Abstract
Background
Iron deficiency is an important co-morbidity in heart failure patients. IV iron may improve quality of life and reduce heart failure hospitalizations, but the results of the clinical trials are varied.
Objective
The purpose of this meta-analysis is to assess not only the effect of IV iron in iron-deficient heart failure patients but also the quality of evidence.
Methods
PubMed and Cochrane databases were searched from inception to Oct 2021. Randomized clinical trials in iron-deficient, heart failure patients assessing the effect of IV iron versus placebo and with at least 12 weeks of follow-up were included. The outcomes were pooled and analyzed using a random-effect model. The quality of evidence was assessed using the GRADE approach.
Results
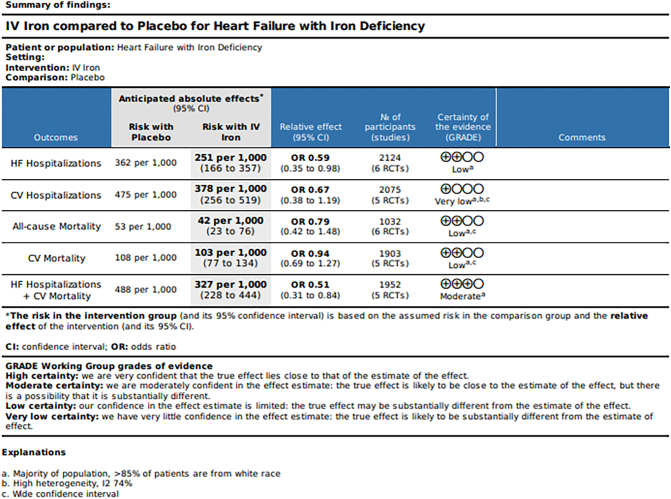
Seven studies were included in our meta-analysis. IV iron was associated with a 13.8 % decreased risk of HF hospitalizations (OR 0.59; 0.35–0.98, p = 0.040, GRADE = Low). All-cause mortality and CV mortality were not different between IV iron and placebo. But a composite outcome of HF hospitalizations or CV mortality was 17.5 % lower with IV iron (OR 0.51;0.31–0.84, p = 0.008, GRADE = Moderate).
Conclusions
Among heart failure patients with iron deficiency, IV iron is associated with lower HF hospitalizations. It is a relatively inexpensive regimen that can potentially improve quality of life and decrease healthcare expenditure.
Keywords: Intravenous Iron, Heart failure, Iron deficiency, Meta-analysis
1. Introduction
Iron deficiency is a common comorbidity in patients with heart failure and is associated with higher mortality and increased hospitalizations [1], [2]. In patients with chronic heart failure, there is impaired iron metabolism, with decreased available free iron for usage even with normal ferritin levels. Decreased available functional iron is associated with decreased oxygen consumption irrespective of the hemoglobin levels [3].
Iron deficiency reduces the oxidative capacity of skeletal muscles [4] and impaired exercise capacity in patients with heart failure, which in turn leads to poor quality of life [5], [6]. While supplementing iron therapy, oral iron administration is likely limited due to gastric and duodenal mucosa edema and increased hepcidin levels which reduces duodenal iron absorption from the duodenum and iron storage release [7], [8].
There are randomized clinical trials that studied the effects of IV iron on mortality, hospitalizations, and quality of life questionnaires but the results are varied [9], [10], [11], [12], [13], [14], [15]. There are previous meta-analyses assessing the effect of IV iron therapy in iron-deficient heart failure patients, but different studies were included in those meta-analyses and the results are without the assessment of the grade of evidence [16], [17], [18]. Therefore, we conducted a meta-analysis assessing the effect of IV iron therapy in hospitalizations and mortality in patients with heart failure, using the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) approach to assess the quality of evidence.
2. Materials and methods
We conducted a systematic review of the literature adhering to the institute of medicine's standards for systematic reviews [19]. This meta-analysis is reported according to the guidelines provided by the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) [20]. It is not registered with the International Prospective Register of Systematic Reviews (PROSPERO). The approval of the institutional review board was not required as the data was publicly available. Our study aims to assess the effect of intravenous iron versus placebo on recurrent hospitalization and mortality in patients with heart failure and iron deficiency.
2.1. Information sources and study selection
One author (PM) devised and performed a systematic search of PubMed and the Cochrane Library using prespecified search terms (see Supplementary material). The databases were searched from inception to Oct 2021. The articles were included if they were
-
–
published randomized human clinical trials, written in English,
-
–
investigating the effect of intravenous iron versus placebo in iron-deficient heart failure patients,
-
–
with a follow-up duration of at least 12 weeks, and
-
–
either of the outcomes assessed: all-cause mortality, cardiovascular mortality, HF hospitalizations, CV hospitalizations, or a composite of HF hospitalizations or CV mortality.
Two independent authors (PM and PN) independently screened studies and employed inclusion criteria. Any disagreement between the two authors was resolved by involving a third author (AT).
2.2. Data extraction
Multiple authors extracted data with oversight of the process by an author (PM). Data were extracted for the baseline trial and the patient characteristics and for the outcomes: all-cause mortality, CV mortality, HF hospitalizations, CV hospitalizations, and a composite endpoint of HF hospitalizations or CV mortality.
