Abstract
Inhibition of p53 function, through either mutation or interaction with viral or cellular transforming proteins, correlates strongly with the oncogenic potential. Only a small percentage of human T-cell lymphotropic virus type 1 (HTLV-1)-transformed cells carry p53 mutations, and mutated p53 genes have been found in only one-fourth of adult T-cell leukemia cases. In previous studies, we demonstrated that wild-type p53 is stabilized and transcriptionally inactive in HTLV-1-transformed cells. Further, the viral transcriptional activator Tax plays a role in both the stabilization and inactivation of p53 through a mechanism involving the first 52 amino acids of p53. Here we show for the first time that phosphorylation of p53 inactivates p53 by blocking its interaction with basal transcription factors. Using two-dimensional peptide mapping, we demonstrate that peptides corresponding to amino acids 1 to 19 and 387 to 393 are hyperphosphorylated in HTLV-1-transformed cells. Moreover, using antibodies specific for phosphorylated Ser15 and Ser392, we demonstrate increased phosphorylation of these amino acids. Since HTLV-1 p53 binds DNA in a sequence-specific manner but fails to interact with TFIID, we tested whether phosphorylation of the N terminus of p53 affected p53-TFIID interaction. Using biotinylated peptides, we show that phosphorylation of Ser15 alone inhibits p53-TFIID interaction. In contrast, phosphorylation at Ser15 and -37 restores TFIID binding and blocks MDM2 binding. Our studies provide evidence that HTLV-1 utilizes the posttranslational modification of p53 in vivo to inactivate function of the tumor suppressor protein.
Mutation of p53 is common in human cancers, being inactivated in over half of all tumors (17). Following an intense period of research into the biochemical function of this critical cellular protein, it is evident that in response to various types of DNA damage and cell stress, the p53 tumor suppressor functions to integrate cellular responses including growth arrest or apoptosis (11, 17), through transcriptional activation of cell cycle control proteins. Consistent with its tumor suppressor function, overexpression of wild-type p53 was found to suppress cell growth of human neoplastic colon (2) and bone tumor (4, 5) cell lines. Further, studies using a human glioblastoma cell line encoding an endogenous mutant p53 gene and a transfected inducible wild-type p53 showed that upon induction of wild-type p53, cells arrested in G1 (27). The biochemical activity required for p53 tumor suppression and presumably the response to DNA damage involves the ability of p53 to bind DNA in a sequence-specific manner and function as a transcriptional activator (7, 8, 34). Clearly, expression of p53 in cells activates, through consensus p53 binding sites, a number of genes involved in p53-induced cell arrest or apoptosis. These include the genes encoding GADD45, WAF1, MDM2, Bax, and cyclin G (17, 21). Although the importance of the DNA binding properties of p53 are evident, the regulation of p53 function remains less well understood.
p53 is a tetrameric, sequence-specific transcription factor with an N-terminal activation domain (amino acids 1 to 50), a sequence-specific DNA binding central core (amino acids 100 to 300), and a multifunctional carboxy-terminal domain (amino acids 300 to 393) (17). Although mutations in p53 that arise in human cancers generally cluster in its DNA binding domain (14), binding of oncoproteins to the amino-terminal region of p53 have also been associated with disease (17). The amino-terminal activation domain of p53 interacts with several general transcription factors including the TATA box binding protein (TBP) and TBP-associated factors (TAFs), components of TFIID (25, 44). Association of the cellular proteins MDM2 and E2F, as well as the viral oncoproteins adenovirus E1B and hepatitis B virus X protein, with the N terminus of p53 have been shown to block its activation function by disrupting p53-TFIID interactions (24, 32, 45). The carboxy terminus of p53 can function as an autonomous domain capable of binding nonspecifically to different forms of DNA, such as damaged DNA, and reannealing complementary single strands of DNA or RNA (17). The carboxy terminus of p53 also contains an oligomerization domain as well as sequences that modulate DNA binding.
The human T-cell lymphotropic virus type 1 (HTLV-1) is the etiologic agent of an aggressive and fatal disease adult T-cell leukemia and the neurodegenerative disease tropical spastic paraparesis/HTLV-1-associated myelopathy (10, 33, 36, 51). HTLV-1 is also associated with arthritis, uveitis, infective dermatitis, and mild immunosuppression (16, 18, 40). Although many transformed uninfected T-cell lines contain a mutated p53 gene, only a minority of HTLV-1-transformed cells carry p53 mutations. In addition, mutated p53 genes have been found in only a fourth of adult T-cell leukemia cases (31, 39). In contrast to untransformed peripheral blood T lymphocytes, we have shown that the half-life of the p53 protein is increased in the majority of HTLV-1-transformed cells, suggesting its functional inactivation (37). In addition, following gamma irradiation, no significant induction of p53 or p53-responsive genes, including those encoding p21WAF1, GADD45, MDM2, and Bax, was observed in HTLV-1-transformed cells compared to HTLV-1-negative cells (3, 35).
To determine the mechanism of Tax-mediated p53 inactivation, we characterized biochemical properties of HTLV-1 p53. Our results demonstrate that p53 from HTLV-1-transformed cells is tetrameric and binds DNA in a sequence-specific manner. The transcriptional activity of p53, however, is regulated by posttranslational modification of the protein. Specifically, we demonstrate that phosphorylation of Ser15 inhibits the interaction of the N-terminal activation domain with the basal transcription factor TFIID. Consistent with recent reports by Shieh et al. (41), we find that phosphorylation of Ser15 and -37 restores TFIID binding. Thus, in addition to uncovering a novel interaction pathway for p53 inactivation in HTLV-1-transformed cells, we demonstrate that the interaction of p53 with TFIID and MDM2 is tightly regulated by the specific phosphorylation pattern of Ser15 and -37. In this report we demonstrate for the first time the use of altered posttranslational modifications of p53 by a viral activator to abrogate p53 function.
MATERIALS AND METHODS
Cell lines.
The human myeloid cell line ML-1 and the HTLV-1-transformed cell lines C81, MT-2, and Hut102 were grown in RPMI medium supplemented with 10% fetal bovine serum. GM47.23 cells were grown in Dulbecco modified Eagle medium supplemented with 10% fetal bovine serum. Wild-type p53 expression was induced in GM47.23 by addition of dexamethasone (50 μg/ml) to cultures 24 h prior to assaying cells.
DNA binding assay.
