Abstract
Background
Randomized controlled trials (RCT) of ultrafiltration (UF) have demonstrated conflicting results regarding its efficacy and safety.
Objective
We reviewed 10 years of data for adjustable UF during heart failure hospitalizations in a real world cohort.
Methods
We performed a retrospective, single center analysis of 335 consecutive patients treated with adjustable rate UF using the CHF Solutions Aquadex Flex Flo System from 2009 to 2019.
Results
Compared to previous RCTs investigating UF, our cohort was older, with worse renal impairment and more antecedent HF hospitalizations in the year preceding therapy. Mean fluid removal with UF was 14.6 l. Mean weight loss with UF was 15.6 lbs (range 0.2–57 lbs) and was sustained at 1–2 week follow-up. Mean creatinine change upon stopping UF, at discharge and follow-up (mean 30 days) was +0.11 mg/dl, +0.07 mg/dl and +0.11 mg/dl, respectively. HF rehospitalizations at 30 days, 90 days and 1 year were 12.4 %, 14.9 % and 27.3 % respectively. On average patients had 1.74 fewer hospitalizations for HF in the year following UF when compared to 12 months preceding UF. Major bleeding defined as requiring discontinuation of anticoagulation occurred in 3.6 % of patients.
Conclusions
Compared with previous UF trials, our study demonstrates that UF compares favorably for HF rehospitalizations, renal function response, and weight/volume loss. Importantly, our real world experience allowed for the adjustment of UF rate during therapy and we believe this is a major contributor to our favorable outcomes. In clinical practice, UF can be a safe and effective strategy for decongestion.
Keywords: Heart failure, Ultrafiltration, Aquapheresis, Decongestion
1. Introduction
Heart failure (HF) is estimated to affect about 6.2 million people in the United States with an annual incidence of 1 million new cases each year, accounting for a cost of >30.7 billion dollars annually to the United States economy [1]. In 2016, there were 809,000 HF hospital discharges in the US with an associated high rate of rehospitalization often due to unresolved congestion [2]. The current standard of care for treating these patients remains the use of loop diuretics. However, with progression of disease, loop diuretics are often associated with declining responsiveness despite escalating doses [3]. Diuretic resistance has therefore been associated with lack of symptom relief due to persistent congestion resulting in higher rates of rehospitalization [2], [3]. Diuretics have also been linked with higher morbidity and mortality possibly attributable to deleterious effects on electrolyte balance, neurohormonal activation, and cardiac and renal function [4].
Recent advancement to the HF management algorithm is the introduction of venovenous ultrafiltration (UF). UF is a mechanical strategy that creates a transmembrane pressure gradient producing the movement of plasma fluid across a semipermeable membrane [5]. The potential advantages of UF include greater control over the volume and rate of fluid loss, a higher overall loss of sodium, and lesser activation of neurohormonal feedback mechanisms [6], [7]. Although current guidelines recommend UF as a reasonable approach for persistent congestion in decompensated HF, data on its safety and efficacy is relatively sparse and conflicting [8]. The Ultrafiltration Versus Intravenous Diuretics for Patients Hospitalized for Acute Decompensated Heart Failure (UNLOAD) trial showed that UF, compared to loop diuretics, was associated with a greater net weight loss and HF rehospitalization of 18 % at 90 days [5]. In contrast, the Cardiorenal Rescue Study in Acute Decompensated Heart Failure (CARRESS-HF) trial favored a stepped pharmacologic therapy algorithm over UF due to higher preservation of renal function [9]. Later, the Aquapheresis Versus Intravenous Diuretics and Hospitalizations for Heart Failure (AVOID-HF) trial showed greater freedom from HF primary events with UF; reducing rehospitalization at 30-days and 90-days [6]. These trials differed somewhat in their inclusion/exclusion criteria and, importantly, in their management strategies as it pertains to UF. Given the conflicting findings of these trials, our study sought to determine the safety and efficacy of UF on a real-world cohort.
2. Methods
2.1. Study design and selection criteria
This was a retrospective, single-center study review of consecutive hospitalized patients treated with UF for decompensated heart failure (HF) between July 2009 and June 2019. A dedicated group of investigators was responsible for the collection and validation of data. We extensively reviewed each patient record using multiple electronic medical record data bases (Sunrise Clinical Manager, Sunrise Allscripts, ALPHA, e Clinical Works), public records as well as outside hospital records when available. If patients opted for hospice care, expired during hospitalization or at any time during the review of data, they were excluded from readmission calculation for the corresponding time period. Data was entered into an Excel spread sheet and verified for accuracy. We utilized an abstraction tool and performed random sample auditing to improve inter-rater reliability. An independent statistician blinded to the study methodology performed the statistical analysis.
All patients were treated with an adjustable UF rate using the CHF Solutions Aquadex Flex Flo System. Institutional Review Board approval was obtained prior to data review and analysis.
