Abstract
In the absence of envelope gene expression, retrovirus packaging cell lines expressing Moloney murine leukemia virus (MLV) gag and pol genes produce large amounts of noninfectious virus-like particles that contain reverse transcriptase, processed Gag protein, and viral RNA (gag-pol RNA particles). We demonstrate that these particles can be made infectious in an in vitro, cell-free system by the addition of a surrogate envelope protein, the G spike glycoprotein of vesicular stomatitis virus (VSV-G). The appearance of infectivity is accompanied by physical association of the G protein with the immature, noninfectious virus particles. Similarly, exposure in vitro of wild-type VSV-G to a fusion-defective pseudotyped virus containing a mutant VSV-G markedly increases the infectivity of the virus to titers similar to those of conventional VSV-G pseudotyped viruses. Furthermore, similar treatment of an amphotropic murine leukemia virus significantly allows infection of BHK cells not otherwise susceptible to infection with native amphotropic virus. The partially cell-free virus maturation system reported here should be useful for studies aimed at the preparation of tissue-targeted retrovirus vectors and will also aid in studies of nucleocapsid-envelope interactions during budding and of virus assembly and virus-receptor interactions during virus uptake into infected cells. It may also represent a potentially useful step toward the eventual development of a completely cell-free retrovirus assembly system.
The maturation of retroviruses in mammalian cells involves a number of processes, including intracellular associations of virus-encoded proteins with viral RNA, transport of subviral ribonucleoprotein complexes through cellular organelles to specific regions of the cellular plasma membrane containing selectively embedded viral envelope (Env) proteins, and finally the budding of fully assembled progeny virus particles into the extracellular space (7, 16). Assembly of virus particles occurs intracellularly in some classes of retroviruses (B and D), while assembly and budding seem to occur simultaneously at the plasma membrane in the case of the type C retroviruses. The precise mechanisms and cellular locations for most of these steps are not completely understood. The spike G glycoprotein of the rhabdovirus vesicular stomatitis virus (VSV) has been used to study many of the processes of mammalian cell membrane biogenesis and assembly of enveloped mammalian viruses. It is a well-characterized marker for intracellular trafficking of membrane-associated proteins (2, 13, 22) and also serves as an efficient surrogate envelope protein during the assembly of retroviruses. For instance, the VSV G protein (VSV-G) has been shown to be incorporated into retrovirus particles that also contain retroviral Env proteins, thereby producing chimeric pseudotype particles (14, 19, 33, 35, 40) with entirely new host range and cell tropism properties. It is also known that detergent-purified VSV-G can be incorporated in vitro into the lipid bilayers of synthetic liposomes to produce agents capable of fusing target cells (15, 20, 24), thereby providing a potentially important approach to studying the formation of a model mammalian cell membrane. However, the relevance of G incorporation into such synthetic liposome structures to the mechanisms of mammalian membrane biogenesis is not entirely clear. Much remains to be learned about the mechanisms responsible for the incorporation of either the native viral Env or the surrogate Env VSV-G into cellular and viral membranes. It would therefore be of great interest to have available an efficient in vitro system for studying the mechanisms of mammalian membrane biogenesis and targeting and incorporation of membrane proteins.
We have reported that VSV-G can entirely replace the Env protein of Moloney murine leukemia virus (MLV)-based retroviruses during retrovirus assembly (5, 9, 21, 38). In these previous studies of VSV-G pseudotype formation, we have identified noninfectious virus-like particles that contain processed Moloney Gag and reverse transcriptase (RT) and viral RNA (gag-pol RNA particles) but that are completely devoid of specific viral envelope proteins and that are produced constitutively in large numbers by retrovirus packaging cell lines. These particles are analogous to those reported to be produced by retrovirus producer cells (3, 28). We have recently found that these noninfectious virus-like particles are produced by envelope-free packaging cell lines and that such particles have many of the morphological and hydrodynamic properties of mature virus, including similar buoyant densities, sedimentation coefficients, viral protein composition, and electron microscope morphology. Furthermore, we have found that they can be made infectious by the addition to the partially purified particles of lipofectins (31), presumably by facilitating the uptake of the assembled nucleoprotein complexes into cells (10). In the present study, we demonstrate that the surrogate viral envelope protein VSV-G is incorporated into the noninfectious RNA-containing virus-like gag-pol RNA particles and that the incorporation of VSV-G is associated with the development of infectivity of the particles. Because this cell-free system permits rapid in vitro characterization of the interactions between a model membrane-embedded protein and enveloped viral ribonucleotide complexes, it should be useful for detailed studies of mechanisms of virus-cell fusion and virus entry into cells and for the illumination of some of the final steps of retrovirus assembly.
MATERIALS AND METHODS
Cell lines, plasmids constructs, and production of viruses.
BHK, CF2, 293, and HeLa cells were obtained from the American Type Culture Collection. 208F, 293, 293GP, 293GP/LZRNL, and 293GP/LLRNL cells and methods for the generation of VSV-G pseudotyped viruses have been previously described (21, 36, 38), as has plasmid pCMV-G, expressing VSV-G from the human cytomegalovirus (CMV) promoter (38). Plasmid pCMV-G-P127R, expressing a fusion-defective VSV-G mutant, VSV-G-P127R, was generated by using a MORPH in vitro mutagenesis kit (5prime→3prime Inc., Boulder, Colo.). The mutagenesis primer was 5′-ATATCCACAACTCTGGCGAGGGAACCCGGGATTCAGCCA-3′, which changes the 127th amino acid from proline to arginine and introduces silent mutations to simplify subsequent cloning steps; mutated sequences are underlined. The fusion-defective pseudotyped virus was produced by the 293GP/LZRNL/G-P127R cell line which was stably transduced with plasmid pCMV-G-P127R. Plasmids pCMV-Ampho and pCMV-Eco, expressing the amphotropic and ecotropic MLV envelope genes, respectively, were constructed by replacing the VSV-G gene in pCMV-G with either the amphotropic or ecotropic env gene (30). The amphotropic LZRNL virus was produced by the 293GP/LZRNL/amphotropic cell line (30).
