Abstract
Introduction
Elevation of cardiac troponin I (cTn-I) is associated with coronary artery disease (CAD) in asymptomatic patients with end-stage renal disease (ESRD) receiving hemodialysis. We aim to investigate the diagnostic value of chronically elevated cTn-I in ESRD patients presenting with an acute rise in serum cTn-I levels.
Methods
We performed a retrospective analysis of 364 patients. Using coronary angiography, we correlated baseline elevation of cTn-I with the severity of CAD when hemodialysis patients present with acute symptomatic elevation in serum cTn-I.
Results
In hemodialysis patients presenting with a rise in serum cTn-I above baseline levels, 59% had severe CAD, and 17% had no angiographic evidence of CAD. Hemodialysis patients with severe CAD had significantly higher baseline cTn-I levels compared to patients with non-severe CAD or normal coronaries (p < 0.0001). Baseline elevation of cTn-I in the severe CAD group was correlated with the degree of CAD occlusion (r2 0.56, p < 0.0001), fitting a positive linear model. Furthermore, baseline cTn-I differentiates between patients with and without severe CAD with a test accuracy of 0.72 (95% CI, 0.69–0.75, p < 0.001). At a value of ≥0.2 ng/mL (cutoff for myocardial necrosis), the specificity of baseline cTn-I for underlying severe CAD was 0.95.
Conclusions
Elevated baseline cTn-I has good accuracy for anticipating more advanced angiographic CAD when hemodialysis patients present with a symptomatic rise in serum cTn-I above baseline levels. Baseline elevation of cTn-I can be used for cardiac disease risk management in hemodialysis patients presenting with symptoms suggestive of CAD.
Keywords: Cardiac troponin I, End-stage renal disease, Coronary artery disease, Coronary angiography
1. Introduction
In patients with end-stage renal disease (ESRD) receiving hemodialysis, coronary artery disease (CAD) is present in about 42% of this population, and is the leading cause of mortality (~50% of deaths) [1,2]. Cardiac troponin I (cTn-I) is the preferred cardiac biomarker for the diagnosis of myocardial infarction (MI) in patients with both normal renal function and those on hemodialysis [3]. Also, cardiac biomarkers are a sensitive prognostic tool for poor survival, a higher risk of cardiac death and all-cause mortality in clinically stable hemodialysis patients [[4], [5], [6], [7]]. Baseline cTn-I is elevated in the majority of hemodialysis patients in the absence of CAD symptoms. Many studies investigated the link between elevated cardiac biomarkers and underlying cardiac pathology in asymptomatic hemodialysis patients. However, only a few investigated the predictive role of chronically elevated cTn-I and the severity of CAD when hemodialysis patients present with symptoms and a rise in serum cTn-I above baseline levels.
Although hemodialysis patients have high CAD-related morbidity and mortality, chronic baseline cTn-I elevation is frequently overlooked due to the frequency of its occurrence. Also, clinicians are often challenged regarding the appropriate workup and management of asymptomatic patients with elevated cTn-I. This study aims to investigate the role of chronic elevation of baseline cTn-I in predicting the severity of angiographic CAD burden in hemodialysis patients presenting with a symptomatic rise in serum cTn-I above baseline levels. We also aim to determine if a specific cTn-I cutoff value can provide further information regarding the risk of having obstructive CAD.
2. Materials and methods
2.1. Study design
We conducted a retrospective analysis at the Detroit Medical Center, a major academic medical center affiliated with Wayne State University. The study protocol (1710000950) was approved by the Ethics Committee of our hospital, and the medical institutional review board approved the study (M1 IRB 110717M1X). The need for informed consent was waived from the ethics committee given the retrospective nature of the analysis.
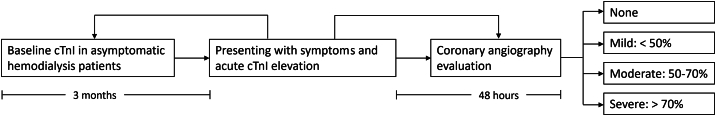
We screened all patients with a history of ESRD on hemodialysis who were hospitalized between July 2014 and September 2017 for inclusion into the study. Inclusion criteria included (1) patients with ESRD above the age of 18 receiving hemodialysis for three or more months, (2) patients admitted to the inpatient ward or intensive care unit with symptoms of chest pain and/or dyspnea, (3) patients who had a rise of serum cTn-I above baseline value with at least one value above the 99th percentile upper reference limit (URL), (4) baseline cTn-I measured after initiation of hemodialysis in the last three months and before the index admission, the measurement was not related to an acute cardiac presentation, and (5) coronary angiography performed within 48 h of hospital admission (Fig. 1). Exclusion criteria included patients with known prior history of CAD, cocaine abuse (historical or documented by positive urine drug screening), acute sepsis, bleeding, severe anemia, and pulmonary embolism.