Cells were lysed in buffer A (50 mM Tris-HCl [pH 7.4], 0.25 M NaCl, 0.1% Triton X-100, 5 mM EDTA, 0.5 mM phenylmethylsulfonyl fluoride [PMSF], 0.5 mM trypsin inhibitor, 0.05 mM microcystin-LR, 1 μg of pepstatin A per ml, 2 mM dithiothreitol [DTT]), and 100 μg was incubated with biotinylated oligonucleotides containing the wild-type (5′-GCCGAATTCGAACATGTCCGAACATGTTGAGATCTGCC-3′; 5′-AATTCTCGAGCAGAACATGTCTAAGCATGCTGGGCTCGAG-3′) and mutant (5′-GCCGAATTCGAAAATTTCCGAATCCTTTGAGATCTGCC-3′; 5′-AATTCTCGAGAAAATTTCTAAGAATTCTGGGCTCGAG-3′) p53 binding sites from WAF1 and GADD45 promoters, respectively. Using magnetic streptavidin beads (Dynal), the bound complexes were captured and washed four times with binding buffer [50 mM Tris (pH 7.6), 50 mM NaCl, 0.5 mM EDTA, 1 mM DTT, 5 mM MgCl2, 0.1% Triton X-100 5% glycerol, 10 μg of poly(dI/dC) per ml, 2.5 mg of bovine serum albumin per ml]. Proteins were separated by electrophoresis on 4 to 20% Tris-glycine gels (Novex), transferred to polyvinylidene fluoride membranes (Millipore), and analyzed by Western blot analysis (35).
Electrophoretic mobility shift assay (EMSA).
Cells (2 × 106 to 5 × 106) were incubated for 15 min on ice in 20 mM HEPES (pH 7.9)–20 mM Na F–1 mM Na3VO4–1 mM Na4P2O7–1 mM EDTA–1 mM EGTA–1 mM DTT–0.5 mM PMSF–1 μg each of leupeptin, aprotinin, and pepstatin per ml. After addition of Nonidet P-40 (NP-40) to 0.2%, the cells were incubated on ice for an additional 15 min and resuspended by vortexing for 15 s, and nuclei were pelleted in a microcentrifuge at 16,000 × g for 20 s. The nuclear pellet was extracted for 15 min in the above buffer that had been adjusted to 0.2% NP-40–0.4 M NaCl and then cleared by centrifugation at 16,000 × g for 15 min. For DNA binding assays, 5 to 30 μg of nuclear extract was incubated for 10 min at 4°C in the presence or absence of antibody PAb421 (Oncogene Research) prior to incubation at room temperature for 30 min in binding buffer (6 mM HEPES [pH 7.9], 1 mM DTT, 6% glycerol, 0.5 mM PMSF) and 0.5 ng of 32P-end-labeled oligonucleotide encoding the WAF1 promoter binding site (see above). Competition for p53 binding activity was carried out in the presence of a 100-fold excess of either unlabeled wild-type or mutant WAF1 oligonucleotide probe (see above).
In vivo labeling and phosphopeptide mapping.
Cells were in vivo labeled with [32P]orthophosphate. Exponentially growing HTLV-1-transformed cells or dexamethasone-induced GM47.23 cells were washed with phosphate-free RPMI supplemented with 10% dialyzed fetal bovine serum, incubated in the same medium for 30 min, and then incubated for 20 min in phosphate-free medium containing 0.6 mCi of [32P]orthophosphate (NEN) per ml. Cells were washed twice with ice-cold phosphate-buffered saline and lysed in buffer A (see above). Lysates were precleared with either preimmune antibody (C81 cells) or antibody PAb421 (GM47.23 cells). p53 protein was immunoprecipitated with PAb1801 and separated by electrophoresis on sodium dodecyl sulfate–8% polyacrylamide gels, and the p53 band was excised from the gel. After alkylation and reduction (47), tryptic/chymotryptic digestion was performed on the samples, which were then treated with performic acid. Synthetic peptides (47) which correspond to peptides 1, 4, 5, and 8 were combined with each sample (equivalent counts per minute) prior to running the two-dimensional map for identification of 32P-labeled peptides. This step allowed us to correlate peptide migration with phosphorylation of specific peptides. Samples were electrophoresed and subjected to chromatography as described previously (47).
Peptide binding assay.
Biotinylated unphosphorylated and monophosphorylated peptides (1 μg) which correspond to amino acids 1 to 39 of p53 were incubated in kinase buffer (13.75 mM HEPES [pH 7.5]–1.3 mM spermidine–7.28 mM MgCl2–11% glycerol–0.55% NP-40–27.5 mM KCl–0.55 mM DTT–0.2 mM ATP in the presence of 400 ng of double-stranded DNA) either with or without DNA-dependent protein kinase (DNA PK; Promega). Wortmannin (0.5 μM; Sigma) was then added to mock-treated or DNA PK-treated peptides to inhibit any further DNA PK activity. Peptide (100 ng) was incubated with a 1 M fraction of a phosphocellulose column or 30 μg of whole-cell extract to a final volume of 200 μl in lysis buffer A (described above) for 2 h at 4°C, with rotation. Bound complexes were captured with magnetic streptavidin beads (Dynal), washed four times with buffer A, separated by electrophoresis, and analyzed by Western blot analysis with antibody to TFIID (Santa Cruz Biotechnology).
Chymotryptic digestion of p53.
Cellular extracts which represented equal amounts of p53 were treated with 200 ng of chymotrypsin for 10 min at room temperature. Reactions were stopped by addition of an equal volume of sodium dodecyl sulfate sample buffer, boiled, and separated by electrophoresis on 4 to 20% Tris-glycine gels. After transfer to a polyvinylidene fluoride membrane, Western blot analysis was done with antibody DO-1 or PAb421 (Oncogene Research).
P-Ser15 and P-Ser37 antibody characterization.
Polyclonal antibodies specific for phosphorylated Ser15 and Ser392 (P-Ser15 and P-Ser392, respectively) were raised against the synthetic peptides Ac-11-22(15P)C and Ac-C385-393(392P) conjugated to keyhole limpet hemocyanin. The peptides used for immunization were Ac-11-22(15P)C [Ac-EPPLS(PO3)QETFSDLC-NH2] and Ac-C385-393(392P) [Ac-CFKTEGPDS(PO3)D-OH]. Antiserum from immunized rabbit was affinity purified by using SulfoLink (Pierce) coupled with phosphorylated peptide. The purified antibody was then passed through a column coupled with unphosphorylated peptide to deplete antibodies that react with unphosphorylated p53. Antibody specificity was analyzed by Western blot analysis and enzyme-linked immunosorbent assay (ELISA). Briefly, 100 ng of peptide (either phosphorylated or unphosphorylated) was bound for 1 h at 37°C to microtiter plate wells at pH 8.0. Antibodies were incubated for 1 h at room temperature. The wells were incubated with peroxidase-conjugated secondary antibody, and specifically bound antibody was detected at 490 nm, using orthophenylenediamine.
RESULTS
HTLV-1 p53 binds to DNA in a sequence-specific manner.
