Abstract
Background:
In a randomized controlled 8-week trial, we examined the efficacy of aerobic and resistance exercise in reducing craving for methamphetamine (MA) among individuals with MA-use disorder during residential treatment.
Methods:
Individuals with MA use disorder (138) who were newly enrolled in residential treatment volunteered for random assignment to either an 8-week exercise intervention (EX) or health education control (HE), with both conditions meeting 3 times weekly; 3 dropped out of the trial, bringing the analysis sample to 135. The majority of participants were male (80%), and 48% were Latino/Hispanic. The mean age of the sample was 31.7 (SD = 6.9) years. Using multivariate mixed models, differences between conditions were examined in: (1) craving for MA, measured by self-reported ratings on a Visual Analog Scale over the 8-week trial and, (2) MA use, measured by self-report and urine drug screens at baseline and 30 and 60 days after discharge from the 8-week study.
Results:
Results revealed significantly lower craving scores among the participants in the EX group than those in the HE group over the 8-week trial (median daily craving score for EX = 13.5, for HE = 21.8; p = 009). In addition, participants with less craving during treatment had significantly lower rates of MA use after discharge than participants with high craving measured at 30-days (p = .004) and 60-days post-discharge (p < .001).
Conclusions:
Results support the utility of incorporating a structured exercise program for individuals with MA-use disorder to reduce craving and improve MA-use outcomes.
Keywords: Methamphetamine, Exercise, Craving, Outcomes
1. Introduction
Overdose deaths involving amphetamines have dramatically increased in the United States in recent years, with age-adjusted mortality rates per 100,000 standard population increasing from 0.8 in 2012 to 3.9 in 2018 (Hedegaard et al., 2020). This increase in mortality has occurred with the increased availability of methamphetamine (MA) in the United States (Drug Enforcement Administration, 2019). MA has been linked to an array of behavioral, cognitive, psychological, and medical co-occurring conditions that negatively affect functioning (Gonzales et al., 2010; Paulus and Stewart, 2020) and worsens the progression and consequences of chronic and infectious diseases, including HIV/AIDS, hepatitis, and others, thereby dampening overall health status and quality of life (National Institute on Drug Abuse, 2014).
To date, there is no approved medication for the treatment of MA use disorder, although a behavioral approach, contingency management, has substantial evidence of efficacy (AshaRani et al., 2020; De Crescenzo et al., 2018; Rawson et al., 2006). However, outside of the VA treatment system, there is minimal use of contingency management in the U.S. treatment system, and additional effective treatments are needed.
Previous reports by the authors have provided support for the usefulness of physical exercise for individuals diagnosed with methamphetamine use disorder (MUD). Individuals with MUD admitted to a residential treatment program were randomly assigned to either an 8-week, 3 times per week, 60-minute exercise intervention or to a 60-minute health-education control session (Mooney, et al., 2013). Those who participated in the exercise condition demonstrated improvements in several physiological outcomes, including improved heart rate variability (Dolezal et al., 2014) and reduced symptoms of anxiety and depression (Rawson, Chudzynski, Gonzales, et al., 2015). Further, participation in the exercise condition resulted in increased dopamine receptor availability (Robertson et al., 2015) and reduced MA use at post-discharge at two follow-ups spanning 1- and 2-month time points (Rawson, Chudzynski, Mooney, et al., 2015).
This paper examines data from the Mooney et al. study concerning the impact of exercise on craving for MA over the course of the 8-week trial. Craving is defined as an intensely strong desire or urge to use a substance that can occur at any time (American Psychiatric Association, 2013). Increasing attention has been directed at targeting psychophysiological processes of craving during treatment, as it has been found to be a clinical predictor of poor treatment outcomes (e.g., relapse) for those using a wide array of substances, including alcohol, nicotine, heroin, cocaine, and MA (Galloway et al., 2008; Tuliao and Liwag, 2011). Studies have shown that relapse occurs more rapidly among high cravers compared to low cravers (Tuliao and Liwag, 2011). Galloway and colleagues (2008), for instance, showed that MA craving was a significant predictor of MA use, such that craving intensity scores were 2.7 times higher before use among those who reported MA use, compared to those who did not report MA use 1 week following treatment admission. Moreover, research has found that craving can persist up to 5 weeks after abstinence from MA (Zorick et al., 2011), rendering the treatment period as a critical time to target craving as a clinical goal (Tiffany et al., 2012).
Although research supports the importance of examining and addressing craving in relation to reducing the risk of relapse, little research has focused on this phenomenon. To date, several treatment studies have identified promising therapeutic practices that have helped patients suffering from substance use disorders minimize or address issues with craving, including cognitive-based skills, non-invasive neurostimulation, and mindfulness (Yen et al., 2004; Field and Cox, 2008; Jansen et al., 2013). Studies using animal models have shown exercise to be an effective modality in attenuating reinstatement of stimulant use, with a focus on neurotransmitters in the pathophysiology of craving (Chen et al., 2008; Engelmann et al., 2013; Lynch et al., 2010; Smith et al., 2012).
Studies on humans have examined this exercise-craving relationship as well. A systematic review (Taylor et al., 2007) found a moderate-to-large acute effect of exercise on cravings to smoke cigarettes, especially by decreasing the strength of the desire to smoke. Even low intensity exercise decreased the duration of craving. Taylor and Katomeri (2007) showed that 15 minutes of low- to moderate-intensity exercise improved cue-related nicotine craving unrelated to stress and that a reduction in craving was associated with a delay in smoking after exercise. Kurti and Dallery (2014) found that among smokers, post-exercise reduction in craving was significantly associated with longer delays in smoking, with the major effect of exercise being on the reward component of craving but not the relief component (Kurti and Dallery, 2014).
