Abstract
The blink reflex (BR) is a protective eye-closure reflex mediated by brainstem circuits. The BR is usually evoked by electrical supraorbital nerve stimulation but can be elicited by a variety of sensory modalities. It has a long history in clinical neurophysiology practice. Less is known, however, about the many ways to modulate the BR. Various neurophysiological techniques can be applied to examine different aspects of afferent and efferent BR modulation. In this line, classical conditioning, prepulse and paired-pulse stimulation, and BR elicitation by self-stimulation may serve to investigate various aspects of brainstem connectivity. The BR may be used as a tool to quantify top-down modulation based on implicit assessment of the value of blinking in a given situation, e.g., depending on changes in stimulus location and probability of occurrence. Understanding the role of non-nociceptive and nociceptive fibers in eliciting a BR is important to get insight into the underlying neural circuitry. Finally, the use of BRs and other brainstem reflexes under general anesthesia may help to advance our knowledge of the brainstem in areas not amenable in awake intact humans. This review summarizes talks held by the Brainstem Special Interest Group of the International Federation of Clinical Neurophysiology at the International Congress of Clinical Neurophysiology 2022 in Geneva, Switzerland, and provides a state-of-the-art overview of the physiology of BR modulation. Understanding the principles of BR modulation is fundamental for a valid and thoughtful clinical application (reviewed in part 2) (Gunduz et al., submitted).
Keywords: Classical conditioning, Prepulse inhibition, Peripersonal space, Pain, Anesthesia, Protective reflex
1. Introduction
The blink reflex (BR) is a protective eye-closure reflex mediated by brainstem circuits and triggered by fast-rising and intense stimuli from a variety of sensory modalities. Figure 1 summarizes the various ways to elicit the BR in clinical practice and research. The most conventional way of BR elicitation is the application of electrical stimuli to the supraorbital nerve (SON), a terminal branch of the first division of the trigeminal nerve, typically in the forehead. Most commonly, stimulus intensity is about 10 times sensory threshold, and the interstimulus interval (ISI) between two consecutive single stimuli should not be less than 10 seconds to avoid habituation. Sensory afferents of the SON project to the principal sensory nucleus (PSN) and the spinal trigeminal nucleus (STN) in the brainstem. From there, fibers take an oligosynaptic route to the ipsilateral facial nucleus through the pons, and a polysynaptic route to both ipsi- and contralateral facial nuclei via the pontomedullary reticular formation (Fig.1). BRs consist of orbicularis oculi muscle (OOc) contraction and levator palpebrae muscle relaxation to allow for lowering of the upper eyelid (Esteban, 1999; Aramideh and Ongerboer de Visser, 2002). However, response analysis is usually limited to the electromyographic (EMG) activity picked up with surface electrodes from the OOc. There are many ways to elicit and to modulate a BR, and each has its history. This two-part review aims to provide an up-to-date summary of the various approaches to elicit and modulate the BR in health and disease.
Figure 1.
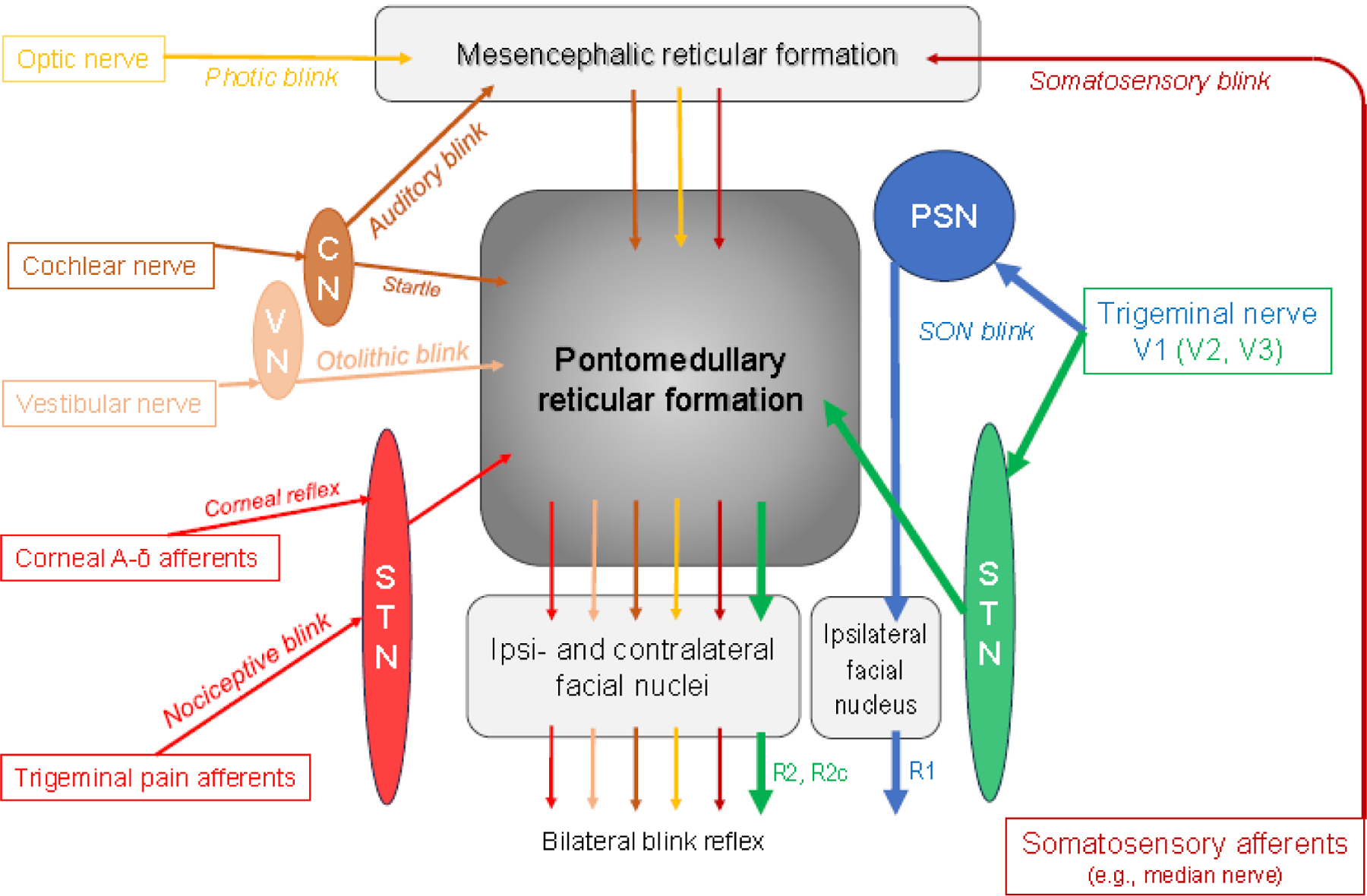
Simplified scheme of the blink reflex circuitry with the pontomedullary reticular formation as the central brainstem structure mediating all kinds of blink reflexes in response to various afferent modalities. CN = cochlear nuclei; PSN = principal sensory nucleus of the trigeminal nerve; STN = spinal trigeminal nucleus of the trigeminal nerve; VN = vestibular nuclei. Note that the early R1 response following supraorbital nerve (SON) stimulation is mediated via the PSN, connecting only with the ipsilateral facial nucleus, whereas the late responses are conveyed via the ipsilateral STN to the pontomedullary reticular formation from where ipsi- (R2) and contralateral (R2c) responses are generated. Blink reflexes evoked by other sensory modalities lack the ipsilateral R1 component. The reticular formation is a complex neuronal network containing numerous diffuse and highly organized regions (Crossman, 2005; Brodal, 2004c). Hence, only the main pathways are shown. Various afferents comprise the trigeminal nerve, in particular the SON (see section 2, Hopf, 1994; Pellegrini et al., 1995; Berardelli et al., 1999; Esteban, 1999; Aramideh and Ongerboer de Visser, 2002; Cruccu et al., 2005; Valls-Solé, 2012, 2019; Kimura, 2013), but also other branches of the trigeminal nerve (Kugelberg, 1952; Oka et al., 1958; Gandiglio and Fra, 1967; Kimura, 1973; Hess et al., 1984; Jääskeläinen, 1995; Valls-Solé et al., 1996; Pavesi et al., 1996). Extratrigeminal sensory afferents have been demonstrated by Gandiglio and Fra (1967), Miwa et al. (1995, 1996, 1998), and Alvarez-Blanco et al. (2009). Visual input is relayed via retinotectal and tectoreticular fibers (Crossman, 2005). Cochlear and vestibular afferents are described in Brodal’s chapters 8 and 9, respectively (Brodal, 2004a, 2004b) and in Gray’s Anatomy (Crossman, 2005). The pathway of the auditory blink reflex is depicted according to Hori et al. (1986), not showing the involvement of the colliculus inferior. The majority of trigeminal pain fibers, but not all, are transmitted through the STN (see section 7 for details).
2. History of the blink reflex and its modulation (Markus Kofler)
2.1. Initial studies applying mechanical stimuli
The first description of the BR dates back to 1896, when Walker Overend’s observations were published in the Lancet (Overend, 1896). He noted: “When the skin of one-half of the forehead is gently tapped with the edge of an ordinary wooden stethoscope a twitch in the lower eyelid of the same side may be observed… If the percussion be made a little stronger the upper portion of the orbicularis also takes part in the response, while severe percussion elicits in addition a simultaneous movement of the opposite lids.” He was the first to describe what we now call the glabella reflex: “Slight tapping in the middle line of the forehead is followed by twitchings on both sides. In many instances, however, and particularly after some amount of education or when the skin of the forehead is abnormally sensitive, gentle stroking alone is sufficient to evoke the reaction.” He observed conditions with increased reflex excitability, suspected “a true skin reflex”, and noted: “The motor path is identical with that of the conjunctival reflex; the sensory channels lie in the supratrochlear and supra-orbital divisions of the frontal nerve, while the centre is probably located in the mid-brain.” He even described absence of the response in “hemianaesthesia” but not in “hemiplegia”. All these observations are still valid to date.
In subsequent years, facial reflexes were clinically described under many different names according to the area tapped, the muscles responding, and the mechanism considered to be responsible. The BR was independently “rediscovered” and reported (McCarthy, 1901, 1902b; Hudovernig, 1901, Editorial 1901; Bechterew, 1901, 1902; Overend, 1902; Weisenburg, 1903; Zeri, 1906), as later reviewed by others (Fine et al., 1992; Pearce, 2008). While first postulating a “true skin reflex” (Overend, 1896), Overend later suggested additional contributions from “periosteal terminal twigs … of all the branches of the ophthalmic nerve” (Overend, 1902), in line with an earlier proposal by Bechterew (1901, 1902). McCarthy (1901) suggested a “pure nerve reflex” identical to tendon reflexes but noted later that warm and cold stimuli applied to the skin in the distribution of the SON were also capable of eliciting the reflex, thus refuting a periosteal reflex generation (McCarthy, 1902a). Other suggestions included “an overflow of the muscular irritability to mechanical irritation into neighbouring muscles innervated by the same nerve” (Hudovernig, 1901), a “defense reflex” neither cutaneous nor periosteal (Kornilow, 1903), a bone reflex (Lewandowsky, 1910), skin and periosteal reflex (Guillain, 1920), perichondreal reflex (Simchowicz, 1922), and finally myotatic or muscle stretch reflex (Weingrow, 1933). It has long been known, though, that facial muscles have no stretch reflexes (Sternberg, 1893; Sommer, 1938), as typical muscle spindles are lacking in human facial muscles (Kadanoff, 1956).
Based on the site stimulated, several reflexes were described in these years: auriculopalpebral (Kisch, 1919) cephalon-palpebral (Galant, 1926), laryngo-palpebral (Gallenga, 1930), palatal palpebral (Imperatori, 1930), and zygomatic-palpebral reflex (Galant, 1932). Finally, Wartenberg (1944) proposed the term “orbicularis oculi reflex” to summarize and replace the long and confusing list of these facial reflexes.
2.2. The blink reflex to electrical trigeminal nerve stimulation
The Swedish neurologist Eric Kugelberg (1952) was the first to record “an electrical discharge coming in two groups” recorded from the OOc. He described the first response as “a well-synchronized volley with a latency of about 12 ms… unilateral… through a simple arc… compatible with a myotatic reflex” and the second response as “long-lasting asynchronous discharge with a variable latency, roughly 21–40 ms… bilateral… reflex arc is multisynaptic… at least some part passes over the spinal tract of the trigeminal nerve… adequate stimuli are pain and probably touch” [for the second but not the first component]. He was also the first to perform intraoperative electrical stimulation of the trigeminal nerve root in a patient with trigeminal neuralgia during trigeminal rhizotomy, confirming Overend’s observations of an absent BR in case of anesthesia (Overend, 1896), here due to a trigeminal nerve lesion. Subsequently, Rushworth (1962), who first noted a possible association with the reticular formation, Bender (1968) and Gandiglio and Fra (1967) largely confirmed Kugelberg’s findings.
Rushworth (1962) compared BRs following mechanical stimulation with those to electrical, corneal (touch), auditory, and photic stimuli, noting many similarities but also some distinct differences in health and disease. As previously suggested (Kornilow, 1903; Böhme, 1927), the nociceptive and protective nature of the BR elicited by various stimulus modalities was noted by several authors (Wartenberg, 1944; Kugelberg, 1952; Gandiglio and Fra, 1967; Shahani, 1970). Shahani and Young (1968) noted similarities of the two-component BR to flexor reflexes in the tibialis anterior muscle following electrical stimulation of the foot sole. They suggested that both BR components would be of cutaneous rather than proprioceptive origin and – based on similar changes at different stages of anesthetic block – be elicited by the same medium sized cutaneous fibers (Shahani, 1970). In this line, patients with Friedreich ataxia (who lack large-diameter afferents) had a preserved BR in the absence of soleus H reflexes (Shahani, 1970). Shahani and Young (1972) noted that “the second component of this reflex has been shown to correlate with closure of the eyelids. The significance of the first component remains to be elucidated”, a statement still valid to date. The terminology “R1” and “R2” for the early and late components appeared first in 1972 (Penders and Delwaide, 1972). The underlying BR pathways and their (patho-)physiology and clinical utility have since been elaborated in animals and humans by several research groups, e.g., led by Evinger, Kimura, Ongerboer de Visser, Esteban, Berardelli, Cruccu, Hopf, and Valls-Solé (reviewed in Hopf, 1994; Pellegrini et al., 1995; Berardelli et al., 1999; Esteban, 1999; Aramideh and Ongerboer de Visser, 2002; Cruccu et al., 2005; Valls-Solé, 2012, 2019; Kimura, 2013). Briefly, R1 is a pontine reflex with a latency of about 10–12 ms, and R2 is a pontomedullary reflex appearing at a latency of some 29–37 ms (Kofler et al., 2013). At low stimulus intensities, R2 may occur substantially later, in our experience up to 50–60 ms. R1 and R2 are mediated by Aβ afferents, but R2 responses can also be triggered by thermal and nociceptive stimuli (Romaniello et al., 2002), suggesting that they are mediated by wide-dynamic-range (WDR) neurons of the STN (Pellegrini et al., 1995; Ellrich and Treede, 1998) (see section 7).
“Sporadic third responses”, possibly an early account on the R3 component of the BR, were first reported by Gandiglio and Fra (1967). The first description of R3 was published in 1972 (Penders and Delwaide, 1972). It was long considered to be a nociceptive reflex component (Rossi et al., 1989; D’Aleo et al., 1999; 2000); however, Ellrich and Hopf (1996) were the first to suggest that R3 might actually be a startle response, as the response disappears after announcing stimulation (Rossi et al., 1993; Ellrich and Hopf, 1996) and emerges at stimulus intensities clearly below pain threshold (Ellrich et al., 2001). Meincke et al. (1999) found increased excitability of the R3 component in patients with schizophrenia but attributed this to attentional deficits rather than a reduced pain threshold. Later, Tellez et al. (2009) reported the preservation of R3 in two patients with congenital indifference to pain. Others confirmed the presence of R3 responses following non-nociceptive but startling stimuli (Kofler et al., 2013; Versace et al., 2020). At present, the R3 is considered a sporadic response, appearing at an approximate latency of 60 to 90 ms and not always clearly separated from the R2,
Electrical stimulation of nerves other than the SON may also elicit a BR, e.g., the infraorbital nerve, although less consistently, as already shown by Kugelberg (1952) and later confirmed by others (Oka et al., 1958; Gandiglio and Fra, 1967; Kimura, 1973; Hess et al., 1984; Valls-Solé et al., 1996) (Fig.1). Gandiglio and Fra (1967) noted that similar two-component responses could be obtained from OOc bilaterally following chin-tapping and electrical stimulation of mental and upper limb nerves (median and ulnar nerves at elbow and wrist), thus refuting the earlier postulated myotatic nature of the first reflex component. Lingual nerve stimulation also elicits an R2-like response in OOc (Pavesi et al., 1996).
2.3. Corneal blink reflexes
As a protective reflex, the corneal reflex resembles to some degree the BR obtained to electrical SON stimuli. The first studies applied completion of an electrical circuit triggering an oscilloscope by means of a knobbed probe or air puff (Kugelberg, 1952), a fine camel’s hair brush moistened in isotonic saline (Magladery and Teasdall, 1961) or a small loop of stainless steel wire (Rushworth, 1962). Other techniques employed saline jet (Thatcher and Van Allen, 1971), weak contact with a small metal ball (Ongerboer de Visser et al., 1977), electrical stimulation through a saline-soaked cotton thread (Accornero et al., 1980; Berardelli et al., 1983; 1985a), or mechanical stimulation by applying an air or water jet from an electrically operated pump (Accornero et al., 1978).
The corneal reflex shows some characteristic differences from the BR to electrical SON stimulation. Foremost, it lacks R1 (Kugelberg, 1952; Magladery and Teasdall, 1961; Rushworth, 1962; Thatcher and Van Allen, 1971). The bilateral responses have longer latencies than R2 with large inter- but little intra-individual variability (Ongerboer de Visser et al., 1977; Berardelli et al., 1983). Unlike for electrical SON stimulation, there is formal evidence of response amplitude growth with increasing intensity for air puff stimulation (Flaten and Blumenthal, 1998). The corneal reflex shows less suppression with paired stimuli (Cruccu et al., 1986) and less habituation (Magladery and Teasdall, 1961; Ongerboer de Visser et al., 1977; Cruccu et al., 1986). It is mediated by Aδ fibers in the ciliary branch of the ophthalmic nerve (Fig.1).
2.4. Auditory stimulation and the blink reflex
Blinking on auditory stimulation is closely related to the startle reaction. Early reports of the ‘cochleo-facial, cochleopalpebral, auriculo-palpebral, auropalpebral or acoustico-palpebral reflex’ (Bechterew, 1896; Stoerk, 1921; Carrari, 1925; Galant, 1926; Veits, 1926) were followed by detailed clinical description and cinematographic analysis of the auditory startle reaction following a pistol shot (Strauss, 1929; Landis and Hunt, 1936).
BRs to clicks had shorter latencies compared to glabella taps (Rushworth, 1962) or photic stimuli (Yates and Brown, 1981; Tackmann et al., 1982) and were less consistent than those to electrical SON stimulation (Rushworth, 1962; Bender, 1968) or photic stimuli (Yates and Brown, 1981). Normative values were also published (Shahani and Young, 1973; Silverstein et al., 1980).
While many authors consider the auditory BR equivalent to, or a consistent part of, the auditory startle reaction (Gogan, 1970; Fox, 1978; Esteban, 1999), others described a separate pathway for the auditory BR involving inferior colliculus and midbrain reticular formation (Buser et al., 1966; Hori et al., 1986), located more rostrally than the auditory startle circuit (Davis et al., 1982). Such a differentiation concurs with differences in the rate of habituation of the two reflexes (Brown et al., 1991b), differential susceptibility to prepulses (Meincke et al., 2002), separation of the two reflexes in case of delayed startles (Brown et al., 1991a), or preservation of the auditory BR in patients with otherwise absent startle reaction (Vidailhet et al., 1992; Kofler et al., 2006) (Fig.1).
2.5. Peripheral nerve stimulation: somatosensory blink reflex
Gandiglio and Fra (1967) provided the first account of BRs elicited by extracephalic nerve stimulation (median and ulnar nerves at elbow and wrist). Miwa et al. (1995, 1996, 1998) elicited EMG responses in OOc following stimulation of the median nerve at the wrist or index finger, which could not be recorded from orbicularis oris, sternocleidomastoid, posterior neck, and pectoralis major muscles. As such, these responses can be regarded a somatosensory BR rather than a somatosensory startle reflex (Fig.1). Stimulation at the ankle (posterior tibial or sural nerve) failed to elicit BRs (Miwa et al., 1995). Alvarez-Blanco et al. (2009) obtained somatosensory blink and startle responses following median nerve stimulation, and only somatosensory startle responses following tibial nerve stimulation, concurring with a higher likelihood to elicit protective BRs with stimuli closer to the face (Sambo et al., 2012b). Analogous to the differentiation of auditory blink from auditory startle reflexes (Brown et al., 1991b), a similar differentiation of somatosensory blink from somatosensory startle reflexes (Brown et al., 1991a) seems plausible.
Somatosensory BRs were rarely studied apart from startle reactions and thus from hyperekplexias. The group of Meral Kiziltan studied associations among median nerve somatosensory and trigeminal BRs, peripheral facial palsy, and postparalytic facial syndrome after excluding startle blinks based on response latencies (Erkol et al., 2009). The topographic utility of median nerve somatosensory versus trigeminal BRs was documented in patients with mesencephalic or medullary vascular lesions, revealing differential dysfunctions depending on lesion location (Leon et al., 2011). The group of Giandomenico Iannetti contributed several publications to the field of somatosensory BRs. They used the so-called “hand-blink reflex” (HBR) as a tool to reveal gradual reflex modulation as a function of the proximity between the stimulated hand and the face (Sambo et al., 2012b; Bufacchi and Iannetti, 2018). A detailed account of BR modulation in the peripersonal space follows in section 5, the modulation of the HBR by prepulses and by self-agency (Versace et al., 2021) is described in section 6.
2.6. The blink reflex to other sensory modalities
2.6.1. Visual / photic stimulation
Landis and Hunt were the first to report startle BRs to visual stimuli (Landis and Hunt, 1939). BR latencies to photic stimuli were longer and more variable as compared to electrical SON stimulation, ranging on average from 45 to more than 75 ms (Rushworth, 1962; Bender, 1968; Hopf et al., 1973; Lowitzsch et al., 1976; Yates and Brown, 1981; Tackmann et al., 1982), depending on the area, intensity and wavelength of the stimulus employed. The exact reflex pathway still remains uncertain, however, the cerebral cortex seems to be bypassed, as reflex blinking may remain intact in hydranencephaly (Hill et al., 1961) and occasionally in neocortical death in humans (Keane, 1979) (Fig.1).
2.6.2. Vestibular and kinematic stimulation
Sudden free fall may serve to elicit a vestibular startle response, including reflex blinking (Bisdorff et al., 1994) (Fig.1). Passive knee flexion of sufficiently high angular velocity may elicit a kinematic startle reflex, including reflex blinking amenable to modulation by prepulse stimuli or by a concomitant motor task (Castellote et al., 2017). When performing the videohead impulse test, ‘contaminating’ blink reflexes may be interfering with the analysis of eye movements (Mantokoudis et al., 2015; Pleshkov et al., 2022).