Fig. 1.
Illustration of the retrospective study design. cTn-I = cardiac troponin I; ESRD = end-stage renal disease; HD = hemodialysis.
2.2. Study variables
CAD severity was quantified according to the coronary angiographic luminal narrowing, based on the original report, into none, mild (coronary stenosis <50%), moderate (coronary stenosis 50–70%), and severe (coronary stenosis >70%, or thrombus) in one or more major epicardial vessels, including the left anterior descending, left circumflex, and right coronary arteries. Demographic information and clinical characteristics were obtained by review of electronic medical records. Hypertension was defined as untreated systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, or being on antihypertensive medications. Hyperlipidemia was defined as total cholesterol ≥240 mg/dL, low high-density lipoprotein cholesterol (≤40 mg/dL in men; ≤50 mg/dL in women), low-density lipoprotein cholesterol ≥160 mg/dL, or by using lipid-lowering therapies. Diabetes mellitus was defined as glycosylated hemoglobin ≥6.5%, or by using glucose-lowering medications. For cTn-I measurements, the institution used Bayer ADVIA Centaur chemiluminometric-immunoassay (Bayer Diagnostics, Tarrytown, New York, USA) to determine serum cTn-I. The assay's limit of detection (LoD) is 0.02 ng/mL, and the 99th percentile URL is 0.2 ng/. cTn-I values between 0.02 and 0.2 ng/mL are considered in the indeterminate range.
2.3. Statistical analysis
Categorical variables were presented as frequencies and percentages, while continuous variables were presented as means ± standard deviation (SD) or standard error of the mean (SEM). cTn-I values were transformed to their corresponding log10 to reduce data skewness and to use other analysis methods. The normality of data distribution was tested using the D'Agostino-Pearson normality test at 95% confidence. All four study groups (none, mild CAD, moderate CAD, and severe CAD) were found to have a normal distribution (P > 0.05). No outliers were identified at a Z-score of ±2. All data points were included in the subsequent analyses.
One-way analysis of variance was used to examine the mean differences between groups of more than two. For post-hoc analysis, Tukey's HSD test was used to compare the pair of means between groups. A Chi-Square test was used to determine differences between categorical variables. Pearson's correlation coefficient and multiple linear regression model were used to correlate the severity of angiographic CAD in the severe group with variation in baseline cTn-I levels. The model was adjusted for age, sex, and CAD risk factors (smoking, diabetes mellitus, hypertension, peripheral artery disease, and hyperlipidemia). We calculated the test's sensitivity and specificity at various cTn-I cutoff values and used the Youden index to identify the optimal cutoff point. To measure test accuracy, we calculated the area under the curve (AUC) in the receiver operating characteristic (ROC) curve. A 2-sided p-value of <0.05 was considered statistically significant. Statistical analysis was conducted using GraphPad Prism 7.04 for Windows (GraphPad Software, La Jolla California, USA).
3. Results
After a careful electronic medical record chart review of 1123 patients, a total of 364 patients met the inclusion criteria and were considered in the final analysis. The mean age of the patients was 62.0 ± 13.0 years, of which 215 (59%) were men, and 317 (87%) were African Americans. Baseline demographic, clinical characteristics, and cTn-I values are summarized in Table 1. Out of the 364 patients, 215 (59%) had severe CAD, 53 (14%) had moderate CAD, 35 (10%) had mild CAD, and 61 (17%) had no angiographic evidence of CAD. No age and sex differences were noted between CAD groups. However, the severe CAD group had a significantly higher prevalence of diabetes, peripheral arterial disease, and hyperlipidemia, but no significant differences in hypertension or smoking.
Table 1.