2.2. Study procedures
All patients that received UF were evaluated for appropriateness of therapy by the Heart Failure Specialty team and ultrafiltration was solely managed by the HF providers. Initial UF rates were determined utilizing clinical judgement and, in the majority of cases, right heart catheterization data. UF rate was adjusted based on clinical data and subsequent patient response. The guideline-directed medical therapy (GDMT) was maintained during ultrafiltration with the exception of transient withholding of blood pressure lowering medications to avoid hypotension, when indicated. Diuretics including aldosterone antagonists were withheld during UF treatment without exception. Patients were treated with intravenous heparin (or intravenous argatroban if heparin was contraindicated) according to protocol to maintain a recommended therapeutic partial thromboplastin time. The designated HF team was responsible for determining the initial UF rate, adjustment of UF rates and duration of UF therapy, as well as, adjunctive therapies such as inotropes for marginal/low cardiac index or right ventricular decompensation. Ultrafiltration was implemented and monitored by specially trained nurses on the dedicated Heart Failure Unit and Cardiac Intensive Care Unit. Nurses closely monitored hourly urine output, serum electrolytes, BUN and creatinine every 12 h and recorded blood pressure (BP) every 1–2 h. The HF team was notified if urine output was <30 cm3/h, if creatinine increased by ≥0.3 mg/dl or significant blood pressure changes occurred that exceeded patient specific parameters.
2.3. Study outcomes
The primary efficacy outcome was the change in weight, volume loss, glomerular filtration rate (GFR), blood urea nitrogen (BUN) and creatinine with UF. The change in the rate of these outcomes was recorded by calculating differences in the mean values at the time of UF, after UF was discontinued and at discharge. Appropriately calibrated standard weight measuring scales were used to determine the weights in pounds (lbs). The net volume loss was a combined measure of the ultrafiltrate and urine volume. Also recorded were the incidence of hypotension episodes, time of UF initiation, inotrope support utilization and need for hemodialysis. The secondary efficacy outcome was the incidence of rehospitalization for HF at 30-days, 90-days and 1-year after the index UF procedure. The primary safety endpoints included renal failure requiring hemodialysis and major bleeding defined as requiring the discontinuation of the anticoagulant therapy, and included persistent minor catheter site bleeding.
2.4. Statistical analysis
The raw data was presented in Excel spreadsheets and was analyzed using the appropriate statistical analysis models. For categorical data, frequency was reported in percentages and data was compared using the chi-square test. Continuous and scale data was reported in means with standard deviations (SD) and was compared using independent t-test analysis. Dichotomous categorical data, unadjusted odds ratios (uOR) were calculated to determine the effect size of the primary and secondary outcomes. A multivariate logistical regression model was used to determine the impact of potential effect modifiers and to calculate the adjusted odds ratios (aOR). A wide range of variables was assessed in a stepwise manner to evaluate the effects of covariates on the readmission rate. A sensitivity analysis based on the exclusion of patients who might have a high impact on outcomes was also performed, such as patients requiring hemodialysis were systematically omitted to measure their contribution to the pooled estimates. A subgroup analysis stratified by left ventricular ejection fraction ≥ 40 % (heart failure with preserved ejection fraction [HFpEF]) or <40 % (heart failure with reduced fraction [HFrEF]) was performed. A two-tailed p-value <0.05 was considered statistically significant. Analysis was performed using SAS 16 and R version 3.04.
3. Results
3.1. Baseline characteristics
A total of 335 consecutive patients treated with adjustable rate UF were included from July 2009 to June 2019. The mean age of the included population was 73.3 years, with 57 % male. Of the included population, 52 % had HFpEF. The mean initial systolic blood pressure (SBP) was 120 ± 22 mm Hg. On average, patients had 2.14 hospitalizations for HF in the 12 months preceding UF. 66 % of patients underwent right heart catheterization prior to UF therapy and in these patients, mean pulmonary capillary wedge pressure was 25 ± 9 mm Hg, right atrial pressure was 17 ± 7 mm Hg and estimated Fick cardiac index was 2.7 ± 0.8 l/min/m2. The mean hospital day of UF initiation was 5.6 days. The average starting UF rate was 151 ml/h with 58 % of patients requiring UF rate adjustments during therapy. The detailed baseline characteristics of the included population are given in Table 1.
Table 1.
Detailed baseline characteristics and hemodynamics of the included population.
| N | % | |
|---|---|---|
| Sex | ||
| Female | 144 | 43 % |
| Male | 190 | 56 % |
| HFpEF or HFrEF | ||
| HFpEF | 173 | 51.8 % |
| HFrEF | 161 | 48.2 % |
| Ascites | ||
| Yes | 19 | 5.6 % |
| No | 315 | 94.3 % |
| Change in UF rate during therapy | ||
| Decrease | 140 | 41.9 % |
| Increase | 55 | 16.4 % |
| Unchanged | 139 | 41.6 % |
| Inotrope use | ||
| Yes | 90 | 27.1 % |
| No | 241 | 72.8 % |
| N | Mean | SD* Standard Deviation | Min | Max | |
|---|---|---|---|---|---|
| Age (years) | 335 | 73 | 11.92 | 38.00 | 99.00 |
| Pre UF SBP (mm/Hg) | 325 | 120.1 | 22.09 | 64.0 | 219.00 |
| Pre UF Cr (mg/dl) | 334 | 1.78 | 0.74 | 0.49 | 4.89 |
| Pre UF BUN (mg/dl) | 326 | 49.09 | 24.99 | 11.0 | 167.0 |
| Pre UF GFR (mg/dl) | 334 | 38.78 | 14.54 | 10.0 | 82.0 |
| Starting UF rate (cm3/h) | 335 | 151 | 47.94 | 50.00 | 400.00 |
| HF admission in past year | 296 | 2.14 | 1.38 | 0.00 | 10.00 |
| Hospital day that UF initiated | 331 | 5.6 | 5.50 | 0.00 | 33.00 |
HFpEF-heart failure with preserved ejection fraction; HfrEF-heart failure with reduced ejection fraction; UF-ultrafiltration; SBP-systolic blood pressure; Cr–creatinine; BUN-blood urea nitrogen; GFR-glomerular filtration rate; HF-heart failure.