There has been at least one prior study demonstrating the beneficial effects of aerobic exercise on MA craving reduction (Wang et al., 2017). This study specifically found that among a sample of 24 patients in China with MUD, acute aerobic exercise at a moderate intensity attenuated MA craving and facilitated both normal and drug-related inhibitory control. However, to date, few studies have examined the impact of exercise on craving among U.S.-based samples of individuals in treatment for MUD.
Given the limited research on the impacts of exercise interventions for MUD with respect to craving measures, in particular, this paper extends upon this previous body of research by examining the effectiveness of an 8-week exercise intervention for reducing MA craving compared to a health education control and the extent to which such differences impact follow-up MA use.
2. Methods
2.1. Participants
One hundred thirty-eight individuals with MA use disorder who were newly enrolled in a social model-based residential treatment consented to random assignment to an 8-week exercise intervention (EX, n = 71) or health education control (HE, n = 67) between 2010 and 2013 in Southern California. Inclusion criteria included having an MA dependence diagnosis (per DSM-IV), being age 18–45 for men or 18–55 for women, English language proficiency, and ability to comply with study procedures. Individuals were excluded if they exhibited medical impairment that compromised their safety as a participant, met criteria for opioid dependence, or had a psychiatric disorder that warranted hospitalization or primary treatment (see Mooney et al., 2013, for details of study procedures). Randomization was stratified, using an urn computerized program, by gender and severity of MA use at treatment entry, defined as days of MA use in the previous 30 days, categorized by median number of days of MA use in the previous 30 days (at baseline), as was done in previous studies (Heinzerling et al., 2014; Shoptaw et al., 2008) and reported in previous work, see Mooney et al., 2014. Specifically, “low severity use” was defined as 18 days or fewer of use and “high-severity use” as use for more than 18 days. There was low study attrition, as only 3 patients dropped from the trial at the 8-week follow-up post-discharge (1 from the HE group and 2 from the EX group), dropping the analysis total sample to 135.
2.2. Procedures
Eligible patients were randomized into either an 8-week exercise (EX) intervention condition, which received 60-minute structured exercise sessions of progressive aerobic and resistance exercise (aerobic treadmill activity and 1–2 sets of weight training for major muscle groups-arms, chest, back, and legs) 3 times a week, or into an 8-week health education (HE) control condition, which received 60-minute health education sessions on a variety of health topics (e.g., stress, sexually transmitted infections, and nutrition) 3 times a week. Each exercise session was led by an exercise physiologist, and each health education session was led by a health education-trained specialist. The true nature (experimental vs. control) of the groups was never divulged to the participants and group preference was not asked or collected. .
Session attendance in both study conditions was not presented as compulsory but rather as voluntary, and patients were told they would not be excluded for missing sessions. Participants were compensated with $10 gift cards per session attended across both conditions. Data collection, which also was voluntary, occurred at baseline, weekly during the 8-week trial, and at follow-ups (30 day/1 month and 60 day/2 month) post-discharge from the trial. In addition to the study conditions, all participants received the same social model-based residential treatment for MA use disorder provided by the facility, which consisted of a combination of individual and group counseling using a relapse-prevention clinical framework with tailored plans to meet individual needs. Other services provided to clients included vocational assistance, family education, neurofeedback, trauma groups, grief counseling, treatment for co-occurring mental health conditions, and daily 12-Step meetings. No medications for stimulant use disorder were provided. However, some patients did have co-occurring psychiatric and medical co-morbidities and were on prescribed medications for these conditions. Additionally, there was no structured post-discharge care provided by the residential facility. Rather, patients from the start of treatment are involved with a 12-step program and when discharged back into the community are recommended to continue 12-step participation.
2.3. Measures
2.3.1. Craving for MA use
A Visual Analog Scale (VAS) was used to measure craving for MA use (Kaplan et al., 1985; McLellan et al., 1992). Patients marked on a line “0” for “not at all” to “100” for “extremely high” in response to the question, “How much do you feel the urge to use MA?” Self-reported craving for MA use was measured at baseline treatment admission (Week 0), once weekly over the 8-week study trial, and upon study discharge (Week 8).
2.3.2. MA use
MA use was measured at baseline (upon admission to the treatment program) and at 1 and 2 months after discharging from the 8-week study trial using urine drug screens (UDS), and self-reported previous-month MA use was collected using the Substance Use Inventory. This data collection protocol aligned with the treatment site used for the study, in that participants were inpatients during the initial 8 weeks of service and did not leave the premises; hence no substance use data was collected during the study 8-week trial. Data on missing observations are listed in all the tables and figures. Since there were very few missing data on craving, no data imputation was needed.
2.3.3. Baseline covariates
The covariates that were controlled for in all analyses included sociodemographic factors (age, gender, education, and employment), substance use severity factors (baseline MA use, route of administration, baseline alcohol use), and psychosocial factors (baseline depression symptoms). The sociodemographic and substance use severity factors were collected using the Addiction Severity Index-ASI (McLellan et al., 1992) as part of a battery of baseline measures administered to participants upon their enrollment in the RCT. In addition, depression symptoms were collected using the Beck Depression Inventory-BDI, a measure that also was a part of the battery of baseline assessments. The BDI is a 21-item self-report questionnaire that calculates a total score (ranging from 0 to 63), with scores of 0 to 13, 14 to 19, 20 to 28, and 29 to 63 indicating minimal, mild, moderate, and severe depression, respectively (Beck et al., 1961), and for this study, the BDI baseline depression score was controlled for in all analyses.
