Abstract
Background
Human myiasis is a parasitic dipteran fly infestation that infects humans and vertebrates worldwide. However, the disease is endemic in Sub-Saharan Africa and Latin America. In Sub-Saharan Africa, it is under-reported and therefore its prevalence is unknown. This systematic review aims to elucidate the prevalence of human myiasis, factors that influence the infection, and myiasis-causing fly species in SSA. The review also dwelled on the common myiasis types and treatment methods of human myiasis.
Methods
Here, we collect cases of human myiasis in Sub-Saharan Africa based on literature retrieved from PubMed, Google Scholar and Science Direct from 1959 to 2022. A total of 75 articles and 157 cases were included in the study. The recommendations of PRISMA 2020 were used for the realization of this systematic review.
Results
In total, 157 cases of human myiasis in SSA were reviewed. Eleven fly species (Cordylobia anthropophaga, Cordylobia rodhaini, Dermatobia hominis, Lucilia cuprina, Lucilia sericata, Oestrus ovis, Sarcophaga spp., Sarcophaga nodosa, Chrysomya megacephala, Chrysomya chloropyga and Clogmia albipuntum) were found to cause human myiasis in SSA. Cordylobia anthropophaga was the most prevalent myiasis-causing species of the reported cases (n = 104, 66.2%). More than half of the reported cases were from travelers returning from SSA (n = 122, 77.7%). Cutaneous myiasis was the most common clinical presentation of the disease (n = 86, 54.7%). Females were more infected (n = 78, 49.6%) than males, and there was a higher infestation in adults than young children.
Conclusion
The findings of this study reveals that international travelers to Sub-Saharan Africa were mostly infested therefore, we recommend that both international travelers and natives of SSA be enlightened by public health officers about the disease and its risk factors at entry points in SSA and the community level respectively. Clinicians in Sub-Saharan Africa often misdiagnose the disease and most of them lack the expertise to properly identify larvae, so we recommend the extensive use of molecular identification methods instead.
Author summary
Human myiasis is a neglected tropical disease in the world especially in SSA. Human myiasis in SSA has infects patients from all dynamics especially those with underlying health issues like primary wounds. The disease can be fatal especially when it involves heavy infestation of the scalp (migratory myiasis) among young children. Subcutaneous myiasis of the eye and genitals can be devastating for patients and can lead to damage in these areas if the disease is misdiagnosed and larvae removal is delayed. The findings and recommendations in our study can be used by government officials in SSA to provide hospitals with state-of-the-art diagnostic tools, trained entomologists, and research funding to comprehensively study human myiasis. Public health officers at entry points in SSA should inform international travelers about the risk factors of the disease and common preventive measures. Clinicians in SSA should report more human myiasis cases to enable researchers to estimate the epidemiology and prevalence of the disease. The natives of SSA should be enlighten more on the symptoms and risk factors of the disease and encourage them to report to health facilities when they experience these symptoms.
1. Introduction
Myiasis, coined from the Greek word ‘myia’ meaning fly, is the infestation of live or dead tissues of vertebrates (humans and animals) by immature stages (maggots) of dipteran flies [1,2]. The disease dates back to 1840 when it was first described by Hope [3] and is still considered a neglected disease in humans, especially in the tropical and sub-tropical regions in SSA, Asia, and Latin America [4]. The disease has a worldwide distribution and has been endemic in Latin America and SSA for years. However, with the increase in global travel, the disease has spread widely, especially in areas with warmer temperatures and high humidity [5]. Myiasis is more common in animals, such as sheep, rodents, and antelope, than humans because humans are accidental hosts. Furuncular myiasis is the most common myiasis reported from travelers returning from endemic regions and is usually caused by the human botfly, Dermatobia hominis in Latin America. In SSA, the tumbu fly or mango fly (Cordylobia anthropophaga) causes year-round infestation which could be dated back to 1904 [6], albeit most of the human myiasis infestation in SSA are caused by this species [7]. The climate condition in SSA is suitable for the breeding of some fly species which makes most places to be endemic of them. Although human myiasis is endemic in SSA, the diversity and prevalence of myiasis-causing flies in SSA is still not clear to date.
Human myiasis can be categorized depending on several factors. According to the host-parasitic relationship (feeding relationship between larva and the host), myiasis can be divided into obligatory myiasis, facultative myiasis, and accidental myiasis. In obligatory myiasis, fly larvae require living tissues for survival and to complete the immature stages of their life cycle. Facultative myiasis on the other hand is caused by free-living fly species (feeding on decaying organic matter and can opportunistically infest living tissues), their larvae do not require a living host to complete their life cycle. While accidental myiasis is a condition in which the larval stages of dipteran flies are accidentally ingested through contaminated food or water [8,9]. Additionally, human myiasis can further be classified into primary and secondary myiasis. When dipteran fly larvae invade healthy tissues or skin it will result in primary myiasis, and when these larvae colonize pre-existing wounds it will result in secondary myiasis [4,10].
According to the anatomical site or clinical presentation, myiasis can be cutaneous which involves the infestation of dermal and sub-dermal layers (tissues) of the skin (humans and animals) or infest any part of the body (nose, eyes, scalp, breast, intestine, leg, urogenital, mouth, arms, and thighs) [11]. Cutaneous myiasis takes account the largest part of clinical presentation in humans which could be categorized into migratory (creeping) myiasis, furuncular myiasis, and wound (traumatic) myiasis [Fig 1] [12]. Similarly, furuncular myiasis is the most common type reported from travelers from endemic regions, and is characterized by the formation of a painful inflammatory nodule with a central punctum on healthy or unbroken skin [1]. It is mainly caused by the tumbu fly or the botfly [13]. Wound (traumatic) myiasis is caused by dipteran fly larvae which colonize pre-existing wounds and enlarge them [8,10]. While migratory (creeping) myiasis is a condition in which dipteran fly larvae burrow in the subcutaneous tissues of the host and migrate, and often causes pruritic lesions within the host tissues. Theppote A., et al., 2020 presented a clinical presentation of cutaneous myiasis [12].
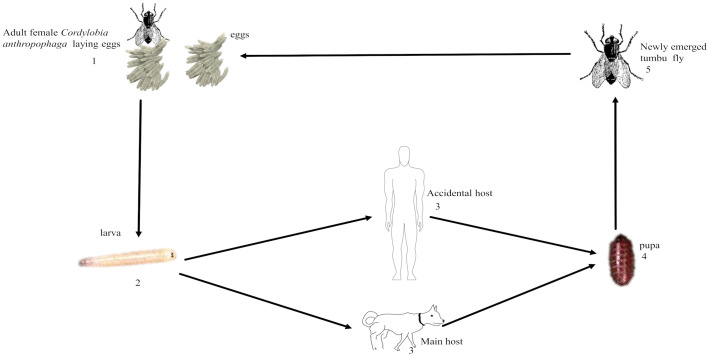
Fig 1. Life cycle of Cordylobia anthropophaga.
1. Adult female Cordylobia anthropophaga lay 100–300 eggs on the on wet clothing or faecal-contaminated soil. 2. Eggs catch to form 1st instar larva d. 3. 1st instar larva penetrates a host which is usually dog, or rodent, but could accidently penetrate a human host and develop to 3rd instar larva. 4. Larva leaves its host to the ground to pupate. 5. pupa metamorphosis to an adult fly.
Compelling scientific literature have revealed that a significant rise in temperature or humidity will increase the growth and redistribution of most myiasis-causing flies, subsequently increasing myiasis infestation in such regions [11,14]. Some factors that influence this dermatosis in SSA are high humidity and temperature (especially during the rainy season) [5]. Poverty, and poor hygiene also add to the vulnerability to human myiasis infestation. Rodents, antelopes, and pet animals, especially dogs, are the hosts of tumbu fly, making farmers, and pet keepers more vulnerable to C. anthropophaga infestation [15,16]. Skin-related diseases belong to the leading travel-related health problems reported. Currently, human myiasis is reported as one of the five most common travel-related skin diseases, which accounts for 7–12% of travel-related diseases globally [1,17]. Hence, an increase in international travel will drastically increase human myiasis infestation in both endemic and non-endemic regions.