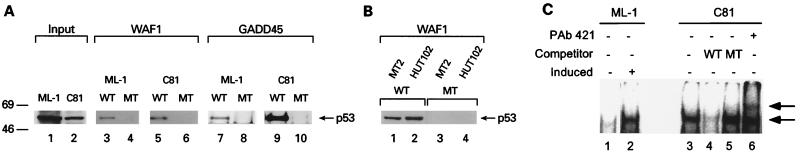
Transforming proteins of several viruses inactivate p53 by protein-protein interaction, blocking sequence-specific DNA binding, transactivation, or targeting p53 for rapid degradation (reference 17 and references therein); however, no in vivo association between Tax and p53 has been demonstrated. To address how Tax inactivates p53 function, we first examined properties of p53 from HTLV-1-transformed cells known to be important for p53 transactivation activity. Using biotinylated-DNA oligomers, we found that p53 from HTLV-1-transformed C81, MT2, or HUT102 cells and untransformed ML-1 cells bound specifically to p53 recognition sequences. p53 from control or HTLV-1-transformed cells bound to oligonucleotides corresponding to recognition sequences from the WAF1 and GADD45 promoters (Fig. 1A, lanes 3, 5, 7, and 9; Fig. 1B, lanes 1 and 2); neither bound to mutated sites (Fig. 1A, lanes 4, 6, 8, and 10; Fig. 1B, lanes 3 and 4).
FIG. 1.
p53 from HTLV-1-transformed cells binds to DNA in a sequence-specific manner. (A) ML-1 and C81 cell lysates were incubated with biotinylated oligonucleotides containing either the wild-type (WT) or mutant (MT) p53 binding sites from WAF1 (lanes 3 to 6) and GADD45 (lanes 7 to 10) promoters. Immunoblot analysis was performed to detect DNA-bound p53. One-fourth of the input amount of p53 is shown in lanes 1 (ML-1) and 2 (C81). Sizes are indicated in kilodaltons. (B) The ability of endogenous p53 from two additional HTLV-1-transformed cell lines (MT-2 and HUT102) to bind to the WAF1 promoter was tested. (C) EMSAs of a 32P-end-labeled WAF1 oligonucleotide probe were performed with nuclear lysates of ML-1 cells untreated (lane 1) or induced with 6 Gy of ionizing radiation (lane 2) and with nuclear extracts of C81 cells (lane 3). Specificity of binding was confirmed by competition with a 100-fold excess of either cold wild-type (WT; lane 4) or mutated (MT; lane 5) WAF1 probe. The specific bound complex is indicated by the lower arrow. PAb421 supershifted this bound complex (lane 6), indicated by the upper arrow.
In addition, we assessed p53 DNA binding by EMSA (Fig. 1C). This assay shows that p53 from C81 cells binds to the WAF1 p53 binding site in a sequence-specific manner (Fig. 1C, lane 3 to 5), similar to that of induced ML-1 cells (Fig. 1C, lane 2). Both the HTLV-1 and ML-1 p53 gel shift complexes were competed by the wild-type but not a mutated WAF1 probe (Fig. 1C, lanes 4 and 5, and data not shown). Further, this bound complex could be supershifted with a p53-specific antibody (Fig. 1C, lane 6). These findings confirm the results obtained in the biotinylated-p53 binding assay.
To further study the properties of p53 in HTLV-1-transformed cells, we analyzed the crude molecular weight of the p53 complex. Consistent with the DNA binding properties, Superose-6B chromatography indicated that HTLV-1 p53 exists as tetramers (data not shown).
DNA bound HTLV-1 p53 fails to bind TBP.
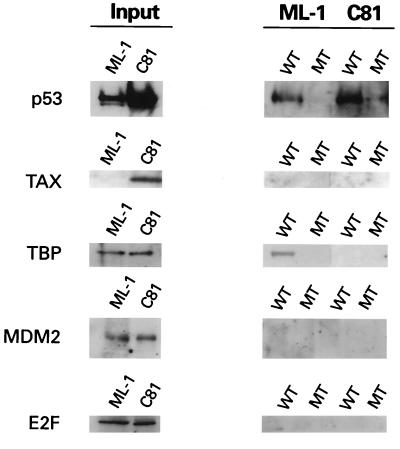
The activation domain of p53 is reported to associate with several cellular proteins, including MDM2, the transcription factor E2F/DP1, TFIIH, CBP/p300, and TAFs (1, 12, 23–25, 32, 44). Interestingly, we observed a distinct difference in the proteins associated with DNA-bound p53 from control and HTLV-1-transformed cells. As expected, DNA-bound p53 from serum-starved ML-1 control cells was associated with TBP (Fig. 2). In contrast, no TBP was detected in DNA-bound p53 complexes from C81 extracts. Although MDM2 and E2F were present in extracts from both cell lines, neither was present in p53 DNA-bound complexes (Fig. 2). Consistent with the immunoprecipitation results of Gartenhaus and Wang (9), Tax protein was not found in DNA-bound p53 complexes (Fig. 2). These data suggest that although p53 from HTLV-1-transformed cells is tetrameric and competent to bind DNA, the ability of the N-terminal activation domain to interact with regulatory proteins is impaired.
FIG. 2.
HTLV-1 p53 DNA complex does not contain TBP or MDM2. DNA-bound p53 complexes isolated as described for Fig. 1 were analyzed by immunoblotting for the presence of p53, Tax, TBP, MDM2, and E2F. One-fourth of the input amount is shown. WT, wild type; MT, mutant.
Hyperphosphorylation of HTLV-1 p53 at Ser15 and Ser392.
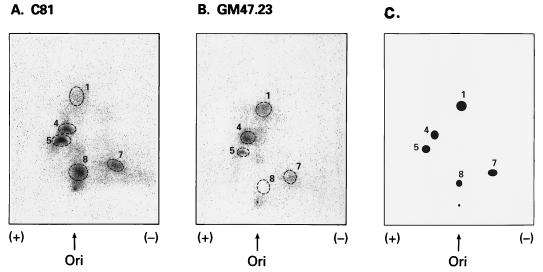
Since the p53 sequence is wild type in HTLV-1-transformed cells, but there is no response to ionizing radiation in respect to p53 cell cycle arrest and induction of p21, MDM2, and GADD45 mRNAs (35), it is possible that inactivation occurs through posttranslational modifications. Phosphopeptide mapping (47) was used to assay for differences in phosphorylation between HTLV-1 (C81) and wild-type (GM47.23) p53. C81 and dexamethasone-induced GM47.23 cells were metabolically labeled with 32P for 20 min. p53 was immunoprecipitated from cell lysates and separated by gel electrophoresis. After alkylation and reduction, the proteins were digested with trypsin and chymotrypsin and subjected to two-dimensional peptide mapping. Equivalent amounts of 32P-labeled p53 from the two cell lines were analyzed. To ensure identification of peptide migration, synthetic peptides corresponding to each of the tryptic/chymotryptic fragments were mixed with each sample. Figure 3C represents a diagram of the stained synthetic peptides. As shown in Fig. 3A and Table 1, a significant increase in HTLV-1 p53 phosphorylation was seen for N-terminal peptide 5 (diphosphorylated amino acids 1 to 19) and C-terminal peptide 8 (monophosphorylated amino acids 387 to 393). Conversely, the ratios of peptide 4 (monophosphorylated amino acids 1 to 19) and peptide 1 (monophosphorylated amino acids 25 to 53) were significantly decreased in C81 cells. Similar results were seen for the HTLV-1-transformed line, MT-2 (data not shown). Interestingly, these changes in phosphorylation patterns are similar to that reported for inactive p53 mutants [Ile237]p53, [His273]p53, and [Ala143]p53 (47).