3.2. Pooled outcomes
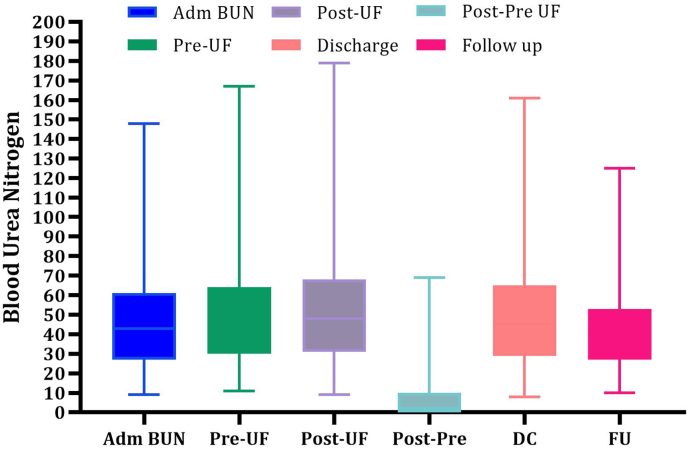
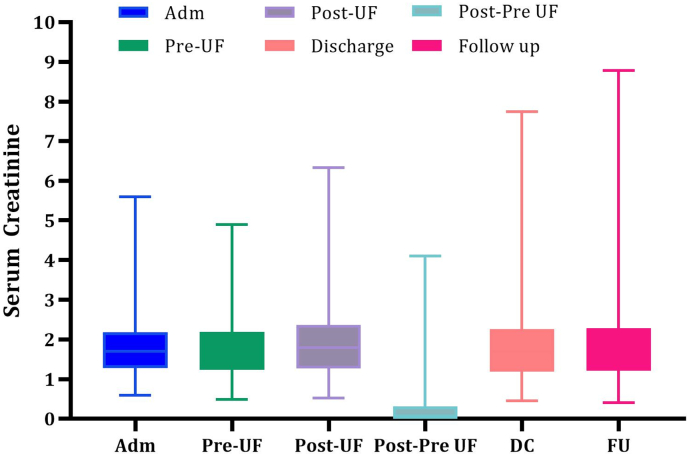
A mean fluid removal of 14.6 ± 8.8 l per person was removed during UF treatment. The mean weight reduction after completion of UF was 15.6 ± 11.1 lbs with 55 % of patients achieving a weight loss >15 lbs with UF. The overall mean increase in BUN with UF was 3.4 mg/dl ± 13.3 mg/dl (pre-UF 49.9 mg/dl ± 24.9 mg/dl and post-UF 52.3 mg/dl ± 27.6 mg/dl). Mean creatinine change upon stopping UF, at discharge and at 30 day follow up was +0.11 mg/dl, +0.07 mg/dl and +0.11 mg/dl respectively (Fig. 1, Fig. 2). Similarly, the GFR change upon stopping UF, at discharge and at follow up was −1.59 ml/min/1.73 m2, −0.19 ml/min/1.73 m2, and −0.89 ml/min/1.73 m2. There was no significant change in the follow-up weight, BUN, creatinine or GFR levels compared with the corresponding values at the time of discharge (Table 2). After exclusion of patients that were lost to follow up, transitioned to hospice or expired, the 30 day, 90 day and 1 year HF rehospitalization rate for patients treated with UF was 12.4 %, 14.9 % and 27.3 % respectively (Table 3). On average, patients had 1.74 fewer hospitalizations for HF in the year following UF when compared to the 12 months preceding UF. The overall need for hemodialysis during index hospitalization due to worsening renal function was 5 % while only 3.6 % of patients had major bleeding events.
Fig. 1.
Range, mean, median and interquartile range of changes in blood urea nitrogen with UF at different time intervals with UF.
ADM-admission; BUN-blood urea nitrogen; UF-ultrafiltration; DC-discharge; FU-follow up.
Fig. 2.
Range, mean, median and interquartile range of changes in serum creatinine level with UF at different time intervals with UF.
ADM-admission; BUN-blood urea nitrogen; UF-ultrafiltration; DC-discharge; FU-follow up.
Table 2.
Summary statistics of the Secondary outcomes in patients receiving UF.