2.3. Statistical analyses
All statistical analyses were performed using the Review Manager (Rev-Man) Version 5.4 (The Cochrane Collaboration, 2020). A random-effects model was used. The results were presented as odds ratios (ORs) with 95 % confidence intervals (CIs) and forest plots were reported.
p-value<0.05 was considered statistically significant. Heterogeneity was assessed from I statistics. I2 > 50 % with a p-value <0.05 is considered significant to further analyzed the cause of heterogeneity. The risks of bias were assessed with Cochrane risk-of-bias tool for randomized trials (RoB 2). The qualities of included studies and the certainty of evidence were rated using the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) approach. The summary of findings figure was created using GRADEpro GDT: GRADEpro Guideline Development Tool [Software]. (McMaster University and Evidence Prime, 2021).
3. Results
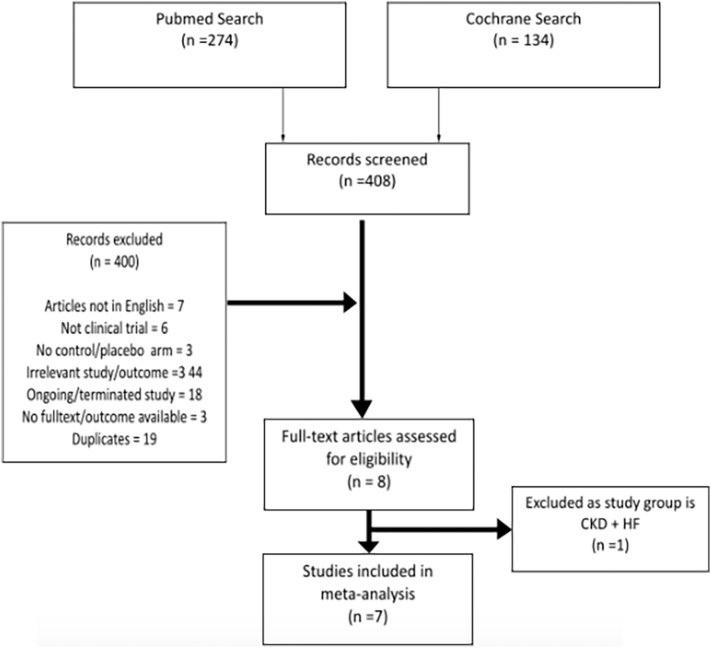
A total of 408 studies were identified from the initial literature search. After screening, 8 full-text articles were assessed for eligibility. The study by Toblli et al. [21] was excluded as it was done exclusively in patients with heart failure and chronic kidney disease and potentially leading to the inconsistency of the results. A total of 7 studies were included in the meta-analysis. The results of our literature search are outlined in the PRISMA flow diagram. Fig. 1 [22].
Fig. 1.
PRISMA Flow Diagram
Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 6(6): e1000097. doi:https://doi.org/10.1371/journal.pmed1000097
For more information, visit www.prisma-statement.org.
Baseline characteristics of the included studies and the patients are summarized in Table 1, Table 2. IRON-HF and FERRIC-HF [12], [14] studied IV iron sucrose but the others IV FCM [9], [10], [11], [13], [15]. Most patients were white [9], [10], [11], [14]. The most frequently used iron deficiency definition was ferritin<100 μg/L (or) 100–299 μg/L with TSAT <20 % [9], [10], [11], [12], [13], [14], [15]. The risk of bias in RCTs was not serious for the outcomes assessed.
Table 1.
Characteristics of the clinical studies included in the analyses.
| Trial Name | AFFIRM-AHF [9] | CONFRIM-HF [10] | IRON-HF [12] | FERRIC-HF [14] | EFFECT-HF [13] | FAIR-HF [11] | PRACTICE-ASIA-HF [15] |
|---|---|---|---|---|---|---|---|
| Year of Publication | 2020 | 2015 | 2013 | 2008 | 2017 | 2009 | 2018 |
| First Author | Ponikowski et al | Ponikowski et al | Luís Beck-da-Silva et al | Okonko et al | van Veldhuisen DJ et al | Anker et al | Yeo TJ et al |
| Country | 121 sites (3 countries) | 41 sites (9 countries) | Brazil, multi-center | UK and Poland | 28 sites (9 countries) | 75 sites (11 countries) | Singapore (2 centers) |
| Study Type | RCT | RCT | RCT | RCT | RCT | RCT | RCT |
| Blinding Status | Double-blind | Double-blind | Double-blind | Open-label, observer-blinded | Open-label, blinded end point | Double-blind | No label but transparent tube, blinded endpoint |
| IV Iron Type | FCM | FCM | Iron sucrose | Iron sucrose | FCM | FCM | FCM |
| Mean IV Iron Dose (mg) | 1352 | 1500 | 1000 | 928 | 1204 | N/A | 1000 |
| Iron Deficiency Definition | ferritin<100 μg/L (or) 100–299 μg/L with TSAT <20 % | ferritin<100 μg/L (or) 100–299 μg/L with TSAT <20 % | ferritin <500 μg/L and TSAT <20 % (Inclusion criteria) | ferritin<100 μg/L (or) 100–299 μg/L with TSAT <20 % | ferritin<100 μg/L (or) 100–300 μg/L with TSAT <20 % | ferritin<100 μg/L (or) 100–300 μg/L with TSAT <20 % | ferritin<100 μg/L (or) 100–299 μg/L with TSAT <20 % |
| Inpatient/outpatient, EF inclusion criteria | Inpatient, LVEF <50 % | Ambulatory, LVEF ≤45 % | Ambulatory, LVEF<40 % | Ambulatory, LVEF ≤45 % | Ambulatory, LVEF ≤45 % | Ambulatory, LVEF ≤40 % for NYHA Class II & LVEF ≤45 % for NYHA Class III | Inpatient, any LVEF |
| Duration of Follow-up | Up to 52 weeks | 52 weeks | 3 months | Up to 18 weeks | Up to 24 weeks | Up to 24 weeks | 12 weeks |
Abbreviations: RCT - Randomized Controlled Trial, FCM - ferric carboxymaltose, TSAT - transferrin saturation, LVEF - left ventricular ejection fraction, NYHA - New York Heart Association, N/A-Not available.