FIG. 3.
Phosphopeptide map of HTLV-1 p53. Tryptic/chymotryptic digestion of purified in vivo 32P-labeled p53 from C81 (A) and dexamethasone-induced GM47.23 (B) cells were performed as described previously (47) and separated by electrophoresis and chromatography. (C) Diagram showing the migration of synthetic peptides where individual phosphopeptides were assigned numbers 1 to 8 (47). Ori, origin.
TABLE 1.
32P radioactivity in phosphopeptides from C81 and GM47.23 (wild-type) cells
| Phosphopeptidea
|
% of total labeled peptideb
|
|||
|---|---|---|---|---|
| Peptide no.a | Sequence | No. of sites phosphorylated (position) | GM47.23 (wild type) | C81 |
| 1 | p53-(25–53) | 1 (S37) | 18.4 ± 5.4 | 9.5 ± 2.9 |
| 4 | Ac-p53-(1–19) | 1 (S9) | 36.9 ± 6.3 | 17.7 ± 0.8 |
| 5 | Ac-p53-(1–19) | 2 (S9 + S15) | 12.4 ± 3.3 | 22.0 ± 1.7 |
| 7 | p53-(307–319) | 1 (S315) | 11.8 ± 1.8 | 15.7 ± 1.3 |
| 8 | p53-(387–393) | 1 (S392) | 8.2 ± 2.1 | 28.5 ± 1.6 |
Numbered as indicated in Fig. 3. Synthetic peptide standards corresponding to phosphopeptides 1, 4, 5, 7, and 8 were used.
The amount of 32P associated with each phosphopeptide was determined by using a PhosphorImager and the ImageQuant program (Molecular Dynamics) and is expressed as the percentage of total radioactivity recovered after digestion and performic acid treatment. Values are given as the mean ± standard deviation of three independent experiments.
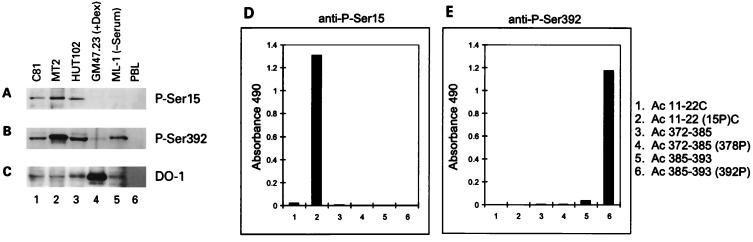
Using affinity-purified antibodies specific for either P-Ser15 or P-Ser392, we confirmed the increased phosphorylation at these sites from three HTLV-1-transformed cell lines compared to control GM47.23 or ML-1 cells (Fig. 4). Although the ML-1 and GM47.23 cells contained at least as much p53 as the three HTLV-1-transformed lines as detected by antibody DO-1 (Fig. 4C), only the HTLV-1-transformed lines reacted strongly with P-Ser15 antibody (Fig. 4A). Interestingly, p53 from serum-starved ML-1 cells, which is capable of binding TBP, has little phosphorylation at Ser15 but is phosphorylated at Ser392 (Fig. 4A and B, lane 5).
FIG. 4.
p53 phosphorylation-specific antibodies confirm increased phosphorylation of Ser15 and Ser392 in HTLV-1-transformed cells. Antibodies specific for P-Ser15 (A) and P-Ser392 (B) and antibody DO-1, which reacts with phosphorylated or unphosphorylated p53 (C), were used in immunoblot analysis of lysates from HTLV-1-transformed cell lines C81 (lane 1), MT-2 (lane 2), and HUT102 (lane 3) or from untransformed dexamethasone-induced GM47.23 cells (lane 4), serum-starved ML-1 cells (lane 5), and peripheral blood leukocytes (PBL; lane 6). (D and E) Characterization of P-Ser15- and P-Ser392-specific antibodies. The graphs of absorbance at 490 nm represent the reactivities of the sera against the synthetic peptides indicated at the right.
The specificity of the phospho-p53 antibodies was determined by Western blot analysis (data not shown) and ELISA against unphosphorylated and monophosphorylated peptides (Fig. 4D and E). Peptides Ac-11-22(15P)C, Ac-11-22C, Ac-C372-385(378P), Ac-C372-385, Ac-C385-393(392P), and Ac-C385-393 were bound to microtiter wells and incubated with the corresponding antibodies, and reactivity was measured with orthophenylenediamine as a substrate. Anti-P-Ser15 antibody reacted specifically with phosphorylated N-terminal peptide Ac-11-22(15P)C (Fig. 4D, lane 2) but not unphosphorylated N-terminal peptide Ac-11-22C (lane 1) or the C-terminal peptides (lanes 3 to 6). Conversely, anti-P-Ser392 antibody reacted specifically with phosphorylated C-terminal peptide Ac-C385-393(392P) (Fig. 4E, lane 6) but not the unphosphorylated C-terminal (lane 5) or N-terminal (lanes 1 and 2) peptides.
HTLV-1 p53 is conformationally distinct.
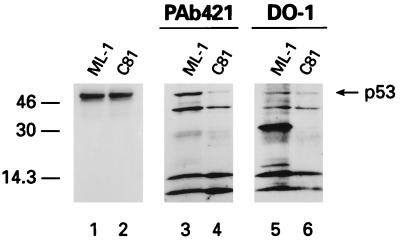
To determine if phosphorylation had an effect on the conformation of p53, chymotryptic digests were performed on cellular extracts from HTLV-1-transformed and control cells. p53 fragments were detected by Western blot analysis using antibodies specific for C-terminal (PAb421) or N-terminal (DO-1) epitopes (Fig. 5). While no significant difference in digestion pattern was observed with the C-terminal antibody (Fig. 5, lanes 3 and 4), antibody DO-1 revealed two major bands of about 30 and 16 kDa from ML-1-digested p53 that were absent in p53 from C81 cells (Fig. 5, lanes 5 and 6). Since the DO-1 epitope corresponds to amino acids 18 to 30, these peptides likely represent N-terminal fragments extending to chymotrypsin sites in the central core domain. The fact that these fragments are not present in the C81 p53 digest suggests that N-terminal chymotrypsin sites are more sensitive to enzyme digestion. Bands at 14.3 kDa and lower, which appear in all digests, likely represent protein fragments which are cross-reactive to the secondary antibody (data not shown). This result is consistent with phosphorylation inducing a conformational change in the N terminus of HTLV-1 p53. Consistent with this suggestion, Shieh et al. have recently reported that phosphorylation of the N terminus of p53 by DNA PK correlates with calpain sensitivity (41).
FIG. 5.