| N | Mean | SD⁎ | min | max | |
|---|---|---|---|---|---|
| Pre UF weight | 334 | 222.3 | 59.87 | 111.9 | 508.5 |
| Post UF weight | 335 | 206.5 | 57.44 | 96.80 | 457.0 |
| D/C weight (lbs) | 331 | 203.9 | 56.43 | 98.00 | 454.8 |
| F/U weight (lbs) | 155 | 208.8 | 57.59 | 103.4 | 378.5 |
| Weight loss (pre UF weight − post UF weight) (lbs) | 334 | 15.63 | 11.17 | −5.80 | 56.30 |
| Weight loss (D/C weight − F/U weight) (lbs) | 155 | 0.60 | 11.97 | −37.1 | 40.10 |
| Pre UF BUN (mg/dl) | 326 | 49.09 | 24.99 | 11.00 | 167.0 |
| Post UF BUN (mg/dl) | 323 | 52.37 | 27.60 | 9.00 | 179.0 |
| Change in BUN (post UF BUN − pre UF BUN) | 315 | 3.44 | 13.36 | −56.0 | 58.00 |
| Pre UF GFR | 334 | 38.78 | 14.54 | 10.00 | 82.00 |
| Post UF GFR | 335 | 37.16 | 15.19 | 9.00 | 60.00 |
| D/C GFR | 330 | 38.63 | 15.63 | 3.00 | 87.00 |
| F/U GFR | 232 | 38.45 | 15.10 | 4.00 | 61.00 |
| Change in GFR (post UF GFR − pre UF GFR) | 334 | −1.59 | 8.42 | −52.0 | 28.00 |
| Change in GFR (DC GFR − pre GFR) | 329 | −0.19 | 10.12 | −59.0 | 52.00 |
| Change in GFR (FU GFR − pre GFR) | 231 | −0.89 | 9.08 | −33.0 | 19.00 |
| Pre UF creatinine level (mg/dl) | 334 | 1.78 | 0.74 | 0.49 | 4.89 |
| Post UF creatinine level (mg/dl) | 335 | 1.89 | 0.86 | 0.52 | 6.34 |
| D/C creatinine level (mg/dl) | 331 | 1.85 | 0.93 | 0.46 | 7.75 |
| F/U creatinine level (mg/dl) | 235 | 1.86 | 1.04 | 0.41 | 8.78 |
| Change in creatinine (post UF creatinine − pre UF creatinine) | 334 | 0.11 | 0.43 | −1.33 | 4.10 |
| Change in creatinine (DC creatinine − pre UF creatinine) | 330 | 0.07 | 0.62 | −2.19 | 5.51 |
| Change in creatinine (FU creatinine − pre UF creatinine) | 234 | 0.11 | 0.76 | −1.36 | 6.44 |
| Total volume removed with UF (UF + UO liters) | 333 | 14.58 | 8.84 | 0.40 | 51.20 |
ADM-admission; BUN-blood urea nitrogen; GFR-glomerular filtration rate; lbs-pounds; UF-ultrafiltration; DC-discharge; FU-follow up.
Standard Deviation.
Table 3.
Summary statistics of the Secondary outcome in patients receiving UF.
| Outcomes | Readmissions |
# of patients excluded from analysis: LTF/hospice/expired | |||
|---|---|---|---|---|---|
| Yes |
No |
||||
| N | % | N | % | ||
| HF readmission at 30 day | 34 | 12.4 | 240 | 87.6 % | 10 E, 32 hos, 18 LTF of 334 total |
| HF readmission at 90 day | 37 | 14.9 % | 212 | 85.1 % | 10 E, 32 hos, 43 LTF/334 |
| HF readmission at 1 year | 63 | 27.3 % | 168 | 72.8 % | 10 E, 32 hos, 61 LTF/334 |
| Major bleeding | 12 | 3.6 % | 322 | 96.4 | NA |
| Worsening renal function requiring HD | 17 | 5.1 % | 318 | 95 % | NA |
HF heart failure; HD-hemodialysis. E-expired; hos-hospice; LTF Lost to follow up
3.3. Sensitivity analysis
A sensitivity analysis based on the exclusion of patients who required hemodialysis followed the findings of the pooled analysis. Marked ascites and high pre-UF BUN levels remained the strongest predictors of HF-related readmissions at 30-days.
3.4. Subgroup analysis
A subgroup analysis of patients who underwent UF with HFpEF compared to HFrEF showed no significant difference between groups. Change in weight, fluid loss and worsening renal function remained similar between patients receiving UF for HFpEF vs. HFrEF (Table 4). The 30-day (OR 0.68, 95 % 0.32–1.32), 90-day (OR 0.97, 95 % CI 0.48–1.96) and 1-year (OR 1.54, 95 % CI 0.85–2.79) readmission rates due to HF exacerbations were similar between the HFpEF and HFrEF patients. There was no difference in the need for dialysis (OR 2.3, 95 % 0.80–6.7) and major bleeding events (OR 0.93, 95 % 0.29–2.97) between the two groups.
Table 4.
Subgroup analysis of patients with HFpEF and HFrEF showing estimates of secondary outcomes.