Table 2.
Patient characteristics of the studies included in the analyses.
| Trial Name | AFFIRM-AHF [9] | CONFRIM-HF [10] | IRON-HF [12] | FERRIC-HF [14] | EFFECT-HF [13] | FAIR-HF [11] | PRACTICE-ASIA-HF [15] |
|---|---|---|---|---|---|---|---|
| No. of Patients (Iron therapy/ Placebo) | 558/ 550 | 150/151 | 10/ 6 | 24/ 11 | 86/ 86 | 304/ 155 | 24/ 25 |
| Mean Age (Yr) (IT/P) | 71.2/ 70.9 | 68.8/ 69.5 | 66.9/ 68.9 | 64/ 62 | 63/ 64 | 67.8/ 67.4 | 61.1/ 64 |
| Women (%) (IT/P) | 44/ 45 | 45/ 49 | 30/ 33.3 | 29/ 27 | 30/ 20 | 52.3/ 54.8 | 25/ 20 |
| White Race (%) (IT/P) | 95/ 95 | 99/ 99 | N/A | 88/ 91 | N/A | 99.7/ 100 | 0/ 0 |
| Ischemic HF (%) (IT/P) | 47/ 47 | 83/ 83 | N/A | N/A | N/A | 80.6/ 79.4 | N/A |
| Anemia (%) (IT/P) | 52/ 57 | N/A | 100/ 100 | 50/ 55 | N/A | N/A | N/A |
| Mean Hb (g/dL) (IT/P) | 12.3/ 12.1 | 12.4/ 12.4 | 11.2/ 10.9 | 12.6/ 12.2 | 12.9/ 13 | 11.9/ 11.9 | 11.6/ 13.1 |
| Ferritin (ng/mL) (IT/P) | 83.9/ 88.5 | 57/ 57.1 | 185/ 95 | 62/ 88 | 48/ 53a | 52.5/ 60.1 | 91.4/ 84.1 |
| Mean TSAT (%) (IT/P) | 15.2/ 14.2 | 20.2/ 18.2 | 18.9/ 13.5 | 20/ 21 | 17.3/ 18.1a | 17.7/ 16.7 | 15.7/ 13.9 |
| Mean LVEF (%) (IT/P) | 32.6/ 32.7 | 37.1/ 36.5 | 25.2/ 30.7 | 30/ 29 | 33/ 31 | 31.9/ 33 | 38.8/ 33.2 |
| Premature Treatment Discontinuation (n) (IT/P) | 157/ 160 | 14/ 19 | N/A | 4/ 1 | 3/ 1 | 16/ 14 | N/A |
Abbreviations: TSAT - transferrin saturation, LVEF - left ventricular ejection fraction, Hb - hemoglobin, Yr - year, n - number, IT/P - Iron therapy/ placebo,
N/A - Not available.
AFFIRM-AHF [9]: A Randomized, Double-blind Placebo-Controlled Trial Comparing the Effect of Intravenous Ferric Carboxymaltose on Hospitalisations and Mortality in Iron Deficient Subjects Admitted for Acute Heart Failure, CONFIRM-HF [10]: Ferric CarboxymaltOse evaluatioN on perFormance in patients with IRon deficiency in coMbination with chronic heart failure, IRON-HF [12]: A randomized trial to assess the effects of iron in heart failure patients with anemia, FERRIC-HF [14]: Intravenous iron sucrose in anemic and non-anemic iron deficient patients with CHF, EFFECT-HF [13]: Effect of Ferric Carboxymaltose on Exercise Capacity in Patients With Chronic Heart Failure and Iron Deficiency, FAIR-HF [11]: Ferinject Assessment in Patients with Iron Deficiency and Chronic Heart Failure, PRACTICE-ASIA-HF [15]: Single-dose intravenous iron in Southeast Asian heart failure patients: A pilot randomized placebo-controlled study.
Median values.
3.1. Functional capacity assessments
Of the seven studies included for analysis, 6 studies assessed improvement in patients' functional capacity, summarized in Table 3. Most studies showed improvement in NHYA class, pVO2, and quality of life assessment scores such as Patient Global Assessment (PGA) and Kanas City Cardiomyopathy Questionnaire (KCCQ) [10], [11], [13], [14], [15]. However, due to significant variations in the types, methods, and timings of the assessments between the studies, quantitative analyses were not done for functional capacity and quality-of-life assessments.
Table 3.
