri maintained SERT at the colon membrane compared with mice receiving phosphate buffered saline vehicle control. These data suggest that L. reuteri metabolites, including ethanol, can upregulate SERT and may be beneficial for maintaining intestinal homeostasis with respect to serotonin signalling.
Keywords: serotonin, serotonin transporter, lactic acid bacteria, probiotics
1. Introduction
Various aspects of human health are influenced by the intestinal microbiota, including the modulation of the immune system, stimulation of the enteric and central nervous systems, production of vitamins and short chain fatty acids, break-down of indigestible food components, and protection against pathogens (Martin et al., 2013). Recent evidence has pointed to the role of the gut microbiota in regulating the serotonergic system (Akiba et al., 2015; Cao et al., 2018a,b; Engevik et al., 2020; Esmaili et al., 2009; Fukumoto et al., 2003; Hata et al., 2017; Nakaita et al., 2013; Neufeld et al., 2011; Nishino et al., 2013; Nzakizwanayo et al., 2015; O’Hara et al., 2006; Reigstad et al., 2015; Wang et al., 2015; Yano et al., 2015). The serotonergic system revolves around the important regulatory amine serotonin (5-HT) that is synthesised by intestinal enterochromaffin (EC) cells. Secreted 5-HT can elicit the activation of receptors on immune cells, goblet cells, epithelial cells, smooth muscle and neurons (Baganz and Blakely, 2013; Bellono et al., 2017; Bertrand and Bertrand, 2010; Gershon and Tack, 2007; Hoffman et al., 2012; Mawe and Hoffman, 2013). The actions of 5-HT are terminated by uptake via the highly selective sodium-chloride coupled serotonin transporter (SERT) SLC6A4 (Broer and Gether, 2012; Chen et al., 1998; Esmaili et al., 2009; Gill et al., 2005; Kristensen et al., 2011; Takayanagi et al., 1995), which is expressed in the intestinal mucosa and the enteric and central nervous system. SERT-mediated uptake of 5-HT results in the degradation and termination of the 5-HT signal (Blakely et al., 1991; Brownstein and Hoffman, 1994; Gershon and Sherman, 1982; Gershon et al., 1990; Hoffman et al., 1991; Kim and Khan, 2014; Martel et al., 2003; Wade et al., 1996). As a result, SERT is postulated to influence intestinal homeostasis by controlling 5-HT concentrations.
Decreased SERT concentrations have been associated with intestinal pathogenesis. SERT is documented to be reduced in experimental models of gastrointestinal (GI) inflammation including dextran sulphate sodium (DSS) (Bertrand et al., 2010) and trinitrobenzene sulphonic acid (TNBS) (Linden et al., 2005) mouse models. Additionally, SERT concentrations are decreased in mouse models of enteric infection by enteropathogenic Escherichia coli, Citrobacter rodentium, Listeria monocytogenes and the parasite Trichinella spiralis (Esmaili et al., 2009; Latorre et al., 2016b; O’Hara et al., 2006; Wheatcroft et al., 2005). In patients, decreased SERT transcript levels have been documented in individuals with a history of diverticulitis or active inflammatory bowel disease (IBD) when compared with controls (Costedio et al., 2008; Tada et al., 2016). Consistent with the importance of SERT in homeostasis, SERT knockout mice exhibit exacerbated inflammation in experimental colitis (interleukin (IL)-10 deficiency and TNBS colitis) and fructose-induced hepatic steatosis in mice (Bischoff et al., 2009; Haub et al., 2010a,b). In parallel with animal studies, reduced SERT levels have been reported in patients with ulcerative colitis (Bellini et al., 2003; Coates et al., 2004). These findings implicate 5-HT signalling and SERT-mediated termination as critical factors in inflammatory conditions of the GI tract.
Commensal lactic acid bacteria (LAB) regulate SERT concentrations in the intestine. Studies have associated Lacticaseibacillus (previously classified as Lactobacillus) rhamnosus, Bifidobacterium longum and Bifidobacterium dentium with an increased abundance of SERT in the intestine (Cao et al., 2018a,b; Engevik et al., 2020; Wang et al., 2015). However, the exact mechanism by which these microbes influence SERT is unknown. Lactic acid bacteria are commonly found in the intestinal mucus layer where fermentation products like ethanol could easily be delivered to the host epithelium (Blomberg et al., 1993; Bohle et al., 2010; Carasi et al., 2014; Coconnier et al., 1992; Henriksson and Conway, 1996; Jacobsen et al., 1999; Jensen et al., 2014; Jonsson et al., 2001; Ma et al., 2006; Macias-Rodriguez et al., 2009; Mackenzie et al., 2010; Miyoshi et al., 2006; Nishiyama et al., 2015; Ouwehand and Conway, 1996; Ramiah et al., 2007; 2009; Van Tassell and Miller, 2011). An inverse relationship between ethanol consumption and 5-HT concentrations has been demonstrated in the central nervous system linking ethanol concentrations to SERT activity (Kelai et al., 2003). Mice treated with ethanol exhibit increased SERT expression in the cingulate cortex, nucleus accumbens, hippocampal CA1-CA3 layers, and mediodorsal nucleus of the thalamus (Shibasaki et al., 2010). Babu and colleagues (2009) demonstrated that ethanol also upregulates SERT in dendritic cells. Interestingly, the microbes associated with a greater abundance of intestinal SERT also produce ethanol (Cao et al., 2018a,b; Engevik et al., 2020; Wang et al., 2015), providing indirect evidence that bacterial generated ethanol may affect SERT activity. Despite these potential connections, little is known regarding bacterial produced ethanol on SERT levels in the intestinal epithelium. In this study, we aimed to identify whether ethanol producing Limosilactobacillus (previously classified as Lactobacillus) reuteri ATCC 6475 and endogenous ethanol could modulate intestinal SERT.
2. Materials and methods
Bacterial strains and microbiological culture conditions
L. reuteri ATCC-PTA 6475 was cultured anaerobically in De Man, Rogosa and Sharpe (MRS) medium (Difco, BD Biosciences, Franklin Lakes, NJ, USA) or a fully defined media, termed LDM4 as previously described (Engevik et al., 2019). L. reuteri cultures were grown anaerobically in a workstation (AS-580; Anaerobe Systems, Morgan Hill, CA, USA) in a mixture of 5% CO2, 5% H2, and 90% N2. Single colonies grown on MRS agar plates were used to inoculate overnight MRS cultures. From MRS, L. reuteri was sub-cultured into LDM4 (OD600nm adjusted to 0.1) and cultured anaerobically at 37 °C for 24 h. Optical density (OD600nm) was measured at 24 h on a Smartspec Plus spectrophotometer (Bio-Rad Laboratories Inc., Hercules, CA, USA) or plate reader (Synergy H1; BioTek, Winooski, VT, USA). Cultures were centrifuged to remove bacterial cells (5,000×g, 5 min), supernatant pH was adjusted to pH 7.0 and sterile filtered through a 0.2 μm membrane filter (Millipore, San Diego, CA, USA). The resulting solution was termed conditioned medium (CM). As a negative control, uninoculated LDM4 bacterial medium was also included. To generate heat killed bacteria, bacterial pellets were resuspended in 10 ml sterile phosphate buffered saline (PBS) and heated at 65 °C for 30 min. For growth curve experiments, L. reuteri was grown in LDM4 in 96-well plates and optical densities were examined at OD600nm on a BioTek Synergy H1 plate reader.
Cell culture and cell treatment
The human colonic epithelial carcinoma cell line, T84 (American Type Culture Collection ATCC CCL-248), was grown in DMEM-F12 media supplemented with 10% foetal bovine serum at 37 °C, 5% CO2. Additionally, the human colonic epithelial immortalised non-cancer NCM460D cell line was grown with enriched M3:10™ medium (Incell Corporation LLC, San Antonio, TX 78249, USA). For both colon lines, approximately 1×105 cells were seeded onto 24-well plates (Corning, Sommerville, MA, USA) with poly-D-Lysine coated coverslips, grown to confluence and various concentrations of uninoculated LDM4, L. reuteri CM or ethanol were applied to the apical membrane at 37 °C, 5% CO2. Cells were incubated with treatment for 16 h and then monolayers were fixed in 4% PFA (ThermoFisher Scientific, Waltham, MA, USA) for 1 h and examined by immunofluorescent microscopy. Alternatively, monolayers were incubated for 6 h and processed in TRIZOL (Life Technologies, Grand Island, NY, USA) for mRNA extraction.
Trinitrobenzene sulphonic acid colitis model
All animal care and experimental procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at Baylor College of Medicine (Houston, TX, USA) in accordance with all guidelines set forth by the U.S. National Institutes of Health. For acute colitis experiments, BALB/c mice (8–12 weeks old) were purchased from Taconic (Rensselaer, NY, USA) and housed at Baylor College of Medicine. After acclimation (1 week), mice were orally gavaged with 109 cfu/ml L. reuteri in PBS or sterile PBS alone (vehicle control; ThermoFisher) daily for 1 week. Following pre-treatment with L. reuteri or PBS, mice were anesthetised by isoflurane inhalation and 5% (w/v) 2,4,6-trinitrobenzenesulfonic acid (TNBS) (#P2297; Sigma, St. Louis, MO, USA) dissolved in an equal volume of absolute ethanol (100 mg/kg of body weight) was rectally delivered by catheter, 4 cm into the colon. Mice continued to receive daily oral gavage of treatment (L. reuteri in PBS or sterile PBS) until euthanasia (3 days post-TNBS). For histology, the colon was excised fixed in Carnoy’s fixative (Sigma) and paraffin embedded. Haematoxylin and eosin stains of colon sections were used for histological scoring of colitis by a blinded board-certified anatomic pathologist.
Mouse organoid culture
C57BL/6 mice, 6–8 weeks of age, were euthanised with isoflurane followed by cervical dislocation. The mid to distal colon was excised and processed to generate organoids as previously described (Engevik et al., 2013a; Fernando et al., 2017). Briefly, the colon was opened lengthwise, washed with Ca2+/Mg2+-free PBS, and small sections were incubated with 3 mM EDTA (ThermoFisher), 0.5 mM DTT (Sigma) and 43.4 mM sucrose (Sigma) for 30 min at 4 °C. After crypt dissociation by shaking and pipetting, crypts were collected in chelation buffer (2% sorbitol, 1% sucrose, 1% bovine serum albumin). Intestinal crypts were then centrifuged at 300×g for 10 min and the crypt pellet was suspended in Matrigel (BD Biosciences). After Matrigel polymerisation, 400 μl CMGF+ (complete media with growth factors) containing 10 μM Y-27632 rock inhibitor was added to each well as previously described (Chang-Graham et al., 2019). All organoid cultures were passaged >2 times to ensure the absence of cellular debris. To differentiate organoids, colonic organoids were grown for 48 h in CMGF+, then grown for 4–5 days in CMGF+ without Wnt, nicotinamide, and only 5% Noggin and 5% R-spondin. After differentiation for 4–5 days, 50% uninoculated LDM4, 50% L. reuteri CM and 5% ethanol was added to the differentiation media. Organoids were incubated overnight at 37 °C, 5% CO2 for immunostaining or incubated for 6 h at 37 °C, 5% CO2 for RNA isolation.