| HFpEF |
HFrEF |
|||||
|---|---|---|---|---|---|---|
| N | Mean | SD | N | Mean | SD | |
| Pre UF weight (weight - lbs; BUN- mg/dl; GFR mg/dl; creat-mg/dl; total volume -liters) | 173 | 234.3 | 65.30 | 161 | 209.4 | 50.54 |
| Post UF weight | 173 | 218.0 | 61.46 | 162 | 194.3 | 50.18 |
| D/C weight | 173 | 214.9 | 59.26 | 158 | 191.9 | 50.69 |
| F/U weight | 82 | 218.1 | 55.43 | 73 | 198.4 | 58.56 |
| Weight loss (pre UF weight − post UF weight) | 173 | 16.30 | 12.88 | 161 | 14.91 | 8.95 |
| Weight loss (D/C weight − F/U weight) | 82 | 1.41 | 12.89 | 73 | −0.30 | 10.87 |
| Pre UF BUN | 169 | 48.73 | 26.13 | 157 | 49.47 | 23.78 |
| Post UF BUN | 167 | 53.36 | 29.27 | 156 | 51.30 | 25.74 |
| Change in BUN (post UF BUN − pre UF BUN) | 164 | 5.10 | 12.84 | 151 | 1.64 | 13.71 |
| Pre UF GFR | 172 | 38.81 | 15.21 | 162 | 38.75 | 13.84 |
| Post UF GFR | 173 | 36.45 | 16.07 | 162 | 37.92 | 14.20 |
| D/C GFR | 170 | 37.74 | 15.85 | 160 | 39.59 | 15.39 |
| F/U GFR | 121 | 37.83 | 15.47 | 111 | 39.13 | 14.72 |
| Change in GFR (post UF GFR − pre UF GFR) | 172 | −2.31 | 7.94 | 162 | −0.83 | 8.87 |
| Change in GFR (DC GFR − pre GFR) | 169 | −1.21 | 8.50 | 160 | 0.89 | 11.51 |
| Change in GFR (FU GFR − pre GFR) | 120 | −1.68 | 9.43 | 111 | −0.04 | 8.65 |
| Pre UF creatinine level | 172 | 1.73 | 0.77 | 162 | 1.84 | 0.70 |
| Post UF creatinine level | 173 | 1.89 | 0.95 | 162 | 1.88 | 0.76 |
| D/C creatinine level | 170 | 1.85 | 1.00 | 161 | 1.86 | 0.85 |
| F/U creatinine level | 124 | 1.89 | 1.22 | 111 | 1.82 | 0.81 |
| Change in creatinine (post UF creatinine − pre UF creatinine) | 172 | 0.16 | 0.48 | 162 | 0.05 | 0.36 |
| Change in creatinine (DC UF creatinine − pre UF creatinine) | 169 | 0.12 | 0.69 | 161 | 0.02 | 0.54 |
| Change in creatinine (FU UF creatinine − pre UF creatinine) | 123 | 0.20 | 0.93 | 111 | 0.02 | 0.48 |
| Total volume removed with UF | 173 | 14.84 | 9.22 | 160 | 14.30 | 8.43 |
HFpEF-heart failure with preserved ejection fraction; HfrEF-heart failure with reduced ejection fraction; UF-ultrafiltration; BUN-blood urea nitrogen; GFR-glomerular filtration rate; HF-heart failure; DC-discharge; FU-follow up.
4. Discussion
The main finding of our study is that ultrafiltration can be used safely and effectively for significant volume removal among patients admitted with decompensated heart failure. Compared to previous trials, the rate and duration of ultrafiltration was slower and longer than that in the UNLOAD and CARRESS trials but similar to that in the AVOID trial [5], [6], [9] (Table 5). In addition, the majority of our patients underwent adjustment of ultrafiltration rate; although not frequently needed, we used adjunctive inotrope support (27 %) when indicated for low cardiac index and/or right ventricular failure. We believe that the “low and slow” approach to ultrafiltration, combined with ultrafiltration rate adjustment as necessary is crucial to safely engendering a large volume decongestion.
Table 5.
Comparison of major ultrafiltration studies utilizing Aquadex.
| Starting rate customized to patient clinical profile | Average UF rate/start rate | Average duration | Allowed adjustments based on clinical response | Inotropes if clinically indicated | Readmissions (%) |
||||
|---|---|---|---|---|---|---|---|---|---|
| 30 day | 60 day | 90 day | 1 year | ||||||
| Abington Jefferson Health-Real World | Y | 151/h average start rate | 91.44 h | Y | Y | 12.41 | NA | 14.86 | 27.27 |
| UNLOAD | Y | Average rate 241/h | 12.3 ± 12 h | Y | N | NA | NA | 18 | NA |
| CARRESS | N | 200 fixed rate per protocol | 40 h (range 28–67) | N | Na | NA | 26 | NA | NA |
| AVOID | Y | Average rate 138 ± 47 ml/h | 80 ± 53 h (range 12–283 h) | Y | N | 9.5 | NA | 25.7b | NA |
Y-yes; N-no.
UNLOAD-Ultrafiltration vs intravenous diuretics in patients hospitalized in acute decompensated heart failure.
CARRESS-Ultrafiltration in decompensated heart failure with cardiorenal syndrome.
AVOID-Aquapheresis vs intravenous diuretics and hospitalizations for heart failure.
Inotropes allowed in diuretic arm only (utilized in 12 % of diuretic arm patients).9
AVOID was stopped prematurely by the sponsor after 27.5 % enrollment.6
Prior UF studies have also shown that weight changes are variable. The UNLOAD (short duration, adjustable UF) and the CARRESS (high rate UF, fixed) trials observed a mean weight loss of 5.0- ± 0.3.1 kg and 5.5 ± 5.1 kg respectively with UF, half that was observed with the AVOID-HF (adjustable UF) trial (10.7 ± 7.2 kg) [5], [6], [9]. Similarly, our study (adjustable UF) showed a mean weight loss of 6.8 ± 5 kg. The net fluid loss in our study ranged from 0.4 to 51.2 l (mean 14.6 ± 8.8 l) which was significantly higher than the UNLOAD trial (4.6 l) and CARRESS-HF (7.4 ± 4.3 l) and somewhat higher than the AVOID-HF trial (12.91 l) [5], [6], [9].