Summary of functional capacity assessments.
| Trial Name | Methods to Assess Patient's functional capacity | Results |
|---|---|---|
| AFFIRM-AHF [9] | No functional capacity assessment. | Not applicable. |
| CONFIRM-HF [10] | - Change in 6MWT distance at 24 weeks - Changes in NYHA class, PGA, health-related QoL Fatigue score at 6, 12, 24, 36, and 52 weeks |
- Significantly prolonged 6MWT distance with FCM compared with placebo - Statistically significant improvement in NYHA class, PGA, QoL, and Fatigue Score in patients treated with FCM compared with placebo from Week 24 onwards. |
| IRON-HF [12] | Variation of peak oxygen consumption (pVO2) over 3-month follow-up | No statistically significant increment in pVO2 between the study groups. |
| FERRIC-HF [14] | - Change in absolute VO2, pVO2, NYHA class, PGA, MLFHQ score, fatigue score at week 18 | - No significant Improvement in absolute VO2 and MLHFQ score with IV iron versus placebo - Significant improvement in pVO2, NYHA class, PGA and fatigue score with IV iron compared with placebo |
| EFFECT-HF [13] | - Change in pVO2 from baseline to 24 weeks - Improvement in NHYA class and PGA at 6,12 and 24 weeks |
- No significant difference in change in pVO2 between IV iron & control at 24 groups - Significant improvement in NYHA class with IV iron versus control at 24 weeks, but not at 6 & 12 weeks - Significant improvement in PGA with IV iron versus control at 12 & 24 weeks but not at 6 weeks |
| FAIR-HF [11] | Change in self-reported PGA & NYHA class, KCCQ, EQ-5D visual analog scale & 6MWT distance at 4,12 & 24 weeks | Significant improvements in self-reported PGA & NYHA class, KCCQ, EQ-5D visual analog scale & 6MWT distance at 4,12 & 24 weeks |
| PRACTICE-ASIA-HF [15] | Change in 6MWT, KCCQ & visual analog scale at 12 weeks | No significant difference in 6MWT, KCCQ & visual analog scale between IV iron at 12 weeks |
Abbreviations: 6MWT- 6-min walk test, NYHA- New York Heart Association, PGA- Patient Global Assessment, QoL- Quality of Life, FCM- Ferric Caroxymaltose, pVO2- Peak VO2, MLFHQ score- Minnesota Living with Heart Failure Questionnaire, IV- intravenous, EQ-5D- European Quality of Life-5 Dimension.
3.2. Assessment of heterogeneity for outcome analyses
Of all the outcome assessments, all-cause mortality and CV mortality have low heterogeneity with an I2 of 0 %. For the analyses of HF hospitalizations and a composite outcome of HF hospitalizations or CV mortality, I2 values are >50 % but heterogeneity p-values were not significant. The heterogeneity of CV hospitalizations assessment was high (I2 74%, p = 0.004) and the EFFECT-HF trial [13] was identified as the cause of heterogeneity, but the reason was unclear. So, the study was included in the analysis.
3.3. Outcome analyses
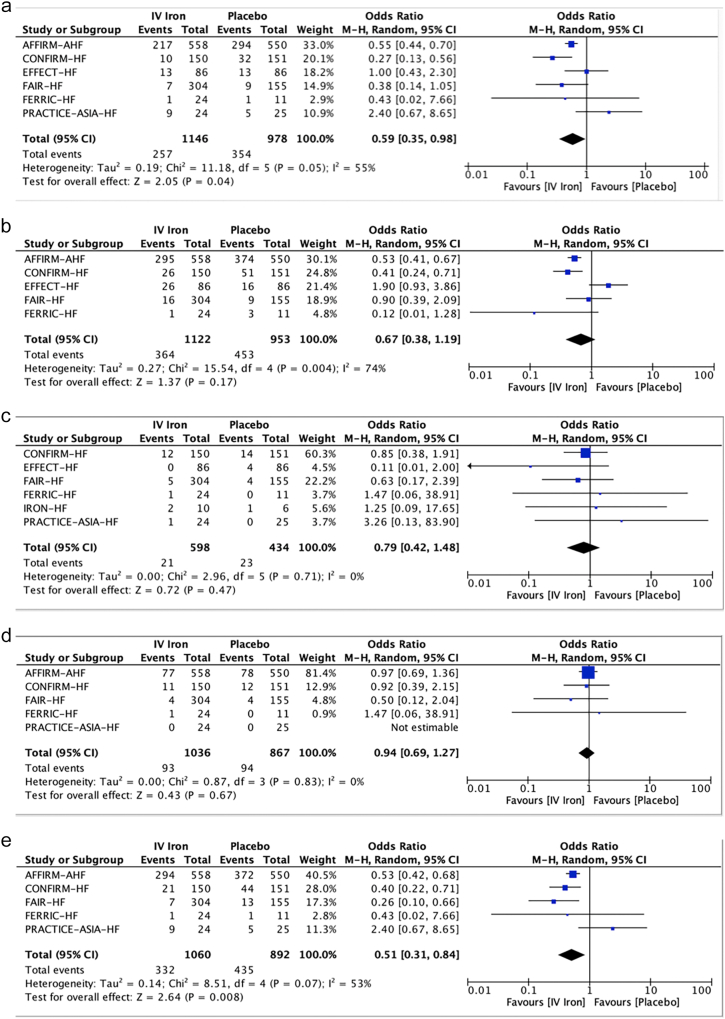
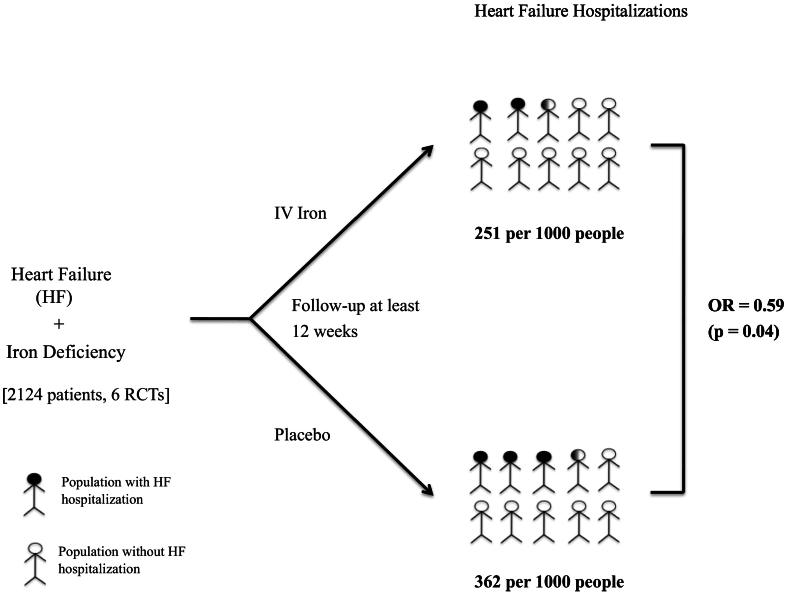
Six studies were included in the analysis of the effect of IV iron versus placebo on HF hospitalization. HF hospitalizations were significantly lower in patients treated with IV iron (22.4 %) compared with placebo (36.2 %) (OR 0.59; 0.35–0.98, p = 0.040, GRADE = Low).
