Abstract
Study objective
This systematic review and meta-analysis aimed to assess the efficacy and safety profile of treatment with inclisiran, a drug that has been recently approved by the United States Food and Drug Administration (FDA) and the European Medicines Agency (EMA).
Design
A systematic literature search was conducted in order to identify randomized controlled trials (RCTs) assessing the effect on lipoproteins and the safety profile of inclisiran.
Results
Data were pooled from 5 RCTs, which included 4226 subjects. Meta-analyses of data suggested that the multiple-dose regimens of inclisiran yielded a significant reduction in serum levels of proprotein convertase subtilisin/kexin type 9 (MD = −78.23%, 95%CI: −86.74, −69.71) and low-density lipoprotein cholesterol (MD = −45.48%, 95%CI: −50.36%, −40.61%) throughout the studies. Furthermore, treatment with inclisiran significantly affected total cholesterol (MD = −13.67%, 95%CI: −20.78%, −6.57%), high-density lipoprotein cholesterol (MD = 8.29%, 95%CI: 4.66%,11.93%), non-HDL cholesterol (MD = −39.45%, 95%CI: −43.6%, −35.31%), apolipoprotein B (MD = −34.58%, 95%CI: −38.78%, −30.78%) and lipoprotein(a) (MD = −20.9%, 95%CI: −25.8%, −15.99%). Multiple-dose regimens of inclisiran were associated with increased risk of injection-site reactions (any reaction: OR = 5.86, 95%CI: 3.44, 9.98; mild reactions: OR = 5.19, 95%CI: 1.68, 16.07; moderate reactions: OR = 13.37, 95%CI: 3.17, 56.46), and bronchitis (OR = 1.58, 95%CI: 1.10, 2.26), while the incidence of the pre-specified exploratory CV endpoint significantly decreased at 18 months (OR = 0.74, 95%CI: 0.58, 0.94).
Conclusion and relevance
Inclisiran has favourable effects on serum lipid levels and an acceptable safety profile. Further well-designed RCTs are needed to explore its longer-term safety.
Keywords: Inclisiran, Proprotein convertase subtilisin/kexin type 9, Silencing ribonucleic acid, Hypercholesterolemia;lipoprotein(a), Low-density lipoprotein cholesterol
Graphical abstract
1. Introduction
Converging data from Mendelian randomization studies, randomized controlled trials (RCTs), and prospective longitudinal cohort studies show that the cumulative lifetime exposure to high levels of low-density lipoprotein cholesterol (LDL-C) causes atherosclerotic cardiovascular disease (ASCVD) [1].
The most recent guidelines for the management of dyslipidemia recommend in subjects at high or very high risk for CV disease an aggressive LDL-C reduction, with LDL-C goal levels that are difficult to achieve with traditional lipid-lowering treatments [2], [3], [4]. Statins remain the cornerstone of LDL-C reduction and CV risk reduction, but the lipid-lowering response to statins shows high inter-individual variation [5]. The addition of ezetimibe to statin therapy is also effective, resulting in an additional 20% reduction in LDL-C and in an incremental reduction in risk for sustaining secondary CV events over 6 years of follow-up [6], [7]. Even though the co-administration of statins with ezetimibe might confer further and significant clinical benefit [8], [9], in cases of statin intolerance or suboptimal control of LDL-C levels, other treatment options are necessary.
Recently, several RCTs were conducted to test the lipid-lowering activity resulting from the inhibition of proprotein convertase subtilisin/kexin type 9 (PCSK9), a chaperone molecule that promotes hepatic LDL receptor degradation in the lysosome [10], [11]. PCSK9 inhibition with the monoclonal antibodies evolocumab and alirocumab significantly reduces LDL-C serum levels by approximately 60–65% [12], and these agents have been shown to reduce risk for acute cardiovascular events on a background of statin therapy with a good safety and tolerability profile [1], [13], [14]. In particular, a recent meta-analysis of 23 RCTs including 88,041 patients, highlighted that treatment with PCSK9 inhibitors in addition to maximally tolerated lipid-lowering therapy is associated with a reduced risk of myocardial infarction [OR = 0.80 (95%CI: 0.71, 0.91), P < 0.0001], stroke [OR = 0.75 (95%CI: 0.65, 0.85), P < 0.0001], and coronary revascularization [OR = 0.82, (95%CI: 0.77, 0.88), P < 0.0001) [15].
Since the introduction of the PCSK9 monoclonal antibodies, the gene silencing drug inclisiran has been developed, which specifically targets hepatic PCSK9 mRNA and inhibits its translation [16]. Inclisiran is a synthetic small interfering RNA (siRNA) duplex oligonucleotide conjugated to triantennary N-acetylgalactosamine that specifically targets inclisiran to hepatocytes. Inside the hepatocytes, it acts by binding to the RNA induced silencing complex (RISC) and blocking the translation of PCSK9 messenger RNA (mRNA), thereby reducing PCSK9 synthesis and its secretion into the extracellular milieu [16]. Based on the available clinical evidence, Inclisiran has recently been approved by the United States Food and Drug Administration (FDA) and the European Medicines Agency (EMA), becoming the first cholesterol-lowering therapy in its class [17], [18], [19].
Given the relatively small number of available RCTs, we aimed to perform a systematic review and meta-analysis to comprehensively evaluate inclisiran's therapeutic efficacy and tolerability profile.
2. Methods
The study was designed in agreement with the 2009 preferred reporting items for systematic reviews and meta-analysis (PRISMA) statement guidelines [20], and it was registered in the PROSPERO database (Registration number CRD42019145876).
Due to study design, neither Institutional Review Board (IRB) approval, nor patient informed consent were required. PRISMA Checklist was included in the supplementary file (Additional File 1).
2.1. Search strategy
PubMed Medline, SCOPUS, Google Scholar and Web of Science by Clarivate databases were searched, with no language restriction, using the following search terms: “Inclisiran” AND (“Clinical trial” OR “Clinical study”). The wild-card term “*” was used to increase the sensitivity of the search strategy, which was limited to studies in humans. The reference list of identified papers was manually checked for additional relevant articles. Literature was searched from inception to December 30th, 2021.
All paper abstracts were screened by two reviewers in an initial process to remove ineligible articles. The remaining articles were obtained in full-text and assessed again by the same two researchers (A.F.G.C. and F.F.) who evaluated each article independently and carried out data extraction and quality assessment. Disagreements were resolved by discussion with another investigator (C.B.).
2.2. Study selection criteria
Original studies were included if they met the following criteria: (i) being an RCT with either multicentre or single-centre design, (ii) having an appropriate controlled design for inclisiran treatment, (iii) investigating the effect of inclisiran on plasma lipids, (iv) testing the safety of inclisiran short-term (<1 year) and medium-term (>1 year) administration, (v) reporting all the adverse events occurred during the treatment.
Exclusion criteria were: (i) lack of a control group for inclisiran administration, (ii) intravenous infusion of the drug and (iii) lack of sufficient information about the prevalence and nature of the adverse events. Studies were also excluded if they contained overlapping subjects with other studies.
