Abstract
In 2022, concurrent outbreaks of hepatitis A, invasive meningococcal disease (IMD), and mpox were identified in Florida, USA, primarily among men who have sex with men. The hepatitis A outbreak (153 cases) was associated with hepatitis A virus genotype IA. The IMD outbreak (44 cases) was associated with Neisseria meningitidis serogroup C, sequence type 11, clonal complex 11. The mpox outbreak in Florida (2,845 cases) was part of a global epidemic. The hepatitis A and IMD outbreaks were concentrated in Central Florida and peaked during March–June, whereas mpox cases were more heavily concentrated in South Florida and had peak incidence in August. HIV infection was more common (52%) among mpox cases than among hepatitis A (21%) or IMD (34%) cases. Where feasible, vaccination against hepatitis A, meningococcal disease, and mpox should be encouraged among at-risk groups and offered along with program services that target those groups.
Keywords: hepatitis A, meningococcal disease, mpox, monkeypox, viruses, bacteria, sexually transmitted infections, meningitis/encephalitis, MSM, outbreaks, Florida, United States
Outbreaks of hepatitis A have been previously reported among gay, bisexual, and other men who have sex with men (MSM) (1). Numerous outbreaks of hepatitis A among MSM were reported in Europe during 1997–2005 (2) and 2016–2017 (3–5). In the United States, hepatitis A outbreaks among MSM were reported in several locations during 2017–2018 (6,7). Outbreaks of invasive meningococcal disease (IMD) have also been previously reported among MSM (8–10). In late 2021, increases of hepatitis A and IMD were observed in Central Florida, primarily among MSM, prompting an investigation to guide the implementation of disease control measures.
During the period those 2 concurrent outbreaks were ongoing in Florida, a global epidemic of mpox emerged, in which sexual and intimate contact, particularly among MSM, was the primary mode of recognized transmission (11,12). In May 2022, a case of mpox associated with the global epidemic was identified in the United States. By November 30, 2022, the United States had >29,600 reported mpox cases and Florida had the fourth highest number of cases in the country (13).
We provide a descriptive, cross-sectional analysis of the concurrent outbreaks of hepatitis A and IMD in Florida in the context of an ongoing global mpox epidemic that also is disproportionally affecting MSM. Through this analysis, we attempted to identify common and distinct features of each outbreak and synergistic factors that might have affected disease progression and control.
Methods
Hepatitis A and IMD are designated as reportable conditions in Florida; in 2022, mpox was reportable in Florida under the broader category of a disease of urgent public health importance (14). Cases for all 3 diseases are investigated by local staff of the Florida Department of Health (FDOH); investigations involve patient interviews to identify risk factors for illness and close contacts for possible prophylaxis or other needed follow-up actions. Interview questionnaires for each disease are standardized statewide; however, some questions differ between diseases. After recognition of an increased number of case reports, we created outbreak case definitions for hepatitis A and IMD (Table 1). We considered all mpox cases in Florida that met the national surveillance case definition to be outbreak related. The analysis period for this investigation included outbreak-associated cases for any of the 3 diseases that had illness onset during November 1, 2021–November 30, 2022.
Table 1. Case definitions used in the investigation of concurrent outbreaks of hepatitis A, invasive meningococcal disease, and mpox, Florida, USA, 2021–2022*.
| Disease | Definition |
|---|---|
| Hepatitis A | |
| Inclusion criteria | Florida residents meeting the surveillance case definition for hepatitis A† with symptom onset during November 1, 2021–November 30, 2022 |
| Confirmed case | A hepatitis A case with laboratory evidence of infection with HAV genotype IA, A17 cluster 21, or epidemiologically linked to a case with HAV A17 cluster 21 infection |
| Probable case | A hepatitis A case who identified as transgender or MSM or had sexual contact with MSM, irrespective of other risk factors for hepatitis A |
| Suspected case | A male hepatitis A case with unknown sexual history, and absence of other risk factors for hepatitis A (e.g., foreign travel, drug use, homelessness, incarceration) |
| Epidemiologically linked | Household or sexual contact during the 15–50 d before symptom onset |
| Exclusions |
Cases with sequencing results indicating infection with HAV other than genotype IA, A17 cluster 21 |
| Invasive meningococcal disease | |
| Inclusion criteria | Florida residents meeting the surveillance case definition for invasive meningococcal disease† with symptom onset during November 1, 2021–November 30, 2022 |
| Confirmed case | An invasive meningococcal disease case who identifies as MSM or had sexual contact with MSM and evidence of serogroup C infection; or laboratory evidence of infection with ST11 CC11 serogroup C, with <100 SNPs difference to the outbreak strain, irrespective of sexual history |
| Probable case | An invasive meningococcal disease case who identifies as MSM or had sexual contact with MSM and for whom no additional serogroup, ST, or CC typing is available; or who had evidence of serogroup C infection regardless of sexual history |
| Exclusions |
Cases with sequencing results indicating infection with Neisseria meningitides other than ST11 CC11 serogroup C or with >100 SNP differences to the outbreak strain |
| Mpox | |
| Inclusion criteria | Florida residents meeting the surveillance case definition for confirmed or probable mpox‡ with symptom onset during May 1–November 30, 2022 |
Hepatitis A Case Investigation
For all persons clinically diagnosed with hepatitis A, hospital and commercial laboratories were asked to forward available specimens to the FDOH Bureau of Public Health Laboratories (BPHL) for hepatitis A virus (HAV) genotyping based on the VP1-P2B junction region (17). Next-generation sequencing was performed on the MiSeq (Illumina) platform, and bioinformatics data processing used the Centers for Disease Control and Prevention (CDC) Global Hepatitis Outbreak Surveillance Technology (GHOST) platform (18). Investigation guidelines in Florida require that a case of hepatitis A in a food employee be further investigated to assess risk for possible food item or food preparation system contamination, which might also involve an environmental assessment. FDOH considered patron notification or suspension orders for food establishments, dependent upon the outcome of the risk assessments conducted.