Human myiasis can affect people (tourists, businesspersons, etc.) traveling to endemic regions, especially in SSA, where it remains a burden and needs urgent attention. Both travelers and natives of SSA lack awareness of how to prevent themselves against human myiasis infestation which adds to the vulnerability of the disease. Wearing long garments to cover legs and hands, especially during the rainy season, and sleeping in bed nets would help prevent insect bites. Lying on the ground, hanging clothes on shady lines or bushes, and lack of ironing of garments or bedding after laundry should be avoided [17]. The use of some fly repellents is encouraged to avoid insect bites. Open wounds should be routinely dressed, good skincare and standard hygiene should be maintained to avoid human myiasis infestation [16]. Animal pets should be properly handled because some of these pets can be reservoirs for the disease and can add to the vulnerability of human myiasis infestation.
This parasitological condition causes harm not only to humans but also to the livestock industry, accruing substantial economic losses for farmers [8,18]. Human myiasis is often misdiagnosed as cellulitis, leishmaniasis, tungiasis, or furunculosis [19] which is a common problem in diagnosing the disease. Therefore, properly extracting fly maggots, and sometimes the use of antibiotics becomes the gold standard for the treatment of human myiasis cases. Morphological identification is considered standard for larval identification in human myiasis cases, however, the use of molecular identification method has been being utilized globally. This method can differentiate closely related species and identify immature stages which could be an effective method in cases where traditional morphological method is ineffective [1,20,21]. Secondary bacterial superinfection and tetanus are some of the severe complications of human myiasis especially if larvae fragmentation occurs during removal [16,21,22]. Subsequently, the need to systematically review the current literature on human myiasis in SSA is an important priority. This review aims to elucidate the prevalence of human myiasis, and highlight the most common myiasis-causing flies and areas where they are endemic in SSA. We wish to uncover the predisposing factors of human myiasis in SSA and highlight the most common clinical forms of myiasis. Our study will equally highlight the common extraction and treatment forms of human myiasis.
Ecology and Life Cycle of Cordylobia anthropophaga
The tumbu fly is the most common myiasis-causing fly in SSA. The adult tumbu fly is yellowish-brown in colour measuring 6–12 mm in length with two bands on the thoracic region and a brownish-black on the abdomen [23]. The female tumbu fly lays approximately 100–300 eggs on urine and faecal-contaminated soil or wet clothing (linens), especially clothes dried on shady lines and or bushes which are favourable oviposition locations [11]. When eggs hatch, the first instar larvae penetrate the host skin [24] and after 7–12 days, the second and third instar larvae of tumbu fly will be formed. These stages are characterized by a cuticular spine, spiracular plates, and peritrenes on both the anterior and posterior ends. The third instar larva of C. anthropophaga leaves the host to the ground to pupate and becomes an adult fly and this cycle is repeated [Fig 1] [25].
2. Materials and methods
2.1 Search strategy
According to the PRISMA Recommendations (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) of 2020 [26], a systematic review of the literature was carried out in pairs. A literature search was conducted in Science Direct, PubMed, and Google scholar using the following search terms: human, myiasis, Sub-Saharan Africa (SSA), and case reports. Boolean operators (AND, OR) were used to combine search terms. We searched manually using the references of retrieved articles and thereby identified articles that were not retrieved from the database search. The search focused on studies conducted in all (48) countries in SSA and the search covered the years between 1959 and 2022 with no filter applied. Case reports from countries in SSA or those acquired from travel to SSA were considered. The following combined keywords were used for the search:
For PubMed, search terms were: human myiasis AND SSA AND case reports;
For Google Scholar, the search terms used were: human myiasis AND SSA AND case reports.
For Science Direct, search terms were: human myiasis AND SSA AND case reports.
It was worth noting that papers were also retrieved using the search terms; human myiasis AND country name AND case reports for all countries in SSA and this was done for more thorough search.
2.2 Inclusion and exclusion criteria
The inclusion criteria were: (1) original articles related to the topic of interest of this study; (2) any case report article from SSA with no language restriction and/or could be translated using Google translate; (3) relevant case reports from SSA found in review studies; (4) studies carried out in SSA; (5) studies published between 1959 to 2022.
The exclusion criteria of this were: (1) articles that are not case reports and articles with case reports that are not from SSA; (2) articles with no author’s name and year of publication; (3) review studies that are with no relevant case reports; (4) unpublished studies; and (5) non-human studies.
2.3 Data extraction
All duplicate articles were removed using the Endnote library. Three separate examiners carried out the first phase of the selection. It allowed us to delete certain selected studies. Thus, after a complete reading, we selected the studies that met the inclusion criteria. Discrepancies were resolved by discussion with a fourth reviewer. The following information was extracted from the articles selected; patient’s age, sex, country of origin, myiasis type, site of infection, country reported, number of patients, and country of infestation [Table 1]. The studies will be grouped according to the sub-regions SSA (West Africa, East Africa, Southern Africa, and Central Africa) based on the United Nations (UN) system classification. A protocol for this study has been upload on protocol.oi with a DOI of DOI: dx.doi.org/10.17504/.
Table 1. Summary of selected studies.
| Central Africa | ||||||||
| Myiasis type | Patient, Age | Sex | Fly species | Site of infection | Country reported | No. of patients | Country of infestation | Ref |
| Furuncular | 29 (average) | M | C. anthropophaga | Various body parts | Central Africa | 16 | Local infestation | [27] |
| Furuncular | 59 | F | C. rodhaini | Scalps | France | 1 | Cameroon | [28] |
| Furuncular | 50 | M | C. anthropophaga | Thorax, back, lower lip | Vienna | 1 | Cameroon | [29] |
| Furuncular | 46 | F | C. anthropophaga | Right lumbar, gluteal region | Panama | 1 | Central Africa | [30] |
| Cutaneous | 55 | M | C. anthropophaga | Left buttock upper thigh | Korea | 1 | Central Africa | [23] |
| Cutaneous | 46 | M | C. anthropophaga | Anterior chest a acromioclavicular joint | Sri-lanka | 1 | Cameroon | [31] |
| Cutaneous | 54 | M | C. anthropophaga | Entire back | Britain | 1 | Congo | [32] |
| Glans (penis) | 22 | M | C. anthropophaga | Near the opening of urethra | Congo | 1 | Local infestation | [33] |
| Furuncular | 5 months | M | C. anthropophaga | Right shoulder | France | 1 | Congo | [34] |
| Furuncular | 61 | F | C. anthropophaga | sub clavicular | Italy | 1 | Cameroon | [35] |
| Furuncular | 37 | M | C. anthropophaga | Chest & shoulder | Morocco | 1 | Congo | [36] |