Immunostaining
Immunofluorescent staining was carried out on 7-μm paraffin sections of mouse organoids or mouse colon tissue. Slides were baked at 60 °C and deparaffinized in a series of xylene and alcohol washes. Antigen retrieval was performed using 0.1 M sodium citrate buffer, pH 6.0 for 20 min in a pressure cooker. Slides were washed with Tris-buffered saline containing Tween 20 (TBS-T), blocked with 10% donkey serum and incubated with a rabbit-anti-SERT antibody (diluted 1:100, Immunostar #24330) overnight at 4 °C. Sections were then stained with AlexaFluor donkey anti-rabbit 555 secondary antibody (diluted 1:1000, Life Technologies #A-31572) for 1 h at room temperature and counterstained with Hoechst 33342 (Invitrogen, Waltham, MA, USA) for 10 min. Coverslips were applied on slides using Fluoromount Aqueous Mounting Medium (Sigma #F4680). For T84 cells on coverslips, cells were permeabilised with 0.1% Triton-X in PBS for 30 min at room temperature, blocked in TBS-T containing 10% donkey serum and incubated with a rabbit-anti-SERT antibody (diluted 1:100; #24330; Immunostar, Hudson, WI, USA) overnight at 4 °C. After washing, cells were incubated with AlexaFluor donkey antirabbit 555 secondary antibody (diluted 1:1000) for 1 h at room temperature and counterstained with Hoechst 33342 for 10 min. Coverslips were mounted inverted on slides using Fluoromount Aqueous Mounting Medium (Sigma #F4680). All slides were imaged on a Nikon Eclipse 90i (Nikon, Tokyo, Japan) microscope. FIJI (Formerly ImageJ, National Institutes of Health) software was used to semi-quantitatively quantify the fluorescence by tabulating mean pixel intensity as previously described (Engevik et al., 2013a,b,c).
Serotonin transporter activity assay
T84 cells were treated with bacterial conditioned media or ethanol overnight. Following incubation, cells were washed and then treated with 10 μM of 4-(4-(dimethylamino) phenyl)-1-methylpyridinium (APP+, Sigma #SML0756). APP+ is a fluorescent substrate for organic cation transporters which has previously been used to calculate SERT activity (Solis et al., 2012; Wilson et al., 2014). T84 cells were incubated with APP+ at 37 °C, 5% CO2 for 30 min and washed three times with Krebs Ringers bicarbonate buffer. Intracellular APP+ was quantified by measuring fluorescence intensity at λex = 505 nm, λem = 550 nm on a plate reader (Biotek Syngery H1).
Alcohol dehydrogenase ELISA
An alcohol dehydrogenase ELISA kit (#ab102533; Abcam, Cambridge, UK) was used to assess the concentrations of alcohol dehydrogenase protein in L. reuteri. The assay is based on the inter-conversion between alcohols and aldehydes or ketones with the reduction of NAD+ to NADH. In Abcam’s Alcohol Dehydrogenase Assay Alcohol NADH will utilise isopropanol as a substrate leading to a proportional colour development, which is measured colorimetrically (λ = 450 nm).
Alcohol measurements in Limosilactobacillus reuteri supernatant
Ethanol production by L. reuteri was determined in LDM4 using an ethanol kit (#10176290035; R-Biopharm, Darmstadt, Germany) according to manufacturer’s details as previously described (Oh et al., 2019). Briefly, L. reuteri was sub-cultured into LDM4 (OD600nm adjusted to 0.1) and cultured anaerobically at 37 °C for 24 h. Cultures were centrifuged to remove bacterial cells (5,000×g, 5 min), the supernatant was sterile filtered through a 0.2 μm membrane filter (Millipore) and then the supernatant was used for colorimetric ethanol analysis.
Quantitative real time PCR (qPCR)
RNA was collected from T84, NCM460, and mouse organoids using TRIZOL reagent (Invitrogen) according to the manufacturer’s details. One microgram of RNA was reverse transcribed to single-stranded cDNA using the SensiFAST cDNA kit (Bioline, Taunton, MA, USA). qPCR was performed using the Quantstudio 3 qPCR machine (Applied Biosystems, Foster City, CA, USA) using FAST SYBR Green PCR master mix (ThermoFisher Scientific) with the following primers: human SERT forward: AATGGGTACTCAGCAGTTCC; human SERT reverse: CCAAGCATAGCCAATCAC; human 18S forward: GATATGCTCATGTGGTGTTG; human 18S reverse: AATCTTCTTCAGTCGCTCCA; mouse Sert forward: TGGGCGCTCTACTACCTCAT; mouse Sert reverse: ATGTTGTCCTGGGCGAAGTA; mouse 18S forward CTTAGAGGGACAAGTGGCG; and mouse 18S reverse: ACGCTGAGCCAGTCAGTGTA. Relative mRNA levels of target genes were normalised to the housekeeping gene 18S and analysed by the ΔΔCT method. All values are presented as fold changes relative to untreated media controls as previously described (Engevik et al., 2013a, 2015).
Statistical analysis
Statistical analyses were performed using GraphPad Prism (version 6) software (GraphPad Inc., La Jolla, CA, USA). Data were examined using a student’s T-test or One-way analysis of variance (ANOVA). Differences between the groups were considered significant at P<0.05.
3. Results
Limosilactobacillus reuteri secretes ethanol
L. reuteri ATCC 6475 is a human commensal gut microbe with a characteristic rod shape (Supplementary Figure S1A). L. reuteri belongs to the heterofermentative group III class of bacteria, which converts glucose to lactate, CO2, and ethanol (Arskold et al., 2008; Elshaghabee et al., 2016; Zaunmuller et al., 2006). Ethanol measurements of 24 h supernatant revealed that L. reuteri produced 83.4±9.0 mM ethanol in our fully-defined LDM4 medium, which equates to 3.8±0.42% of ethanol per volume (Supplementary Figure S1B). These data support previous work which demonstrates that L. reuteri secretes ethanol in vitro (Oh et al., 2019). Bacteria manage ethanol tolerance through alcohol dehydrogenase enzymes, which facilitate the interconversion between alcohols and aldehydes with the reduction of NAD+ to NADH. We measured alcohol dehydrogenase activity in L. reuteri by measuring the conversion of isopropanol to NADH. Using this assay, we identified elevated alcohol dehydrogenase activity in L. reuteri (4.82±0.24 mU/ml), suggesting that L. reuteri is equipped to generate and tolerate ethanol in the environment. We challenged L. reuteri with increasing concentrations of ethanol and observed that L. reuteri was capable of growing in the presence of 1% ethanol without any loss of viability (Supplementary Figure S1C). L. reuteri exhibited a slight decrease in growth at 5% ethanol (0% ethanol: 0.93±0.04 vs 5% ethanol: 0.78±0.04 at 16 h). However, addition of 10% ethanol significantly inhibited L. reuteri growth (10% ethanol: 0.25±0.02). These data indicate that L. reuteri secretes ethanol and possesses the necessary enzyme activity to tolerate ethanol.
Limosilactobacillus reuteri metabolites, including ethanol, upregulate epithelial serotonin transporter
Ethanol has been shown to upregulate SERT in the central nervous system and in dendritic cells (Babu et al., 2009; Daws et al., 2006; Shibasaki et al., 2010), but little information exists on the effects of ethanol on SERT in the intestine. To address this question, we tested the effects of L. reuteri metabolites and ethanol on human colonic T84 cells. L. reuteri was cultured in LDM4 for 24 h and varying concentrations of cell-free supernatants were used to treat T84 cells for 6 h. Uninoculated LDM4 and 5% ethanol were used as negative and positive controls, respectively. qPCR analysis revealed that L. reuteri conditioned media (25 and 50%) or 5% ethanol significantly enhanced the expression of SERT in T84 cells (Figure 1A). Of note, heat killed L. reuteri had no effect on SERT mRNA levels (data not shown), suggesting that secreted factors were responsible for SERT upregulation. To test the functional activity of SERT, T84 cells were incubated with APP+, a fluorescent substrate for organic cation transporters commonly used to measure uptake by SERT (Solis et al., 2012; Wilson et al., 2014). Increased intracellular APP+ was observed in L. reuteri conditioned media and ethanol-treated T84 cells, compared to untreated or 50% uninoculated LDM4 controls (Figure 1B). Consistent with mRNA expression and functional activity, SERT upregulation was also seen at the protein level (Figure 1C,D). Immunostaining of SERT in T84 cells on coverslips revealed that L. reuteri conditioned media and 5% ethanol were able to increase the abundance of SERT.
Figure 1.

Limosilactobacillus reuteri conditioned LDM4 and exogenous ethanol upregulate serotonin transporter (SERT) in human colonic T84 cells. (A) SERT mRNA expression analysis of T84 cells treated with media (DMEM), 50% uninoculated LDM4, 10% L. reuteri conditioned LDM4, 25% L. reuteri conditioned LDM4, 50% L. reuteri conditioned LDM4, or 5% ethanol. Cells were incubated for 6 h and examined for SERT expression by qPCR. * P<0.05; ANOVA. n=4 replicates/experiment, repeated 3× independent times. (B) SERT functional assay as assessed by APP+ cell fluorescence via plate reader (em:505/ex:550). (C) Quantification of T84 immunostaining for SERT in media controls (DMEM), 50% uninoculated LDM4, 50% L. reuteri conditioned LDM4, or 5% ethanol after 16 h treatment using FIJI software. (D) Representative images of T84 cells incubated with the same conditions as (C) with SERT staining in pink and nuclei in blue. Scale bar = 50 μm.
Although cancer-derived cell lines are commonly used to model the intestine, these cells do not entirely recapitulate the functions and signalling pathways of the normal human epithelium. Multiple groups have begun to use organoids as a more physiologically relevant model for the gut. Organoids are derived from intestinal stem cells and mirror the native intestinal epithelium (Sato et al., 2009, 2011). They contain enterocytes, goblet cells, Paneth cells, stem cells and enteroendocrine cells and they retain their segment specificity. We first compared baseline SERT expression in T84 cells and NCM460 cells (an immortalised human colon cell line) to mouse and human colon-derived organoids. Examination of SERT message in these various models revealed that human and mouse organoids expressed greater concentrations of SERT mRNA when compared to either the immortalised human NCM460 cells and cancer-derived human T84 cells (Figure 2A). To query this further, we examined the effects of L. reuteri metabolites and ethanol on mouse colonic organoids. Incubation of 3D colonic organoids with 50% LDM4, 50% L. reuteri conditioned media or 5% ethanol revealed that L. reuteri metabolites or ethanol could increase Sert at the level of mRNA (Figure 2B). Moreover, immunostaining showed L. reuteri metabolites or ethanol were able to increase the abundance of SERT protein by >2 fold at the apical membrane of mouse colonic organoids compared to untreated organoid controls (Figure 2C,D). Together these data demonstrate that L. reuteri secreted products such as ethanol can upregulate cellular SERT quantities.
Figure 2.
