Abstract
None of the clinical trials of omega-3 fatty acids using combinations of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) were able to show any effect on cardiovascular outcomes, despite reductions in triglyceride levels. In contrast, the Reduction of Cardiovascular Events With Icosapent Ethyl–Intervention Trial (REDUCE-IT), which employed high-dose (4 g) purified EPA, demonstrated a 25% reduction in atherosclerotic cardiovascular disease–related events compared with placebo (hazard ratio 0.75; 95% confidence interval 0.68–0.83; P < 0.001). Moreover, REDUCE-IT is the first clinical trial using a lipid-lowering agent as adjuvant therapy to a statin to show a significant reduction in cardiovascular mortality. Significant reductions in stroke, need for revascularization, and myocardial infarction were also observed. The pharmacology of EPA is distinct from that of DHA, with a differential effect on membrane structure, lipoprotein oxidation, and the production of downstream metabolites that promote the resolution of inflammation. Attained plasma levels of EPA may be an important determinant of efficacy, with a substudy of REDUCE-IT suggesting that the threshold for clinical benefit of EPA is approximately 100 μg/mL, a level achieved in only a minority of patients in other studies. No similar clinical trials of DHA monotherapy have been conducted, so no such threshold has been established. The results of the REDUCE-IT and the Japan EPA Lipid Intervention Study (JELIS) together affirm the efficacy of EPA therapy for cardiovascular disease risk reduction in certain patient populations.
Keywords: Eicosapentaenoic acid, Docosahexaenoic acid, Omega-3 fatty acid, Icosapent ethyl, Triglyceride, Cardiovascular diseases
Graphical abstract
Highlights
-
•
Mixed EPA + DHA therapies failed to demonstrate consistent reduction in CV events.
-
•
Only EPA alone plus statin reduced major CV events, as shown in REDUCE-IT and JELIS.
-
•
Plasma EPA levels may help explain CV benefit seen with EPA-only therapies.
-
•
EPA and DHA are distinct biochemically and exert different pharmacologic effects.
-
•
Future research should evaluate DHA monotherapy in patients with CV disease.
1. Introduction
Atherosclerotic cardiovascular disease (ASCVD) is the leading cause of death worldwide, and resulted in approximately 1.7 million deaths in the European Union (EU) in 2016 [1]. Although high levels of low-density lipoprotein cholesterol (LDL-C) are recognized as one of the most readily modifiable risk factors for ASCVD, hypertriglyceridemia is also associated with increased risk independent of LDL-C [2], [3], [4]. Statins are central to the management of cardiovascular (CV) disease through lowering of LDL-C levels, but have a relatively modest impact on triglyceride (TG) levels in patients with TG levels <250 mg/dL [5]. Recent longitudinal studies have re-emphasized the need for effective TG lowering, with many patients having residual CV risk despite control of LDL-C levels with statins [6]. However, TG-lowering therapies such as niacin and fibrates have also failed to show any meaningful reduction in the risk of ASCVD events when added to statins [7], [8].
Epidemiologic studies have suggested that populations with even a low to moderate intake of omega-3 fatty acids from fish in their diet have an unexpectedly low rate of CV disease and mortality [9], [10]. As a result, the potential for omega-3 fatty acids to reduce CV risk has long been of interest [11], and a number of omega-3 fatty acid products have been approved and are available (Table 1) [12], [13], [14], [15]. However, mixed omega-3 fatty acid products are not indicated to reduce CV events, with many high-profile trials of mixed omega-3 fatty acid products (docosahexaenoic acid [DHA] + eicosapentaenoic acid [EPA]) failing to show a reduction in ASCVD [16]. In the recent REDUCE-IT trial (Reduction of Cardiovascular Events With Icosapent Ethyl–Intervention Trial), a highly purified formulation of EPA demonstrated a significant risk reduction of ASCVD events in statin-treated patients ≥45 years of age with established CV disease, or ≥ 50 years of age with diabetes mellitus and at least one additional risk factor [17], leading to regulatory approvals for CV event risk reduction [12], [14].
Table 1.
Prescription omega-3 fatty acids.
| Formulation | Product | EPA/DHA composition | Dose | Indication |
|---|---|---|---|---|
| Icosapent ethyl (ethyl ester of EPA) | Vascepaa (US) [14] | 1-g capsules containing 998 mg icosapent ethyl | 2 × 1-g capsules twice daily with or following a meal | To reduce the risk of CV events in adult statin-treated patients with elevated TG (≥150 mg/dL) and established CV disease, or diabetes and at least two other CV risk factors |
| Vazkepa (EU) [12] | To reduce the risk of CV events in adult statin-treated patients at high CV risk with elevated TG (≥150 mg/dL) and established CV disease, or diabetes and at least one other CV risk factor | |||
| Omega-3-ethyl esters | Lovaza (US) [15]b | 1-g capsules containing ≥900 mg PUFA, including EPA 460 mg and DHA 380 mg | 4 capsules daily | To reduce TG levels in adult patients with severe (≥500 mg/dL) hypertriglyceridemia in adjunct to diet |
| Omacor (EU) [13]c | 2–4 capsules daily | Treatment of endogenous hypertriglyceridemia as a supplement to diet when dietary measures alone are insufficient to produce an adequate response: as monotherapy or in combination with statins when control of TG is insufficient |
CV, cardiovascular; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; EU, European Union; PUFA, polyunsaturated fatty acid; TG, triglycerides; US, United States.
Generic versions of Vascepa are available in the US; these generic versions are approved for treatment of severe hypertriglyceridemia (≥500 mg/dL) only.
Generic versions of Lovaza are available in the US.
Generic versions of Omacor are available in the EU.
2. Early evidence for omega-3 fatty acids and cv risk
It is well established that prescription omega-3 fatty acids can reduce TG levels by ≈20% to 30% in patients with TG levels 200 to 499 mg/dL and by ≥30% in patients with TG levels ≥500 mg/dL [18]. However, early CV outcomes trials failed to show a consistent benefit in CV risk reduction. A likely reason for this is that the dose of omega-3 fatty acids studied was too low. In a recent meta-analysis of 13 trials of omega-3 fatty acids (11 trials of DHA + EPA and 2 trials of EPA monotherapy), including some of the more recent trials reviewed below, the median dose of omega-3 fatty acids was 1000 mg (median EPA, 500 mg; median DHA, 380 mg) [19]. The same meta-analysis found that 1 g/day DHA, taken from combination DHA + EPA products, was associated with a nonsignificant 4% (95% confidence interval [CI] 0.89–1.03; P = 0.27) reduction in major CV events. A statistically significant 7% (95% confidence interval [CI] 0.91–0.95; P < 0.001) reduction in major CV events was observed for a 1 g/day dose of EPA, but more than half of studies did not employ a dose that high [19]. Given the apparent lack of benefit of combination DHA + EPA products in preventing CV disease and death, in 2019 the European Medicines Agency took steps that led to the removal of their indications for secondary prevention of CV disease [20].
Although most of the trials in the meta-analysis failed to demonstrate any CV benefit, the presumed health benefits of EPA and DHA, including supporting the health of the skin, eyes, and CV and central nervous systems, as well as reduced inflammation have led to a proliferation of fish oil dietary supplements. Because the regulation of dietary supplements is less stringent than that required for pharmaceutical products, such as prescription and over-the-counter medications [21], the quality and EPA content of these products can be quite variable. Contaminants such as saturated fats and oxidized lipids, including peroxides and secondary oxidation products, may counter the purported beneficial effects of omega-3 fatty acids by exerting atherogenic and proinflammatory effects within the arterial wall [22], [23].
There continues to be great interest in the use of omega-3 fatty acids for reducing CV risk, including prescription medications and dietary supplements. Here we review recent clinical trial data that shed new light on the best approach for use of omega-3 fatty acids to reduce ASCVD, and offer potential explanations as to why early trials failed, while more recent ones have finally shown promise.
3. Contemporary omega-3 fatty acid outcomes trials
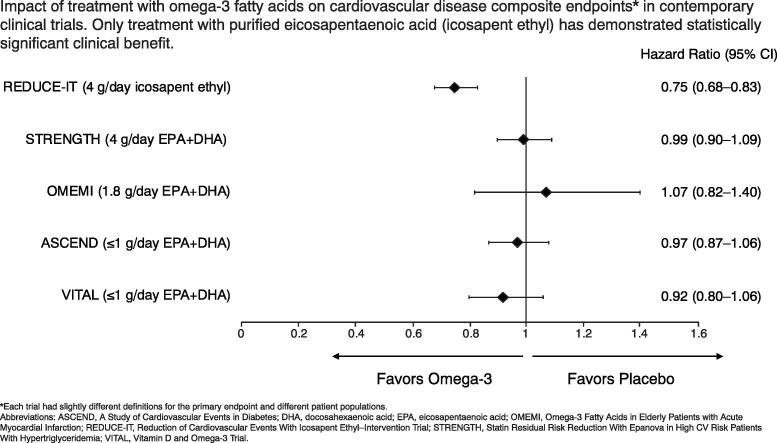
Contemporary CV outcomes trials using omega-3 fatty acids are summarized in Table 2 [17], [24], [25], [26], [27]. Findings from ASCEND (A Study of Cardiovascular Events in Diabetes) and VITAL (Vitamin D and Omega-3 Trial), which used low-dose (≤1 g) formulations, were consistent with earlier studies [26], [27]. ASCEND was conducted in patients with diabetes mellitus, while VITAL was a primary prevention study. Neither trial prospectively included patients with established CV disease or dyslipidemia, and only 75.2% and 34.9% were taking a statin, respectively. Both ASCEND and VITAL failed to show any reduction in CV endpoints (Table 2).