Western blot analysis of chymotryptic digestion of p53 from serum-starved ML-1 and C81 cells stained with either antibody PAb421, a C-terminal epitope (lanes 3 and 4), or DO-1, an N-terminal epitope (lanes 5 and 6). Lanes 1 and 2 show the amount of p53 in each extract prior to digestion. Sizes are indicated in kilodaltons.
Phosphorylation of the activation domain of p53 at Ser15 impairs TFIID binding.
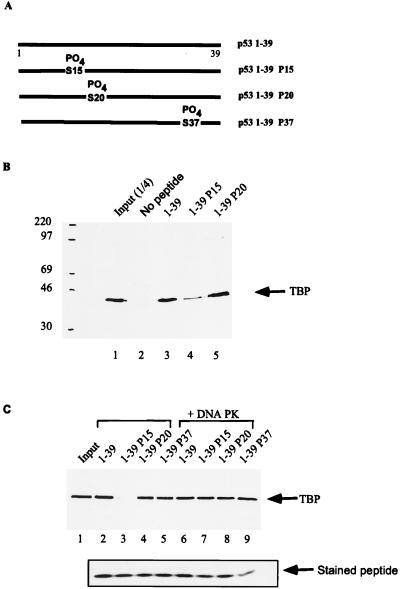
To directly determine whether phosphorylation of p53 at Ser15 interfered with its binding to TFIID, we synthesized a series of p53 activation domain peptides (Fig. 6A). The peptides contained amino acids 1 to 39 with no modifications or with phosphoserine substitution at Ser15, -20, or -37. The biotinylated peptides were incubated with the 1 M phosphocellulose TFIID fraction from HeLa extracts, and p53 complexes were precipitated with streptavidin agarose beads. The 1 M fraction is highly enriched for TFIID complexes but contains no detectable DNA PK activity (data not shown). Analysis of TBP in the precipitates represents the interaction of p53 with holo-TFIID, which could be mediated through interaction with TBP or TAFs. Zhou et al. (52) have demonstrated that the majority of TBP present in this phosphocellulose fraction is a component of TFIID.
FIG. 6.
Phosphorylation at p53 Ser15 inhibits TFIID binding. (A) Diagram of biotinylated peptides which correspond to the activation domain (amino acids 1 through 39) of human p53. Biotinylated peptides were incubated with the 1 M phosphocellulose TFIID fraction from HeLa cell extracts. Bound complexes were captured with magnetic streptavidin beads and analyzed by Western blotting for the presence of TBP, using an anti-TFIID antibody (Santa Cruz). (B) Lane 1, one-fourth of input TFIID fraction; lane 2, no peptide; lane 3, unphosphorylated 1-39 peptide; lane 4, 1-39 peptide phosphorylated at Ser15; lane 5, 1-39 peptide phosphorylated at Ser20. Sizes are indicated in kilodaltons. (C) Peptides were either mock treated (lanes 2 to 5) or treated with DNA PK. The reaction was stopped by addition of 0.5 μM wortmannin. Lane 1, one-fourth of input TFIID fraction; lane 2, unphosphorylated 1-39 peptide; lane 3, 1-39 peptide phosphorylated at Ser15; lane 4, 1-39 peptide phosphorylated at Ser20; lane 5, 1-39 peptide phosphorylated at Ser37; lane 6, DNA PK-treated peptide 1-39; lane 7, DNA PK-treated peptide 1-39P15; lane 8, DNA PK-treated peptide 1-39P20; lane 9, DNA PK-treated peptide 1-39P37. Below lanes 2 to 9 are captured peptides stained with Coomassie blue to determine the amount of peptide recovered in the binding assays.
As shown in Fig. 6B, the wild-type p53 peptide bound to TFIID 36-fold above background, as determined by densitometric scanning of Western blots for TBP, the basic subunit of TFIID (Fig. 6B, lanes 2 and 3). In contrast, the p53 peptide containing P-Ser15 was significantly diminished in its ability to interact with TFIID (Fig. 6B, lane 4; 85% reduction in TBP binding). As a control, we show that a p53 peptide containing P-Ser20 interacted with TFIID equivalently to wild-type peptide (Fig. 6B, lane 5).
These results are in apparent contrast to recent observations by Shieh et al., who demonstrated that treatment of p53 with DNA PK, which phosphorylates Ser15 and Ser37 (41), did not inhibit interaction of p53 with TFIID as measured by in vitro transcription. To directly test whether there was a difference in TFIID binding between Ser15 and Ser15/Ser37 peptides, the peptides in Fig. 6A were treated with DNA PK. After treatment, the DNA PK inhibitor wortmannin was added to all samples to prevent further DNA PK activity. Comparison of TFIID binding activity before and after DNA PK treatment demonstrates that, in fact, there is a dramatic difference between TFIID binding to the P-Ser15 and P-Ser15/37 peptides (Fig. 6C, lanes 3 and 7). The lack of TFIID binding was not due to the inability of the peptides to be captured on streptavidin-conjugated beads since equivalent amounts of peptide were recovered (Fig. 6C, lanes 2 to 9), as determined by Coomassie blue staining.
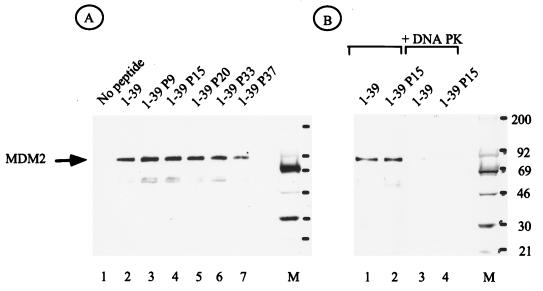
Interaction of MDM2 with the p53 N terminus is regulated by phosphorylation at Ser15 and -37.
The ability of the peptides to interact with MDM2 was also analyzed. As shown in Fig. 7A (lane 2), the wild-type p53 peptide bound to MDM2. When each of the monophosphorylated p53 peptides was analyzed, no significant reduction in MDM2 binding was observed (Fig. 7A, lanes 3 to 7). In particular, the Ser15 peptide, which failed to interact with TFIID, displayed no defect in MDM2 binding. Strikingly, when either the wild-type or Ser15 peptide was treated with DNA PK, resulting in phosphorylation of Ser15 and Ser37, a dramatic decrease in MDM2 binding was observed (Fig. 7B, lanes 1 to 4). The ability of these same peptides to interact with TFIID (Fig. 6C) provides an internal control to demonstrate that the peptides are not inactivated or degraded during DNA PK treatment.
FIG. 7.
Phosphorylation of p53 by DNA PK abolishes MDM2 binding. (A) Western blot analysis of MDM2 from whole-cell extracts bound to peptides as indicated at the top. (B) MDM2 binding to peptides 1-39 (lane 1), 1-39 P15 (lane 2), 1-39 treated with DNA PK (lane 3), and 1-39 P15 treated with DNA PK (lane 4). The lane M indicates positions of molecular weight standard in kilodaltons.