Limosilactobacillus reuteri conditioned LMD4 and exogenous ethanol upregulate erotonin transporter (SERT) in mouse colonic organoids. (A) SERT mRNA expression analysis of human colonic T84 cells (cancer-derived), human colonic NCM460 cells (immortalised), human colonic organoids (colonoids) and mouse colonic organoids by qPCR. (B) Sert mRNA expression analysis by qPCR of mouse colonic organoids after incubation with organoid media, 50% uninoculated LDM4, 50% L. reuteri conditioned LDM4, or 5% ethanol for 6 h. * P<0.05; ANOVA. n=4 replicates/experiment, repeated 2× independent times. (C) Quantification of mouse colonic organoid immunostaining for SERT in media controls, 50% L. reuteri conditioned LDM4, or 5% ethanol after 16 h treatment using FIJI software. (D) Representative images of mouse colonic organoids incubated with the same conditions as (C) with SERT staining in pink and nuclei in blue. Scale bar = 50 μm.
Limosilactobacillus reuteri promotes epithelial serotonin transporter in a TNBS-model of colitis
TNBS acute colitis is characterised by decreased levels of SERT in both mice and guinea pigs as compared to healthy controls (Linden et al., 2003, 2005; O’Hara et al., 2004). To identify whether L. reuteri can regulate SERT levels during colitis, BALB/c mice were rectally administered TNBS and orally gavaged with PBS (vehicle control) or 109 cfu L. reuteri. As a control, BALB/c mice which did not receive TNBS were also used for comparison. Consistent with the literature (Linden et al., 2003, 2005; O’Hara et al., 2004), we observed decreased levels of SERT by immunostaining at the apical membrane of the mid-colon in TNBS mice compared to control mice which did not receive TNBS (Figure 3A). In mice that received TNBS and oral gavage of L. reuteri, we observed retention of SERT at the apical membrane, resulting in a higher abundance of SERT in the L. reuteri treated animals compared to PBS treated mice. Quantification of the immunostaining using the FIJI software confirmed that these findings were statistically significant (Figure 3B). Additionally, retention of SERT in the L. reuteri group mirrored colitis scores (Figure 3C). Compared to untreated mice, mice receiving TNBS and PBS exhibited greater histologic scores, which reflect disruption of crypt architecture, inflammatory cell infiltration, muscle thickening, goblet cell depletion, crypt abscesses and necrosis. In contrast, mice receiving TNBS with L. reuteri exhibited ~7-fold lower histological scores than TNBS mice supplemented with PBS. Consistent with our histological scores, we observed decreased colonic levels of pro-inflammatory cytokine expression (tumour necrosis factor (TNF), chemokine (C-X-C motif) ligand 1 (KC) and interferon (IFN)-γ) in our TNBS mice treated with L. reuteri compared to our TNBS mice supplemented with PBS (Figure 3D); indicating that L. reuteri treatment reduced colitis. Collectively, these results indicate that L. reuteri metabolites, including ethanol, regulate intestinal SERT levels and ameliorate colitis (Figure 4).
Figure 3.
Limosilactobacillus reuteri promotes serotonin transporter (SERT) at the apical membrane in the setting of TNBS colitis. (A) Representative immunostaining of mouse colon 3 days following TNBS administration in mice treated with either phosphate buffered saline (PBS) (control) or 109 live L. reuteri. SERT is identified in pink, nuclei in blue, and SERT apical localisation is outlined in white. Scale bar = 50 μm. (B) Quantification of mouse colon immunostaining for SERT in TNBS + PBS or TNBS + L. reuteri treated mice using FIJI software. (C) Histological scoring performed by a blinded board-certified pathologist. (D) qPCR analysis of pro-inflammatory cytokines tumour necrosis factor (TNF), chemokine (C-X-C motif) ligand 1 (KC; interleukin-8 homologue), and interferon (IFN)-γ in TNBS + PBS or TNBS + L. reuteri treated mice. * P<0.05, ANOVA. n=6 mice/group.
Figure 4.
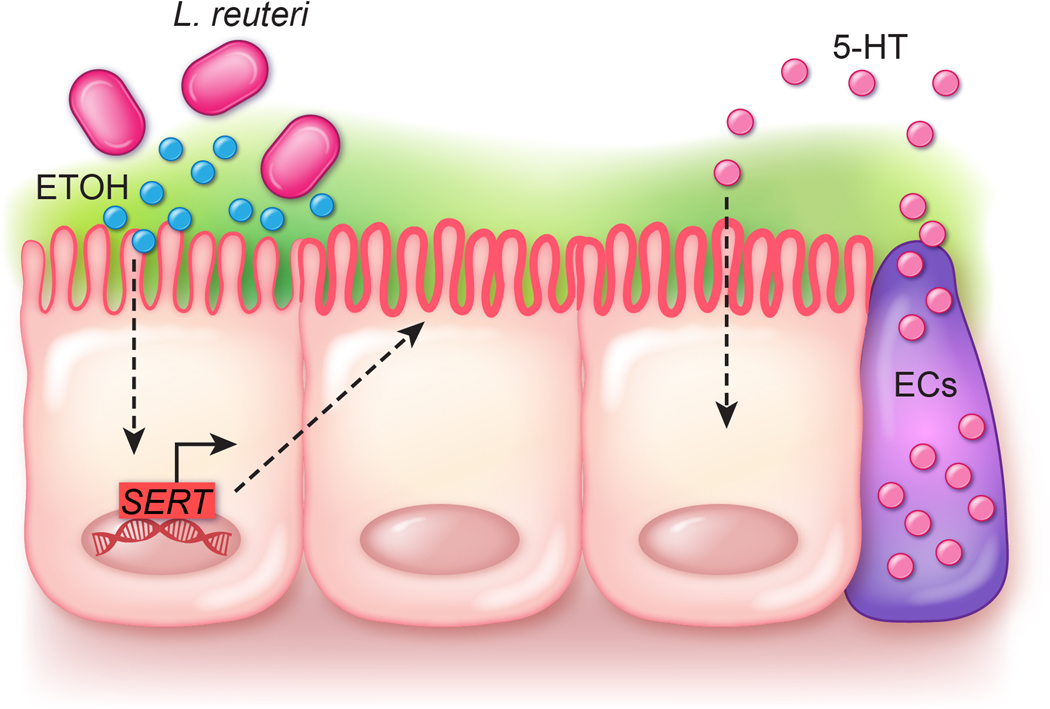
Proposed model for Limosilactobacillus reuteri serotonin transporter (SERT) modulation. Our data suggest that L. reuteri secretes ethanol and we predict that ethanol drives the transcription of SERT at the level of mRNA. We speculate SERT is trafficked to the apical membrane where it can uptake enterochromaffin (EC) released serotonin (5-HT), thereby aiding intestinal homeostasis.
4. Discussion
Our data demonstrate that the human gut commensal L. reuteri is capable of secreting ethanol which can upregulate SERT in human colon cell lines as well as mouse organoids. We confirmed the functional activity of SERT using the APP+ assay in our human colon lines. Moreover, we identified that L. reuteri was able to modulate cellular quantities of SERT in the setting of colitis. Based on the pivotal role of the gut microbiome in gut homeostasis, these findings may have practical applications for human health. We speculate that modulation of intestinal SERT by commensal bacteria has the potential to be harnessed for the management of gastrointestinal disorders.
Commensal microbes and particularly probiotic organisms are being examined for their ability to modulate specific GI targets (Yano et al., 2015). Currently, several probiotic strains have clinical efficacy in the treatment or management of GI disorders (Clarke et al., 2012; Committee et al., 2006; Goldin and Gorbach, 2008; Kopp-Hoolihan, 2001; O’Sullivan et al., 2005; Shanahan, 2009). As a result, these beneficial microbes (or probiotics) and their metabolites have garnered great interest with respect to their therapeutic potential to promote health. Probiotic strains, particularly Lactobacillus (now reclassified as Lacticaseibacillus, Lactiplantibacillus, Limosilactobacillus, etc.) and Bifidobacterium, have been hypothesised to beneficially influence diseases associated with the serotonergic system (Guglielmetti et al., 2011; Kato et al., 2004; O’Mahony et al., 2005; O’Sullivan and O’Morain, 2000; Oliva et al., 2012; Zocco et al., 2006). Since these regulatory functions of lactic acid bacteria are highly strain-specific, further characterisation is required to identify specific microbes with SERT modulating functions. Our work has identified a common theme among LAB that regulate SERT: the ability to secrete ethanol.
Ethanol production by human microbes is well characterised. One study demonstrated steady ethanol levels (~15 mmol/l) in infant faecal suspensions over ~150 days (Wolin et al., 1998). Additionally, using C13labeled glucose, the authors identified that ethanol could be produced by fermentation (Wolin et al., 1998). This is consistent with another study that found ethanol in the faeces of infants (Stansbridge et al., 1993). The authors also found that the levels of ethanol were higher in stool from infants fed a formula containing lactobacilli compared to infants receiving a formula without lactobacilli. Select lactobacilli can generate ethanol. It has been demonstrated that Lacticaseibacillus paracasei, Lacticaseibacillus rhamnosus, Lactiplantibacillus plantarum, Limosilactobacillus fermentum and L. reuteri can generate ethanol (Elshaghabee et al., 2016; Narendranath et al., 1997; Oh et al., 2019). Interestingly, the ability of lactobacilli to produce ethanol depends on the carbohydrate source.
For example, L. fermentum produced high amounts of ethanol (80 mM) from glucose, but no ethanol from other carbohydrates (fructose, arabinose, ribose, lactose, lactulose, or inulin) (Elshaghabee et al., 2016). Similarly, Oh and colleagues identified that L. reuteri generated high ethanol levels (100 mM) with glucose, but no ethanol with a mixture of glucose and fructose (20:80) or fructose alone (Oh et al., 2019). These data suggest that diet could potentially regulate the ability of lactobacilli to produce ethanol and thus beneficially modulate SERT.
Although we have found that ethanol can upregulate SERT, ethanol has also been shown to interfere with the intestinal barrier. Studies have found that long-term ethanol abusers, defined as individuals with a consumption of more than four drinks (>80 g alcohol) per day, have increased intestinal permeability (Elamin et al., 2013; Holford, 1987; Parlesak et al., 2000). Similarly, rodent studies have demonstrated that both short- and long-term ethanol administration can increase intestinal permeability and activate intestinal inflammation (Bode et al., 1991; Keshavarzian et al., 2001, 2009; Lambert et al., 2003). Despite these findings, administration of lactobacilli, which can produce ethanol locally, attenuates ethanol-induced injury (Fang et al., 2019; Forsyth et al., 2009; Gu et al., 2020; Li et al., 2016; Liu et al., 2004). We speculate that ethanol-producing lactobacilli are also actively secreting anti-inflammatory compounds which prevent ethanol-induced injury. For example, we have shown that L. reuteri secretes metabolites which stimulate anti-inflammatory IL-10 production from dendritic cells (Engevik et al., 2021). We have also found that L. reuteri generates small compounds such as y-glutamyl-cysteine which can suppress epithelial oxidative stress. As a result, we hypothesise that anti-oxidative stress and anti-inflammatory compounds may suppress the deleterious effects of ethanol and allow for upregulation of SERT without intestinal damage.