Detection of VSV-G by Western blotting.
To detect intracellular and extracellular VSV-G in cell lines transfected with pCMV-G, cells were grown in six-well plates and transfected with 4 μg of plasmid pCMV-G per well by established calcium phosphate coprecipitation methods, with the exception of HeLa cells, which were transfected with 1 μg of pCMV-G by Lipofectin (GIBCO BRL). Cells and conditioned medium were harvested 48 h after transfection. One milliliter of each conditioned medium was then centrifuged at 14,000 rpm with a Beckman F2402 rotor for 1 h at 4°C, and the pellets were resuspended into 50 μl of sodium dodecyl sulfate (SDS) sample buffer (25 mM Tris HCl, 5% glycerol, 1% SDS, 1% β-mercaptoethanol, 0.05% bromphenol blue). Cells were lysed by three rapid freeze-thaw cycles, and cell debris was removed by low-speed centrifugation. Two micrograms of protein in the cell lysates and 5 μl of pellet suspension from the conditioned medium were applied to denaturing polyacrylamide gels and examined by established Western blotting methods, using monoclonal antibody P5D4 (Sigma, St. Louis, Mo.) to visualize VSV-G. To examine the distribution of the VSV-G in the sucrose velocity gradient centrifugation, 10-μl aliquots of each fraction were mixed with 10 μl of 2× SDS sample buffer (see above) and applied to electrophoresis followed by the Western blotting as described above.
Preparation of VSV-G and gag-pol RNA particles for in vitro assembly.
To prepare VSV-G, 293 and 293GP cells in 10-cm-diameter culture dishes were transfected with 20 μg of pCMV-G by calcium phosphate coprecipitation. Cells and conditioned medium were harvested 48 h after transfection.
To prepare solubilized and partially purified VSV-G, 100 to 200 ml of conditioned medium from 293 cells transfected with pCMV-G was centrifuged at 24,000 rpm in a Beckman SW28 rotor for 90 min at 4°C. The pellet was resuspended in 100 μl of 60 mM octyl-β-d-glycoside (β-OG) or 100 mM dodecyl octaethyl monoether (C12H8) in 50 mM Tris-HCl (pH 7.5) containing 100 mM NaCl and 0.5 mM EDTA. After centrifugation at 14,000 rpm for 30 min in a microcentrifuge, the supernatant containing solubilized VSV-G was collected. Detergent depletion was performed by dialysis against phosphate-buffered saline (PBS) or with SM-2 beads as described before (20). The resulting VSV-G was concentrated (Centricon 30; Amicon, Beverly, Mass.), and the purity of the preparation was determined by polyacrylamide gel electrophoresis and silver staining (1). Approximately 5 μg of VSV-G at a purity of >90% was obtained from 200 ml of conditioned medium (data not presented).
Virus assays.
Virus titers were determined by infecting 208F or BHK cells in the presence of Polybrene (4 μg/ml), selecting the infected cells with G418 (400 μg/ml), and counting resulting G418-resistant colonies 14 days after infection (36). Alternatively, in the case of assaying luciferase-expressing virus, virus titers were determined by measuring luciferase activity expressed as relative light units in the infected BHK cells 2 days after infection as previously described (36). RT activity in the virus particles was measured by established methods (12).
Sucrose gradient centrifugation.
Continuous 5 to 30% sucrose gradients in PBS with a 40% sucrose cushion were centrifuged at 30,000 rpm in a Beckman SW41Ti rotor for 28 min at 4°C. Aliquots of 0.5 ml each were collected from the top and analyzed for RT activity, virus titer, and amount of VSV-G as described above.
RESULTS
Efficient secretion of sedimentable VSV-G particle into the culture medium.
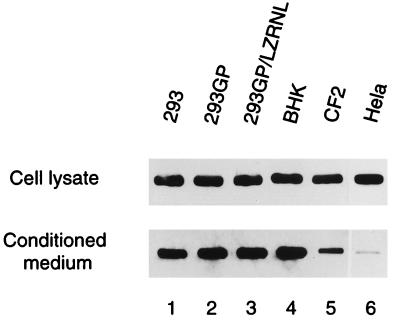
To determine an appropriate source of VSV-G for subsequent assembly and infectivity studies, a variety of cell lines, including the human embryonic renal cell line 293, the packaging cell line 293GP expressing Moloney MLV gag and pol genes, and the producer cell line 293GP/LZRNL expressing the retrovirus LZRNL (38), as well as BHK, canine thymus fibroblast CF2, and human HeLa cells were transfected with plasmid pCMV-G, which expresses VSV-G from the strong human CMV promoter-enhancer (38). Cell lysates and pellets from conditioned media were examined by Western blotting techniques for the presence of VSV-G (Fig. 1). Expression of G protein was detected at comparably high levels in all cell lysates, and in all cases except the CF2 and HeLa cells, VSV-G was also found at high levels in the pellets from conditioned media, indicating that VSV-G was efficiently secreted into the media in those cells and that it was present in those media as sedimentable particles. Rolls et al. (27) have previously reported that in the presence of Semliki Forest virus RNA, VSV-G is released into culture medium in the form of assembled particles. The results presented in the present study demonstrate that VSV-G produced in some cells is efficiently secreted into the conditioned medium even in the absence of viral RNA and is found in the form of sedimentable particles. We have detected large amounts of 50- to 160-nm-diameter particles in the media by electron microscopy (data not shown).
