Abstract
The Nef protein of human immunodeficiency virus type 1 (HIV-1) promotes virion infectivity through mechanisms that are yet ill defined. Some Nef is incorporated into particles, where it is cleaved by the viral protease between amino acids 57 and 58. The functional significance of this event, which liberates the C-terminal core domain of the protein from its membrane-associated N terminus, is unknown. To address this question, we examined the modalities of Nef virion association and processing. We found that although significant levels of Nef were detected in HIV-1 virions partly in a cleaved form, cell-specific variations existed in the efficiency of Nef proteolytic processing. The virion association of Nef was strongly enhanced by myristoylation but did not require other HIV-1-specific proteins, since Nef was efficiently incorporated into and cleaved inside murine leukemia virus particles. Substituting alanine for tryptophan57 decreased the efficiency of Nef processing, while mutating leucine58 had little effect. In contrast, replacing both of these residues simultaneously almost completely prevented this process. However, when the resulting mutants were compared with a wild-type control in viral infectivity assays, no correlation was found between the levels of cleavage and the ability to stimulate virion infectivity. Furthermore, simian immunodeficiency virus Nef, which lacks the sequence recognized by the protease and as a consequence is not cleaved despite its incorporation into virions, could stimulate the infectivity of a nef-defective HIV-1 variant as efficiently as HIV-1 Nef. On these bases, we conclude that the proteolytic processing of Nef is not required for the ability of this protein to enhance virion infectivity.
In addition to the gag, pol, and env genes found in all retroviruses, the genome of human immunodeficiency virus type 1 (HIV-1) contains several additional reading frames which encode critical virulence factors (34, 46, 47). Among these, the nef gene is found only in primate lentiviruses and codes for a short myristoylated cytoplasmic protein that associates with membranes and the cytoskeleton (23, 38). Initial experiments suggested that Nef reduces the rate of viral replication by inhibiting transcription from the proviral long terminal repeat (LTR), hence, its acronym for “negative factor” (1, 28, 36). However, these early results were not confirmed, and it was instead found that Nef is essential for high levels of viral replication in vivo and for AIDS pathogenesis (14, 25, 26). In vitro, at least three functions of Nef have been described: (i) the downregulation of CD4 and to a lesser degree of major histocompatibility complex class I (3, 6, 18, 22, 31, 40, 42), (ii) the alteration of T-cell activation pathways (8, 29, 37, 44), and (iii) the enhancement of viral infectivity (5, 12, 32, 41, 45).
First observed in activated peripheral blood lymphocytes (PBL) (15), Nef-induced stimulation of HIV-1 infectivity is particularly pronounced when resting T cells are first infected and subsequently activated (32, 45). However, the effect of Nef is also manifested in single-round infectivity assays. Nef acts when supplied in producer cells but not in target cells, at least partly irrespective of the presence of CD4, and in a dose-dependent manner (5, 12, 13, 32, 33). The consequences of Nef action are manifested immediately after viral entry, that is, before integration and viral gene expression. Functionally, they translate into an increased efficiency of proviral DNA synthesis, although the enzymatic activity of reverse transcriptase per se is not affected (5, 12, 41). Most likely, Nef facilitates uncoating or stabilizes the reverse transcription complex.
Nef could exert this effect indirectly, by modifying during viral assembly a protein that is subsequently involved in facilitating the early steps of infection. Alternatively, Nef could act directly as a component of the reverse transcription complex. Consistent with this latter model, recent experiments demonstrated the presence of some Nef in HIV-1 particles (39, 48). It was also noted that a significant proportion of virion-associated Nef molecules is cleaved by the viral protease (39, 48). This phenomenon previously had been observed in vitro (16, 17); the cleavage site was mapped to the peptide bond between tryptophan57 and leucine58 of Nef.
In the present study, we investigated the modalities of Nef virion incorporation, examining in particular whether the proteolytic processing of Nef is important for its ability to stimulate HIV-1 infectivity. Our results suggest that this is not the case.
MATERIALS AND METHODS
DNA constructs.
The pCMXNef, R7, and ΔNXR7 constructs have been described elsewhere (3). The ΔNefR7 plasmid is the previously described ΔNR7 (3). pCMXNefG2A is a derivative of pCMXNef that expresses a Nef myristoylation mutant, and pCMXPL2 is the empty vector. R9 and ΔNefR9 were generated from R7 and ΔNXR7, respectively, by replacing the BssHII-BamHI fragment from R7 and R7ΔNX with the corresponding fragment from HIV-1NL4-3. Plasmids pCMXNefW57A, pCMXNefL58A, and pCMXNefWL58AA were generated by PCR-mediated site-directed mutagenesis of nef in the context of pCMXNef. pCMXNefFLAG (a gift from Vincent Piguet) was derived from pCMXNef by fusing the sequence encoding a FLAG epitope (amino acid sequence: DYKDDDDK) to the 3′ end of nef. pCMXSIVNef has been described elsewhere (5). Construction of R9 Nef mutants was carried out in a series of steps. First, R9Δ3′ was generated by PCR introduction of an XbaI restriction site immediately outside the 3′ LTR of R9. Plasmid BS/XXNefW57A was generated by ligating into pBluescript/KS− opened with XhoI and XbaI the XhoI-DraIII fragment from pCMXNefW57A linked to the appropriate DraIII-XbaI fragment containing the full-length 3′ LTR fragment from R9. Plasmids BS/XXNefL58A and BS/XXNefWL58AA were made in a similar manner. NefW57AR9, NefL58AR9, and NewWL58AAR9 were constructed by replacing the XhoI-XbaI fragment from R9Δ3′ with the corresponding fragments from BS/XXNefW57A, BS/XXNefL58A, and BS/XXNefWL58AA, respectively. The pET-20bNef construct was made by inserting a PCR-generated nef gene from R7, with BamHI and HindIII restriction sites flanking the sequence, into pET-20b (Novagen) opened with the same restriction enzymes. The pCMV-GAGPOL plasmid expresses the gag and pol genes of murine leukemia virus (MLV) from the cytomegalovirus promoter (35). The SV-E-MLV-env plasmid, expressing the ecotropic MLV env gene, was obtained from Ned Landau.
Cell lines, transfection, and electroporation.