Multiple pathways could be responsible for the effects of ethanol on SERT. Ethanol enhances intracellular cyclic AMP (cAMP) levels in a number of cell types (Atkinson et al., 1977; Rabe et al., 1990; Uhlemann et al., 1979). cAMP in turn has been shown to regulate SERT (Yammamoto et al., 2013). Thus, it is possible that ethanol-induced cAMP could be promoting increased SERT transcription and thus protein quantities. Alternatively, Szabo and colleagues reported that ethanol was effective in inducing transforming growth factor (TGF)-β (Szabo et al., 1992) and in a separate study, TGF-β was found to upregulate SERT protein levels in Caco-2 cells (Nazir et al., 2015). Application of L. reuteri conditioned media to HT29 cells, which have also been used for SERT studies, was found to increase phosphorylated p38 (data not shown). Previous studies have demonstrated that p38 MAPK signalling pathways were involved in rapid SERT up-regulation (Zhu et al., 2005); suggesting that p38 signalling may be involved in L. reuteri-mediated SERT upregulation. It is also possible that ethanol enhances the activity of other L. reuteri metabolites through unknown pathways or that other L. reuteri metabolites are primarily responsible for SERT regulation and ethanol is expendable.
In our TNBS mouse model, we speculate that multiple signalling pathways are involved in L. reuteri-mediated retention of SERT at the apical membrane. L. reuteri has been shown to shift the intestinal microbiome (He et al., 2017, 2019; Romani Vestman et al., 2015), increasing ‘beneficial’ genera Bifidobacterium, Prevotella, and Lactobacillus, while decreasing Anaeroplasma, Rikenellaceae, and Clostridium. Shifts in the microbiome, particularly gram-negative microbes, may affect TLR signalling. In Caco-2 cells, TLR2 activation reduced SERT protein levels at the apical membrane (Latorre et al., 2016a). Tlr2−/− mice were also found to have augmented intestinal SERT expression, providing evidence of the roles of microbial pattern recognition receptors in modulating intestinal SERT (Latorre et al., 2016a). Interestingly, we did not observe any effect of heat-killed L. reuteri on SERT levels in our T84 cell model, indicating that L. reuteri activation of TLR2 may not be responsible for our observed SERT regulation. However, L. reuteri-driven shifts in the microbiome may indirectly affect SERT levels via TLR2 activation by other microbes. L. reuteri is also known to be immune-modulatory and suppress pro-inflammatory cytokines. Our data demonstrate that L. reuteri reduced levels of pro-inflammatory TNF, KC (IL-8 homologue) and IFN-γ. In Caco-2 cells, application of the pro-inflammatory cytokines TNF and IFN-γ decreased SERT function and expression (Foley et al., 2007). These findings suggest that by regulating inflammation, L. reuteri may also contribute to the maintenance of SERT at the membrane. Although the exact mechanism is unknown, our work demonstrates that L. reuteri regulates SERT in vitro and in vivo.
An important consideration for future work is whether L. reuteri can regulate SERT levels once SERT has been diminished; such as in diverticulitis or IBD patients. In our studies, we pre-treated mice with L. reuteri and then administered TNBS. While this may be beneficial for prevention, it doesn’t indicate the effectiveness of this strategy for treatment. Additionally, it is unclear whether ETOH alone before or after colitis would beneficially regulate SERT. Based on animal models with ethanol alone (Bode et al., 1991; Keshavarzian et al., 2001, 2009; Lambert et al., 2003) which demonstrate adverse intestinal affects, we speculate that microbial-produced ethanol, complemented with anti-inflammatory compounds, would be necessary for ethanol-mediated upregulation of SERT in vivo. Further studies are necessary to identify the ability of L. reuteri to upregulate diminished SERT and the ability of ethanol to directly modulate SERT levels in vivo.
Another interesting finding in our work was the strong apical expression of SERT in our mouse colonic organoids and mouse colon. We have previously shown that serotonin is secreted both apically and basolaterally in response to stimuli using human enteroids/organoids (Chang-Graham et al., 2019). In this study, we observed that serotonin levels were elevated in both the apical and basal chambers in response to rotavirus infection, suggesting that apically released serotonin may be physiologically relevant. Consistent with our findings, Gill et al. (2008) observed a similar high apical SERT staining and low basolateral staining in human surgical resections that were paraffin embedded. When frozen sections were stained, the authors still observed strong apical localisation of SERT, but observed less positive staining of goblet cell mucin vacuoles; suggesting that some signal was an artifact of staining paraffin-embedded tissue. Since our organoids and colon were paraffin embedded we recognise that our positive staining of goblet cells and the mucus layer above the epithelium is likely an artifact. However, we believe that the prominent apical SERT staining and low basolateral SERT in mouse colons are valid. In the future, we are interested in examining SERT in frozen sections and think that tissue preparation is an important consideration for SERT related research.
Serotonin (5-hydroxytryptamine; 5-HT) is actively produced by enterochromaffin cells in the small and large intestine. Serotonin released into the lamina propria can interact with neighbouring epithelial cells, goblet cells smooth muscle, enteric nerves or immune cells (Chen et al., 1998, 2001; Gershon, 2013; Ghia et al., 2009; Karsenty and Gershon, 2011; Li et al., 2011b; Wade et al., 1996; Yadav et al., 2010). Serotonin release in response to damage appears to be a conserved mechanism. Mice treated with dinitrobenzenesulfonic acid (DNBS) (Khan et al., 2006), TNBS (Linden et al., 2005), or DSS (Bertrand et al., 2010) exhibit increased serotonin after damage. Likewise, in guinea pigs, TNBS-induced colitis (Linden et al., 2003) and ileitis (O’Hara et al., 2004) both exhibited increased serotonin. In addition to colitis models, increased serotonin has been observed in response to acetic acid induced gastric ulcers (Amirov and Trubitsyna, 1981), indomethacin induced colonic wounds (Kato et al., 2012) and enteric infection by Nippostrongylus brasiliensis (Farmer and Laniyonu, 1984). The effects of serotonin are largely dependent on the receptor they activate. Spohn and colleagues demonstrated that activation of 5-HTR4 by the agonist tegaserod minimised DSS and TNBS driven colitis in mice (Spohn et al., 2016). Consistent with the protective role of 5-HTR4, Kato et al. (2012) demonstrated that a 5-HTR4 receptor agonist, mosapride, attenuated indomethacin-induced intestinal lesions in mice. In contrast to the protective role of 5-HTR4, blockade of 5-HTR3 receptor with antagonists ondansetron and ramosetron dose-dependently reduced damage, indicating that 5-HTR3 drives inflammation. These findings suggest that serotonin exerts a dual role in colitis. This is supported by the fact that aberrant upregulation of serotonin by loss of the transporter SERT or supplementation of endogenous serotonin exacerbates colitis (Chen et al., 2016; Ghia et al., 2009; Khan et al., 2006; Li et al., 2011a; Margolis and Pothoulakis, 2009; Regmi et al., 2014). It is likely that high levels of serotonin, not necessarily localised to damaged sites, may activate pro-inflammatory receptors on immune cells (Li et al., 2011a). We speculate that aberrantly high serotonin levels due to the loss of SERT, which is seen in colitis models and IBD patients, activates several pro-inflammatory cytokines on immune cells, thereby promoting a cycle of inflammation. We believe that L. reuteri upregulates SERT and suppresses inflammation, thereby restoring normal serotonin levels and aiding the transition back to intestinal homeostasis. In healthy mice with a complete gut microbiota, we did not observe upregulation of SERT with L. reuteri administration (data not shown). Since mice are normally colonised with L. reuteri strains, additional L. reuteri supplementation (particularly by a human-derived L. reuteri strain) is likely unable to further upregulate SERT. Alternatively, it is possible that enterocytes in the healthy setting already harbour maximal SERT levels which don’t allow for further upregulation. Using tissue-derived organoids in a reductionist approach, we have demonstrated that L. reuteri secreted products are capable of upregulating SERT levels. However, it is difficult to distinguish whether the SERT levels we observe in untreated organoids are representative of the gut, since normally microbes and microbial products are present. In the future, we believe gnotobiotic animals and additional organoid experiments will shed light into the precise role of microbial-induced SERT regulation.
With this work, we have now identified another pathway by which commensal microbes can modulate the host. Our work suggests that L. reuteri can secrete ethanol and regulate SERT. Given the importance of serotonin in host health, we speculate that this regulation of SERT may have important implications in both GI and systemic homeostasis.
Supplementary Material
Acknowledgements
This study was supported by the NIH K01 K123195-01 (MAE), K01DK121869 (ACE), and NIH T32DK007664-28 (KAE, WR), NIH P30-DK-56338 (Texas Medical Center Digestive Disease Center), NIH U01CA170930 (JV), and unrestricted research support from BioGaia AB (Stockholm, Sweden) (JV).
Footnotes
Conflict of interest
JV receives unrestricted research support from the Swedish Probiotic Company BioGaia AB. JV also serves on the scientific advisory boards of Seed (USA-based probiotics/prebiotics company), Biomica (Israeli informatics enterprise) and Plexus Worldwide (USA-based nutrition company). All other authors have no finical disclosures.
Supplementary material
Supplementary material can be found online at https://doi.org/10.3920/BM2020.0216.
Figure S1. Limosilactobacillus reuteri ATCC PTA 6475 secretes ethanol and is ethanol tolerant.