FIG. 1.
Intracellular and extracellular VSV-G in cell lines transfected with pCMV-G. Cells were grown in six-well plates and transfected with plasmid pCMV-G. VSV-G samples in the conditioned medium and cell lysate were examined by established Western blotting methods, using P5D4 monoclonal antibody to visualize VSV-G protein as described in Materials and Methods.
Conversion of noninfectious particles to infectious particles by in vitro exposure to VSV-G.
For in vitro assembly and infectivity studies, we determined if the noninfectious virus-like gag-pol RNA particles in conditioned medium from the packaging cell line 293GP/LZRNL could be converted to infectious particles by exposure to VSV-G. Noninfectious gag-pol RNA particles were used in the form of either unpelleted conditioned medium derived from 293GP/LZRNL cells or the same conditioned medium pelleted by centrifugation (31). The G protein was added either as conditioned medium or as lysates of 293 or 293GP cells transfected with the plasmid pCMV-G or purified VSV-G obtained from detergent-lysed pellets of conditioned medium of 293 cells transfected with pCMV-G (VSV-G pellets). In some cases, assays for infectivity were carried out with preparations in which the conditioned media were pelleted either immediately before or immediately after mixing.
As is evident from Table 1, the noninfectious, virus-like gag-pol RNA particles present in conditioned media of 293GP/LZRNL packaging cells become infectious after exposure to various forms of VSV-G, including cell membrane-bound VSV-G, secreted and pelletable VSV-G in conditioned medium of pCMV-G-transfected cells, or VSV-G solubilized from cellular membranes. Infectious particles were produced when the G protein was solubilized from VSV-G pellets with the detergent C12H8, an agent known to allow formation of fusogenic VSV-G liposomes after detergent removal by SM-2 beads but not by dialysis (20). In contrast, no infectious virus was detected after exposure of gag-pol RNA particles to VSV-G solubilized by the detergent β-OG followed by removal of excess detergent with SM-2 beads (20). We are examining the possibility that the differences between the C12H8 and β-OG solubilization procedures may be due to loss of fusogenic activity of the isolated VSV-G or to inefficient removal of detergent by SM-2 beads or dialysis.
TABLE 1.
Titers of infectious LZRNL virus preparations prepared by exposure of noninfectious gag-pol RNA particles to VSV-G in vitro by mixing of conditioned media, mixing of media followed by pelleting, or mixing of pelleted mediaa
| Source of VSV-G | Virus titer (CFU/ml)b
|
||
|---|---|---|---|
| Conditioned media mixed | Media mixed before pelleting | Media pelleted, then mixed | |
| 293/pCMV-G | |||
| Conditioned medium | 6.0 × 102 | 8.4 × 103 | 3.6 × 103 |
| Conditioned medium + I1 antibody | <1 | <1 | <1 |
| Cell lysate | ND | 4.4 × 103 | ND |
| 293GP/pCMV-G | |||
| Conditioned medium | 5.8 × 102 | 1.1 × 104 | 4.3 × 103 |
| Conditioned medium + I1 antibody | <1 | <1 | <1 |
| Cell lysate | ND | 6.1 × 103 | ND |
| Detergent-purified VSV-G | |||
| β-OG | ND | ND | <1 |
| C12H8 | ND | ND | 2.0 × 102 |
| 293/pCMV-Ampho | |||
| Conditioned medium | <1 | <1 | <1 |
| 293/pCMV-Eco | |||
| Conditioned medium | <1 | <1 | <1 |
| Cell lysate | <1 | <1 | <1 |
Sources of VSV-G included 293 or 293GP cells transfected with pCMV-G as described in Materials and Methods. For samples mixed after centrifugation, 1 ml of each medium was centrifuged, the pellets were resuspended in 10 μl of PBS and mixed, and serial dilutions were assayed for infection on rat 208F cells. For assays involving mixing prior to pelleting, 0.5-ml aliquots of the media were mixed and centrifuged as above. The pellets were resuspended in 50 μl of PBS, and serial dilutions were assayed for infectivity on rat 208F cells. Prior to infection of 208F cells, an aliquot of each sample was incubated with VSV-G neutralizing antibody I1 or with anti-VSV-G polyclonal antibody for 15 min at room temperature. Results of antibody I1-treated samples are shown. The virus titer is indicated as the total infectious virus in 1 ml of the starting 293GP/LZRNL conditioned medium. The titer of a conventionally prepared pseudotyped LZRNL virus produced under identical conditions was 2.3 × 105 CFU/ml. Solubilized and partially purified VSV-G samples were prepared as described in Materials and Methods. One microgram of purified VSV-G was mixed with the resuspended pellet from 293GP/LZRNL conditioned medium and assayed for infectivity as described above. Conditioned media and cell lysates of 293 cells transfected with pCMV-Ampho or pCMV-Eco were also examined by the same procedure as those of pCMV-G-transfected cells.