H9, CEM, and SupT1 human T-lymphoid cells were grown in RPMI 1640 medium supplemented with 10% fetal calf serum. 293T cells, a gift from Gary Nolan, Stanford University, are derivatives of the 293 human kidney cell line that stably express the simian virus 40 large T antigen. HeLa-derived P4 cells (11) were a gift from F. Clavel. 293T and P4 cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal calf serum.
Transfection of 293T cells was accomplished with the previously described calcium phosphate method (7). The medium was changed after overnight incubation with DNA. Two days following transfection, supernatants containing virus were harvested and the cells were lysed. For cell lysate preparation, 8 × 106 transfected cells were washed twice with phosphate-buffered saline (PBS) and then resuspended in 500 μl of cell lysis buffer (10 mM NaCl, 10 mM Tris-HCl [pH 7.5], 0.5% Nonidet P-40, 100 μg of phenylmethylsulfonyl fluoride per ml, 1 μg of aprotinin per ml, 1 μg of pepstatin A per ml, 2 μg of leupeptin per ml) for 5 min on ice. The lysates were subsequently centrifuged at 14,000 × g in a tabletop microcentrifuge for 5 min at 4°C to remove nuclei, and the supernatants were harvested and stored at −75°C until needed. Total protein content of the lysates was measured with the bicinchoninic acid assay (Pierce).
H9 and SupT1 cells were infected by coculturing with transfected 293T cells. Specifically, 2.5 × 107 H9 or SupT1 cells in 100 ml of DMEM were laid over 4 × 106 293T cells that had been transfected 2 days earlier. RPMI 1640 medium (300 ml) was added to the coculture after 5 h, and the cells were further incubated for 3 days. H9 or SupT1 cells in 100 ml of RPMI 1640 medium were then transferred to another flask. Supernatants were harvested 3 days later.
CEM cells (107) were electroporated (250 mV, 960 μF) with proviral constructs (40 μg) as previously described (7). Virus-containing supernatants were harvested at the peaks of virus production, between 8 and 10 days postelectroporation. Peripheral blood mononuclear cells were isolated from seronegative donors by banding of whole blood on Ficoll-Paque (Pharmacia) and cultured in RPMI 1640 medium supplemented with 10% fetal calf serum at 2 × 105 to 5 × 105 cells/ml. Monocytes were removed from the cultures over a period of 3 days by adherence to plastic, with the cultures being placed into new flasks every 24 h. The resulting PBL were removed and maintained in cultures for an additional 4 days before infection.
Virus preparation and infection.
Virus-containing supernatants were harvested from transfected 293T cells, infected H9 and SupT1 cells, and electroporated CEM cells and filtered through a 0.45-μm-pore-size nitrocellulose membrane. The filtered supernatant was then subjected to ultracentrifugation through a 20% (wt/vol) sucrose cushion (in PBS) at 26,000 rpm in an SW28 rotor (Beckman) for 1.5 h. The resulting virus-containing pellet was resuspended in PBS at 4°C for 2 h. When needed, the virus was further purified over a sucrose gradient. The sucrose gradient was generated with a gradient maker by mixing a 60% (wt/wt) sucrose solution with a 20% (wt/wt) sucrose solution, both in PBS. The resuspended virus was carefully laid over the 20 to 60% linear sucrose gradient and, after ultracentrifugation at 20,000 rpm in an SW55 rotor (Beckman) for 18 h, fractions of approximately 400 μl each were collected and analyzed for viral content by an HIV-1 p24 capsid (CA) enzyme-linked immunosorbent assay (ELISA) (DuPont).
The P4 infection assay has been described elsewhere (5).
Quantitation of MLV virions was accomplished with a modified exogenous reverse transcription assay originally described by Goff et al. (19). Briefly, 10 μl of concentrated virus was added to 20 μl of assay buffer containing 50 mM Tris-HCl (pH 7.9), 75 mM KCl, 2 mM dithiothreitol, 5 mM MnCl2, 25 μg of poly(A) · oligo(dT12–18), 0.05% Nonidet P-40, and 50 μCi of 3H-TTP per ml and incubated for 2 h at 37°C. The reaction mixtures then were spotted onto 2.3-cm-diameter DE81 paper disks (Whatman). The disks were washed three times for 5 min each time in 2× SSC (1× SSC is 150 mM NaCl plus 15 mM sodium citrate) and two times for 5 min each time in 95% ethanol and air dried, and tritium incorporation was determined by liquid scintillation counting. One-week-old PBL (2 × 107) were infected with 750 ng of virus produced from 293T cells in 10 ml of RPMI 1640 medium for 12 h. Following incubation with the virus, the PBL were washed with and resuspended in 10 ml of medium, and a time-zero sample was taken for the p24 CA ELISA. One day later, the cultures were activated with 3 μg of phytohemagglutinin (Sigma) per ml. At day 3, recombinant human interleukin-2 (IL-2) (Sigma) was added to the cultures at a concentration of 10 U/ml. The cultures were subsequently maintained in RPMI 1640 medium supplemented with 10 U of IL-2 per ml and split 1:2 every other day.
Western blot analysis.
Cell extracts and virion fractions were resolved on sodium dodecyl sulfate–15% polyacrylamide gels. The proteins were then transferred to polyvinylidene fluoride membranes (Micron Separations Inc.) in a buffer containing 25 mM Tris-HCl (pH 8.0), 192 mM glycine, 0.035% sodium dodecyl sulfate, and 20% methanol. The membranes were blocked in 10% milk for 1 h and then incubated with a 1:2,000 dilution of rabbit αNef polyclonal antiserum (4), a 1:10,000 dilution of αp24 CA monoclonal antibody purified from hybridoma 183-H12-5C (a gift from Bruce Chesebro and obtained through the National Institutes of Health AIDS Repository), a 1:1,000 dilution of HIV-1 αp17 MA monoclonal antibody (Advanced Biotechnologies, Inc.), a 1:2,000 dilution of αFLAG monoclonal antibody (Kodak), a 1:500 dilution of goat αRauscher leukemia virus p15 antiserum (obtained from Quality Biotech Inc. through the National Cancer Institute), or a 1:100 dilution of rabbit αSIVNef polyclonal antibody (a gift from Janice Clements, Johns Hopkins University). The membranes were washed in a buffer containing 10 mM Tris-HCl (pH 8.0), 150 mM NaCl, and 0.05% Tween 20. Detection was performed with horseradish peroxidase-conjugated rabbit, mouse, or goat (Dako) immunoglobulins by enhanced chemiluminescence (ECL Western Blotting Kit; Amersham) according to the manufacturer’s instructions.