2.4. Statistical analyses
Statistical analyses were conducted using SAS 9.4 and STATA 16 software. For all statistical analyses, alpha was set at 0.05.
2.4.1. Participant characteristics:
Statistical diagnostics were performed on participant characteristics, including baseline covariates (sociodemographic, substance-use severity, and psychosocial factors). T-tests were used to test for differences in participant characteristics for the continuous variables, which were age, years of education, days of methamphetamine and alcohol use, and Beck Depression baseline score. Chi-square tests were used to test for differences in the binary categorical variables of gender (males vs. females) and employment (employed vs. not employed). Results showed no statistically significant differences in participant characteristics between the study conditions (see Table 1).
Table 1.
Differences in Participant Characteristics by Study Condition
| Health Education Condition (N = 66) | Exercise Condition (N = 69) | P-value | |
|---|---|---|---|
| Mean Age (SD) | 31.4 (6.5) | 31.9 (7.4) | 0.61 |
| % Females | 28.90% | 30.40% | 0.83 |
| Mean Years Education (SD) | 12.1 (1.9) | 12.2 (1.9) | 0.76 |
| % Employed | 18.20% | 14.50% | 0.84 |
| Baseline Past-Month Mean Days Methamphetamine Use (SD) | 16.6 (10.6) |
15.9 (9.1) | 0.61 |
| Baseline Past-Month Mean Days Alcohol Consumption (SD) | 5.2 (7.6) | 5.0 (7.3) | 0.86 |
| Mean Beck Depression Baseline Scores (SD) | 13.7 (8.3) | 12.0 (7.3) | 0.21 |
2.4.2. Craving and differences by study conditions.
Given that craving was measured at repeated time points, using a continuous scale from 0 to 100, different analyses were performed. First, the Mann-Whitney U testing was used to compare differences in craving scores between the conditions, given the fluctuations in craving reported weekly between the groups throughout the 8-week trial using weekly craving scores. Second, we tested differences in craving at each of the weekly time points (one test for each week), as well as the difference in craving score between groups and overall average score for each group using bivariate Mann-Whitney U testing. Third, changes in mean weekly craving scores for MA use by study condition were examined across time (from baseline to Week 8) using longitudinal mixed-effects repeated-measures regression. The longitudinal mixed-effects repeated-measures regression model was used to test the main effects of study condition (dummy coded with 1 = exercise, 0 = education), time (in weeks), and time x condition interaction.
2.4.3. Craving and MA use outcome differences.
For these analyses, craving was examined in different ways. Chi-square analyses were conducted to examine differences in MA use at follow-up data visits (1 month/30-days and 2 month/60-days post–8-week study discharge) using urine drug screen data measured dichotomously (0 = negative MA use; 1 = positive MA use) and craving severity measured dichotomously (0 = low craving [scores less than 16]; 1 = high craving scores [greater than 16]). Craving groups (low vs. high) were determined using the median as cutoff, with those scoring above the median classified as the high group and those below the median classified as the low group. The rationale for using the median as cutoff is based on prior research with MA-use severity measured as low versus high (see Heinzerling et al., 2014; Shoptaw et al., 2008), which used MA-use baseline median split to categorize patients into “low severity use” and “high-severity use.” In addition to the chi-square analyses mentioned above, which examines differences in MA use at 1-month and 2-month post-discharge versus high/low craving, multiple logistic regression was used to examine craving (high vs. low as the outcome) and MA use, controlling for age, gender, education, employment and baseline depression score (measured by the Beck Depression Inventory-BDI) at 1 months and 2 months post-discharge. Finally, the average craving score was examined longitudinally from baseline to 1-month and 2-month post-discharge using mixed-models repeated measures analysis. The interaction effect of MA use, time, and treatment condition was examined in the model.
2.4.4. Study participation and attrition.
An intent-to-treat model was used that included participants who were randomized and received at least one treatment session in the exercise or education condition during the 8-week trial. Study participation, in terms of session attendance, was high for both study conditions. Specifically, of the 69 participants assigned to the thrice weekly exercise condition, average attendance was 17.4 (SD = 7.3) out of the 24 exercise scheduled sessions. Similarly, of the 66 participants assigned to the thrice weekly health education condition, average attendance was 18.5 (SD = 7.0) out of the 24 health education scheduled sessions. As noted in the Procedures section, sessions were not compulsory but rather voluntary; however, participants were compensated with $10 gift cards per session for each exercise or education session attended, which may have helped with attendance. In addition, given that the study took place in a social model-based residential setting where patients were active in treatment, high attendance may have been an artifact of participating in something novel in addition to treatment. The dropout rate was very low during the 8-week trial; only 3 patients dropped out, 2 from the exercise condition and 1 from the health education condition.
3. Results
3.1. Participant characteristics
The 135 study participants had an average age of 31.7 (SD = 6.9), ranging from 18 to 47 years old. The majority of participants (80%) were males, and most were Hispanic/Latino (48.1%) or Caucasian/White (41.5%), with fewer African American/Blacks (4.4%), Asians (3.7%), or members of other ethnic groups represented (2.2%). Most participants were unemployed (83.7%) in the month prior to treatment entry, and 67.4% had a high school education.