2.3. Data extraction
All data extraction and database typing were reviewed by the principal investigator before the final analysis, and doubts were resolved by mutual agreement among the authors.
2.4. Quality assessment
A systematic assessment of risk of bias in the included studies was performed using the Cochrane criteria [21]. Risk-of-bias assessment was performed independently by 2 reviewers; disagreements were resolved by a consensus-based discussion. The following items were used: adequacy of sequence generation, allocation concealment, blinding addressing of dropouts (incomplete outcome data), selective outcome reporting, and other probable sources of bias [22].
2.5. Data synthesis
Meta-analysis was entirely conducted using Comprehensive Meta-Analysis (CMA) V3 software (Biostat, NJ) [23].
Net changes in the investigated parameters (change scores) were calculated by subtracting the value at baseline from the one after intervention, in the active-treated group and in the control one. All values were calculated-reported as percent change from baseline. Standard deviations (SDs) of the mean difference were obtained as reported by Follman and colleagues: SD = √[(SDpre-treatment)2 + (SDpost-treatment)2 − (2R ⨯ SDpre-treatment ⨯ SDpost-treatment)], assuming a correlation coefficient (R) = 0.5 [24]. If the outcome measures were reported as median and range (or 95% confidence interval (CI)) or mean and 95%CI, values were estimated using the method described by Wan et al. [25]. To avoid a double-counting problem, in trials comparing multiple treatment arms versus a single control group, the number of subjects in the control group was divided by the required comparisons. Treatment arms testing 25 mg and 800 mg inclisiran were excluded from the meta-analysis as the control arm was not large enough for comparison. The findings from studies were combined using a fixed-effect model or a random-effect model (using the DerSimonian-Laird method) and the generic inverse variance method, based on the level of inter-study heterogeneity, which was quantitatively assessed using the Higgins index (I2). In particular, findings were combined using a random-effect model when I2 was high (>50%); on the other hand, when heterogeneity was low (I2 < 50%) a fixed-effect model was used [26]. Effect sizes for lipid changes were expressed as percentage mean difference (MD) and 95% confidence interval (CI). For safety analysis, odd ratio (OR) and 95%CI intervals were calculated using the Mantel-Haenszel method [27]. If one or more outcomes could not be extracted from a study, the study was removed only from the analysis involving those outcomes. Safety analysis was performed by excluding studies with zero events in both arms. Adverse events were considered for the analysis only if occurring in at least two of the included RCTs. Primary outcomes were treatment-emergent adverse events (TEAE), including pre-specified exploratory CV endpoint, which comprised a Medical Dictionary for Regulatory Activities–defined cardiovascular basket of non-adjudicated terms including those classified within cardiac death, and any signs or symptoms of cardiac arrest, nonfatal myocardial infarction, or stroke. The severity of injection site reactions was classified as “mild” in the presence of discomfort, but no disruption to daily activity; “moderate” in the presence of discomfort sufficient to reduce or affect normal daily activity; and “severe” in case of inability to work or perform normal daily activity.
In order to evaluate the influence of each study on the overall effect size, sensitivity analysis was conducted using the leave-one-out method [28]. Two-sided P-values ≤0.05 were considered statistically significant for all tests.
2.6. Publication biases
Potential publication biases were explored using visual inspection of Begg's funnel plot asymmetry, Begg's rank correlation test, and Egger's weighted regression test [29]. Two-sided P values ≤0.05 were considered statistically significant.
3. Results
3.1. Flow and characteristics of the included studies
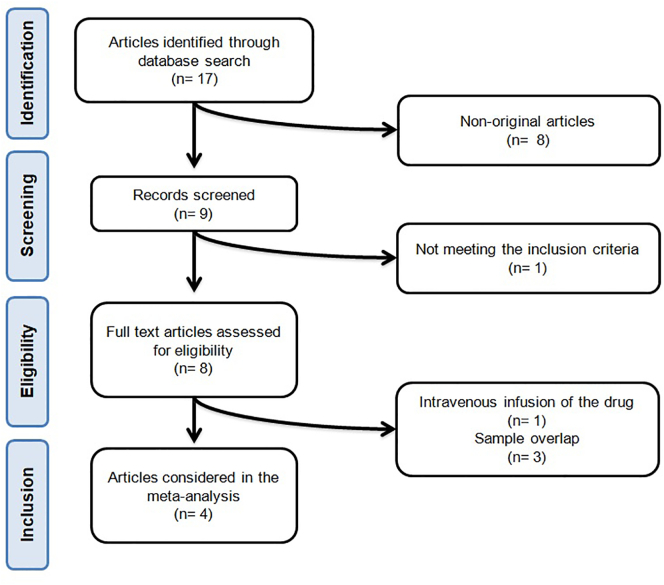
After database searches and assessment of eligible studies, 4 articles were included in the meta-analysis [30], [31], [32], [33]. The study selection process is shown in Fig. 1.
Fig. 1.
- Flow chart of the number of studies identified and included into the meta-analysis.
Complete information about excluded clinical studies was included in the supplementary file (Additional File 2).
Data were pooled from five RCTs comprising 24 treatment arms, which included 4226 subjects, 2254 in the active-treated arms and 1972 in the control ones.
Eligible studies were published between 2017 and 2020. Follow-up periods ranged between 56 and 540 days and different treatment schedules were tested. Baseline characteristics of the evaluated studies are summarized in Table 1.
Table 1.
Main characteristics of the clinical trials testing safety and efficacy of treatment with inclisiran.