RESULTS
HIV-1 Nef virion incorporation and cleavage.
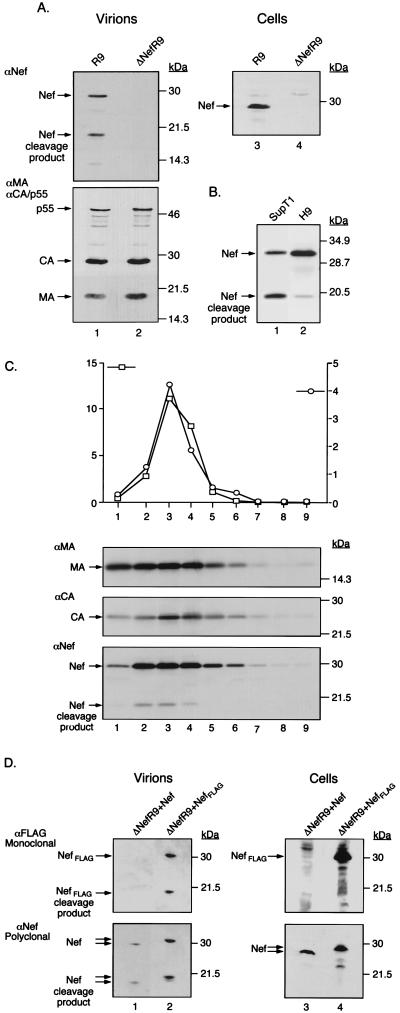
A Western blot analysis of virions produced from 293T cells transfected with either a wild-type or a nef-defective proviral DNA revealed the presence of two protein species that reacted with a Nef-specific antiserum in wild-type but not mutant particles (Fig. 1A). The apparent molecular masses of these proteins, 27 and 19 kDa, were consistent with those of full-length Nef and of its previously described C-terminal cleavage product (16, 17). To examine this phenomenon further, wild-type virions were produced from T-lymphoid cells and analyzed by Western blotting. A side-by-side comparison of H9- and SupT1-produced virions revealed that particles released by H9 cells contained higher levels of full-length Nef, while virions from SupT1 cells contained an almost equal distribution of cleaved and uncleaved Nef products (Fig. 1B).
FIG. 1.
Virion incorporation and cleavage of HIV-1 Nef. (A) Left panel: Cell-free supernatants from 293T cells transiently transfected with R9 and ΔNefR9 constructs were concentrated by ultracentrifugation through a 20% sucrose cushion, normalized for p24 content (1 μg), and analyzed by Western blotting with an αNef polyclonal antiserum (top) and with a mixture of αp17 MA and αp24 CA antibodies, which also recognize the p55 Gag precursor (bottom). Molecular mass markers are shown on the right. Right panel: Cytoplasmic extracts from transfected 293T cells normalized for total protein content (250 μg) were probed with αNef. (B) Wild-type (R9) virus produced from infected SupT1 and H9 cells was examined by Western blotting with αNef antiserum. Lanes: 1, SupT1 cell-produced virus (83.8 ng of p24); 2, H9 cell-produced virus (119.1 ng of p24). (C) H9 cell-produced wild-type virus was fractionated on a linear 20 to 60% (wt/wt) sucrose gradient. Fractions (approximately 400 μl) were evaluated for reverse transcriptase (RT) activity (103 counts per minute per microliter) (squares) by an exogenous RT assay as well as for p24 CA content (103 nanograms per milliliter) (circles) by an ELISA (graph). Each fraction (100 μl) was subjected to Western blot analysis with αMA (upper panel), αCA (middle panel), and αNef (lower panel). Numbers at the bottom correspond to fractions collected from the top to the bottom of the gradient. (D) Western blot analysis of virions from 293T cells cotransfected with ΔNefR9 and either pCMXNef or pCMXNefFLAG. Left panel: Cell-free supernatants were concentrated by ultracentrifugation through a 20% sucrose cushion, normalized for p24 content (1 μg), and probed with an αFLAG monoclonal antibody (top) and with αNef antiserum (bottom). Right panel: Cytoplasmic extracts from transfected 293T cells normalized for total protein content (250 μg) were similarly analyzed with αFLAG (top) and αNef (bottom).
To demonstrate that both full-length Nef and its cleavage product were indeed virion associated and were not just contaminants of the virus concentration process, H9-produced wild-type virus was fractionated on a 20 to 60% sucrose gradient. The presence of viral proteins in the various fractions was assessed by a combination of immunological and enzymatic methods (Fig. 1C). In fractions that also contained peaks of p24 antigen and reverse transcriptase activities (at an approximate density of 1.18 g/ml), both full-length Nef and its 19-kDa cleavage product were detected. Of note, we repeatedly failed to detect significant amounts of Nef in the supernatant of cells expressing a budding defective proviral construct or nef alone, ruling out the possibility that the Nef protein observed here is associated with membrane vesicles rather than with virions.
The 19-kDa species corresponded to the C-terminal core domain of Nef, because when the distal end of the viral protein was tagged with an eight-amino-acid-long sequence (FLAG), the sizes of both the full-length and the lower-molecular-weight Nef-reactive proteins were increased (Fig. 1D). Finally, in virions produced from a pro-defective HIV-1 provirus, only full-length Nef was detected (data not shown), consistent with previous data indicating that the viral protease is responsible for cleaving Nef (39, 48).
Effects of mutations around the Nef cleavage site.