Baseline demographics, clinical characteristics, and cTn-I levels in the study subjects.
| Variables | Angiographic CAD classification |
P-value⁎ | ||||
|---|---|---|---|---|---|---|
| None (n = 61) | Mild (n = 35) | Moderate (n = 53) | Severe (n = 215) | Total (n = 364) |
||
| Demographics | ||||||
| Age, mean (SD, years) | 53 (12) | 57 (15) | 68 (11) | 63 (12) | 62 (13) | <0.001 |
| Female, n (%) | 28 (46) | 14 (40) | 23 (43) | 84 (39) | 149 (41) | 0.78 |
| African American, n (%) | 56 (92) | 34 (97) | 48 (91) | 177 (82) | 315 (86) | 0.005 |
| CAD Risk factors | ||||||
| Smoking, n (%) | 34 (56) | 14 (40) | 20 (38) | 116 (54) | 184 (51) | 0.08 |
| Diabetes mellitus, n (%) | 21 (34) | 14 (40) | 33 (62) | 164 (76) | 232 (64) | <0.0001 |
| Hypertension, n (%) | 55 (90) | 31 (89) | 48 (91) | 199 (93) | 333 (91) | 0.40 |
| Peripheral arterial disease, n (%) | 9 (15) | 4 (11) | 8 (15) | 72 (33) | 93 (26) | 0.0005 |
| Hyperlipidemia, n (%) | 13 (21) | 4 (11) | 21 (40) | 111 (52) | 149 (41) | <0.0001 |
| Baseline cTn-I | ||||||
| LoD 0.02 ng/mL, n (%) | 27 (44) | 7 (20) | 8 (15) | 15 (7) | 57 (16) | <0.0001 |
| Indeterminate 0.02–0.19 ng/mL, n (%) | 33 (54) | 26 (74) | 42 (79) | 131 (61) | 232 (64) | 0.078 |
| 99th percentile URL ≥ 0.20 ng/mL, n (%) | 1 (2) | 2 (6) | 3 (6) | 69 (32) | 75 (20) | 0.0001 |
CAD, coronary artery disease; cTn-I, cardiac troponin I; LoD, limit of detection; n, number; SD, standard deviation; URL, upper reference limit.
P-value <0.05 was considered statistically significant.
Baseline cTn-I was detectable in 307 (84%) hemodialysis patients; of those, 232 (64%) had indeterminate cTn-I elevations, and 75 (20%) had positive cTn-I elevation (at or above the 99th percentile URL). Patients with negative baseline cTn-I levels were more likely to be in the CAD-free group (p < 0.0001). On the other hand, patients with positive baseline cTn-I elevation were more likely to have severe CAD (p < 0.0001).
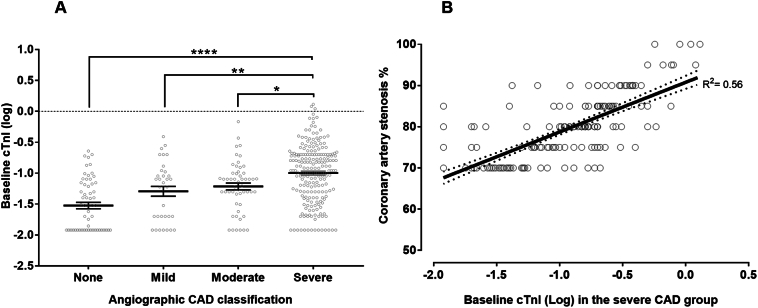
The baseline elevation of cTn-I levels in hemodialysis patients was positively correlated with the angiographic CAD burden. One-way ANOVA comparing mean baseline cTn-I levels in CAD subgroups showed a significant difference in baseline cTn-I levels (p < 0.0001). Post-hoc Tukey's HSD test revealed that mean cTn-I level in the severe CAD group (mean = 0.17 ng/mL and SEM = 0.013) was significantly higher than the moderate CAD group (mean = 0.09 ng/mL and SEM = 0.015, p = 0.01), mild CAD group (mean = 0.08 ng/mL and SEM = 0.02, p = 0.002), and the CAD free group (mean = 0.05 ng/mL and SEM = 0.007, p < 0.0001) (Fig. 2A).
Fig. 2.
(A) Means and individual data points of baseline cTn-I in all CAD groups plotted against the log transformant of cTn-I levels measured in ng/ml. Patients with severe CAD had significantly higher baseline cTn-I than all other groups. (B) Linear regression of baseline cTn-I and the percentage of coronary artery stenosis in the severe CAD group. CAD = coronary artery disease; cTn-I = cardiac troponin I; * indicates statistical significance. Error bars represent the standard error of the mean (SEM).
