Abstract
Objective
The aim of this cross-sectional, retrospective, descriptive study was to review and classify cardiac masses systematically and to determine their frequencies.
Methods
The medical records of 64,862 consecutive patients were investigated within 12 years. Every patient with a cardiac mass imaged by transthoracic echocardiography (TTE) and confirmed with an advanced imaging modality such as transesophageal echocardiography (TEE), computed tomography (CT) and/or cardiac magnetic resonance imaging (CMR) was included. Acute coronary syndromes triggering thrombus formation, vegetations, intracardiac device and catheter related thrombi were excluded.
Results
Data demonstrated 127 (0.195%) intracardiac masses consisting of 33 (0.050%) primary benign, 3 (0.004%) primary malignant, 20 (0.030%) secondary tumors, 3 (0.004%) hydatid cysts and 68 (0.104%) thrombi respectively. The majority of primary cardiac tumors were benign (91.67%), predominantly myxomas (78.79%), and the less malignant (8.33%). Secondary cardiac tumors were common than the primary malignant tumors (20:3), with male dominancy (55%), lymphoma and lung cancers were the most frequent. Intracardiac thrombi was the majority of the cardiac masses, thrombi accompanying malignancies were in the first range (n = 17, 25%), followed by autoimmune diseases (n = 13, 19.12%) and ischemic heart disease with low ejection fraction (n = 12, 17.65%).
Conclusions
This retrospective analysis identified 127 patients with cardiac masses. The majority of benign tumors were myxoma, the most common tumors that metastasized to the heart were lymphoma and lung cancers, and the thrombi associated with malignancies and autoimmune diseases were the most frequent.
Keywords: I-MASS Study, Primary cardiac tumors, Secondary cardiac tumors, Cardiac cysts, Cardiac thrombus
Highlights
-
•
The majority of primary cardiac tumors are benign, predominantly myxomas
-
•
Primary malignant cardiac tumors are very rare
-
•
Secondary cardiac malignant tumors are much more common than the primary malignant tumors with male dominancy
-
•
The most common tumors that metastasize to the heart are lymphoma and lung cancer
-
•
Intracardiac thrombi is the majority of cardiac masses, thrombi accompanying malignancies are the most frequent followed by autoimmune diseases predominantly located in the right atrium
1. Introduction
Cardiac masses include primary and secondary tumors, cysts, thrombi, and vegetations. The studies published to date include either autopsy data [1], clinical features and treatment options in specific groups of cardiac masses [2], [3], [4], [5], [6], imaging modalities [5], [7], [8], [9], or global reviews [10], [11], [12], [13], [14], [15] and case reports. The incidence rates of cardiac masses differ between studies and autopsy series. No study addressing cardiac masses of the heart cavity collectively including tumors, cysts and thrombi has yet been reported. The aim of this cross-sectional, retrospective, descriptive study was to determine the frequencies of intracardiac masses consisting of primary and secondary tumors, cysts and thrombi in hospitalized and out-patients referred to the echocardiography laboratory within twelve years.
2. Methods
We retrospectively reviewed the imaging and medical records of 64,862 consecutive patients referred to the Istanbul Medical Faculty, Department of Cardiology, Echocardiography Laboratory between 2006 and 2017. These patients were hospitalized or outpatients on follow up by Department of Cardiology and other clinics located on the same campus of the Istanbul Medical Faculty. The echocardiographic video recordings of the whole group were retrospectively reviewed by 12 cardiologists and their residents. Every patient with a cardiac mass imaged by transthoracic echocardiography (TTE) and confirmed with an advanced imaging modality such as transesophageal echocardiography (TEE), computed tomography (CT), cardiac magnetic resonance imaging (CMR) was included. CT and CMR images were evaluated by collaboration with Department of Radiology, reports of patients who had undergone surgery with Department of Cardiovascular Surgery and those who had histopathological examinations with Department of Pathology. Patients with a history of acute myocardial infarction within six months and left ventricular apical thrombus, left atrial appendage thrombus revealed by TEE before cardioversion for atrial fibrillation, cardiac masses associated with mechanical or bio-prosthetic valves, vegetations of proven infective endocarditis and intracardiac device and/or catheter/lead induced thrombi were excluded, de-novo thrombi were evaluated.
Data collection and evaluation was started at the beginning of 2018, and completed by the end of 2019.
Echocardiographic examinations were performed using Vivid 7 Dimension (GE Vingmed Ultrasound AS, Horten, Norway) and iE33 xMatrix - DS ultrasound imaging system (Philips Ultrasound, Bothell, WA). The first step examination for all patients with a definite mass in the heart, including the aortic and pulmonary roots was 2D and/or 3D TTE and TEE with standard views performed according to the guidelines [16], [17]. The anatomical localization of the mass, mobility, echogenicity, tissue properties, pedicle, if present, relation to adjacent cardiac structures, invasions and extensions, effects on blood flow and valve functions were examined and recorded and the patient was referred to CT and/or CMR before the appropriate treatment decision.
Contrast-enhanced CT was performed with a 4-MDCT scanner (Somatom Sensation 4, Siemens Medical Solutions) and a helical CT scanner (Somatom Plus-S, Siemens Medical Solutions) to investigate the size, location, morphology and tissue characteristics. For right atrial (RA) and right ventricular (RV) masses, intravenous contrast injection was performed to obtain optimal images and low-dose non-contrast and delayed CT imaging was performed in distinguishing intracardiac thrombus from tumor.
CMR was performed with a 1.5 T system (Symphony, Siemens Medical Solutions, Erlangen, Germany) with ECG triggering, tissue characterization with T1- and T2-weighted images. Contrast enhanced CMR, cine images, T1- and T2-weighted turbo spin echo, first-pass perfusion were used in patients referred to CMR with a diagnosis of a cardiac mass and TI scout sequences mainly for the diagnosis of tumors.
The researchers were allowed to obtain data from the general archives of the imaging devices and the electronic archives of the hospital with the authority granted by the institution. Patients and/or public were not involved in the design. The study was approved by Istanbul Medical Faculty Ethics Committee (75486), and conforms the principles outlined in the Declaration of Helsinki.