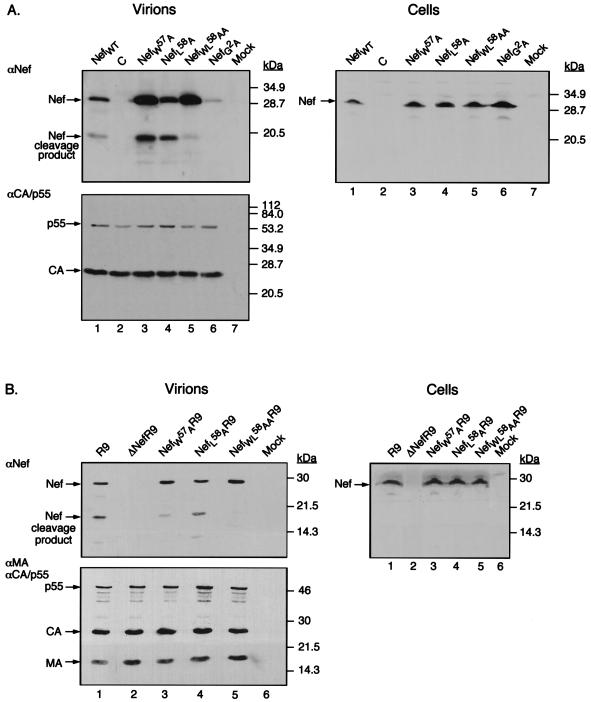
In vitro experiments with recombinant proteins revealed that the HIV-1 protease cleaves Nef between tryptophan57 (W57) and leucine58 (L58) (16, 17). Accordingly, site-directed mutagenesis was used to change these residues to alanine, either individually or together, within the context of a cytomegalovirus-based nef expression vector. 293T cells were cotransfected with the nef-defective ΔNefR9 proviral construct, together with plasmids expressing either wild-type Nef, a NefG2A (glycine at position 2 changed to alanine) myristoylation mutant, or the NefW57A, NefL58A, or NefWL58AA variant. Two days later, cytoplasmic extracts and virions purified from the supernatant were analyzed by Western blotting with antibodies against Nef or p24 CA (Fig. 2A). The G2A mutation almost completely abolished Nef virion incorporation, as previously demonstrated (9). Mutation W57A resulted in a moderate decrease in the ratio between the cleaved and the uncleaved forms of Nef in particles, while the L58A change did not alter cleavage. However, replacing both W57 and L58 with alanine almost completely abrogated the processing of Nef. Interestingly, no 19-kDa Nef product was detected in cell extracts, consistent with the viral protease becoming active only once it is incorporated into virions (24).
FIG. 2.
Nef mutants exhibit various degrees of cleavage. (A) Western blot analysis of virions produced by cotransfection of 293T cells with ΔNefR9 and either pCMXNef (lane 1), empty control vector pCMXPL2 (C) (lane 2), pCMXNefW57A (lane 3), pCMXNefL58A (lane 4), pCMXNefWL58AA (lane 5), pCMXNefG2A (lane 6), or nothing (lane 7). Molecular mass markers are shown on the right. Left panel: Immunoblot analysis of virions (1 μg) with αNef (top) and αp24 CA antibodies, which also recognize the p55 Gag precursor (bottom). Right panel: Transfected cell extracts (250 μg of total protein) probed with αNef antiserum. (B) Western blot analysis, similar to that in panel A, of virions produced from transfection of 293T cells with proviral construct R9 (lane 1), ΔNefR9 (lane 2), NefW57AR9 (lane 3), NefL58AR9 (lane 4), or NefWL58AAR9 (lane 5) or nothing (lane 7). Left panel: Immunodetection with αNef (top) and a mixture of αp17 MA and αp24 CA antibodies, which also recognize the p55 Gag precursor (bottom). Right panel: Cell extracts probed with αNef.
To confirm these results, the three nef mutations were introduced into full-length HIV-1 proviral construct R9, and the resulting viruses were similarly analyzed (Fig. 2B). The patterns observed when Nef was expressed in trans were recapitulated, with the WL58AA mutation having the most dramatic effect on Nef cleavage.
Incorporation of Nef into and cleavage of Nef inside MLV particles.
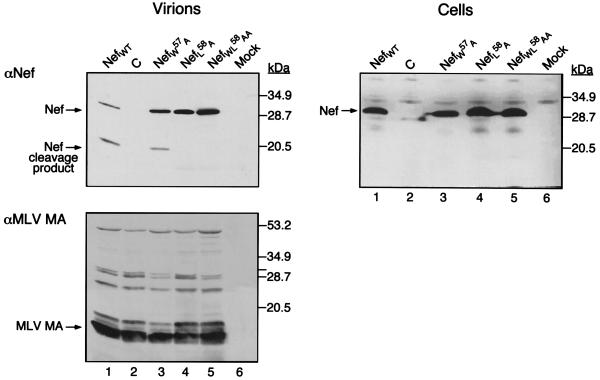
To determine if other HIV-1-specific components are necessary for Nef virion association, we asked whether Nef could be incorporated into MLV, a simple retrovirus which does not encode accessory factors such as Nef. MLV particles were produced by transient transfection of 293T cells expressing wild-type or mutated forms of Nef. Cytoplasmic extracts and purified virions were analyzed by Western blotting with antibodies against Nef or the p15 matrix (MA) protein of MLV (Fig. 3). The results revealed that Nef was efficiently incorporated into MLV particles, where it underwent proteolytic cleavage. The patterns observed with the NefW57A, NefL58A, and NefWL58AA mutants were reminiscent of those observed for HIV-1 virions, with one exception. Whereas the HIV-1 protease efficiently cleaved the NefL58A mutant, the MLV protease did not. As noted with HIV-1, cleavage was observed in viruses but not in cell lysates.
FIG. 3.
HIV-1 Nef incorporation and cleavage in MLV particles. 293T cells were cotransfected with pCMX-GAGPOL, pSV-E-MLV-env, and the pCMXNef constructs encoding the Nef cleavage mutants or the pCMXPL2 control. The resultant virions were concentrated by ultracentrifugation through a 20% sucrose cushion and normalized for reverse transcriptase activity. Markers are shown on the right. Left panels: Immunoblot analysis with αNef (top) and αMLV MA (bottom) of viral particles containing wild-type HIV-1 Nef (lane 1), no Nef (lane 2), NefW57A (lane 3), NefL58A (lane 4), or NefWL58AA (lane 5). Lane 6, pelleted cell supernatant from mock-transfected cells. Right panel: Cell extracts from the same experiment normalized for total protein content (250 μg) and probed with αNef antiserum.
Absence of correlation between Nef cleavage and enhancement of HIV-1 infectivity.