References
- Akiba Y, Inoue T, Kaji I, Higashiyama M, Narimatsu K, Iwamoto K, Watanabe M, Guth PH, Engel E, Kuwahara A and Kaunitz JD, 2015. Short-chain fatty acid sensing in rat duodenum. Journal of Physiology 593: 585–599. 10.1113/jphysiol.2014.280792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amirov N and Trubitsyna IE, 1981. [Role of serotonin in the pathogenesis of ulcer development]. Biulleten Eksperimentalnoi Biologii i Meditsiny 92: 23–26. [PubMed] [Google Scholar]
- Arskold E, Lohmeier-Vogel E, Cao R, Roos S, Radstrom P. and van Niel EW, 2008. Phosphoketolase pathway dominates in Lactobacillus reuteri ATCC 55730 containing dual pathways for glycolysis. Journal of Bacteriology 190: 206–212. 10.1128/JB.01227-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson JP, Sullivan TJ, Kelly JP and Parker CW, 1977. Stimulation by alcohols of cyclic AMP metabolism in human leukocytes. Possible role of cyclic AMP in the anti-inflammatory effects of ethanol. Journal of Clinical Investigation 60: 284–294. 10.1172/JCI108776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babu DK, Diaz A, Samikkannu T, Rao KV, Saiyed ZM, Rodriguez JW and Nair MP, 2009. Upregulation of serotonin transporter by alcohol in human dendritic cells: possible implication in neuroimmune deregulation. Alcoholism, Clinical and Experimental Research 33: 1731–1738. 10.1111/j.1530-0277.2009.01010.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baganz NL and Blakely RD, 2013. A dialogue between the immune system and brain, spoken in the language of serotonin. ACS Chemical Neuroscience 4: 48–63. 10.1021/cn300186b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellini M, Rappelli L, Blandizzi C, Costa F, Stasi C, Colucci R, Giannaccini G, Marazziti D, Betti L, Baroni S, Mumolo MG, Marchi S. and Del Tacca M, 2003. Platelet serotonin transporter in patients with diarrhea-predominant irritable bowel syndrome both before and after treatment with alosetron. American Journal of Gastroenterology 98: 2705–2711. 10.1111/j.1572-0241.2003.08669.x [DOI] [PubMed] [Google Scholar]
- Bellono NW, Bayrer JR, Leitch DB, Castro J, Zhang C, O’Donnell TA, Brierley SM, Ingraham HA and Julius D, 2017. Enterochromaffin cells are gut chemosensors that couple to sensory neural pathways. Cell 170: 185–198 e116. 10.1016/j.cell.2017.05.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand PP and Bertrand RL, 2010. Serotonin release and uptake in the gastrointestinal tract. Autonomic Neuroscience 153: 47–57. 10.1016/j.autneu.2009.08.002 [DOI] [PubMed] [Google Scholar]
- Bertrand PP, Barajas-Espinosa A, Neshat S, Bertrand RL and Lomax AE, 2010. Analysis of real-time serotonin (5-HT) availability during experimental colitis in mouse. American Journal of Physiology – Gastrointestinal and Liver Physiology 298: G446–G455. 10.1152/ajpgi.00318.2009 [DOI] [PubMed] [Google Scholar]
- Bischoff SC, Mailer R, Pabst O, Weier G, Sedlik W, Li Z, Chen JJ, Murphy DL and Gershon MD, 2009. Role of serotonin in intestinal inflammation: knockout of serotonin reuptake transporter exacerbates 2,4,6-trinitrobenzene sulfonic acid colitis in mice. American Journal of Physiology – Gastrointestinal and Liver Physiology 296: G685–G695. 10.1152/ajpgi.90685.2008 [DOI] [PubMed] [Google Scholar]
- Blakely RD, Berson HE, Fremeau RT Jr., Caron MG, Peek MM, Prince HK and Bradley CC, 1991. Cloning and expression of a functional serotonin transporter from rat brain. Nature 354: 66–70. 10.1038/354066a0 [DOI] [PubMed] [Google Scholar]
- Blomberg L, Henriksson A. and Conway PL, 1993. Inhibition of adhesion of Escherichia coli K88 to piglet ileal mucus by Lactobacillus spp. Applied and Environmental Microbiology 59: 34–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bode C, Vollmer E, Hug J. and Bode JC, 1991. Increased permeability of the gut to polyethylene glycol and dextran in rats fed alcohol. Annals of the New York Academy of Sciences 625: 837–840. 10.1111/j.1749-6632.1991.tb33931.x [DOI] [PubMed] [Google Scholar]
- Bohle LA, Brede DA, Diep DB, Holo H. and Nes IF, 2010. Specific degradation of the mucus adhesion-promoting protein (MapA) of Lactobacillus reuteri to an antimicrobial peptide. Applied and Environmental Microbiology 76: 7306–7309. 10.1128/AEM.01423-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broer S. and Gether U, 2012. The solute carrier 6 family of transporters. British Journal of Pharmacology 167: 256–278. 10.1111/j.1476-5381.2012.01975.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownstein MJ and Hoffman BJ, 1994. Neurotransmitter transporters. Recent Progress in Hormone Research 49: 27–42. [DOI] [PubMed] [Google Scholar]
- Cao YN, Feng LJ, Liu YY, Jiang K, Zhang MJ, Gu YX, Wang BM, Gao J, Wang ZL and Wang YM, 2018a. Effect of Lactobacillus rhamnosus GG supernatant on serotonin transporter expression in rats with post-infectious irritable bowel syndrome. World Journal of Gastroenterology 24: 338–350. 10.3748/wjg.v24.i3.338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao YN, Feng LJ, Wang BM, Jiang K, Li S, Xu X, Wang WQ, Zhao JW and Wang YM, 2018b. Lactobacillus acidophilus and Bifidobacterium longum supernatants upregulate the serotonin transporter expression in intestinal epithelial cells. Saudi Journal of Gastroenterology 24: 59–66. 10.4103/sjg.SJG_333_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carasi P, Ambrosis NM, De Antoni GL, Bressollier P, Urdaci MC and Serradell Mde L, 2014. Adhesion properties of potentially probiotic Lactobacillus kefiri to gastrointestinal mucus. Journal of Dairy Research 81: 16–23. 10.1017/S0022029913000526 [DOI] [PubMed] [Google Scholar]
- Chang-Graham AL, Danhof HA, Engevik MA, Tomaro-Duchesneau C, Karandikar UC, Estes MK, Versalovic J, Britton RA and Hyser JM, 2019. Human intestinal enteroids with inducible neurogenin-3 expression as a novel model of gut hormone secretion. Cellular and Molecular Gastroenterology and Hepatology 8: 209–229. 10.1016/j.jcmgh.2019.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JJ, Li Z, Pan H, Murphy DL, Tamir H, Koepsell H. and Gershon MD, 2001. Maintenance of serotonin in the intestinal mucosa and ganglia of mice that lack the high-affinity serotonin transporter: Abnormal intestinal motility and the expression of cation transporters. Journal of Neuroscience 21: 6348–6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JX, Pan H, Rothman TP, Wade PR and Gershon MD, 1998. Guinea pig 5-HT transporter: cloning, expression, distribution, and function in intestinal sensory reception. American Journal of Physiology 275: G433–448. [DOI] [PubMed] [Google Scholar]
- Chen M., Gao L., Chen P., Feng D., Jiang Y., Chang Y., Ji J., Chu FF. and Gao Q., 2016. Serotonin-exacerbated DSS-induced colitis is associated with increase in MMP-3 and MMP-9 expression in the mouse colon. Mediators of Inflammation 2016: 5359768. 10.1155/2016/5359768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke G, Cryan JF, Dinan TG and Quigley EM, 2012. Review article: probiotics for the treatment of irritable bowel syndrome-focus on lactic acid bacteria. Alimentary Pharmacology and Therapeutics 35: 403–413. 10.1111/j.1365-2036.2011.04965.x [DOI] [PubMed] [Google Scholar]
- Coates MD, Mahoney CR, Linden DR, Sampson JE, Chen J, Blaszyk H, Crowell MD, Sharkey KA, Gershon MD, Mawe GM and Moses PL, 2004. Molecular defects in mucosal serotonin content and decreased serotonin reuptake transporter in ulcerative colitis and irritable bowel syndrome. Gastroenterology 126: 1657–1664. [DOI] [PubMed] [Google Scholar]
- Coconnier MH, Klaenhammer TR, Kerneis S, Bernet MF and Servin AL, 1992. Protein-mediated adhesion of Lactobacillus acidophilus BG2FO4 on human enterocyte and mucus-secreting cell lines in culture. Applied and Environmental Microbiology 58: 2034–2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Committee NNR, Michail S, Sylvester F, Fuchs G. and Issenman R, 2006. Clinical efficacy of probiotics: review of the evidence with focus on children. Journal of Pediatric Gastroenterology and Nutrition 43: 550–557. 10.1097/01.mpg.0000239990.35517.bf [DOI] [PubMed] [Google Scholar]
- Costedio MM, Coates MD, Danielson AB, Buttolph TR 3rd, Blaszyk HJ, Mawe GM and Hyman NH, 2008. Serotonin signaling in diverticular disease. Journal of Gastrointestinal Surgery 12: 1439–1445. 10.1007/s11605-008-0536-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daws LC, Montanez S, Munn JL, Owens WA, Baganz NL, Boyce-Rustay JM, Millstein RA, Wiedholz LM, Murphy DL and Holmes A, 2006. Ethanol inhibits clearance of brain serotonin by a serotonin transporter-independent mechanism. Journal of Neuroscience 26: 6431–6438. 10.1523/JNEUROSCI.4050-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elamin EE, Masclee AA, Dekker J. and Jonkers DM, 2013. Ethanol metabolism and its effects on the intestinal epithelial barrier. Nutrition Reviews 71: 483–499. 10.1111/nure.12027 [DOI] [PubMed] [Google Scholar]
- Elshaghabee FM, Bockelmann W, Meske D, de Vrese M, Walte HG, Schrezenmeir J. and Heller KJ, 2016. Ethanol production by selected intestinal microorganisms and lactic acid bacteria growing under different nutritional conditions. Frontiers in Microbiology 7: 47. 10.3389/fmicb.2016.00047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engevik MA, Aihara E, Montrose MH, Shull GE, Hassett DJ and Worrell RT, 2013a. Loss of NHE3 alters gut microbiota composition and influences Bacteroides thetaiotaomicron growth. American Journal of Physiology – Gastrointestinal and Liver Physiology 305: G697–G711. 10.1152/ajpgi.00184.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engevik MA, Engevik KA, Yacyshyn MB, Wang J, Hassett DJ, Darien B, Yacyshyn BR and Worrell RT, 2015. Human Clostridium difficile infection: inhibition of NHE3 and microbiota profile. American Journal of Physiology – Gastrointestinal and Liver Physiology 308: G497–G509. 10.1152/ajpgi.00090.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engevik MA, Faletti CJ, Paulmichl M, Worrell RT, 2013b. Prebiotic properties of galursan HF 7K on mouse gut microbiota. Cellular Physiology and Biochemistry 32: 96–110. [DOI] [PubMed] [Google Scholar]