Total CFU of virus produced from 1 ml of conditioned medium from 293GP/LZRNL cells. ND, not determined.
Table 1 indicates that under currently optimal conditions, the level of infectious particles achieved titers of up to 104 CFU/ml, representing up to approximately 10% of that of conventionally produced VSV-G pseudotyped virus. In vitro maturation and assembly were maximal in samples that had been pelleted compared with samples in which nonpelleted conditioned media were mixed. In contrast to VSV-G results, mixing experiments in which VSV-G was replaced by conditioned media or lysates from 293 and 293GP cells expressing the amphotropic or ecotropic Moloney envelope proteins from CMV-driven plasmids did not result in the appearance of infectious virus. The phenomenon of in vitro conversion of noninfectious immature virus-like particles into infectious forms was completely abrogated when VSV-G neutralizing monoclonal antibody I1 (5) or anti-VSV polyclonal antibody (9) was added to the mixtures, confirming the essential role of VSV-G in the infection activation process and indicating that the Moloney viral envelope proteins are not present in lysates or conditioned media in a form that permits the appropriate complex formation with gag-pol RNA particles.
Physical association of VSV-G particles with immature virus-like particles in vitro.
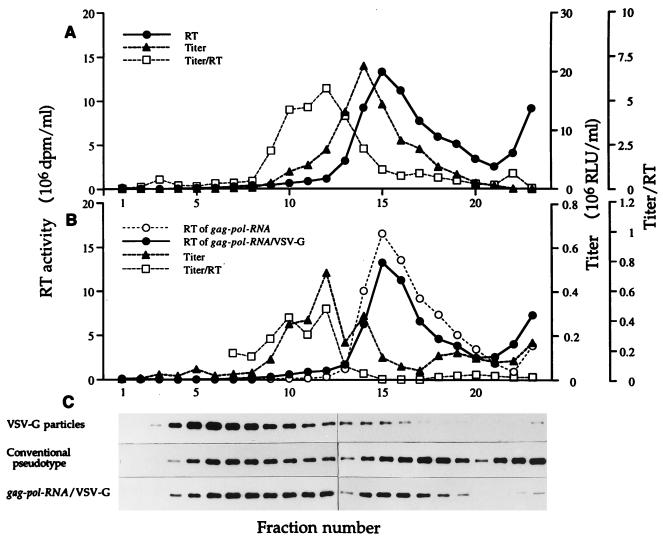
Because the buoyant density of the starting noninfectious gag-pol RNA particles is almost identical to that of mature assembled virus (31), we further characterized the infectious particles made as described above by velocity sedimentation sucrose gradient centrifugation. Figure 2 shows the sedimentation profiles of RT activity, infectivity titer, and titer/RT ratio, as well as localization of VSV-G protein of conventional VSV-G pseudotyped virus (Fig. 2A) and of the in vitro-assembled infectious particles (Fig. 2B). In both cases, the major peak of extracellular RT activity sediments at a gradient position corresponding to a sedimentation coefficient of approximately 580S (31). The major infectivity peak of the conventionally produced VSV-G pseudotype sediments slightly to the slowly sedimenting side of the major RT peak, and the titer/RT ratio is several fractions slower still.
FIG. 2.
Analysis of virus particles by sucrose velocity gradient centrifugation. (A) Authentic pseudotyped LLRNL virus prepared by conventional methods from 293GP/LLRNL cells. (B) Untreated gag-pol RNA particles and gag-pol RNA particles made infectious by exposure to VSV-G for which 0.5-ml volumes of conditioned medium of 293 cells transfected with pCMV-G and of 293GP/LLRNL cells were mixed and centrifuged at 24,000 rpm at 4°C in Beckman SW28 rotor for 90 min. Pellets were resuspended with 1 ml of PBS and loaded onto the sucrose gradients. (C) Western blot analysis of VSV-G in gradient fractions containing the media from 293 cells transfected with pCMV-G, authentic mature pseudotyped LLRNL virus, and gag-pol RNA particles from 293GP/LLRNL cells made infectious by treatment with VSV-G as described above. RLU, relative luciferase units.
VSV-G alone, in the form of particles in the conditioned medium from transfected cells, sediments as a single major peak, predominantly in fractions 4 to 8, as detected by Western blotting (Fig. 2). In the case of preparations of conventionally produced pseudotyped virus, the VSV-G protein is found in two principal peaks, one corresponding approximately to the major RT peak and the other to a slower-sedimenting peak, separate from both the RT and infectivity activities and similar to that seen in the unmixed conditioned medium of cells transfected with pCMV-G alone. These results indicate that the infectious particles present in preparations of conventionally produced VSV-G pseudotyped virus are structurally heterogeneous and that infectivity is maximal in slower-sedimenting, and therefore possibly smaller, particles. The RT activity of the in vitro-assembled particles made infectious by exposure to VSV-G sediments indistinguishably from the starting, noninfectious gag-pol RNA particles. However, the major peak of infectivity is shifted to an even slower-sedimenting fraction very low in RT activity and in a position close to that of the titer/RT maximum. Interestingly, the magnitude and direction of the shift of the titer/RT ratio from the RT peak of both the conventionally prepared pseudotyped virus and the in vitro-reconstituted infectious particles are similar. The addition of VSV-G to noninfectious gag-pol RNA particles therefore generates particles that are heterogeneous but that resemble the particles with the highest infectivity/RT ratio produced by standard methods of pseudotyped vector production.