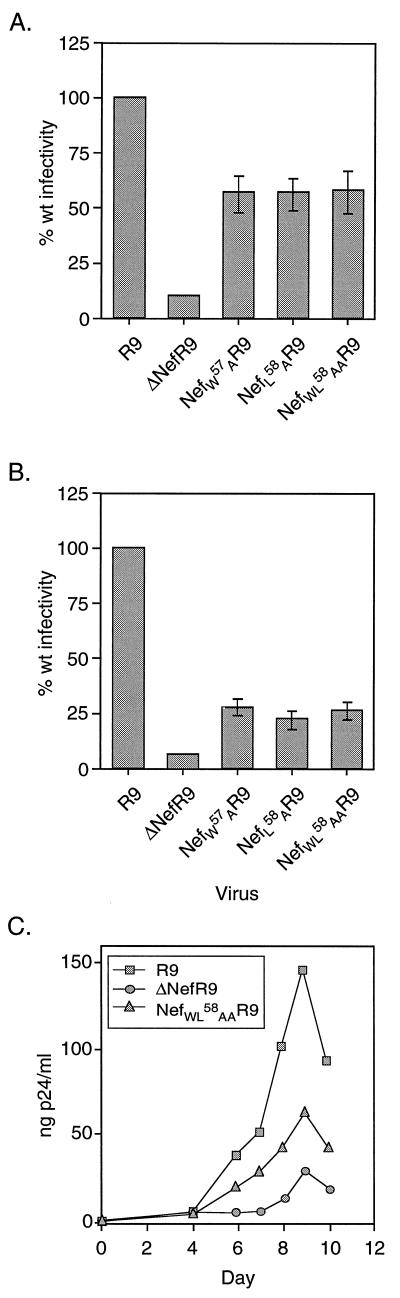
The NefWL58AA variant, which was stably expressed and efficiently incorporated into virions yet resisted cleavage by the viral protease, gave us the opportunity to ask whether the proteolytic processing of Nef is necessary for the stimulation of HIV-1 infectivity. To probe this issue, virions produced from transiently transfected 293T cells and expressing various forms of Nef were subjected to a single-round infectivity assay with P4 cells as targets. P4 cells are CD4-positive HeLa cells which contain a lacZ reporter gene under the control of the HIV-1 LTR. Upon infection, Tat production induces LacZ expression, which can be scored by 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) staining. The wild-type R9 virus was approximately 10 times more infectious than its nef deletion counterpart (ΔNefR9) in this assay (Fig. 4A), in agreement with previous results (5). Despite their clearly distinct levels of sensitivity to proteolytic cleavage, mutants NefW57AR9, NefL58AR9, and NefWL58AAR9 exhibited levels of infectivity that were identical and approximately 60% the wild-type level. When virions were produced from CD4-positive CEM cells, the mutants again displayed similar levels of infectivity. However, in this case, the mutations had a more pronounced influence, reducing viral infectivity to roughly 25% of the wild-type level. Nevertheless, all three mutants were still significantly more active than ΔNefR9 (5%) in this setting (Fig. 4B). Finally, in PBL infected prior to activation, the NefWL58AAR9 mutant exhibited kinetics of growth that were intermediate between those of wild-type and nef deletion viruses (Fig. 4C).
FIG. 4.
Nef cleavage does not correlate with virion infectivity. (A) The viruses produced in Fig. 2B from 293T cells were normalized for p24 content and used to infect P4 indicator cells. The infectivity of the Nef mutants is expressed as a percentage of wild-type (wt) infectivity. In a typical experiment, wild-type virus yielded between 1,000 and 4,000 infectious units/ng of p24. (B) Wild-type (R9), nef deletion (ΔNefR9), and Nef mutant (NefW57AR9, NefL58AR9, and NefWL58AAR9) viruses produced from electroporated CEM T-lymphoid cells were concentrated, normalized for p24 content, and assayed for infectivity as described for panel A. (C) Growth curves for wild-type (R9), nef deletion (ΔNefR9), and Nef cleavage-defective (NefWL58AAR9) viruses from Fig. 2B in PBL. PBL were activated with phytohemagglutinin 48 h after infection and maintained in IL-2. p24 samples were taken from the cultures on the indicated days.
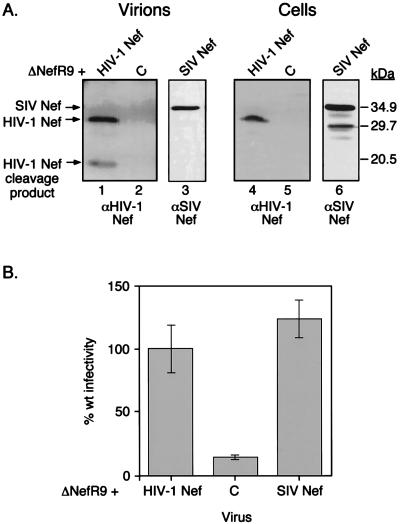
These data suggested that the cleavage of Nef is not essential for its ability to enhance virion infectivity. To confirm this point, we examined the virion incorporation and processing of simian immunodeficiency virus (SIV) Nef. This aspect was of interest because SIV Nef does not contain the sequence recognized by the protease in HIV-1 Nef, specifically, the WL cleavage site. SIV Nef was efficiently incorporated into HIV-1 virions, but in contrast to its HIV-1 counterpart, it did not undergo proteolytic cleavage to any appreciable extent (Fig. 5A). However, SIV Nef stimulated the infectivity of ΔNef HIV-1 virions in P4 cells as efficiently as HIV-1 Nef (Fig. 5B), confirming that these two proteins are functionally interchangeable (5, 43). The presence of a cleaved Nef product is thus not necessary for the stimulation of HIV-1 infectivity.
FIG. 5.
SIV Nef is not cleaved by HIV-1 protease but stimulates HIV-1 infectivity. (A) Western blot analysis of virions (1 μg of p24) produced from 293T cells cotransfected with ΔNefR9 and pCMXNef (lane 1), pCMXPL2 (C) (lane 2), or pCMXSIVNef (lane 3) and of the corresponding cell extracts (250 μg of total protein) (lanes 4 through 6). Lanes 1, 2, 4, and 5 were probed with αHIV-1 Nef antiserum, and lanes 3 and 6 were probed with αSIV Nef antiserum. (B) P4 cell infectivity assay with the viruses produced in panel A. Infectivity is expressed as a percentage of wild-type (wt) activity.