IMD Case Investigation
For persons with diagnosed IMD, isolates of Neisseria meningitidis were forwarded to BPHL for serogrouping by slide agglutination, and further characterization by whole-genome sequencing on MiSeq or NextSeq550 (Illumina) systems. Select specimens were also forwarded to CDC laboratories for additional characterization or confirmation. We analyzed sequencing data by using BPHL’s FLAQ-AMR pipeline (https://github.com/BPHL-Molecular/flaq_amr) to assess sequence quality and identify the sequence type (ST) and submitted data to CDC’s Bacterial Meningitis Genome Analysis Platform (BMGAP; https://github.com/CDCgov/BMGAP) to identify the clonal complex (CC) and to verify serogroup for each isolate (19). We identified outbreak-associated cases by serogroup, ST, and CC, and constructed phylogenetic trees.
Mpox Case Investigation
In May 2022, FDOH notified healthcare providers and laboratories that mpox was considered reportable under existing rules. FDOH adopted the national surveillance case definition. That definition initially included clinical manifestations as a criterion, but on July 22, 2022, was modified to focus on laboratory results and epidemiologic risk factors for probable and confirmed cases (20).
Initially, all testing for orthopoxvirus and mpox virus infections was conducted at BPHL and CDC using previously described procedures (21,22). In July 2022, use of the nonvariola orthopoxvirus assay expanded to commercial laboratories (23). Molecular subtyping of isolates from mpox patients in Florida was not routinely done for surveillance purposes during the study period; however, CDC performed partial or full sequencing for a subset of samples (24,25).
Epidemiology and Analyses
FDOH Bureau of Epidemiology operates an electronic patient-based reportable disease surveillance system known as Merlin. Using data from Merlin, we identified persons meeting the outbreak case definition for >1 disease in the 3 concurrent outbreaks. We used data from other surveillance systems operated by the Bureau of Communicable Diseases for sexually transmitted infections (STIs) and HIV to ascertain history and risk factors for reportable STIs (e.g., chlamydia, gonorrhea, or syphilis) and HIV infection. We matched patient profiles between systems by using name, sex, date of birth, and address information.
We calculated descriptive statistics to characterize outbreak-related cases by person, place, and time. We assessed pairwise differences in median age via Dunn test and conducted χ2 tests to compare cases of each disease by HIV infection, history of recent STI, and Orange County residency. We used 2-sided tests in all statistical analyses and considered p<0.05 statistically significant.
To assess the role of the public health response in controlling the concurrent outbreaks, we analyzed data from the statewide immunization registry by vaccine antigen and month of first dose administered. To distinguish outbreak response efforts from routine childhood or adolescent immunization, we limited data to vaccines administered to adults >18 years of age. We stratified data by persons receiving vaccine from a county health department (CHD) provider. To further characterize synergies in outbreak response efforts, we identified instances of vaccine administration to the same person, on the same day, against >1 of the diseases in the concurrent outbreaks. This activity was reviewed by the Ethics and Human Research Protection Program of FDOH and by CDC and was determined by both institutions to be public health practice, outbreak investigation, not requiring review and approval by an institutional review board.
Results
Hepatitis A Cases
During the analysis period, a total of 322 hepatitis A cases among Florida residents were reported to FDOH; 153 (48%) met the outbreak case definition. Of nonoutbreak cases during that period, ≈50% had an identifiable risk factor for hepatitis A, among which 60% were associated with recent international travel. Among the 153 outbreak-associated cases, 95% were in male persons and 5% in female persons, 74% were in MSM, and 21% were in persons with HIV; 1 death occurred (Table 2). Among persons for whom sexual history was obtained, 25% reported no sexual contacts in the previous 3 months; 8% reported >5 sexual partners, and 22% reported recent sexual contact with a person whose identity was not known. Seventeen cases were identified among food employees, but no foodborne transmission was documented. Environmental assessments performed at food establishments where exposure might have occurred did not necessitate FDOH orders for patron notification or suspension of service.
Table 2. Description of outbreak-related cases during concurrent outbreaks of hepatitis A, invasive meningococcal disease, and mpox, Florida, USA, 2021–2022*.
| Characteristics | Value |
||
|---|---|---|---|
| Hepatitis A | Invasive meningococcal disease | Mpox† | |
| Total no. |
153 |
44 |
2,845 |
| Median age, y (range) |
35 (9–75) |
32 (8–77) |
36 (0–81) |
| Race or ethnicity | |||
| White | 102/153 (67) | 29/44 (66) | 1,957/2,845 (69) |
| Hispanic |
53/150 (35) |
22/44 (50) |
1,270/2,845 (45) |
| Sex | |||
| M | 145/153 (95) | 36/44 (82) | 2,786/2,845 (98) |
| F | 8/153 (5) | 8/44 (18) | 59/2,845 (2) |
| Transgender |
4/153 (3) |
1/44 (2) |
22/2,845 (1) |
| MSM‡ |
113/152 (74) |
31/43 (72) |
2,507/2,836 (88) |
| No. sexual partners during past 3 mo‡ | |||
| 0 | 25/100 (25) | 4/31 (13) | 27/1,680 (2)§ |
| 1 | 44/100 (44) | 17/31 (55) | 914/1,680 (54)§ |
| 2–5 | 23/100 (23) | 8/31 (26) | 631/1,680 (38)§ |
| >5 |
8/100 (8) |
2/31 (6) |
108/1,680 (6)§ |
| Sex with unknown person in previous 3 mo‡ |
22/101 (22) |
6/30 (20) |
225/956 (23)§ |
| HIV positive |
32/153 (21) |
14/44 (34) |
1,473/2,836 (52) |
| Taking HIV preexposure prophylaxis |
20/130 (15) |
5/44 (11) |
Unknown |
| STI in previous 2 y |
42/153 (27) |
9/44 (20) |
1,567/2,836 (55) |
| Mpox virus infection in 2022 |
4 |
3 |
All |
| Recent international travel |
12/153 (8) |
1/44 (2) |
199/2,845 (7) |
| Recent homelessness |
8/153 (5) |
2/44 (5) |
25/928 (3) |
| Recent incarceration |
1/153 (<1) |
0/44 (0) |
1/928 (<1) |
| Recent injection drug use |
3/153 (2) |
2/44 (5) |
Unknown |
| Orange County resident |
53/153 (35) |
15/44 (34) |
295/2,845 (10) |
| Food employee |
17/121 (15) |
7/44 (16) |
Unknown |
| Hospitalized |
119/153 (78) |
41/44 (93) |
162/2,845 (6) |
| Died |
1/153 (<1) |
9/44 (20) |
3/2,845 (<1) |
| Outbreak case classification | |||
| Confirmed | 67/153 (44) | 40/44 (91) | 1,720/2,845 (60) |
| Probable | 73/153 (48) | 4/44 (9) | 1,125/2,845 (40) |
| Suspect |
13/153 (8) |
NA |
NA |
| Laboratory evidence of infection with outbreak genotype/sequence | 60/153 (39) | 34/44 (77) | NA |
*Values are no. cases/no. reported (%) except as indicated. All risk factor questions relate to exposures in the 3 mo before symptom onset, except sexual history for mpox cases, which refers to the 3 weeks before symptom onset. Denominators vary based on available data for each variable. Percentages may not add to 100 due to rounding. MSM, men who have sex with men. †Outbreak ongoing, data through November 30, 2022. ‡Minors age <18 y excluded from numerator and denominator. §Number of sexual partners in 3 weeks before onset (mpox incubation period).