- Engevik MA, Hickerson A, Shull GE, Worrell RT, 2013c. Acidic conditions in the NHE2−/− mouse intestine result in an altered mucosa-associated bacterial population with changes in mucus oligosaccharides. Cellular Physiology and Biochemistry 32: 111–128. [DOI] [PubMed] [Google Scholar]
- Engevik MA, Luck B, Visuthranukul C, Ihekweazu FD, Engevik C, Shi Z, Danhof HA, Chang-Graham A, Hall A, Endres T, Haidacher SJ, Horvath TD, Haag AM, Devaraj S, Garey KW, Britton RA, Hyser JM, Shroyer NF and Versalovic J, 2020. Human-derived Bifidobacterium dentium modulates the mammalian serotonergic system and gut-brain axis. Cellular and Molecular Gastroenterology and Hepatology. 10.1016/j.jcmgh.2020.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engevik MA, Morra CN, Roth D, Engevik K, Spinler JK, Devaraj S, Crawford SE, Estes MK, Kalkum M. and Versalovic J, 2019. Microbial metabolic capacity for intestinal folate production and modulation of host folate receptors. Frontiers in Microbiology 10: 2305. 10.3389/fmicb.2019.02305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engevik MA, Ruan W, Esparza M, Fultz R, Shi Z, Engevik KA, Engevik AC, Ihekweazu FD, Visuthranukul C, Venable S, Schady DA and Versalovic J, 2021. Immunomodulation of dendritic cells by Lactobacillus reuteri surface components and metabolites. Physiol Rep 9: e14719. 10.14814/phy2.14719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esmaili A, Nazir SF, Borthakur A, Yu D, Turner JR, Saksena S, Singla A, Hecht GA, Alrefai WA and Gill RK, 2009. Enteropathogenic Escherichia coli infection inhibits intestinal serotonin transporter function and expression. Gastroenterology 137: 2074–2083. 10.1053/j.gastro.2009.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang TJ, Guo JT, Lin MK, Lee MS, Chen YL and Lin WH, 2019. Protective effects of Lactobacillus plantarum against chronic alcohol-induced liver injury in the murine model. Applied Microbiology and Biotechnology 103: 8597–8608. 10.1007/s00253-019-10122-8 [DOI] [PubMed] [Google Scholar]
- Farmer SG and Laniyonu AA, 1984. Effects of p-chlorophenylalanine on the sensitivity of rat intestine to agonists and on intestinal 5-hydroxytryptamine levels during Nippostrongylus brasiliensis infection. British Journal of Pharmacology 82: 883–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernando EH, Dicay M, Stahl M, Gordon MH, Vegso A, Baggio C, Alston L, Lopes F, Baker K, Hirota S, McKay DM, Vallance B. and MacNaughton WK, 2017. A simple, cost-effective method for generating murine colonic 3D enteroids and 2D monolayers for studies of primary epithelial cell function. American Journal of Physiology – Gastrointestinal and Liver Physiology 313: G467–G475. 10.1152/ajpgi.00152.2017 [DOI] [PubMed] [Google Scholar]
- Foley KF., Pantano C., Ciolino A. and Mawe GM., 2007. IFN-gamma and TNF-alpha decrease serotonin transporter function and expression in Caco2 cells. American Journal of Physiology – Gastrointestinal and Liver Physiology 292: G779–G784. 10.1152/ajpgi.00470.2006 [DOI] [PubMed] [Google Scholar]
- Forsyth CB, Farhadi A, Jakate SM, Tang Y, Shaikh M. and Keshavarzian A, 2009. Lactobacillus GG treatment ameliorates alcohol-induced intestinal oxidative stress, gut leakiness, and liver injury in a rat model of alcoholic steatohepatitis. Alcohol 43: 163–172. 10.1016/j.alcohol.2008.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumoto S, Tatewaki M, Yamada T, Fujimiya M, Mantyh C, Voss M, Eubanks S, Harris M, Pappas TN and Takahashi T, 2003. Short-chain fatty acids stimulate colonic transit via intraluminal 5-HT release in rats. American Journal of Physiology – Regulatory Integrative and Comparative Physiology 284: R1269–R1276. 10.1152/ajpregu.00442.2002 [DOI] [PubMed] [Google Scholar]
- Gershon MD, 2013. 5-Hydroxytryptamine (serotonin) in the gastrointestinal tract. Current Opinion in Endocrinology, Diabetes, and Obesity 20: 14–21. 10.1097/MED.0b013e32835bc703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon MD and Sherman DL, 1982. Identification of and interactions between noradrenergic and serotonergic neurites in the myenteric plexus. Journal of Comparative Neurology 204: 407–421. 10.1002/cne.902040411 [DOI] [PubMed] [Google Scholar]
- Gershon MD and Tack J, 2007. The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology 132: 397–414. 10.1053/j.gastro.2006.11.002 [DOI] [PubMed] [Google Scholar]
- Gershon MD, Sherman DL and Pintar JE, 1990. Type-specific localization of monoamine oxidase in the enteric nervous system: relationship to 5-hydroxytryptamine, neuropeptides, and sympathetic nerves. Journal of Comparative Neurology 301: 191–213. 10.1002/cne.903010205 [DOI] [PubMed] [Google Scholar]
- Ghia JE, Li N, Wang H, Collins M, Deng Y, El-Sharkawy RT, Cote F, Mallet J. and Khan WI, 2009. Serotonin has a key role in pathogenesis of experimental colitis. Gastroenterology 137: 1649–1660. 10.1053/j.gastro.2009.08.041 [DOI] [PubMed] [Google Scholar]
- Gill RK, Pant N, Saksena S, Singla A, Nazir TM, Vohwinkel L, Turner JR, Goldstein J, Alrefai WA and Dudeja PK, 2008. Function, expression, and characterization of the serotonin transporter in the native human intestine. American Journal of Physiology – Gastrointestinal and Liver Physiology 294: G254–G262. 10.1152/ajpgi.00354.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill RK, Saksena S, Tyagi S, Alrefai WA, Malakooti J, Sarwar Z, Turner JR, Ramaswamy K. and Dudeja PK, 2005. Serotonin inhibits Na+/H+ exchange activity via 5-HT4 receptors and activation of PKC alpha in human intestinal epithelial cells. Gastroenterology 128: 962–974. [DOI] [PubMed] [Google Scholar]
- Goldin BR and Gorbach SL, 2008. Clinical indications for probiotics: an overview. Clinical Infectious Diseases 46 Suppl 2: S96–100; discussion S144–151. 10.1086/523333 [DOI] [PubMed] [Google Scholar]
- Gu Z, Wu Y, Wang Y, Sun H, You Y, Piao C, Liu J. and Wang Y, 2020. Lactobacillus rhamnosus granules dose-dependently balance intestinal microbiome disorders and ameliorate chronic alcohol-induced liver injury. Journal of Medicinal Food 23: 114–124. 10.1089/jmf.2018.4357 [DOI] [PubMed] [Google Scholar]
- Guglielmetti S, Mora D, Gschwender M. and Popp K, 2011. Randomised clinical trial: Bifidobacterium bifidum MIMBb75 significantly alleviates irritable bowel syndrome and improves quality of life – a double-blind, placebo-controlled study. Alimentary Pharmacology and Therapeutics 33: 1123–1132. 10.1111/j.1365-2036.2011.04633.x [DOI] [PubMed] [Google Scholar]
- Hata T, Asano Y, Yoshihara K, Kimura-Todani T, Miyata N, Zhang XT, Takakura S, Aiba Y, Koga Y. and Sudo N, 2017. Regulation of gut luminal serotonin by commensal microbiota in mice. PLoS ONE 12: e0180745. 10.1371/journal.pone.0180745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haub S, Kanuri G, Volynets V, Brune T, Bischoff SC and Bergheim I, 2010a. Serotonin reuptake transporter (SERT) plays a critical role in the onset of fructose-induced hepatic steatosis in mice. American Journal of Physiology – Gastrointestinal and Liver Physiology 298: G335–G344. 10.1152/ajpgi.00088.2009 [DOI] [PubMed] [Google Scholar]
- Haub S, Ritze Y, Bergheim I, Pabst O, Gershon MD and Bischoff SC, 2010b. Enhancement of intestinal inflammation in mice lacking interleukin 10 by deletion of the serotonin reuptake transporter. Neurogastroenterology and Motility 22: 826–834, e229. 10.1111/j.1365-2982.2010.01479.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B, Hoang TK, Tian X, Taylor CM, Blanchard E, Luo M, Bhattacharjee MB, Freeborn J, Park S, Couturier J, Lindsey JW, Tran DQ, Rhoads JM and Liu Y, 2019. Lactobacillus reuteri reduces the severity of experimental autoimmune encephalomyelitis in mice by modulating gut microbiota. Frontiers in Immunology 10: 385. 10.3389/fimmu.2019.00385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B, Hoang TK, Wang T, Ferris M, Taylor CM, Tian X, Luo M, Tran DQ, Zhou J, Tatevian N, Luo F, Molina JG, Blackburn MR, Gomez TH, Roos S, Rhoads JM and Liu Y, 2017. Resetting microbiota by Lactobacillus reuteri inhibits T reg deficiency-induced autoimmunity via adenosine A2A receptors. Journal of Experimental Medicine 214: 107–123. 10.1084/jem.20160961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksson A. and Conway PL, 1996. Adhesion of Lactobacillus fermentum 104-S to porcine stomach mucus. Current Microbiology 33: 31–34. [DOI] [PubMed] [Google Scholar]
- Hoffman BJ, Mezey E. and Brownstein MJ, 1991. Cloning of a serotonin transporter affected by antidepressants. Science 254: 579–580. [DOI] [PubMed] [Google Scholar]
- Hoffman JM, Tyler K, MacEachern SJ, Balemba OB, Johnson AC, Brooks EM, Zhao H, Swain GM, Moses PL, Galligan JJ, Sharkey KA, Greenwood-Van Meerveld B. and Mawe GM, 2012. Activation of colonic mucosal 5-HT(4) receptors accelerates propulsive motility and inhibits visceral hypersensitivity. Gastroenterology 142: 844–854 e844. 10.1053/j.gastro.2011.12.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holford NH, 1987. Clinical pharmacokinetics of ethanol. Clinical Pharmacokinetics 13: 273–292. 10.2165/00003088-198713050-00001 [DOI] [PubMed] [Google Scholar]
- Jacobsen CN., Rosenfeldt Nielsen V., Hayford AE., Moller PL., Michaelsen KF., Paerregaard A., Sandstrom B., Tvede M. and Jakobsen M., 1999. Screening of probiotic activities of forty-seven strains of Lactobacillus spp. by in vitro techniques and evaluation of the colonization ability of five selected strains in humans. Applied and Environmental Microbiology 65: 4949–4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen H, Roos S, Jonsson H, Rud I, Grimmer S, van Pijkeren JP, Britton RA and Axelsson L, 2014. Role of Lactobacillus reuteri cell and mucus-binding protein A (CmbA) in adhesion to intestinal epithelial cells and mucus in vitro. Microbiology 160: 671–681. 10.1099/mic.0.073551-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson H, Strom E. and Roos S, 2001. Addition of mucin to the growth medium triggers mucus-binding activity in different strains of Lactobacillus reuteri in vitro. FEMS Microbiology Letters 204: 19–22. [DOI] [PubMed] [Google Scholar]