DISCUSSION
In this work, we confirmed previous findings that Nef is incorporated into HIV-1 virions and cleaved by the viral protease (39, 48). Using a semiquantitative analysis previously described (10), we estimated that there are on average 60 to 200 copies of Nef per H9 cell-produced virion (data not shown), within the range defined for the products of the pol gene. Through techniques comparable to ours, Pandori et al. (39) detected approximately 70 molecules of Nef per particle, predominantly in the cleaved form, when analyzing virus released from CEM cells. Welker et al. (48), on the other hand, counted 5 to 10 molecules per MT4 cell-produced particle, with equal amounts of full-length versus cleaved proteins. However, in the latter case, the use of immunoprecipitation could have led to an underestimate if the antibody was only partially effective at capturing Nef.
We also observed that the extent of Nef cleavage varied depending upon the cell type producing the HIV-1 virions. Virions produced from H9 cells had a higher ratio of full-length Nef to cleaved Nef than those produced from SupT1 or 293T cells. The fact that H9 cell-produced particles also had a comparatively higher ratio of full length Gag to cleaved Gag suggests that this differential Nef cleavage might have been due in part to the activity of the protease enzyme.
The specificity of Nef virion incorporation remains questionable, since Nef can also associate with MLV particles (Fig. 3) (9). The dependence of Nef on myristoylation for efficient virion incorporation suggests that the viral protein might be passively engulfed by budding particles owing to its association with the plasma membrane. However, this suggestion does not exclude the possibility that at least part of the Nef effect might be linked to its presence in virions, since nonmyristoylated Nef fails to stimulate viral infectivity (5).
While it is unclear whether Nef promotes HIV-1 replication through direct or indirect mechanisms, our results conclusively demonstrate that proteolytic cleavage of the viral protein is not necessary for this effect. First, three mutations that affect this process to clearly distinct degrees had similarly mild consequences on the infectivity of HIV-1 particles, as measured in a single-round assay. Second, a mutation which completely abrogated the processing of Nef resulted only in partial impairment of its ability to stimulate viral infectivity, either in CD4-positive HeLa cells or in PBL. Finally, the defective phenotype of a nef-deleted HIV-1 strain was rescued as efficiently by SIV Nef as by HIV-1 Nef, even though SIV Nef does not contain the sequence recognized by the viral protease and, as a consequence, does not undergo readily detectable proteolytic processing. This latter result corroborates the positive effect of SIV Nef on the infectivity of SIV virions (43).
Substituting alanine for tryptophan57 and leucine58 almost completely prevented the cleavage of Nef, confirming the previous mapping of the protease target site between these two amino acids (16). The MLV protease cleaved Nef as efficiently as the HIV-1 protease (Fig. 3), even though mutations around the enzyme target site resulted in subtle differences. For instance, while replacing both W57 and L58 of Nef resulted in the abrogation of processing by both proteases, NefL58A was significantly more resistant to cleavage in MLV than in HIV-1 virions. Both proteases have poor consensus recognition sites in which either aliphatic long-chain residues or aromatic amino acids immediately flanking the cleavage site are preferred. The accessibility of this site is probably a major determinant of susceptibility. In that respect, it is notable that the nuclear magnetic resonance structure analysis of HIV-1 Nef reveals that amino acids 57 and 58 of the protein are within an exposed region easily accessible to solvent (20).
W57 and L58 of Nef also appear to participate in the binding of the CD4 cytoplasmic tail (21). Correspondingly, NefWL58AA is defective for CD4 downregulation (30). It is noteworthy that the phenotype of a virus expressing this Nef variant was more pronounced when it was released from CD4-positive CEM cells than when it was produced from CD4-negative 293T cells (compare Fig. 4A and B). One potential explanation for this difference is that the infectivity of HIV-1 virions might be decreased when producer cells express high levels of CD4 on their surface. Nef would then counteract this negative influence by downregulating CD4, playing a role somehow analogous to that fulfilled by neuraminidase in influenza virus. Our preliminary results support this model (27), even though Nef appears to exert a major part of its effect in a CD4-independent manner, as previously described (3, 12, 41).
Nef augments the infectivity of HIV-1 virions coated with the amphotropic MLV envelope (3). In contrast, the Nef mutant phenotype is rescued by mediation of viral entry via the G protein of vesicular stomatitis virus (VSV) (2). This effect is not due to the higher intrinsic activity of VSV G protein-coated particles, because in the presence of limiting amounts of this envelope protein, infectivity decreases to a level similar to that of HIV-1 virions yet remains unaffected by Nef (2). Instead, it suggests that the Nef requirement is restricted to virions penetrating cells via direct fusion at the plasma membrane, the major route of entry for the HIV-1 and amphotropic MLV envelope proteins, whereas it is alleviated when this process occurs through receptor-mediated endocytosis and fusion in the endocytic compartment, the pathway targeted by the VSV G protein. Interestingly, in the latter case an important step is the acidification of the endosomal compartment, which triggers fusion between the viral and cellular membranes and facilitates the uncoating process. Whether Nef functionally replaces this event when uncoating occurs at the plasma membrane remains to be determined.
ACKNOWLEDGMENTS
D.C. and Y.-L.C. contributed equally to this work.
We thank Vincent Piguet, François Clavel, Ned Landau, Gary Nolan, and Bruce Chesebro for gifts of various reagents and Leslie Barden for helping with the artwork. We are particularly grateful to Janice Clements for providing an as-yet-unpublished SIV Nef-specific antiserum.
This study was supported by grant R37 AI34306 from the NIH to D.T.