2.1. Statistics
All statistical tests were performed using SPSS software, version 16. Descriptive statistic was applied in calculation of the frequencies.
3. Results
Data from 64.862 consecutive patients within 12 years demonstrated 127 (0.195%) intracardiac masses consisting of; 36 primary tumors (0.056%), 33 (0.050%) primary benign, 3 (0.004%) primary malignant; 20 (0.030%) secondary tumors; 3 (0.004%) cysts and 68 (0.104%) thrombi respectively (Table 1).
Table 1.
Frequency of cardiac masses, and the percentages of primary and secondary tumors, cysts and thrombi in the study group.
| N: 64,862 patients | |||
|---|---|---|---|
| Cardiac mass | No of patients | Cardiac mass % | Study group % |
| Primary tumor | 36 | 28.35 | 0.056 |
| Primary benign tumor | 33 | 25.98 | 0.050 |
| Primary malignant tumor | 3 | 2.36 | 0.004 |
| Secondary malignant tumor | 20 | 15.75 | 0.030 |
| Cyst | 3 | 2.36 | 0.004 |
| Thrombus | 68 | 53.54 | 0.104 |
| Total cardiac mass | 127 | 0.195 | |
3.1. Primary benign cardiac tumors
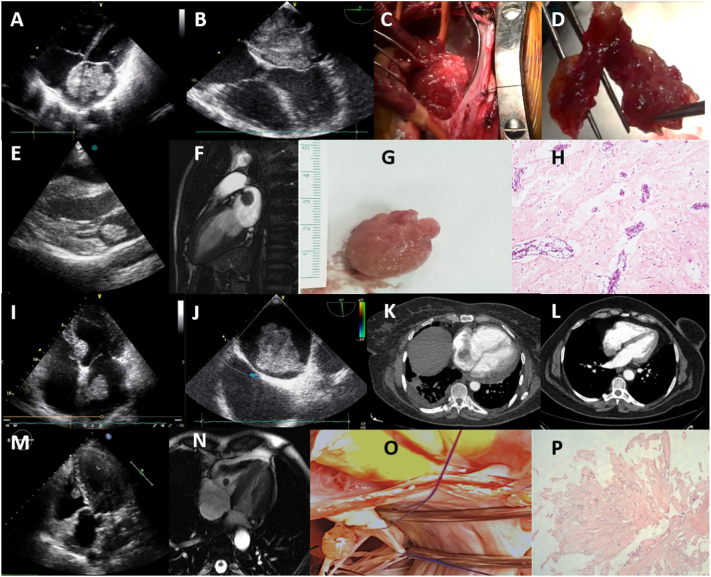
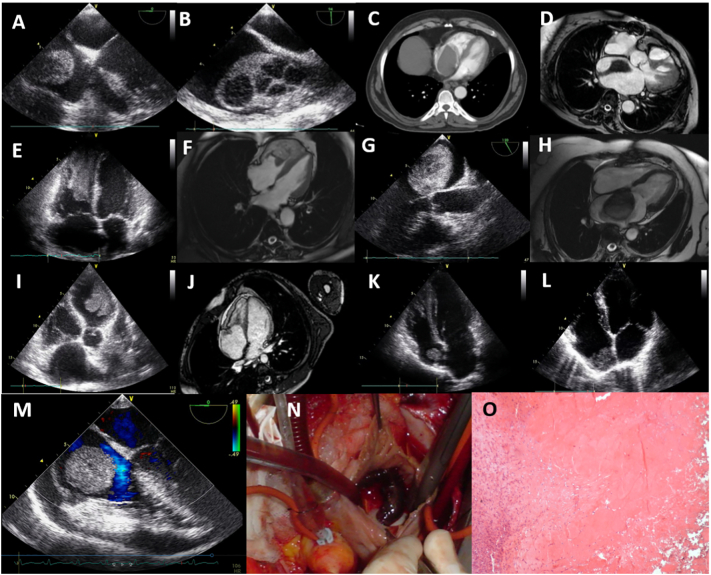
Twenty six myxomas were identified (78.79% of benign tumors; 20.47% of cardiac masses; 0.04% of the study group), followed by 5 papillary fibroelastomas (15.15% of benign tumors; 3.94% of cardiac masses; 0.007% of the study group), 1 fibroma and 1 lipoma (3.03% of benign tumors; 0.78% of cardiac masses; 0.001% of the study group for both), all confirmed with surgery and histology. Seventeen (65.38%) myxomas were located in the left atrium (LA) (Fig. 1A–J), 7 (26.92%) in the RA (Fig. 1K, L), 2 (7.69%) with atypical localizations, one at the mitral anterior leaflet chorda, the other originating from the pulmonary valve, 19 (73.07%) with pedicles originating from fossa ovalis, 6 (23.07%) sessile masses from atrial walls (Fig. 1).
Fig. 1.
Primary benign tumors of the heart. (A) 2D TTE giant left atrial myxoma; (B) 2D TEE polylobulated myxoma, attached with a pedicle to the fossa ovalis (C) intraoperative view; (D) gross examination of the tumor, a dark red, fragile, polylobulated mass; (E) 2D TTE left atrial mobile, well circumscribed, ball shaped myxoma; (F) CMR SSFP cine, a hypointense mass with a short pedicle originating from the superior wall of the left atrium; (G) gross view of the surgically resected mass, smooth surface, bright pink colour (H) histology shows intense perivascular benign neoplastic cells and rare same type cells in myxoid stroma, compatible with myxoma, hematoxylin-eosin × 100; (I) 2D TTE pediculated left atrial myxoma originating from the interatrial septum; (J) 2D TEE, pediculated left atrial myxoma adjacent to the superior vena cava and patent foramen ovale; (K) CT, right atrial lobulated myxoma originating from fossa ovalis; (L) CT, myxoma originating from the posterior right atrial wall; (M) 2D TTE, a well-defined, nodular, mobile papillary fibroelastoma, originating from the anterior tricuspid leaflet chorda in the right ventricle; (N) CMR SSFP cine of the papillary fibroelastoma; (O) gross examination of the surgically resected papillary fibroelastoma, a translucent and gelatinous mass; (P) histology shows a benign tumor with multiple papillary fronds of different size that consisted of an acellular matrix and surrounded by single layer of endothelial cells, hematoxylin-eosin × 100.