- Karsenty G. and Gershon MD, 2011. The importance of the gastrointestinal tract in the control of bone mass accrual. Gastroenterology 141: 439–442. 10.1053/j.gastro.2011.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K, Mizuno S, Umesaki Y, Ishii Y, Sugitani M, Imaoka A, Otsuka M, Hasunuma O, Kurihara R, Iwasaki A. and Arakawa Y, 2004. Randomized placebo-controlled trial assessing the effect of bifidobacteria-fermented milk on active ulcerative colitis. Alimentary Pharmacology and Therapeutics 20: 1133–1141. 10.1111/j.1365-2036.2004.02268.x [DOI] [PubMed] [Google Scholar]
- Kato S, Matsuda N, Matsumoto K, Wada M, Onimaru N, Yasuda M, Amagase K, Horie S. and Takeuchi K, 2012. Dual role of serotonin in the pathogenesis of indomethacin-induced small intestinal ulceration: pro-ulcerogenic action via 5-HT3 receptors and anti-ulcerogenic action via 5-HT4 receptors. Pharmacological Research 66: 226–234. 10.1016/j.phrs.2012.06.002 [DOI] [PubMed] [Google Scholar]
- Kelai S, Aissi F, Lesch KP, Cohen-Salmon C, Hamon M. and Lanfumey L, 2003. Alcohol intake after serotonin transporter inactivation in mice. Alcohol 38: 386–389. [DOI] [PubMed] [Google Scholar]
- Keshavarzian A, Choudhary S, Holmes EW, Yong S, Banan A, Jakate S. and Fields JZ, 2001. Preventing gut leakiness by oats supplementation ameliorates alcohol-induced liver damage in rats. Journal of Pharmacology and Experimental Therapeutics 299: 442–448. [PubMed] [Google Scholar]
- Keshavarzian A, Farhadi A, Forsyth CB, Rangan J, Jakate S, Shaikh M, Banan A. and Fields JZ, 2009. Evidence that chronic alcohol exposure promotes intestinal oxidative stress, intestinal hyperpermeability and endotoxemia prior to development of alcoholic steatohepatitis in rats. Journal of Hepatology 50: 538–547. 10.1016/j.jhep.2008.10.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan WI, Motomura Y, Wang H, El-Sharkawy RT, Verdu EF, Verma-Gandhu M, Rollins BJ and Collins SM, 2006. Critical role of MCP-1 in the pathogenesis of experimental colitis in the context of immune and enterochromaffin cells. American Journal of Physiology – Gastrointestinal and Liver Physiology 291: G803–811. 10.1152/ajpgi.00069.2006 [DOI] [PubMed] [Google Scholar]
- Kim JJ and Khan WI, 2014. 5-HT7 receptor signaling: improved therapeutic strategy in gut disorders. Frontiers in Behavioral Neuroscience 8: 396. 10.3389/fnbeh.2014.00396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp-Hoolihan L, 2001. Prophylactic and therapeutic uses of probiotics: a review. Journal of the American Dietetic Association 101: 229–238; quiz 239–241. 10.1016/S0002-8223(01)00060-8 [DOI] [PubMed] [Google Scholar]
- Kristensen AS, Andersen J, Jorgensen TN, Sorensen L, Eriksen J, Loland CJ, Stromgaard K. and Gether U, 2011. SLC6 neurotransmitter transporters: structure, function, and regulation. Pharmacological Reviews 63: 585–640. 10.1124/pr.108.000869 [DOI] [PubMed] [Google Scholar]
- Lambert JC, Zhou Z, Wang L, Song Z, McClain CJ and Kang YJ, 2003. Prevention of alterations in intestinal permeability is involved in zinc inhibition of acute ethanol-induced liver damage in mice. Journal of Pharmacology and Experimental Therapeutics 305: 880–886. 10.1124/jpet.102.047852 [DOI] [PubMed] [Google Scholar]
- Latorre E, Layunta E, Grasa L, Castro M, Pardo J, Gomollon F, Alcalde AI and Mesonero JE, 2016a. Intestinal serotonin transporter inhibition by Toll-like receptor 2 activation. A feedback modulation. PLoS ONE 11: e0169303. 10.1371/journal.pone.0169303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latorre E, Pradilla A, Chueca B, Pagan R, Layunta E, Alcalde AI and Mesonero JE, 2016b. Listeria monocytogenes inhibits serotonin transporter in human intestinal Caco-2 cells. Microbial Ecology 72: 730–739. 10.1007/s00248-016-0809-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Duan K, Wang C, McClain C. and Feng W, 2016. Probiotics and alcoholic liver disease: treatment and potential mechanisms. Gastroenterology Research and Practice 2016: 5491465. 10.1155/2016/5491465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Ghia JE, Wang H, McClemens J, Cote F, Suehiro Y, Mallet J. and Khan WI, 2011a. Serotonin activates dendritic cell function in the context of gut inflammation. American Journal of Pathology 178: 662–671. 10.1016/j.ajpath.2010.10.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Chalazonitis A, Huang YY, Mann JJ, Margolis KG, Yang QM, Kim DO, Cote F, Mallet J. and Gershon MD, 2011b. Essential roles of enteric neuronal serotonin in gastrointestinal motility and the development/survival of enteric dopaminergic neurons. Journal of Neuroscience 31: 8998–9009. 10.1523/JNEUROSCI.6684-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden DR, Chen JX, Gershon MD, Sharkey KA and Mawe GM, 2003. Serotonin availability is increased in mucosa of guinea pigs with TNBS-induced colitis. American Journal of Physiology Gastrointestinal and Liver Physiology 285: G207–216. 10.1152/ajpgi.00488.2002 [DOI] [PubMed] [Google Scholar]
- Linden DR, Foley KF, McQuoid C, Simpson J, Sharkey KA and Mawe GM, 2005. Serotonin transporter function and expression are reduced in mice with TNBS-induced colitis. Neurogastroenterology and Motility 17: 565–574. 10.1111/j.1365-2982.2005.00673.x [DOI] [PubMed] [Google Scholar]
- Liu Q, Duan ZP, Ha DK, Bengmark S, Kurtovic J. and Riordan SM, 2004. Synbiotic modulation of gut flora: effect on minimal hepatic encephalopathy in patients with cirrhosis. Hepatology 39: 1441–1449. 10.1002/hep.20194 [DOI] [PubMed] [Google Scholar]
- Ma YL, Guo T, Xu ZR, You P. and Ma JF, 2006. Effect of Lactobacillus isolates on the adhesion of pathogens to chicken intestinal mucus in vitro. Letters in Applied Microbiology 42: 369–374. 10.1111/j.1472-765X.2006.01844.x [DOI] [PubMed] [Google Scholar]
- Macias-Rodriguez ME, Zagorec M, Ascencio F, Vazquez-Juarez R. and Rojas M, 2009. Lactobacillus fermentum BCS87 expresses mucus- and mucin-binding proteins on the cell surface. Journal of Applied Microbiology 107: 1866–1874. 10.1111/j.1365-2672.2009.04368.x [DOI] [PubMed] [Google Scholar]
- Mackenzie DA, Jeffers F, Parker ML, Vibert-Vallet A, Bongaerts RJ, Roos S, Walter J. and Juge N, 2010. Strain-specific diversity of mucus-binding proteins in the adhesion and aggregation properties of Lactobacillus reuteri. Microbiology 156: 3368–3378. 10.1099/mic.0.043265-0 [DOI] [PubMed] [Google Scholar]
- Margolis KG and Pothoulakis C, 2009. Serotonin has a critical role in the pathogenesis of experimental colitis. Gastroenterology 137: 1562–1566. 10.1053/j.gastro.2009.09.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel F, Monteiro R. and Lemos C, 2003. Uptake of serotonin at the apical and basolateral membranes of human intestinal epithelial (Caco-2) cells occurs through the neuronal serotonin transporter (SERT). Journal of Pharmacology and Experimental Therapeutics 306: 355–362. 10.1124/jpet.103.049668 [DOI] [PubMed] [Google Scholar]
- Martin R, Miquel S, Ulmer J, Kechaou N, Langella P. and Bermudez-Humaran LG, 2013. Role of commensal and probiotic bacteria in human health: a focus on inflammatory bowel disease. Microbial Cell Factories 12: 71. 10.1186/1475-2859-12-71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mawe GM and Hoffman JM, 2013. Serotonin signalling in the gut--functions, dysfunctions and therapeutic targets. Nature Reviews: Gastroenterology and Hepatology 10: 473–486. 10.1038/nrgastro.2013.105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi Y, Okada S, Uchimura T. and Satoh E, 2006. A mucus adhesion promoting protein, MapA, mediates the adhesion of Lactobacillus reuteri to Caco-2 human intestinal epithelial cells. Bioscience Biotechnology and Biochemistry 70: 1622–1628. 10.1271/bbb.50688 [DOI] [PubMed] [Google Scholar]
- Nakaita Y, Kaneda H. and Shigyo T, 2013. Heat-killed Lactobacillus brevis SBC8803 induces serotonin release from intestinal cells. Food and Nutrition Sciences 4: 767–771. [Google Scholar]
- Narendranath NV, Hynes SH, Thomas KC and Ingledew WM, 1997. Effects of lactobacilli on yeast-catalyzed ethanol fermentations. Applied and Environmental Microbiology 63: 4158–4163. 10.1128/AEM.63.11.4158-4163.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazir S, Kumar A, Chatterjee I, Anbazhagan AN, Gujral T, Priyamvada S, Saksena S, Alrefai WA, Dudeja PK and Gill RK, 2015. Mechanisms of intestinal serotonin transporter (SERT) upregulation by TGF-beta1 induced non-smad pathways. PLoS ONE 10: e0120447. 10.1371/journal.pone.0120447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld KM, Kang N, Bienenstock J. and Foster JA, 2011. Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterology and Motility 23: 255–264, e119. 10.1111/j.1365-2982.2010.01620.x [DOI] [PubMed] [Google Scholar]
- Nishino R, Mikami K, Takahashi H, Tomonaga S, Furuse M, Hiramoto T, Aiba Y, Koga Y. and Sudo N, 2013. Commensal microbiota modulate murine behaviors in a strictly contamination-free environment confirmed by culture-based methods. Neurogastroenterology and Motility 25: 521–528. 10.1111/nmo.12110 [DOI] [PubMed] [Google Scholar]
- Nishiyama K, Nakamata K, Ueno S, Terao A, Aryantini NP, Sujaya IN, Fukuda K, Urashima T, Yamamoto Y. and Mukai T, 2015. Adhesion properties of Lactobacillus rhamnosus mucus-binding factor to mucin and extracellular matrix proteins. Bioscience Biotechnology and Biochemistry 79: 271–279. 10.1080/09168451.2014.972325 [DOI] [PubMed] [Google Scholar]
- Nzakizwanayo J, Dedi C, Standen G, Macfarlane WM, Patel BA and Jones BV, 2015. Escherichia coli Nissle 1917 enhances bioavailability of serotonin in gut tissues through modulation of synthesis and clearance. Scientific Reports 5: 17324. 10.1038/srep17324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh JH, Alexander LM, Pan M, Schueler KL, Keller MP, Attie AD, Walter J. and van Pijkeren JP, 2019. Dietary fructose and microbiota-derived short-chain fatty acids promote bacteriophage production in the gut symbiont Lactobacillus reuteri. Cell Host and Microbe 25: 273–284 e276. 10.1016/j.chom.2018.11.016 [DOI] [PubMed] [Google Scholar]
- O’Hara JR, Ho W, Linden DR, Mawe GM and Sharkey KA, 2004. Enteroendocrine cells and 5-HT availability are altered in mucosa of guinea pigs with TNBS ileitis. American Journal of Physiology – Gastrointestinal and Liver Physiology 287: G998–1007. 10.1152/ajpgi.00090.2004 [DOI] [PubMed] [Google Scholar]
