Abstract
Background
There is no clear consensus on the optimal choice of anticoagulant in patients with left ventricular thrombi (LVT). Given the potentially fatal complications associated with this disease entity, we performed a systematic review and meta-analysis of recent randomized clinical trials (RCTs) to synthesize the latest evidence on this topic.
Methods
We performed a comprehensive search of electronic databases to identify RCTs comparing warfarin to direct oral anticoagulants (DOACs) in patients with LVT. A random-effects Bayesian analysis using a binomial-normal hierarchical model was performed to compare the two treatment options with regards to the risk of mortality, stroke, LVT resolution, and major bleeding.
Results
In an analysis comprising 3 RCTs (N = 139), there were no statistically significant differences regarding mortality (OR: 0.68; 95% CrI: 0.10 to 4.43), stroke (OR: 0.14; 95% CrI: 0.01 to 1.27), or LVT resolution (OR: 1.17; 95% CrI: 0.37 to 3.45). Major bleeding was significantly lower in the DOAC group (OR: 0.16; 95% CrI: 0.02 to 0.82).
Conclusion
In patients with LVT, the currently available evidence from RCTs supports the use of DOACs rather than warfarin due to lower major bleeding risks and no evidence of inferiority with respect to mortality, stroke or LVT resolution.
Keywords: Oral anticoagulants, Warfarin, Left ventricular thrombi, Novel oral anticoagulants, Direct oral anticoagulants, Bleeding, Stroke, Mortality
Highlights
-
•
Direct oral anticoagulants (DOAC) result in fewer major bleeding events in patients with left ventricular thrombi (LVT).
-
•
There is no evidence of DOAC inferiority to warfarin in terms of mortality or stroke in patients with LVT.
-
•
There is no evidence of DOAC inferiority to warfarin in terms of LVT resolution.
Left ventricular thrombi (LVT) most commonly occur as a complication of myocardial infarctions and carry a significant risk of embolization and stroke. Currently, the ACCF/AHA guidelines [1] recommend Warfarin as the first-choice treatment and direct oral anticoagulants (DOACs) as second-line options. European guidelines [2] do not recommend one option over the other. Recent findings from randomized controlled trials (RCT) have challenged these recommendations, although the small sample size of these trials is a limitation. Accordingly, we undertook a systematic review and meta-analysis of RCTs comparing DOACs to warfarin in the setting of left ventricular thrombi.
This review was registered on PROSPERO (CRD: 42021258194). We performed a comprehensive search of electronic databases (MEDLINE, CENTRAL, and clinicaltrials.gov) to retrieve RCTs comparing DOACs to warfarin for the treatment of left ventricular thrombi. Our pre-specified outcomes of interest included mortality, stroke, resolution of LVT, and major bleeding. For studies where data regarding these outcomes was not clearly outlined in the study report, we attempted to contact the authors to retrieve the required data.
We used a random-effects binomial-normal hierarchical model provided by the MetaStan package [3] on R, version 4.1.1. [4] using weakly informative priors [5] to obtain odds ratios (OR) and credible intervals (CrI). The prior distributions used for relative treatment effects and between-study variation are provided in Fig. 2. We also quantified the probability that one treatment option was superior to the other using the posterior distribution of relative treatment effects. For outcomes with a statistically significant difference, we calculated the number needed to treat to benefit (NNTB). The NNTB requires the specification of a baseline risk; we thus calculated three estimates of the NNTB based on the least, highest, and median risks in the warfarin control groups. We assessed model convergence using the reduction scale factor, trace-plots, and ensuring the absence of any divergent transitions.
Fig. 2.
Forest plots for the outcomes of mortality, stroke, thrombus resolution, and major bleeding.
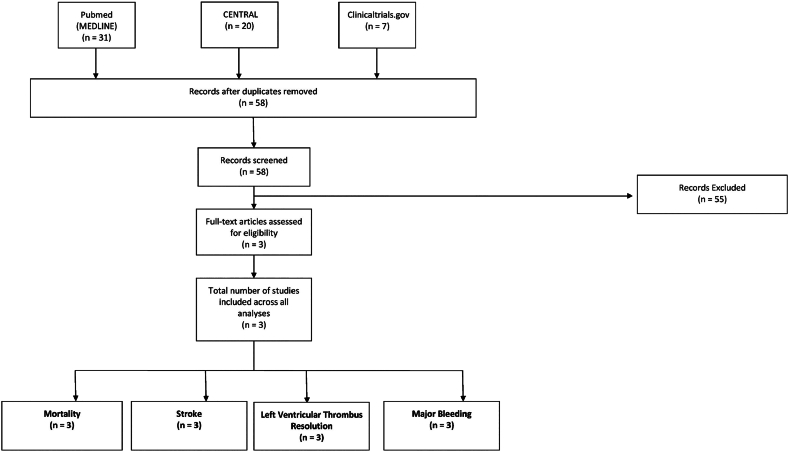
A total of 58 articles were identified by our search strategy, of which three RCTs [6], [7], [8] (N = 139; DOACs: 71; Warfarin: 68) qualified for inclusion in our analysis (Fig. 1). Males made up 66.2% of the study population, the mean age was 53.3 years (range: 49.6 to 59.8 years), and the mean follow-up duration was 4.65 months (range: 2.8 to 6.0 months). Regarding the choice of DOAC, two studies [6], [8] utilized apixaban, whereas one study [7] utilized rivaroxaban.