Renal dysfunction characterized by a rise in post UF serum creatinine levels > 0.3 mg/dl was used to determine the safety of UF in prior RCT's [11]. We found rises in post UF serum creatinine levels above 0.3 mg/dl in 25.1 % of patients with a mean post UF increase of 0.11 ± 0.43 mg/dl. This decline in renal function was strikingly lower than the UF-patient population of the CARRESS trial (0.23 ± 0.70 mg/dl) [9]. Similarly, there was no clinically significant decline in the GFR (mean −1.59 ± 8.42) or rise in BUN observed (3.44 ± 13.3 mg/dl). The lack of significant correlation between weight loss and rise in serum creatinine suggests that judicious adjustments in UF rates in response to clinical variables (predominantly blood pressure, urine output and renal function trends) combined with the appropriate use of adjunctive inotropic support when required, contributed to significant decongestion without compromising renal perfusion. The risk of complications including major bleeding and renal failure requiring hemodialysis was observed in <4 % of patients, despite our inclusion of patients with baseline creatinine greater than those enrolled in prior UF RCTs [5], [6], [9].
We also observed lower readmission rates than has been published in previous clinical trials. Compared with the UNLOAD and AVOID HF trials in which a 90 day HF rehospitalization occurred in 18 % and 25 % of UF patients, respectively, only 14.9 % of our study population required rehospitalization for HF [5], [6]. Similarly, our 90 day HF rehospitalization rate of patients treated with UF was lower than the 60 day readmission rate of the CARRESS trial at 26 % [9]. More importantly, while all these trials focused on the short-term follow-up outcomes, our study also demonstrated a 27 % HF-related rehospitalization rate at 1-year after index UF (Table 5). Although patients who were lost to follow-up, died, or enrolled in hospice were eliminated from the readmission calculations, it is possible that patients were admitted to other hospital systems that we did not capture (Table 3). Of the 335 patients analyzed, 5 (1.5 %) expired during the hospitalization and 39 (11.6 %) were discharged to hospice. We compared these 44 patients to the remaining 291 in the analyzed cohort. We found that these patients were older, had worse renal function, more precedent hospitalizations for HF and required lower average UF rates. Of note, these patients tended to have ultrafiltration therapy started later in the hospital stay (Table 6). These 44 patients were included in the analysis of secondary outcomes in tables 2 and 4 but were not included in the readmission analyses in Table 3. Of note, of the sickest 44 patients, 19 received documented palliative ultrafiltration, meaning we were asked to provide ultrafiltration for symptom relief of known end stage disease (Table 6). Therefore, we cannot compare our readmission rate to those of published clinical trials, nor can we conclude definitively that we lowered hospitalization rates with ultrafiltration compared to clinical trials. However, among those patients in whom there was follow-up for 12 months, there were 1.74 fewer rehospitalizations for HF in the year following UF when compared to the 12 months preceding UF.
Table 6.
Subgroup analysis of patients that went to hospice or expired at discharge vs remaining UF patients.
| Remaining (N = 291) non-hospice and/or expired |
Hospice or expired at discharge (N = 44) |
|||||
|---|---|---|---|---|---|---|
| Mean | Standard deviation | Mean | Standard deviation | Mean difference | p-Value from t-test | |
| Age (years) | 72.8 | 12.05 | 77.0 | 10.44 | −4.21 | 0.03 |
| Pre UF SBP mm/Hg | 122.2 | 22.10 | 105.7 | 15.84 | 16.54 | <0.01 |
| UF starting Rate | 154 | 48.01 | 130.9 | 42.91 | 22.91 | <0.01 |
| HF admission in past year | 2.03 | 1.23 | 2.84 | 2.02 | −0.81 | 0.02 |
| Hospital day that UF initiated | 5.28 | 5.11 | 8.07 | 7.31 | −2.79 | 0.02 |
| Pre UF GFR | 40.16 | 14.51 | 29.7 | 11.22 | 10.45 | <0.01 |
UF-ultrafiltration; sbp systolic blood pressure; HF-heart failure; GRF-glomerular filtration rate
Our cohort had greater volume removal than described in published RCTs [5], [6], [9] and this may account for the lower rehospitalization rates we observed. The treatment targets for UF and objective measure of volume status were not pre-specified in the UNLOAD trial. Only 27.5 % of the intended 810 patients were enrolled in the AVOID-HF trial which was stopped unilaterally due to a change in sponsor support [5], [6]. Together, these might have resulted in underestimating the potential benefits of UF. Similarly, a small sample size, higher UF rate (200 ml/h) and lack of UF rate adjustment based on patient vital signs, urine output and renal function could explain the higher rate of rehospitalization and worsening renal function in the CARRESS trial. In addition, inotropic support was prohibited in the UF arm yet allowed in the diuretic arm (12 %) in CARRESS; 39 % of patients randomized to UF either did not receive UF or received a mix of UF and IV diuretics confounding outcome attribution (Table 5). Additionally, the study population in the CARRESS trial was restricted to patients with cardiorenal syndrome where UF was a rescue treatment for patients who already had an acute rise in serum creatinine levels in response to standard-of-care therapy [9].