2.6.3. Pain and heat stimulation
Nociceptive stimulation may evoke protective reflexes and may initiate complex protective behavior. According to Kornilow (1903) the BR belongs to the “defense reflexes”, which was also suggested by others (Böhme, 1927; Broser et al., 1964). Later, noxious electrical stimuli (“train-of-four”) to the back of the hand were compared to auditory startle blinks, revealing a similar pattern of excitation followed by inhibition when delivering two stimuli closely separated, be it both auditory, both electrical or mixed. In contrast, spontaneous and voluntary blinks were not followed by such a period of suppression (Fox, 1978). Duranti et al. (1983) used noxious electrical stimulation through wire electrodes inserted into the vastus medialis muscle to elaborate on BR characteristics with varying repetition rates and intensities of electrical train stimuli. BRs, corresponding to the electrically evoked R2 and R3 components, were elicited by noxious laser stimuli applied to the ophthalmic nerve dermatome (Ellrich et al., 1997). The description of the involvement of WDR neurons of the STN in the generation of the R2 component of the BR (Pellegrini et al., 1995; Ellrich et al., 1998; Ellrich and Treede, 1998) led eventually to the development of a special type of concentric electrode to selectively activate Aδ afferents (Kaube et al., 2000; Katsarava et al., 2002) (Fig.1). This technique has been mostly applied in headaches (Coppola et al., 2007; Magis et al., 2007, 2013) and has been found to be generally useful and reliable (von Dincklage et al., 2010; Costa et al., 2017) (see section 7 and part 2 of this review (Gunduz et al., submitted)).
2.7. Brief history of blink reflex modulation
Obvious influences on BRs relate to stimulus location and modality (see above) (Soliven et al., 1988), electrical stimulus intensity (Kimura et al., 1969; Ellrich and Treede, 1998) or contraction of the target muscle, allowing for unmasking subliminal R1 and R2 responses (Leis et al., 1993) and the appearance of contralateral R1 responses (Willer et al., 1984; Soliven et al., 1988). Fear, anxiety, and mental tasks were reported to affect BRs (Esteban, 1999). BR did not differ significantly between males and females, but R2 and R2c latencies increased with age (Kofler et al., 2013). Attention to the blink-eliciting stimulus was reported to increase R2 (Schicatano, 2016). However, other authors reported the opposite: anticipation facilitates R1 and suppresses R2 (Ison et al., 1990), whereas distraction facilitates R2 and R3 responses (Rossi et al., 1993). Self-triggered stimulation, which combines attention, expectation, and sense of agency, also leads to R1 enhancement and R2 inhibition (see section 6). A facilitatory effect on R2 is seen when stimuli are applied closer to the eye, within the so-called peripersonal space (Sambo et al., 2012b; Versace et al., 2020), an effect that will be fully described in section 5. Sleep affects both R1 and R2 with higher thresholds in “synchronous sleep” (Ferrari and Messina, 1972) and markedly smaller and fewer responses in sleep stages II – IV, with a relative increase of excitability in REM sleep similar to stage I, but still less than during wakefulness (Kimura and Harada, 1972). A reduction of BRs was also observed with auditory (Silverstein et al., 1980) and photic (Hoshina and Sakuma, 1991) stimulation applied during sleep. Reflex elicitation during anesthesia has been used not only for intraoperative monitoring (Deletis and Fernandez-Conejero, 2016) but has also shed light on certain aspects of physiology that are impossible to study in awake humans (see section 8).
Pharmacological influences on the BR and its modulation may also aid in the localization of the drug’s site of action. E.g., serial BR recordings following intrathecal application of baclofen, an agonist of gamma-amino-butyric acid (GABA) that is used to treat severe spasticity, showed changes that paralleled the time course and degree of spasticity reduction, concurring with a brainstem site of action (Kumru et al., 2011; Kumru and Kofler, 2012).
High-frequency repetitive transcranial magnetic stimulation suppressed the R2 component of the BR (Kumru et al., 2019), and also other non-invasive stimulation techniques may serve to modulate brainstem reflex excitability, as recently reviewed by Kumru et al. (2021).
The size of the BR can be experimentally modulated using various maneuvers. In his 1863 monograph Reflexes of the Brain, Sechenov (1863) (reviewed in Stuart et al., 2014) proposed an inhibitory center in the brain and thus was among the first to postulate that reflexes can be centrally modulated. This occurs in fact in various neurological disorders, such as idiopathic Parkinson’s disease (IPD), dystonia, stroke, multiple sclerosis, and others.
In a narrower sense, the term “modulation” may refer to the change in a reflex response when modifying inputs coincide with the response-eliciting stimulus in the CNS, increasing or decreasing its reflex gain, depending upon the nature, intensity, and timing of those inputs. In this line, various neurophysiological techniques may serve to document modulation of the BR. A preceding conditioning stimulus of the same characteristics as the test stimulus may be applied to the SON for the paired-pulse paradigm. The first report of suppression of the second relative to the first R2 response following paired-pulse electrical stimulation dates back to 1968 (Ferro Milone and Perfetti, 1968). The authors observed a prolonged inhibition period in patients with corticospinal lesions and the opposite with basal ganglia lesions.
Penders and Delwaide (1969) were the first to elaborate on R1 excitability enhancement between ISIs of 20 – 80 ms. The clinical applicability of this methodology took off after Kimura (1973) established the procedure of paired-pulse testing using intervals between 100 and 1000 ms. These authors demonstrated enhanced BR excitability recovery in patients with IPD. Since then, other authors have used the same technique to show BR excitability abnormalities in various disorders, i.e., focal, segmental, and generalized dystonic disorders (Berardelli et al., 1985b; Tolosa et al., 1988; Cohen et al., 1989; Nakashima et al., 1990; Valls-Solé et al., 1991; Eekhof et al., 1996), hemifacial spasm (Valls-Sole and Tolosa, 1989; Eekhof et al., 1996), Gilles de la Tourette syndrome (Smith and Lees, 1989), functional tic disorder (Versace et al., 2019b), and others. Paired-pulse stimulation was also applied in other modalities, e.g., acoustic and visual, revealing facilitation at short ISIs (30 ms for auditory, 50 ms for visual) and suppression at long intervals (250 ms), similar to trigeminal nerve stimulation (Rimpel et al., 1982). The authors also noted cross-modal modulation in a similar time range.
A preceding stimulus of any modality may cause inhibition of the R2 component of the BR, even if it is of an intensity low enough not to elicit an overt response by itself. The technique of using a low-intensity conditioning stimulus to inhibit the BR is known as prepulse inhibition (PPI) (see section 4). PPI was first described in the startle reflex (Graham, 1975). The use of the BR as a test stimulus was first published by Ison et al. (1990), who showed the disparate modulation of the BR components, i.e., facilitation of R1 and suppression of R2 and described cross-modal modulation, concurring with a “central effect” rather than “on their shared endpoints”. PPI is a topic of ongoing research interest for many authors (Garcia-Rill et al., 2019; Gunduz et al., 2019; Insola et al., 2021; Kofler et al., 2023a). Interactions and similarities between PPI and paired-pulse stimulation have been noted for more than 20 years (Swerdlow et al., 2002; Coppola et al., 2007; Kofler et al., 2023a) but have still not been entirely resolved.
A related but not extensively studied form of BR modulation is the habituation following serial stimulation. The first account of BR habituation to auditory stimuli in humans dates back to 1937 (Oldfield, 1937). Penders and Delwaide (1971) were the first to quantify reduced BR habituation in IPD. Dimitrijevic et al. (1972) performed the first systematic study comparing sequential with stochastic SON stimulation. Distraction from the stimulus diminished habituation (Gregoric, 1973), which was more pronounced in Huntington’s disease than IPD (Esteban and Gimenez-Roldan, 1975; Caraceni et al., 1976; Ferguson et al., 1978). BR habituation was more pronounced following mental nerve than SON stimulation (Jääskeläinen, 1995).
The classical conditioning paradigm, based on Pavlov’s seminal experiments (reviewed in (Maren, 2001) and section 3), is a special case of BR modulation. Subjects are presented with the combination of a warning stimulus and a BR-eliciting stimulus, either an air-puff or an electrical SON stimulus, with fixed ISIs. After systematically repeating this combination of stimuli for several blocks, subjects learn to produce a new reflex response timed just to precede the presentation of the reflex-eliciting stimulus. Notably, for this form of learning the cerebellum and its associated brainstem circuitry is both necessary and sufficient, whereas the hippocampus is not necessary (Thompson, 1990). Pavlov’s discovery implies in fact a new concept in the generation of reflexes, which should not be considered just the mechanistic responses to stimuli, but the result of behavioral adaptation to environmental conditions (Windholz, 1986). Many studies of classical conditioning have been carried out in animals and humans (Takehara-Nishiuchi, 2018) and its clinical application to health and disease are discussed in section 3. Readers who are interested in the history of classical conditioning are referred to a review by Clark (2004).
The rest of this article is devoted to describing advances acquired in our knowledge of the physiological mechanisms implicated in some of the techniques referred to above.
3. Blink reflex conditioning (Mark Hallett)
The BR can be used to study classical conditioning, a form of motor learning. This type of learning depends heavily on the cerebellum, and, therefore, it is a good way to evaluate cerebellar function. In this type of learning, a person (or other animal) learns to make a (reflex) response to a stimulus that ordinarily would not produce such a response. The learning is produced over time by pairing a stimulus that does produce the response with the one that does not ordinarily produce the response (Fig. 2). The stimulus that is innately wired to produce the response is called the unconditioned stimulus (US), and the stimulus that produces the response after pairing is called the conditioned stimulus (CS). In BR conditioning, the BR to air puff to the eye or electrical stimulation of the SON is commonly used to produce the unconditioned response (UR). The air puff or electrical stimulus would be the US. The CS is commonly an auditory tone that is subthreshold for producing an auditory BR itself. The CS is given, then the paired US, and after many trials, a blink is produced by the auditory tone; this would be the conditioned response (CR).
Figure 2.
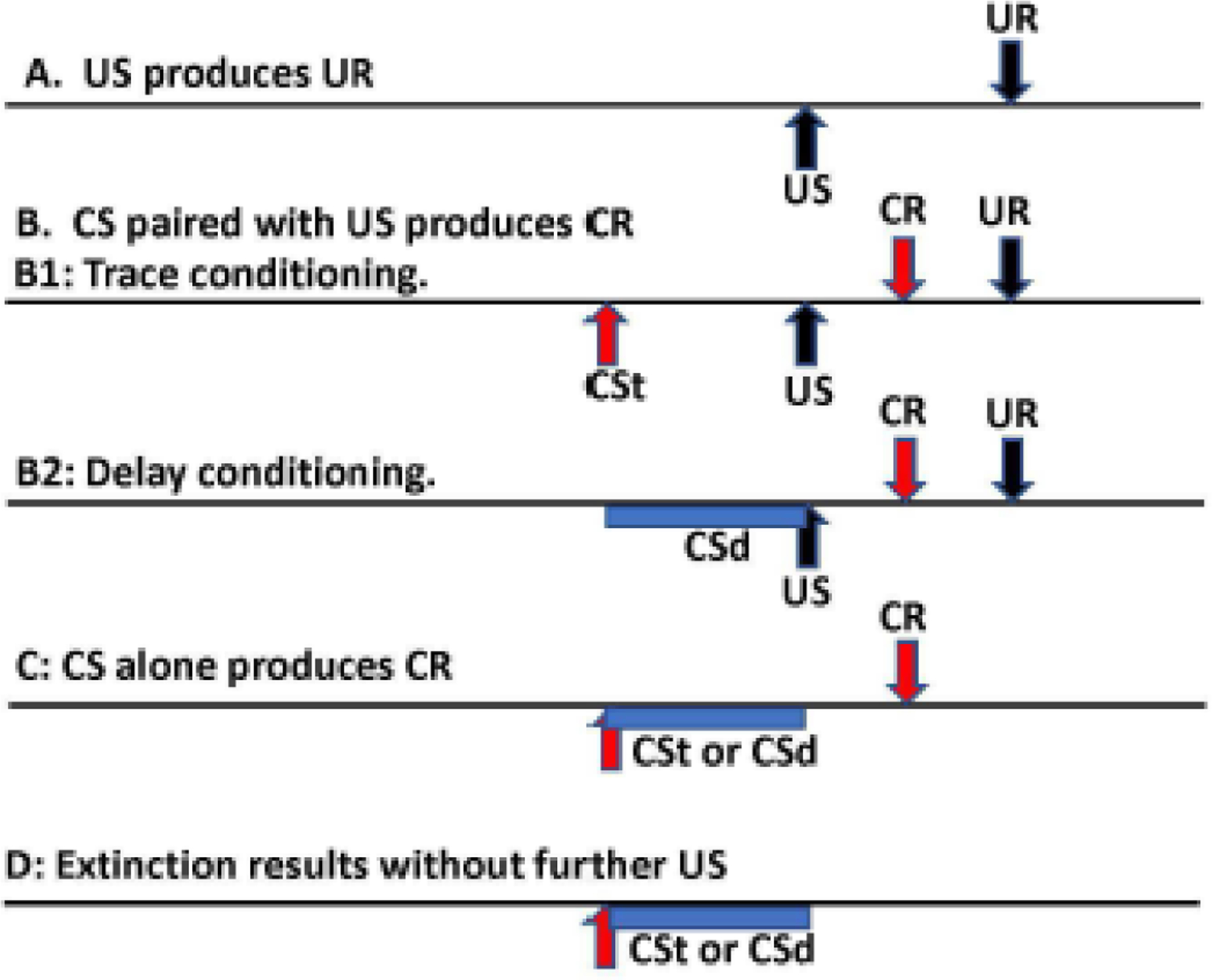
Method of blink reflex conditioning. The horizontal axis is time with events marked. A. An unconditioned stimulus (US) produces an unconditioned response (UR). B. If a conditioned stimulus (CS) precedes the US multiple times, if there is learning, there will be a conditioned response (CR) coming near the time of the UR, usually just before it. B1 is trace conditioning with the CS ending before the delivery of the US (labelled CSt). B2 is delay conditioning with the CS ending at the same time as the US (labelled CSd). C. After successful conditioning, delivery of the CS will produce a CR even without any US. D. If the CS is delivered multiple times without the US, the CR will eventually be extinguished.
Depending on the exact timing of the CS and US there are two different types of conditioning. In delay conditioning, the US occurs during the end of the CS and coterminates with it. In trace conditioning, there is a gap between the end of the CS and the US. Notably, the terminology is odd since there is a delay (or interval) in trace conditioning, but not in delay conditioning.
The BR, specifically the R2, produced by the US is a brainstem reflex. The pathway is the trigeminal nerve to the STN, the medullary reticular formation, and the facial nerve (Valls-Solé, 2019). Conditioning of the BR depends heavily on the cerebellum, particularly lobule VI and the interpositus nucleus (Takehara-Nishiuchi, 2018). The trigeminal information in the medulla also connects with the inferior olivary nucleus which then sends climbing fibers to the Purkinje cells of the cerebellum. The CS activates the auditory nerve, which synapses in the pontine nuclei, activating mossy fibers that stimulate granule cells which send their parallel fibers to the Purkinje cells. The concordance of the climbing fiber and parallel fiber input leads to synaptic changes at the parallel fiber-Purkinje cell synapse that represent the learning (Robleto et al., 2004). The output of the Purkinje cells is to the interpositus nucleus and then via the red nucleus to the facial nucleus which causes the blink. Some of the learning could also be at the Purkinje cell-interpositus synapse.
There is also involvement of the hippocampus, and this is much more for trace conditioning than delay conditioning, presumably because a short memory is needed due to the gap between the CS and US in trace conditioning. Evaluating the activity of individual parvalbumin-positive inhibitory interneurons in the hippocampus showed that they supported trace but not delay conditioning (Li et al., 2022). Most of the data come from animal studies, but these results have been documented in humans as well. For example, lesion studies show the importance of the cerebellum for eyeblink conditioning (Gerwig et al., 2007). In a fMRI study, similar involvement of the cerebellum was shown for both delay and trace conditioning, but hippocampal involvement was mainly for trace conditioning (Cheng et al., 2008). Using several novel techniques, a cerebellar activity could be monitored with EEG during conditioning of the otolith BR (Todd et al., 2021) and the maxillary nerve stimulus BR (Todd et al., 2023). Even though the BR is a “subcortical” reflex, it is under some control by the cortex. If conditioning is done while the subject is doing a working memory task, the amount of conditioning is less (Etemadi et al., 2023).
Once conditioned, the BR can be extinguished by giving the CS frequently without any USpairings (Fig. 2). If the same CS is conditioned a second time after extinction, the learning is faster (Hu et al., 2015). This implies that the original learning is not completely erased. Additionally, it would be implied that the process of extinction is not just a simple reversal of the conditioning process. Experiments show that extinction is a type of inhibitory learning that is mediated by the hippocampus for both delay and trace conditioning (Hu et al., 2015; Robleto et al., 2004). Further evidence that extinction is distinct from conditioning is that different hippocampal cells are activated for the two processes (Mount et al., 2021).
Emotion affects most functions in the brain including BR conditioning (Loi et al., 2021). Seeing pictures of sad faces will reduce delay conditioning, while happy or neutral faces have no effect. Extinction, on the other hand, will be shortened by happy and sad faces. This finding can be taken as further evidence for the difference between conditioning and extinction. There is also an effect of personality with extraversion leading to poorer conditioning (Eysenck, 1965; Todd et al., 2023).
BR conditioning is an excellent probe for cerebellar function. A deficit in eyeblink conditioning in patients with cerebellar degeneration was first demonstrated in 1993 (Topka et al., 1993). Patients (n=12) and healthy controls (n=13) were studied with delay conditioning and a marked difference was found between groups (Fig. 3). The number of CRs grew rapidly in the controls and only minimally in patients. Taken all together there were CRs in 48.9% of trials in controls and only 7.6% of trials in patients. In the tone-alone trials, the controls had CRs in 67.5% of trials, compared with only 25.7% in patients. In experiments, care needs to be given to be sure what blinks are really CRs. The investigators considered only blinks <200 ms before the expected UR to be CRs. Blinks for the 200 ms after the start of the CS were considered alpha blinks; that is, blinks related to the CS itself, too remote from the expected time of a true CR. A similar result with delay conditioning was published in 1993 by another group in 7 patients with a mixture of degenerations, stroke, and cerebellar tumor postresection (Daum et al., 1993).
Figure 3.
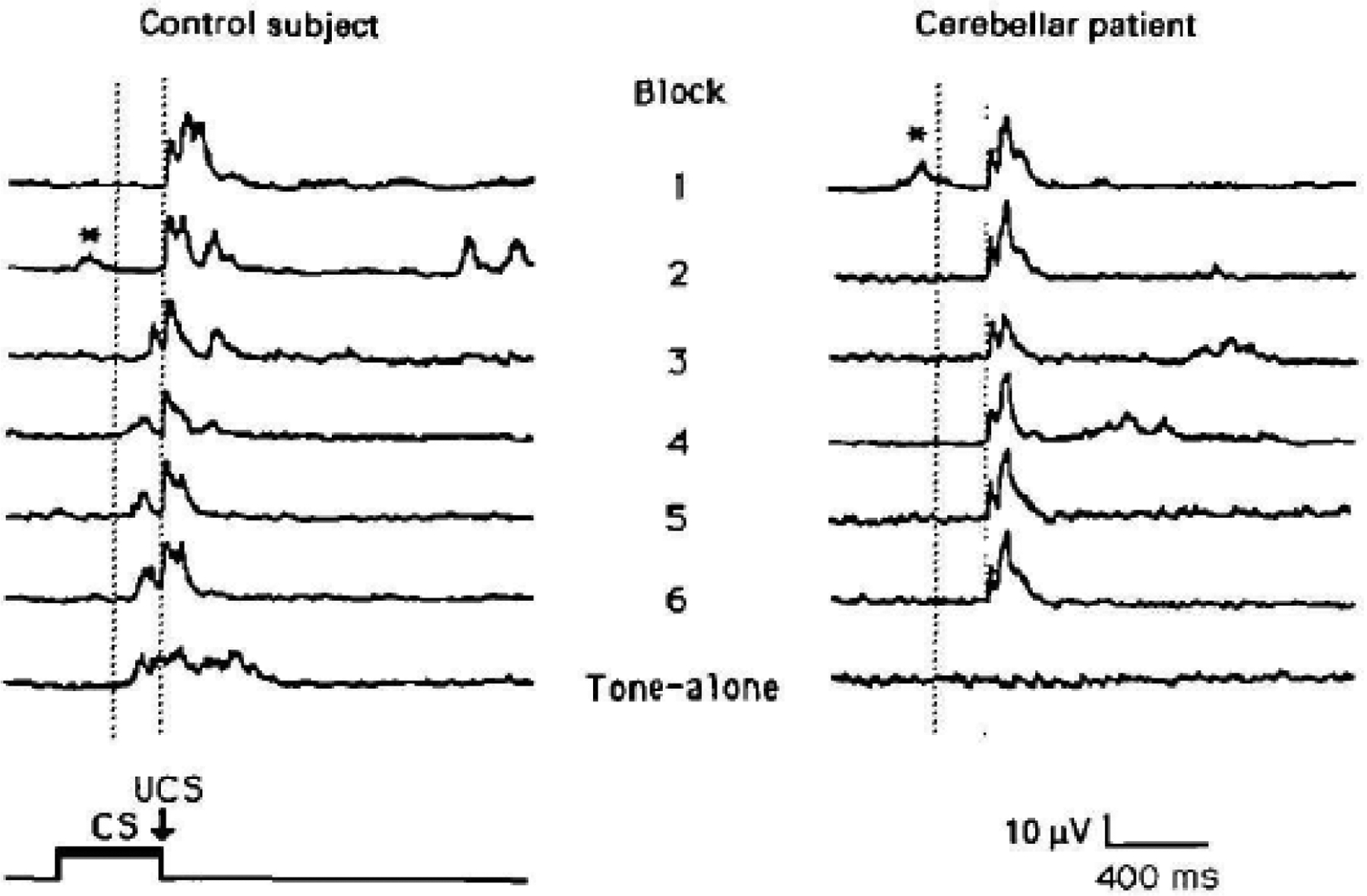
Delay conditioning in a control subject and a patient with cerebellar degeneration. Timing of the stimuli is noted in the lower left, CS is the conditioned stimulus and US is the unconditioned stimulus. The paradigm has 6 blocks with 10 trials per block, 8 with CS-US pairing, 1 with CS alone, and 1 with US alone, with a 10 second intertrial interval. The CS was a tone lasting 400 ms. Traces are rectified electromyographic activity in orbicularis oculi muscle. The first 6 traces are examples of responses to the pair of CS-US stimuli in the respective blocks, and the seventh trace is the CS, tone-alone, trace from the sixth block. In blocks 1 and 2 for the control subject and in all the blocks of the patient, the only response seen is the unconditioned response (UR). Conditioned responses (CR) can be seen preceding the UR in blocks 3 to 6 and the tone-alone block of the control subject. Only blinks in the interval between the vertical dotted lines are considered CR. Early blinks after delivery of the CS are called alpha blinks and are indicated with *. From Topka et al. (1993) with permission.
There is considerable evidence for cerebellar dysfunction in patients with essential tremor. Two groups found delay BR conditioning to be abnormal (Kronenbuerger et al., 2007; Shill et al., 2009). In one of the studies (Kronenbuerger et al., 2007), some of the patients had mild cerebellar signs and their results did not differ from the other patients.
There have been two studies of delay BR conditioning in IPD. Both in the on- and off-state conditioning appears fully normal (Daum et al., 1996; Sommer et al., 1999). Studies are also normal in isolated dystonia (Sadnicka et al., 2022). BR conditioning is abnormal, however, in progressive supranuclear palsy (Sommer et al., 2001) and multiple system atrophy (von Lewinski et al., 2013) (Fig. 4). Hence, BR conditioning might be a good test to differentiate IPD from other parkinsonisms.
Figure 4.