Table 2.
| REDUCE-IT [17] | STRENGTH [24] | OMEMI [25] | ASCEND [26] | VITAL [27] | |
|---|---|---|---|---|---|
| Intervention | 2 g icosapent ethyl twice daily vs 2 g twice daily mineral oil placebo | 4 g/day omega-3 carboxylic acids or 4 g/day corn oil placebo | 1.8 g/day (930 mg EPA + 660 mg DHA) vs 1.8 g/day corn oil placebo | 1 g/day (460 mg EPA + 380 mg DHA) or 1 g/day olive oil placebo | 1 g/day (460 mg EPA + 380 mg DHA) or 1 g/day olive oil placebo |
| Total number of patients | 8179 | 13,078 | 1027 | 15,480 | 25,871 |
| Entry criteria |
≥50 years of age with diabetes mellitus and ≥1 additional CV disease risk factor
|
Type 1 or 2 diabetes (≥40 years of age for men and ≥50 years of age for women) with ≥1 additional CV risk factor OR High-risk primary prevention patients (≥50 years of age for men and ≥60 years of age for women) with ≥1 additional CV risk factor
|
|
|
|
| Patients with established CVD, % | 70.7 | 55.9 | 100 | – | – |
| Concomitant statin, % | 100 | 100 | 95.2 | 75.2 | 34.9 |
| Median baseline metabolic parameters | |||||
| TG, mg/dL | 216 | 240 | 111.4a | – | – |
| LDL-C, mg/dL | 75 | 75 | 76.1a | – | – |
| Median follow-up | 4.9 years | 42 months | 24 months | 7.4 years | 5.3 years |
| Primary endpoint | Composite of CV death, nonfatal MI, nonfatal stroke, coronary revascularization, or hospitalization for unstable angina | Composite of CV death, nonfatal MI, nonfatal stroke, coronary revascularization, or hospitalization for unstable angina | First major CV event, consisting of nonfatal MI, unscheduled revascularization, stroke, or all-cause deathb | First serious vascular event (composite of nonfatal MI or stroke, transient ischemic attack, or vascular death excluding intracranial hemorrhage) | Major CV events (composite of MI, stroke, and death from any CV cause) |
| Primary endpoint result, HR (95% CI) | 0.75 (0.68–0.83; P < 0.001) | 0.99 (0.90–1.09; P = 0.84) | 1.07 (0.82–1.40; P = 0.62) | 0.97 (0.87−1.08; P = 0.55)c | 0.92 (0.80−1.06) |
| Components of the primary endpoint, HR (95% CI) | |||||
| All-cause mortality | – | – | 1.01 (0.54–1.88; P = 0.98) | – | – |
| CV death | 0.80 (0.66–0.98) | 1.09 (0.90–1.31; P = 0.37) | – | 0.81 (0.67−0.99)d | 0.96 (0.76−1.21) |
| MI | – | – | – | – | 0.72 (0.59−0.90) |
| Nonfatal MI | 0.70 (0.59–0.82) | 0.97 (0.81–1.17; P = 0.77) | 1.14 (0.72–1.80; P = 0.57) | 0.93 (0.76−1.14) | – |
| Stroke | – | – | 1.37 (0.65–2.88; P = 0.41) | – | 1.04 (0.83−1.31) |
| Nonfatal stroke | 0.71 (0.54–0.94) | 1.14 (0.90–1.45; P = 0.28) | – | 1.01 (0.84−1.22) | – |
| Transient ischemic attack | – | – | – | 1.03 (0.84–1.26) | – |
| Coronary revascularization | 0.66 (0.58–0.76) | 0.94 (0.83–1.08; P = 0.41) | 0.66 (0.34–1.30; P = 0.23) | – | – |
| Hospitalization for unstable angina | 0.68 (0.53–0.87) | 0.84 (0.63–1.12; P = 0.23) | – | – | – |
| Hospitalization for heart failure | – | – | 1.19 (0.62–2.26; P = 0.61) | – | – |
| Secondary endpoint | Composite of CV death, nonfatal MI, or nonfatal stroke | Composite of CV death, nonfatal MI, nonfatal stroke, coronary revascularization, or hospitalization for unstable angina in patients with established CV disease at baseline | New-onset atrial fibrillation | Serious vascular event or revascularization | Major CV events plus coronary revascularization |
| Secondary endpoint result, HR (95% CI) | 0.74 (0.65–0.83; P < 0.001) | 0.94 (0.84–1.05; P = 0.27) | 1.84 (0.98–3.44; P = 0.056) | 1.00 (0.91−1.09) | 0.93 (0.82−1.04) |
| Median placebo-corrected percent change in metabolic parameters after 1 year | |||||
| TG, mg/dL | −20.1 | −18 | −13.2% | – | – |
| LDL-C, mg/dL | −6.6 | +3 | −0.7% | – | – |
ASCEND, A Study of Cardiovascular Events in Diabetes; ASCVD, atherosclerotic cardiovascular disease; CI, confidence interval; CV, cardiovascular; CVD, cardiovascular disease; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; HDL-C, high-density lipoprotein cholesterol; HR, hazard ratio; LDL-C, low-density lipoprotein cholesterol; MI, myocardial infarction; OMEMI, Omega-3 Fatty acids in Elderly with Myocardial Infarction; qd, daily; REDUCE-IT, Reduction of Cardiovascular Events With Icosapent Ethyl–Intervention Trial; STRENGTH, Statin Residual Risk Reduction With Epanova in High CV Risk Patients With Hypertriglyceridemia; TG, triglyceride; VITAL, Vitamin D and Omega-3 Trial.
Mean.
Hospitalization for heart failure was added to the definition of major cardiovascular events.
Rate ratio.
Vascular death.
The OMEMI (Omega-3 Fatty acids in Elderly with Myocardial Infarction) and STRENGTH (Statin Residual Risk Reduction With Epanova in High CV Risk Patients With Hypertriglyceridemia) trials investigated higher doses of mixed EPA + DHA formulations (1.8 g/day and 4 g/day, respectively), while REDUCE-IT employed a higher dose of pure EPA (icosapent ethyl 4 g/day) [17], [24], [25]. Results from RESPECT-EPA (Randomized Trial for Evaluation in Secondary Prevention Efficacy of Combination Therapy–Statin and Eicosapentaenoic Acid), which is investigating EPA 1.8 g/day in 3900 statin-treated patients with stable coronary artery disease, are expected in 2022 [28]. Of note, all CV outcomes trials conducted to date have investigated EPA alone or EPA + DHA formulations; there have been no studies of purified DHA, so it is not possible to effectively evaluate its role in prevention of CV disease.
3.1. OMEMI
OMEMI was a multicenter, placebo-controlled, double-blind trial conducted in Norway [25]. A total of 1027 patients, aged between 70 and 82 years with baseline TG approximately 110 mg/dL, and who had been admitted for acute myocardial infarction (MI) were randomized to EPA + DHA 1.8 g/day or placebo; almost all patients were taking a statin at baseline. In the EPA + DHA group, 108 patients (21.0%) experienced a composite primary endpoint consisting of nonfatal MI, unscheduled revascularization, stroke, or all-cause death compared with 102 patients (19.8%) in the placebo group (hazard ratio [HR] 1.07; 95% confidence interval [CI] 0.82–1.40; P = 0.62). There was also no difference between groups for the various components of the primary endpoint. Elderly patients with a recent acute MI who were treated with EPA + DHA 1.8 g/day, therefore, did not have a lower incidence of major adverse CV events or death than those treated with placebo.
3.2. STRENGTH
The double-blind, randomized, multicenter STRENGTH trial randomized 13,078 statin-treated patients at high CV risk to receive EPA + DHA 4 g/day or placebo [24]. High CV risk was defined as either the presence of established ASCVD or type 1 or 2 diabetes (age ≥ 40 years for men and age ≥ 50 for women) with at least one additional risk factor (chronic smoking, hypertension, high sensitivity C-reactive protein [hsCRP] ≥2 mg/L, or moderately high albuminuria). The trial also included high-risk primary prevention patients (age ≥ 50 years for men and age ≥ 60 for women) with at least one additional risk factor (including a family history of premature coronary artery disease, chronic smoking, hsCRP ≥2 mg/L, impaired kidney function, or coronary calcium score > 300 Agatston units). Median baseline TG level was 240 mg/dL. After 54 months of follow-up, there was no difference in the primary composite endpoint of CV death, nonfatal MI, nonfatal stroke, coronary revascularization, and hospitalization for unstable angina; overall, 785 patients (12.0%) treated with EPA + DHA and 795 (12.2%) treated with corn oil placebo experienced the primary endpoint (HR 0.99; 95% CI 0.90–1.09; P = 0.84). Therefore, the mixed EPA + DHA formulation had no statistically significant effect on the incidence of major CV events in patients at increased CV risk compared with placebo. Furthermore, patients treated with the mixed EPA + DHA compound had an increased rate of investigator-reported atrial fibrillation compared with those treated with corn oil placebo (2.2% vs 1.3%; HR 1.69; 95% CI 1.29–2.21; P < 0.001).