Next, we correlated the severity of angiographic CAD in the severe group with baseline cTn-I levels. Pearson's correlation showed a significant positive correlation between the two variables (r = 0.56; P < 0.0001) (Fig. 2B). We conducted a multiple linear regression, using baseline cTn-I as the independent variable and CAD severity as the dependent variable. We adjusted for age as a potential confounding variable in our regression model. There was a significant regression equation (p < 0.0001); for each ten-unit increase in cTn-I (measured in the log transformant), the % predicted severity of CAD stenosis increased by 12.05%. After adjustment for age, sex, and CAD risk factors, CAD remained statistically significantly associated with baseline cTn-I (p < 0.0001).
cTn-I at a cutoff value of 0.10 ng/mL was found to be the optimal point with the best sensitivity-to-specificity balance. At that value, the sensitivity of elevated baseline cTn-I for a diagnosis of severe CAD was 0.8 (0.73–0.86), the specificity was 0.59 (0.52–0.65), the positive predictive value was 0.57 (0.50–0.68), and the negative predictive value was 0.81 (0.74–0.85). At a value of 0.20 ng/mL or more (cutoff value for myocardial necrosis), the sensitivity of elevated baseline cTn-I for a diagnosis of severe CAD was 0.23, and the specificity was 0.95 (Table 2). Overall, baseline cTn-I differentiates between patients with and without advanced CAD (AUC = 0.72, 95% CI, 0.69–0.75, p < 0.001) (Fig. 3).
Table 2.
Sensitivity and specificity of severe CAD at various baseline cTn-I cutoff values.
| Cutoff value | Sensitivity | Specificity | Youden index⁎ |
|---|---|---|---|
| ≥0.02 ng/mL | 0.93 | 0.28 | 0.21 |
| ≥0.04 ng/mL | 0.77 | 0.44 | 0.22 |
| ≥0.06 ng/mL | 0.70 | 0.58 | 0.27 |
| ≥0.08 ng/mL | 0.63 | 0.72 | 0.36 |
| a≥0.10 ng/mL | 0.59 | 0.80 | 0.38 |
| ≥0.12 ng/mL | 0.52 | 0.83 | 0.35 |
| ≥0.14 ng/mL | 0.44 | 0.89 | 0.32 |
| ≥0.16 ng/mL | 0.35 | 0.89 | 0.25 |
| ≥0.18 ng/mL | 0.33 | 0.90 | 0.22 |
| b≥0.20 ng/mL | 0.23 | 0.95 | 0.17 |
| ≥0.22 ng/mL | 0.20 | 0.95 | 0.15 |
| ≥0.24 ng/mL | 0.18 | 0.95 | 0.13 |
| ≥0.26 ng/mL | 0.17 | 0.96 | 0.13 |
| ≥0.28 ng/mL | 0.14 | 0.97 | 0.12 |
| ≥0.30 ng/mL | 0.13 | 0.98 | ≤0.11 |
CAD coronary artery disease, cTn-I cardiac troponin I.
Youden index: (Sensitivity + Specificity) − 1.
Optimal cutoff with the best sensitivity-to-specificity balance.
cTn-I cutoff value for myocardial necrosis.
Fig. 3.
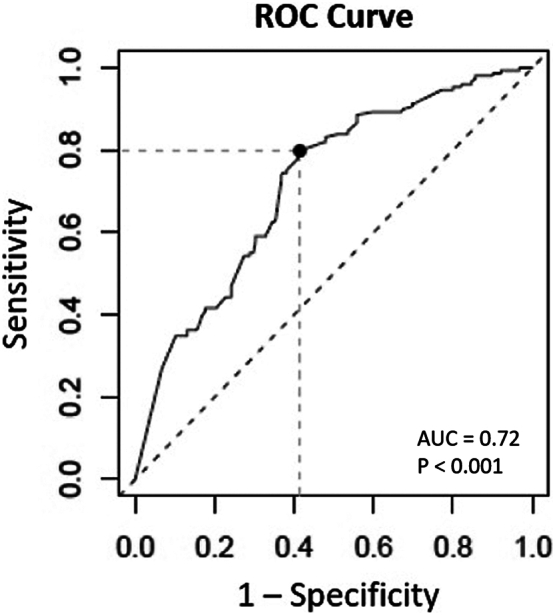
ROC curve for baseline cTn-I in hemodialysis patients for diagnosis of severe CAD. AUC = area under the curve; CAD = coronary artery disease; cTn-I = cardiac troponin I; ROC = receiver operating characteristic.