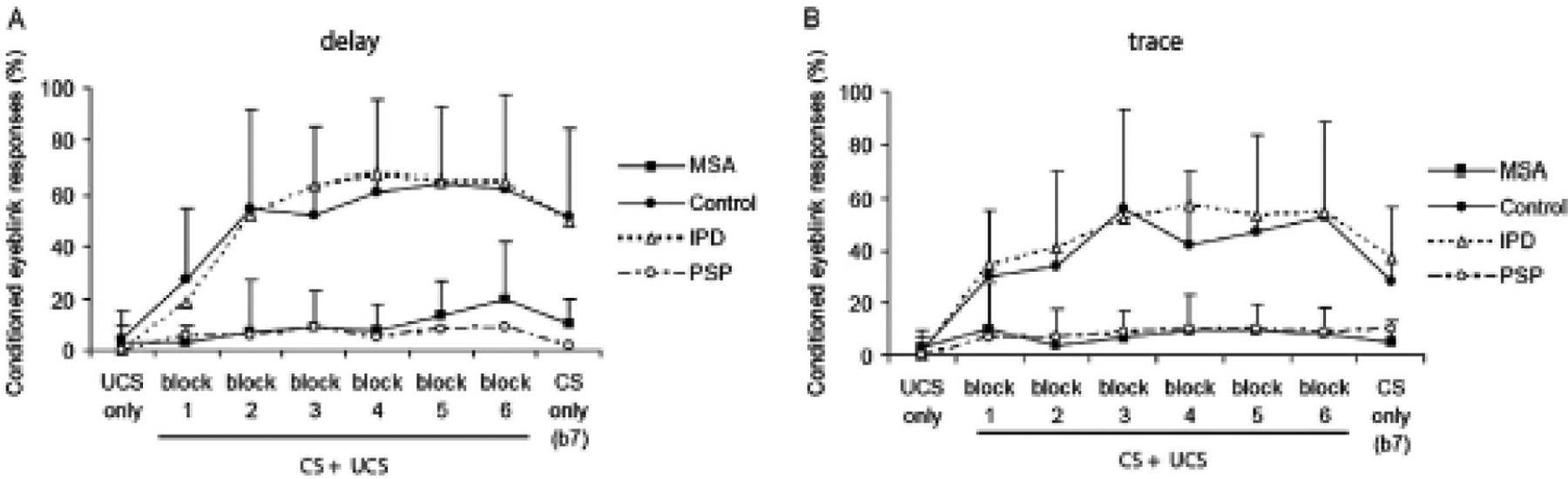
Blink reflex conditioning in idiopathic Parkinson’s disease (IPD), healthy controls, multiple system atrophy (MSA), and progressive supranuclear palsy (PSP). Delay conditioning on the left and trace conditioning on the right. The graphs show percent of conditioned responses (CR) in each of 7 blocks. The unconditioned stimulus (US) is a shock to the supraorbital nerve, the conditioned stimulus (CS) was a 400 ms tone. In delay conditioning the tone ended at the time of the shock; in trace conditioning there was a gap of 600 ms between the end of the tone and the shock. There were 6 blocks with pairing of US and CS in trials 1 to 9, an US only in trial 10, and a CS alone in trial 11. In the 7th block, there were 11 trials of the CS only (that would begin an extinction process). Note that conditioning is normal in IPD, but markedly diminished in MSA and PSP in both types of conditioning. From von Lewinski et al. (2013) with permission.
In Alzheimer disease, BR conditioning is reduced presumably because of hippocampal involvement. This was first reported in 1990 in 20 patients compared with 20 healthy agematched controls (Woodruff-Pak et al., 1990). The US was an air puff, and the CS was a 400 ms tone in a delay paradigm. There were 90 trials, 80 with paired CS-US and 10 with CS alone. There was a big difference in conditioning. Using a criterion of 25% CRs, 17 normal controls were above criterion while only 1 of the patients was; this gave a sensitivity of 95% and specificity of 65%. A second study of 15 patients compared with 17 healthy controls was published the next year with a similar study design. In a delay paradigm, the US was an air puff, and the CS was a 500 ms tone. There were 70 trials in blocks of 10 with the first trial CS alone and the other trials with paired CS-US. Using a cutoff of 20% CRs, the investigators obtained a sensitivity of 80% and specificity of 80%. In a comparison of Alzheimer disease with vascular dementia, more abnormality was seen in Alzheimer disease presumably due to the hippocampal pathology (Woodruff-Pak et al., 1996). Recognizing that trace conditioning should be a more sensitive test than delay conditioning, a study was done comparing 750 ms trace to 400 ms delay paradigms (Woodruff-Pak and Papka, 1996). The 750 ms interval was thought to be optimal based on animal experiments where trace conditioning was clearly superior to delay conditioning. Surprisingly however, delay conditioning was more sensitive. There is no clear explanation of this finding, and the issue deserves more study.
In conclusion, BR conditioning is a good simple model to study motor learning with correlative studies in animals and humans. It appears to have useful clinical applications identifying, with specificity, conditions with cerebellar or hippocampal pathology. Currently, it is not utilized as much as it could be for clinical and research purposes.
4. Gating and prepulse effects. Physiology and techniques (Josep Valls-Solé)
PPI is usually regarded as an expression of gating. While this is certainly consistent with present physiological knowledge (Garcia-Rill et al., 2019), there are important differences between PPI and gating. Both PPI and sensory gating relate to the same physiological phenomenon implicated in the control of sensory inputs, however, reflex responses are required for the expression of PPI but not for sensory gating. Also for the expression of PPI, the relevant parameter governing the modulation of subsequent responses remains the afferent sensory volley (Kofler et al., 2023b). PPI can in fact be considered a special case of sensory gating.
While PPI is a phenomenological effect implying responses in the motor system, gating is a physiological mechanism implicated in the control of sensory inputs.
4.1. Prepulse inhibition and reflex responses
PPI is expressed on reflexes. The largest amount of work has been done with either the startle reflex or the BR, although a few authors have reported PPI in other reflexes, such as the masseteric inhibitory reflex (Gomez-Wong and Valls-Solé, 1996) or autonomic responses (Eder et al., 2009). The startle reflex is the test of choice in animal experimentation studies, whereas the BR is often used in humans, where a discrete recording from the OOc is sufficient for most studies. Although blinking to some stimulation modalities may be a local manifestation of the startle reaction, the BR elicited by electrical stimulation of the SON has its own circuitry, the main distinctive feature being the presence of the R1 in the ipsilateral side of the stimulus. This early response (10–12 ms), which may also be occasionally seen with infraorbital nerve stimulation (Aramideh and Ongerboer de Visser, 2002), results from activation of the facial motoneurons after an oligosynaptic relay in the PSN and thus it is not conveyed through the reticular formation as it is the case with the R2 and R2c or the OOc responses to other stimulation modalities. R2 and R2c responses follow the trigemino-facial chain of interneurons lying in the pontomedullary reticular formation and are, therefore, susceptible to modulation through all inputs reaching the reticular formation within a certain preceding time period, i.e., prepulses. In fact, such modulation is likely to occur constantly in the human nervous system, where reflex response elicitation results from the combined effect of stimulus salience above background noise and prepulse modulation from other environmental inputs. A main influence on prepulse modulatory effects originates in the pedunculopontine tegmental nucleus (Garcia-Rill et al., 2019). Although the exact configuration of the ‘prepulse circuit’ is still unclear, Mamiya et al. (2005) found that the output from cholinergic neurons of the pedunculopontine nucleus caused hyperpolarization of neurons in the nucleus reticularis pontis caudalis, limiting in this way the expression of incoming inputs.
While the reflex responses for which PPI may be demonstrated are essentially limited to the BR and the startle reflex, a diversity of stimulus modalities may play the role of either prepulses or reflex-eliciting stimuli (Fig. 5). A prepulse, by definition, precedes the responseeliciting stimulus and the time interval in between is essential for the effect. The peak of the inhibitory effect is 100 ms for an electrical stimulus to digital nerves, it occurs slightly sooner for a mechanical tap to the hand, understandably later for laser or contact-heat stimuli, and shows almost no delay for intracranial electrical stimulation, in accordance with the distance between the stimulation site and the structures mediating PPI (Valls-Solé et al., 2000; Costa et al., 2006; Correa et al., 2019; Insola et al., 2021). The long interval needed with contactheat stimuli to reach the brainstem was used as an advantage by Correa et al. (2019) to examine the effects that a prepulse stimulus might have on perception of the sensory volley. The rationale of such a study was to search for neurophysiological signs of gating using PPI methods: since PPI is caused by gating of the afferent volley, there should be an effect of prepulses on perception. To this end, the authors used Libet’s clock (Libet, 2004), asking participating volunteers to report the time at which they felt the stimuli. The onset times of such conscious perception in baseline trials were 353.4 ms (SD=52.7 ms) for the SON stimulus and 733.6 ms (SD=75.6 ms) for the thermoalgesic stimulus (note the longer time needed for perception of the thermoalgesic stimulus due to conduction in poorly myelinated peripheral nerve fibers and through the spinothalamic tract). Therefore, possible effects on perception should take place when the SON stimulus is delivered more than 380 ms after the thermoalgesic stimulus. In fact, what the authors found was a period of significant bidirectional changes in perception (shortening in the perception of the SON stimulus and delay in the perception of the thermoalgesic stimulus) between 450 and 700 ms. This result suggests that, with the methods employed by Correa et al. (2019), the effect of PPI on perception occurred in both directions, although in real life, the effect may depend on the specific focus of attention.
Figure 5.
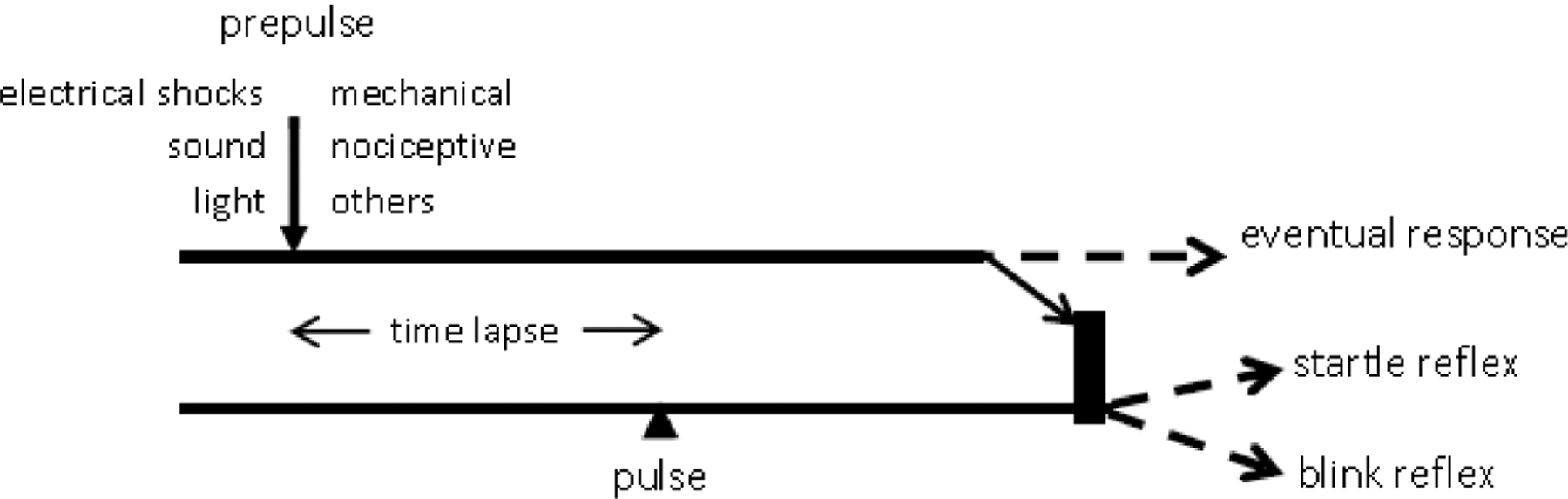
Simplified scheme of prepulse inhibition A prepulse stimulus of any of the modalities listed under ‘prepulse’ precedes a pulse stimulus, either a startling sound or a supraorbital nerve electrical stimulus, by a specific time lapse. The volley generated by the prepulse reaches the brainstem where it causes inhibition of the responses expected from the pulse stimulus, i.e., the startle reflex or the blink reflex (BR). The inhibition shows usually in a significant size reduction of either the startle reflex or the BR, depending on the pulse stimulus modality. The prepulse volley may also generate a response by itself, such as a BR, depending on the prepulse stimulus intensity. Similar mechanisms may apply in part also to circuits mentioned in Sections 5 and 6.
The works of Inui et al. (2012, 2018, 2022) deserve special mention. These authors used the term PPI to report on the effect of a low-intensity sound prepulse on the cortical responses to suprathreshold auditory stimuli. Although such observation could be used to widen the scope of prepulse effects to include non-reflex responses, it fits closer to the idea of sensory gating (see below) than that of prepulse, even though no analysis of perception was reported by Inui et al. (2012).
A variety of factors may influence the degree of PPI, such as gender (Kofler et al., 2013), body posture (Versace et al., 2019a), stimulation site (Versace et al., 2019a), cognition (Versace et al., 2021), and the subject’s emotional state such as attention (Dawson et al., 1993) or fear (Gunduz et al., 2019). The information gathered in these studies should be useful to strengthen its clinical utility (see part 2 of this review (Gunduz et al., submitted).
4.2. On the relationship between prepulse inhibition and gating
The term ‘gating’ is employed in various areas of neurophysiology, including ion channels in axonal membranes, afferent inputs in their way towards conscious appraisal, sensory interference with attention focusing, and others. The usage of the term more akin to PPI is that of filtering out potentially disturbing inputs carrying irrelevant information for the ongoing signal processing. The interference between two sensory volleys in their way to the central nervous system (CNS) occurs at various levels of the neuraxis, starting at the dorsal horn (Cohen and Starr, 1985; Stachowski and Dougherty, 2021), and being already substantial with direct recordings from thalamic nuclei (Costa et al., 2008).
However, a physiologically and clinically more interesting form of gating relates to inhibiting afferent input during movement. When we execute an action, the sensory inputs generated from the joints, muscles, and skin participating in the movement are actively attenuated. A comparator system between these sensory signals and those predicted by the efference copy helps shape the final movement outcome. When the result of the comparator is zero, sensory attenuation occurs and a sense of being ourselves the agents of the movement, i.e., sense of agency, is generated (Haggard, 2017; Blakemore et al., 2000). This mechanism is likely altered in patients with functional movement disorders, whose probable deficit in sensory attenuation may lead to absent recognition of themselves as movement agents. Abnormal movement-related gating has been demonstrated already by recording the somatosensory evoked potentials (SEPs) at onset of self-paced movements (Parees et al., 2014; Macerollo et al., 2015). But the PPI of the BR may also be an important tool for expanding our knowledge on the pathophysiology of the defective sensory attenuation of patients with functional movement disorders (Hanzlikova et al., 2019) (see part 2 of this review (Gunduz et al., submitted).
The neurophysiological study of gating and prepulse may contribute to deepen our knowledge of the pathophysiological mechanisms of many neurological disorders. Gating and prepulse effects are windows to CNS circuits devoted to control the inflow of sensory signals, the main source of information for us to interact with our surroundings.
5. The blink reflex and peripersonal space (Giandomenico Iannetti)
A bilateral reflex response in OOc can be elicited by electrical stimulation of nerves different from the trigeminal nerve. The responses obtained to upper and lower limb nerve stimulation show characteristics similar to the R2 of the trigeminal BR (Valls-Solé et al., 1994; Miwa et al., 1995, 1998; Alvarez-Blanco et al., 2009). As detailed in section 2.5, the response elicited by median nerve stimulation (the HBR), is larger than the one obtained with stimuli applied to the lower limb. This difference is usually explained with the shorter conduction distance and the consequently more synchronized afferent volley generated by stimuli applied to the upper limb (Alvarez-Blanco et al., 2009). However, considering the protective value of blinking (Sherrington, 1906), and the modulation of subcortical reflexes by higher centers to maximize fitness (Sechenov, 1863), an alternative explanation for the larger magnitude of the BR elicited by median nerve stimulation is the greater proximity of the upper limb to the face compared to the lower limb. It makes intuitive sense that stimuli closer to the face have a stronger potential to harm the eye and elicit a larger BR.
5.1. Hand-blink reflex magnitude depends on top-down cortical modulation
The size of the BR may indicate how the nervous system implicitly estimates the potential to harm of the eliciting stimulus (Sambo et al., 2012a, 2012b; Bufacchi et al., 2016) (see also section 5.4). Among the many factors determining the potential to harm of environmental stimuli, the spatial proximity of stimuli to the eye is straightforward to modulate, especially using the HBR, because a change of the position of the stimulated hand in egocentric coordinates does not alter the intensity of the sensory input eliciting the BR. In contrast, applying, e.g., acoustic stimuli to elicit an auditory BR would present the major drawback of different stimulus intensities when the stimulus is in different spatial locations. A seminal experiment that has been now reproduced by several research groups, demonstrated an HBR double in magnitude when the stimulated hand was close to the face rather than far away (Fig. 6A) (Sambo et al., 2012b; Sambo and Iannetti, 2013). Importantly, this enhancement occurs irrespectively of whether the proximity of the hand to the face was altered by changing the position of the arm or by rotating the head while keeping the arm position constant. Thus, the HBR enhancement is not due to changes in peripheral input (Sambo et al., 2012b) nor to the effort necessary to keep the hand close to the face (but see (Bufacchi et al., 2019). These results indicate a remarkably fine top-down modulation of the HBR by higher-order cortical areas. This modulation could take place at different levels of the reflex circuit: presynaptic disinhibition of primary Aβ neurons, specific facilitation of the HBR interneurons in the lower medulla, or general facilitation of facial motoneurons. There is empirical evidence that neither the N20 component of median nerve SEPs nor the BR elicited by electrical SON stimulation are affected by hand position (Sambo et al., 2012b). These observations rule out a disinhibition of the afferent lemniscal pathways (i.e., in the cuneate nucleus, before Aβ afferents from the hand enter the brainstem circuits subserving the HBR) or the facial motoneurons, and provide instead compelling evidence that the brainstem circuits mediating the HBR undergo tonic and selective top-down modulation from higher order cortical areas.
Figure 6.
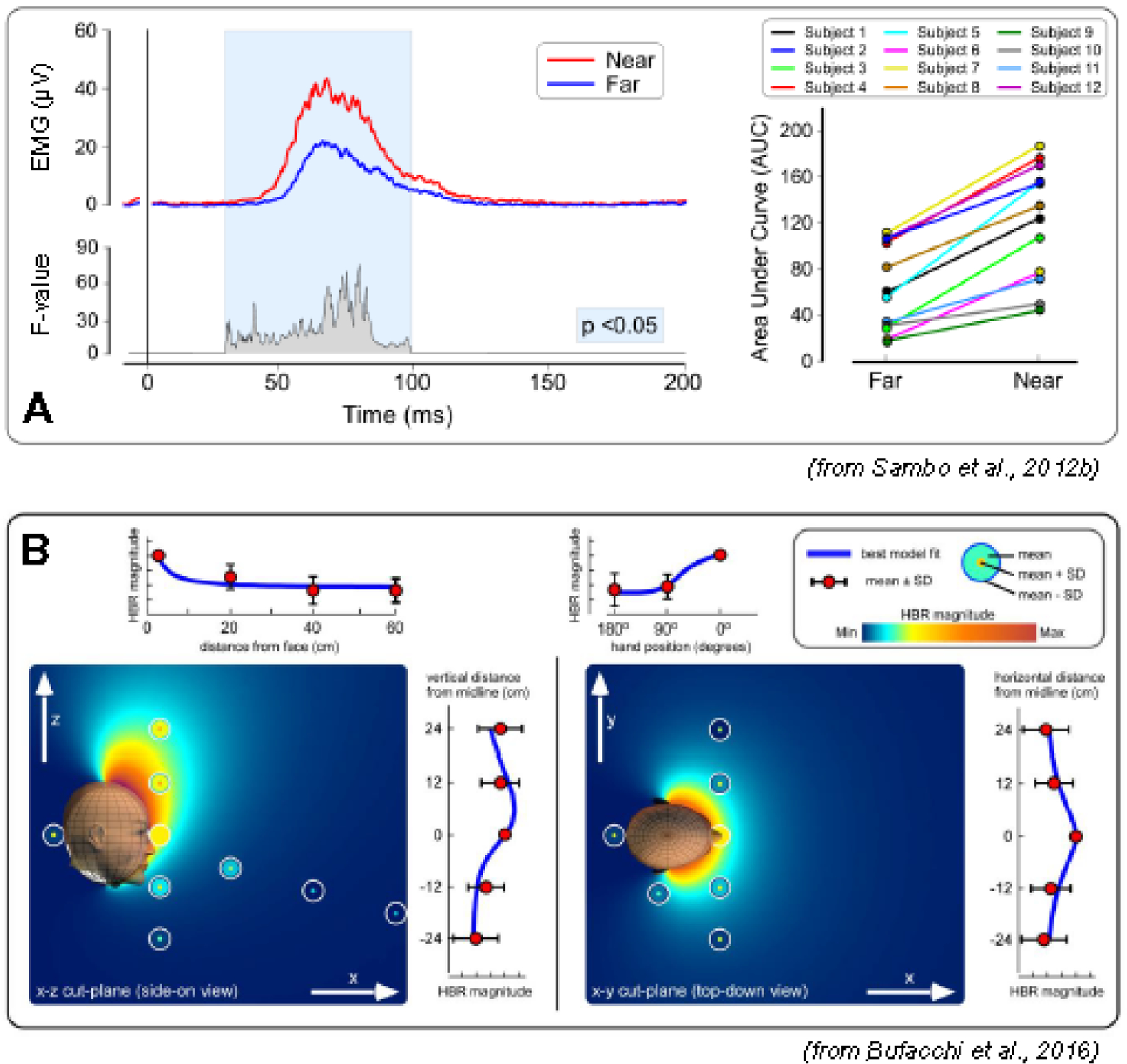
The magnitude of the blink reflex elicited by median nerve stimulation (hand-blink reflex, HBR) is modulated by the proximity between the stimulated hand and the face. A: the top waveforms are the rectified group average HBR for the hand position ”far” (blue) and “near” (red). The bottom waveform expresses the ANOVA F-value for each time point, in the significant time windows (P <0.05). The right panel shows a consistent effect across participants (single-subject HBR magnitudes are expressed as area under the curve, AUC) (modified from Sambo et al. (2012b)). B: geometric modelling of HBR strength as a function of stimulus position. Plots are a combined description of the experimental data with the bestfitting geometric model. Measured HBR data are represented as concentric circles located where the measurements were taken. Background color represents the HBR magnitude predicted by the best-fitting geometric model. Line graphs at the side of each color plot show HBR magnitudes along each axis, together with the best-fitting geometric model (blue line). HBR magnitude increases monotonically with the proximity between the stimulus and the face, and it is symmetrical on the axial plane, but asymmetrical along the rostro-caudal axis, with stronger HBR elicited by stimuli occurring above than below the face (from Bufacchi et al. (2016)).
5.2. A peripersonal map of blink reflex magnitude
While most studies investigated the HBR magnitude as a function of two stimulus positions (typically ‘far’ and ‘near’ the face), when a larger number of stimulus positions are explored it becomes possible to use geometrical models to derive fine-grained topographical maps of BR strength (Fig. 6B). This modelling approach allows testing a number of physiological assumptions on why the BR increases as a function of hand position. Specifically, when the hand position covers large portions of space, the HBR magnitude reflects the probability of the face being hit by a threat. These maps show that the HBR increases monotonically with the proximity between the stimulus and the face (Bufacchi et al., 2016). Importantly, while HBR strength is symmetrical on the axial plane, it is elongated asymmetrically along the rostro-caudal axis, with stronger HBR elicited by stimuli occurring above than below the face (Fig. 6B). Furthermore, HBR modulation when systematically altering body posture, i.e., with participants being upright, supine, and lying sideways, suggests that the nervous system adjusts the strength of the BR taking gravity into account when estimating the probability of being hit by a threat (Bufacchi and Iannetti, 2016). Indeed, the vertical asymmetry of the HBR magnitude field is invariant to body posture: stimuli coming from above in earth-centered coordinates always result in stronger HBR compared to stimuli at the same distance from the face but coming from below. Thus, the brain takes gravitational cues to automatically update threat value in an adaptive mechanism that accounts for the simple fact that objects fall down.