3.2. Craving by study condition during the 8-week trial
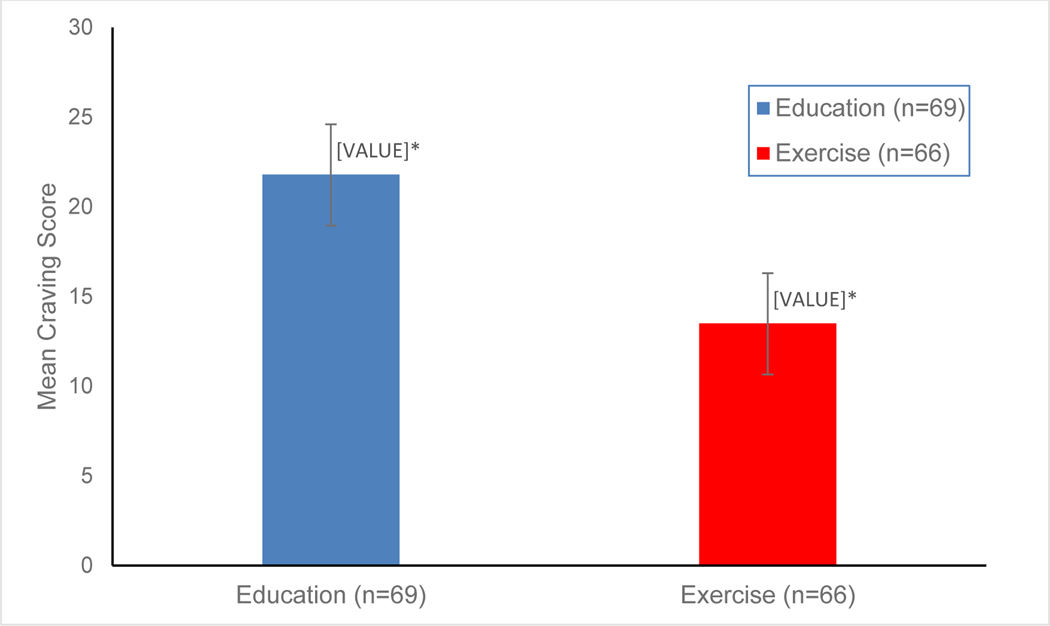
As a preliminary analysis, we examined the total average craving scores across the 8 weeks by treatment condition using the Mann-Whitney U Test, to determine if there were any overall significant differences across study conditions. Results show that participants in the HE control group had a significantly higher total mean weekly craving score (mean = 21.8) during the 8-week trial, compared to the EX condition (mean = 13.5; P = .009; see Figure 1).
Figure 1. Mean craving score by study condition during 8-week trial.
* P = .009 (Mann-Whitney U test)
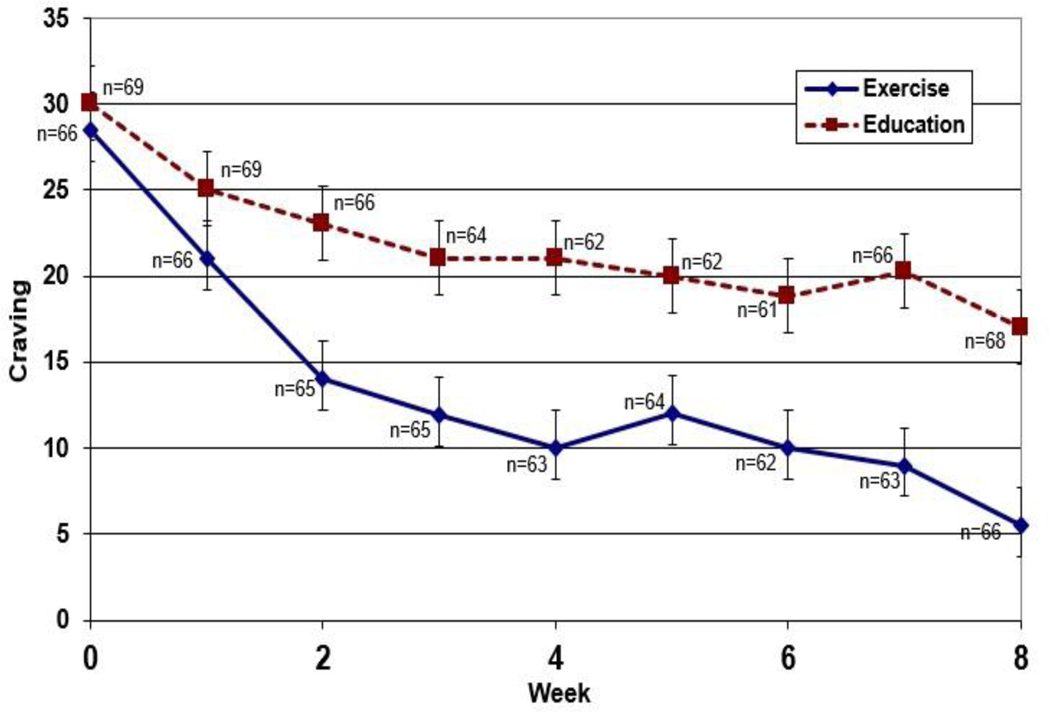
As a next step, we examined the average weekly craving score from Weeks 1 to 8 by study condition. Figure 2 displays the results across the 8 weeks showing the average craving rating from Week 1 to Week 8 by study condition. Since the exercise group appears to have a lower craving score across time compared to the health education group, advanced mixed-modeling analyses were performed to examine whether there were significant statistical longitudinal effects of craving ratings on MA use.