| Author, year | Study | Location | Design | Main inclusion criteria | Follow-up visits | Study group | Enrolled subjects⁎ (n) | Concomitant lipid-lowering treatment |
Age (years; mean ± SD) | Male (n (%)) | Baseline LDL-C (mg/dL) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Statin (%) | High-intensity statin (%) | Ezetimibe (%) | |||||||||||
| Multiple-dose studies | |||||||||||||
| Raal, 2020 [29] | ORION-9 | Canada, Czechia, Denmark, Netherlands, South Africa, Spain, Sweden, United States | Randomized, double-blind, placebo-controlled, parallel-group, phase 3 clinical trial |
|
On days 30, 150, 330, 510 (end-of-treatment visit), 540 (last trial visit) | 300 mg inclisiran sodium (corresponding to 284 mg inclisiran free acid) administered on days 1, 90, 270, and 450 | 242 | 90.5 | 76.4 | 55.8 | 56 (47–63)§ | 112 (46.3) | 151.4 ± 50.4 |
| Placebo | 240 | 90.4 | 71.2 | 50 | 56 (46–64)§ | 115 (47.9) | 154.7 ± 58 | ||||||
| Ray, 2020 [30] | ORION-10 | United States | Randomized, double-blind, placebo-controlled, parallel-group, phase 3 clinical trial |
|
On days 30, 150, 330, 510 (end-of-treatment visit), 540 (last trial visit) | 300 mg inclisiran sodium (corresponding to 284 mg inclisiran free acid) administered on days 1, 90, 270, and 450 | 781 | 89.8 | 67.2 | 10.2 | 66.4 ± 8.9 | 535 (68.5) | 104.5 ± 39.6 |
| Placebo | 780 | 88.7 | 68.8 | 9.5 | 65.7 ± 8.9 | 548 (70.3) | 104.8 ± 37 | ||||||
| ORION-11 | Europe and South Africa | Randomized, double-blind, placebo-controlled, parallel-group, phase 3 clinical trial |
|
On days 30, 150, 330, 510 (end-of-treatment visit), 540 (last trial visit) | 300 mg inclisiran sodium (corresponding to 284 mg inclisiran free acid) administered on days 1, 90, 270, and 450 | 810 | 94.6 | 79 | 6.3 | 64.8 ± 8.3 | 579 (71.5) | 107.2 ± 41.8 | |
| Placebo | 807 | 94.9 | 78.2 | 7.7 | 64.8 ± 8.7 | 581 (72) | 103.7 ± 36.4 | ||||||
| Fitzgerald, 2017 [31] | United Kingdom | Randomized, single-blind, placebo-controlled, parallel-group, dose-ranging, phase 1 clinical trial |
|
On days 56 and 180 (last trial visit) | Inclisiran 125 mg 4 weekly doses, 250 2 biweekly doses, 300 mg 2 monthly doses or 500 mg 2 monthly doses | 24 | 0 | 0 | NA | 51 ± 12 | 17; 71 | 139.3 ± 32.3 | |
| Placebo | 8 | 0 | 0 | NA | 51 ± 14 | 6; 75 | 131.5 ± 20.9 | ||||||
|
Inclisiran 300 mg 2 monthly doses or 500 mg 2 monthly doses | 9 | 100 | NA | NA | 54 ± 16 | 4; 44 | 143.4 ± 29.8 | |||||
| Placebo | 4 | 100 | NA | NA | 58 ± 3 | 2; 50 | 143.1 ± 89.7 | ||||||
| Ray, 2017 [32] | ORION-1 | Canada, Europe and United States | Randomized, double-blind, placebo-controlled, parallel-group, dose-ranging, phase 2 clinical trial |
|
On days 14, 30, 60, 90 (end-of-treatment visit), 120, 150, 180, 210 (last trial visit) | Inclisiran 100 mg administered on days 1 and 90 | 61 | 71 | 47 | 31 | 65.2 ± 9.4 | 38 (62) | 128.5 ± 49.5 |
| Inclisiran 200 mg administered on days 1 and 90 | 62 | 67 | 38 | 33 | 62.3 ± 10.9 | 39 (63) | 138.8 ± 76.9 | ||||||
| Inclisiran 300 mg administered on days 1 and 90 | 61 | 73 | 34 | 25 | 64.1 ± 9.4 | 45 (74) | 131.3 ± 60.3 | ||||||
| Placebo | 62 | 77 | 36 | 28 | 62.8 ± 10.3 | 33 (53) | 125.0 ± 44.3 | ||||||
| Single-dose Studies | |||||||||||||
| Fitzgerald, 2017 [31] | United Kingdom | Randomized, single-blind, placebo-controlled, parallel-group, dose-ranging, phase 1 clinical trial |
|
On day 56 (last trial visit) | Inclisiran 25 mg administered on day 0 | 3 | 0 | 0 | 0 | 47 ± 14.2 | 3 (100) | 177 ± 50.7 | |
| Inclisiran 100 mg administered on day 0 | 3 | 0 | 0 | 0 | 48 ± 6.2 | 3 (100) | 150.8 ± 35.6 | ||||||
| Inclisiran 300 mg administered on day 0 | 3 | 0 | 0 | 0 | 48 ± 12.7 | 3 (100) | 162.4 ± 36.7 | ||||||
| Inclisiran 500 mg administered on day 0 | 3 | 0 | 0 | 0 | 39 ± 14 | 3 (100) | 119.9 ± 17 | ||||||
| Inclisiran 800 mg administered on day 0 | 6 | 0 | 0 | 0 | 49 ± 6.7 | 5 (83.3) | 158.5 ± 28.6 | ||||||
| Placebo | 6 | 0 | 0 | 0 | 48 ± 14.2 | 2 (33.3) | 131.5 ± 19.3 | ||||||
| Ray, 2017 [32] | ORION-1 | Canada, Europe and United States | Randomized, double-blind, placebo-controlled, parallel-group, dose-ranging, phase 2 clinical trial |
|
On days 14, 30, 60, 90, 120, 150, 180, 210 (last trial visit) | Inclisiran 200 mg administered on day 1 | 60 | 83 | 52 | 38 | 63.9 ± 11.4 | 39 (65) | 122.3 ± 34.7 |
| Inclisiran 300 mg administered on day 1 | 61 | 75 | 38 | 28 | 64.1 ± 12.8 | 41 (67) | 120.1 ± 41.8 | ||||||
| Inclisiran 500 mg administered on day 1 | 65 | 65 | 33 | 35 | 62.1 ± 12.4 | 46 (71) | 134.8 ± 45.7 | ||||||
| Placebo | 65 | 70 | 41 | 33 | 62 ± 11.4 | 42 (65) | 126.9 ± 52 | ||||||
Intention-to-treat (ITT) population; reported as mean (interquartile range); ASCVD = Atherosclerotic cardiovascular disease; CHD = Coronary heart disease; CV = Cardiovascular; CVD = Cardiovascular disease; eGFR = Estimated glomerular filtration rate; HeFH = Heterozygous familial hypercholesterolemia; LDL-C = Low-density lipoprotein cholesterol; LLT = Lipid lowering treatment; PAD = Peripheral artery disease; SD = Standard deviation; T2D = Type 2 diabetes; TG = Triglycerides.
3.2. Risk of bias assessment
Almost all of the included studies were characterized by sufficient information regarding sequence generation, allocation concealment, personal and outcome assessments. However, all phase three RCTs showed high risk of bias because of incomplete outcome data and selective outcome reporting. Details of the quality of bias assessment are reported in Table S1.