5.3. What does the hand-blink reflex enhancement truly reflect?
The clear dependence of HBR modulation on proximity between the stimulus and the face has induced many authors to consider the HBR modulation an index of how the nervous system represents the space surrounding the body (“peripersonal space”). However, there is ample evidence that the HBR is strongly influenced also by factors other than proximity, and in many instances the HBR is largely modulated even when the stimulus position with respect to the face remains constant. One example is the above-mentioned effect of gravity: the magnitude of the HBR elicited by a stimulus at the same Euclidean distance from the face changes when the subject posture is altered (Bufacchi and Iannetti, 2016). Several other non-spatial factors affect the HBR magnitude: the presence of a screen between the stimulated hand and the eye (Sambo et al., 2012a), changes in the probability or control of stimulus occurrence (Sambo et al., 2012a; Versace et al., 2020) – see also section 6), whether the stimulus is approaching or receding (Wallwork et al., 2016; Bisio et al., 2017), and the presence of other moving and static environmental objects (Fossataro et al., 2016; Somervail et al., 2019). It is therefore incorrect to relate HBR magnitude to proximity and peripersonal space, as we and others have done in the past (Sambo and Iannetti, 2013; Wallwork et al., 2017; Bisio et al., 2017). This unjustified primacy of proximity shows the issues consequent to interpreting HBR modulations in spatial terms: given that many factors other than proximity can cause the observation that the HBR magnitude is increased, interpreting such HBR increases as reflecting changes in how the nervous system represents stimulus location in egocentric coordinates is likely incorrect. For a more exhaustive discussion on the topic, we refer to Bufacchi and Iannetti (2018, 2021).
5.4. Hand-blink reflex modulation reflects the potential of the stimulus to harm the eye rather than its spatial configuration
Thus, HBR magnitude fields do not reflect representations of stimulus configuration in facecentered coordinates. Rather, they are better understood as mappings onto behavior. Specifically, HBR magnitude represents a case of a class of neural and behavioral responses that reflect the value of actions aiming to create or avoid contact between objects and the body (for an extensive discussion on the topic and on the different definitions given (and often interchangeably used) to the term “peripersonal space” see Bufacchi and Iannetti (2018, 2021)).
The BR is a prototypical contact action, as it aims to avoid contact between a dangerous stimulus and the eye through the interposition of the eyelid; thus, it is behaviorally useful that its magnitude depends on the likelihood that a stimulus hits the eye, a likelihood that, in turn, depends on the proximity between the stimulus and the face (although by no means only on the proximity) (Sambo et al., 2012a; Bufacchi et al., 2016). The action value perspective (i.e., that the HBR magnitude reflects the output of a neural estimate of how necessary it is to blink in a given condition (Bufacchi and Iannetti, 2018, 2021)) parsimoniously explains why factors other than proximity affect HBR magnitude. This is in striking contrast to previous interpretations, which often considered non-proximity effects as interesting exceptions to the spatial proximity rule – but nevertheless interpreted in spatial terms, as indicating, for example, that “far becomes near” and that the “space representation is dynamically shaped” by the contingent factors affecting the HBR (Bisio et al., 2017). Finally, the action value perspective allows speculating on the cortical structures exerting the top-down modulation of HBR-specific circuitry in the brainstem. Parieto-premotor circuits describe the relevance of potential actions within the interactive behavior framework (Cisek and Kalaska, 2010), and precisely in these areas there are bimodal visuo-tactile neurons with visual body-part centered receptive fields (Clery et al., 2015). Also, in addition to the HBR, many behaviors whose magnitude displays a body-part centered field have been linked to neural activity occurring within this loop (Brozzoli et al., 2014). It is therefore likely that the parietal and premotor cortices are the cortical sites where the value of potential actions, including the HBR, are specified.
6. Self-triggering of blink reflexes (Viviana Versace)
The excitability of brainstem circuitries mediating defensive blinking in response to abrupt sensory inputs is continuously modulated in part by the estimated threat that these inputs pose to the eyes (see section 5). In fitting with the idea that control over a stimulus reduces its threat value and self-induced sensory perturbations render reflexive protective eye-closure less necessary, few authors have shown that when BRs are elicited by selfstimulation, the R2 response is reduced at the same time that the R1 response is potentiated (Ison et al., 1990; Meincke et al., 1992, 2003; Leis et al., 1993; Versace et al., 2020, 2023). However, the exact physiological mechanisms that underlie these effects are still unclear.
The effect of self-inflicted unpleasant stimuli has rarely been studied in the context of brainstem reflexes other than the BR to SON stimulation, with some examples for auditory startle reactions (Kawachi et al., 2014), and somatosensory BR following high-intensity median nerve stimulation (the HBR) (Versace et al., 2021). Some spinal reflexes are similarly depressed by self-stimulation, e.g., the cutaneous flexor reflex response (Young, 1973), the stretch reflexes (Rothwell et al., 1986), and cutaneous reflexes evoked during human walking (Baken et al., 2006; Hoogkamer et al., 2015).
Volitional activity, sensory inputs, and motivational and emotional factors may all influence reflexes (Sechenov, 1863) by gaining access to polysensory integrative brainstem centers, where they may modify the excitability of reflex pathways (Fig. 5).
Both self-produced sensations and self-generated motor actions (e.g., those resulting in delivery of self-directed stimuli) are similarly “attenuated”. Based on an internal cognitively mediated forward model, the act of self-eliciting a stimulus brings about an efference copy of the motor command. This efference copy is thought to reflect the predicted sensation of the self-initiated motor act, which may not only lead to sensory attenuation but also to inhibition of reflex responses.
Not all findings related to sensory attenuation, however, can be explained by forward models. Hence, another theoretical framework, i.e., predictive processing, has more recently been developed (Kiepe et al., 2021). This model suggests sensory attenuation to be a result of attention orienting based on predictions that are not necessarily dependent upon motor behavior. Predictive processing states that we constantly make use of prior information, either self- or externally generated, in order to create predictions about upcoming changes in sensory input in the form of a generative model. In this framework, only the predictability of a stimulus determines its potential to elicit sensory attenuation. This theory is again unable to explain all the evidence, leaving room for hybrid models, which combine the efference-based forward model with a global predictive mechanism.
Several studies have shown that self-administration of a moderately painful stimulus, relative to the administration of the same stimulus by external agents, reduces the perceived intensity and unpleasantness (Wang et al., 2011; Muller, 2012) and modulates neural activity in the anterior cingulate cortex (Mohr et al., 2005; Wang et al., 2011), primary somatosensory cortex (Helmchen et al., 2006; Wang et al., 2011), posterior insula and prefrontal cortex (Mohr et al., 2008), all areas related to saliency detection (Mouraux and Iannetti, 2009; Mouraux et al., 2011). Such a reduction in perceived unpleasantness was also observed for self-induced BRs (Meincke et al., 1992; Versace et al., 2023).
Interestingly, self-stimulation and observation of stimulus triggering suppressed the R2 component, despite the stimulation probe being close to the person’s face, a condition known to facilitate R2 (Versace et al., 2020). Indeed, the perception of a threat near the face potentiates R2 despite unchanged properties of the SON stimuli, while self-stimulation can overrule this effect.
A peri-liminal (barely perceptible) sensory stimulus, which does not produce a response by itself, delivered prior to the reflex-eliciting SON-stimulus at appropriate ISIs facilitates R1 and dramatically suppresses R2 (Rossi and Scarpini, 1992) (see section 4, Fig. 5). The apparently similar modulation of R1 and R2 induced by a prepulse and by self-stimulation suggests the possibility of a common mechanism (Versace et al., 2020). However, certain disparities led to refute this assumption (Versace et al., 2023): recent experiments demonstrated that prepulse effects are evident in a time window ranging from 40 to at least 500 ms ISIs with a maximum effect at ISI 100 ms between prepulse and SON stimulus, concurring with a time-locked mechanism of presynaptic inhibition at the brainstem level. In contrast, R2 suppression and R1 facilitation due to self-stimulation of SON already occur in a 2-s period before the act of self-triggering (Versace et al., 2023), suggesting a tonic “cognitive” tuning of the excitability of brainstem circuits.
While a top-down facilitatory influence of the “readiness to act” on the excitability of the neurons in the pontine facial nucleus (responsible for R1) is certainly not easy to explain from a physiological point of view, it seems easier to explain a top-down inhibitory influence on the pontomedullary interneurons (responsible for R2) that regulate the magnitude of protective blinking, according to ongoing needs.
The excitability of the respective interneurons in the brainstem reticular formation is crucial for the size of the BR to SON stimulation, or of the startle eyeblink, as they can rapidly tune their excitability depending on descendent projections from higher-order areas (Sambo et al., 2012b; Valls-Solé, 2012; Kawachi et al., 2014). The higher-order control mediating “readiness to act” and the “sense of agency” may share corticofugal modulatory influence on the brainstem neural circuits responsible for protective eye closure. A concrete example is the difference in blinking when administering eye drops oneself versus having someone else doing it. “Knowing that you are not posing a threat to your eyes” does not require to close them promptly.
7. The blink reflex and pain (Jens Ellrich)
7.1. Functional anatomy and physiology of the trigeminal system
Trigeminal afferent nerve fibers project via the trigeminal ganglion to the mesencephalic nucleus, the PSN, the interstitial nucleus of the spinal trigeminal tract, and the STN in the brainstem (Clara, 1942; Olszewski, 1950; Phelan and Falls, 1989; Shults, 1992; Nieuwenhuys et al., 2008). The STN, extending from the pons to the upper cervical spinal cord, is divided into subnucleus oralis, interpolaris, and caudalis (Olszewski, 1950; Shults, 1992). Based upon the responsiveness to mechanical stimulation applied to the orofacial skin, secondary sensory neurons within the subnuclei of the STN are classified in lowthreshold mechanoreceptive (LTM), WDR, and nociceptive-specific (NS) neurons (Willis, 1985; Sessle et al., 1986; Ellrich and Messlinger, 1999). Whereas LTM and WDR neurons respond to light tactile stimuli via Aβ fiber afferents, only WDR neurons increase discharge rates when stimulus intensity becomes noxious and involves afferent input from Aδ and C fiber afferents. NS neurons do not respond to innocuous tactile input but only to noxious stimuli via nociceptive afferent nerve fibers. Nociceptive neurons, i.e., WDR and NS, were localized in the interstitial nucleus of the spinal trigeminal tract and in all subnuclei of the STN indicating their involvement in trigeminal nociception and pain processing (Hayashi et al., 1984; Sessle et al., 1986; Hayashi and Tabata, 1989; Dallel et al., 1990). Studies in patients with circumscribed brainstem lesions confirmed that nociceptive processing within the trigeminal system involves these brainstem nuclei (Ongerboer de Visser and Kuypers, 1978; Kimura et al., 1994; Hopf, 1994; Valls-Solé et al., 1996; Jerath and Kimura, 2019). Reflex pattern alterations in patients with solitary and circumscribed brainstem lesions enabled inferring which brainstem nuclei are part of the BR arcs, allowing for topodiagnosis in clinical neurophysiology. The interneurons are located in the PSN for the R1 and in the medullary STN for the R2 components of the electrically elicited BR (Fig. 7). The location of reflex interneurons was confirmed by reflex studies in patients with small circumscribed brainstem lesions (Hopf, 1994; Cruccu et al., 2005). A unilateral ischemic lesion in the dorsolateral medulla, the so-called Wallenberg syndrome, caused an abnormal R2 in more than 90% of patients, while the R1 remained unchanged. Stimulation on the healthy side elicited a normal reflex pattern (Kimura, 2013; Valls-Solé et al., 1996).
Figure 7.
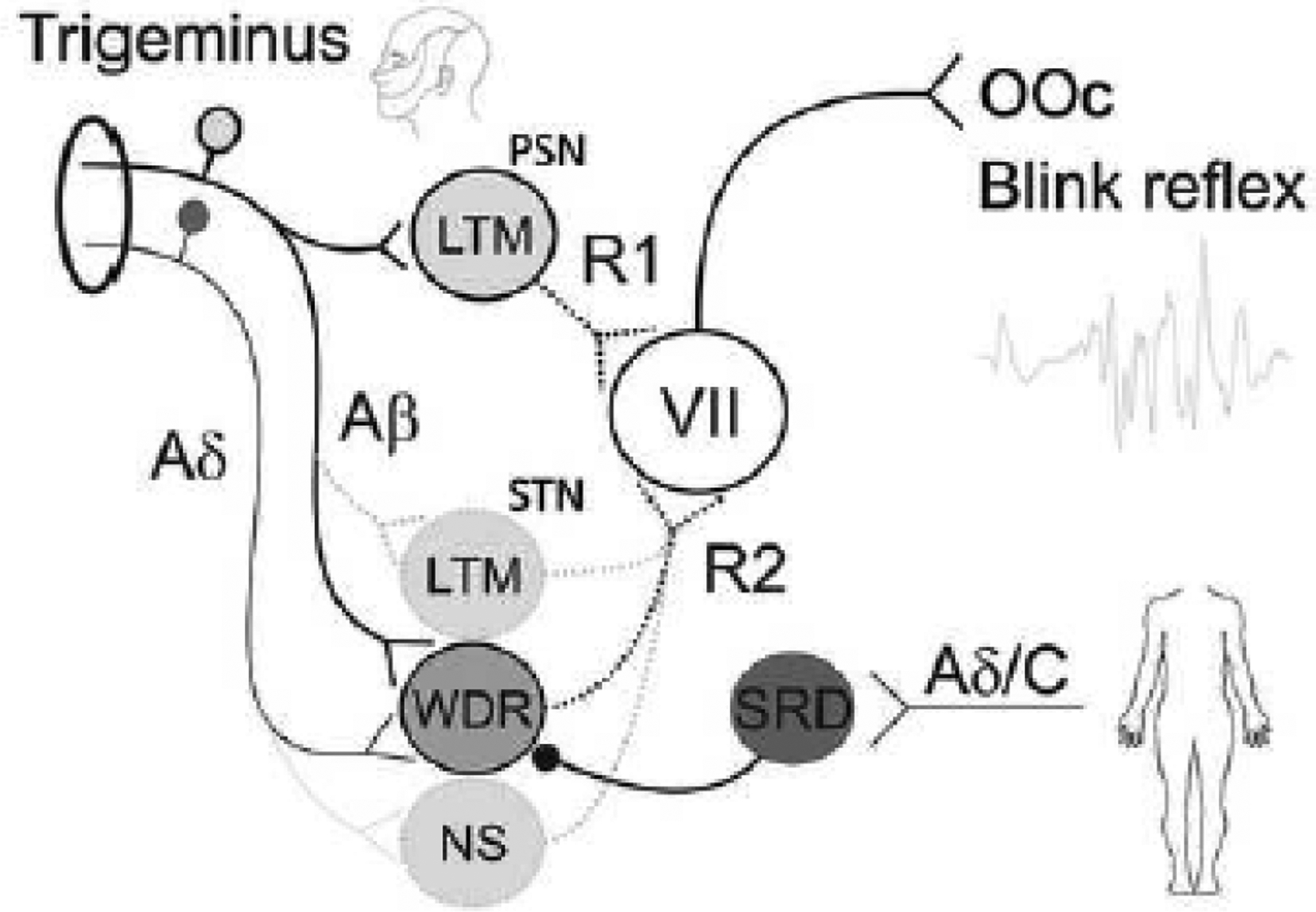
Neuronal network model of the blink reflex (BR). The principal sensory nucleus (PSN) mediates the R1 component, and the spinal trigeminal nucleus (STN) mediates the R2 component of the BR. Trigeminal non-nociceptive and thick-myelinated Aβ afferents project on low-threshold mechanoreceptive (LTM) neurons of the PSN generating the R1 reflex of the orbicularis oculi muscle (OOc) via motor neurons of the facial nerve (VII). Trigeminal tactile Aβ and nociceptive Aδ afferents converge onto common wide-dynamic-range (WDR) interneurons of the STN generating the R2 reflex via motor neurons of the VII. Noxious stimulation to remote body sites such as the extremities, activate multireceptive neurons of the subnucleus reticularis dorsalis (SRD) inhibiting WDR neurons and, hence, the R2 reflex responses. Additionally, the R2 reflex may be evoked or modulated by Aβ fiber input on LTM neurons of the STN or noxious input from Aδ afferents on nociceptive specific neurons (NS) of the STN.
7.2. Afferent inputs and thresholds for elicitation of the blink reflex
R1 and R2 can be elicited by phasic innocuous mechanical or electrical stimuli indicating that these components are mediated by thick-myelinated Aβ afferents (Kimura, 2013; Ellrich and Treede, 1998). Average electrical thresholds with stimulation of the SON at the supraorbital foramen via two identical surface electrodes utilizing square wave pulses with a duration of 200 μs in healthy volunteers were reported to be 2.2 mA for detection (touch sensation), 2.4 mA for R2, and 5.3 mA for R1. These thresholds are far below the reported pricking pain threshold of 16.2 mA indicating the non-nociceptive origin of R1 and R2 evoked by this common type of electrical stimulation (Ellrich and Treede, 1998). Aβ fiber afferents project to LTM and WDR neurons. Thus, the R1 is mediated by afferent input from Aβ fibers to LTM neurons of the PSN that does not contain any WDR neurons (Fig. 7). Consequently, Aβ afferents may project to LTM and/or WDR neurons of the STN generating the R2 reflex (Fig. 7). If WDR neurons of the STN are involved in the R2, noxious stimulation should be able to evoke the reflex as well.
Selective activation of trigeminal Aδ fiber nociceptors of the forehead by heat pulses of an infrared laser causing a pricking painful sensation elicits a BR (Ellrich et al., 1997; Romaniello et al., 2002). This noxious phasic stimulus evokes a bilateral BR with an onset latency of approximately 70 ms following trigeminal stimulation. Considering the nociceptor activation time of about 40 ms (transduction), the onset latencies of the electrically evoked R2 with innocuous intensity and the laser-evoked BR correspond very well. Notably, this component is the earliest one, no component corresponding to the electrically evoked R1 is elicited by noxious heat (Ellrich et al., 1997; Romaniello et al., 2002).
The BR can be evoked by electrical rectangular pulses applied by a custom-made concentric electrode to the forehead (Kaube et al., 2000). This electrode consists of a small central cathode (diameter Ø 1 mm) and a large external ring anode (inner Ø 8 mm, outer Ø 24 mm). With stimulus intensities below 1 mA but high current density, this electrode allows preferential activation of cutaneous nociceptive Aδ-fibers (Bromm and Meier, 1984; Kaube et al., 2000). This kind of noxious electrical stimulation of supraorbital nociceptive afferents evokes R2 reflexes with latencies of approximately 42 ms. Local anesthesia of the forehead skin is able to suppress the BR confirming its mediation by nociceptive skin afferents (Kaube et al., 2000).
Elicitation of R2 by both noxious stimulation techniques confirms the involvement of nociceptive afferent input in the R2 reflex arc. Different reflex arcs are conceivable (Fig. 7): (1) Discrete afferent reflex arc: electrically or mechanically activated low-threshold afferent input via Aβ fibers projects onto LTM neurons and thermally or electrically evoked nociceptive input projects via Aδ afferents onto NS neurons; In this case, the non-nociceptive and the nociceptive R2 are mediated by different interneurons. (2) Both innocuous and noxious inputs converge onto common WDR interneurons, i.e., both reflexes share the same interneurons. If afferent input from thick-myelinated, non-nociceptive Aβ fibers and thinmyelinated, nociceptive Aδ fibers converge on the same WDR interneurons, homotopic subthreshold noxious stimulation should facilitate the R2 reflex elicited by low-intensity electrical stimuli corresponding to the phenomenon of spatial summation.
7.3. Blink reflex modulation by noxious stimuli
When a conditioning noxious heat pulse, which does not evoke any BR, is homotopically applied to the left forehead preceding by 75 ms an innocuous BR-eliciting electrical stimulus to the SON, the R1 remains unchanged while the R2 is facilitated by about 30% (Ellrich et al., 1998). These results suggest that both afferent inputs, the electrically evoked Aβ input and the heat evoked Aδ input, facilitate the R2 reflex by spatial summation (Fig. 7). These data confirm the mediation of the R2 by WDR neurons and of the R1 by LTM neurons. The simultaneous occurrence of R1 and R2 components may help to differentiate nonnociceptive from nociceptive processes within the trigeminal system.
If WDR neurons significantly contribute to the signal processing of R2 after weak electrical stimulation, the BR should be inhibited by activation of the system of diffuse noxious inhibitory controls. Nociceptive afferent input from anywhere on the body activates nociceptive neurons in the subnucleus reticularis dorsalis (SRD) of the brainstem causing inhibition of WDR neurons in the spinal cord and the trigeminal system (Villanueva et al., 1996; Ellrich and Messlinger, 1999; Le Bars, 2002). Thus, if the R2 is mediated by WDR neurons, it should be suppressed by remote painful stimuli via diffuse noxious inhibitory controls. Actually, the BR elicited by weak electrical stimuli is modulated by noxious conditioning heat applied to the extremities: the R2 decreased while the R1 remains unchanged (Ellrich and Treede, 1998). This inhibition of the R2 by remote noxious heat indicates not only an involvement of WDR neurons in the generation of the Aβ fiber-mediated R2 but also a convergence of Aβ and Aδ afferents onto common WDR neurons within the medullary STN (Fig. 7). Constancy of R1 seems to confirm the mediation of this response by pontine LTM neurons of the PSN. Nociceptive R2 BRs elicited by intra-epidermal or superficial electrical stimulation were inhibited by remote cold or heat stimulation of the hand or the forearm, respectively, in a similar manner (Drummond et al., 2018; Kinukawa et al., 2021).
In summary, the “traditional” electrically evoked BR following SON stimulation is mediated by trigeminal Aβ fibers that project to LTM and WDR neurons of the PSN and the STN. There is evidence, however, that nociceptive neurons are involved in the BR as well. The R2, but not the R1, is elicited by selective or highly preferential nociceptive afferent input from infrared laser pulses or high current density electrical stimulation. R2 facilitation by homotopic noxious input and R2 inhibition by remote noxious heat indicate considerable involvement of multireceptive WDR neurons of the STN. Trigeminal nociception can therefore be investigated by applying this brainstem reflex. According to the investigation of spinal nociception by cutaneomuscular reflexes, pathophysiological mechanisms of central sensitization, hyperalgesia, allodynia and referred pain can be investigated in the trigeminal system by using the BR (Kofler and Halder, 2014; Pillai et al., 2020; Khan, 2021; Thoma et al., 2022). An experimental study in humans addressed the convergence of meningeal and facial input on the STN, probably a condition for referred pain, and demonstrated in healthy volunteers the facilitation of the R2 by raising intracranial pressure (Ellrich et al., 1999). The R2 qualifies for exploring nociception and pain in the trigeminal system and thus may be an appropriate model to test analgesic drug effects on trigeminal nociception.
8. The blink reflex and other brainstem reflexes under general anesthesia (Maria J. Téllez)
Virtually all functional circuits of the CNS are subject to synaptic modulation and plasticity by pharmacologic agents like anesthetic drugs. Remarkably, how and where anesthetic drugs disrupt synaptic networks remains to be determined. The classic view predicates that anesthetic drugs bind to protein receptors extensively distributed in the CNS, inducing global synaptic suppression. A recent proposition suggests that dedicated axonal pathways and specific brainstem locations may convey a suppressive signal to remote parts of the CNS (Sukhotinsky et al., 2016). The BR has been for a long time used to identify the transition from patient responsiveness to unresponsiveness after induction of general anesthesia and to assess the depth of anesthesia maintenance. The BR, elicited with a single electrical stimulus over the SON, exhibits a dose-dependent relationship with infusion pumps and volatile anesthetic agents, gradually reducing the BR R1 and R2 amplitudes until abolishing both responses in a few minutes (Marelli and Hillel, 1989; Mourisse et al., 2004).