| Furuncular | 59 | F | C. rodhaini | Scalp, left parietal temporal part, right flank, upper lip intranasal lesion and abdomen | Morocco | 1 | Cameroon | [37] |
| Cutaneous | - | - | D. hominis | leg | Morocco | 1 | Congo | [38] |
| East Africa | ||||||||
| Myiasis type | Patient Age | Sex | Fly species | Site of infection | Country reported | No. of patients | Country of infestation | Ref |
| Furuncular | 45 | F | C. anthropophaga | Left thigh | Sudan | 1 | Local infestation | [39] |
| Cutaneous | 31, 28 | M, F | C. anthropophaga | Trunk penis lower leg, left buttock | Germany | 2 | East Africa | [40] |
| Furuncular | 21 | F | C. anthropophaga | Left finger | Japan | 1 | Uganda | [41] |
| Cutaneous | 46 | F | C. anthropophaga | Upper right arm | Sudan | 1 | Local infestation | [42] |
| Furuncular | 61 | F | C. anthropophaga | Left arm, abdomen, left, thigh | Ethiopia | 1 | Local infestation | [43] |
| Furuncular | 59, 58 | M, F | C. rodhaini | Left shoulder, abdomen Pubis | Italy | 2 | Uganda, | [13] |
| Furuncular | 52 | F | C. rodhaini | Right leg | Italy | 1 | Ethiopia | [13] |
| Furuncular | 42,5 | M, M | C. rodhaini | Left buttock, top right side of head & maxilla | Ethiopia | 2 | Local infestation | [44] |
| Furuncular | 22 | F | C. anthropophaga | Right gluteus | Italy | 1 | Kenya | [17] |
| Furuncular | 56 | - | C. anthropophaga | Upper thigh, lower abdomen, lower back | USA | 1 | Ethiopia | [45] |
| Ocular | 59 | M | C. anthropophaga | Right eye lid | Italy | 1 | Kenya | [46] |
| Furuncular | - | M | C. rodhaini | Scalp, right arm, left arm, torso | Italy | 1 | Uganda | [8] |
| Furuncular | 33 | M | C. anthropophaga | Left leg | Korea | 1 | Uganda | [1] |
| Furuncular | - | - | C. anthropophaga | Left thigh | - | 1 | Tanzania | [47] |
| Cutaneous | 30 | M | C. anthropophaga | Left groin | UK | 1 | Sierra Leone | [48] |
| Furuncular | 57 | F | C. rodhaini | Right thigh | Australia | 1 | East Africa | [49] |
| Furuncular | - | M | C. rodhaini | All over the body | Italy | 1 | Ethiopia | [50] |
| Cutaneous | 52 | F | C. rodhaini | - | Germany | 1 | Tanzania | [51] |
| Cutaneous | 38 | M | C. anthropophaga | Left lower back | China | 1 | Ethiopia | [52] |
| Cutaneous | 55 | F | C. rodhaini | Fore head | UK | 1 | Uganda | [53] |
| Cutaneous | 26 | F | C. anthropophaga | Left upper arm | China | 1 | Uganda | [20] |
| Cutaneous | 26 | F | C. rodhaini | Left upper arm | Canada | 1 | Ethiopia | [54] |
| Furuncular | 4month, 13month | F, - | C. anthropophaga | Aural flanks, chin, and fore legs, Lower trunk, forearm and right arm | Ireland | 2 | Kenya | [55] |
| Furuncular | 26 | M | C. anthropophaga | Fifth digit on foot | USA | 1 | Tanzania | [56] |
| Cutaneous | 26 | M | C. anthropophaga | Back (mid scapular region) | UK | 1 | Kenya | [57] |
| West Africa | ||||||||
| Myiasis type | Patient Age | Sex | Fly species | Site of infection | Country reported | No. of patients | Country of infestation | Ref |
| Cutaneous | 17 | F | C. rodhaini | Left breast | UK | 1 | Ghana | [58] |
| Furuncular | 42 | F | C. anthropophaga | Left buttocks | France | 1 | Senegal | [28] |
| Glans penis | 10 | M | C. anthropophaga | penis | Denmark | 1 | Senegal | [59] |
| Cutaneous | - | F | C. anthropophaga | Breast, upper & lower lips | Nigeria | 28 | Local infestation | [60] |
| Furuncular | 45 | M | C. anthropophaga | Limbs & trunk | India | 1 | Nigeria | [61] |
| Cutaneous | 29 | F | C. anthropophaga | - | US | 1 | Sierra Leone | [62] |
| Furuncular | - | F | C. rodhaini | On the thigh, below the breast | Israel | 2 | Ghana | [63] |
| Furuncular | 16, 17 | M, F | C. anthropophaga | Bilateral legs, ankle, left thigh, buttocks | USA | 2 | Senegal | [64] |
| Cutaneous | 48, 47, 14 | M, F, F | C. anthropophaga | Back & nose, shoulder, wrist, back | Slovenia | 3 | Ghana | [65] |
| Furuncular | 32 | F | C. anthropophaga | Thigh, left flank | Britain | 1 | Gambia | [66] |
| Cutaneous | 30 | F | C. anthropophaga | Right leg | Italy | 1 | Senegal | [67] |
| Furuncular | 45 | C. anthropophaga | Aural flanks, chin, and fore legs | Vienna | 1 | Senegal | [68] | |
| Cutaneous | 6 weeks | Child | C. anthropophaga | Scalp, dorsal part of trunk, hands, leg | Gambia | 1 | Local infestation | [11] |
| Vulva | 16 | F | C. anthropophaga | vulva | - | 1 | Senegal | [69] |
| Cutaneous | 50 | M | C. anthropophaga | Sole of the left foot | Italy | 1 | Senegal | [70] |
| Furuncular | - | - | D. hominis | - | Nigeria | 1 | Local infestation | [71] |
| furuncular | 29 | F | D. hominis | buttocks | Spain | 1 | Guinea Bissau | [72] |
| Ocular | 24 | F | C. anthropophaga | Upper eye lid | France | 1 | Cape Verde | [73] |
| Furuncular | 12 | F | C. anthropophaga | Lower lid of the right eye and forearm | Thailand | 1 | Ghana | [74] |
| Cutaneous | 55 | M | C. anthropophaga | Trunks, arms and legs | USA | 1 | Nigeria | [22] |
| Nasopharyngeal | Adult | M | Clogmia albipunctatum | Nose | UK | 1 | Nigeria | [75] |
| Cutaneous | 27 | M | C. anthropophaga | - | Netherlands | 1 | Gambia | [76] |
| Furuncular | 11month, 10y | -, F | C. anthropophaga | Right arm, thigh | Nigeria | 2 | Local infestation | [77] |
| Cutaneous | 52 | M | D. hominis | Upper lip | UK | 1 | Gambia | [78] |
| Furuncular | 30 | F | C. anthropophaga | Lower back | USA | 1 | Guinea | [79] |
| Cutaneous | 70 | F | C. anthropophaga | Right breast | Nigeria | 1 | Local infestation | [80] |
| Cutaneous | 24, 23 | F, F | C. anthropophaga | Left flank, right thigh | Italy | 2 | Senegal | [81] |
| Cutaneous | 34, 21 | F, F | C. anthropophaga | Breast, breast | Nigeria | 2 | Local infestation | [60] |
| South Africa | ||||||||
| Myiasis type | Patient Age | Sex | Fly species | Site of infection | Country reported | No. of patients | Country of infestation | Ref |
| - | 22 &57 | - | Sarcophaga spp & L. cuprina | Various body part | South Africa | 2 | Local infestation | [82] |
| Ocular | 10 | M | Oestrus ovis | Right and left eye | India | 1 | South Africa | [83] |
| - | - | Infants | C. anthropophaga | Arms, buttocks, trunk | South Africa | 6 | - | [84] |
| Ocular | 27 | F | Oestrus ovis | eyes | Greece | 1 | South Africa | [85] |
| Furuncular | 6-month | F | C. anthropophaga | Thighs, trunk | Malawi | 1 | Local infestation | [19] |
| Furuncular | 39 | M | C. anthropophaga | Left eye, right thigh, left buttocks | UK | 1 | Angola | [86] |
| Wound or traumatic | 51 | M | L. cuprina | foot | South Africa | 1 | Local infestation | [87] |
| Cutaneous | 28 | F | C. anthropophaga | Upper arms, wrist & inner thigh | Australia | 1 | South Africa | [88] |
| Furuncular | 57 | F | - | Left shoulder, back, left arm, right thigh, popliteal fossa | UK | 1 | South Africa | [89] |
| Furuncular | 38 | M | C. anthropophaga | Right side of his scalp | UK | 1 | South Africa | [90] |
| Cutaneous | 56 (average) | M(14), F(11) | L. cuprina, L. sericata, C.megacephala, C. chloropyga, Sarcophaga, 2UI | Different body parts | South Africa | 25 | Local infestation | [91] |
2.4 Quality assessment
The risk of bias was assessed by the Joanna Briggs Institute (JBI) checklist for Case Reports Critical Appraisal Tool [92]. Two reviewers independently assessed selected articles, and discrepancies were resolved by discussion or by the other reviewers. The assessment of the quality of the selected studies is presented in the S1 Table. The selected studies were homogeneous, and 73 of 75 studies were of high quality, one study was moderate and one study was below quality according to the JBI-MAStARI.