Stability of VSV-G, gag-pol RNA particles, and pseudotyped virus.
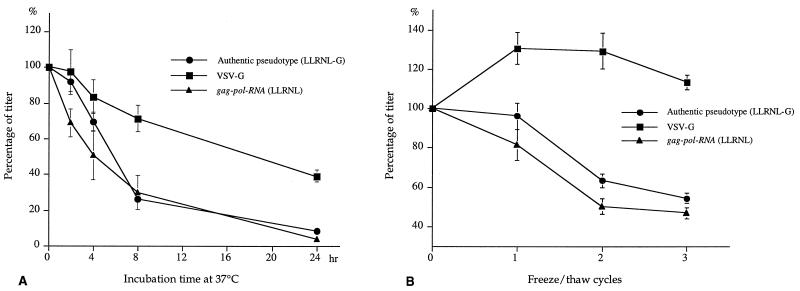
The present in vitro virus maturation system allows identification of optimum conditions for each of the separate components of in vitro retrovirus assembly and maturation. We examined the stability of VSV-G particles and gag-pol RNA virus-like particles by incubating at 4 and 37°C or by repeating freeze-thaw of each component separately and then mixing each treated component with the freshly prepared counterpart. As shown in Fig. 3, the MLV gag-pol RNA capsid particles are relatively unstable to conditions of incubation at 37°C or freeze-thaw compared with the VSV-G particles. The stability profiles of authentic VSV-G pseudotyped virus are similar to those of gag-pol RNA particles in the same experiments (Fig. 3), suggesting that the half-life of the pseudotyped virus is limited by the gag-pol RNA particles rather than by the VSV-G envelope. Incubation experiment of the components at 4°C showed stability profiles similar to those at 37°C (data not shown).
FIG. 3.
Stability of VSV-G, gag-pol RNA, and authentic pseudotyped virus particles. (A) Stability in incubation at 37°C. VSV-G-containing conditioned medium from 293 cells transfected with pCMV-G and gag-pol RNA (GP/LLRNL)-containing conditioned medium from 293GP/LLRNL cells (0.5 ml of each) were independently incubated at 37°C for the indicated time. After that, they were mixed with equal volumes of the other fresh components, centrifuged at 14,000 rpm for 1 h, and used to infect BHK cells. Authentic pseudotyped virus was made by the conventional method and incubated at 37°C. The activity of each component was determined by luciferase assay and expressed as the percentage of fresh-sample activity. An identical experiment in which samples were incubated at 4°C instead of 37°C resulted in a similar profile (data not shown). (B) Stability during freeze-thaw. Each sample (0.5 ml) was subjected to freezing at −80°C for 15 min and thawing at 37°C for 5 min, and each sample was kept at 4°C until the last samples were prepared. Then VSV-G or gag-pol RNA particles were mixed with equal volumes of fresh other components, centrifuged at 14,000 rpm for 1 h, and subjected to infection. Each value represents the percentage of fresh-sample activity. Results are the mean ± standard error of three independent experiments.
In vitro interaction of VSV-G with enveloped MLV particles.
We have examined the interaction in vitro of VSV-G with retrovirus particles containing the specific membrane glycoproteins such as amphotropic envelope or the fusion-defective VSV-G mutant (VSV-G-P127R). VSV-G consists of a single polypeptide containing both binding and fusion properties. The fusogenic region(s) of VSV-G has previously been partially characterized, and in particular, the presence of mutations within a sequence encoding a stretch of 19 uncharged amino acid residues (118 to 136) near proline 127 has indicated a vital fusogenic role for this domain (11, 18, 41). We introduced several mutations into position 127 and found that one such mutant, VSV-G-P127R, in which proline is replaced with arginine, was efficiently expressed on the cell surface and was also secreted efficiently into the culture medium (data not shown). Using this mutant, we generated a fusion-defective pseudotyped virus, LZRNL-G-P127R. While wild-type VSV-G is highly cytotoxic, hampering constitutive production of pseudotyped virus by a stable packaging cell line (6, 23, 37, 38), VSV-G-P127R is only minimally cytotoxic. Using this mutant, we have therefore been able to establish a stable cell line that constitutively produces large amounts of the fusion-defective pseudotyped virus LZRNL-G-P127R. Such a pseudotyped virus produced by a stable packaging cell line and the amphotropic LZRNL virus produced by an amphotropic packaging cell line derived from 293GP cells (30) were both exposed in vitro to wild-type VSV-G prepared as sedimentable vesicles from the conditioned medium of 293 cells transfected with pCMV-G expressing wild-type VSV-G. As shown in Table 2, when the fusion-defective LZRNL-G-P127R virus was treated with wild-type VSV-G, the infectivity was efficiently reconstituted to titers comparable to those of wild-type pseudotyped virus produced by conventional methods. Under the same conditions, the amphotropic virus, generally not infectious in BHK cells, became infectious to BHK cells with an efficiency similar to that of conventional wild-type VSV-G pseudotyped virus.
TABLE 2.