Subgroup analysis showed equal efficacy of UF in both HFrEF and HFpEF patients, as has been demonstrated previously [10]. This may be due to the significant volume removal with UF while simultaneously reducing total body sodium and excess isotonic fluid more effectively than the isolated removal of hypotonic fluid by diuretics. Additionally, unlike diuretics, UF judiciously applied, is independent of renin release and avoids the neurohormonal activation of the Na-K-2Cl cotransporters [7]. The “diuretic holiday” during UF therapy also increases diuretic responsiveness as indicated by the effectiveness of lower diuretic requirement post-UF. In our study 37 % of patients were discharged with a decreased dose of diuretic and 31 % on an increased dose. This compares favorably to the ASCEND HF trial where only 13 % of the patients were discharged on a reduced diuretic dose and 56 % on an increased diuretic dose [12]. Our results demonstrate strong evidence of decongestion reflected by weight and fluid loss without provoking significant renal dysfunction. The ability to promote adequate decongestion in our study led to favorable reductions in HF rehospitalizations at 30 day, 90 day and 1 year time points. These findings reflect those of recent studies demonstrating the imperative to effect decongestion during hospitalization in order to improve outcomes [13], [14], [15]. We believe that an individualized determination of initial UF rates with appropriate and dynamic UF adjustments by a dedicated multidisciplinary team according to patient's clinical response is crucial to effective UF management. Of note, the CARRESS trial mandated a fixed initial UF rate and did not allow for dynamic rate adjustment [9].
4.1. Limitations
The major limitation of our study is that it was a single-center analysis of retrospective data. Patients were not randomized and we did not compare UF patients to a cohort receiving other decongestive therapies. Therefore, we cannot comment on the superiority of UF compared to other therapies. As discussed, our readmission rates may be underestimated despite our extensive review of records. Patients were selected based on HF specialty team's clinical assessment and judgement; similarly, dynamic UF rate management was based on clinician judgement. Further study of patient characteristics and responsiveness to therapy is warranted.
5. Conclusions
Compared with previous trials with UF (UNLOAD, CARRESS, and AVOID), this real world experience demonstrates that UF compares favorably for weight/volume loss and renal function response, and this may be associated with a lower HF rehospitalization rate. We found ultrafiltration to be safe with regards to renal function despite the cohort in this study being sicker than those studied in other clinical trials. Importantly, this real world experience allowed for the adjustment of UF rate during the therapy and the use of inotropes when indicated. We believe these factors are major contributors to our favorable outcomes. In clinical practice, UF can be a safe and effective strategy for decongestion wherein the benefits outweigh the potential risks of kidney dysfunction requiring hemodialysis and major bleeding events.
Clinical perspective
Competency in Medical Knowledge: Pateints with HF are often discharged with persistent volume overload. Decongesting patients admitted with acute decompensated heart failure is a key factor in improving patient outcomes and reducing HF readmissions.
Competency in Patient Care: Adjustable ultrafiltration is an effective way to remove volume. Ultrafiltration is most successful when managed by a team that is familiar with the therapy; this includes the prescribers and the clinical team that will monitor the patient.
Translational Outlook 1: Additional research is needed to define the best hemodynamic profile that will yield the best outcomes with ultrafiltration.
Translational Outlook II: Additional research is needed to understand the incorporation of other diagnostic modalities to quantify volume status to guide volume removal.
Source of support
Nuwellis Inc. (previously CHF Solutions Inc.) provided funding for an independent statistician.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Maureen Hummel reports financial support was provided by CHF Solutions (Nuwellis) for independent data analysis. Maureen Hummel reports a relationship with CHF Solutions (Nuwellis) that includes: speaking and lecture fees and travel reimbursement.
Acknowledgement
Richard Slobodien, Jefferson Health Abington.
Contributor Information
Donald C. Haas, Email: donald.haas@jefferson.edu.
Patricia Barrella, Email: patricia.barrella@jefferson.edu.
References
- 1.Virani S.S., Alonso A., Benjamin E.J., Bittencourt M.S., Callaway C.W., Carson A.P., Chamberlain A.M., Chang A.R., Cheng S., Delling F.N., Djousse L., Elkind M.S.V., Ferguson J.F., Fornage M., Khan S.S., Kissela B.M., Knutson K.L., Kwan T.W., Lackland D.T., Lewis T.T., Lichtman J.H., Longenecker C.T., Loop M.S., Lutsey P.L., Martin S.S., Matsushita K., Moran A.E., Mussolino M.E., Perak A.M., Rosamond W.D., Roth G.A., Sampson U.K.A., Satou G.M., Scroeder E.B., Shah S.H., Shay C.M., Spartano N.L., Stokes A., Tirschwell D.L., VanWagner L.B., Tsao C.W. Heart disease and stroke statistics: 2020 update-a report for the American Heart Association. Circ. 2020 Mar 3;141(9) doi: 10.1161/CIR.0000000000000757. e139- e596. [DOI] [PubMed] [Google Scholar]