Nevertheless, several methodologies and anesthetic routines have been successfully developed to elicit and sustain brainstem reflexes under general anesthesia for intraoperative monitoring guidance in complex surgeries. Recording brainstem reflexes under general anesthesia have significantly advanced how various surgical procedures are monitored. It is now possible to monitor cranial nerves’ sensory and motor functions, pathways, and nuclei within the brainstem. In some cases, the elicitation of a brainstem reflex requires facilitation by stimulating sensory afferents with a short train of electrical stimuli. In other cases, we hypothesize that the effect of anesthetic drugs may facilitate the elicitation of brainstem reflexes. There is evidence that propofol-induced unconsciousness disrupts the signaling between cortical and brainstem structures, thereby modifying the salience network and its connectivity with the brainstem (Guldenmund et al., 2013).
This section summarizes the methodologies and behavior of brainstem reflexes elicited under general anesthesia, each presenting diverse susceptibility to anesthetic modulation.
8.1. General Anesthesia
Propofol, etomidate, and barbiturates are the most common agents to induce general anesthesia. After induction, the patient loses consciousness and the oculocephalic and corneal reflexes at approximately 10 to 30 seconds. As anesthesia induction occurs, the patient presents muscle atonia and apnea, suggesting that these GABAergic agents disrupt synapses at the brainstem level where arousal and respiratory centers are located. A decrement in cortical activity follows, and the flow of communication between the brainstem, subcortical and cortical pathways is also interrupted (Brown et al., 2010, 2011; Feldman and Del Negro, 2006).
The primary goals of general anesthesia maintenance are providing an adequate level of unconsciousness and antinociception to the patient.
Maintaining general anesthesia with volatile halogenated anesthetics (isoflurane, desflurane, and sevoflurane) profoundly affects brainstem reflexes (Møller and Jannetta, 1986). For instance, at a median alveolar concentration >1 (Eger, 1974), the BR and the laryngeal adductor reflex (LAR) are not elicitable. Additionally, there is a dramatic delay in the reappearance of the reflexes, despite the end-tidal anesthetic concentrations returning to zero by mass spectroscopy (Marelli and Hillel, 1989). At a median alveolar concentration <1, both reflexes have proven to be elicitable, presenting smaller amplitude (Deletis et al., 2009; Sinclair et al., 2017a). The mean or median alveolar concentration is not given in specific units since it represents a ratio or proportion of the concentration of anesthetic gas to other factors (Eger, 1974).
Total intravenous anesthesia (TIVA) is a more appropriate preparation for maintaining anesthesia if brainstem reflexes have to be monitored during surgery. TIVA incorporates an infusion pump with propofol (100–300 μg/kg/min) and opioids (remifentanil at 0.1–0.3 μg/kg/min, fentanyl at 1–2 μg/kg/hr, or sufentanil at 0.1–0.3 μg/kg/hr) adjusted throughout surgery according to the patient’s anesthesia depth.
8.2. The blink reflex elicited under general anesthesia
A single electrical stimulus applied to the SON does not elicit the BR in fully anesthetized humans, except if the trigeminal or facial nerves are hyperexcitable or the patient is only slightly anesthetized (Møller and Jannetta, 1986; Sindou et al., 1994; Fernandez-Conejero et al., 2012). Instead, a short train of stimuli (3–9 pulses, 2 ms ISI) is needed to overcome the inhibitory action of anesthetics (Deletis et al., 2009).
The short train of stimuli was initially introduced to elicit motor-evoked potentials in anesthetized humans (Taniguchi et al., 1993). The repetitive discharge of electrical current applied to cortical neurons is known to increase spinal cord excitability (Phillips and Porter, 1964). In analogy, building-up of facial motoneuron excitability is likely effective for BR elicitation under general anesthesia.
The elicitability of the BR varies from 80 to 90 % of the patients when a subject is fully anesthetized (Deletis et al., 2009; Ying et al., 2021; Aydinlar et al., 2022). Unlike awake subjects, only the oligosynaptic R1 component of the BR is present (Fig. 8A). During surgery, the BR can help detect impending injuries upon the trigeminal and facial nerves and uppermid pons. BR changes during posterior fossa surgery strongly correlate with the status of facial nerve motor function (Aydinlar et al., 2022) and trigeminal nerve sensory function (Ying et al., 2021) at postoperative and third-month evaluations.
Figure 8.
A. Blink reflex (BR) elicited in a patient under total intravenous anesthesia (TIVA, two trials shown). The BR was elicited by applying a short train of 9 electrical pulses (2 ms inter-stimulus-interval, at 36 mA intensity) to the supraorbital nerve, ipsilateral to the recorded side (Deletis et al., 2009). B. Laryngeal adductor reflex (LAR) elicited in a patient under TIVA (two trials shown). The LAR was elicited by applying a short train of 3 electrical pulses (1–2 ms inter-stimulus-interval, at 9 mA intensity) applied to the laryngeal mucosa contralateral to the recording side (Sinclair et al., 2017b). C. Masseter H reflex elicited in a patient under TIVA. The first trial was elicited by stimulating the third branch of the trigeminal nerve (under the zygomatic arc) with a single pulse at a slightly higher intensity than the second to show M/H and isolated H, respectively (Ulkatan et al., 2017). D. Trigeminal-hypoglossal reflex (THR) elicited in a patient under TIVA (two trials shown). The THR was elicited by stimulating the third branch of the trigeminal nerve (under the zygomatic arc) with a short train of 3 electrical pulses at 30 mA.
8.3. Differences between the blink reflex and other brainstem reflexes obtained during general anesthesia
Other brainstem reflexes elicited under general anesthesia include the LAR, the H reflex of the masseter muscle, and the trigeminal-hypoglossal reflex (THR).
The LAR is a vagal, life-sustaining protective reflex that shields the lower airway from inhaled foreign bodies (Sasaki et al., 2003; Sinclair et al., 2017b). The LAR is evoked by applying an electrical stimulus in the supraglottic mucosa, utilizing surface electrodes placed on an EMGendotracheal tube (routinely used intra-operatively by endocrine neck surgeons) (Sinclair et al., 2017b). It can be recorded bilaterally from vocal cord adductor muscles (thyroarytenoid and lateral cricoarytenoid), showing short-latency R1 and long-latency R2 components, which, in contrast to the BR, are both bilateral regardless of the stimulated side (Fig. 8B). These observations have been made both, in awake subjects (Tellez et al., 2018) and in patients under general anesthesia (Sinclair et al., 2017a, 2018).
Unlike the BR R1, which to date has no known physiological function, a recent study provided evidence that the bilateral LAR R1 response is the electrical event that initiates vocal cord adduction (Tellez et al., 2021). In contrast, eyelid closure in humans predominantly relies on the BR R2 component. Thus, the BR and the LAR diverge from each other at this critical point. To effectively maintain visual surveillance of the surroundings, the human brain can supersede the response of closing the eyes when circumstances demand it. Hence, the properties of the bilateral, long-latency BR R2 component are highly modulated by supratentorial influences and conscious state.
Conversely, the larynx must have a short-latency, reflex mechanism that can override any other voluntary action to protect the human airway. In this line, conditioning protocols in awake humans have shown that the short-latency LAR R1 overrides phonation, respiratory, and swallowing tasks. On the contrary, the LAR R2 response is highly modulated and decreases during multitasking, such as swallowing, respiration, and phonation (Barkmeier et al., 2000; Kearney et al., 2005; Henriquez et al., 2007; Ludlow, 2011). Even more, the ipsi-and contralateral LAR R1 components can be elicited at all anesthesia depths if TIVA is used in 100% of patients. These findings could have significant implications in patients with a high risk of aspiration, undergoing sedation or anesthesia, provided the LAR R1 response contributes to airway protection by initiating vocal cord closure.
The performance of the BR and the LAR diverges under general anesthesia (Ambalavanar et al., 2002). A likely explanation relates to the pyramidal projections to the STN that facilitate the sensory transmission of the BR through the brainstem circuits (Kimura, 1974; Chase et al., 1980; Berardelli et al., 1983). Anesthetic agents conceivably impair these facilitatory pyramidal projections, making the BR less elicitable than the LAR under general anesthesia. The lateral tegmental field of the reticular formation carries the polysynaptic R2 component of the BR (Kimura and Lyon, 1972; Ongerboer de Visser and Kuypers, 1978; Aramideh et al., 1997) and likely the R2 component of the LAR (Sessle, 1973; Tanaka et al., 1995; Ambalavanar et al., 2004; Adachi et al., 2010) to the corresponding motor nuclei (facial and ambiguous, respectively). However, the BR R2 is abolished in deeply anesthetized subjects in contrast to the LAR R2 response. N-methyl-D-aspartate (NMDA) receptors, essential targets of intravenous anesthetics that enhance GABA and reduce NMDA activation, may play a role in this distinct presentation of the R2 between BR and LAR. NMDA receptors are involved in respiratory reflexes in the brainstem, modulating the medullary respiratory network (Pierrefiche et al., 1994; Haji et al., 1998). Classically conditioned eyeblink responses (Kishimoto et al., 1997) and elicitation of the LAR R2 response depend on NMDA receptor activation (Ambalavanar et al., 2004).
In addition to the BR, two other trigeminal-elicited brainstem reflexes have been investigated under general anesthesia. The homonymous and heteronymous H reflex in the masseter and temporalis muscles, respectively, have been recorded in patients under TIVA (Ulkatan et al., 2017) (Fig. 8C), despite the lack of classic facilitation by the teeth clenching. The THR or jaw-tongue reflexes coordinate the tongue’s position in the mouth relative to jaw movement. Rarely documented in awake subjects, they were successfully recorded under general anesthesia (TIVA) with an elicitability of 82.1% of the patients (Mirallave Pescador et al., 2022) (Fig. 8D). Interestingly, these authors elicited the THR in four patients by SON stimulation (V1 branch) while simultaneously evoking the trigeminal BR, probably due to trigeminal hyperexcitability secondary to offending vascular structures over the nerve. In a more appealing proposition, we hypothesize that a modified salience network (Guldenmund et al., 2013), deprived of higher neuron control input due to anesthetic drugs, may uncover how integrating sensory information across several inputs produces excitatory and inhibitory responses across different effectors through the reticular formation. Additional neurophysiological evidence of a link between trigeminal and hypoglossal networks in humans under anesthesia was provided by another group (Szelenyi and Fava, 2022). Trigeminal-hypoglossal connections are known to exist following two possible pathways: a monosynaptic connection between neurons of the mesencephalic trigeminal nucleus and hypoglossal motoneurons (Zhang et al., 2001, 2003) and through a polysynaptic network relaying on pontomedullary structures of the reticular formation (Tomioka et al., 1999; Zhang et al., 2003; Dong et al., 2005; Luo et al., 2006; Urban, 2015).
In summary, the ability to elicit and monitor brainstem reflexes during surgery has practical implications for improving neurosurgical outcomes. It also opens new opportunities for understanding the underlying physiology of these brainstem reflexes. The disruption of signaling between modulating supranuclear structures and the brainstem by anesthetic drugs may facilitate the examination of brainstem reflexes rarely recorded in awake subjects.
9. Concluding remarks and outlook
BR testing has been part of the neurophysiological armamentarium for over 50 years. Its topodiagnostic value is well established. Being a brainstem reflex, however, puts the BR in a strategically challenging position: on the one hand, the BR serves to reflect some aspects of brainstem functioning, but on the other hand, its circuitry is also subject to a multitude of afferent and efferent influences. Understanding the ways and mechanisms of BR modulation is the basis to understand human brainstem physiology and may serve to open new diagnostic avenues in clinical neurophysiology. Some of these aspects have already arrived in, or are on the verge of, clinical practice and are described and discussed in part 2 of this review (Gunduz et al., submitted).
Highlights.
Review of the history of the blink reflex (BR) and its modulation
Review of current and new developments in BR modulation
Understanding the principles of BR modulation may serve for future clinical applications
Acknowledgements
MH is supported by the NINDS Intramural Program.
Abbreviations
- BR
blink reflex
- CNS
central nervous system
- CR
conditioned response
- CS
conditioned stimulus
- EMG
electromyographic
- GABA
gamma-amino-butyric acid
- HBR
hand-blink reflex
- IPD
idiopathic Parkinson’s disease
- ISI
interstimulus interval
- LAR
laryngeal adductor reflex
- LTM
low-threshold mechanoreceptive
- NMDA
N-methyl-D-aspartate
- NS
nociceptive-specific
- OOc
orbicularis oculi muscle
- PPI
prepulse inhibition
- PSN
principal sensory nucleus
- SEP
somatosensory evoked potential
- SON
supraorbital nerve
- SRD
subnucleus reticularis dorsalis
- STN
spinal trigeminal nucleus
- THR
trigeminal-hypoglossal reflex
- TIVA
total intravenous anesthesia
- UR
unconditioned response
- US
unconditioned stimulus
- WDR
wide-dynamic-range
Footnotes
Conflict of interest
MH: Relevant: none. Irrelevant: MH gets a share of patent royalties given to NIH by Brainsway for licensing a patent on the H-coil, a device for magnetic stimulation. He serves on the Medical Advisory Board of Brainsway, QuantalX and VoxNeuro. He has consulted for Janssen Pharmaceutical.
None of the remaining authors has any conflict of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Accornero N, Berardelli A, Bini G, Cruccu G, Manfredi M. Corneal reflex elicited by electrical stimulation of the human cornea. Neurology 1980;30(7 Pt 1):782–5. 10.1212/wnl.30.7.782 [DOI] [PubMed] [Google Scholar]
- Accornero N, Berardelli A, Bini G, Cruccu G, Manfredi M, Caruso R, et al. [Method of recording the corneal reflex in humans, using physiological stimulation]. Boll Soc Ital Biol Sper 1978;54(3):249–51. [PubMed] [Google Scholar]
- Adachi M, Nonaka S, Katada A, Arakawa T, Ota R, Harada H, et al. Carbachol injection into the pontine reticular formation depresses laryngeal muscle activities and airway reflexes in decerebrate cats. Neurosci Res 2010;67(1):40–50. 10.1016/j.neures.2010.01.009 [DOI] [PubMed] [Google Scholar]
- Alvarez-Blanco S, Leon L, Valls-Solé J. The startle reaction to somatosensory inputs: different response pattern to stimuli of upper and lower limbs. Exp Brain Res 2009;195(2):285–92. 10.1007/s00221-009-1784-7 [DOI] [PubMed] [Google Scholar]
- Ambalavanar R, Purcell L, Miranda M, Evans F, Ludlow CL. Selective suppression of late laryngeal adductor responses by N-methyl-D-aspartate receptor blockade in the cat. J Neurophysiol 2002;87(3):1252–62. 10.1152/jn.00595.2001 [DOI] [PubMed] [Google Scholar]
- Ambalavanar R, Tanaka Y, Selbie WS, Ludlow CL. Neuronal activation in the medulla oblongata during selective elicitation of the laryngeal adductor response. J Neurophysiol 2004;92(5):2920–32. 10.1152/jn.00064.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aramideh M, Ongerboer de Visser BW. Brainstem reflexes: electrodiagnostic techniques, physiology, normative data, and clinical applications. Muscle Nerve 2002;26(1):14–30. 10.1002/mus.10120 [DOI] [PubMed] [Google Scholar]
- Aramideh M, Ongerboer de Visser BW, Koelman JH, Majoie CB, Holstege G. The late blink reflex response abnormality due to lesion of the lateral tegmental field. Brain 1997;120 ( Pt 9)(9):1685–92. 10.1093/brain/120.9.1685 [DOI] [PubMed] [Google Scholar]
- Aydinlar EI, Kocak M, Soykam HO, Mat B, Dikmen PY, Sezerman OU, et al. Intraoperative neuromonitoring of blink reflex during posterior fossa surgeries and its correlation with clinical outcome. J Clin Neurophysiol 2022;39(4):299–306. 10.1097/WNP.0000000000000777 [DOI] [PubMed] [Google Scholar]
- Baken BC, Nieuwenhuijzen PH, Bastiaanse CM, Dietz V, Duysens J. Cutaneous reflexes evoked during human walking are reduced when self-induced. J Physiol 2006;570(Pt 1):113–24. 10.1113/jphysiol.2005.095240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkmeier JM, Bielamowicz S, Takeda N, Ludlow CL. Modulation of laryngeal responses to superior laryngeal nerve stimulation by volitional swallowing in awake humans. J Neurophysiol 2000;83(3):1264–72. 10.1152/jn.2000.83.3.1264 [DOI] [PubMed] [Google Scholar]
- Bechterew W Die Funktion der Nervenzentren unter den einfachen Reflexen des verlängerten Markes. Die Leitungsbahnen im Gehirn und Rückenmark. 1. St. Petersburg; 1896. p. 172. [Google Scholar]
- Bechterew W Über die Reflexe im Antlitz und Kopfgebiete. Neurol Centralbl 1901;20:930–6. [Google Scholar]
- Bechterew W Über den Augenreflex oder das Augenphenomen. Neurol Centralbl 1902;21:107–11. [Google Scholar]
- Bender LF. Blink reflex test. Electroencephalogr Clin Neurophysiol 1968;25(4):409. [PubMed] [Google Scholar]
- Berardelli A, Accornero N, Cruccu G, Fabiano F, Guerrisi V, Manfredi M. The orbicularis oculi response after hemispheral damage. J Neurol Neurosurg Psychiatry 1983;46(9):837–43. 10.1136/jnnp.46.9.837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berardelli A, Cruccu G, Kimura J, Ongerboer de Visser BW, Valls-Solé J. The orbicularis oculi reflexes. In: Deuschl G, Eisen A, editors. Electroencephalogr Clin Neurophysiol Suppl Recommendations for the Practice of Clinical Neurophysiology: Guidelines of the International Federation of Clinical Neurophysiology. 52. Ansterdam: Elsevier Science B.V.; 1999. p. 249–53. [PubMed] [Google Scholar]
- Berardelli A, Cruccu G, Manfredi M, Rothwell JC, Day BL, Marsden CD. The corneal reflex and the R2 component of the blink reflex. Neurology 1985a;35(6):797–801. 10.1212/wnl.35.6.797 [DOI] [PubMed] [Google Scholar]
- Berardelli A, Rothwell JC, Day BL, Marsden CD. Pathophysiology of blepharospasm and oromandibular dystonia. Brain 1985b;108 ( Pt 3):593–608. 10.1093/brain/108.3.593 [DOI] [PubMed] [Google Scholar]
- Bisdorff AR, Bronstein AM, Gresty MA. Responses in neck and facial muscles to sudden free fall and a startling auditory stimulus. Electroencephalogr Clin Neurophysiol 1994;93(6):409–16. 10.1016/0168-5597(94)90146-5 [DOI] [PubMed] [Google Scholar]
- Bisio A, Garbarini F, Biggio M, Fossataro C, Ruggeri P, Bove M. Dynamic shaping of the defensive peripersonal space through predictive motor mechanisms: when the “near” becomes “far”. J Neurosci 2017;37(9):2415–24. 10.1523/JNEUROSCI.0371-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore SJ, Wolpert D, Frith C. Why can’t you tickle yourself? Neuroreport 2000;11(11):R11–6. 10.1097/00001756-200008030-00002 [DOI] [PubMed] [Google Scholar]
- Böhme A Spezielle Physiologie des Zentralnervensystems der Wirbeltiere. In: Bethe A, Bergmann G, Embden G, Ellinger A, editors. Handbuch der normalen und pathologischen Physiologie. 10. Berlin: Springer; 1927. p. p982. [Google Scholar]
- Brodal P The auditory system (Section II: Sensory systems). In: Brodal P, editor. The central nervous system - structure and function. 3 ed. New York, Oxford: Oxford University Press; 2004a. p. 208–20. [Google Scholar]
- Brodal P The sense of equilibrium (Section II: Sensory systems). In: Brodal P, editor. The central nervous system - structure and function. 3 ed. New York, Oxford: Oxford University Press; 2004b. p. 221–32. [Google Scholar]
- Brodal P Reticular formation (Section IV: The brain stem and the cranial nerves). In: Brodal P, editor. The central nervous system - structure and function. 3 ed. New York, Oxford: Oxford University Press; 2004c. p. 333–48. [Google Scholar]
- Bromm B, Meier W. The intracutaneous stimulus: a new pain model for algesimetric studies. Methods Find Exp Clin Pharmacol 1984;6(7):405–10. [PubMed] [Google Scholar]
- Broser F, Hopf HC, Hufschmidt HJ. Die Ausbreitung des Trigeminusreflexes beim Menschen. Dtsch Z Nervenheilkd 1964;186(2). 10.1007/bf00243617 [DOI] [PubMed] [Google Scholar]