3.3. Lipid-lowering effect of inclisiran
3.3.1. Primary outcomes
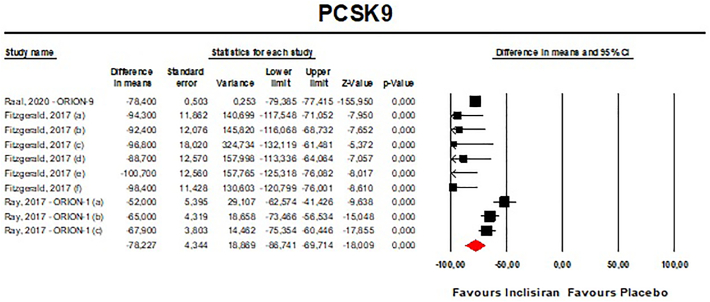
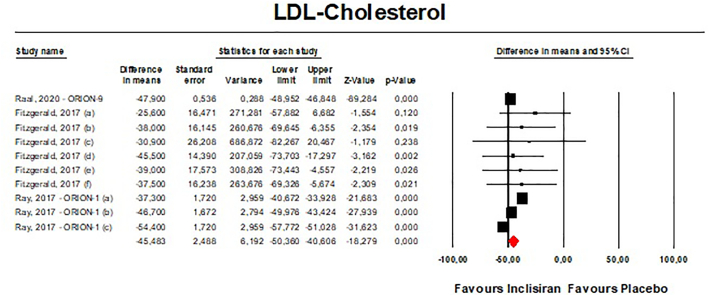
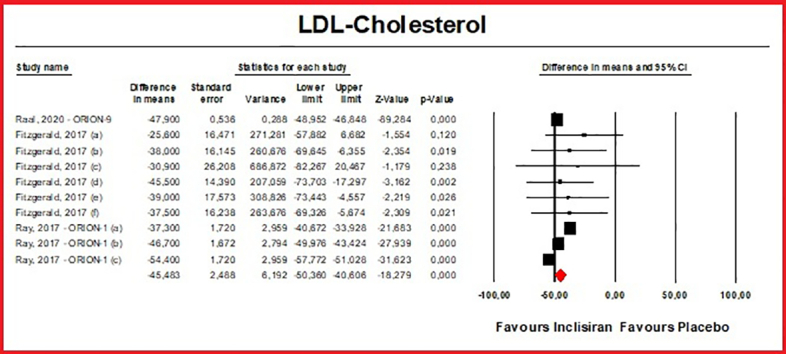
Meta-analyses of data suggested that the multiple-dose regimen of inclisiran yielded a significant reduction in serum levels of PCSK9 [n = 765, MD = −78.23% (95%CI: −86.74%, −69.71%); I2 = 82.5%] (Fig. 2) and LDL-C [n = 765, MD = −45.48% (95%CI: −50.36%, −40.61%); I2 = 83.7%] (Fig. 3) throughout the included RCTs. Pooled data from phase 3 RCTs showed that at 510 days (about 17 months) the success rate for LDL-C goal achievement was significant in the inclisiran group (Table S2).
Fig. 2.
- Forest plot displaying mean difference and 95% confidence intervals for the effect of inclisiran on plasma levels of PCSK9. Fitzgerald et al. (2017) tested inclisiran at a dose of 125 mg without statin treatment (a), 250 mg without statin treatment (b), 300 mg with statin treatment (c), 300 mg without statin treatment (d), 500 mg with statin treatment (e) and 500 mg without statin treatment (f). Ray et al. (2017) tested inclisiran at a dose of 100 mg (a), 200 mg (b), and 300 mg (c).
Fig. 3.
- Forest plot displaying mean difference and 95% confidence intervals for the effect of inclisiran on plasma levels of LDL-C.
Visual inspection of Begg's funnel plot suggested potential publication biases for the effect of inclisiran on both PCSK9 and LDL-C serum concentrations (Fig. S3). These asymmetries were imputed to 3 potentially missing studies on the right-side of the funnel plot decreasing the estimated effect size on PCSK9 to −73.56% (95%CI: −81.84%,65.29%) and 5 potentially missing studies on the left-side of the funnel plot which increased the estimated effect size on LDL-C to −49.13% (95%CI: −54.51%,43.75%). However, both Begg's rank correlation and Egger's linear regression tests did not detect any potential bias in the analyses (P > 0.05 always).
3.3.2. Secondary outcomes
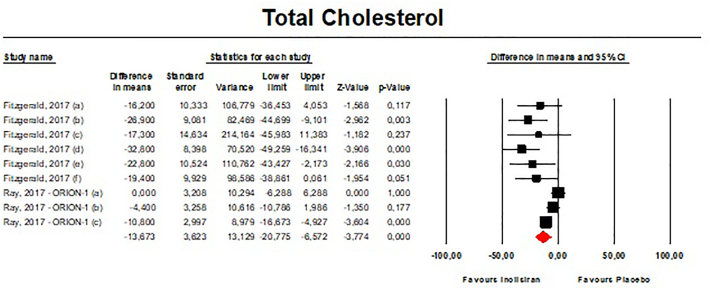
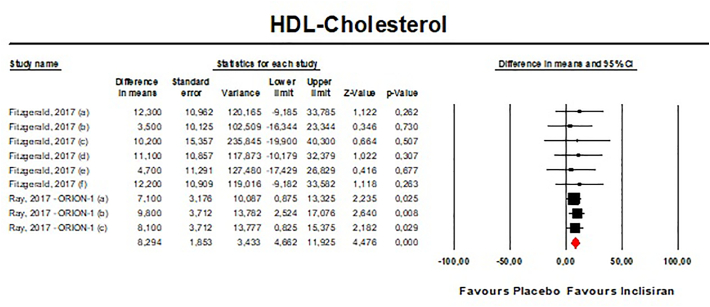
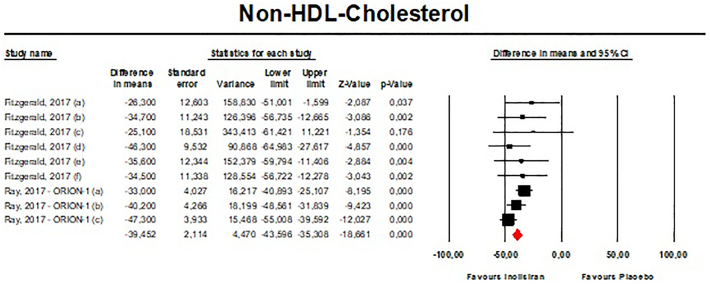
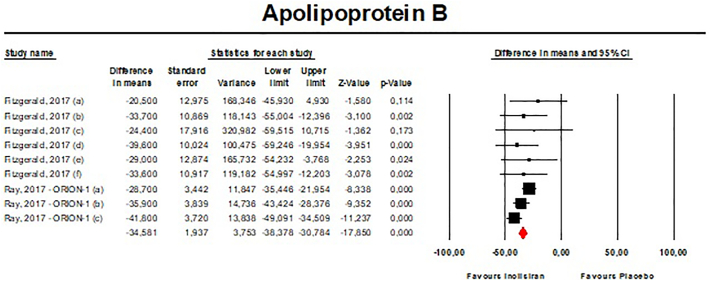
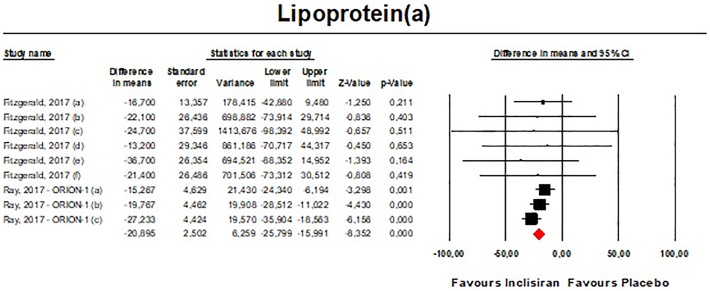
Meta-analysis of available data showed that inclisiran significantly affected total cholesterol (TC) [n = 283, MD = −13.67% (95%CI: −20.78%, −6.57%); I2 = 68.8%] (Fig. 4), high-density lipoprotein cholesterol (HDL—C) [n = 283, MD = 8.29% (95%CI: 4.66%,11.93%); I2 = 0%] (Fig. 5), non-HDL cholesterol (non-HDL-C) [n = 283, MD = −39.45% (95%CI: −43.6%, −35.31%); I2 = 13.5%] (Fig. 6), apolipoprotein B (apoB) [n = 283, MD = −34.58% (95%CI: −38.78%, −30.78%); I2 = 8.7%] (Fig. 7), lipoprotein(a) [Lp(a)] [n = 283, MD = −20.9% (95%CI: −25.8%, −15.99%); I2 = 0%] (Fig. 8).