Among outbreak-associated cases, 67 (44%) met the confirmed case classification, among which 60 cases had matching HAV genotype IA, GHOST cluster A17 cluster 21 (a.k.a. SC076 US-Mexican); the remaining 7 confirmed cases had an epidemiologic link to a laboratory-confirmed case. We excluded 4 cases from the outbreak that otherwise met the probable or suspected outbreak case classification because HAV sequencing results did not match the outbreak cluster type. Of the 60 cases with the matching HAV outbreak cluster type, 2 patients appear to be outliers and had no identifiable risk factors or recognized exposures to HAV. One case was in a 74-year-old heterosexual female person and the other was in a 9-year-old male child. Of the 7 confirmed cases not genotyped but confirmed through epidemiologic linkage, 2 were linked to the 9-year-old patient, his parents, who later became ill, likely because of secondary household transmission; the remaining 5 cases were sexual or household contacts of persons who identified as MSM.
IMD Cases
During the analysis period, 71 IMD cases among Florida residents were reported to FDOH. Of those, 44 (62%) cases were classified as outbreak associated. Among the outbreak cases, 72% were in persons who identified as MSM, 34% were in persons with HIV, and 20% of cases resulted in death (Table 2). The distribution of outbreak-associated cases by type of infection was 55% bacteremia, 20% meningitis, 5% septic arthritis, and 20% with >1 clinical syndrome. Case-fatality was highest (33%) among patients with bacteremia.
Among outbreak-associated cases, 40 (91%) met the confirmed case classification. Of those, 33 were caused by the ST11 CC11 outbreak strain; the other 7 confirmed cases were in persons who identified as MSM and had a serogroup C infection, but further strain characterization was not possible. Ten confirmed cases of the outbreak strain were in persons who did not identify as MSM, including a 77-year-old heterosexual woman with no known epidemiologic link to any other cases and 3 women who had no direct connection to one another but who were sexually active with the same male partner. Those 3 cases represent the only identified epidemiologic linkages among all the IMD cases in this outbreak.
Mpox Cases
During May 10–November 30, 2022, Florida reported 2,845 confirmed or probable mpox cases among residents. Most cases were locally acquired, but 14% of patients reported out-of-state travel during the 3-week incubation period. Most cases were among adult (>99%) and male (98%) persons. Among cases in adults, 88% were in persons who identified as MSM, but transmission through sexual contact between heterosexual persons was also identified. Among Florida cases, uncommon transmission routes were identified and have been previously reported by FDOH (26–28), including nonsexual household contact (n = 8) and occupational exposure among healthcare workers (n = 2).
Among mpox cases, 52% were in persons living with HIV and 55% in persons who had a history of >1 STI in the previous 2 years (Table 2). For the 1,414 cases with information available regarding previous HIV diagnoses, 10% were diagnosed within the previous year; 93% of cases were in persons who received HIV care within the previous year, 81% were in persons whose HIV infections were virally suppressed, and 6% were in persons who had a recent CD4 count of <200 cells/µL. Few (6%) cases were hospitalized because of mpox; 3 deaths occurred for which mpox was considered a causative or contributing factor. All 3 deaths were in persons who had a previous AIDS diagnosis, and 2 were unhoused.
CDC sequenced 17 mpox virus isolates and identified 16 as clade IIb subclade B.1 (25). One isolate collected early in the outbreak, from a patient with exposure in the United Arab Emirates, was identified as clade IIb subclade A.2 (24).
Outbreak Overlap
We observed temporal overlap for the 3 outbreaks; hepatitis A cases occurred somewhat earlier, and peak incidence was during late March and early April 2022 (Figure 1). Peak incidence for IMD occurred a few weeks later, and 13 cases were reported over a 6-week period during May–June. The number of hepatitis A and IMD cases declined as mpox cases rapidly increased; mpox incidence peaked during late July to early August, then rapidly declined. We did not identify any instances of the same person being part of both the hepatitis A and IMD outbreaks. However, among mpox cases, 4 patients were also part of the hepatitis A outbreak, and 3 others were part of the IMD outbreak (Appendix Table 1).
Figure 1.