3.3. JELIS
Thus far, our discussion of clinical trials involving omega-3 fatty acids has largely focused on mixed formulations of EPA plus DHA, which predominantly failed to demonstrate benefit in CV outcomes. Conversely, JELIS (Japan EPA Lipid Intervention Study) emerged as the first prospective randomized clinical trial of a highly purified, EPA-only omega-3 fatty acid to show significant reduction in risk of CV events [29]. The open-label trial included 18,645 Japanese patients with mean TG levels of 1.7 mmol/L who were randomized to receive 1800 mg EPA plus statin or statin alone. The primary endpoint was occurrence of any major coronary event, including sudden cardiac death, fatal and nonfatal MI, and other nonfatal events including unstable angina pectoris, angioplasty, stenting, or coronary artery bypass grafting [30]. The 5-year cumulative rate of major coronary events was 2.8% in the EPA-plus-statin group versus 3.5% in the statin-only group, translating to a 19% (95% CI 0.69–0.95; P = 0.011) reduction in CV events, including a 24% (95% CI 0.62–0.95; P = 0.014) reduction in unstable angina and a 19% (95% CI 0.68–0.96; P = 0.015) reduction in nonfatal coronary events. Overall, reduction in CV events was not significant in the primary prevention group; however, there was a significant 19% (95% CI 0.66–1.00; P = 0.048) reduction in CV events in the secondary prevention group [30].
It is important to note several unique aspects of JELIS, which subsequently helped to inform the design of REDUCE-IT. First, the dose of EPA was relatively low [31], and was administered to patients whose dietary intake of EPA is comparatively higher than in those in other countries. In addition, JELIS was an open-label design without a placebo group. Furthermore, the trial used a low-intensity statin. Finally, the trial was conducted in a single country, which limits generalizability to a wider patient population [30].
3.4. REDUCE-IT
REDUCE-IT was a randomized, double-blind, placebo-controlled trial in 8179 statin-treated patients ≥45 years of age with established CV disease or ≥ 50 years of age with diabetes mellitus and at least one additional risk factor [17]. This study built on the earlier JELIS study that established the potential role of a higher 4 g dose of EPA [30]. Enrolled patients had a fasting LDL-C level of 41–100 mg/dL and the initial fasting TG level inclusion range was 150–499 mg/dL, while the protocol allowed for a 10% lower TG level from the target lower limit to account for intraindividual variability of TG levels, which permitted patients to be enrolled if they had a TG level of at least 135 mg/dL. The first protocol amendment changed the lower limit of the acceptable TG level from 150 mg/dL to 200 mg/dL, with no allowance for variability. Median baseline TG level was 216 mg/dL and median baseline LDL-C level was 75 mg/dL. After a median 4.9 years of follow-up, pharmaceutical-grade, stable EPA (icosapent ethyl) at 4 g/day reduced ASCVD events by 25% compared with placebo (HR 0.75; 95% CI 0.68–0.83; P < 0.001) and reduced total (first and recurrent) events by 30% (HR 0.70; 95% CI 0.62-0.78; P < 0.0001) [32]. In addition, icosapent ethyl was associated with reductions in a number of components of the composite primary endpoint, including death due to CV causes (20% reduction; 95% CI 0.66–0.98; P = 0.03), nonfatal MI (30% reduction; 95% CI 0.59–0.82), and nonfatal stroke (29% reduction; 95% CI 0.54–0.94).
In contrast to ASCEND, patients with diabetes in REDUCE-IT derived significant clinical benefit from icosapent ethyl, with the risk of first and total occurrence of the primary composite endpoint decreased by 23% (95% CI 0.68–0.87; P < 0.001) and 24% (95% CI 0.66–0.88; P < 0.001), respectively, compared with placebo [33]. This result is particularly important because patients with diabetes in the placebo arm had a 1.5-fold greater rate of the primary endpoint than those without diabetes. Patients with established CV disease also had a significant clinical benefit with icosapent ethyl. For those with established CV disease and diabetes, there was a 30% (95% CI 0.59–0.84; P < 0.001) and 28% (95% CI 0.62–0.84; P < 0.001) reduction in the risk of first and total primary events, respectively. Among patients with established CV disease without diabetes, the risk of first composite primary endpoint was reduced by 41% (95% CI 0.49–0.70; P < 0.001), and the risk of total endpoints by 27% (95% CI 0.62–0.85; P < 0.001). Patients with diabetes and/or established CV disease should therefore be considered good candidates for treatment with icosapent ethyl 4 g/day. Further subgroup analyses have been published; a REDUCE-IT analysis in patients with a history of coronary artery bypass grafting (n = 1837) found that icosapent ethyl was associated with a significant reduction in the primary endpoint (HR 0.76; 95% CI 0.63–0.92; P = 0.004) and key secondary endpoint (HR 0.69; 95% CI 0.56–0.87; P = 0.001) [34]. Among patients in REDUCE-IT with prior MI, a similar relative reduction was found for the primary endpoint (HR 0.74; 95% CI 0.65–0.85; P < 0.001) as well as the key secondary endpoint (HR 0.71; 95% CI 0.61–0.84; P < 0.001) [35]. An analysis comparing the treatment effect of icosapent ethyl across the range of kidney function encountered in REDUCE-IT found benefit on the primary endpoint and key secondary endpoint in all estimated glomerular filtration rate (eGFR) groups (eGFR <60 mL/min/1.73m2, 60 to <90 mL/min/1.73m2, ≥90 mL/min/1.73m2), with patients in the <60 mL/min/1.73m2 group having the largest risk reduction in the primary (HR 0.71; 95% CI 0.59–0.85; P = 0.0002) and secondary endpoint (HR 0.71; 95% CI 0.57–0.88; P = 0.001) [36].
The use of a mineral oil placebo in REDUCE-IT has generated controversy, with some commentators suggesting that mineral oil may reduce absorption of certain drugs and raise levels of atherogenic lipoproteins and inflammatory markers such as hsCRP [16], [37]. However, a comprehensive review of 281 papers, including 80 in which mineral oil was used as a placebo, concluded that mineral oil is biologically inert, except when taken in high doses as a lubricant laxative [38]. Critically, mineral oil was found to have no systemic effects. In trials reporting changes in blood lipids and inflammatory markers, changes were mixed and inconsistent, ranging from −16% to +18.5% for TGs and − 6.1% to +9.2% for LDL-C when taken orally. Concerns about the effect of mineral oil on drug absorption are theoretical, with no clinically important drug–drug interactions with statins reported, particularly if the statin and mineral oil are administered separately. An analysis of REDUCE-IT data comparing the primary and key secondary endpoint in subgroups based on lipophilic versus lipophobic statin category found no difference in terms of benefit from icosapent ethyl between groups as evidenced by similar event rates and treatment with statin-type group interaction P values of 0.67 for the primary endpoint and 0.74 for the key secondary endpoint [39]. Findings in mice showed that coadministration of mineral oil (10 or 30 μL) with a single dose of atorvastatin (5 or 10 mg/kg) was associated with a reduction in statin plasma exposure (AUC0-∞ ratio 0.81-0.87), whereas repeated dosing of atorvastatin 10 mg/kg with 30 μL of mineral oil resulted in increased statin exposure (AUC0-∞ ratio 1.5); the lack of consistent effects suggest mineral oil does not affect the absorption of atorvastatin [40]. Independent regulatory reviews and approvals by the US Food and Drug Administration (FDA), European Medicines Agency, Medicines Healthcare Products Regulatory Agency, and Health Canada support that any effect of mineral oil placebo on the outcomes in REDUCE-IT were clinically insignificant [38], [41], [42].
Based on the results of REDUCE-IT, icosapent ethyl has been approved in the US to reduce the risk of CV events in adult statin-treated patients at high CV risk with elevated TG levels (≥150 mg/dL) and established CV disease or diabetes and at least two other CV risk factors [14]. In the EU, icosapent ethyl has been approved to reduce the risk of CV events in adult statin-treated patients at high CV risk with elevated TG levels (≥150 mg/dL) and established CV disease or diabetes and at least one other CV risk factor [12], [43]. Icosapent ethyl has also been adopted globally in major medical society guidelines as a recommended treatment for patients with persistent CV risk despite statin treatment, including the American Diabetes Association (ADA), the National Lipid Association (NLA), the European Society of Cardiology (ESC)/European Association for the Study of Diabetes, and the ESC/European Atherosclerosis Society [44], [45], [46], [47], [48].
An exploration of the difference in outcomes between REDUCE-IT and other CV outcomes trials is warranted. There were some differences in baseline TG levels and the observed reductions in TG levels across trials. Baseline TG levels were low in OMEMI (111 mg/dL) but were higher in STRENGTH (239 mg/dL) than in REDUCE-IT (216 mg/dL); the percent reduction in TG levels was similar in REDUCE-IT and STRENGTH. Furthermore, REDUCE-IT investigators demonstrated that the difference in clinical benefit likely cannot be attributed to reductions in TG levels alone [49], [50]. The biggest apparent difference among these trials that may help to explain the positive result observed in REDUCE-IT is that a highly purified ethyl ester of EPA (icosapent ethyl) was used as opposed to the mixed EPA + DHA formulations of different structural forms in OMEMI and STRENGTH. The high dose of pure EPA resulted in a substantial increase in blood EPA levels, which correlated with the CV benefits seen in REDUCE-IT [49]. Additionally, potential antiplatelet/antithrombotic effects may also play a potential beneficial mechanistic role for CV risk reduction as noted by a nonsignificant trend in increased bleeding events that was not seen in STRENGTH or OMEMI [51].