2D TTE: two-dimensional transthoracic echocardiography; 2D TEE: two-dimensional transesophageal echocardiography; CMR: cardiac magnetic resonance imaging; CT: computed tomography; SSFP: steady state free processing.
Five fibroelastomas (11.54% of benign tumors; 3.94% of cardiac masses, 0.007% of the study group) were identified incidentally. Three of them small, sessile, immobile masses, two located on the atrial side of the mitral anterior leaflet, one on the atrial side of the posterior leaflet. The other two fibroelastomas were small, mobile masses with short pedicles, one on the pulmonary valve and the other attached to the tricuspid anterior leaflet chorda (Fig. 1M–P).
One lipoma and one fibroma were also incidentally observed. Lipoma was located at the left ventricular (LV) posterior wall, originating from epicardium, it was the only benign cardiac tumor definitely diagnosed by CMR. Fibroma was located on the ventricular basis of posterior mitral leaflet with undetermined preliminary diagnosis (3.03% of benign tumors, 0.79% of cardiac masses, 0.001% of the study group for both) (Supplementary Table 1, Fig. 1).
3.2. Primary malignant cardiac tumors
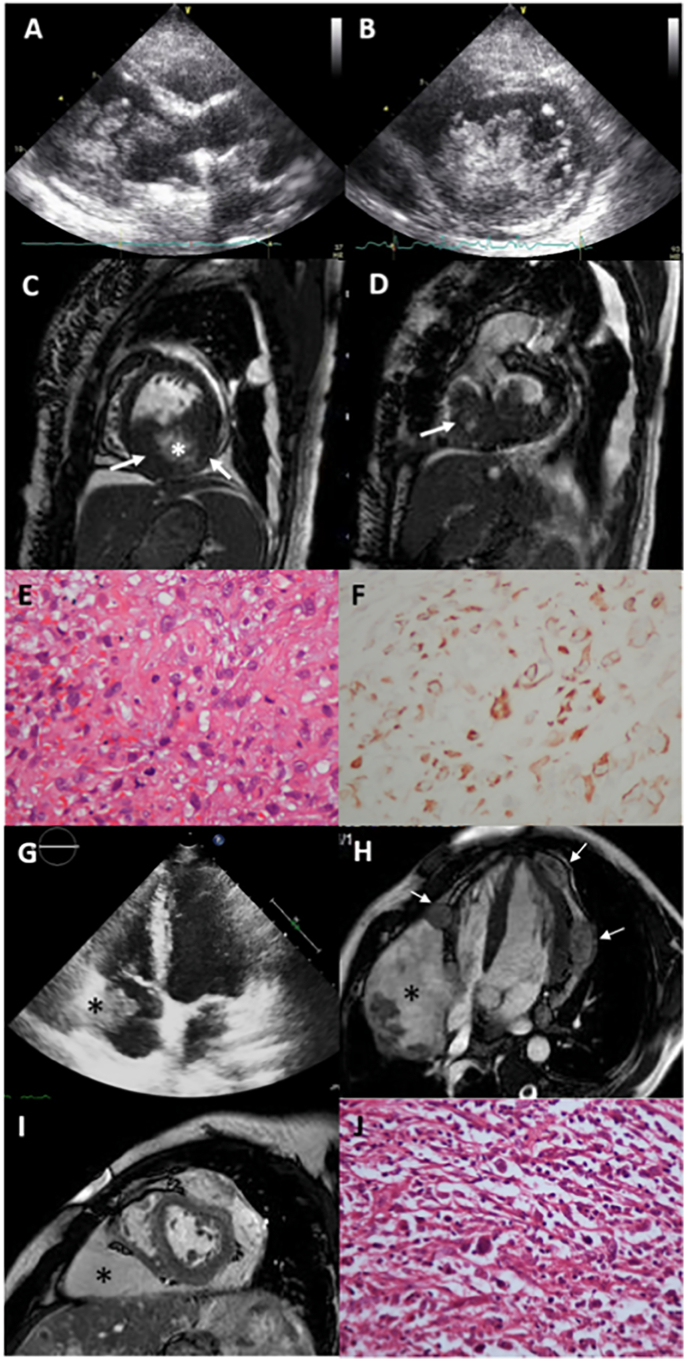
Three primary malignant tumors of the heart were identified (0.004% of the study group). One rhabdomyosarcoma (33.33% of malignant tumors, 0.79% of cardiac masses, 0.001% of the study group) and 2 angiosarcomas (66,67% of malignant tumors, 1.574% of cardiac masses, 0.003% of the study group) (Supplementary Table 2, Fig. 2). The rhabdomyosarcoma was a poly-lobulated, mobile mass originating from the posterior wall of the LV myocardium, completely filling the LV cavity and prolapsing into the LV outflow tract towards the aortic lumen, also infiltrating the right ventricle (RV) (Fig. 2A–D), with desmin-positive histology (Fig. 2E, F). Both angiosarcomas were huge masses originating from right atrioventricular groove infiltrating RA wall and cavity and RV lateral wall and pericardium (Fig. 2G–I), confirmed by histology (Fig. 2J), accompanied by pericardial effusion.
Fig. 2.
Primary malignant tumors of the heart.
Left ventricular endocavitary rhabdomyosarcoma; (A) 2D TTE, a polylobulated, mobile tumor filling the left ventricle; (B) 2D TTE short-axis view, tumor mass and extensions; (C) CMR, tumor originating from the inferior wall of the left ventricle (arrow) with cystic and necrotic areas at the centrum (*); (D) tumor also infiltrates the right ventricle (arrow); (E) histology: pleomorphic malign tumor cells of rhabdomyosarcoma, mitotic figures and atypical cells, hematoxylin-eosin × 200; (F) positive desmin immunochemistry in tumoral cells × 200.