Fig. 4.
- Forest plot displaying mean difference and 95% confidence intervals for the effect of inclisiran on plasma levels of TC.
Fig. 5.
- Forest plot displaying mean difference and 95% confidence intervals for the effect of inclisiran on plasma levels of HDL-C.
Fig. 6.
- Forest plot displaying mean difference and 95% confidence intervals for the effect of inclisiran on plasma levels of Non-HDL-C.
Fig. 7.
- Forest plot displaying mean difference and 95% confidence intervals for the effect of inclisiran on plasma levels of apoB.
Fig. 8.
– Forest plot displaying mean difference and 95% confidence intervals for the effect of inclisiran on plasma levels of Lp(a).
The effect sizes were robust in the leave-one-out sensitivity analysis and not mainly driven by a single study (Figs. S4-S8).
3.3.3. Safety analysis
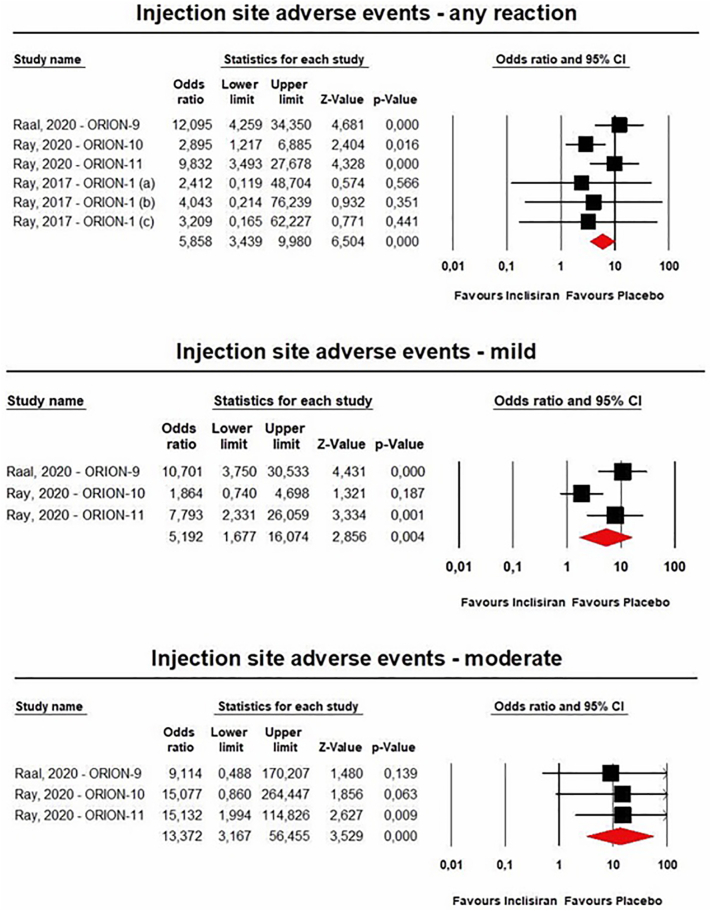
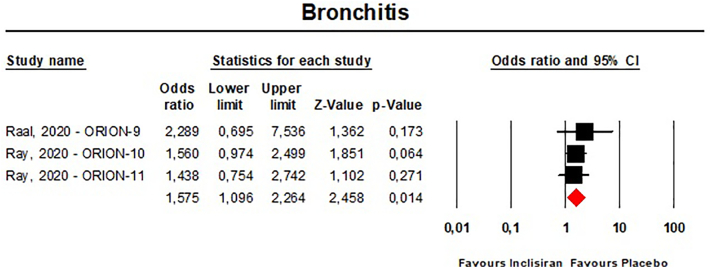
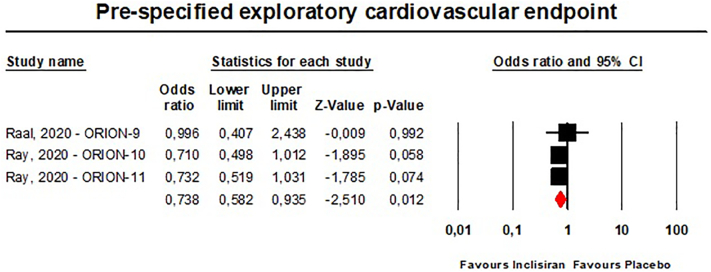
Single-dose administration of inclisiran was safe and not associated with any emergent adverse event. Multiple-dose regimens of inclisiran were associated with in-creased risk of injection-site reactions [any reaction: n = 3902, OR = 5.86 (95%CI: 3.44,9.98), I2 = 15.4%; mild reactions: n = 3655, OR = 5.19 (95%CI: 1.68,16.07), I2 = 71%; moderate reactions: n = 3655, OR = 13.37 (95%CI: 3.17,56.46), I2 = 0%] (Fig. 9), and bronchitis [n = 3655, OR = 1.58 (95%CI: 1.10,2.26), I2 = 0%] (Fig. 10), while the incidence of the pre-specified exploratory CV endpoint significantly decreased at 540 days [n = 3655, OR = 0.74 (95%CI: 0.58,0.94), I2 = 0%] (Fig. 11). These findings were robust in the leave-one-out sensitivity analyses (Figs. S9-S11).
Fig. 9.
- Forest plots for the risk of injection site adverse events occurred in patients on the multiple-dose regimens of inclisiran.
Fig. 10.
- Forest plot for the risk of bronchitis occurred in patients on the multiple-dose regimens of inclisiran.
Fig. 11.
- Forest plots for the incidence of pre-specified exploratory CV endpoint occurred in patients on the multiple-dose regimens of inclisiran.
Visually, the funnel plot of standard error by log odds ratio was slightly asymmetric only for the risk of injection site reactions (i.e., any reaction) (Fig. S12). This asymmetry was imputed to 3 potentially missing studies on the right-side of the funnel plot, which increased the estimated effect size to 10.28 (95%CI: 6.59, 16.02) (Fig. S12). However, Egger's linear regression and Begg's rank correlation did not confirm the presence of any publication bias in the safety meta-analysis (P > 0.05 always).
The incidence of the other AEs did not differ between groups (Table S3).