4. Pharmacology of omega-3 fatty acids
Both EPA and DHA (and mixed EPA + DHA combinations) have been shown to reduce circulating TG levels, explaining why many trials have focused on mixed combinations [52], [53]. However, the two omega-3 fatty acids have different pharmacologic effects (Fig. 1, Fig. 2), including effects on other circulating lipoproteins, formation of cholesterol domains, cell membrane structure, and as precursors for bioactive compounds involved in platelet function and inflammation [54], [55]. EPA is associated with reduced circulating very-low-density lipoprotein cholesterol (VLDL-C) and TGs, as well as apolipoprotein (apo) B, apo-CIII, and remnant-like particle cholesterol [53]. EPA may also inhibit phosphatidic acid phosphatase/phosphohydrolase and diacylglycerol transferase to reduce TG synthesis [56], increase clearance of TG-rich lipoproteins via lipoprotein lipase activity, and reduce expression of platelet endothelial cell adhesion molecule-1 on endothelial cells [57], [58]. Furthermore EPA, but not DHA, induces expression of heme oxygenase-1 and increases nitric oxide levels in endothelial cells under inflammatory conditions; this may help to preserve vascular endothelial cell function [59]. Endothelial nitric oxide synthase coupling efficiency, as measured by the nitric oxide to peroxynitrite ratio, was greater in endothelial cells pretreated with EPA than in cells pretreated with DHA, further supporting a preferential benefit of EPA on endothelial function [60].
Fig. 1.
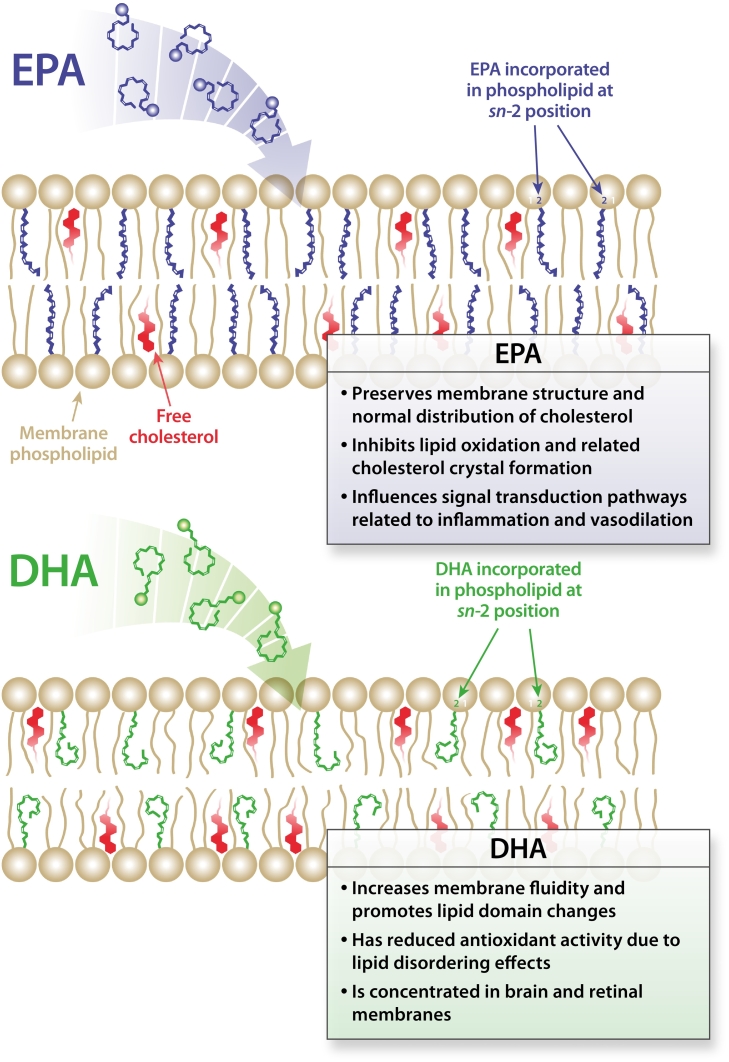
Molecular membrane interactions of omega-3 fatty acids. Schematic illustration of the proposed location and contrasting effects of EPA and DHA on membrane structure. The insertion of EPA and DHA affects distinct regions of the membrane lipid bilayer due to differences in their hydrocarbon length and number of double bonds. The longer hydrocarbon length of DHA leads to more rapid isomerization and conformational changes that result in increased membrane fluidity and promotion of cholesterol domains. EPA has a more stable and extended structure that contributes to membrane stability as well as inhibition of lipid oxidation and cholesterol domain formation. DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid. Reprinted with permission from Mason RP, Libby P, Bhatt DL. Emerging mechanisms of cardiovascular protection for the omega-3 fatty acid eicosapentaenoic acid. Arterioscler Thromb Vasc Biol 2020;40(5):1135–1147; www.ahajournals.org/journal/atvb[54].
Fig. 2.
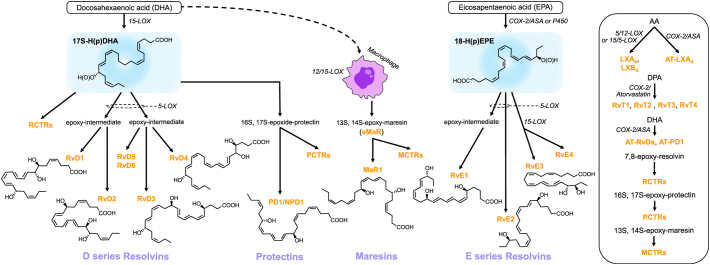
Specialized pro-resolving mediator biosynthetic pathways [65]. (A) Biosynthesis of the D series resolvins, protectins, and maresins from docosahexaenoic acid (DHA) and the E series resolvins from eicosapentaenoic acid (EPA). (B) Biosynthesis of lipoxins and aspirin-triggered (AT)-lipoxins from arachidonic acid (AA), the T series resolvins AT-RvDs and AT-PD1 from DHA, and the resolvin, protectin, and maresin conjugates in tissue regeneration (RCTRs, PCTRs, MCTRs) from their epoxy precursors. 15-LOX, 15-lipoxygenase; 5-LOX, 5-lipoxygenase; 12-LOX, 12-lipoxygenase; COX2-ASA, aspirin-acetylated cyclooxygenase-2; P450, cytochrome P450; RvD1, resolvin D1; RvD2, resolvin D2; RvD3, resolvin D3; RvD4, resolvin D4; PD1/NPD1, protectin D1/neuroprotectin D1; MaR1, maresin 1; RvE1, resolvin E1; RvE2, resolvin E2; RvE3, resolvin E3; LXA4, lipoxin A4; LXB4, lipoxin B4; AT-LXA4, aspirin-triggered lipoxin A4. Reprinted with permission from Panigrahy D, Gilligan MM, Serhan CN, Kashfi K. Resolution of inflammation: An organizing principle in biology and medicine. Pharmacol Ther 2021;227:107879. Copyright 2021, with permission from Elsevier.
EPA has a minimal or neutral effect on LDL-C; DHA, by contrast is associated with increases in LDL-C levels. For example, in a study comparing treatment with purified EPA versus DHA, DHA was associated with a significant 8% increase in serum LDL-C levels, while there was a nonsignificant decrease of 3.5% with EPA; both omega-3 fatty acids reduced TG levels by approximately 20% [61].
Omega-3 fatty acids have a role in maintaining membrane fluidity and preventing development of atherosclerotic plaque, with both EPA and DHA being incorporated into the cholesterol domain of cell membranes (Fig. 1) [54]. However, EPA and DHA have different effects on membrane structure [54], [62]. When omega-3 fatty acids are in low supply, membrane structure can facilitate formation of extracellular cholesterol crystals, which are associated with atherogenesis and plaque rupture. EPA preserves membrane structure and promotes normal distribution of cholesterol, inhibits lipid oxidation and cholesterol crystal formation, and influences signal transduction pathways related to inflammation and vasodilation. Compared to DHA, EPA has a more stable extended molecular structure that allows it to scavenge reactive oxygen species, hence promoting membrane stability [54]. In fact, EPA inhibits oxidation of apo-B–containing lipoprotein particles of different sizes, an effect that has been attributed to quenching of reactive oxygen species in the lipid membrane [63]. DHA, on the other hand, increases membrane fluidity and promotes lipid domain changes, has reduced antioxidant activity due to lipid-disordering effects, and is concentrated in brain and retinal membranes [54], [62]. Interestingly, when EPA and DHA are combined, the resulting effects on membrane structure are attenuated compared to their separate actions, providing a potential mechanistic explanation for contrasting results between EPA-only and EPA + DHA clinical trials [64].
Another key difference between EPA and DHA is in how they are metabolized by cyclooxygenase and lipoxygenase and other downstream enzymes to produce anti-inflammatory and antithrombotic compounds (Fig. 2) [55], [65]. Metabolism of EPA leads to production of 3-series prostaglandins, thromboxane A3, 5-series leukotrienes, and E-series resolvins RvE1 and RvE2. DHA by contrast is converted to D-series resolvins, protectins, and maresins. EPA competes with arachidonic acid (AA) for cyclooxygenase and lipoxygenase, leading to production of less inflammatory and less prothrombotic bioactive lipid metabolites, whereas AA results in the predominantly proinflammatory 2-series prostaglandins, thromboxane A2, and 4-series leukotrienes [66]. As a result, increased serum EPA, resulting in a higher EPA:AA ratio, shifts the balance toward a less inflammatory milieu; a higher EPA:AA ratio is associated with lower CV disease risk [66]. The downstream effects of this less inflammatory state have been demonstrated in mouse models. Administration of EPA resulted in attenuated chronic post-MI cardiac modeling related to modulation of proinflammatory M1 macrophage activity [67]. Compared with the EPA:AA ratio, the DHA:AA ratio has a much weaker association with CV disease risk [66]. Increasing the EPA:AA ratio has anti-aggregatory effects through increased production of antithrombotic thromboxane A3 and prostacyclin I3 versus the prothrombotic thromboxane A2 and prostacyclin I2 [68], [69]. The production of resolvin RvE1 may also reduce platelet activation and thrombosis [68], [70].