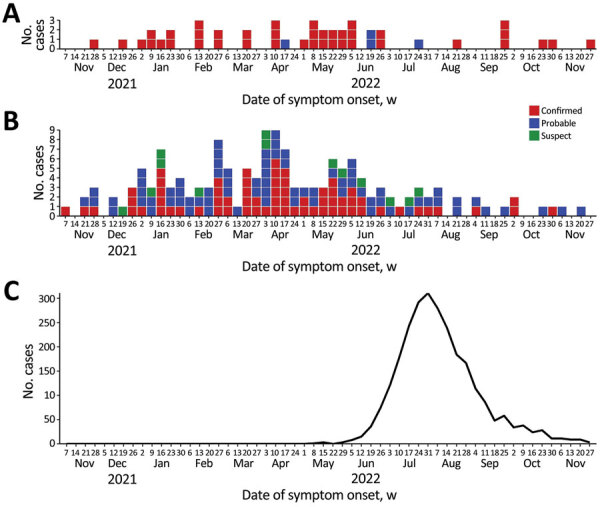
Epidemiologic curve of concurrent outbreaks of hepatitis A, invasive meningococcal disease, and mpox, by week of symptom onset, Florida, USA, November 1, 2021—November 30, 2022. A) Invasive meningococcal disease; B) hepatitis A; C) mpox. The case definition for invasive meningococcal disease cases had 2 categories: confirmed and probable. The case definition for hepatitis A cases had 3 categories: confirmed, probable, and suspect. The graph for mpox includes all confirmed and probable cases.
The greatest concentration of outbreak-associated hepatitis A and IMD cases occurred in the central region of Florida (Figure 2). Orange County had the highest number of cases for both diseases: 53 (35%) cases of hepatitis A and 15 (34%) cases of IMD. On the basis of patient residence, we identified 12 postal (ZIP) codes with >1 case of both hepatitis A and IMD, 3 of which had >2 cases of each disease (Figure 2). For hepatitis A, several cases occurred in west-central Florida (Hillsborough and Pinellas Counties), where IMD was not observed. Several IMD cases occurred in southeastern Florida (Miami-Dade, Broward, and Palm Beach Counties), where outbreak-associated hepatitis A was not frequently observed.
Figure 2.
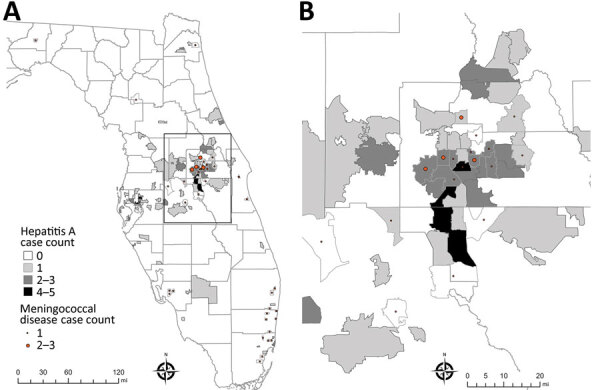
Spatial distribution of outbreak-associated cases of hepatitis A and invasive meningococcal disease in an investigation of concurrent outbreaks of hepatitis A, invasive meningococcal disease, and mpox, Florida, USA, 2021–2022. A) Locations of cases by postal (ZIP) code of patient residence; B) detail of box from central Florida in panel A, in which >1 case of each disease were reported in the same postal code. Outside the area represented in that panel, no cases of both diseases were identified in the same postal code. Invasive meningococcal disease cases were georeferenced by postal code centroid. Small polygons are outlines of postal code areas. Light gray lines indicat county borders; dark gray lines indicate postal areas where instances were identified.
Mpox cases in Florida were more widely dispersed; cases were reported in 45 of 67 counties. The highest concentration was observed in the southeast region, and 56% of all cases were reported in Broward and Miami-Dade Counties (Figure 3). Orange County reported the third highest (295; 10%) number of mpox cases. The 7 most populated counties in Florida accounted for 87% of mpox cases reported in the state. Exposure occurred exclusively outside the United States for 5% of Florida mpox cases, and an additional 2% of cases reported multiple exposure locations that included >1 location outside the United States.
Figure 3.
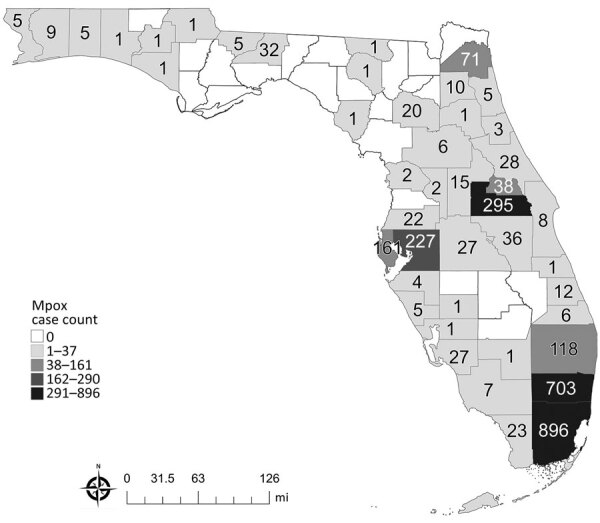
Distribution of mpox cases by county of residence in an investigation of concurrent outbreaks of hepatitis A, invasive meningococcal disease, and mpox, Florida, USA, 2021–2022. Numbers on map indicate numbers of persons with mpox reported to Florida Department of Health, by the person’s county of residence.
Comparing cases by disease, the median age of IMD case-patients was 32 years, which was significantly younger than for hepatitis A (35 years; p = 0.0145) and mpox (36 years; p = 0.0043) case-patients. We detected statistically significant differences when comparing cases of each disease by HIV infection, history of recent STI, and Orange County residence (p<0.0001). HIV infection was more prevalent among mpox (52%) cases than among IMD (34%) or hepatitis A (21%) cases. In addition, STI in the previous 2 years was more prevalent among mpox (55%) cases than among hepatitis A (27%) or IMD (20%) cases. A lower percentage (10%) of mpox cases were among Orange County residents than were IMD (34%) and hepatitis A (35%) cases.