4. Discussion
Pooling data from the available RCTs, this meta-analysis suggests that the multiple-dose regimen of inclisiran significantly reduces serum levels of PCSK9 (−78.2%) and LDL-C (−45.5%), remarkably increasing the 17-month success rate for LDL-C goal achievement. One of the most widely recognized predictors of failure to achieve risk-stratified LDL-C goals is poor long-term therapeutic adherence [34]. An attractive advantage of inclisiran treatment is the number of administrations compared to mAbs (twice a year versus 12–26 injections per year) [35]. This twice/year dosing regimen for Inclisiran might contribute to higher adherence rates and provide better long-term reductions of acute cardiovascular events [36] contributing to the CV residual risk attributable to poor therapeutic adherence. Of course, the advantage could be attenuated by the need to administrate Inclisiran during a clinic visit, and the compliance to treatment could be not impressively higher than the one with PCSK9is, that is already acceptable.
A recently released report showed that the use of inclisiran is not cost effective for statin-treated patients with CVD, assuming acquisition costs of current PCSK9 inhibitors [37]. However, that cost-effectiveness analysis referred to the Australian healthcare system, and it is not easily decontextualized from that setting. Furthermore, the effect of ezetimibe was not considered. More importantly, the model referred to an elderly population (mean aged 66 years), but younger patients might experience greater benefit from treatment with Inclisiran. Cost-effectiveness of treatment with inclisiran in other clinical settings (e.g., in individuals with high polygenic risk for CVD, or high risk patients with heterozygous familial hypercholesterolemia or established ASCVD) remains to be assessed based on outcomes trials assessing MACE reduction with inclisiran use.
Clinical and genetic factors for predicting treatment response should be one objective of future investigations, in order to identify the patient group that might benefit from treatment with inclisiran the most. For example, inclisiran might be useful to reduce the risk of ASCVD events in individuals with chronic kidney disease (CKD), since it does not require dose adjustment and has no known drug interactions [38]. Moreover, adults with severe heterozygous familial hypercholesterolemia could be other candidates for the treatment.
According to our results, inclisiran exerts consistent, favourable effects on several lipid/lipoprotein parameters, including TC, LDL-C, HDL-C, non-HDL-C, apoB and Lp(a). However, there is little or no evidence with respect to the impact of inclisiran treatment on triglycerides, apolipoprotein A, very low-density lipoprotein cholesterol and high-sensitivity C-reactive protein (hsCRP): more data are needed to correctly quantify the effect of Inclisiran on these parameters. Our findings substantially strengthen and deepen those previously reported by Wang et al., which however only included 583 patients, in some cases treated with intravenous infusion of the drug [39]. The current study brings significant changes, also with respect to the meta-analysis by Khan et al. that focused on a smaller number of outcomes and considered only phase 3 RCTs [40]. Moreover, Khan et al. did not perform a PRISMA compliant systematic review and meta-analysis since the study's protocol was not deposited in any international registry (e.g. PROSPERO) [41]. Unfortunately, in our study we could not include findings from phase 3 RCTs when they were not adequately reported in the primary literature. Therefore, the sample size for some analyses was reduced. However, the methodology we have applied is more rigorous than the previous analyses.
Even though the favourable effect on cumulative CV outcomes was already investigated by Asbeutah and colleagues in a low-quality study [42], our data confirm a lower incidence of the pre-specified CV endpoint, even though the treatment was not shown to modify the occurrence of angina pectoris, myocardial infarction, and stroke. The absolute and relative risk reduction in CV events with inclisiran is challenging to estimate based on the available short-term data. There is a linear association between LDL-C reduction and decrease in CV events, as reported originally by the Cholesterol Treatment Trialists meta-analyses of the statin trials where a 1 mmol/L LDL-C reduction was associated with a 21–23% relative risk reduction in CV events over a mean 5-year follow-up period [41]. Robust and growing evidence highlights that this linear association is observed regardless of the LDL-C lowering approach adopted, i.e., lipid lowering diet, ezetimibe, bile acid binding resins, PCSK9 inhibitors, etc. [43]. It is therefore plausible to expect a similar 21–23% CV event reduction per 1 mmol/L of LDL-C reduction in patients treated with inclisiran. However, the longer-term impact of Inclisiran therapy on CV outcomes is being prospectively evaluated in the HPS-4/TIMI 65/ORION-4 trial (https://clinicaltrials.gov/ct2 /show/NCT03705234), whose primary completion date is expected for December 2024.
Our meta-analysis confirms that Inclisiran is overall well tolerated and safe, even if associated with a slightly increased risk of injection-site reactions and bronchitis. In particular, the increased incidence of bronchitis during the treatment (with an observed relative risk of 1.55 but an absolute risk of 0.04) is a first observation that will require further investigation and monitoring. This finding is consistent with similar observations with the PCSK9 monoclonal antibodies, whose use is associated with increased risk for upper respiratory tract infection and influenza like reaction, likely attributable to mast cell degranulation in the airway. However, a recent publication has clearly highlighted that inclisiran did not exert any significant effect on immune cells (including leucocytes, monocytes, and neutrophils) and inflammatory biomarkers such as interleukin 6 (IL-6) and tumor necrosis factor alpha (TNF-α) [44].
Our meta-analysis has some limitations, such as the high degree of heterogeneity observed in the analysis for LDL-C and PCSK9 changes that could be due to the different drug regimens tested across the studies. Moreover, this was not a patient level meta-analysis. Another limitation regards the relatively small number of subjects involved in some RCTs (especially in the single-dose RCTs) and, in particular, the small number of patients randomized to placebo. Indeed, treatment arms testing 25 mg and 800 mg inclisiran in the context of the study by Fitzgerald et al. [36] were excluded from the current analysis, as the relative control arm was not large enough for appropriate comparison. However, it is reasonable to assume that this should not have affected the observed degree of heterogeneity. Finally, we could not include findings from phase 3 RCTs (e.g., ORION-10 and ORION-11) when they were not adequately reported in the primary literature. However, in these studies the effect sizes for the intervention had the same direction as reported herein. In the ORION-10 trial, the percentage change in LDL-C at day 510 was 1.0% in the placebo group and −51.3% in the inclisiran group, resulting in a between-group difference of −52.3%. In the ORION-11 trial, the percentage change in LDL-C at day 510 was 4.0% in the placebo group and −45.8% in the inclisiran group, resulting in a between-group difference of −49.9%. In the ORION-10 trial, the percentage change in PCKS9 at day 510 (key secondary endpoint) was 13.5% with placebo and −69.8% with inclisiran, representing a between group difference of −83.3%. Similarly, in the ORION-11 trial, the percentage change at day 510 was 15.6% with placebo and −63.6% with inclisiran, representing a between-group difference of −79.3%. The accuracy of our results should not be compromised even though the sample size has been reduced by the stringent and rigorous inclusion and exclusion criteria of the meta-analysis.
The maximal tolerated dose of statins used in conjunction with ezetimibe and bempedoic acid [45] would facilitate LDL-C target achievement in a relatively large number of high-risk patients. Inclisiran could be an interesting option for high-risk statin-intolerant patients and patients at very high CV risk requiring the largest LDL-C reduction, especially if beneficial effects on hard outcomes are confirmed.
5. Conclusion
In conclusion, the current meta-analysis demonstrates the multiple positive effects of inclisiran on lipid/lipoprotein serum concentrations, a trend towards CV events reduction, and an acceptable safety profile. Adequately powered longer-term CV outcomes trials such as ORION-4 will address the question of whether inclisiran reduces CV events and mortality in addition to background statin therapy and further address long-term safety.