EPA demonstrated consistent antiatherosclerotic effects in imaging studies; in combination with a statin, EPA has been associated with a decrease in features associated with vulnerable plaque [71]. Recent evidence for a role for icosapent ethyl comes from the randomized, double-blind, placebo-controlled EVAPORATE study (Effect of Vascepa on Progression of Coronary Atherosclerosis in Persons With Elevated Triglycerides [200–499] on Statin Therapy) [72]. Patients with coronary atherosclerosis involving at least one angiographic stenosis with ≥20% narrowing and on stable statin therapy with LDL-C levels 40–115 mg/dL and TG levels 135–499 mg/dL underwent multidetector computed tomography during 18 months of follow-up. After 18 months, there were significant decreases versus placebo in low-attenuation plaque (P = 0.006), total noncalcified plaque (P = 0.0005), and total plaque (P = 0.0002) in icosapent ethyl–treated patients. In the CHERRY trial (Combination Therapy of Eicosapentaenoic Acid and Pitavastatin for Coronary Plaque Regression Evaluated by Integrated Backscatter Intravascular Ultrasonography), addition of EPA 1800 mg/day to pitavastatin 4 mg/day led to significant reductions in normalized total atheroma volume over ≈8 months in patients with stable angina pectoris and acute coronary syndrome. Moreover, reductions were significantly greater than in patients receiving pitavastatin alone [73], providing further evidence that observed effects from EPA are not driven by changes due to a placebo. By contrast, there was no significant difference in the volume of noncalcified plaque—the most sensitive measure of plaque volume—after a longer follow-up of 30 months in the randomized, controlled, parallel-design HEARTS trial (Slowing Heart Disease with Lifestyle and Omega-3 Fatty Acids) of EPA + DHA (1.86 g and 1.5 g, respectively) versus control (no EPA + DHA) in 285 subjects with coronary artery disease and ≥ 50% stenosis in at least one coronary artery at cardiac catheterization, prior MI or percutaneous coronary intervention (both ≥6 months previously), coronary bypass surgery (≥12 months previously), abnormal exercise treadmill test or reversible ischemia on nuclear imaging, and/or pharmacologic stress or stress echocardiography with subsequent revascularization [74]. Although this study was negative overall, a substudy of HEARTS further demonstrated the importance of EPA [75]. Higher plasma levels of EPA and DHA were associated with an increased ratio of resolvin E1 plus 18-hydroxy-EPA (both derived from EPA) to leukotriene B4 and significant plaque regression. Similar to the HEARTS trial, the AQUAMARINE study assessed the effect of 2 g of mixed EPA/DHA, 4 g of mixed EPA/DHA, or no treatment for 12 months in addition to statin treatment in 84 patients with coronary artery disease and found no difference in plaque-to-myocardial ratio changes among the groups [76].
5. EPA plasma levels
These differences in the pharmacology of EPA and DHA may therefore help to explain differences in clinical outcomes between patients taking EPA versus EPA+DHA. JELIS provided the first data in a cardiovascular outcomes trial with EPA that the on-treatment EPA plasma level was related to the risk of a major coronary event [29]. Compared to an EPA level of <150 μg/mL, patients with plasma EPA ≥150 μg/mL had an 18% reduction in major coronary events (95% CI 0.68–0.98; P = 0.032). In REDUCE-IT, the crucial relationship between higher levels of on-treatment EPA and reduced CV disease events rate were again seen. The on-treatment EPA levels in patients taking icosapent ethyl correlated strongly with the primary endpoint, the key secondary endpoint, CV death, MI, stroke, coronary revascularization, unstable angina, sudden cardiac death, new heart failure, and all-cause mortality [49]. Time-varying covariate analyses revealed that changes in other biomarkers such as TGs, LDL-C, and high-density lipoprotein cholesterol levels had only a limited impact on the primary and secondary endpoint. In fact, on-treatment EPA levels accounted for essentially all of the 25% (95% CI 0.68–0.83; P < 0.001) relative risk reduction in the primary endpoint and 26% (95% CI 0.65–0.83; P < 0.001) relative risk reduction in the secondary endpoint. Furthermore, dose-response analyses showed that the higher the on-treatment serum EPA level, the greater the ASCVD event risk reduction; therefore, some ASCVD events may require higher EPA thresholds to observe beneficial effects. Further evidence comes from a comparison of achieved EPA levels between REDUCE-IT and STRENGTH. In REDUCE-IT, median EPA levels increased by almost 400% to 144 μg/mL after 1 year of treatment with icosapent ethyl compared with a 269% increase to only 90 μg/mL in STRENGTH (DHA levels increased by 40%) [24], [49].
6. Conclusions
Dyslipidemia management has evolved to utilize a range of drugs beyond statins that target LDL-C. These include proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors, ezetimibe, and bempedoic acid, which also target LDL-C, and omega-3 fatty acids, niacin, and fibrates, which target TGs [18], [46]. As reviewed here, there is now clear, strong evidence-based data to support use of EPA 4 g/day for reduction of CV disease risk beyond its effect on TGs alone, and it has now been included in a number of national and international guidelines [44], [45], [46]. Based on REDUCE-IT, EPA is currently the only lipid-modifying agent available that has been shown to reduce CV mortality over and above statin therapy in high-risk patients.
It is important to note that there is an apparent need to differentiate omega-3 fatty acids and drug formulations and their roles in conferring CV benefit. EPA and DHA have distinct biochemical properties and exert different pharmacologic effects, so it cannot be assumed that both will have the same effects on CV outcomes. Although products containing mixed EPA + DHA and EPA alone have been approved to reduce TGs in patients with hypertriglyceridemia, only EPA-alone formulations have demonstrated clinical benefit in reducing major CV disease events and death when added to contemporary medical therapy, including statins. The ongoing RESPECT-EPA trial, which is assessing the effect of 1.8 g EPA per day versus no EPA on CV outcomes in 3900 patients with established ASCVD and on statin therapy will provide further insight into EPA-only treatment [77], [78]. While EPA may have pleiotropic pharmacologic effects, it is not clear which of these are clinically relevant. A current limitation is the lack of available data on use of DHA monotherapy, including at higher doses. Future research should evaluate the impact of DHA alone in patients with CV disease. Further evaluation of the importance of plasma EPA levels both as a tool for measuring adherence as well as efficacy is also warranted. This would include further establishing a threshold above which maximum clinical benefit can be achieved.
Role of the funding source
Medical writing assistance was provided by Peloton Advantage, LLC, an OPEN Health company, Parsippany, NJ, and funded by Amarin Pharma Inc.
CRediT authorship contribution statement
Peter P. Toth: Writing – original draft, Writing – review & editing. M. John Chapman: Writing – original draft, Writing – review & editing. Klaus G. Parhofer: Validation, Writing – review & editing. John R. Nelson: Validation, Writing – review & editing.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
PP Toth serves on the speaker's bureaus of Amarin, Amgen, Esperion, and Novo-Nordisk and serves as a consultant to Amarin, Amgen, Merck, Novartis, Resverlogix, and Theravance.
MJ Chapman has received research funding from CSL, Kowa, Merck, and Pfizer and has participated in advisory boards and/or speaker's bureaus for Akcea, Alexion, Amarin, Amgen, AstraZeneca, Daiichi-Sankyo, Kowa, Medscape, Novartis, Pfizer, Sanofi, Regeneron, and Servier.
KG Parhofer has received research funding and/or honoraria for consultancy and/or speaker's bureaus and/or DMC activity from Akcea, Amarin, Amgen, Boehringer-Ingelheim, Dr. Schär, Daiichi-Sankyo, Ionis, MSD, Novartis, Pfizer, Regeneron, Sanofi, and Silence Therapeutics.
JR Nelson is on the speaker's bureau and/or advisory board of Amarin, Amgen, Esperion, and Regeneron. He is a stockholder of Amgen.
References
- 1.Eurostat CVD statistics. 2020. https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Cardiovascular_diseases_statistics Available at.