Control Measures
JYNNEOS (Bavarian Nordic) vaccine for preventing mpox became available in limited supplies in Florida in late May and June 2022. As the vaccine supply increased, persons vaccinated with their first dose increased markedly; in July and August, >53,000 adults in Florida received >1 dose (Appendix Table 2). Immunization against hepatitis A and IMD also increased markedly among adults during that timeframe (Figure 4). In August 2022, of the 28,592 persons receiving a first dose of JYNNEOS vaccine, 7,305 (26%) received >1 other vaccine against either hepatitis A or IMD on the same day; 2,979 (10%) received vaccines against all 3 diseases on the same day. More than half of adult immunizations for hepatitis A and IMD in August were administered by CHD providers. In contrast, during months outside the August–October period, <38% of adults receiving a vaccine for either disease received that dose from a CHD provider.
Figure 4.
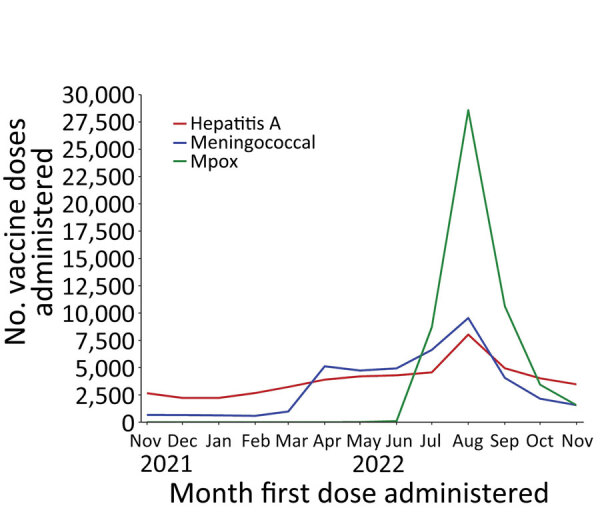
Persons vaccinated by month and antigen during concurrent outbreaks of hepatitis A, invasive meningococcal disease, and mpox, Florida, USA, 2021–2022. The figure shows the number of adult persons (>18 years of age) vaccinated with their first dose, by month the first dose was administered. For hepatitis A, the number includes persons vaccinated with any Food and Drug Administration (FDA)–approved vaccine against hepatitis A virus. For meningococcal disease, the number includes persons vaccinated with any FDA-approved serogroup ACWY vaccine (Menveo [GlaxoSmithKline], Menactra [Sanofi Pasteur, Inc.], MenQuadfi [Sanofi Pasteur, Inc.]). For mpox, the number includes persons vaccinated with JYNNEOS (Bavarian Nordic). FDA, Food and Drug Administration.
The hepatitis A and IMD outbreaks concluded by the end of 2022, but mpox cases continued to be reported in Florida as of January 2024. By that time, a total of 2,957 mpox cases had been reported since the start of the outbreak, which is considered ongoing.
Discussion
Outbreaks of hepatitis A and IMD among MSM were ongoing in Florida when the emerging mpox outbreak added further complexity and urgency to the public health response. In addition, the introduction of mpox might have hastened the decline of cases in the hepatitis A and IMD outbreaks, possibly due to multiple factors. First, the negative perceptions and fear of acquiring mpox might have led to changes in sexual behaviors among MSM, particularly persons outside of monogamous relationships. In a national survey of 703 MSM and transgender women conducted in August 2022, more than half reported a change in their sexual behavior because of the mpox outbreak; 40% reported limiting the number of sexual partners, and 24% avoided having any type of sex (29). Similarly, in another survey of MSM conducted in August 2022, a total of 50% of respondents reported reducing 1-time sexual encounters and 50% reported reducing sex with partners met on dating apps or at sex venues (30).
The response to mpox might also have hastened the end of the hepatitis A and IMD outbreaks through coordinated immunization outreach efforts targeting overlapping risk groups. Before August 2022, demand for JYNNEOS vaccine was strong, but supplies were limited (31,32). As the vaccine became more readily available (33), CHDs in Florida conducted outreach activities to provide mpox vaccination to gay, bisexual, and other MSM, which also helped provide vaccination against hepatitis A or IMD to the same persons, where indicated (34,35). The strong interest and demand for JYNNEOS vaccine among MSM likely led some persons to get vaccinated against hepatitis A and IMD who might not otherwise have been vaccinated. Vaccines administered by CHDs were provided free of charge to the client and, in some counties, made available outside nightclubs and at social venues or community events. That strategy leveraged lessons learned from HIV prevention programs, including making services available to persons who would most benefit, where they most likely would be, and when they would most likely be comfortable (36).
We observed a high percentage of concurrent HIV infection among hepatitis A, IMD, and most notably, mpox case-patients. We observed a similar pattern regarding history of an STI in the previous 2 years. The duration of HAV viremia and stool shedding might be longer in persons with HIV (37), and persons with HIV who have low CD4 cell counts are also more susceptible to invasive disease when exposed to N. meningitidis (8,10). Some researchers have proposed the concept of syndemics to refer to multiple diseases occurring in the same population that have a synergistic effect on the progression or outcome of each disease (38,39). HIV and STIs are frequently cited examples of infectious disease syndemics. We observed current or recent infections in the same person, including HIV, >1 STI, mpox, and hepatitis A or IMD, in a setting of concurrent outbreaks. A single case report involving a similar combination with severe hepatitis A was previously reported (40).
Of note, 25% of adult hepatitis A case-patients in the outbreak reported no sexual activity in the previous 3 months. That might be the result of unreliable information regarding sexual history provided by case-patients. However, it also might be explained by possible undetected spillover into foodborne transmission or environmental surface contamination at venues that cater to an exclusively or predominantly lesbian, gay, bisexual, and transgender clientele, a possibility that has been noted in similar outbreaks (41). No common venues were identified in our outbreaks but interviewed case-patients frequently declined to identify specific venues. Moreover, some confirmed cases in the hepatitis A outbreak in persons who were not sexually active, such as the elderly woman and young boy, might be explained by limitations of HAV subgenomic sequencing to detect variations in phylogenetic relationships (42).