The following are the supplementary data related to this article.
Supplementary material
PRISMA Checklist
Studies excluded from the meta-analysis after assessment.
Conflict of interest statement
Claudio Borghi has served as a consultant to Menarini and Servier; Arrigo F.G. Cicero has served as a consultant to Mylan and Menarini; Federica Fogacci has served as a consultant to Mylan and Roelmi HPC; Peter P. Toth has served on the speakers bureau of Amarin, Amgen, Kowa, Merck, Novo-Nordisk, Regeneron, Sanofi, and has served as a consultant to Amarin, Amgen, AstraZeneca, Kowa, Merck, Nov-Nordisk, and Theravance; Alberto Zambon has served on the speakers bureau of Abbott, Mylan, Amarin, Amgen, Merck, Alfasigma, Lilly, Servier, Sanofi, Chiesi, and has served as a consultant to Amarin, AKCEA, Mylan, Novartis.
Funding sources
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Ference B.A., Ginsberg H.N., Graham I., et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2017;38:2459–2472. doi: 10.1093/eurheartj/ehx144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.ESC Committee for Practice Guidelines (CPG) ESC National Cardiac Societies 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Atherosclerosis. 2019;290:140–205. doi: 10.1016/j.atherosclerosis.2019.08.014. [DOI] [PubMed] [Google Scholar]
- 3.Grundy S.M., Stone N.J., Bailey A.L., et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the Management of blood cho-lesterol: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. J. Am. Coll. Cardiol. 2019;73:e285–e350. doi: 10.1016/j.jacc.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 4.Ferrières J., Roubille F., Farnier M., et al. Control of low-density lipoprotein cholesterol in secondary prevention of coronary artery disease in real-life practice: the DAUSSET study in French cardiologists. J. Clin. Med. 2021;10:5938. doi: 10.3390/jcm10245938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reiner Ž. Statins in the primary prevention of cardiovascular disease. Nat. Rev. Cardiol. 2013;10:453–464. doi: 10.1038/nrcardio.2013.80. [DOI] [PubMed] [Google Scholar]
- 6.Cannon C.P., Blazing M.A., Giugliano R.P., et al. IMPROVE-IT investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N. Engl. J. Med. 2015;372:2387–2397. doi: 10.1056/NEJMoa1410489. [DOI] [PubMed] [Google Scholar]
- 7.Murphy S.A., Cannon C.P., Blazing M.A., et al. Reduction in total cardiovascular events with ezetimibe/simvastatin post-acute coronary syndrome: the IMPROVE-IT trial. J. Am. Coll. Cardiol. 2016;67:353–361. doi: 10.1016/j.jacc.2015.10.077. [DOI] [PubMed] [Google Scholar]
- 8.Bove M., Fogacci F., Cicero A.F.G. Pharmacokinetic drug evaluation of ezetimibe + simvastatin for the treatment of hyper-cholesterolemia. Expert Opin. Drug Metab. Toxicol. 2017;10:1099–1104. doi: 10.1080/17425255.2017.1381085. [DOI] [PubMed] [Google Scholar]
- 9.Strilchuk L., Tocci G., Fogacci F., Cicero A.F.G. An overview of rosuvastatin/ezetimibe association for the treatment of hypercholesterolemia and mixed dyslipidemia. Expert. Opin. Pharmacother. 2020;21:531–539. doi: 10.1080/14656566.2020.1714028. [DOI] [PubMed] [Google Scholar]
- 10.Norata G.D., Tavori H., Pirillo A., Fazio S., Catapano A.L. Biology of proprotein convertase subtilisin kexin 9: beyond low-density lipoprotein cholesterol lowering. Cardiovasc. Res. 2016;112:429–442. doi: 10.1093/cvr/cvw194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giugliano R.P., Sabatine M.S. Are PCSK9 inhibitors the next breakthrough in the cardiovascular field? J. Am. Coll. Cardiol. 2015;65:2638–2651. doi: 10.1016/j.jacc.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 12.Dicembrini I., Giannini S., Ragghianti B., Mannucci E., Monami M. Effects of PCSK9 inhibitors on LDL cholesterol, cardi-ovascular morbidity and all-cause mortality: a systematic review and meta-analysis of randomized controlled trials. J. Endocrinol. Investig. 2019;42:1029–1039. doi: 10.1007/s40618-019-01019-4. [DOI] [PubMed] [Google Scholar]
- 13.Cicero A.F., Tartagni E., Ertek S. Safety and tolerability of injectable lipid-lowering drugs: a review of available clinical data. Expert Opin. Drug Saf. 2014;13:1023–1030. doi: 10.1517/14740338.2014.932348. [DOI] [PubMed] [Google Scholar]
- 14.Pucci G., Cicero A.F., Borghi C., Schillaci G. Emerging biologic therapies for hypercholesterolaemia. Expert. Opin. Biol. Ther. 2017;17:1077–1087. doi: 10.1080/14712598.2017.1341485. [DOI] [PubMed] [Google Scholar]
- 15.Al Turki A., Marafi M., Dawas A., et al. Meta-analysis of randomized controlled trials assessing the impact of proprotein convertase subtilisin/kexin type 9 antibodies on mortality and cardiovascular outcomes. Am. J. Cardiol. 2019;124:1869–1875. doi: 10.1016/j.amjcard.2019.09.011. [DOI] [PubMed] [Google Scholar]
- 16.Catapano A.L., Pirillo A., Norata G.D. New pharmacological approaches to target PCSK9. Curr. Atheroscler. Rep. 2020;22:24. doi: 10.1007/s11883-020-00847-7. [DOI] [PubMed] [Google Scholar]
- 17.Strilchuk L., Fogacci F., Cicero A.F. Safety and tolerability of injectable lipid-lowering drugs: an update of clinical data. Expert Opin. Drug Saf. 2019;18:611–621. doi: 10.1080/14740338.2019.1620730. [DOI] [PubMed] [Google Scholar]
- 18.Bove M., Cicero A.F.G., Borghi C. Emerging drugs for the treatment of hypercholesterolemia. Expert Opin. Emerg. Drugs. 2019;24:63–69. doi: 10.1080/14728214.2019.1591372. [DOI] [PubMed] [Google Scholar]
- 19.Aguilar-Salinas C.A., Gómez-Díaz R.A., Corral P. New therapies for primary hyperlipidaemia. J. Clin. Endocrinol. Metab. 2021 Dec 9 doi: 10.1210/clinem/dgab876. [DOI] [PubMed] [Google Scholar]
- 20.Page M.J., McKenzie J.E., Bossuyt P.M., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. J. Clin. Epidemiol. 2021 Jun;134:178–189. doi: 10.1016/j.jclinepi.2021.03.001. [DOI] [PubMed] [Google Scholar]
- 21.Higgins J., Green S. John Wiley and Sons Ltd.; Chichester, UK: 2010. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.0. 2. 2009. Ref Type: Report. [Google Scholar]
- 22.Fogacci F., Ferri N., Toth P.P., Ruscica M., Corsini A., Cicero A.F.G. Efficacy and safety of mipomersen: a systematic review and meta-analysis of randomized clinical trials. Drugs. 2019;79:751–766. doi: 10.1007/s40265-019-01114-z. [DOI] [PubMed] [Google Scholar]