For CV hospitalizations, five studies were included. CV hospitalizations occurred in 32.4 % with IV iron and 47.5 % with placebo (OR 0.67; 0.38–1.19, p = 0.170, GRADE = Very Low).
Six studies were included for all-cause mortality and five studies for CV mortality analyses. All-cause mortality was 3.5 % with IV iron and 5.3 % with placebo (OR 0.79; 0.42–1.48, p = 0.47, GRADE = low). CV mortality was 9.0 % with IV iron versus 10.8 % with placebo (OR 0.94;0 0.69–1.27, p = 0.670, GRADE = Low).
Based on the analysis of five studies, the composite outcome of HF hospitalizations or CV mortality is significantly lower in patients treated with IV iron (31.3 %) compared with placebo (48.8 %) (OR 0.51; 0.31–0.84, p = 0.008, GRADE = Moderate).
Forest plots of the analyses were shown in Fig. 2a, b, c, d, e, the Summary of findings in Fig. 3 and Central illustration in Fig. 4.
Fig. 2.
Forest Plots of the Meta-analyses of
(a). Heart Failure Hospitalizations
(b). CV Hospitalizations
(c). All-cause Mortality
(d). CV Mortality
(e). Composite of HF Hospitalizations or CV Mortality
IV-intravenous, CI-confidence interval.
Fig. 3.
Summary of Findings of the Meta-analyses
IV-intravenous, HF-heart failure, CV-cardiovascular,
GRADE- Grading of Recommendations, Assessment, Development, and Evaluation.
Fig. 4.
Central Illustration: Effect of IV Iron on HF Hospitalizations in Heart Failure Patients with Iron Deficiency
IV-intravenous, HF-heart failure, RCTs-Randomized controlled trials, OR-Odds ratio, p-p-value.
4. Discussion
Despite advances in treatments including device therapies, heart failure remains a major public health problem and healthcare burden associated with significant hospitalizations, admission rates, mortality, and costly healthcare expenditure [23], [24], [25], [26]. Most recent data suggested that around 6 million Americans were diagnosed with heart failure, and it is estimated that >8 million Americans may be living with heart failure by 2030 [24], [27]. Based on the data from 2014, the estimated cost of primary heart failure hospitalization was $11,552 per individual, with a total estimated cost of >11 billion $ [28].
Studies not only suggest that iron deficiency is associated with reduced quality of life and exercise capacity in heart failure patients, but also have shown that IV iron therapy improves 6MWT and quality of life [10], [11], [12], [29]. However, there was no large study that showed a significant reduction in HF admissions until AFFIRM-AHF [9].
Our meta-analysis findings, based on a total study population of 2124 from 6 RCTs, proved that there was a statistically significant reduction in HF hospitalization rate in IV iron arm compared to the placebo arm despite there being no statistically significant reduction in total CV hospitalizations. The potential cause of statistical insignificance in total CV hospitalizations is the inclusion of the EFFECT-HF study. Though there is an unclear reason for high heterogeneity with the EFFECT-HF study, one could argue that there may be other potential significant baseline characteristic differences in the study [13]. Our data suggest that IV iron therapy does not have the CV and total mortality benefit effect.