Simultaneous response to these 3 concurrent outbreaks highlighted differences in public health surveillance, investigation, and response activities for diseases considered sexually transmitted compared with other communicable diseases, such as hepatitis A or IMD, that have multiple transmission modes that also can include sexual contact. Many US public health departments, including FDOH, are organizationally separated between general communicable diseases and control programs related to STIs and HIV. Barriers often exist for integration of staff and surveillance information resources across those program areas. Nevertheless, we found added value in close collaboration and integration of case-related data in response to these concurrent outbreaks. Further integration across program areas could provide added benefit in disease control efforts, particularly those involving overlapping risk groups.
When the global mpox epidemic emerged in 2022, and transmission among MSM was quickly recognized, investigation methods in the United States (e.g., questionnaires and case interviews) rapidly aligned to methods typically used for STIs and HIV (36). In Florida, methods included using experienced disease intervention specialists (DISs) who have advanced skills in eliciting sensitive details regarding sexual history. In contrast, case investigations for most reportable diseases in Florida, including hepatitis A and IMD, do not routinely include detailed information on gender identity, sexual orientation, or sexual history, nor are those investigations routinely conducted by trained DIS staff with specialized interviewing skills. Thus, data collected during these concurrent outbreak investigations were not standardized nor consistently gathered across diseases, limiting direct comparisons. Furthermore, we did not directly compare MSM status across the diseases because MSM was a criterion within the outbreak case definitions for hepatitis A and IMD but not for mpox. Nor did we compare race or ethnicity across the 3 diseases because population demographics differ by region in Florida and mpox was much more heavily concentrated in southern Florida than the other 2 diseases.
This investigation was also limited by an inability in many instances to identify close contacts, particularly known or anonymous sexual contacts. Thus, epidemiologic linkages between cases were often difficult or impossible to establish. The lack of available specimens from all patients also limited efforts to fully use molecular and genomic analyses to identify connections between cases or to rule out probable or suspected cases that might not have been truly related to the hepatitis A or IMD outbreaks.
Recognition of factors associated with an emerging syndemic early in its course facilitated improved data sharing and leveraged limited resources among public health programs responsible for disease control in Florida. In addition, combined vaccination efforts targeting overlapping at-risk populations likely contributed to the control of these outbreaks.
In conclusion, we found that cross-training between DIS and general communicable disease surveillance investigators might improve the quality of information collected through case interviews, particularly information related to sexual history. Disease prevention opportunities, also provided by DIS, might be enhanced through an integrated approach to screening, education, and linkage to care for multiple infectious diseases across program areas. Finally, vaccination against hepatitis A, meningococcal disease, and mpox should be encouraged among MSM, consistent with national guidelines (33–35) and, where feasible, offered with other program services to the same at-risk population.
Additional information on concurrent outbreaks of hepatitis A, invasive meningococcal disease, and mpox, Florida, USA, 2021–2022.
Acknowledgments
We gratefully acknowledge the assistance of patients and medical providers in Florida for providing information for this investigation. We also acknowledge the assistance of numerous staff of county health departments and public health laboratories in Florida, as well as Jeremy Adams, Labake Ajayi, Tom Bendle, Tricia Foster, Lorene Maddox, Ann Schmitz, Craig Wilson, Namratha Tarigopula, Omer Tekin, Brenna McGruder Rawson, Ronique Bain, Candiss Ducksworth, Andres Echeverri, Alexandria Fletcher, Jackie Pho, Mariella Rivera, Antonisha Seay, Celia Vickery, Anne Barrera, Reynald Jean, Diana Paladino, Julio Pelaez, Johanna Segura, Nicholas DelVecchio, Jeanete Figueroa, Patrick Jenkins, Rafael Mendoza, Ryan Oms, Jonathan Pope, Chelsee Rose, Maria Diaz, Erin Bates, Ulyee Choe, Shelly Geisler, Gayle Guidash, Colin Hilliard, Rachel Ilic, JoAnne Lamb, Russell Kopit, Karen Thomas, Chowdhury Bari, Jacob Crosby. We also acknowledge CDC’s Bacterial Meningitis Laboratory (National Center for Immunization and Respiratory Diseases) and Viral Hepatitis Laboratory (Division of Viral Hepatitis, National Center for HIV, Viral Hepatitis, STD, and TB Prevention) for assistance with genomic analysis used to categorize outbreak associated cases.
Outbreak investigation team members: Alvina Chu, Michelle Persaud, Alvaro Mejia-Echeverry, Edhelene Rico, Shannon Patelsky, Jenniffer Rivas, Chelsea Kendrick, Maria Tarnoi, Rebecca Bohinc, Alissa Brown, Dell Walters, Purva Patel, Muniba McCabe, Patricia Kirkland.
Biography
Dr. Doyle is a CDC Career Epidemiology Field Officer assigned to the Florida Department of Health. His primary research interests include applied infectious disease epidemiology, disease surveillance, and public health workforce development.
Footnotes
Suggested citation for this article: Doyle TJ, Gumke M, Stanek D, Moore J, Buck B, Locksmith T, et al.; the outbreak investigation team. Concurrent outbreaks of hepatitis A, invasive meningococcal disease, and mpox, Florida, USA, 2021–2022. Emerg Infect Dis. 2024 Apr [date cited]. https://doi.org/10.3201/eid3004.231392
Members of the team are listed at the end of the article.