3. Results
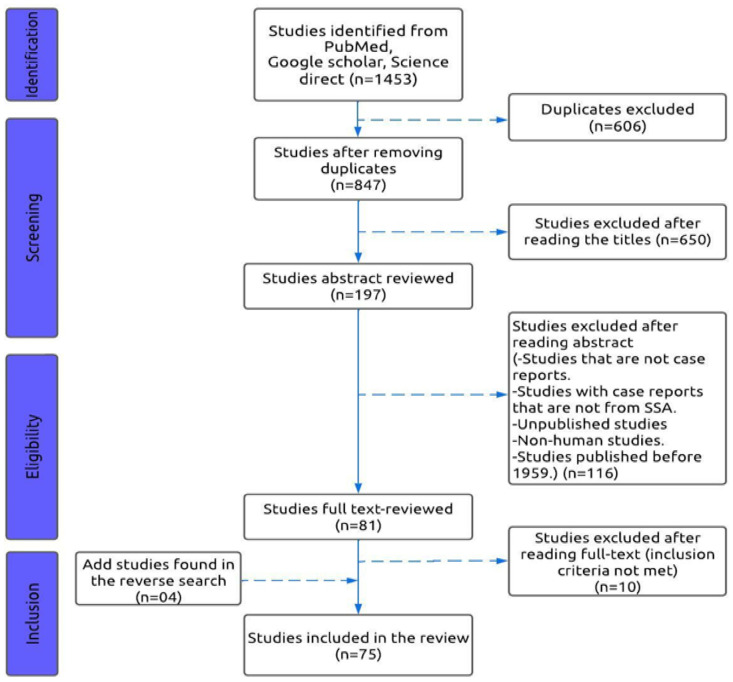
There were 1,453 original articles identified in the three databases, of which 847 articles were retained after the deletion of duplicates. A selection based on title led to the exclusion of 650 articles. Abstracts of the remaining 197 articles were reviewed, excluding 116 more articles. Reading the full text of the remaining 81 articles allowed the exclusion of 10 articles. Finally, 71 articles which met the inclusion criteria were selected. A reverse search was performed on the 71 included articles by searching the terms in the references of the selected articles to identify the articles which had not been initially selected and which fulfilled the inclusion criteria. Thus, other articles were identified and included, totaling 75 articles for this systematic review. The representative search design and number of eligible studies are shown in Fig 2.
Fig 2. Flowchart for the selection of studies based on the PRISMA 2020 guidelines.
3.1. Characteristics of included studies
Table 1 summarized all included studies in this systematic review and were grouped by sub-regions. Thus, 13 studies were identified from Central Africa, 23 in East Africa, 28 in West Africa, and 11 in Southern Africa. There were 75 studies in total and the cases from these study included both males and females of different age groups. Some cases do not contain gender information. There were 7 classifications of human myiasis (cutaneous, furuncular, ocular, glancpenis, vulva, wound, and nasopharyngeal myiasis) detected in this study. The sample size ranged from 1 to 157 patients, and patients’ ages ranged from 6 weeks to 70 years (the average age is 17 years).
3.2. Evidence from reviewed studies
Table 1 summarizes the different types of myiasis, fly species, and site of infection. This table also shows the countries where these infections were reported, the number of patients, and the country of infestation.
Regarding myiasis types, cutaneous myiasis was described by more than half (54.7%) of the studies examined (n = 86). Furuncular myiasis was described by 38.2% of the articles retained (n = 60). Three (n = 03) articles reviewed genital myiasis (glans penis, and vulva) representing 1.2% and 0.6%, respectively. Very few studies (n = 4) described ocular myiasis (2.5%). In addition, two (n = 2) (1.2%) studies reviewed had described nasopharyngeal myiasis and wound myiasis. There were 2 (1.2%) cases with un-identified myiasis type.
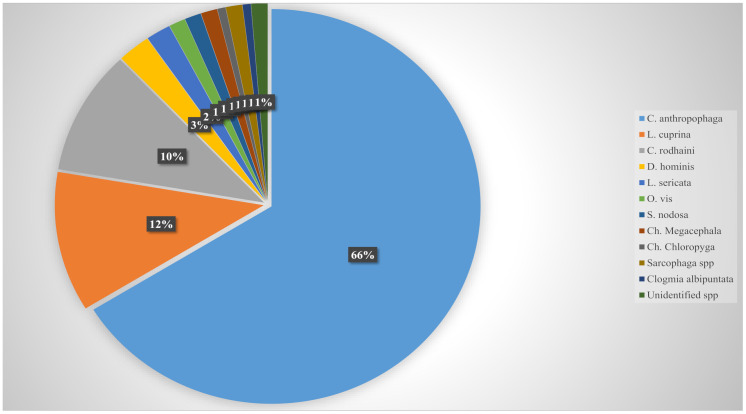
Based on reviewed cases, a total of 11 fly species were found to cause human myiasis in SSA which included Cordylobia anthropophaga, Cordylobia rodhaini, Dermatobia hominis, Lucilia cuprina, Lucilia sericata, Oestrus ovis, Sarcophaga spp., Sarcophaga nodosa, Chrysomya megacephala, Chrysomya chloropyga and Clogmia albipuntum. The obligatory cutaneous parasite C. anthropophaga, was the most commonly encountered fly species (n = 104), which accounted for 66% of the total cases. Lucilia cuprina, which is usually responsible for facultative myiasis, recorded 18 cases which accounts for 11% of the total cases. There were 16 cases of Cordylobia rodhaini which accounted for 10% of the cases detected. These three fly species were responsible for the majority of the human myiasis cases in SSA. The other species detected during this study accounted for either 2% or 1% and accumulated to 12% of the total reported cases. The prevalence (percentage) of all the myiasis-causing flies from this study are shown in Fig 3.
Fig 3. Diversity and prevalence of human myiasis–causing fly species.
Anatomically, the most prevalent sites of infestation were from the lower abdomen down to the lower limbs (abdomen, genital regions, buttocks, both lower and upper thighs, both lower and upper legs, feet). The second most frequent infection sites found in this study were the thoracic and back region (thorax, chest, both shoulders, both breasts, both lower and upper arms including wrists, and upper back). However, the least common infested sites in this study were the head and neck regions (scalp, parietal and temporal lobes, intranasal lesions, forehead, chin, both lower and upper eyelids, truck, nose, maxilla). Three (n = 3) of the studies indicated unspecified infestation sites (various both parts).
Regarding patients’ gender, 78 out of 157 patients were female (49.6%). Males accounted for 38.9% (n = 61) of the studies, and 11.5% (n = 18) were un-identified gender. There were 35 (22.3%) cases reported in SSA with no travel history outside of this area. While 122 (77.7%) of the cases were infected in SSA but reported from countries out of SSA.
4. Discussion
Human myiasis is a dermatological condition that can affect people from different areas. However, the most common underlying factor for human myiasis infestation in this study is international travel to endemic regions in SSA [42] [Table 1]. The cases infected and reported in SSA are those patients who are inhabitants (natives) of SSA, and were infected and diagnosed in SSA without traveling out of SSA. While the cases reported outside SSA but are infected in SSA are travelers who visited SSA and got infected in SSA. When they returned to their countries (outside SSA) and got diagnosed, the cases were reported from their countries. With the increase in international travel, there is a higher risk of re-introducing some of these myiasis-causing flies into non-endemic regions of the world that were successful in eradicating these flies [93].
4.1 Human myiasis from travelers visiting SSA
In this study, cases were reported from different continents of the world ranging from Europe (mainly Italy) [81], North America (mainly USA) [13,62,79], Asia [20,52,61], North Africa [36–38] to Australia [49] however, North Africa was not included as part of SSA. More than half of the reported cases in this study came from Europe (mainly Italy) [28,40]. This could be because more European travelers visited SSA, also clinicians in these countries report more human myiasis cases due to the availability of entomologist compared to other regions of the world, especially in SSA. While in SSA, only a few countries like Nigeria, Ghana, and South Africa seem to have entomologists [71,74,91].