Fig. 1.
PRISMA flow diagram outlining the study selection process.
There were no statistically significant differences with regard to mortality (4.23% with DOACs vs 5.88% with warfarin; OR: 0.68; 95% CrI: 0.10 to 4.43), stroke (1.43% with DOACs vs 7.35% with warfarin; OR: 0.14; 95% CrI: 0.01 to 1.27), or LVT resolution (78.6% with DOACs vs 76.5% with warfarin; OR: 1.17; 95% CrI: 0.37 to 3.45). Major bleeding was significantly lower in the DOAC group (2.86% with DOACs vs 13.2% with warfarin; OR: 0.16; 95% CrI: 0.02 to 0.82) (Fig. 2). The baseline bleeding risk varied across the three warfarin control groups, being lowest in the study by Isa et al. (7.69%; corresponding NNTB: 16), highest in the study by Abdelnabi et al. (15.00%; corresponding NNTB: 9), and the median risk belonged to the study by Alcalai et al. (13.33%; corresponding NNTB: 10). Heterogeneity was low across all outcomes (I2 = 3% for mortality, 14% for stroke, and 0% for LVT resolution and major bleeding).
The findings of our analysis show that there is no evidence to support the use of warfarin over DOACs with respect to the outcomes of mortality, stroke, or LVT resolution. In addition, there is evidence that DOACs result in fewer major bleeding events in this population. Moreover, warfarin is known to have a greater potential for interacting with a number of medications and dietary foods and its use often imposes a stricter degree of monitoring to ensure an optimal INR. On the other hand, warfarin is a cheaper drug that is more widely available in certain resource-limited settings.
We must note two important limitations regarding these trials: first, they were all relatively small in size. Second, a rigorous assessment of their risk of bias could not be conducted given that only two of the three articles have been published as full-length manuscripts [6,8]. Given the above, it is imperative to conduct a large-scale multicenter study less vulnerable to the biases that might arise in small studies. Nevertheless, our findings of a reduction in major bleeding are in accord with previous knowledge from other RCTs comparing DOACs and warfarin.
In sum, these findings suggest that, given the currently available evidence from RCTs, DOACs may be preferable to warfarin as first-choice agents for the treatment of LVT, with a proven reduction in the risk of major bleeding and no evidence of inferiority with respect to mortality, stroke, or thrombus resolution.
Ethical approval
This study was exempted from the institutional review board's approval because it uses study (not patient) level data, publicly available in the literature.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Kernan W.N., Ovbiagele B., Black H.R., Bravata D.M., Chimowitz M.I., Ezekowitz M.D., et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:2160–2236. doi: 10.1161/STR.0000000000000024. [DOI] [PubMed] [Google Scholar]
- 2.Ibanez B., James S., Agewall S., Antunes M.J., Bucciarelli-Ducci C., Bueno H., et al. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur. Heart J. 2018;39(2):119–177. doi: 10.1093/eurheartj/ehx393. [DOI] [PubMed] [Google Scholar]
- 3.Guenhan B.K. 2020. MetaStan: Bayesian Meta-analysis via “Stan”. R Package Version 0.2.0. [Google Scholar]
- 4.Team RC . 2021. R: A Language and Environment for Statistical Computing.https://www.r-project.org/ Available from: [Google Scholar]
- 5.Günhan B.K., Röver C., Friede T. Random-effects meta-analysis of few studies involving rare events. Res. Synth. Methods. 2020;11(1):74–90. doi: 10.1002/jrsm.1370. [DOI] [PubMed] [Google Scholar]
- 6.Isa WyH W., Hwong N., Mohamed Yusof A., Yusof Z., Loong N., Wan-Arfah N., et al. Apixaban versus warfarin in patients with left ventricular thrombus: a pilot prospective randomized outcome blinded study investigating size reduction or resolution of left ventricular thrombus. J. Clin. Prev. Cardiol. 2020;9(4):150. [Google Scholar]
- 7.Mahmoud A., Yehia S., Ahmed F., Alexander N., Ling W., Mahmoud M., et al. Comparative study of oral anticoagulation in left ventricular thrombi (No-LVT trial) J. Am. Coll. Cardiol. 2021 Mar 30;77(12):1590–1592. doi: 10.1016/j.jacc.2021.01.049. Available from: [DOI] [PubMed] [Google Scholar]
- 8.Alcalai R., Butnaru A., Moravsky G., Yagel O., Rashad R., Ibrahimli M., et al. Apixaban vs. warfarin in patients with left ventricular thrombus: a prospective multicentre randomized clinical trial‡. Eur. Heart J. Cardiovasc. Pharmacother. 2021 Jul 19 doi: 10.1093/ehjcvp/pvab057. Available from: [DOI] [PubMed] [Google Scholar]