DISCUSSION
The p53 tumor suppressor protein is critical for controlled cell proliferation and protection against oncogenic transformation. We previously demonstrated that expression of the HTLV-1 Tax protein was sufficient for stabilization and transcriptional inactivation of wild-type p53 (35). In this report, we demonstrate that the p53 transcriptional inactivation in HTLV-1-transformed cells is not due to its inability to bind DNA in a sequence-specific manner but rather results from a failure of DNA-bound HTLV-p53 to interact with the basal transcription factor TFIID. Further, the lack of TFIID binding can be attributed to phosphorylation of p53 at Ser15.
p53 contains distinct phosphorylated regions in the N and C termini, and phosphorylation has been proposed as a mechanism for regulating the transition of p53 between inactive and active conformations (30, 46). The N-terminal transactivation domain of human p53 has potential phosphorylation sites at Ser6, -9, -15, -20, -33, and -37. Casein kinase I phosphorylates N-terminal residues Ser6 and -9 (29), DNA PK phosphorylates Ser15 and -37 (19), and Jun N-terminal kinase phosphorylates murine Ser34, which corresponds to human p53 Ser33 (28). Three phosphorylation sites and their respective kinases have been identified in the C terminus of human p53: Ser315, p34cdc2 and cdk2; Ser378, protein kinase C; and Ser392, casein kinase II (reference 43 and references therein).
Although p53 can be phosphorylated in vitro, the role of phosphorylation at specific sites has yet to be shown for the function and regulation of p53 in vivo. Fiscella and colleagues (7a) have reported that mutation of Ser15 to alanine results in partial failure of p53 to inhibit cell cycle progression. In addition, Mayr and coworkers (26) have shown that simultaneous mutation of several N-terminal serine residues of p53 causes a reduction in the ability of recombinant p53 to suppress transformation, as well as decrease its ability to transactivate a reporter construct. Further, several laboratories find that phosphorylation of the C terminus of p53 acts to regulate the sequence-specific DNA binding activity of p53 (11, 15, 17, 38). It is important to point out, however, that mutation of potential phosphorylation sites may not be the same as the effect of phosphorylation. For example, Ser15 lies within the N-terminal activation domain but, unlike amino acids 19, 22, and 23, is not a point of contact with TFIID. Thus, mutation of the amino acid may have little effect on transactivation. We provide evidence that phosphorylation of Ser15 significantly influences the ability of the p53 activation domain to interact with TFIID.
The N-terminal transactivation domain of p53 has been shown to interact with subunits of TFIID (TBP, human TAF32, and human TAF70), TFIIH (p62), CBP/p300, and MDM2 (12, 20, 23, 25, 44, 49). The fact that p53 phosphorylation sites at amino acids 9, 15, 33, and 37 overlap the binding site for these proteins suggests the strong possibility that phosphorylation at these sites regulates the activity of this domain. Recent reports by Shieh et al. (41) and Siliciano et al. (42) suggest that upon DNA damage, p53 undergoes phosphorylation at Ser15 and additional N-terminal sites, converting p53 into a transcriptionally active protein. Consistent with this hypothesis, our results demonstrate that Ser15 and Ser37 phosphorylation allows TFIID binding while inhibiting MDM2 binding. Remarkably, our results further show that Ser15 phosphorylation alone inhibits the interaction of TFIID with p53, blocking its transcription function. Thus, in addition to uncovering a novel interaction pathway for p53 inactivation in HTLV-1-transformed cells, our results demonstrate that the interaction of p53 with TFIID and MDM2 is tightly regulated by the specific phosphorylation pattern of Ser15 and -37.
It is of interest that HTLV-1 p53 failed to interact with MDM2 in cell extracts. Clearly, our peptide binding results demonstrate that MDM2 interacts with p53 which is phosphorylated at Ser15. It is possible that in vivo, the interaction between MDM2 and HTLV-1 p53 is blocked by some other p53 binding protein(s) present in the HTLV-1-transformed cells. Along these lines, it has been shown that the N terminus of p53 interacts with the p62 subunit of TFIIH, a dual transcription-DNA repair protein (20, 48, 50), the single-stranded DNA binding protein RP-A (6, 13, 22), and the transcription coactivator and acetyltransferase p300/CBP (12, 23). It will be of interest to determine if the binding of these proteins is stimulated by p53 phosphorylation.
Tax could regulate p53 hyperphosphorylation through several pathways. Tax itself is a transcriptional activator and, as such, could activate transcription and expression of individual kinases. Alternatively, Tax could alter the activity of the kinase(s). The kinase responsible for phosphorylation of p53 at Ser15 in vivo has not been determined; however, DNA PK has been shown to phosphorylate both Ser15 and Ser37 in vitro. It will be important to determine the complex cascade of kinases and/or phosphatases involved in Tax-mediated p53 inactivation. Targeting the phosphorylation state of p53 represents a novel mechanism of viral protein inactivation of the tumor suppressor and likely plays a critical role in HTLV-1-induced transformation and leukemogenesis.
ACKNOWLEDGMENTS
We thank Carl Anderson and Daniel Masison for discussion and suggestions, excellent editorial assistance, and technical contributions.