4.2 Reported myiasis causing-species in SSA
Cordylobia anthropophaga was the most common myiasis-causing fly species in SSA mainly in West Africa and this information is consistent with previously published literature [52] and the geo-climatic condition in SSA is a suitable breeding ground for this fly (Cordylobia anthropophaga). Cordylobia rodhaini was most prevalent in East Africa than other parts of SSA [42] however, this species have also been recorded from other parts of SSA which suggest that this fly can survive in different region of the world. The results show that L. cuprina was most prevalent in Southern Africa. This could be because most of the published literature is from South Africa, and or because the cold weather in some parts of Southern Africa (especially South Africa) is a favourable condition for the breeding of this particular fly species [91]. Although the human botfly (D. hominis) is not endemic in SSA, there were reported cases of D. hominis infestation with no travel history to an endemic region (e.g., Latin America) [38,71,72,78]. Although, there is no evidence for this proposition, this phenomenon could be due to climatic changes in SSA, studies on the temperature tolerance, epidemiology, and occurrence pattern of the botfly (D. hominis) in SSA should be explored to establish the cause for this phenomenon. To enable proper dipteran fly larvae studies, there is a need to extract, identify, and preserve these larvae. Larvae are usually identified morphologically, but sometimes molecular identification is used. Preserving the larvae is very important, especially for molecular identification. Improper preservation can result in DNA degradation that can affect molecular analysis. For morphological identification, larvae are usually killed in warm water of >80°C for 30 seconds to avoid larval decay. Larvae could also be immersed in normal saline or fix in 10% formaldehyde for morphological identification [8,20]. For molecular identification, larvae cannot be killed in hot water. Instead, larvae could be stored in 70%-95% ethanol for a short period or samples can be frozen to -20°C or -80°C for long-term preservation [9,23,44].
4.3 Reported myiasis type in SSA
The most common clinical manifestation of myiasis in humans is cutaneous myiasis and this is consistent with the results of this study [11]. As the larvae of Cordylobia species are laid in wet environments, they can invade exposed parts of the body (limbs, buttocks, genitals) and subsequently more of these areas would be infected [42]. Several fly species have been found to cause cutaneous myiasis in our study. Different parts of the body (ocular, genitals, nasopharyngeal) could be infested by different fly species resulting in different clinical presentations.
4.4 Common identification and treatment (removal) for human myiasis
Human myiasis is usually unpleasant to both patients and health workers, and it is not generally fatal. However, the disease could be serious when it involves delicate parts of the body especially the scalp of young children [6]. Treatment normally involves the extraction of larvae and sometimes the use of antibiotics which has been evident in our study. There are three main larvae removal methods (manual, mechanical, and surgical extraction) described in previous studies and all these extraction methods have been encountered in our study. Manual extraction is usually done with a gentle press on the furuncle with hands (fingers) and paraffin or ointments are usually applied on the central punctum to suffocate the larvae. Dehecq and colleauges [34] manually extracted two larvae from a 9-month-old boy and the lesions healed shortly after extraction [23,66]. There was 45.3% of the reviewed literature which used manual extraction to successfully remove larvae [9,17,30,33,36,37]. None of these cases reported larval fragmentation during the extraction process. In cases involving cutaneous tissues, larvae could be mechanically removed by using specialized devices to aid in larvae extraction. Only 12% of cases in this research used mechanical extraction. Pezzi M and colleauges [8] mechanically extracted 15 larvae from a 45-year-old man, however, 5 of the larvae were damaged. The patient was given a treatment of doxycycline for a week and recovered later [22,66,73]. Of the reported cases, 28% did not specify the extraction method used. Human myiasis can be very severe especially when subcutaneous tissues of the eyes, nose, ears, genitals, and scalp regions of the body are infested [94]. In such extreme cases involving delicate parts of the body, a surgical incision under local anaesthesia (lidocaine) is used to remove larvae. Georgalas I and colleauges [85] used surgical incision to remove a larva from the sub retinal space from a 27-year-old female. Two months after removal, the patient was asymptomatic with visual acuity (VA) of 6/6. Out of the reported cases, 14.6% used surgical extraction to successfully remove larvae and all patients recovered well after incision [35,46,70,74,83].
When larvae are very difficult to detect in subcutaneous tissues, ultrasonography (USG) can be used to detect larvae by scanning the infested area. In such cases, surgical extraction could be used to remove larvae because when other extraction methods are used [83], larvae fragmentation can occur and it could lead to secondary infection or tetanus. Punch biopsy is also an uncommon but very effective method used for larvae detection in subcutaneous lesions [33,64]. Fundoscopy can be used to detect larvae especially with ocular myiasis, and larvae can also be removed through surgical incision [83,85]. Ultrasonography (USG), Punch biopsy, and Fundoscopy were all used in our study to detect larvae in subcutaneous tissues and procedures were successful. These procedures are recorded for use in patients with subcutaneous infestation.
There is no published literature suggesting that sex is a predisposing factor to human myiasis infestation. However, our results have shown that there were more females infected with the disease than males. This could be because more females reported to the hospital when they are sick. Observational and cross-sectional studies should be conducted to ascertain this proposition.
5. Limitations
Some reported cases (published papers) of human myiasis from SSA could not be accessed due to lack of institutional access. Therefore, we conclude that the prevalence of human myiasis in SSA could be slightly under-reported.
6. Conclusion
Human myiasis is a parasitological condition, which is under-reported and neglected in SSA. Therefore, the actual prevalence and epidemiology of this parasitic infestation is not enough to be estimated. Cases of D. hominis infestation in SSA with no travel history to Latin America have been reported, therefore, research to study the underlying reasons why this species is resurfacing in SSA should be explored. The molecular identification method should be extensively utilized to identify myiasis-causing flies for its importance in determining myiasis species diversity and epidemic studies. There should be research done to explore dipteran flies’ endemicity in SSA; and the relationship between their prevalence and climatic conditions in the sub-region. Both travelers and natives of SSA (tourists, business people, etc.) should be notified, and provided with adequate information about the prevention of human myiasis at entry points and at a community level in endemic regions. There is also a need to raise awareness for inhabitants, clinicians, and travelers to these regions about some of the symptoms and predisposing factors for human myiasis.
Supporting information
Sheet A-Number and Percentage of human myiasis-causing fly species. Sheet B-Number and percentage of the Sex of infected patients. Sheet C-Number and percentage of human myiasis types. Sheet D- Summary of selected studies.
(XLSX)
The items were collapsed into 8 quality-appraisal criteria (Q1-Were patient’s demographic characteristics clearly described? Q2-Was the patient’s history clearly described and presented as a timeline? Q3-Was the current clinical condition of the patient on presentation clearly described? Q4-Were diagnostic tests or assessment methods and the results clearly described? Q5-Was the intervention(s) or treatment procedure(s) clearly described? Q6-Was the post-intervention clinical condition clearly described? Q7-Were adverse events (harms) or unanticipated events identified and described? Q8-Does the case report provide takeaway lessons?). JBI-MAStARI was used to assess risk of bias. Articles that scored between 1 and 2 were classified as low methodological quality, articles with scores between 3 and 4 were classified as moderate quality, and those with scores ≥ 5 were classified as high quality. N: no, NA: not applicable, U: unclear, Y: yes.
(DOCX)
(DOCX)
Acknowledgments
Mr Paul A Correa, Assistant Lecturer at the University of The Gambia (UTG) and PhD Candidate in the department of Biosciences at Comsats University Islamabad (CUI), Pakistan, assistance with academic writing organizers like Mendeley and endnote 20.
Data Availability
All relevant data are in the manuscript and its Supporting information files.