In vitro interaction of gag-pol RNA particles, fusion-defective pseudotyped virus, and amphotropic virus with VSV-Ga
| Virus | Neor titer (CFU/ml) on:
|
|
|---|---|---|
| 208F cells | BHK cells | |
| LZRNL (G) | 1.0 × 106 | 8.0 × 105 |
| LZRNL (gag-pol RNA) | ||
| Alone | <1 | <1 |
| + VSV-G | 4.2 × 104 | 3.4 × 104 |
| LZRNL (G-P127R) | ||
| Alone | 1.8 × 102 | 2.0 × 102 |
| + VSV-G | 8.0 × 105 | 6.0 × 105 |
| LZRNL (A) | ||
| Alone | 6.0 × 105 | <1 |
| + VSV-G | 8.0 × 105 | 4.4 × 105 |
Preparations of VSV-G pseudotyped virus LZRNL (G) were made by conventional methods (37). Conditioned medium of 293GP/LZRNL containing LZRNL (gag-pol RNA), 293GP/LZRNL/G-P127R containing LZRNL (G-P127R), and 293GP/LZRNL/amphotropic containing LZRNL (A) were harvested and used to infect 208F and BHK cells before and after exposure to wild-type VSV-G. For the in vitro assembly of infectious particles, 0.5 ml of each of these conditioned media was mixed with 0.5 ml of conditioned medium of 293 cells transfected with the pCMV-G and centrifuged at 14,000 rpm in Beckman F2402 rotor at 4°C for 1 h. Pellets were resuspended with PBS and used to infect 208F and BHK cells. Titers of the viruses treated in vitro with VSV-G represent total infectious virus recovered from 1 ml of corresponding conditioned medium.
DISCUSSION
These studies demonstrate that the noninfectious virus-like particles produced by provirus-expressing packaging cell lines can readily be converted, at least partially, into infectious virus in vitro by addition of a surrogate envelope protein, VSV-G, in a cell-free system (Table 1). The in vitro conversion of immature virus-like particles to infectious virus is associated with the physical association of the immature virus-like particles with VSV-G (Fig. 2). Similarly, complex formation by the same in vitro cell-free treatment of wild-type VSV-G with MLV-based particles containing specific envelope proteins such as a fusion-defective VSV-G, G-P127R, or the amphotropic envelope significantly altered the infectivity of the particles. Low titers characteristic of the fusion-defective VSV-G particles were enhanced by more than 3 orders of magnitude by exposure in vitro to wild-type VSV-G to titers equivalent to those of conventional VSV-G pseudotyped virus (Table 2). Even more impressive is the appearance in BHK cells of high titers of infectivity of otherwise completely noninfectious amphotropic virus after exposure to VSV-G.
The precise mechanisms by which VSV-G interacts with the envelopes of immature virus-like particles or with the envelope protein components of the particles are not yet characterized. The alteration of infectivity and host range of the LZRNL-G-P127R or of amphotropic MLV exposed to VSV-G could have resulted either from the introduction of VSV-G homotrimers directly into the virus envelope or from the formation of heterotrimers between wild-type VSV-G and the envelope protein resident in the particle. Subunit exchange of VSV-G subunits from trimers in membranes and lipid bilayers has been shown to occur (34). The formation of even more complex structures is also possible.
Systems of in vitro Gag assembly into virus-like particles have previously been reported for Mason-Pfizer monkey virus, a retrovirus whose mechanisms of assembly differ from those of MLV (28, 32). Type C retroviruses, represented by MLV, are thought to mature at the plasma membrane by simultaneous capsid assembly and budding (4, 26, 39), whereas other retroviruses represented by mammary tumor virus (type B) and Mason-Pfizer virus (type D) mature by intracytoplasmic assembly of virus capsids (25, 29). Unfortunately, because of their markedly different mechanisms of assembly, the in vitro system suitable for studying type D virus maturation has not yet been extended to the study of the assembly of type C viruses (8, 17). The isolated, in vitro and partially cell-free infectivity reconstitution system reported here therefore may provide the beginnings of an in vitro virus maturation-assembly system suitable for type C viruses such as MLV. Like other cell-free in vitro systems, the present system has the important property of making possible the in vitro biochemical study of each of the components involved in the interaction of VSV-G as a model intrinsic membrane protein with a relatively purified cellular membrane, in the form of an immature viral nucleoprotein complex. The system therefore has the potential to be useful for the direct elucidation of virus-cell interactions and the mechanisms of virus-cell membrane fusion during virus entry, for studies of the assembly of enveloped viruses by cell surface budding, and possibly for the design and study of potentially therapeutic agents that might interfere with virus maturation or virus entry into cells. At a practical level, these kinds of studies may also eventually permit the preparation of retrovirus vectors at titers higher than presently possible through the conversion of the large amounts of noninfectious virus-like material in conditioned medium of retrovirus packaging and producer cells into infectious virus particles. Such conversion at a late stage of vector assembly may also make large-scale vector preparation safer. Furthermore, the present cell-free system should facilitate studies of the incorporation of modified envelope proteins into vector particles for tissue-specific targeting of gene transfer vectors.
ACKNOWLEDGMENTS
This study was supported by grants DK49023 and HL53680 from the National Institutes of Health, a grant from the Del Webb Foundation, and a grant to A.A. from the Sankyo Foundation of Life Science.
We thank Sanjai Sharma and Fukashi Murai for helpful discussions and Henrik Steinberg for skillful technical assistance.