- 2.Lala A., McNulty S.E., Mentz R.J., Dunlay S.M., Vader J.M., AbouEzzedine OF. DeVore A.D., Khazanie P., Redfield M.M., Goldsmith S.R., Bradley B.A., Anstrom K.J., Felker G.M., Hernandez A.F., Stevenson L.W. Relief and recurrence of congestion during and after hospitalization for acute heart failure. Insights from Diuretic Optimization Strategy Evaluation in Acute Decompensate Heart Failure (DOSE-AHF) and Cardiorenal Rescue Study in Acute Decompensated Heart Failure (CARRESS-HF) Circ. Heart Fail. 2015;8:741–748. doi: 10.1161/CIRCHEARTFAILURE.114.001957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singh D., Shrestha K., Testani J.M., Vebrugge F.H., Dupont M., Mullens W., Tang W.H.W. Insufficient natruietic response to continuous intravenous furosemide is associated with poor long term outcomes in acute decompensated heart failure. J. Card. Fail. 2014;20(6):392–399. doi: 10.1016/j.cardfail.2014.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schrier R.W. Role of diminished renal function in cardiovascular mortality: marker or pathogenetic factor? J. Am. Coll. Cardiol. 2006;47(1):1–8. doi: 10.1016/j.jacc.2005.07.067. [DOI] [PubMed] [Google Scholar]
- 5.Costanzo M.R., Guglin M.E., Saltzberg M.T., Jessup M.L., Bart B.A., Teerlink J.R., Jaski B.E., Fang J.C., Feller E.D., Haas G.J., Anderson A.S., Schollmeyer M.P., Sobotka P.A. Ultrafiltration versus intravenous diuretics patients hospitalized for acute decompensated heart failure. J. Am. Coll. Cardiol. 2007;49(6):675–683. doi: 10.1016/j.jacc.2006.07.073. [DOI] [PubMed] [Google Scholar]
- 6.Costanzo M.R., Negoianu D., Jaski B.E., Bart B.A., Heywood J.T., Anand I.S., Smelser J.M., Kaneshige A.M., Chomsky D.B., Adler E.D., Haas G.J., Watts J.A., Nabut J.L., Schollmeyer M.P., Fonarow G.C. Aquapheresis versus intravenous diuretics and hospitalizations for heart failure. J. Am. Coll. Cardiol. 2016;4(2):95–105. doi: 10.1016/j.jchf.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 7.Costanzo M.R., Ronco C., Abraham W.T., Agostini P., Barasch J., Fonarrow G.C., Gottlieb S.S., Jaski B.E., Kazory A., Levin A.P., Levin H.R., Marenzi G., Mullens W., Negoianu D., Redfield M.M., Tang W.W.H., Testani J.M., Voors A.A. Extracorporeal ultrafiltration for fluid overload in heart failure. Current status and prospects for further research. J. Am. Coll. Cardiol. 2017;69(19):2428–2445. doi: 10.1016/j.jacc.2017.03.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hunt S.A., Abraham W.T., Chin M.H., Feldman A.M., Francis G.S., Ganiats T.G., Jessup M., Konstam M.A., Mancini D.M., Michi K., Oates J.A., Rahko P.S., Silver M.A., Stevenson L.W., Yancy C.W. 2009 focused update incorporated into the ACC/AHA 2005 guidelines for the diagnosis and Management of Heart Failure in adults: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;119(14):e391–e479. doi: 10.1161/CIRCULATIONAHA.109.192065. [DOI] [PubMed] [Google Scholar]
- 9.Bart B.A., Goldsmith S.R., Lee K.L., Givertz M.M., O’Connor C.M., Bull D.A., Redfield M.M., Deswal A., Rouleau J.L., LeWinter M.M., Ofili E.O., Stevenson L.W., Semigran M.J., Felker G.M., Chen H.H., Hernandez A.F., Anstrom K.J., McNulty S.E., Velazquez E.J., Ibarra J.C., Mascette A.M., Brunwald E. Ultrafiltration in decompensated heart failure with cardiorenal syndrome. N. Engl. J. Med. 2012 Dec 13;367(24):2296–2304. doi: 10.1056/NEJMoa1210357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jefferies J.L., Bartone C., Menon S., Egnaczyk G.F., O’Brien T.M., Chung E.S. Ultrafiltration in heart failure with preserved ejection fraction: comparison with systolic heart failure patients. Circ. Heart Fail. 2013;6:733–739. doi: 10.1161/CIRCHEARTFAILURE.112.000309. [DOI] [PubMed] [Google Scholar]
- 11.Mehta R.L., Kellum J.A., Shah S.V., Molitoris B.A., Ronco C., Warnock D.G., Levin A. Acute kidney injury network: report of an initiative to improve outcomes in acute kidney injury. Crit. Care. 2007;11(2) doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeVore A.D., Hasselblad V., Mentz R.J., O’Connor C.M., Armstong P.W., McMurray J.J., Ezekowitz J.A., Tang W.H.W., Starling R.C., Voors A.A., Califf R.M., Hernandez A.F. Loop diuretic dose adjustment after a hospitalization for heart failure: insights from ASCEND-HF. Eur. J. Heart Fail. 2015;17:340–346. doi: 10.1002/ejhf.235. [DOI] [PubMed] [Google Scholar]
- 13.McCallum W., Tighiouart H., Testani J.M., Griffin M., Konstam M.A., Udelson J.E., Sarnak M.J. Acute kidney function declines in the context of decongestion in acute decompensated heart failure. J. Am. Coll. Cardiol. July 2020;8(7):537–547. doi: 10.1016/j.jchf.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kociol R.D., McNulty S.E., Hernandez A.F., Lee K.L., Redfield M.M., Tracy R.P., Brunwald E., O’Connor C.M., Felker G.M. Markers of decongestion, dyspnea relief, and clinical outcomes among patients hospitalized with acute heart failure. Circ. Heart Fail. 2013;6:240–245. doi: 10.1161/CIRCHEARTFAILURE.112.969246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Testani J.M., Chen J., McCauley B.D., Kimmel S.E., Shannon R.P. Potential effects of aggressive decongestion during the treatment of decompensated heart failure on renal function and survival. Circ. 2010;122:265–272. doi: 10.1161/CIRCULATIONAHA.109.933275. [DOI] [PMC free article] [PubMed] [Google Scholar]