We analyzed only HF hospitalizations and mortality but did not conduct the analysis of the pooled data for quality-of-life scoring, 6MWT, and NYHA since not all the included studies assessed the functional status and the scoring methods varied. AFFIRM-AHF data suggested that treatment with IV iron reduced the risk of HF hospitalization with no significant effect on the risk of CV mortality [9]. CONFIRM-HF results showed treatment with IV iron showed improved QoL, 6MWT, and NYHA class at 52 weeks as well as decreased heart failure hospitalization [10]. FAIR-HF concluded that treatment with IV iron improved symptoms, functional capacity, and quality but had no significant effect on HF hospitalization and CV mortality. EFFECT-HF trial suggested that treatment with IV iron improved peak VO2 at 24 weeks but had no significant effect on HF hospitalization and CV mortality. However, HF hospitalization and CV mortality in both Fair-HF and EFFECT-HF were only assessed as a safety endpoint but not as a primary or secondary endpoint [11], [13]. In FERRIC-HF study, IV iron improved NYHA class, and peak VO2 level and had no effect on HF hospitalization and CV mortality. But it is a small study, and HF hospitalization and CV mortality were not evaluated as the primary outcomes [14]. PRACTICE-ASIA-HF showed that IV iron therapy has no significant effect on 6MWT, QoL, and HF admission at 12 weeks among a total of 50 subjects with 25 in each arm which is again a small and underpowered study.
There were a handful of prior meta-analyses conducted for IV iron in HF [16], [17], [18], [30]. Compared to most prior meta-analyses except Graham et al. [30], our study highlights a statistically significant reduction of HF hospitalization with a certainty assessment. Unlike Graham et al., our study did not include Toblli et al. since it was done exclusively in HF patients with CKD which could lead to inconsistency in results and the data may not be applicable to the general HF population (serious indirectness). In contrast to Graham et al., we conducted an analysis for all-cause mortality which however did not show any significant difference [17], [21]. In contrast to Yamani et al., our study included EFFECT-HF and PRACTICE-ASIA-HF studies to give a more comprehensive data review and excluded Toblli et al. given the reason mentioned above [18].
The clinical trials included in our meta-analyses used either IV iron sucrose (IRON-HF & FERRIC-HF) [12], [14] or IV FCM [9], [10], [11], [13], [15]. There are studies comparing different IV iron formulations: IV iron sucrose versus IV FCM [31], [32] with varying results in outcomes such as hemoglobin, and IV iron isomaltoside versus IV iron sucrose [33], [34] showing potential higher efficacy and convenience of iron isomaltose over iron sucrose. However, these studies are not specific to iron-deficient heart failure patients so further studies are warranted before we favor one IV iron formulation over another.
Again, the exact mechanism of intravenous iron therapy improves functional status and clinical outcomes in heart failure patients remain unclear [29]. But studies have proved that oral iron preparation was not an effective way to manage ID in HF due to pathophysiological considerations (i.e., overactivation of inflammatory mediator which interferes with iron absorption and transport) and there was some evidence of bowel well thickness and edema due to congestion and chronic inflammatory state in HF patients which could theoretically interfere with the iron absorption [35]. In clinical trials studying the effect of oral iron in heart failure patients (IRON-HF and IRONOUT-HF), oral iron did not show significant improvement in peak VO2 (venous oxygen concentration), 6-min walk test distance, or KCCQ score (Kansas City Cardiomyopathy Questionnaire) compared with placebo [36], [12].
2022 ACC/AHA/HFSA guidelines provided a Class IIA recommendation for IV iron replacement in iron-deficient heart failure patients with reduced ejection fraction to improve functional status and QoL [37]. Most studies in our meta-analysis used EF ≤ 45 % or < 40 % as the inclusion criteria [10], [11], [12], [13], [14] except the AFFIRM-HF trial which included patients <50 % [9] and the PRACTICE-ASIA-HF trial which included patients with any EF [15]. Currently, there is limited data for IV iron use in heart failure with preserved ejection fraction.
5. Limitations
Our meta-analysis has a handful of limitations. First, most patients in the included studies, except PRACTICE-ASIS-HF, are white despite there being a significant number of HF patients of other ethnicities, especially African Americans [9], [10], [11], [12], [13], [14], [15]. Therefore, our meta-analysis data may not be generalizable to the global HF population. There are also a couple of reasons which could have contributed to heterogeneity in our study. One is that there is no exact data for the reason for total hospital admission and mortality. Another reason is the patients' baseline characteristics could vary especially social status and medication adherence. The mortality results were mostly determined by the well-powered AFFIRM-AHF trial, other included trials are smaller, and some only evaluated mortality as a safety endpoint but not as a primary or secondary endpoint. Last but not the least, the data for the exact IV iron doses were not available for all studies except AFFIRM-AHF and FERRIC-HF. Therefore, the effectiveness of the potential underdosing of IV iron in some studies may have altered the outcomes including HF admissions, long-term mortality, and functional capacity.
6. Conclusion
IV iron is a relatively inexpensive regimen, and its cost-effectiveness outweighs readmission costs. Our meta-analysis proved that IV iron supplementation in iron-deficient heart failure patients decreases HF admission. Ongoing clinical trials with quality-of-life assessments, CV mortality and all-cause mortality endpoints are yet to be completed on the efficacy of IV iron in iron-deficient heart failure patients with reduced as well as preserved ejection fraction.
CRediT authorship contribution statement
Phyo Thazin Myint: Conceptualization, Methodology, Investigation, Formal analysis, Writing – original draft, Writing – review & editing. Phoo Pwint Nandar: Conceptualization, Investigation, Writing – original draft. Aye M. Thet: Investigation, Writing – original draft. Gabriela Orasanu: Supervision.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This research has not received any external funding. So, there is no funding body that we could acknowledge.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ahjo.2022.100204.