REFERENCES
- 1.Abe A, Miyanohara A, Friedmann T. Enhanced gene transfer with fusogenic liposomes containing vesicular stomatitis virus G glycoprotein. J Virol. 1998;72:6159–6163. doi: 10.1128/jvi.72.7.6159-6163.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balch W E, McCaffery J M, Plutner H, Farquhar M G. Vesicular stomatitis virus glycoprotein is sorted and concentrated during export from the endoplasmic reticulum. Cell. 1994;76:841–852. doi: 10.1016/0092-8674(94)90359-x. [DOI] [PubMed] [Google Scholar]
- 3.Bassin R H, Phillips L A, Kramer M J, Haapala D K, Peebles P T, Nomura S, Fischinger P J. Transformation of mouse 3T3 cells by murine sarcoma virus: release of virus-like particles in the absence of replicating murine leukemia helper virus. Proc Natl Acad Sci USA. 1971;68:1520–1524. doi: 10.1073/pnas.68.7.1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bolognesi D P, Montelaro R C, Frank H, Schafer W. Assembly of type C oncornaviruses: a model. Science. 1978;199:183–186. doi: 10.1126/science.202022. [DOI] [PubMed] [Google Scholar]
- 5.Burns J C, Friedmann T, Driever W, Burrascano M, Yee J K. Vesicular stomatitis virus G glycoprotein pseudotyped retroviral vectors: concentration to very high titer and efficient gene transfer into mammalian and nonmammalian cells. Proc Natl Acad Sci USA. 1993;90:8033–8037. doi: 10.1073/pnas.90.17.8033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen S T, Iida A, Guo L, Friedmann T, Yee J K. Generation of packaging cell lines for pseudotyped retroviral vectors of the G protein of vesicular stomatitis virus by using a modified tetracycline inducible system. Proc Natl Acad Sci USA. 1996;93:10057–10062. doi: 10.1073/pnas.93.19.10057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dickson C, Eisenman R, Fan H, Hunter E, Teich N. Protein biosynthesis and assembly. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1984. [Google Scholar]
- 8.Einfeld D. Maturation and assembly of retroviral glycoproteins. Curr Top Microbiol Immunol. 1996;214:133–176. doi: 10.1007/978-3-642-80145-7_5. [DOI] [PubMed] [Google Scholar]
- 9.Emi N, Friedmann T, Yee J K. Pseudotype formation of murine leukemia virus with the G protein of vesicular stomatitis virus. J Virol. 1991;65:1202–1207. doi: 10.1128/jvi.65.3.1202-1207.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Felgner P L, Gadek T R, Holm M, Roman R, Chan H W, Wenz M, Northrop J P, Ringold G M, Danielsen M. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc Natl Acad Sci USA. 1987;84:7413–7417. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fredericksen B L, Whitt M A. Vesicular stomatitis virus glycoprotein mutations that affect membrane fusion activity and abolish virus infectivity. J Virol. 1995;69:1435–1443. doi: 10.1128/jvi.69.3.1435-1443.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goff S, Traktman D, Baltimore D. Isolation and properties of Moloney murine leukemia virus mutants: use of a rapid assay for release of virion reverse transcriptase. J Virol. 1981;38:239–248. doi: 10.1128/jvi.38.1.239-248.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hobman T C, Lundstrom M L, Mauracher C A, Woodward L, Gillam S, Farquhar M G. Assembly of rubella virus structural proteins into virus-like particles in transfected cells. Virology. 1994;202:574–585. doi: 10.1006/viro.1994.1379. [DOI] [PubMed] [Google Scholar]
- 14.Huang A S, Besmer P, Chu L, Baltimore D. Growth of pseudotypes of vesicular stomatitis virus with N-tropic murine leukemia virus coats in cells resistant to N-tropic viruses. J Virol. 1973;12:659–662. doi: 10.1128/jvi.12.3.659-662.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hug P, Sleight R G. Fusogenic virosomes prepared by partitioning of vesicular stomatitis virus G protein into preformed vesicles. J Biol Chem. 1994;269:4050–4056. [PubMed] [Google Scholar]
- 16.Hunter E. Macromolecular interactions in the assembly of HIV and other retroviruses. Semin Virol. 1994;5:71–83. [Google Scholar]
- 17.Hunter E, Swanstrom R. Retrovirus envelope glycoproteins. Curr Top Microbiol Immunol. 1990;157:187–253. doi: 10.1007/978-3-642-75218-6_7. [DOI] [PubMed] [Google Scholar]
- 18.Li Y, Drone C, Sat E, Ghosh H P. Mutational analysis of the vesicular stomatitis virus glycoprotein G for membrane fusion domains. J Virol. 1993;67:4070–4077. doi: 10.1128/jvi.67.7.4070-4077.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Livingston D M, Howard T, Spence C. Identification of infectious virions which are vesicular stomatitis virus pseudotypes of murine type C virus. Virology. 1976;70:432–439. doi: 10.1016/0042-6822(76)90284-1. [DOI] [PubMed] [Google Scholar]
- 20.Metsikko K, van Meer G, Simmons K. Reconstitution of the fusiogenic activity of vesicular stomatitis virus. EMBO J. 1986;5:3429–3435. doi: 10.1002/j.1460-2075.1986.tb04665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miyanohara A, Yee J K, Bouic K, LaPorte P, Friedmann T. Efficient in vivo transduction of the neonatal mouse liver with pseudotyped retroviral vectors. Gene Ther. 1995;2:138–142. [PubMed] [Google Scholar]