4. Discussion
In this study, we investigated the relationship between baseline elevation of cTn-I in asymptomatic hemodialysis patients and the angiographic CAD burden when they present with symptomatic elevation in serum cTn-I above baseline levels. For such a presentation, our findings demonstrated that in hemodialysis patients with no known prior CAD, 59% had severe angiographic CAD, and only 17% had normal coronary angiography. Moreover, baseline elevation of cTn-I in asymptomatic hemodialysis patients was significantly correlated with the angiographic CAD burden, and with the increased baseline cTn-I levels, the specificity of this cardiac biomarker increases to predict more advanced angiographic CAD.
The assessment of CAD in ESRD is challenging [8]. We included patients with or without baseline elevation of cTn-I and excluded patients with known underlying CAD. Our study's most important finding is that despite baseline elevation in asymptomatic hemodialysis patients, cTn-I is still reliably associated with the presence and severity of coronary stenosis. Baseline cTn-I was elevated in 84% and above the 99th percentile URL in 20% of the study population, similar to the previously reported elevation of cTn-I in asymptomatic hemodialysis patients [9]. Baseline cTn-I in asymptomatic hemodialysis patients predicted severe CAD with a specificity of 0.95 and an overall accuracy of 0.72. The specificity increased dramatically with increased baseline cTn-I. Therefore, the higher the baseline cTn-I values, the more likely the presentation is due to more advanced CAD. Moreover, a previous study showed that not only a negative cTn-I identifies patients at low risk of MI and 30-day cardiac mortality, but also those with cTn-I levels at or above the 99th percentile had a 2-fold increased risk of MI and cardiac death at one year [10,11]. In our study, patients with baseline cTn-I above the 99th percentile had a high test accuracy to indicate more advanced CAD. Therefore, earlier cardiac workup and more strict risk management might be suggested, given that hemodialysis patients with MI suffer dismal long-term survival [12,13].
Our study adds to the growing literature demonstrating the diagnostic power of cTn-I cardiac for predicting the severity of CAD in hemodialysis patients. Our findings should help clarify the challenging clinical problem of interpreting the significance of the symptomatic rise of cTn-I when there is an existing elevation of cTn-I in hemodialysis patients. We show a low false-positive elevation of cTn-I to predict advanced CAD. Various risk stratification models that are routinely used in clinical practice have been shown to improve clinical outcomes and reduce the financial burden on the healthcare system. Improving our ability to forecast risk, especially in a high-risk population like hemodialysis patients, has tangible benefits. Therefore, our results suggest the potential value of including baseline cTn measurements to the existing scoring systems for risk stratification of patients hospitalized for suspected acute coronary syndrome (ACS).
The pathophysiology behind cTn-I elevations in hemodialysis patients is not fully understood [14,15]. The elevation of cardiac biomarkers is predictive of all-cause and cardiovascular mortality, suggesting true underlying cardiac pathology [5,7,16]. Several studies have implicated epicardial CAD as a potentially major cause of increased cTn-I in hemodialysis patients [16,17]. Other cardiac pathologies like microvascular dysfunction, diffuse CAD, vascular calcification, and arteriosclerosis can potentially explain the chronic enzyme elevation [8,18]. Results from our study support this evidence by showing that the severity of coronary stenosis is associated with higher baseline cTn-I.
Concerning CAD risk factors, we noted, unsurprisingly, a higher prevalence of diabetes mellitus, hyperlipidemia, and peripheral artery diseases in hemodialysis patients with severe CAD. Hypertension was present in most hemodialysis patients (91%) regardless of their CAD status. This was similar to previous reports documenting hypertension as high as 86% in chronic hemodialysis patients [19,20]. Likewise, smoking prevalence was equally high in all groups. We demonstrate that more CAD risk factors are associated with an increased risk of obstructive CAD in hemodialysis patients. Therefore, optimal medical management for CAD risk factors in hemodialysis patients is essential. Very few clinical trials have investigated outcomes of conservative strategy compared to invasive strategy in coronary disease in patients with advanced kidney disease [21,22]. However, none of these studies have differentiated the outcomes in hemodialysis patients with or without cTn-I elevation.