REFERENCES
- 1.Ahmad N, Venkatesan S. Nef protein of HIV-1 is a transcriptional repressor of HIV-1 LTR. Science. 1988;241:1481–1485. doi: 10.1126/science.3262235. [DOI] [PubMed] [Google Scholar]
- 2.Aiken C. Pseudotyping human immunodeficiency virus type 1 (HIV-1) by the glycoprotein of vesicular stomatitis virus targets HIV-1 entry to an endocytic pathway and suppresses both the requirement for Nef and the sensitivity to cyclosporin A. J Virol. 1997;71:5871–5877. doi: 10.1128/jvi.71.8.5871-5877.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aiken C, Konner J, Landau N R, Lenburg M E, Trono D. Nef induces CD4 endocytosis: requirement for a critical dileucine motif in the membrane-proximal CD4 cytoplasmic domain. Cell. 1994;76:853–864. doi: 10.1016/0092-8674(94)90360-3. [DOI] [PubMed] [Google Scholar]
- 4.Aiken C, Krause L, Chen Y-L, Trono D. Mutational analysis of HIV-1 Nef: identification of two mutants that are temperature-sensitive for CD4 downregulation. Virology. 1996;217:293–300. doi: 10.1006/viro.1996.0116. [DOI] [PubMed] [Google Scholar]
- 5.Aiken C, Trono D. Nef stimulates human immunodeficiency virus type 1 proviral DNA synthesis. J Virol. 1995;69:5048–5056. doi: 10.1128/jvi.69.8.5048-5056.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson S, Shugars D C, Swanstrom R, Garcia J V. Nef from primary isolates of human immunodeficiency virus type 1 suppresses surface CD4 expression in human and mouse T cells. J Virol. 1993;67:4923–4931. doi: 10.1128/jvi.67.8.4923-4931.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ausubel F, Brent R, Kingston R E, Moore D D, Smith J A, Seidman J G, Struhl K. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons, Inc.; 1987. [Google Scholar]
- 8.Baur A, Sawai E T, Dazin P, Fantl W J, Cheng-Mayer C, Peterlin B M. HIV-1 Nef leads to inhibition or activation of T-cells depending on its intracellular location. Immunity. 1994;1:373–384. doi: 10.1016/1074-7613(94)90068-x. [DOI] [PubMed] [Google Scholar]
- 9.Bukovsky A A, Dorfman T, Weimann A, Göttlinger H G. Nef association with human immunodeficiency virus type 1 virions and cleavage by the viral protease. J Virol. 1997;71:1013–1018. doi: 10.1128/jvi.71.2.1013-1018.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Camaur D, Trono D. Characterization of human immunodeficiency virus type 1 Vif particle incorporation. J Virol. 1996;70:6106–6111. doi: 10.1128/jvi.70.9.6106-6111.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Charneau P, Mirambeau G, Roux P, Paulus S, Buc H, Clavel F. HIV-1 reverse transcription: a termination step at the center of the genome. J Mol Biol. 1994;241:651–662. doi: 10.1006/jmbi.1994.1542. [DOI] [PubMed] [Google Scholar]
- 12.Chowers M Y, Pandori M W, Spina C A, Richman D D, Guatelli J C. The growth advantage conferred by HIV-1 Nef is determined at the level of viral DNA formation and is independent of CD4 downregulation. Virology. 1995;212:451–457. doi: 10.1006/viro.1995.1502. [DOI] [PubMed] [Google Scholar]
- 13.Chowers M Y, Spina C A, Kwoh T J, Fitch N J S, Richman D D, Guatelli J C. Optimal infectivity in vitro of human immunodeficiency virus type 1 requires an intact nef gene. J Virol. 1994;68:2906–2914. doi: 10.1128/jvi.68.5.2906-2914.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deacon N J, Tsykin A, Soloman A, Smith K, Ludford-Menting M, Hooker D J, McPhee D A, Greenway A L, Ellett A, Chatfield C, Lawson V A, Crowe S, Maerz A, Sonza S, Learmont J, Sullivan J S, Cunningham A, Dwyer D, Dowton D, Mills J. Genomic structure of an attenuated quasi species of HIV-1 from a blood transfusion donor and recipients. Science. 1995;270:988–991. doi: 10.1126/science.270.5238.988. [DOI] [PubMed] [Google Scholar]
- 15.deRonde A, Klaver B, Keulen W, Smit L, Goudsmit J. Natural HIV-1 Nef accelerates virus replication in primary human lymphocytes. Virology. 1992;188:391–395. doi: 10.1016/0042-6822(92)90772-h. [DOI] [PubMed] [Google Scholar]
- 16.Freund J, Kellner R, Konvalinka J, Wolber V, Krausslich H-G, Kalbitzer H R. A possible regulation of negative factor (Nef) activity of human immunodeficiency virus type 1 by the viral protease. Eur J Biochem. 1994;223:589–593. doi: 10.1111/j.1432-1033.1994.tb19029.x. [DOI] [PubMed] [Google Scholar]
- 17.Gaedigk-Nitschko K, Schön A, Wachinger G, Erfle V, Kohleisen B. Cleavage of recombinant and cell derived human immunodeficiency virus 1 (HIV-1) Nef protein by HIV-1 protease. FEBS Lett. 1995;357:275–278. doi: 10.1016/0014-5793(94)01370-g. [DOI] [PubMed] [Google Scholar]
- 18.Garcia J V, Miller A D. Serine phosphorylation independent downregulation of cell-surface CD4 by Nef. Nature. 1991;350:508–511. doi: 10.1038/350508a0. [DOI] [PubMed] [Google Scholar]
- 19.Goff S, Traktman P, Baltimore D. Isolation and properties of Moloney murine leukemia virus mutants: use of a rapid assay for release of virion reverse transcriptase. J Virol. 1981;38:239–248. doi: 10.1128/jvi.38.1.239-248.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grzesiek S, Bax A, Clore G M, Gronenborn A M, Hu J-S, Kaufman J, Palmer I, Stahl S J, Wingfield P T. The solution structure of HIV-1 Nef reveals an unexpected fold and permits delineation of the binding surface for the SH3 domain of Hck tyrosine protein kinase. Nat Struct Biol. 1996;3:340–345. doi: 10.1038/nsb0496-340. [DOI] [PubMed] [Google Scholar]
- 21.Grzesiek S, Stahl S J, Wingfield P T, Bax A. The CD4 determinant for downregulation by HIV-1 Nef directly binds to Nef. Mapping of the Nef binding surface by NMR. Biochemistry. 1996;35:10256–10261. doi: 10.1021/bi9611164. [DOI] [PubMed] [Google Scholar]
- 22.Guy B, Kieny M P, Riviere Y, Peuch C L, Dott K, Girard M, Montagnier L, Lecocq J-P. HIV F/3′ orf encodes a phosphorylated GTP-binding protein resembling an oncogene product. Nature. 1987;330:266–269. doi: 10.1038/330266a0. [DOI] [PubMed] [Google Scholar]
- 23.Kaminchik J, Bashan N, Itach A, Sarver N, Gorecki M, Panet A. Genetic characterization of human immunodeficiency virus type 1 nef gene products translated in vitro and expressed in mammalian cells. J Virol. 1991;65:583–588. doi: 10.1128/jvi.65.2.583-588.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaplan A H, Manchester M, Swanstrom R. The activity of the protease of human immunodeficiency virus type 1 is initiated at the membrane of infected cells before the release of viral proteins and is required for release to occur with maximum efficiency. J Virol. 1994;68:6782–6786. doi: 10.1128/jvi.68.10.6782-6786.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kestler H W, Ringler D J, Mori K, Panicali D L, Desrosiers R C. Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell. 1991;65:651–662. doi: 10.1016/0092-8674(91)90097-i. [DOI] [PubMed] [Google Scholar]
- 26.Kirchoff F, Greenough T C, Brettler D B, Sullivan J L, Desrosiers R C. Absence of intact nef sequences in a long-term survivor with nonprogressive HIV-1 infection. N Engl J Med. 1995;332:228–232. doi: 10.1056/NEJM199501263320405. [DOI] [PubMed] [Google Scholar]
- 27.Lama, J., and D. Trono. Submitted for publication.