- 2.Sarwar N., Danesh J., Eiriksdottir G., Sigurdsson G., Wareham N., Bingham S., Boekholdt S.M., Khaw K.T., Gudnason V. Triglycerides and the risk of coronary heart disease: 10,158 incident cases among 262,525 participants in 29 Western prospective studies. Circulation. 2007;115:450–458. doi: 10.1161/CIRCULATIONAHA.106.637793. [DOI] [PubMed] [Google Scholar]
- 3.Toth P.P., Philip S., Hull M., Granowitz C. Association of elevated triglycerides with increased cardiovascular risk and direct costs in statin-treated patients. Mayo Clin. Proc. 2019;94:1670–1680. doi: 10.1016/j.mayocp.2019.03.028. [DOI] [PubMed] [Google Scholar]
- 4.Ginsberg H.N., Packard C.J., Chapman M.J., Borén J., Aguilar-Salinas C.A., Averna M., Ference B.A., Gaudet D., Hegele R.A., Kersten S., Lewis G.F., Lichtenstein A.H., Moulin P., Nordestgaard B.G., Remaley A.T., Staels B., Stroes E.S.G., Taskinen M.R., Tokgözoğlu L.S., Tybjaerg-Hansen A., Stock J.K., Catapano A.L. Triglyceride-rich lipoproteins and their remnants: metabolic insights, role in atherosclerotic cardiovascular disease, and emerging therapeutic strategies-a consensus statement from the European Atherosclerosis Society. Eur. Heart J. 2021;42:4791–4806. doi: 10.1093/eurheartj/ehab551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stein E.A., Lane M., Laskarzewski P. Comparison of statins in hypertriglyceridemia. Am. J. Cardiol. 1998;81:66b–69b. doi: 10.1016/s0002-9149(98)00041-1. [DOI] [PubMed] [Google Scholar]
- 6.Nichols G.A., Philip S., Reynolds K., Granowitz C.B., Fazio S. Increased cardiovascular risk in hypertriglyceridemic patients with statin-controlled LDL cholesterol. J. Clin. Endocrinol. Metab. 2018;103:3019–3027. doi: 10.1210/jc.2018-00470. [DOI] [PubMed] [Google Scholar]
- 7.The HPS2-THRIVE Collaborative Group Effects of extended-release niacin with laropiprant in high-risk patients. N. Engl. J. Med. 2014;371:203–212. doi: 10.1056/NEJMoa1300955. [DOI] [PubMed] [Google Scholar]
- 8.The ACCORD Study Group. Ginsberg H.N., Elam M.B., Lovato L.C., Crouse J.R., III L.A.Leiter, Linz P., Friedewald W.T., Buse J.B., Gerstein H.C., Probstfield J., Grimm R.H., Ismail-Beigi F., Bigger J.T., Goff D.C., Jr., Cushman W.C., Jr., Simons-Morton D.G., Jr., Byington R.P., Jr. Effects of combination lipid therapy in type 2 diabetes mellitus. N. Engl. J. Med. 2010;362:1563–1574. doi: 10.1056/NEJMoa1001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daviglus M.L., Stamler J., Orencia A.J., Dyer A.R., Liu K., Greenland P., Walsh M.K., Morris D., Shekelle R.B. Fish consumption and the 30-year risk of fatal myocardial infarction. N. Engl. J. Med. 1997;336:1046–1053. doi: 10.1056/NEJM199704103361502. [DOI] [PubMed] [Google Scholar]
- 10.Albert C.M., Hennekens C.H., O'Donnell C.J., Ajani U.A., Carey V.J., Willett W.C., Ruskin J.N., Manson J.E. Fish consumption and risk of sudden cardiac death. JAMA. 1998;279:23–28. doi: 10.1001/jama.279.1.23. [DOI] [PubMed] [Google Scholar]
- 11.GISSI Prevenzione Investigators Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto miocardico. Lancet. 1999;354:447–455. [PubMed] [Google Scholar]
- 12.Amarin Pharmaceuticals Ireland; Dublin, Ireland: 2021. Vazkepa [Summary of Product Characteristics] [Google Scholar]
- 13.Mylan; Hertfordshire, United Kingdom: 2019. Omacor [Summary of Product Characteristics] [Google Scholar]
- 14.Amarin Pharma Inc.; Bridgewater, NJ: 2019. Vascepa [Package Insert] [Google Scholar]
- 15.GlaxoSmithKline; Research Triangle Park, NC: 2020. Lovaza [Package Insert] [Google Scholar]
- 16.Kapoor K., Alfaddagh A., Stone N.J., Blumenthal R.S. Update on the omega-3 fatty acid trial landscape: a narrative review with implications for primary prevention. J. Clin. Lipidol. 2021;15:545–555. doi: 10.1016/j.jacl.2021.06.004. [DOI] [PubMed] [Google Scholar]
- 17.Bhatt D.L., Steg G., Miller M., Brinton E.A., Jacobson T.A., Ketchum S.B., Doyle R.T., Jr., Juliano R.A., Jiao L., Granowitz C., Tardif J.C., Ballantyne C.M. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N. Engl. J. Med. 2019;380:11–22. doi: 10.1056/NEJMoa1812792. [DOI] [PubMed] [Google Scholar]
- 18.Skulas-Ray A.C., Wilson P.W.F., Harris W.S., Brinton E.A., Kris-Etherton P.M., Richter C.K., Jacobson T.A., Engler M.B., Miller M., Robinson J.G., Blum C.B., Rodriguez-Leyva D., de Ferranti S.D., Welty F.K. Omega-3 fatty acids for the management of hypertriglyceridemia: a science advisory from the American Heart Association. Circulation. 2019;140:e673–e691. doi: 10.1161/CIR.0000000000000709. [DOI] [PubMed] [Google Scholar]
- 19.Marston N.A., Giugliano R.P., Im K., Silverman M.G., O'Donoghue M.L., Wiviott S.D., Ference B.A., Sabatine M.S. Association between triglyceride lowering and reduction of cardiovascular risk across multiple lipid-lowering therapeutic classes: a systematic review and meta-regression analysis of randomized controlled trials. Circulation. 2019;140:1308–1317. doi: 10.1161/CIRCULATIONAHA.119.041998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.European Medicines Agency EMA confirms omega-3 fatty acid medicines are not effective in preventing further heart problems after a heart attack [press release], Amsterdam, The Netherlands. 2019. https://www.ema.europa.eu/en/news/ema-confirms-omega-3-fatty-acid-medicines-are-not-effective-preventing-further-heart-problems-after Available at.
- 21.European Food Safety Authority Food supplements. 2021. https://www.efsa.europa.eu/en/topics/topic/food-supplements Available at.
- 22.Mason R.P., Sherratt S.C.R. Omega-3 fatty acid fish oil dietary supplements contain saturated fats and oxidized lipids that may interfere with their intended biological benefits. Biochem. Biophys. Res. Commun. 2017;483:425–429. doi: 10.1016/j.bbrc.2016.12.127. [DOI] [PubMed] [Google Scholar]
- 23.Borén J., Chapman M.J., Krauss R.M., Packard C.J., Bentzon J.F., Binder C.J., Daemen M.J., Demer L.L., Hegele R.A., Nicholls S.J., Nordestgaard B.G., Watts G.F., Bruckert E., Fazio S., Ference B.A., Graham I., Horton J.D., Landmesser U., Laufs U., Masana L., Pasterkamp G., Raal F.J., Ray K.K., Schunkert H., Taskinen M.R., van de Sluis B., Wiklund O., Tokgozoglu L., Catapano A.L., Ginsberg H.N. Low-density lipoproteins cause atherosclerotic cardiovascular disease: pathophysiological, genetic, and therapeutic insights: a consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2020;41:2313–2330. doi: 10.1093/eurheartj/ehz962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nicholls S.J., Lincoff A.M., Garcia M., Bash D., Ballantyne C.M., Barter P.J., Davidson M.H., Kastelein J.J.P., Koenig W., McGuire D.K., Mozaffarian D., Ridker P.M., Ray K.K., Katona B.G., Himmelmann A., Loss L.E., Rensfeldt M., Lundström T., Agrawal R., Menon V., Wolski K., Nissen S.E. Effect of high-dose omega-3 fatty acids vs corn oil on major adverse cardiovascular events in patients at high cardiovascular risk: the STRENGTH randomized clinical trial. JAMA. 2020;324:2268–2280. doi: 10.1001/jama.2020.22258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kalstad A.A., Myhre P.L., Laake K., Tveit S.H., Schmidt E.B., Smith P., Nilsen D.W.T., Tveit A., Fagerland M.W., Solheim S., Seljeflot I., Arnesen H. Effects of n-3 fatty acid supplements in elderly patients after myocardial infarction: a randomized controlled trial. Circulation. 2020;143:528–539. doi: 10.1161/CIRCULATIONAHA.120.052209. [DOI] [PubMed] [Google Scholar]
- 26.ASCEND Study Collaborative Group. Bowman L., Mafham M., Wallendszus K., Stevens W., Buck G., Barton J., Murphy K., Aung T., Haynes R., Cox J., Murawska A., Young A., Lay M., Chen F., Sammons E., Waters E., Adler A., Bodansky J., Farmer A., McPherson R., Neil A., Simpson D., Peto R., Baigent C., Collins R., Parish S., Armitage J. Effects of n-3 fatty acid supplements in diabetes mellitus. N. Engl. J. Med. 2018;379:1540–1550. doi: 10.1056/NEJMoa1804989. [DOI] [PubMed] [Google Scholar]
- 27.Manson J.E., Cook N.R., Lee I.M., Christen W., Bassuk S.S., Mora S., Gibson H., Albert C.M., Gordon D., Copeland T., D'Agostino D., Friedenberg G., Ridge C., Bubes V., Giovannucci E.L., Willett W.C., Buring J.E. Marine n-3 fatty acids and prevention of cardiovascular disease and cancer. N. Engl. J. Med. 2019;380:23–32. doi: 10.1056/NEJMoa1811403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mason R.P. New insights into mechanisms of action for omega-3 fatty acids in atherothrombotic cardiovascular disease. Curr. Atheroscler. Rep. 2019;21 doi: 10.1007/s11883-019-0762-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Itakura H., Yokoyama M., Matsuzaki M., Saito Y., Origasa H., Ishikawa Y., Oikawa S., Sasaki J., Hishida H., Kita T., Kitabatake A., Nakaya N., Sakata T., Shimada K., Shirato K., Matsuzawa Y. Relationships between plasma fatty acid composition and coronary artery disease. J. Atheroscler. Thromb. 2011;18:99–107. doi: 10.5551/jat.5876. [DOI] [PubMed] [Google Scholar]