Funding Statement
This work is funded by The National Natural Science Foundation China (81901923; 32370554 to FM) and the Natural Science Foundation of Hunan province (2022JJ30693 to FM). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Song SM, Kim SW, Goo YK, Hong Y, Ock M, Cha HJ, et al. A Case of Furuncular Myiasis Due to Cordylobia anthropophaga in a Korean Traveler Returning from Uganda. Korean J Parasitol. 2017;55(3):327–31. doi: 10.3347/kjp.2017.55.3.327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Devi R, Rao N, Sivakumar T, Kalpana G, Jacob E, Menaka K. Case Report of Maggot Infestation on Diabetic Foot Ulcer. Journal of Pharmaceutical Research International. 2021:194–7. [Google Scholar]
- 3.Hope F. On insect and their larvae occacionally found in human body. Trans R Ent Soc. 1840;2:256–71. [Google Scholar]
- 4.Hall M. Screwworm flies as agents of wound myiasis. World animal review. 1991;1991:8–17. [Google Scholar]
- 5.Ogbalu OK, Achufusi TGO, Orlu EE. Epidemiology of human furuncular myiasis of Cordylobia anthropophaga (Grunberg) in Nigeria. International Journal of Dermatology. 2013;52(3):331–6. doi: 10.1111/j.1365-4632.2012.05641.x [DOI] [PubMed] [Google Scholar]
- 6.Rosen I, Neuberger D. Myiasis Dermatobia hominis, Linn: report of a case and review of the literature. Cutis. 1977;19(1):63–6. [PubMed] [Google Scholar]
- 7.Solomon M, Lachish T, Schwartz E. Cutaneous myiasis. Current infectious disease reports. 2016;18(9):1–7. doi: 10.1007/s11908-016-0537-6 [DOI] [PubMed] [Google Scholar]
- 8.Pezzi M, Cultrera R, Chicca M, Leis M. Furuncular Myiasis Caused by Cordylobia rodhaini (Diptera: Calliphoridae): A Case Report and a Literature Review. J Med Entomol. 2015;52(2):151–5. doi: 10.1093/jme/tju027 [DOI] [PubMed] [Google Scholar]
- 9.Bernhardt V, Finkelmeier F, Tal A, Bojunga J, Derwich W, Meier S, et al. Multispecies blow fly myiasis combined with hypothermia in a man assumed to be dead. Parasitol Res. 2018;117(2):579–83. doi: 10.1007/s00436-017-5691-8 [DOI] [PubMed] [Google Scholar]
- 10.Gashout A, Amro A, Hamarsheh O, Al-Dwibe H. Urogenital Myiasis Caused by Psychoda albipennis in a Female Child in Libya. Turkiye Parazitol Derg. 2019;43(3):152–4. doi: 10.4274/tpd.galenos.2019.6135 [DOI] [PubMed] [Google Scholar]
- 11.Onyeama C, Njai P. Cutaneous myiasis (Tumbu fly larvae): A case report. Nigerian Journal of Paediatrics. 2005;32(1):26–7. [Google Scholar]
- 12.Theppote A, Laborde Y, Knoepp L, Thomas S, Nnedu ON. Cutaneous Myiasis in Rural Haiti. Ochsner Journal. 2020;20(3):331–3. doi: 10.31486/toj.19.0073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Veraldi S, Serini SM, Süss L. Three cases of cutaneous myiasis caused by Cordylobia rodhaini. J Infect Dev Ctries. 2014;8(2):249–51. doi: 10.3855/jidc.3825 [DOI] [PubMed] [Google Scholar]
- 14.Andreatta E, Bonavina L. Wound myiasis in Western Europe: prevalence and risk factors in a changing climate scenario. European Surgery. 2022;54(6):289–94. [Google Scholar]
- 15.Kouam MK, Meutchieye F, Miegoue E, Nguafack TT, Tchoumboue J, Teguia A. Prevalence and husbandry-related risk factors of myiasis in domestic cavies in the western highlands of Cameroon. Epidemiol Infect. 2017;145(2):339–46. doi: 10.1017/S0950268816002466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McGraw TA, Turiansky GW. Cutaneous myiasis. Journal of the American Academy of Dermatology. 2008;58(6):907–26. doi: 10.1016/j.jaad.2008.03.014 [DOI] [PubMed] [Google Scholar]
- 17.Oliva E, Bargiggia G, Quinzan G, Lanza P, Farina C. Furuncular myiasis in Italian traveler returning from Kenya. J Infect Dev Ctries. 2020;14(1):114–6. doi: 10.3855/jidc.11560 [DOI] [PubMed] [Google Scholar]
- 18.Obanda V, Ndambiri EM, Kingori E, Gakuya F, Lwande OW, Alasaad S. Traumatic myiasis in free-ranging eland, reported from Kenya. Parasit Vectors. 2013;6:89. doi: 10.1186/1756-3305-6-89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Musaya J, Mponda K. Case Report: Furuncular Myiasis in Malawi. Wellcome Open Research. 2020;5(41):41. [Google Scholar]
- 20.Ruan W, Feng Y, Zhang L, Sun J, Yao L. Health problems associated with international travel: a case of cutaneous myiasis in China due to Cordylobia anthropophaga imported from Uganda. Biosci Trends. 2014;8(6):346–9. doi: 10.5582/bst.2014.01132 [DOI] [PubMed] [Google Scholar]
- 21.Toberer F, Hanner S, Haus G, Haenssle HA. Furuncular Myiasis of the Lower Leg. Acta Dermatovenerol Croat. 2019;27(3):190–1. [PubMed] [Google Scholar]
- 22.Wangia M, Glenn C, Mitchell C, Fisher S. Florid Cordylobia anthropophaga furuncular myiasis from travel in Nigeria. J Dermatol. 2012;39(12):1099–100. doi: 10.1111/j.1346-8138.2012.01648.x [DOI] [PubMed] [Google Scholar]
- 23.Ko JY, Lee IY, Park BJ, Shin JM, Ryu JS. A Case of Cutaneous Myiasis Caused by Cordylobia anthropophaga Larvae in a Korean Traveler Returning from Central Africa. Korean J Parasitol. 2018;56(2):199–203. doi: 10.3347/kjp.2018.56.2.199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yusuf MA, Pritt BS, McMichael JR. Cutaneous myiasis in an elderly woman in Somaliland. International Journal of Women’s Dermatology. 2019;5(3):187–9. doi: 10.1016/j.ijwd.2019.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mayabi L, Badawy M, Abdallah A. Cordylobia Anthropophaga: Furuncular Myiasis in a Family of 3. Annals of African Surgery. 2014;11(2). [Google Scholar]
- 26.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Bmj. 2021;372:n71. doi: 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pathania V, Kashif AW, Aggarwal RN. Cutaneous myiasis: Think beyond furunculosis. Medical Journal Armed Forces India. 2018;74(3):268–72. doi: 10.1016/j.mjafi.2017.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blaizot R, Vanhecke C, Le Gall P, Duvignaud A, Receveur MC, Malvy D. Furuncular myiasis for the Western dermatologist: treatment in outpatient consultation. Int J Dermatol. 2018;57(2):227–30. doi: 10.1111/ijd.13815 [DOI] [PubMed] [Google Scholar]
- 29.Schubert L, Tobudic S, Sillaber C, Winkler S. The swollen lip: unusual presentation of furuncular myiasis in a returning traveller. J Travel Med. 2022;29(5). [DOI] [PubMed] [Google Scholar]
- 30.Suárez JA, Ying A, Orillac LA, Cedeño I, Sosa N. First case of Furuncular Myiasis due to Cordylobia anthropophaga in a Latin American resident returning from Central African Republic. Braz J Infect Dis. 2018;22(1):70–3. doi: 10.1016/j.bjid.2017.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Naotunna TdS, Ismail M, Ihalamulla R. The second case of cutaneous myiasis caused by Cordylobia anthropophaga (Tumbu fly) in Sri Lanka. 2000.