- 28.Luciw P A, Cheng-Mayer C, Levy J A. Mutational analysis of the human immunodeficiency virus: the orf-B region downregulates virus replication. Proc Natl Acad Sci USA. 1987;84:1434–1438. doi: 10.1073/pnas.84.5.1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luria S, Chambers I, Berg P. Expression of the type 1 human immunodeficiency virus Nef protein in T-cells prevents antigen receptor-mediated induction of interleukin 2 mRNA. Proc Natl Acad Sci USA. 1991;88:5326–5330. doi: 10.1073/pnas.88.12.5326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mangasarian, A., V. Piguet, J.-K. Wang, J.-L. Carpentier, and D. Trono. Unpublished data.
- 31.Mariani R, Skowronski J. CD4 down-regulation by nef alleles isolated from human immunodeficiency virus type 1-infected individuals. Proc Natl Acad Sci USA. 1993;90:5549–5553. doi: 10.1073/pnas.90.12.5549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller M D, Warmerdam M T, Gaston I, Greene W C, Feinberg M B. The human immunodeficiency virus type 1 nef gene product: a positive factor for viral infection and replication in primary lymphocytes and macrophages. J Exp Med. 1994;179:101–113. doi: 10.1084/jem.179.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller M D, Warmerdam M T, Page K A, Feinberg M B, Greene W C. Expression of the human immunodeficiency virus type 1 (HIV-1) nef gene during HIV-1 production increases progeny particle infectivity independent of gp160 or viral entry. J Virol. 1995;69:579–584. doi: 10.1128/jvi.69.1.579-584.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller R H, Sarver N. HIV accessory proteins as therapeutic targets. Nat Med. 1997;3:389–394. doi: 10.1038/nm0497-389. [DOI] [PubMed] [Google Scholar]
- 35.Naldini L, Blömer U, Gallay P, Ory D, Mulligan R, Gage F H, Verma I M, Trono D. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996;272:263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- 36.Niederman T M, Thielan B J, Ratner L. Human immunodeficiency virus type 1 negative factor is a transcriptional silencer. Proc Natl Acad Sci USA. 1989;86:1128–1132. doi: 10.1073/pnas.86.4.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Niederman T M J, Garcia V J, Hastings W R, Luria S, Ratner L. Human immunodeficiency virus type 1 Nef protein inhibits NF-κB induction in human T cells. J Virol. 1992;66:6213–6219. doi: 10.1128/jvi.66.10.6213-6219.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Niederman T M J, Hastings W R, Ratner L. Myristoylation-enhanced binding of the HIV-1 Nef protein to T cell skeletal matrix. Virology. 1993;197:420–425. doi: 10.1006/viro.1993.1605. [DOI] [PubMed] [Google Scholar]
- 39.Pandori M W, Fitch N J S, Craig H M, Richman D D, Spina C A, Guatelli J C. Producer-cell modification of human immunodeficiency virus type 1: Nef is a virion protein. J Virol. 1996;70:4283–4290. doi: 10.1128/jvi.70.7.4283-4290.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rhee S S, Marsh J W. Human immunodeficiency virus type 1 Nef-induced down-modulation of CD4 is due to rapid internalization and degradation of surface CD4. J Virol. 1994;68:5156–5163. doi: 10.1128/jvi.68.8.5156-5163.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schwartz O, Marechal V, Danos O, Heard J-M. Human immunodeficiency virus type 1 Nef increases the efficiency of reverse transcription in the infected cell. J Virol. 1995;69:4053–4059. doi: 10.1128/jvi.69.7.4053-4059.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schwartz O, Marechal V, Gall S L, Lemonnier F, Heard J-M. Endocytosis of major histocompatibility complex class I molecules is induced by HIV-1 Nef protein. Nat Med. 1996;2:338–342. doi: 10.1038/nm0396-338. [DOI] [PubMed] [Google Scholar]
- 43.Sinclair E, Barbosa P, Feinberg M B. The nef gene products of both simian and human immunodeficiency viruses enhance virus infectivity and are functionally interchangeable. J Virol. 1997;71:3641–3651. doi: 10.1128/jvi.71.5.3641-3651.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Skowronski J, Parks D, Mariani R. Altered T cell activation and development in transgenic mice expressing the HIV-1 nef gene. EMBO J. 1993;12:703–713. doi: 10.1002/j.1460-2075.1993.tb05704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Spina C, Kwoh T J, Chowers M Y, Guatelli J C, Richman D. The importance of nef in the induction of human immunodeficiency virus type 1 replication from primary quiescent CD4 lymphocytes. J Exp Med. 1994;179:115–123. doi: 10.1084/jem.179.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Subbramanian R A, Cohen E A. Molecular biology of the human immunodeficiency virus accessory proteins. J Virol. 1994;68:6831–6835. doi: 10.1128/jvi.68.11.6831-6835.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Trono D. HIV accessory proteins: leading roles for the supporting cast. Cell. 1995;82:189–192. doi: 10.1016/0092-8674(95)90306-2. [DOI] [PubMed] [Google Scholar]
- 48.Welker R, Kottler H, Kalbitzer H R, Krausslich H-G. Human immunodeficiency virus type 1 Nef protein is incorporated into virus particles and specifically cleaved by the viral protease. Virology. 1996;219:228–236. doi: 10.1006/viro.1996.0240. [DOI] [PubMed] [Google Scholar]