We recognize some limitations in our study. The retrospective design, the majority of the study population, consisted of African Americans, who share a disproportionately higher prevalence of ESRD and are overall understudied; this can affect the generalizability of the results. There may be selection bias as hemodialysis patients with no measured baseline cTn-I level were automatically excluded, and the study was limited to hospitalized patients. Also, there were no cardiac structural and functional correlations with the baseline elevation of cTn-I, focusing only on the association with the angiographic CAD burden. Lastly, the study was not designed to evaluate the prognostic value of elevated baseline cTn-I.
5. Conclusions
The evaluation of CAD in hemodialysis patients with elevated baseline cTn-I is challenging. Clinical judgment remains a critical component for assessing the asymptomatic elevation of baseline cTn-I in hemodialysis patients when they present with a rise in serum cTn-I levels and symptoms suggestive of ACS. Along with traditional CAD risk factors, baseline cTn-I is predictive of advanced CAD and can be incorporated into existing scoring systems for risk stratification of hemodialysis patients hospitalized for suspected ACS. These results can also suggest the potential utilization of baseline cTn-I to identify the cohort with more advanced CAD who would likely derive benefit from an early aggressive management strategy before the onset of symptoms. Ultimately, well-conducted clinical trials are needed for a more optimal evaluation of cTn's and treatment strategies of CAD in this group of patients.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgment
None.
Funding
None.
References
- 1.Tong J., Liu M., Li H., Luo Z., Zhong X., Huang J., et al. Mortality and associated risk factors in dialysis patients with cardiovascular disease. Kidney Blood Press. Res. 2016;41(4):479–487. doi: 10.1159/000443449. [DOI] [PubMed] [Google Scholar]
- 2.Saran R., Robinson B., Abbott K.C., Agodoa L.Y.C., Bragg-Gresham J., Balkrishnan R., et al. US renal data system 2017 annual data report: epidemiology of kidney disease in the United States. Am. J. Kidney Dis. 2018;71:A7. doi: 10.1053/j.ajkd.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roffi M., Patrono C., Collet J.P., Mueller C., Valgimigli M., Andreotti F., et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent st-segment elevation: task force for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation of the european society of cardiology (ESC) Eur. Heart J. 2016;37:267–315. doi: 10.1093/eurheartj/ehv320. [DOI] [PubMed] [Google Scholar]
- 4.Aviles R.J., Askari A.T., Lindahl B., Wallentin L., Jia G., Magnus Ohman E., et al. Troponin T levels in patients with acute coronary syndromes, with or without renal dysfunction. N. Engl. J. Med. 2002;346(26):2047–2052. doi: 10.1056/NEJMoa013456. [DOI] [PubMed] [Google Scholar]
- 5.Apple F.S., Murakami M.A.M., Pearce L.A., Herzog C.A. Predictive value of cardiac troponin I and T for subsequent death in end-stage renal disease. Circulation. 2002;106(23):2941–2945. doi: 10.1161/01.cir.0000041254.30637.34. [DOI] [PubMed] [Google Scholar]
- 6.Michos E.D., Wilson L.M., Yeh H.C., Berger Z., Suarez-Cuervo C., Stacy S.R., et al. Prognostic value of cardiac troponin in patients with chronic kidney disease without suspected acute coronary syndrome: a systematic review and meta-analysis. Ann. Intern. Med. 2014;161:491–501. doi: 10.7326/M14-0743. [DOI] [PubMed] [Google Scholar]
- 7.Khan N.A., Hemmelgarn B.R., Tonelli M., Thompson C.R., Levin A. Prognostic value of troponin T and I among asymptomatic patients with end-stage renal disease: a meta-analysis. Circulation. 2005;112(20):3088–3096. doi: 10.1161/CIRCULATIONAHA.105.560128. [DOI] [PubMed] [Google Scholar]