- 30.Yokoyama M., Origasa H., Matsuzaki M., Matsuzawa Y., Saito Y., Ishikawa Y., Oikawa S., Sasaki J., Hishida H., Itakura H., Kita T., Kitabatake A., Nakaya N., Sakata T., Shimada K., Shirato K. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet. 2007;369:1090–1098. doi: 10.1016/S0140-6736(07)60527-3. [DOI] [PubMed] [Google Scholar]
- 31.Bhatt D.L., Steg P.G., Brinton E.A., Jacobson T.A., Miller M., Tardiff J.-C., Ketchum S.B., Doyle R.T., Jr., Murphy S.A., Soni P.N., Braeckman R.A., Juliano R.A., Ballantyne C.M. Rationale and design of REDUCE-IT: reduction of cardiovascular events with icosapent ethyl-intervention trial. Clin. Cardiol. 2017;40:138–148. doi: 10.1002/clc.22692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bhatt D.L., Steg P.G., Miller M., Brinton E.A., Jacobson T.A., Ketchum S.B., Doyle R.T., Jr., Juliano R.A., Granowitz C., Tardif J.C., Gregson J., Pocock S.J., Ballantyne C.M. The REDUCE-IT investigators, effects of icosapent ethyl on total ischemic events: from REDUCE-IT. J. Am. Coll. Cardiol. 2019;73:2791–2802. doi: 10.1016/j.jacc.2019.02.032. [DOI] [PubMed] [Google Scholar]
- 33.Bhatt D.L., Brinton E.A., Miller M., Steg G., Jacobson T.A., Ketchum S.B., Juliano R.A., Jiao L., Doyle R.T., Jr., Granowitz C., Ganda O., Welty F.K., Busch R.S., Goldberg A.C., Herrington D.M., Budoff M., Tardif J.C., Ballantyne C.M. Annual Scientific Sessions of the American Diabetes Association; 2020. Icosapent Ethyl Provides Consistent Cardiovascular Benefit in Patients With Diabetes in REDUCE-IT [Presentation] [Google Scholar]
- 34.Verma S., Bhatt D.L., Steg P.G., Miller M., Brinton E.A., Jacobson T.A., Dhingra N.K., Ketchum S.B., Juliano R.A., Jiao L., Doyle R.T., Jr., Granowitz C., Gibson C.M., Pinto D., Giugliano R.P., Budoff M.J., Mason R.P., Tardif J.C., Ballantyne C.M. Icosapent ethyl reduces ischemic events in patients with a history of prior coronary artery bypass grafting: REDUCE-IT CABG. Circulation. 2021;144:1845–1855. doi: 10.1161/CIRCULATIONAHA.121.056290. [DOI] [PubMed] [Google Scholar]
- 35.Gaba P., Bhatt D.L., Steg P.G., Miller M., Brinton E.A., Jacobson T.A., Ketchum S.B., Juliano R.A., Jiao L., Doyle R.T., Jr., Granowitz C., Tardif J.C., Giugliano R.P., Martens F., Gibson C.M., Ballantyne C.M. Prevention of cardiovascular events and mortality with icosapent ethyl in patients with prior myocardial infarction. J. Am. Coll. Cardiol. 2022;79:1660–1671. doi: 10.1016/j.jacc.2022.02.035. [DOI] [PubMed] [Google Scholar]
- 36.Majithia A., Bhatt D.L., Friedman A.N., Miller M., Steg P.G., Brinton E.A., Jacobson T.A., Ketchum S.B., Juliano R.A., Jiao L., Doyle R.T., Jr., Granowitz C., Budoff M., Mason R.P., Tardif J.C., Boden W.E., Ballantyne C.M. Benefits of icosapent ethyl across the range of kidney function in patients with established cardiovascular disease or diabetes: REDUCE-IT RENAL. Circulation. 2021;144:1750–1759. doi: 10.1161/CIRCULATIONAHA.121.055560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kastelein J.J.P., Stroes E.S.G. FISHing for the miracle of eicosapentaenoic acid. N. Engl. J. Med. 2019;380:89–90. doi: 10.1056/NEJMe1814004. [DOI] [PubMed] [Google Scholar]
- 38.Olshansky B., Chung M.K., Budoff M.J., Philip S., Jiao L., Doyle R.T., Jr., Copland C., Giaquinto A., Juliano R.A., Bhatt D.L. Mineral oil: safety and use as placebo in REDUCE-IT and other clinical studies. Eur. Heart J. 2020;22(suppl J):J32–J48. doi: 10.1093/eurheartj/suaa117. Suppl. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Singh N., Bhatt D.L., Miller M., Steg P.G., Brinton E.A., Jacobson T.A., Jiao L., Tardif J.-C., Mason R.P., Ballantyne C.M. Consistency of benefit of icosapent ethyl by background statin type in REDUCE-IT. J. Am. Coll. Cardiol. 2022;79:220–222. doi: 10.1016/S0735-1097(22)01211-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gopaul V.S., Pieterman E.J., Princen H.M.G., Bergenholm L., Lundborg E., Cavallin A., Johansson M.J., Hawthorne G., Björkbom A., Hammarberg M., Li X., Jarke A., Bright J., Svensson L., Jansson-Löfmark R., Abrahamsson B., Agrawal R., Hurt-Camejo E. Effects of mineral oil administration on the pharmacokinetics, metabolism and pharmacodynamics of atorvastatin and pravastatin in mice and dogs. Eur. J. Pharm. Sci. 2021;161 doi: 10.1016/j.ejps.2021.105776. [DOI] [PubMed] [Google Scholar]
- 41.Curfman G., Shehada E. Icosapent ethyl: scientific and legal controversies. Open Heart. 2021;8 doi: 10.1136/openhrt-2021-001616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.US Food and Drug Administration; 2019. Endocrinologic and Metabolic Drugs Advisory Committee Briefing Document: Vascepa® (icosapent ethyl; AMR101) REDUCE-IT® (Reduction of Cardiovascular Events With EPA – Intervention Trial) NDA Number: 202057 Amarin Backgrounder. [Google Scholar]
- 43.Chapman M.J., Zamorano J.L., Parhofer K.G. Reducing residual cardiovascular risk in Europe: therapeutic implications of European medicines agency approval of icosapent ethyl/eicosapentaenoic acid. Pharmacol. Ther. 2022;237 doi: 10.1016/j.pharmthera.2022.108172. [DOI] [PubMed] [Google Scholar]
- 44.Cosentino F., Grant P.J., Aboyans V., Bailey C.J., Ceriello A., Delgado V., Federici M., Filippatos G., Grobbee D.E., Hansen T.B., Huikuri H.V., Johansson I., Juni P., Lettino M., Marx N., Mellbin L.G., Ostgren C.J., Rocca B., Roffi M., Sattar N., Seferovic P.M., Sousa-Uva M., P.W. Valensi D.C. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: the Task Force for diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and the European Association for the Study of Diabetes (EASD) Eur. Heart J. 2020;41:255–323. doi: 10.1093/eurheartj/ehz486. [DOI] [PubMed] [Google Scholar]
- 45.Averna M., Banach M., Bruckert E., Drexel H., Farnier M., Gaita D., Magni P., März W., Masana L., Mello E.S.A., Reiner Z., Ros E., Vrablik M., Zambon A., Zamorano J.L., Stock J.K., Tokgözoğlu L.S., Catapano A.L. Practical guidance for combination lipid-modifying therapy in high- and very-high-risk patients: a statement from a European Atherosclerosis Society Task Force. Atherosclerosis. 2021;325:99–109. doi: 10.1016/j.atherosclerosis.2021.03.039. [DOI] [PubMed] [Google Scholar]
- 46.Mach F., Baigent C., Catapano A.L., Koskinas K.C., Casula M., Badimon L., Chapman M.J., De Backer G.G., Delgado V., Ference B.A., Graham I.M., Halliday A., Landmesser U., Mihaylova B., Pedersen T.R., Riccardi G., Richter D.J., Sabatine M.S., Taskinen M.R., Tokgozoglu L., Wiklund O. ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk: the Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS) Eur. Heart J. 2019;41(2020):111–188. doi: 10.1093/eurheartj/ehz455. [DOI] [PubMed] [Google Scholar]
- 47.Orringer C.E., Jacobson T.A., Maki K.C. National Lipid Association Scientific Statement on the use of icosapent ethyl in statin-treated patients with elevated triglycerides and high or very-high ASCVD risk. J. Clin. Lipidol. 2019;13:860–872. doi: 10.1016/j.jacl.2019.10.014. [DOI] [PubMed] [Google Scholar]
- 48.Draznin B., Aroda V.R., Bakris G., Benson G., Brown F.M., Freeman R., Green J., Huang E., Isaacs D., Kahan S., Leon J., Lyons S.K., Peters A.L., Prahalad P., Reusch J.E.B., Young-Hyman D., Das S., Kosiborod M. Cardiovascular disease and risk management: standards of medical care in diabetes-2022. Diabetes Care. 2022;45:S144–s174. doi: 10.2337/dc22-S010. [DOI] [PubMed] [Google Scholar]
- 49.Bhatt D.L., Miller M., Steg G., Brinton E.A., Jacobson T.A., Ketchum S.B., Juliano R.A., Jiao L., Doyle R.T., Jr., Copland C., Dunbar R.L., Granowitz C., Martens F.M.A.C., Budoff M., Nelson J.R., Mason R.P., Libby P., Ridker P., Tardiff J.-C., Ballantyne C.M. Annual Scientific Session of the American College of Cardiology; Chicago, IL: 2020. EPA Levels And Cardiovascular Outcomes in the Reduction of Cardiovascular Events With Icosapent Ethyl-intervention Trial [Oral Presentation] [Google Scholar]
- 50.Mason R.P., Eckel R.H. Mechanistic insights from REDUCE-IT STRENGTHen the case against triglyceride lowering as a strategy for cardiovascular disease risk reduction. Am. J. Med. 2021;134:1085–1090. doi: 10.1016/j.amjmed.2021.03.014. [DOI] [PubMed] [Google Scholar]