Appendix A. Supplementary data
Supplementary material (Search Terms for Literature Search).
References
- 1.Klip I.T., Comin-Colet J., Voors A.A., et al. Iron deficiency in chronic heart failure: an international pooled analysis. Am. Heart J. Apr 2013;165(4):575–582 e3. doi: 10.1016/j.ahj.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 2.Jankowska E.A., Rozentryt P., Witkowska A., et al. Iron deficiency: an ominous sign in patients with systolic chronic heart failure. Eur. Heart J. Aug 2010;31(15):1872–1880. doi: 10.1093/eurheartj/ehq158. [DOI] [PubMed] [Google Scholar]
- 3.Okonko D.O., Mandal A.K., Missouris C.G., Poole-Wilson P.A. Disordered iron homeostasis in chronic heart failure: prevalence, predictors, and relation to anemia, exercise capacity, and survival. J. Am. Coll. Cardiol. 2011;58(12):1241–1251. doi: 10.1016/j.jacc.2011.04.040. [DOI] [PubMed] [Google Scholar]
- 4.Dziegala M., Josiak K., Kasztura M., et al. Iron deficiency as energetic insult to skeletal muscle in chronic diseases. J. Cachexia. Sarcopenia Muscle. Oct 2018;9(5):802–815. doi: 10.1002/jcsm.12314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ebner N., Jankowska E.A., Ponikowski P., et al. The impact of iron deficiency and anaemia on exercise capacity and outcomes in patients with chronic heart failure. Results from the studies investigating co-morbidities aggravating heart failure. Int. J. Cardiol. 2016;205:6–12. doi: 10.1016/j.ijcard.2015.11.178. [DOI] [PubMed] [Google Scholar]
- 6.Jankowska E.A., Rozentryt P., Witkowska A., et al. Iron deficiency predicts impaired exercise capacity in patients with systolic chronic heart failure. J. Card. Fail. Nov 2011;17(11):899–906. doi: 10.1016/j.cardfail.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 7.Anand I.S., Gupta P. Anemia and iron deficiency in heart failure: current concepts and emerging therapies. Circulation. 2018;138(1):80–98. doi: 10.1161/CIRCULATIONAHA.118.030099. [DOI] [PubMed] [Google Scholar]
- 8.Lopez A., Cacoub P., Macdougall I.C., Peyrin-Biroulet L. Iron deficiency anaemia. Lancet. 2016;387(10021):907–916. doi: 10.1016/S0140-6736(15)60865-0. [DOI] [PubMed] [Google Scholar]
- 9.Ponikowski P., Kirwan B.A., Anker S.D., et al. Ferric carboxymaltose for iron deficiency at discharge after acute heart failure: a multicentre, double-blind, randomised, controlled trial. Lancet. 2020;396(10266):1895–1904. doi: 10.1016/S0140-6736(20)32339-4. [DOI] [PubMed] [Google Scholar]
- 10.Ponikowski P., van Veldhuisen D.J., Comin-Colet J., et al. Beneficial effects of long-term intravenous iron therapy with ferric carboxymaltose in patients with symptomatic heart failure and iron deficiencydagger. Eur. Heart J. 2015;36(11):657–668. doi: 10.1093/eurheartj/ehu385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anker S.D., Comin Colet J., Filippatos G., et al. Ferric carboxymaltose in patients with heart failure and iron deficiency. N. Engl. J. Med. 2009;361(25):2436–2448. doi: 10.1056/NEJMoa0908355. [DOI] [PubMed] [Google Scholar]
- 12.Beck-da-Silva L., Piardi D., Soder S., et al. IRON-HF study: a randomized trial to assess the effects of iron in heart failure patients with anemia. Int. J. Cardiol. 2013;168(4):3439–3442. doi: 10.1016/j.ijcard.2013.04.181. [DOI] [PubMed] [Google Scholar]
- 13.van Veldhuisen D.J., Ponikowski P., van der Meer P., et al. Effect of ferric carboxymaltose on exercise capacity in patients with chronic heart failure and iron deficiency. Circulation. 2017;136(15):1374–1383. doi: 10.1161/CIRCULATIONAHA.117.027497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okonko D.O., Grzeslo A., Witkowski T., et al. Effect of intravenous iron sucrose on exercise tolerance in anemic and nonanemic patients with symptomatic chronic heart failure and iron deficiency FERRIC-HF: a randomized, controlled, observer-blinded trial. J. Am. Coll. Cardiol. 2008;51(2):103–112. doi: 10.1016/j.jacc.2007.09.036. [DOI] [PubMed] [Google Scholar]
- 15.Yeo T.J., Yeo P.S.D., Hadi F.A., et al. Single-dose intravenous iron in Southeast Asian heart failure patients: a pilot randomized placebo-controlled study (PRACTICE-ASIA-HF) ESC Heart Fail. Apr 2018;5(2):344–353. doi: 10.1002/ehf2.12250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anker S.D., Kirwan B.A., van Veldhuisen D.J., et al. Effects of ferric carboxymaltose on hospitalisations and mortality rates in iron-deficient heart failure patients: an individual patient data meta-analysis. Eur. J. Heart Fail. Jan 2018;20(1):125–133. doi: 10.1002/ejhf.823. [DOI] [PubMed] [Google Scholar]