References
- 1.Cotter SM, Sansom S, Long T, Koch E, Kellerman S, Smith F, et al. Outbreak of hepatitis A among men who have sex with men: implications for hepatitis A vaccination strategies. J Infect Dis. 2003;187:1235–40. 10.1086/374057 [DOI] [PubMed] [Google Scholar]
- 2.Stene-Johansen K, Tjon G, Schreier E, Bremer V, Bruisten S, Ngui SL, et al. Molecular epidemiological studies show that hepatitis A virus is endemic among active homosexual men in Europe. J Med Virol. 2007;79:356–65. 10.1002/jmv.20781 [DOI] [PubMed] [Google Scholar]
- 3.Ndumbi P, Freidl GS, Williams CJ, Mårdh O, Varela C, Avellón A, et al. ; Members of the European Hepatitis A Outbreak Investigation Team. Hepatitis A outbreak disproportionately affecting men who have sex with men (MSM) in the European Union and European Economic Area, June 2016 to May 2017. Euro Surveill. 2018;23:1700641. 10.2807/1560-7917.ES.2018.23.33.1700641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beebeejaun K, Degala S, Balogun K, Simms I, Woodhall SC, Heinsbroek E, et al. Outbreak of hepatitis A associated with men who have sex with men (MSM), England, July 2016 to January 2017. Euro Surveill. 2017;22:5. 10.2807/1560-7917.ES.2017.22.5.30454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Werber D, Michaelis K, Hausner M, Sissolak D, Wenzel J, Bitzegeio J, et al. Ongoing outbreaks of hepatitis A among men who have sex with men (MSM), Berlin, November 2016 to January 2017 - linked to other German cities and European countries. Euro Surveill. 2017;22:30457. 10.2807/1560-7917.ES.2017.22.5.30457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Foster MA, Hofmeister MG, Albertson JP, Brown KB, Burakoff AW, Gandhi AP, et al. Hepatitis A virus infections among men who have sex with men—eight U.S. states, 2017–2018. MMWR Morb Mortal Wkly Rep. 2021;70:875–8. 10.15585/mmwr.mm7024a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Latash J, Dorsinville M, Del Rosso P, Antwi M, Reddy V, Waechter H, et al. Notes from the field: increase in reported hepatitis A infections among men who have sex with men—New York City, January–August 2017. MMWR Morb Mortal Wkly Rep. 2017;66:999–1000. 10.15585/mmwr.mm6637a7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bozio CH, Blain A, MacNeil J, Retchless A, Weil LM, Wang X, et al. Meningococcal disease surveillance in men who have sex with men—United States, 2015–2016. MMWR Morb Mortal Wkly Rep. 2018;67:1060–3. 10.15585/mmwr.mm6738a4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taha MK, Claus H, Lappann M, Veyrier FJ, Otto A, Becher D, et al. Evolutionary events associated with an outbreak of meningococcal disease in men who have sex with men. PLoS One. 2016;11:e0154047. 10.1371/journal.pone.0154047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Folaranmi TA, Kretz CB, Kamiya H, MacNeil JR, Whaley MJ, Blain A, et al. Increased risk for meningococcal disease among men who have sex with men in the United States, 2012–2015. Clin Infect Dis. 2017;65:756–63. 10.1093/cid/cix438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kava CM, Rohraff DM, Wallace B, Mendoza-Alonzo JL, Currie DW, Munsey AE, et al. Epidemiologic features of the monkeypox outbreak and the public health response—United States, May 17–October 6, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:1449–56. 10.15585/mmwr.mm7145a4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blackburn D, Roth NM, Gold JAW, Pao LZ, Olansky E, Torrone EA, et al. Epidemiologic and clinical features of mpox in transgender and gender-diverse adults—United States, May–November 2022. MMWR Morb Mortal Wkly Rep. 2022;71:1605–9. 10.15585/mmwr.mm715152a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention. Mpox: 2022. U.S. map & case count [cited 2022 Dec 27]. https://www.cdc.gov/poxvirus/mpox/response/2022/us-map.html
- 14.Florida Department of Health. Disease reporting and surveillance [cited 2022 Nov 10]. https://www.floridahealth.gov/diseases-and-conditions/disease-reporting-and-management/disease-reporting-and-surveillance/index.html
- 15.Florida Department of Health. Surveillance case definitions for reportable diseases in Florida [cited 2022 Nov 10]. https://www.floridahealth.gov/diseases-and-conditions/disease-reporting-and-management/disease-reporting-and-surveillance/_documents/surveillance-case-def-select-rd-fl.pdf
- 16.Centers for Disease Control and Prevention. Case definitions for use in the 2022. monkeypox response [cited 2022 Nov 10]. https://www.cdc.gov/poxvirus/monkeypox/clinicians/case-definition.html
- 17.Probert WS, Gonzalez C, Espinosa A, Hacker JK. Molecular genotyping of hepatitis A virus, California, USA, 2017–2018. Emerg Infect Dis. 2019;25:1594–6. 10.3201/eid2508.181489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramachandran S, Xia GL, Dimitrova Z, Lin Y, Montgomery M, Augustine R, et al. Changing molecular epidemiology of hepatitis A virus infection, United States, 1996–2019. Emerg Infect Dis. 2021;27:1742–5. 10.3201/eid2706.203036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buono SA, Kelly RJ, Topaz N, Retchless AC, Silva H, Chen A, et al. Web-based genome analysis of bacterial meningitis pathogens for public health applications using the Bacterial Meningitis Genomic Analysis Platform (BMGAP). Front Genet. 2020;11:601870. 10.3389/fgene.2020.601870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Minhaj FS, Ogale YP, Whitehill F, Schultz J, Foote M, Davidson W, et al. ; Monkeypox Response Team 2022. Monkeypox Response Team 2022. Monkeypox outbreak—nine states, May 2022. MMWR Morb Mortal Wkly Rep. 2022;71:764–9. 10.15585/mmwr.mm7123e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Y, Olson VA, Laue T, Laker MT, Damon IK. Detection of monkeypox virus with real-time PCR assays. J Clin Virol. 2006;36:194–203. 10.1016/j.jcv.2006.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Y, Zhao H, Wilkins K, Hughes C, Damon IK. Real-time PCR assays for the specific detection of monkeypox virus West African and Congo Basin strain DNA. J Virol Methods. 2010;169:223–7. 10.1016/j.jviromet.2010.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.US Health and Human Services. HHS expanding monkeypox testing capacity to five commercial laboratory companies [cited 2022 Dec 27]. https://www.hhs.gov/about/news/2022/06/22/hhs-expanding-monkeypox-testing-capacity-five-commercial-laboratory-companies.html