High grade angiosarcoma; (G) 2D TTE tumor mass (*) in the right atrial cavity, infiltrating right atrial wall, (H) contrast-enhanced SSFP cine CMR, nodular lesions of the tumor (arrows) infiltrating left and right ventricular myocardium and epicardium, with a large mass of tumor in the atrioventricular groove and right ventricular myocardium (*) narrowing the right heart chambers; (I) CMR, short axis SSFP, large tumor mass (*); (J) High grade angiosarcoma, irregular malignant spindle cells haphazardly arranged, hematoxylin-eosin × 40.
2D TTE: two-dimensional transthoracic echocardiography; CMR: cardiac magnetic resonance imaging, SSFP: steady state free processing.
3.3. Secondary malignant cardiac tumors
These patients were histologically proven primary noncardiac cancers referred for echocardiographic examination of cardiac functions before initiation of chemotheraphy while they were on follow up in other clinics. In most of the patients, echocardiographic examinations were suboptimal due to deteriorated general condition. Because of limited body motion and difficulty to achieve standard echocardiographic imaging planes, CMR was predominantly used for diagnosis.
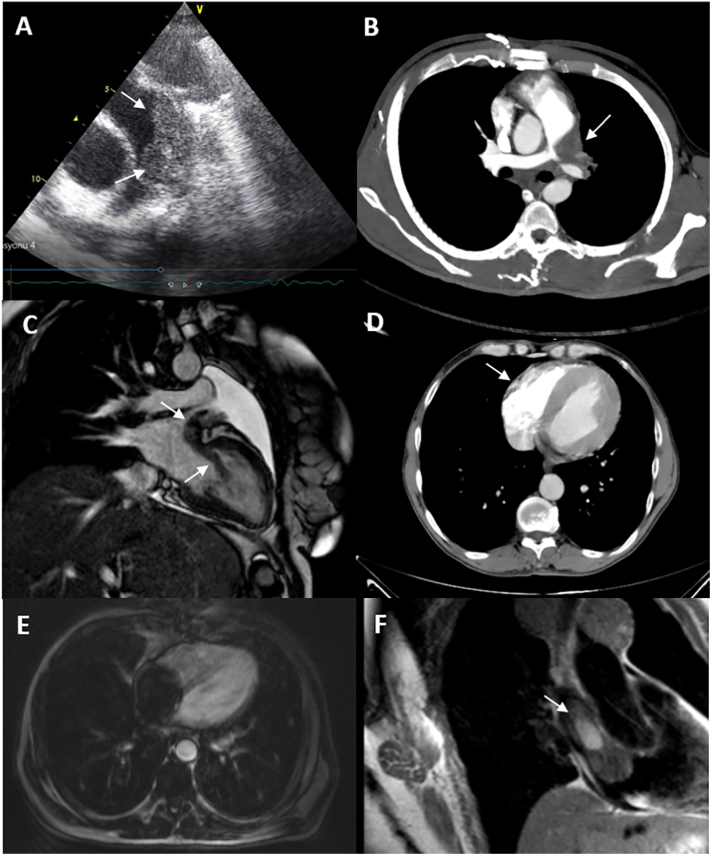
Cardiac metastases or invasions of cancers were detected in 20 patients (15.75% of cardiac masses, 0.03% of the study group), with male dominancy (55%). One of the cases was published before [18]. Malignancies with cardiac metastases consisted of lymphoma (n = 6, 30%), mainly B-cell lymphomas (n = 5) (Fig. 3A, B); lung cancer (n = 5, 25%) (Fig. 3C); sarcomas of bone and soft tissue (n = 3, 15%); breast cancer (n = 2, 10%); renal cell carcinoma (n = 1, 5%) (Fig. 3D); hepatocellular carcinoma (n = 1, 5%) (Fig. 3E); stage IV thymoma (n = 1, 5%); papillary thyroid cancer (n = 1, 5%) (Fig. 3F). Secondary cardiac tumors were predominantly observed in the atria (n = 12), majority in the RA (n = 9). Surgery was performed for only 7 patients with large RA masses causing symptoms of obstruction, and histology proved invasions or metastases of the primary tumor to the atrium (Supplementary Table 3, Fig. 3).
Fig. 3.
Secondary malignant tumors of the heart.
(A) 2D TTE short axis, main PA infiltration of the B-cell lymphoma with granular appearance; (B) contrast CT, lymphoma infiltrating the main PA; (C) CMR, malignant mesenchymal intrabronchial tumor, cardiac invasion of the tumor via left pulmonary vein (18, with permission); (D) CT, right ventricular wall invasion of the renal cell carcinoma; (E) CMR, hepatocellular carcinoma, almost filling the whole right atrial cavity; (F) CMR, right atrial invasion of the papillary thyroid cancer.
2D TTE: two-dimensional transthoracic echocardiography; PA: pulmonary artery; CMR: cardiac magnetic resonance imaging; CT: computed tomography.