Figure 2.
Average weekly craving scores by study conditions during 8-week trial
3.3. Craving and MA use outcome differences post-discharge from 8-week trial among all study participants
Given that these analyses examine craving by MA-use outcomes post the 8-week trial, we used multiple regression to examine differences among all participants regardless of condition. Results show that weekly craving scores (averaged per participant) during the 8-week trial were significantly associated with MA use outcomes post-discharge at 1 month/30-days and 2 month/60-days data visits for all participants. As shown in Tables 2 and 3, lower craving average scores during the 8-week trial were significantly associated with less MA use at 1 month/30-days post-discharge (β = .38, p < .001) and 2 month/60-days post-discharge (β = .45, p < 0.001), after controlling for participant demographics, substance use severity, and psychosocial factors (shown in Table 1).
Table 2.
MA use outcomes by 8-week average craving score at 1 month/30-days post-discharge among all study participants (n=66 in EX and n=69 in HE group)
| Coefficient | P-value | |
|---|---|---|
| Average MA Craving Score | 0.3763 | <0.001 |
| MA, baseline | 0.0649 | 0.511 |
| Age | 0.0392 | 0.764 |
| Education, yrs | 0.4909 | 0.317 |
| Females | −1.9298 | 0.356 |
| Mean Beck Depression Baseline Score | −0.0541 | 0.654 |
| Employed full time | −0.4514 | 0.259 |
P < .05, Significant effect of craving; those with low craving average scores over the 8-week trial had significantly lower MA use at the 1 month/30-day post-discharge follow-up.
Table 3.
MA use outcomes by 8-week average craving score at 2 month/60-days post-discharge among all study participants (n=66 in EX and n=69 in HE group)
| Coefficient | P-value | |
|---|---|---|
| Average MA Craving Score | 0.4524 | <0.001 |
| MA, baseline | 0.2442 | 0.380 |
| Age | −0.2239 | 0.158 |
| Education, yrs | −0.4017 | 0.489 |
| Females | −1.855 | 0.440 |
| Mean Beck Depression Baseline Score | −0.0493 | 0.730 |
| Employed full time | −0.1365 | 0.766 |
P < .05, Significant effect of craving; those with low craving average score over the 8-week trial had significantly lower MA use at the 2 month/60-day post-discharge follow-up.
3.4. Craving and MA use outcome differences post-discharge from 8-week trial by study condition
Given that this set of analyses examined craving by MA-use outcomes post the 8-week trial by study condition, different analyses were performed. In this first step, we used chi-square tests to examine differences in craving by MA-use outcomes. Results show that at 1 month/30-days post-discharge, 77.3% of participants who had high craving scores during the 8-week study period tested positive for MA use in their urine drug screens, compared to 22.7% of participants with low craving scores (chi-square = 8.1, p = .004). At 2 month/60-days post-discharge, 88.2% of the participants with high craving scores tested positive for MA use compared to 11.8% among participants with low craving scores (chi-square = 12.9, p = < .001). In the second set of analyses, using logistic regression, results indicate that participants with high craving scores were more likely to test positive for MA at 30-days (OR = 3.7, CI 1.2–11.6) and at 60-days post-discharge (OR = 13.4, CI 2.5, 31.1), after controlling for participant demographics and severity factors. See Table 4.
Table 4.
High vs. low craving Scores by urine drug screen test results for methamphetamine
| 1 Month/30-Days Post-Discharge | UDS Positive for MA (n=22) | UDS Negative for MA (n=77) | Chi-Square | P-value | Odds Ratio* (Confidence Intervals) |
|---|---|---|---|---|---|
| High Craving, ≥ 16 (n=50) | 77.3% (17/22) | 42.9% (33/77) | 8.1 | 0.004 | 3.7 (1.2, 11.6) |
| Low Craving, < 16(n=49) | 22.7% (5/22) | 57.1% (44/77) | |||
| Total | 100.0% | 100.0% | |||
| 2 Month/60-Days Post-Discharge |
(n=17) | (n=82) | |||
| High Craving, ≥ 16 (n=33) | 88.2% (15/17) | 40.2% (33/82) | 12.9 | <0.001 | 13.4 (2.5,31.1) |
| Low Craving, < 16 (n=49( | 11.8% 2/17) | 59.8% (49/82) | |||
| Total | 100.0% | 100.0% |
Odds Ratios adjusted for age, gender, education, employment, and mean beck depression baseline score; N=99, with n=26 missing UDS data for both 1 month/30- and 2 month/60-days post-discharge.
Lastly, advanced mixed-modeling analyses were performed to examine the longitudinal effects of craving ratings on MA-use outcomes from baseline to post-discharge (1 month/30-days and 2 month/60-days) data points by study condition, controlling for participant factors. Results showed a significant interaction effect of treatment condition (exercise) by MA use on craving (β = - 0.769, p = 0.002) across time, indicating that MA users in the exercise condition had significantly lower craving scores compared to those in the health education condition (see Table 5).
Table 5.
Mean Craving across time by treatment condition
| Coefficient | P-value | |
|---|---|---|
| Age | 0.140 | 0.551 |
| Education, yrs | 0.749 | 0.385 |
| Females | 6.455 | 0.092 |
| Mean Beck Depression Baseline Score | 0.199 | 0.342 |
| Employed full time | −1.493 | 0.023 |
| MA use | −4.296 | 0.392 |
| Time in weeks | 0.085 | 0.482 |
| Study condition: exercise | −9.826 | 0.029 |
| Time x study condition: exercise | −0.289 | 0.04 |
| Time x study condition: exercise x MA use | −0.769 | 0.002 |
N= 129 with n=6 missing observation for MA use and craving score at 30- and 60-days post-discharge.