REFERENCES
- 1.Avantaggiati M L, Ogryzko V, Gardner K, Giordano A, Levine A S, Kelly K. Recruitment of p300/CBP in p53-dependent signal pathways. Cell. 1997;89:1175–1184. doi: 10.1016/s0092-8674(00)80304-9. [DOI] [PubMed] [Google Scholar]
- 2.Baker S J, Markowitz S, Fearon E R, Willson J K, Vogelstein B. Suppression of human colorectal carcinoma cell growth by wild-type p53. Science. 1990;249:912–915. doi: 10.1126/science.2144057. [DOI] [PubMed] [Google Scholar]
- 3.Cereseto A, Diella F, Mulloy J C, Cara A, Michieli P, Grassmann R, Franchini G, Klotman M E. p53 functional impairment and high p21waf1/cip1 expression in human T-cell lymphotropic/leukemia virus type I-transformed T cells. Blood. 1996;88:1551–1560. [PubMed] [Google Scholar]
- 4.Chen P L, Chen Y M, Bookstein R, Lee W H. Genetic mechanisms of tumor suppression by the human p53 gene. Science. 1990;250:1576–1580. doi: 10.1126/science.2274789. [DOI] [PubMed] [Google Scholar]
- 5.Diller L, Kassel J, Nelson C E, Gryka M A, Litwak G, Gebhardt M, Bressac B, Ozturk M, Baker S J, Vogelstein B. p53 functions as a cell cycle control protein in osteosarcomas. Mol Cell Biol. 1990;10:5772–5781. doi: 10.1128/mcb.10.11.5772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dutta A, Ruppert S M, Aster J C, Winchester E. Inhibition of DNA replication factor RPA by p53. Nature. 1993;365:79–82. doi: 10.1038/365079a0. [DOI] [PubMed] [Google Scholar]
- 7.El-Deiry W S, Kern S E, Pietenpol J A, Kinzler K W, Vogelstein B. Definition of a consensus binding site for p53. Nat Genet. 1992;1:45–49. doi: 10.1038/ng0492-45. [DOI] [PubMed] [Google Scholar]
- 7a.Fiscella M, Ullrich S J, Zambrano N, Shields M T, Lin D, Lees-Miller S P, Anderson C W, Mercer W E, Appella E. Mutation of the serine 15 phosphorylation site of human p53 reduces the ability of p53 to inhibit cell cycle progression. Oncogene. 1993;8:1519–1528. [PubMed] [Google Scholar]
- 8.Funk W D, Pak D T, Karas R H, Wright W E, Shay J W. A transcriptionally active DNA-binding site for human p53 protein complexes. Mol Cell Biol. 1992;12:2866–2871. doi: 10.1128/mcb.12.6.2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gartenhaus R B, Wang P. Functional inactivation of wild-type p53 protein correlates with loss of IL-2 dependence in HTLV-I transformed human T lymphocytes. Leukemia. 1995;9:2082–2086. [PubMed] [Google Scholar]
- 10.Gessain A, Barin F, Vernant J C, Gout O, Maurs L, Calender A, de The G. Antibodies to human T-lymphotropic virus type-I in patients with tropical spastic paraparesis. Lancet. 1985;2:407–410. doi: 10.1016/s0140-6736(85)92734-5. [DOI] [PubMed] [Google Scholar]
- 11.Gottlieb T M, Oren M. p53 in growth control and neoplasia. Biochim Biophys Acta. 1996;1287:77–102. doi: 10.1016/0304-419x(95)00019-c. [DOI] [PubMed] [Google Scholar]
- 12.Gu W, Shi X, Roeder R G. Synergistic activation of transcription by CBP and p53. Nature. 1997;387:819–823. doi: 10.1038/42972. [DOI] [PubMed] [Google Scholar]
- 13.He Z, Brinton B T, Greenblatt J, Hassell J A, Ingels C J. The transactivator proteins VP16 and GAL4 bind replication factor A. Cell. 1993;73:1223–1232. doi: 10.1016/0092-8674(93)90650-f. [DOI] [PubMed] [Google Scholar]
- 14.Hollstein M, Rice K, Greenblatt M S, Soussi T, Fuchs R, Sorlie T, Hovig E, Smith-Sorensen B, Montesano R, Harris C C. Database of p53 gene somatic mutations in human tumors and cell lines. Nucleic Acids Res. 1994;22:3551–3555. [PMC free article] [PubMed] [Google Scholar]
- 15.Hupp T R, Meek D W, Midgley C A, Lane D P. Regulation of the specific DNA binding function of p53. Cell. 1992;71:875–886. doi: 10.1016/0092-8674(92)90562-q. [DOI] [PubMed] [Google Scholar]
- 16.Ijichi S, Matsuda T, Maruyama I, Izumihara T, Kojima K, Niimura T, Maruyama Y, Sonoda S, Yoshida A, Osame M. Arthritis in a human T lymphotropic virus type I (HTLV-I) carrier. Ann Rheum Dis. 1990;49:718–721. doi: 10.1136/ard.49.9.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ko L J, Prives C. p53: puzzle and paradigm. Genes Dev. 1996;10:1054–1072. doi: 10.1101/gad.10.9.1054. [DOI] [PubMed] [Google Scholar]
- 18.LaGrenade L, Hanchard B, Fletcher V, Cranston B, Blattner W. Infective dermatitis of Jamaican children: a marker for HTLV-I infection. Lancet. 1990;336:1345–1347. doi: 10.1016/0140-6736(90)92896-p. [DOI] [PubMed] [Google Scholar]
- 19.Lees-Miller S P, Sakaguchi K, Ullrich S J, Appella E, Anderson C W. Human DNA-activated protein kinase phosphorylates serines 15 and 37 in the amino-terminal transactivation domain of human p53. Mol Cell Biol. 1992;12:5041–5049. doi: 10.1128/mcb.12.11.5041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leveillard T, Andera L, Bissonnette N, Schaeffer L, Bracco L, Egly J M, Wasylyk B. Functional interactions between p53 and the TFIIH complex are affected by tumour-associated mutations. EMBO J. 1996;15:1615–1624. [PMC free article] [PubMed] [Google Scholar]
- 21.Levine A J. p53, the cellular gatekeeper for growth and division. Cell. 1997;88:323–331. doi: 10.1016/s0092-8674(00)81871-1. [DOI] [PubMed] [Google Scholar]
- 22.Li R, Botchan M R. The acidic transcriptional activation domains of VP16 and p53 bind the cellular replication protein A and stimulate in vitro BPV-1 DNA replication. Cell. 1993;73:1207–1221. doi: 10.1016/0092-8674(93)90649-b. [DOI] [PubMed] [Google Scholar]
- 23.Lill N L, Grossman S R, Ginsberg D, DeCaprio J, Livingston D M. Binding and modulation of p53 by p300/CBP coactivators. Nature. 1997;387:823–827. doi: 10.1038/42981. [DOI] [PubMed] [Google Scholar]
- 24.Lin J, Chen J, Elenbaas B, Levine A J. Several hydrophobic amino acids in the p53 amino-terminal domain are required for transcriptional activation, binding to mdm-2 and the adenovirus 5 E1B 55-kD protein. Genes Dev. 1994;8:1235–1246. doi: 10.1101/gad.8.10.1235. [DOI] [PubMed] [Google Scholar]
- 25.Lu H, Levine A J. Human TAFII31 protein is a transcriptional coactivator of the p53 protein. Proc Natl Acad Sci USA. 1995;92:5154–5158. doi: 10.1073/pnas.92.11.5154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mayr G A, Reed M, Wang P, Wang Y, Schweds J F, Tegtmeyer P. Serine phosphorylation in the NH2 terminus of p53 facilitates transactivation. Cancer Res. 1995;55:2410–2417. [PubMed] [Google Scholar]