3.4. Cysts
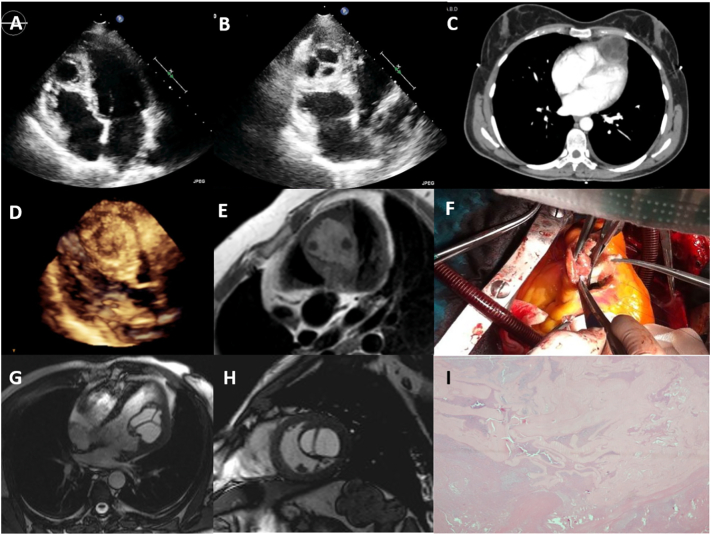
Intracardiac cysts were found in 3 patients (2.36% of cardiac masses, 0.004% of the study group), all were hydatid cysts. The localizations of the cysts were bi-ventricular apical region (Fig. 4A–C), interventricular septum (Fig. 4D–F), LV lateral wall (Fig. 4G, H), all were confirmed by CT and/or CMR, with positive indirect hemagglutination tests. All patients underwent surgery (Fig. 4F), and histology proved the diagnosis (Fig. 4I) (Supplementary Table 4, Fig. 4). Two of three cases were published before [19], [20].
Fig. 4.
Cardiac hydatid cysts with various myocardial locations. (A and B) 2D TTE, (C) contrast-enhanced thoracic CT scan, a multiseptated anechoic cyst located at the apex; (D) 3D TTE, a granulated spheric mass, with patchy hypoechoic regions located within the interventricular septum, (19, with permission); (E) CMR, hydatid cyst located within the interventricular septum with water content and a few daughter cysts; (F) gross examination of the surgically resected hydatid cyst; (G) SSFP cine (20, with permission) (H) CMR, showing left ventricular hydatid cyst with septations originating from the lateral wall into the left ventricular cavity (I) histological examination of the echinococcal lesion with cuticular membranes in cystic content, hematoxylin-eosin × 40.
2D TTE: two-dimensional transthoracic echocardiography; CT: computed tomography; 3D TTE: three-dimensional transthoracic echocardiography; CMR: cardiac magnetic resonance imaging; SSFP: steady state free processing.
3.5. Thrombi
An intracardiac thrombus was identified in 68 patients (53.54% of cardiac masses, 0.104% of the study group). Thirty one patients (45.59%) had underlying cardiac diseases, 37 patients (54.41%) with non-cardiac disorders. Ischemic heart disease with low ejection fraction (EF %), with dilated left ventricle and prominent regional wall motion abnormalities (n = 12, 38.71%, EF 20–40%) was the first range of cardiac disease related intracardiac thrombi, followed by coexistence of hypertension and atrial fibrillation (n = 8, 25.8%), valvular heart disease (n = 6, 19.35%), and a few patients with hypertrophic obstructive cardiomyopathy, peri-myocarditis, biventricular non-compaction, ruptured sinus of Valsalva, generalized atherosclerosis (n = 1, 3.22% for each). The majority of intracardiac thrombi with non-cardiac disorders was associated with malignancies (n = 17, 45.94%) followed by autoimmune diseases (n = 13, 35.13%); chronic kidney disease (n = 4, 10.81%), deep venous thrombosis related with immobilization (n = 2, 5.40%), and hereditary spherocytosis (n = 1, 2.70%) (Fig. 5, Supplementary Table 5).
Fig. 5.
Intracardiac thrombi with noncardiac disorders.
Non small cell lung cancer: (A) 2D TEE, a large thrombus in the right atrium with lytic areas; (B) 2D TEE, thrombus extending from vena cava superior through the right atrial cavity; renal cell carcinoma: (C) CT, thrombus almost completely filling the left atrium; endometrial cancer: (D) CMR, a thrombus extending from vena cava inferior through the right atrium; esophageal cancer: (E) 2D TTE, and (F) CMR, a thrombus filling the right ventricular cavity and extending through the mid ventricular level; ovarian cancer: (G) 2D TEE, and (F) CMR, left atrial thrombus with hypoechoic regions; Behçet's disease: (I) 2D TTE, a massive, lobulated, mobile left ventricular thrombus; (J) CMR, an immobile thrombus infiltrating the right atrial side of the tricuspid valve and endocardial fibroelastosis at the right ventricular wall; rheumatoid arthritis: (K) 2D TTE, right atrial immobile thrombus; systemic lupus erythematosus with antiphospholipid syndrome: (L) 2D TTE, right atrial immobile thrombus; chronic kidney disease, hemodialysis: (M) 2D TEE, right atrial giant, ball shaped thrombus, moderate pericardial effusion; (N) surgical image (O) thrombus histology with organized fibrin mass, hematoxylin-eosin × 200.
2D TTE: two-dimensional transthoracic echocardiography; 2D TEE: two-dimensional transesophageal echocardiography; CMR: cardiac magnetic resonance imaging.
The thrombi developed in patients with cardiac diseases were mainly located in the left heart chambers; those with systolic dysfunction in the LV (n = 16), hypertension and valvular heart disease accompanied by atrial fibrillation in the LA (n = 8). All of the patients received anticoagulant treatment, five patients were operated on.
Intracardiac thrombi of the patients associated with malignancies (n = 17) were predominantly located in the RA (n = 11), RV was the secondary heart chamber (n = 3). Malignancies accompanied by thrombi were; lung cancer (n = 4) (Fig. 5A, B), renal cell carcinoma (n = 3) (Fig. 5C), lymphoma (n = 3), and single cases of endometrial (Fig. 5D), esophageal (Fig. 5E, F), breast, ovarian (Fig. 5G, H), gastric cancers, leiomyosarcoma and chronic lymphocytic leukemia. All of the patients received anticoagulant treatment, only a patient with breast cancer with a very large thrombus in the RA was operated. The autoimmune diseases (n = 13) predominantly consisted of Behçet's disease (n = 7) (Fig. 5I, J) followed by rheumatoid arthritis (n = 2) (Fig. 5K), lupus erythematosus with antiphospholipid syndrome (n = 2) (Fig. 5L), Takayasu arteritis (n = 1) and Wegener granulomatosis (n = 1). The most frequently involved cardiac chamber in autoimmune diseases was RA (n = 9), and infrequently LA (n = 2), LV (n = 1) and ascending aorta (n = 1). Wegener granulomatosis, which was published before [21] with a large thrombus in the left ventricle underwent surgery, and others received medical treatment. Four chronic kidney disease patients on hemodialysis had 2 RA (Fig. 5M–O), 1 LA and 1 RV large thrombi, three of whom were operated. Two patients with deep vein thrombosis, due to trauma and tibia fracture in one and diabetic foot ulcer in the other had RV thrombi followed by pulmonary embolism, were treated medically (details can be viewed from Supplementary Table 5).