4. Discussion
There has been growing interest in the role craving plays within the context of addiction treatment. This study examined differences in reports of craving for MA among a clinical sample of patients in a social model-based residential treatment for MA use disorder who participated in a RCT comparing the effects of an 8-week exercise intervention to a health education control and the extent to which differences in craving impacted MA-use outcomes after the 8-week trial.
This study has replicated some previous animal and human research studies that have found positive impacts of exercise interventions on craving measures, showing that exercise during periods of abstinence is associated with reductions in craving and that there is a positive association of reduced craving with improved treatment outcomes (Chen et al., 2008; Engelman et al., 2013; Kurti and Dallery, 104; Lynch et al., 2010; Smith et al., 2012; Taylor et al., 2007; Yamaguchi et al., 2011). Findings are also consistent with a prior 12-week trial of exercise for MA dependence in China, which was also conducted in a residential setting and reported reductions in MA craving associated with exercise from Weeks 6 to 12 of the intervention period (Wang et al., 2017).
Overall, this paper extends prior literature on the benefits of exercise with respect to craving among patients with MA-use disorder in social model-based residential treatment. The current study demonstrated that, after controlling for patient covariates (sociodemographics, substance-use severity, and baseline Beck Depression Inventory scores), exercise was associated with significantly more craving reduction over the 8-week trial (p = 0.04), compared to the health education control condition. Moreover, we found that participants with low craving during the trial were significantly more likely than those with higher craving to have reduced MA-use outcomes at study follow-ups at 1-month/30-days (p = 0.004) and 2-months/60-days post-discharge from the treatment program (p = 0.001).
4.1. Limitations
This study was limited to understanding the benefits of exercise on MA use craving and MA-use outcomes among patients in a social model-based residential treatment program and, therefore, may not be generalizable to individuals with MA-use disorder in other types of treatment settings. In addition, because patients were in a captive inpatient setting, adherence to the exercise and health education session protocols and attendance was high. In studies with outpatients, much lower adherence with protocols have been reported (Trivedi et al., 2017). In addition, post-discharge MA consumption outcomes were limited to the immediate effects of the trial (30- and 60-days post-discharge) and craving reports. Furthermore, this study limited the measure of craving to a self-report technique rather than other objective measures that test craving using cue-elicited protocols.
5. Conclusions
Results support the utility of providing structured exercise for MA-use disorder to reduce craving and improve MA-use treatment outcomes. This study not only found that a structured exercise intervention had significant effects on reducing MA cravings over the 8-week trial, compared to a health education control, but also that reduced craving was associated with significant reductions in MA-use outcomes after treatment at the 30-day (p < .004) and 60-day (p < .001) data-collection time points. These findings provide value to the field as they support the clinical utility and promising effects of patients who engage in routine physical exercise, including reductions in cravings/urges for MA and decreased risk of relapse to MA use. The explanation for the success of exercise in affecting craving and MA-use behaviors may be related to exercise-related dopamine system recovery affecting reward and reinforcement (Robertson et al., 2015) and the broad health benefits and enjoyment associated with exercise, which may counteract strong cravings/urges and reduce the tendency of succumbing to behavioral urges (Giménez-Meseguer, et al., 2020).
Together with previously published research from this study (Dolezal et al., 2014; Rawson, Chudzynski, Gonzales et al., 2015; Rawson, Chudzynski, Mooney et al., 2015; Robertson et al., 2015), results suggest that exercise provides a broad array of benefits to individuals in the early months of abstinence from MA use. Clinicians seeking treatment strategies to assist patients struggling with high craving should consider recommending exercise to their patients, along with proper medical screening and supervision. Future studies should be conducted for wider replication as well as to explore effects (craving by condition on outcomes) by potential mechanisms, such as mental health functioning, medical status (e.g., HIV) and tobacco use. In addition, to develop a clearer understanding of protracted treatment outcomes associated with exercise in individuals with MA use disorder, further long-term follow-up studies involving clinical populations with MA-use disorder who receive exercise interventions are warranted to examine long-term effects of exercise interventions on continued exercise behaviors as well as relationships between such behaviors and craving, MA consumption, and recovery outcomes.
HIGHLIGHTS.
Exercise significantly reduced craving during treatment for methamphetamine use.
Participants with less craving during treatment had less MA use after discharge.
Exercise may be a useful intervention for people in treatment for MA use disorder.
Acknowledgments
This study was supported by grant R01 DA027633 from the National Institute on Drug Abuse (NIDA). The authors would like to thank the administrative and treatment staff at the participating treatment program for their support, and they also thank research staff Vanessa Novoa, Marlon Abrazado, and Patricia Ballesteros for contributing to the implementation of the study.
Role of Funding Source
Funding for this study was provided by the National Institute on Drug Abuse (NIDA) by grant R01 DA027633. NIDA had no further role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Conflict of Interest
No conflict declared by the authors.