- Brown EN, Lydic R, Schiff ND. General anesthesia, sleep, and coma. N Engl J Med 2010;363(27):2638–50. 10.1056/NEJMra0808281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown EN, Purdon PL, Van Dort CJ. General anesthesia and altered states of arousal: a systems neuroscience analysis. Annu Rev Neurosci 2011;34:601–28. 10.1146/annurev-neuro-060909-153200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown P, Rothwell JC, Thompson PD, Britton TC, Day BL, Marsden CD. The hyperekplexias and their relationship to the normal startle reflex. Brain 1991a;114 ( Pt 4):1903–28. 10.1093/brain/114.4.1903 [DOI] [PubMed] [Google Scholar]
- Brown P, Rothwell JC, Thompson PD, Britton TC, Day BL, Marsden CD. New observations on the normal auditory startle reflex in man. Brain 1991b;114(4):1891–902. 10.1093/brain/114.4.1891 [DOI] [PubMed] [Google Scholar]
- Brozzoli C, Ehrsson HH, Farne A. Multisensory representation of the space near the hand: from perception to action and interindividual interactions. Neuroscientist 2014;20(2):122–35. 10.1177/1073858413511153 [DOI] [PubMed] [Google Scholar]
- Bufacchi RJ, Iannetti GD. Gravitational cues modulate the shape of defensive peripersonal space. Curr Biol 2016;26(21):R1133–R4. 10.1016/j.cub.2016.09.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bufacchi RJ, Iannetti GD. An action field theory of peripersonal space. Trends Cogn Sci 2018;22(12):1076–90. 10.1016/j.tics.2018.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bufacchi RJ, Iannetti GD. What do ‘peripersonal space measures’ really reflect? The action field perspective. The World at Our Fingertips. New York, NY, US: Oxford University Press; 2021. p. 155–80. 10.1093/oso/9780198851738.003.0009 [DOI] [Google Scholar]
- Bufacchi RJ, Liang M, Griffin LD, Iannetti GD. A geometric model of defensive peripersonal space. J Neurophysiol 2016;115(1):218–25. 10.1152/jn.00691.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bufacchi RJ, Ponticelli S, Novembre G, Kilintari M, Guo Y, Iannetti GD. Muscular effort increases hand-blink reflex magnitude. Neurosci Lett 2019;702:11–4. 10.1016/j.neulet.2018.11.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buser P, St Laurent J, Menini C. [Role of the inferior colliculus in the elicitation and specific cortical control of sound-induced clonic discharges in chloralose-anesthetized cats]. Exp Brain Res 1966;1(1):102–26. 10.1007/BF00235212 [DOI] [PubMed] [Google Scholar]
- Caraceni T, Avanzini G, Spreafico R, Negri S, Broggi G, Girotti F. Study of the excitability cycle of the blink reflex in Huntington’s chorea. Eur Neurol 1976;14(6):465–72. 10.1159/000114774 [DOI] [PubMed] [Google Scholar]
- Carrari GG. Über den Auriculo-palpebralreflex von Kisch und über den Rachenreflex. Arch Ital Otol Rinol Laringol 1925;36:507–14. [Google Scholar]
- Castellote JM, Kofler M, Mayr A, Saltuari L. Evidence for startle effects due to externally induced lower limb movements: implications in neurorehabilitation. Biomed Res Int 2017;2017:8471546. 10.1155/2017/8471546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase MH, Chandler SH, Nakamura Y. Intracellular determination of membrane potential of trigeminal motoneurons during sleep and wakefulness. J Neurophysiol 1980;44(2):349–58. 10.1152/jn.1980.44.2.349 [DOI] [PubMed] [Google Scholar]
- Cheng DT, Disterhoft JF, Power JM, Ellis DA, Desmond JE. Neural substrates underlying human delay and trace eyeblink conditioning. Proc Natl Acad Sci U S A 2008;105(23):8108–13. 10.1073/pnas.0800374105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisek P, Kalaska JF. Neural mechanisms for interacting with a world full of action choices. Annu Rev Neurosci 2010;33:269–98. 10.1146/annurev.neuro.051508.135409 [DOI] [PubMed] [Google Scholar]
- Clara M Das Nervensystem des Menschen. Leipzig: Johann Ambrosius Barth Verlag, 1942. [Google Scholar]
- Clark RE. The classical origins of Pavlov’s conditioning. Integr Physiol Behav Sci 2004;39(4):279–94. 10.1007/BF02734167 [DOI] [PubMed] [Google Scholar]
- Clery J, Guipponi O, Wardak C, Ben Hamed S. Neuronal bases of peripersonal and extrapersonal spaces, their plasticity and their dynamics: knowns and unknowns. Neuropsychologia 2015;70:313–26. 10.1016/j.neuropsychologia.2014.10.022 [DOI] [PubMed] [Google Scholar]
- Cohen LG, Ludlow CL, Warden M, Estegui M, Agostino R, Sedory SE, et al. Blink reflex excitability recovery curves in patients with spasmodic dysphonia. Neurology 1989;39(4):572–7. 10.1212/wnl.39.4.572 [DOI] [PubMed] [Google Scholar]
- Cohen LG, Starr A. Vibration and muscle contraction affect somatosensory evoked potentials. Neurology 1985;35(5):691–8. 10.1212/wnl.35.5.691 [DOI] [PubMed] [Google Scholar]
- Coppola G, Di Clemente L, Fumal A, Magis D, De Pasqua V, Pierelli F, et al. Inhibition of the nociceptive R2 blink reflex after supraorbital or index finger stimulation is normal in migraine without aura between attacks. Cephalalgia 2007;27(7):803–8. 10.1111/j.1468-2982.2007.01323.x [DOI] [PubMed] [Google Scholar]
- Correa LI, Cardenas K, Casanova-Molla J, Valls-Solé J. Thermoalgesic stimuli induce prepulse inhibition of the blink reflex and affect conscious perception in healthy humans. Psychophysiology 2019;56(4):e13310. 10.1111/psyp.13310 [DOI] [PubMed] [Google Scholar]
- Costa J, Valls-Solé J, Valldeoriola F, Pech C, Rumia J. Single subthalamic nucleus deep brain stimuli inhibit the blink reflex in Parkinson’s disease patients. Brain 2006;129(Pt 7):1758–67. 10.1093/brain/awl143 [DOI] [PubMed] [Google Scholar]
- Costa J, Valls-Sole J, Valldeoriola F, Rumia J. Subcortical interactions between somatosensory stimuli of different modalities and their temporal profile. J Neurophysiol 2008;100(3):1610–21. 10.1152/jn.90412.2008 [DOI] [PubMed] [Google Scholar]
- Costa YM, Baad-Hansen L, Bonjardim LR, Conti PCR, Svensson P. Reliability of the nociceptive blink reflex evoked by electrical stimulation of the trigeminal nerve in humans. Clin Oral Investig 2017;21(8):2453–63. 10.1007/s00784-016-2042-6 [DOI] [PubMed] [Google Scholar]
- Crossman AR. Brain stem. In: Standring S, editor. Gray’s Anatomy The Anatomical Basis of Medicine and Surgery. Section 2 - Neuroanatomy. 39 ed. Edinburgh: Elsevier; 2005. p. 327–51. [Google Scholar]
- Cruccu G, Agostino R, Berardelli A, Manfredi M. Excitability of the corneal reflex in man. Neurosci Lett 1986;63(3):320–4. 10.1016/0304-3940(86)90378-2 [DOI] [PubMed] [Google Scholar]
- Cruccu G, Iannetti GD, Marx JJ, Thoemke F, Truini A, Fitzek S, et al. Brainstem reflex circuits revisited. Brain 2005;128(Pt 2):386–94. 10.1093/brain/awh366 [DOI] [PubMed] [Google Scholar]
- D’Aleo G, Rifici C, Sessa E, Di Bella P, Bramanti P. R3 nociceptive reflex in multiple sclerosis patients with paroxysmal symptoms treated with gabapentin. Funct Neurol 2000;15(4):205–9. [PubMed] [Google Scholar]
- D’Aleo G, Sessa E, D’Aleo P, Rifici C, Di Bella P, Petix M, et al. Nociceptive R3 reflex in relapsingremitting multiple sclerosis patients. Funct Neurol 1999;14(1):43–7. [PubMed] [Google Scholar]
- Dallel R, Raboisson P, Woda A, Sessle BJ. Properties of nociceptive and non-nociceptive neurons in trigeminal subnucleus oralis of the rat. Brain Res 1990;521(1–2):95–106. 10.1016/0006-8993(90)91529-p [DOI] [PubMed] [Google Scholar]
- Daum I, Schugens MM, Ackermann H, Lutzenberger W, Dichgans J, Birbaumer N. Classical conditioning after cerebellar lesions in humans. Behav Neurosci 1993;107(5):748–56. 10.1037//0735-7044.107.5.748 [DOI] [PubMed] [Google Scholar]
- Daum I, Schugens MM, Breitenstein C, Topka H, Spieker S. Classical eyeblink conditioning in Parkinson’s disease. Mov Disord 1996;11(6):639–46. 10.1002/mds.870110608 [DOI] [PubMed] [Google Scholar]
- Davis M, Gendelman DS, Tischler MD, Gendelman PM. A primary acoustic startle circuit: lesion and stimulation studies. J Neurosci 1982;2(6):791–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson ME, Hazlett EA, Filion DL, Nuechterlein KH, Schell AM. Attention and schizophrenia: impaired modulation of the startle reflex. J Abnorm Psychol 1993;102(4):633–41. 10.1037//0021-843x.102.4.633 [DOI] [PubMed] [Google Scholar]
- Deletis V, Fernandez-Conejero I. Intraoperative monitoring and mapping of the functional integrity of the brainstem. J Clin Neurol 2016;12(3):262–73. 10.3988/jcn.2016.12.3.262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deletis V, Urriza J, Ulkatan S, Fernandez-Conejero I, Lesser J, Misita D. The feasibility of recording blink reflexes under general anesthesia. Muscle Nerve 2009;39(5):642–6. 10.1002/mus.21257 [DOI] [PubMed] [Google Scholar]
- Dimitrijevic MR, Faganel J, Gregoric M, Nathan PW, Trontelj JK. Habituation: effects of regular and stochastic stimulation. J Neurol Neurosurg Psychiatry 1972;35(2):234–42. 10.1136/jnnp.35.2.234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong CC, Macdonald DB, Akagami R, Westerberg B, Alkhani A, Kanaan I, et al. Intraoperative facial motor evoked potential monitoring with transcranial electrical stimulation during skull base surgery. Clin Neurophysiol 2005;116(3):588–96. 10.1016/j.clinph.2004.09.013 [DOI] [PubMed] [Google Scholar]
- Drummond PD, Bell A, Vo L. Painful stimulation of a sensitized site in the forearm inhibits ipsilateral trigeminal nociceptive blink reflexes. Exp Brain Res 2018;236(7):2097–105. 10.1007/s00221-018-5255-x [DOI] [PubMed] [Google Scholar]
- Duranti R, Galletti R, Pantaleo T. Relationships between characteristics of electrical stimulation, muscle pain and blink responses in man. Electroencephalogr Clin Neurophysiol 1983;55(6):637–44. 10.1016/0013-4694(83)90273-0 [DOI] [PubMed] [Google Scholar]
- Eder DN, Elam M, Wallin BG. Sympathetic nerve and cardiovascular responses to auditory startle and prepulse inhibition. Int J Psychophysiol 2009;71(2):149–55. 10.1016/j.ijpsycho.2008.09.001 [DOI] [PubMed] [Google Scholar]
- Editorial. The facial or supra-orbital reflex. Lancet 1901;158(4083):1513–4. 10.1016/s0140-6736(01)74564-3 [DOI] [Google Scholar]
- Eekhof JL, Aramideh M, Bour LJ, Hilgevoord AA, Speelman HD, Ongerboer de Visser BW. Blink reflex recovery curves in blepharospasm, torticollis spasmodica, and hemifacial spasm. Muscle Nerve 1996;19(1):10–5. [DOI] [PubMed] [Google Scholar]
- Eger EI, II. 1 ed. Baltimore/London: Williams & Wilkins, 1974. [Google Scholar]
- Ellrich J, Andersen OK, Messlinger K, Arendt-Nielsen L. Convergence of meningeal and facial afferents onto trigeminal brainstem neurons: an electrophysiological study in rat and man. Pain 1999;82(3):229–37. 10.1016/S0304-3959(99)00063-9 [DOI] [PubMed] [Google Scholar]
- Ellrich J, Andersen OK, Treede RD, Arendt-Nielsen L. Convergence of nociceptive and nonnociceptive input onto the medullary dorsal horn in man. Neuroreport 1998;9(14):3213–7. 10.1097/00001756-199810050-00015 [DOI] [PubMed] [Google Scholar]
- Ellrich J, Bromm B, Hopf HC. Pain-evoked blink reflex. Muscle Nerve 1997;20(3):265–70. [DOI] [PubMed] [Google Scholar]
- Ellrich J, Hopf HC. The R3 component of the blink reflex: normative data and application in spinal lesions. Electroencephalogr Clin Neurophysiol 1996;101(4):349–54. 10.1016/0924-980x(96)95684-2 [DOI] [PubMed] [Google Scholar]
- Ellrich J, Katsarava Z, Przywara S, Kaube H. Is the R3 component of the human blink reflex nociceptive in origin? Pain 2001;91(3):389–95. [DOI] [PubMed] [Google Scholar]
- Ellrich J, Messlinger K. Afferent input to the medullary dorsal horn from the contralateral face in rat. Brain Res 1999;826(2):321–4. 10.1016/s0006-8993(99)01305-0 [DOI] [PubMed] [Google Scholar]
- Ellrich J, Treede RD. Characterization of blink reflex interneurons by activation of diffuse noxious inhibitory controls in man. Brain Res 1998;803(1–2):161–8. 10.1016/s0006-8993(98)00646-5 [DOI] [PubMed] [Google Scholar]
- Erkol G, Kızıltan ME, Uluduz D, Uzun N. Somatosensory eye blink reflex in peripheral facial palsy. Neurosci Lett 2009;460(3):201–4. 10.1016/j.neulet.2009.05.077 [DOI] [PubMed] [Google Scholar]
- Esteban A A neurophysiological approach to brainstem reflexes. Blink reflex. Neurophysiol Clin 1999;29(1):7–38. 10.1016/S0987-7053(99)80039-2 [DOI] [PubMed] [Google Scholar]
- Esteban A, Gimenez-Roldan S. Blink reflex in Huntingon’s chorea and Parkinson’s disease. Acta Neurol Scand 1975;52(2):145–57. 10.1111/j.1600-0404.1975.tb05768.x [DOI] [PubMed] [Google Scholar]
- Etemadi L, Jirenhed DA, Rasmussen A. Effects of working memory load and CS-US intervals on delay eyeblink conditioning. NPJ Sci Learn 2023;8(1):16. 10.1038/s41539-023-00167-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eysenck HJ. Extraversion and the acquisition of eyeblink and GSR conditioned responses. Psychol Bull 1965;63(4):258–70. 10.1037/h0021921 [DOI] [PubMed] [Google Scholar]
- Feldman JL, Del Negro CA. Looking for inspiration: new perspectives on respiratory rhythm. Nat Rev Neurosci 2006;7(3):232–42. 10.1038/nrn1871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson IT, Lenman JA, Johnston BB. Habituation of the orbicularis oculi reflex in dementia and dyskinetic states. J Neurol Neurosurg Psychiatry 1978;41(9):824–8. 10.1136/jnnp.41.9.824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Conejero I, Ulkatan S, Sen C, Deletis V. Intra-operative neurophysiology during microvascular decompression for hemifacial spasm. Clin Neurophysiol 2012;123(1):78–83. 10.1016/j.clinph.2011.10.007 [DOI] [PubMed] [Google Scholar]
- Ferrari E, Messina C. Blink reflexes during sleep and wakefulness in man. Electroencephalogr Clin Neurophysiol 1972;32(1):55–62. 10.1016/0013-4694(72)90227-1 [DOI] [PubMed] [Google Scholar]
- Ferro Milone F, Perfetti CC. An EMG study on the late reflex component (nociceptive) of the orbicularis oculi muslce. Electroencephalogr Clin Neurophysiol 1968;25(4):409. [PubMed] [Google Scholar]
- Fine EJ, Sentz L, Soria E. The history of the blink reflex. Neurology 1992;42(2):450–4. 10.1212/wnl.42.2.450 [DOI] [PubMed] [Google Scholar]
- Flaten MA, Blumenthal TD. A parametric study of the separate contributions of the tactile and acoustic components of airpuffs to the blink reflex. Biol Psychol 1998;48(3):227–34. 10.1016/s0301-0511(98)00018-0 [DOI] [PubMed] [Google Scholar]
- Fossataro C, Sambo CF, Garbarini F, Iannetti GD. Interpersonal interactions and empathy modulate perception of threat and defensive responses. Sci Rep 2016;6:19353. 10.1038/srep19353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox JE. Excitatory and inhibitory components of the eyeblink responses to startle evoking stimuli, studied in the human subject. Electroencephalogr Clin Neurophysiol 1978;44(4):490–501. 10.1016/0013-4694(78)90033-0 [DOI] [PubMed] [Google Scholar]
- Galant JS. Der cephalopalpebrale Reflex, der cephalo-palpebro-nasale Reflex und ihnen verwandte Reflexe, die vom Schädeldach ausgelöst werden. Psychiatrisch-neurologische Wochenschrift 1926;28:490–2. [Google Scholar]
- Galant JS. Über den periostalen Zygomaticusreflex. Kinderarztl Prax 1932;3:453–5. [Google Scholar]
- Gallenga R Sopra un riflesso palpebrale da stimolazione del nervo laringeo superiore. Arch Ottalm 1930;87:410–5. [Google Scholar]
- Gandiglio G, Fra L. Further observations on facial reflexes. J Neurol Sci 1967;5(2):273–85. 10.1016/0022-510x(67)90136-0 [DOI] [PubMed] [Google Scholar]
- Garcia-Rill E, Saper CB, Rye DB, Kofler M, Nonnekes J, Lozano A, et al. Focus on the pedunculopontine nucleus. Consensus review from the May 2018 brainstem society meeting in Washington, DC, USA. Clin Neurophysiol 2019;130(6):925–40. 10.1016/j.clinph.2019.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerwig M, Kolb FP, Timmann D. The involvement of the human cerebellum in eyeblink conditioning. Cerebellum 2007;6(1):38–57. 10.1080/14734220701225904 [DOI] [PubMed] [Google Scholar]
- Gogan P The startle and orienting reactions in man. A study of their characteristics and habituation. Brain Res 1970;18:117–35. [DOI] [PubMed] [Google Scholar]
- Gomez-Wong E, Valls-Solé J. Effects of a prepulse stimulus on the masseteric inhibitory reflex in humans. Neurosci Lett 1996;208(3):183–6. 10.1016/0304-3940(96)12574-x [DOI] [PubMed] [Google Scholar]
- Graham FK. Presidential Address, 1974. The more or less startling effects of weak prestimulation. Psychophysiology 1975;12(3):238–48. 10.1111/j.1469-8986.1975.tb01284.x [DOI] [PubMed] [Google Scholar]
- Gregoric M Habituation of the blink reflex. Role of selective attention. In: Desmedt JE, editor. New developments in electromyography and clinical neurophysiology. 3. Basel: Karger; 1973. p. 673–7. [Google Scholar]
- Guillain G Le réflex naso-palpébral (réflexe trijumeau-facial) et sa valeur pronostique dans la paralysie faciale. C R Soc Biol (Paris) 1920;83:1394–6. [Google Scholar]
- Guldenmund P, Demertzi A, Boveroux P, Boly M, Vanhaudenhuyse A, Bruno MA, et al. Thalamus, brainstem and salience network connectivity changes during propofol-induced sedation and unconsciousness. Brain Connect 2013;3(3):273–85. 10.1089/brain.2012.0117 [DOI] [PubMed] [Google Scholar]
- Gunduz A, Kocak S, Gez S, Kızıltan ME. Fear leads to a deficit of prepulse inhibition of blink reflex in healthy humans. Neurol Sci 2019;40(12):2581–6. 10.1007/s10072-019-04028-6 [DOI] [PubMed] [Google Scholar]
- Gunduz A, Valls-Solé J, Serranová T, Coppola G, Kofler M, Jääskeläinen SK. The blink reflex and its modulation – Part 2: Pathophysiology and clinical utility. Clin Neurophysiol 2023. Submitted. [DOI] [PubMed] [Google Scholar]
- Haggard P Sense of agency in the human brain. Nat Rev Neurosci 2017;18(4):196–207. 10.1038/nrn.2017.14 [DOI] [PubMed] [Google Scholar]
- Haji A, Okazaki M, Yamazaki H, Takeda R. NMDA receptor-mediated inspiratory off-switching in pneumotaxic-disconnected cats. Neurosci Res 1998;32(4):323–31. 10.1016/s0168-0102(98)00103-5 [DOI] [PubMed] [Google Scholar]
- Hanzlikova Z, Kofler M, Slovak M, Vechetova G, Fecikova A, Kemlink D, et al. Prepulse inhibition of the blink reflex is abnormal in functional movement disorders. Mov Disord 2019;34(7):1022–30. 10.1002/mds.27706 [DOI] [PubMed] [Google Scholar]
- Hayashi H, Sumino R, Sessle BJ. Functional organization of trigeminal subnucleus interpolaris: nociceptive and innocuous afferent inputs, projections to thalamus, cerebellum, and spinal cord, and descending modulation from periaqueductal gray. J Neurophysiol 1984;51(5):890–905. 10.1152/jn.1984.51.5.890 [DOI] [PubMed] [Google Scholar]
- Hayashi H, Tabata T. Physiological properties of sensory neurons of the interstitial nucleus in the spinal trigeminal tract. Exp Neurol 1989;105(2):219–20. 10.1016/0014-4886(89)90123-4 [DOI] [PubMed] [Google Scholar]
- Helmchen C, Mohr C, Erdmann C, Binkofski F, Buchel C. Neural activity related to self- versus externally generated painful stimuli reveals distinct differences in the lateral pain system in a parametric fMRI study. Hum Brain Mapp 2006;27(9):755–65. 10.1002/hbm.20217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriquez VM, Schulz GM, Bielamowicz S, Ludlow CL. Laryngeal reflex responses are not modulated during human voice and respiratory tasks. J Physiol 2007;585(Pt 3):779–89. 10.1113/jphysiol.2007.143438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess K, Kern S, Schiller HH. Blink reflex in trigeminal sensory neuropathy. Electromyogr Clin Neurophysiol 1984;24(3):185–90. [PubMed] [Google Scholar]
- Hill K, Gogan DG, Dodge PR. Ocular signs associated with hydranencephaly. Am J Ophthalmol 1961;51(2):267–75. 10.1016/0002-9394(61)91949-3 [DOI] [PubMed] [Google Scholar]
- Hoogkamer W, Van Calenbergh F, Swinnen SP, Duysens J. Cutaneous reflex modulation and selfinduced reflex attenuation in cerebellar patients. J Neurophysiol 2015;113(3):915–24. 10.1152/jn.00381.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopf HC. Topodiagnostic value of brain stem reflexes. Muscle Nerve 1994;17(5):475–84. 10.1002/mus.880170502 [DOI] [PubMed] [Google Scholar]
- Hopf HC, Bier J, Brener B, Scheerer W. The blink reflex induced by photic stimuli. In: Desmedt JE, editor. New developments in electromyography and clinical neurophysiology. 3. Basel: Karger; 1973. p. 666–72. [Google Scholar]
- Hori A, Yasuhara A, Naito H, Yasuhara M. Blink reflex elicited by auditory stimulation in the rabbit. J Neurol Sci 1986;76(1):49–59. 10.1016/0022-510x(86)90141-3 [DOI] [PubMed] [Google Scholar]
- Hoshina Y, Sakuma Y. Changes in photically evoked blink reflex during sleep and wakefulness. Jpn J Ophthalmol 1991;35(2):182–7. [PubMed] [Google Scholar]
- Hu C, Zhang LB, Chen H, Xiong Y, Hu B. Neurosubstrates and mechanisms underlying the extinction of associative motor memory. Neurobiol Learn Mem 2015;126:78–86. 10.1016/j.nlm.2015.07.009 [DOI] [PubMed] [Google Scholar]
- Hudovernig K Zur Frage des Supraorbitalreflexes. Neurol Centralbl 1901;20:933–6. [Google Scholar]
- Imperatori CJ. Palatal palpebral reflex. Ann Otol Rhinol Laryngol 1930;39:458–62. [Google Scholar]
- Insola A, Mazzone P, Della Marca G, Capozzo A, Vitale F, Scarnati E. Pedunculopontine tegmental nucleus-evoked prepulse inhibition of the blink reflex in Parkinson’s disease. Clin Neurophysiol 2021;132(10):2729–38. 10.1016/j.clinph.2021.06.028 [DOI] [PubMed] [Google Scholar]