Advanced imaging method was CMR in all patients with suspected thrombus in TTE, 10 patients underwent surgery and the thrombus was confirmed with histopathology, whereas 58 patients on anticoagulant treatment were observed to develop volume decrease in serial controls.
In 127 patients with an intracardiac mass diagnosed by TTE, the presence of the mass was confirmed with an advanced imaging modality and, no false-positives. TEE was performed in 94 (74%), CT in 17 (13.4%), and CMR in 109 (85.8%) patients. The imaging diagnosis were controversial in 5 of the 56 (44.1%) operated patients, and the definitive diagnosis was achieved by surgery and histopathology, and also the imaging diagnoses were confirmed histopathologically in 51 patients. Seventy one patients did not undergo surgery or biopsy, 58 of them were patients with intracardiac thrombus receiving anticoagulant therapy, and the remaining 13 were in poor general condition or in the terminal phase for whom invasive interventions were not appropriate.
4. Discussion
This retrospective, cross-sectional, descriptive study demonstrated 127 (0.195%) cardiac masses, consisted of 33 (0.050%) primary benign, 3 (0.004%) primary malignant, 20 (0.030%) secondary tumors, 3 (0.004%) cysts and 68 (0.104%) thrombi respectively.
Primary tumors of the heart are rare, based upon the data of autopsy series, the frequency of primary cardiac tumors ranges from 0.001% to 0.03%, 10% malignant, 90% benign [1], [3], [12], [15]. The majority of benign tumors are myxomas followed by papillary fibroelastomas, and very rare fibroma and lipoma [15]. In a report of 323 operated consecutive patients of primary cardiac tumors, 94% were benign and 50% were myxomas [12]. Primary malignant cardiac tumors are predominantly sarcomas; angiosarcoma and undifferentiated sarcomas are the most prevalent [4]. Although our series consisted of patients referred to the echocardiography laboratory in a university hospital, the frequencies of the primary benign and malignant tumors are in consistent with autopsy and surgery based data and literature.
Specific cardiac tumors have a predilection for certain locations, 75–85% of myxomas arise in the LA, 15–20% from the right atrial septum, usually solitary, with a pedicle attached to the atrial septum at the border of the fossa ovalis membrane, and the remainder from a variety of sites [10], [12], [14]. A retrospective analysis of 194 myxomas operated within fifty years have experienced that, the LA was the most common location and atria were significantly more involved than the ventricles [6]. In our series, in accordance with these previous statements, majority of the myxomas were located in the LA, less in the RA, most of them originating from fossa ovalis with a pedicle, and few sessile masses. Myxomas with atypical locations are rare in the literature [22].
Papillary fibroelastomas are small benign endocardial papillomas, the second most common primary benign cardiac tumors that invariably develop on aortic and mitral valves, left ventricular endocardium and tricuspid valves, but may arise anywhere in the heart [12], [14]. An analysis of 725 histopathologically confirmed papillary fibroelastomas have demonstrated that, 77% of them originate on the valves, 23% in the endocardial nonvalvular surface [2]. In the present study, fibroelastomas were located at the mitral leaflets and tricuspid valve chorda, incidentally no fibroelastomas in the aortic valve. The lipoma and fibroma of the heart are rare tumors, lipoma with a predilection for the epicardium or endocardium, fibroma invariably located in the ventricles [12], [14]. In our series, the single lipoma was imaged at the LV posterior wall originating from the epicardium and fibroma at the ventricular basis of posterior mitral leaflet, a rather atypical location.
Angiosarcomas account for approximately 37–40% of cardiac sarcomas and typically present in the right atrioventricular groove, with frequent involvement of the pericardium and RA wall, with short survival [23]. Rhabdomyosarcomas derived from striated muscle are distinctly rare, accounting for 0% to 5% of primary cardiac sarcomas and most cases appear in children with poor survival [23]. The two angiosarcoma and one rhabdomyosarcoma in our series were in full agreement with the literature in terms of frequency, localization, tumor characteristics and surveillance.
Although primary cardiac tumors are rare, the frequency of secondary metastatic tumors to the heart in autopsy studies is between 0.7% and 3.5%, up to 9.1% in patients with known malignancies [13], [24], [25]. Tumors can reach the heart via 4 pathways: hematogenous or lymphatic spread, transvenous or direct extension [13]. Spread by the hematogenous route generally gives rise to myocardial or endocardial metastases and is common with melanoma, lymphoma, and sarcoma, whereas spread by the lymphatic route will often result in pericardial and epicardial tumor involvement, as with many epithelial tumors such as lung and breast [13]. Certain tumors can extend into the inferior vena cava and grow into the RA via transvenous extension [13]. In the present study, cardiac metastases were diagnosed in 20 patients, when evaluated in terms of frequency; lymphoma, lung cancer and sarcoma were the majority of the secondary metastatic tumors, followed by breast cancer. Lymphoma metastases were remarkably in the LV suggesting hematogenous spread, whereas lung cancer metastases predominantly in RA suggesting transvenous extension, in fact, secondary cardiac tumors were mostly observed in the atria, most of them in the RA which supports the transvenous extension.
Cardiac echinococcosis is an uncommon disease, with an estimated prevalence ranging from 0.5% to 2% [26]. In published series, involvement of all sites of cardiac structures has been reported [19], [27], [28], [29]. Three intracardiac cysts in this study were hydatid cysts with myocardial involvement, which revealed the fact of our endemic character, incidentally no other cysts were encountered.