- 17.Osman M., Syed M., Balla S., Kheiri B., Faisaluddin M., Bianco C. A meta-analysis of intravenous iron therapy for patients with iron deficiency and heart failure. Am. J. Cardiol. 2021;141:152–153. doi: 10.1016/j.amjcard.2020.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamani N., Ahmed A., Gosain P., et al. Effect of iron supplementation in patients with heart failure and iron deficiency: a systematic review and meta-analysis. Int. J. Cardiol. Heart Vasc. Oct 2021;36 doi: 10.1016/j.ijcha.2021.100871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.L Eden L.L., Berg A., et al. Finding What Works in Health Care: Standards for Systematic Reviews: 2011. 2011. pp. 45–234. [PubMed] [Google Scholar]
- 20.Page M.J., McKenzie J.E., Bossuyt P.M., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. PLoS Med. Mar 2021;18(3) doi: 10.1371/journal.pmed.1003583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Toblli J.E., Lombrana A., Duarte P., Di Gennaro F. Intravenous iron reduces NT-pro-brain natriuretic peptide in anemic patients with chronic heart failure and renal insufficiency. J. Am. Coll. Cardiol. 2007;50(17):1657–1665. doi: 10.1016/j.jacc.2007.07.029. [DOI] [PubMed] [Google Scholar]
- 22.Moher D., Liberati A., Tetzlaff J., Altman D.G., Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. Jul 21 2009;6(7) doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Go Alan S., Roger Veronique L., Bravata Dwan M., Dai Shifan. Heart disease and stroke statistics—2013 update a report from the American Heart Association. Circulation. 2012;2013(127):e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heidenreich P.A., Trogdon J.G., Khavjou O.A., et al. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation. 2011;123(8):933–944. doi: 10.1161/CIR.0b013e31820a55f5. [DOI] [PubMed] [Google Scholar]
- 25.Roger V.L. Epidemiology of heart failure. Circ. Res. 2013;113(6):646–659. doi: 10.1161/CIRCRESAHA.113.300268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roger V.L. Epidemiology of heart failure: a contemporary perspective. Circ. Res. 2021;128(10):1421–1434. doi: 10.1161/CIRCRESAHA.121.318172. [DOI] [PubMed] [Google Scholar]
- 27.Mozaffarian D., Benjamin E.J., Go A.S., et al. Heart disease and stroke statistics–2015 update: a report from the American Heart Association. Circulation. 2015;131(4):e29–e322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 28.Jackson S.L., Tong X., King R.J., Loustalot F., Hong Y., Ritchey M.D. National burden of heart failure events in the United States, 2006 to 2014. Circ. Heart Fail. Dec 2018;11(12) doi: 10.1161/CIRCHEARTFAILURE.117.004873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.von Haehling S., Ebner N., Evertz R., Ponikowski P., Anker S.D. Iron deficiency in heart failure: an overview. JACC Heart Fail. Jan 2019;7(1):36–46. doi: 10.1016/j.jchf.2018.07.015. [DOI] [PubMed] [Google Scholar]
- 30.Graham F.J., Pellicori P., Ford I., Petrie M.C., Kalra P.R., Cleland J.G.F. Intravenous iron for heart failure with evidence of iron deficiency: a meta-analysis of randomised trials. Clin. Res. Cardiol. Aug 2021;110(8):1299–1307. doi: 10.1007/s00392-021-01837-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Basha A., Ibrahim M.I.M., Hamad A., et al. Efficacy and cost effectiveness of intravenous ferric carboxymaltose versus iron sucrose in adult patients with iron deficiency anaemia. PLoS One. 2021;16(8) doi: 10.1371/journal.pone.0255104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Naqash A., Ara R., Bader G.N. Effectiveness and safety of ferric carboxymaltose compared to iron sucrose in women with iron deficiency anemia: phase IV clinical trials. BMC Womens Health. Jan 5 2018;18(1):6. doi: 10.1186/s12905-017-0506-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Auerbach M., Henry D., Derman R.J., Achebe M.M., Thomsen L.L., Glaspy J. A prospective, multi-center, randomized comparison of iron isomaltoside 1000 versus iron sucrose in patients with iron deficiency anemia; the FERWON-IDA trial. Am. J. Hematol. Sep 2019;94(9):1007–1014. doi: 10.1002/ajh.25564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Derman R., Roman E., Modiano M.R., Achebe M.M., Thomsen L.L., Auerbach M. A randomized trial of iron isomaltoside versus iron sucrose in patients with iron deficiency anemia. Am. J. Hematol. Mar 2017;92(3):286–291. doi: 10.1002/ajh.24633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anker S.D., von Haehling S. Inflammatory mediators in chronic heart failure: an overview. Heart. Apr 2004;90(4):464–470. doi: 10.1136/hrt.2002.007005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lewis G.D., Malhotra R., Hernandez A.F., et al. Effect of oral iron repletion on exercise capacity in patients with heart failure with reduced ejection fraction and iron deficiency: the IRONOUT HF randomized clinical trial. JAMA. 2017;317(19):1958–1966. doi: 10.1001/jama.2017.5427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heidenreich P.A., Bozkurt B., Aguilar D., et al. AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines. Circulation. 2022 doi: 10.1161/CIR.0000000000001063. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material (Search Terms for Literature Search).