- Inui K, Itoh Y, Bayasgalan B, Shingaki M, Taniguchi T, Motomura E, et al. Target site of prepulse inhibition of the trigeminal blink reflex in humans. J Neurosci 2023;43(2):261–9. 10.1523/JNEUROSCI.1468-22.2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inui K, Takeuchi N, Sugiyama S, Motomura E, Nishihara M. GABAergic mechanisms involved in the prepulse inhibition of auditory evoked cortical responses in humans. PLoS One 2018;13(1):e0190481. 10.1371/journal.pone.0190481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inui K, Tsuruhara A, Kodaira M, Motomura E, Tanii H, Nishihara M, et al. Prepulse inhibition of auditory change-related cortical responses. BMC Neurosci 2012;13:135. 10.1186/1471-2202-13-135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ison JR, Sanes JN, Foss JA, Pinckney LA. Facilitation and inhibition of the human startle blink reflexes by stimulus anticipation. Behav Neurosci 1990;104(3):418–29. 10.1037//0735-7044.104.3.418 [DOI] [PubMed] [Google Scholar]
- Jääskeläinen SK. Electrophysiological study of blink reflex in humans: differences in mental and supraorbital nerves. Acta Physiol Scand 1995;154(2):143–50. 10.1111/j.1748-1716.1995.tb09896.x [DOI] [PubMed] [Google Scholar]
- Jerath N, Kimura J. F wave, A wave, H reflex, and blink reflex. Handb Clin Neurol 2019;160:225–39. 10.1016/B978-0-444-64032-1.00015-1 [DOI] [PubMed] [Google Scholar]
- Kadanoff D Die sensiblen Nervenendigungen in der mimischen Muskulatur des Menschen. Z Mikrosk Anat Forsch 1956;62:1–15. [PubMed] [Google Scholar]
- Katsarava Z, Ellrich J, Diener HC, Kaube H. Optimized stimulation and recording parameters of human ‘nociception specific’ blink reflex recordings. Clin Neurophysiol 2002;113(12):1932–6. 10.1016/s1388-2457(02)00307-3 [DOI] [PubMed] [Google Scholar]
- Kaube H, Katsarava Z, Kaufer T, Diener H, Ellrich J. A new method to increase nociception specificity of the human blink reflex. Clin Neurophysiol 2000;111(3):413–6. 10.1016/s1388-2457(99)00295-3 [DOI] [PubMed] [Google Scholar]
- Kawachi Y, Matsue Y, Shibata M, Imaizumi O, Gyoba J. Auditory startle reflex inhibited by preceding self-action. Psychophysiology 2014;51(1):97–102. 10.1111/psyp.12150 [DOI] [PubMed] [Google Scholar]
- Keane JR. Blinking to sudden illumination. A brain stem reflex present in neocortical death. Arch Neurol 1979;36(1):52–3. 10.1001/archneur.1979.00500370082023 [DOI] [PubMed] [Google Scholar]
- Kearney PR, Poletto CJ, Mann EA, Ludlow CL. Suppression of thyroarytenoid muscle responses during repeated air pressure stimulation of the laryngeal mucosa in awake humans. Ann Otol Rhinol Laryngol 2005;114(4):264–70. 10.1177/000348940511400403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan ZA. Revisiting the corneal and blink reflexes for primary and secondary trigeminal facial pain differentiation. Pain Res Manag 2021;2021:6664736. 10.1155/2021/6664736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiepe F, Kraus N, Hesselmann G. Sensory attenuation in the auditory modality as a window into predictive processing. Front Hum Neurosci 2021;15:704668. 10.3389/fnhum.2021.704668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura J Disorder of interneurons in Parkinsonism. The orbicularis oculi reflex to paired stimuli. Brain 1973;96(1):87–96. 10.1093/brain/96.1.87 [DOI] [PubMed] [Google Scholar]
- Kimura J Effect of hemispheral lesions on the contralateral blink reflex. Neurology 1974;24(2):168–74. 10.1212/wnl.24.2.168 [DOI] [PubMed] [Google Scholar]
- Kimura J Studies of the facial nerve and the blink reflex. In: Kimura J, editor. Electrodiagnosis in diseases of nerve and muscle: principles and practice. 4 ed. Oxford: Oxford University Press; 2013. p. 180–207. [Google Scholar]
- Kimura J, Daube J, Burke D, Hallett M, Cruccu G, Ongerboer de Visser BW, et al. Human reflexes and late responses. Report of an IFCN committee. Electroencephalogr Clin Neurophysiol 1994;90(6):393–403. 10.1016/0013-4694(94)90131-7 [DOI] [PubMed] [Google Scholar]
- Kimura J, Harada O. Excitability of the orbicularis oculi reflex in all night sleep: its suppression in nonrapid eye movement and recovery in rapid eye movement sleep. Electroencephalogr Clin Neurophysiol 1972;33(4):369–77. 10.1016/0013-4694(72)90117-4 [DOI] [PubMed] [Google Scholar]
- Kimura J, Lyon LW. Orbicularis oculi reflex in the Wallenberg syndrome: alteration of the late reflex by lesions of the spinal tract and nucleus of the trigeminal nerve. J Neurol Neurosurg Psychiatry 1972;35(2):228–33. 10.1136/jnnp.35.2.228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura J, Powers JM, Van Allen MW. Reflex response of orbicularis oculi muscle to supraorbital nerve stimulation. Study in normal subjects and in peripheral facial paresis. Arch Neurol 1969;21(2):193–9. 10.1001/archneur.1969.00480140093009 [DOI] [PubMed] [Google Scholar]
- Kinukawa TA, Inui K, Taniguchi T, Takeuchi N, Sugiyama S, Nishihara M, et al. Conditioned pain modulation: comparison of the effects on nociceptive and non-nociceptive blink reflex. Neuroscience 2021;468:168–75. 10.1016/j.neuroscience.2021.06.019 [DOI] [PubMed] [Google Scholar]
- Kisch B Ein unbekannter Lidschlag- und Tränenreflex. Pflugers Arch Gesamte Physiol Menschen Tiere 1919;173:224–42. [Google Scholar]
- Kishimoto Y, Kawahara S, Kirino Y, Kadotani H, Nakamura Y, Ikeda M, et al. Conditioned eyeblink response is impaired in mutant mice lacking NMDA receptor subunit NR2A. Neuroreport 1997;8(17):3717–21. 10.1097/00001756-199712010-00012 [DOI] [PubMed] [Google Scholar]
- Kofler M, Halder W. Alterations in excitatory and inhibitory brainstem interneuronal circuits in fibromyalgia: evidence of brainstem dysfunction. Clin Neurophysiol 2014;125(3):593–601. 10.1016/j.clinph.2013.08.009 [DOI] [PubMed] [Google Scholar]
- Kofler M, Kumru H, Schaller J, Saltuari L. Blink reflex prepulse inhibition and excitability recovery: influence of age and sex. Clin Neurophysiol 2013;124(1):126–35. 10.1016/j.clinph.2012.07.001 [DOI] [PubMed] [Google Scholar]
- Kofler M, Müller J, Valls-Solé J. Auditory startle responses as a probe of brainstem function in healthy subjects and patients with movement disorders. In: Cruccu G, Hallett M, editors. Clin Neurophysiol Suppl Brainstem Function and Dysfunction. 58: Elsevier Science B.V.; 2006. p. 232–48. [DOI] [PubMed] [Google Scholar]
- Kofler M, Valls-Sole J, Thurner M, Pucks-Faes E, Versace V. In the spotlight: How the brainstem modulates information flow. Clin Neurophysiol 2023a;148:52–64. 10.1016/j.clinph.2023.01.013 [DOI] [PubMed] [Google Scholar]
- Kofler M, Valls-Solé J, Thurner M, Pucks-Faes E, Versace V. Prepulse inhibition of the blink reflex –The role of prepulse stimulus intensity versus prepulse-induced blink reflex size. Clin Neurophysiol 2023b;150. 10.1016/j.clinph.2023.03.057 [DOI] [Google Scholar]
- Kornilow A Ueber cerebrale und spinale Reflexe. Dtsch Z Nervenheilkd 1903;23:216–42. [Google Scholar]
- Kronenbuerger M, Gerwig M, Brol B, Block F, Timmann D. Eyeblink conditioning is impaired in subjects with essential tremor. Brain 2007;130(Pt 6):1538–51. 10.1093/brain/awm081 [DOI] [PubMed] [Google Scholar]
- Kugelberg E [Facial reflexes]. Brain 1952;75(3):385–96. 10.1093/brain/75.3.385 [DOI] [PubMed] [Google Scholar]
- Kumru H, Kofler M. Effect of spinal cord injury and of intrathecal baclofen on brainstem reflexes. Clin Neurophysiol 2012;123(1):45–53. 10.1016/j.clinph.2011.06.036 [DOI] [PubMed] [Google Scholar]
- Kumru H, Kofler M, Valls-Sole J. Modulation of brainstem reflexes induced by non-invasive brain stimulation: is there a future? Neural Regen Res 2021;16(10):2004–5. 10.4103/1673-5374.308083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumru H, Kofler M, Valls-Solé J, Vidal J. Brainstem reflex excitability after high-frequency repetitive transcranial magnetic stimulation in healthy and spinal cord injury subjects. Brain Res Bull 2019;147:86–91. 10.1016/j.brainresbull.2019.02.002 [DOI] [PubMed] [Google Scholar]
- Kumru H, Stetkarova I, Schindler C, Vidal J, Kofler M. Neurophysiological evidence for muscle tone reduction by intrathecal baclofen at the brainstem level. Clin Neurophysiol 2011;122(6):1229–37. 10.1016/j.clinph.2010.09.010 [DOI] [PubMed] [Google Scholar]
- Landis C, Hunt WA. Studies of the startle pattern: III. Facial pattern. J Psychol 1936;2(1):215–9. 10.1080/00223980.1936.9917454 [DOI] [Google Scholar]
- Landis C, Hunt WA. The startle pattern. New York: Farrar & Rinehart, 1939. [Google Scholar]
- Le Bars D The whole body receptive field of dorsal horn multireceptive neurones. Brain Res Brain Res Rev 2002;40(1–3):29–44. 10.1016/s0165-0173(02)00186-8 [DOI] [PubMed] [Google Scholar]
- Leis AA, Kofler M, Stokic DS, Grubwieser GJ, Delapasse JS. Effect of the inhibitory phenomenon following magnetic stimulation of cortex on brainstem motor neuron excitability and on the cortical control of brainstem reflexes. Muscle Nerve 1993;16(12):1351–8. 10.1002/mus.880161213 [DOI] [PubMed] [Google Scholar]
- Leon L, Casanova-Molla J, Lauria G, Valls-Solé J. The somatosensory blink reflex in upper and lower brainstem lesions. Muscle Nerve 2011;43(2):196–202. 10.1002/mus.21810 [DOI] [PubMed] [Google Scholar]
- Lewandowsky M Die Störungen der Reflexe. In: Lewandowsky M, editor. Handbuch der Neurologie. Berlin: Springer; 1910. p. 584. [Google Scholar]
- Li R, Zhang W, Zhang J, Zhang H, Chen H, Hu Z, et al. Sustained activity of hippocampal parvalbumin-expressing interneurons supports trace eyeblink conditioning in mice. J Neurosci 2022;42(44):8343–60. 10.1523/JNEUROSCI.0834-22.2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libet B Mind time. The temporal factor in consciousness. London, UK: Harvard University Press, 2004. Jan. [Google Scholar]
- Loi N, Ginatempo F, Doppiu C, Deriu F. Emotional face expressions influence the delay eye-blink classical conditioning. Neuroscience 2021;471:72–9. 10.1016/j.neuroscience.2021.07.019 [DOI] [PubMed] [Google Scholar]
- Lowitzsch K, Kuhnt U, Sakmann C, Maurer K, Hopf HC, Schott D, et al. Visual pattern evoked responses and blink reflexes in assessment of MS diagnosis. A clinical study of 135 multiple sclerosis/pathol. J Neurol 1976;213(1):17–32. 10.1007/BF00316336 [DOI] [PubMed] [Google Scholar]
- Ludlow CL. Central nervous system control of interactions between vocalization and respiration in mammals. Head Neck 2011;33 Suppl 1:S21–5. 10.1002/hed.21904 [DOI] [PubMed] [Google Scholar]
- Luo P, Zhang J, Yang R, Pendlebury W. Neuronal circuitry and synaptic organization of trigeminal proprioceptive afferents mediating tongue movement and jaw-tongue coordination via hypoglossal premotor neurons. Eur J Neurosci 2006;23(12):3269–83. 10.1111/j.1460-9568.2006.04858.x [DOI] [PubMed] [Google Scholar]
- Macerollo A, Chen JC, Parees I, Kassavetis P, Kilner JM, Edwards MJ. Sensory attenuation assessed by sensory evoked potentials in functional movement disorders. PLoS One 2015;10(6):e0129507. 10.1371/journal.pone.0129507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magis D, Ambrosini A, Bendtsen L, Ertas M, Kaube H, Schoenen J, et al. Evaluation and proposal for optimalization of neurophysiological tests in migraine: part 1--electrophysiological tests. Cephalalgia 2007;27(12):1323–38. 10.1111/j.1468-2982.2007.01440.x [DOI] [PubMed] [Google Scholar]
- Magis D, Vigano A, Sava S, d’Elia TS, Schoenen J, Coppola G. Pearls and pitfalls: electrophysiology for primary headaches. Cephalalgia 2013;33(8):526–39. 10.1177/0333102413477739 [DOI] [PubMed] [Google Scholar]
- Magladery JW, Teasdall RD. Corneal reflexes. An electromygraphic study in man. Arch Neurol 1961;5:269–74. 10.1001/archneur.1961.00450150035004 [DOI] [PubMed] [Google Scholar]
- Mamiya K, Bay K, Skinner RD, Garcia-Rill E. Induction of long-lasting depolarization in medioventral medulla neurons by cholinergic input from the pedunculopontine nucleus. J Appl Physiol (1985) 2005;99(3):1127–37. 10.1152/japplphysiol.00253.2005 [DOI] [PubMed] [Google Scholar]
- Mantokoudis G, Saber Tehrani AS, Kattah JC, Eibenberger K, Guede CI, Zee DS, et al. Quantifying the vestibulo-ocular reflex with videooculography: nature and frequency of artifacts. Audiol Neurootol 2015;20(1):39–50. 10.1159/000362780 [DOI] [PubMed] [Google Scholar]
- Marelli RA, Hillel AD. Effects of general anesthesia on the human blink reflex. Head Neck 1989;11(2):137–49. 10.1002/hed.2880110207 [DOI] [PubMed] [Google Scholar]
- Maren S Neurobiology of Pavlovian fear conditioning. Annu Rev Neurosci 2001;24:897–931. 10.1146/annurev.neuro.24.1.897 [DOI] [PubMed] [Google Scholar]
- McCarthy DJ. Der Supraorbitalreflex. Ein neuer Reflex im Gebiet des 5. und 7. Nervenpaares. Neurol Centralbl 1901;20:800–1. [Google Scholar]
- McCarthy DJ. The supraorbital reflex: an explanatory note. Phil Med J 1902a;9:588–90. [Google Scholar]
- McCarthy DJ. Weiteres zur Kenntnis des Supraorbitalreflexes. Neurol Centralbl 1902b;21:843. [Google Scholar]
- Meincke U, Ferbert A, Vielhaber S, Buchner H. Exzitabilität des Blinkreflexes bei Selbst- und Fremdauslösung. EEG EMG Z Elektroenzephalogr Elektromyogr Verwandte Geb 1992;23:43–7. 10.1055/s-2008-1060699 [DOI] [PubMed] [Google Scholar]
- Meincke U, Morth D, Voss T, Gouzoulis-Mayfrank E. Electromyographical differentiation between the acoustic blink and startle reflex. Implications for studies investigating startle behavior. Eur Arch Psychiatry Clin Neurosci 2002;252(3):141–5. 10.1007/s00406-002-0375-4 [DOI] [PubMed] [Google Scholar]
- Meincke U, Topper R, Gouzoulis-Mayfrank E. The electrically elicited R2 blink reflex consists of distinct subcomponents. Electromyogr Clin Neurophysiol 2003;43(2):91–5. [PubMed] [Google Scholar]
- Meincke U, Topper R, Hoff P. The electrically elicited startle blink reflex in patients with schizophrenia: A threshold study of different reflex components. Neuropsychobiology 1999;39(2):76–80. 10.1159/000026564 [DOI] [PubMed] [Google Scholar]
- Mirallave Pescador A, Tellez MJ, Sanchez Roldan MLA, Samusyte G, Lawson EC, Coelho P, et al. Methodology for eliciting the brainstem trigeminal-hypoglossal reflex in humans under general anesthesia. Clin Neurophysiol 2022;137:1–10. 10.1016/j.clinph.2022.02.004 [DOI] [PubMed] [Google Scholar]
- Miwa H, Imamura N, Kogahara K, Ohori T, Mizuno Y. Somatosensory evoked blink response: findings in patients with Miller Fisher syndrome and in normal subjects. J Neurol Neurosurg Psychiatry 1995;58(1):95–9. 10.1136/jnnp.58.1.95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miwa H, Nohara C, Hotta M, Shimo Y, Amemiya K. Somatosensory-evoked blink response: investigation of the physiological mechanisms. Brain 1998;121 ( Pt 2):281–91. 10.1093/brain/121.2.281 [DOI] [PubMed] [Google Scholar]
- Miwa H, Yamaji Y, Abe H, Mizuno Y. Evaluation of the somatosensory evoked blink response in patients with neurological disorders. J Neurol Neurosurg Psychiatry 1996;60(5):539–43. 10.1136/jnnp.60.5.539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr C, Binkofski F, Erdmann C, Buchel C, Helmchen C. The anterior cingulate cortex contains distinct areas dissociating external from self-administered painful stimulation: a parametric fMRI study. Pain 2005;114(3):347–57. 10.1016/j.pain.2004.12.036 [DOI] [PubMed] [Google Scholar]
- Mohr C, Leyendecker S, Helmchen C. Dissociable neural activity to self- vs. externally administered thermal hyperalgesia: a parametric fMRI study. Eur J Neurosci 2008;27(3):739–49. 10.1111/j.1460-9568.2008.06036.x [DOI] [PubMed] [Google Scholar]
- Møller AR, Jannetta PJ. Blink reflex in patients with hemifacial spasm. Observations during microvascular decompression operations. J Neurol Sci 1986;72(2–3):171–82. 10.1016/0022-510x(86)90005-5 [DOI] [PubMed] [Google Scholar]
- Mount RA, Sridhar S, Hansen KR, Mohammed AI, Abdulkerim M, Kessel R, et al. Distinct neuronal populations contribute to trace conditioning and extinction learning in the hippocampal CA1. Elife 2021;10. 10.7554/eLife.56491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouraux A, Diukova A, Lee MC, Wise RG, Iannetti GD. A multisensory investigation of the functional significance of the “pain matrix”. Neuroimage 2011;54(3):2237–49. 10.1016/j.neuroimage.2010.09.084 [DOI] [PubMed] [Google Scholar]
- Mouraux A, Iannetti GD. Nociceptive laser-evoked brain potentials do not reflect nociceptive-specific neural activity. J Neurophysiol 2009;101(6):3258–69. 10.1152/jn.91181.2008 [DOI] [PubMed] [Google Scholar]
- Mourisse J, Lerou J, Zwarts M, Booij L. Electromyographic assessment of blink reflexes correlates with a clinical scale of depth of sedation/anaesthesia and BIS during propofol administration. Acta Anaesthesiol Scand 2004;48(9):1174–9. 10.1111/j.1399-6576.2004.00485.x [DOI] [PubMed] [Google Scholar]
- Muller MJ. Will it hurt less if I believe I can control it? Influence of actual and perceived control on perceived pain intensity in healthy male individuals: a randomized controlled study. J Behav Med 2012;35(5):529–37. 10.1007/s10865-011-9382-0 [DOI] [PubMed] [Google Scholar]
- Nakashima K, Rothwell JC, Thompson PD, Day BL, Berardelli A, Agostino R, et al. The blink reflex in patients with idiopathic torsion dystonia. Arch Neurol 1990;47(4):413–6. 10.1001/archneur.1990.00530040055019 [DOI] [PubMed] [Google Scholar]
- Nieuwenhuys R, Voogd J, van Huijzen C. The human central nervous system. 4th edition ed: Springer, 2008. [Google Scholar]
- Oka M, Tokunaga A, Murao T, Yokoi H, Okumura T, Hirata T, et al. Trigemino-facial reflex, its evoked electromyographic study on several neurologic disorders. Med J Osaka Univ 1958;9:389–96. [Google Scholar]
- Oldfield RC. Some recent experiments bearing on ‘internal inhibition’. Br J Psychol 1937;28(1):28–42. 10.1111/j.2044-8295.1937.tb00860.x [DOI] [Google Scholar]
- Olszewski J On the anatomical and functional organization of the spinal trigeminal nucleus. J Comp Neurol 1950;92(3):401–13. 10.1002/cne.900920305 [DOI] [PubMed] [Google Scholar]
- Ongerboer de Visser BW, Kuypers HG. Late blink reflex changes in lateral medullary lesions. An electrophysiological and neuro-anatomical study of Wallenberg’s Syndrome. Brain 1978;101(2):285–94. 10.1093/brain/101.2.285 [DOI] [PubMed] [Google Scholar]
- Ongerboer de Visser BW, Melchelse K, Megens PH. Corneal reflex latency in trigeminal nerve lesions. Neurology 1977;27(12):1164–7. 10.1212/wnl.27.12.1164 [DOI] [PubMed] [Google Scholar]
- Overend W Preliminary note on a new cranial reflex. Lancet 1896;147(3784):619. 10.1016/s0140-6736(01)93291-x [DOI] [Google Scholar]
- Overend W A further note concerning the frontal or supra-orbital reflex. Lancet 1902;1(4091):219. 10.1016/s0140-6736(01)80830-8 [DOI] [Google Scholar]
- Parees I, Brown H, Nuruki A, Adams RA, Davare M, Bhatia KP, et al. Loss of sensory attenuation in patients with functional (psychogenic) movement disorders. Brain 2014;137(Pt 11):2916–21. 10.1093/brain/awu237 [DOI] [PubMed] [Google Scholar]
- Pavesi G, Medici D, Macaluso GM. Trigeminofacial reflex in lingual neuropathy. Muscle Nerve 1996;19(12):1636–7. 10.1002/mus.880191206 [DOI] [PubMed] [Google Scholar]
- Pearce JM. Observations on the blink reflex. Eur Neurol 2008;59(3–4):221–3. 10.1159/000114053 [DOI] [PubMed] [Google Scholar]
- Pellegrini JJ, Horn AK, Evinger C. The trigeminally evoked blink reflex. I. Neuronal circuits. Exp Brain Res 1995;107(2):166–80. 10.1007/bf00230039 [DOI] [PubMed] [Google Scholar]
- Penders C, Delwaide P. Analyse électrophysiologique du réflexe de clignement chez l’homme normal et en clinique neurologique. Rev Electroencephalogr Neurophysiol Clin 1972;2(3):354–61. 10.1016/s0370-4475(72)80046-7 [DOI] [PubMed] [Google Scholar]
- Penders CA, Delwaide PJ. Êtude chez l’homme du cycle d’excitabilité de la réponse précoce du réflexe de clignement. C R Soc Biol 1969;163:228–32. [PubMed] [Google Scholar]
- Penders CA, Delwaide PJ. Blink reflex studies in patients with Parkinsonism before and during therapy. J Neurol Neurosurg Psychiatry 1971;34(6):674–8. 10.1136/jnnp.34.6.674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelan KD, Falls WM. The interstitial system of the spinal trigeminal tract in the rat: anatomical evidence for morphological and functional heterogeneity. Somatosens Mot Res 1989;6(4):367–99. 10.3109/08990228909144682 [DOI] [PubMed] [Google Scholar]
- Phillips CG, Porter R. The pyramidal projection to motoneurones of some muscle groups of the baboon’s forelimb. Prog Brain Res 1964;12:222–45. 10.1016/s0079-6123(08)60625-1 [DOI] [PubMed] [Google Scholar]
- Pierrefiche O, Foutz AS, Champagnat J, Denavit-Saubie M. NMDA and non-NMDA receptors may play distinct roles in timing mechanisms and transmission in the feline respiratory network. J Physiol 1994;474(3):509–23. 10.1113/jphysiol.1994.sp020041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai RS, Thai CM, Zweers L, Koutris M, Lobbezoo F, Costa YM, et al. Feasibility and reliability of intraorally evoked “nociceptive-specific” blink reflexes. Clin Oral Investig 2020;24(2):883–96. 10.1007/s00784-019-02966-1 [DOI] [PubMed] [Google Scholar]
- Pleshkov M, Zaitsev V, Starkov D, Demkin V, Kingma H, van de Berg R. Comparison of EOG and VOG obtained eye movements during horizontal head impulse testing. Front Neurol 2022;13:917413. 10.3389/fneur.2022.917413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimpel J, Geyer D, Hopf HC. Changes in the blink responses to combined trigeminal, acoustic and visual repetitive stimulation, studied in the human subject. Electroencephalogr Clin Neurophysiol 1982;54(5):552–60. 10.1016/0013-4694(82)90040-2 [DOI] [PubMed] [Google Scholar]