- 27.Mercer W E, Shields M T, Amin M, Sauve G J, Appella E, Romano J W, Ullrich S J. Negative growth regulation in a glioblastoma tumor cell line that conditionally expresses human wild-type p53. Proc Natl Acad Sci USA. 1990;87:6166–6170. doi: 10.1073/pnas.87.16.6166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Milne D M, Campbell L E, Campbell D G, Meek D W. p53 is phosphorylated in vitro and in vivo by an ultraviolet radiation-induced protein kinase characteristic of the c-Jun kinase, JNK1. J Biol Chem. 1995;270:5511–5518. doi: 10.1074/jbc.270.10.5511. [DOI] [PubMed] [Google Scholar]
- 29.Milne D M, Palmer R H, Campbell D G, Meek D W. Phosphorylation of the p53 tumour-suppressor protein at three N-terminal sites by a novel casein kinase I-like enzyme. Oncogene. 1992;7:1361–1369. [PubMed] [Google Scholar]
- 30.Milner J. A conformation hypothesis for the suppressor and promoter functions of p53 in cell growth control and in cancer. Proc R Soc Lond Ser B. 1991;245:139–145. doi: 10.1098/rspb.1991.0100. [DOI] [PubMed] [Google Scholar]
- 31.Nagai H, Kinoshita T, Imamura J, Murakami Y, Hayashi K, Mukai K, Ikeda S, Tobinai K, Saito H, Shimoyama M. Genetic alteration of p53 in some patients with adult T-cell leukemia. Jpn J Cancer Res. 1991;82:1421–1427. doi: 10.1111/j.1349-7006.1991.tb01815.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Connor D J, Lam E W, Griffin S, Zhong S, Leighton L C, Burbidge S A, Lu X. Physical and functional interactions between p53 and cell cycle co-operating transcription factors, E2F1 and DP1. EMBO J. 1995;14:6184–6192. doi: 10.1002/j.1460-2075.1995.tb00309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Osame M, Usuku K, Izumo S, Ijichi N, Amitani H, Igata A, Matsumoto M, Tara M. HTLV-I associated myelopathy, a new clinical entity. Lancet. 1986;1:1031–1032. doi: 10.1016/s0140-6736(86)91298-5. . (Letter.) [DOI] [PubMed] [Google Scholar]
- 34.Pietenpol J A, Tokino T, Thiagalingam S, El-Deiry W S, Kinzler K W, Vogelstein B. Sequence-specific transcriptional activation is essential for growth suppression by p53. Proc Natl Acad Sci USA. 1994;91:1998–2002. doi: 10.1073/pnas.91.6.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pise-Masison C A, Choi K-S, Radonovich M, Dittmer J, Kim S-J, Brady J N. Inhibition of p53 transactivation function by the human T-cell leukemia virus type 1 Tax protein. J Virol. 1998;72:1165–1170. doi: 10.1128/jvi.72.2.1165-1170.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Poiesz B J, Ruscetti F W, Gazdar A F, Bunn P A, Minna J D, Gallo R C. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci USA. 1980;77:7415–7419. doi: 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reid R L, Lindholm P F, Mireskandari A, Dittmer J, Brady J N. Stabilization of wild-type p53 in human T-lymphocytes transformed by HTLV-I. Oncogene. 1993;8:3029–3036. [PubMed] [Google Scholar]
- 38.Sakaguchi K, Sakamoto H, Lewis M S, Anderson C W, Erickson J W, Appella E, Xie D. Phosphorylation of serine 392 stabilizes the tetramer formation of tumor suppressor protein p53. Biochemistry. 1997;36:10117–10124. doi: 10.1021/bi970759w. [DOI] [PubMed] [Google Scholar]
- 39.Sakashita A, Hattori T, Miller C W, Suzushima H, Asou N, Takatsuki K, Koeffler H P. Mutations of the p53 gene in adult T-cell leukemia. Blood. 1992;79:477–480. [PubMed] [Google Scholar]
- 40.Sasaki K, Morooka I, Inomata H, Kashio N, Akamine T, Osame M. Retinal vasculitis in human T-lymphotropic virus type I associated myelopathy. Br J Ophthalmol. 1989;73:812–815. doi: 10.1136/bjo.73.10.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shieh S, Ikeda M, Taya Y, Prives C. DNA damage-induce phosphorylation of p53 alleviates inhibition by MDM2. Cell. 1997;91:325–334. doi: 10.1016/s0092-8674(00)80416-x. [DOI] [PubMed] [Google Scholar]
- 42.Siliciano J D, Canman C E, Taya Y, Sakaguchi K, Appella E, Kastan M B. DNA damage induces phosphorylation of the amino terminus of p53. Genes Dev. 1997;11:3471–3481. doi: 10.1101/gad.11.24.3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Steegenga W T, van der Eb A J, Jochemsen A G. How phosphorylation regulates the activity of p53. J Mol Biol. 1996;263:103–113. doi: 10.1006/jmbi.1996.0560. [DOI] [PubMed] [Google Scholar]
- 44.Thut C J, Chen J L, Klemm R, Tjian R. p53 transcriptional activation mediated by coactivators TAFII40 and TAFII60. Science. 1995;267:100–104. doi: 10.1126/science.7809597. [DOI] [PubMed] [Google Scholar]
- 45.Truant R, Antunovic J, Greenblatt J, Prives C, Cromlish J A. Direct interaction of the hepatitis B virus HBx protein with p53 leads to inhibition by HBx of p53 response element-directed transactivation. J Virol. 1995;69:1851–1859. doi: 10.1128/jvi.69.3.1851-1859.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ullrich S J, Anderson C W, Mercer W E, Appella E. The p53 tumor suppressor protein, a modulator of cell proliferation. J Biol Chem. 1992;267:15259–15262. [PubMed] [Google Scholar]
- 47.Ullrich S J, Sakaguchi K, Lees-Miller S P, Fiscella M, Mercer W E, Anderson C W, Appella E. Phosphorylation at Ser-15 and Ser-392 in mutant p53 molecules from human tumors is altered compared to wild-type p53. Proc Natl Acad Sci USA. 1993;90:5954–5958. doi: 10.1073/pnas.90.13.5954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang X W, Yeh H, Schaeffer L, Roy R, Moncollin V, Egly J M, Wang Z, Freidberg E C, Evans M K, Taffe B G. p53 modulation of TFIIH-associated nucleotide excision repair activity. Nat Genet. 1995;10:188–195. doi: 10.1038/ng0695-188. [DOI] [PubMed] [Google Scholar]
- 49.Xiao H, Pearson A, Coulombe B, Truant R, Zhang S, Regier J L, Triezenberg S J, Reinberg D, Flores O, Ingles C J. Binding of basal transcription factor TFIIH to the acidic activation domains of VP16 and p53. Mol Cell Biol. 1994;14:7013–7024. doi: 10.1128/mcb.14.10.7013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xiao H, Pearson A, Coulombe B, Truant R, Zhang S, Regier J L, Triezenberg S J, Reinberg D, Flores O, Ingles C J, Greenblatt J. Binding of basal transcription factor TFIIH to the acidic activation domains of VP16 and p53. Mol Cell Biol. 1994;14:7013–7024. doi: 10.1128/mcb.14.10.7013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yoshida M, Seiki M, Yamaguchi K, Takatsuki K. Monoclonal integration of human T-cell leukemia provirus in all primary tumors of adult T-cell leukemia suggests causative role of human T-cell leukemia virus in the disease. Proc Natl Acad Sci USA. 1984;81:2534–2537. doi: 10.1073/pnas.81.8.2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhou Q, Lieberman P M, Boyer T G, Berk A J. Holo-TFIID supports transcriptional stimulation by diverse activators and from a TATA-less promoter. Genes Dev. 1992;6:1964–1974. doi: 10.1101/gad.6.10.1964. [DOI] [PubMed] [Google Scholar]