- 22.Olkkonen V M, Dupree P, Simons K, Liljestrom P, Garoff H. Expression of exogenous proteins in mammalian cells with the Semliki Forest virus vector. Methods Cell Biol. 1994;43(Part A):43–53. doi: 10.1016/s0091-679x(08)60597-x. [DOI] [PubMed] [Google Scholar]
- 23.Ory D S, Neugeboren B A, Mulligan R C. A stable human-derived packaging cell line for production of high titer retrovirus/vesicular stomatitis virus G pseudotypes. Proc Natl Acad Sci USA. 1996;93:11400–11406. doi: 10.1073/pnas.93.21.11400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petri W A J, Wagner R R. Reconstitution into liposomes of the glycoprotein of vesicular stomatitis virus by detergent dialysis. J Biochem. 1979;254:4313–4316. [PubMed] [Google Scholar]
- 25.Rhee S S, Hui H X, Hunter E. Preassembled capsids of type D retroviruses contain a signal sufficient for targeting specifically to the plasma membrane. J Virol. 1990;64:3844–3852. doi: 10.1128/jvi.64.8.3844-3852.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rhee S S, Hunter E. A single amino acid substitution within the matrix protein of a type D retrovirus converts its morphogenesis to that of a type C retrovirus. Cell. 1990;63:77–86. doi: 10.1016/0092-8674(90)90289-q. [DOI] [PubMed] [Google Scholar]
- 27.Rolls M M, Webster P, Balba N H, Rose J K. Novel infectious particles generated by expression of the vesicular stomatitis virus glycoprotein from a self-replicating RNA. Cell. 1994;79:497–506. doi: 10.1016/0092-8674(94)90258-5. [DOI] [PubMed] [Google Scholar]
- 28.Sakalian M, Parker S D, Weldon R A J, Hunter E. Synthesis and assembly of retrovirus Gag precursors into immature capsids in vitro. J Virol. 1996;70:3706–3715. doi: 10.1128/jvi.70.6.3706-3715.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schochetman G, Kortright K, Schlom J. Mason-Pfizer monkey virus: analysis and localization of virion proteins and glycoproteins. J Virol. 1975;16:1208–1219. doi: 10.1128/jvi.16.5.1208-1219.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sharma S, Cantwell M, Kipps T J, Friedmann T. Efficient infection of a human T-cell line and of human primary peripheral blood leukocytes with a pseudotyped retrovirus vector. Proc Natl Acad Sci USA. 1996;93:11842–11847. doi: 10.1073/pnas.93.21.11842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sharma S, Murai F, Miyanohara A, Friedmann T. Noninfectious virus-like particles produced by Moloney murine leukemia virus-based retrovirus packaging cells deficient in viral envelope become infectious in the presence of lipofection reagents. Proc Natl Acad Sci USA. 1997;94:10803–10808. doi: 10.1073/pnas.94.20.10803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sommerfelt M A, Rhee S S, Hunter E. Importance of p12 protein in Mason-Pfizer monkey virus assembly and infectivity. J Virol. 1992;66:7005–7011. doi: 10.1128/jvi.66.12.7005-7011.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weiss R A, Boettiger D, Murphy H M. Pseudotypes of avian sarcoma viruses with the envelope properties of vesicular stomatitis virus. Virology. 1977;76:808–825. doi: 10.1016/0042-6822(77)90261-6. [DOI] [PubMed] [Google Scholar]
- 34.Wilcox M D, McKenzie M O, Parce J W, Lyles D S. Subunit interactions of vesicular stomatitis virus envelope glycoprotein influenced by detergent micelles and lipid bilayers. Biochemistry. 1992;31:10458–10464. doi: 10.1021/bi00158a007. [DOI] [PubMed] [Google Scholar]
- 35.Witte O N, Baltimore D. Mechanism of formation of pseudotypes between vesicular stomatitis virus and murine leukemia virus. Cell. 1977;11:505–511. doi: 10.1016/0092-8674(77)90068-x. [DOI] [PubMed] [Google Scholar]
- 36.Xu L, Yee J K, Wolff J A, Friedmann T. Factors affecting long-term stability of Moloney murine leukemia virus-based vectors. Virology. 1989;171:331–341. doi: 10.1016/0042-6822(89)90600-4. [DOI] [PubMed] [Google Scholar]
- 37.Yang Y, Vanin E F, Whitt M A, Fornerod M, Zwart R, Schneiderman R D, Grosveld G, Nienhuis A W. Inducible, high-level production of infectious murine leukemia retroviral vector particles pseudotyped with vesicular stomatitis virus G envelope protein. Hum Gene Ther. 1995;6:1203–1213. doi: 10.1089/hum.1995.6.9-1203. [DOI] [PubMed] [Google Scholar]
- 38.Yee J K, Miyanohara A, LaPorte P, Bouic K, Burns J C, Friedmann T. A general method for the generation of high-titer, pantropic retroviral vectors: highly efficient infection of primary hepatocytes. Proc Natl Acad Sci USA. 1994;91:9564–9568. doi: 10.1073/pnas.91.20.9564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yeger H, Kalnins V I, Stephenson J R. Type-C retrovirus maturation and assembly: post-translational cleavage of the gag-gene coded precursor polypeptide occurs at the cell membrane. Virology. 1978;89:34–44. doi: 10.1016/0042-6822(78)90037-5. [DOI] [PubMed] [Google Scholar]
- 40.Zavada J. The pseudotype paradox. J Gen Virol. 1982;63:15–24. doi: 10.1099/0022-1317-63-1-15. [DOI] [PubMed] [Google Scholar]
- 41.Zhang L, Ghosh H P. Characterization of the putative fusogenic domain in vesicular stomatitis virus glycoprotein G. J Virol. 1994;68:2186–2193. doi: 10.1128/jvi.68.4.2186-2193.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]