- 32.Hakeem MJML Bhattacharyya DN. Exotic human myiasis. Travel Medicine and Infectious Disease. 2009;7(4):198–202. doi: 10.1016/j.tmaid.2009.05.007 [DOI] [PubMed] [Google Scholar]
- 33.Koźmińska-Kubarska A. Cordylobia anthropophaga infestation. Int J Dermatol. 1981;20(7):495–6. doi: 10.1111/j.1365-4362.1981.tb04912.x [DOI] [PubMed] [Google Scholar]
- 34.Dehecq E, Nzungu PN, Cailliez JC, Guevart E, Delhaes L, Dei-Cas E, et al. Cordylobia anthropophaga (Diptera: Calliphoridae) outside Africa: a case of furuncular myiasis in a child returning from Congo. J Med Entomol. 2005;42(2):187–92. doi: 10.1093/jmedent/42.2.187 [DOI] [PubMed] [Google Scholar]
- 35.Pica R, Castellano C, Pignata D, Ipri D. Human cutaneous myiasis: a case report. La Clinica Terapeutica. 2008;159(6):431–3. [PubMed] [Google Scholar]
- 36.Ajili F, Abid R, Bousseta N, Mrabet A, Karoui G, Louzir B, et al. [Antibiotic resistant furuncles: think myiasis]. Pan Afr Med J. 2013;15:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vanhecke C, Nguimfack RN, Lemarchand J, Reichart V, Le Gall P. [Facial edema caused by multifocal myiasis of Cordylobia rodhaini in Yaounde—Cameroon]. Presse Med. 2015;44(5):564–6. [DOI] [PubMed] [Google Scholar]
- 38.Frikh R, Hjira N, Frikh M, Baba N, Ghfir M, Lmimouni B, et al. Furuncular myiasis: unusual case of African Dermatobia hominis. Dermatol Online J. 2009;15(9):11. [PubMed] [Google Scholar]
- 39.Rotte M, Fields M. That’s Not An Abscess! Furuncular myiasis. Ann Emerg Med. 2013;62(1):98, 103. doi: 10.1016/j.annemergmed.2012.10.035 [DOI] [PubMed] [Google Scholar]
- 40.Jelinek T, Nothdurft HD, Rieder N, Loscher T. Cutaneous myiasis: review of 13 cases in travelers returning from tropical countries. International journal of dermatology. 1995;34(9):624–6. doi: 10.1111/j.1365-4362.1995.tb01088.x [DOI] [PubMed] [Google Scholar]
- 41.Hasegawa M, Harada T, Kojima Y, Nakamura A, Yamada Y, Kadosaka T, et al. An imported case of furuncular myiasis due to Cordylobia anthropophaga which emerged in Japan. British Journal of Dermatology. 2000;143(4):912–4. doi: 10.1046/j.1365-2133.2000.03809.x [DOI] [PubMed] [Google Scholar]
- 42.Musa HA, Allah EW. Cutaneous myiasis caused by Cordylobia Anthropophaga: description of a case from Gazira State–Sudan. Sudanese J Pub Health. 2008;3(2):91–3. [Google Scholar]
- 43.Dires A, Kebede A, Gedamu S, Dires T. Case of multiple furuncular myiasis in Northeast Ethiopia. Clin Case Rep. 2022;10(7):e6015. doi: 10.1002/ccr3.6015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tolera TB. Human cutaneous myiasis under-reported in Dilla, Ethiopia. Journal of Clinical & Medical Case Reports. 2017;3(23):1–5. [Google Scholar]
- 45.Yasukawa K, Dass K. Myiasis due to Cordylobia anthropophaga. Am J Trop Med Hyg. 2020;102(2):251. doi: 10.4269/ajtmh.19-0579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sivelli P, Vinciguerra R, Tondini L, Cavalli E, Galli A, Chelazzi P, et al. Eyelid myiasis caused by Cordylobia anthropophaga. Ocular Immunology and Inflammation. 2015;23(3):259–60. doi: 10.3109/09273948.2014.893366 [DOI] [PubMed] [Google Scholar]
- 47.Novati S, Sacchi L, Chichino G, Scaglia M. [Furuncular myiasis caused by Cordylobia anthropophaga: description of a case from Tanzania]. Parassitologia. 1994;36(3):265–7. [PubMed] [Google Scholar]
- 48.Parkhouse D. Cutaneous myiasis due to the Tumbu fly during Operation Keeling. J R Army Med Corps. 2004;150(1):24–6. doi: 10.1136/jramc-150-01-05 [DOI] [PubMed] [Google Scholar]
- 49.Geary MJ, Russell RC, Hudson BJ, Hardy A. Exotic myiasis with Lund’s fly (Cordylobia rodhaini). Medical journal of Australia. 1999;171(11–12):654–5. doi: 10.5694/j.1326-5377.1999.tb123838.x [DOI] [PubMed] [Google Scholar]
- 50.Pampiglione S, Schiavon S, Candiani G, Fioravanti ML. [Clinical and parasitological observations on a case of disseminated furuncular myiasis caused by Cordylobia rodhaini in a man in Ethiopia]. Parassitologia. 1991;33(2–3):159–67. [PubMed] [Google Scholar]
- 51.Strohbücker L, Dissemond J, Körber A. [Inflammatory papules and nodi in a 52-year-old woman after a vacation in Zanzibar]. Hautarzt. 2016;67(8):667–9. [DOI] [PubMed] [Google Scholar]
- 52.Deng Y, Liu F, Chen X, Lu S. The first imported cutaneous myiasis due to Cordylobia anthropophaga in China. Int J Dermatol. 2013;52(1):120–2. doi: 10.1111/j.1365-4632.2010.04823.x [DOI] [PubMed] [Google Scholar]
- 53.Wade N, Shahi F, Mawer D, Brown N. Rare cutaneous myiasis of the face due to Lund’s fly (Cordylobia rodhaini) in a British traveller returning from Uganda. BMJ Case Rep. 2019;12(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hannam P, Khairnar K, Downey J, Powis J, Ralevski F, Pillai DR. Cutaneous myiasis in traveler returning from Ethiopia. Emerg Infect Dis. 2011;17(12):2385–6. doi: 10.3201/eid1712.111062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Roberts LW, Boyce WL, Lyerly WH, Jr. Cordylobia anthropophaga (Diptera: Calliphoridae) myiasis in an infant and dog and a technique for larval rearing. J Med Entomol. 1982;19(3):350–1. doi: 10.1093/jmedent/19.3.350 [DOI] [PubMed] [Google Scholar]
- 56.Palmieri JR, North D, Santo A. Furuncular myiasis of the foot caused by the tumbu fly, Cordylobia anthropophaga: report in a medical student returning from a medical mission trip to Tanzania. Int Med Case Rep J. 2013;6:25–8. doi: 10.2147/IMCRJ.S44862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.James AS, Stevenson J. Cutaneous myiasis due to Tumbu fly. Arch Emerg Med. 1992;9(1):58–61. doi: 10.1136/emj.9.1.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Grassi V, Butterworth JW, Latiffi L. Cordylobia rodhaini infestation of the breast: Report of a case mimicking a breast abscess. Int J Surg Case Rep. 2016;27:122–4. doi: 10.1016/j.ijscr.2016.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Petersen C, Zachariae C. Acute balanoposthitis caused by infestation with Cordylobia anthropophaga. Acta dermato-venereologica. 1999;79(2). doi: 10.1080/000155599750011525 [DOI] [PubMed] [Google Scholar]
- 60.Ogbalu OK, Achufusi TGO, Adibe C. Incidence of multiple myiases in breasts of rural women and oral infection in infants from the human warble fly larvae in the humid Tropic-Niger Delta. International journal of dermatology. 2006;45(9):1069–70. doi: 10.1111/j.1365-4632.2006.02983.x [DOI] [PubMed] [Google Scholar]
- 61.Sharma P, Pai HS, Pai GS. Furuncular myiasis mimicking pyoderma. 2008. [DOI] [PubMed]
- 62.Schechter E, Lazar J, Nix ME, Mallon WK, Moore CL. Identification of subcutaneous myiasis using bedside emergency physician performed ultrasound. The Journal of emergency medicine. 2011;40(1):e1–e3. doi: 10.1016/j.jemermed.2007.11.095 [DOI] [PubMed] [Google Scholar]
- 63.Tamir J, Haik J, Schwartz E. Myiasis with Lund’s fly (Cordylobia rodhaini) in travelers. Journal of travel medicine. 2003;10(5):293–5. doi: 10.2310/7060.2003.2732 [DOI] [PubMed] [Google Scholar]
- 64.Rimoin L, Jackson J, Yang A, Goh C, Soriano T. Furuncular myiasis in 2 American travelers returning from Senegal. Cutis. 2014;94(6):281–4. [PubMed] [Google Scholar]
- 65.Logar J, Soba B, Parac Z. Cutaneous myiasis caused by Cordylobia anthropophaga. Wien Klin Wochenschr. 2006;118(5–6):180–2. doi: 10.1007/s00508-006-0535-z [DOI] [PubMed] [Google Scholar]