Clinical trial registration details: ClinicalTrials.gov Identifier: NCT01103531
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychiatric Association, 2013. Diagnostic and statistical manual of mental disorders, fifth ed. Author, Arlington, VA. [Google Scholar]
- AshaRani P, Hombali A, Seow E, Jie W, Ong TJH, Subramaniam M, 2020. Nonpharmacological interventions for methamphetamine use disorder: a systematic review. Drug Alcohol Depend. 212. 10.1016/j.drugalcdep.2020.108060. [DOI] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh, 1961. An inventory for measuring depression. Arch Gen Psychiatry. 4, 561–571. 10.1001/archpsyc.1961.01710120031004 [DOI] [PubMed] [Google Scholar]
- Beckson M, Tucker D, 2014. Commentary: craving diagnostic validity in DSM-5 substance use disorders. J. Am. Acad. Psychiatry Law 42, 453–458. https://pubmed.ncbi.nlm.nih.gov/25492071/. [PubMed] [Google Scholar]
- Chen HI, Kuo YM, Liao CH, Jen CJ, Huang AM, Cherng CG, Su SW, Yu L, 2008. Long-term compulsive exercise reduces the rewarding efficacy of 3, 4 methylenedioxymethamphetamine. Behav. Brain Res. 187 (1), 185–189, 10.1016/j.bbr.2007.09.014. [DOI] [PubMed] [Google Scholar]
- De Crescenzo F, Ciabattini M, D’Alò GL., De Giorgi R, Del Giovane C, Cassar C, Janiri L, Clark N, Ostacher MJ, & Cipriani A, 2018. Comparative efficacy and acceptability of psychosocial interventions for individuals with cocaine and amphetamine addiction: a systematic review and network meta-analysis. PLoS Med. 15 (12), e1002715. 10.1371/journal.pmed.1002715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolezal BA, Chudzynski J, Dickerson D, Mooney L, Rawson RA, Garfinkel A, Cooper CB, 2014. Exercise training improves heart rate variability after methamphetamine dependency. Med. Sci. Sports Exerc. 46 (6), 1057–1066. 10.1249/mss.0000000000000201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drug Enforcement Administration, 2019. Drug Enforcement Administration National Drug Threat Assessment. U.S. Department of Justice. https://www.dea.gov/sites/default/files/2020-02/DIR-007-20%202019%20National%20Drug%20Threat%20Assessment%20-%20low%20res210.pdf. [Google Scholar]
- Engelmann AJ, Aparicio MB, Kim A, Sobieraj JC, Yuan CJ, Grant Y, Mandyam CD, 2013. Chronic wheel running reduces maladaptive patterns of methamphetamine intake: regulation by attenuation of methamphetamine-induced neuronal nitric oxide synthase. Brain Struct. Funct. 219 (2), 657–672, 10.1007/s00429-013-0525-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field M, Cox WM, 2008. Attentional bias in addictive behaviors: a review of its development, causes, and consequences. Drug Alcohol Depend. 97, 1–20, 10.1016/j.drugalcdep.2008.03.030. [DOI] [PubMed] [Google Scholar]
- Galloway GP, Singleton EG, and the Methamphetamine Treatment Project Corporate Authors, 2008. How long does craving predict use of methamphetamine? Assessment of use one to seven weeks after the assessment of craving. Subst. Abuse 1, 63–79, 10.4137/SART.S775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giménez-Meseguer J, Tortosa-Martínez J, Cortell-Tormo JM (2020). The benefits of physical exercise on mental disorders and quality of life in substance use disorders patients. Systematic review and meta-analysis. Int. J. Environ. Res. Public Health, 17(10), 3680. 10.3390/ijerph17103680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales R, Mooney L, Rawson R, 2010. The methamphetamine problem in the United States. Ann. Rev. Public Health 31, 385–398, https://dx.doi.org/10.1146%2Fannurev.publhealth.012809.103600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedegaard H, Minino AM, Warner M, 2020. Drug overdose deaths in the United States, 1999–2018. NCHS Data Brief 356, 1–8. https://pubmed.ncbi.nlm.nih.gov/32487285/. [PubMed] [Google Scholar]
- Heinzerling KG, Swanson AN, Hall TM, Yi Y, Wu Y, Shoptaw SJ, 2014. Randomized, placebo-controlled trial of Bupropion in methamphetamine-dependent participants with less than daily methamphetamine use. Addiction 109 (11), 18781886, 10.1111/add.12636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen JM, Daams JG, Koeter MWJ, Veltman DJ, Van Den Brink W, Goudriaan AE, 2013. Effects of non-invasive neurostimulation on craving: a meta-analysis. Neurosci. Biobehav Rev. 37, 2472–2480, 10.1016/j.neubiorev.2013.07.009. [DOI] [PubMed] [Google Scholar]
- Kaplan RF, Cooney NL, Baker LH, Gillespie RA, Meyer RE, Pomerleau OF, 1985. Reactivity to alcohol-related cues: physiological and subjective responses in alcoholics and non-problem drinkers. J. Stud. Alcohol 46, 267–272, 10.15288/jsa.1985.46.267. [DOI] [PubMed] [Google Scholar]
- Kurti AN, Dallery J, 2014. Effects of exercise on craving and cigarette smoking in the human laboratory. Addict. Behav. 39 (6), 1131–1137, 10.1016/j.addbeh.2014.03.004. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Piehl KB, Acosta G, Peterson AB, Hemby SE, 2010. Aerobic exercise attenuates reinstatement of cocaine-seeking behavior and associated neuroadaptations in the prefrontal cortex. Biol. Psychiatry 68 (8), 774–777, 10.1016/j.biopsych.2010.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, Pettinati H, Argeriou M, 1992. The Fifth Edition of the Addiction Severity Index. J. Subst. Abuse Treat, 9 (3), 199–213, 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- Mooney LJ, Cooper C, London ED, Chudzynski J, Dolezal B, Dickerson D, Brecht M-L, Peñate J, Rawson RA, 2013. Exercise for methamphetamine dependence: rationale, design, and methodology. Contemp. Clin. Trials 37 (1), 139–147, 10.1016/j.cct.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute on Drug Abuse (NIDA), 2019. Methamphetamine DrugFacts. https://www.drugabuse.gov/publications/drugfacts/methamphetamine.