- 24.Gigante CM, Korber B, Seabolt MH, Wilkins K, Davidson W, Rao AK, et al. Multiple lineages of monkeypox virus detected in the United States, 2021-2022. Science. 2022;378:560–5. 10.1126/science.add4153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention. Update on managing monkeypox in patients receiving therapeutics [cited 2022 Nov 17]. https://emergency.cdc.gov/han/2022/han00481.asp
- 26.Saunders KE, Van Horn AN, Medlin HK, Carpenter A, Lee PA, Gutierrez L, et al. Monkeypox in a young infant—Florida, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:1220–1. 10.15585/mmwr.mm7138e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hennessee I, Shelus V, McArdle CE, Wolf M, Schatzman S, Carpenter A, et al. ; California Department of Public Health Monkeypox Pediatric Working Group; CDC Monkeypox Pediatric Working Group. CDC Monkeypox Pediatric Working Group. Epidemiologic and clinical features of children and adolescents aged <18 years with monkeypox—United States, May 17–September 24, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:1407–11. 10.15585/mmwr.mm7144a4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mendoza R, Petras JK, Jenkins P, Gorensek MJ, Mableson S, Lee PA, et al. Monkeypox virus infection resulting from an occupational needlestick—Florida, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:1348–9. 10.15585/mmwr.mm7142e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hubach RD, Owens C. Findings on the monkeypox exposure mitigation strategies employed by men who have sex with men and transgender women in the United States. Arch Sex Behav. 2022;51:3653–8. 10.1007/s10508-022-02423-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Delaney KP, Sanchez T, Hannah M, Edwards OW, Carpino T, Agnew-Brune C, et al. Strategies adopted by gay, bisexual, and other men who have sex with men to prevent monkeypox virus transmission—United States, August 2022. MMWR Morb Mortal Wkly Rep. 2022;71:1126–30. 10.15585/mmwr.mm7135e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kriss JL, Boersma PM, Martin E, Reed K, Adjemian J, Smith N, et al. Receipt of first and second doses of JYNNEOS vaccine for prevention of monkeypox—United States, May 22–October 10, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:1374–8. 10.15585/mmwr.mm7143e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Millman AJ, Denson DJ, Allen ML, Malone JA, Daskalakis DC, Durrence D, et al. ; Atlanta Black Gay Pride Festival Monkeypox Response Team. A health equity approach for implementation of JYNNEOS vaccination at large, community-based LGBTQIA+ events—Georgia, August 27–September 5, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:1382–883. 10.15585/mmwr.mm7143e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Owens LE, Currie DW, Kramarow EA, Siddique S, Swanson M, Carter RJ, et al. JYNNEOS vaccination coverage among persons at risk for mpox—United States, May 22, 2022–January 31, 2023. MMWR Morb Mortal Wkly Rep. 2023;72:342–7. 10.15585/mmwr.mm7213a4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mbaeyi SA, Bozio CH, Duffy J, Rubin LG, Hariri S, Stephens DS, et al. Meningococcal Vaccination: Recommendations of the Advisory Committee on Immunization Practices, United States, 2020. MMWR Recomm Rep. 2020;69:1–41. 10.15585/mmwr.rr6909a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nelson NP, Weng MK, Hofmeister MG, Moore KL, Doshani M, Kamili S, et al. Prevention of hepatitis A virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices, 2020. MMWR Recomm Rep. 2020;69:1–38. 10.15585/mmwr.rr6905a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Daskalakis D, Romanik N, Jha AK. Lessons from the mpox response. JAMA. 2024;331:387–8; Epub ahead of print. 10.1001/jama.2023.27868 [DOI] [PubMed] [Google Scholar]
- 37.Lin KY, Chen GJ, Lee YL, Huang YC, Cheng A, Sun HY, et al. Hepatitis A virus infection and hepatitis A vaccination in human immunodeficiency virus-positive patients: A review. World J Gastroenterol. 2017;23:3589–606. 10.3748/wjg.v23.i20.3589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Singer M, Bulled N, Ostrach B, Mendenhall E. Syndemics and the biosocial conception of health. Lancet. 2017;389:941–50. 10.1016/S0140-6736(17)30003-X [DOI] [PubMed] [Google Scholar]
- 39.Tsai AC, Mendenhall E, Trostle JA, Kawachi I. Co-occurring epidemics, syndemics, and population health. Lancet. 2017;389:978–82. 10.1016/S0140-6736(17)30403-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oprea C, Ionuț Popa, Ianache I, Păun A, Vasile S, Grațiela Țârdei, et al. Monkeypox, severe hepatitis A, and syphilis in an HIV returning traveler from Spain to Romania. Travel Med Infect Dis. 2022;50:102455. 10.1016/j.tmaid.2022.102455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Friesema IH, Sonder GJ, Petrignani MW, Meiberg AE, van Rijckevorsel GG, Ruijs WL, et al. Spillover of a hepatitis A outbreak among men who have sex with men (MSM) to the general population, the Netherlands, 2017. Euro Surveill. 2018;23:1800265. 10.2807/1560-7917.ES.2018.23.23.1800265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vaughan G, Goncalves Rossi LM, Forbi JC, de Paula VS, Purdy MA, Xia G, et al. Hepatitis A virus: host interactions, molecular epidemiology and evolution. Infect Genet Evol. 2014;21:227–43. 10.1016/j.meegid.2013.10.023 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional information on concurrent outbreaks of hepatitis A, invasive meningococcal disease, and mpox, Florida, USA, 2021–2022.