Thrombi are the most common intracardiac masses, located within the atria, and/or both ventricle cavities with an underlying cardiac disease or hypercoagulable state. Left ventricular thrombi usually occur in the setting of systolic dysfunction, with or without aneurysm formation in ischemic heart disease and dilated cardiomyopathy [15]. Thrombi in the absence of underlying cardiac disease are much less common [15]. Conditions such as acute coronary syndrome within 6 months, newly diagnosed atrial fibrillation, patients with prosthetic valves where thrombus formation is expected and device and catheter induced thrombi were excluded in our series. De-novo intracardiac thrombus was identified in 68 patients and the majority of intracardiac thrombi were associated with malignancies, followed by autoimmune diseases, ischemic heart disease with systolic dysfunction and low ejection fraction, hypertension associated with atrial fibrillation and valvular heart disease. It should be noted here that, the most frequent cause of cardiac thrombus in patients with autoimmune diseases was Behcet's disease which has a peculiar geographical distribution in our country. The majority of thrombi were in the RA and LV and the less in the LA. The RA thrombi predominantly were associated with malignancy and autoimmune diseases, whereas LV thrombi were detected in patients with LV systolic dysfunction and, LA with valvular heart disease, hypertension and atrial fibrillation. The RV thrombi were seen in patients with deep vein thrombosis and malignancies. In the literature, other than case reports, there is no clinical series regarding the localization and etiology of intracardiac thrombi. An autopsy study reporting the largest series of intracardiac thrombi of 11,724 autopsies performed in 15 years has found 276 patients (2.4%) with intracardiac thrombi, of these, 45 patients had intracardiac thrombi that were unrelated to primary cardiac disease, the most recognizable risk factor was underlying cancers (24.4%) which supports our results [30].
This study has limitations. I-Mass Study is not an epidemiological study, it aimed to specify the frequency of cardiac tumors and masses other than tumors, with a descriptive analysis in consecutive patients referred to the echocardiography laboratory in a multidisciplinary university hospital. There may be criticisms that, some images used in the article are not clear enough, this was largely due to the patients not being able to position themselves adequately or being unable to stay in a fixed position enough time for a standard imaging due to pain, fatigue, shortness of breath, and some cases had poor imaging quality. We tried to share the clearest images to the extent allowed by the technical features of the imaging devices. In some of the patients, surgical and histological examinations for definitive diagnosis was incomplete due to the deterioration of the general condition or the diagnosis was in the terminal period, in this case invasive procedures such as surgery or biopsy could not be performed and we focused on noninvasive imaging.
5. Conclusion
Although cardiac masses are rare entities, they are being increasingly recognized in the current era by means of advanced imaging modalities. In the current analysis, we classified and presented various categories of cardiac masses systematically in relation to concomitant disease, and their locations in the heart chambers. The majority of benign tumors were myxoma, the most common secondary malignant tumors that metastasized to the heart were lymphoma and lung cancers, and the most frequent thrombi other than cardiac diseases were associated with malignancies and autoimmune diseases. This descriptive analysis may, therefore, contribute to appropriate diagnosis of patients with cardiac mass, which principally rely on correct recognition of the masses made generally based on their localizations and imaging characteristics and methods.
Funding source
None declared.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgement
Special thanks to Mukadder Kale and Valentina Senay Can for their contribution on reviewing the patient medical records system.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ahjo.2021.100081.
Appendix A. Supplementary data
Supplementary tables
References
- 1.Reynen K. Frequency of primary tumors of the heart. Am. J. Cardiol. 1996;77:107. doi: 10.1016/s0002-9149(97)89149-7. [DOI] [PubMed] [Google Scholar]
- 2.Gowda R.M., Khan I.A., Nair C.K., Mehta N.J., Vasavada B.C., Sacchi T.J. Cardiac papillary fibroelastoma: a comprehensive analysis of 725 cases. Am. Heart J. 2003;146:404–410. doi: 10.1016/S0002-8703(03)00249-7. [DOI] [PubMed] [Google Scholar]
- 3.Strecker T., Rösch J., Weyand M., Agaimy A. Primary and metastatic cardiac tumors: imaging characteristics, surgical treatment, a histopathological spectrum: a 10 year-experience at a german heart center. Cardiovasc. Pathol. 2012;21:436–443. doi: 10.1016/j.carpath.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 4.Hudzik B., Miszalski-Jamka K., Glowacki J., Glowacki J., Lekston A., Gierlotka M., Zembala M., Polonski L., Gasior M. Malignant tumors of the heart. Cancer Epidemiol. 2015;39:665–672. doi: 10.1016/j.canep.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 5.Yu L., Gu T., Shi E. Echocardiographic findings and clinical correlation with cardiac myxoma. J. Am. Coll. Cardiol. Imaging. 2016;9:618–620. doi: 10.1016/j.jcmg.2015.04.029. [DOI] [PubMed] [Google Scholar]