- 8.Poli F.E., Gulsin G.S., McCann G.P., Burton J.O., Graham-Brown M.P. The assessment of coronary artery disease in patients with end-stage renal disease [Internet] Clin. Kidney J. 2019;12:721–734. doi: 10.1093/ckj/sfz088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tarapan T., Musikatavorn K., Phairatwet P., Takkavatakarn K., Susantitaphong P., Eiam-Ong S., et al. High sensitivity troponin-I levels in asymptomatic hemodialysis patients. Ren. Fail. 2019;41(1):393–400. doi: 10.1080/0886022X.2019.1603110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dierkes J., Domröse U., Westphal S., Ambrosch A., Bosselmann H.P., Neumann K.H., et al. Cardiac troponin T predicts mortality in patients end-stage renal disease. Circulation. 2000;102(16):1964–1969. doi: 10.1161/01.cir.102.16.1964. [DOI] [PubMed] [Google Scholar]
- 11.Miller-Hodges E., Anand A., Shah A.S.V., Chapman A.R., Gallacher P., Lee K.K., et al. High-sensitivity cardiac troponin and the risk stratification of patients with renal impairment presenting with suspected acute coronary syndrome. Circulation. 2018;137(5):425–435. doi: 10.1161/CIRCULATIONAHA.117.030320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herzog C.A. Acute myocardial infarction in patients with end-stage renal disease. Kidney Int. Suppl. 1999;56(71):S130–S133. doi: 10.1046/j.1523-1755.1999.07132.x. [DOI] [PubMed] [Google Scholar]
- 13.Banerjee D., Perrett C., Troponins Banerjee A. Acute coronary syndrome and renal disease: from acute kidney injury through end-stage kidney disease. Eur. Cardiol. Rev. 2019;14(3):187–190. doi: 10.15420/ecr.2019.28.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lauer M.S. Cardiac troponins and renal failure: the evolution of a clinical test. Circulation. 2005;112:3036–3037. doi: 10.1161/CIRCULATIONAHA.105.579888. [DOI] [PubMed] [Google Scholar]
- 15.Freda B.J., Tang W.H.W., Van Lente F., Peacock W.F., Francis G.S. Cardiac troponins in renal insufficiency: review and clinical implications. J. Am. Coll. Cardiol. 2002;40:2065–2071. doi: 10.1016/s0735-1097(02)02608-6. [DOI] [PubMed] [Google Scholar]
- 16.DeFilippi C., Wasserman S., Rosanio S., Tiblier E., Sperger H., Tocchi M., et al. Cardiac troponin T and C-reactive protein for predicting prognosis, coronary atherosclerosis, and cardiomyopathy in patients undergoing long-term hemodialysis. J. Am. Med. Assoc. 2003;290(3):353–359. doi: 10.1001/jama.290.3.353. [DOI] [PubMed] [Google Scholar]
- 17.Sharma R., Gaze D.C., Pellerin D., Mehta R.L., Gregson H., Streather C.P., et al. Cardiac structural and functional abnormalities in end stage renal disease patients with elevated cardiac troponin T. Heart. 2006;92(6):804–809. doi: 10.1136/hrt.2005.069666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hirose K., Chikamori T., Hida S., Tanaka N., Yamashita J., Igarashi Y., et al. Application of pressure-derived myocardial fractional flow reserve in chronic hemodialysis patients. J. Cardiol. 2018;71(1):52–58. doi: 10.1016/j.jjcc.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 19.Agarwal R., Nissenson A.R., Batlle D., Coyne D.W., Trout J.R., Warnock D.G. Prevalence, treatment, and control of hypertension in chronic hemodialysis patients in the United States. Am. J. Med. 2003;115(4):291–297. doi: 10.1016/s0002-9343(03)00366-8. [DOI] [PubMed] [Google Scholar]
- 20.Longenecker J.C., Coresh J., Powe N.R., Levey A.S., Fink N.E., Martin A., et al. Traditional cardiovascular disease risk factors in dialysis patients compared with the general population: the CHOICE Study. J. Am. Soc. Nephrol. 2002;13(7):1918–1927. doi: 10.1097/01.asn.0000019641.41496.1e. [DOI] [PubMed] [Google Scholar]
- 21.Chernin G., Chen S., Ozan O., Liu M., Haberman D., Perlman G., et al. Percutaneous coronary intervention does not lower cardiovascular outcomes in patients with chronic kidney disease. Am. J. Nephrol. 2019;50(6):465–472. doi: 10.1159/000503916. [DOI] [PubMed] [Google Scholar]
- 22.Bangalore S., Maron D.J., O’Brien S.M., Fleg J.L., Kretov E.I., Briguori C., et al. Management of coronary disease in patients with advanced kidney disease. N Engl. J. Med. 2020;382(17):1608–1618. doi: 10.1056/NEJMoa1915925. [DOI] [PMC free article] [PubMed] [Google Scholar]