- 23.Borenstein M., Hedges L., Higgins J., Rothstein H. Comprehensive Meta-analysis Version 3. Biostat; Englewood, NJ: 2005. p. 104. [Google Scholar]
- 24.Follmann D., Elliott P., Suh I., Cutler J. Variance imputation for overviews of clinical trials with continuous response. J. Clin. Epidemiol. 1992;45:769–773. doi: 10.1016/0895-4356(92)90054-q. [DOI] [PubMed] [Google Scholar]
- 25.Wan X., Wang W., Liu J., Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014;14:135. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Melsen W.G., Bootsma M.C., Rovers M.M., Bonten M.J. The effects of clinical and statistical heterogeneity on the predictive values of results from meta-analyses. Clin. Microbiol. Infect. 2014;20:123–129. doi: 10.1111/1469-0691.12494. [DOI] [PubMed] [Google Scholar]
- 27.Haenszel W., Hon N.B. Statistical approaches to the study of cancer with particular reference to case registers. J. Chronic Dis. 1956;4:589–599. doi: 10.1016/0021-9681(56)90049-2. [DOI] [PubMed] [Google Scholar]
- 28.Fogacci S., Fogacci F., Banach M., et al. Lipid and Blood Pressure Meta-analysis Collaboration (LBPMC) Group Lipid and Blood Pressure Meta-analysis Collaboration (LBPMC) Group. Vitamin D supplementation and incident preeclampsia: a systematic review and meta-analysis of randomized clinical trials. Clin. Nutr. 2020;39(6):1742–1752. doi: 10.1016/j.clnu.2019.08.015. [DOI] [PubMed] [Google Scholar]
- 29.Fogacci F., Banach M., Mikhailidis D.P., et al. Lipid and Blood Pressure Meta-analysis Collaboration (LBPMC) Group; International Lipid Expert Panel (ILEP). Safety of red yeast rice supplementation: a systematic review and me-ta-analysis of randomized controlled trials. Pharmacol. Res. 2019;143:1–16. doi: 10.1016/j.phrs.2019.02.028. [DOI] [PubMed] [Google Scholar]
- 30.Raal F.J., Kallend D., Ray K.K., et al. ORION-9 Investigators. Inclisiran for the treatment of heterozygous familial hypercholesterolemia. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa1913805. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 31.Ray K.K., Wright R.S., Kallend D., et al. ORION-10 and ORION-11 Investigators. Two phase 3 trials of inclisiran in patients with elevated LDL cholesterol. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa1912387. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 32.Fitzgerald K., White S., Borodovsky A., et al. A highly durable RNAi therapeutic inhibitor of PCSK9. N. Engl. J. Med. 2017;376:41–51. doi: 10.1056/NEJMoa1609243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ray K.K., Landmesser U., Leiter L.A., et al. Inclisiran in patients at high cardiovascular risk with elevated LDL cholesterol. N. Engl. J. Med. 2017;376:1430–1440. doi: 10.1056/NEJMoa1615758. [DOI] [PubMed] [Google Scholar]
- 34.Korneva V., Kuznetsova T., Julius U. Efficiency and problems of statin therapy in patients with heterozygous familial hypercholesterolemia. Atheroscler. Suppl. 2019;40:79–87. doi: 10.1016/j.atherosclerosissup.2019.08.029. [DOI] [PubMed] [Google Scholar]
- 35.Kosmas C.E., Muñoz Estrella A., Sourlas A., et al. Inclisiran: a new promising agent in the management of hypercholesterolemia. Diseases. 2018;6:63. doi: 10.3390/diseases6030063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cupido A.J., Kastelein J.J.P. Inclisiran for the treatment of hypercholesterolaemia: implications and unanswered questions. Cardiovasc. Res. 2020;116(11):e136–e139. doi: 10.1093/cvr/cvaa212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kam N., Perera K., Zomer E., Liew D., Ademi Z. Inclisiran as adjunct lipid-lowering therapy for patients with cardiovascular disease: a cost-effectiveness analysis. PharmacoEconomics. 2020;38:1007–1020. doi: 10.1007/s40273-020-00948-w. [DOI] [PubMed] [Google Scholar]
- 38.Goonasekera M.A., Mafham M.M., Haynes R.J. LDL-cholesterol reduction in chronic kidney disease: options beyond statins. Curr. Opin. Nephrol. Hypertens. 2020;29:480–488. doi: 10.1097/MNH.0000000000000628. [DOI] [PubMed] [Google Scholar]
- 39.Wang Y., Wang J., Wang S. Comparative effectiveness of inclisiran 100, 300, and 500 mg in a population with hyperlipidemia: a network meta-analysis of randomized controlled trials. Am. J. Cardiovasc. Drugs. 2018;18:271–282. doi: 10.1007/s40256-018-0270-7. [DOI] [PubMed] [Google Scholar]
- 40.Khan S.A., Naz A., Qamar Masood M., Shah R. Meta-analysis of inclisiran for the treatment of hypercholesterolemia. Am. J. Cardiol. 2020;134:69–73. doi: 10.1016/j.amjcard.2020.08.018. [DOI] [PubMed] [Google Scholar]
- 41.Baigent C., Keech A., Kearney P.M., et al. Cholesterol Treatment Trialists' (CTT) collaborators. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267–1278. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- 42.Asbeutah A.A.A., Asbeutah S.A., Abu-Assi M.A. A meta-analysis of cardiovascular outcomes in patients with hypercholesterolemia treated with inclisiran. Am. J. Cardiol. 2020;128:218–219. doi: 10.1016/j.amjcard.2020.05.024. [DOI] [PubMed] [Google Scholar]
- 43.Silverman M.G., Ference B.A., Im K., et al. Association between lowering LDL-C and cardiovascular risk reduction among different therapeutic interventions: a systematic review and meta-analysis. JAMA. 2016;316:1289–1297. doi: 10.1001/jama.2016.13985. [DOI] [PubMed] [Google Scholar]
- 44.Landmesser U., Haghikia A., Leiter L.A., et al. Effect of inclisiran, the small-interfering RNA against proprotein convertase subtilisin/kexin type 9, on platelets, immune cells, and immunological biomarkers: a pre-specified analysis from ORION-1. Cardiovasc. Res. 2021;117:284–291. doi: 10.1093/cvr/cvaa077. [DOI] [PubMed] [Google Scholar]
- 45.Cicero A.F.G., Fogacci F., Hernandez A.V., Banach M. Lipid and Blood Pressure Meta-analysis Collaboration (LBPMC) Group and the International Lipid Expert Panel (ILEP). Efficacy and safety of bempedoic acid for the treatment of hypercholesterolemia: a systematic review and meta-analysis. PLoS Med. 2020;17 doi: 10.1371/journal.pmed.1003121. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
PRISMA Checklist
Studies excluded from the meta-analysis after assessment.