- 51.Maki K.C. Investigating contrasting results in REDUCE-IT and STRENGTH: partial answers but questions remain. Eur. Heart J. 2021;42:4818–4820. doi: 10.1093/eurheartj/ehab643. [DOI] [PubMed] [Google Scholar]
- 52.Harris W.S., Ginsberg H.N., Arunakul N., Shachter N.S., Windsor S.L., Adams M., Berglund L., Osmundsen K. Safety and efficacy of omacor in severe hypertriglyceridemia. J. Cardiovasc. Risk. 1997;4:385–391. [PubMed] [Google Scholar]
- 53.Ballantyne C.M., Bays H.E., Philip S., Doyle R.T.J., Braeckman R.A., Stirtan W.G., Soni P.N., Juliano R.A. Icosapent ethyl (eicosapentaenoic acid ethyl ester): effects on remnant-like particle cholesterol from the MARINE and ANCHOR studies. Atherosclerosis. 2016;253:81–87. doi: 10.1016/j.atherosclerosis.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 54.Mason R.P., Libby P., Bhatt D.L. Emerging mechanisms of cardiovascular protection for the omega-3 fatty acid eicosapentaenoic acid. Arterioscler. Thromb. Vasc. Biol. 2020;40:1135–1147. doi: 10.1161/ATVBAHA.119.313286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Calder P.C. Omega-3 polyunsaturated fatty acids and inflammatory processes: nutrition or pharmacology? Br. J. Clin. Pharmacol. 2013;75:645–662. doi: 10.1111/j.1365-2125.2012.04374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bays H.E., Tighe A.P., Sadovsky R., Davidson M.H. Prescription omega-3 fatty acids and their lipid effects: physiologic mechanisms of action and clinical implications. Expert. Rev. Cardiovasc. Ther. 2008;6:391–409. doi: 10.1586/14779072.6.3.391. [DOI] [PubMed] [Google Scholar]
- 57.Oscarsson J., Hurt-Camejo E. Omega-3 fatty acids eicosapentaenoic acid and docosahexaenoic acid and their mechanisms of action on apolipoprotein B-containing lipoproteins in humans: a review. Lipids Health Dis. 2017;16:149. doi: 10.1186/s12944-017-0541-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mason R.P., Sherratt S.C.R., Dawoud H., Malinski T., Bhatt D. Platelet endothelial cell adhesion molecule-1 (PECAM-1) and nitroxidative stress reduced by eicosapentaenoic acid (EPA) during cytokine exposure in endothelial cells [abstract] J. Am. Coll. Cardiol. 2021;77(18 suppl 1):1831. [Google Scholar]
- 59.Mason R.P., Sherratt S.C.R., Dawoud H., Malinski T., Bhatt D. Eicosapentaenoic acid (EPA) increases heme oxygenase-1 expression in endothelial cells under conditions of inflammation unlike docosahexaenoic acid (DHA) [abstract] J. Am. Coll. Cardiol. 2021;77(18 suppl 1):1829. [Google Scholar]
- 60.Sherratt S.C.R., Dawoud H., Bhatt D.L., Malinski T., Mason R.P. Omega-3 and omega-6 fatty acids have distinct effects on endothelial fatty acid content and nitric oxide bioavailability. Prostaglandins Leukot. Essent Fatty Acids. 2021;173 doi: 10.1016/j.plefa.2021.102337. [DOI] [PubMed] [Google Scholar]
- 61.Mori T.A., Burke V., Puddey I.B., Watts G.F., O'Neal D.N., Best J.D., Beilin L.J. Purified eicosapentaenoic and docosahexaenoic acids have differential effects on serum lipids and lipoproteins, LDL particle size, glucose, and insulin in mildly hyperlipidemic men. Am. J. Clin. Nutr. 2000;71:1085–1094. doi: 10.1093/ajcn/71.5.1085. [DOI] [PubMed] [Google Scholar]
- 62.Jacobs M.L., Faizi H.A., Peruzzi J.A., Vlahovska P.M., Kamat N.P. EPA and DHA differentially modulate membrane elasticity in the presence of cholesterol. Biophys. J. 2021;120:2317–2329. doi: 10.1016/j.bpj.2021.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mason R.P., Sherratt S.C.R., Jacob R.F. Eicosapentaenoic acid inhibits oxidation of apoB-containing lipoprotein particles of different size in vitro when administered alone or in combination with atorvastatin active metabolite compared with other triglyceride-lowering agents. J. Cardiovasc. Pharmacol. 2016;68:33–40. doi: 10.1097/FJC.0000000000000379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sherratt S.C.R., Juliano R.A., Copland C., Bhatt D.L., Libby P., Mason R.P. EPA and DHA containing phospholipids have contrasting effects on membrane structure. J. Lipid Res. 2021;62 doi: 10.1016/j.jlr.2021.100106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Panigrahy D., Gilligan M.M., Serhan C.N., Kashfi K. Resolution of inflammation: an organizing principle in biology and medicine. Pharmacol. Ther. 2021;227 doi: 10.1016/j.pharmthera.2021.107879. [DOI] [PubMed] [Google Scholar]
- 66.Nelson J.R., Raskin S. The eicosapentaenoic acid:arachidonic acid ratio and its clinical utility in cardiovascular disease. Postgrad. Med. 2019;131:268–277. doi: 10.1080/00325481.2019.1607414. [DOI] [PubMed] [Google Scholar]
- 67.Takamura M., Kurokawa K., Ootsuji H., Inoue O., Okada H., Nomura A., Kaneko S., Usui S. Long-term administration of eicosapentaenoic acid improves post-myocardial infarction cardiac remodeling in mice by regulating macrophage polarization. J. Am. Heart Assoc. 2017;6 doi: 10.1161/JAHA.116.004560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nelson J.R., Budoff M.J., Wani O.R., Le V., Patel D.K., Nelson A., Nemiroff R.L. EPA's pleiotropic mechanisms of action: a narrative review. Postgrad. Med. 2021;133:651–664. doi: 10.1080/00325481.2021.1921491. [DOI] [PubMed] [Google Scholar]
- 69.Dyerberg J., Bang H.O., Stoffersen E., Moncada S., Vane J.R. Eicosapentaenoic acid and prevention of thrombosis and atherosclerosis? Lancet. 1978;2:117–119. doi: 10.1016/s0140-6736(78)91505-2. [DOI] [PubMed] [Google Scholar]
- 70.Dona M., Fredman G., Schwab J.M., Chiang N., Arita M., Goodarzi A., Cheng G., von Andrian U.H., Serhan C.N. Resolvin E1, an EPA-derived mediator in whole blood, selectively counterregulates leukocytes and platelets. Blood. 2008;112:848–855. doi: 10.1182/blood-2007-11-122598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nelson J.R., Wani O., May H.T., Budoff M. Potential benefits of eicosapentaenoic acid on atherosclerotic plaques. Vasc. Pharmacol. 2017;91:1–9. doi: 10.1016/j.vph.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 72.Budoff M.J., Bhatt D.L., Kinninger A., Lakshmanan S., Muhlestein J.B., Le V.T., May H.T., Shaikh K., Shekar C., Roy S.K., Tayek J., Nelson J.R. Effect of icosapent ethyl on progression of coronary atherosclerosis in patients with elevated triglycerides on statin therapy: final results of the EVAPORATE trial. Eur. Heart J. 2020;41:3925–3932. doi: 10.1093/eurheartj/ehaa652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Watanabe T., Ando K., Daidoji H., Otaki Y., Sugawara S., Matsui M., Ikeno E., Hirono O., Miyawaki H., Yashiro Y., Nishiyama S., Arimoto T., Takahashi H., Shishido T., Miyashita T., Miyamoto T., Kubota I. A randomized controlled trial of eicosapentaenoic acid in patients with coronary heart disease on statins. J. Cardiol. 2017;70:537–544. doi: 10.1016/j.jjcc.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 74.Alfaddagh A., Elajami T.K., Ashfaque H., Saleh M., Bistrian B.R., Welty F.K. Effect of eicosapentaenoic and docosahexaenoic acids added to statin therapy on coronary artery plaque in patients with coronary artery disease: a randomized clinical trial. J. Am. Heart Assoc. 2017;6 doi: 10.1161/JAHA.117.006981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Welty F.K., Schulte F., Alfaddagh A., Elajami T.K., Bistrian B.R., Hardt M. Regression of human coronary artery plaque is associated with a high ratio of (18-hydroxy-eicosapentaenoic acid + resolvin E1) to leukotriene B(4) FASEB J. 2021;35 doi: 10.1096/fj.202002471R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nakao K., Noguchi T., Miura H., Asaumi Y., Morita Y., Takeuchi S., Matama H., Sawada K., Doi T., Hosoda H., Nakashima T., Honda S., Fujino M., Yoneda S., Kawakami S., Nagai T., Nishihira K., Kanaya T., Otsuka F., Nakanishi M., Kataoka Y., Tahara Y., Goto Y., Kusano K., Yamamoto H., Omae K., Ogawa H., Yasuda S. 2022. Effect of eicosapentaenoic acid/docosahexaenoic acid on coronary high-intensity plaques detected using non-contrast T1-weighted imaging: a randomized trial, SSRN. Available at. [Google Scholar]
- 77.Trivedi K., Le V., Nelson J.R. The case for adding eicosapentaenoic acid (icosapent ethyl) to the ABCs of cardiovascular disease prevention. Postgrad. Med. 2021;133:28–41. doi: 10.1080/00325481.2020.1783937. [DOI] [PubMed] [Google Scholar]
- 78.UMIN Clinical Trials Registry; 2018. Randomized Trial for Evaluation in Secondary Prevention Efficacy of Combination Therapy - Statin And Eicosapentaenoic Acid UMIN000012069. [DOI] [PubMed] [Google Scholar]