- O’Hara JR, Skinn AC, MacNaughton WK, Sherman PM and Sharkey KA, 2006. Consequences of Citrobacter rodentium infection on enteroendocrine cells and the enteric nervous system in the mouse colon. Cellular Microbiology 8: 646–660. 10.1111/j.1462-5822.2005.00657.x [DOI] [PubMed] [Google Scholar]
- Oliva S, Di Nardo G, Ferrari F, Mallardo S, Rossi P, Patrizi G, Cucchiara S. and Stronati L, 2012. Randomised clinical trial: the effectiveness of Lactobacillus reuteri ATCC 55730 rectal enema in children with active distal ulcerative colitis. Alimentary Pharmacology and Therapeutics 35: 327–334. 10.1111/j.1365-2036.2011.04939.x [DOI] [PubMed] [Google Scholar]
- O’Mahony L, McCarthy J, Kelly P, Hurley G, Luo F, Chen K, O’Sullivan GC, Kiely B, Collins JK, Shanahan F. and Quigley EM, 2005. Lactobacillus and Bifidobacterium in irritable bowel syndrome: symptom responses and relationship to cytokine profiles. Gastroenterology 128: 541–551. [DOI] [PubMed] [Google Scholar]
- O’Sullivan GC, Kelly P, O’Halloran S, Collins C, Collins JK, Dunne C. and Shanahan F, 2005. Probiotics: an emerging therapy. Current Pharmaceutical Design 11: 3–10. [DOI] [PubMed] [Google Scholar]
- O’Sullivan MA and O’Morain CA, 2000. Bacterial supplementation in the irritable bowel syndrome. A randomised double-blind placebo-controlled crossover study. Digestive and Liver Disease 32: 294–301. [DOI] [PubMed] [Google Scholar]
- Ouwehand AC and Conway PL, 1996. Purification and characterization of a component produced by Lactobacillus fermentum that inhibits the adhesion of K88 expressing Escherichia coli to porcine ileal mucus. Journal of Applied Bacteriology 80: 311–318. [DOI] [PubMed] [Google Scholar]
- Parlesak A, Schafer C, Schutz T, Bode JC and Bode C, 2000. Increased intestinal permeability to macromolecules and endotoxemia in patients with chronic alcohol abuse in different stages of alcohol-induced liver disease. Journal of Hepatology 32: 742–747. 10.1016/s0168-8278(00)80242-1 [DOI] [PubMed] [Google Scholar]
- Rabe CS, Giri PR, Hoffman PL and Tabakoff B, 1990. Effect of ethanol on cyclic AMP levels in intact PC12 cells. Biochemical Pharmacology 40: 565–571. [DOI] [PubMed] [Google Scholar]
- Ramiah K, van Reenen CA and Dicks LM, 2007. Expression of the mucus adhesion genes Mub and MapA, adhesion-like factor EF-Tu and bacteriocin gene plaA of Lactobacillus plantarum 423, monitored with real-time PCR. International Journal of Food Microbiology 116: 405–409. 10.1016/j.ijfoodmicro.2007.02.011 [DOI] [PubMed] [Google Scholar]
- Ramiah K., Van Reenen CA. and Dicks LM., 2009. Expression of the mucus adhesion gene Mub, surface layer protein Slp and adhesion-like factor EF-TU of Lactobacillus acidophilus ATCC 4356 under digestive stress conditions, as monitored with real-time PCR. Probiotics and Antimicrobial Proteins 1: 91. 10.1007/s12602-009-9009-8 [DOI] [PubMed] [Google Scholar]
- Regmi SC, Park SY, Ku SK and Kim JA, 2014. Serotonin regulates innate immune responses of colon epithelial cells through Nox2-derived reactive oxygen species. Free Radical Biology and Medicine 69: 377–389. 10.1016/j.freeradbiomed.2014.02.003 [DOI] [PubMed] [Google Scholar]
- Reigstad CS, Salmonson CE, Rainey JF 3rd, Szurszewski JH, Linden DR, Sonnenburg JL, Farrugia G. and Kashyap PC, 2015. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB Journal 29: 1395–1403. 10.1096/fj.14-259598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romani Vestman N, Chen T, Lif Holgerson P, Ohman C. and Johansson I, 2015. Oral microbiota shift after 12-week supplementation with Lactobacillus reuteri DSM 17938 and PTA 5289; a randomized control trial. PLoS ONE 10: e0125812. 10.1371/journal.pone.0125812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, Stange DE, Ferrante M, Vries RG, Van Es JH, Van den Brink S, Van Houdt WJ, Pronk A, Van Gorp J, Siersema PD and Clevers H, 2011. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology 141: 1762–1772. 10.1053/j.gastro.2011.07.050 [DOI] [PubMed] [Google Scholar]
- Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ and Clevers H, 2009. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459: 262–265. 10.1038/nature07935 [DOI] [PubMed] [Google Scholar]
- Shanahan F, 2009. Therapeutic implications of manipulating and mining the microbiota. Journal of Physiology 587: 4175–4179. 10.1113/jphysiol.2009.174649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibasaki M, Inoue M, Kurokawa K, Ogou S. and Ohkuma S, 2010. Expression of serotonin transporter in mice with ethanol physical dependency. Journal of Pharmacological Sciences 114: 234–237. [DOI] [PubMed] [Google Scholar]
- Solis E Jr., Zdravkovic I, Tomlinson ID, Noskov SY, Rosenthal SJ and De Felice LJ, 2012. 4-(4-(dimethylamino)phenyl)-1-methylpyridinium (APP+) is a fluorescent substrate for the human serotonin transporter. Journal of Biological Chemistry 287: 8852–8863. 10.1074/jbc.M111.267757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spohn SN, Bianco F, Scott RB, Keenan CM, Linton AA, O’Neill CH, Bonora E, Dicay M, Lavoie B, Wilcox RL, MacNaughton WK, De Giorgio R, Sharkey KA and Mawe GM, 2016. Protective actions of epithelial 5-hydroxytryptamine 4 receptors in normal and inflamed colon. Gastroenterology 151: 933–944, e933. 10.1053/j.gastro.2016.07.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stansbridge EM, Walker V, Hall MA, Smith SL, Millar MR, Bacon C. and Chen S, 1993. Effects of feeding premature infants with Lactobacillus GG on gut fermentation. Archives of Disease in Childhood 69: 488–492. 10.1136/adc.69.5_spec_no.488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo G, Verma BK, Fogarasi M. and Catalano DE, 1992. Induction of transforming growth factor-beta and prostaglandin E2 production by ethanol in human monocytes. Journal of Leukocyte Biology 52: 602–610. 10.1002/jlb.52.6.602 [DOI] [PubMed] [Google Scholar]
- Tada Y, Ishihara S, Kawashima K, Fukuba N, Sonoyama H, Kusunoki R, Oka A, Mishima Y, Oshima N, Moriyama I, Yuki T, Ishikawa N, Araki A, Harada Y, Maruyama R. and Kinoshita Y, 2016. Downregulation of serotonin reuptake transporter gene expression in healing colonic mucosa in presence of remaining low-grade inflammation in ulcerative colitis. Journal of Gastroenterology and Hepatology 31: 1443–1452. 10.1111/jgh.13268 [DOI] [PubMed] [Google Scholar]
- Takayanagi S, Hanai H, Kumagai J. and Kaneko E, 1995. Serotonin uptake and its modulation in rat jejunal enterocyte preparation. Journal of Pharmacology and Experimental Therapeutics 272: 1151–1159. [PubMed] [Google Scholar]
- Uhlemann ER, Robberecht P. and Gardner JD, 1979. Effects of alcohols on the actions of VIP and secretin on acinar cells from guinea pig pancreas. Gastroenterology 76: 917–925. [PubMed] [Google Scholar]
- Van Tassell ML and Miller MJ, 2011. Lactobacillus adhesion to mucus. Nutrients 3: 613–636. 10.3390/nu3050613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade PR, Chen J, Jaffe B, Kassem IS, Blakely RD and Gershon MD, 1996. Localization and function of a 5-HT transporter in crypt epithelia of the gastrointestinal tract. Journal of Neuroscience 16: 2352–2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YM, Ge XZ, Wang WQ, Wang T, Cao HL, Wang BL and Wang BM, 2015. Lactobacillus rhamnosus GG supernatant upregulates serotonin transporter expression in intestinal epithelial cells and mice intestinal tissues. Neurogastroenterology and Motility 27: 1239–1248. 10.1111/nmo.12615 [DOI] [PubMed] [Google Scholar]
- Wheatcroft J, Wakelin D, Smith A, Mahoney CR, Mawe G. and Spiller R, 2005. Enterochromaffin cell hyperplasia and decreased serotonin transporter in a mouse model of postinfectious bowel dysfunction. Neurogastroenterology and Motility 17: 863–870. 10.1111/j.1365-2982.2005.00719.x [DOI] [PubMed] [Google Scholar]
- Wilson JN., Ladefoged LK., Babinchak WM. and Schiott B., 2014. Binding-induced fluorescence of serotonin transporter ligands: A spectroscopic and structural study of 4-(4-(dimethylamino)-phenyl)-1-methylpyridinium (APP(+)) and APP(+) analogues. ACS Chemical Neuroscience 5: 296–304. 10.1021/cn400230x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolin MJ, Yerry S, Miller TL, Zhang Y. and Bank S, 1998. Changes in production of ethanol, acids and H2 from glucose by the fecal flora of a 16- to 158-d-old breast-fed infant. Journal of Nutrition 128: 85–90. 10.1093/jn/128.1.85 [DOI] [PubMed] [Google Scholar]
- Yadav VK, Balaji S, Suresh PS, Liu XS, Lu X, Li Z, Guo XE, Mann JJ, Balapure AK, Gershon MD, Medhamurthy R, Vidal M, Karsenty G. and Ducy P, 2010. Pharmacological inhibition of gut-derived serotonin synthesis is a potential bone anabolic treatment for osteoporosis. Nature Medicine 16: 308–312. 10.1038/nm.2098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yammamoto H, Tanaka S, Tanaka A, Hide I, Seki T. and Sakai N, 2013. Long-term exposure of RN46A cells expressing serotonin transporter (SERT) to a cAMP analog up-regulates SERT activity and is accompanied by neural differentiation of the cells. Journal of Pharmacological Sciences 121: 25–38. [DOI] [PubMed] [Google Scholar]
- Yano JM, Yu K, Donaldson GP, Shastri GG, Ann P, Ma L, Nagler CR, Ismagilov RF, Mazmanian SK and Hsiao EY, 2015. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 161: 264–276. 10.1016/j.cell.2015.02.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaunmuller T, Eichert M, Richter H. and Unden G, 2006. Variations in the energy metabolism of biotechnologically relevant heterofermentative lactic acid bacteria during growth on sugars and organic acids. Applied Microbiology and Biotechnology 72: 421–429. 10.1007/s00253-006-0514-3 [DOI] [PubMed] [Google Scholar]
- Zhu CB, Carneiro AM, Dostmann WR, Hewlett WA and Blakely RD, 2005. p38 MAPK activation elevates serotonin transport activity via a trafficking-independent, protein phosphatase 2A-dependent process. Journal of Biological Chemistry 280: 15649–15658. 10.1074/jbc.M410858200 [DOI] [PubMed] [Google Scholar]
- Zocco MA, dal Verme LZ, Cremonini F, Piscaglia AC, Nista EC, Candelli M, Novi M, Rigante D, Cazzato IA, Ojetti V, Armuzzi A, Gasbarrini G. and Gasbarrini A, 2006. Efficacy of Lactobacillus GG in maintaining remission of ulcerative colitis. Alimentary Pharmacology and Therapeutics 23: 1567–1574. 10.1111/j.1365-2036.2006.02927.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.