- 6.Shah I.K., Dearani J.A., Daly R.C., Daly R.C., Suri R.M., Park S.J., Joyce L.D., Li Z., Schaff H.V. Cardiac myxomas: a 50-year experience with resection and analysis of risk factors for recurrence. Ann. Thorac. Surg. 2015;100:495–500. doi: 10.1016/j.athoracsur.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 7.Kassop D., Donovan M.S., Cheezum M.K., Nguyen B.T., Gambill N.B., Blankstein R., Villines T.C. Cardiac masses on CT: a review. Curr. Cardiovasc. Imaging Rep. 2014;7:9281. doi: 10.1007/s12410-014-9281-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parvani P., Co M., Ramesh T., Akhter N., Iliescu C., Palaskas N., Kim P., Gladish G., Stojavonska J., Abramov D., Lopez-Mattel J. Differentiation of cardiac masses by cardiac magnetic resonance imaging. Curr. Cardiovasc. Imaging Rep. 2020;13:1. [Google Scholar]
- 9.Esposito A., De Cobelli F., Ironi G., Marra P., Canu T., Mellone R., Del Maschio A. CMR in assessment of cardiac masses, primary benign tumors. J. Am. Coll. Cardiol. Imaging. 2014;7:733–736. doi: 10.1016/j.jcmg.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 10.Reynen K. Cardiac myxomas. N. Engl. J. Med. 1995;333:1610–1617. doi: 10.1056/NEJM199512143332407. [DOI] [PubMed] [Google Scholar]
- 11.Orlandi A., Ferlosio A., Roselli M., Chiariello L., Spagnoli L.G. Cardiac sarcomas, an update. J. Thorac. Oncol. 2010;5:1483–1489. doi: 10.1097/JTO.0b013e3181e59a91. [DOI] [PubMed] [Google Scholar]
- 12.Bruce C.J. Cardiac tumours: diagnosis and management. Heart. 2011;97:151–160. doi: 10.1136/hrt.2009.186320. [DOI] [PubMed] [Google Scholar]
- 13.Goldberg A.D., Blankstein R., Padera R.F. Tumors metastatic to the heart. Circulation. 2013;128:1790–1794. doi: 10.1161/CIRCULATIONAHA.112.000790. [DOI] [PubMed] [Google Scholar]
- 14.Burke A., Tavora F. The 2015 WHO classification of tumors of the heart and pericardium. J. Thorac. Oncol. 2015;11:441–452. doi: 10.1016/j.jtho.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 15.Basso C., Rizzo S., Valente M., Thiene G. Cardiac masses and tumours. Heart. 2016:1–16. doi: 10.1136/heartjnl-2014-306364. [DOI] [PubMed] [Google Scholar]
- 16.Cheitlin M.D., Armstrong W.F., Aurigemma G.P., Beller G.A., Davis J.L., Douglas P.S., Faxon D.P., et al. ACC/AHA/ASE 2003 guideline update for the clinical application of echocardiography: summary article. A report of the American College of Cardiology/American Heart Association task force on practice guidelines (ACC/AHA/ASE Committee to update the 1997 guidelines for the clinical application of echocardiography. J. Am. Soc. Echocardiogr. 2003;16:1091–1110. doi: 10.1016/S0894-7317(03)00685-0. [DOI] [PubMed] [Google Scholar]
- 17.Lang R.M., Badano L.P., Tsang W., Adams D.H., Agricola E., Buck T., et al. EAE/ASE recommendations for image acquisition and display using three-dimensional echocardiography. Eur. Heart J. Cardiovasc. Imaging. 2012;13:1–46. doi: 10.1093/ehjci/jer316. [DOI] [PubMed] [Google Scholar]
- 18.Emet S., Kocaaga M., Bugra Z., Umman B. Cardiac invasion of the intrabronchial malignant mesenchymal tumor from left pulmonary vein. Arch. Turk. Soc. Cardiol. 2014;42:688. doi: 10.5543/tkda.2014.96641. [DOI] [PubMed] [Google Scholar]
- 19.Bayramov F., Emet S., Dadashov M., Umman B., Bugra Z. A case of an atypically located cardiac hydatid cyst. Anatol. J. Cardiol. 2015;15:E24–E25. doi: 10.5152/AnatolJCardiol.2015.6548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dursun M., Terzibasioglu E., Yilmaz R., Cekrezi B., Olgar S., Nisli K., et al. A pictorial essay: cardiac hydatid disease : CT and MRI findings. AJR. 2008;190:226–232. doi: 10.2214/AJR.07.2035. [DOI] [PubMed] [Google Scholar]
- 21.Elitok A., Emet S., Onur I., Karaayvaz E.B., Sayın O.A., Umman B., et al. A rare cardiac manifestation of Wegener’s granulomatosis. Anatol. J. Cardiol. 2015;15:170–171. doi: 10.5152/akd.2014.5820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Turhan S., Kilickap M., Candemir B., Berkalp B., Eren N.T., Akgün G. Three unusual myxomas originating from the left atrial appendage: a case report. J. Am. Soc. Echocardiogr. 2005;18:694. doi: 10.1016/j.echo.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 23.Burke A. Primary malignant cardiac tumors. Semin. Diagn. Pathol. 2008;25:39–46. doi: 10.1053/j.semdp.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 24.Reynen K., Köckeritz U., Strasser R.H. Metastases to the heart. Ann. Oncol. 2004;15:375–381. doi: 10.1093/annonc/mdh086. [DOI] [PubMed] [Google Scholar]
- 25.Bussani R., De-Giorgio F., Abbate A., Silvestri F. Cardiac metastases. J. Clin. Pathol. 2007;60:27–34. doi: 10.1136/jcp.2005.035105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yaman N.D., Sirlak M. Cardiac hydatid cyts, review of recent literature. J. Vet. Med. Res. 2017;4:1102–1107. [Google Scholar]
- 27.Atilgan D., Kudat H., Tukek T., Ozcan M., Yildirim O.B., Elmaci T.T., et al. Role of transesophageal echocardiography in diagnosis and management of cardiac hydatid cyst: report of three cases and review of the literature. J. Am. Soc. Echocardiogr. 2002;15:271–274. doi: 10.1067/mje.2002.120507. [DOI] [PubMed] [Google Scholar]
- 28.Ugurlucan M., Sayın O.A., Surmen B., Cinar T., Yekeler E., Dursun M. Hydatid cyst of the interventricular septum. Circulation. 2006;113:e869–e870. doi: 10.1161/CIRCULATIONAHA.105.595298. [DOI] [PubMed] [Google Scholar]
- 29.Fennira S., Kamoun S., Bebes B., Ben Mrad I., Zairi I., Ben Moussa F., et al. Cardiac hydatid cyst in the interventricular septum: a literature review. Int. J. Infect. Dis. 2019;88:120–126. doi: 10.1016/j.ijid.2019.09.004. [DOI] [PubMed] [Google Scholar]
- 30.Vaideeswar P., Divate S., Harke M. Intracardiac thrombi in extracardiac disorders: an autopsy study. Cardiovasc. Pathol. 2012;21:e1–e9. doi: 10.1016/j.carpath.2011.11.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary tables