- Robleto K, Poulos AM, Thompson RF. Brain mechanisms of extinction of the classically conditioned eyeblink response. Learn Mem 2004;11(5):517–24. 10.1101/lm.80004 [DOI] [PubMed] [Google Scholar]
- Romaniello A, Valls-Solé J, Iannetti GD, Truini A, Manfredi M, Cruccu G. Nociceptive quality of the laser-evoked blink reflex in humans. J Neurophysiol 2002;87(3):1386–94. 10.1152/jn.00041.2001 [DOI] [PubMed] [Google Scholar]
- Rossi A, Scarpini C. Gating of trigemino-facial reflex from low-threshold trigeminal and extratrigeminal cutaneous fibres in humans. J Neurol Neurosurg Psychiatry 1992;55(9):774–80. 10.1136/jnnp.55.9.774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi B, Risaliti R, Rossi A. The R3 component of the blink reflex in man: a reflex response induced by activation of high threshold cutaneous afferents. Electroencephalogr Clin Neurophysiol 1989;73(4):334–40. 10.1016/0013-4694(89)90111-9 [DOI] [PubMed] [Google Scholar]
- Rossi B, Vignocchi G, Mazzoni M, Pardossi L, Bianchi F, Muratorio A. Causes of the instability of R3 component of electrically evoked blink reflex: role of the attention to the stimulus. Electromyogr Clin Neurophysiol 1993;33(1):49–53. [PubMed] [Google Scholar]
- Rothwell JC, Day BL, Berardelli A, Marsden CD. Habituation and conditioning of the human long latency stretch reflex. Exp Brain Res 1986;63(1):197–204. 10.1007/bf00235664 [DOI] [PubMed] [Google Scholar]
- Rushworth G Observations on blink reflexes. J Neurol Neurosurg Psychiatry 1962;25(2):93–108. 10.1136/jnnp.25.2.93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadnicka A, Rocchi L, Latorre A, Antelmi E, Teo J, Parees I, et al. A critical investigation of cerebellar associative learning in isolated dystonia. Mov Disord 2022;37(6):1187–92. 10.1002/mds.28967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambo CF, Forster B, Williams SC, Iannetti GD. To blink or not to blink: fine cognitive tuning of the defensive peripersonal space. J Neurosci 2012a;32(37):12921–7. 10.1523/JNEUROSCI.0607-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambo CF, Iannetti GD. Better safe than sorry? The safety margin surrounding the body is increased by anxiety. J Neurosci 2013;33(35):14225–30. 10.1523/JNEUROSCI.0706-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambo CF, Liang M, Cruccu G, Iannetti GD. Defensive peripersonal space: the blink reflex evoked by hand stimulation is increased when the hand is near the face. J Neurophysiol 2012b;107(3):880–9. 10.1152/jn.00731.2011 [DOI] [PubMed] [Google Scholar]
- Sasaki CT, Jassin B, Kim YH, Hundal J, Rosenblatt W, Ross DA. Central facilitation of the glottic closure reflex in humans. Ann Otol Rhinol Laryngol 2003;112(4):293–7. 10.1177/000348940311200401 [DOI] [PubMed] [Google Scholar]
- Schicatano EJ. The effects of attention on the trigeminal blink reflex. Percept Mot Skills 2016;122(2):444–51. 10.1177/0031512516640673 [DOI] [PubMed] [Google Scholar]
- Sechenov I Refleksy golovnogo mozga [“Reflexes of the brain“]. Meditsinsky vestnik 1863:47–8. [Google Scholar]
- Sessle BJ. Excitatory and inhibitory inputs to single neurones in the solitary tract nucleus and adjacent reticular formation. Brain Res 1973;53(2):319–31. 10.1016/0006-8993(73)90217-5 [DOI] [PubMed] [Google Scholar]
- Sessle BJ, Hu JW, Amano N, Zhong G. Convergence of cutaneous, tooth pulp, visceral, neck and muscle afferents onto nociceptive and non-nociceptive neurones in trigeminal subnucleus caudalis (medullary dorsal horn) and its implications for referred pain. Pain 1986;27(2):219–35. 10.1016/0304-3959(86)90213-7 [DOI] [PubMed] [Google Scholar]
- Shahani B The human blink reflex. J Neurol Neurosurg Psychiatry 1970;33(6):792–800. 10.1136/jnnp.33.6.792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahani B, Young RR. A note on blink reflexes. J Physiol 1968;198(2):103p–4p. 10.1113/jphysiol.1968.sp008635 [DOI] [PubMed] [Google Scholar]
- Shahani BT, Young RR. Human orbicularis oculi reflexes. Neurology 1972;22(2):149–54. 10.1212/wnl.22.2.149 [DOI] [PubMed] [Google Scholar]
- Shahani BT, Young RR. Blink reflex in orbicularis oculi. In: Desmedt JE, editor. New developments in electromyography and clinical neurophysiology. 3. Basel: Karger; 1973. p. 641–8. [Google Scholar]
- Sherrington CS. The integrative action of the nervous system. New Haven, CT: Yale University Press, 1906. [Google Scholar]
- Shill HA, De La Vega FJ, Samanta J, Stacy M. Motor learning in essential tremor. Mov Disord 2009;24(6):926–8. 10.1002/mds.22479 [DOI] [PubMed] [Google Scholar]
- Shults RC. Nociceptive neural organization in the trigeminal nuclei. In: Light AR, editor. The initial processing of pain and its descending control: Spinal and trigeminal systems. Pain and headache. 12. Basel: S. Karger AG; 1992. [Google Scholar]
- Silverstein LD, Graham FK, Calloway JM. Preconditioning and excitability of the human orbicularis oculi reflex as a function of state. Electroencephalogr Clin Neurophysiol 1980;48(4):406–17. Doi 10.1016/0013-4694(80)90133-9 [DOI] [PubMed] [Google Scholar]
- Simchowicz T Über den Nasenaugenreflex und den Nasenkinnreflex. Dtsch Z Nervenheilkd 1922;75(6):342–55. 10.1007/bf02549982 [DOI] [Google Scholar]
- Sinclair CF, Tellez MJ, Tapia OR, Ulkatan S. Contralateral R1 and R2 components of the laryngeal adductor reflex in humans under general anesthesia. Laryngoscope 2017a;127(12):E443–E8. 10.1002/lary.26744 [DOI] [PubMed] [Google Scholar]
- Sinclair CF, Tellez MJ, Tapia OR, Ulkatan S, Deletis V. A novel methodology for assessing laryngeal and vagus nerve integrity in patients under general anesthesia. Clin Neurophysiol 2017b;128(7):1399–405. 10.1016/j.clinph.2017.03.002 [DOI] [PubMed] [Google Scholar]
- Sinclair CF, Tellez MJ, Ulkatan S. Noninvasive, tube-based, continuous vagal nerve monitoring using the laryngeal adductor reflex: Feasibility study of 134 nerves at risk. Head Neck 2018;40(11):2498–506. 10.1002/hed.25377 [DOI] [PubMed] [Google Scholar]
- Sindou M, Fobe JL, Berthier E, Vial C. Facial motor responses evoked by direct electrical stimulation of the trigeminal root. Localizing value for radiofrequency thermorhizotomy. Acta Neurochir (Wien) 1994;128(1–4):57–67. 10.1007/BF01400654 [DOI] [PubMed] [Google Scholar]
- Smith SJ, Lees AJ. Abnormalities of the blink reflex in Gilles de la Tourette syndrome. J Neurol Neurosurg Psychiatry 1989;52(7):895–8. 10.1136/jnnp.52.7.895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soliven B, Meer J, Uncini A, Petajan J, Lovelace R. Physiologic and anatomic basis for contralateral R1 in blink reflex. Muscle Nerve 1988;11(8):848–51. 10.1002/mus.880110808 [DOI] [PubMed] [Google Scholar]
- Somervail R, Bufacchi RJ, Guo Y, Kilintari M, Novembre G, Swapp D, et al. Movement of environmental threats modifies the relevance of the defensive eye-blink in a spatially-tuned manner. Sci Rep 2019;9(1):3661. 10.1038/s41598-019-40075-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer J Hat die mimische Gesichtsmuskulatur Eigenreflexe oder nicht? Dtsch Z Nervenheilkd 1938;146. [Google Scholar]
- Sommer M, Grafman J, Clark K, Hallett M. Learning in Parkinson’s disease: eyeblink conditioning, declarative learning, and procedural learning. J Neurol Neurosurg Psychiatry 1999;67(1):27–34. 10.1136/jnnp.67.1.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer M, Grafman J, Litvan I, Hallett M. Impairment of eyeblink classical conditioning in progressive supranuclear palsy. Mov Disord 2001;16(2):240–51. 10.1002/mds.1050 [DOI] [PubMed] [Google Scholar]
- Stachowski NJ, Dougherty KJ. Spinal inhibitory interneurons: gatekeepers of sensorimotor pathways. Int J Mol Sci 2021;22(5). 10.3390/ijms22052667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg M Die Sehnenreflexe und ihre Bedeutung für die Pathologie der Nervensystems. Leipzig: F. Deuticke, 1893. [Google Scholar]
- Stoerk O Klinische und pathologische Beiträge zu den Erkrankungen der oberen Luftwege und des Ohres. Ist der Ohr-Lidschlagreflex ein pathognomisch verwertbares Symptom? Z Laryngol Rhinol Otol 1921;10:417–26. [Google Scholar]
- Strauss H Das Zusammenschrecken. Journal für Psychologie und Neurologie 1929;39:111–231. [Google Scholar]
- Stuart DG, Schaefer AT, Massion J, Graham BA, Callister RJ. Pioneers in CNS inhibition: 1. Ivan M. Sechenov, the first to clearly demonstrate inhibition arising in the brain. Brain Res 2014;1548:20–48. 10.1016/j.brainres.2013.12.006 [DOI] [PubMed] [Google Scholar]
- Sukhotinsky I, Minert A, Soja P, Devor M. Mesopontine switch for the induction of general anesthesia by dedicated neural pathways. Anesth Analg 2016;123(5):1274–85. 10.1213/ANE.0000000000001489 [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Shoemaker JM, Stephany N, Wasserman L, Ro HJ, Geyer MA. Prestimulus effects on startle magnitude: sensory or motor? Behav Neurosci 2002;116(4):672–81. 10.1037//0735-7044.116.4.672 [DOI] [PubMed] [Google Scholar]
- Szelenyi A, Fava E. Long latency responses in tongue muscle elicited by various stimulation sites in anesthetized humans - New insights into tongue-related brainstem reflexes. Brain Stimul 2022;15(3):566–75. 10.1016/j.brs.2022.03.003 [DOI] [PubMed] [Google Scholar]
- Tackmann W, Ettlin T, Barth R. Blink reflexes elicited by electrical, acoustic and visual stimuli. I. Normal values and possible anatomical pathways. Eur Neurol 1982;21(3):210–6. 10.1159/000115483 [DOI] [PubMed] [Google Scholar]
- Takehara-Nishiuchi K The anatomy and physiology of eyeblink classical conditioning. Curr Top Behav Neurosci 2018;37:297–323. 10.1007/7854_2016_455 [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Yoshida Y, Hirano M. Expression of Fos-protein activated by tactile stimulation on the laryngeal vestibulum in the cat’s lower brain stem. J Laryngol Otol 1995;109(1):39–44. 10.1017/s0022215100129196 [DOI] [PubMed] [Google Scholar]
- Taniguchi M, Cedzich C, Schramm J. Modification of cortical stimulation for motor evoked potentials under general anesthesia: technical description. Neurosurgery 1993;32(2):219–26. 10.1227/00006123-199302000-00011 [DOI] [PubMed] [Google Scholar]
- Tellez MJ, Axelrod F, Kaufmann H. The R3 component of the electrically elicited blink reflex is present in patients with congenital insensitivity to pain. Pain 2009;141(1–2):178–80. 10.1016/j.pain.2008.10.030 [DOI] [PubMed] [Google Scholar]
- Tellez MJ, Sinclair CF, Diaz-Baamonde A, Pelaez-Cruz R, Ulkatan S. The short-latency R1 response of the electrical laryngeal adductor reflex contributes to airway protection by initiating glottic closure. Clin Neurophysiol 2021;132(12):3160–5. 10.1016/j.clinph.2021.09.017 [DOI] [PubMed] [Google Scholar]
- Tellez MJ, Ulkatan S, Blitzer A, Sinclair CF. Unearthing a consistent bilateral R1 component of the laryngeal adductor reflex in awake humans. Laryngoscope 2018;128(11):2581–7. 10.1002/lary.27249 [DOI] [PubMed] [Google Scholar]
- Thatcher DB, Van Allen MW. Corneal reflex latency. Neurology 1971;21(7):735–7. 10.1212/wnl.21.7.735 [DOI] [PubMed] [Google Scholar]
- Thoma P, Dramel N, Grothe M, Lotze M, Fleischmann R, Strauss S. Impaired pain processing at a brainstem level is involved in maladaptive neuroplasticity in patients with chronic complex regional pain syndrome. Int J Mol Sci 2022;23(23). 10.3390/ijms232315368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RF. Neural mechanisms of classical conditioning in mammals. Philos Trans R Soc Lond B Biol Sci 1990;329(1253):161–70. 10.1098/rstb.1990.0161 [DOI] [PubMed] [Google Scholar]
- Todd NPM, Govender S, Colebatch JG. Non-invasive recording from the human cerebellum during a classical conditioning paradigm using the otolith-evoked blink reflex. Neurosci Lett 2021;765:136270. 10.1016/j.neulet.2021.136270 [DOI] [PubMed] [Google Scholar]
- Todd NPM, Govender S, Keller PE, Colebatch JG. Electrophysiological activity from over the cerebellum and cerebrum during eye blink conditioning in human subjects. Physiol Rep 2023;11(6):e15642. 10.14814/phy2.15642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolosa E, Montserrat L, Bayes A. Blink reflex studies in patients with focal dystonias. Adv Neurol 1988;50:517–24. [PubMed] [Google Scholar]
- Tomioka S, Nakajo N, Takata M. Inhibition of styloglossus motoneurons during the palatally induced jaw-closing reflex. Neuroscience 1999;92(1):353–60. 10.1016/s0306-4522(98)00758-1 [DOI] [PubMed] [Google Scholar]
- Topka H, Valls-Sole J, Massaquoi SG, Hallett M. Deficit in classical conditioning in patients with cerebellar degeneration. Brain 1993;116 ( Pt 4):961–9. 10.1093/brain/116.4.961 [DOI] [PubMed] [Google Scholar]
- Ulkatan S, Jaramillo AM, Tellez MJ, Goodman RR, Deletis V. Feasibility of eliciting the H reflex in the masseter muscle in patients under general anesthesia. Clin Neurophysiol 2017;128(1):123–7. 10.1016/j.clinph.2016.10.092 [DOI] [PubMed] [Google Scholar]
- Urban PP. Trigeminal-hypoglossal silent period: a pontomedullary brainstem reflex. Muscle Nerve 2015;51(4):538–40. 10.1002/mus.24349 [DOI] [PubMed] [Google Scholar]
- Valls-Solé J Assessment of excitability in brainstem circuits mediating the blink reflex and the startle reaction. Clin Neurophysiol 2012;123(1):13–20. 10.1016/j.clinph.2011.04.029 [DOI] [PubMed] [Google Scholar]
- Spontaneous Valls-Solé J., voluntary, and reflex blinking in clinical practice. J Clin Neurophysiol 2019;36(6):415–21. 10.1097/WNP.0000000000000561 [DOI] [PubMed] [Google Scholar]
- Valls-Solé J, Cammarota A, Alvarez R, Hallett M. Orbicularis oculi responses to stimulation of nerve afferents from upper and lower limbs in normal humans. Brain Res 1994;650(2):313–6. 10.1016/0006-8993(94)91797-3 [DOI] [PubMed] [Google Scholar]
- Valls-Sole J, Tolosa ES. Blink reflex excitability cycle in hemifacial spasm. Neurology 1989;39(8):1061–6. 10.1212/wnl.39.8.1061 [DOI] [PubMed] [Google Scholar]
- Valls-Solé J, Tolosa ES, Ribera G. Neurophysiological observations on the effects of botulinum toxin treatment in patients with dystonic blepharospasm. J Neurol Neurosurg Psychiatry 1991;54(4):310–3. 10.1136/jnnp.54.4.310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valls-Solé J, Veciana M, Serra J, Cruccu G, Romaniello A. Prepulse inhibition of the blink reflex by laser stimuli in normal humans. Neurosci Lett 2000;286(2):79–82. 10.1016/s0304-3940(00)01085-5 [DOI] [PubMed] [Google Scholar]
- Valls-Solé J, Vila N, Obach V, Alvarez R, González LE, Chamorro A. Brain stem reflexes in patients with Wallenberg’s syndrome: Correlation with clinical and magnetic resonance imaging (MRI) findings. Muscle Nerve 1996;19(9):1093–9. [DOI] [PubMed] [Google Scholar]
- Veits L Nachprüfungen über den Ohr-Lidschlagreflex. Zeitschrift für Hals-, Nasen- und Ohrenheilkunde 1926;14:308–11. [Google Scholar]
- Versace V, Campostrini S, Dezi S, Sebastianelli L, Ortelli P, Saltuari L, et al. Conscious agency vs. pre-conscious sensory filtering: Disparate suppression of trigeminal blink reflex by self-stimulation and by prepulses. Psychophysiology 2023;60(3):e14190. 10.1111/psyp.14190 [DOI] [PubMed] [Google Scholar]
- Versace V, Campostrini S, Sebastianelli L, Saltuari L, Valls-Sole J, Kofler M. Threat vs control: Potentiation of the trigeminal blink reflex by threat proximity is overruled by self-stimulation. Psychophysiology 2020;57(10):e13626. 10.1111/psyp.13626 [DOI] [PubMed] [Google Scholar]
- Versace V, Campostrini S, Sebastianelli L, Saltuari L, Valls-Sole J, Kofler M. Prepulse inhibition vs cognitive modulation of the hand-blink reflex. Sci Rep 2021;11(1):4618. 10.1038/s41598-021-84241-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versace V, Campostrini S, Sebastianelli L, Saltuari L, Valls-Solé J, Kofler M. Influence of posture on blink reflex prepulse inhibition induced by somatosensory inputs from upper and lower limbs. Gait Posture 2019a;73:120–5. 10.1016/j.gaitpost.2019.07.194 [DOI] [PubMed] [Google Scholar]
- Versace V, Campostrini S, Sebastianelli L, Soda M, Saltuari L, Lun S, et al. Adult-onset Gilles de la Tourette syndrome: psychogenic or organic? The challenge of abnormal neurophysiological findings. Front Neurol 2019b;10:461. 10.3389/fneur.2019.00461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidailhet M, Rothwell JC, Thompson PD, Lees AJ, Marsden CD. The auditory startle response in the Steele-Richardson-Olszewski syndrome and Parkinson’s disease. Brain 1992;115 ( Pt 4):1181–92. 10.1093/brain/115.4.1181 [DOI] [PubMed] [Google Scholar]
- Villanueva L, Bouhassira D, Le Bars D. The medullary subnucleus reticularis dorsalis (SRD) as a key link in both the transmission and modulation of pain signals. Pain 1996;67(2–3):231–40. 10.1016/0304-3959(96)03121-1 [DOI] [PubMed] [Google Scholar]
- von Dincklage F, Koppe P, Kotsch J, Baars JH, Rehberg B. Investigation of threshold and magnitude criteria of the nociceptive blink reflex. Clin Neurophysiol 2010;121(6):945–9. 10.1016/j.clinph.2010.01.012 [DOI] [PubMed] [Google Scholar]
- von Lewinski F, Schwan M, Paulus W, Trenkwalder C, Sommer M. Impairment of brainstem implicit learning paradigms differentiates multiple system atrophy (MSA) from idiopathic Parkinson syndrome. BMJ Open 2013;3(9):e003098. 10.1136/bmjopen-2013-003098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallwork SB, Bufacchi RJ, Moseley GL, Iannetti GD. Rethinking blinking: No cognitive modulation of reflex eye protection in early onset blindness. Clin Neurophysiol 2017;128(1):16–7. 10.1016/j.clinph.2016.10.089 [DOI] [PubMed] [Google Scholar]
- Wallwork SB, Talbot K, Camfferman D, Moseley GL, Iannetti GD. The blink reflex magnitude is continuously adjusted according to both current and predicted stimulus position with respect to the face. Cortex 2016;81:168–75. 10.1016/j.cortex.2016.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Wang JY, Luo F. Why self-induced pain feels less painful than externally generated pain: distinct brain activation patterns in self- and externally generated pain. PLoS One 2011;6(8):e23536. 10.1371/journal.pone.0023536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wartenberg R Studies in reflexes. Arch Neurol Psychiatry 1944;51(2). 10.1001/archneurpsyc.1944.02290260003001 [DOI] [Google Scholar]
- Weingrow SM. Facial reflexes. Arch Pediat 1933;50:234–54. [Google Scholar]
- Weisenburg TH. A consideration of the facial reflexes and nerve and muscle phenomena in the distribution of the fifth and seventh nerves. Univ Pa Med Bull 1903;16:63. [Google Scholar]
- Willer JC, Boulu P, Bratzlavsky M. Electrophysiological evidence for crossed oligosynaptic trigeminofacial connections in normal man. J Neurol Neurosurg Psychiatry 1984;47(1):87–90. 10.1136/jnnp.47.1.87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis WD. The pain system. Basel: Karger, 1985. [Google Scholar]
- Windholz G A comparative analysis of the conditional reflex discoveries of Pavlov and Twitmyer, and the birth of a paradigm. Pavlov J Biol Sci 1986;21(4):141–7. 10.1007/BF02734512 [DOI] [PubMed] [Google Scholar]
- Woodruff-Pak DS, Finkbiner RG, Sasse DK. Eyeblink conditioning discriminates Alzheimer’s patients from non-demented aged. Neuroreport 1990;1(1):45–8. 10.1097/00001756-199009000-00013 [DOI] [PubMed] [Google Scholar]
- Woodruff-Pak DS, Papka M. Alzheimer’s disease and eyeblink conditioning: 750 ms trace vs. 400 ms delay paradigm. Neurobiol Aging 1996;17(3):397–404. 10.1016/0197-4580(96)00022-x [DOI] [PubMed] [Google Scholar]
- Woodruff-Pak DS, Papka M, Romano S, Li YT. Eyeblink classical conditioning in Alzheimer’s disease and cerebrovascular dementia. Neurobiol Aging 1996;17(4):505–12. [PubMed] [Google Scholar]
- Yates SK, Brown WF. Light-stimulus-evoked blink reflex: methods, normal values, relation to other blink reflexes, and observations in multiple sclerosis. Neurology 1981;31(3):272–81. 10.1212/wnl.31.3.272 [DOI] [PubMed] [Google Scholar]
- Ying T, Bao B, Yuan Y, Zhong W, Zhu J, Tang Y, et al. Blink reflex monitoring in microvascular decompression for trigeminal neuralgia. Neurol Res 2021;43(7):591–4. 10.1080/01616412.2021.1900705 [DOI] [PubMed] [Google Scholar]
- Young RR. The clinical significance of exteroceptive reflexes. In: Desmedt JE, editor. New developments in electromyography and clinical neurophysiology. 3. Basel: Karger; 1973. p. 697–712. [Google Scholar]
- Zeri A Du réflexe trigemino-facial. Annali dell’ Instituto psichiatrico della Regia Università di Roma 1904. Rev Neurol 1906(1):15. [Google Scholar]
- Zhang J, Luo P, Pendlebury WW. Light and electron microscopic observations of a direct projection from mesencephalic trigeminal nucleus neurons to hypoglossal motoneurons in the rat. Brain Res 2001;917(1):67–80. 10.1016/s0006-8993(01)02911-0 [DOI] [PubMed] [Google Scholar]
- Zhang J, Pendlebury WW, Luo P. Synaptic organization of monosynaptic connections from mesencephalic trigeminal nucleus neurons to hypoglossal motoneurons in the rat. Synapse 2003;49(3):157–69. 10.1002/syn.10227 [DOI] [PubMed] [Google Scholar]