- 66.How EH, Yap D, Mbakada N. An exotic abscess within the United Kingdom from The Gambia: a case report. J Med Case Rep. 2017;11(1):310. doi: 10.1186/s13256-017-1472-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lodi A, Bruscagin C, Gianni C, Mancini LL, Crosti C. Myiasis due to Cordylobia anthropophaga (Tumbu-fly). Int J Dermatol. 1994;33(2):127–8. doi: 10.1111/j.1365-4362.1994.tb01542.x [DOI] [PubMed] [Google Scholar]
- 68.Bardach H, Aspöck H. [Furunculoid myiasis due to Cordylobia anthropophaga in a traveler returning from Africa and review of the literature]. Z Hautkr. 1981;56(4):216–20. [PubMed] [Google Scholar]
- 69.Kovaleva A, Climent PC, Bécares CV, Martín Azaña MJ, Irishina N, Goy EI. Urogenital myiasis by Cordylobia anthropophaga. J Pediatr Adolesc Gynecol. 2013;26(6):e123–5. doi: 10.1016/j.jpag.2013.04.008 [DOI] [PubMed] [Google Scholar]
- 70.Fusco FM, Nardiello S, Brancaccio G, Rossiello R, Gaeta GB. [Cutaneous myiasis from Cordylobia anthropophaga in a traveller returning from Senegal: a case study]. Infez Med. 2005;13(2):109–11. [PubMed] [Google Scholar]
- 71.Nwosu PU, Dakul DA. Report of a case of cutaneous (furuncular) and gastrointestinal myiasis (dermatobia hominis) in a Nigerian child. West Afr J Med. 2013;32(2):149–52. [PubMed] [Google Scholar]
- 72.Rodríguez-Cerdeira C, Gregorio MC, Guzman RA. Dermatobia Hominis Infestation Misdiagnosed as Abscesses in a Traveler to Spain. Acta Dermatovenerol Croat. 2018;26(3):267–9. [PubMed] [Google Scholar]
- 73.Devambez H, Richeux M, Guericolas M, Choquet C, Casalino E, Ghazali AD. Eyelid inflammation: An uncommon cause in occidental countries. Am J Emerg Med. 2017;35(11):1789.e3–.e5. doi: 10.1016/j.ajem.2017.08.021 [DOI] [PubMed] [Google Scholar]
- 74.Choontanom R, Thanos S, Busse H, Stupp T. A souvenir from Ghana. J Pediatr. 2008;153(2):297. doi: 10.1016/j.jpeds.2008.02.036 [DOI] [PubMed] [Google Scholar]
- 75.Mohammed N, Smith KG. Letter: Nasopharyngeal myiasis in man caused by larve of Clogmia (= Telmetoscopus) albipunctatus Williston (Psychodidae, Dipt.). Trans R Soc Trop Med Hyg. 1976;70(1):91. doi: 10.1016/0035-9203(76)90022-5 [DOI] [PubMed] [Google Scholar]
- 76.Schouten W, Kager P. Diagnostic image (109). A man with furuncles. Cutaneous myiasis. Nederlands tijdschrift voor geneeskunde. 2002;146(41):1937. [PubMed] [Google Scholar]
- 77.Ogunniyi IO. Furuncular swelling caused by larva of Cordylobia anthropophaga in Kaduna, Nigeria. Trans R Soc Trop Med Hyg. 1981;75(5):752. doi: 10.1016/0035-9203(81)90171-1 [DOI] [PubMed] [Google Scholar]
- 78.Messahel A, Sen P, Wilson A, Patel M. An unusual case of myiasis. Journal of Infection and Public Health. 2010;3(1):43–5. doi: 10.1016/j.jiph.2009.08.001 [DOI] [PubMed] [Google Scholar]
- 79.Malek AE, Ostrosky-Zeichner L. Furuncular myiasis in a traveller to West Africa. J Travel Med. 2021;28(6). [DOI] [PubMed] [Google Scholar]
- 80.Adisa CA, Mbanaso A. ’Furuncular myiasis of the breast caused by the larvae of the Tumbu fly (Cordylobia anthropophaga)’. BMC Surg. 2004;4:5. doi: 10.1186/1471-2482-4-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Veraldi S, Brusasco A, Süss L. Cutaneous myiasis caused by larvae of Cordylobia anthropophaga (Blanchard). Int J Dermatol. 1993;32(3):184–7. doi: 10.1111/j.1365-4362.1993.tb02789.x [DOI] [PubMed] [Google Scholar]
- 82.Kuria S, Kingu H, Vasaikar S, Mkhize J, Iisa J, Dhaffala A. New fly species causing human myiasis identified in Eastern Cape, South Africa. SAMJ: South African Medical Journal. 2010;100(9):580–1. doi: 10.7196/samj.4271 [DOI] [PubMed] [Google Scholar]
- 83.Parikh V, Biswas J, Vaijayanthi K, Das D, Raval V. Bilateral ocular myiasis interna caused by botfly (Oestrus ovis): a case report. Ocular Immunology and Inflammation. 2011;19(6):444–7. doi: 10.3109/09273948.2011.622455 [DOI] [PubMed] [Google Scholar]
- 84.Van Niekerk G, Henning M, Coetzee M. Outbreak of myiasis. South African Medical Journal. 2007;97(2):112–4. [PubMed] [Google Scholar]
- 85.Georgalas I, Ladas I, Maselos S, Lymperopoulos K, Markomichelakis N. Intraocular safari: ophthalmomyiasis interna. Clinical & experimental ophthalmology. 2011;39(1):84–5. doi: 10.1111/j.1442-9071.2010.02407.x [DOI] [PubMed] [Google Scholar]
- 86.Lee E, Robinson F. Furuncular myiasis of the face caused by larva of the Tumbu fly (Cordylobia anthropophaga). Eye. 2007;21(2):268–9. doi: 10.1038/sj.eye.6702508 [DOI] [PubMed] [Google Scholar]
- 87.Kingu HJ, Kuria SK, Villet MH, Mkhize JN, Dhaffala A, Iisa JM. Cutaneous myiasis: is Lucilia cuprina safe and acceptable for maggot debridement therapy? 2012.
- 88.Ng SOC, Yates M. Cutaneous myiasis in a traveller returning from Africa. Australasian journal of dermatology. 1997;38(1):38–9. doi: 10.1111/j.1440-0960.1997.tb01098.x [DOI] [PubMed] [Google Scholar]
- 89.Weightman NC, Mitra S, Kipling PT. Clinical microbiological case: Itchy furunculosis on return from South Africa. Clin Microbiol Infect. 2003;9(12):1249, 67–8. doi: 10.1111/j.1469-0691.2003.00800.x [DOI] [PubMed] [Google Scholar]
- 90.Lowe P, Naseem S, Bailey C. Cordylobia anthropophaga: a rare surgical emergency in the UK. BMJ Case Rep. 2013;2013. doi: 10.1136/bcr-2013-008659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kuria SK, Kingu HJ, Villet MH, Dhaffala A. Human myiasis in rural South Africa is under-reported. S Afr Med J. 2015;105(2):129–33. doi: 10.7196/samj.8118 [DOI] [PubMed] [Google Scholar]
- 92.Moola S, Munn Z, Tufanaru C, Aromataris E, Sears K, Sfetcu R, et al. Chapter 7: Systematic reviews of etiology and risk. Joanna briggs institute reviewer’s manual The Joanna Briggs Institute. 2017;5. [Google Scholar]
- 93.Hall MJ, Wall RL, Stevens JR. Traumatic Myiasis: A Neglected Disease in a Changing World. Annu Rev Entomol. 2016;61:159–76. doi: 10.1146/annurev-ento-010715-023655 [DOI] [PubMed] [Google Scholar]
- 94.Zhou X, Kambalame DM, Zhou S, Guo X, Xia D, Yang Y, et al. Human Chrysomya bezziana myiasis: A systematic review. PLoS Negl Trop Dis. 2019;13(10):e0007391. doi: 10.1371/journal.pntd.0007391 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sheet A-Number and Percentage of human myiasis-causing fly species. Sheet B-Number and percentage of the Sex of infected patients. Sheet C-Number and percentage of human myiasis types. Sheet D- Summary of selected studies.
(XLSX)
The items were collapsed into 8 quality-appraisal criteria (Q1-Were patient’s demographic characteristics clearly described? Q2-Was the patient’s history clearly described and presented as a timeline? Q3-Was the current clinical condition of the patient on presentation clearly described? Q4-Were diagnostic tests or assessment methods and the results clearly described? Q5-Was the intervention(s) or treatment procedure(s) clearly described? Q6-Was the post-intervention clinical condition clearly described? Q7-Were adverse events (harms) or unanticipated events identified and described? Q8-Does the case report provide takeaway lessons?). JBI-MAStARI was used to assess risk of bias. Articles that scored between 1 and 2 were classified as low methodological quality, articles with scores between 3 and 4 were classified as moderate quality, and those with scores ≥ 5 were classified as high quality. N: no, NA: not applicable, U: unclear, Y: yes.
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are in the manuscript and its Supporting information files.