- Paulus MP, Stewart JL, 2020. Neurobiology, clinical presentation and treatment of methamphetamine use disorder: a review. JAMA Psychiatry 77, 959–966. 10.1001/jamapsychiatry.2020.0246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawson RA, Chudzynski J, Gonzales R, Mooney L, Dickerson D, Ang A, Dolezal B, Cooper CB, 2015. The impact of exercise on depression and anxiety symptoms among abstinent methamphetamine-dependent individuals in a residential treatment setting. J. Subst. Abuse Treat. 57, 36–40, 10.1016/j.jsat.2015.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawson RA, Chudzynski J, Mooney L, Gonzales R, Ang A, Dickerson D, Penate J, Salem BA, Dolezal B, Cooper CB, 2015. Impact of an exercise intervention on methamphetamine use outcomes post-residential treatment care. Drug Alcohol Depend. 156, 21–28. https://psycnet.apa.org/doi/10.1016/j.drugalcdep.2015.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawson RA, McCann MJ, Flammino F, Shoptaw S, Miotto K, Reiber C, Ling W, 2006. A comparison of contingency management and cognitive-behavioral approaches for stimulant-dependent individuals. Addiction 101, 267–274, 10.1111/j.1360-0443.2006.01312.x [DOI] [PubMed] [Google Scholar]
- Robertson CL, Ishibashi K, Chudzynski J, Mooney LJ, Rawson RA, Dolezal BA, Cooper CB, Brown AK, Mandelkern MA, London ED, 2016. Effect of exercise training on striatal dopamine D2/D3 receptors in methamphetamine users during behavioral treatment. Neuropsychopharmacol. 41 (6), 1629–1636. 10.1038/npp.2015.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoptaw S, Heinzerling KG, Rotheram-Fuller E, Steward T, Wang J, Swanson AN, De La Garza R, Newton T, Ling W, 2008. Randomized, placebo-controlled trial of Bupropion for the treatment of methamphetamine dependence. Drug Alcohol Depend. 96 (3), 222–32, 10.1016/j.drugalcdep.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Pennock MM, Walker KL, Lang KC, 2012. Access to a running wheel decreases cocaine-primed and cue-induced reinstatement in male and female rats. Drug Alcohol Depend. 121 (1–2), 54–61, 10.1016/j.drugalcdep.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A, Katomeri M, 2007. Walking reduces cue-elicited cigarette cravings and withdrawal symptoms, and delays ad libitum smoking. Nicotine Tob. Res. 9 (11), 1183–1190, 10.1080/14622200701648896. [DOI] [PubMed] [Google Scholar]
- Taylor AH, Ussher MH, Faulkner G, 2007. The acute effects of exercise on cigarette cravings, withdrawal symptoms, affect and smoking behavior: a systematic review. Addiction 102 (4), 534–543, 10.1111/j.1360-0443.2006.01739.x. [DOI] [PubMed] [Google Scholar]
- Tiffany ST, Friedman L, Greenfield SF, Hasin DS, Jackson R, 2012. Beyond drug use: a systematic consideration of other outcomes in evaluations of treatments for substance use disorders. Addiction 107, 709–718, 10.1111/j.1360-0443.2011.03581.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi MH, Greer TL, Rethorst C, Carmody T, Grannemann B, Walker R, Nunes E, 2017. Randomized trial comparing exercise to health education for stimulant use disorder: results from stimulant reduction intervention using dosed exercise (CTN-0037; STRIDE). J. Clin. Psychiatry 78 (8), 1075–1082, https://dx.doi.org/10.4088%2FJCP.15m10591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuliao AP, Liwag ME, 2011. Predictors of relapse in Filipino male methamphetamine users: a mixed methods approach. J. Ethn. Subst. Abuse 10 (2), 162–179, 10.1080/15332640.2011.573319. [DOI] [PubMed] [Google Scholar]
- Wang D, Zhu T, Zhou C, Chang Y-K, 2017. Aerobic exercise training ameliorates craving and inhibitory control in methamphetamine dependencies: a randomized controlled trial and event-related potential study. Psychol Sport Exerc. 30, 82–90, 10.1016/j.psychsport.2017.02.001. [DOI] [Google Scholar]
- Yamaguchi T, Wang HL, Li X, Ng TH, Morales M, 2011. Mesocorticolimbic glutamatergic pathway. J. Neurosci. 31 (23), 8476–8490, 10.1523/jneurosci.1598-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen CF, Wu HY, Yen JY, Ko CH, 2004. Effects of brief cognitive-behavioral interventions on confidence to resist the urges to use heroin and methamphetamine in relapse-related situations. J. Nerv. Ment. Dis. 192 (11), 788–791. DOI: 10.1097/01.nmd.0000144699.80765.7f. [DOI] [PubMed] [Google Scholar]
- Zorick T, Sugar CA, Hellemann G, Shoptaw S, London ED, 2011. Poor response to sertraline in methamphetamine dependence is associated with sustained craving for methamphetamine. Drug Alcohol Depend. 118, 500–503, https://dx.doi.org/10.1016%2Fj.drugalcdep.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]